Abstract
Multiple N-methyl-d-aspartate (NMDA) receptor enhancing agents have had promising effects on cognition among patients with dementia. However, the results remain inconsistent. This exploratory meta-analysis investigated the effectiveness of NMDA receptor enhancing agents for cognitive function. PubMed, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews were searched for randomized controlled trials (RCTs). Controlled trials assessing add-on NMDA receptor enhancing agent treatment in patients with dementia and using cognition rating scales were eligible and pooled using a random-effect model for comparisons. The standardized mean difference (SMD) was calculated in each study from the effect size; positive values indicated that NMDA receptor enhancing agent treatment improved cognitive function. Funnel plots and the I2 statistic were evaluated for statistical heterogeneity. Moderators were evaluated using meta-regression. We identified 14 RCTs with 2224 participants meeting the inclusion criteria. Add-on NMDA receptor enhancing agents had small positive significant effects on overall cognitive function among patients with dementia (SMD = 0.1002, 95% CI 0.0105–0.1900, P = 0.02860). Subgroup meta-analysis showed patients with Alzheimer’s Disease and trials using the Alzheimer Disease Assessment Scale-cognitive subscale as the primary outcome had small positive significant effects (SMD = 0.1042, 95% CI 0.0076–0.2007, P = 0.03451; SMD = 0.1267, 95% CI 0.0145–0.2388, P = 0.2686). This exploratory meta-analysis showed a very small, positive, and significant effect on overall cognition function in patients with dementia. Studies with larger samples are needed to evaluate different cognitive domains and phases of dementia.
Subject terms: Diseases, Medical research, Molecular medicine, Neurology
Introduction
Dementia is a progressive neuropsychiatric disorder that affects memory and other cognitive functions, thus interfering with life function1. Alzheimer disease (AD) is the leading cause of dementia among elderly people2,3, and the prevalence of dementia due to AD is 4.02% among adults aged over 60 years4. Approximately 35.6 million adults worldwide developed dementia in 2010, and the number is projected to increase to 65.7 million in 20305. Worldwide spending on AD was approximately 422 billion US dollars in 20096. The main pharmacologic treatments for patients with AD are cholinesterase inhibitors and the N-methyl-d-aspartate receptor (NMDAR) partial antagonist memantine. However, these treatments have adverse effects, and the response is unsatisfactory7. Mild cognitive impairment (MCI), characterized by objective cognitive impairment, is defined as the predementia stage on the continuum of cognitive decline8,9. Several studies have reported that patients with MCI generally develop AD10,11. However, no treatment has yet been approved for MCI. Therefore, the development of alternative medications is urgently required for AD and MCI.
NMDAR has been demonstrated to play a critical role in controlling synaptic plasticity and memory function12. Studies have reported that overactivation of NMDAR may cause neurotoxicity, especially in the late phase of AD13,14. NMDAR antagonists have been developed for the treatment of AD, and memantine, an uncompetitive NMDAR partial antagonist, has been approved as an antidementia medication for moderate to severe AD15,16; memantine is not approved for mild AD and MCI because of unsatisfactory efficacy17. However, NMDAR antagonists, such as ketamine, have been demonstrated to impair spatial learning and verbal information ability in healthy humans18. Moreover, human studies have reported an age-related decrease in the density of NMDARs in the cerebral cortex and hippocampus19. These findings implicate the hypoactivation of NMDAR in cognitive impairment.
Several trials of NMDAR enhancing agents have been performed to evaluate their effect on cognitive impairment among patients with dementia. Sodium benzoate, an inhibitor of D-amino acids oxidase, can increase d-serine and thus enhance NMDAR activation20,21. A randomized, double-blind, placebo-controlled trial of 60 patients with early-phase AD or MCI reported a larger improvement in the AD Assessment Scale-cognitive subscale (ADAS-cog) score compared with placebo22. D-cycloserine, a partial agonist at the glycine site of NMDAR, has been reported to improve ADAS-cog score in patients with AD23. However, other trials with D-cycloserine did not report significant improvement in cognition24,25. Therefore, we conducted a meta-analysis on the cognitive effects of NMDAR enhancing agents in dementia.
Methods
Search strategy and inclusion criteria
Two independent authors (Chun-Hung Chang and Shaw-Ji Chen) performed a systematic literature search from the study’s inception until August 7th, 2021. We followed the drug keywords used by previous meta-analytic reviews about NMDA receptor positive modulators26–29. The search strategy comprised the following keywords: (Dementia OR Alzheimer*) AND (acetylcysteine OR α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid OR AMPA OR benzoate OR CX516 OR D-cycloserine OR D-serine OR glutamine OR glutamate OR glutamate carboxypeptidase 2 OR GCP2 OR glycine OR glycine transporter type 1 OR GlyT1 OR glutamate receptor ionotropic kainate OR GRIK OR kynurenine aminotransferase OR KAT OR metabotropic glutamate receptor OR mGluR OR minocycline OR N-acetyl-aspartylglutamate OR NAAG OR N-methyl-d-aspartate OR NMDA OR pregnenolone OR sarcosine) AND controlled trial 26. The authors independently evaluated the titles and abstracts of all retrieved papers for eligibility. Full-text articles of potentially eligible trials were reviewed and compiled in a list of studies for inclusion. Disagreements between the two authors regarding the inclusion of a study were settled by a third reviewer (Professor Tsai). The Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) were followed (Fig. 1, Supplementary Table S1)30–32.
Figure 1.
PRISMA flow diagram of the search and identification of included studies. Database: PubMed (n = 304), Cochrane Central Register of Controlled Trials (n = 208), Cochrane Database of Systematic Reviews (n = 7). Keyword: (Dementia OR Alzheimer*) AND (acetylcysteine OR α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid OR AMPA OR benzoate OR CX516 OR D-cycloserine OR D-serine OR glutamine OR glutamate OR glutamate carboxypeptidase 2 OR GCP2 OR glycine OR glycine transporter type 1 OR GlyT1 OR glutamate receptor ionotropic kainate OR GRIK OR kynurenine aminotransferase OR KAT OR metabotropic glutamate receptor OR mGluR OR minocycline OR N-acetyl-aspartylglutamate OR NAAG OR N-methyl-d-aspartate OR NMDA OR pregnenolone OR sarcosine) AND controlled trial. Date: date available to August 7th, 2020.
Eligibility criteria
The inclusion criteria were as follows: (a) studies on participants who received a diagnosis of dementia, (b) studies including randomized placebo-controlled trials, and (c) studies on the use of NMDAR enhancing agents as a monotherapy or adjunctive treatment to concomitant antidementia drugs. The exclusion criteria were as follows: (1) abstracts from ClinicalTrials.gov, (2) conference abstracts, (3) reviews and comments, (4) studies on treatment other than NMDAR enhancing modulators, (5) studies on a different population or outcome, and (6) trial protocols.
Outcome measures
The effects of glutamate positive modulators on cognitive deficits in patients with dementia were investigated. Overall cognitive function (primary outcome) was compared between patients receiving NMDAR enhancing agents and those receiving placebo. The common cognitive measures in dementia are the Mini-Mental State Examination (MMSE)33 and the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog)34. The MMSE is a cognitive test commonly used to screen for dementia and measure cognitive impairment in older adults35. The MMSE total score ranges from 0 to 30 (highest to lowest level of cognitive impairment). The ADAS-cog consists of 11 tasks, and its score ranges from 0 to 70 (lowest to highest level of cognitive impairment).
Data extraction
Two independent reviewers (CH Chang and SJ Chen) screened and identified articles. We recorded information including the first author, published year, number of participants, gender proportion, mean age, duration, NMDA receptor enhancing agents, and summary of neurocognitive measures (Table 1). We followed the PRISMA guidelines. The primary outcome was the difference in effect sizes between the NMDAR enhancing agent and control groups (calculated in terms of the standard mean difference [SMD] with the corresponding 95% confidence interval [CI] and P value). The primary author of a study was contacted to request the original data if these data were not available in the corresponding article. If no relevant data were reported in the article, other compatible statistical parameters (e.g., P value, sample size, or odds ratio) were used to estimate the effect size, following the Comprehensive Meta-Analysis manuals protocol and the Comprehensive Meta-Analysis website guide (https://www.meta-analysis.com/downloads/Meta-analysis%20Converting%20among%20effect%20sizes.pdf); the estimated effect sizes were then converted and pooled into SMDs. Variables of interest were extracted when possible, including first author, year, sample size, proportion of men, mean age, therapy duration, add-on NMDAR enhancing agents, and cognitive outcome measures.
Table 1.
Summary of the characteristics of studies included in the meta-analysis.
| Study (first author, year) | N | Gender (%male) |
Mean age (years) |
Duration | Add-on therapy | Diagnosis | Primary cognition outcome measures | Other cognitive measures | Mean cognitive score at baseline | Study design | Double-blind |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Howard et al. (2020) | 544 | 55.7 | 74.3(8.2) | 24 m | Minocycline | Alzheimer disease | MMSE | NR | MMSE:26.4 ± 1.9 | RCT | Yes |
| Bernard et al. (2019) | 520 | 30.3 | 71.1(7.3) | 24w | S47445 | Alzheimer disease | ADAS-cog | MMSE |
ADAS-cog: 23.6 ± 9.0 MMSE:19.7 ± 2.8 |
RCT | Yes |
| Lin et al. (2019) | 97 | 45.1 | 75.5(7.8) | 6w | Benzoate | Dementia | ADAS-cog | CDR, MMSE | ADAS-cog:29.3 ± 12.6 | RCT | Yes |
| Kouzuki et al. (2019) | 159 | 12.6 | 87.1(0.7) | 12w | Monosodium L-glutamate | Dementia | TDAS | GBSS |
TDAS:55.2 ± 3.6 GBSS:64.7 ± 3.9 |
RCT | No |
| Lin et al. (2014) | 60 | 38.3 | 70.2(8.5) | 24w | Benzoate | Alzheimer disease | ADAS-cog | CIBIC-plus, cognitive composite | ADAS-cog:15.3 ± 7.5 | RCT | Yes |
| Tsai et al. (2014) | 30 | 63.3 | 76.8(6.0) | 8w | Sarcosine | Parkinson’s disease with dementia | CASI | CASI, MMSE, CDR |
CASI:57.4 ± 23.9 MMSE:17.3 ± 6.7 CDR:2.2 ± 1.2 |
RCT | Yes |
| Chappell et al. (2007) | 181 | 47.5 | 74.5(9.0) | 11w | LY451395 | Alzheimer disease | ADAS-cog |
CIBIC, Trail Making Part A, Stylus Tapping Test (STT), Single Digit Modality Test (SDMT) |
ADAS-cog:19.4 ± 9.0 MMSE:20.3 ± 3.3 | RCT | Yes |
| Adair et al. (2001) | 43 | NR | NR | 6 m | NAC | Alzheimer disease | MMSE | Four separate batteriesa | MMSE:19.0 ± 3.7 | RCT | Yes |
| Tsai et al. (1999) | 17 | 64.7 | 72.2(7.3) | 4w | D-Cycloserine | Alzheimer disease | ADAS-cog | NR |
ADAS-cog:23.5 ± 9.0 MMSE:18.8 ± 5.3 |
RCT, crossover | Yes |
| Tsai et al. (1998) | 10 | 40.0 | 74.7(8.5) | 4w | D-Cycloserine | Alzheimer disease | ADAS-cog | MMSE |
ADAS-cog:25.5 ± 2.7 MMSE:20.0 ± 5.2 |
RCT, crossover | Yes |
| Schwartz et al. (1996) | 108 | 53.8 | 74.4(8.6) | 10w | D-Cycloserine | Alzheimer disease | NR | MMSE, DRS, Implicit memory performance of words | NR | RCT | Yes |
| Mohr et al. (1995) | 40 | NR | NR | 24w | Cycloserine | Alzheimer disease | Cognitive Drug Research Computerized Assessment System (CDR System) | Mattis Dementia Rating Scale (MDRS), CIBIC, Brief Cognitive Rating Scale (BCRS) | NR | RCT | Yes |
| Fakouhi et al. (1995) | 403 | 46.1 | 73.6(8.0) | 26w | Cycloserine | Alzheimer disease | Cognitive Drug Research (CDR) computerized test , DRS | MMSE | NR | RCT | Yes |
| Randolph et al. (1994) | 12 | 70.0 | 65.0(8.4) | 2w | D-Cycloserine | Alzheimer disease | ADAS-cog, RBAD | MMSE | MMSE:21.0 ± 3.3 | RCT, crossover | Yes |
ADAS-cog Alzheimer’s Disease Assessment Scale-cognitive subscale; CASI Cognitive Abilities Screening Instrument; CDR Clinical Dementia Rating; CIBIC-plus Clinician’s Interview-Based Impression of Change plus Caregiver Input; DRS Dementia Rating Scale; GBSS Gottfries–Bråne–Steen Scale; HVLT Hopkins Verbal Learning Task; MMSE Mini-Mental Status Examination; NR no reported; RBAD Repeatable Battery for the Assessment of Dementia; TDAS the Touch Panel-type Dementia Assessment Scale; WMS, Wechsler Memory Scale.
aBoston Naming Test, Gesture to Command, WMS Figure Reproduction(immediate), HVLT Recall (immediate), HVLT Recognition, Letter fluency, Category fluency, Judgment of Line Orientation.
Quality assessment
Two authors (CH Chang and SJ Chen) evaluated the methodological quality of the included studies. The Jadad scoring system was used for randomized controlled trials (RCTs)36. The Jadad scoring system contains three items that assess randomization (2 points), blinding (2 points), and an account of all patients (1 point). Therefore, the score ranges from 0 to 5. A higher score indicates higher methodological quality.
Meta-analysis procedure
A random-effects model was used for the meta-analysis37. The SMD with 95% CI was selected to compare the ESs of our primary outcome, rather than the difference in means, because each study was presumed to use different units. Furthermore, when the SMD value was higher than 0, the effect size was defined as indicating a positive effect in the NMDAR enhancing agent group compared with the placebo group. After data from included trials were extracted, Comprehensive Meta-Analysis software version 3 (Biostat, Englewood, NJ, USA) was used to perform meta-analyses. Statistical significance was defined as a two-tailed P < 0.05. Of note, the correction of multiple comparison was not made in this study due to this was an exploratory study rather than confirmatory study.
Heterogeneity and publication bias
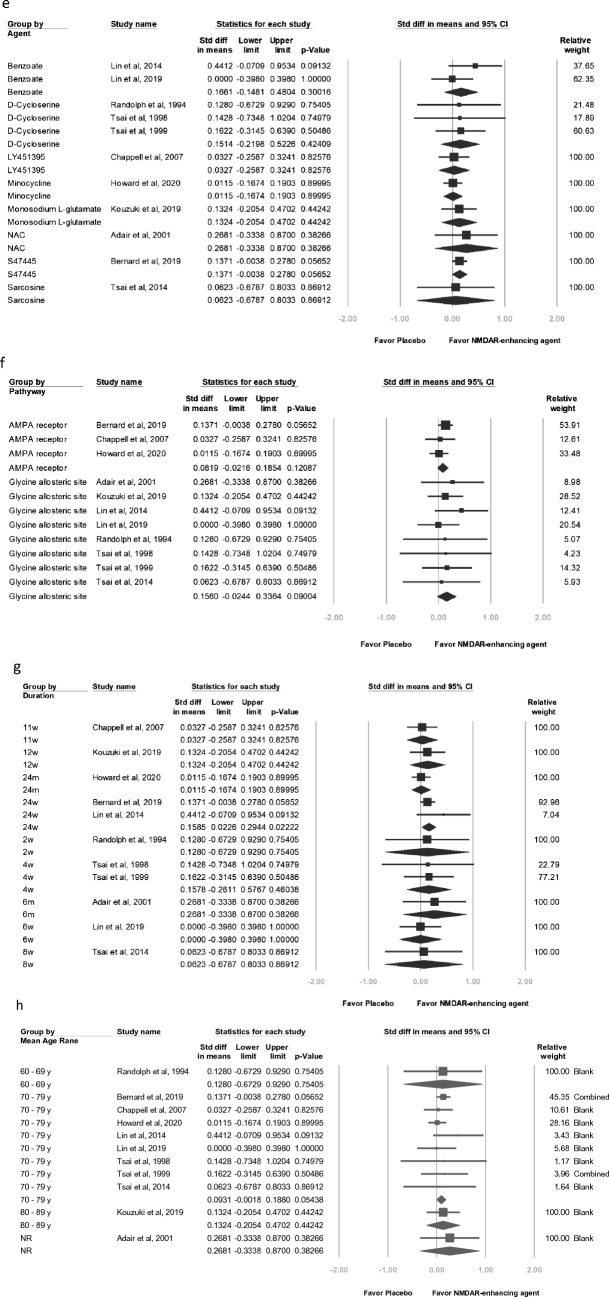
The I2 statistic was used as a measure of heterogeneity37, which was assessed using the Cochran Q test and corresponding P value38. Publication bias was assessed using funnel plots39 and Egger's regression test40 If publication bias was discovered, Duval and Tweedie's trim-and-fill procedure, a validated model for ES estimation, was used41.
Sensitivity test
Studies were individually removed from the meta-analysis to verify that the results were not caused by outliers in the included studies.
Subgroup meta-analysis and meta-regression
Subgroup analyses were performed when at least two independent data sets were available41. Samples were segmented by variables, including diagnosis of dementia, cognitive measure, study design, NMDAR enhancing agent, drug pathway in enhancing glutamatergic neurotransmission, duration of therapy, and age range. Furthermore, meta-regression analyses were performed for each potential moderator, including baseline mean ADAS-cog total score, proportion of men, and mean age.
Results
Characteristics of included studies and patients
The final quantitative analysis included 2224 participants from 14 trials22–25,42–51. The PRISMA flow diagram is displayed in Fig. 1, and the study characteristics are summarized in Table 1 and Supplementary Table S2. The mean number of subjects in each study was 158.86 ± 188.73 (range: 10–544), and the mean duration of the studies was 20.21 ± 25.69 weeks (range: 2–104 weeks). The mean age of the subjects was 74.10 ± 5.14 years. The mean proportion of men was 47.27% ± 16.02%. Nine NMDAR enhancing agents were investigated. The numbers of studies and subjects for each compound investigated were as follows: benzoate, two studies, n = 157; cycloserine, two studies, n = 443; D-cycloserine, four studies, n = 147; LY451395, one study, n = 181; minocycline, one study, n = 544; monosodium L-glutamate, one study, n = 159; N-acetylcysteine (NAC), one study, n = 43; S47445, one study, n = 520; and sarcosine, one study, n = 30.
Meta-analyses of overall cognitive function
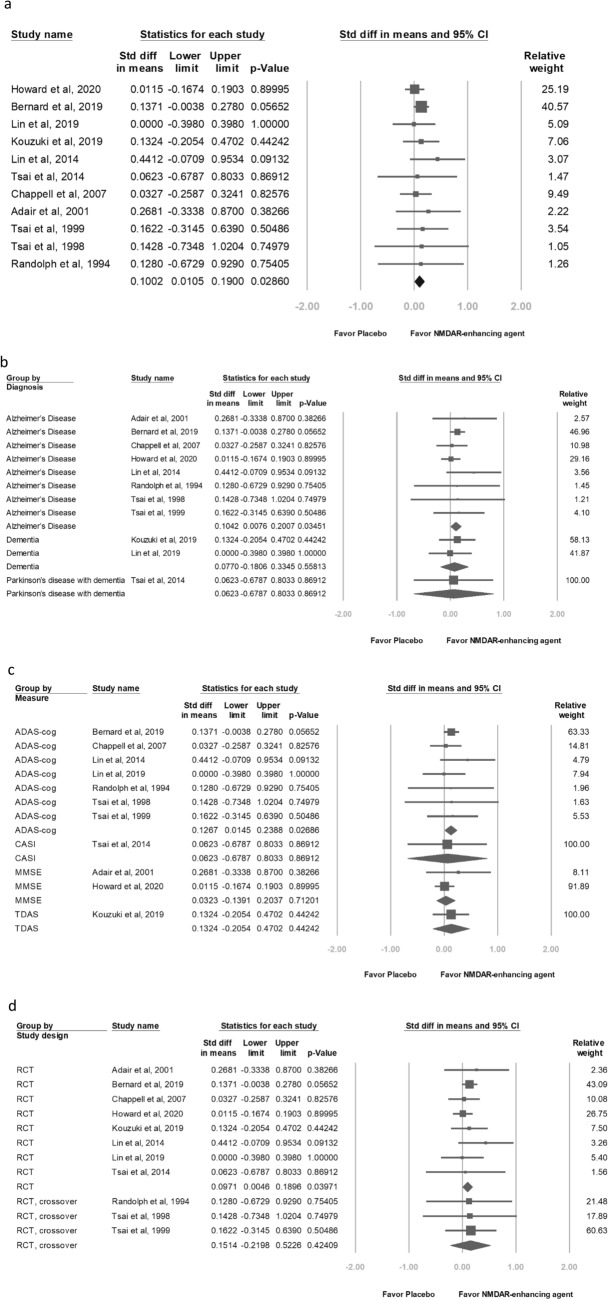
Overall cognitive function data were reported in 11 studies22–25,42–44,48,49,51,52. Effects were conventionally categorized as small (SMD = 0.2), moderate (SMD = 0.5) or large (SMD = 0.8), with positive values indicating improvements in cognitive function. Overall, NMDAR enhancing modulators had a small positive significant effect on overall cognitive function among patients with dementia (SMD = 0.1002, 95% CI 0.0105–0.1900, P = 0.02860; Fig. 2a).
Figure 2.
Meta-analyses of (a) overall cognitive function, (b) diagnosis of dementia groups, (c) cognitive measure groups, (d) study design groups, (e) NMDAR enhancing agent groups, (f) drug pathway in enhancing glutamatergic neurotransmission groups, (g) duration of therapy groups, and (h) age range groups.
Subgroup analyses
Diagnosis of dementia
This meta-analysis which pooled eight trials22–25,43,44,48,51 showed that the patients with AD revealed a significant but small effect (SMD = 0.1042, 95% CI 0.0076–0.2007, P = 0.03451; Fig. 2b).
Cognitive measure as the primary outcome
This meta-analysis which summarized seven trials22–25,43,49,51 that used the ADAS-cog as their cognitive measure reported small effects (SMD = 0.1267, 95% CI 0.0145–0.2388, P = 0.2686), whereas two trials44,48 that used the MMSE as their cognitive measure reported nonsignificant effects (SMD = 0.0323, 95% CI − 0.1391–0.2037, P = 0.71201; Fig. 2c).
Study design
This meta-analysis which pooled eight RCTs22,42–44,48–51 reported significant positive effects (SMD = 0.0971, 95% CI 0.0046–0.1896, P = 0.03971). Three trials23–25 were crossover RCTs and discovered nonsignificant effects (SMD = 0.1514, 95% CI − 0.2198–0.5226, P = 0.42409; Fig. 2d).
NMDAR enhancing agents
Furthermore, we performed a subgroup meta-analysis of the studies with add-on NMDAR enhancing agents to evaluate the overall cognitive effect. Nine NMDAR enhancing agents were investigated: benzoate, cycloserine, D-cycloserine, LY451395, minocycline, monosodium L-glutamate, NAC, S47445, and sarcosine. Subgroup meta-analyses for each NMDAR enhancing agent revealed small positive but nonsignificant differences in effect on overall cognitive function (Fig. 2e).
Pathway through which the drugs enhance glutamatergic neurotransmission
Glycine allosteric site of NMDARs
A small positive but nonsignificant SMD was observed in the effect of drugs (benzoate, monosodium L-glutamate, sarcosine, NAC, and D-cycloserine) and placebo on overall cognition (SMD = 0.1560, 95% CI − 0.0244–0.3364, P = 0.09004; Fig. 2f).
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors
A small positive but nonsignificant SMD was observed in the effects of drugs (minocycline, S47445, and LY451395) and placebo on overall cognition (SMD = 0.0819, 95% CI − 0.0216–0.1854, P = 0.12087; Fig. 2f).
Treatment duration
This meta-analysis which summarized two trials22,51 with 24-week treatment duration discovered small positive significant effect (SMD = 0.1585, 95% CI 0.0226–0.2944, P = 0.02222; Fig. 2g).
Patient age
This meta-analysis which pooled eight trials22,23,25,42,43,48,49,51 enrolling patients aged 70–79 years obtained nonsignificant small effect (SMD = 0.0931, 95% CI − 0.0018–0.1880, P = 0.05438; Fig. 2h).
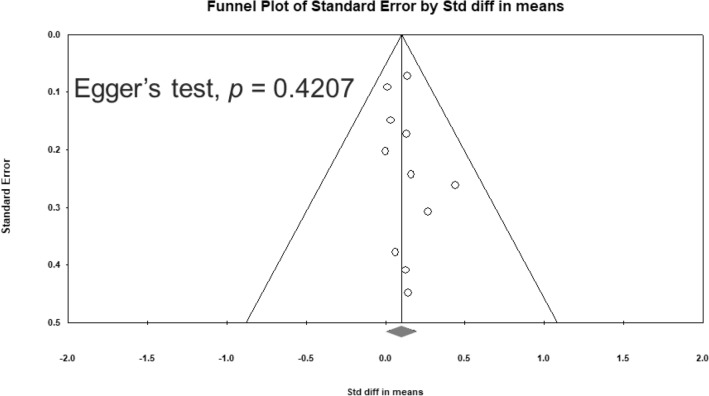
Meta-regression analyses of overall cognitive function
Higher baseline mean ADAS-cog total score was associated but nonsignificant with higher proportion of men, higher mean age, and weaker effect of NMDAR modulators on overall cognitive function (baseline mean ADAS-cog score, slope = − 0.0174, P = 0.3984; proportion of men, slope = − 0.2973, P = 0.3580; age, slope = − 0.0044, P = 0.7029; Fig. 3).
Figure 3.
Meta-regression of effects of NMDAR enhancing agents on overall cognitive function in relation to (a) baseline mean ADAS-cog total score, (b) proportion of men, and (c) mean age.
Heterogeneity and publication bias
No significant heterogeneity was observed in overall cognitive function among the studies (Q = 3.784, df = 10, I2 = 0.000%, P = 0.957). Egger’s test revealed no significant publication bias regarding the overall cognitive effects (P = 0.4207). The funnel plots for the effect sizes of overall cognitive function are displayed in Fig. 4.
Figure 4.
Funnel plots of overall cognitive function SMD.
Sensitivity analysis
The positive effect of NMDAR enhancing agents on overall cognitive function became nonsignificant when the study by Bernard51 or Lin22 was removed.
Methodological quality of the included studies
Among the fourteen RCTs, the average Jadad score was 3.14 with a standard deviation (SD) of 0.36 (Supplementary Table S2).
Discussion
To our knowledge, this is the first study to investigate the efficacy of NMDA enhancing agents on cognition among patients with dementia. The main findings of this analysis are that (1) NMDAR enhancing agents had small positive significant effects on overall cognitive function compared with placebo, (2) trials on patients with AD obtained small positive significant effects, (3) trials using the ADAS-cog as the primary outcome obtained small positive significant effects.
These findings accord with those of most related studies. Two of the studies reported significant improvement in overall cognitive function in patients with AD22,23. Lin and colleagues treated patients with mild AD with 250–750 mg/d of sodium benzoate or placebo for 24 weeks, and the patients treated with sodium benzoate displayed significantly greater improvement in ADAS-cog score (P = 0.0031)22. Tsai and colleagues treated 17 patients with AD with 50 or 100 mg/d of D-cycloserine or placebo for 4 weeks; the patients treated with D-cycloserine displayed significantly greater improvement in ADAS-cog score (placebo: 24.05 ± 10.99; 50 mg of D-cycloserine: 23.86 ± 10.19; 100 mg of D-cycloserine: 21.12 ± 8.82)23. Seven studies reported nonsignificant improvement in overall cognitive functions24,25,43,44,48,50,51. For example, Howard reported that patients treated with minocycline had mean MMSE score 0.1 points higher than those treated with placebo; however, the mean was nonsignificantly different (95% CI − 1.1–1.2; P = 0.90)48. Bernard reported that patients treated with S47445 had nonsignificant improvement in ADAS-cog score; the adjusted mean change in ADAS-cog total score from baseline to week 24 was 0.35 ± 5.53 in the placebo group, − 0.20 ± 4.91 in the 5 mg of S47445 group, − 0.74 ± 5.43 in the 15 mg of S47445 group, and − 0.05 ± 5.82 in the 50 mg of S47445 group51.
Subgroup analyses revealed that the meta-analysis which pooled eight trials enrolling patients with AD22–25,43,44,48,51 showed a small positive significant effect (SMD = 0.1042, 95% CI 0.0076–0.2007, P = 0.03451; Fig. 2b), while trials enrolling patients with non-AD dementia did not. This finding may imply that patients with AD may more benefit from NMDAR enhancing agents than patients with non-AD dementia. Lin and colleagues treated patients with mild AD receiving with 250–750 mg/d of sodium benzoate or placebo for 24 weeks; those treated with sodium benzoate displayed significantly greater improvement in ADAS-cog score (P = 0.0031)22. Their findings suggest that patients with mild AD may benefit more from NMDAR enhancing agents than patients with moderate to severe AD. However, in our meta-regression, no significant association between effect size and mean ADAS-cog was observed. Further studies are needed to investigate these findings.
Moreover, we noted that this meta-analysis summarizing seven trials22,23,25,43,49,51 in which the ADAS-cog was employed as the cognitive measure revealed small positive significant effects (SMD = 0.1267, 95% CI 0.0145–0.2388, P = 0.02686), whereas two trials44,48 using the MMSE reported nonsignificant effects (SMD = 0.0323, 95% CI − 0.1391–0.2037, P = 0.71201; Fig. 2c). The MMSE and ADAS-cog are most common cognitive measures in these trials. The MMSE is often employed to screen for dementia and measure cognitive impairment in older adults35. The MMSE total score ranges from 0 to 30 (highest to lowest level of cognitive impairment) while the ADAS-cog consists of 11 tasks, with a total score ranging from 0 to 70 (lowest to highest level of cognitive impairment)53. MMSE and ADAS-cog have shown to be relatively psychometrically poor at detecting small changes54–56. Therefore, researchers may use more sensitive instruments like Addenbrooke's Cognitive Examination57 or Montreal Cognitive Assessment58,59 to explore minor changes in cognition in the early stages of dementia receiving NMDAR enhancing agents.
The mechanism of the positive effect of NMDAR enhancing agents on cognition remains unclear. Meta-analytic studies have investigated NMDAR enhancing agents in patients with schizophrenia. Tsai and colleagues reported that the overall effect of NMDAR enhancing agents (glycine, D-serine, and sarcosine) on cognitive symptoms was 0.28 (95% CI 0.10–0.47, P = 0.002, 13 studies, n = 485)29, whereas two other meta-analytic studies did not report a significant effect on overall cognition in schizophrenia26,28. An updated meta-analysis enrolling 25 trials and 1951 participants revealed no significant effect of NMDA-enhancing agents on overall cognition. However, subgroup analysis suggested that NMDAR-enhancing agents may benefit young patients with schizophrenia, and NAC may have effect on working memory27. Human studies have reported an age-related decrease in the density of NMDARs in the cerebral cortex and hippocampus19. NMDAR activation improves memory function12. Several trials with NMDAR enhancing agents, such as benzoate and D-cycloserine, have reported promising effects, especially in early-stage AD22,23.
Side effects
The side effects were mostly mild and relieved after discontinuing the agents. The side effect rate of NMDAR enhancing agents ranged from 0 to 61.1%. Minocycline was reported side effects including dermatologic symptoms (hyperpigmentation, photosensitivity, rash), gastrointestinal symptoms (diarrhea, nausea, sore mouth, vomiting), neurologic symptoms (headache, visual or auditory disturbances, dizziness), infections (oral or genital candidiasis, vaginitis, anal irritation, bacterial enteritis, staphylococcal, or Clostridium difficile). Six skin toxic effects were considered severe48. The most common adverse effects of the study with AMPA modulator S47445 are nasopharyngitis, blood creatine phosphokinase increased, diarrhea, fall, headache, type 2 diabetes mellitus, abdominal pain51. D-cycloserine was reported no side effects23,25. Detailed information about the adverse events addressed in the included studies is summarized in Supplementary Table S2. The attrition rate ranged from 0 to 0.49. The effect size in this exploratory meta-analysis was very small. Most side effects were mild. The benefit may weigh up against the side effects.
Implication
Cognitive impairment is a critical problem in central nerve disease such as dementia. AD is the leading cause of dementia. In 2010, the number of patients with AD was estimated at 36.6 million and is projected to double every 20 years2. Dementia is a considerable economic burden on both patients and society6. However, understanding of the complex mechanisms and treatment response remains unsatisfactory3. Therefore, developing alternative therapeutic approaches is crucial. Our meta-analysis revealed a positive and significant effect of NMDAR positive modulators on overall cognition among patients with dementia. Further trials with better design and larger samples are needed to explore NMDAR enhancing agents in MCI or early-phase dementia.
Strength
The present study had several strengths. First, this is the first meta-analytic study to evaluate the treatment effect of NMDAR positive modulators among patients with dementia. Second, nine compounds were included in this meta-analysis. Third, we noted that NMDAR enhancing agents may have a small positive significant effect on overall cognitive function compared with placebo.
Limitations
There are several limitations in the present study. First, this is an exploratory systematic review and meta-analysis of randomized controlled trials. We could observe related phenomena but not clarify the underlying pathophysiology because of the methodological limitations of meta-analyses. Therefore, caution should be exercised when drawing conclusions. Second, the number of participants was small in some included trials. Third, some trials were conducted over a short period (less than 6 months). Therefore, the long-term cognitive effects of NMDAR enhancing agents and the persistent effects after treatment remain unclear. Kouzuki and colleagues reported improvement in the Touch Panel-type Dementia Assessment Scale total score during a 4-week follow-up assessment50. Fourth, the trials used different standard cognitive tests. Fifth, nine NMDAR enhancing agents were investigated. These agents may have different mechanisms of action involving the N-methyl-d-aspartate (NMDA) system. Further studies are needed to explore the relationships between neurocognitive effects and specific NMDAR enhancing mechanisms. Some modulators may be involved in mechanisms other than the NMDA system. Sixth, the effects of the stage of dementia and concomitant antidementia drugs remain unclear.
Conclusion
The findings from this meta-analysis indicated that NMDAR enhancing agents showed a very small positive effect on overall cognitive function in patients with dementia. Further studies with a larger sample are warranted to explore the role of the NMDA system on specific cognitive domains in subgroups of patients with early-stage dementia.
Supplementary Information
Acknowledgements
This work was supported by grants from An Nan Hospital, China Medical University Hospital (ANHRF108-01 and ANHRF108-12), China Medical University Hospital (DMR-109-246), the National Health Research Institutes (NHRI-EX109-10731NI), and TCMF-MP 108-01-03, Buddhist Tzu Chi Medical Foundation.
Author contributions
C.-H.C., S.-J.C., and C.-Y.L. proposed the research ideas, performed the statistical analysis, processed the database, and drafted the initial manuscript. H.-C.T. was in charge of this study, critically reviewed the draft of the manuscript, and approved the final submitted version of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-02040-5.
References
- 1.Mitchell SL. Advanced dementia. N. Engl. J. Med. 2015;373:1276–1277. doi: 10.1056/NEJMc1509349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Querfurth HW, LaFerla FM. Alzheimer's disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.Scheltens P, et al. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 4.Fiest KM, et al. The Prevalence and Incidence of Dementia Due to Alzheimer's Disease: a Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. 2016;43(Suppl 1):S51–S82. doi: 10.1017/cjn.2016.36. [DOI] [PubMed] [Google Scholar]
- 5.Prince M, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's Dementia: J Alzheimer's Assoc. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Wimo A, Winblad B, Jonsson L. The worldwide societal costs of dementia: estimates for 2009. Alzheimer's Dementia. 2010;6:98–103. doi: 10.1016/j.jalz.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Shafqat S. Alzheimer disease therapeutics: perspectives from the developing world. J. Alzheimer's Dis.: JAD. 2008;15:285–287. doi: 10.3233/JAD-2008-15211. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC. Clinical practice. Mild cognitive impairment. N. Engl. J. Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 9.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manly JJ, et al. Frequency and course of mild cognitive impairment in a multiethnic community. Ann. Neurol. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia–meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Tsien JZ. Memory and the NMDA receptors. N. Engl. J. Med. 2009;361:302–303. doi: 10.1056/NEJMcibr0902052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 14.Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet. Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reisberg B, et al. Memantine in moderate-to-severe Alzheimer's disease. N. Engl. J. Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 16.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 17.Schneider LS, Dagerman KS, Higgins JP, McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch. Neurol. 2011;68:991–998. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- 18.Rowland LM, et al. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology. 2005;30:633–639. doi: 10.1038/sj.npp.1300642. [DOI] [PubMed] [Google Scholar]
- 19.Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech. Ageing Dev. 2001;122:1–29. doi: 10.1016/S0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- 20.Lin CH, Lane HY, Tsai GE. Glutamate signaling in the pathophysiology and therapy of schizophrenia. Pharmacol. Biochem. Behav. 2012;100:665–677. doi: 10.1016/j.pbb.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Howley E, et al. Assessment of the target engagement and D-serine biomarker profiles of the D-amino acid oxidase inhibitors sodium benzoate and PGM030756. Neurochem. Res. 2017;42:3279–3288. doi: 10.1007/s11064-017-2367-9. [DOI] [PubMed] [Google Scholar]
- 22.Lin CH, et al. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: a randomized, double-blind, placebo-controlled trial. Biol. Psychiatry. 2014;75:678–685. doi: 10.1016/j.biopsych.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Tsai GE, Falk WE, Gunther J, Coyle JT. Improved cognition in Alzheimer's disease with short-term D-cycloserine treatment. Am. J. Psychiatry. 1999;156:467–469. doi: 10.1176/ajp.156.3.467. [DOI] [PubMed] [Google Scholar]
- 24.Randolph C, et al. D-cycloserine treatment of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1994;8:198–205. doi: 10.1097/00002093-199408030-00006. [DOI] [PubMed] [Google Scholar]
- 25.Tsai GE, Falk WE, Gunther J. A preliminary study of D-cycloserine treatment in Alzheimer's disease. J. Neuropsychiatry Clin. Neurosci. 1998;10:224–226. doi: 10.1176/jnp.10.2.224. [DOI] [PubMed] [Google Scholar]
- 26.Iwata Y, et al. Effects of glutamate positive modulators on cognitive deficits in schizophrenia: a systematic review and meta-analysis of double-blind randomized controlled trials. Mol. Psychiatry. 2015;20:1151–1160. doi: 10.1038/mp.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang CH, et al. Effect of N-methyl-d-aspartate -receptor-enhancing agents on cognition in patients with schizophrenia: a systematic review and meta-analysis of double-blind randomised controlled trials. J. Psychopharmacol. 2019;33:436–448. doi: 10.1177/0269881118822157. [DOI] [PubMed] [Google Scholar]
- 28.Choi KH, Wykes T, Kurtz MM. Adjunctive pharmacotherapy for cognitive deficits in schizophrenia: meta-analytical investigation of efficacy. Br. J. Psychiatry. 2013;203:172–178. doi: 10.1192/bjp.bp.111.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai GE, Lin PY. Strategies to enhance N-methyl-d-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr. Pharm. Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- 30.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am. J. Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 35.Creavin ST, et al. Mini-mental state examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev. 2016 doi: 10.1002/14651858.CD011145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 37.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res. Synth. Methods. 2017;8:5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 39.Sterne JA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 40.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 42.Tsai CH, et al. Activation of N-methyl-d-aspartate receptor glycine site temporally ameliorates neuropsychiatric symptoms of Parkinson's disease with dementia. Psychiatry Clin. Neurosci. 2014;68:692–700. doi: 10.1111/pcn.12175. [DOI] [PubMed] [Google Scholar]
- 43.Chappell AS, et al. AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology. 2007;68:1008–1012. doi: 10.1212/01.wnl.0000260240.46070.7c. [DOI] [PubMed] [Google Scholar]
- 44.Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer's disease. Neurology. 2001;57:1515–1517. doi: 10.1212/WNL.57.8.1515. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz BL, Hashtroudi S, Herting RL, Schwartz P, Deutsch SI. d-Cycloserine enhances implicit memory in Alzheimer patients. Neurology. 1996;46:420–424. doi: 10.1212/WNL.46.2.420. [DOI] [PubMed] [Google Scholar]
- 46.Mohr E, et al. Cognitive and quantified electroencephalographic correlates of cycloserine treatment in Alzheimer's disease. Clin. Neuropharmacol. 1995;18:28–38. doi: 10.1097/00002826-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Fakouhi TD, et al. Evaluation of cycloserine in the treatment of Alzheimer's disease. J. Geriatr. Psychiatry Neurol. 1995;8:226–230. doi: 10.1177/089198879500800405. [DOI] [PubMed] [Google Scholar]
- 48.Howard R, et al. Minocycline at 2 different dosages vs placebo for patients with mild Alzheimer disease: a randomized clinical trial. JAMA Neurol. 2020;77:164–174. doi: 10.1001/jamaneurol.2019.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin CH, Chen PK, Wang SH, Lane HY. Sodium benzoate for the treatment of behavioral and psychological symptoms of dementia (BPSD): a randomized, double-blind, placebo-controlled, 6-week trial. J. Psychopharmacol. 2019;33:1030–1033. doi: 10.1177/0269881119849815. [DOI] [PubMed] [Google Scholar]
- 50.Kouzuki M, et al. Effect of monosodium L-glutamate (umami substance) on cognitive function in people with dementia. Eur. J. Clin. Nutr. 2019;73:266–275. doi: 10.1038/s41430-018-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernard K, et al. A 24-week double-blind placebo-controlled study of the efficacy and safety of the AMPA modulator S47445 in patients with mild to moderate Alzheimer's disease and depressive symptoms. Alzheimers Dement (N Y) 2019;5:231–240. doi: 10.1016/j.trci.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kouzuki M, et al. Effect of monosodium L-glutamate (umami substance) on cognitive function in people with dementia. Eur. J. Clin. Nutr. 2018 doi: 10.1038/s41430-018-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wesnes KA. Assessing change in cognitive function in dementia: the relative utilities of the Alzheimer's disease assessment scale-cognitive subscale and the cognitive drug research system. Neurodegener. Dis. 2008;5:261–263. doi: 10.1159/000113719. [DOI] [PubMed] [Google Scholar]
- 54.Kim JW, et al. Improvement of screening accuracy of mini-mental state examination for mild cognitive impairment and non-Alzheimer's disease dementia by supplementation of verbal fluency performance. Psychiatry Investig. 2014;11:44–51. doi: 10.4306/pi.2014.11.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J. Psychiatr. Res. 2009;43:411–431. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 56.Verma N, et al. New scoring methodology improves the sensitivity of the Alzheimer's disease assessment scale-cognitive subscale (ADAS-Cog) in clinical trials. Alzheimer's Res. Therapy. 2015;7:64. doi: 10.1186/s13195-015-0151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beishon LC, et al. Addenbrooke's Cognitive Examination III (ACE-III) and mini-ACE for the detection of dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2019;12:CD013282. doi: 10.1002/14651858.CD013282.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinto TCC, et al. Is the montreal cognitive assessment (MoCA) screening superior to the mini-mental state examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's disease (AD) in the elderly? Int. Psychogeriatr. 2019;31:491–504. doi: 10.1017/S1041610218001370. [DOI] [PubMed] [Google Scholar]
- 59.Ciesielska N, et al. Is the montreal cognitive assessment (MoCA) test better suited than the mini-mental state examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis . Psychiatr. Pol. 2016;50:1039–1052. doi: 10.12740/PP/45368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.