Abstract
Nonalcoholic fatty liver disease (NAFLD) is a multifactorial metabolic disorder that was first described in 1980. It has been prevalent and on the rise for many years and is associated with other metabolic disorders such as obesity and type 2 diabetes mellitus (T2DM). NAFLD can be best described as a metabolic dysfunction that stems from insulin resistance-induced hepatic lipogenesis. This lipogenesis increases oxidative stress and hepatic inflammation and is often potentiated by genetic and gut microbiome dysfunction. As NAFLD progresses from simple steatosis to non-alcoholic steatohepatitis (NASH) and to cirrhosis and hepatocellular carcinoma (HCC), the odds of complications including cardiovascular disease (CVD), chronic kidney disease (CKD), and overall mortality increase. The aim of this review is to describe the metabolic causes and consequences of NAFLD while examining the risks that each stage of NAFLD poses. In this review, the etiology of “lean” NAFLD, the impact of obesity, T2DM, genetics, and microbiome dysbiosis on NAFLD progression are all explored. This review will also discuss the core issue behind the progression of NAFLD: insulin resistance (IR). Upon describing the causes and consequences of NAFLD, the effectiveness of diet modification, lifestyle changes, and glucagon-like peptide 1 receptor (GLP-1) agonists to retard NAFLD progression and stem the rate of complications is examined.
1. Definition and prevalence
Fueled by overnutrition and sedentary lifestyles, obesity, T2DM, and NAFLD are all on the rise [1]. NAFLD is often understood to be the hepatic expression of metabolic syndrome and it has become clear that NAFLD is one of the most common diseases of modern humanity [2].
Until 1980, NAFLD and alcoholic fatty liver disease were indistinguishable and physicians often admonished patients who presented with hepatic steatosis for abusing alcohol despite patients indicating that they never abused alcohol [3]. In 1980, Ludwig et al. coined the term “nonalcoholic steatohepatitis (NASH)” for patients whose liver biopsies were similar to those with alcoholic liver disease, but who did not abuse alcohol and were not subject to other known causes of liver disease such as medications or bypass surgery. In this study, 20 patients with the condition were identified from a population of 535 cases between 1969 and 1979 [3].
Over the past decade, there has been debate about the suitability of NAFLD as the appropriate name for liver disease that occurs in the presence of metabolic dysfunction and in the absence of alcohol abuse. Critics say the term is misguided as it does not accurately describe the characteristics of this subtype of liver disease. Not only does the name NAFLD imply a diagnosis of exclusion, but it can also lead to stigma and trivialization as patients may erroneously assume that NAFLD is more benign because it is not directly caused by alcohol abuse [4]. Therefore, experts posit that NAFLD should be renamed “metabolic dysfunction associated-liver disease” (MAFLD). The diagnostic criteria of MAFLD have been defined as: (1) the presence of hepatic steatosis diagnosed by ultrasound AND either a (2a) diagnosis of obesity, (2b) a diagnosis of diabetes mellitus (DM), or (2c) metabolic dysfunction, which may include one or more of the following criteria: waist circumference greater than 102 cm (cm) in males and 88 cm in females, blood pressure greater than 130/85 mmHg, triglyceride content above 1.70 mmol/L, HDL-C content less than 1 mmol/L in males and less than 1.3 mmol/L in females, prediabetes, insulin resistance scores (HOMA-IR) greater or equal to 2.5, or C-reactive protein levels above 2 mg/L [5]. The criteria described as metabolic dysfunction allow MAFLD to include lean patients who do not have DM or obesity (these patients are discussed later in this review). Despite the potential merits of renaming NAFLD into MAFLD, this review will use the still commonly accepted name NAFLD.
Currently, NAFLD affects about 25% of the worldwide population with estimates ranging from 13% to 32% [2]. NAFLD can be described as the presence of excess adipose tissue accumulation in the liver, also known as hepatic steatosis, that is not caused by excessive alcohol intake. More specifically, nonalcoholic fatty liver disease is defined as at least 5% hepatic steatosis without hepatocellular injury [6]. NAFLD represents a spectrum of diseases ranging from simple steatosis to NASH to cirrhosis and hepatocellular carcinoma [7]. Nonalcoholic steatohepatitis, which is commonly abbreviated as NASH, is a more severe form of NAFLD than simple steatosis. It affects about 2–6% of all NAFLD patients and can lead to several consequences [8].
The severity of NAFLD varies among different ethnic groups. In a study of 1026 individuals with NAFLD, compared with population demographics of the USA where African-Americans (AA) make up 12% of the population, AA were underrepresented making up about 3% of the sample population. In terms of severity, NASH had a lower prevalence in AA (52%) and a higher prevalence in Hispanics (63%). About 62% of Caucasians and 52% of Asians with NAFLD exhibited NASH histology. These differences may be secondary to a different frequency of genetic variants such as a variant containing a certain allele (rs738409; I148 M) of the patatin-like phospholipase domain-containing protein 3 (PNPLA-3) gene in different ethnic groups [[9], [10], [11]]. This gene codes for a lipase protein that is involved in energy consumption and storage in adipocytes. Those with the errant allele of the PNPLA-3 gene can exhibit hepatic lipid content that is two times greater than in those without the allele [10].
2. Causes
For many years, the “two-hit hypothesis” was the most widespread model of NAFLD pathogenesis. The “first hit” is defined as lipid accumulation in the hepatocytes. This hit increases the vulnerability of the liver to many factors that constitute the “second hit” and promote hepatic injury, inflammation, and fibrosis [12].
However, the traditional “two-hit” hypothesis of NAFLD pathogenesis has been replaced by the “multiple-hit” hypothesis in order to explain the several molecular and metabolic changes of NAFLD [6]. The “multiple hit” hypothesis has provided a more accurate explanation of NAFLD pathogenesis as it includes multiple interlocking processes rather than just two hits. IR, lipotoxicity, innate immune activation, and microbiome on a background of genetics (PNPLA3) as well as diet (saturated fat and fructose) and sedentary lifestyle are the multiple factors that lead to NAFLD progression [12].
The metabolic syndrome is a constellation of cardiometabolic risk factors including increased visceral adiposity and an increase in IR causing impaired glucose tolerance and T2DM, dyslipidemia, and hypertension [13]. Previous studies have demonstrated that abnormal serum concentrations of sex hormones, thyroid hormones, and growth hormone can trigger the development of metabolic syndrome [[14], [15], [16], [17]]. NAFLD, as a hepatic manifestation of metabolic syndrome, can be associated with a number of endocrine diseases including polycystic ovary syndrome (PCOS), hypogonadism, primary hypothyroidism, and growth hormone deficiency [18,19].
Despite being closely linked with obesity, NAFLD can also manifest itself in non-obese individuals. In fact, about 10–20% of non-obese Americans may present with NAFLD [20]. “Lean” NAFLD is most commonly seen in Asian individuals in whom the majority of “lean” NAFLD studies have been carried out. Between 7 and 18% of the non-obese population in Asia (including China, Korea, and Japan) may have NAFLD [20]. In Japan, one study of 3271 individuals reported that 68.5% of obese patients and 15.2% of non-obese patients developed NAFLD [20]. While “lean” NAFLD is still not fully understood, a number of metabolic factors have been linked with this diagnosis. For example, despite exhibiting a “healthy” body weight, “lean” NAFLD patients exhibited the same pattern of IR and free fatty acid (FFA) distribution as obese individuals [20].
Interestingly, many of these individuals exhibit a lipodystrophic phenotype in which subcutaneous lipid storage is impaired and hepatic lipid storage increases, which coincides with an increase in IR [20,21]. Several genes have been shown to be involved in this type of errant metabolic profile including PPARγ, c-fos, p85α, Phosphate Cytidylyltransferase 1 Alpha, and WRN21. The metabolic development of “lean” NAFLD can be most concisely explained by the idea that increased lipolysis overwhelms the body's ability to store lipids subcutaneously and this leads to free fatty acid accumulation in visceral areas of the body, including in the liver. This errant lipid metabolism drives IR and inflammation leading to NAFLD progression and is similar to NAFLD progression in more classic obesity-driven and T2DM-driven pathogenesis [21].
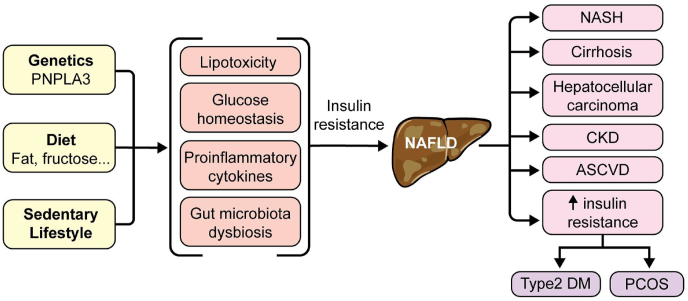
In both lean and obese individuals, one common theme of NAFLD metabolism is the prevalence of IR. In fact, among patients with T2DM, which is comorbid with IR, about 60% also exhibit NAFLD [22,23]. IR is a hallmark of T2DM and studies have shown how T2DM increases the risk of developing NAFLD [22]. Since obesity, T2DM, IR, and NAFLD are interlinked and display similar physiological developments (Fig. 1) it has been difficult to ascertain which disorder comes first or which causes another [22,23]. In support of the bi-directional relationship between T2DM and NAFLD, the odds of developing T2DM are two times higher in patients with NAFLD than in those without NAFLD [22,23]. The mechanism by which IR influences NAFLD is still being studied, but several key connections have been established. For example, adipocytokines can improve insulin sensitivity through adiponectin secretion; NAFLD seems to alter this pathway and decrease the production of adiponectin, thus enhancing IR [23].
Fig. 1.
NAFLD (non-alcoholic fatty liver disease) stems from a combination of genetics, diet, and lifestyle, which drives dysbiosis and other metabolic malfunction. The result is insulin resistance (IR) that drives NAFLD progression. As NAFLD progresses to NASH (non-alcoholic steatohepatitis) and HCC (hepatocellular carcinoma), IR progresses as well and contributes to the development of ASCVD (atherosclerotic cardiovascular disease), PCOS (polycystic ovary syndrome), T2DM (type 2 diabetes-mellitus), and CKD (chronic kidney disease).
The mechanism by which NAFLD alters adiponectin production is still being studied, but research has established that adiponectin concentrations are influenced by genetics, diet, nutrition, exercise, and abdominal adipose tissue, all of which have been implicated in NAFLD pathogenesis. Adiponectin decreases IR through several methods: it inhibits the production of inflammatory cytokines that contribute to IR such as tumor necrosis factor-alpha (TNF-α) and interleukin-18 (IL-18) [24]. IL-18 is a known mediator of hepatic cellular injury and its inhibition can prevent the destruction and dysfunction of hepatic cells. Adiponectin also possesses anti-fibrotic activity by inhibiting the synthesis of key proteins and genes involved in fibrotic tissue development. In fact, adiponectin levels have been shown to be a diagnostic indication of NAFLD and upregulating adiponectin experimentally could represent a future treatment for NAFLD, although more studies are needed to corroborate such an assertion [24].
Obesity is a significant independent risk factor for NAFLD development and progression. A study of 381,655 individuals reported that obesity increased the odds of NAFLD 3.5 fold [25]. This robust meta-analysis was controlled for confounding conditions including diabetes, hypertension, alcohol intake, and physical activity. In addition, each unit increase in BMI was positively correlated in a dose-dependent fashion to NAFLD risk [26]. Similar to claims by other authors, Li et al. ‘s reasoning for obesity-mediated NAFLD risk is based on increased IR and inflammation [26]. Obesity stimulates inflammation via TNF-α, which enhances IR [23]. Abnormal mitochondrial activity in the liver has also been shown to increase inflammation and, subsequently, enhance IR [23]. The mechanism by which increased adipose tissue in the liver produces increased inflammation and IR involves the proliferation of M1 macrophages that secrete pro-inflammatory biomarkers including IL-6 and TNF-α [22]. These biomarkers activate downstream signaling cascades, which have been linked with IR.
Visceral adiposity plays an important role in the pathogenesis of NAFLD. Adipose tissue secretes pro-inflammatory cytokines including TNF-α and IL-6. Previous studies showed that the severity of steatohepatitis and fibrosis correlates with a higher level of TNF-α. Il-6 and TNF-α contribute to IR by interfering with the activation of the insulin receptor substrates. IR causes increased lipolysis of visceral fat by reducing the glucose uptake into the muscle [[27], [28], [29], [30], [31]].
Excess dietary carbohydrates and fatty acids from adipose tissue or de novo lipogenesis in the setting of IR play an important role in the pathogenesis of NASH [32]. Excess carbohydrates are converted to fatty acids through the multi-enzyme process. Excessive accumulation of fatty acids may lead to the production of lipotoxic agents which cause endoplasmic reticulum stress, mitochondrial dysfunction, hepatocellular injury, inflammation, and apoptosis. Hepatocellular response to lipotoxic stress is regulated by the gut microbiome, cholesterol, uric acid, and possibly periodic hypoxia [33].
Oxidative stress has been suggested as the main triggering factor for the progression of steatosis to steatohepatitis and also as a prominent feature of NASH [[34], [35], [36]]. Bergichio and et al. proposed that genetic and environmental factors are potential contributors to hepatic steatosis and inflammation via the production of reactive oxygen and nitrogen species (ROS/RNS) [36].
The spleen is an important organ in the regulation of immune function and physiological inflammation. It is, therefore, worth identifying the effect, if any, that NAFLD can have on splenic function. Several studies have shown how patients with NASH exhibit increased splenic volumes and higher levels of several inflammatory biomarkers including IL-6 and hepatocyte growth factor [37]. An interesting study demonstrated that splenectomy in obese mice led to a decrease in IR and reduced growth of adipose tissue. However, in a contrasting study, splenectomy of obese mice resulted in facilitated progression of NAFLD [37]. It is thus difficult to ascertain whether the spleen serves in a protective or antagonistic capacity in regards to NAFLD progression. Nevertheless, the literature suggests that splenic volume is affected by NAFLD and these changes could be used as an ancillary method for diagnosing NAFLD [37].
Finally, gut microbiota dysbiosis may play a significant role in NAFLD. Although many studies identifying NAFLD-related gut microbiota abnormalities have been performed in mice, key human studies have shown that gut dysbiosis is apparent in NAFLD patients. A study in obese juvenile patients with NAFLD showed that Gammaproteobacteria and Prevotella were at increased concentrations compared to obese juvenile patients without NAFLD [38]. Moreover, the microbiome changes as NAFLD progresses and research shows that increases in Proteobacteria and decreases in Firmicutes can accompany NAFLD progression [38].
Additionally, patients with NASH exhibit altered concentrations of Prevotella copri and Bacteriodes vulgatus when compared with individuals without NASH [39]. Bacteriodes concentration showed a positive link with NASH severity, while Prevotella was decreased in patients with NASH [40]. As the severity of fibrotic lesions in NASH patients increased, the concentrations of Bacteriodes and Ruminococcus increased while Prevotella decreased. Furthermore, when patients were stratified into three groups by Ruminococcus concentration, it was observed that those with the highest concentration of Ruminococcus exhibited twice as much fibrosis as those in the bottom two groups [40].
Interestingly, microbiota modulation including the administration of probiotics enriched with Lactobacillus casei decreased inflammation and improved hepatic metabolism in a murine model [41]. Antibiotic treatment has also been shown to decrease bacterial overgrowth and stem NAFLD progression. However, antibiotic side effects must be carefully weighed and any usage of antibiotics to target microbiome dysfunction needs more research and clarification [38]. It is clear that gut dysbiosis may contribute to and enhance poor outcomes in NAFLD patients [26]. However, gut microbiota composition can vary among population groups and among different stages of NAFLD, making any conclusive or causational claims about gut microbiota categorization in NAFLD patients challenging [26,38]. Nevertheless, some hypotheses on the ways dysbiosis can directly affect liver functioning have been developed.
While NAFLD is independent of exogenous alcohol consumption, bacteria in the gut have been known to produce alcohol through ethanol fermentation. Alcohol is known to adversely affect hepatic function and several studies have shown how obese patients with NASH often exhibit an elevated blood-alcohol content (BAC) when compared to obese patients without NASH. This suggests that gut bacteria can exacerbate NAFLD progression by way of direct ethanol production [39]. Other mechanisms through which dysbiosis can directly impact hepatic function is through the migration of bacteria directly into the liver through enhanced small intestine permeability, which is increased in patients with NAFLD [42]. Bacteria that migrate to the liver secrete proinflammatory toxins that can trigger hepatic damage. However, it is not clear if gut permeability is a cause or effect of NAFLD-related dysbiosis.
3. Consequences
As discussed above, NAFLD represents a spectrum of diseases from simple steatosis to NASH to cirrhosis and HCC. The consequences of NAFLD are mainly driven by the severity of IR as NAFLD progresses toward NASH and HCC. The worsening hepatic pathology drives further increases in IR, creating a vicious cycle of NAFLD progression (Fig. 1).
The cellular pathways involved in the consequences of NAFLD are based on the development and sustained effect of IR leading to an increased level of free fatty acids in the blood, which in turn, leads to the development of NASH characterized by hepatic cellular dysfunction and cellular death [43]. Impaired lipid clearance via lipid autophagy also results in an increased hepatic lipid content [43,44]. Signaling pathways triggered by this excess lipid accumulation lead to the ‘unfolded protein response’--a well-characterized type of endoplasmic reticulum (ER) stress. Other types of ER stress also contribute to hepatocyte malfunction, and this malfunction is the essence of NASH physiology.
One of the main consequences of NASH is an increased likelihood of progression to cirrhosis and hepatocellular carcinoma (HCC), as NASH represents a preliminary step towards severe liver damage displayed by cirrhosis and HCC [45]. Patients who exhibit NASH-related liver fibrosis are at greater risk of death with mortality risk rising with each stage of fibrosis severity [46]. As mentioned previously, there is evidence of a bidirectional relationship between NASH and obesity: 51.3% of patients with NAFLD are obese while 81.3% of patients with NASH are obese [47]. Targeting obesity has been shown to retard NASH progression as weight loss of 7% or greater in obese patients results in a 65–90% improvement in NASH [47]. Furthermore, a 5% weight loss has been linked to a two-fold decrease in intrahepatic triglyceride content (IHTG) [48]. However, weight loss is very difficult to maintain for many patients and bodyweight fluctuation has been shown to lead to more adverse outcomes for patients with NAFLD. Kim and et al. studied 726,736 patients with NAFLD. They found that patients with NAFLD who had large body weight fluctuations were at increased risk of stroke, myocardial infarct, and all causes of mortality [49].
Diet modulation plays a significant role in the prevention of NAFLD consequences. For example, overconsumption of saturated fat and fructose leads to greater increases in hepatic triglyceride accumulation, even if the patient is not consuming more total calories [50]. Interestingly, substituting protein for excess fat intake led to a 22% decrease in hepatic lipid content [50]. Comparison of diets with high carbohydrate/low-fat content versus low carbohydrate/high-fat content has produced contrasting claims in regards to their effect on hepatic lipid accumulation, but both types of diets proved effective when total caloric intake was reduced [48,50]. Studies have shown how overconsumption of fructose and simple sugars could exacerbate NAFLD severity through several pathways including the promotion of certain transcription factors implicated in de-novo lipogenesis, promotion of maladaptive microbiome composition, and inhibition of fatty acid oxidation. Ketogenic diet studies corroborate this claim as the elimination of fructose and simple sugars retards NAFLD progression [48].
The abnormal glucose and lipid metabolism in patients with NASH further exacerbates NAFLD progression and thus targeting glucose metabolism and IR through the use of GLP-1 receptor agonists has been shown to reduce NASH severity [43]. GLP-1 receptor agonists can rescue this impaired metabolic phenotype by improving lipid transport and increasing the liver's ability to metabolize fatty acids. Most importantly, GLP-1 receptor agonists reduce IR [43]. In fact, one study showed that 39% of patients with NASH who were treated with GLP-1 receptor agonists exhibited reversal in NASH hepatic morphology [51].
Cirrhosis is also a consequence of NAFLD and there is increasing evidence that in the near future NAFLD will be the leading cause of cirrhosis [52]. Cirrhosis is categorized by fibrotic lesions in the liver and the development of scar tissue that impairs liver function. Inflammation caused by lipotoxicity leads to an increase in cellular hepatic injury, which results in impaired bilirubin metabolism--a hallmark of cirrhosis [52]. In fact, elevated concentrations of IL-6 in patients with NAFLD and cirrhosis give credence to the theory that inflammatory processes play a key role in cirrhotic progression [53]. Cirrhosis is often diagnosed subsequently to NASH and an analysis that included 18 million patients from Europe showed that there is a much higher risk of cirrhosis when a patient already has NASH compared to patients with NAFLD without NASH [54]. The same study showed that about 0.6% of patients with a known NASH diagnosis progress to cirrhosis or hepatocellular carcinoma (HCC) within three years. This makes sense as fibrosis is a necessary precursor to liver cirrhosis [53].
HCC is a consequence of NAFLD that can arise with or without cirrhosis. HCC is among the top five causes of cancer mortality in the world and NAFLD-related HCC incidence is expected to increase [[55], [56], [57]]. As HCC represents the most severe form of hepatic damage, it is no surprise that severe fibrosis scores are predictive of HCC [54]. Interestingly, the best single predictor for HCC apart from fibrosis scores is a concurrent diagnosis of diabetes. HCC is also reported as the most common cancer in T2DM. Diabetes has an important role in developing HCC in patients with NAFLD especially when associated with obesity.
Piscaglia and et al. noted that HCC secondary to NAFLD is usually detected at a later tumor stage and could arise also in the absence of cirrhosis in comparison to HCC secondary to HCV infection with a similar survival rate [58]. Some key metabolic adipokines and hepatokines have been described as being correlated with HCC. For example, low levels of adiponectin and high levels of leptin have been associated with HCC development and severity [59]. In fact, there is evidence that adiponectin can mitigate oncogenesis and HCC development [59].
Cardiovascular disease is the leading cause of morbidity and mortality in patients with NAFLD [60]. A strong association was reported between markers of atherosclerosis including coronary artery calcification, impaired vasodilation, increased carotid intima-media thickness, arterial stiffness, and NAFLD in a comprehensive meta-analysis [61]. Since NAFLD involves the dysfunctional metabolism of lipids, elevated triglycerides, elevated VLDL, and lower HDL are all common in patients with NAFLD and these metabolic factors contribute to cardiovascular disease [61].
Two recent cohort studies reported that NAFLD is independently associated with a higher incidence of heart failure (HF) due to left ventricular dysfunction, myocardial hypertrophy, change in phosphate metabolism, and IR in the myocardium [62,63]. Patients with T2DM and NAFLD have a higher risk of atrial fibrillation (AF) over 10 years of follow-up compared to patients with T2DM without NAFLD, and ultrasound-diagnosed NAFLD was associated with a fivefold increased risk of incident AF independent of several clinical AF risk factors [64].
Recently, attention has been also focused on NAFLD-related chronic kidney disease (CKD). A recent meta-analysis showed that NAFLD increases the risk of CKD two-fold. Moreover, NASH was associated with a higher incidence and prevalence of CKD compared to simple steatosis [65]. The prevalence of NAFLD-related CKD is reported to be approximately 20–55%. Importantly, the severity of NAFLD is associated with CKD stages, independently of established cardio-renal risk factors [66,67].
Previous studies noted an association between NAFLD and other chronic diseases associated with IR including colorectal cancer, osteoporosis, psoriasis, and some endocrine disorders including PCOS, hypothyroidism, and GH deficiency [68].
Selected articles addressing the metabolic consequences of NAFLD are summarized in Table 1.
Table 1.
Selected studies of metabolic consequences in patients with NAFLD.
| Type of consequences | Authors | Study population | Country | Main findings |
|---|---|---|---|---|
| Insulin resistance | Lonardo et al. [13] | A systematic review of 47 longitudinal studies which provided evidence for NAFLD as a risk factor for the future development of diabetes and metabolic syndrome | Italy | NAFLD is a strong determinant for the development of diabetes and metabolic syndrome. |
| Khan et al. [23] | A systematic review of insulin resistance in NAFLD | Multi countries | There is a close relationship between insulin resistance (IR) and NAFLD, with the prevalence of NAFLD being 5-fold higher in patients with diabetes compared to those without. | |
| Li et al. [25] | A systematic review of twenty-one cohort studies including 13 prospective studies and 8 retrospective studies with 381,655 total patients | China | Obese individuals have a 3.5-fold increased risk of developing NAFLD. | |
| Cirrhosis/HCC | Alexander et al. [54] | Cohort study of 136,703 patients with diagnosis of NAFLD or NASH (NAFLD/NASH) were followed up about incident cirrhosis and HCC diagnosis | Multi countries | NAFLD/NASH increases risk of life-threatening liver outcomes. Diabetes is an independent predictor of advanced liver disease. |
| Piscagila et al. [58] | Observational prospective study of 756 patients with either NAFLD (145) or HCV-related chronic liver disease (611) | Italy | HCC secondary to NAFLD is usually detected at a later tumor stage and could arise in the absence of cirrhosis. | |
| ASCVD | Targher et al. [60] | Observational, prospective, and retrospective studies with 34,043 total adult patients | Multi countries | NAFLD is associated with an increased risk of fatal and non-fatal CVD events. |
| Zeb et al. [69] | Prospective cohort study of 4119 adult participants who were free of CVD and known liver diseases at baseline | United States | NAFLD was independently associated with incident CHD events (HR 1.42, 95% CI 1.00 to 2.03) | |
| Mantovani et al. [70] | Retrospective cohort of 286 adults with type 1 diabetes without known liver diseases | Italy | NAFLD was independently associated with an increased risk of non-fatal CVD events (HR 6.73, 95% CI 1.2 to 38 | |
| Targher et al. [64] | Prospectively cohort of 400 patients with type 2 diabetes, who were free from Atrial fibrillation at baseline | Italy | NAFLD is strongly associated with an increased incidence of AF in patients with type 2 diabetes. | |
| CKD | Musso et al. [65] | Meta-analysis of thirty-three studies (63,902 participants, 16 population-based, 17 hospital-based, 20 cross-sectional, and 13 longitudinal) | Multi countries | NAFLD was associated with an increased risk of prevalent (odds ratio [OR] 2.12, 95% CI 1.69–2.66) and incident (hazard ratio [HR] 1.79, 95% CI 1.65–1.95) CKD. The presence and severity of NAFLD are associated with an increased risk and severity of CKD. |
| Yasui et al. [66] | A cross-sectional study of 174 patients | Japan | CKD was present in 24 (14%) of 174 NAFLD patients. The prevalence of CKD was significantly higher in NASH patients (19 of 92; 21%) than non-NASH patients (5 of 82; 6%). | |
| Machado et al. [67] | Prospective and consecutive recruitment of 148 morbidly obese patients undergoing bariatric surgey | Portugal | NASH patients were older, with higher body mass index and had more frequent metabolic syndrome and lower eGFR ((97 ± 22 vs 106 ± 16 ml/min/1.73 (2), p = 0.035) | |
| All-cause and liver-related mortality | Kim et al. [49] | Cohort of 726,736 individuals with NAFLD who underwent a health examination | Korea | Body weight variability was associated with increased risks of MI, stroke, and all-cause mortality in NAFLD patients. |
| Matteoni et al. [7] | Retrospective cohort of 132 patients with NAFLD and elevated serum liver enzymes | United States | Patients with NASH had higher rates of all-cause and liver-related mortality than those without. CVD death rate did not differ between the groups. | |
| Polyzos et al. [47] | A systematic review of obesity and nonalcoholic fatty liver disease | Multi countries | Obesity increases liver-specific mortality in NAFLD patients. |
4. Conclusion
From its initial classification in 1980 until now, our understanding of NAFLD has grown and evolved. Over the years, the literature has shown that obesity, T2DM, and other lifestyle factors are influential in the development and progression of NAFLD. Factors such as gut microbiota and gene identification have given us a better understanding of NAFLD pathophysiology and causes. Based on studies in lean patients, we have learned that IR is the main physiologic finding across all types of NAFLD patients. Because of NAFLD consequences, which include NASH, CVD, cirrhosis, and HCC, targeting weight loss and IR is important in order to mitigate the progression of NAFLD and improve patient health. GLP-1 receptor agonists have shown promise in reducing IR and improving NAFLD severity, but lifestyle modifications remain the mainstay of NAFLD therapy due to NAFLD's extensive linkage with other metabolic disorders such as obesity and T2DM. The complex nature of the disease requires a multidisciplinary approach by a healthcare team including a hepatologist, endocrinologist, and cardiologist for proper primary and secondary NAFLD diagnosis, treatment, and prevention of its causes and consequences.
CRediT authorship contribution statement
Paria Zarghamravanbakhsh: Conceptualization, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing. Michael Frenkel: Conceptualization, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing. Leonid Poretsky: Conceptualization, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Supervision, Project administration.
References
- 1.Poretsky L. Looking beyond overnutrition for causes of epidemic metabolic disease. Proc Natl Acad Sci Unit States Am. 2012;109(39):15537–15538. doi: 10.1073/pnas.1213503109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi Z., Anstee Q.M., Marietti M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig J., Viggiano T.R., McGill D.B., Oh B.J. Nonalcoholic steatohepatitis: mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–438. [PubMed] [Google Scholar]
- 4.Fouad Y., Waked I., Bollipo S., Gomaa A., Ajlouni Y., Attia D. What's in a name? Renaming “NAFLD” to “MAFLD. Liver Int. Off. J. Int. Assoc. 2020;40(6):1254–1261. doi: 10.1111/liv.14478. [DOI] [PubMed] [Google Scholar]
- 5.Lin S., Huang J., Wang M., et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. Off. J. Int. Assoc. 2020;40(9):2082–2089. doi: 10.1111/liv.14548. [DOI] [PubMed] [Google Scholar]
- 6.Maurice J., Manousou P. Non-alcoholic fatty liver disease. Clin. Med. Lond. Engl. 2018;18(3):245–250. doi: 10.7861/clinmedicine.18-3-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 8.Roeb E., Geier A. Nonalcoholic steatohepatitis (NASH) - current treatment recommendations and future developments. Z Gastroenterol. 2019;57(4):508–517. doi: 10.1055/a-0784-8827. [DOI] [PubMed] [Google Scholar]
- 9.Bambha K., Belt P., Abraham M., et al. Ethnicity and nonalcoholic fatty liver disease. Hepatol. Baltim. Md. 2012;55(3):769–780. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeo S., Kozlitina J., Xing C., et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browning J.D., Szczepaniak L.S., Dobbins R., et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatol. Baltim. Md. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 12.Paschos P., Paletas K. Non alcoholic fatty liver disease two-hit process: multifactorial character of the second hit. Hippokratia. 2009;13(2):128. [PMC free article] [PubMed] [Google Scholar]
- 13.Lonardo A., Ballestri S., Marchesini G., Angulo P., Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Stud. Liver. 2015;47(3):181–190. doi: 10.1016/j.dld.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Iwen K.A., Oelkrug R., Kalscheuer H., Brabant G. Metabolic syndrome in thyroid disease. Front Horm Res. 2018;49:48–66. doi: 10.1159/000485996. [DOI] [PubMed] [Google Scholar]
- 15.Rastrelli G., Filippi S., Sforza A., Maggi M., Corona G. Metabolic syndrome in male hypogonadism. Front Horm Res. 2018;49:131–155. doi: 10.1159/000485999. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer A.A., Quinton R. The metabolic syndrome in central hypogonadotrophic hypogonadism. Front Horm Res. 2018;49:156–169. doi: 10.1159/000485998. [DOI] [PubMed] [Google Scholar]
- 17.Pasquali R. Metabolic syndrome in polycystic ovary syndrome. Front Horm Res. 2018;49:114–130. doi: 10.1159/000485995. [DOI] [PubMed] [Google Scholar]
- 18.Lonardo A., Carani C., Carulli N., Loria P. “Endocrine NAFLD” a hormonocentric perspective of nonalcoholic fatty liver disease pathogenesis. J Hepatol. 2006;44(6):1196–1207. doi: 10.1016/j.jhep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Loria P., Carulli L., Bertolotti M., Lonardo A. Endocrine and liver interaction: the role of endocrine pathways in NASH. Nat Rev Gastroenterol Hepatol. 2009;6(4):236–247. doi: 10.1038/nrgastro.2009.33. [DOI] [PubMed] [Google Scholar]
- 20.Younes R., Bugianesi E. NASH in lean individuals. Semin Liver Dis. 2019;39(1):86–95. doi: 10.1055/s-0038-1677517. [DOI] [PubMed] [Google Scholar]
- 21.Ficarella R., Laviola L., Giorgino F. Lipodystrophic diabetes mellitus: a lesson for other forms of diabetes? Curr Diabetes Rep. 2015;15(3):12. doi: 10.1007/s11892-015-0578-5. [DOI] [PubMed] [Google Scholar]
- 22.Tanase D.M., Gosav E.M., Costea C.F., et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD) J. Diabetes Res. 2020:3920196. doi: 10.1155/2020/3920196. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan R.S., Bril F., Cusi K., Newsome P.N. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology. 2019 Aug;70(2):711–724. doi: 10.1002/hep.30429. Epub 2019 Jul 19. PMID: 30556145. [DOI] [PubMed] [Google Scholar]
- 24.Polyzos S.A., Toulis K.A., Goulis D.G., Zavos C., Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2011;60(3):313–326. doi: 10.1016/j.metabol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Li L., Liu D.-W., Yan H.-Y., Wang Z.-Y., Zhao S.-H., Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes. Rev. Off. J. Int. Assoc. Stud. Obes. 2016;17(6):510–519. doi: 10.1111/obr.12407. [DOI] [PubMed] [Google Scholar]
- 26.Safari Z., Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD) Cell. Mol. Life Sci. CMLS. 2019;76(8):1541–1558. doi: 10.1007/s00018-019-03011-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsuki A., Sumida Y., Murashima S., et al. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(3):859–862. doi: 10.1210/jcem.83.3.4618. [DOI] [PubMed] [Google Scholar]
- 28.Giby V.G., Ajith T.A. Role of adipokines and peroxisome proliferator-activated receptors in nonalcoholic fatty liver disease. World J Hepatol. 2014;6(8):570–579. doi: 10.4254/wjh.v6.i8.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hui J.M., Hodge A., Farrell G.C., Kench J.G., Kriketos A., George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatol. Baltim. Md. 2004;40(1):46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 30.Ueki K., Kondo T., Tseng Y.-H., Kahn C.R. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci U S A. 2004;101(28):10422–10427. doi: 10.1073/pnas.0402511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotamisligil G.S., Peraldi P., Budavari A., Ellis R., White M.F., Spiegelman B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271(5249):665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 32.Li Z., Clark J., Diehl A.M. The liver in obesity and type 2 diabetes mellitus. Clin Liver Dis. 2002;6(4):867–877. doi: 10.1016/s1089-3261(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 33.Neuschwander-Tetri B.A. Non-alcoholic fatty liver disease. BMC Med. 2017;15(1):45. doi: 10.1186/s12916-017-0806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albano E., Mottaran E., Occhino G., Reale E., Vidali M. Review article: role of oxidative stress in the progression of non-alcoholic steatosis. Aliment Pharmacol Ther. 2005;22(Suppl 2):71–73. doi: 10.1111/j.1365-2036.2005.02601.x. [DOI] [PubMed] [Google Scholar]
- 35.Seki S., Kitada T., Yamada T., Sakaguchi H., Nakatani K., Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37(1):56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 36.Begriche K., Igoudjil A., Pessayre D., Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6(1):1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Tarantino G., Citro V., Balsano C. Liver-spleen axis in nonalcoholic fatty liver disease. Expet Rev Gastroenterol Hepatol. 2021;15(7):759–769. doi: 10.1080/17474124.2021.1914587. [DOI] [PubMed] [Google Scholar]
- 38.Kolodziejczyk A.A., Zheng D., Shibolet O., Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11(2) doi: 10.15252/emmm.201809302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu L., Baker S.S., Gill C., et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatol. Baltim. Md. 2013;57(2):601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 40.Boursier J., Mueller O., Barret M., et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatol. Baltim. Md. 2016;63(3):764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okubo H., Sakoda H., Kushiyama A., et al. Lactobacillus casei strain Shirota protects against nonalcoholic steatohepatitis development in a rodent model. Am J Physiol Gastrointest Liver Physiol. 2013;305(12):G911–G918. doi: 10.1152/ajpgi.00225.2013. [DOI] [PubMed] [Google Scholar]
- 42.Miele L., Valenza V., La Torre G., et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatol. Baltim. Md. 2009;49(6):1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 43.Marra F., Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68(2):280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Singh R., Kaushik S., Wang Y., et al. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierantonelli I., Svegliati-Baroni G. Nonalcoholic fatty liver disease: basic pathogenetic mechanisms in the progression from NAFLD to NASH. Transplantation. 2019;103(1):e1–e13. doi: 10.1097/TP.0000000000002480. [DOI] [PubMed] [Google Scholar]
- 46.Kanwal F., Shubrook J.H., Younossi Z., et al. Preparing for the NASH epidemic: a call to action. Obes. Silver. Spring . Md. 2021;29(9):1401–1412. doi: 10.1002/oby.23250. [DOI] [PubMed] [Google Scholar]
- 47.Polyzos S.A., Kountouras J., Mantzoros C.S. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Sandby K., Geiker N.R.W., Dalamaga M., Grønbæk H., Magkos F. Efficacy of dietary manipulations for depleting intrahepatic triglyceride content: implications for the management of non-alcoholic fatty liver disease. Curr. Obes. Rep. 2021;10(2):125–133. doi: 10.1007/s13679-021-00430-4. [DOI] [PubMed] [Google Scholar]
- 49.Kim M.N., Han K., Yoo J., et al. Body weight variability and the risk of cardiovascular outcomes in patients with nonalcoholic fatty liver disease. Sci Rep. 2021;11(1):9154. doi: 10.1038/s41598-021-88733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hydes T., Alam U., Cuthbertson D.J. The impact of macronutrient intake on non-alcoholic fatty liver disease (NAFLD): too much fat, too much carbohydrate, or just too many calories? Front. Nutr. 2021;8:640557. doi: 10.3389/fnut.2021.640557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armstrong M.J., Hull D., Guo K., et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2016;64(2):399–408. doi: 10.1016/j.jhep.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith A., Baumgartner K., Bositis C. Cirrhosis: diagnosis and management. Am Fam Physician. 2019;100(12):759–770. [PubMed] [Google Scholar]
- 53.Zhou W.-C., Zhang Q.-B., Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20(23):7312–7324. doi: 10.3748/wjg.v20.i23.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander M., Loomis A.K., van der Lei J., et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17(1):95. doi: 10.1186/s12916-019-1321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. Ca - Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 56.Davila J.A., Morgan R.O., Shaib Y., McGlynn K.A., El-Serag H.B. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54(4):533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baffy G., Brunt E.M., Caldwell S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56(6):1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 58.Piscaglia F., Svegliati-Baroni G., Barchetti A., et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatol. Baltim. Md. 2016;63(3):827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 59.Kim H., Lee D.S., An T.H., et al. Metabolic spectrum of liver failure in type 2 diabetes and obesity: from NAFLD to NASH to HCC. Int J Mol Sci. 2021;22(9):4495. doi: 10.3390/ijms22094495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Oni E.T., Agatston A.S., Blaha M.J., et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230(2):258–267. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 62.Dhingra R., Gona P., Wang T.J., Fox C.S., D'Agostino R.B., Vasan R.S. Serum gamma-glutamyl transferase and risk of heart failure in the community. Arterioscler Thromb Vasc Biol. 2010;30(9):1855–1860. doi: 10.1161/ATVBAHA.110.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wannamethee S.G., Whincup P.H., Shaper A.G., Lennon L., Sattar N. Γ-glutamyltransferase, hepatic enzymes, and risk of incident heart failure in older men. Arterioscler Thromb Vasc Biol. 2012;32(3):830–835. doi: 10.1161/ATVBAHA.111.240457. [DOI] [PubMed] [Google Scholar]
- 64.Targher G., Valbusa F., Bonapace S., et al. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Musso G., Gambino R., Tabibian J.H., et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7) doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yasui K., Sumida Y., Mori Y., et al. Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism. 2011;60(5):735–739. doi: 10.1016/j.metabol.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 67.Machado M.V., Gonçalves S., Carepa F., Coutinho J., Costa A., Cortez-Pinto H. Impaired renal function in morbid obese patients with nonalcoholic fatty liver disease. Liver Int. Off. J. Int. Assoc. 2012;32(2):241–248. doi: 10.1111/j.1478-3231.2011.02623.x. [DOI] [PubMed] [Google Scholar]
- 68.Musso G., Cassader M., Olivetti C., Rosina F., Carbone G., Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Stud. Obes. 2013;14(5):417–431. doi: 10.1111/obr.12020. [DOI] [PubMed] [Google Scholar]
- 69.Zeb I., Li D., Budoff M.J., et al. Nonalcoholic fatty liver disease and incident cardiac events: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2016;67(16):1965–1966. doi: 10.1016/j.jacc.2016.01.070. [DOI] [PubMed] [Google Scholar]
- 70.Mantovani A., Mingolla L., Rigolon R., et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular disease in adult patients with type 1 diabetes. Int J Cardiol. 2016;225:387–391. doi: 10.1016/j.ijcard.2016.10.040. [DOI] [PubMed] [Google Scholar]