Abstract
Objective
In the antenatal late preterm steroids (ALPS) trial betamethasone significantly decreased short-term neonatal respiratory morbidity but increased the risk of neonatal hypoglycemia, diagnosed only categorically (<40 mg/dL). We sought to better characterize the nature, duration, and treatment for hypoglycemia.
Study Design
Secondary analysis of infants from ALPS, a multicenter trial randomizing women at risk for late preterm delivery to betamethasone or placebo. This study was a reabstraction of all available charts from the parent trial, all of which were requested. Unreviewed charts included those lost to follow-up or from sites not participating in the reabstraction. Duration of hypoglycemia (<40 mg/dL), lowest value and treatment, if any, were assessed by group. Measures of association and regression models were used where appropriate.
Results
Of 2,831 randomized, 2,609 (92.2%) were included. There were 387 (29.3%) and 223 (17.3%) with hypoglycemia in the betamethasone and placebo groups, respectively (relative risk [RR]: 1.69, 95% confidence interval [CI]: 1.46–1.96). Hypoglycemia generally occurred in the first 24 hours in both groups: 374/385 (97.1%) in the betamethasone group and 214/222 (96.4%) in the placebo group (p = 0.63). Of 387 neonates with hypoglycemia in the betamethasone group, 132 (34.1%) received treatment, while 73/223 (32.7%) received treatment in placebo group (p = 0.73). The lowest recorded blood sugar was similar between groups. Most hypoglycemia resolved by 24 hours in both (93.0 vs. 89.3% in the betamethasone and placebo groups, respectively, p = 0.18). Among infants with hypoglycemia in the first 24 hours, the time to resolution was shorter in the betamethasone group (2.80 [interquartile range: 2.03–7.03) vs. 3.74 (interquartile range: 2.15–15.08) hours; p = 0.002]. Persistence for >72 hours was rare and similar in both groups, nine (2.4%, betamethasone) and four (1.9%, placebo, p = 0.18).
Conclusion
In this cohort, hypoglycemia was transient and most received no treatment, with a quicker resolution in the betamethasone group. Prolonged hypoglycemia was uncommon irrespective of steroid exposure.
Keywords: late preterm, Antenatal corticosteroids, late preterm steroids, antenatal late preterm steroids, hypoglycemia
Late preterm delivery, between 340/7 and 366/7 weeks of gestation, is associated with increased neonatal morbidities compared with term delivery, with respiratory morbidity, hyperbilirubinemia, and hypoglycemia being the most common.1,2 Late preterm birth is a recognized risk factor for hypoglycemia due to immature glucose homeostasis, such that the American Academy of Pediatrics includes this group, along with infants of diabetic mothers and small or large for gestational age infants, in those that should be screened for hypoglycemia at birth.3 In fact, neonatal rates of hypoglycemia after late preterm birth have been reported to be as high at 80% in the first 24 hours of life.4 The concern with neonatal hypoglycemia stems from an association with increased adverse neurodevelopment if persistent or prolonged.3 There is no single accepted definition of persistent or prolonged hypoglycemia, nor is there an established threshold below which adverse neurodevelopmental outcomes are common.5,6 Lower blood glucose levels after birth are common in most mammals and may represent a unique aspect of their extrauterine transitional physiology. Such hypoglycemia is often transient and nonpathologic.7,8
To address respiratory morbidity among infants born late preterm, the antenatal late preterm steroids (ALPS) trial randomized women at risk for late preterm delivery to receive betamethasone or a placebo, and investigators found that betamethasone given to women at risk for late preterm delivery significantly decreased short-term respiratory morbidity.9 There was also an unexpected increase in the risk of neonatal hypoglycemia in the betamethasone group, a finding that differed from prior steroid trials in which hypoglycemia was assessed.9,10 Therefore, we chose to characterize further the extent, duration, and treatment of hypoglycemia in neonates exposed to betamethasone versus placebo from the ALPS trial.
Materials and Methods
This secondary analysis of neonates whose mothers were enrolled in the ALPS trial was conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network from October 2010 to February 2015.9 Women at risk for late preterm delivery were randomized to betamethasone or placebo. Hypoglycemia was defined as <40 mg/dL. Timing and method of glucose measurement was at the discretion of each center; no further data were collected in the original trial. The current study was a reabstraction of all available charts from the parent trial, with a goal of assessing the lowest recorded glucose value, the duration of hypoglycemia, and the treatment, if any, by parent trial treatment assignment. These were not collected as part of the parent trial but became pertinent after our unexpected finding related to hypoglycemia. Institutional review board (IRB) approval was obtained at each participating clinical site and at the data coordinating center. Exclusions for the current study were subjects lost to follow-up, and subjects recruited at 9 of the 32 sites that were no longer part of the MFMU Network at the time of this study. The duration of hypoglycemia was assessed for infants noted to have hypoglycemia in the first 24 hours of life. Treatment for hypoglycemia was characterized as none, intravenous (IV) dextrose maintenance, IV dextrose bolus, oral glucose gel, total parenteral nutrition with dextrose, and other medication to treat refractory hypoglycemia. While information on infant feeding was captured in the parent trial, it was not possible to ascertain whether feeding was a specific treatment for hypoglycemia, and as such, this was not included as a treatment. Prolonged, persistent hypoglycemia was defined as hypoglycemia which persisted for ≥72 hours after birth.11 The lowest reported blood glucose concentration in the infant’s hospital course was assessed. If a blood glucose was not recorded in the chart, and the chart documented no neonatal complications, the neonate was coded as not having hypoglycemia. We also evaluated the time to resolution, treatments, and time from last dose to delivery in those with hypoglycemia. To analyze the lowest glucose measured per randomization arm, we used multiple imputation to impute values reported as “less than” a certain cut-off.12 Perinatal characteristics related to the development of hypoglycemia were estimated by using multivariable regression models. A random selection of approximately 2% of reabstracted charts were again reabstracted by study subcommittee members to ensure accuracy of the data. General estimating equations were used to test for differences in characteristics between the total cohort and the subsample to control for subjects who were members of both distributions. Chi-square, Fisher’s exact, and Wilcoxon’s rank-sum tests were used as appropriate. Logistic regression models were used to test the association between perinatal characteristics and neonatal hypoglycemia by treatment group. Nominal two-sided p-values of less than 0.05 were considered to indicate statistical significance; no adjustments were made for multiple comparisons.
Results
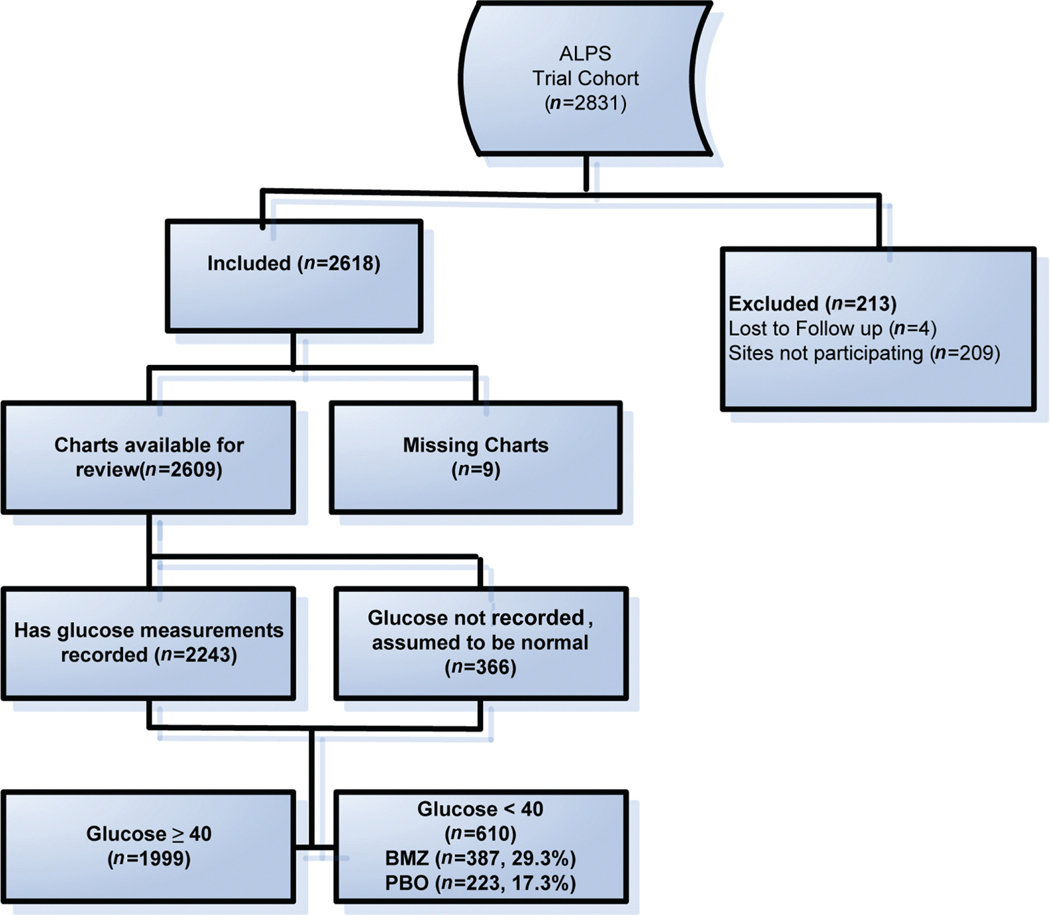
Of 2,831 women included in the ALPS trial, 4 (0.1%) were lost to follow-up, 209 (7.4%) were from nonparticipating sites, and 9 (0.3%) had missing charts (►Fig. 1), leaving 2,609 (92.2%) subjects available for analysis. Differences between the initial trial cohort and the reabstraction cohort were noted in ►Table 1. Of the 2,609 included in this study, there were 610 infants with hypoglycemia, 387 (29.3%) in the betamethasone group and 223 (17.3%) in the placebo group (relative risk [RR]: 1.69, 95% confidence interval [CI]: 1.46–1.96).
Fig. 1.
Patients included in the analysis.
Table 1.
Comparison of antenatal late preterm steroids trial cohort to subset reabstracted for hypoglycemia analysis
| Characteristic | Trial (n = 2,831) | Subset (n = 2,609) | p-Valuea |
|---|---|---|---|
| Randomized to betamethasone, n (%) | 1,429 (50.5) | 1,321 (50.6) | 0.57 |
| Indication for trial entry, n (%) | 0.22 | ||
| Preterm labor with intact membranes | 792 (28.0) | 715 (27.4) | |
| Ruptured membranes | 620 (21.9) | 576 (22.1) | |
| Expected delivery for gestational hypertension or preeclampsia | 727 (25.7) | 683 (26.2) | |
| Expected delivery for fetal growth restriction | 90 (3.2) | 84 (3.2) | |
| Expected deliver for oligohydramnios | 90 (3.2) | 83 (3.2) | |
| Expected delivery for other indication | 512 (18.1) | 468 (17.9) | |
| Gestational age at trial entry, n (%) | 0.27 | ||
| ≤346/7 wk | 768 (27.1) | 703 (26.9) | |
| 350/7–356/7 wk | 1,103 (39.0) | 1,014 (38.9) | |
| ≥360/7 wk | 960 (33.9) | 892 (34.2) | |
| Mean (± SD) maternal age, y | 28.2 ± 6.2 | 28.3 ± 6.2 | 0.03 |
| Race, n (%)b | <0.001 | ||
| Black | 757 (26.7) | 736 (28.2) | |
| White | 1,628 (57.5) | 1,444 (55.3) | |
| Asian | 96 (3.4) | 88 (3.4) | |
| Other, unknown, or more than one race | 350 (12.4) | 341 (13.1) | |
| Latino ethnicity, n (%)b | 853 (30.2) | 755 (29.0) | <0.001 |
| Nulliparous, n (%) | 1,137 (40.2) | 837 (32.1) | <0.001 |
| Smoking during current pregnancy, n (%) | 390 (13.8) | 355 (13.6) | 0.40 |
| Preeclampsia or gestational hypertension, n (%) | 875 (30.9) | 818 (31.4) | 0.07 |
| Gestational diabetes, n (%) | 306 (10.8) | 283 (10.8) | 0.82 |
| Hypoglcyemia | 553 (19.6) | 500 (19.1) | 0.047 |
p-values from generalized estimating equations correcting for repeated measures.
Self-reported race or ethnic group.
Among the 610 infants with hypoglycemia, there was no statistical difference between randomization groups in the time interval in which hypoglycemia was identified (p = 0.15, ►Table 2). Hypoglycemia occurred in the first 24 hours in the majority of both groups: 374 of 385 (97.1%) in the betamethasone group and 214 of 222 (96.4%) in the placebo group (p = 0.63). To assess the lowest glucose value between groups, imputation was employed when a value was listed as “less than” a certain number. This resulted in 11 imputed observations in the betamethasone arm and 9 in the placebo arm. Nonetheless, the lowest recorded blood sugar was similar between both groups (►Table 2). Median length of stay in a special care nursery was shorter in betamethasone group (7 days [interquartile range {IQR]: 3–11]) vs. the placebo group (8 days [IQR: 4–12], p = 0.01). There were two reported neonatal seizures, both in the placebo group. Of 387 infants with hypoglycemia in the betamethasone group, 132 (34.1%) received a treatment, while 73 of 223 (32.7%) received treatment in the placebo group (►Table 2). Because feeding as a treatment modality could not be assessed, the most common treatment in this cohort was IV dextrose given either as a bolus or as maintenance fluids, followed by total parenteral nutrition and oral glucose gel. Of the 108 women with both gestational diabetes and an infant with hypoglycemia, the frequency of treatment for hypoglycemia was similar between betamethasone (58; 41.1%) and placebo (50; 35.2%) groups, RR of 1.17 (95% CI: 0.87–1.57).
Table 2.
Hypoglycemia characteristics among infants who developed hypoglycemia (n=610)
| Hypoglycemia characteristics | Betamethasone (n = 387) | Placebo (n = 223) | p-Value |
|---|---|---|---|
| Time interval of first occurrence of hypoglycemia (glucose <40 mg/dL), n (%)a | 0.15b | ||
| 0 to <24 h | 374 (97.1) | 214 (96.4) | |
| 24 to <48 h | 7(1.8) | 4(1.8) | |
| 48 to <72 h | 1 (0.3) | 4(1.8) | |
| ≥72 h | 3 (0.8) | 0(0) | |
| Median (interquartile range) lowest glucose value, mg/dLc | 31 (24–35) | 31 (25–36) | 0.51d |
| Received treatment for hypoglycemia, n (%)e | 132 (34.1) | 73 (32.7) | 0.73f |
Two infants in betamethasone group and one infant in placebo group missing time of the first occurrence of hypoglycemia.
Fisher’s exact test.
Data were imputed if value was reported at less than the lowest threshold related to the site’s laboratory.
Wilcoxon’s rank sum test normal approximation.
Treatments were characterized as none, oral glucose gel, IV dextrose bolus, IV dextrose maintenance, total parenteral nutrition with dextrose, and other medication to treat refractory hypoglycemia.
Chi-square test.
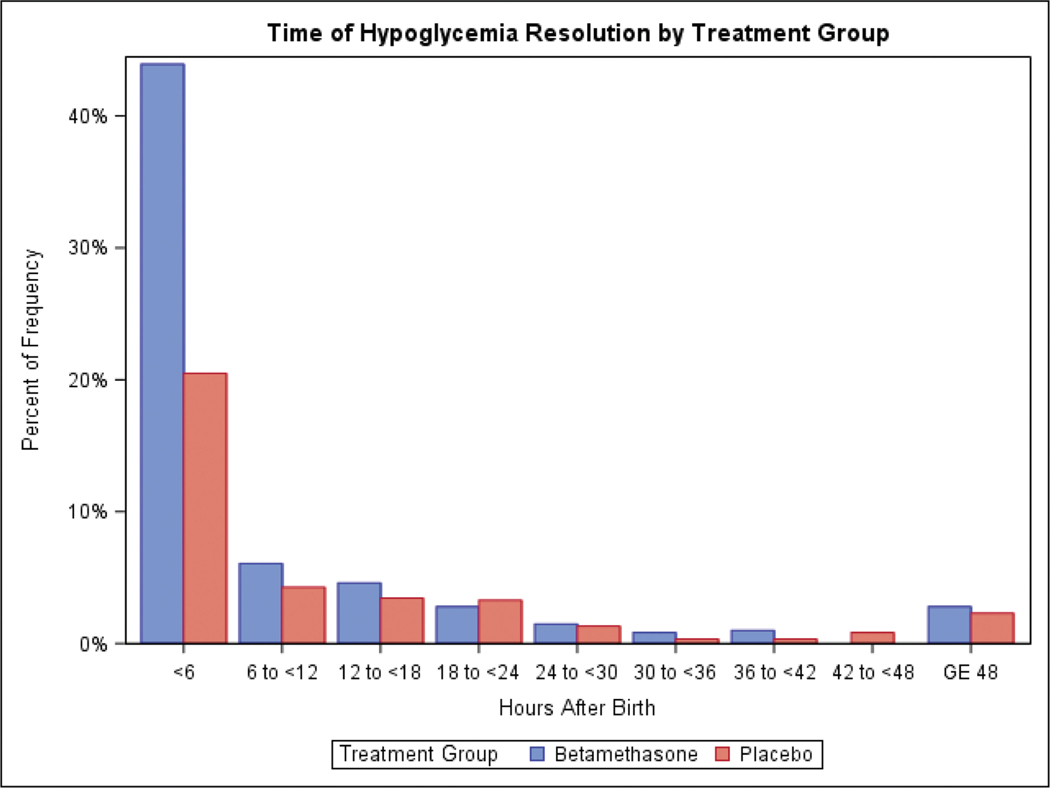
Among infants who developed hypoglycemia in the first 24 hours after birth, the time interval categories in which hypoglycemia ended was similar between the betamethasone and placebo groups with the majority resolved by 24 hours in both groups (p = 0.18, ►Table 3). However, comparing time to resolution between the two groups among infants who developed hypoglycemia in the first 24 hours demonstrates that hypoglycemia resolved sooner in the betamethasone group compared with the placebo group (p = 0.002, ►Table 3) and largely within the first 6 hours in the betamethasone group (►Fig. 2).
Table 3.
Time hypoglycemia (glucose <40 mg/dL) ended among infants who developed hypoglycemia in the first 24 hours after birth (n=588)
| Time hypoglycemia ended | Betamethasone (n = 374)a | Placebo (n = 214) | p-Value |
|---|---|---|---|
| Time interval, n (%) | 0.18b | ||
| 0 to <24 h | 346 (93.0) | 191 (89.3) | |
| 24 to <48 h | 14 (3.8) | 14 (6.5) | |
| 48 to <72 h | 3 (0.8) | 5(2.3) | |
| ≥72 h | 9 (2.4) | 4(1.9) | |
| Median (interquartile range), h | 2.80 (2.03–7.03) | 3.74 (2.15–15.08) | 0.002c |
Two infants with missing time hypoglycemia ended.
Fisher’s exact test.
Wilcoxon’s rank sum test.
Fig. 2.
Time to resolution.
We then assessed perinatal characteristics that were related to the development of neonatal hypoglycemia in each treatment group. Because gestational age was of interest, gestational age was added to the parsimonious models. In the betamethasone group, nonblack race (odds ratio [OR]: 1.40, 95% CI: 1.06–1.85), gestational diabetes (OR: 1.80, 95% CI: 1.25–2.59), and cesarean delivery (OR: 1.72, 95% CI: 1.34–2.22) were associated with neonatal hypoglycemia (►Table 4). Other neonatal characteristics associated with hypoglycemia are listed in ►Supplementary Table S1 (available in the online version). Associations were similar in the placebo group, aside from race which was not associated with hypoglycemia and chronic hypertension which was associated with hypoglycemia. Gestational age at randomization was not associated with the development of hypoglycemia in either group.
Table 4.
Perinatal characteristics associated with neonatal hypoglycemia
| Characteristic | Odds ratio (95% CI) |
|---|---|
| Betamethasone | |
| Non-black race vs. African American or black | 1.40 (1.06–1.85) |
| Gestational diabetes vs. none | 1.80 (1.25–2.59) |
| Cesarean vs. vaginal delivery | 1.72 (1.34–2.22) |
| Gestational age at randomization | |
| ≥36 vs. ≤34 wk | 0.96 (0.70–1.30) |
| 35 vs. ≤34 wk | 0.75 (0.55–1.02) |
| Placebo | |
| Gestational diabetes vs. none | 2.68 (1.82–3.97) |
| Cesarean delivery vs. vaginal | 1.44 (1.06–1.95) |
| Chronic hypertension vs. none | 1.72 (1.14–2.60) |
| Gestational age at randomization | |
| ≥36 vs. ≤34 wk | 1.26 (0.85–1.87) |
| 35 vs. ≤34 wk | 1.38 (0.94–2.02) |
Abbreviation: CI, confidence interval.
Discussion
In this cohort of neonates born to mother enrolled in the ALPS trial, consistent with our prior findings, we found a higher rate of hypoglycemia in the betamethasone group. However, among neonates with hypoglycemia, the majority had a resolution by 24 hours, and less than one percent had prolonged or persistent hypoglycemia. Neonates in the steroid group also had a faster time to resolution of hypoglycemia compared with the placebo group. These findings suggest that the majority of hypoglycemia in this cohort was transient with a quick resolution.
Understanding the consequences of neonatal hypoglycemia is challenging because it is both common and normally adaptive and yet can be associated with adverse neurodevelopment if persistent or prolonged.3 Diminished neonatal cerebral glucose has been associated with decreased cerebral oxygenation, though not consistently so.6,13 This may provide the explanation for and variability in adverse neurologic outcomes.6 Further, the threshold to define hypoglycemia is controversial, with some advocating for a definition of <30 mg/dL and others suggesting increasing the threshold to 60 mg/dL.5 Moreover, the timing of assessment for hypoglycemia is particularly relevant, as concentrations as low as 30 mg/dL are common in the first 1 to 2 hours of life in healthy neonates.3,14
It is accepted that persistent and prolonged neonatal hypoglycemia can lead to neurologic injury; however, the glucose concentration beneath which this injury occurs is not defined.3,6,11,15 Clinical data suggestive of the association are difficult to interpret due to differing definitions of hypoglycemia and incomplete information on duration.3 Therefore, clinicians have developed a conservative threshold, generally below 36 and 45mg/dL, to initiate treatment.3,6
The landmark study of antenatal betamethasone for neonatal respiratory distress syndrome by Liggins and Howie assessed neonatal hypoglycemia.10 The majority of infants in this study were delivered between 32 and 36 weeks, and blood glucose concentrations were assessed three times daily for the first 3 days of life. There were no significant differences in hypoglycemia by treatment assignment. These reassuring findings may explain why many of the subsequent randomized clinical trials assessing antenatal corticosteroids did not include hypoglycemia as a study outcome.16–19
Our study has several limitations. We were unable to reabstract data on hypoglycemia on the full cohort, as one center and a few subsites either chose not to participate or were no longer in the MFMU Network. The smaller sample size, without 100% abstraction, explains the slight difference in estimates of hypoglycemia from the parent trial. However, there is unlikely to be selection bias in the included subjects, both evidenced by the comparison of the original to the included cohort in ►Table 1 and because it was entire sites rather than selected participants who were excluded. Further, the parent trial randomization was stratified by center, such that exclusion of one center would not alter the overall randomization scheme. The most common intervention for low neonatal blood sugar, feeding, could not be assessed. However, because less than half of infants with hypoglycemia required an IV, we can likely conclude that feeding improved the blood sugar in the untreated group. Our study also has several strengths. Over 90% of the initial cohort had charts reabstracted and reviewed by a centralized committee that was blinded to treatment allocation. The cohort is well characterized from a randomized clinical trial. Finally, we were able to collect data regarding glucose concentrations and treatment based on information in the neonatal charts.
Conclusion
In women at risk of late preterm birth, antenatal corticosteroid use was associated with increased risk of hypoglycemia. However, hypoglycemia was transient, and mostly resolved within the first 24 hours after birth, and resolved more rapidly in the betamethasone group. The majority, two-thirds of hypoglycemic neonates required no treatment. Prolonged and persistent hypoglycemia was uncommon irrespective of steroid exposure.
Supplementary Material
Key Points.
Hypoglycemia received no treatment.
Neonates in the ALPS trial who received betamethasone had a shorter time to resolution than those with hypoglycemia in the placebo group.
Prolonged hypoglycemia occurred in approximately 2 out of 100 late preterm newborns, irrespective of antenatal steroid exposure.
Acknowledgments
The authors thank Felecia Ortiz, RN, BSN, and Sabine Bousleiman, RNC, MSN, MPH, for protocol development and coordination between clinical research centers and Ronald Wapner, MD, Elizabeth A. Thom, PhD, Carol Blais-dell, MD, and Catherine Spong, MD for protocol development and oversight.
Funding
This study received support by grants (HL098554 and HL098354) from the NHLBI, by grants (HD21410, HD27915, HD27917, HD27869, HD34116, HD34208, HD40485, HD40500, HD40512, HD40544, HD40545, HD40560, HD53097, HD53118, HD68268, HD68258, HD68282, and HD36801) from the NICHD, and by a grant (UL1 TR000040) from the National Center for Advancing Translational Sciences, National Institutes of Health. The comments and views expressed in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health. C.G.B. reports grants from NICHD, grants from NHLBI, during the conduct of the study; grants from SMFM/AMAG, personal fees from Sera Prognostics, outside the submitted work.
Footnotes
Additional members of this network are listed in ►Supplementary Material (available in the online version).
Conflict of Interest
None declared.
References
- 1.Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics 2004;114(02):372–376 [DOI] [PubMed] [Google Scholar]
- 2.Escobar GJ, Clark RH, Greene JD. Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions. Semin Perinatol 2006;30(01):28–33 [DOI] [PubMed] [Google Scholar]
- 3.Adamkin DHCommittee on Fetus and Newborn. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 2011; 127(03):575–579 [DOI] [PubMed] [Google Scholar]
- 4.Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr 2012;161(05): 787–791 [DOI] [PubMed] [Google Scholar]
- 5.Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics 2000;105(05):1141–1145 [DOI] [PubMed] [Google Scholar]
- 6.Rozance PJ, Hay WW. Hypoglycemia in newborn infants: features associated with adverse outcomes. Biol Neonate 2006;90(02): 74–86 [DOI] [PubMed] [Google Scholar]
- 7.Kalhan S, Parimi P. Gluconeogenesis in the fetus and neonate. Semin Perinatol 2000;24(02):94–106 [DOI] [PubMed] [Google Scholar]
- 8.Hawdon JM, Ward Platt MP, Aynsley-Green A. Patterns of metabolic adaptation for preterm and term infants in the first neonatal week. Arch Dis Child 1992;67(4 Spec No):357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. ;NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med 2016;374 (14):1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50(04):515–525 [PubMed] [Google Scholar]
- 11.Thompson-Branch A, Havranek T. Neonatal hypoglycemia. Pediatr Rev 2017;38(04):147–157 [DOI] [PubMed] [Google Scholar]
- 12.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardiner RM. The effects of hypoglycaemia on cerebral blood flow and metabolism in the new-born calf. J Physiol 1980; 298:37–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heck LJ, Erenberg A. Serum glucose levels in term neonates during the first 48 hours of life. J Pediatr 1987;110(01):119–122 [DOI] [PubMed] [Google Scholar]
- 15.Sinclair JC. Approaches to the definition of neonatal hypoglycemia. Acta Paediatr Jpn 1997;39(Suppl 1):S17–S20 [PubMed] [Google Scholar]
- 16.Morales WJ, Diebel ND, Lazar AJ, Zadrozny D. Effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am J Obstet Gynecol 1981; 141(03):276–287 [DOI] [PubMed] [Google Scholar]
- 17.Balci O, Ozdemir S, Mahmoud AS, Acar A, Colakoglu MC. The effect of antenatal steroids on fetal lung maturation between the 34th and 36th week of pregnancy. Gynecol Obstet Invest 2010;70(02): 95–99 [DOI] [PubMed] [Google Scholar]
- 18.Garite TJ, Rumney PJ, Briggs GG, et al. A randomized, placebo-controlled trial of betamethasone for the prevention of respiratory distress syndrome at 24 to 28 weeks’ gestation. Am J Obstet Gynecol 1992;166(02):646–651 [DOI] [PubMed] [Google Scholar]
- 19.Gamsu HR, Mullinger BM, Donnai P, Dash CH. Antenatal administration of betamethasone to prevent respiratory distress syndrome in preterm infants: report of a UK multicentre trial. Br J Obstet Gynaecol 1989;96(04):401–410 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.