Abstract
Introduction
With the approval of the adjuvanted recombinant zoster vaccine (RZV; Shingrix, GSK) in October 2017, GSK established enhanced safety surveillance measures to allow prompt identification of potential safety signals not observed during clinical development. In Germany, cases of vesicular and bullous cutaneous eruptions following RZV vaccination were reported.
Objective
Our objective was to search and analyse 2.5 years of worldwide spontaneously reported post-marketing data for vesicular and bullous cutaneous eruptions, represented by adverse events suggestive of (1) herpes zoster (HZ) and (2) non-HZ vesicular and bullous cutaneous eruptions, that occurred following RZV vaccination.
Methods
We conducted a descriptive analysis of all identified reports of HZ and non-HZ vesicular and bullous cutaneous eruptions following RZV vaccination and an observed versus expected (O/E) analysis of reports of HZ that met criteria of varicella zoster virus (VZV) reactivations following RZV vaccination (i.e., time to onset [TTO] of the event < 30 days or missing after any dose).
Results
Until the data lock point, 32,597,779 RZV doses had been distributed globally. There were 2423 reports of HZ (including complications) identified, of which 645 met the criteria of possible vaccination failure (i.e., TTO of the event ≥ 30 days or missing following a complete RZV vaccination schedule). The O/E analysis of 1928 reports assessed as possible VZV reactivations indicated that the observed number of cases was lower than that expected in the general population. Additionally, 810 reports of non-HZ vesicular and bullous cutaneous eruptions were identified, including injection site rashes attributed to the vaccine’s reactogenicity.
Conclusion
This review of spontaneously reported post-marketing data did not raise safety concerns regarding the occurrence of vesicular and bullous cutaneous eruptions following vaccination with RZV.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-021-01118-3.
Plain Language Summary
Shingles is a disease caused by reactivation of the chickenpox virus. It mostly affects adults aged 50 years and older and patients of all ages who have an impaired immune system. Diagnosis of shingles is often based only on the presence of symptoms such as a typical rash and pain. However, rashes can have various other causes (e.g., allergies, autoimmune diseases, and infections). Consequently, rashes with other causes may be misdiagnosed as shingles. Adults at increased risk of shingles and/or aged 50 years and older may be vaccinated with Shingrix (GSK, Belgium) to protect them from shingles and its complications. Since Shingrix became available in Germany, blister-like skin rashes have been reported that occurred shortly after vaccination. We searched the GSK safety database for reports of blister-like skin rashes that occurred following vaccination with Shingrix and that were spontaneously reported from countries where Shingrix was first marketed. To analyse these reports of rashes, we described the reports that we retrieved, we performed a statistical analysis to quantify whether the number of events assessed as reactivations of the chickenpox virus following Shingrix vaccination was higher than the number of reactivations that would be expected in the general population, and we described possible explanations for the observed rashes and underlying disease mechanisms. Our analyses did not raise safety concerns related to the onset of these rashes after vaccination with Shingrix. This paper raises awareness about the varying causes of rashes since a shingles-like rash that onsets shortly after vaccination with Shingrix is not necessarily caused by vaccination. In conclusion, this analysis shows that caution is needed when evaluating rashes in older adults and that all potential contributing factors (e.g., pre-existing diseases, medication, vaccination) should be considered.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-021-01118-3.
Key Points
| The adjuvanted recombinant zoster vaccine (RZV) was first approved in October 2017 to prevent herpes zoster and its complications in adults aged ≥ 50 years. |
| Following the marketing of RZV in Germany in 2018, reports of rashes, including those clinically compatible with herpes zoster, were received following vaccination with RZV. |
| We analysed 2.5 years of worldwide spontaneously reported post-marketing data of vesicular and bullous cutaneous eruptions following RZV vaccination. |
| The analyses did not raise safety concerns related to the onset of vesicular and bullous cutaneous rashes following vaccination with RZV. |
| This paper raises awareness about the varying causes of rashes: even though herpes zoster is a disease characterised by rash, a rash that onsets shortly after vaccination with RZV is not necessarily caused by vaccination. |
Introduction
Varicella zoster virus (VZV) causes varicella (chickenpox), a highly contagious disease that affects most people in the absence of vaccination [1]. After infection, VZV remains dormant in sensory nerve ganglia, from where it may reactivate to cause herpes zoster (HZ; shingles) [2]. The incidence of HZ has been estimated to range between 5.2 and 10.9 cases per 1000 person-years in those aged ≥ 50 years [3]. Generally, the incidence increases with age [3–6], which is primarily attributed to an age-related decline in immunity (immune senescence) and a higher likelihood of the presence of immunosuppressive conditions [5]. HZ is characterised by a painful, localised rash and can be associated with serious complications of the nervous system such as ophthalmic HZ and post-herpetic neuralgia (PHN) [2, 7–9].
To prevent HZ and its complications, adults aged ≥ 50 years may be vaccinated with a live attenuated HZ vaccine or the adjuvanted recombinant zoster vaccine (RZV; Shingrix, GSK, Belgium) [10, 11]. Additionally, RZV can be used in adults aged ≥ 18 years who are at increased risk of HZ [12]. RZV consists of a truncated form of the VZV glycoprotein E (gE) antigen adjuvanted with the AS01B system [10]. In adults aged ≥ 50 years, two doses of RZV had a vaccine efficacy against HZ ranging between 89.8 and 97.2%, depending on the participants’ age, as demonstrated in two large, parallel, phase III, randomised, observer-blind, controlled trials of RZV (ZOE-50 [NCT01165177] and ZOE-70 [NCT01165229]) [13, 14]. Pooled safety data from ZOE-50/70 showed that RZV was more reactogenic than placebo (i.e., individuals vaccinated with RZV reported more injection site reactions and common systemic symptoms such as fever, myalgia, fatigue, chills, headache, or gastrointestinal symptoms than individuals vaccinated with placebo). The occurrence of serious adverse events (SAEs), potential immune-mediated diseases (pIMDs [15]), and deaths was similar between vaccine and placebo recipients during the median 4 years of follow-up. Skin and subcutaneous tissue disorders (system organ class) and various types of cutaneous eruptions by Medical Dictionary for Regulatory Activities (MedDRA®) preferred term (PT) also occurred at the same rate between vaccine and placebo recipients [16]. Overall, these data demonstrated no safety concern related to RZV vaccination in adults aged ≥ 50 years [13, 14, 16, 17]. Furthermore, clinical trials in adults with a compromised immune system, who are at increased risk of HZ, also did not identify safety concerns of RZV vaccination in this population [18–21].
With the approval of RZV for immunisation of adults aged ≥ 50 years from October 2017 onwards [12, 22, 23], GSK established enhanced post-marketing safety surveillance measures to promptly identify safety signals [24], for which data were continuously shared with regulatory authorities. A review of the first 1.5 years of post-marketing safety surveillance data showed that the post-marketing safety profile of RZV was consistent with that previously observed in pre-licensure clinical trials and reflected in the RZV patient leaflet [25].
Following the marketing of RZV in Germany in 2018, the Drug Commission of the German Medical Association (DCGMA) and Paul-Ehrlich-Institut (PEI) received reports of vesicular and bullous cutaneous eruptions, including blistering eruptions clinically compatible with HZ rash, that occurred in close temporal association with RZV vaccination. The DCGMA and PEI initiated a study to further investigate such reports [26]. Here, the occurrence of vesicular and bullous cutaneous eruptions following vaccination with RZV was evaluated, based on the available post-marketing data comprising 2.5 years of spontaneously reported data.
Materials and Methods
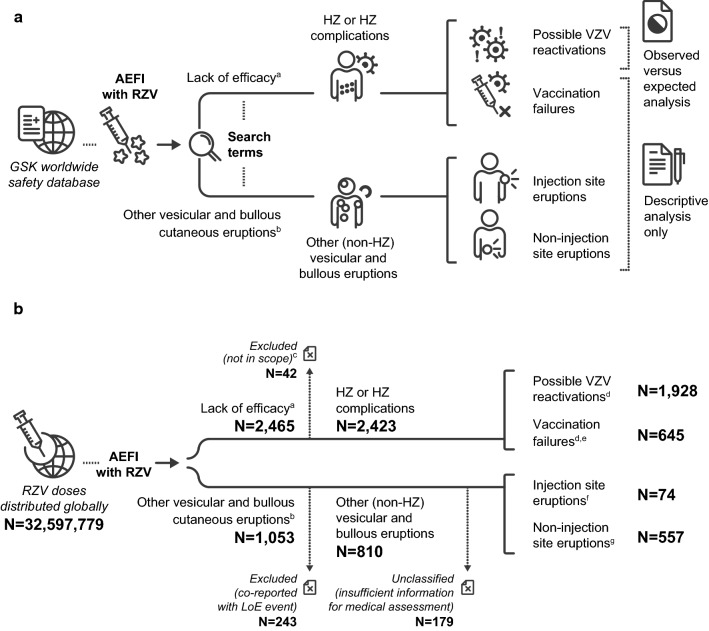
A summary of the study methodology is shown in Fig. 1a. In short, spontaneous reports of adverse events (AEs) suggestive of (1) HZ and (2) non-HZ vesicular and bullous cutaneous eruptions following vaccination with RZV were searched. A descriptive analysis of all identified reports (regardless of time to onset [TTO] and categorisation [detailed in Sect. 2.3]) and an observed versus expected (O/E) analysis of reports of HZ assessed as possible VZV reactivation following vaccination with RZV (i.e., a TTO of the event < 30 days [day 0–29] or unknown after any dose) were performed.
Fig. 1.
a Study methodology and b number of reports identified. Note: One report could contain more than one adverse event reported by the same individual. aIdentified with the Medical Dictionary for Regulatory Activities (MedDRA®) preferred terms listed in ESM 2. bIdentified with the MedDRA® preferred terms listed in ESM 3. cExcluded because linked to a co-administered vaccine and not to the adjuvanted recombinant zoster vaccine. dReports with unknown time to onset were included among both “possible VZV reactivations” and “vaccination failures.” eIncluded suspected and confirmed vaccination failures. fDetails on these reports are provided in ESM 6. gDetails on these reports are provided in Table 2. AEFI adverse events following immunisation, ESM electronic supplementary material, HZ herpes zoster, LoE lack of efficacy, N total number of reports in a given category, RZV adjuvanted recombinant zoster vaccine, VZV varicella zoster virus
Data Source
The occurrence of any AE suggestive of vesicular and bullous cutaneous eruptions (including HZ and non-HZ rashes) following vaccination with RZV was analysed using data recorded in the GSK worldwide safety database, which contains spontaneous reports of AEs following immunisation (AEFIs) with RZV. The seriousness of the AEFIs was based on details provided by the reporter in accordance with the definition of SAEs defined by the International Council for Harmonisation (ICH) regulatory guidelines [27] (see the electronic supplementary material [ESM] 1). The analytical period ran from the date of RZV launch (October 2017) until 12 April 2020.
Search Strategy
Customised search criteria with selected PTs from the MedDRA® terminology developed by the ICH were used [28]. To identify reports of AEs suggestive of HZ (including HZ complications), a customised search was performed using the standardised MedDRA® query for lack of efficacy (LoE) combined with a GSK-customised MedDRA® query for RZV-specific LoE (ESM 2). To identify reports of AEs suggestive of other (non-HZ) vesicular and bullous cutaneous eruptions, we proposed a customised list of MedDRA® PTs (ESM 3).
Medical Review and Categorisation of Identified Reports
All identified spontaneous reports were reviewed, regardless of whether the reports were medically confirmed (i.e., confirmed by a healthcare professional [HCP] or by medical documentation).
Based on the medical review, all identified reports were categorised as (1) AEs suggestive of HZ or HZ complications or (2) AEs suggestive of other vesicular and bullous cutaneous eruptions (non-HZ):
(1) AEs suggestive of HZ and HZ complications: Reports identified using the PTs indicative of LoE (ESM 2) were considered to be secondary to or diagnosed as HZ or an HZ complication. It was further assessed whether these reports met the criteria for (1.1) possible VZV reactivations or (1.2) vaccination failures as follows:
(1.1) Possible VZV reactivations: Reports with a TTO < 30 days (based on the proposed pathophysiological mechanism underlying possible VZV reactivations, which is explained in Sect. 4) or an unknown TTO (conservative approach) after any vaccine dose were considered possible VZV reactivations and were included in the O/E analysis.
(1.2) Vaccination failures:
Confirmed vaccination failure The case presented with HZ clinical symptoms that occurred ≥ 30 days after receiving the full vaccination schedule (two doses) and had a laboratory-confirmed VZV infection (based on a positive polymerase chain reaction, culture, immunohistochemical staining, or other test strongly suggestive of VZV and performed in the course of a medical evaluation).
Suspected vaccination failure The case presented with HZ clinical symptoms that occurred ≥ 30 days after receiving the full vaccination schedule (two doses) but did not have a laboratory-confirmed VZV infection. Reports with an unknown TTO after receiving the full vaccination schedule (two doses) were conservatively considered to meet criteria for suspected vaccination failure. Reports that had a TTO < 30 days or included only one vaccine dose administered were considered to not meet criteria for vaccination failure (confirmed or suspected).
(2) AEs suggestive of other vesicular and bullous cutaneous eruptions (non-HZ): Reports identified using the PTs indicative of other (non-HZ) vesicular and bullous cutaneous eruptions (ESM 3) were considered to present aetiologies unrelated to HZ. Reports that were co-reported with AEs suggestive of HZ or HZ complications (i.e., identified using the PTs indicative of LoE listed in ESM 2) were excluded from this group and were discussed only among AEs suggestive of HZ or HZ complications, as detailed earlier. Based on medical review of the case narrative, all identified reports of AEs suggestive of other (non-HZ) vesicular and bullous cutaneous eruptions were further classified as injection site eruptions or non-injection site eruptions.
Analyses
Descriptive Analysis
A descriptive analysis was performed for all identified reports of AEs suggestive of vesicular and bullous cutaneous eruptions (i.e., reports identified with both searches in ESM 2 and 3). All reports were categorised as detailed in Sect. 2.3. Additionally, it was assessed whether the reports of AEs suggestive of HZ or HZ complications (identified using the PTs indicative of LoE; ESM 2) occurred in individuals with a history of HZ. Data were shown as the number and/or proportion of individuals who reported the event.
Observed vs. Expected (O/E) Analysis for Possible Varicella Zoster Virus Reactivations
An O/E analysis was performed to compare the observed number of possible VZV reactivations that occurred in the pre-defined risk period with the estimated expected number in the general population. The O/E analysis was conducted as previously described [29].
As detailed earlier, AEs suggestive of HZ or HZ complications that were retrieved using the MedDRA® PTs listed in ESM 2 were further categorised as possible VZV reactivations if the TTO of the event was < 30 days (day 0–29) or unknown after any vaccine dose. Reports with HZ clinical symptoms occurring ≥ 30 days after receiving the full vaccination schedule (two doses; i.e., that met the criteria for confirmed or suspected vaccination failure) were excluded from the O/E analysis. As a conservative approach, if the TTO was unknown after any dose, the case was considered to have occurred in the risk period. Confidence intervals (CIs) for the reported number of cases were calculated from an exact Poisson distribution [30], which depends only on the observed number of cases.
The observed number of possible VZV reactivations was calculated by dividing the reported number of these possible VZV reactivations by the reporting fraction (RF). The RF is the proportion of possible VZV reactivations reported among all those events that actually occurred in the vaccinated general population within the risk period, regardless of the causality. Exact 95% CIs were calculated for the observed number of cases.
The age-adjusted expected number of cases (Ne) was calculated as
with the following definitions:
Incidence of the diseasen the background incidence of HZ in the general population in age stratum n, expressed in person-years. Background incidences for countries where RZV was first marketed (i.e., Germany, Canada, and the USA) were estimated from systematic literature reviews of epidemiological studies that provided age-stratified incidence rates (ESM 4) [31–34]. To account for an increasing incidence of HZ with age, background incidences were standardised according to the age distribution of RZV recipients in each country, which was inferred from all spontaneous reports of AEFIs with RZV available in the GSK safety database.
Ndosesn total number of doses administered in age stratum n, which was estimated based on sales data for RZV from launch until 12 February 2020. A time lag of 2 months compared with the data lock point for the reported number of cases was used to account for the average time lag between dose shipment and dose administration and to minimise the risk of inaccuracy as a consequence of sales database updates.
Risk period the time at risk after vaccination, expressed in years. The risk period ran from the day of vaccination until day 29 post-vaccination (i.e., TTO < 30 days). Each vaccine dose independently contributed to the total time at risk.
The observed and age-adjusted expected number of cases were computed for worldwide and for the USA, Canada, and Germany, which are the countries where RZV was first marketed. The O/E analysis was conducted separately for worldwide and the included countries so as not to dilute any possible effects in Canada and Germany because the majority (90%) of RZV doses had been sold in the USA. The analysis was performed for the different background HZ incidence rates while estimating the expected number of cases from the background incidence rates of the country-specific references (ESM 4) [31–34].
Based on the potential biological mechanisms underlying VZV reactivation, clinical symptoms are expected to occur only from 7 days post-vaccination (rationale is provided in Sect. 4). Therefore, a sensitivity analysis was run with a risk period of 22 days (TTO between days 8 and 29). If the TTO was unknown after any dose, it was imputed based on the country-specific distribution of known TTOs.
The O/E analyses were performed using Statistical Analysis System (SAS) version 9.4.
Results
Descriptive Analysis
A total of 3274 reports were retrieved from the GSK worldwide safety database by both searches combined (ESM 2 and 3); one report could contain more than one AE reported by the same individual and/or a report could have been retrieved by both searches. The categorisation of retrieved reports is graphically presented in Fig. 1b. Most of the reports were from HCPs (69.3%), from the USA (53.4%), from events occurring in females (59.0%) and in individuals aged 50–69 years (36.4%). Approximately one-quarter of all reports were assessed as serious (27.3%). Among the reports for which the outcome was known (32.6%), most were recovered/resolved (11.9%) at the time of reporting (Table 1).
Table 1.
Characteristics of spontaneous reports retrieved
| Characteristics | N | % |
|---|---|---|
| Total reports | 3274 | 100 |
| Sex | ||
| Female | 1933 | 59.0 |
| Male | 934 | 28.5 |
| Unknown | 407 | 12.4 |
| Reporter | ||
| Consumer/non-HCP | 1006 | 30.7 |
| HCP (other)a | 1358 | 41.5 |
| HCP (physician) | 910 | 27.8 |
| Country of occurrence | ||
| Canada | 921 | 28.1 |
| Germany | 597 | 18.2 |
| USA | 1748 | 53.4 |
| Other | 8 | 0.2 |
| Age group, years | ||
| < 50 | 46 | 1.4 |
| 50–69 | 1193 | 36.4 |
| > 70 | 922 | 28.2 |
| Unknown | 1113 | 34.0 |
| Case outcome | ||
| Fatal | 4 | 0.1 |
| Not recovered/not resolved | 344 | 10.5 |
| Not reported | 164 | 5.0 |
| Recovered/resolved | 390 | 11.9 |
| Recovering/resolving | 153 | 4.7 |
| Resolved with sequelae | 11 | 0.3 |
| Unchanged | 1 | 0.0 |
| Unknown | 2207 | 67.4 |
| Seriousness | ||
| Non-serious | 2381 | 72.7 |
| Serious | 893 | 27.3 |
HCP healthcare professional, N number of retrieved reports in a given category, % percentage of retrieved reports in a given category
aIncludes reports by nurses, pharmacists, and unspecified HCPs
Adverse Events (AEs) Suggestive of Herpes Zoster (HZ) or HZ Complications
The search using MedDRA® PTs indicative of LoE (ESM 2) retrieved 2465 reports, 42 of which were assessed as not related to LoE after medical review (i.e., AEs suggestive of HZ or HZ complications linked to a co-administered vaccine and not to RZV; out of scope of the analysis), resulting in 2423 reports of AEs suggestive of HZ or HZ complications retained for analysis (Fig. 1b). Of these 2423 reports, 803 were serious. TTO was < 30 days (day 0–29) for 1096 reports (included in the O/E analysis), ≥ 30 days for 495 reports (included in the descriptive analysis), and unknown for 832 reports (included in both the descriptive and the O/E analyses) (ESM 5).
Vaccination Failures
Most of the 2423 retained reports of AEs suggestive of HZ or HZ complications did not meet the criteria for vaccination failure (n = 1778 [73.4%]), whereas the remainder met the criteria of confirmed (n = 2 [0.1%]) or suspected (n = 643 [26.5%]) vaccination failure (Fig. 1b). The 645 reports that met the criteria of vaccination failure (suspected or confirmed) corresponded to a reporting rate of 2.0 cases per 100,000 RZV doses distributed. They were most frequently reported from Canada (n = 316 [49.0%]) and by a non-HCP source (55.0%). The median age of the individuals was 67 years, where age was reported.
Characteristics of Reporting
Most of the 2423 retained reports of AEs suggestive of HZ or HZ complications were recorded in the GSK safety database using the PT “herpes zoster” (n = 2344; reporting rate of 7.19 per 100,000 doses distributed). Other reports included AEs suggestive of HZ complications, with the most frequently reported complications following vaccination with RZV being PHN (n = 92; reporting rate of 0.28 per 100,000 doses distributed), HZ ophthalmicus (n = 81; reporting rate of 0.25 per 100,000 doses distributed), and HZ oticus (n = 12; reporting rate of 0.04 per 100,000 doses distributed). Less frequent HZ complications were cutaneous disseminated HZ (n = 4), disseminated HZ (n = 3), and neurological HZ infection (n = 2). One report could contain more than one AE, reported by the same individual. Among the 81 HZ ophthalmicus reports, 18 were suspected vaccination failures. From the remaining 63 HZ ophthalmicus reports, 54 contained insufficient information for evaluation and nine had reported historical medical conditions that could have contributed to the event (five cases with a previous history of HZ or HZ ophthalmicus, one case with a history of keratitis and corneal scarring, and three cases with a history of malignancy or autoimmune disease). Among the other HZ complications (i.e., PHN, HZ oticus, [cutaneous] disseminated HZ, and neurological HZ; n = 113), 90 reports did not meet vaccination failure criteria, and the other 23 reports were assessed as suspected vaccination failures.
Among the 2423 retained reports of AEs suggestive of HZ or HZ complications, 447 occurred in individuals who, prior to vaccination with RZV, reportedly had at least one episode of HZ (n = 434), HZ ophthalmicus (n = 11), and/or PHN (n = 5). This corresponded to a reporting rate of 1.37 cases per 100,000 doses distributed. Of these 447 reports, 129 constituted suspected or confirmed vaccination failures, and the majority were reported from the USA (n = 203), followed by Germany (n = 146) and Canada (n = 95). The age of the individuals ranged from 34 to 96 years.
AEs Suggestive of Other (Non-HZ) Vesicular and Bullous Cutaneous Eruptions
The search using MedDRA® PTs indicative of other (non-HZ) vesicular and bullous cutaneous eruptions (ESM 3) retrieved 1053 reports. For 243 of these, the events were co-reported with LoE and were therefore excluded from this analysis (Fig. 1b). These are discussed among the reports of AEs suggestive of HZ or HZ complications (Sect. 3.1.1). From the remaining 810 reports, 81 (10.0%) were assessed as serious by the reporter (n = 3) or by the medical reviewer (n = 78), the latter based on the case being medically significant (n = 73), requiring hospitalisation (n = 4), or leading to disability or incapacity (n = 1).
From the 810 retained reports, 179 contained insufficient information for medical assessment and remained unclassified. The other 631 reports were classified as injection site eruptions (n = 74) and non-injection site eruptions (n = 557) and were further reviewed (Fig. 1b).
Injection Site Eruptions
All 74 injection site eruptions were assessed as secondary to reactogenicity symptoms. Of these, three cases were assessed as serious; for 22 cases, the outcome was recorded as recovered/resolved. The PTs that were recorded for these cases are listed in ESM 6.
Non-Injection Site Eruptions
The 557 non-injection site eruptions were classified as having the following alternative aetiologies: pIMDs (n = 19), non-injection site hypersensitivity rashes (n = 102; including one case of anaphylaxis), and other aetiologies (n = 436) (Table 2).
Table 2.
Events of interest among the other (non-herpes zoster) vesicular and bullous cutaneous eruptions identified with the search using the MedDRA® preferred terms indicative of other vesicular and bullous cutaneous eruptions,a that contained sufficient information for assessment and were classified as non-injection site eruptions based on medical review of the case narrative
| Event typeb | Number of reportsc (N = 557) | Reporting rate per 100,000 doses distributed | Number of serious reportsc | TTOd (post-vaccination) |
|---|---|---|---|---|
| pIMDs | 19 | 0.06 | 17 | 3 h–< 9 months |
| Autoimmune bullous skin disease (pemphigus, pemphigoid) | 11 | 0.03 | 11 | 1 day–< 2 years |
| Stevens–Johnson syndrome | 3 | 0.01 | 3 | 3 h–7 days |
| Erythema multiforme | 2 | 0.01 | 2 | 21 days |
| Psoriasis (rash vesicular) | 2 | 0.01 | 0 | 2 days; < 9 months |
| Systemic lupus erythematosus (blister) | 1 | 0.00 | 1 | 2 days |
| Non-injection site hypersensitivity rashese | 102 | 0.31 | 12 | Immediately–160 days |
| Rash vesicular | 51 | 0.16 | 5 | Immediately–2 months |
| Blister | 38 | 0.12 | 2 | Immediately–160 days |
| Pustule | 9 | 0.03 | 2 | < 1 day–18 days |
| Rash pustular | 5 | 0.02 | 0 | 4 h–8 days |
| Anaphylactic reaction (rash vesicular) | 1 | 0.00 | 1 | 2 h |
| Other aetiologies | 436 | 1.34 | 39 | Immediately–< 2 years |
| Oral herpes | 149 | 0.46 | 8 | 1 day–< 1 year |
| Blister | 120 | 0.37 | 7 | Immediately–< 2 years |
| Rash vesicular | 80 | 0.25 | 5 | Immediately–< 2 years |
| Herpes simplex | 45 | 0.14 | 11 | 1 day–< 1 year |
| Varicella | 39 | 0.12 | 3 | 1 day–45 days |
| Herpes virus infection | 13 | 0.04 | 1 | Immediately–< 4 months |
| Genital herpes | 13 | 0.04 | 0 | Immediately–< 2 years |
| Rash pustular | 10 | 0.03 | 0 | 2 days–4 months |
| Ophthalmic herpes simplex | 9 | 0.03 | 9 | 4 days–< 2 months |
| Herpes ophthalmic | 8 | 0.02 | 8 | 2 days–< 1 year |
| Acne (acne pustular, rash vesicular) | 3 | 0.01 | 0 | 1 day–14 days |
| Injection site vesicles | 1 | 0.00 | 0 | 24 days |
ESM electronic supplementary material, MedDRA® Medical Dictionary for Regulatory Activities, N total number of reports classified as non-injection site eruptions, pIMD potential immune-mediated disease, PT preferred term, TTO time to onset
aSee ESM 3
bEvents of interest were reviewed and classified manually based on the diagnosis or possible aetiology. Consequently, the reported event type may not be a MedDRA® PT. In such cases, the corresponding MedDRA® PT (ESM 3) is reported between brackets
cOne report could contain more than one adverse event reported by the same individual
dTime to onset was not available for all reports
eAssessed as immediate- or delayed-type hypersensitivity reaction based on the event description and considering the time to onset
There were 19 reports of AEs suggestive of non-HZ vesicular and bullous cutaneous eruptions that were reported in the context of the following pIMD diagnoses: autoimmune bullous skin disease (n = 11), Stevens–Johnson syndrome (SJS; n = 3), erythema multiforme (n = 2), psoriasis (n = 2), and systemic lupus erythematosus (n = 1). Of these reports, 17 were assessed as serious (Table 2). For most cases, the case narratives contained insufficient information for a full assessment (Table 2; described in more detail in ESM 7). Among the two cases reported in the context of psoriasis, one had a TTO of 2 days post-vaccination. The other report had a TTO of < 9 months post-vaccination but contained insufficient information for full assessment. The one case reported in the context of systemic lupus erythematosus developed 2 days post-vaccination.
The 102 reports that were classified as a non-injection site hypersensitivity rash included the following events of interest: vesicular rash (n = 51), blister (n = 38), pustule (n = 9), pustular rash (n = 5), and anaphylaxis (n = 1). Of these reports, 12 were assessed as serious (Table 2). The majority (n = 87) were reports of delayed-type hypersensitivity reactions as per the TTO and medical review of the case narrative. The report categorised as anaphylaxis occurred 2 h post-vaccination. The individual was treated with an antihistamine.
The remaining 436 reports of non-injection site eruptions were categorised as other aetiologies, which included other non-allergic and non-injection site localised rashes or events. Of these 436 reports, 39 were assessed as serious (Table 2). These reports of non-injection site eruptions included the following events of interest: blister (n = 120), vesicular rash (n = 80), pustular rash (n = 10), acne (n = 3; including one report of pustular acne), and injection site vesicles (n = 1). These reports were categorised as other aetiologies mainly because they were co-reported with viral infections, including oral herpes (n = 149), herpes simplex (n = 45), varicella (n = 39), herpes virus infection (n = 13), genital herpes (n = 13), ophthalmic herpes simplex (n = 9), and ophthalmic herpes (n = 8). When documented (n = 355), the TTO ranged from immediately (1 h) to less than 2 years post-vaccination. The one report of injection site vesicles occurred 24 days post-vaccination. The individual also developed injection site haemorrhage, skin rash, and altered sensitivity to touch. When taking into consideration the TTO, other conditions such as viral exanthema or delayed-type hypersensitivity reactions may be considered. Among all reports of AEs suggestive of other vesicular and bullous cutaneous eruptions that were categorised as other aetiologies, there was no indication for a presumed underlying immunoglobulin E-mediated immune mechanism; alternative factors may have explained the event occurrence. Therefore, other aetiologies such as viral exanthems, localised skin infection, or irritation due to minor trauma (e.g., skin rubs against skin) may be considered; however, there was insufficient information for diagnosis ascertainment.
O/E Analysis
During the analytical period, 32,597,779 RZV doses were distributed globally, including 1,045,785 doses in Germany, 2,170,178 doses in Canada, and 29,380,658 doses in the USA.
There were 1928 spontaneous reports of AEs suggestive of HZ or HZ complications identified from the search using MedDRA® PTs indicative of LoE (ESM 2) that met the VZV reactivation criteria (TTO < 30 days [day 0–29] or unknown after any dose) (Fig. 1b and ESM 5).
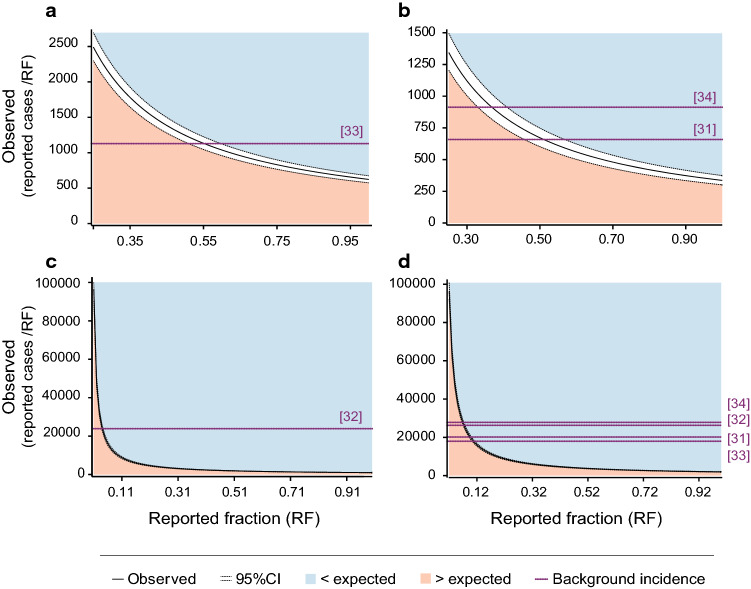
When considering an RF of 75%, the observed number of possible VZV reactivations was lower than the expected number in all countries (ESM 8). The observed number of possible VZV reactivations was higher than the expected number for RFs < 10% for worldwide and the USA, < 56% for Canada, and < 53% for Germany (Fig. 2 and ESM 9).
Fig. 2.
Significance area for the equality test of observed and expected incidences of herpes zoster within 30 days (day 0–29) after vaccination with the adjuvanted recombinant zoster vaccine in a Canada, b Germany, c the USA, and d worldwide. Number of doses distributed was 2,170,178 for Canada, 1,045,785 for Germany, 29,380,658 for the USA, and 32,597,779 worldwide. Background incidence represents the herpes zoster incidence rate in the general population and is adjusted for the age distribution of recipients of the adjuvanted recombinant zoster vaccine. CI confidence interval
The sensitivity analysis, which considered a shorter risk period (days 8–29 post-vaccination), was in line with the main analysis and indicated that the observed number of possible VZV reactivations was higher than the expected number for RFs < 22% (ESMs 8, 10, and 11).
Discussion
In this analysis, spontaneous reports of AEs suggestive of HZ or HZ complications and other (non-HZ) vesicular and bullous cutaneous eruptions that occurred following vaccination with RZV were assessed (Fig. 1a). It is important to note that cutaneous eruptions can have multiple aetiologies, such as viral infections, autoimmune diseases, vaccination, and allergic-type reactions, even when occurring in close temporal relation with vaccination. In particular, the elderly, one of the target populations for RZV, can show a marked susceptibility to dermatological disorders manifesting with eruptions. This is because of structural and physiological changes in their cutaneous membranes that may occur as a consequence of ageing and a lifetime of exposure to environmental and lifestyle factors and that can result in xerosis and reduced strength and elasticity [35]. The elderly also have an increased risk of cutaneous drug reactions because of such comorbidities as well as polypharmacy, which can complicate accurate diagnosis and safety assessment of dermatological disorders reported in this patient group [35, 36].
Our search in the GSK worldwide safety database using the MedDRA® PTs indicative of LoE (ESM 2) identified 2423 reports of AEs suggestive of HZ or HZ complications. Among these, only two reports were laboratory-confirmed cases, and 643 reports were suspected cases based on the presence of HZ clinical symptoms and TTO post-vaccination. Conservatively, suspected cases were included in the analysis despite limited diagnostic certainty. Most confirmed and suspected vaccination failures (49%) were reported from Canada via a Canadian RZV social media page. These cases were not medically confirmed, and the provided clinical details and possibility for follow-up were very limited. The reporting rate for vaccination failures was low (2.0 cases per 100,000 RZV doses distributed), in line with the high efficacy of RZV in adults aged ≥50 years demonstrated in clinical trials [13, 14, 16, 17]. A reporting rate of 1.37 cases per 100,000 RZV doses distributed for AEs suggestive of HZ or HZ complications that occurred in individuals who previously had an episode of HZ was observed. HZ recurrence incidence rates of 1–10 cases per 1000 person-years have been reported in immunocompetent populations [7, 37, 38]. Together, the reporting rates observed in our analysis did not raise any safety concerns.
Our search using the MedDRA® PTs suggestive of other (non-HZ) vesicular and bullous cutaneous eruptions (ESM 3) identified a total of 810 non-HZ reports (i.e., that were not co-reported with an LoE event). Most of the non-HZ reports that included sufficient information for full assessment were non-allergic and non-injection site localised eruptions. This may be explained by RZV being first licensed for immunisation of older adults, who have a higher prevalence of and susceptibility for dermatological disorders in general [35, 36] and for hypersensitivity rashes including vesicular, blistering, or pustular rashes as clinical manifestations. Hypersensitivity rashes are rarely reported with vaccines in general and are also recognised and reflected in the patient leaflet for RZV [10, 12]. Local injection site reactions secondary to the vaccine’s reactogenicity occurred in 74 reports and are also reflected in the patient leaflet for RZV [10, 12]. Among the 19 AEs suggestive of other (non-HZ) vesicular and bullous cutaneous eruptions that were reported in the context of a pIMD diagnosis, four reports (one autoimmune bullous skin disease, one SJS, one psoriasis, and one systemic lupus erythematosus report) had a TTO of < 5 days. Studies have shown that pro-inflammatory cytokine production induced by the adjuvant system AS01 is transient, with a peak on day 1 and return to baseline by day 2–3, and localised at the injection site and draining lymph node [39]. Based on this, it would likely take more than 5–7 days for symptoms of a new pIMD to manifest [15], indicating that those four pIMDs were unlikely a consequence of the vaccination. In the remaining reports, the TTO was unknown, and/or no medical assessment was possible because clinical information was limited or because assessment of a causal relationship with vaccination was confounded and alternative aetiologies may be considered. Overall, the review of spontaneous reports of AEs suggestive of other (non-HZ) vesicular and bullous cutaneous eruptions following vaccination with RZV did not raise safety concerns.
There were 1928 reports among those identified using the MedDRA® PTs indicative of LoE (ESM 2) that met criteria for possible VZV reactivations (TTO < 30 days or unknown after any vaccine dose). An O/E analysis demonstrated that, generally, the observed incidence of HZ cases following RZV vaccination was below the background incidence in the general population [31–34], and this may be explained by the protection conferred by the vaccine or underreporting of cases. Worldwide and in the USA, the observed number of cases was higher than expected only for very low levels of RF (< 10%). In Germany and Canada, the observed number of cases was higher than the expected number for RFs < 56%. Estimates have indicated that between 50 and 81% of AEFIs are generally reported [29], meaning that the RFs estimated for Germany and Canada are at the lower end of this range. Consequently, it is likely that the actual RFs for these countries are higher than the RF limits estimated in our analysis. There is some indication for a higher likelihood to be vaccinated with RZV within 30 days after an HZ episode (data on file; publication in preparation). Therefore, the risk of recurrent HZ may be higher than the background incidence rates identified from the literature, which decreases the O/E ratio, and the RF limits reported here may therefore be an overestimation. Overall, RF limits below which the observed number of cases was higher than the expected number were lower in the sensitivity analysis than in the main analysis. The risk period of the sensitivity analysis (starting 7 days post-vaccination) seems more reflective of an actual HZ episode from a pathophysiological perspective. More specifically, when VZV is reactivated, it is transported along microtubules within sensory axons to infect epithelial cells. At these sites, VZV replicates and causes the typical vesicular rash usually about 10–21 days after infection [40]. Although very unlikely, if postulating that inflammatory cytokines, of which levels peaked 1 day following administration of a vaccine containing the adjuvant system AS01 in animal models [39], could theoretically induce VZV reactivation, a period of more than 7 days would likely be needed for the apparition of HZ vesicular rash.
Two possible biological mechanisms have been hypothesised for VZV reactivation to occur following vaccination with RZV [26]. The first hypothesis would be that vaccination with RZV may cause immune exhaustion, which is the result of chronic T-cell stimulation and causes suboptimal control of infections [26, 41]. However, to date, such chronic stimulation has not been described following HZ disease or vaccination with RZV. On the contrary, it has been demonstrated that VZV-specific T cells that functioned as effector T cells at the peak of an HZ episode reverted to a polyfunctional memory phenotype within 4 months of recovery [42]. Additionally, considering that gE is not expressed during VZV latency [43] and that the gE antigen in RZV rapidly degrades following vaccination [44], it is unlikely that chronic antigenic stimulation of gE-specific T cells occurs following vaccination with RZV. Furthermore, telomere shortening, which has been linked with immune exhaustion for several viruses [45], has not been described for VZV-specific T cells; in contrast, one report demonstrated increased telomere size in the VZV-specific T cells of one patient [46]. Lastly, in line with what has been shown for the live attenuated HZ vaccine [47], RZV may mobilise a naive pool of T cells in addition to re-stimulating memory cells. Such naive cells are thus not impacted by previous VZV exposure or previous episodes of HZ. However, it has also been speculated that RZV vaccination during subclinical VZV reactivation could lead to symptomatic HZ because the excess gE might saturate T-cell-mediated immunity that would normally control such subclinical reactivation [26]. The second hypothesis would be that massive inflammation and cytokine production in response to vaccination with RZV may increase the risk of VZV reactivation [26]. The authors of a case report of HZ ophthalmicus following RZV vaccination in a patient who had had HZ ophthalmicus 20 years prior to the reported episode speculated that, since histopathological studies had found VZV DNA in the human cornea up to 8 years after the onset of HZ ophthalmicus, an immune response to the vaccine might trigger an immune response to the VZV DNA in the eye, leading to disease [48] (although detection of VZV DNA does not imply presence of VZV gE). Pre-clinical studies have demonstrated that specific cytokines (i.e., interferon [IFN]-γ, IFNα, interleukin [IL]-6, and tumour necrosis factor-α) inhibit VZV replication and promote latency [49, 50] and that the use of anti-nerve growth factor antibodies reactivates VZV [51, 52]. A study in macaques showed that administration of gE adjuvanted with AS01 resulted in transient increases in IL-6 and low levels of IFNγ with a peak on day 1 [53]. Similar data following administration of RZV in humans are currently not available; however, immunisation of adults with hepatitis B surface antigen adjuvanted with AS01B induced a rapid and transient response of five (IL-6, IFNγ, IFNγ-induced protein 10, monocyte chemotactic protein 2, and macrophage inflammatory protein 1β) of 24 measured cytokines, all peaking between 12 and 24 h post-vaccination [54]. This indicates that no signs of massive inflammation and cytokine production could be observed following immunisation with RZV and that it is very unlikely that an increase in cytokines could be the trigger for VZV reactivation and HZ. Overall, the O/E analysis and discussion of potential underlying pathophysiological mechanisms supported the medical review of the reports retrieved and did not raise safety concerns.
This analysis included the use of a large data set based on 32,597,779 distributed RZV doses from three countries where RZV was first marketed. As such, the analysis was based on a data set that was representative of the population currently exposed to RZV. This analysis had some limitations that are inherent to analyses using spontaneously reported data as a passive reporting system from a population of unknown size, e.g., underreporting, missing information, and misclassification [55]. These limitations may have biased the analyses towards specific regions or patient groups and may have hindered medical review and assessment of the risk period. Furthermore, most identified reports of AEs suggestive of HZ or HZ complications included only clinical diagnosis without any confirmation using laboratory testing. As such, case identification could be biased because of a close temporal relation with RZV vaccination. To formally test whether RZV vaccination increased the frequency of VZV reactivations, an O/E analysis was performed. However, this O/E analysis had uncertainties surrounding the number of doses distributed, the true risk period, and the relevance of the background incidence rates extrapolated from the literature.
Conclusions
Although post-marketing safety surveillance that relies on spontaneous reporting of AEFIs has known limitations, this review of the available data on RZV use in the post-marketing setting did not raise safety concerns regarding the onset of vesicular and bullous cutaneous eruptions following vaccination with RZV. Among the reported non-HZ eruptions, there were anticipated injection site reactions attributed to the known reactogenicity of the vaccine and non-injection site hypersensitivity rashes. The analyses reported here provide important safety information and allow ongoing evaluation of eruptions that are reported following RZV immunisation in the target population. GSK will continue to closely monitor HZ and non-HZ vesicular and bullous cutaneous eruptions occurring in close temporal relation with RZV vaccination as part of routine pharmacovigilance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Froilan Celzo, François Haguinet, and Neeraj Parmar for their contributions to the project. The authors also thank the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK. Lotte Mathé and Kristel Vercauteren provided medical writing support, Gil Costa designed the figures, and Sander Hulsmans coordinated the manuscript development and provided editorial support.
Declarations
Funding
This work was supported by GlaxoSmithKline Biologicals SA, including all costs associated with the development and publication of this manuscript.
Conflicts of interest
Maribel Co, Caroline Hervé, Nicolas Lecrenier, Paola Pirrotta, Jens-Ulrich Stegmann, and Fernanda Tavares-Da-Silva were employees of the GSK group of companies at the time the analysis was designed, initiated, and/or conducted. Caroline Hervé is currently an employee of UCB Pharma and has stock options in UCB Pharma. Jens-Ulrich Stegmann and Fernanda Tavares-Da-Silva hold shares in the GSK groups of companies as part of their employee remuneration. The authors have no other non-financial relationships and activities or conflicts of interest.
Ethics approval
Not applicable
Consent
Not applicable
Trademark statement
Shingrix is a trademark owned by or licensed to the GSK group of companies. MedDRA® is a trademark registered by IFPMA on behalf of ICH.
Availability of data and material
The data that support the findings of this work are available from the corresponding author on reasonable request.
Code availability
Not applicable
Authors’ contributions
Conceptualisation: MC, CH, NL, PP, JUS, and FTDS. Validation: MC, CH, NL, PP, JUS, and FTDS. Formal analysis: MC and PP. Investigation: MC, CH, NL, PP, and FTDS. Resources: MC, NL, PP, and FTDS. Writing (original draft): PP. Visualisation: NL, PP, and FTDS. Supervision: JUS and FTDS. All authors contributed to the writing (review and editing) of the manuscript and approved the final version.
References
- 1.Warren-Gash C, Forbes H, Breuer J. Varicella and herpes zoster vaccine development: lessons learned. Expert Rev Vaccines. 2017 doi: 10.1080/14760584.2017.1394843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JI. Clinical practice: Herpes zoster. N Engl J Med. 2013 doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Oorschot D, Vroling H, Bunge E, Diaz-Decaro J, Curran D, Yawn B. A systematic literature review of herpes zoster incidence worldwide. Hum Vaccin Immunother. 2021 doi: 10.1080/21645515.2020.1847582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014 doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinchinat S, Cebrian-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis. 2013 doi: 10.1186/1471-2334-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013 doi: 10.1212/WNL.0b013e3182a3516e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng HF, Bruxvoort K, Ackerson B, Luo Y, Tanenbaum H, Tian Y, et al. The epidemiology of herpes zoster in immunocompetent, unvaccinated adults ≥50 years old: incidence, complications, hospitalization, mortality, and recurrence. J Infect Dis. 2020 doi: 10.1093/infdis/jiz652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000 doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- 9.Gross G, Schofer H, Wassilew S, Friese K, Timm A, Guthoff R, et al. Herpes zoster guideline of the German Dermatology Society (DDG) J Clin Virol. 2003 doi: 10.1016/s1386-6532(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. Shingrix: Package insert. 2018. https://www.fda.gov/media/108597/download. Accessed 21 Aug 2020.
- 11.European Medicines Agency. Zostavax: Summary of product characteristics. 2009. https://www.ema.europa.eu/en/documents/product-information/zostavax-epar-product-information_en.pdf. Accessed 16 Feb 2021.
- 12.European Medicines Agency. Shingrix - herpes zoster vaccine (recombinant, adjuvant). 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/shingrix. Accessed 22 Dec 2020.
- 13.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016 doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 14.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015 doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 15.Tavares Ds Silva F, De Keyser F, Lambert PH, Robinson WH, Westhovens R, Sindic C. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Fauqued M, Campora L, Delannois F, El Idrissi M, Oostvogels L, De Looze FJ, et al. Safety profile of the adjuvanted recombinant zoster vaccine: Pooled analysis of two large randomised phase 3 trials. Vaccine. 2019 doi: 10.1016/j.vaccine.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Ocran-Appiah J, Boutry C, Herve C, Soni J, Schuind A, Zoster-056 Study Group Safety of the adjuvanted recombinant zoster vaccine in adults aged 50 years or older. A phase IIIB, non-randomized, multinational, open-label study in previous ZOE-50 and ZOE-70 placebo recipients. Vaccine. 2021 doi: 10.1016/j.vaccine.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Berkowitz EM, Moyle G, Stellbrink HJ, Schurmann D, Kegg S, Stoll M, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015 doi: 10.1093/infdis/jiu606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagnew AF, Ilhan O, Lee WS, Woszczyk D, Kwak JY, Bowcock S, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019 doi: 10.1016/S1473-3099(19)30163-X. [DOI] [PubMed] [Google Scholar]
- 20.Vink P, Delgado Mingorance I, Maximiano Alonso C, Rubio-Viqueira B, Jung KH, Rodriguez Moreno JF, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer. 2019 doi: 10.1002/cncr.31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastidas A, de la Serna J, El Idrissi M, Oostvogels L, Quittet P, Lopez-Jimenez J, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019 doi: 10.1001/jama.2019.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Government of Canada. Regulatory Decision Summary-Shingrix - Health Canada. 2017. https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00293. Accessed 24 Aug 2020.
- 23.U.S. Food and Drug Administration. Shingrix - Biologics license application approval. 2017. https://www.fda.gov/media/108274/download. Accessed 24 Aug 2020.
- 24.Tavares-Da-Silva F, Mahaux O, Van Holle L, Haguinet F, Seifert H, Stegmann JU. Post-marketing safety surveillance for the adjuvanted recombinant zoster vaccine: methodology. Drug Saf. 2020 doi: 10.1007/s40264-020-00989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavares-Da-Silva F, Co MM, Dessart C, Herve C, Lopez-Fauqued M, Mahaux O, et al. Review of the initial post-marketing safety surveillance for the recombinant zoster vaccine. Vaccine. 2020 doi: 10.1016/j.vaccine.2019.11.058. [DOI] [PubMed] [Google Scholar]
- 26.Arznei¬mittel¬kommission der deutschen Ärzteschaft „Aus der UAW-Datenbank“ – Fallberichte von Herpes zoster bzw. Zoster-artigen Hautläsionen nach Shingrix ® -Impfung Literatur. Deutsches Ärtzeblatt International. 2020;117(26):1356. [Google Scholar]
- 27.International Conference on Harmonisation. Topic E2A: Clinical safety data management: Definitions and standards for expedited reporting. 1994. https://database.ich.org/sites/default/files/E2A_Guideline.pdf. Accessed 25 Aug 2020.
- 28.Medical Dictionary for Regulatory Activities (MedDRA). 2020. https://www.meddra.org/. Accessed 25 Aug 2020.
- 29.Mahaux O, Bauchau V, Van Holle L. Pharmacoepidemiological considerations in observed-to-expected analyses for vaccines. Pharmacoepidemiol Drug Saf. 2016 doi: 10.1002/pds.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garwood F. Fiducial limits for the poisson distribution. Biometrika. 1936 doi: 10.1093/biomet/28.3-4.437. [DOI] [Google Scholar]
- 31.Gonzalez Chiappe S, Sarazin M, Turbelin C, Lasserre A, Pelat C, Bonmarin I, et al. Herpes zoster: Burden of disease in France. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.09.074. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis. 2015 doi: 10.1186/s12879-015-1262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanuseputro P, Zagorski B, Chan KJ, Kwong JC. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Ultsch B, Siedler A, Rieck T, Reinhold T, Krause G, Wichmann O. Herpes zoster in Germany: quantifying the burder of disease. BMC Infect Dis. 2011 doi: 10.1186/1471-2334-11-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009 doi: 10.2165/00128071-200910020-00001. [DOI] [PubMed] [Google Scholar]
- 36.Blume-Peytavi U, Kottner J, Sterry W, Hodin MW, Griffiths TW, Watson REB, et al. Age-associated skin conditions and diseases: current perspectives and future options. Gerontologist. 2016 doi: 10.1093/geront/gnw003. [DOI] [PubMed] [Google Scholar]
- 37.Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013 doi: 10.1016/j.jinf.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Tseng HF, Chi M, Smith N, Marcy SM, Sy LS, Jacobsen SJ. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis. 2012 doi: 10.1093/infdis/jis334. [DOI] [PubMed] [Google Scholar]
- 39.Didierlaurent AM, Laupeze B, Di Pasquale A, Hergli N, Collignon C, Garcon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017 doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- 40.Ku CC, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med. 2004 doi: 10.1084/jem.20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wherry EJ. T cell exhaustion. Nat Immunol. 2011 doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 42.Schub D, Janssen E, Leyking S, Sester U, Assmann G, Hennes P, et al. Altered phenotype and functionality of varicella zoster virus-specific cellular immunity in individuals with active infection. J Infect Dis. 2015 doi: 10.1093/infdis/jiu500. [DOI] [PubMed] [Google Scholar]
- 43.Gershon AA, Chen J, Davis L, Krinsky C, Cowles R, Reichard R, et al. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans Am Clin Climatol Assoc. 2012;123:17–35. [PMC free article] [PubMed] [Google Scholar]
- 44.Didierlaurent AM, Collignon C, Bourguignon P, Wouters S, Fierens K, Fochesato M, et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J Immunol. 2014 doi: 10.4049/jimmunol.1400948. [DOI] [PubMed] [Google Scholar]
- 45.Bellon M, Nicot C. Telomere dynamics in immune senescence and exhaustion triggered by chronic viral infection. Viruses. 2017 doi: 10.3390/v9100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Bryan JM, Woda M, Co M, Mathew A, Rothman AL. Telomere length dynamics in human memory T cells specific for viruses causing acute or latent infections. Immun Ageing. 2013 doi: 10.1186/1742-4933-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi Q, Cavanagh MM, Le Saux S, NamKoong H, Kim C, Turgano E, et al. Diversification of the antigen-specific T cell receptor repertoire after varicella zoster vaccination. Sci Transl Med. 2016 doi: 10.1126/scitranslmed.aaf1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jabbour S, Shekhawat NS, Chen A, Woreta FA. Presumed herpes zoster ophthalmicus reactivation following recombinant zoster vaccination. Cornea. 2020 doi: 10.1097/ICO.0000000000002537. [DOI] [PubMed] [Google Scholar]
- 49.Como CN, Pearce CM, Cohrs RJ, Baird NL. Interleukin-6 and type 1 interferons inhibit varicella zoster virus replication in human neurons. Virology. 2018 doi: 10.1016/j.virol.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sen N, Sung P, Panda A, Arvin AM. Distinctive roles for type I and type II interferons and interferon regulatory factors in the host cell defense against varicella-zoster virus. J Virol. 2018 doi: 10.1128/JVI.01151-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadaoka T, Depledge DP, Rajbhandari L, Venkatesan A, Breuer J, Cohen JI. In vitro system using human neurons demonstrates that varicella-zoster vaccine virus is impaired for reactivation, but not latency. Proc Natl Acad Sci USA. 2016 doi: 10.1073/pnas.1522575113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baird NL, Zhu S, Pearce CM, Viejo-Borbolla A. Current in vitro models to study varicella zoster virus latency and reactivation. Viruses. 2019 doi: 10.3390/v11020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coccia M, Collignon C, Herve C, Chalon A, Welsby I, Detienne S, et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNgamma response promoting vaccine immunogenicity. NPJ Vaccines. 2017 doi: 10.1038/s41541-017-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burny W, Marchant A, Herve C, Callegaro A, Caubet M, Fissette L, et al. Inflammatory parameters associated with systemic reactogenicity following vaccination with adjuvanted hepatitis B vaccines in humans. Vaccine. 2019 doi: 10.1016/j.vaccine.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther. 1998 doi: 10.1016/s0149-2918(98)80007-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.