Abstract
Background
White blood cells (WBC) are commonly measured to investigate suspected infection and inflammation in pregnant women, but the pregnancy-specific reference interval is variably reported, increasing diagnostic uncertainty in this high-risk population. It is essential that clinicians can interpret WBC results in the context of normal pregnant physiology, given the huge global burden of infection on maternal mortality.
Methods
We performed a longitudinal, repeated measures population study of 24,318 pregnant women in Oxford, UK, to map the trajectory of WBC between 8-40 weeks of gestation. We defined 95% reference intervals (RI) for total WBC, neutrophils, lymphocytes, eosinophils, basophils, and monocytes for the antenatal and postnatal periods.
Findings
WBC were measured 80,637 times over five years. The upper reference limit for total WBC was elevated by 36% in pregnancy (RI 5.7-15.0×109/L), driven by a 55% increase in neutrophils (3.7-11.6×109/L) and 38% increase in monocytes (0.3-1.1×109/L), which remained stable between 8-40 weeks. Lymphocytes were reduced by 36% (1.0-2.9×109/L), while eosinophils and basophils were unchanged. Total WBC was elevated significantly further from the first day after birth (similar regardless of the mode of delivery), which resolved to pre-delivery levels by an average of seven days, and to pre-pregnancy levels by day 21.
Interpretation
There are marked changes in WBC in pregnancy, with substantial differences between cell subtypes. WBC are measured frequently in pregnant women in obstetric and non-obstetric settings, and results should be interpreted using a pregnancy-specific RI until delivery, and between days 7-21 after childbirth.
Funding
None.
Keywords: leucocyte, white blood cell, pregnancy, gestational, reference interval, range
Research in context.
Evidence before this study
It is well reported that white blood cells (WBC) increase during pregnancy, although the extent of this elevation is unclear. Reference intervals for WBC in pregnancy vary widely between sources, and most studies have very small cohorts, from which we cannot draw conclusions about how to interpret WBC results in this very high-risk population.
Added value of this study
This is the largest known study of WBC in pregnancy to date, in which we used highly sensitive, modern statistical techniques to define reference intervals for all WBC subtypes, with a subgroup analysis for important clinical characteristics. These may be applied directly in clinical practice, supporting clinicians to make decisions based on robust evidence.
Implications of all the available evidence
WBC should be interpreted using these pregnancy-specific reference intervals until delivery, and between days 7-21 after childbirth.
Alt-text: Unlabelled box
1. Introduction
Infections are responsible for more than half of maternal deaths worldwide, so it is essential that clinicians know how to interpret investigations for suspected infection in pregnant women [1]. White blood cells (WBC) are commonly measured in pregnancy to investigate infection or inflammation, and most are requested and interpreted by clinicians (of all specialties) in emergency departments, general practice, and hospital outpatient settings. It is reported that the total WBC count is elevated in pregnancy, and even further during labour and the puerperium,[2] so the non-pregnant reference interval (RI) is not reliable in the context of the known, marked changes in maternal physiology. However, the upper limit of the reference interval (RI) in pregnancy has been variably reported between 13.8-19.6×109/L in previous studies, but all have reported on much smaller populations, with wide variation in ethnicity and gestational age at the time of sampling [3], [4], [5], [6], [7].
C-reactive protein, another commonly used inflammatory marker, is also elevated in pregnancy, with an upper reference limit almost three times higher than the non-pregnant standard [8]. Importantly, using this pregnancy-specific threshold significantly increases the diagnostic accuracy for infections in pregnancy. This prompted us to consider how we should use WBC to improve the safety of pregnant women and their babies.
The primary objective of this study was to define pregnancy specific RIs for total WBC and its constituent cell subtypes (neutrophils, lymphocytes, eosinophils, basophils, and monocytes), and to evaluate whether key characteristics (gestational age, maternal BMI, and ethnicity) affect these limits, using the largest cohort of women to date. Secondly, we investigated the trajectory of WBC in the first four weeks after delivery, to evaluate how these results should be interpreted in the immediate postpartum period, since this is when infection is commonly suspected, investigated and treated.
1. Methods
2.1. Setting and participants
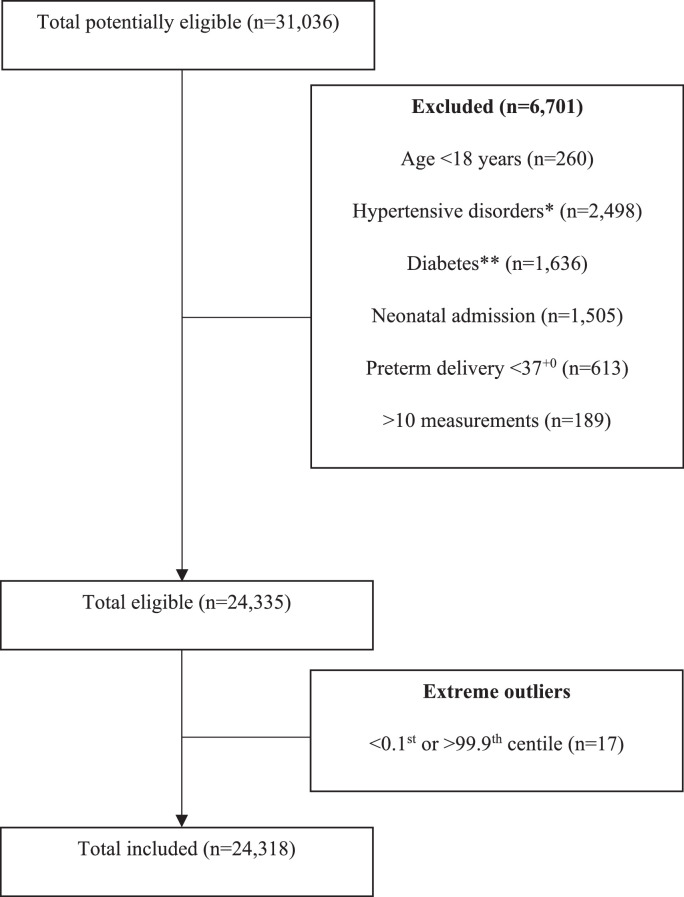
We performed a retrospective, longitudinal study of 24,318 pregnant women in Oxford, UK, using data collected as part of routine antenatal care. Potential participants were identified by searching electronic records for women who delivered live, singleton babies at the John Radcliffe Hospital or midwife-led units in Oxford between 1st January 2016 and 20th February 2021, who had a full blood count taken at least once in pregnancy. Where there were multiple pregnancies in the study period, only the first was included. To obtain a healthy reference cohort, [9] participants were excluded for whom there was an increased likelihood of maternal or fetal disease, which might increase suspicion of infection or other factors affecting WBC. Exclusion criteria were maternal age <18 years old, hypertensive disorders of pregnancy (chronic hypertension, gestational hypertension, pre-eclampsia, eclampsia, or gestational proteinuria, ICD-10 O10-16), diabetes mellitus (type 1, 2, or gestational, ICD-10 O24), preterm delivery (<37 weeks) and babies requiring neonatal admission for any reason or duration. Women who had blood tests measured on more than ten occasions were excluded due to the increased likelihood that these were taken to investigate an abnormality.
2.2. Outcome measures
Blood results were extracted from electronic hospital records, with the corresponding gestational age in weeks and days since the last menstrual period (e.g., 37+3), and were limited to samples collected between 8+0-40+0, with complete data on the total WBC, neutrophils, lymphocytes, eosinophils, basophils, and monocytes. Venous blood samples had been collected as part of routine clinical care, drawn into 4.5 mL potassium EDTA tubes, and were analysed using the Sysmex XN analyser (lower detection limit 0.1×109/L). The same analytical method was used throughout the five-year study period. Where there were multiple results, each was treated as an individual record, and we subsequently investigated the effect of using repeated measures by repeating the analysis using a single, randomly chosen measurement from each pregnancy. Data were available on maternal age and ethnicity, body mass index (BMI) at booking in early pregnancy, gestational age at delivery, neonatal birthweight and birthweight centiles, fetal/neonatal sex, and mode of birth. Where possible, data were used as continuous variables, otherwise ethnicity was categorised using ONS groups (white, mixed, Asian, black, other), [10] body mass index was categorised according to WHO guidance, [11] and the mode of birth was defined as spontaneous vaginal, operative vaginal, elective Caesarean or emergency Caesarean.
2.3. Statistics
It is widely understood that RIs for laboratory tests should be derived from at least 120 participants, to allow the limits to be estimated accurately [12]. We collected all available data during a five-year period, and confirmed that the requisite number of WBC results were available according to each week of gestational age.
A natural logarithmic transformation was applied to each subset of the WBC data to approximate it to the normal distribution, and these distributions were confirmed by inspecting quantile-quantile plots. The distributions were examined for extreme outliers which might represent underlying disease or analytical error, in accordance with international guidance on defining RIs [12]. Outliers were defined as values more extreme than 4 standard deviations above or below the mean in any cell subtype. Outliers were excluded from all analyses.
Firstly, we investigated the trajectory of WBC as pregnancy progressed, to assess whether single or multiple RIs were required in each case. The mean WBC count was estimated for each gestational age () using fractional polynomial regression, where the potential powers were single terms or combinations of , , , , , x, x2 and x3, and the chosen model was that which minimised the negative log-likelihood (i.e., the best fit). The residuals from this model were scaled (multiplied by to optimise approximation to the normal distribution) and regressed linearly against gestational age to define the standard deviation. The gestational age-specific RI for WBC was then calculated as the mean ± 1.96 standard deviations. In Supplemental Figure 1 we have provided an infographic, outlining the steps of this method, which has been used extensively for this purpose [13]. If WBC or the constituent cell types changed during pregnancy then gestational-age specific limits were estimated, otherwise a single RI was defined based on the average and standard deviation calculated across the whole population. Conventional (non-parametric) RIs were presented alongside for comparison, as these are recommended in CLSI/IFCC guidance [12]. Goodness of fit was assessed by inspecting the quantile-quantile plots of z-scores and the distribution of z-scores against gestational age.
We then undertook a series of further analyses, in which:
-
1)
The analysis was restricted to women whose BMI was normal or only slightly raised (≥18.5 but <30 kg/m2)
-
2)
The analysis was restricted to only a single WBC measurement per pregnancy, in which the result was chosen using a random number generator, to investigate the potential effect of using repeated measures
-
3)
Separate models were fitted for different ethnic groups, where a mean difference of >20% was considered clinically relevant
-
4)
The postnatal RI was defined using data from samples taken between 1-28 days after delivery, to compare it with the antenatal and pre-pregnancy RIs, investigating group differences according to mode of birth.
RIs for WBC in non-pregnant populations vary between sources, so we drew reference to those reported similarly in the Oxford Handbook of Clinical Medicine[14] and Tietz Clinical Guide to Laboratory Tests, [15] as pragmatic comparators relevant for general clinical practice in the UK. Normally distributed data were summarised as the mean ± standard deviation (SD), and group differences were investigated using Student's t-tests. For other distributions the median and interquartile range (IQR) are presented, and differences were investigated using Wilcoxon Rank Sum tests. Women with missing data were excluded from subgroup analyses investigating that variable, as this was <5% of the total in all cases, and such a small proportion of missing data is unlikely to affect the results [16]. All analyses were performed using Stata SE (version 17.0, StataCorp LLC, 2021). This study was conducted and reported in accordance with STROBE recommendations [17].
2.4. Ethics
Research ethics approval was granted by the Health Research Authority Research Ethics Committee, Oxford South Central C (Ref: 08/H0606/139). Informed consent was not explicitly required or collected for this retrospective observational study using anonymised, routinely collected clinical data.
2.5. Role of funding source
No funding was sought or received to undertake this study. The corresponding author (SD) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Participants and characteristics
In total, 31,036 women delivered live, singleton babies in Oxford between 2016-2021, and had complete data on WBC. Once all the exclusion criteria were applied, 24,318 women were included (see Figure 1). On average, women were 31 years old (SD ± 5.3), with a BMI of 25 kg/m2 (± 5.2), and they delivered well-grown babies (3512 g ± 473) at full term gestations (40.1 weeks ± 1.2). With regards to these features, we may conclude that this cohort is approximately demographically representative of the wider UK population, as reported by the Office for National Statistics, [18] the Royal College of Obstetricians and Gynaecologists, [19] and standardised growth charts [20]. Demographic characteristics of included participants are presented in Table 1.
Figure 1.
Flowchart for inclusion and exclusion of participants
Table 1.
Demographic characteristics of the study cohort
| Characteristic | Summary statistics* | |
|---|---|---|
| All women, n | 24,318 | |
| Blood measurements, n | 80,637 | |
| Maternal age, years | 30.8 ± 5.3 | |
| Body mass index, kg/m2 | 25.1 ± 5.1 | |
| Gestational age at delivery, weeks | 40.1 ± 1.2 | |
| Neonatal birthweight, g | 3512 ± 473 | |
| Neonatal birthweight centile⁎⁎ | 65.8 (39.7-85.7) | |
| Neonatal sex | Male | 12,417 (51.1%) |
| Female | 11,896 (48.9%) | |
| Ethnicity, n (%) | White | 20,031 (82.4%) |
| Mixed | 465 (1.9%) | |
| Asian | 1,915 (7.9%) | |
| Black | 510 (2.1%) | |
| Other | 438 (1.8%) | |
| Mode of birth, n (%) | Spontaneous vaginal | 15,178 (62.4%) |
| Operative vaginal | 4,228 (17.4%) | |
| Elective Caesarean | 2,121 (8.7%) | |
| Emergency Caesarean | 2,791 (11.5%) |
Data were missing on BMI in 288 women (1.2%), neonatal sex in 5 (<0.1%), and ethnicity in 959 (3.9%). Summary statistics for the available data are reported as the mean ± standard deviation, median (interquartile range), or frequencies (n) and proportions (%).
3.2. Blood results
WBC were measured a median of two times (IQR 1-3) between eight weeks and delivery, making a total of 80,637 results. Samples were mostly taken as part of the recommended antenatal schedule, with peaks at 28 weeks (n=11,982) and 34 weeks (n=12,164) of gestation (see Supplemental Figure 2). The fewest samples were taken between 15+0-15+6 weeks (n=132), thus there were more than the 120 required for the generation of a RI at all gestational ages. In total, 17 women were excluded as having outliers in at least one WBC subgroup. After these exclusions, the total WBC data were normally distributed and were suitable for further analysis (see Supplemental Figure 2).
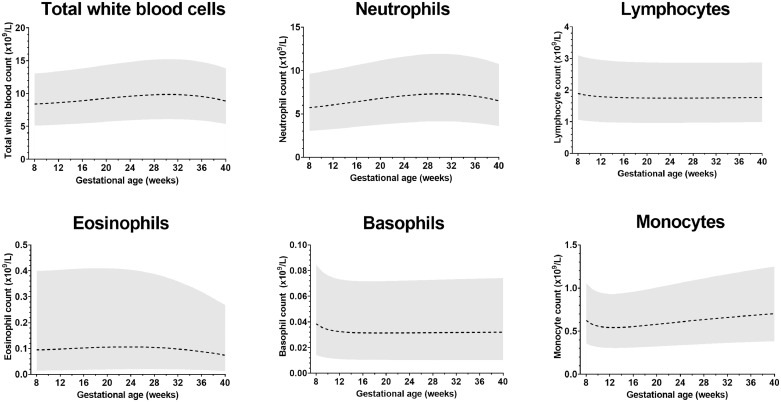
There was little variation in the average total WBC or any cell subtype between 8-40 weeks (see Figure 2). The upper reference limit for monocytes increased marginally with gestational age (+0.3×109/L), and for eosinophils this fell slightly (-0.1×109/L), but these differences were so small they are unlikely to be of clinical significance. Therefore, a single pregnancy-specific 95% RI was estimated for each WBC subtype, which are presented in Table 2 alongside the non-pregnant standards. Importantly, the upper reference limit for WBC was elevated by 36% in pregnancy, which was primarily driven by the rise in neutrophils (upper reference limit 55% higher). The upper limit for lymphocytes was 36% lower in pregnancy, whereas for monocytes this increased by 38%. Basophils were unchanged in pregnant and non-pregnant women.
Figure 2.
Gestational age-specific reference intervals for white blood cell subtypes (n=80,637 results)
Table 2.
Reference intervals for white blood cells
| Cell type | Non-pregnant 95% reference intervals [14](×109/L) | Parametric pregnancy-specific 95% reference intervals*(×109/L) | Non-parametric pregnancy-specific 95% reference intervals⁎⁎ (×109/L) |
|
|---|---|---|---|---|
| 2.5th centile (90% CI) | 97.5th centile (90% CI) | |||
| Total white blood cells | 4.0-11.0 | 5.7-15.0 | 5.7 (5.6-5.7) | 15.0 (14.9-15.1) |
| Neutrophils | 2.0-7.5 | 3.7-11.6 | 3.7 (3.6-3.7) | 11.6 (11.5-11.7) |
| Lymphocytes | 1.0-4.5 | 1.0-2.9 | 1.0 (1.0-1.0) | 2.9 (2.9-3.0) |
| Eosinophils | 0.04-0.44 | 0.02-0.39 | 0.02 (0.02-0.02) | 0.39 (0.39-0.40) |
| Basophils | 0.0-0.1 | 0.1-0.1 | 0.0 (0.0-0.0) | 0.1 (0.1-0.1) |
| Monocytes | 0.2-0.8 | 0.3-1.1 | 0.3 (0.3-0.3) | 1.1 (1.1-1.1) |
Parametric RIs were estimated as the mean ± 1.96 multiples of the standard deviation, using logarithmically transformed data between 8-40 weeks.
For reference, conventional non-parametric reference intervals are presented with 90% confidence intervals, in accordance with CLSI/IFCC guidance,12 showing strong concordance between the two methods.
3.3. Subgroups and sensitivity analyses
There were no material differences when the analysis was restricted to a single measurement per pregnancy or when excluding obese and underweight women, which supports the use of repeated measures employed in this study, and the inclusion of women with a high or low maternal BMI (see Supplemental Figure 2). WBC varied slightly between ethnic groups, with the largest difference seen between white and black women, although this difference was marginal (-20%) and does not support the need for different thresholds, which is consistent with current clinical practice.
3.4. Postnatal changes
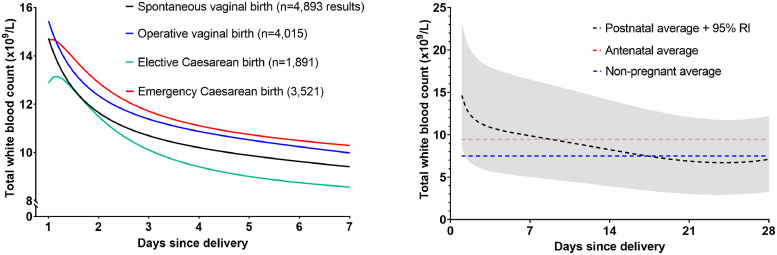
In total, 9,271 (38.2%) women had WBC measured within 28 days of delivery, with a median of one measurement each (IQR 1-2), totaling 14,320 results. On the first day after delivery, total WBC counts increased substantially but variably (95% RI 8.4-23.2×109/L). The average WBC in women who were delivered by elective Caesarean section (and thus who did not labour) was elevated to a lesser degree, although the absolute difference in comparison with operative vaginal delivery was small (15.4 vs. 12.9×109/L, t-test p<0.0001). The mean WBC then resolved to the antenatal average by approximately day seven, whereafter WBC fell consistently until reaching non-pregnant levels within 21 days (see Figure 3).
Figure 3.
Total white blood cells in the postnatal period (n=14,320 results). Left: the trajectory of the average white blood cell count in the first seven days postnatally, according to the mode of birth. Right: the mean and 95% reference interval for WBC in the first 28 days after of any delivery, with reference lines to highlight the antenatal (pre-delivery) and the pre-pregnancy averages.
4. Discussion
Based on 80,637 blood results from 24,318 women, this is the largest known study to have defined pregnancy specific RIs for WBC in pregnancy, with an investigation of how these change during and after pregnancy. We report substantial differences in the reference limits for WBC, compared with non-pregnant adults, and this information may improve how clinicians investigate suspected infection and inflammation in these high-risk patients.
4.1. Total WBC and neutrophils
Total WBC was persistently elevated between 8-40 weeks of gestational age (5.7-15.0×109/L) [2,21]. This is primarily driven by increased neutrophils (3.7-11.6×109/L), which remained stable throughout pregnancy, consistent with previous studies [22,23]. This confirms the need for a pregnancy-specific RI, but refutes the need for partitioned, gestational-age specific limits. This demonstrates a similar pattern to CRP, the other main inflammatory marker used in pregnancy, which is persistently raised from the first trimester, and which is most accurately interpreted using a single pregnancy-specific RI at any stage of pregnancy [8].
Small studies have investigated the value of a raised neutrophil count for diagnosing infection in pregnancy when using this upper threshold (15×109/L) and, while the sensitivity and specificity of using this a standalone tool were limited (53% and 73%, respectively),[24] this was an overall improvement on studies that used lower limits.[25] While neutropenia is technically anything below the lower reference limit, the threshold for treatment for febrile neutropenia is substantially lower (0.5-1.0×109/L). Importantly, a severe neutropenia has been reported in several cases of maternal COVID-19, and clinicians should remain vigilant for a very low neutrophil count in pregnant women [26].
4.2. Lymphocytes
Previous studies have reported a reduction in total lymphocytes in pregnancy, which is consistent with our findings of a lower RI in pregnancy (1.0-2.9×109/L) but, in contrast with other reports, [2] we did not observe an increase in the third trimester.
4.3. Eosinophils, basophils, and monocytes
Eosinophils have been variably reported to decrease [27] or remain unchanged in pregnancy, [21,28] although many of these studies were small and relatively early, and the analytical methods and sensitivities were not clearly defined. We found that the RIs for eosinophils and basophils were approximately consistent with non-pregnant values and, while there was less variation in eosinophils results towards late pregnancy (i.e., a narrower RI), there were only marginal differences in the absolute values as pregnancy progressed. In contrast, there is a well described monocytosis in pregnancy, which has been proposed to prevent fetal allograft rejection, [29] constituting an important protective mechanism for the developing fetus, by modification of the maternal innate immune response. While there was a small increase in monocyte levels with advancing gestation, and there is compelling evidence that monocyte activation and function increases towards term, [30] the absolute difference in circulating cells was small and unlikely to be of implementable clinical value, so we have defined a pragmatic single RI (0.3-1.1×109/L).
4.4. Postnatal
In this study, we deliberately omitted WBC results in the week before delivery, primarily to mitigate the effects of labour or the administration of corticosteroids. Whereas other studies have reported separate RIs for pregnancy and labour, we propose that the highly variable conditions and events in labour preclude the definition of a robust RI, as has been similarly reported by Joyce, et al. in an investigation of CRP [31]. WBC have been reported to be elevated in the postnatal period, with small differences according to mode of delivery, [32] although it was previously unclear how long it takes for this to resolve to pre-pregnancy values, which introduces diagnostic uncertainty when interpreting results in postnatal women. We have described the course of WBC in the immediate postnatal period, which demonstrates resolution to pre-labour (pregnant) levels within seven days, with only small differences according to the mode of delivery. After this time, WBC are approximately consistent with the antenatal RI until resolving to pre-pregnancy levels within 21 days.
In this study we aimed to build upon the existing (often contradictory) literature, to demonstrate the nuanced trajectory of WBC in pregnancy with a greater degree of accuracy than has been previously possible. We achieved this by refining a very large cohort of pregnant women with healthy term babies, forming a population more than twice as large as the next largest study, [33] with the added benefit of complete data on all the major leucocyte subtypes, and longitudinal repeated sampling through pregnancy and the puerperium. We used modern, highly sensitive regression techniques to define RIs, which may be used in clinical practice to improve how routine blood results are interpreted in the context of normal maternal physiology.
There were inevitable challenges in this study. In the absence of a universal definition of maternal infection, or a unified reporting system, it is difficult to identify and exclude women with infection using clinical coding from retrospective hospital data. Therefore, there is a risk of selection bias, as women may be more likely to have had WBC measured if infection or inflammation was suspected. To address this, we used indirect methods to identify women with the highest risk, by i) restricting the population (excluding preterm deliveries, women with several measurements, women with babies requiring neonatal admission), and ii) excluding extreme WBC outliers. Other methods may include identifying and excluding women with HIV or viral hepatitis, which may affect WBC, but the prevalence of both conditions is likely to be low in this cohort. Importantly, results taken at scheduled times (e.g., 28 weeks as per the NICE antenatal pathway) did not differ from those taken at other times, which suggests that the results were not biased by undiagnosed infection or other inflammatory disease in this retrospective study. Furthermore, a previous study showed similar RIs for WBC in a prospective cohort of healthy pregnant women [34].
Corticosteroids (which are usually indicated in the days preceding planned or suspected preterm delivery) are known to increase WBC with a peak around 24 hours after administration, but it is difficult to identify women to whom these had been given. We addressed this by excluding preterm deliveries, and any samples taken in the week before delivery when this may be relevant. WBC and other inflammatory markers have been reported to show diurnal variation according to the time of sampling, as well as the season [35]. Interestingly, neutrophils and CRP peaked in mid-afternoon but had a nadir in the summer months, but lymphocytes and monocytes rose progressively throughout the day and peaked in spring. In general, the absolute differences were small within a given day or year. It is beyond the scope of this study to investigate RIs for WBC in the context of the sampling time, although this may be a focus of future research. Finally, WBC may be elevated in women with non-infectious inflammatory conditions. Previous studies have demonstrated that, with a suitably large population, RIs can be accurately estimated in this way from unselected populations [36]. Other methods may be used to limit the selection criteria further, including using proxy biomarkers (e.g., haemoglobin or platelets) to identify women with other haematological conditions. However, as conditions like pancytopenia are rare, but uncomplicated iron deficiency anaemia and gestational thrombocytopaenia are common, this may over-restrict the cohort to exclude women in whom WBC are likely to be normal. In other words, some women will have been included with these conditions, many of which are undiagnosed or unreported. However, the results are broadly consistent with previous studies and, given that the prevalence is likely to be small, this is unlikely to have materially affected the results of this very large, pragmatic study.
5. Conclusion
This study shows the substantial changes in WBC in the antenatal and postnatal periods, with marked differences between cell subtypes. We have established pregnancy-specific RIs for each white blood cell type, for use between 8-40 weeks of gestational age and 7-21 days postnatally, to improve how clinicians identify women and babies with the highest risk of mortality and morbidity, given the huge global burden of infection in this population.
Supplemental Figure 1: Infographic outlining the method for defining reference intervals using fractional polynomial and linear regression
Supplemental Figure 2: Distributions of white blood cell data and goodness of fit of for the fitted model for total WBC (n=80,637 results). Top: quantile-quantile plot of white blood cell data to investigate adherence to the normal distribution (left) and the distribution of z-scores against gestational age (right). Bottom: quantile-quantile plot of z-scores (left), and the frequency distribution of gestational age at the time of sampling (right).
Supplemental Figure 3: Subgroups and sensitivity analyses. Left: the 2.5th, 50th and 97.5th centiles for WBC when using all (repeated) measurements (n=80,837) compared with a single measurement per participant (n=24,318). Middle: investigating the difference in reference centiles when the analysis was restricted to exclude women who were underweight or obese (body mass index <18.5 or ≥30 kg/m2) (n=65,071). Right: Group-differences in reference centiles according to maternal ethnicity (white n=66,079 results, mixed n=1,579, Asian n=6,498, black 1,918, other 3,114).
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
Acknowledgements
We would like to acknowledge James Bland (Nuffield Department of Women's and Reproductive Health, University of Oxford) for helping with data extraction, Oxford University Hospitals NHS Foundation Trust for providing clinical data, and the women who contributed their data to this study.
Data sharing statement
Individual participant data that underlie the results reported in this article may be requested from the corresponding author. This will be provided after de-identification (data underlying text, tables, figures, and appendices).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103715.
Appendix. Supplementary materials
References
- 1.Bonet M, Brizuela V, Abalos E, Cuesta C, Baguiya A, Chamillard M, et al. Frequency and management of maternal infection in health facilities in 52 countries (GLOSS): a 1-week inception cohort study. The Lancet Global Health. 2020;8(5):e661–ee71. doi: 10.1016/S2214-109X(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandra S, Tripathi AK, Mishra S, Amzarul M, Vaish AK. Physiological Changes in Hematological Parameters During Pregnancy. Indian Journal of Hematology and Blood Transfusion. 2012;28(3):144–146. doi: 10.1007/s12288-012-0175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and Laboratory Studies: A Reference Table for Clinicians. Obstetrics & Gynecology. 2009;114(6):1326–1331. doi: 10.1097/AOG.0b013e3181c2bde8. [DOI] [PubMed] [Google Scholar]
- 4.Li A, Yang S, Zhang J, Qiao R. Establishment of reference intervals for complete blood count parameters during normal pregnancy in Beijing. Journal of clinical laboratory analysis. 2017;31(6):e22150. doi: 10.1002/jcla.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balloch AJ, Cauchi MN. Reference ranges for haematology parameters in pregnancy derived from patient populations. Clinical & Laboratory Haematology. 1993;15(1):7–14. doi: 10.1111/j.1365-2257.1993.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 6.Jin Y, Lu J, Jin H, Fei C, Xie X, Zhang J. Reference intervals for biochemical, haemostatic and haematological parameters in healthy Chinese women during early and late pregnancy. Clinical Chemistry and Laboratory Medicine (CCLM) 2018;56(6):973–979. doi: 10.1515/cclm-2017-0804. [DOI] [PubMed] [Google Scholar]
- 7.Milman N, Bergholt T, Byg K-E, Eriksen L, Hvas A-M. Reference intervals for haematological variables during normal pregnancy and postpartum in 434 healthy Danish women. European Journal of Haematology. 2007;79(1):39–46. doi: 10.1111/j.1600-0609.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- 8.Dockree S, Brook J, James T, Shine B, Impey L, Vatish M. Pregnancy-specific reference intervals for C-reactive protein improve diagnostic accuracy for infection: A longitudinal study. Clinica Chimica Acta. 2021;517:81–85. doi: 10.1016/j.cca.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 9.PetitClerc C, Solberg HE. Approved recommendation (1987) on the theory of reference values. Part 2. Selection of individuals for the production of reference values. Clinica Chimica Acta. 1987;170(2):S1–S11. doi: 10.1016/0009-8981(88)90074-5. [DOI] [PubMed] [Google Scholar]
- 10.Office for National Statistics. Ethnic group, national identity and religion [Available from: https://www.ons.gov.uk/methodology/classificationsandstandards/measuringequality/ethnicgroupnationalidentityandreligion.
- 11.World Health Organisation Body mass index - BMI. 2021 https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi [Available from: [Google Scholar]
- 12.Solberg HE. Approved recommendation (1987) on the theory of reference values. Part 5. Statistical treatment of collected reference values. Determination of reference limits. Clinica Chimica Acta. 1987;170(2):S13–S32. [Google Scholar]
- 13.Royston P, Wright EM. A method for estimating age-specific reference intervals (‘normal ranges’) based on fractional polynomials and exponential transformation. Journal of the Royal Statistical Society: Series A (Statistics in Society) 1998;161(1):79–101. [Google Scholar]
- 14.Wilkinson I, Raine T, Wiles K, Hall C, Goodhart A, O'Neill H. Oxford University Press; 2017. Oxford handbook of clinical medicine. [Google Scholar]
- 15.Wu AH. Elsevier Health Sciences; 2006. Tietz clinical guide to laboratory tests-E-book. [Google Scholar]
- 16.Lee KJ, Tilling KM, Cornish RP, Little RJA, Bell ML, Goetghebeur E, et al. Framework for the treatment and reporting of missing data in observational studies: The Treatment And Reporting of Missing data in Observational Studies framework. Journal of Clinical Epidemiology. 2021;134:79–88. doi: 10.1016/j.jclinepi.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bmj. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Office for National Statistics . 2019. Births by parents' characteristics.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthsbyparentscharacteristics [Available from: [Google Scholar]
- 19.Denison FC, Aedla NR, Keag O, Hor K, Reynolds RM, Milne A, et al. Care of Women with Obesity in Pregnancy. BJOG: An International Journal of Obstetrics & Gynaecology. 2019;126(3):e62–e106. doi: 10.1111/1471-0528.15386. [DOI] [PubMed] [Google Scholar]
- 20.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 21.Fleming AF. 1 - Haematological Changes in Pregnancy. Clinics in Obstetrics and Gynaecology. 1975;2(2):269–283. [Google Scholar]
- 22.Belo L, Santos-Silva A, Rocha S, Caslake M, Cooney J, Pereira-Leite L, et al. Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2005;123(1):46–51. doi: 10.1016/j.ejogrb.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Pramanik SS, Pramanik T, Mondal SC, Chanda R. Number, maturity and phagocytic activity of neutrophils in the three trimesters of pregnancy. Eastern Mediterranean Health Journal. 2007;13(4):862–867. [PubMed] [Google Scholar]
- 24.Oludag T, Gode F, Caglayan E, Saatli B, Okyay RE. Altunyurt S. Value of maternal procalcitonin levels for predicting subclinical intra-amniotic infection in preterm premature rupture of membranes. Journal of Obstetrics and Gynaecology Research. 2014;40(4):954–960. doi: 10.1111/jog.12273. [DOI] [PubMed] [Google Scholar]
- 25.Cataño Sabogal CP, Fonseca J, García-Perdomo HA. Validation of diagnostic tests for histologic chorioamnionitis: Systematic review and meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2018;228:13–26. doi: 10.1016/j.ejogrb.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 26.Vlachodimitropoulou Koumoutsea E, Vivanti AJ, Shehata N, Benachi A, Le Gouez A, Desconclois C, et al. COVID-19 and acute coagulopathy in pregnancy. Journal of Thrombosis and Haemostasis. 2020;18(7):1648–1652. doi: 10.1111/jth.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasu M, Fujiyasu S, Iwatani Y, Amino N, Tanizawa O, Miyai K. Changes of differential leukocyte counts during pregnancy and in the postpartum period. Rinsho Byori. 1992;40(12):1292–1296. [PubMed] [Google Scholar]
- 28.Andrews WC, Bonsnes RW. The leucocytes during pregnancy. American Journal of Obstetrics and Gynecology. 1951;61(5):1129–1135. doi: 10.1016/0002-9378(51)90315-8. [DOI] [PubMed] [Google Scholar]
- 29.Oberbarnscheidt MH, Zeng Q, Li Q, Dai H, Williams AL, Shlomchik WD, et al. Non-self recognition by monocytes initiates allograft rejection. Journal of Clinical Investigation. 2014;124(8):3579–3589. doi: 10.1172/JCI74370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luppi P, Haluszczak C, Betters D, Richard CA, Trucco M, DeLoia JA. Monocytes are progressively activated in the circulation of pregnant women. Journal of Leukocyte Biology. 2002;72(5):874–884. [PubMed] [Google Scholar]
- 31.Joyce CM, Deasy S, Abu H, Lim YY, O'Shea PM, O'Donoghue K. Reference values for C-reactive protein and procalcitonin at term pregnancy and in the early postnatal period. Annals of Clinical Biochemistry. 2021 doi: 10.1177/00045632211005807. [DOI] [PubMed] [Google Scholar]
- 32.Arbib N, Aviram A, Gabbay Ben-Ziv R, Sneh O, Yogev Y, Hadar E. The effect of labor and delivery on white blood cell count. Journal of Maternal-Fetal and Neonatal Medicine. 2016;29(18):2904–2908. doi: 10.3109/14767058.2015.1110572. [DOI] [PubMed] [Google Scholar]
- 33.Markus C, Flores C, Saxon B, Osborn K. Pregnancy-specific continuous reference intervals for haematology parameters from an Australian dataset: A step toward dynamic continuous reference intervals. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2021;61(2):223–231. doi: 10.1111/ajo.13260. [DOI] [PubMed] [Google Scholar]
- 34.Klajnbard A, Szecsi PB, Colov NP, Andersen MR, Jørgensen M, Bjørngaard B, et al. Laboratory reference intervals during pregnancy, delivery and the early postpartum period. Clinical Chemistry and Laboratory Medicine. 2010;48(2):237–248. doi: 10.1515/CCLM.2010.033. [DOI] [PubMed] [Google Scholar]
- 35.Wyse C, O'Malley G, Coogan AN, McConkey S, Smith DJ. Seasonal and daytime variation in multiple immune parameters in humans: Evidence from 329,261 participants of the UK Biobank cohort. iScience. 2021;24(4) doi: 10.1016/j.isci.2021.102255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shine B. Use of routine clinical laboratory data to define reference intervals. Annals of Clinical Biochemistry. 2008;45(5):467–475. doi: 10.1258/acb.2008.008028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.