Abstract
The global spread of antimicrobial-resistant infectious diseases and cancer are the most widespread public health issue and has led to high mortality rates. This study aims to evaluate and verify the antibacterial and antitumor activities of Shaoka and Manuka honey against pathogenic bacteria, human hepatocarcinoma (HepG2) and breast cancer (MCF-7) cell lines. Shaoka hone was analyzed using HPLC, UV–vis, and GC/MC, while antibacterial activity was measured by agar diffusion, broth microdilution methods, and Transmission Electron Microscopy (TEM). Antitumor activity was investigated morphologically and by MTT assay. According to the presented data of HPLC analysis, Shaoka honey was generally richer in polyphenolic components, the antibacterial activity showed that Shaoka honey is equivalent or relatively more active than Manuka honey against a broad spectrum of multi-drug-resistant bacteria. It inhibited the growth of ESBL Escherichia coli in the absence or presence of catalase enzyme with a concentration approximately 8.5%–7.3% equivalent to phenol, which supported the highest level of non-peroxide-dependent activity. The minimum bactericidal concentrations (MBCs) ranged between 5.0% and 15.0% honey (w/v). TEM observation revealed distorted cell morphology, cytoplasmic shrinkage, and cell wall destruction of treated bacteria. The selected honey exerted cytotoxicity on both cancer cell lines, inhibiting cell proliferation rate and viability percent in HepG2 and MCF-7 cancer cells, by different degrees depending on the honey quality, Shaoka honey competed Manuka inhibitory effects against both cancer cells. The obtained data confirmed the potential for use of Saudi Shaoka honey as a remedy, this well introduces a new honey template as medical-grade honey for treating infectious disease and cancer.
Keywords: Shaoka honey, Manuka honey, Antibacterial, Anticancer, MDR bacteria, Saudi honey
1. Introduction
With the increase of drug-resistant bacteria among pathogens, causing difficulty in treatment regimes, more attention has been given recently to alternative medicine in controlling infectious disease and cancer. Honey is a natural product with nutritive value that can play an important role in human health. It is scientifically proven to prevent and treat many diseases, such as cancers, microbial infections, oxidative stress, diabetes, burns and wounds, infertility, high blood pressure, and immunodeficiency (Oryan et al., 2016, Eteraf-Oskouei and Najafi, 2013). It has been established as a bactericidal drug with broad-spectrum activity against Gram-positive and Gram-negative bacterial species (Yaacob et al., 2020, Almasaudi et al., 2017).
Generally, honey is very rich in antioxidants such as phenolic and flavonoid compounds (Cianciosi et al., 2018, Jibril et al., 2019). Shaoka honey is derived from the Shaoka tree (Fagonia bruguieri), a small montane tree from the family Zygophyllaceae that grows native to Saudi Arabia and is classified as having medicinal plant value (Al-Sodany et al., 2013, Shawky and Alzamel, 2020). Shaoka honey has a dark color with a special taste and unique aromatic scent. In traditional medicine, Saudi citizens often used Shaoka honeys for wound healing, gastrointestinal distress, ulcers, anemia, cancers, burns, hepatitis, and pulmonary disease. (Adgaba et al., 2017, Hosny et al., 2010).
Multiple factors have been shown to contribute to honey’s anticancer and antibacterial activities. The first factor is its high sugar concentration, which exerts osmotic pressure on bacterial cells, causing shrinkage and stopping the growth of many microbes (Albaridi, 2019). The second factor is the acidity of honey due to the presence of organic acids, especially gluconic acid (Cavia et al., 2007, Majewska et al., 2019). Hydrogen peroxide (), the third important factor of antibacterial activity of honey, acts as an oxidizing and sanitizing agent, killing bacteria. (Brudzynski et al., 2011, Poli et al., 2018). The fourth factor is the presence of phytochemical compounds in honey, which act as antibacterial and antioxidant agents (these are referred to as having “non-peroxide activity”). Studies done on different types of honey have shown that honey has high levels of phenolic acid and flavonoids, and has been marketed as a good alternative therapeutic drug (Combarros-Fuertes et al., 2019, Halagarda et al., 2020). The polyphenol level in honey can be affected by its botanical origin, nectar, and geographical region, (Alqarni et al., 2016, Velásquez et al., 2019). From New Zealand, the superior Manuka honey has phenolic acid content ranging between 430 and 2709 mg/kg and Kanuka honey 424–1575 mg/kg (Girma et al., 2019). The fifth factor is the presence of antimicrobial peptides from bee saliva, such as defensin-1, which is found in honey that has antibacterial activity (Valachova et al., 2016, Bucekova et al., 2017). The sixth factor, which has been demonstrated to enhance anticancer and antibacterial activity, is that honey at low concentration (1%) can stimulate monocytes in cell culture to release cytokines, tumor necrosis factor (TNF) alpha, and interleukins 1L-1 and 1L-6, which are responsible for activating the immune response to cancer and infection (Zohair et al., 2015, Tonks et al., 2003).
The outline of cancer chemoprevention is the employment of natural, artificial, or biological chemical agents to prevent or suppress carcinogenic progression. Many studies support that honey has the potency to prevent, inhibit, or reverse the development of cancer at different stages, so it can be called a cancer chemopreventive agent (Erejuwa et al., 2014, Badolato et al., 2017, Afrin et al., 2018). A large amount of data has been made available on different kinds of honey from around the world and their bioactive effects, although some local honey in different areas have not yet been evaluated.
There are untapped resources of medical-grade honey in Saudi Arabia. Therefore, this study was undertaken as the first evaluation of Saudi Shaoka honey (Fagonia bruguieri DC), the study aims to evaluate the:(i) chemical and bioactive components, (ii) antibacterial, and (iii) anticancer activities of Shaoka honey in comparison to Manuka honey, to determine the key factors of Saudi Shaoka honey that could make it a new kind of medical-grade honey.
2. Material and methods
2.1. Honey samples
Twelve Shaoka honey samples were purchased from local markets in various locations in the Western region of Saudi Arabia. Two samples of Manuka honey (Leptospermum scoparium) with Unique Manuka Factor (UMF10+) were purchased from Comvita Factory in New Zealand-Australia (Comvita Ltd., New Zealand). All samples were stored at (−25 ± 2 °C) until further analysis (El Sohaimy et al., 2015).
2.2. Chemical analysis of honey
For evaluating the main components in Shaoka honey, techniques were used as described by Sereia et al., (2017) The analysis was done at the analytical chemistry unit (ACAL), Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt. Chemical analyses of honey include:
2.2.1. Detection of total carbohydrate, protein, and polyphenol
Using a UV–vis Lambda 2 spectrophotometer (Perkin-Elmer, UK), total carbohydrates, proteins, and polyphenols were detected to differentiate honey types and quality.
2.2.2. Detection of amino acids by high-performance liquid chromatography (HPLC)
Separation and quantification of main amino acids were done using a chromatographic system 1260 (Agilent Technologies, CA, USA), which consisted of a 9012Q pump, 9100 auto-injectors, and 9075 fluorescence detector.
2.2.3. Detection of volatile compounds in Shaoka honey by gas chromatography-mass spectrometry (GC-MS)
Analysis of honey was performed by dissolving samples with phosphoric acid (1:1), followed by hexane sonication for 10 min to detect their floral markers and volatile organic compounds (VOCs). The organic layer was injected into an Agilent GC-MS model 6890 (Agilent Technologies, CA, USA).
2.2.4. Analysis of phenolic compounds by HPLC
HPLC analysis was carried out to measure the polyphenolic content of Shaoka and Manuka honey by an Agilent 1260 Infinity Quaternary LC, USA. The separation was executed using an Eclipse plus c18 column (4.6 mm × 250 mm i.d., 5 μm). The movable phase is composed of water (A) and 0.02% trifluoroacetic acid in acetonitrile (B) at a flow rate of 1 mL/min. This mobile stage was programmed consecutively in a linear gradient as follows: 0 min (80% A); 0–5 min (80% A); 5–8 min (40% A); 8–12 min (50% A); 12–14 min (80% A), and 14–16 min (80% A). Then, 10 µl of each sample solution was injected into the multi-wavelength detector at 280 nm. The temperature of the column was maintained at 35 °C (Chan et al., 2013). The phenolic compounds were identified by comparing the retention time and UV-spectra with 14 standard phenolic compounds selected for comparison based on their common presence in honey.
2.2.5. Measurement of non -peroxide activity (NPA) of honeys
To measure the non-peroxide activity for two different kinds of honey, 32% (w/v) of the honey solutions were freshly prepared in sterile distilled water, and further diluted to 16%(v/v) with equal volumes of sterile distilled water containing 40 mg/20 mL catalase solution (4000 units/ mg, Sigma- Canada) and kept for 2 h at room temperature to remove hydrogen peroxide activity as described by Allen, et al. (1991), and then applied to wells to compare the percentage of phenol content. The proportion of peroxide and non-peroxide activity in Shaoka and Manuka were calculated by equivalent percent phenols against tested bacteria.
2.3. Antibacterial activity of honey
2.3.1. Test bacteria
Eight clinical isolates of multi-drug-resistant bacteria were used for the antibacterial assay, including Mycobacterium tuberculosis, methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus mutans, Streptococcus pneumoniae, Helicobacter pylori, Salmonella typhi, Escherichia coli, and Pseudomonas aeruginosa, obtained from stock cultures from the Department of Biology, Science College, Taif University.
2.3.2. Agar well diffusion assay
Prepared water solutions of 16% (w/v) honey samples and phenol (Sigma-Aldrich) standards of 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9% and 10 % (v/v) were prepared according to the method reported by Irish, et al. (2011). β-lactamase-resistant E. coli, at a count of CFU/mL were seeded on Muller-Hinton Agar and used as the test organism for the phenol assays. Phenol and samples of both kinds of honey (50 µl) before and after prepared with catalase, as above, were applied to wells (8 mm in diameter) based on a standard template of quasi-Latin squares to randomize the sample distribution. The mean diameters were calculated after 20 h of incubation at 37 °C. A standard graph was then plotted to calculate the diameter of inhibition zones of phenol percent equivalent to the antibacterial activity of each honey sample.
2.3.3. Determination of minimum inhibitory concentration (MICs) and minimum bactericidal concentration (MBCs) of honey samples
The antibacterial activity of the honey was assessed using a broth microdilution assay. The minimum inhibitory concentration was detected using serially diluted honey samples with nutrient broth (5.0%, 7.5%, 10.0%,12.5%,15.0%, 17.5%, 20.0%, 22.5%, 25.0% and 30.0%). 200 µl of each dilution was placed in a 96-well microplate (Costar, Fisher, Canada). Then, each dilution was seeded with 20 µl of CFU/ml of tested bacteria, the microplate was incubated at 37 °C for 20 h, and the MIC was determined. From each inhibitory concentration observed by turbidity in a spectrophotometer or no visible sign of growth, sub-culturing was done on nutrient agar to determine the minimum bactericidal concentration. Each honey was tested in triplicate for each bacterial strain and the obtained medians were recorded.
2.4. Transmission Electron Microscopy (TEM)
The effect of Shaoka honey on the bacterial structure was investigated as described by Henriques and Jenkins (Henriques et al., 2010) using a CX100-JEM transmission electron microscope-TEM (JEOL, Japan). 100 mL overnight cultures of Escherichia coli in Mueller-Hinton (MH) broth (Oxoid, UK), were centrifuged and suspended in 0.05 mM Tris buffer with and without honey (at approximately 1 × MIC value). Cells were suspended at times 0, 3, and 6 h of incubation at 37 °C for electron microscopy. The specimens were fixed in 2.5% glutaraldehyde and post-fixed in cacodylate buffer, then dehydrated with alcohol, and finally embedded in Araldite resin according to the method of Lemar and Turner (Lemar et al., 2002)
2.5. Anticancer activity of honey
2.5.1. HepG-2 and MCF-7 cell line cultures
Human hepatocellular carcinoma (HepG2) and breast cancer cell (MCF-7) lines were kindly provided by Dr. K. Fekry from the National Research Center in Egypt. Cancer cell lines were purchased originally from ATCC (The American Type Culture Collection). HepG2 and MCF-7 cells were grown in monolayer cultures in DMEM medium (Gibco, Life Technologies, USA) supplemented with 10% pre-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, Life Technologies, USA). Then the cell lines were incubated at 37 °C in 5% CO2 with 95% humidity.
2.5.2. Preparation of honey for anticancer activity
2.5.2.1. Determination of cell viability
Pure Shaoka and Manuka honey were diluted with a DMEM medium to a concentration of 6.25%, 12.5%, 25%, and 50% solution concentrations. The cell viability was determined as the potency of live cells to accept Trypan blue at 0.2% concentration (Szende et al., 2001). The surviving cells were counted in a hemocytometer and the cell survival percentage of treated cells at various concentrations was calculated by the type of honey at different durations.
2.5.2.2. Determination of HepG2 and MCF-7 cell line proliferation by MTT assay
Both types of cancerous cells were seeded in 96-well culture plates (2,000 cells/well) for 24 h, and then supplemented with the honey at tested concentrations. Meanwhile, a group of untreated cells was added to an equal volume of culture medium. For each concentration of selected honey, three replicate wells were set up, and the experiment was repeated three times (Mosmann, 1983). After the cells were incubated for another 48 and 72 h, 20 µl of MTT (Bio Basic INC, Canada) at 5 mg/mL was added to each well and incubated for an additional 4 h. (Badakhshan et al., 2009). The values were measured using a microplate reader at a wavelength of 492 nm.
2.6. Microscopic observation of cell morphology
Either MCF-7 or HepG2 cells were seeded in 50 mL culture flasks at 1 × 106 cells/flask under routinely cultured conditions for 24 h. After the cells were adhesive, the supernatant was discarded, and 1 mL of honey in media, at different concentrations, was added to the media. Meanwhile, an equal volume (1 mL) of the medium was added to a negative control group. After 48 and 72 h, morphological changes of cancer cells were examined under an inverted microscope and photographed.
2.7. Statistical analysis
Comparison between means in antibacterial activity was calculated using ANOVA analysis using mini-Tab software (BIO-TEK Instruments, USA). Statistical analyses for anticancer activity were performed using SPSS (version 11.0). Results were presented as Mean ± SD one-way ANOVA (analysis of variance) followed by multiple Duncan's tests (Walter and Duncan, 1969, Custódio et al., 2009). The results were considered statistically significant when p < 0.05.
3. Results
3.1. Analysis of the components in Shaoka honey
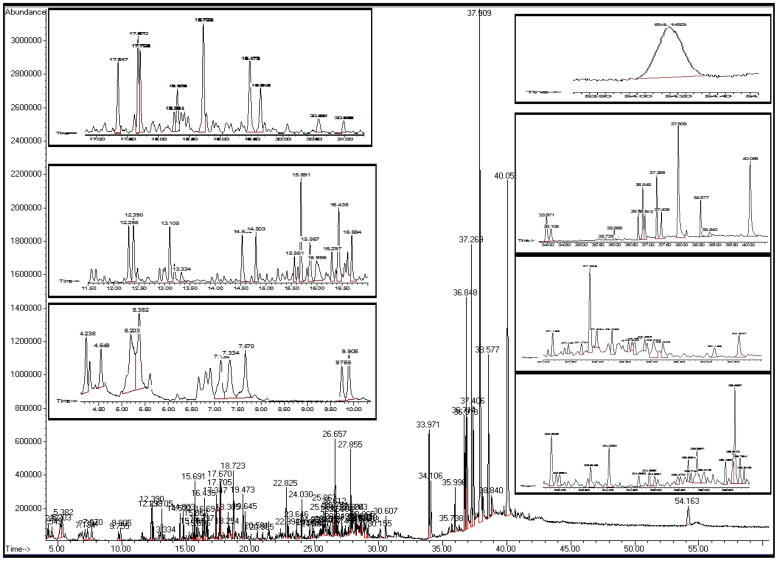
Shaoka honey was analyzed for the first time to detect the total composition of phytochemical constituents. The data obtained from GC-MS, UV/vis, and HPLC are shown in Table 1 and Fig. 1, Fig. 2. The total polyphenolic compounds, carbohydrates, proteins, and amino acids were 273.45, 1016.44, 19526.00, and 344.68 mg/100 g, respectively. Volatile organic compounds as unsaturated fatty acids (esters) were found in concentrations of 31.64 mg/100 g, alkaloids 12.45 mg/100 g, alkane 13.71 mg/100 g, ketones 14.48 mg/100 g, alcohol 9.71 mg/100 g, and terpenes 1.12 mg/100 g. Out of 72 extracted volatile compounds, the highest two values recorded were hexadecenoic acid (2 hydroxy-1-(hydroxymethyl) ethyl) esters, with a concentration 16.008% and retention time (RT) 37.91 min and octadecanoic acid 2,3-dihydroxy propyl ester 12.624% at R.T 40.055 min, as shown in Fig. 1. These volatile components give the dark amber color and aroma to Shoaka honey. These common components in Shaoka honey depend on the nectar contents of Fagonia bruguieri distributed in Arabian regions.
Table 1.
Average composition of common components identified of Shaoka honey samples (mg /100 g).
| Component | Average (%) |
|---|---|
| Total Polyphenol | 273.45 |
| Total protein | 1016.44 |
| Total carbohydrate | 19526.00 |
| Amino acids | 344.68 |
| Alanine | 72.00 |
| Tyrosine | 52.40 |
| Tryptophan | 34.58 |
| Phenylalanine | 185.70 |
| Volatile compounds | 83.11 |
| Esters | 31.64 |
| Ketone | 14.48 |
| Alkane | 13.71 |
| Alkaloids | 12.45 |
| Alcohol | 9.71 |
| Terpene | 1.12 |
Fig. 1.
Total profiles of shaoka honey volatiles obtained by GC-MS.
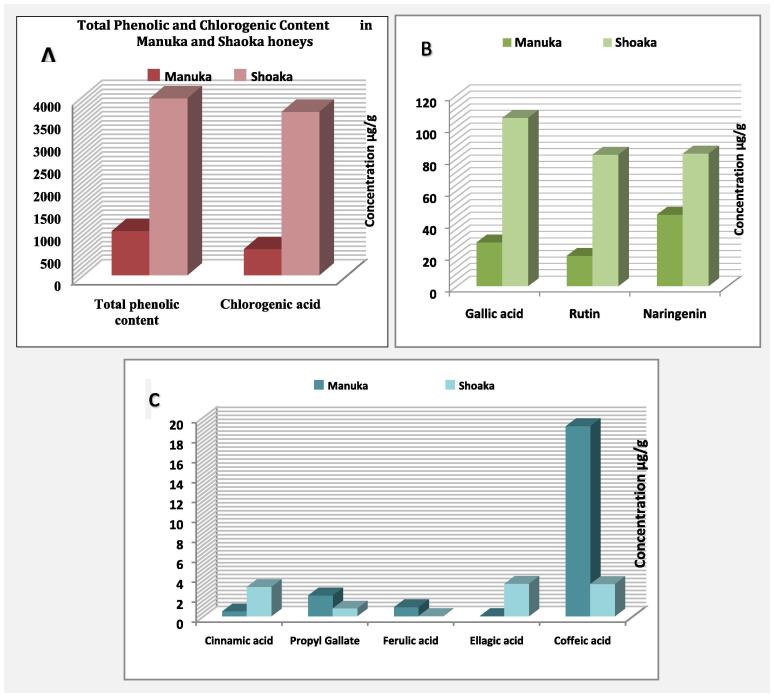
Fig. 2.
Histograms representing the main (A&B) and the minor polyphenolic components (C) in Shaoka and Manuka honey.
3.2. HPLC analysis of Shaoka honey polyphenols comparable to Manuka honey
HPLC analysis of polyphenols and flavonoids for Shaoka and Manuka honey revealed the occurrence of five main polyphenols, including gallic acid(GA), rutin, naringenin, quercetin, and caffeic acid, in concentrations of 105.42 ± 8.3, 82.30 ± 5.2, 82.93 ± 6.0, 18.20 ± 2.8, and 3.21 ± 0.42, µg/g, respectively, for Shaoka honey and in concentrations of 27.25 ± 4.0, 18.81 ± 1.9, 44.67 ± 3.4, 292.33 ± 18.9 and 19.07 ± 2.7, µg/g, respectively, for Manuka honey (Fig. 2b & c). The highest polyphenolic component in both kinds of honey was chlorogenic acid (CGA) in a concentration of 2453.57 ± 51.38 µg/g in Shaoka honey, depending on the geographic region, and 579.46 ± 19.85 µg/g in Manuka honey. This means that the chlorogenic acid in Shaoka represents 4.2-fold that of Manuka honey, and the total polyphenolic content of Shaoka honey was 2.8-fold that of Manuka honey (2734.48 and 985.01 µg/g for Shaoka and Manuka honey, respectively) (Fig. 2a).
3.3. Antibacterial activity of Shaoka and Manuka honey by well diffusion assay
Honey sensitivity was calculated for 12 honey samples against E. coli. The largest average zone of inhibition produced by Shaoka honey ranged from 29.9 ± 0.17 to 33.2 ± 0.13 mm, while it ranged from 29.0 ± 0.63 to 30.5 ± 0.51 in Manuka honey. The total antibacterial activity was evaluated in response to phenol percent. In all Shaoka types, the phenol percent ranged from 7.9% to 8.5% (Table 2). Compared to Manuka honey, the inhibition zones produced were equivalent to 6.9–7.0% phenol, which appear slightly lower in activity than Shaoka (Table 2).
Table 2.
Non-peroxide antibacterial activities of Shaoka and Manuka honey against MDR Escherichia coli.
| Sample | Honey type | Source | In. zone (mm ± SD) | Total activity Equiv. to Phenol (w/v) | Non-peroxide Activity Equiv. to Phenol (w/v) | The proportion of Non-peroxide Activity (%) |
|---|---|---|---|---|---|---|
| 1 | Shaoka | Alshafa | 32.0 ± 0.27 | 8.4 ± 0.14 | 7.1 ± 0.52 | 91.4 ± 0.21 |
| 2 | Shaoka | Alshafa | 30.0 ± 0.56 | 8.1 ± 0.18 | 7.0 ± 0.44 | 91.2 ± 0.32 |
| 3 | Shaoka | Alshafa | 31.0 ± 0.13 | 7.9 ± 0.15 | 6.9 ± 0.35 | 91.3 ± 0.11 |
| 4* | Shaoka | Alshafa | 33.0 ± 1.73 | 8.5 ± 0.13 | 7.2 ± 0.32 | 92.6 ± 1.10 |
| 5 | Shaoka | Alshafa | 31.2 ± 0.85 | 8.2 ± 0.06 | 7.0 ± 0.15 | 90.0 ± 1.01 |
| 6 | Shaoka | Alshafa | 33.0 ± 0.16 | 8.4 ± 0.16 | 7.1 ± 0.16 | 91.9 ± 1.23 |
| 7 | Shaoka | Tohama | 31.6 ± 0.70 | 7.9 ± 0.30 | 6.9 ± 0.12 | 90.0 ± 2.50 |
| 8 | Shaoka | Tohama | 33.2 ± 0.13 | 8.4 ± 0.01 | 7.0 ± 0.04 | 91.3 ± 1.42 |
| 9 | Shaoka | Tohama | 31.1 ± 0.91 | 8.2 ± 0.04 | 7.0 ± 0.00 | 91.4 ± 0.45 |
| 10 | Shaoka | Aljanub | 29.9 ± 0.17 | 7.9 ± 0.14 | 7.3 ± 0.17 | 90.2 ± 0.52 |
| 11 | Shaoka | Aljanub | 33.0 ± 0.13 | 8.5 ± 0.16 | 7.1 ± 0.16 | 92.3 ± 0.90 |
| 12 | Shaoka | Aljanub | 32.0 ± 0.37 | 8.5 ± 0.14 | 7.2 ± 0.14 | 91.1 ± 0.16 |
| 13 | Monuka | comivita | 30.5 ± 0.51 | 7.0 ± 0.11 | 7.0 ± 0.11 | 100.0 ± 0.8 |
| 14 | Monuka | comivita | 29.0 ± 0.63 | 6.9 ± 0.32 | 6.9 ± 0.12 | 100.0 ± 0.1 |
*Sample 4, the sample used for further antibacterial and anticancer studies In. zone: Inhibition zone
3.4. Peroxide and non-peroxide antibacterial activity of honeys
Screened honey was tested before and after treatment with catalase enzyme to estimate the non-peroxide activity. The inhibition zone produced by the total activity of Shaoka honey before treatment shows higher antibacterial activity, ranging from 30 ± 0.27 to 33.0 ± 0.13 mm, which is equivalent to 7.9–8.5% (w/v) phenols according to the presence of peroxide and non-peroxide activity. In contrast, the antibacterial activity of Shaoka decreased to 6.9% and 7.3% (w/v) equivalent to phenol after treatment, with the activity appearing to lessen slightly compared to total antimicrobial activity before catalase treatment (7.9–8.5%), which means Shaoka honey had peroxide activity when present in low concentrations. Since there is an insignificant change in the total activity of Manuka honey after treatment with catalase, it means that the activity of Manuka honey depends totally on non-peroxide effects. The inhibition zones were equivalent to 6.9% and 7.0% phenol, ranging from 29.0 ± 0.63 and 30.5 ± 0.51 mm (Table 2).
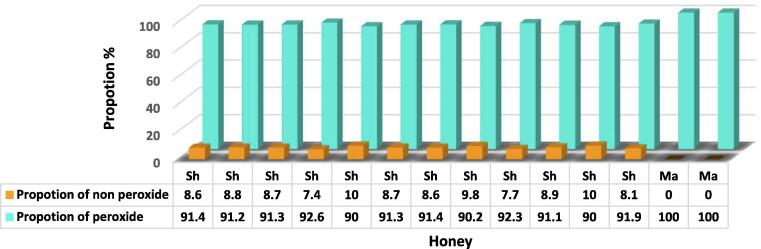
Before the inactivation of peroxide, the activity of Shaoka honey was significantly more active than Manuka honey (P < 0.0007). When the proportion of peroxide activity was deduced from the total phenol percent as antibacterial activity, Shaoka honey had activity comparative to non-peroxide Manuka honey, 6.9–7.3% and 6.9–7.0% for Shaoka and Manuka, respectively, as shown in Table (2). The proportion of non-peroxide activity showed no significant difference (p > 0.05) between Shaoka 92.6 ± 1.10 and Manuka 100 ± 0.10. In contrast, the proportion of peroxide activity of total activity in Shaoka honey was low, 7.4–10% (Fig. 3).
Fig. 3.
Propotion of peroxide and non-peroxide activity in shaoka and manuka honeys.
3.5. Minimum inhibitory and bactericidal concentration of honey samples
Minimum inhibitory concentrations (MIC) for tested honey were determined against the tested pathogenic bacteria, mainly those found in wound infections, pneumonia, and tuberculosis. The MICs ranged from 5.0% and 17.0%, while the minimum bactericidal concentration (MBC) of different bacteria ranged from 7.5 ± 2.5% and 22.0 ± 3.5% w/v. Staphylococcus aureus was the most sensitive Gram-positive bacteria which was inhibited by 5. 0 ± 0.0% w/v of Shaoka honey, while Gram-positive Streptococcus mutans appeared sensitive to Manuka, with the MIC and MBC 5.0 ± 0.0% and 10.0 ± 0.0%, respectively. The most sensitive Gram-negative bacteria for both kinds of honey was Pseudomonas aeruginosa with MIC and MBC 5.0 ± 0.0 and 7.5 ± 0.0%, respectively, when treated with Shaoka honey, and from 7.5 ± 3.5 and 7.5 ± 0.0 when treated with Manuka honey (Table 3). In general, Shaoka honey had lower MIC and MBC on tested organisms than Manuka honey.
Table 3.
Antibacterial activity of Shaoka and Manuka honey against Gram-negative and Gram-positive pathogens.
| Bacteria | Honey (W/V) |
|||
|---|---|---|---|---|
| Shaoka (%) |
Manuka (%) |
|||
| MIC | MBC | |||
| Mycobacterium tuberculosis | 7.5 ± 3.5 | 10.0 ± 0.11 | 12.5 ± 0.0 | 20.0 ± 3.5 |
| Staphylococcus aureus (MRSA) | 5.0 ± 0.0 | 7.5 ± 3.5 | 7.5 ± 3.5 | 17.5 ± 0.0 |
| Streptococcus mutans | 7.5 ± 3.5 | 10.0 ± 0.0 | 5.0 ± 0.0 | 10.0 ± 0.0 |
| Helicobacter pylori | 10.0 ± 0.0 | 15.0 ± 0.0 | 15.0 ± 2.5 | 22.0 ± 0.0 |
| Salmonella typhi | 10.0 ± 0.0 | 12.5 ± 0.0 | 17.5 ± 0.0 | 20.5 ± 3.5 |
| Streptococcus pneumoniae | 12.5 ± 3.5 | 15.0 ± 3.5 | 15.0 ± 0.0 | 17.5 ± 3.5 |
| Escherichia coli | 7.5 ± 0.0 | 12.5 ± 3.5 | 10.0 ± 3.5 | 12.5 ± 0.0 |
| Pseudomonas aeruginosa | 5.0 ± 0.0 | 7.5 ± 0.0 | 7.5 ± 3.5 | 7.5 ± 0.0 |
1Minimum inhibitory concentration.
2Minimum bactericidal concentration.
3.6. Morphological effect of honey
Fig. 4 shows the TEM images of E. coli cells treated with Shaoka honey, showing a marked change in cell shape and bacterial structure. The images were compared with the untreated control to identify structural changes (Fig. 4A). Honey-treated cultures of E. coli displayed unpreserved morphology, cell wall thickening, and non-homogeneous cytoplasm. The most significant changes observed were distorted cell morphologies, including shrinkage and decreased size which increased over time, reduction in length, leakage of intracellular content, cytoplasmic shrinkage, and cell wall destruction leading to cell death (Fig. 4B & C). The presence of cellular debris in TEM images indicated cell lysis (Fig. 4C).
Fig. 4.
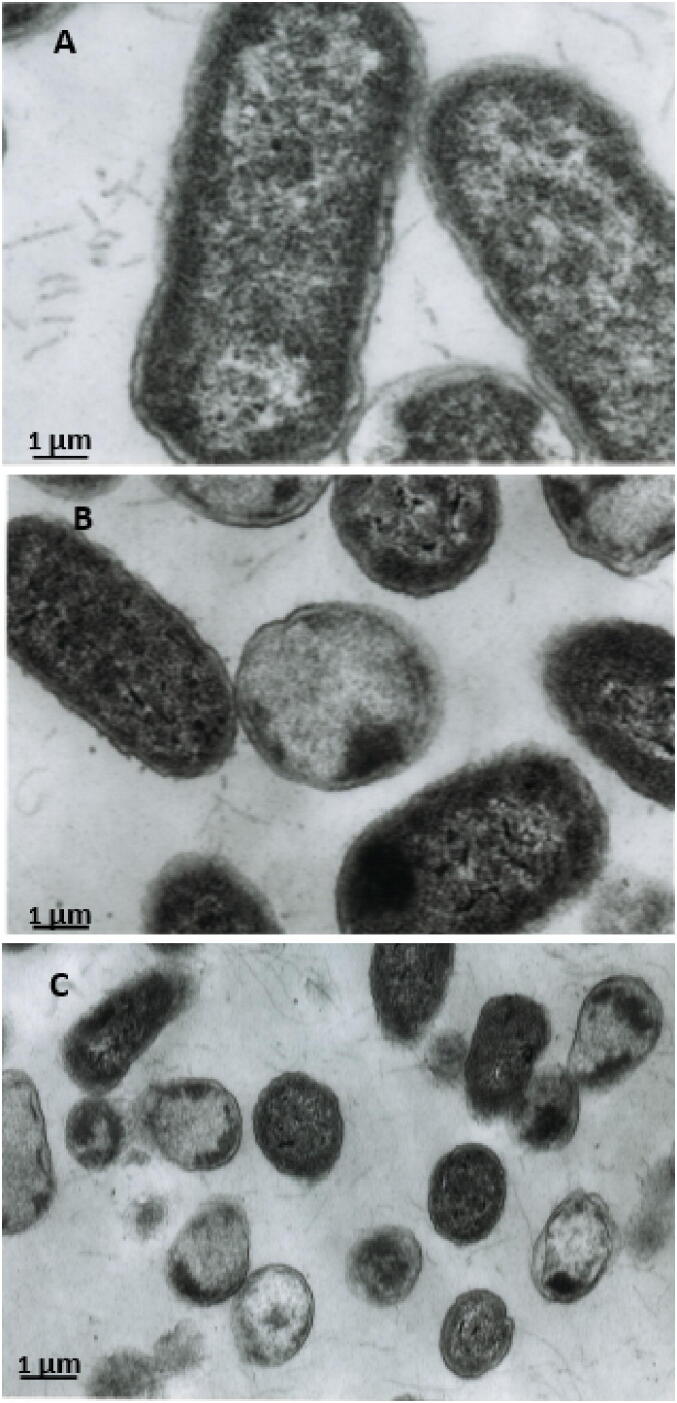
Electron microscopic analysis. (A) In untreated cells of E. coli, cells exhibited uniformly dense and homogenous structures. (B) The Shaoka honey’s effect on cellular structure appears as distorted cell morphologies, including shrinkage in shape and decreased size after 3 h of honey treatment at 37 °C. (C) Loss of structural integrity and cell wall destruction after 6 h of exposure to honey. The sample was examined using TEM at 10,000x magnification).
3.7. Anticancer activity of Shaoka and Manuka honey
3.7.1. Proliferation percent of MCF-7 and HepG2 cells treated with different kinds of honey
The cytotoxic effect and the most effective concentration value of targeted bee honey (Shaoka and Manuka honey) against breast adenocarcinoma (MCF-7) and hepatocellular carcinoma (HepG2) were investigated using different concentrations of these kinds of honey for various exposure times (Table 4). The data are presented as mean values ± Standard Error. The screening results proved that Shaoka honey was highly active against both tested cell lines and inhibited the proliferation percentage by 70% for MCF-7 cells and 52% for HepG2 cells at a concentration of 25% when treated for 48 h, whereas Manuka honey had lower effects against MCF-7 and induced inhibition of the growth by 50% and 51% in HepG2 cancer cells when treated at the same concentration and duration time (Table 4).
Table 4.
Effect of selected honeys treated at different concentration on the proliferative percentage of mcf-7 and hepg2 cultured cells for different duration times.
 |
Means with different superscripts (a, b, c, d, e, f, and g) between groups in the same column are significantly different (P < 0.05). Cell numbers were counted and data are expressed as the percentage of untreated control.
3.7.2. MTT assay for the inhibitory effect of honey on HepG2 and MCF-7 cell viability
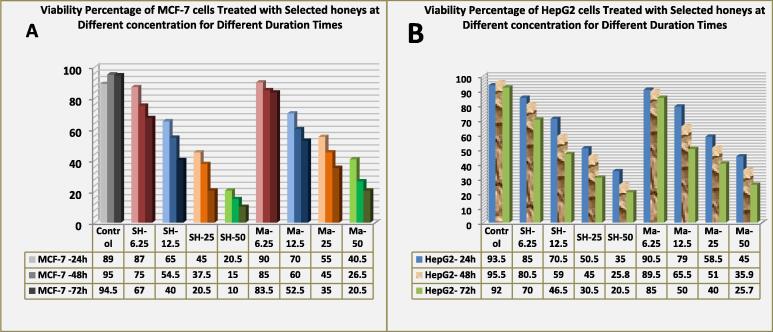
MTT assay results showed that both kinds of honey had a marked inhibitory effect on the viability of both types of cancer cells. MCF-7 cancer cells were more sensitive than HepG2, and the inhibition rate increased with increasing honey concentration and exposure time. After treatment for 72 h, MCF-7 and HepG2 cell inhibition rates reached 90% and 80%, respectively, at 25% concentration Shaoka honey treatment group, which was significantly different from the control group, as shown in Fig. 5A & B. In comparison, the obtained data showed that Manuka honey treatment had moderate inhibitory effects against both cancer cells.
Fig. 5.
The viability percentage of MCF-7 (a) and HepG2 (b) cells treated with different kinds of honey at the different concentrations for various times.
3.7.3. Morphological changes in MCF-7 and HepG2 cells treated with selected honey
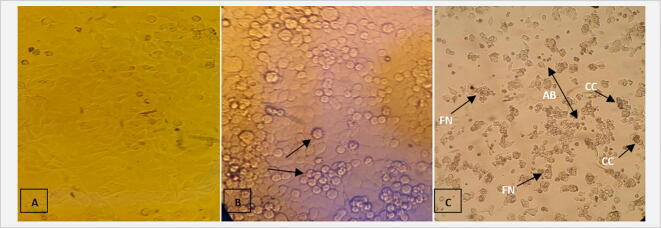
Under the inverted microscope, MCF-7 and/or HepG2 cells in the control group were seen to be firmly adherent and in irregular polygonal and fusiform shape. Cells were bright and fully stretched, with intact membrane and uniform cytoplasmic distribution (Fig. 6a). In comparison, treatments with different types of honey affected the cells’ morphology. At certain concentrations of honey, changes in cellular morphology became increasingly evident. Most cells were detached, chromatins were condensed and compacted, and typical apoptotic characteristics such as cell shrinkage and apoptotic bodies were seen. Additionally, 72-h treatment-induced obvious cytotoxic effects such as shrunken cells and decreases in cellular crowding and cell number (Fig. 6b)
Fig. 6.
Inverted microscopic photograph of (A) represents untreated MCF-7 cells with normal angular or polygonal shape. (B) Treated MCF-7 cells with Manuka honeys at 25% concentration for 48 h. that has lost its normal shape and shows a shrunken cytoplasm. (C) Treated MCF-7 cells with 25% concentration of Shaoka honeys after 48 h. that shows evident morphologically signs of apoptosis. The apoptotic changes including, condensed chromatin (CC), fragmented nuclei (FN) and apoptotic bodies (AB). A Nikon Eclipse 50i Nikon microscope with a 20 objective lens was used to investigate the cells.
4. Discussion
In the present work, phytochemical analysis of Shaoka honey identified more than 72 compounds from different chemical classes, most of which also occur in other kinds of honey in different concentrations (Ciucure and Geană, 2019, Gašić et al., 2014). Shaoka honey has a unique flavor and a strong amber aroma, which is actually due to the volatile and semi-volatile organic compounds present (Bayraktar and Onoğur, 2011).
In this study, the HPLC analysis identified nine phenolic compounds, mainly phenolic acids and flavonoids, in either Shaoka or Manuka honey; the total phenolic content in Shaoka honey was approximately three times higher than in Manuka honey. The results demonstrated a strong positive relationship between Shaoka honey’s phytochemical constituents and its biological activity. Previous studies documented that polyphenols, mostly phenolic acids/flavonoids, are used as markers for honey in quality authentication and their direct effects as an antibacterial and anticancer agent (Pauliuc et al., 2020). This phenolic content may contribute to the antioxidant and antimicrobial potential of Shaoka honey, which agrees with a study on buckwheat honey, which has higher phenolic content than Manuka (Deng et al., 2018).
The present results found that CGA and GA are the dominant phenolic compounds in Shaoka honey, while the flavonoids present in the highest amounts are rutin and naringenin. Greater amounts of quercetin than CGA were detected in Manuka honey, which agrees with the results of other studies (Alvarez-Suarez et al., 2014, Oelschlaegel et al., 2012, Deng et al., 2018). It is known that CGA is a polyphenol that has important roles as an antioxidant, antibacterial, antiviral, and anti-inflammatory agent and causes hepatoprotective activity (Naveed et al., 2018). Previous data showed that CGA significantly inhibits the growth of Gram-positive and Gram-negative bacteria, with MIC values ranging from 20 to 80 µg/mL (Lou et al., 2011). Gallic acid was also shown to be present in high levels in Shaoka honey, which exhibits various properties such as antimutagenic, antibacterial, antivirus, antitumor, and antioxidant activities (Velásquez et al., 2020, Kumar and Goel, 2019). Yao et al. found that GA is the main phenolic acid in various Australian honey types (Yaoa et al., 2005). Others have reported that GA is highly antimicrobial, and can act directly against Gram-negative pathogens (Borges et al., 2013).
Interestingly, Shaoka honey is also rich in flavonoid naringenin, which has several biological properties (Salehi et al., 2019). Growing evidence from both in vitro and in vivo studies has shown that naringenin has pharmaceutical value for the control and management of infectious and malignant diseases (Céliz et al., 2011, Lim et al., 2017, Salehi et al., 2019). These active polyphenolic components give Shaoka honey superior properties when they work together synergistically against multi-drug-resistant bacteria and cancer cells. Manuka honey is rich in methylglyoxal and quercetin, which act effectively against multidrug-resistant bacteria, (Tyagi et al., 2015, Nolan et al., 2020).
Furthermore, a low level of hydrogen peroxide detected in Shaoka honey is considered to be an important antibacterial agent in diluted honey (Girma et al., 2019) Molan previously reported the maximum level of hydrogen peroxide produced can be obtained when honey is diluted by 30–50% (Molan, 1992, Brudzynski, 2006). In this study, Shaoka honey was diluted to 16% and the maximum proportion of peroxide activity (PA) detected was ≤10%, which means ≥90 % of the activity is related to the non-peroxide activity (NPA). The antibacterial activity of Shaoka honey was equivalent to 7.2% phenol, approximately the same activity as that exhibited by Manuka (7.0%). Both Shaoka and Manuka honey inhibited the growth of ESBL E. coli successfully and with a similar inhibition zone (30–33 mm), implying a bacteriostatic potency of Shaoka honey equal to that of Manuka. Interestingly, Shaoka was still as active or relatively more active than Manuka even after catalase treatment destroyed any hydrogen peroxide. These findings showed that Shaoka honey has low peroxide activity and high non-peroxide activity, which can work together synergistically to successfully inhibit and kill bacteria and cancer cells. In contrast, the proportion of peroxide activity in Manuka honey was recorded as zero before and after treating honey with catalase, so the proportion of non-peroxide activity was 100%. These results are in agreement with a previous study (Adams et al., 2009). However, some-dependent honey-like honeydew and blossom honey showed antibacterial efficacy as effective as Manuka honey, but this activity was markedly reduced by treatment with catalase (Bucekova et al., 2019).
Recently, researchers have suggested that polyphenols could be involved in the generation of hydrogen peroxide at low concentration by autoxidation of polyphenols (Bucekova et al., 2019, Grzesik et al., 2019), and the slow release of in honey may improve its antibacterial activity (Dogan-Guner et al., 2018). A new finding documented a significant correlation between the and total phenol content in the overall antibacterial activity of the honey (Grzesik et al., 2019)
Another area of interest is the antibacterial effects of both types of honey on broad-spectrum-antibiotic-resistant bacteria, which are common causes of severe infections. The antibacterial activity of Shaoka honey was either comparable or stronger than Manuka honey, with different sensitivity depending on the type of bacteria. The results of this study are in line with our previous work, which indicated that Shaoka and Sidr honey surpasses all local and imported honey available in Saudi markets in their antibacterial activities (Halawani and Shohayeb, 2011). In this regard, other studies have reported that Australian Manuka honey inhibits the growth of Staphylococcus aureus, Bacillus subtilis, Salmonella typhi, MRSA, Pseudomonas, Klebsiella pneumonia, and ESBL E. coli (Deng et al., 2018, Girma et al., 2019).
Electron microscopic observation indicated that E. coli cells treated with diluted Shaoka honey showed distorted cell morphologies, including shrinkage in cells, and related to cell permeability change, there was evidence of decrease and leakage of intracellular content and cell-wall destruction, which lead to cell death. Similar results have been shown in the mechanisms of actions of phenolic compounds, which caused cell death by the loss of cellular membrane integrity of function (Yaacob et al., 2020). Other researchers have also reported that the antibacterial mechanisms of flavonoids are assumed to have effects including inhibition of nucleic acid synthesis, alteration of cytoplasmic membrane and function, alteration of membrane permeability, and attenuation of pathogenicity (Xie et al., 2015, Jibril et al., 2019). Furthermore, Eumkeb and Chukrathok have suggested that naringenin detected in Shaoka honey can alter the outer and cytoplasmic membrane of E. coli and inhibit DNA gyrase (Eumkeb and Chukrathok, 2013). Researchers have also found that a minimum level of hydrogen peroxide causes DNA degradation and growth inhibition in E. coli. There is also a synergistic effect with high levels of polyphenols in the presence of hydrogen peroxide that enhances oxidative stress on bacterial cells (Nolan et al., 2019, Saranraj and Sivasakthi, 2018, Brudzynski et al., 2017, Finnegan et al., 2010). These results agree with the E. coli cell degradation observed in the present study.
The technique adopted as part of this work intended to detect components in Shaoka and Manuka honey that exhibit anticancer effects in different cancer cells at non-cytotoxic concentrations. The results obtained show that anticancer activities investigated by MTT and morphological examination in HepG2 and MCF-7 cancer cell lines are comparable regardless of the modes of action on cancer cells. The results showed that Shaoka honey had the highest cytotoxic effects on both tested cell lines and inhibited proliferation by 70% for MCF-7 cells and 52% for HepG2 cells at 25% concentration when treated for 48 h, whereas Manuka honey had smaller effects on MCF-7 and induced growth inhibition by 50% and 51% in HepG2 cancer cells when treated at the same concentration and duration time. Both honey samples having cytostatic effects against cancer cells were revealed to be abundant with phenolic compounds, which are well documented as anticancer agents (Erejuwa et al., 2014, Martinello and Mutinelli, 2021). Our findings are consistent with several published studies that have documented cytostatic activities of honey in colon, breast, and hepatic cancer cells based on the phenolic content (Erejuwa et al., 2014, Wen et al., 2012, Jaganathan and Mandal, 2019). Chlorogenic acid and gallic acid represented the major components in both tested honey. These have a wide scope of pharmacokinetic properties, such as cancer prevention and potential therapeutic effects on cancer cells, depending on its concentration, exposure dose, and duration time (Maalik et al., 2016, Belkaid et al., 2006). Burgos-Morón et al. (2012) reported that CGA treatment resulted in damage of DNA and formed a complex of topoisomerase-DNA at concentrations of 0.5 to 5 mM (Burgos-Morón et al., 2012). The topoisomerases topo I and topo II are key players in DNA fragmentation during the process of apoptosis (Deweese et al., 2009). When cells are exposed to CGA for 24 h, it induces significant levels of topo-DNA complexes (Burgos-Morón et al., 2012).
5. Conclusion
This study is to prove the importance of the non-peroxide-dependent activity of Shaoka honey that exhibits wide spectrum antibacterial action on multidrug-resistant bacteria and superior anticancer activity on hepatocarcinoma and breast cancer cells. This dual activity of Saudi Shaoka honey is either comparable or stronger than New-Zealand Manuka honey, both of whose antibacterial and antioxidant activities were found to be correlated with their high polyphenolic compound content.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by Taif University Researchers Supporting Project number (TURSP-2020/273), Taif University, Taif, Saudi Arabia. The author would like to thank professor, Aziza M. Hassan for her cooperation and constructive support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adams C.J., Manley-Harris M., Molan P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009;344:1050–1053. doi: 10.1016/j.carres.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Adgaba N., Al-Ghamdi A., Tadesse Y., Getachew A., Awad A.M., Ansari M.J., Owayss A.A., Mohammed S.E.A., Alqarni A.S. Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi Journal of Biological Sciences. 2017;24(1):180–191. doi: 10.1016/j.sjbs.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrin S., Giampieri F., Gasparrini M., Hernandez T.F., Cianciosi D., Rodríguez P.R., Amici A., Quiles J.L., Battino M. Inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cells growth. Part 1: suppression of proliferation, promotion of apoptosis, and arrest of the cell cycle. Food Funct. 2018;9:2145–2157. doi: 10.1039/c8fo00164b. [DOI] [PubMed] [Google Scholar]
- Al-Sodany Y.M., Bazaid S.A., Mossalam H.A. Medicinal plants in Saudi Arabia: I. Sarrwat mountains at Taif, KSA. Acad. J. Plant Sci. 2013;6:134–145. [Google Scholar]
- Albaridi A.N. Antibacterial potency of honey. Int. J. Microbiol. 2019;1:1–10. doi: 10.1155/2019/2464507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K.L., Molan P.C., Reid G.M. The variability of the antibacterial activity of honey. Apiacta. 1991;26:114–121. doi: 10.1080/0005772X.1992.11099118. [DOI] [Google Scholar]
- Almasaudi S.B., Al-Nahari A.A.M., Abd El-Ghany E.S.M., Barbour E., Al Muhayawi S.M., Al-Jaouni S., Azhar E., Qari M., Qari Y.A., Harakeh S. Antimicrobial effect of different types of honey on Staphylococcus aureus. Saudi J. Bio. Sci. 2017;24(6):1255–1261. doi: 10.1016/j.sjbs.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqarni A.S., Owayss A.A., Mahmoud A.A. Physicochemical characteristics, total phenols, and pigments of national and international honeys in Saudi Arabia. Arabian J. Chem. 2016;9(1):114–120. doi: 10.1016/j.arabjc.2012.11.013. [DOI] [Google Scholar]
- Alvarez-Suarez J.M., Gasparrini M., Forbes-Hernández T.Y., Mazzoni L., Giampieri F. The composition and biological activity of honey: A focus on Manuka honey. Foods. 2014;3(3):420–432. doi: 10.3390/foods3030420. PMID: 28234328; PMCID: PMC5302252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badakhshan M.P., Sreenivasan S., Jegathambigai R.N., Surash R. Anti-leukemia activity of methanolic extracts of Lantana camara. Pharmacogn. Res. 2009;1:274–279. [Google Scholar]
- Badolato M., Carullo G., Cione E., Aiello F., Caroleo M.C. From the hive: Honey, a novel weapon against cancer. Eur. J. Med. Chem. 2017;142:290–299. doi: 10.2307/2286085. [DOI] [PubMed] [Google Scholar]
- Bayraktar D., Onoğur T.A. Investigation of the aroma impact volatiles in Turkish pine honey samples produced in Marmaris, Datça and Fethiye regions by SPME/GC/MS technique. Int. J. Food Sci. Technol. 2011;46:1060–1065. doi: 10.1111/j.1365-2621.2011.02588.x. [DOI] [Google Scholar]
- Belkaid A., Currie J.C., Desgagnés J., Annabi B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006;6:7. doi: 10.1186/1475-2867-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges A., Ferreira C., Saavedra M.J., Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013;19(4):256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- Brudzynski K. Effect of hydrogen peroxide on antibacterial activities of Canadian honey. Can. J. Microbiol. 2006;52:1228–1237. doi: 10.1139/w06-086. [DOI] [PubMed] [Google Scholar]
- Brudzynski K., Abubaker K., Martin L., Castle A. Reexamining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011;2(1–9):2011. doi: 10.3389/fmicb.2011.00213. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski K., Miotto D., Kim L., Sjaarda C., Maldonado-Alvarez L., Fuk’s H. Active macromolecules of honey form colloidal particles essential for honey antibacterial activity and hydrogen peroxide production. Sci. Rep. 2017;7:1–15. doi: 10.1038/s41598-017-08072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucekova, M., Jardekova, L., Juricova, V., Bugarova, V., Di Marco, G., Gismondi, A., et al., 2019. Antibacterial activity of different blossom honeys: New findings molecules, 24 (8), 1573. 10.3390/molecules24081573 doi: 10.3390/molecules24081573. [DOI] [PMC free article] [PubMed]
- Bucekova M., Sojka M., Valachova I., Martinotti S., Ranzato E., Szep Z., Majtan V., Klaudiny J., Majtan J. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-07494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Morón E., Calderón-Montaño J.M., Orta M.L., et al. The coffee constituent chlorogenic acid induces cellular DNA damage and the formation of topoisomerase I- and II-DNA complexes in cells. J. Agric. Food Chem. 2012;60:7384–7391. doi: 10.1021/jf300999e. [DOI] [PubMed] [Google Scholar]
- Cavia M.M., Fernández-Muiño M.A., Alonso-Torre S.R., Huidobro J.F., Sancho M.T. Evolution of acidity of honeys from continental climates: influence of induced granulation. Food Chem. 2007;100(4):1728–1733. doi: 10.1016/j.foodchem.2005.10.019. [DOI] [Google Scholar]
- Céliz G., Daz M., Audisio M.C. Antibacterial activity of naringenin derivatives against pathogenic strains. J. App. Microbiol. 2011;111:731–738. doi: 10.1016/j.foodchem.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Chan C.W., Deadman B.J., Manley-Harris M., Wilkins A.L., Alber D.G., Harry E. Analysis of the flavonoid component of bioactive New Zealand manuka (Leptospermum scoparium) honey and the isolation, characterization, and synthesis of an unusual pyrrole. Food Chem. 2013;141:1772–1781. doi: 10.1016/j.foodchem.2013.04.092. [DOI] [PubMed] [Google Scholar]
- Cianciosi Danila, Forbes-Hernández Tamara, Afrin Sadia, Gasparrini Massimiliano, Reboredo-Rodriguez Patricia, Manna Piera, Zhang Jiaojiao, Bravo Lamas Leire, Martínez Flórez Susana, Agudo Toyos Pablo, Quiles José, Giampieri Francesca, Battino Maurizio. Phenolic compounds in honey and their associated health benefits: A. Review. Mol. 2018;23(9):2322. doi: 10.3390/molecules23092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucure Corina Teodora, Geană Elisabeta‐Irina. Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal. 2019;30(4):481–492. doi: 10.1002/pca.v30.410.1002/pca.2831. [DOI] [PubMed] [Google Scholar]
- Combarros-Fuertes Patricia, Estevinho Leticia M., Dias Luis G., Castro José M., Tomás-Barberán Francisco A., Tornadijo M. Eugenia, Fresno-Baro José M. Bioactive components and antioxidant and antibacterial activities of different varieties of honey: A screening prior to clinical application. J. Ag. Food Chem. 2019;67(2):688–698. doi: 10.1021/acs.jafc.8b0543610.1021/acs.jafc.8b05436.s001. [DOI] [PubMed] [Google Scholar]
- Custódio Luísa, Fernandes Eliana, Escapa Ana Luisa, López-Avilés Sandra, Fajardo Alba, Aligué Rosa, Alberício Fernando, Romano Anabela. Antioxidant activity and in vitro inhibition of tumor cell growth by leaf extracts from the carob tree (Ceratonia siliqua L.) Pharm. Biol. 2009;47(8):721–728. doi: 10.1080/13880200902936891. [DOI] [Google Scholar]
- Deng J., Liu R., Lu Q., Hao P., Xu A., Zhang J., Tan J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018;252:243–249. doi: 10.1016/j.foodchem.2018.01.115. [DOI] [PubMed] [Google Scholar]
- Deweese Joseph E., Osheroff Michael A., Osheroff Neil. DNA topology and topoisomerases: teaching a “knotty” subject. Biochem. Mol. Biol. Educ. 2009;37(1):2–10. doi: 10.1002/bmb.v37:110.1002/bmb.20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan-Guner E.M., Mohamed H., Orbey N., Goodyear N. Stabilization and controlled release of micro-encapsulated hydrogen peroxide for wound treatment applications. J. Appl. Microbiol. 2018;126:965–972. doi: 10.1111/jam.14177. [DOI] [PubMed] [Google Scholar]
- El Sohaimy S.A., Masry S.H.D., Shehata M.G. Physicochemical characteristics of honey from different origins. Ann. Ag. Sci. 2015;60(2):279–287. doi: 10.1016/j.aoas.2015.10.015. [DOI] [Google Scholar]
- Erejuwa O.O., Sulaiman S.A., Ab Wahab M.S. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules. 2014;19(2):2497–2522. doi: 10.3390/molecules19022497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eteraf-Oskouei T., Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran. J. Basic Med. Sci. 2013;16(6):731–742. PMID: 23997898. [PMC free article] [PubMed] [Google Scholar]
- Eumkeb G., Chukrathok S. Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant Enterobacter cloacae. Phytomedicine. 2013;20(3–4):262–269. doi: 10.1016/j.phymed.2012.10.008. Epub 2012 Dec 3 PMID: 23218402. [DOI] [PubMed] [Google Scholar]
- Finnegan M., Linley E., Denyer S.P., McDonnell G., Simons C., Maillard J.-Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010;65(10):2108–2115. doi: 10.1093/jac/dkq308. [DOI] [PubMed] [Google Scholar]
- Gašić U., Šikoparija B., Tosti T., Trifković J., Milojković-Opsenica D., Natić M., Tešić Ž. Phytochemical fingerprints of lime honey collected in Serbia. J. AOAC Int. 2014;97(5):1259–1267. doi: 10.5740/jaoacint.SGEGasic. PMID: 25902974. [DOI] [PubMed] [Google Scholar]
- Girma Alodia, Seo Wonjae, She Rosemary C., Giarratana Filippo. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS ONE. 2019;14(10):e0224495. doi: 10.1371/journal.pone.0224495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesik M., Bartosz G., Stefaniuk I., Pichla M., Namiesnik J., Sadowska-Bartosz I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem. 2019;278:692–699. doi: 10.1016/j.foodchem.2018.11.109. [DOI] [PubMed] [Google Scholar]
- Halagarda M., Groth S., Popek S., Rohn S., Pedan V. Antioxidant activity and phenolic profile of selected organic and conventional honeys from Poland. Antioxidants (Basel) 2020;9:44. doi: 10.3390/antiox9010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halawani E., Shohayeb M. Survey of the antibacterial activity of Saudi and some international honeys. J. Microbiol. Antimicrobials. 2011;3(4):94–101. http://www.academicjournals.org/JMA [Google Scholar]
- Henriques A.F., Jenkins R.E., Burton N.F., Cooper R.A. The intracellular effects of manuka honey on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29(1):45–50. doi: 10.1007/s10096-009-0817-2. [DOI] [PubMed] [Google Scholar]
- Hosny, A., Mosallam, M., Bazaid. S. and Salman, S., 2010. Plant life and habitat diversity in Saudi Arabia, first ed. King Fahad National Library: 510.
- Irish Julie, Blair Shona, Carter Dee A., Otto Michael. The antibacterial activity of honey derived from Australian flora. PLoS ONE. 2011;6(3):e18229. doi: 10.1371/journal.pone.0018229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaganathan S.K., Mandal M. Honey constituents and their apoptotic effect in colon cancer cells. J. ApiProduct ApiMedical Sci. 2019;1(2):29–36. [Google Scholar]
- Jibril F.I., Hilmi A.B.M., Manivannan L. Isolation and characterization of polyphenols in natural honey for the treatment of human diseases. Bull. Natl. Res. Cent. 2019;43:4. doi: 10.1186/s42269-019-0044-7. [DOI] [Google Scholar]
- Kumar Naresh, Goel Nidhi. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotech. Rep. 2019;24:e00370. doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemar K.M., Turner M.P., Lloyd D. Garlic (Allium sativum) as an anti-Candida agent: a comparison of the efficacy of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002;93(3):398–405. doi: 10.1046/j.1365-2672.2002.01707.x. [DOI] [PubMed] [Google Scholar]
- Lim Whasun, Park Sunwoo, Bazer Fuller W., Song Gwonhwa. Naringenin-induced apoptotic cell death in prostate cancer cells is mediated via the PI3K/AKT and MAPK signaling pathways. J. Cell. Biochem. 2017;118(5):1118–1131. doi: 10.1002/jcb.25729. [DOI] [PubMed] [Google Scholar]
- Lou Z.X., et al. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011;76(6):398–403. doi: 10.1111/j.1750-3841.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- Maalik A., Bukhari S.M., Zaidi A., Shah K.H., Khan F.A. Chlorogenic acid: A pharmacologically potent molecule. Acta Pol. Pharm. 2016;74:851–854. https://pubmed.ncbi.nlm.nih.gov/29648710/ [PubMed] [Google Scholar]
- Majewska Ewa, Drużyńska Beata, Wołosiak Rafał. Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotechnol. 2019;28(5):1307–1314. doi: 10.1007/s10068-019-00598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinello M., Mutinelli F. Antioxidant activity in bee products: A review. Antioxidants. 2021;10:71. doi: 10.3390/antiox10010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molan P.C. The antibacterial activity of honey. The nature of the antibacterial activity. Bee world. 1992;73(1):5–28. https://hdl.handle.net/10289/2094 [Google Scholar]
- Mosmann Tim. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays, 1983. J. Immunol. Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M., Ahmad F., Babazadeh D., FangFang X., Modar-resi-Ghazani F., WenHua L., XiaoHui Z. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- Nolan V.C., Harrison J., Cox J.A.G. Dissecting the antimicrobial composition of honey. Antibiotics. 2019;8:1–16. doi: 10.3390/antibiotics8040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan V.C., Harrison J., Wright J.E.E., Cox J.A.G. Clinical significance of manuka and medical-grade honey for antibiotic-resistant infections: A systematic review, 2020. Antibiotics. 2020;9(11):766. doi: 10.3390/antibiotics9110766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelschlaegel Stefanie, Gruner Margit, Wang Pang-Ning, Boettcher Anja, Koelling-Speer Isabelle, Speer Karl. Classification and characterization of manuka honeys based on phenolic compounds and methylglyoxal. J. Ag. and Food Chem. 2012;60(29):7229–7237. doi: 10.1021/jf300888q. [DOI] [PubMed] [Google Scholar]
- Oryan A., Alemzadeh E., Moshiri A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability. 2016;25(2):98–118. doi: 10.1016/j.jtv.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Pauliuc D., Dranca F., Oroian M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitable for Romanian honey authentication. Foods. 2020;9(3):306. doi: 10.3390/foods9030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli J.-P., Guinoiseau E., Luciani A., Yang Y., Battesti M.-J., Paolini J., Costa J., Quilichini Y., Berti L., Lorenzi V. Key role of hydrogen peroxide in antimicrobial activity of spring, honeydew maquis and chestnut grove Corsican honeys on Pseudomonas aeruginosa DNA. Lett. Appl. Microbiol. 2018;66(5):427–433. doi: 10.1111/lam.2018.66.issue-510.1111/lam.12868. [DOI] [PubMed] [Google Scholar]
- Salehi B., Fokou P.V.T., Sharifi-Rad M., Zucca P., Pezzani R., Martins N., Sharifi-Rad J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals. 2019;12(1):11. doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranraj P., Sivasakthi S. A comprehensive review on honey: Biochemical and medicinal properties. J. Acad. Ind. Res. 2018;6:165–181. https://www.researchgate.net/publication/324835659 [Google Scholar]
- Sereia, M.J., Março, P.H., Perdoncini, M.R.G., Parpinelli, R.S., Lima, E.G., Anjo, F.A., 2017. Techniques for the evaluation of the physicochemical quality and bioactive compounds in honey. In: Toledo, V.A.A. (Ed.), Honey Analysis. InTech Open, London, pp. 193–214. http://dx.doi.org/10.5772/66839.
- Shawky R., Alzamel N. Survey on medicinal plants in the flora of Al Riyadh Region, Saudi Arabia. Eurasian J. Biosci. 2020;14:3795–3800. https://www.semanticscholar.org [Google Scholar]
- Szende B., Tyihak E., Trezl L. Role of arginine and its methylated derivatives in cancer biology and treatment. Cancer Cell Int. 2001;17:1–3. doi: 10.1186/1475-2867-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks A.J., Cooper R.A., Jones K.P., et al. Honey stimulates inflammatory cytokine production from monocytes. Cytokine. 2003;21(5):242–247. doi: 10.1016/s1043-4666(03)00092-9. [DOI] [PubMed] [Google Scholar]
- Tyagi B., Dubey A., Verma A., Tiwari S. Antibacterial activity of phenolic compounds against pathogenic bacteria. Int. J. Pharm. Sci. Rev. Res. 2015;35:16–18. doi: 10.1007/s00284-009-9501-0. [DOI] [Google Scholar]
- Valachova I., Bucekova M., Majtan J. Quantification of bee-derived defensin-1 in honey by competitive enzyme-linked immunosorbent assay, a new approach in honey quality control. Czech J. Food Sci. 2016;34:233–243. doi: 10.17221/422/2015-CJFS. [DOI] [Google Scholar]
- Velásquez P., Montenegro G., Giordano A., Retamal M., Valenzuela L.M. Bioactivities of phenolic blend extracts from Chilean honey and bee pollen. CyTA-J. Food. 2019;17(1):754–762. doi: 10.1080/19476337.2019.1646808. [DOI] [Google Scholar]
- Velásquez P., Montenegro G., Leyton F., Ascar L., Ramirez O., Giordano A. Bioactive compounds and antibacterial properties of monofloral Ulmo honey. CYTA-J. Food. 2020;18(1):11–19. doi: 10.1080/19476337.2019.1701559. [DOI] [Google Scholar]
- Walter R.A., Duncan D.B. A Bayes rule for the symmetric multiple comparison problems. J. Am. Stat. Assoc. 1969;64:1484–1503. [Google Scholar]
- Wen C.T.P., Hussein S.Z., Abdullah S., Karim N.A., Makpol S., Yusof Y.A.M. Gelam and nenas honeys inhibit proliferation of HT 29 colon cancer cells by inducing DNA damage and apoptosis while suppressing inflammation. Asian Pac. J. Cancer Prev. 2012;13(4):1605–1610. doi: 10.7314/APJCP.2012.13.4.1605. [DOI] [PubMed] [Google Scholar]
- Xie Y., Yang W., Tang F., Chen X., Ren L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015;22:132–149. doi: 10.2174/0929867321666140916113443. [DOI] [PubMed] [Google Scholar]
- Yaacob S.N.S., Wahab R.A., Huyop F., Lani M.N., Zin N.M. Morphological alterations in Gram-positive and Gram-negative bacteria exposed to minimal inhibitory and bactericidal concentration of raw Malaysian stingless bee honey Biotechnol. Equip. 2020;34(1):575–586. doi: 10.1080/13102818.2020.1788421. [DOI] [Google Scholar]
- Yaoa L., Jiang Y., Singanusong R., Datta N., Raymont K. Phenolic acids in Australian Melaleuca, Guioa, Laphostemon, Banksia and Helianthus honeys and their potential for floral authentication. Food Res. Int. 2005;38:651–658. doi: 10.1016/j.foodres.2005.01.002. [DOI] [Google Scholar]
- Zohair K., Harisa G., Abo-Salem O., Ahmed S. The honey bee is a potential antioxidant against cyclophosphamide-induced genotoxicity in albino male mice. Pak. J. Pharm. Sci. 2015;28(3):973–981. PMID: 26004732. [PubMed] [Google Scholar]
Further Reading
- Mancuso E., Tonda-Turo C., Ceresa C., Pensabene V., Connell S.D., Fracchia L., Gentile P. Potential of Manuka honey as a natural polyelectrolyte to develop biomimetic nanostructured meshes with antimicrobial properties. Front. Bioeng. Biotechnol. 2019;7:344. doi: 10.3389/fbioe.2019.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senedecor G.W., Cochran W.G. seventh ed. Iowa State University Press; Ames, Iowa: 1980. Statistical Methods; pp. 334–364. [Google Scholar]