Abstract
Background
Alterations in the hematological profile have been linked to disease activity in rheumatoid arthritis (RA). We aimed to evaluate the levels of hematological parameters in different phases of rheumatoid arthritis (RA) and determine whether hematological parameters could be used to predict RA remission.
Materials and methods
The medical records of 365 RA patients were reviewed. Multivariate logistic regression analysis was used to compare hematological parameters among RA patients who were categorized into 4 groups according to disease activity: disease remission or low, moderate or high disease activity. Receiver operating characteristic curves were used to determine the predictive performances of significant parameters for RA remission.
Results
Complete data were obtained from 325 patients. The 4 groups of patients had different levels of hemoglobin (Hb), red blood cell, white blood cell, and platelet values. In multivariate analysis, Hb level, neutrophil-to-lymphocyte ratio (NLR), and mean platelet volume (MPV) were independent factors associated with disease activity. The combination of these 3 parameters yielded a sensitivity of 95.2% (95% confidence interval [CI] 88.7–98.2), specificity of 23.6% (95% CI 18.3–29.9), positive predictive value of 37.3% (95% CI 31.6–43.4), and negative predictive value of 91.2% (95% CI 80.0–96.7) in predicting disease remission.
Conclusion
Hb level, NLR and MPV were independently associated with RA disease activity. The high sensitivity and negative predictive value of the model consisting of Hb level, NLR and MPV may serve as a simple and inexpensive tool to identify patients who are less likely to have disease remission.
Keywords: Disease activity, Hematological parameters, Remission, Rheumatoid arthritis
Highlights
-
•
Patients with high disease activity had a significantly shorter duration of RA.
-
•
Hematological parameters are associated with RA disease activity.
-
•
A combined hematological parameter-based model could predict RA remission.
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease that primarily affects the synovium, resulting in progressive joint damage and subsequent anatomical deformity [1,2]. A treatment goal in RA is to control inflammation by suppressing disease activity, with the aim of achieving disease remission or at least low disease activity [3]. In practice, patient with RA would be evaluated for disease activity at regular intervals. Several clinical tools are currently used to monitor disease activity, such as the Disease Activity Score, the 28-joint count Disease Activity Score (DAS28), and the Simplified Disease Activity Index [4].
Emerging evidence indicates that hematological parameters, in addition to white blood cells (WBCs), including hemoglobin (Hb) and platelets, are associated with chronic inflammation [[5], [6], [7]]. Some authors have found lower Hb levels [8] and mean platelet volumes (MPVs) [9,10] but higher neutrophil-to-lymphocyte ratios (NLRs) [10,11] and platelet counts [9] in RA patients with high disease activity than in those with disease remission. One prior study also reported high NLR as a predictor of RA relapse [11]. However, such a study was limited by its small sample size and showed only a modest predictive performance [11].
Considering that the complete blood count (CBC) is a simple and inexpensive laboratory test, using parameters of CBC might be a useful adjunctive tool in assessing RA disease activity. To date, no studies have investigated whether multiple hematological parameters could be incorporated into a prediction model for identifying patients with RA remission. The objective of this study was to evaluate the levels of hematological parameters, including Hb, red blood cell (RBC), WBC, and platelet components, among RA patients with different disease activity levels. The performance of combined hematological parameters in predicting the remission phase of RA was also determined.
2. Materials and methods
2.1. Study design
This study was undertaken after approval from the Vajira Institutional Review Board (certificate of approval no. 062/2563). A retrospective chart review was performed of RA patients who attended our rheumatology outpatient clinic between September 2014 and February 2020. The inclusion criteria were patients aged ≥18 years who had available data on CBC and DAS28. Both tests must be performed in the institutional laboratory and within 1–2 weeks. The exclusion criteria were patients who had other autoimmune diseases, including systemic lupus erythematosus, vasculitis, scleroderma or seronegative spondyloarthropathy. Patients with hematologic conditions, such as thalassemia or idiopathic thrombocytopenia, or signs or symptoms of infection were also excluded.
2.2. Patient care
At our institution, the diagnosis of RA was made using the 2010 American College of Rheumatology criteria [12]. Medication therapy was determined and prescribed by an attending rheumatologist according to the disease activity. The therapy may be a single agent of steroids, disease-modifying antirheumatic drugs (DMARDs) or combined drugs. RA disease activity, as assessed by DAS28, was monitored at regular intervals of 3 months. Other laboratory tests, including CBC, were also monitored regularly to determine patients’ general health status and as surveillance for any extra-articular manifestations of RA.
2.3. Data collection and study outcome
The data collected included age, sex, duration of RA, positivity or negativity of anti-cyclic citrullinated peptide antibody and rheumatoid factor, extra-articular symptoms, number of DMARDs used, DAS28, and CBC.
The primary outcome was hematological parameters involving Hb level, red blood cell distribution width, WBC count and differential, NLR, platelet count, platelet-to-lymphocyte ratio and MPV. All CBC specimens were measured within a week prior to or after the measurement of DAS28 using an automated hematology analyzer (model: UniCel DxH 800; Beckman Coulter, Inc., Brea, CA, USA). Erythrocyte sedimentation rate (ESR) determination was assessed by the conventional Westergren method in which blood is mixed with sodium citrate as an anticoagulant. The laboratory personnel calibrated the machine three times daily for quality control.
DAS28 was calculated using the formula that included tender joint count, swollen joint count and ESR [4]. The DAS28 scores were classified into 4 groups according to RA disease activity: disease remission (DAS28 < 2.6), low disease activity (2.6≤ DAS28 < 3.2), moderate disease activity (3.2≤ DAS28 ≤ 5.1), and high disease activity (DAS28 > 5.1) [4].
2.4. Statistical analysis
The data were analyzed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corporation, Armonk, NY, USA). Categorical variables are presented as numbers with percentages, and continuous variables are reported as the median and interquartile range (IQR) due to their non-normal distributions. The data were compared using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Hematological parameters associated with disease activity were analyzed by multivariate logistic regression analysis to determine the odds ratios and 95% confidence intervals. Collinearity among significant variables was assessed using variance inflation factor [13]. The receiver operating characteristic (ROC) curves of significant hematological parameters were plotted and evaluated for the area under the ROC curves (AUCs) to find the optimal cutoff level of each parameter. Combinations of significant hematological parameters (using their optimal cutoff levels) were subsequently performed to evaluate for the AUCs. The predictive performances (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV] with their associated 95% confidence intervals) among single and combined hematological parameters at their optimal cutoff levels to identify RA remission were thus compared. A value of p < 0.05 was considered statistically significant.
The study has been reported in accordance with the STARD (Standards for the Reporting of Diagnostic Accuracy studies) criteria.
3. Results
3.1. Characteristics of the study population
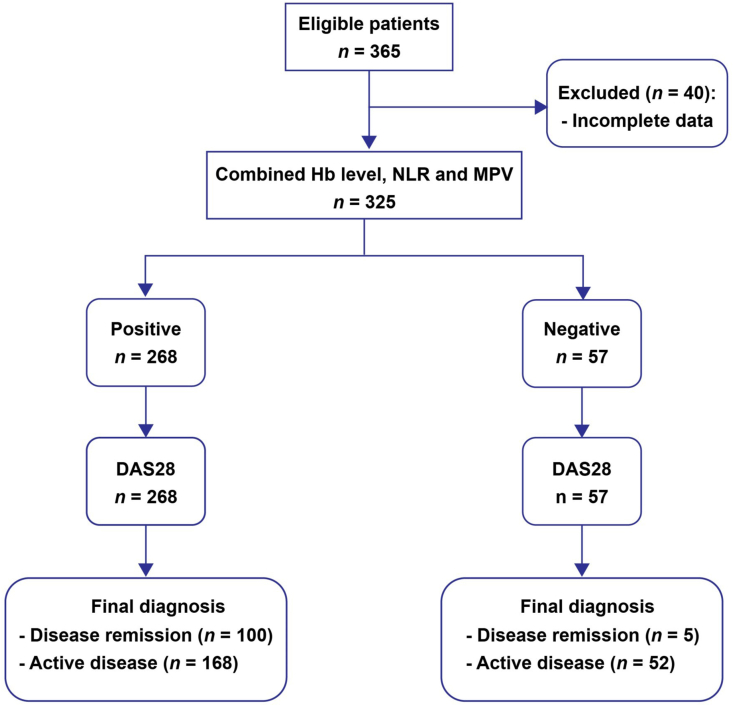
A total of 365 RA patients attended our rheumatology clinic during the study period. Of these patients, 40 were excluded due to incomplete data (Fig. 1). Among 325 patients recruited into the study, 287 (88.3%) were female, and 38 (11.7%) were male. The median age was 55 years (IQR 47–62 years). The median duration of RA was 4 years (IQR 2–8 years).
Fig. 1.
STARD (Standards for the Reporting of Diagnostic Accuracy studies) flow diagram for the evaluation of combined Hb level, NLR and MPV on the prediction of rheumatoid arthritis remission. Hb, hemoglobin; MPV, mean platelet volume; NLR, neutrophil-to-lymphocyte ratio.
At the time of the study, moderate disease activity was the most frequent (37.2%, n = 121), followed by disease remission (32.3%, n = 105), low disease activity (20.9%, n = 68), and high disease activity (9.6%, n = 31). Table 1 presents the baseline characteristics of the patients according to their disease activity. Patients with high disease activity had a significantly shorter duration of RA than patients with low to moderate disease activity or disease remission. On the other hand, the number of DMARDs used was significantly lower in patients with disease remission than in the other patients. Other characteristics were not significantly different among all groups of patients.
Table 1.
Characteristics of the study population stratified by rheumatoid arthritis disease activity (n = 325).
| Characteristic | Total | Patients with disease remission | Patients with low disease activity | Patients with moderate disease activity | Patients with high disease activity | P value |
|---|---|---|---|---|---|---|
| Number of patients | 325 | 105 | 68 | 121 | 31 | |
| Age in years | 55 (47–62) | 54 (47–61) | 57 (46–65) | 55 (47–63) | 56 (47–61) | 0.567a |
| Sex | 0.533b | |||||
| Female | 287 (88.3) | 90 (85.7) | 61 (89.7) | 110 (90.9) | 26 (83.9) | |
| Male | 38 (11.7) | 15 (14.3) | 7 (10.3) | 11 (9.1) | 5 (16.1) | |
| Duration of RA diagnosis in years | 4 (2–8) | 5 (3–9) | 4 (2–6) | 4 (2–9) | 2 (1–6) | 0.001a |
| Anti-CCP antibody | 0.588b | |||||
| Positive | 204 (62.8) | 65 (61.9) | 39 (57.4) | 78 (64.5) | 22 (71.0) | |
| Negative | 121 (37.2) | 40 (38.1) | 29 (42.6) | 43 (35.5) | 9 (29.0) | |
| Rheumatoid factor | 0.300b | |||||
| Positive | 138 (42.5) | 44 (41.9) | 26 (38.2) | 50 (41.3) | 18 (58.1) | |
| Negative | 187 (57.5) | 61 (58.1) | 42 (61.8) | 71 (58.7) | 13 (41.9) | |
| Extra-articular symptoms | 0.826b | |||||
| Yes | 159 (48.9) | 48 (45.7) | 36 (52.9) | 60 (49.6) | 15 (48.4) | |
| No | 166 (51.1) | 57 (54.3) | 32 (47.1) | 61 (50.4) | 16 (51.6) | |
| Number of DMARDs used | 2 (2–3) | 2 (2–3) | 3 (1–3) | 3 (2–4) | 3 (2–3) | 0.008a |
| DAS28 score | 3.1 (2.4–4.1) | 2.3 (2.0–2.4) | 3.0 (2.8–3.1) | 3.9 (3.6–4.3) | 5.6 (5.3–6.3) | <0.001a |
Data are expressed as the median (IQR) or n (%).
CCP, cyclic citrullinated peptide; DAS28, 28-joint count Disease Activity Score; DMARDs, disease-modifying antirheumatic drugs; IQR, interquartile range; RA, rheumatoid arthritis.
Kruskal-Wallis test.
Chi-square test.
3.2. Hematological parameters of the study population
Table 2 shows the hematological parameters of the 4 groups of patients. Hb level, red blood cell distribution width, WBC and platelet counts, neutrophil and lymphocyte percentages, NLR, platelet-to-lymphocyte ratio, and MPV were significantly different among the 4 groups of patients.
Table 2.
Hematological parameters of the 4 groups of patients according to their rheumatoid arthritis disease activity.
| Characteristic | Total | Patients with disease remission | Patients with low disease activity | Patients with moderate disease activity | Patients with high disease activity | P valuea |
|---|---|---|---|---|---|---|
| Number of patients | 325 | 105 | 68 | 121 | 31 | |
| Hb (g/dL) | 11.9 (11.0–12.7) | 12.4 (11.5–13.1) | 12.1 (11.4–12.6) | 11.5 (10.7–12.2) | 10.8 (9.8–11.8) | <0.001 |
| RDW (%) | 14.9 (14.1–16.5) | 14.8 (14.0–16.2) | 14.6 (13.8–15.3) | 15.2 (14.3–16.6) | 16.5 (14.5–17.3) | 0.001 |
| WBC count (cells/μL) | 7400 (5700–9095) | 6800 (5500–8450) | 6900 (5625–8600) | 7900 (5850–9400) | 8500 (6400–10100) | 0.013 |
| WBC differential (%) | ||||||
| Neutrophil | 61.1 (53.9–68.4) | 58.3 (51.2–64.0) | 60.2 (52.2–68.0) | 63.4 (55.9–70.1) | 66.7 (60.2–73.8) | <0.001 |
| Lymphocyte | 28.0 (20.8–33.6) | 31.2 (24.3–36.0) | 28.9 (22.1–34.9) | 27.0 (20.1–33.0) | 23.0 (18.0–30.4) | 0.002 |
| Eosinophil | 2.0 (1.0–3.2) | 2.0 (1.3–3.4) | 2.0 (0.9–3.4) | 1.6 (0.9–3.5) | 1.9 (0.7–2.7) | 0.221 |
| Monocyte | 7.3 (5.9–9.2) | 7.9 (6.1–9.7) | 7.3 (5.7–9.2) | 7.2 (6.0–8.6) | 7.3 (5.4–9.0) | 0.189 |
| Basophil | 0.5 (0.2–0.7) | 0.5 (0.2–0.8) | 0.4 (0.2–0.6) | 0.5 (0.3–0.7) | 0.4 (0.1–0.6) | 0.131 |
| NLR | 2.2 (1.6–3.2) | 1.9 (1.4–2.6) | 1.5 (2.1–3.1) | 2.3 (1.7–3.5) | 3.0 (1.9–4.0) | 0.001 |
| Platelet count (103/μL) | 286 (241–332) | 268 (227–306) | 269 (229–305) | 311 (249–360) | 339 (280–429) | <0.001 |
| PLR | 10.8 (7.4–15.0) | 9.2 (6.4–12.5) | 10.2 (7.4–13.4) | 11.4 (8.2–16.7) | 15.6 (10.9–19.6) | <0.001 |
| MPV (fL) | 8.0 (7.3–8.8) | 8.3 (7.6–9.2) | 8.0 (7.2–8.6) | 8.0 (7.4–8.6) | 7.5 (7.1–8.6) | 0.022 |
Data are expressed as the median (IQR).
Hb, hemoglobin; IQR, interquartile range; MPV, mean platelet volume; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; RDW, red blood cell distribution width; WBC, white blood cell.
Kruskal-Wallis test.
The significant hematological parameters in Table 2 were entered into a multivariate logistic regression model. The analysis showed that Hb level, NLR and MPV retained significance as independent factors associated with disease activity (Table 3). Both Hb level and MPV had an inverse relationship, whereas NLR had a positive relationship with disease activity. The variance inflation factors of all independent variables in the regression model were approximately 1, indicating a negligible effect of collinearity.
Table 3.
Multivariate analysis to determine significant hematological parameters associated with rheumatoid arthritis remissiona.
| Parameter | Adjusted ORb (95% CI) | P valuec |
|---|---|---|
| Hb (g/dL) | 0.56 (0.44–0.71) | <0.001 |
| RDW (%) | 0.98 (0.89–1.08) | 0.730 |
| WBC count (cells/μL) | 0.99 (0.87–1.13) | 0.894 |
| Neutrophil (%) | 1.03 (0.94–1.12) | 0.552 |
| Lymphocyte (%) | 1.06 (0.98–1.14) | 0.185 |
| NLR | 1.24 (1.03–1.48) | 0.021 |
| Platelet count (103/μL) | 1.01 (1.00–1.02) | 0.071 |
| PLR | 0.90 (0.75–1.08) | 0.254 |
| MPV (fL) | 0.72 (0.57–0.90) | 0.005 |
CI, confidence interval; Hb, hemoglobin; MPV, mean platelet volume; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PLR, platelet-to-lymphocyte ratio; RDW, red blood cell distribution width; WBC, white blood cell.
Level of measurement was defined as yes/no.
Adjusted for the other variables in the table.
P < 0.05 was considered statistically significant.
3.3. Predictive performances of hematological parameters for RA remission
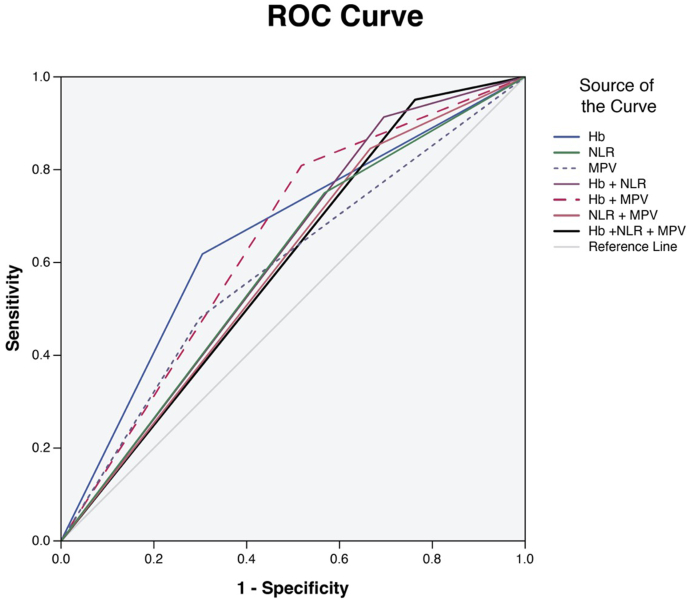
ROC curves were constructed to determine the optimal cutoff points that divided the patients into 2 groups of disease remission or active disease. The optimal cutoff values for Hb were ≥12.2 g/dL for women and ≥12.4 g/dL for men (AUC of 0.657 for both sexes). The cutoff value for NLR was ≤2.6 (AUC of 0.592), and the cutoff value for MPV was ≥8.4 fL (AUC of 0.591).
We further made various combinations of hematological parameters and evaluated whether the combined parameters would yield better predictive performances for disease remission than a single parameter. Fig. 2 depicts the ROC curves of these parameters. The single parameter of Hb level produced the best AUC, but the sensitivity was only 61.9%. However, when it was combined with NLR and MPV, the sensitivity of a positive test (either Hb level ≥12.2 g/dL in women or ≥12.4 g/dL in men, NLR ≤2.6, or MPV ≥8.4 fL) increased up to 95.2% along with a high NPV of 91.2% (Fig. 1). Details of the predictive performances of various combinations of hematological parameters for RA remission are presented in Table 4.
Fig. 2.
Receiver operating characteristic curves of single and combined hematological parameters in predicting rheumatoid arthritis remission.
Table 4.
Predictive performances of single versus combined hematological parameters in identifying patients with rheumatoid arthritis remission.
| Hematological parameter |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
AUC |
|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Hb level ≥12.2 g/dL for women and ≥12.4 g/dL for men | 61.9 (51.9–71.1) | 69.5 (62.9–75.5) | 49.2 (40.5–58.0) | 79.3 (72.7–84.6) | 0.657 (0.593–0.722) |
| NLR ≤2.6 | 75.2 (65.7–82.9) | 43.2 (36.6–50.0) | 38.7 (32.1–45.8) | 78.5 (69.9–85.2) | 0.592 (0.527–0.657) |
| MPV ≥8.4 fL | 48.6 (38.8–58.5) | 69.5 (62.9–75.5) | 43.2 (34.2–52.7) | 73.9 (67.3–79.6) | 0.591 (0.524–0.658) |
| Combination of Hb level and NLRa | 91.4 (83.9–95.8) | 30.5 (24.5–37.1) | 38.6 (32.5–44.9) | 88.2 (78.2–94.1) | 0.609 (0.547–0.672) |
| Combination of Hb level and MPVb | 81.0 (71.9–87.7) | 48.2 (41.4–55.0) | 42.7 (35.8–49.9) | 84.1 (76.3–89.8) | 0.646 (0.584–0.708) |
| Combination of NLR and MPVc | 84.8 (76.1–90.8) | 33.2 (27.1–39.9) | 37.7 (31.6–44.3) | 82.0 (72.1–89.1) | 0.590 (0.526–0.654) |
| Combination of Hb level, NLR and MPVd | 95.2 (88.7–98.2) | 23.6 (18.3–29.9) | 37.3 (31.6–43.4) | 91.2 (80.0–96.7) | 0.594 (0.532–0.657) |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; Hb, hemoglobin; MPV, mean platelet volume; NLR, neutrophil-to-lymphocyte ratio; NPV, negative predictive value; PPV, positive predictive value.
Either Hb level ≥ cutoff point (≥12.2 g/dL for women and ≥12.4 g/dL for men) or NLR ≤2.6.
Either Hb level ≥ cutoff point (≥12.2 g/dL for women and ≥12.4 g/dL for men) or MPV ≥8.4 fL.
Either NLR ≤2.6 or MPV ≥8.4 fL.
Hb level ≥ cutoff point (≥12.2 g/dL for women and ≥12.4 g/dL for men) or NLR ≤2.6 or MPV ≥8.4 fL.
4. Discussion
The findings of this study indicated that Hb level, NLR and MPV were independent factors for disease activity in RA. Our results were consistent with the findings of previous studies, which reported a significantly lower Hb level but higher NLR in patients with high disease activity than in those with low disease activity or disease remission [8,10,11,14,15]. With respect to MPV, there were conflicting results between our study and previous studies [[8], [9], [10]]. The study of Talukdar et al. [8] found a significantly higher MPV in the high disease activity group. In contrast, our study and the studies of Işık et al. [9] and Tekeoğlu et al. [10] observed a significantly higher MPV in patients with disease remission. The different findings among studies might be due to the differences in sample size and the dissimilar phases of disease activity of the populations included. The patients included in the study of Talukdar et al. [8] were limited to only those with certain disease activity levels (low to high phases), whereas our study and the studies of Işık et al. and Tekeoğlu et al. included a wider spectrum of disease activity, ranging from remission to highly active disease.
The mechanisms by which RA-related chronic inflammation may enhance alterations in the levels of various hematological parameters involve the effects of proinflammatory cytokines, including TNF-α, IL-1, IL-6 and IFN-γ [16]. In vivo and in vitro studies have shown that these cytokines could suppress erythropoietin production [17,18], interfere with iron homeostasis [19], and reduce erythroid responsiveness and red cell survival [20,21], leading to a decrease in Hb levels. The chronic inflammatory state in RA also upregulates the level of IL-6, which subsequently increases neutrophil efflux from the bone marrow to the circulating pools [22,23], resulting in an increase in NLR. Furthermore, the upregulation of IL-6 can stimulate the release and migration of large platelets to the inflammatory site [24], where they become activated and worn out [25]. A decline in MPV in patients with ongoing inflammation is therefore explained by the pathways of activation and consumption of large platelets at the site of inflammation.
To date, only one study has reported the predictive performance of a single hematological parameter, specifically NLR, for RA relapse [11]. Chandrashekara et al. [11], who performed ROC analysis using data from 88 Indian patients, found that NLR yielded an AUC of only 0.584. In their study, the optimal cutoff value of NLR was >2.9, which provided a sensitivity of only 30.4% and specificity of 80.0% for predicting active RA. In line with the findings of Chandrashekara et al., our results demonstrated that at an optimal NLR cutoff value of ≤2.6, it yielded a sensitivity of 75.2%, a specificity of 43.2%, and an AUC of 0.592 for predicting RA remission, which indicated a modest predictive performance. Our study, which explored various hematological parameters, found that compared to NLR, Hb level yielded a better predictive performance, as assessed by the AUC value. Nevertheless, its AUC remained modest at 0.657. At the optimal cutoff points (≥12.2 g/dL for women and ≥12.4 g/dL for men), Hb level yielded a high false-negative rate (low sensitivity of 61.9%) and a high false-negative rate (low specificity of 69.5%). Hence, these results suggest that the single Hb parameter is inappropriate for both ruling in patients with disease remission and ruling out those without remission.
In this study, incorporating multiple hematological parameters, including Hb level, NLR and MPV, into a prediction model yielded an AUC of 0.594, which was less than that obtained from the single Hb parameter. However, the combination of these three parameters produced a much better sensitivity of 95.2% and a high NPV of 91.2%. From a statistical viewpoint, a negative result in a test that yields higher sensitivity and NPV signifies a higher probability of ruling out patients with the event. Thus, these combined hematological parameters of low Hb (<12.2 g/dL for women and <12.4 g/dL for men), NLR >2.6, and MPV <8.4 fL) would be useful for identifying patients who are less likely to have disease remission or, in other words, are more likely to have active disease or RA relapse.
Currently, CBC is commonly performed on an automated hematology analyzer as part of a medical assessment in daily rheumatology practice. Using the CBC to detect patients at risk for active RA is thus more feasible with a lower cost than the measurement of C-reactive protein and with more ease than the measurement of ESR, which requires a longer time to perform the test. The data of this study may enable a rheumatologist to apply this basic blood test to RA patients. Under any circumstance when an individual has persistently low Hb levels and MPV values but high NLR, the doses of drug treatment can be maintained or possibly increased. In the event that an individual with a normal Hb level and/or normal NLR and/or normal MPV turns out to have a low Hb level and MPV along with a high NLR, he or she should be counseled about the probability of acquiring active disease or RA relapse, be fully evaluated for the development of active disease, and be prepared for further treatment.
This is the first study to develop a combined hematological parameter-based model for predicting RA remission. The strength of this study was that it included a large number of patients. In addition, all analyses were performed using the same automated hematology analyzer with regular machine calibration. Hence, the test results were accurate and reliable. Furthermore, the optimal cutoff point for Hb level was defined separately for men and women due to the sex difference in Hb levels. This is therefore more proper to apply than using one cutoff point for both sexes.
Given the retrospective design of this study, some data might have been missing, such as anti-cyclic citrullinated peptide antibody, rheumatoid factor, DAS28, and CBC data. Furthermore, diagnostic accuracy of this multiple hematological parameter-based model might be limited in patients with any other medical conditions that affect levels of Hb, NLR and MPV. Additionally, this hematological model has not yet been validated in other ethnic/population groups. Thus, further validation studies are crucial to establish a clinical implication.
Based on our study results, it may be concluded that parameters of the CBC might be used as an adjunctive tool in the monitoring of disease activity in RA.
5. Conclusion
The levels of hematological parameters, including Hb level, NLR and MPV, were independently associated with RA disease activity. The combination of Hb level, NLR and MPV may serve as a simple and inexpensive tool to predict the disease activity.
Ethics approval
This study was approved by the Research Ethics Committee of the Faculty of Medicine Vajira Hospital, Navamindradhiraj University (certificate of approval no. 062/2563).
Sources of funding
This research was supported by the Navamindradhiraj University Research Fund.
Author contribution
PD: study design, data collection, analysis and interpretation, and manuscript writing, proofreading and validation; and RP: manuscript proofreading and validation. All authors have critically revised the manuscript and approved the final version.
Guarantor
Pornchai Dechanuwong.
Consent
Not applicable.
Declaration of competing interest
The authors declared that there is no conflict of interest.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Registration of research studies
-
1.
Name of the registry: Thai Clinical Trials Registry
-
2.
Unique Identifying number or registration ID: TCTR20210923003
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.thaiclinicaltrials.org/page_user/#
Acknowledgments
The author thanks Drs. Chadakarn Phaloprakarn and Siriwan Tangjitgamol for their scientific advice and manuscript preparation.
Acronyms and abbreviations
- AUC
Area under the receiver operating characteristic curve
- CBC
Complete blood count
- DAS28
28-joint count Disease Activity Score
- DMARD
Disease-modifying antirheumatic drug
- ESR
Erythrocyte sedimentation rate
- Hb
Hemoglobin
- IQR
Interquartile range
- MPV
Mean platelet volume
- NLR
Neutrophil-to-lymphocyte ratio
- RA
Rheumatoid arthritis
- ROC
Receiver operating characteristic
- SPSS
Statistical Package for the Social Sciences
- WBC
White blood cell
References
- 1.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.Tipsing W., Dechanuwong P., Manavanchai S. Prevalence of atlanto-axial subluxation in patients with rheumatoid arthritis. J. Med. Assoc. Thai. 2019;102(9):70–75. [Google Scholar]
- 3.Smolen J.S., Landewé R.B.M., Bijlsma J.W.J., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020;79(6):685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 4.Anderson J.K., Zimmerman L., Caplan L., Michaud K. Measures of rheumatoid arthritis disease activity: patient (PtGA) and provider (PrGA) global assessment of disease activity, disease activity score (DAS) and disease activity score with 28-joint counts (DAS28), simplified disease activity index (SDAI), clinical disease activity index (CDAI), patient activity score (PAS) and patient activity score-II (PASII), routine assessment of patient index data (RAPID), rheumatoid arthritis disease activity index (RADAI) and rheumatoid arthritis disease activity index-5 (RADAI-5), chronic arthritis systemic index (CASI), patient-based disease activity score with ESR (PDAS1) and patient-based disease activity score without ESR (PDAS2), and mean overall index for rheumatoid arthritis (MOI-RA) Arthritis Care Res. 2011;63(Suppl 112011) doi: 10.1002/acr.20621. S14–S36. [DOI] [PubMed] [Google Scholar]
- 5.Ganz T. Anemia of inflammation. N. Engl. J. Med. 2019;381(12):1148–1157. doi: 10.1056/NEJMra1804281. [DOI] [PubMed] [Google Scholar]
- 6.Kounis N.G., Soufras G.D., Tsigkas G., Hahalis G. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin. Appl. Thromb. Hemost. 2015;21(2):139–143. doi: 10.1177/1076029614531449. [DOI] [PubMed] [Google Scholar]
- 7.Stokes K.Y., Granger D.N. Platelets: a critical link between inflammation and microvascular dysfunction. J. Physiol. 2012;590(5):1023–1034. doi: 10.1113/jphysiol.2011.225417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talukdar M., Barui G., Adhikari A., Karmakar R., Ghosh U.C., Das T.K. A study on association between common haematological parameters and disease activity in rheumatoid arthritis. J. Clin. Diagn. Res. 2017;11(1) doi: 10.7860/JCDR/2017/23524.9130. EC01–EC04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Işık M., Şahin H., Hüseyin E. New platelet indices as inflammatory parameters for patients with rheumatoid arthritis. Eur. J. Rheumatol. 2014;1(4):144–146. doi: 10.5152/eurjrheumatol.2014.140023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tekeoğlu İ., Gürol G., Harman H., Karakeçe E., Çiftçi İ.H. Overlooked hematological markers of disease activity in rheumatoid arthritis. Int. J. Rheum. Dis. 2016;19(11):1078–1082. doi: 10.1111/1756-185X.12805. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekara S., Rajendran A., Bai Jaganath A., Krishnamurthy R. Neutrophil-lymphocyte ratio, pain perception, and disease activity score may serve as important predictive markers for sustained remission in rheumatoid arthritis. Reumatismo. 2015;67(3):109–115. doi: 10.4081/reumatismo.2015.838. [DOI] [PubMed] [Google Scholar]
- 12.Aletaha D., Neogi T., Silman A.J., et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. 2010. [DOI] [PubMed] [Google Scholar]
- 13.Vatcheva K.P., Lee M., McCormick J.B., Rahbar M.H. Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiology. 2016;6(2):227. doi: 10.4172/2161-1165.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furst D.E., Chang H., Greenberg J.D., et al. Prevalence of low hemoglobin levels and associations with other disease parameters in rheumatoid arthritis patients: evidence from the CORRONA registry. Clin. Exp. Rheumatol. 2009;27(4):560–566. [PubMed] [Google Scholar]
- 15.Chen Y.F., Xu S.Q., Xu Y.C., et al. Inflammatory anemia may be an indicator for predicting disease activity and structural damage in Chinese patients with rheumatoid arthritis. Clin. Rheumatol. 2020;39(6):1737–1745. doi: 10.1007/s10067-019-04873-y. [DOI] [PubMed] [Google Scholar]
- 16.McInnes I.B., Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 17.Vannucchi A.M., Grossi A., Rafanelli D., Statello M., Cinotti S., Rossi-Ferrini P. Inhibition of erythropoietin production in vitro by human interferon gamma. Br. J. Haematol. 1994;87(1):18–23. doi: 10.1111/j.1365-2141.1994.tb04864.x. [DOI] [PubMed] [Google Scholar]
- 18.Frede S., Fandrey J., Pagel H., Hellwig T., Jelkmann W. Erythropoietin gene expression is suppressed after lipopolysaccharide or interleukin-1 beta injections in rats. Am. J. Physiol. 1997;273(3 Pt 2):R1067–R1071. doi: 10.1152/ajpregu.1997.273.3.R1067. [DOI] [PubMed] [Google Scholar]
- 19.Ludwiczek S., Aigner E., Theurl I., Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101(10):4148–4154. doi: 10.1182/blood-2002-08-2459. [DOI] [PubMed] [Google Scholar]
- 20.Macdougall I.C., Cooper A.C. Erythropoietin resistance: the role of inflammation and pro-inflammatory cytokines. Nephrol. Dial. Transplant. 2002;17(Suppl 11):39–43. doi: 10.1093/ndt/17.suppl_11.39. [DOI] [PubMed] [Google Scholar]
- 21.Libregts S.F., Gutiérrez L., de Bruin A.M., et al. Chronic IFN-γ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118(9):2578–2588. doi: 10.1182/blood-2010-10-315218. [DOI] [PubMed] [Google Scholar]
- 22.Wright H.L., Cross A.L., Edwards S.W., Moots R.J. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology. 2014;53(7):1321–1331. doi: 10.1093/rheumatology/keu035. [DOI] [PubMed] [Google Scholar]
- 23.Suwa T., Hogg J.C., English D., Van Eeden S.F. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am. J. Physiol. Heart Circ. Physiol. 2000;279(6):H2954–H2960. doi: 10.1152/ajpheart.2000.279.6.H2954. [DOI] [PubMed] [Google Scholar]
- 24.Senchenkova E.Y., Komoto S., Russell J., et al. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am. J. Pathol. 2013;183(1):173–181. doi: 10.1016/j.ajpath.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamath S., Blann A.D., Lip G.Y. Platelet activation: assessment and quantification. Eur. Heart J. 2001;22(17):1561–1571. doi: 10.1053/euhj.2000.2515. [DOI] [PubMed] [Google Scholar]