Abstract
Urbanization processes are increasing globally. Anthropogenic alterations in the environment have profound effects on biodiversity. Decreased biodiversity due to biotic homogenization processes as a consequence of urbanization often result in increased levels of mosquito vector species and vector-borne pathogen transmission. Understanding how anthropogenic alterations in the environment will affect the abundance, richness, and composition of vector mosquito species is crucial for the implementation of effective and targeted mosquito control strategies. We hypothesized that anthropogenic alterations in the environment are responsible for increasing the abundance of mosquito species that are adapted to urban environments such as Aedes aegypti and Culex quinquefasciatus. Therefore, our objective was to survey mosquito relative abundance, richness, and community composition in Miami-Dade County, Florida, in areas with different levels of urbanization. We selected 24 areas, 16 remote areas comprised of natural and rural areas, and 8 urban areas comprised of residential and touristic areas in Miami-Dade County, Florida. Mosquitoes were collected weekly in each area for 24 h for 5 consecutive weeks from August to October 2020 using BG-Sentinel traps baited with dry ice. A total of 36,645 mosquitoes were collected, from which 34,048 were collected in the remote areas and 2,597 in the urban areas. Our results show a clear and well-defined pattern of abundance, richness, and community composition according to anthropogenic modifications in land use and land cover. The more urbanized a given area the fewer species were found and those were primary vectors of arboviruses, Ae. aegypti and Cx. quinquefasciatus.
Subject terms: Ecological epidemiology, Ecosystem ecology, Evolutionary ecology
Introduction
There are over one billion cases of mosquito-borne diseases reported worldwide every year1. The epidemiology of mosquito-borne diseases is affected by how modifications of the natural environment alter the interactions between vector mosquito species, hosts, and pathogens2. Anthropogenic changes in the environment in the form of climate change, urbanization, and biodiversity loss favor the proliferation of some vector mosquito species, such as Aedes aegypti and Aedes albopictus, and are important drivers for arbovirus transmission in urban areas3–11.
The epidemiology of mosquito-borne diseases continues to be uncertain since the extent to which mosquito species are dispersing across spatiotemporal scales and how urbanization processes are affecting their presence and abundance remains unclear2,12,13. Recent estimates have shown that 129 of the world’s countries and territories have conducive environments for the proliferation of mosquito vector species and are at risk of dengue transmission1,14.
Urbanization processes are ubiquitous in the contemporary world. Land use and land cover transformation to better suit the needs of the human population have led to the degradation of natural environments15,16. The human footprint can be found in most areas of the planet, including uninhabited areas17–19. The human population continues to grow and is expected to reach 8.5 billion people in 2030, 10 billion in 2050, and 11 billion in 210020.
The urbanization of natural areas unavoidably leads to habitat fragmentation and shifts in resource availability15. The availability of resources in urban areas, and most importantly, the absence of resources that were available prior to their urbanization are responsible for either supporting or halting the proliferation of populations of vector mosquito species3,21–23. Many vector mosquito species are abundant in natural areas but due to their ecology and behavior are unable to invade and colonize urban areas9,24. On the other hand, mosquito vectors of anthroponotic vector-borne diseases represent a more pressing matter from the epidemiological standpoint due to their increased contact with humans25,26. Therefore, understanding how mosquito community composition and abundance are impacted by urbanization is key for the development of effective mosquito control strategies.
Many drivers are responsible for the proliferation of mosquito vectors of anthroponotic vector-borne diseases in urban areas. A previous study showed that, in Africa, urbanization processes are increasing the preference of Ae. aegypti to humans, being mainly driven by two factors: dry season intensity and human population density27. Furthermore, the levels of human exposure to mosquito vector species, especially in populations that spend a disproportioned amount of time outdoors such as the construction workforce28, was found to be an important driver for Zika virus transmission29,30. In this context, many socio-ecological drivers are important for mosquito proliferation in urban areas and play an important role in disease transmission31,32. Low-income neighborhoods and underserved populations are often more vulnerable to bites of mosquito vectors and consequently are at a higher risk of being exposed to arboviruses33–35.
Understanding the processes by which mosquito vector species adapt and thrive in urban environments is vital not only for the implementation of mosquito control strategies but also for improving and guiding policy to prevent outbreaks22,36,37. This is especially true for arboviral diseases such as dengue, Zika, and chikungunya, which are transmitted primarily by mosquito vector species that can thrive in urban environments such as Ae. aegypti and Ae. albopictus38–40.
Miami-Dade County, Florida has been the most affected county in the contiguous United States by Aedes-borne diseases41–46. Multiple introductions of the Zika virus to Miami-Dade in 201647, have led to 256 locally transmitted human cases48, and in 2020, 6 cases of locally transmitted dengue virus were reported in Miami-Dade by the Florida Department of Health49. Furthermore, the Florida Department of Health reported 59 human cases of West Nile virus in Miami-Dade in 202050, most of them reported in highly urbanized areas inhabited by underserved populations. Miami-Dade has suitable conditions for the proliferation of mosquitoes3,9. It has a warm climate and abundant rainfall added to rapid urban development to accommodate growing immigrant populations as well as transient underserved populations51.
Vector mosquito species such as Ae. aegypti and Cx. quinquefasciatus are abundant in Miami-Dade year-round9, and are important mosquito vectors of arboviruses in urban areas52–54. These species are able to exploit and benefit from a vast range of widely available resources that are present in urban areas3,22,55. Consistent with global population trends, current predictions indicate that approximately 700,000 people are expected to move to Miami-Dade by 203056. Accordingly, Miami-Dade is experiencing increased levels of urbanization, and many natural areas are being transformed into urban areas57.
Understanding how such anthropogenic alterations in the environment will affect the abundance, richness, and composition of vector mosquito species is crucial for the implementation of effective and targeted mosquito control strategies. We hypothesized that urbanization processes are responsible for increasing the abundance of mosquito vectors of anthroponotic vector-borne diseases (e.g., Zika, chikungunya, dengue) by favoring mosquito species that are adapted to urban environments such as Ae. aegypti and Cx. quinquefasciatus. Therefore, our objective was to assess differences in the mosquito community composition between urbanized and natural and rural areas in Miami-Dade County, Florida.
Results
Mosquitoes were collected in 24 collection sites across Miami-Dade County, from which 16 were located in remote areas and 8 in urban areas. A total of 36,645 mosquitoes were collected, from which 34,048 were collected in the remote areas and 2,597 in the urban areas. A total of 26 species were collected in the remote sites and 20 in the urban sites. The species richness per collection site ranged from 8 to 19 species among the remote sites with an average of 11 species per site, whereas among the urban sites the species richness ranged from 3 to 18 with an average of 6 species per site. However, the urban site U8 is a new urban development and borders natural areas. If the urban site U8 had been removed from the analyses the species richness among the urban sites would have ranged from 3 to 7 species with an average of 5 species per site.
All species collected in the urban sites were also collected in the remote sites. However, the opposite was not true, Aedes bahamensis, Anopheles atropos, Coquillettidia perturbans, Psorophora ferox, Uranotaenia lowii, and Uranotaenia sapphirina were collected in the remote sites but not on the urban sites. The most abundant species in the remote areas were Cx. nigripalpus (13,338 females and 3 males), followed by Anopheles crucians (6,509 females and 7 males) and Culex erraticus (6,086 females and 18 males). On the other hand, the most abundant species in the urban areas were Ae. aegypti (762 females and 399 males), followed by Cx. nigripalpus (547 females and 7 males) and Cx. quinquefasciatus (247 females and 185 males) (Table 1).
Table 1.
Total number of mosquitoes collected at the remote and urban collection sites in Miami-Dade County, Florida. F female, M male.
| Collection Site | Ae. aegypti | Ae. albopictus | Ae. atlanticus | Ae. bahamensis | Ae. infirmatus | Ae. taeniorhynchus | Ae. tortilis | Ae. triseriatus | An. atropos | An. crucians | An. quadrimaculatus | Cq. perturbans | Cx. coronator | Cx. erraticus | Cx. interrogator | Cx. nigripalpus | Cx. quinquefasciatus | De. cancer | Ma. dyari | Ma. titillans | Ps. columbiae | Ps. ferox | Ur. lowii | Ur. sapphirina | Wy. mitchellii | Wy. vanduzeei | Species Richness | Relative Abundance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | F | F | F | M | F | F | F | F | M | F | M | F | F | F | M | F | F | M | F | M | F | F | F | F | F | F | F | F | F | |||
| R1 | 8 | 1 | 1 | 2 | 1 | 122 | 2 | 21 | 70 | 3 | 119 | 6 | 1 | 2 | 12 | 359 | ||||||||||||||||||||
| R2 | 2 | 1 | 6 | 156 | 47 | 2 | 328 | 5 | 52 | 11 | 6 | 7 | 4 | 12 | 627 | |||||||||||||||||||||
| R3 | 4 | 2 | 16 | 3 | 328 | 5 | 12 | 10 | 2 | 103 | 1 | 56 | 1 | 567 | 3 | 2 | 53 | 1 | 14 | 1,169 | ||||||||||||||||
| R4 | 41 | 4 | 16 | 90 | 500 | 1 | 363 | 946 | 1 | 1 | 8 | 1,963 | ||||||||||||||||||||||||
| R5 | 29 | 51 | 12 | 2 | 3 | 29 | 16 | 5 | 21 | 71 | 735 | 15 | 39 | 13 | 10 | 1,041 | ||||||||||||||||||||
| R6 | 2 | 18 | 1 | 33 | 103 | 2 | 61 | 5 | 800 | 19 | 38 | 5 | 8 | 1,087 | ||||||||||||||||||||||
| R7 | 3 | 277 | 12 | 915 | 4 | 79 | 1,814 | 2 | 1 | 1 | 10 | 3,108 | ||||||||||||||||||||||||
| R8 | 4 | 2,484 | 69 | 2 | 15 | 17 | 2,788 | 7,092 | 694 | 29 | 6 | 80 | 160 | 13 | 13,440 | |||||||||||||||||||||
| R9 | 137 | 682 | 109 | 3 | 222 | 164 | 13 | 2 | 4 | 9 | 1,336 | |||||||||||||||||||||||||
| R10 | 68 | 1,078 | 1 | 1 | 472 | 6 | 24 | 1 | 4 | 8 | 1,655 | |||||||||||||||||||||||||
| R11 | 4 | 1 | 56 | 716 | 15 | 1,070 | 3 | 18 | 3 | 2 | 9 | 1,888 | ||||||||||||||||||||||||
| R12 | 115 | 2,140 | 1 | 1 | 208 | 17 | 1 | 3 | 2 | 8 | 2,488 | |||||||||||||||||||||||||
| R13 | 1 | 14 | 7 | 14 | 35 | 24 | 1 | 20 | 17 | 14 | 20 | 85 | 9 | 2 | 8 | 14 | 271 | |||||||||||||||||||
| R14 | 3 | 11 | 465 | 172 | 16 | 14 | 5 | 47 | 96 | 14 | 583 | 6 | 3 | 94 | 4 | 6 | 4 | 39 | 2 | 12 | 19 | 1,596 | ||||||||||||||
| R15 | 2 | 2 | 98 | 67 | 4 | 21 | 60 | 170 | 2 | 11 | 142 | 1 | 8 | 2 | 62 | 13 | 652 | |||||||||||||||||||
| R16 | 17 | 18 | 8 | 1 | 17 | 549 | 279 | 13 | 19 | 232 | 180 | 5 | 5 | 15 | 10 | 12 | 1,368 | |||||||||||||||||||
| Total | 62 | 140 | 56 | 14 | 2,825 | 25 | 8 | 1,434 | 824 | 22 | 34 | 6,509 | 7 | 979 | 6 | 10 | 105 | 6,086 | 18 | 14 | 13,338 | 3 | 82 | 92 | 176 | 717 | 55 | 98 | 41 | 9 | 1 | 82 | 176 | 26 | 34,048 | |
| U1 | 16 | 14 | 7 | 37 | 11 | 9 | 4 | 94 | ||||||||||||||||||||||||||||
| U2 | 154 | 159 | 1 | 1 | 1 | 73 | 65 | 5 | 454 | |||||||||||||||||||||||||||
| U3 | 139 | 108 | 1 | 40 | 45 | 3 | 333 | |||||||||||||||||||||||||||||
| U4 | 169 | 27 | 2 | 1 | 1 | 10 | 4 | 1 | 6 | 215 | ||||||||||||||||||||||||||
| U5 | 7 | 2 | 8 | 3 | 17 | |||||||||||||||||||||||||||||||
| U6 | 88 | 14 | 2 | 14 | 3 | 13 | 24 | 1 | 46 | 7 | 205 | |||||||||||||||||||||||||
| U7 | 151 | 57 | 48 | 29 | 8 | 2 | 15 | 15 | 15 | 7 | 340 | |||||||||||||||||||||||||
| U8 | 38 | 20 | 4 | 99 | 1 | 36 | 3 | 14 | 11 | 13 | 15 | 38 | 2 | 530 | 7 | 51 | 21 | 1 | 8 | 25 | 1 | 1 | 18 | 939 | ||||||||||||
| Total | 762 | 399 | 5 | 99 | 1 | 88 | 3 | 44 | 14 | 11 | 13 | 16 | 46 | 2 | 547 | 7 | 247 | 185 | 2 | 8 | 25 | 1 | 1 | 71 | 20 | 2,597 | ||||||||||
| Grand Total | 824 | 539 | 61 | 14 | 2,924 | 25 | 9 | 1,522 | 3 | 868 | 36 | 34 | 6,520 | 7 | 992 | 6 | 10 | 121 | 6,132 | 18 | 16 | 13,885 | 10 | 329 | 277 | 178 | 725 | 80 | 99 | 41 | 9 | 1 | 83 | 247 | 26 | 36,645 |
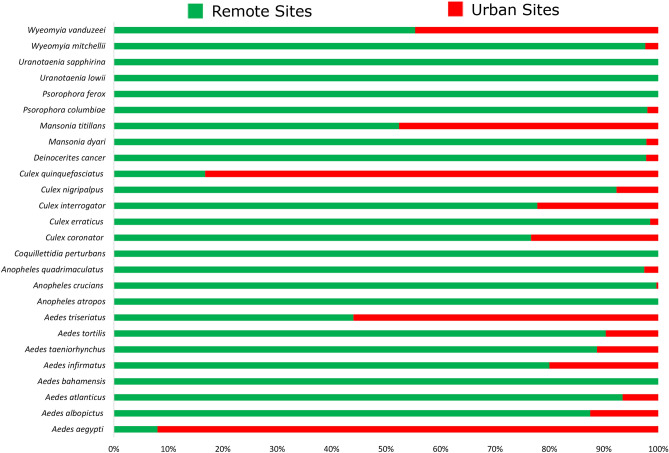
The mean number of mosquitoes collected in the remote and urban collection sites revealed that most of the species were more commonly found in the remote areas. From the 26 species collected, only 5 species had a mean number higher than 20% in the urban areas, Ae. aegypti, Ae. triseriatus, Cx. quinquefasciatus, Mansonia titillans, and Wyeomyia vanduzeei. Furthermore, Ae. aegypti was the most commonly found species in urban areas with a mean value higher than 90%. On the other hand, Ae. albopictus, An. crucians, Anopheles quadrimaculatus, and Cx. erraticus were almost exclusively collected in the remote areas (Fig. 1).
Figure 1.
Relative proportion of mosquitoes collected at the remote and urban collection sites in Miami-Dade County, Florida.
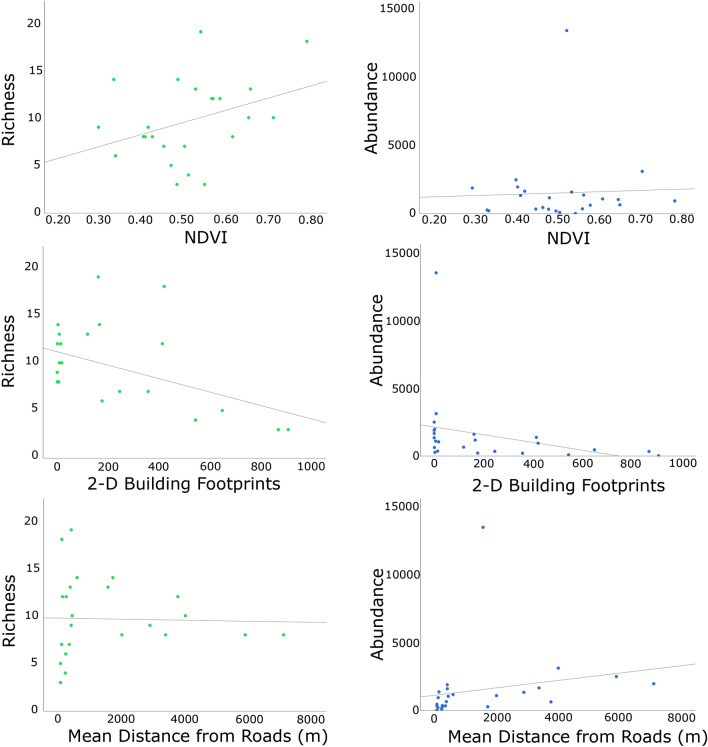
Our results indicate a robust pattern of mosquito relative abundance and species richness and composition between remote and urban collection sites. The remote collection sites had a higher species richness with many abundant species that were not commonly found in the urban collection sites such as Aedes atlanticus, An. quadrimaculatus, and Cx. erraticus. On the other hand, Ae. aegypti and Cx. quinquefasciatus were more commonly found in the urban collection sites. Furthermore, the species richness and abundance were higher in areas less impacted by urbanization, being inversely proportional to the presence of buildings and proximity to roads (Fig. 2).
Figure 2.
Impact of urbanization on species richness and mosquito relative abundance. Left: Species richness relationship with NDVI, building footprint, and mean distance from roads (in meters); Right: Relative abundance relationship with NDVI, building footprint, and mean distance from roads (in meters).
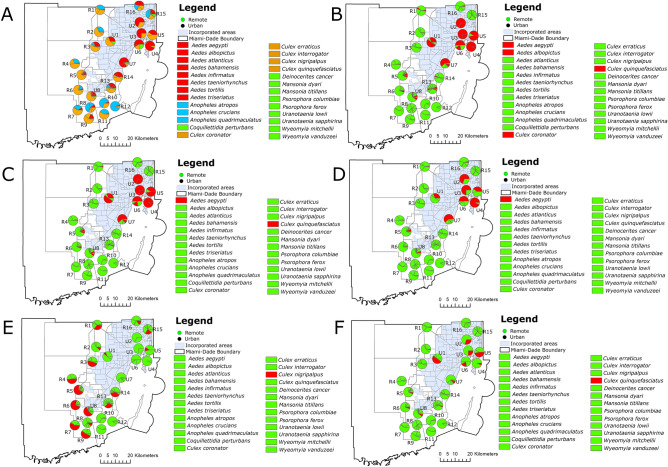
The analysis of the percent composition of vector mosquito species in the remote and urban collection sites revealed that the urban collection sites had fewer and more dominant mosquito vector species than the remote collection sites. Aedes aegypti and Cx. quinquefasciatus comprised most of the mosquitoes collected in the urban sites with Ae. aegypti being the most abundant species collected in these sites except collection site U8, which was located in a natural-urban transition zone. On the other hand, Ae. aegypti and Cx. quinquefasciatus were not collected in 6 of the remote sites and apart from the remote sites R4 and R5 were collected in negligible numbers in the remote collection sites. On the remote collection sites, Cx. nigripalpus was the dominant species but not as dominant as Ae. aegypti and Cx. quinquefasciatus in the urban collection sites. These results showed a clear pattern of species richness and relative abundance in both the remote and urban collection sites according to the environment and respective resource availability. The mosquito community composition was substantially different in remote and urban collection sites disregarding geographical proximity as observed in the comparison between the collection sites U1 and R2 and R3, U2 and R15 and R16, and U7 and R14 (Fig. 3).
Figure 3.
Percent composition of vector mosquito species in remote and urban collection site locations in Miami-Dade County, Florida. Species were organized by (A) red = Aedes, blue = Anopheles, yellow = Culex, green = Coquillettidia, Deinocerites, Mansonia, Psorophora, Uranotaenia, and Wyeomyia; (B) red = Aedes aegypti, Aedes albopictus, Culex coronator, and Culex quinquefasciatus, green = all other species; (C) red = Aedes aegypti and Culex quinquefasciatus, green = all other species; (D) red = Aedes aegypti, green = all other species; (E) red = Culex nigripalpus, green = all other species; and (F) red = Culex quinquefasciatus, green = all other species. The figure was produced using ArcGIS 10.2 (Esri, Redlands, CA), using freely available layers from the Miami-Dade County’s Open Data Hub—https://gis-mdc.opendata.arcgis.com/.
The Permutational Multivariate Analysis of Variance (PERMANOVA) yielded significant results for the comparison between mosquito species composition in remote and urban areas (F = 8.707; P = 0.0001). The PERMANOVA analysis also revealed significant differences in the mosquito species composition in areas with different land uses (F = 3.205; P < 0.0003). The subsequent SIMPER (Similarity Percentage) analysis comparing remote and urban areas showed that Cx. nigripalpus, An. crucians, Cx. erraticus, and Ae. aegypti contributed the most to the observed differences, whereas in the analysis considering different land uses Cx. nigripalpus, An. crucians, Cx. erraticus, and Ae. atlanticus contributed the most to the observed differences (Supplementary Table 2).
Results from the Moran's Index of Spatial Autocorrelation were not statistically significant failing to reject the null hypothesis, therefore, indicating that autocorrelation is not present in the samples analyzed here (Observed: − 0.002; Expected: − 0.043; Standard Deviation: 0.050; and P-value: 0.420). The Generalized Linear Mixed Methods (GLMM) regression results showed a statistically significant association between the Normalized Difference Vegetation Index (NDVI) and the mean distance from major roads and their interaction with species richness (Table 2).
Table 2.
Generalized linear mixed methods (GLMM) regression results.
| Covariates | Species Richness | Relative Abundance | ||||
|---|---|---|---|---|---|---|
| Wald Chi-Square | df | P value | Wald Chi-Square | df | P value | |
| Model | 0.277 | 1 | 0.598 | 7.603 | 1 | 0.01 |
| NDVI | 17.78 | 1 | < .001 | 2.734 | 1 | 0.1 |
| Building Footprint | 0.527 | 1 | 0.468 | 0.211 | 1 | 0.65 |
| Mean Distance from Roads | 12.422 | 1 | < .001 | 0.758 | 1 | 0.38 |
| NDVI * Building Footprint | 0.173 | 1 | 0.677 | 1.637 | 1 | 0.2 |
| NDVI * Mean Distance from Roads | 15.639 | 1 | < .001 | 0.65 | 1 | 0.42 |
| Building Footprint * Mean Distance from Roads | 3.158 | 1 | 0.076 | 1.756 | 1 | 0.19 |
Species richness and relative abundance served as the dependent variables and were analyzed using a normal distribution for each urbanization measurement (Normalized Difference Vegetation Index (NDVI), 2-D Building Footprint, and the mean distance from major roads) and their interaction as independent variables, and week as a random effect with collection sites nested in time (weeks).
Discussion
Our results show a clear and well-defined pattern of abundance, richness, and community composition according to anthropogenic modifications in land use and land cover in Miami-Dade. The more urbanized a given area the fewer species were found and Ae. aegypti and Cx. quinquefasciatus were the most dominant species. Our study shows that land use and land cover transformation of natural areas into urban areas of South Florida can affect mosquito relative abundance, richness, and community composition by favoring mosquito vector species that are adapted to thrive in urban environments. As a consequence, human populations will have increased contact with mosquito vector species, especially Ae. aegypti and Cx. quinquefasciatus, increasing the risk of vector-borne disease transmission.
Our results also show that the community composition, species richness, and relative abundance were not related to the geographic proximity of the remote and urban collection sites. Even adjacent remote and urban collection sites had completely distinct mosquito community composition, species richness, and relative abundance. These results support the hypothesis that the availability of resources at the micro-geographic scale (i. e., neighborhood-level) is a major driver for the proliferation or decrease of populations of vector mosquito species according to their specific ecology and behavior22,58. For example, both Ae. aegypti and Cx. quinquefasciatus were collected in relatively high numbers in the remote areas R4 and R5. The remote area R4 is a conservation zone closed to the general public, however, an abandoned checkpoint flooded with rainwater was a potentially conducive habitat for the proliferation of Ae. aegypti and Cx. quinquefasciatus. A similar scenario was observed in the remote area R5, in which the presence of a human-made ditch to divert rainwater also made available suitable habitats for these vector species.
The transformation of natural environments into urban spaces and the increased density of urban land cover surfaces (e.g., asphalt, concrete, etc.) within existing urbanized locations is a major driver for biodiversity loss6,59,60. As a result, the steep increase in urbanization in the last decades has led to biodiversity loss on a global scale15.
In this context, the mosquito community composition is greatly affected by urbanization, in which mosquito abundance and species richness are significantly affected and tend to decrease proportionally with urbanization levels61–64. However, some mosquito vector species can thrive in urban environments and greatly benefit from the resources made available by the rise of transient underserved populations, absence of natural predators, human-made aquatic habitats, increased human population density, and warmer temperatures due to global warming. Aedes aegypti and Cx. quinquefasciatus are among those species that can thrive in urban areas, and coincidentally are the primary vectors of chikungunya, dengue, yellow fever, West Nile, and Zika viruses48,65–69. This is in agreement with the results from the GLMM analysis, in which NDVI and mean distance from roads were significantly associated with reduced species richness.
Our results also revealed the presence of many mosquito vector species in the remote areas of Miami-Dade. Anopheles quadrimaculatus, the primary vector of human malaria in the southeast United States and Cx. erraticus, the bridge vector of Eastern Equine Encephalitis in the southern United States, were almost exclusively found in the remote areas and were only collected in high numbers in the urban area U8, a new urban development bordering natural areas. Aedes albopictus is not commonly found in urban areas in Miami-Dade County9,22, and is relegated to restricted and well-defined areas such as cemeteries70. Therefore, it was not unexpected that approximately 90% of Ae. albopictus specimens were collected in the remote areas. However, despite the epidemiological importance of these species, their distant relationship with humans and inability of invading and thriving in urban areas of Miami-Dade greatly decreases their relevance in disease transmission when compared with mosquito vectors of anthroponotic vector-borne diseases. In this context, as our results revealed, Ae. aegypti is considerably more abundant in urban areas and is the primary vector of arboviruses that use humans as amplification hosts without the need for bridge vectors and primary hosts, such as dengue and Zika, representing a much greater public health threat71.
Urbanization has a major impact on the epidemiology of vector-borne disease transmission. It not only provides all the resources necessary for the survival of vector mosquito species such as Ae. aegypti and Cx. quinquefasciatus, but also provides shelter from the elements. Urban features such as tire shops or underground subway stations may allow populations of vector mosquito species to survive scorching and freezing temperatures that normally would kill them72,73. Furthermore, these highly productive urban environments that are responsible for the proliferation of vector mosquitoes are often located in populous areas, increasing, even more, the contact between humans and mosquito vectors28,70.
The impact of urbanization in the proliferation of vector mosquito species and arbovirus transmission must be considered under the Integrated Vector Management (IVM) framework74. Environmental ordinances and good practices, as well as simple modifications in the urban built environment at the early stages of development, can substantially decrease human exposure to mosquito vectors by attenuating social inequities and consequently social determinants of health75,76.
Even though several new technologies for controlling vector mosquito species in urban areas are being developed (e.g., genetically modified mosquitoes and Wolbachia-infect mosquitoes), they have not been validated to be used on large scales under real-world conditions77,78, and further entomological and epidemiological validation is still needed before they can be included and implemented under the IVM framework79. In this context, the World Health Organization (WHO) recommendation to control vector mosquito populations relies on the removal of aquatic habitats for immature mosquitoes and targeted insecticide application when needed. However, the current levels of proliferation of vector mosquito species in urban areas mediated by an overabundance of resources3,9,22 make it virtually impossible to achieve the desired results of a safe mosquito abundance threshold to avoid arbovirus transmission80.
This study is not without limitations. We did not collect data across all weather and season variations that would have brought further insight into the natural variation in the mosquito community composition and abundance. We have collected mosquitoes using BG-Sentinel traps, which are the gold standard for collecting Aedes Stegomyia species. Even though BG-Sentinel traps have been proven effective to collect other less anthropophilic mosquito species and the fact that we have collected 26 mosquito species during this study, including many species that are notably not anthropophilic, we may have underestimated the presence and relative abundance of mosquito species that are less attracted by the BG-Sentinel traps.
Conclusion
The relationships between mosquito vectors, human hosts, and pathogens are driven by environmental conditions. Vector-borne diseases are conditioned by the environment, and anthropogenic changes have a direct influence on their epidemiology7. Climate change and urbanization increase the risk of arbovirus transmission by increasing the presence and abundance of mosquito vector species, therefore, increasing their contact with human populations. The findings of this study shed light on the effect of urbanization on the community composition of mosquitoes by reducing species richness and increasing the abundance of Ae. aegypti and Cx. quinquefasciatus in a non-random process of biotic homogenization. Large urban areas hold diverse socio-ecological conditions that can be highly conducive to both mosquito vector proliferation and local arbovirus transmission. Miami-Dade is one of the most critical entry points into the United States, with an elevated influx of people arriving and departing from endemic areas. Miami-Dade is, therefore, a sentinel or harbinger of what other cities in the contiguous United States will experience this century with climate change, population growth, regional trade, and human movement. The findings from this study highlight the importance of understanding how anthropogenic changes in the environment create an overabundance of resources that are responsible for sustaining the invasion, spread, and colonization of urban areas by vector mosquito species.
Methods
Study design
In this study, we identified and selected 24 areas: (i) 16 remote areas with high normalized difference vegetation index (NDVI) values. These areas were comprised of natural and rural areas with low population density or complete absence of humans, and no or minimum urban development allowed; and (ii) 8 urban areas with low NDVI values comprised of a university campus, and residential, and touristic areas with high human population density in Miami-Dade County, Florida (Fig. 4). We quantified the change in the NDVI obtained from Landsat satellite imagery mapped at 30 m spatial resolution. NDVI, which ranges from − 1 to + 1, provides a direct measurement of photosynthetic activity and is positively correlated with moisture availability, evapotranspiration, and vegetation biomass81. NDVI values less than or equal to ~ 0.1 are typically associated with urbanized surfaces (e.g., pavement, bare soils, and rooftops) or water bodies. Thus, decreases in NDVI through time may indicate vegetation loss or degradation associated with urban and suburban development characteristics of South Florida. For example, Al Rifat and Liu82 found that urbanization in Miami-Dade County advanced at a rate of 96.57 km2 yr−1 between 1996–2001 but had decreased to 11.45 km2 yr−1 between 2011–2016 as available land areas for development became increasingly limited. Further, their analysis revealed that urbanized surfaces in Miami-Dade County also became more compact (i.e., dense) since the 1990s82.
Figure 4.
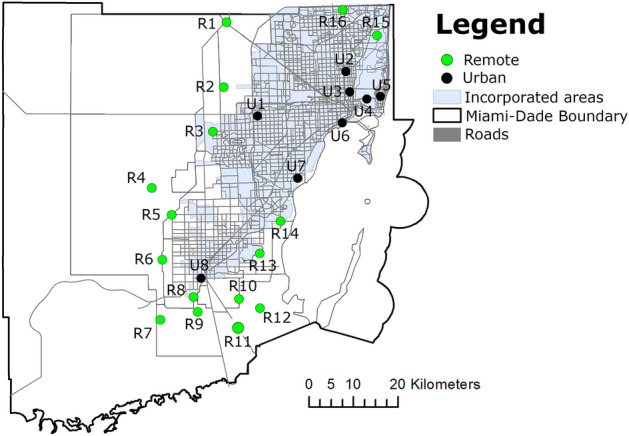
Map showing the location of the remote and urban collection sites in Miami-Dade, Florida (latitude, 25.761681; longitude, -80.191788). The figure was produced using ArcGIS 10.2 (Esri, Redlands, CA) using freely available layers from the Miami-Dade County’s Open Data Hub— https://gis-mdc.opendata.arcgis.com/.
We selected a cloud-free, atmospherically corrected Landsat from 2017 from https://earthexplorer.usgs.gov/ during the month of November, which coincides with the approximate start of the dry season in South Florida82,83. To assess vegetation conditions around the traps, we utilized GIS software to create 1-km buffers around each trap and calculated the mean and standard deviation (SD) of NDVI within 1-km radii, which coincides approximately with the maximum flight distance of many species collected at the traps. The NDVI for each trap buffer provides a measure of spatial variation in vegetation state around each trap. For traps that were proximate to the shore (i.e., within 1 km of a shoreline), we masked out any water portions of the radius so that only NDVI values from land areas were included in NDVI. The masking process reduced the sample area from 3.15 km2 for inland locations (i.e., greater than 1 km from shorelines) to a minimum of 0.76 km2 for one island site with an extensive shoreline (Table 3). Then, we used the same 1-km buffers around each trap to calculate the number of pixels with buildings in each buffer around the trap sites to create a 2-D Building Footprint as one measure of urban development. We also calculated the mean distance from major roads in meters for each of the buffers (i.e., mean for all the pixels that comprise a buffer) as a proxy for urbanization. The layers are freely available at the Miami-Dade County’s Open Data Hub— https://gis-mdc.opendata.arcgis.com/.
Table 3.
Description of the remote and urban collection sites in Miami-Dade, Florida.
| Collection Site | Location | Latitude | Longitude | Description | NDVI 2017 | 2-D Building Footprints | Mean Distance from Roads (m) |
|---|---|---|---|---|---|---|---|
| U1 | Sweetwater | 25.757452 | − 80.376182 | University | 0.506 | 543 | 240 |
| U2 | Little River | 25.844456 | − 80.20358 | Residential | 0.465 | 648 | 90 |
| U3 | Wynwood | 25.80472 | − 80.196006 | Touristic | 0.479 | 868 | 89 |
| U4 | San Marino | 25.791004 | − 80.16252 | Residential | 0.334 | 176 | 254 |
| U5 | Convention Center | 25.796003 | − 80.135516 | Touristic | 0.544 | 907 | 86 |
| U6 | Vizcaya | 25.744416 | − 80.210485 | Touristic | 0.497 | 357 | 123 |
| U7 | Tarpon Dr | 25.636065 | − 80.297609 | Residential | 0.448 | 245 | 363 |
| U8 | Naranja | 25.440493 | − 80.48667 | Residential | 0.785 | 420 | 131 |
| R1 | Okeechobee | 25.94113 | − 80.43672 | Natural | 0.561 | 14 | 267 |
| R2 | West Doral | 25.81421 | − 80.44214 | Natural | 0.580 | 1 | 3,775 |
| R3 | Bird Road | 25.72724 | − 80.46365 | Natural | 0.481 | 166 | 611 |
| R4 | East Everglades | 25.61732 | − 80.58278 | Natural | 0.404 | 2 | 7,099 |
| R5 | Hainlin Mill | 25.56462 | − 80.54359 | Rural | 0.648 | 18 | 455 |
| R6 | Everglades Trail | 25.47662 | − 80.56225 | Rural | 0.610 | 6 | 2,016 |
| R7 | Southern Glades | 25.35947 | − 80.56582 | Natural | 0.706 | 8 | 4,012 |
| R8 | Alligator Farm | 25.40366 | − 80.50124 | Rural | 0.523 | 8 | 1,581 |
| R9 | Detention Center | 25.37453 | − 80.49284 | Natural | 0.411 | 0 | 2,898 |
| R10 | SW 137 Ave | 25.40004 | − 80.41216 | Natural | 0.421 | 0 | 3,390 |
| R11 | Card Sound | 25.34158 | − 80.41219 | Natural | 0.294 | 0 | 424 |
| R12 | Cooling Canals | 25.38174 | − 80.37111 | Natural | 0.400 | 0 | 5,892 |
| R13 | Air Force Base | 25.48889 | − 80.37162 | Rural | 0.330 | 3 | 1,733 |
| R14 | Black Point | 25.55204 | − 80.33118 | Natural | 0.535 | 161 | 424 |
| R15 | Oleta Park | 25.915682 | − 80.142643 | Natural | 0.652 | 119 | 393 |
| R16 | NW 207 St | 25.96551 | − 80.209737 | Natural | 0.564 | 413 | 154 |
Collection of mosquitoes
Mosquitoes were collected from August to October 2020 using BG-Sentinel traps (Biogents AG, Regensburg, Germany) baited with dry ice84. Mosquitoes were collected weekly at each collection site for 24 h for 5 consecutive weeks. Traps were placed in shaded areas that were protected from direct solar radiation, wind, and precipitation to enhance mosquito collections. The collected mosquitoes were transported to the Miami-Dade County Mosquito Control Laboratory and subsequently morphologically identified to species using taxonomic keys85.
Statistical analyses
To compare the mosquito species composition in remote and urban areas as well as in areas with different land uses we performed a PERMANOVA with 9,999 permutations based on Bray–Curtis distances86,87. First, we subsetted the data into two groups to compare the mosquito species composition between rural and urban areas, and then we subsequently subsetted the data into 5 groups according to land use: natural, rural, university, residential, and touristic areas. Then we used the SIMPER method for assessing which species has contributed the most to the observed differences between groups of samples88. Analyses were done using PAST v3.289.
We assessed spatial autocorrelation between samples using Moran's Index of Spatial Autocorrelation using ArcMap v10.5, in which failing to accept the alternative hypothesis indicates autocorrelation is not present in the data90. We checked for multicollinearity between the covariates NDVI, 2-D Building Footprint, and the mean distance from major roads using the variance inflation factor (VIF) values. VIF values below 10.00 indicate the assumptions were met and the covariates were not colinear91,92. There was no collinearity between the covariates, as the highest VIF value yielded 1.433 (Supplementary Table 3). We used the Eta squared (η2) as the proportion of variance in the continuous target field explained by an effect to determine effect size93 (Supplementary Table 4). Then, we performed a Generalized Linear Mixed Methods (GLMM) regression using a normal distribution considering species richness and relative abundance as the dependent variables and each urbanization measurement (Normalized Difference Vegetation Index (NDVI), 2-D Building Footprint, and the mean distance from major roads) and their interaction as independent variables, and week as a random effect with collection sites nested in time (weeks). Statistical analyses were done in SPSS V28.0.
Supplementary Information
Acknowledgements
We thank the staff of the Miami-Dade County Mosquito Control Division for their help in the processing and identification of the mosquitoes.
Author contributions
A.B.B.W., C.V., D.O.F., J.C.B. conceived of and designed the study. A.B.B.W., and C.V., were responsible for the mosquito collection. M.M. was responsible for the taxonomic identification. A.B.B.W., D.O.F., developed the study methodology and data analysis methodologies. A.B.B.W. and D.O.F., collected and analyzed the data and prepared the original figures. G.C. was responsible for the statistical analyses. A.B.B.W. wrote the original draft of the paper. All authors contributed to reviewing and editing the paper. C.V., J.C.B., W.D.P. were responsible for the project administration, funding acquisition, resources, supervision and validation of this study.
Funding
This research was supported by the Miami-Dade Mosquito Control Division and by the CDC (https://www.cdc.gov/) grant 1U01CK000510-05: Southeastern Regional Center of Excellence in Vector-Borne Diseases: The Gateway Program. CDC had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-02061-0.
References
- 1.World Health Organization. Vector-borne diseases. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (2020).
- 2.Wilke ABB, Beier JC, Benelli G. Complexity of the relationship between global warming and urbanization—an obscure future for predicting increases in vector-borne infectious diseases. Curr. Opin. Insect Sci. 2019;35:1–9. doi: 10.1016/j.cois.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Wilke ABB, et al. Proliferation of Aedesaegypti in urban environments mediated by the availability of key aquatic habitats. Sci. Rep. 2020;10:12925. doi: 10.1038/s41598-020-69759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilke ABB, Wilk-da-Silva R, Marrelli MT. Microgeographic population structuring of Aedesaegypti (Diptera: Culicidae) PLoS ONE. 2017;12:e0185150. doi: 10.1371/journal.pone.0185150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubler DJ. Dengue, urbanization and globalization: The unholy trinity of the 21st Century. Trop. Med. Health. 2011;39:S3–S11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson MTJ, Munshi-South J. Evolution of life in urban environments. Science. 2017;358:8327. doi: 10.1126/science.aam8327. [DOI] [PubMed] [Google Scholar]
- 7.Zohdy S, Schwartz TS, Oaks JR. The coevolution effect as a driver of spillover. Trends Parasitol. 2019;35:399–408. doi: 10.1016/j.pt.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Rochlin I, Faraji A, Ninivaggi DV, Barker CM, Kilpatrick AM. Anthropogenic impacts on mosquito populations in North America over the past century. Nat. Commun. 2016;7:13604. doi: 10.1038/ncomms13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilke ABB, et al. Community composition and year-round abundance of vector species of mosquitoes make Miami-Dade County, Florida a receptive gateway for arbovirus entry to the United States. Sci. Rep. 2019;9:8732. doi: 10.1038/s41598-019-45337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkett-Cadena ND, Vittor AY. Deforestation and vector-borne disease: Forest conversion favors important mosquito vectors of human pathogens. Basic Appl. Ecol. 2018;26:101–110. doi: 10.1016/j.baae.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochlin I, Harding K, Ginsberg HS, Campbell SR. Comparative analysis of distribution and abundance of West Nile and eastern equine encephalomyelitis virus vectors in Suffolk County, New York, using human population density and land use/cover data. J. Med. Entomol. 2008;45:563–571. doi: 10.1603/0022-2585(2008)45[563:caodaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Monaghan AJ, et al. Consensus and uncertainty in the geographic range of Aedesaegypti and Aedesalbopictus in the contiguous United States: Multi-model assessment and synthesis. PLoS Comput. Biol. 2019;15:1–19. doi: 10.1371/journal.pcbi.1007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilke ABB, Benelli G, Beier JC. Beyond frontiers: On invasive alien mosquito species in America and Europe. PLoS Negl. Trop. Dis. 2020;14:e0007864. doi: 10.1371/journal.pntd.0007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraemer MUG, et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data. 2015;2:150035. doi: 10.1038/sdata.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dirzo R, et al. Defaunation in the anthropocene. Science. 2014;345:401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 16.Lewis SL, Maslin MA. Defining the anthropocene. Nature. 2015;519:171–180. doi: 10.1038/nature14258. [DOI] [PubMed] [Google Scholar]
- 17.Law KL, Thompson RC. Microplastics in the seas. Science. 2014;345:144–145. doi: 10.1126/science.1254065. [DOI] [PubMed] [Google Scholar]
- 18.Jambeck JR, et al. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 19.Turner WR, Oppenheimer M, Wilcove DS. A force to fight global warming. Nature. 2009;462:278–279. doi: 10.1038/462278a. [DOI] [PubMed] [Google Scholar]
- 20.United Nations. World population prospects 2019. Department of Economic and Social Affairs. World Population Prospects 2019. (2019).
- 21.Multini LC, de Souza AL, da S., Marrelli, M. T. & Wilke, A. B. B. The influence of anthropogenic habitat fragmentation on the genetic structure and diversity of the malaria vector Anophelescruzii (Diptera: Culicidae) Sci. Rep. 2020;10:18018. doi: 10.1038/s41598-020-74152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilke ABB, et al. Urbanization creates diverse aquatic habitats for immature mosquitoes in urban areas. Sci. Rep. 2019;9:15335. doi: 10.1038/s41598-019-51787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pernat N, Kampen H, Jeschke JM, Werner D. Buzzing homes: Using citizen science data to explore the effects of urbanization on indoor mosquito communities. Insects. 2021;12:1–13. doi: 10.3390/insects12050374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blosser EM, Burkett-cadena ND. Acta Tropica Culex (Melanoconion) panocossa from peninsular Florida, USA. Acta Trop. 2017;167:59–63. doi: 10.1016/j.actatropica.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun K, et al. Quantifying the risk of local Zika virus transmission in the contiguous US during the 2015–2016 ZIKV epidemic. BMC Med. 2018;16:195. doi: 10.1186/s12916-018-1185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose NH, et al. Climate and urbanization drive mosquito preference for humans. Curr. Biol. 2020;30:3570–3579.e6. doi: 10.1016/j.cub.2020.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilke ABB, et al. Mosquito adaptation to the extreme habitats of urban construction sites. Trends Parasitol. 2019;35:607–614. doi: 10.1016/j.pt.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Ajelli M, et al. Host outdoor exposure variability affects the transmission and spread of Zika virus: Insights for epidemic control. PLoS Negl. Trop. Dis. 2017;11:e0005851. doi: 10.1371/journal.pntd.0005851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutebi J-P, et al. Zika virus MB16-23 in mosquitoes, Miami-Dade County, Florida, USA, 2016. Emerg. Infect. Dis. 2018;24:808–810. doi: 10.3201/eid2404.171919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little E, et al. Socio-ecological mechanisms supporting high densities of Aedesalbopictus (Diptera: Culicidae) in Baltimore, MD. J. Med. Entomol. 2017;54:1183–1192. doi: 10.1093/jme/tjx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkett-Cadena ND, McClure CJW, Estep LK, Eubanks MD. What drives the spatial distribution of mosquitoes? Ecosphere. 2013;4:1–16. [Google Scholar]
- 33.LaDeau SL, Leisnham PT, Biehler D, Bodner D. Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: Understanding ecological drivers and mosquito-borne disease risk in temperate cities. Int. J. Environ. Res. Public Health. 2013;10:1505–1526. doi: 10.3390/ijerph10041505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowling Z, et al. Linking mosquito infestation to resident socioeconomic status, knowledge, and source reduction practices in Suburban Washington, DC. EcoHealth. 2013;10:36–47. doi: 10.1007/s10393-013-0818-6. [DOI] [PubMed] [Google Scholar]
- 35.Scavo NA, Barrera R, Reyes-Torres LJ, Yee DA. Lower socioeconomic status neighborhoods in Puerto Rico have more diverse mosquito communities and higher Aedesaegypti abundance. J. Urban Ecol. 2021;7:1–11. [Google Scholar]
- 36.Trewin BJ, et al. The elimination of the dengue vector, Aedesaegypti, from Brisbane, Australia: The role of surveillance, larval habitat removal and policy. PLoS Negl. Trop. Dis. 2017;11:e0005848. doi: 10.1371/journal.pntd.0005848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Multini LC, de Souza AL, da S., Marrelli, M. T. & Wilke, A. B. B. Population structuring of the invasive mosquito Aedesalbopictus (Diptera: Culicidae) on a microgeographic scale. PLoS ONE. 2019;14:e0220773. doi: 10.1371/journal.pone.0220773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leta S, et al. Global risk mapping for major diseases transmitted by Aedesaegypti and Aedesalbopictus. Int. J. Infect. Dis. 2018;67:25–35. doi: 10.1016/j.ijid.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benelli G, Wilke ABB, Beier JC. Aedesalbopictus (Asian Tiger Mosquito) Trends Parasitol. 2020;36:942–943. doi: 10.1016/j.pt.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Benelli G, Mehlhorn H. Declining malaria, rising of dengue and Zika virus: Insights for mosquito vector control. Parasitol. Res. 2016;115:1747–1754. doi: 10.1007/s00436-016-4971-z. [DOI] [PubMed] [Google Scholar]
- 41.Danauskas JX, Ehrenkranz NJ, Davies JE, Pond WL. Arboviruses and human disease in South Florida. Am. J. Trop. Med. Hyg. 1966;15:205–210. doi: 10.4269/ajtmh.1966.15.205. [DOI] [PubMed] [Google Scholar]
- 42.Gill J, Stark LM, Clark GG. Dengue surveillance in Florida, 1997–98. Emerg. Infect. Dis. 2000;6:30–35. doi: 10.3201/eid0601.000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rey J. Dengue in Florida (USA) Insects. 2014;5:991–1000. doi: 10.3390/insects5040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitek CJ, Richards SL, Mores CN, Day JF, Lord CC. Arbovirus transmission by Culexnigripalpus in Florida, 2005. J. Med. Entomol. 2008;45:483–493. doi: 10.1603/0022-2585(2008)45[483:atbcni]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messenger AM, et al. Serological evidence of ongoing transmission of dengue virus in permanent residents of Key West, Florida. Vector Borne Zoonotic Dis. 2014;14:783–787. doi: 10.1089/vbz.2014.1665. [DOI] [PubMed] [Google Scholar]
- 46.Patterson KD. Yellow fever epidemics and mortality in the United States, 1693–1905. Soc. Sci. Med. 1992;34:855–865. doi: 10.1016/0277-9536(92)90255-o. [DOI] [PubMed] [Google Scholar]
- 47.Grubaugh ND, et al. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature. 2017;546:401–405. doi: 10.1038/nature22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Likos A, et al. Local mosquito-borne transmission of zika virus—Miami-Dade and Broward Counties, Florida, June–August 2016. Morb. Mortal. Wkly. Rep. 2016;65:1032–1038. doi: 10.15585/mmwr.mm6538e1. [DOI] [PubMed] [Google Scholar]
- 49.Florida Department of Health. Available at: http://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/_documents/week52arbovirusreport-12-31-16.pdf (2016).
- 50.Florida Department of Health. Available at: http://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/_documents/alert-dade-wnv-human-10-19-20.pdf (2020)
- 51.Wilke ABB, et al. Local conditions favor dengue transmission in the contiguous United States. Entomol. Gen. 2021;41:523–529. [Google Scholar]
- 52.Alto BW, Connelly CR, O’Meara GF, Hickman D, Karr N. Reproductive biology and susceptibility of Florida Culexcoronator to infection with West Nile virus. Vector-Borne Zoonotic Dis. 2014;14:606–614. doi: 10.1089/vbz.2013.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honório NA, Wiggins K, Câmara DCP, Eastmond B, Alto BW. Chikungunya virus vector competency of Brazilian and Florida mosquito vectors. PLoS Negl. Trop. Dis. 2018;12:1–16. doi: 10.1371/journal.pntd.0006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richards SL, Anderson SL, Lord CC. Vector competence of Culexpipiensquinquefasciatus (Diptera: Culicidae) for West Nile virus isolates from Florida. Trop. Med. Int. Heal. 2014;19:610–617. doi: 10.1111/tmi.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hribar LJ, Smith JM, Vlach JJ, Verna TN. Survey of container-breeding mosquitoes from the Florida Keys, Monroe County, Florida. J. Am. Mosq. Control Assoc. 2001;17:245–248. [PubMed] [Google Scholar]
- 56.United States Environmental Protection Agency. Growing for a sustainable future: Miami-Dade County urban development boundary assessment. Available at: http://www.epa.gov/smartgrowth/pdf/Miami-Dade_Final_Report_12-12-12.pdf (2012).
- 57.Miami-Dade County Building Permits. Available at, http://www.miamidade.gov/permits/.
- 58.Wilke ABB, Carvajal A, Vasquez C, Petrie WD, Beier JC. Urban farms in Miami-Dade County, Florida have favorable environments for vector mosquitoes. PLoS ONE. 2020;15:e0230825. doi: 10.1371/journal.pone.0230825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reba M, Reitsma F, Seto KC. Spatializing 6,000 years of global urbanization from 3700 BC to AD 2000. Sci. Data. 2016;3:1–16. doi: 10.1038/sdata.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ceretti-Júnior W, et al. Mosquito faunal survey in a central park of the city of São Paulo, Brazil. J. Am. Mosq. Control Assoc. 2015;31:172–176. doi: 10.2987/14-6457R. [DOI] [PubMed] [Google Scholar]
- 61.Ferraguti M, et al. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 2016;6:29002. doi: 10.1038/srep29002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zahouli JBZ, et al. Effect of land-use changes on the abundance, distribution, and host-seeking behavior of Aedes arbovirus vectors in oil palm-dominated landscapes, southeastern Côte d’Ivoire. PLoS ONE. 2017;12:e0189082. doi: 10.1371/journal.pone.0189082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Westby KM, Adalsteinsson SA, Biro EG, Beckermann AJ, Medley KA. Aedesalbopictus populations and larval habitat characteristics across the landscape: Significant differences exist between urban and rural land use types. Insects. 2021;12:196. doi: 10.3390/insects12030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Estallo EL, et al. Modelling the distribution of the vector Aedesaegypti in a central Argentine city. Med. Vet. Entomol. 2018;32:451–461. doi: 10.1111/mve.12323. [DOI] [PubMed] [Google Scholar]
- 65.Messina JP, et al. A global compendium of human dengue virus occurrence. Sci. Data. 2014;1:140004. doi: 10.1038/sdata.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cunha MS, et al. Epizootics due to yellow fever virus in São Paulo State, Brazil: viral dissemination to new areas (2016–2017) Sci. Rep. 2019;9:5474. doi: 10.1038/s41598-019-41950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ronca SE, Murray KO, Nolan MS. Cumulative incidence of West Nile virus infection, continental United States, 1999–2016. Emerg. Infect. Dis. 2019;25:325–327. doi: 10.3201/eid2502.180765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poletti P, et al. Transmission potential of chikungunya virus and control measures: The case of Italy. PLoS ONE. 2011;6:e18860. doi: 10.1371/journal.pone.0018860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilk-da-Silva R, de Souza Leal Diniz, M. M. C., Marrelli, M. T. & Wilke, A. B. B. Wing morphometric variability in Aedesaegypti (Diptera: Culicidae) from different urban built environments. Parasit. Vectors. 2018;11:561. doi: 10.1186/s13071-018-3154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilke ABB, et al. Cemeteries in Miami-Dade County, Florida are important areas to be targeted in mosquito management and control efforts. PLoS ONE. 2020;15:e0230748. doi: 10.1371/journal.pone.0230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weaver SC. Urbanization and geographic expansion of zoonotic arboviral diseases: Mechanisms and potential strategies for prevention. Trends Microbiol. 2013;21:360–363. doi: 10.1016/j.tim.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilke ABB, Vasquez C, Petrie W, Beier JC. Tire shops in Miami-Dade County, Florida are important producers of vector mosquitoes. PLoS ONE. 2019;14:2. doi: 10.1371/journal.pone.0217177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kothera L, Godsey M, Mutebi JP, Savage HM. A comparison of aboveground and belowground populations of Culexpipiens (Diptera: Culicidae) mosquitoes in Chicago, Illinois, and New York City, New York, using microsatellites. J. Med. Entomol. 2010;47:805–813. doi: 10.1603/me10031. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization . Handbook for Integrated Vector Management. World Health Organization; 2012. [Google Scholar]
- 75.Lizzi KM, Qualls WA, Brown SC, Beier JC. Expanding Integrated Vector Management to promote healthy environments. Trends Parasitol. 2014;30:394–400. doi: 10.1016/j.pt.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Souza RL, et al. Effect of an intervention in storm drains to prevent Aedesaegypti reproduction in Salvador, Brazil. Parasit. Vectors. 2017;10:1–6. doi: 10.1186/s13071-017-2266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilke ABB, Beier JC, Benelli G. Transgenic mosquitoes—Fact or fiction? Trends Parasitol. 2018;34:456–465. doi: 10.1016/j.pt.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 78.Beier JC, Wilke ABB, Benelli G. Newer approaches for malaria vector control and challenges of outdoor transmission. Towards Malaria Elimination - A Leap Forward. 2018 doi: 10.5772/intechopen.75513. [DOI] [Google Scholar]
- 79.World Health Organization. Tenth Meeting of the WHO Vector Control Advisory Group. (2019).
- 80.Wilke ABB, et al. Effectiveness of adulticide and larvicide in controlling high densities of Aedesaegypti in urban environments. PLoS ONE. 2021;16:e0246046. doi: 10.1371/journal.pone.0246046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vicente-Serrano SM, et al. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. 2013;110:52–57. doi: 10.1073/pnas.1207068110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rifat SA, Al & Liu, W. Quantifying spatiotemporal patterns and major explanatory factors of urban expansion in Miami metropolitan area during 1992–2016. Remote Sens. 2019;11:2493. [Google Scholar]
- 83.Fuller DO, Wang Y. Recent trends in satellite vegetation index observations indicate decreasing vegetation biomass in the southeastern saline Everglades wetlands. Wetlands. 2014;34:67–77. [Google Scholar]
- 84.Wilke ABB, et al. Assessment of the effectiveness of BG-Sentinel traps baited with CO2 and BG-Lure for the surveillance of vector mosquitoes in Miami-Dade County. Florida. PLoS One. 2019;14:e0212688. doi: 10.1371/journal.pone.0212688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darsie, R. F. Jr. & Morris, C. D. Keys to the adult females and fourth-instar larvae of the mosquitoes of Florida (Diptera, Culicidae). 1st ed. Vol. 1. Tech Bull Florida Mosq Cont Assoc (2000).
- 86.Anderson, M. J. Permutational Multivariate Analysis of Variance (PERMANOVA). WileyStatsRef:StatisticsReferenceOnline. 1–15 (2017) DOI:10.1002/9781118445112.stat07841.
- 87.Alencar J, et al. Culicidae community composition and temporal dynamics in Guapiaçu ecological reserve, Cachoeiras de Macacu, Rio de Janeiro, Brazil. PLoS ONE. 2015;10:1–16. doi: 10.1371/journal.pone.0122268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 1993;18:117–143. [Google Scholar]
- 89.Hammer Ø, Harper DATT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:9. [Google Scholar]
- 90.Ryan PA, Lyons SA, Alsemgeest D, Thomas P, Kay BH. Spatial statistical analysis of adult mosquito (Diptera: Culicidae) counts: An example using light trap data, in Redland Shire, southeastern Queensland, Australia. J. Med. Entomol. 2004;41:1143–1156. doi: 10.1603/0022-2585-41.6.1143. [DOI] [PubMed] [Google Scholar]
- 91.O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 2007;41:673–690. [Google Scholar]
- 92.Wilke ABB, Medeiros-Sousa AR, Ceretti-Junior W, Marrelli MT. Mosquito populations dynamics associated with climate variations. Acta Trop. 2016;166:343–350. doi: 10.1016/j.actatropica.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 93.Cohen J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educ. Psychol. Meas. 1973;33:107–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.