Short telomere syndromes (STS) are the most common premature aging disorders; mutations in telomerase and other telomere maintenance genes underlie their etiology(1). Their biology is defined by short telomere length which provokes senescence and apoptosis, leading to organ failure. The majority of STS are autosomal dominant, but X-linked, de novo and recessive forms exist. Mutations in fourteen genes are identifiable in 70–80% of STS, with mutant telomerase reverse transcriptase, TERT, accounting for nearly half the cases(1). End-stage liver disease is the third most common degenerative complication after idiopathic pulmonary fibrosis (IPF) and aplastic anemia(2). While aplastic anemia generally manifests in children and young adults, and IPF is adult-onset, liver disease presents in intermediate age groups. PF on the other hand manifests in the vast majority of cases after the fourth decade(1). Liver disease has two primary manifestations in STS: 1) non-cirrhotic portal hypertension with hepatopulmonary syndrome (NCPH-HPS), usually diagnosed in children/younger adults and often in association with nodular regenerative hyperplasia (NRH) (2). HPS is the predominant manifestation in this subset of patients while other complications of portal hypertension are rare(2); 2) Cryptogenic cirrhosis (with or without HPS) in older adults preceding or co-occurring with IPF(3). The optimal management of liver disease, especially in the presence of these syndromic comorbidities, remains unclear.
Recent success of reduced intensity hematopoietic stem cell transplantation (HSCT) regimens for aplastic anemia has uncovered the natural history of STS with nearly all HSCT survivors developing HPS. However, the role of liver transplantation (LT) in these patients remains unknown, especially given the pulmonary vulnerability and potential for exacerbation of parenchymal pulmonary fibrosis as well as increased relative risk of squamous cancers and myelodysplastic syndromes(4). For patients requiring LT, finding suitable donors remain a challenge, given the low Model for End-Stage Liver Disease (MELD) score at initial presentation. Patients with HPS are eligible for MELD exception points to improve access to transplantation; however, this is often in later stages of disease, risking the sequelae of prolonged hypoxemia. To ensure timely LT for these patients, Public Health Service (PHS)-designated “increased risk” donors can be considered to increase the pool. Herein, we present our experience with STS patients who underwent LT for NCPH-HPS following HSCT. The liver allografts came from HCVAb+ donors (5). LT completely eliminated the patients’ supplemental oxygen dependence.
CASE-1
Twenty-three-year-old female with the classic STS dyskeratosis congenita. She presented with progressive dyspnea, recurrent syncope with cyanosis and other stigmata of HPS(2). She had severe aplastic anemia requiring non-myeloablative allogeneic HSCT at age 19, and two primary stage-1 tongue squamous cell carcinoma diagnosed at 22. Telomere length at diagnosis was below the first percentile, but she had no identifiable pathogenic mutations (Figure1A). A brother also had aplastic anemia. At presentation, the patient was on 10L of oxygen and the carbon monoxide diffusion capacity (DLCO) was 12% of predicted. She had shunting on agitated saline echocardiogram and transjugular liver biopsy showed no fibrosis. At the time of LT evaluation, she had complete donor T cell engraftment (>95%) and she was CMV IgG−. High resolution chest CT showed mild reticular changes consistent with early PF but spirometry showed only a mild restrictive defect with FEV1 and FVC of 76% and 79%, respectively. Nuclear lung perfusion/ventilation study using Tc-99m MAA IV and a single-breath Xenon-133 inhalation estimated 34% shunting.
Figure 1. Telomere length, timeline of transplants relative to patient’s history and explant pathology.
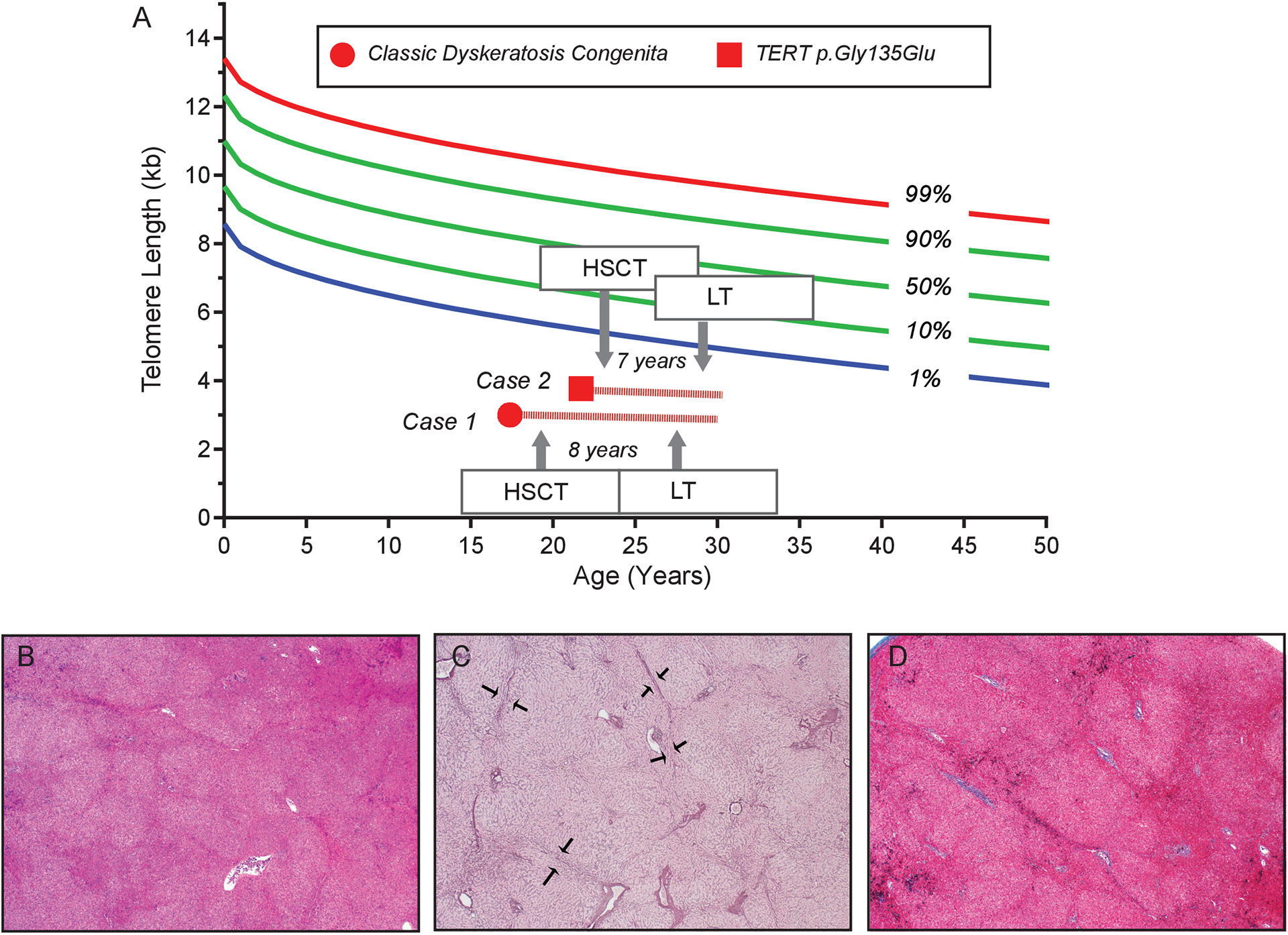
A. Telogram shows lymphocyte telomere length from patients 1 and 2 as measured by flow cytometry and fluorescence in situ hybridization (flowFISH). In both patients, it falls below the first age-adjusted percentile at diagnosis. The granulocyte telomere length, which is not shown, was also concordant falling in a similar respective range. The timeline shown schematizes the timing of hematopoietic stem cell transplantation (HSCT) relative to liver transplantation (LT) with the number of years intervening and the followup. B. Representative liver explant hematoxylyn and eosin stained section demonstrating nodularity in the hepatic parenchyma (x20). C. Reticulin stain highlights compression of hepatocytes at the edges of nodules (arrows) (x20). D. Masson trichrome stain reveals no significant fibrosis (x20).
The patient met MELD exception point criteria (score 29) when her room air PaO2 decreased to 55 mmHg and she was allocated a PHS increased risk, HCVNAT+, CMVIgG+, deceased donor liver transplant (DDLT) four years after initial evaluation. Immunosuppressive (IS) management was standard with Tacrolimus, Mycophenolate and steroid taper. She had a prolonged intensive care unit stay complicated by recurrent hypoxemia and slow recovery. She was discharged home 60 days later and weaned off of supplemental oxygen 4 months post-LT. Allograft-related HCV was treated with 12-week Glecaprevir/Pibrentasvir, achieving SVR. At 2 years post-LT, she continues to do well with stable liver allograft and lung function, and remains cancer-free.
CASE-2
Twenty-nine-year-old male with premature graying presented for LT evaluation for progressive HPS diagnosed at age 22. He had bone marrow failure and primary immunodeficiency that were treated with allogeneic non-myeloablative HSCT at age 23. He carried a pathogenic mutation in TERT, with telomere length below the first percentile at diagnosis (Figure 1A). Family history was remarkable for bone marrow failure and IPF. HSCT was complicated by ganciclovir-resistant CMV viremia. HPS was diagnosed during evaluation for dyspnea and findings of shunting on agitated saline echocardiography and a liver biopsy showing nodular regenerative hyperplasia (NRH) without cirrhosis.
Nine months prior to LT, he developed a sharp PaO2 drop after knee surgery for avascular necrosis. Nuclear lung perfusion study with Tc-99m MAA IV showed 29% shunt and DLCO was 37% of predicted. He was granted MELD exception points (score 26) after his PaO2 dropped to 56 mmHg, and he received a DDLT 18 months after initial evaluation, from a PHS increased risk HCVAb+, HCVNA−, CMVIgG+ donor. Explant confirmed NRH without cirrhosis (Figure1B–D). Peri-operatively he developed an intra-abdominal bleed which required exploratory laparotomy and a biliary anastomotic leak 6 days post-LT which was managed endoscopically. He received standard IS regimen, was weaned off of supplemental oxygen one week after LT and discharged 14 days post-LT. His serum HCV RNA remained undetectable. He has stable allograft and lung function 6 months post-LT.
DISCUSSION
To our knowledge this is the first report successful LT in STS patients following HSCT using HCV+ donors. Oxygen dependence was reversed and the concurrent pulmonary fibrosis remained stable during follow-up in both cases. Usage of PHS increased risk donors, facilitated the prioritization of LT. This early intervention may have contributed to faster recovery and the relatively benign post-operative course. The systemic nature of STS requires multidisciplinary input from pulmonology, hematology and infectious disease. Table 1 summarizes the factors we consider in the evaluation/management of STS patients seen at our center. We highlight the importance of perioperative management to minimize lung injury.
Table 1.
Considerations in liver transplant evaluation and management of short telomere syndrome patients
| Organ system involved | Considerations |
|---|---|
| Liver disease |
*Follow DLCO and clinical symptoms of hypoxemia as early signs of HPS
*Evaluate for portal hypertension/risk of gastrointestinal bleed *Note synthetic function usually retained and cirrhosis rare in young adults *Timely evaluation for PHS Increased risk or living donor transplant to reduce wait-list time |
| Pulmonary complications |
*Avoid lung biopsy in diagnostic evaluation due to risk of respiratory failure
*Minimize oxygen exposure to avoid alveolar damage *Vigilance for opportunistic infection post-operatively *Assess diffusion capacity decline relative to spirometry *Follow with pulmonary consultant familiar with STS-associated IPF |
| Infectious/Immune complications |
*Consider CMV recipient risk and prophylaxis in non-HSCT patients
*Consider reduction of IST in non-HSCT patients to reduce infectious risks *Follow with infectious disease and hematology consultants to manage risk of immune/hematologic complications |
| Musculoskeletal |
*High index of suspicion for AVN with shoulder/hip pain symptoms
*Refer to orthopedics early for definitive management |
| Malignancy |
*Risk for MDS/AML increased in adults over 50 (non-HSCT)
*Risk for squamous cell cancers especially in males with DKC1 mutations |
Abbreviations: AVN: avascular necrosis; CMV: cytomegalovirus; DKC1: dyskeratosis congenita 1 gene (X-linked); DLCO: diffusion capacity for carbon monoxide; HPS: hepatopulmonary syndrome; IPF: Idiopathic Pulmonary Fibrosis; IST: immunosuppressive therapy; MDS/AML: myelodysplastic syndrome/acute myeloid leukemia; non-HSCT: patients who have not undergone hematopoietic stem cell transplant; PHS: The Public Health Service; STS: short telomere syndrome
The biology of telomere-mediated liver disease is incompletely understood. Vascular endothelial senescence is hypothesized to play a role in the pathophysiology of NRH, which also occurs at increasing frequency with age and may represent premature aging in STS patients(2). Hepatocyte apoptosis/senescence, on the other hand, likely contributes to the fibrotic phenotype seen in older adults. The percentage of end-stage liver disease patients presenting with STS is not fully known, but the diagnosis should be suspected based on a history of bone marrow failure or IPF in the patient or family. Telomere length testing along with genetic evaluation, provide molecular evidence for the diagnosis.
The patients described had successful bone marrow and T cell engraftment pre-LT; however, in patients where NCPH-HPS is the first manifestation, there is a risk for hematologic and infectious complications with standard treatments that require management. The interval between HSCT and LT in both patients was 7–8 years consistent with an indolent presentation in this subset of post-HSCT patients. Tracking symptoms of hypoxemia and shunt physiology and/or portal hypertension, along with referral to centers familiar with STS, can facilitate early LT evaluation. Table1 lists the factors we consider in evaluating and monitoring STS patients at our center. In older adults, usually over the age of 40, NCPH-HPS may be diagnosed concurrently with PF, and in these patients dual organ transplant may be feasible in some cases(6), although these patients are potentially prone to hematologic and immune complications of STS including myelodysplastic syndrome/acute myeloid leukemia(4). Consideration of PHS increased risk organs including those from HCV infected individuals, has the potential to improve outcomes by shortening waitlist time, although these patients remain at risk for progression of their parenchymal lung disease.
Funding Statement:
MA receives funding support from NIH - RO1CA225027
Abbreviations:
- DAA
Direct-acting antiviral
- DDLT
Diseased donor liver transplant
- DLCO
Diffusing Capacity for Carbon Monoxide
- FISH
Fluorescence in situ hybridization
- HPS
Hepatopulmonary syndrome
- HSCT
Hematopoietic stem cell transplantation
- IPF
Idiopathic Pulmonary Fibrosis
- IST
Immunosuppressive Therapy
- NAT
Nucleic acid test
- NCPH
Non-cirrhotic portal hypertension
- PHS
Public Health Service
- STS
Short telomere syndromes
- SVR
Sustained viral response
- TERT
Telomerase reverse transcriptase gene
Footnotes
Conflicts of Interest:
NONE Reported
References
- 1.McNally EJ, Luncsford PJ, Armanios M. Long telomeres and cancer risk: the price of cellular immortality. J Clin Invest. 2019;130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorgy AI, Jonassaint NL, Stanley SE, Koteish A, DeZern AE, Walter JE, et al. Hepatopulmonary syndrome is a frequent cause of dyspnea in the short telomere disorders. Chest. 2015;148(4):1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schratz KE, Haley L, Danoff SK, Blackford A, DeZern A, Gocke CD, et al. Cancer spectrum and outcomes in the Mendelian short telomere syndromes. Blood. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ting PS, Hamilton JP, Gurakar A, Urrunaga NH, Ma M, Glorioso J, et al. Hepatitis C-positive donor liver transplantation for hepatitis C seronegative recipients. Transpl Infect Dis. 2019;21(6):e13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moschouri E, Vionnet J, Giostra E, Daccord C, Lazor R, Sciarra A, et al. Combined Lung and Liver Transplantation for Short Telomere Syndrome. Liver Transpl. 2020;26(6):840–4. [DOI] [PubMed] [Google Scholar]


