Abstract
Objectives.
We previously identified HLA-DR-presented epitopes from a 27-kD protein of Prevotella copri (Pc) obtained from the PBMC of one RA patient. Herein, we sought to identify other HLA-DR-presented Pc peptides and source proteins from the PBMC of additional patients to better understand Pc immune responses and RA disease pathogenesis.
Methods.
Using tandem mass spectrometry, we searched for HLA-DR-presented Pc peptides in PBMC from RA and Lyme arthritis (LA) patients. The identified peptides and source proteins were tested for reactivity in RA patients, those with other arthritides, or the general population; the results were correlated with clinical findings.
Results.
Including Pc-p27, we have identified 5 HLA-DR-presented Pc peptides, each derived from a different Pc protein, in 3 of 4 RA patients, but none in 2 LA patients. When tested in our RA cohort, 14 of 19 patients (74%) had T cell responses and 47 of 89 patients (53%) had IgG or IgA responses with ≥1 of the 5 Pc peptides or proteins, most commonly IgA reactivity with Pc-p27. Additionally, 74% of RA patients with IgA antibodies to ≥1 Pc protein had anti-citrullinated protein antibodies (ACPA) compared with 49% of patients who lacked IgA Pc antibody responses (P=0.05), and IgA Pc antibody levels correlated with ACPA values.
Conclusions.
The majority of our RA patients had Pc immune responses. The correlation of IgA Pc antibodies, particularly to Pc-p27, with ACPA supports the hypothesis that specific microbial antigens in the mucosa have a role in shaping or amplifying immune responses in RA joints.
Keywords: Rheumatoid arthritis, Prevotella copri, T cell epitope, mass spectrometry
There is increasing evidence that mucosal immune responses to microbial agents in the periodontium, lung, or intestine may shape immune responses in the joints of patients with rheumatoid arthritis (RA) (1, 2). However, identification of microbial agents and immune responses that may connect mucosal and joint immunity remain incomplete. In a seminal study of the gut microbiota in RA patients, Scher et al reported an overabundance or Prevotella spp., particularly Prevotella copri (Pc), in stool samples from patients with new-onset RA (NORA) (3), which was the stimulus for our Pc immune response studies.
We developed a novel technique to identify HLA-DR-presented microbial or self-peptides from synovial tissue, synovial fluid mononuclear cells (SFMC), or peripheral blood mononuclear cells (PBMC) of arthritis patients using nanoUPLC-tandem mass spectrometry (nano-LC-MS/MS), followed by determination of the antigenicity of the peptides and their source proteins using patients’ samples (4). With this technique, we first searched for Pc peptides and self-peptides in 9 such samples (2 from PBMC) from RA patients. From the PBMC of 1 of the 2 patients, one HLA-DR-presented Pc peptide was identified, which was derived from a 27-kD Pc protein (Pc-p27) (5). Pc peptides were not identified from synovia or SFMC. When testing was done in our entire cohort of RA patients, 42% of 40 patients had Th1 responses to the Pc-p27 peptide, and 24% of 127 patients had IgG or IgA antibodies to the Pc-p27 protein.
From the same patient in whom the Pc peptide was identified, 2 novel, immunogenic HLA-DR-presented self-peptides, one derived from N-acetylglucosamine-6-sulfatase (GNS) and the other from filamin A (FLNA), were identified from her synovial tissue (6). These 2 self-proteins have sequences homologous with Prevotella epitopes; and patients who had T cell reactivity with 1 or both self-peptides also had responses to the corresponding Prevotella peptides (6), implicating molecular mimicry between these microbial and self-proteins as a possible link between gut microbial immunity and autoimmunity in joints.
In the current study, we searched for HLA-DR-presented Pc peptides from the PBMC of 2 new RA patients and, for comparison, from 2 Lyme arthritis (LA) patients. We report here the identification of 4 new HLA-DR-presented Pc peptides (T cell epitopes) from the 2 new RA patients. When samples from our recent RA cohort were tested for reactivity with these 4 Pc proteins and the previously identified Pc-p27, the majority of patients had T and/or B cell responses to ≥1 of these 5 Pc antigens. Moreover, the correlation between IgA responses to Pc proteins and ACPA support the hypothesis that specific microbial antigens in the mucosa may shape immune responses in RA.
PATIENTS AND METHODS
Patients.
The study was approved by the Human Investigations Committee at Massachusetts General Hospital (MGH); all subjects gave written informed consent. All RA patients met the 2010 American College of Rheumatology/ European League Against Rheumatism criteria for RA (7). HLA-DR typing was performed at the American Red Cross in Dedham, Massachusetts.
Isolation and identification of HLA-DR presented peptides.
We have previously published methods for immunoprecipitation of HLA-DR molecules from patient samples, followed by the elution and identification of HLA-DR-presented peptides using nano-LC-MS/MS (4). Here, only PBMC were analyzed, as we did not previously identify Pc proteins from synovia or SFMC. Spectra-to-peptide assignments were made by searching each patient’s MS/MS dataset against a UniProt Pc database (assembled in-house) using 3 search engines, Mascot, OMSSA, and X!Tandem. A consensus match among ≥2 programs was required for identification of a peptide sequence, with a Mascot score ≥20, OMSSA e-value ≤0.01, and X!Tandem ≤10. To rule out erroneous assignment of a human sequence as a Pc sequence, each microbial sequence was screened against the most recent version of the UniProt human database.
Enzyme-linked immunospot (ELISpot) T cell assay.
Each HLA-DR-presented candidate microbial antigen was synthesized and HPLC-purified in the Core Proteomics Laboratory at MGH. Each peptide was used first to stimulate the matching patient’s PBMC in an IFNγ ELISpot assay. Immunogenic peptides were then tested in larger numbers of patients, as previously described (5). A positive T cell response was defined as 3 standard deviations (SD) above the mean value of healthy subjects.
Determinations of Pc antibodies.
Recombinant preparations of the 5 Pc proteins were made by GenScript using an E. coli expression vector (pET30a). Target protein purity was estimated to be about 90% based on densitometric analysis using SDS-PAGE gels.
ELISA assays were performed, with modifications of previously described methods (5). After coating overnight at 4°C with each recombinant Pc protein (1 μg/ml), the plates were incubated at room temperature with blocking buffer (PBST, 5% milk) for 1 hour. Depending on the Pc protein, each patient’s serum sample (diluted 1:50 or 1:100) was added for 2 hours, followed by horseradish-peroxidase (HRP)-conjugated goat anti-human IgG or IgA (Dako) (diluted 1:2,000 or 1:3,000) for 1.5 hours and then TMB substrate (BD) for 10 to 15 minutes. A positive antibody response was defined as 2 standard deviations (SD) above the mean value of the general population.
Statistical analysis.
Quantitative data were analyzed using the Mann-Whitney test or t test with Welch correction, categorical data using Fisher’s exact test, and correlations using Spearman’s correlation test. All analyses were performed using GraphPad Prism 8. All P values were two-tailed. P values ≤ 0.05 were considered statistically significant.
RESULTS
Identification of naturally presented HLA-DR Pc peptides.
When HLA-DR-presented peptides were eluted from PBMC from 2 new RA patients and analyzed by nano-LC-MS/MS, 4 new Pc peptides were identified. When combined with the 2 previously reported RA patients (5), 3 of the 4 RA patients tested had 1-to-3 HLA-DR-presented Pc peptides, and each of these 3 patients had Pc IgG or IgA antibodies. Pc peptides or antibodies were not identified in the remaining RA patient or in the 2 LA patients. Clinical data for all 6 RA and LA patients is presented in suppl table 1.
The 5 HLA-DR-presented Pc peptides identified, to date, were each derived from a different Pc protein: 27-kD protein (Pc-p27), ribonuclease HII protein (Pc-ribo), DNA binding protein (DNAbind), glutamate-5-kinase protein (Pc-glut), and type III restriction endonuclease protein (Pc-endo) (suppl Figure 1). Peptide length ranged from 10-to-19 amino acids, which is typical of HLA-DR-class II-presented peptides. Each peptide had 100% sequence homology with the corresponding Pc protein, but had limited sequence homology with any human peptide, suggesting that they were not human peptides erroneously assigned with a microbial database.
T cell reactivity to Pc peptides.
As reported previously, when PBMC from 40 NORA patients were stimulated with the peptide sequence from Pc-p27, 17 (42%) secreted IFN-γ levels that were >3 SD above the mean value of healthy controls (P=0.0002) (Figure 1). To conserve cells, samples from these patients were not retested for Pc-p27 responses. However, the 4 new Pc peptides were tested for this study using PBMC from 20 of the 40 NORA patients in whom enough cells remained.
Figure 1. T cell responses to P. copri HLA-DR-presented peptides in RA patients.
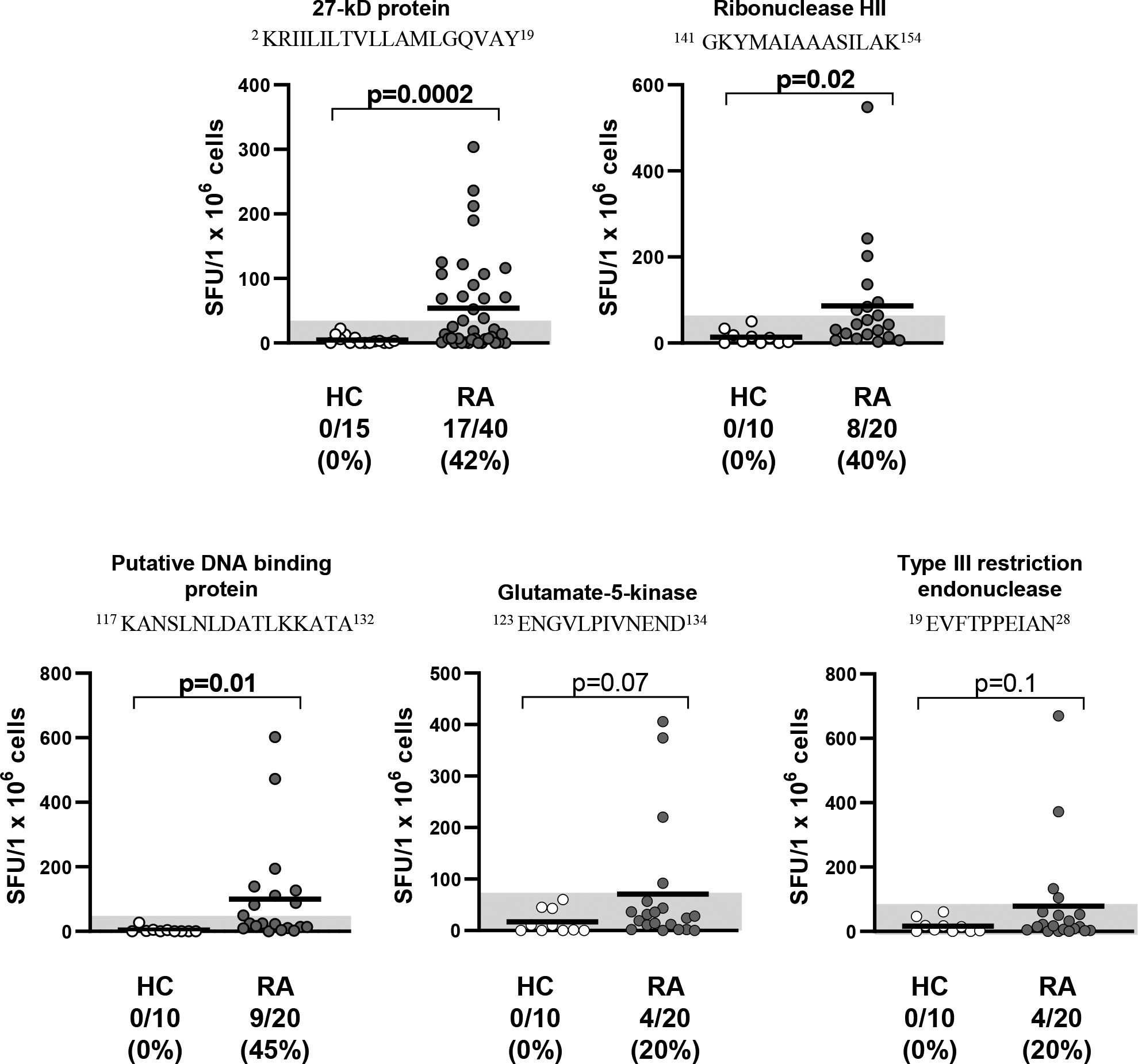
Five peptides derived from 5 P. copri (Pc) proteins were synthesized and used to stimulate PBMC from RA patients by IFN-γ ELISpot assays. Using TEPITOPE, the Pc-p27, Pc-ribonuclease HII, and Pc-DNAbinding peptides were predicted to be promiscuous binders of ≥20 of the 25 HLA-DR molecules modeled in the program. The predicted binding of the Pc-glutamate-5-kinase and Pc-type III restriction endonuclease peptides was restricted primarily to DRB1*04 molecules. The superscript numbers around each peptide sequence show the location of the amino acids within the source protein. The horizontal bar represents the mean value, and the grey area shows >3SD above the mean value in healthy control subjects (hospital personnel). The groups were compared using unpaired t test with Welch correction. SFU = spot forming units per 1 × 106 cells. RA = rheumatoid arthritis, and HC = healthy controls.
Of 20 NORA patients, 40% responded to the promiscuous binding Pc-ribo and 45% reacted with the Pc-DNAbind peptide (Figure 1). A smaller percentage of patients (20%) responded to the 2 peptides with more restricted HLA-DR binding profiles (Pc-glut and Pc-endo). Of the 19 patients in whom testing was done with all 5 proteins, 14 (74%) had T cell reactivity with ≥1 of the 5 Pc peptides.
B cell reactivity to Pc proteins.
IgG and IgA antibody responses to the 5 Pc proteins were determined in 89 RA patients, including 54 NORA and 35 chronic RA (CRA) patients (Figure 2). The 89 patients included 17 of the 20 patients in whom T cell testing was done and 72 patients in our new RA cohort enrolled during the past 2 years. Because the results were similar in NORA and CRA patients, these data were combined for presentation here. For comparison, serum samples were tested from 37 patients with other chronic inflammatory arthritides (IA), including spondyloarthropathies, psoriatic arthritis, or sarcoidosis; from 80 patients with Lyme arthritis (LA), and from 45 individuals in the general population (GP), including hospital personnel and blood donors.
Figure 2. Antibody responses to P. copri proteins in RA patients, those with other forms of arthritis, and those in the general population.
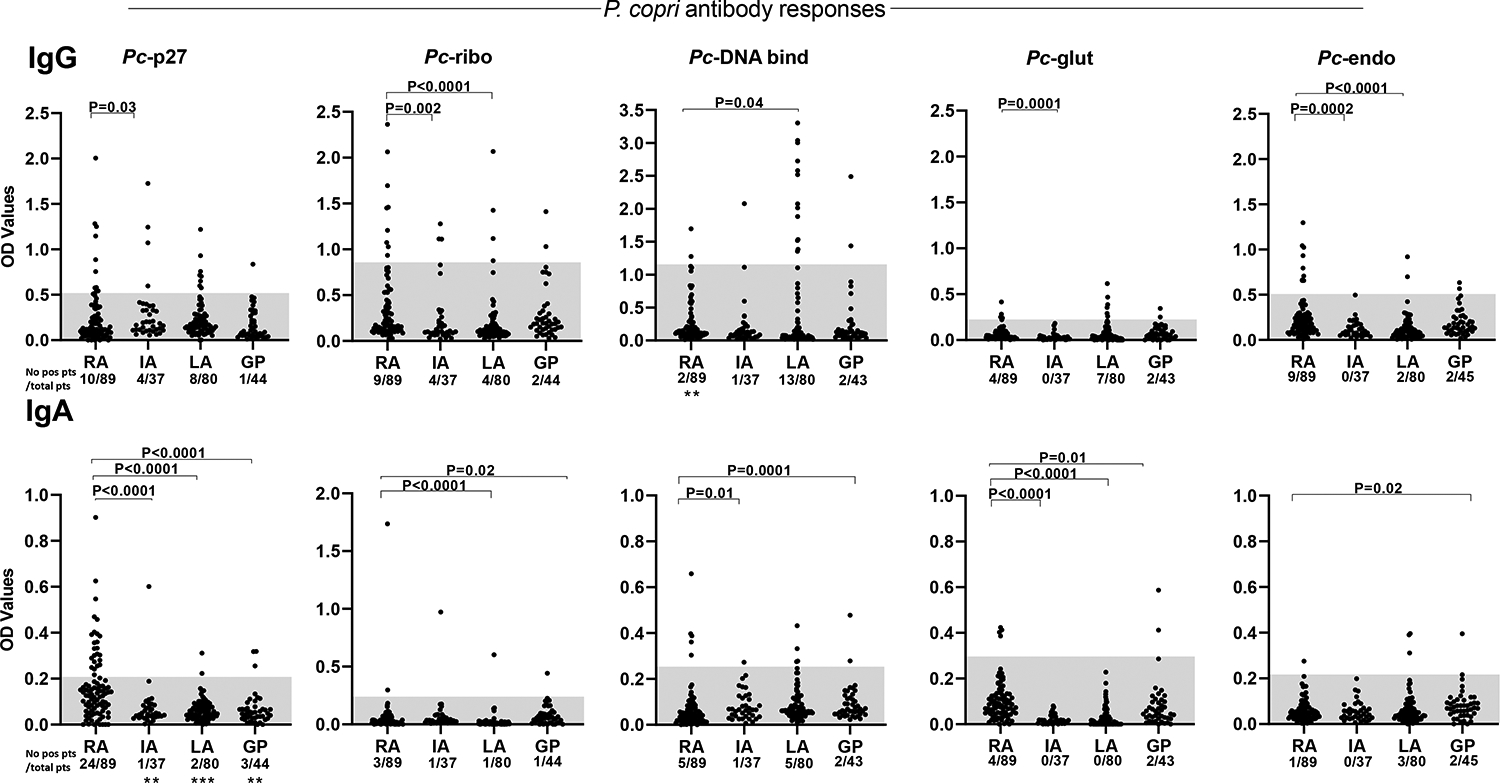
IgG and IgA antibody responses to the 5 Pc proteins are shown. The shaded areas represent 2 SD above the mean value in the general population. Quantitative values were compared between RA patients and those in each of the other groups using Mann-Whitney test; the P values for these comparisons are shown above the data points. The number of individuals in each group with positive responses were compared between RA patients and those in each of the other groups by Fisher’s exact test; P values for these comparisons are shown at the bottom of each panel (**=0.001 and ***=0.0001). Only significant P values are shown. OD = optical density, RA = rheumatoid arthritis, IA = other chronic inflammatory arthritides, LA = Lyme arthritis, and GP = general population.
Of the 89 RA patients, 24 (27%) had IgA antibody responses to Pc-p27, which were >2 SD above the mean value in the general population (Figure 2). The number of patients with positive IgA responses to Pc-p27 and quantitative Pc-p27 values were significantly greater than those in comparison groups. Although only small numbers of patients had positive IgA responses to the 4 new Pc proteins, quantitative values were frequently greater in the RA cohort than in other groups.
Among the 89 RA patients, 12% had positive IgG Pc-p27 responses, 10% had IgG antibodies to Pc-ribo, and 10% had IgG Pc-endo antibodies, which tended to be higher than that in the other groups, but the number of patients who had positive values were not significantly different among the groups. However, quantitative values for each Pc protein, except for Pc-DNAbind, were significantly greater in RA patients than in the IA group. Surprisingly, 13 of the 80 LA patients (16%) had elevated IgG responses to Pc-DNA binding protein, which was a higher percentage than that in RA patients, raising the question of whether Pc-DNAbind has a cross-reactive antibody epitope with a spirochetal protein. Unlike RA patients, only a small number of LA patients had IgA responses to Pc-DNAbind or other Pc proteins.
Most patients in RA or comparison groups had IgG or IgA responses to only a single Pc protein. Only 8 RA patients had positive IgG or IgA responses to >1 Pc protein, but none had both IgG and IgA responses to the same protein. Altogether, 29 RA patients (33%) had IgA responses to ≥1 of the 5 Pc proteins compared with 18 of 161 patients (11%) in all other groups (P<0.0001), and 26 of the 89 RA patients (29%) had IgG antibody responses to ≥1 of the 5 Pc proteins compared with 39 of 161 patients (24%) in the other groups (P=0.45). A total of 47 of the 89 RA patients (53%) had IgG or IgA responses to ≥1 of the 5 Pc proteins.
Clinical correlations.
Among the 89 RA patients, the majority (60%) had NORA, the sex ratio was 4 to 1 in favor of women; 60% had ACPA, 38% had rheumatoid factor (RF), and 62% had ACPA or RF, percentages typical of early RA cohorts (8). Among patients with IgA Pc-p27 antibodies, 75% had ACPA compared with 55% of those without IgA Pc-p27 antibodies (P=0.1), and there was a similar trend for RF (Table 1). Moreover, IgA antibody responses to Pc-p27, Pc-ribo, or Pc-glut correlated directly with ACPA levels; and IgA antibodies to Pc-p27, Pc-ribo, Pc-glut or Pc-endo correlated with RF levels (Table 2). Overall, ACPA were found in 74% of those who had IgA responses to ≥1 of 5 Pc proteins compared with 49% of those who lacked such responses (P=0.05). Conversely, among the 34 patients who lacked ACPA or RF, 6 (18%) had IgA and 3 (8%) had IgG Pc-p27 antibodies. Finally, patients with Pc tended to have higher levels of inflammatory markers and a higher frequency of shared epitope HLA-DRB1 alleles than patients without Pc antibodies, but the differences were not statistically significant.
Table 1:
Correlation of Demographic and Clinical Parameters with Prevotella copri Antibodies
| Characteristic | Pc-p27 IgG (N=10) | Pc-p27 IgA (N=24) | No Pc-p27 IgA or IgG (N=55) | Any Pc IgG (N=26) | Any Pc IgA (N=31) | No Pc IgG/IgA (N=41) | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, median, (range) | 52 (24–75) | 46 (19–91) | 51 (19–80) | 53 (24–75) | 49 (19–91) | 52 (19–75) | |
| Sex, female/ male | 5/5 P=0.03 |
20/4 | 46/9 | 17/9 P=0.1 |
25/6 | 34/7 | |
| Smoking, n. (%) | |||||||
| Current | 1 (10) | 1 (4) | 8 (15) | 3 (12) | 2 (7) | 6 (15) | |
| Former | 2 (20) | 5 (21) | 16 (29) | 5 (19) | 7 (23) | 14 (34) | |
| Never | 7 (70) | 18 (75) P=0.1 |
31 (56) | 18 (69) | 22 (71) P=0.1 |
21 (51) | |
| Autoantibodies | |||||||
| RF, n. positive (%) | 5 (50) | 11 (46) | 19 (35) | 13(50) | 14 (45) | 13 (32) | |
| ACPA, n. pos. (%) | 7 (70) | 18 (75) P=0.1 |
30 (55) | 18(69) P=0.1 |
23 (74) P=0.05 |
20 (49) | |
| HLA-DRB1 Alleles | |||||||
| SE, n. patients pos./n. tested (%) | 4/7 (57) | 12/19 (63) | 21/38 (55) | 12/18 (67) | 15/24 (63) | 16/29 (55) | |
| Disease Activity | |||||||
| ESR, mm/hr, median (range) | 22 (2–67) | 23 (4–107) | 14 (2–60) | 21 (2–107) P=0.08 |
23 (4–107) P=0.07 |
12 (2–60) | |
| CRP, mg/L, median (range) | 16 (0.5–92) | 3.7(0.1–126) | 4.7 (0.2–96) | 9.6 (0.5–126) P=0.09 |
7.7 (0.1–126) | 4.3 (0.2–96) | |
| DAS-28-ESR | 4.5 (2.0–7.0) | 3.6 (1.3–8.2) | 3.3 (1.0–7.0) | 3.7 (1.6–8.2) | 3.7 (1.3–8.2) P=0.1 |
3.17 (1.0–7.0) | |
| DAS-28-CRP | 3.8 (2–7) | 3.1 (1.5–7.6) | 3.2 (1.1–6.8) | 3.5 (1.3–7.6) | 3.5 (1.1–7.6) | 3.17 (1.1–6.8) |
SE, shared epitope, alleles *0101, 0102, 0401, 0404, 0405, 0408, 1001; RF, rheumatoid factor; ACPA, anti-citrullinated protein antibodies; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; DAS-28, disease activity score-28.P values shown are for comparison with neither Pc-p27 IgA/IgG antibodies or Neither Pc peptide IgG/IgA group respectively.
Table 2:
Correlation of RA Autoantibodies with Prevotella copri Antibodies
| ACPA | Rheumatoid Factor | ||
|---|---|---|---|
| Pc Antibody IgG | |||
| Pc-p27 IgG | r = 0.116, p = 0.3 | r = 0.086, p = 0.4 | |
| Pc-ribo IgG | r = 0.172, p = 0.1 | r = 0.135, p=0.2 | |
| Pc-DNA bind IgG | r= 0.098, p=0.4 | r= 0.026, p=0.8 | |
| Pc-glut IgG | r= 0.051, p=0.6 | r= −.0752, p=0.5 | |
| Pc-endo IgG | r= −0.137, p=0.2 | r= −.0631, p=0.6 | |
| Pc Antibody IgA | |||
| Pc-p27 IGA | r = 0.283, p = 0.008 | r = 0.194, p= 0.07 | |
| Pc-ribo IgA | r = 0.251, p = 0.018 | r = 0.245, p = 0.02 | |
| Pc-DNA bind IgA | r = 0.006, p = 0.95 | r = 0.087, p = 0.42 | |
| Pc-glut IgA | r = 0.271, p = 0.01 | r = 0.260, p = 0.014 | |
| Pc-endo IgA | r = 0.112, p = 0.3 | r = 0.212, p = 0.047 | |
ACPA, anti-citrullinated protein antibodies. Correlations were performed using a Spearman Test. Values in bold reflect statistically significant correlations.
DISCUSSION
Using a novel approach in which HLA-DR-presented peptides were identified directly from patient samples, we have now identified 5 immunogenic HLA-DR-presented Pc peptides from PBMC in 3 of 4 RA patients tested. The large sample volumes and the complexity of the technique precluded evaluation of large numbers of patients. However, when serum samples from our current RA cohort and comparison groups were tested for IgG or IgA reactivity with each of the 5 Pc proteins, the most robust difference between the groups was IgA Pc-p27 reactivity. Of the 89 RA patients, 24 (27%) had IgA responses to this protein, a significantly higher percentage than that in the other groups, making it an attractive diagnostic target. However, in contrast with our initial study (5), the number of patients with positive IgG Pc responses was not significantly greater in RA patients, though quantitative values were often higher in the RA group than in the other groups.
Because of the importance of anti-citrullinated protein antibodies (ACPA) in RA (9, 10), we searched our MS/MS spectra carefully for evidence of peptides with the one-Dalton gain that could indicate an arginine-to-citrulline conversion. Of the 5 Pc T cell epitopes studied here, only Pc-p27 contained an arginine, and that peptide was not citrullinated. However, ACPA correlated significantly with Pc-p27 antibodies, suggesting that another portion of the protein may become citrullinated. Moreover, when we previously citrullinated 2 autoantigens, GNS and FLNA, which had T cell epitopes with sequence similarity with Prevotella sp., RA patients had higher antibody responses to citrullinated GNS than to its non-citrullinated proteoform (6), suggesting that the GNS self-protein may be citrullinated in vivo.
In the current study, the correlation of IgA Pc responses with ACPA values support a central hypothesis in RA pathogenesis that specific microbial antigens in the mucosa, which may cross-react with like self-proteins (6), may shape immune responses in RA joints (1). ACPA appear to be beneficial in controlling microbes in the mucosa but may become detrimental in joints (1). In addition, we previously found Prevotella DNA in joint fluid in 3 of 5 patients with IgG Pc antibody responses (5), suggesting that Pc or their products may sometimes reach joints where they may further amplify inflammatory responses. There is a provocative, emerging literature about distant spread of commensal organisms resulting not only in autoimmunity, but also in malignancies and adverse treatment outcomes (11, 12).
Limitations of this study include the small number of patients in whom it was possible to test for HLA-DR-presented Pc peptides. However, this initial assessment shows that nano-LC-MS/MS is now sensitive enough to identify immunogenic in vivo-HLA-DR-presented microbial peptides directly from PBMC, which can then be tested in large numbers of patients. Second, the reasons for gut dysbiosis and mucosal Pc immunoreactivity are not yet defined in RA patients. However, in a recent analysis of ileal biopsies in 50 HLA-B27-positive patients with ankylosing spondylitis, adherent, invading rod-shaped bacteria, identified primarily as E. coli or Prevotella sp., were often seen in the epithelial layer of the gut mucosa along with significant down-regulation of tight junction proteins, resulting in a loosening of the epithelial and gut vascular barriers (13). A similar process may occur in RA patients.
Greater understanding of the interactions between gut commensals and joint autoimmunity will likely influence the diagnosis and treatment of RA. In addition to DMARDs, adjunctive treatment aimed at the control of gut “pathobionts”, such as targeted non-absorbable antibiotic therapy, probiotic strategies, dietary changes, or fecal matter transplants, may prove to be effective and safe. Moreover, the identification of T cell epitopes to microbial and related self-proteins may lead to therapies with blocking peptides. Animal models have shown that blocking peptides may ameliorate pathogenic responses (14), and reestablishment of tight junctions in the gut restores gut homeostasis, which may reverse autoimmune processes (15). In the future, biomarkers, such as those identified here, may contribute to the diagnosis and treatment of patients with gut-associated RA.
Supplementary Material
Supplemental Figure 1. Tandem mass spectra for HLA-DR-presented P. copri peptides. From the spectra of patient RA2, 1 Pc peptide was identified (shown in panel A), and from the spectra of patient RA3, 3 Pc peptides identified (panels B, C, and D). The spectra from patient RA1 were published previously (5). (A) The peptide from Pc-ribo was consistently identified by OMSSA and X!Tandem from CID MS2 spectrum recorded using a 6550 QTOF MS. The post-translational modification of methionine oxidation at M4 was assigned by two protein database search programs. (B) The peptide from Pc-DNAbind was consistently identified by OMSSA and X!Tandem from HCD MS2 spectrum recorded with a Q Exactive plus MS. The post-translational modification of asparagine deamidation at N3 was assigned by two protein database search programs. (C) The peptide from Pc-glut was consistently identified by Mascot, OMSSA, and X!Tandem from CID MS2 spectrum recorded using the 6550 QTOF MS; (D) The peptide from Pc-endo was consistently identified by OMSSA and X!Tandem from CID MS2 spectrum recorded using the 6550 QTOF MS.
ACKNOWLEDGMENTS
We thank Dr. John Branda, Judith M. Holden, Gail McHugh for help with microbial cultures, Robert Seward and Chunxiang Yao for help with mass spectrometry analyses, and Drs. Deborah Collier and Marcy Bolster for help with patient care.
Supported by NIH grant R01-AI-144365, the American College of Rheumatology Innovative Grant Program,“Within our Reach, Finding a Cure for RA”; the Ounsworth-Fitzgerald Foundation; Mathers Foundation; English, Bonter, Mitchell Foundation; Littauer Foundation; Lillian B. Davey Foundation, and the Eshe Fund (to A.C.S.), and by NIH grants P41 GM104603, R24 GM134210, S10 RR020946 and S10 OD010724 (to C.E.C.). K.S. was supported by NIAMS grant K01-AR-062098, and S.A. received support from a Rheumatology Research Foundation Scientist Development Award. Dr. Steere is an inventor of the patent, T cell testing for Pc-p27. The other authors report no conflicts of interest.
REFERENCES
- 1.Holers VM, Demoruelle MK, Kuhn KA, Buckner JH, Robinson WH, Okamoto Y, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol. 2018;14:542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21:895–905. [DOI] [PubMed] [Google Scholar]
- 3.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Drouin EE, Yao C, Zhang J, Huang Y, Leon DR, et al. Immunogenic HLA-DR-presented self-peptides identified directly from clinical samples of synovial tissue, synovial fluid, or peripheral blood in patients with rheumatoid arthritis or Lyme arthritis. J Proteome Res. 2017;16:122–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pianta A, Arvikar S, Strle K, Drouin EE, Wang Q, Costello CE, et al. Evidence of the immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pianta A, Arvikar SL, Strle K, Drouin EE, Wang Q, Costello CE, et al. Two rheumatoid arthritis-specific autoantigens correlate microbial immunity with autoimmune responses in joints. J Clin Invest. 2017;127:2946–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 8.Kuriya B, Xiong J, Boire G, Haraoui B, Hitchon C, Pope J, et al. Earlier time to remission predicts sustained clinical remission in early rheumatoid arthritis--results from the Canadian Early Arthritis Cohort (CATCH). J Rheumatol. 2014;41:2161–6. [DOI] [PubMed] [Google Scholar]
- 9.De Rycke L, Nicholas AP, Cantaert T, Kruithof E, Echols JD, Vandekerckhove B, et al. Synovial intracellular citrullinated proteins colocalizing with peptidyl arginine deiminase as pathophysiologically relevant antigenic determinants of rheumatoid arthritis-specific humoral autoimmunity. Arthritis Rheum. 2005;52:2323–30. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359:1156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciccia F, Guggino G, Rizzo A, Alessandro R, Luchetti MM, Milling S, et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 2017;76:1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11:S69–76. [DOI] [PubMed] [Google Scholar]
- 15.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:71–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Tandem mass spectra for HLA-DR-presented P. copri peptides. From the spectra of patient RA2, 1 Pc peptide was identified (shown in panel A), and from the spectra of patient RA3, 3 Pc peptides identified (panels B, C, and D). The spectra from patient RA1 were published previously (5). (A) The peptide from Pc-ribo was consistently identified by OMSSA and X!Tandem from CID MS2 spectrum recorded using a 6550 QTOF MS. The post-translational modification of methionine oxidation at M4 was assigned by two protein database search programs. (B) The peptide from Pc-DNAbind was consistently identified by OMSSA and X!Tandem from HCD MS2 spectrum recorded with a Q Exactive plus MS. The post-translational modification of asparagine deamidation at N3 was assigned by two protein database search programs. (C) The peptide from Pc-glut was consistently identified by Mascot, OMSSA, and X!Tandem from CID MS2 spectrum recorded using the 6550 QTOF MS; (D) The peptide from Pc-endo was consistently identified by OMSSA and X!Tandem from CID MS2 spectrum recorded using the 6550 QTOF MS.


