Abstract
Fanconi anemia, the most frequent genetic cause of bone marrow failure, is characterized by an extreme predilection towards multiple malignancies, including a greater than 500-fold incidence of head and neck squamous cell carcinoma (HNSCC) relative to the general population. Fanconi anemia-associated HNSCC and esophageal SCC (FA-HNSCC) often present at advanced stages with poor survival. Surgical resection remains the primary treatment for FA-HNSCC, and there is often great reluctance to administer systemic agents and/or radiation therapy (RT) to these patients given their susceptibility to DNA damage. The paucity of FA-HNSCC case reports limits evidence-based management, and such cases have not been analyzed collectively in detail. We present a systematic review of FA-HNSCC treatments reported from 1966 to 2020, defining a cohort of 119 FA-HNSCC patients including 16 esophageal SCCs (131 total primary tumors), who were treated with surgery, RT, systemic therapy (including cytotoxic agents, EGFR inhibitors, or immune checkpoint inhibitors), or a combination of modalities. We summarize the clinical responses and regimen-associated toxicities by treatment modality. The collective evidence suggests that when possible, surgical resection with curative intent should remain the primary treatment modality for FA-HNSCC. Radiation can be administered with acceptable toxicity in the majority of cases, including patients who have undergone stem cell transplantation (SCT). While there is little justification for cytotoxic chemotherapy, EGFR inhibitors and tyrosine kinase inhibitors (TKIs) may be both safe and effective. Immunotherapy may also be considered. Most oncologists have little personal experience with FA-HNSCC. This review is intended as a comprehensive resource for clinicians.
Keywords: Fanconi Anemia, head and neck, oral, oropharynx, esophageal, HNSCC
Introduction
Fanconi anemia (FA) is an inherited disease of genomic instability characterized by progressive bone marrow failure, congenital growth defects, and increased predisposition to cancer. While FA patients are at high risk for many hematologic and solid malignancies, the proclivity for mucosal squamous cell carcinomas, especially head and neck squamous carcinoma (HNSCC), is particularly striking. FA-associated HNSCC (FA-HNSCC) occurs with an estimated incidence that is 500–800 fold greater than HNSCC in the general population, frequently developing at young ages with generally poor survival1,2. With the substantial advances in hematologic management of FA and the associated improvement in life expectancy for these patients, the number of patients at risk to develop FA-HNSCC will likely increase.
FA patients demonstrate an intrinsic sensitivity to DNA-damaging agents including chemotherapy and radiation therapy, at least in part because the FA-BRCA pathway plays a critical role in DNA repair3. This poses unique challenges to the treatment of FA-HNSCC since chemoradiation (CRT) is a standard approach for pharyngeal and laryngeal cancers and adjuvant radiation or CRT is commonly employed following surgical resection of oral cavity cancers with cervical lymph node involvement. While surgery is generally well tolerated in this population, cytotoxic chemotherapy and radiation therapy (RT) are less commonly utilized due to legitimate concerns of severe toxicity4. There are few reports of administration of the FDA-approved agents cetuximab, pembrolizumab and/or nivolumab to FA-HNSCC patients and the efficacy of these agents in treating HNSCC that arises in the setting of FA remains unclear. To date, there is a paucity of evidence in the literature to guide therapy of FA-HNSCC, and the details of existing individual case reports have not been comprehensively synthesized to inform treatment decisions for future FA-HNSCC patients. In this systematic review, we identified all reported cases of FA-HNSCC in the peer-reviewed literature that described both the treatment regimen and patient outcome from 1966–2020, including treatment-related toxicity and survival. We now summarize these cases with a focus on clinical responses to the regimen(s) employed and treatment-associated toxicities.
Parents of FA children worldwide gravitate towards academic centers of excellence which, over time, have accumulated substantial expertise in managing their child’s bone marrow failure. However, because FA is a rare genetic disorder and mucosal squamous cell carcinomas, including HNSCCs, tend to arise years later in adulthood, the multidisciplinary cancer-oriented teams who manage these malignancies rarely have any experience with FA patients. Surgical resection remains the primary treatment modality for primary oral cavity cancers and this is no different for FA-HNSCC. But few centers have administered systemic therapy and/or radiation as primary or adjuvant therapy to these individuals. To our knowledge, there are no reports of clinical trials dedicated to FA-HNSCC and no ongoing therapeutic studies, underscoring the challenges of developing evidence-based treatment approaches. This review is intended to serve as a guide for clinicians who encounter a FA patient with HNSCC.
Genetics, Epidemiology, and Cancer Predisposition
FA arises from predominantly biallelic germline mutations in any of 22 identified genes of the Fanconi anemia complementation group: FANCA, FANCB, FANCC, FANCD1 (BRCA2), FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ (BRIP1), FANCL, FANCM, FANCN (PALB2), FANCO (RAD51C), FANCP (SLX4), FANCQ (ERCC4/XPF), FANCR (RAD51), FANCS (BRCA1), FANCT (UBE2T), FANCU (XRCC2), FANCV (MAD2L2), or FANCW (RFWD3)5. Broadly, gene products of the FANC family function in concert to orchestrate repair of DNA interstrand crosslinks (ICLs), lesions which halt DNA synthesis and transcription3,6. Notably, the identification of multiple FANC genes as prominent susceptibility loci for breast cancer (FANCD1 as BRCA2 and FANCS as BRCA1) resulted in the naming of this integrated repair pathway FA-BRCA7. Recognition of stalled replication forks triggers recruitment of the FA core complex, a multimeric ubiquitin ligase comprised of at least 8 FANC protein subunits8–10. Association of the core complex with an E2-conjugating ligase allows for monoubiquitination of a FANCD2-I heterodimer, a key step which initiates downstream formation of the ICL repairosome11,12. Endonucleases are subsequently recruited to cleave DNA at the ICL site, after which trans-lesion synthesis and homologous recombination occur to restore the original DNA duplexes13–15. This process is illustrated in greater detail in Figure 1.
Figure 1. The FA-BRCA Pathway.
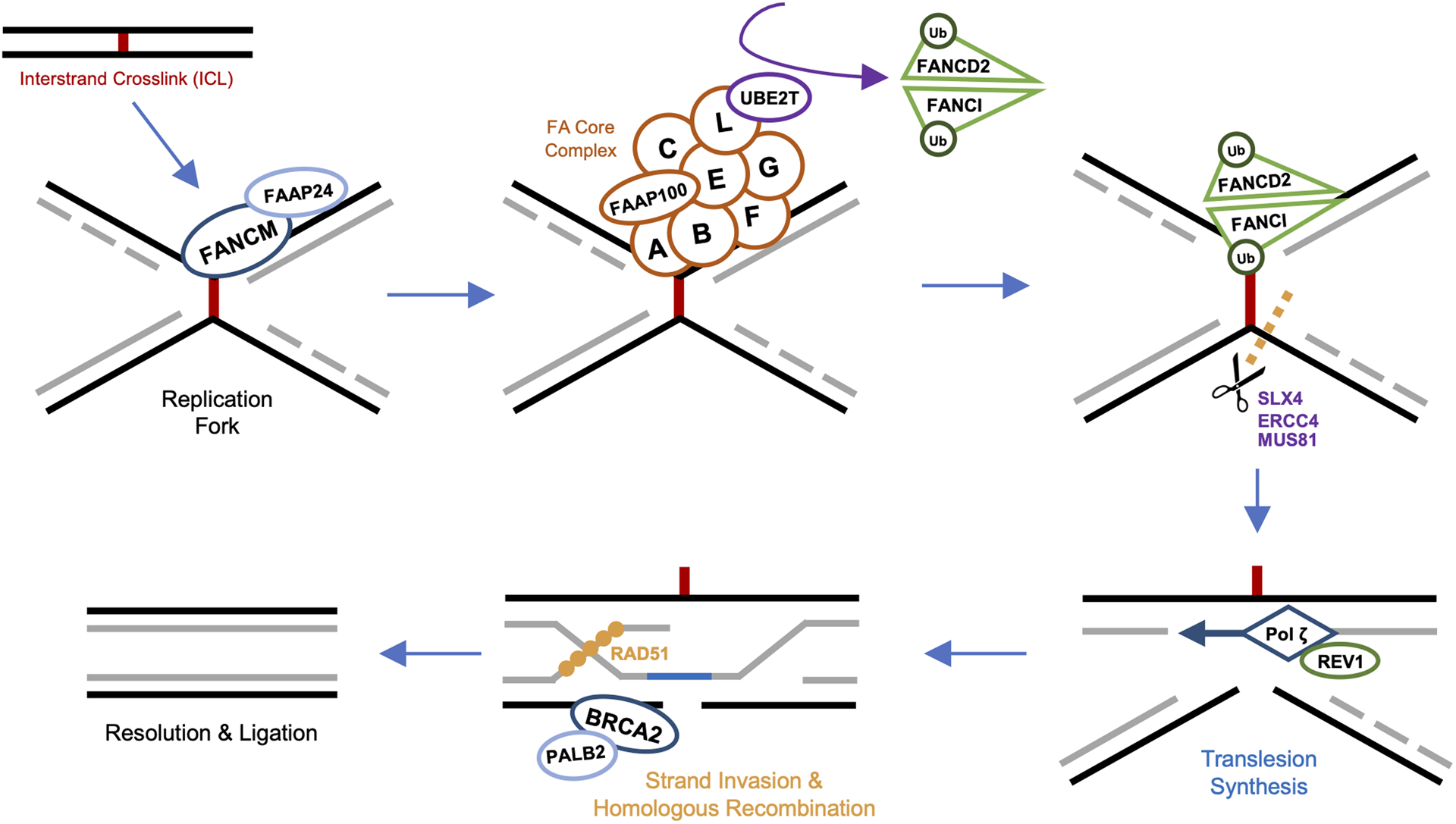
Interstrand crosslink (ICL) stalled replication forks are recognized by FANCM and its binding partner FAAP24, allowing for precise localization of the FA core complex to damaged chromatin. The FA core complex, a multimeric ubiquitin ligase, is comprised of at least 8 subunits including FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL and FAAP100. Once bound to the FANCM/FAAP24 docking platform, FANCL of the core complex associates with E2-conjugating ligase UBE2T (FANCT) to monoubiquitinate a heterodimer of FANCD2 and FANCI (FANCD2-I). Ubiquitination of FANCD2-I triggers assembly of the downstream ICL repairosome, with recruitment of SLX4 (FANCP), activating various endonucleases, including ERCC4 (FANCQ), MUS81, and SLX1. Cleavage at ICL sites by these enzymes separates the DNA strands, to which the FA core complex recruits REV1, REV3, and REV7 (FANCV) to form the translesion synthesis polymerase complex (REV1–pol ζ). Pol ζ bypasses the ICL, with the nascent strand re-establishing one of the original DNA duplexes. The double-stranded break of the remaining damaged duplex is then repaired via homologous recombination. First, BRCA2 (FANCD1) and its binding partner PALB2 (FANCN) localize to the break. BRCA2 initiates the homologous strand exchange process by recruiting recombinase RAD51 (FANCR), which facilitates invasion of the cleaved strand into the intact sister chromatid. This RAD51-guided recombination filament searches for and identifies the complementary sister sequence upon which repair synthesis and ligation are completed.
Defective ICL repair and genomic instability underly the central pathophysiology of FA. Without the ability to repair ICLs, cells are prone to DNA breakage and rearrangement, increasing the risk for oncogene alteration and loss of tumor suppressor genes3. Additionally, deficiencies in FANC proteins lead to further cellular dysregulation resulting in excess cytokine production, inflammasome activation, cell-cycle defects, and increased sensitivity to free radicals and aldehydes16. This constellation of genetic and cellular alterations is thought to contribute to the high incidence of hematologic disorders and markedly elevated cancer predisposition in FA individuals.
While FA is rare, with an incidence of approximately 1 in 130,000 births, it is the most frequent genetic cause of bone marrow failure17–20. The carrier frequency is estimated at 1:181 in the United States, but is notably higher in Israel with a frequency of approximately 1:9321. In addition to the increased frequency in Ashkenazi Jews, FA is also more common in Spanish Gypsies and Afrikaners17,21–24. For unknown reasons, FA preferentially affects males, with a male to female ratio of 1.2:125. The vast majority of pathologic FANC variants are inherited in an autosomal recessive pattern with the exception of FANCB which causes an X-linked recessive form of FA, and FANCR which manifests as an autosomal dominant FA-like syndrome26. The most commonly mutated gene in cases of FA is FANCA, responsible for 60–70% of cases, followed by FANCC (10–15%), and FANCG (10%)26,27.
Many FA patients display characteristic congenital abnormalities, including short stature, skeletal malformations (especially of the upper extremities), abnormal skin pigmentation, decreased fertility, and renal anomalies28. However, FA is a highly heterogenous disease, and different FANC mutations are associated with remarkably disparate clinical syndromes29,30. Mutations of various FANC genes result not only in diverse external phenotypes, but distinct propensities for the development of myelodysplasias and cancer31,32. Without medical management, FA patients are at high risk of severe hematologic disorders including bone marrow failure, aplastic anemia, myelodysplastic syndrome, and acute myelogenous leukemia33. The only treatment which can restore adequate hematopoiesis in FA remains hematopoietic stem cell transplantation (SCT)34. While early attempts (over 20 years ago) at SCT for FA were associated with a high mortality, recent advances in patient selection, HLA donor typing, and conditioning regimens have led to 5-year survival rates currently as high as 70–94%35–38. These improvements in hematologic management have substantially increased the survival of FA patients into adulthood. The management of solid tumors, specifically HNSCC, now represents the foremost threat to life for these FA patients.
The overall risk of developing any solid tumor is approximately 40-fold greater in FA relative to individuals without FA, with a cumulative incidence exceeding 75% by age 4539–41. Occurrence of head and neck, esophageal, and vulvar squamous cell carcinomas in particular is substantially higher among FA patients compared to the general population40. The increased risk of developing HNSCC in individuals with FA (500–800 fold) is especially notable1,2. However, the mechanisms underlying this extreme predilection for developing squamous cell carcinomas at these particular mucosal sites are poorly understood42. It remains conceivable that the increase in oral cavity cancer in young non-smokers can be attributed to under-recognized germline mutations in FANC related genes43. Premalignant and malignant lesions affecting the lateral tongue and buccal mucosa often occur adjacent to dentition, supporting a possible relationship to dental trauma and metabolites of the microbiome, which emphasizes the importance of attention to oral examination and hygiene in this population.
While the diagnosis of FA is made prior to the development of HNSCC in the majority of cases, the phenotypic heterogeneity of FA necessitates a high index of suspicion in all teenage or young adult patients who unexpectedly develop HNSCC. Strikingly, some patients with FA display no obvious physical manifestations of the disease25,44,45. Thus, the presentation of FA-related cancer and susceptibility to DNA damage in the absence of bone marrow failure may be related to differences in effects of genetic alterations in FA pathway, modifiers, or mosaicism30. We identified four case reports of young patients without an antecedent FA diagnosis, who were subsequently diagnosed with FA as adults based on toxic reactions to the HNSCC or esophageal SCC treatment regimen. These patients ranged in age from 24–51, and developed severe toxicity including sepsis from neutropenia with as little as a single dose of platinum chemotherapy administered with RT46–49. In 3 of the 4 patients, the toxicity proved fatal. These sobering reports highlight the importance of considering FA in the differential diagnosis of young HNSCC patients.
Human papillomavirus (HPV) and FA-HNSCC
Exposure to tobacco and alcohol are well characterized risk factors for developing head and neck cancers50. However, despite declining tobacco usage, the overall incidence of oropharyngeal squamous cell carcinoma (OPSCC) is increasing rapidly in the United States and Western Europe51. This rise is largely attributed to human papillomavirus (HPV) infection, with a vast majority arising from high risk strain HPV strains, especially HPV1652. In the United States, the incidence of HPV-positive OPSCC is now estimated to be 2.5 fold higher than that of HPV-negative OPSCC53. To date, three studies have evaluated the HPV status of FA-HNSCC tumors using molecular methods. One group assayed 18 FA-HNSCC tumors (15 oral cavity, 2 pharynx, and 1 larynx) using PCR, finding that 15/18 FA-HNSCC cases were positive for DNA from HPV16 or HPV1854. However, these findings remain controversial. A subsequent study also utilizing PCR reported that none of the 16 FA-HNSCC tumors assessed had detectable HPV DNA55. The third study, from 2013, also found no HPV DNA via PCR in the 5 FA-HNSCC cases analyzed (3 oral cavity, 2 oropharynx)56. Detailed genomic analysis of FA-HNSCC will be necessary to further delineate the role of HPV in the pathogenesis of these cancers.
The anatomic subsite distribution of FA-HNSCC is consistent with HPV-negative HNSCC. In the general population, HPV-positive HNSCC occurs most commonly in the oropharynx, particularly affecting the lingual and palatine tonsils57. FA-HNSCC generally occurs in the oral cavity, at subsites typically associated with HPV-negative disease58,59. The low incidence of oropharyngeal cancer relative to oral cavity tumors in FA patients suggests that FA-HNSCC is driven by lack of proper repair of DNA lesions, rather than increased HPV-mediated carcinogenesis60. Nevertheless, it is essential for FA patients to receive preventive HPV vaccination, just as it is for any young adult. There are currently three HPV vaccines approved by the FDA in various demographics: bivalent, quadrivalent, and nonavalent, with the latter indicated for both males and females aged 9–45. Since the majority of vulvar (and vaginal) SCCs arising in FA patients are associated with HPV, all FA patients should receive the HPV vaccine as children, prior to engaging in sexual activity and, hence, HPV exposure.
Clinical Presentation and Management of HNSCC in FA patients
The median age of diagnosis for sporadic HNSCC is 66 for HPV-negative cancers and 53 for HPV-positive cancers61. In individuals with FA, HNSCC develops much earlier, with a median onset of approximately 30 years of age1,4,58. FA patients who have undergone SCT present with HNSCC at even younger ages than untransplanted individuals with a median age of 18–20, possibly due to the carcinogenic effect of cytotoxic conditioning regimens employed in preparation for SCT, initiating and promoting effects of inflammation by graft-versus-host disease (GVHD), or decreased cancer immune surveillance59,62. Notably, HNSCC (and specifically oropharyngeal cancer) is also the most frequent secondary malignancy among non-FA patients who receive SCT, developing at a 7–16 times greater incidence than is seen in the general population63. The oral cavity is the most common site of FA-HNSCC, with approximately 60% of FA-associated oral cavity carcinomas affecting the oral tongue58,59. FA-HNSCC frequently presents at advanced stages with correspondingly poor survival, which underscores the need for active surveillance including frequent oral cavity exams in all FA patients and definitive management of high-risk premalignant lesions. Early detection of cancer allows for treatment with surgery alone, conferring the highest chance of survival, as well as the potential to avoid radiation and chemotherapy treatments. However, the largest cohort study to date, utilizing the International Fanconi Anemia Registry (IFAR), found that of 35 FA-HNSCC cases, 15 (43%) presented as Stage IV, of which two-thirds of these Stage IV cases (10/15) had N2b disease or greater, indicating advanced cancer spread to the neck. Furthermore, after surgical resection, definitive RT, and/or adjuvant RT/CRT, recurrence of FA-HNSCC occurred in 48% (17/35) of patients with a 5-year Kaplan-Meier survival estimate of only 39%4.
Multiple factors complicate developing a treatment plan for FA-HNSCC. Individuals with FA are likely to experience toxicity when treated with agents which cross-link DNA, generating great concern over the use of many of the FDA-approved chemotherapy agents (notably platinum-based compounds) as well as RT. As a result, FA-HNSCC patients often undergo ablative surgeries with the goal of completely resecting locoregional disease, without adjuvant RT or CRT. Surgery itself is generally well-tolerated, but late presentation and a reluctance to administer adjuvant chemotherapy or RT (or CRT) in FA-HNSCC results in discouragingly high rates of recurrence4,64,65. In contrast to surgical management, the efficacy and tolerability of chemotherapy or RT in FA-HNSCC patients remains unclear. Here, we review the cases of FA-HNSCC treated with surgery, RT, and/or systemic agents and summarize the associated toxicities and clinical outcomes.
Systematic Review of FA-HNSCC Case Reports
To define the review cohort, a PubMed search was performed using the terms: “(Fanconi anemia) AND (oral OR head OR neck OR mouth OR tongue OR buccal OR pharynx OR pharyngeal OR oropharynx OR oropharyngeal OR larynx OR laryngeal OR esophagus OR esophageal) AND (carcinoma OR cancer).” Reports which pertained to FA-associated squamous cell carcinomas of the oral cavity, oropharynx, larynx, or esophagus were selected and aggregated for analysis without restrictions on publication date, which ranged from February 1966 to December 2020. All patients who were treated with surgical resection, RT, systemic therapy, or a combination of modalities were included. Overall, we identified a total of 119 FA-HNSCC patients (including 16 with esophageal SCC) with these criteria, with demographic and clinical information summarized in Table 1. The median age of diagnosis at first primary was 28 years old, which is consistent with the reported onset of FA-HNSCC in prior epidemiologic studies1,58. The cohort was 52% (62/119) female, an intriguing observation given that both FA and HNSCC are, in general, more common in men than women25. Approximately half (58/119) of the patients received a bone marrow transplantation prior to their HNSCC diagnosis. Of the 47 patients with available complementation group information, the two most common mutations were FANCA (68%) and FANCC (17%), though mutational data was not reported for 60% (72/119) of the cohort.
Table 1:
Clinical and Demographic Characteristics of FA-HNSCC Review Cohort
| Patients | Total | 119 |
| Age at diagnosis (first primary) | Median | 28.0 |
| 10–20 | 26 | |
| 21–30 | 50 | |
| 31–40 | 29 | |
| ≥ 41 | 13 | |
| NA | 1 | |
| Sex | Male | 57 |
| Female | 62 | |
| SCT | Yes | 58 |
| No | 54 | |
| NA | 7 | |
| FANC Group | A | 32 |
| C | 8 | |
| D2 | 1 | |
| F | 1 | |
| G | 3 | |
| J | 1 | |
| P | 1 | |
| NA | 72 | |
| Primary Tumors | Total | 131 |
| Primary Tumor Subsite | Oral Cavity | 91 |
| Pharynx | 15 | |
| Larynx | 8 | |
| Esophagus | 16 | |
| NA | 1 | |
| Stage | I | 24 |
| II | 13 | |
| III | 14 | |
| IV | 33 | |
| NA | 47 | |
| HPV Status (Primary Tumor) | Negative | 11 |
| Positive | 16 | |
| NA | 104 | |
| Treatment (Primary Tumor) | Resection | 107 |
| RT | 48 | |
| Systemic | 25 | |
| Patients with recurrence | Total | 43 |
The 119 individual patients identified developed a total of 131 separate primary HNSCC tumors, with 11 patients having multiple distinct primaries of the head, neck, and/or esophagus (synchronous in 1 patient and metachronous in 10 patients, with one patient developing three metachronous oral cavity tumors). Of these primaries, 69% (91/131) were oral cavity tumors. Eighty-four primaries had staging data, of which 39% (33/84) were Stage IV. Twenty-seven tumors were assessed for HPV status, of which 11 (41%) were HPV-negative (1 oropharynx, 10 non-oropharynx) and 16 (59%) were deemed HPV-positive (2 oropharynx, 14 non-oropharynx). However, it is important to note that 15/16 HPV-positive tumors were reported from the same controversial study described above, which reported all HPV+ samples as HPV16 with a very high number in non-oropharyngeal locations (13 were oral cavity primaries)4,54.
Out of 131 primary tumors, 106 were treated with primary surgical resection. Of these, 22 cases were received adjuvant RT, 8 had adjuvant CRT, 1 was treated with neoadjuvant CRT, and 1 received adjuvant chemotherapy therapy alone. When the primary disease was deemed “unresectable,” 9 tumors were treated with primary RT, 9 with primary CRT, and 6 with chemotherapy only. One patient underwent salvage surgery only after prematurely terminating chemotherapy due to toxicity. These patients and their outcomes are summarized in Tables 2–7. Of the 119 total patients, 43 were reported to experience at least one recurrence of their disease (distinct from the 12 second primary tumors). Given that FA patients generally tolerate surgery well, FA-HNSCC primaries treated with surgical resection alone will not be discussed further. There are 26 reports detailing one or more of these cases4,42,48,64,66–87.
Table 2:
Adjuvant RT for FA-HNSCC Primaries
| Patient | Age | Sex | FA Group | SCT (Age) | Tumor site (subsite) | Stage | RT Dose | Toxicities | Interruption or Termination of RT | Outcome | Recurrence | Second Primary |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anak #1 (2020)106 | 15 | M | NA | Yes | Oral cavity (retromolar trigone) | T3N1M0 | 42.5 Gy total (in 25 fractions) | Mucositis, wound site breakdown, cerebral edema, somnolence | Yes (terminated) | DOD (6 mo pD) | - | - |
| Beckham #6 (2019)65 | 29 | F | C | Yes | Oral cavity (tongue) | T2N1 | 66 Gy total to oral cavity (in 33 fractions), 50.4 Gy total to bilateral neck | Mucositis (grade 2), dermatitis (grade 2) | No | DOD (13 mo pD, 12 mo pS, 9 mo pRT) | Yes | - |
| Nolan 2017100 | 31 | M | NA | Yes (12) | Oral cavity (buccal mucosa) | NA | Dose NA | NA | No | DOD (9 yr pD) | Yes* [Table 7] | - |
| Kutler #10 (2016)4 | 48.5 | M | J | No | Oral cavity (retromolar trigone) | T4N2bM0 | 40 Gy total (200 cGy x20 fractions) over 33 days | Mucositis (high grade), dysphagia, cytopenia, sepsis | Yes | DOD (10 mo pS) | - | - |
| Kutler #1 (2016)4 | 30.2 | F | A | No | Oral cavity (buccal gingiva) | T4N0M0 | 25 Gy total | Sepsis | Yes | DOD (16 mo pS) | Yes | - |
| Kutler #18 (2016)4 | 43.9 | M | A | No | Oral cavity (alveolus) | T4N2bM0 | 61 Gy total over 55 days | Mucositis (high grade), cytopenia, graft site breakdown, mandibular hardware removal | No | DOD (172 mo pS) | Yes | - |
| Kutler #17 (2016)4 | 36.9 | F | A | No | Oral cavity (alveolus) | T4N1M0 | 56 Gy total | Mucositis (high grade), dysphagia, cytopenia, hemorrhage, pleural thickening, sepsis | No | DOD (171 mo pS) | - | - |
| Kutler #20 (2016)4 | 26.8 | F | C | Yes | Oral cavity (submandibular) | T4N2cM0 | Dose NA | Dysphagia, dyspnea, cardiac arrest | Yes | DOD (20 mo pS) | - | - |
| Kutler #12 (2016)4 | 42.1 | F | A | No | Oral cavity (alveolus) | T4N2bM0 | 64.6 Gy total (170 cGy x30 fractions) over 70 days | Dysphagia, cytopenia | No | DOD (113 pS) | - | - |
| Kutler #30 (2016)4 | 41.1 | F | A | No | NA | TXN2bM0 | 60 Gy total (200 cGy x30 fractions) over 39 days | Mucositis (high grade), dysphagia, cytopenia, hemorrhage, trismus, xerostomia, fibrosis, esophageal stenosis | No | NED (178 mo pS) | - | - |
| Kutler #9 (2016)4 | 20.9 | M | P | No | Oral cavity (tongue) | T4N2cM0 | 70.2 Gy total (180 cGy x39 fractions) over 50 days | Mucositis (high grade), dermatitis, cytopenia, sepsis | No | DOD (34 mo pS) | - | - |
| Kutler #2 (2016)4 | 42.1 | M | A | Yes | Larynx (supraglottis) | T2N2bM0 | 55.8 Gy total (180 cGy x22 fractions) over 31 days | Mucositis (high grade), dysphagia, laryngeal edema, fibrosis, esophageal stenosis | No | DOD (341 mo pS) | Yes | - |
| Kutler #33 (2016)4 | 29 | F | C | Yes | Oral cavity (tongue) | T2N1M0 | 50 Gy total (200 cGy x25 fractions) over 31 days | Trismus, erythema | No | DOD (31 mo pS) | - | - |
| Kutler #34 (2016)4 | 44 | F | A | No | Oral cavity (tongue) | T1N0M0 | 25 Gy total | Erythema | No | DOD (73 mo pS) | - | - |
| Bonfim #4 (2016)84 | 24 | M | NA | Yes | Oral cavity | T4aN2M0 | Dose NA | NA | NA | AWD (NA) | - | - |
| Bonfim #8 (2016)84 | 12 | F | NA | Yes | Oral cavity | T2N0M0 | Dose NA | NA | NA | DOD (NA) | - | - |
| Kaplan 201187 | 43 | F | NA | NA | Oral cavity (floor of mouth) | NA | Dose NA | NA | No | NED (19 mo pS) | - | Pharynx |
| Chao 2010117 | 43 | F | NA | NA | Oral cavity (floor of mouth, mandible) | NA | Dose NA | NA | Ongoing | NED (4 mo pS) | - | - |
| Masserot #2 (2008)64 | 16.9 | M | NA | Yes (11.2) | Oral cavity (tongue) | T2N+M0 | 22 Gy total | NA | Yes | DOD (5.5 mo pD) | - | - |
| Bremer #2 (2003)49 | 32 | M | NA | No | Pharynx (lateral and posterior oropharyngeal wall) | T4cN2M0 | 8 Gy total (100 cGy x5 fractions, 150 cGy x2 fractions) | Thrombocytopenia (grade 5) | Yes (terminated) | DOD (NA) | - | - |
| Kozhevnikov 1986118 | 14 | M | NA | No | Oral cavity (gingiva) | T3N0M0 | 80 Gy total | Local bleeding | NA | DOD (6 mo pRT) | - | - |
| Esparza 196695 | 26 | F | NA | No | Esophagus (middle) | NA | Dose NA | NA | NA | DOD (4 mo pRT) | - | - |
Table 7:
Treatment of Recurrent FA-HNSCC
| Patient | Age (time to recurrence) | Sex | FA Group | SCT (Age) | Recurrence site | Surgical Resection | Systemic Agents (Dose) | RT Dose | Toxicities | Interruption or Termination of CRT | Outcome | Second Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lewis 202097 [Recurrence #1] |
27 (8 mo pS1) | F | G | No | Level 1B lymph nodes with masseter invasion (oral cavity primary) | Yes (S2) | Pembrolizumab every 21 days | [IMRT] 60 Gy total (200 cGy daily x30 fractions), escalated from 50 cGy x5 fractions | Mucositis (grade 2), dermatitis (grade 1), oropharyngeal pain, xerostomia, mild trismus | No | (see below) | Yes* [Table 7] |
| Lewis 202097 [Recurrence #2] |
28 (10 mo pS2, 7 mo pRT) | F | G | No | Maxillary gingiva (oral cavity primary) | Yes (S3) | Pembrolizumab every 21 days | - | Well tolerated | Ongoing | NED (4 mo pS3) | - |
| Lach #1 (2020)48 | 51 (6 mo pS) | M | A | No | Mediastinum (esophageal primary) | No | Capecitabine initially, then oxaliplatin and 5-FU (doses NA) | - | Diarrhea, acute pericarditis | Yes (CT terminated) | DOD (<6 mo pCT) | - |
| Beckham #5 (2019)65 | 37 (8 mo pS) | M | - | No | Pretracheal soft tissue, dermal metastasis (oral cavity primary) | No | Concurrent cetuximab ×2 doses; cetuximab alone after RT termination (dose NA) | 42.4 Gy total (in 20 fractions) | Mucositis (grade 3), dermatitis (grade 4), pancytopenia, xerostomia, abscess, bleeding | Yes (cetuximab and RT terminated) | DOD (20 mo pD, 17 mo pS, 7 mo pRT) | - |
| Beckham #8 (2019)65 | 33 (3 mo pCRT) | M | C | Yes (17) | Cavernous sinus (pharyngeal primary) | No | Nivolumab (3 mg/kg x3 doses) | [IMRT] 30 Gy total (in 10 fractions) | Nivolumab encephalitis, aspiration pneumonia | Yes (died during nivolumab therapy), RT completed | DOD (12 mo pD, 10 mo pS, 7 mo pRT) | - |
| Beckham #9 (2019)65 | 36 (NA pS1) | F | A | No | Retropharyngeal space and base of skull (oral cavity primary) | Yes (S2) | Cetuximab (dose NA) with RT cycles 1 + 2, cetuximab and paclitaxel (20 mg/m^2) with RT cycle 3, tremelimumab (1 mg/kg) and durvalumab (20 mg/kg) after RT completion | 3.7 Gy BID ×2 days (3 cycles) | Carotid bleed | No | DOD (77 mo pD, 8 mo pS2, 2 mo pRT) | - |
| Nolan 2017100 | 39 (8 yr pRT) | M | - | Yes (12) | Tongue with BOT extension (oral cavity primary) | Yes (S2) | [Neoadjuvant] Cisplatin, 5-FU, Cetuximab (doses NA, “80% reduced”) x2 cycles | - | Neutropenic sepsis | Yes (CT and CTX terminated) | DOD (9 yr pD) | Yes |
| Wong 201386 | 21 (3 mo pS1) | M | - | No | Oral tongue and BOT with extensive invasion (oral cavity primary) | Yes (S2) | Cetuximab (400 mg/m^2 loading dose, 200 mg/m^2 once weekly x8 total infusions) | 70.2 Gy total to oral cavity, 50.4 Gy total to neck (fractions of 180 cGy) | Mucositis (grade 3) by 4500 cGy, dermatitis with desquamation (grade 3) by 5000 cGy, neutropenia (after cetuximab loading dose), acneiform rash (after second infusion) | Yes (cetuximab terminated), RT completed | DOD (10 wk pRT) | Yes |
| Masserot #7 (2008)64 | 18 (6 mo pS) | M | - | Yes (11.2) | Pelvilingual (oral cavity primary) | No | Agent(s) and dose(s) NA | 60 Gy total | “severe toxicity” | Yes (CT terminated) | DOD (16 mo pD) | Yes |
| Oksüzoğlu 200268 | 29 (7.5 mo pS) | F | - | No | Tongue (oral cavity primary) | No | - | Dose NA | Mucositis (severe), thrombocytopenia, neutropenia, pleural effusion, sepsis | Yes | DOD (9 mo pS) | - |
| Millen 199771 | 18 (1 mo pS) | F | - | Yes (9) | Buccal mucosa with extension into masseter and parotid (oral cavity primary) | No | - | Dose NA (palliative) | Eye “deterioration” | NA | DOD (3 mo pS) | - |
| Lustig 199573 | 32 (2 mo pS) | F | - | No | Submandibular mass (oral cavity primary) | No | - | 3.2 Gy total | Mucositis, local bleeding, progressive pancytopenia | Yes (RT terminated) | DOD (9 mo pS, 6 mo pRT) | - |
| Snow 199174 | 30 (8 mo pS) | F | - | No | Supraclavicular fossa and anterior neck (esophageal primary) | No | - | 32.5 Gy total (palliative) | Well tolerated | No | DOD (12 mo pD) | - |
Radiation Therapy in FA-HNSCC
Surgical resection is the preferred primary treatment modality for oral cavity tumors, and carries a high rate of cure for early stage disease88,89. Unilateral or bilateral neck dissections are often performed to allow for more accurate pathological staging and remove nodal metastases90. Adverse pathologic features (such as extranodal extension, positive resection margins, multiple involved nodes, and lymphovascular or perineural invasion) inform the decision to administer adjuvant treatment. In patients who have undergone surgery with curative intent, advanced lymph node involvement or the presence of the aforementioned adverse pathologic features typically warrant a course of adjuvant RT, or in rare cases of highly concerning features, adjuvant CRT. In contrast to oral cavity tumors, cancers of the oropharynx, larynx, and hypopharynx are commonly treated with definitive CRT as primary therapy, particularly in cases where surgery would result in unacceptable functional deficit90.
When RT is employed, intensity-modulated radiotherapy (IMRT) is the current standard of care in HNSCC, and demonstrates an improved toxicity profile with better locoregional control compared to historical three-dimensional conformal RT91. Completing a course of RT, typically administered 5 days a week over the course of 6–7 weeks without interruption, is key for maximal efficacy, as breaks in RT are associated with poorer outcomes92,93. However, radiation toxicities of varying degrees are common among all HNSCC patients, including dermatitis, edema, mucositis, pain, dysphagia, and xerostomia94. Dose-limiting side effects can easily interfere with the delivery of uninterrupted RT, especially in patients with FA who are intrinsically sensitive to DNA damage. Thus, planning for enhanced supportive care, with ability to adapt the treatment in case of poor tolerance, must be a part of the pretreatment discussion. A key limitation in reviewing the evidence of RT tolerability in FA-HNSCC is the lack of detail specifying the precise radiation modality utilized in the majority of patients, as reports of RT-treated FA-HNSCC date back to the 1960s95.
Initial use of IMRT began in the 1990s and became the standard of care for HNSCC with widespread adoption in the 2000s, with efforts to standardize IMRT across institutions in the 2010s. A major advantage of IMRT is enabling initial fields to encompass the lesion and monitor toxicity and response, before widening the field to provide lower cumulative doses planned for adjacent mucosa or neck. The use of IMRT is mentioned specifically in only three patient cases65,96,97, but is presumed to be the current standard approach for delivery of external beam RT for all HNSCC, including FA-HNSCC. Proton beam RT has been reported in a single case treated with 70.4 CGE to the high-risk volume and 60 CGE to the remaining ipsilateral neck, with concurrent cetuximab. The patient tolerated the regimen but developed an in-field recurrence 16 months after completing therapy65. Proton therapy is highly intriguing due to its increased conformality to targeted areas and is frequently used for these reasons in pediatric and young adult radiation treatments. However, because of the potential for less dose-sparing of the superficial skin and mucosal surfaces, the advantages of this radiation modality must be more clearly established in FA-HNSCC patients before it can be recommended as a rule.
Adjuvant RT
Post-operative RT is typically administered once daily, five times per week at 1.8–2 Gy as conventionally fractionated irradiation; a typical goal is to achieve a total dose of 60–66 Gy over the course of 6–6.5 weeks (RTOG 9501 and EORTC 22931 trials)98,99. Twenty-two FA-HNSCC patients (21 HNSCC, 1 esophagus) underwent surgery followed by adjuvant RT for primary tumors (Table 2). RT dosage was reported for 15/22 patients, who achieved a median total dose of 51.8 Gy. Eleven of 22 patients completed the planned RT course, while 6/22 had their treatment interrupted or terminated due to RT toxicities. Completion status was not available for 5 patients. Of the 11 patients who completed adjuvant RT, 8 died of disease without further treatment, 1 underwent subsequent surgery and adjuvant chemotherapy for recurrence (Nolan 2017)100, 1 was alive 19 months postoperatively without recurrence of her initial tumor but with a new primary (Kaplan 2011)87, and 1 was alive with no evidence of disease (NED) over 14 years postoperatively (Kutler #30)4. The 6 patients who interrupted or terminated RT early all succumbed to disease within 20 months of their initial surgery. Of the remaining 5 patients without RT completion status, three died of disease, one was still undergoing treatment, while one was alive with cancer progression at time of publication and presumably succumbed to disease. It should be noted that patients for whom postoperative RT is recommended are those with more advanced cancers, and the outcomes are expected to be worse than the surgery-alone population.
Primary RT
Definitive, or curative-intent, RT is typically administered once daily, five times per week at 2 Gy as conventionally fractionated irradiation aiming to achieve a total dose of 70 Gy over the course of 7 weeks. Nine patients (7 HNSCC, 2 esophagus) received RT without surgery for primary disease, 56% of whom (5/9) completed the course and 44% of whom terminated treatment early due to toxicity (1 during RT) (Table 3). The average total dose achieved was 52 Gy. Of the 5 who completed primary RT, 4 died of disease (one from a separate primary) and 1 was alive with recurrence 14 months post-diagnosis (Budrukkar 2009)101. Of the 4 patients who terminated RT, 3 succumbed to disease, while 1 was alive with disease 2 months post-RT completion (Marcou 2001)102. Presumably, all patients alive with disease at the time of case report publication ultimately died of HNSCC.
Table 3:
Primary RT for FA-HNSCC Primaries
| Patient | Age | Sex | FA Group | SCT (Age) | Tumor site (subsite) | Stage | RT Dose | Toxicities | Interruption or Termination of RT | Outcome | Recurrence | Second Primary |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anak #4 (2020)106 | 32 | F | NA | Yes | Esophagus (middle third) | T2N0M0 | 45.9 Gy total (in 27 fractions) | Mucositis, dysphagia, retrosternal pain, pneumonitis, esophageal stenosis | No | DOD (10 mo pD, 3 mo pRT) | - | Oral cavity |
| Lach #2 (2020)48 | 52 | M | A | No | Larynx | T2N0M0 | 69 Gy total | NA | No | Died of SP (esophagus) | - | Esophagus, Anus |
| Budrukkar 2009101 | 27 | M | NA | No | Pharynx (BOT) | T2N0M0 | 70 Gy total (200 cGy x35 fractions) over 51 days | Mucositis (grade 3), dermatitis (grade 3), leukopenia with fever | No | AWD (14 mo pD) | Yes | - |
| Han 200996 | 32 | F | NA | No | Oral cavity (retromolar trigone and hard palate), HPV+ | NA | [IMRT] 64.8 Gy total (in 27 fractions) | Mucositis (grade 3), leukopenia (grade 4) | No | DOD (NA) | - | Vulva, cervix |
| Masserot #6 (2008)64 | 19.7 | M | NA | Yes (7.3) | Pharynx (oropharynx) | T4N2cMX | 70 Gy total | Mucositis (grade 3), severe dehydration | No | DOD (4.5 mo pD) | - | - |
| Masserot #8 (2008)64 | 10.1 | M | NA | Yes (4.6) | Oral cavity (tongue) | T3N0MX | 25 Gy total over 4 weeks (vs. 65 Gy planned) | Mucositis (severe) | Yes (terminated) | DOD (2.5 mo pD) | - | - |
| Horta 2006119 | 24 | F | NA | No | Pharynx (pyriform sinus) | NA | 19.8 Gy total | Dysphagia, odynophagia, neutropenia, pneumonia | Yes (terminated) | DOD (NA) | - | - |
| Marcou 2001102 | 32 | F | A | No | Pharynx (tonsil) | NA | 34 Gy total in 17 fractions (vs. 60 Gy planned) | Mucositis, ulceration, dysphagia, septicemia | Yes (terminated) | AWD (2 mo pRT) | - | - |
| Kozarek 1981120 | 26 | M | NA | No | Esophagus (cervical) | NA | 70 Gy total | Laryngeal edema, pneumonia | Yes (died during RT) | DOD (during RT) | - | - |
In 4 patients, recurrent disease was treated with RT alone (Table 7). All died within 1 year of recurrence, with premature RT termination in 2 and unavailable completion information for 1 patient. The remaining patient completed an RT course of 32.5 Gy with palliative effect, although failure to control disease led to widespread metastasis and death (Snow 1991)74.
In summary, RT was relatively well tolerated in FA-HNSCC patients both in the definitive and adjuvant treatment setting. For recurrent FA-HNSCC, RT was less well tolerated with only 1 out of 3 patients completing the planned course. Overall, about 60% of patients successfully completed RT with average total dose of 59.2 Gy, while 40% of patients who had to terminate early received total average dose of 31.8 Gy. Although the numbers of cases reported are relatively small, there is no evidence that prior SCT impacts radiation tolerability or toxicity.
Conventional Chemotherapy in FA-HNSCC
CRT is indicated as primary treatment for stage III/IV HNSCC where surgery is not feasible or as adjuvant therapy after surgical resection of tumors demonstrating pathologic high-risk features, such as positive margin and/or extranodal extension. The standard chemotherapy agent used for CRT is cisplatin. Although cetuximab has been used in definitive CRT, its use has declined after a number of recent clinical trials demonstrated its inferiority compared to cisplatin in HPV-positive HNSCC103,104. Patients described in this section received only conventional cytotoxic chemotherapy with or without RT. Patients who received molecular targeted agents with or without conventional chemotherapy or RT are presented in a subsequent section.
Adjuvant CRT with conventional cytotoxic chemotherapy
Five patients received CRT with conventional cytotoxic chemotherapy in conjunction with surgical resection of primary tumors (Table 4; rows 1–5). This included 4 HNSCC patients receiving adjuvant treatment, and 1 esophageal carcinoma patient who underwent neoadjuvant CRT. Agents administered included cisplatin and 5-FU (1 patient), cisplatin, bleomycin and methotrexate (1 patient), methotrexate single agent (1 patient), bleomycin single agent (1 patient) for HNSCC and cisplatin and 5-FU for esophageal SCC. Of these 5 patients, 1 completed the CRT course, 3 terminated treatment due to toxicity, and completion status was not available for 1 patient. Four patients died of disease from 3 to 33 months following therapy. Only 1 patient was alive without disease at the time of publication (Hosoya 2010)66. Although CRT was terminated early due to toxicity, her primary esophageal SCC “showed an excellent response to the pre-operative chemoradiotherapy66.” She developed a second primary of the oral cavity five years later which was cured with resection, and remarkably, was alive with no evidence of either cancer at publication (6 years following esophagectomy, and 1 year after oral cavity surgery).
Table 4:
Adjuvant CRT (Cytotoxic or Cetuximab) for FA-HNSCC Primaries
| Patient | Age | Sex | FA Group | SCT (Age) | Tumor site (subsite) | Stage | Systemic Agents (Dose) | RT Dose | Toxicities | Interruption or Termination of CRT | Outcome | Recurrence | Second Primary |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anak #2 (2020)106 | 21 | M | NA | Yes | Oral cavity (retromolar trigone) | T3N1M0 | Cisplatin (30–40 mg/m^2) every 21 days, 5FU (1 g/m^2/per cycle) | 32.3 Gy total (in 19 fractions) | Mucositis, dermatitis, pancytopenia, dysphagia, local edema, fatigue, nausea | Yes (CT and RT terminated) | DOD (6 mo pD) | - | - |
| Kutler #13 (2016)4 | 28.2 | M | NA | No | Pharynx (tonsil) | T3N2bM0 | Cisplatin, bleomycin, methotrexate (dose NA) | 56 Gy total over 52 days | Mucositis, cytopenia, tracheal stenosis, radiation pneumonitis, aspiration pneumonia | Yes | DOD (33 mo pS) | Yes | - |
| Kutler #14 (2016)4 | 15.4 | F | A | Yes | Oral cavity (tongue) | T1N0M0 | Methotrexate (dose NA) | Dose NA | NA | No | DOD (18 mo pS) | Yes | - |
| Hosoya 201066 | 35 | F | NA | Yes (23) | Esophagus (middle thoracic), HPV− | T3N1M0 | [Neoadjuvant] Cisplatin (5 mg/day, 3.3 mg/m2), 5-FU (500 mg/day, 330 mg/m2) x5 days with 2-day rest periods. Total dose of 50 mg cisplatin, 5000 mg 5-FU. | 30.4 Gy total (in 21 fractions) | Bone marrow suppression, diarrhea (grade 3) | Yes (CT and RT terminated) | NED (6 yr pS) | - | Oral cavity |
| Vaitiekaitis 1980121 | 19 | F | NA | No | Oral cavity (gingiva and mandible) | NA | Bleomycin (dose NA) | 68 Gy | NA | NA | DOD (3 mo pRT) | - | - |
| Beckham #7 (2019)65 | 33.6 | M | A | No | Oral cavity (maxilla) | pT1Nx | Cetuximab (dose NA) | [proton beam] 70.4 CGE (70.4 Gy) to primary, 60 CGE (60 Gy) to neck | Mucositis, dermatitis (grade 2), xerostomia | No | AWD (26 mo pD, 20 mo pS, 16 mo pCRT) | Yes | - |
| Beckham #8 (2019)65 | 33 | M | C | Yes (17) | Pharynx (pyriform sinus) | pT3N2c | Weekly Cetuximab (dose NA) | 70 Gy (in 35 fractions) to primary, 54 Gy to the high-risk nodes, 45 Gy to neck | Mucositis (grade 2), dermatitis (grade 3), dysphagia, xerostomia | No | DOD (12 mo pD, 10 mo pS, 7 mo pCRT) | Yes* [Table 7] | - |
| Kutler #19 (2016)4 | 34.8 | M | A | No | Oral cavity (tongue) | T4N1M0 | Cetuximab (dose NA) | 42.4 Gy total (200 cGy x20 fractions) over 28 days | Mucositis (high grade), dermatitis, cytopenia, dysphagia, wound breakdown, hemorrhage | Yes | DOD (121 mo pS) | - | - |
| Kutler #21 (2016)4 | 29.9 | M | A | No | Larynx (aryepiglottic fold) | T1N0M0 | Cetuximab (dose NA) | Dose NA | NA | No | DOD (198 mo pS) | Yes | - |
Primary CRT with conventional cytotoxic chemotherapy
An additional 7 patients with primary tumors (6 HNSCC, 1 esophageal) received primary CRT using conventional cytotoxic chemotherapy (cisplatin, carboplatin, gemcitabine, and/or 5-FU), described in Table 5 (rows 1–8). These 7 patients account for 8 total tumors, as one patient had multiple primaries treated with primary CRT. Of the 8 tumors treated with cytotoxic CRT, only 2 treatment courses, involving non-platinum based concurrent chemotherapy regimen with single agent 5-FU or single agent gemcitabine, were completed without interruption or termination (1/8 had completion status not available). All 7 patients receiving CRT died of disease, but the clinical course of one patient (Bremer #1)49 is particularly notable. This patient first presented with a base of tongue tumor at age 24, for which he received CRT with carboplatin (60 mg/m2). However, carboplatin was discontinued after just one cycle due to pancytopenia. He completed 67.0 Gy RT total with one interruption at 38.4 Gy due to mucositis, after which he underwent surgical salvage (neck dissection) for persistent enlarged cervical lymph nodes. Remarkably, the patient remained free of disease for five years, at which time he developed two new primaries, of the oral cavity and anal canal. He received CRT with gemcitabine (100 mg/m2) and 25.2 Gy RT to the oral cavity, both of which were completed and well tolerated, but the patient succumbed to tumor progression 3 months later.
Table 5:
Primary CRT (Cytotoxic or Cetuximab) for FA-HNSCC Primaries
| Patient | Age | Sex | FA Group | SCT (Age) | Tumor site (subsite) | Stage | Systemic Agents (Dose) | RT Dose | Toxicities | Interruption or Termination of CRT | Outcome | Recurrence | Second Primary |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anak #3 (2020)106 | 16 | M | NA | Yes | Oral cavity (retromolar trigone) | T3N3M0 | Cisplatin (30–40 mg/m^2) every 21 days, 5FU (1 g/m^2/per cycle) | 34 Gy total (in 20 fractions) | Mucositis, stomatitis, neutropenia, thrombocytopenia, dysphagia, cerebral edema | Yes (CT and RT terminated) | DOD (6 mo pD) | - | - |
| Spanier 201247 | 27 | M | NA | No | Pharynx (palatine tonsil with extensive infiltration), HPV− | T4N2bM0 | Cisplatin (40 mg/m^2) x1 cycle | 1.4 Gy x4 fractions, 1.8 Gy x4 fractions, reduction to 1.4 Gy fractions (total dose NA) | Pancytopenia (grade 4), diarrhea (grade 1), laryngeal edema, dyspnea, oral candidiasis, emphysema of upper body, bilateral jugular vein thrombosis | Yes (CT and RT terminated) | DOD (NA) | - | - |
| Beehuat Tan 201146 | 32 | F | A | No | Pharynx (pyriform sinus) | T2N2b | Cisplatin (100 mg/m^2) - single dose | Dose NA | Bone marrow failure, neutropenic sepsis, alveolar hemorrhage | Yes (CT and RT terminated) | DOD (NA) | - | - |
| Tipples 2008122 | 25 | F | NA | Yes (10) | Esophagus (upper third) | T3N0 | 5-FU (“25% of normal dose”) | 52.2 Gy total (fractions of 120 cGy increased to 180 cGy) | Dermatitis with desquamation (grade 2), dysphagia | No | DOD (12 mo pD) | Yes | - |
| Salum 2006123 | 16 | M | NA | Yes (5) | Oral cavity (dorsum of tongue) | T3N0M0 | Drug and dose NA (palliative) | Dose NA (palliative) | NA | NA | DOD (4 mo pCRT) | Yes | - |
| Bremer #1 (2003)49 | 24 | M | A | No | Pharynx (BOT) | T4cN2G2M0 | Carboplatin (60 mg/m^2) x1 cycle | 67 Gy total [break at 38.4 Gy], fractions of 160–180 cGy | Mucositis (confluent) after 2720 cGy, pancytopenia (grade 3) | Yes (RT interrupted, CT terminated) | Died of SP after 5 yr (see below) | - | Oral cavity* [Table 5], anal |
| Bremer #1 (2003)49 | 29 | M | A | No | Oral cavity (floor of mouth) | NA | Gemcitabine (100 mg/m^2) once weekly x3 weeks | 25.2 Gy total (fractions of 180 cGy) | Mucositis (grade 2), thrombocytopenia, anemia | No | DOD (3 mo pCRT) | - | - |
| Bradford 199075 | 29 | F | NA | Yes (20) | Pharynx (tonsillar fossa and BOT) | T4N0M0 | Cisplatin (100 mg/m^2) and 5-FU (500 mg/m^2) x2 cycles | 72.2 Gy total [3 breaks] | Mucositis, stomatitis, conjunctivitis, pneumonia | Yes (RT interrupted, CT terminated) | DOD (6 mo pRT) | Yes | - |
| Kutler #26 (2016)4 | 24.2 | M | A | No | Oral cavity (tongue) | NA | Cetuximab (dose NA) | Dose NA | NA | No | DOD (NA) | - | - |
One patient with recurrent disease received conventional CRT with agents and doses NA, but chemotherapy was terminated early due to “severe toxicity,” and the patient developed a second recurrence before dying of disease64 (Table 7).
Conventional chemotherapy alone
Six patients (3 HNSCC, 3 esophageal) received conventional chemotherapy alone without concurrent RT for primary tumors (5 as intended primary therapy and 1 as adjuvant treatment), detailed in Table 6 (rows 1–6). Regimens administered included cisplatin and 5-FU, carboplatin and paclitaxel, cisplatin and 5-FU, 5-FU and cis-retinoic acid, and pepleomycin, a bleomycin-like drug. All 6 patients receiving cytotoxic chemotherapy either terminated treatment early, or information about treatment completion was unavailable. All 6 died of disease within 18 months of diagnosis. One additional patient received cis-retinoic acid alone without cytotoxic agents and developed recurrence (Table 6; row 7)105. It is notable that in the three patients with toxicity information available, all developed pancytopenia, one additionally complicated by sepsis (received cisplatin and 5-FU), and another experiencing liver failure and C. difficile colitis after 2 cycles of carboplatin and paclitaxel65,106.
Table 6:
Systemic agents alone for FA-HNSCC Primaries
| Patient | Age | Sex | FA Group | SCT (Age) | Tumor site (subsite) | Stage | Surgical Resection | Systemic Agents (Dose) | Toxicities | Interruption or Termination of CT | Outcome | Recurrence | Second Primary |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anak #5 (2020)106 | 25 | M | - | Yes | Esophagus (cervical) | T3N1M0 | Yes | Cisplatin (30–40 mg/m^2) every 21 days, 5-FU (1 g/m^2 per cycle) | Mucositis, pancytopenia, sepsis | Yes (terminated) | DOD (6 mo pD) | Yes | - |
| Lach #1 (2020)48 | 51 | M | A | No | Esophagus (distal) | NA | Yes (salvage after CT) | Carboplatin and paclitaxel x1 cycle (dose NA, “low”) | Pancytopenia | Yes (terminated) | DOD (<1 yr pD) | Yes* [Table 7] | - |
| Beckham #4 (2019)65 | 32.6 | F | - | No | Esophagus (cervical) | T2N0 | No | Carboplatin and paclitaxel x2 cycles (dose NA) | Pancytopenia, liver failure, C. difficile colitis | Yes (terminated) | DOD (18 mo pD) | - | - |
| Masserot #3 (2008)64 | 17.5 | M | - | Yes (9.7) | Pharynx (hypopharynx) | T4N2cMX | No | Cisplatin (8 mg), 5-FU (60 mg) | NA | NA | DOD (6 mo pD) | - | - |
| Murayama 1990124 | 11 | M | - | Yes (8) | Oral cavity (dorsum of tongue) | NA | No | 5-FU (dose NA), cis-retinoic acid | NA | NA | DOD (3 mo pCT) | - | - |
| Fukuoka 1989125 | 39 | F | - | NA | Pharynx (pyriform sinus) | NA | No | Pepleomycin (dose NA) | NA | Yes (terminated) | DOD (NA) | - | - |
| Koo 1996105 | 44 | F | - | NA | Oral cavity | NA | No | Cis-retinoic acid (dose NA) | NA | NA | NA | Yes | - |
| Jung 2005107 | 27 | F | - | No | Oral cavity (tongue) | IVA | No | Gefitinib 250 mg/day, 2 month total duration | Well tolerated | No | DOD (from hepatocellular carcinoma, 2 mo pCT) | - | Liver, anal |
One patient with recurrent disease received conventional chemotherapy alone, initially with capecitabine and then with oxaliplatin and 5-FU, but terminated therapy early due to toxicity (acute pericarditis and diarrhea), and died of disease within 6 months48 (Table 7).
In summary, cytotoxic chemotherapy (usually with a platinum-based regimen) with or without radiation was not well tolerated in the majority of FA-HNSCC patients. Only 30% of patients who received primary CRT were able to complete the planned treatment course. All patients who received cytotoxic chemotherapy alone (n=7) had to terminate treatment early and died of disease.
EGFR inhibitors in FA-HNSCC
Cetuximab was FDA approved in 2006 for the treatment of primary HNSCC in combination with RT and subsequently approved for recurrent or metastatic disease in combination with chemotherapy (the EXTREME regimen). In contrast, EGFR tyrosine kinase inhibitors have failed to improve outcomes in unselected HNSCC populations. Ten FA-HNSCC patients received EGFR inhibitors (9 cetuximab, 1 gefitinib) either alone or in combination with other agents for treatment of primary or recurrent FA-HNSCC. Four patients (2 oral cavity tumors, 1 pharynx cancer, and 1 larynx cancer) received adjuvant RT with concurrent cetuximab after surgical resection of primary disease, of which 3 completed the entire course of treatment with the standard toxicities of mucositis, dermatitis, dysphagia, and xerostomia using a mean RT dose of 70 Gy (Table 4; rows 6–9). All 3 patients who completed adjuvant cetuximab plus RT developed recurrences, one of whom died of disease without further treatment (Kutler #21)4, one who died following immunotherapy for recurrence (Beckham #8)65, and one who was alive with recurrence at 16 months post-CRT of his primary and presumably succumbed to the cancer (Beckham #7)65. The patient who terminated adjuvant cetuximab and RT (total dose of 42.4 Gy) early experienced mucositis, dermatitis, and dysphagia along with cytopenia, wound breakdown and hemorrhage (Kutler #19)4. Additionally, one patient (with primary oral cavity disease) received cetuximab with RT as primary treatment without surgery (Table 5; row 9). Although interruption or termination of CRT was not reported, detailed toxicity information was not available, he died of disease (Kutler #26).
Cetuximab was also administered to 4 patients with recurrent FA-HNSCC (Table 7). One patient (Beckham #5)65 received 10 fractions of RT with 2 doses of concurrent cetuximab before developing grade 3 mucositis and an abscess. After a break in therapy, he resumed RT without cetuximab, which was subsequently terminated at a cumulative dose of 42.4 Gy due to bleeding. Following RT termination, he began cetuximab therapy once again for three months with a “temporary” tumor response noted, before eventually dying of disease 7 months after RT cessation. Beckham #9 underwent adjuvant RT with 2 cycles of concurrent cetuximab, developing a carotid sentinel bleed before undergoing a third cycle with RT and paclitaxel. Following completion of CRT, she continued cetuximab with two further doses of paclitaxel before receiving immunotherapy. While follow-up revealed a tumor response, she died shortly after administration of immunotherapy (discussed in the following section)65. Another patient received two cycles of chemotherapy consisting of cisplatin, 5-FU, and cetuximab (Nolan 2017)100. All systemic agents were terminated early after the patient developed sepsis and died. One patient received adjuvant RT with concurrent cetuximab for his recurrence (Wong 2013)86. He experienced neutropenia following a loading dose of cetuximab, and the cetuximab was discontinued for the final two weeks of RT (after 8 total infusions) due to worsening radiation dermatitis. While cetuximab was terminated in this patient, he completed the planned RT course, with the authors reporting that both “RT and cetuximab were well tolerated with manageable toxicities.86” The patient subsequently developed an extensive second recurrence and Pseudomonas bacteremia, dying 10 weeks after RT completion.
The European Medicines Agency approved the use of the EGFR TKIs gefitinib or afatinib for FA-HNSCC in 2018 based on compelling results in relevant preclinical models. There was one reported case of FA-HNSCC treatment with the small molecule EGFR inhibitor gefitinib (Table 6; row 8). This patient, a 27-year-old female with tongue SCC, completed a two-month course of gefitinib treatment without notable toxicity, including no rash or diarrhea (Jung 2005)107. Initially, her tumor size was substantially reduced (from 2.6×3.6 cm to 1.5×1.3 cm), but she later progressed on gefitinib. The patient subsequently received transhepatic chemoembolization for a hepatocellular carcinoma and died two months later of gastrointestinal bleeding.
In summary, concurrent cetuximab with radiation was well tolerated as primary treatment in FA-HNSCC patients, although the same regimen proved toxic in recurrent FA-HNSCC patients. The approval of EGFR TKI for FA-HNSCC in Europe highlights the potential of these agents for this difficult to treat population.
Immune Checkpoint Inhibition in FA-HNSCC
Advances in the use of immune checkpoint inhibitors (ICIs) has dramatically changed the landscape of systemic R/M HNSCC treatment. There are currently two FDA-approved ICIs for HNSCC, pembrolizumab and nivolumab, both of which were approved in 2016108. These monoclonal antibodies target programmed cell death protein 1 (PD1) to stimulate lymphocyte-mediated anti-tumor activity, and can induce potent and durable responses in a subset of HNSCC patients109. ICI is now routinely used in combination with platinum and 5-FU, and in 2019, pembrolizumab was FDA-approved as a first line monotherapy for unresectable R/M HNSCC in patients with programmed death-ligand 1 (PD-L1) expressing tumors88. Patients who cannot receive first-line immunotherapy usually receive platinum-based combination chemotherapy incorporating cetuximab and a taxane or antifolate90.
Due to the unique hematologic considerations of FA patients, notably a high prevalence of allogeneic SCT, there is a general reticence to attempt cancer immunotherapy in this population. In particular, ICI administration post-SCT can trigger or exacerbate severe graft-versus-host disease (GVHD). A recent analysis of 150 non-FA patients who received ICIs for hematologic malignancies after previous SCT found that 13% developed acute GVHD and 11% developed chronic GVHD, in addition to common hematologic side effects including neutropenia. However, of these hematologic malignancy patients, an overall response rate of 48% was reported, with 28% achieving complete response and 20% demonstrating partial response110. While these responses may not recapitulate the response of FA-HNSCC patients to ICI after SCT, it suggests that SCT itself does not universally portend a poor outcome to immunotherapy.
There are only three reports of FA-HNSCC patients who received ICI (all for disease recurrence; Table 7), of which one (Beckham #8) previously underwent SCT. Beckham #8 completed a course of palliative IMRT, after which he began nivolumab. However, after only three doses of 3 mg/kg, the patient was hospitalized for aspiration pneumonia and nivolumab-induced encephalitis, to which he succumbed65. Beckham #9 received a single dose of 1 mg/kg tremelimumab (anti-CTLA4) and 20 mg/kg durvalumab (anti-PDL1) following 3 CRT cycles with cetuximab and paclitaxel (described in the EGFR inhibitor section above). However, she died 10 days after administration of these ICI agents65. The most recent report (Lewis 2020) detailed a case in which immune checkpoint inhibition with pembrolizumab was administered as part of adjuvant treatment for two locoregional recurrences of an oral cavity tumor. In this patient, pembrolizumab was administered every three weeks in combination with IMRT for the first recurrence and as a single agent for the second recurrence. ICI was well tolerated in this patient, and was still being administered at the time of publication with no evidence of disease 4 months after the most recent surgery97. Nonetheless, care should be taken in drawing conclusions based on so few reports, and there is a need for rigorous evaluation of the safety and efficacy of ICI in FA-HNSCC patients. Furthermore, studies characterizing the prevalence and extent of PD-L1 expression in FA-HNSCC tumors are warranted. Only after substantial evidence of ICI toxicity profiles in this population is collected is it appropriate to conclude whether or not immune checkpoint inhibition is a viable treatment option for FA-HNSCC.
Discussion
The paucity of aggregated reports or clinical trials describing the efficacy and toxicity of specific therapeutic regimens represents a major barrier in determining the appropriate treatment strategy for FA-HNSCC patients. Complete surgical resection is accepted as the standard of care for these individuals given their heightened sensitivity to DNA-damaging agents including radiation and chemotherapy and the prevalence of oral cavity tumors which are commonly resected in sporadic HNSCC. However, surgery alone is rarely curative for advanced stage HNSCC and when the tumor arises in the hypopharynx or larynx, surgery may include total laryngopharyngectomy, which is dramatically life altering in young patients. We reviewed the case reports and case series of FA-HNSCC patients reported in the peer-reviewed literature from 1966 through 2020 with a focus on descriptions of individuals who were treated with systemic chemotherapy and/or radiation in both adjuvant and definitive contexts for primary and/or recurrent disease. This review is intended as a guide for treatment discussions and decisions, and to stimulate the development of much needed clinical trials for this vulnerable population.
It is notable that the majority of FA-HNSCC patients who received cetuximab experienced substantially less toxicity than those who underwent treatment with conventional cytotoxic chemotherapy. However, there are only 9 total patients reported in the literature who received this EGFR-targeted monoclonal antibody (5 with primary tumors and 4 for recurrent disease), all of whom also received treatment with RT, ICI, and/or cytotoxic chemotherapy agents (Beckham #5, 7, 8, 9; Kutler #19, 21, 26; Nolan 2017; Wong 2013)4,65,86,100. Of note, the majority of chemotherapy agents used were either cisplatin- or carboplatin-based regimen. There is an ongoing study with concurrent docetaxel and cetuximab with radiation in adjuvant treatment of HNSCC (RTOG 1216, NCT01810913), which may be better tolerated in FA-HNSCC patients, although data in this population are lacking. One patient received low dose single-agent gemcitabine (100 mg/m2 weekly) and another had low dose 5-FU. Both patients tolerated the planned dose of RT, which suggests that alternative chemotherapy may need to be investigated further. Disentangling the toxicities of individual agents used in these regimens is difficult, and more information on the use of cetuximab in FA-HNSCC are needed to determine its role in disease management. However, given that the completion of RT is crucial for RT efficacy in HNSCC combined with the dismal tolerance of cytotoxic chemotherapy for FA-HNSCC, it seems prudent to consider cetuximab/RT for these patients. Importantly, the one patient who received gefitinib experienced substantial regression of a Stage IV oral cavity tumor without any notable toxicities reported during the treatment (Jung 2005)107. It is notable that she was the only FA-HNSCC patient who completed a course of systemic therapy alone, as all patients who received conventional chemotherapy without RT terminated treatment early due to toxicity. This case report is augmented by preclinical findings using small molecule EGFR inhibitors in FA-HNSCC xenograft models in mice. In this study, gefitinib or afatinib significantly inhibited the growth of two distinct, cell line-derived xenograft models of FA-HNSCC, with in vivo toxicity studies in FANCA-deficient mice showing minimal side effects and no bone marrow toxicity111. Based on these findings, the European Medicines Agency granted orphan drug designation to gefitinib (EU/3/18/2075) and afatinib (EU/3/18/2110) for the treatment of FA-HNSCC in late 2018. The remarkable evolution from data in one patient and a relevant preclinical model to drug approval is a testament to the power of single patient studies for rare diseases. Additional evaluation of the efficacy of gefitinib and/or afatinib in FA-HNSCC patients is critical to determine whether these drugs can improve survival and quality of life in this especially vulnerable population. The tolerability of cetuximab coupled with the approval of EGFR TKIs suggest that EGFR may represent a viable therapeutic target for FA-HNSCC. Studies to date in sporadic HNSCC have failed to identify predictive biomarkers for cetuximab, which was FDA-approved in 2006 and is used in patients who cannot tolerate platinum chemotherapy. In addition, EGFR TKIs have proven ineffective in unselected HNSCC populations, although responses have been reported112–114. Identification of the precise molecular alterations which drive HNSCC carcinogenesis in FA patients remains crucial to developing effective targeted therapies with acceptable toxicity profiles.
Immune checkpoint inhibitors are widely used in variety of cancers and recent studies suggest that defects in homology-dependent recombination, could lead to higher predicted neoantigen load, increased tumor-infiltrating lymphocytes, and enhanced PD-1/PD-L1 expression115. A recent report shows that patients with germline or somatic BRCA2 mutations are more likely to receive clinical benefit from ICIs across multiple tumor types116. At least for FA-HNSCC patients who have not received allogenic SCT, ICIs may be a reasonable option to consider, although more investigation is needed to elucidate PD-L1 expression along with the genomic landscape and tumor mutational burden in these patients. Studies are needed to determine if prior SCT is an exclusion criterion for treatment with ICIs. Moreover, it is noteworthy that systemic agents are often reserved for recurrent or metastatic disease, which may be too late to observe benefit. Consideration of adjuvant therapy with curative intent immediately following surgery may reveal benefits that are inapparent in the face of widespread disease.
The collective evidence in these case reports and case series suggests that when possible, surgical resection with curative intent should remain the primary treatment modality for FA-HNSCC. Radiation therapy has been successfully administered with acceptable toxicity in the majority of cases in the modern era. There is likely no role for platinum-based cytotoxic chemotherapy. The role of non-platinum based chemotherapy has not been explored and further investigation is needed. In lieu of platinum agents, EGFR inhibitors including cetuximab and TKIs may be both safe and effective. Immunotherapy may also be considered if the patient has not undergone SCT. The suggested FA-HNSCC treatment pathway is summarized in Figure 2.
Figure 2.
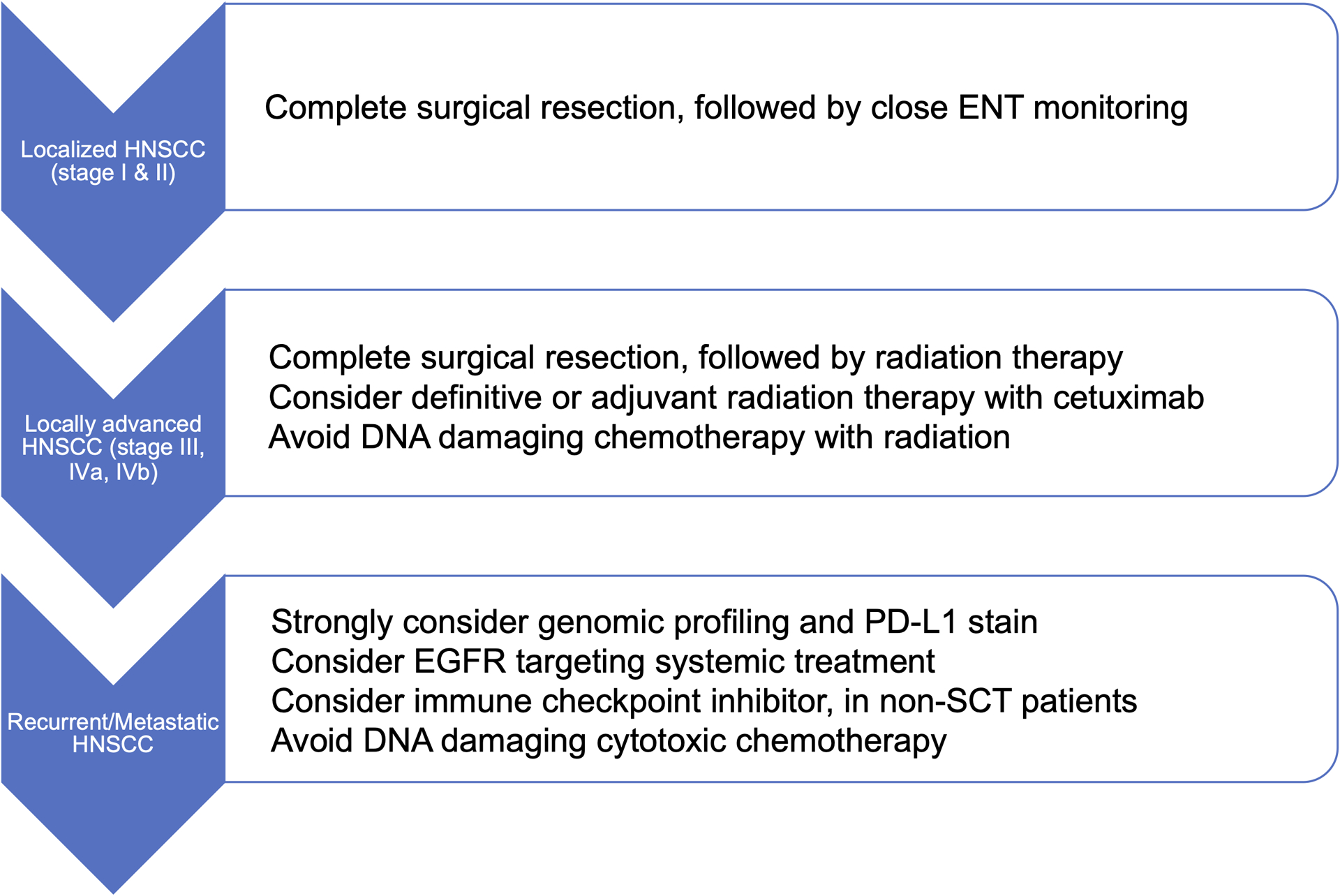
Suggested FA-HNSCC Treatment Pathway.
Finally, it is crucial to reiterate the importance of physician awareness in detecting and treating FA-HNSCC. The variability (or absence) of physical anomalies in FA patients requires a high degree of suspicion for all teenagers or young patients presenting with head and neck cancers without established risk factors. Conversely, known FA carriers should undergo frequent dedicated screening examinations for cancer. The recognition of FA-HNSCC as a distinct entity will further allow individual institutions to compile and share treatment strategies and outcomes with the entire medical community. With global collaboration, we can begin to construct an accessible database complete with safety and efficacy data, which would provide an invaluable resource for guiding thoughtful, evidence-based therapies for future FA patients with HNSCC.
Acknowledgments:
This work was supported by National Institutes of Health grants R01 DE023685 (JRG and DEJ), R35 CA231998 (JRG), R01 DE028289 (DEJ and JRG), R01 CA204127 (AS), and V Foundation translational grant T2019-013 (AS). AS is HHMI Faculty Scholar.
Competing interests:
HK received compensation as a consultant from MitoImmune and PIN Therapeutics and honorarium for serving on scientific advisory boards from Bayer and Achilles Therapeutics. HK receives research grants to support clinical trials from Kura Oncology, Exeliris, Eli Lilly, Elevar therapeutics, Ayala pharmaceuticals, and PDS Biotechnology.
SSY receives research grant support from Genentech, Bristol-Myers Squibb, Merck, and BioMimetix, and personal fees from Springer and UpToDate. Rocket Pharmaceuticals provided research funding and partial salary support to AS for an unrelated project. JRG and DEJ are co-inventors of cyclic STAT3 decoy and have financial interests in STAT3 Therapeutics, Inc. STAT3 Therapeutics, Inc. holds an interest in the cyclic STAT3 decoy oligonucleotide.
REFERENCES
- 1.Kutler DI et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch. Otolaryngol. - Head Neck Surg 129, 106–112 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Alter BP Inherited bone marrow failure syndromes: considerations pre- and posttransplant. Blood 130, 2257–2264 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deans AJ & West SC DNA interstrand crosslink repair and cancer. Nature Reviews Cancer 11, 467–480 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kutler DI et al. Natural history and management of Fanconi anemia patients with head and neck cancer: A 10-year follow-up. Laryngoscope 126, 870–879 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Rockefeller University » Fanconi Anemia Mutation Database. Available at: http://www2.rockefeller.edu/fanconi/. (Accessed: 26th March 2020)
- 6.Knipscheer P et al. The fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science (80-.) 326, 1698–1701 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlett NG et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science (80-.) 297, 606–609 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, Kee Y, Gurtan A & D’Andrea AD Cell cycle-dependent chromatin loading of the Fanconi anemia core complex by FANCM/FAAP24. Blood 111, 5215–5222 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gari K, Décaillet C, Delannoy M, Wu L & Constantinou A Remodeling of DNA replication structures by the branch point translocase FANCM. Proc. Natl. Acad. Sci. U. S. A 105, 16107–16112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shakeel S et al. Structure of the Fanconi anaemia monoubiquitin ligase complex. Nature 575, 234–237 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajendra E et al. The Genetic and Biochemical Basis of FANCD2 Monoubiquitination. Mol. Cell 54, 858–869 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swuec P et al. The FA Core Complex Contains a Homo-dimeric Catalytic Module for the Symmetric Mono-ubiquitination of FANCI-FANCD2. Cell Rep. 18, 611–623 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budzowska M, Graham TG, Sobeck A, Waga S & Walter JC Regulation of the Rev1–pol ζ complex during bypass of a DNA interstrand cross-link. EMBO J. 34, 1971–1985 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long DT, Räschle M, Joukov V & Walter JC Mechanism of RAD51-dependent DNA interstrand cross-link repair. Science (80-.) 333, 84–87 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michl J, Zimmer J & Tarsounas M Interplay between Fanconi anemia and homologous recombination pathways in genome integrity. EMBO J. 35, 909–923 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogliolo M & Surrallés J Fanconi anemia: A model disease for studies on human genetics and advanced therapeutics. Current Opinion in Genetics and Development 33, 32–40 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Dokal I & Vulliamy T Inherited bone marrow failure syndromes. Haematologica 95, 1236–1240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zierhut HA, Tryon R & Sanborn EM Genetic Counseling for Fanconi Anemia: Crosslinking Disciplines. Journal of Genetic Counseling 23, 910–921 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Velmurugan KR, Michalak P, Kang L, Fonville NC & Garner HR Dysfunctional DNA repair pathway via defective FANCD2 gene engenders multifarious exomic and transcriptomic effects in Fanconi anemia. Mol. Genet. Genomic Med 6, 1199–1208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aymun U et al. Screening for mutations in two exons of FANCG gene in Pakistani population. Biomed. Pap 161, 158–163 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg PS, Tamary H & Alter BP How high are carrier frequencies of rare recessive syndromes? Contemporary estimates for Fanconi Anemia in the United States and Israel. Am. J. Med. Genet. Part A 155, 1877–1883 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tipping AJ et al. Molecular and genealogical evidence for a founder effect in Fanconi anemia families of the Afrikaner population of South Africa. Proc. Natl. Acad. Sci. U. S. A 98, 5734–5739 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitney MA, Jakobs P, Kaback M, Moses RE & Grompe M The Ashkenazi Jewish Fanconi anemia mutation: Incidence among patients and carrier frequency in the at-risk population. Hum. Mutat 3, 339–341 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Callén E et al. A common founder mutation in FANCA underlies the world’s highest prevalence of Fanconi anemia in Gypsy families from Spain. Blood 105, 1946–1949 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Shimamura A & Alter BP Pathophysiology and management of inherited bone marrow failure syndromes. Blood Reviews 24, 101–122 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta P & Tolar J Fanconi Anemia - NCBI GeneReviews®. GeneReviews® [Internet]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK1401/. (Accessed: 28th March 2020) [Google Scholar]
- 27.Gille JJP et al. Diagnosis of Fanconi Anemia: Mutation Analysis by Multiplex Ligation-Dependent Probe Amplification and PCR-Based Sanger Sequencing. Anemia 2012, 603253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chirnomas SD & Kupfer GM The inherited bone marrow failure syndromes. Pediatric Clinics of North America 60, 1291–1310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neveling K, Endt D, Hoehn H & Schindler D Genotype-phenotype correlations in Fanconi anemia. Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis 668, 73–91 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Fiesco-Roa MO, Giri N, McReynolds LJ, Best AF & Alter BP Genotype-phenotype associations in Fanconi anemia: A literature review. Blood Reviews 37, 100589 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faivre L et al. Association of complementation group and mutation type with clinical outcome in Fanconi anemia. Blood 96, 4064–4070 (2000). [PubMed] [Google Scholar]
- 32.Gillio AP, Verlander PC, Batish SD, Giampietro PF & Auerbach AD Phenotypic consequences of mutations in the Fanconi anemia FAC gene: An International Fanconi Anemia Registry study. Blood 90, 105–110 (1997). [PubMed] [Google Scholar]
- 33.Kutler DI et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood 101, 1249–1256 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Anur P et al. Late effects in patients with Fanconi anemia following allogeneic hematopoietic stem cell transplantation from alternative donors. Bone Marrow Transplant. 51, 938–944 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gluckman E et al. Bone marrow transplantation for Fanconi anemia. Blood 86, 2856–62 (1995). [PubMed] [Google Scholar]
- 36.Chaudhury S et al. Fludarabine-based cytoreductive regimen and T-cell-depleted grafts from alternative donors for the treatment of high-risk patients with Fanconi anaemia. Br. J. Haematol 140, 644–655 (2008). [DOI] [PubMed] [Google Scholar]
- 37.MacMillan ML et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood 125, 3798–3804 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebens CL, DeFor TE, Tryon R, Wagner JE & MacMillan ML Comparable Outcomes after HLA-Matched Sibling and Alternative Donor Hematopoietic Cell Transplantation for Children with Fanconi Anemia and Severe Aplastic Anemia. Biol. Blood Marrow Transplant 24, 765–771 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alter BP Cancer in Fanconi anemia, 1927–2001. Cancer 97, 425–440 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg PS, Greene MH & Alter BP Cancer incidence in persons with Fanconi anemia. Blood 101, 822–826 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Alter BP Fanconi anemia and the development of leukemia. Best Practice and Research: Clinical Haematology 27, 214–221 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy AW & Hart WR Multiple squamous-cell carcinomas in Fanconi’s anemia. Cancer 50, 811–4 (1982). [DOI] [PubMed] [Google Scholar]
- 43.Patel SC et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J. Clin. Oncol 29, 1488–1494 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Giampietro PF, Verlander PC, Davis JG & Auerbach AD Diagnosis of Fanconi anemia in patients without congenital malformations: An International Fanconi Anemia Registry study. Am. J. Med. Genet 68, 58–61 (1997). [PubMed] [Google Scholar]
- 45.Huck K et al. Delayed diagnosis and complications of Fanconi anaemia at advanced age - A paradigm. Br. J. Haematol 133, 188–197 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Tan IB et al. Fanconi’s anemia in adulthood: Chemoradiation-induced bone marrow failure and a novel FANCA mutation identified by targeted deep sequencing. J. Clin. Oncol 29, e591–4 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Spanier G et al. Fatal course of tonsillar squamous cell carcinoma associated with Fanconi anaemia: A mini review. J. Cranio-Maxillofacial Surg 40, 510–515 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Lach FP et al. Esophageal cancer as initial presentation of Fanconi anemia in patients with a hypomorphic FANCA variant. Cold Spring Harb. Mol. Case Stud 6, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bremer M et al. Fanconi’s Anemia and Clinical Radiosensitivity: Report on Two Adult Patients with Locally Advanced Solid Tumors Treated by Radiotherapy. Strahlentherapie und Onkol. 179, 748–753 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Argiris A, Karamouzis MV, Raben D & Ferris RL Head and neck cancer. Lancet 371, 1695–1709 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaturvedi AK et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol 31, 4550–4559 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aupérin A Epidemiology of head and neck cancers: an update. Curr. Opin. Oncol 32, 178–186 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Mahal BA et al. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol. Biomarkers Prev 28, 1660–1667 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Kutler DI et al. Human Papillomavirus DNA and p53 Polymorphisms in Squamous Cell Carcinomas From Fanconi Anemia Patients. J. Natl. Cancer Inst 95, 1718–1721 (2003). [DOI] [PubMed] [Google Scholar]
- 55.van Zeeburg HJT et al. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. J. Natl. Cancer Inst 100, 1649–53 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alter BP et al. Squamous cell carcinomas in patients with Fanconi anemia and dyskeratosis congenita: A search for human papillomavirus. Int. J. Cancer 133, 1513–1515 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi K et al. A Review of HPV-Related Head and Neck Cancer. J. Clin. Med 7, 241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furquim CP, Pivovar A, Amenábar JM, Bonfim C & Torres-Pereira CC Oral cancer in Fanconi anemia: Review of 121 cases. Critical Reviews in Oncology/Hematology 125, 35–40 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Alter BP, Giri N, Savage SA & Rosenberg PS Cancer in the national cancer institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica 103, 30–39 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alter BP & Rosenberg PS In reference to Natural history and management of fanconi anemia patients with head and neck cancer: A 10-year follow-up. Laryngoscope 126, E229–E229 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson DE et al. Head and neck squamous cell carcinoma. Nature Reviews Disease Primers 6, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg PS, Socié G, Alter BP & Gluckman E Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood 105, 67–73 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Inamoto Y et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplantation 50, 1013–1023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masserot C et al. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer 113, 3315–3322 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Beckham TH et al. Treatment modalities and outcomes of Fanconi anemia patients with head and neck squamous cell carcinoma: Series of 9 cases and review of the literature. Head Neck 41, 1418–1426 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hosoya Y et al. Successful treatment of esophageal squamous cell carcinoma in a patient with fanconi anemia. Jpn. J. Clin. Oncol 40, 805–810 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Gasparini G, Longobardi G, Boniello R, di Petrillo A & Pelo S Fanconi anemia manifesting as a squamous cell carcinoma of the hard palate: A case report. Head Face Med. 2, 1 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Öksüzoǧlu B & Yalçin Ş Squamous cell carcinoma of the tongue in a patient with Fanconi’s anemia: A case report and review of the literature. Annals of Hematology 81, 294–298 (2002). [DOI] [PubMed] [Google Scholar]
- 69.Jansisyanont P, Pazoki A & Ord RA Squamous cell carcinoma of the tongue after bone marrow transplantation in a patient with Fanconi’s anemia. J. Oral Maxillofac. Surg 58, 1454–1457 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Doerr TD, Shibuya TY & Marks SC Squamous cell carcinoma of the supraglottic larynx in a patient with Fanconi’s anemia. Otolaryngol. Neck Surg 118, 523–525 (1998). [DOI] [PubMed] [Google Scholar]
- 71.Millen FJ et al. Oral squamous cell carcinoma after allogeneic bone marrow transplantation for Fanconi anaemia. Br. J. Haematol 99, 410–414 (1997). [DOI] [PubMed] [Google Scholar]
- 72.Somers GR et al. Squamous cell carcinoma of the tongue in a child with fanconi anemia: A case report and review of the literature. Fetal Pediatr. Pathol 15, 597–607 (1995). [DOI] [PubMed] [Google Scholar]
- 73.Lustig JP, Lugassy G, Neder A & Sigler E Head and neck carcinoma in Fanconi’s anaemia-report of a case and review of the literature. Eur. J. Cancer. Part B Oral Oncol 31, 68–72 (1995). [DOI] [PubMed] [Google Scholar]
- 74.Snow DG, Campbell JB & Smallman LA Fanconi’s anaemia and post-cricoid carcinoma. J. Laryngol. Otol 105, 125–127 (1991). [DOI] [PubMed] [Google Scholar]
- 75.Bradford CR et al. Squamous Carcinoma of the Head and Neck in Organ Transplant Recipients. Laryngoscope 100, 190–194 (1990). [DOI] [PubMed] [Google Scholar]
- 76.Gendal ES, Mendelson DS, Janus CL, Schlossberg I & Vogel JM Squamous cell carcinoma of the esophagus in Fanconi’s anemia. Dysphagia 2, 178–179 (1988). [DOI] [PubMed] [Google Scholar]
- 77.Kaplan M, Sabio H, Wanebo H & Cantrell R Squamous Cell Carcinoma in the Immunosuppressed Patient: Fanconi’s Anemia. Laryngoscope 95, 771–5 (1985). [PubMed] [Google Scholar]
- 78.Reed K, Ravikumar TS, Gifford RRM & Grage TB The association of Fanconi’s anemia and squamous cell carcinoma. Cancer 52, 926–928 (1983). [DOI] [PubMed] [Google Scholar]
- 79.Sarna G et al. Multiple neoplasms in two siblings with a variant form of Fanconi’s anemia. Cancer 36, 1029–1033 (1975). [DOI] [PubMed] [Google Scholar]
- 80.Soravia C & Spiliopoulos A Epidermoid carcinoma of the esophagus and Fanconi’s anemia. Schweiz Med Wochenschr. 124, 725–728 (1994). [PubMed] [Google Scholar]
- 81.Schofield I & Worth A Malignant mucosal change in Fanconi’s anemia. J Oral Surg 38, 619–622 (1980). [PubMed] [Google Scholar]
- 82.Itskoviz D et al. Endoscopic findings and esophageal cancer incidence among Fanconi Anemia patients participating in an endoscopic surveillance program. Dig. Liver Dis 51, 242–246 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Onishi S et al. Superficial esophageal cancer in a fanconi anemia patient that was treated successfully by endoscopic submucosal resection. Intern. Med 58, 529–533 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonfim C et al. Long-term Survival, Organ Function, and Malignancy after Hematopoietic Stem Cell Transplantation for Fanconi Anemia. Biol. Blood Marrow Transplant 22, 1257–1263 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Torres-Pereira CC et al. Oral squamous cell carcinoma in two siblings with Fanconi anemia after allogeneic bone marrow transplantation. Special Care in Dentistry 34, 212–215 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Wong WM et al. Squamous cell carcinoma of the oral tongue in a patient with Fanconi anemia treated with radiotherapy and concurrent cetuximab: A case report and review of the literature. Head Neck 35, E292–8 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Kaplan KA, Reiffel AJ, Kutler DI, Rohde CH & Spector JA Sequential second free flap for head and neck reconstruction in a patient with fanconi anemia and metachronous squamous cell carcinoma. Plastic and Reconstructive Surgery 128, (2011). [DOI] [PubMed] [Google Scholar]
- 88.Chow LQM Head and neck cancer. New England Journal of Medicine 382, 60–72 (2020). [DOI] [PubMed] [Google Scholar]
- 89.D’Cruz AK et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N. Engl. J. Med 373, 521–529 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Colevas AD et al. NCCN guidelines ® insights: Head and neck cancers, version 1.2018 featured updates to the NCCN guidelines. JNCCN Journal of the National Comprehensive Cancer Network 16, 479–490 (2018). [DOI] [PubMed] [Google Scholar]
- 91.Gutiontov SI, Shin EJ, Lok B, Lee NY & Cabanillas R Intensity-modulated radiotherapy for head and neck surgeons. Head and Neck 38, E2368–E2373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barton MB, Keane TJ, Gadalla T & Maki E The effect of treatment time and treatment interruption on tumour control following radical radiotherapy of laryngeal cancer. Radiother. Oncol 23, 137–143 (1992). [DOI] [PubMed] [Google Scholar]
- 93.Alden ME et al. Elapsed radiation therapy treatment time as a predictor of survival in patients with advanced head and neck cancer who receive chemotherapy and radiation therapy. Radiology 201, 675–680 (1996). [DOI] [PubMed] [Google Scholar]
- 94.Bhide SA, Newbold KL, Harrington KJ & Nutting CM Clinical evaluation of intensity-modulated radiotherapy for head and neck cancers. Br. J. Radiol 85, 487–494 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esparza A & Thompson W Familial hypoplastic anemia with multiple congenital anomalies (Fanconi’s syndrome)--report of three cases. R. I. Med. J 49, 103–110 (1966). [PubMed] [Google Scholar]
- 96.Han TJ et al. Synchronous multifocal HPV-related neoplasm involving both the genital tract and the head-and-neck area: A case report of Fanconi anemia. Radiother. Oncol 92, 138–141 (2009). [DOI] [PubMed] [Google Scholar]
- 97.Lewis LM et al. Successful use of a therapeutic trial of graduated volume and dose escalation for postoperative head and neck radiotherapy in a Fanconi anemia patient. Head Neck (2020). doi: 10.1002/hed.26395 [DOI] [PubMed] [Google Scholar]
- 98.Bernier J et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med 350, 1945–1952 (2004). [DOI] [PubMed] [Google Scholar]
- 99.Cooper JS et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med 350, 1937–1944 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Nolan M, Courtney R, Sexton P, Barry T & McCann P Aggressive Recurrence of Oral Squamous Cell Carcinoma in a patient with Fanconi’s Anaemia (FA). Ir. Med. J 110, 533 (2017). [PubMed] [Google Scholar]
- 101.Budrukkar A et al. Squamous cell carcinoma of base of tongue in a patient with Fanconi’s anemia treated with radiation therapy: Case report and review of literature. Head Neck 32, 1422–1427 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Marcou Y, D’Andrea A, Jeggo PA & Plowman PN Normal cellular radiosensitivity in an adult Fanconi anaemia patient with marked clinical radiosensitivity. Radiother. Oncol 60, 75–79 (2001). [DOI] [PubMed] [Google Scholar]
- 103.Mehanna H et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 393, 51–60 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gillison ML et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 393, 40–50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koo WH, Knight LA & Ang PT Fanconi’s anaemia and recurrent squamous cell carcinoma of the oral cavity: a case report. Ann Acad Med Singapore 25, 289–292 (1996). [PubMed] [Google Scholar]
- 106.Anak S et al. Squamous cell carcinoma development in Fanconi anemia patients who underwent hematopoietic stem cell transplantation. Pediatr. Transplant 24, (2020). [DOI] [PubMed] [Google Scholar]
- 107.Jung HS et al. Gefitinib Trial in a Fanconi’s Anemia Patient with Multiple Squamous Cell Carcinomas and Hepatocellular Carcinoma. Cancer Res. Treat 37, 370 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bauml JM, Aggarwal C & Cohen RB Immunotherapy for head and neck cancer: where are we now and where are we going? Ann. Transl. Med 7, S75–S75 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Economopoulou P, Perisanidis C, Giotakis EI & Psyrri A The emerging role of immunotherapy in head and neck squamous cell carcinoma (HNSCC): Anti-tumor immunity and clinical applications. Annals of Translational Medicine 4, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ijaz A et al. Significant Risk of Graft-versus-Host Disease with Exposure to Checkpoint Inhibitors before and after Allogeneic Transplantation. Biol. Blood Marrow Transplant 25, 94–99 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Montanuy H et al. Gefitinib and Afatinib Show Potential Efficacy for Fanconi Anemia-Related Head and Neck Cancer. Clin. Cancer Res 26, 3044–3057 (2020). [DOI] [PubMed] [Google Scholar]
- 112.Soulieres D et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J. Clin. Oncol 22, 77–85 (2004). [DOI] [PubMed] [Google Scholar]
- 113.Stewart JSW et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J. Clin. Oncol 27, 1864–1871 (2009). [DOI] [PubMed] [Google Scholar]
- 114.Machiels JPH et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 16, 583–594 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Bever KM & Le DT DNA repair defects and implications for immunotherapy. Journal of Clinical Investigation 128, 4236–4242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Samstein RM et al. Mutations in BRCA1 and BRCA2 differentially affect the tumor microenvironment and response to checkpoint blockade immunotherapy. Nat. Cancer 1, 1188–1203 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chao JW, Cohen BD, Rohde CH, Kutler DI & Spector JA Free fibular flap reconstruction of the mandible in a patient with fanconi anemia. Plastic and Reconstructive Surgery 125, (2010). [DOI] [PubMed] [Google Scholar]
- 118.Kozhevnikov V & Khodorenko S Cancer of the mucous membrane of the left side of the mouth associated with congenital hypoplastic Fanconi’s anemia in a 14-year-old boy. Vestn. Khir. Im. I. I. Grek 136, 105–106 (1986). [PubMed] [Google Scholar]
- 119.E Horta HDLE, Guimarães FF, Rocha LOS, Guimarães RES & Valadares ER Squamous cell carcinoma of the hypopharynx in a young woman with Fanconi’s anemia. Brazilian Journal of Otorhinolaryngology 72, 845–848 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kozarek RA & Sanowski RA Carcinoma of the esophagus associated with fanconi’s anemia. J. Clin. Gastroenterol 3, 171–174 (1981). [DOI] [PubMed] [Google Scholar]
- 121.Vaitiekaitis AS & Grau WH Squamous cell carcinoma of the mandible in Fanconi anemia: report of case. J. Oral Surg. (Chic) 38, 372–373 (1980). [PubMed] [Google Scholar]
- 122.Tipples K & Raouf S Treatment of Oesophageal Squamous Cell Carcinoma in a Patient with Fanconi Anaemia. Clinical Oncology 20, 383–384 (2008). [DOI] [PubMed] [Google Scholar]
- 123.Salum FG et al. Squamous cell carcinoma of the tongue after bone marrow transplantation in a patient with fanconi anemia. Braz. Dent. J 17, 161–165 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Murayama S, Manzo RP, Kirkpatrick DV & Robinson AE Squamous cell carcinoma of the tongue associated with fanconi’s anemia: MR characteristics. Pediatr. Radiol 20, 347 (1990). [DOI] [PubMed] [Google Scholar]
- 125.Fukuoka K et al. Fanconi’s anemia with squamous cell carcinoma--a case report and a review of literature. Rinsho Ketsueki 30, 1992–1996 (1989). [PubMed] [Google Scholar]


