Abstract
The efficacy of immunotherapy for advanced non‐small cell lung cancer (NSCLC) remains unsatisfactory, as the majority of patients either do not experience an objective response or acquire secondary resistance. As a result, several methods to enhance the systemic efficacy of immunotherapy have been investigated, including a large area of active research by combining immunotherapy with radiation therapy (RT). Given the rapidly burgeoning concept of combining immunotherapy and RT for increasing therapeutic benefit, we review the progress in this field thus far and explore further avenues for enhancing this combination. This review commences with a discussion of the only two existing randomized trials (and a pooled analysis) showing that the addition of RT to immunotherapy improves the abscopal response rate, progression‐free survival, and overall survival in metastatic NSCLC patients. We then discussed factors and biomarkers that may be associated with a proportionally greater benefit to additional RT, such as low programmed cell death protein ligand 1 (PD‐L1) status, tumor mutational burden (TMB), and patient's immune function. Next, the implementation of RT to overcome immunotherapy resistance is discussed, including a mechanistic discussion and methods with which these mechanisms could be exploited. Lastly, the emerging role of low‐dose RT is discussed, which may help to overcome inhibitory signals in the tumor stroma that limit T‐cell infiltration. Taken together, given the current state of this rapidly expanding realm, these futuristic strategies may be reflected upon to further enhance the efficacy of immunotherapy for a wider group of patients.
Keywords: immune checkpoint inhibitors, immunotherapy, immunotherapy combined with radiotherapy, low‐dose radiotherapy, non‐small cell lung cancer, radiotherapy
In recent years, immunotherapy has been considered the most promising method to overcome cancer and extend survival. It has been widely reported in preclinical and clinical studies that radiotherapy induces immunomodulatory effects by releasing TAAs and activating immunoregulation‐related signaling pathways, initiating tumor‐specific cytotoxic T cells, and promoting T cells to enter into tumor tissues. In this manuscript, we summarize the current status of immunotherapy and iRT and the development and additional combination strategies to enhance the efficacy of iRT.
Abbreviations
- ACR
abscopal control rate
- ARR
abscopal response rate
- cGAS
cyclic GMP‐AMP synthase
- DC
dendritic cell
- DCs
dendritic cells
- dMMR
mismatch repair‐deficient
- dsDNA
double‐stranded DNA
- ICIs
immune checkpoint inhibitors
- IFNβ
interferonβ
- iNOS+
inducible nitric oxide synthase‐positive
- iRT
immunoradiotherapy
- iRT
immunotherapy combined with radiotherapy
- LDI
low‐dose irradiation
- MDACC
MD Anderson Cancer Center
- NKI
Netherlands Cancer Institute
- NSCLC
non‐small cell lung cancer
- OS
overall survival
- OXPHOS
novel oxidative phosphorylation
- PD‐1
programmed cell death protein 1
- PD‐L1
programmed cell death protein ligand 1
- PFS
progression‐free survival
- RT
radiation therapy
- SBRT
stereotactic body RT
- STING
stimulator of interferon genes
- TAAs
tumor‐associated antigens
- TMB
tumor mutational burden
1. BACKGROUND
Immunotherapy, most notably immune checkpoint inhibitors (ICIs) such as anti‐programmed cell death protein 1/programmed cell death protein ligand 1 (PD‐1/PD‐L1) compounds, have improved the survival of patients with advanced non‐small‐cell lung cancer (NSCLC) [1, 2, 3, 4]. However, the efficacy of ICIs remains unsatisfactory, as a majority of patients do not experience an objective response [1, 5] and most patients initially develop primary resistance or acquire secondary resistance soon after therapy [6, 7]. As a result, many methods to enhance the systemic efficacy of ICIs have been explored [8, 9, 10], and a large area of active research is to combine ICIs with radiation therapy (RT), termed immunoradiotherapy (iRT) [11, 12, 13].
The concept of RT enhancing the effects of systemic therapy is known as the “abscopal effect”. Although this phenomenon has been known for decades [14], it is also known that RT‐induced abscopal responses are rare. The first case detailing an abscopal effect produced by iRT was reported in 2012 [15].
iRT could potentially be utilized for any stage of NSCLC. For metastatic cases, there is randomized evidence to support the addition of RT to immunotherapy alone [16, 17, 18]. For locally advanced non‐metastatic cases, the randomized PACIFIC trial demonstrated the efficacy of combining definitive RT with subsequent immunotherapy [19]. Lastly, for early‐stage NSCLC, there are a number of randomized trials aiming to evaluate stereotactic RT with or without adjuvant immunotherapy (e.g., NCT03110978, NCT03446547, NCT03833154, NCT03924869, NCT04214262). Representative clinical trials for NSCLC are presented in Table 1.
TABLE 1.
Representative ongoing or completed clinical trials using PD‐1/PD‐L1/CTLA‐4 inhibitors and RT for NSCLC
| ClinicalTrials.gov identifier | Trial Phase | Drug classification | Inventions | Sponsors | Estimated/Actual study completion date | Status |
|---|---|---|---|---|---|---|
| Early‐stage NSCLC | ||||||
| NCT03801902 | 1 | PD‐L1 inhibitors |
Arm I: 13 cycles × durvalumab with ACRT (60 Gy in 15 fractions) Arm II: 13 cycles × durvalumab with standard RT (60 Gy in 30 fractions) |
National Cancer Institute (NCI) | Dec 31, 2021 | Active, not recruiting |
| NCT03383302 | 1/2 | PD‐1 inhibitors | 1 year × nivolumab following SBRT (54 Gy in 3 fractions or 55 Gy in 5 fractions) | Royal Marsden NHS Foundation Trust | Jan 01, 2022 | Recruiting |
| NCT03110978 | 2 | PD‐1 inhibitors |
Arm I: 1‐2 weeks × SBRT Arm II: 1‐3 cycles × nivolumab with 1‐2 weeks × SBRT |
M.D. Anderson Cancer Center | Jun 30, 2022 | Recruiting |
| NCT03148327 | 1/2 | PD‐L1 inhibitors |
Arm I: 5 cycles × durvalumab with SBRT (54Gy, 50Gy or 65Gy in 3, 4 or 10 fractions) Arm II: SBRT (54Gy, 50Gy or 65Gy in 3, 4 or 10 fractions) |
Jonsson Comprehensive Cancer Center | Jun 01, 2023 | Active, not recruiting |
| NCT03924869 | 3 | PD‐1 inhibitors |
Arm 1:17 cycles × pembrolizumab with SBRT (45‐54 Gy in 3‐5 fractions) Arm 2:17 cycles × placebo with SBRT (45‐54 Gy in 3‐5 fractions) |
Merck Sharp & Dohme Corp | Jul 01, 2026 | Recruiting |
| NCT04271384 | 2 | PD‐1 inhibitors |
3 cycles × nivolumab with SAR (54 Gy in 3 fractions or 50 Gy in 5 fractions or 60 Gy in 8 fractions) |
Hospital Israelita Albert Einstein | Jun 29, 2023 | Recruiting |
| NCT03833154 | 3 | PD‐L1 inhibitors |
Arm 1:24 months × durvalumab with SBRT (in 3, 4, 5 or 8 fractions) Arm 2:24 months × placebo with SBRT (in 3, 4, 5 or 8 fractions) |
AstraZeneca | Oct 31, 2025 | Recruiting |
| Locally‐advanced NSCLC | ||||||
| NCT03801902 | 1 | PD‐L1 inhibitors |
Arm I: 13 cycles × durvalumab with ACRT (60 Gy in 15 fractions) Arm II: 13 cycles × durvalumab with standard RT (60 Gy in 30 fractions) |
National Cancer Institute (NCI) | Dec 31, 2021 | Active, not recruiting |
| NCT04013542 | 1 |
PD‐1 and CTLA‐4 inhibitors |
Concurrent therapy:8 cycles × nivolumab and 4 cycles × ipilimumab with 6‐7 weeks × RT Maintenance therapy:8 cycles × nivolumab |
M.D. Anderson Cancer Center | Feb 01, 2022 | Recruiting |
| NCT03818776 | 1 | PD‐L1 inhibitors |
Arm I: 13 cycles × durvalumab with Proton beam therapy RT (60 CGyE in 20 fractions) Arm II: 13 cycles × durvalumab with Proton beam therapy RT (69 CGyE in 23 fractions) |
Case Comprehensive Cancer Center | Nov 01, 2022 | Recruiting |
| NCT04765709 | 2 | PD‐1 inhibitors |
Part 1: Induction with durvalumab and platinum‐based chemotherapy (cisplatin or carboplatin plus vinorelbine or pemetrexed) Part 2: Eligible for durvalumab and RT Part 3: Eligible for durvalumab maintenance |
Mario Negri Institute for Pharmacological Research | Jun 01, 2026 | Not yet recruiting |
| NCT03519971 | 3 | PD‐L1 inhibitors |
Arm I: Durvalumab + platinum‐based chemotherapy (cisplatin/etoposide, carboplatin/paclitaxel, pemetrexed/cisplatin, pemetrexed/carboplatin) and RT Arm II: Placebo + platinum‐based chemotherapy (cisplatin/etoposide, carboplatin/paclitaxel, pemetrexed/cisplatin, pemetrexed/carboplatin) and RT |
AstraZeneca | Nov 13, 2023 | Active, not recruiting |
| NCT03523702 | 2 | PD‐1 inhibitors |
PembroRT Cohort: 15 cycles × pembrolizumab with 4 weeks × RT ChemoRT Cohort: Chemotherapy (carboplatin and paclitaxel) with 4‐7 weeks × RT |
Albert Einstein College of Medicine | Sep 01, 2022 | Recruiting |
| NCT04230408 | 2 | PD‐L1 inhibitors |
Induction chemo‐immunotherapy phase: 2 cycles × paclitaxel, carboplatin and durvalumab Concurrent chemo‐immuno‐radiotherapy phase: RT with paclitaxel, carboplatin and durvalumab Consolidation immunotherapy:12 cycles × durvalumab |
Latin American Cooperative Oncology Group | May 01, 2024 | Recruiting |
| NCT03102242 | 2 | PD‐L1 inhibitors |
Induction immunotherapy:4 cycles × atezolizumab Chemoradiotherapy:6 cycles × carboplatin and paclitaxel with RT (60 Gy in 30 fractions) Adjuvant immunotherapy: 1 year × atezolizumab |
Alliance Foundation Trials, LLC | Mar 01, 2020 | Active, not recruiting |
| NCT03237377 | 2 |
PD‐L1 and CTLA‐4 inhibitors |
Arm 1: 3 cycles × durvalumab with RT (45Gy in 25 fractions) Arm 2: 3 cycles × durvalumab and tremelimumab with RT (45Gy in 25 fractions) |
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | Sep 01, 2021 | Recruiting |
| NCT04597671 | 3 | PD‐L1 inhibitors |
Arm I: Durvalumab with low‐dose PCI (15 Gy in 10 fractions) Arm II: Durvalumab with observation |
Association NVALT Studies | Nov 01, 2027 | Not yet recruiting |
| NCT03774732 | 3 | PD‐1 inhibitors |
Arm 1: Pembrolizumab and chemotherapy (carboplatin/paclitaxel, cisplatin/pemetrexed, carboplatin/pemetrexed) Arm 2: Pembrolizumab and chemotherapy (carboplatin/paclitaxel, cisplatin/pemetrexed, carboplatin/pemetrexed) with 3D‐CRT (18 Gy in 3 fractions) or SBRT |
UNICANCER | May 15, 2023 | Recruiting |
| NCT04577638 | 2 | PD‐1 inhibitors | 3 cycles × nivolumab with IMRT (66 Gy in 24 fractions)+ 6 months × nivolumab maintenance | Center Eugene Marquis | April 1, 2023 | Not yet recruiting |
| Advanced NSCLC | ||||||
| NCT03168464 | 1/2 |
PD‐1 and CTLA‐4 inhibitors |
Ipilimumab and nivolumab with RT (30 Gy in 5 fractions) | Weill Medical College of Cornell University | Dec 30, 2022 | Recruiting |
| NCT03158883 | 1 | PD‐L1 inhibitors | Avelumab with SAR (50 Gy in 5 fractions) | Megan Daly, MD | Jun 01, 2022 | Recruiting |
| NCT03275597 | 1 |
PD‐L1 and CTLA‐4 inhibitors |
Durvalumab and tremelimumab with SBRT (30 ‐ 50 Gy in 5 fractions) | University of Wisconsin, Madison | Oct 01, 2022 | Recruiting |
| NCT03035890 | Not Applicable | PD‐1 or PD‐L1 inhibitors | RT (24‐45 Gy in 3 fractions or 30‐50 Gy in 5fractions) with nivolumab or pembrolizumab or atezolizumab | West Virginia University | Jun 30, 2023 | Active, not recruiting |
| NCT04081688 | 1 | PD‐L1 inhibitors | 18 cycles × atezolizumab and varlilumab with 2 cycles × SBRT | Rutgers, The State University of New Jersey | Jun 30, 2023 | Recruiting |
| NCT03825510 | Not Applicable | PD‐1 inhibitors | Nivolumab or pembrolizumab with SBRT | Crozer‐Keystone Health System | Aug 28, 2021 | Recruiting |
| NCT03915678 | 2 | PD‐L1 inhibitors | Atezolizumab and BDB001 with RT (27‐60 Gy in 3‐5 fractions) | Institut Bergonié | Mar 01, 2025 | Not yet recruiting |
| NCT03774732 | 3 | PD‐1 inhibitors |
Arm 1:Pembrolizumab and chemotherapy (carboplatin/paclitaxel, cisplatin/pemetrexed, carboplatin/pemetrexed) |
UNICANCER | May 15, 2023 | Recruiting |
|
Arm 2:Pembrolizumab and chemotherapy (carboplatin/paclitaxel, cisplatin/pemetrexed, carboplatin/pemetrexed) with 3D‐CRT (18 Gy in 3 fractions) or SBRT |
||||||
| NCT03509584 | 1 |
PD‐1 and CTLA‐4 inhibitors |
Part 1a: Nivolumab with hypofractionated RT (24 Gy in 3 fractions)(bone metatase) Part 1b: Nivolumab and ipilimumab with hypofractionated RT (24 Gy in 3 fractions)(bone metatase) Part 2a: Nivolumab with hypofractionated RT (24 Gy in 3 fractions)(outside the brain) Part 2b: Nivolumab and ipilimumab with hypofractionated RT (24 Gy in 3 fractions)(outside the brain) |
Assistance Publique Hopitaux De Marseille | April 2021 | Not yet recruiting |
| NCT02221739 | 1/2 | CTLA‐4 inhibitors | 3 cycles × ipilimumab with RT (IMRT or 3‐D CRT)(30 Gy in 5 fractions or 28.5 Gy in 3 fractions) | NYU Langone Health | Oct 27, 2015 | Completed |
| NCT03223155 | 1 |
PD‐1 and CTLA‐4 inhibitors |
Sequential Arm: SBRT (in 3‐5 fractions)and nivolumab and ipilimumab Concurrent Arm: Nivolumab and ipilimumab with SBRT (in 3‐5 fractions) |
University of Chicago | Dec 01, 2024 | Recruiting |
| NCT02888743 | 2 |
PD‐L1 and CTLA‐4 inhibitors |
Arm I:4 cycles × tremelimumab and 13 cycles × durvalumab Arm II: 4 cycles × tremelimumab and 13 cycles × durvalumab with High‐dose RT Arm III: 4 cycles × tremelimumab and 13 cycles × durvalumab with Low‐dose RT |
National Cancer Institute (NCI) | Dec 31, 2021 | Active, not recruiting |
| NCT02463994 | 1 | PD‐L1 inhibitors | MPDL3280A + HIGRT | University of Michigan Rogel Cancer Center | Nov 07, 2018 | Completed |
RT, Radiotherapy; ACRT, Accelerated Hypofractionated Radiotherapy; SBRT, Stereotactic Body Radiotherapy; SAR, Stereotactic Ablative Radiotherapy; PCI, Prophylactic Cranial Irradiation; 3D‐CRT, Conformal 3D Radiotherapy; IMRT, Intensity‐Modulated Radiotherapy; HIGRT, Hypofractionated Image‐guided Radiotherapy.
Given this rapidly burgeoning concept of combining immunotherapy and RT for further therapeutic benefit, we herein review the progress in this field thus far and explore further avenues to further enhance this combination. We first provide a discussion of the only two existing randomized trials of immunotherapy with or without RT. Next, we discuss factors and biomarkers associated with a potentially higher benefit to adding RT to immunotherapy. Then, we describe the utility of RT for overcoming immunotherapy resistance. Lastly, we review the emerging role of low‐dose RT in efforts to promote immune infiltration of tumor tissue.
2. CURRENT STATUS
Currently, there are only two published randomized trials evaluating immunotherapy with or without RT for metastatic NSCLC, the PEMBRO‐RT study from the Netherlands Cancer Institute (NKI) [16], and the phase I/II trial from the MD Anderson Cancer Center (MDACC) [18]. The NKI PEMBRO‐RT randomized trial showed that anti‐PD‐1 antibodies combined with stereotactic body RT (SBRT) produced a non‐significant trend towards better response rate than anti‐PD‐1 antibodies alone (P = 0.07), particularly in PD‐L1‐negative patients. In the MDACC trial, although the survival rates of patients treated with SBRT or traditional radiotherapy were not different from the overall population (n = 80), SBRT was associated with increased treatment response rate and improved progression‐free survival (PFS).
Although a beneficial trend for iRT was found in both studies, it was not statistically significant because of the small sample sizes in both trials (n = 72 in NKI trial [16] and n = 80 in MDACC trial [18]). As such, a pooled analysis of these two clinical trials was performed to better evaluate response rates and PFS [17]. Overall, 148 patients were included in the final analysis. The iRT cohort was found to experience a higher abscopal response rate (ARR) (41.7%) as compared to the immunotherapy alone group (19.7%) (P = 0.0039). There were also significant advantages for abscopal control rate (ACR) (65.3% vs. 43.4%, P = 0.0071). The improved control of systemic disease translated to a higher PFS (9.0 months vs. 4.4 months, P = 0.045) and overall survival (OS) (19.2 months vs. 8.7 months, P = 0.0004) with iRT. Additionally, from that pooled analysis, an exploratory subgroup analysis of different radiotherapy regimens showed that the ARR in SBRT patients was higher than that of non‐stereotactic dosing. This pooled analysis publication remains the only known study showing an improvement in survival‐related endpoints with the addition of RT to immunotherapy for metastatic NSCLC. However, those findings (as well as translational/correlational data [10]) illustrate that, even with iRT, the efficacy was only 41.7%, highlighting the need for further optimization.
3. PATIENT SELECTION AND BIOMARKERS: UPDATES AND CHALLENGES
It is intuitive that the addition of RT to immunotherapy may not benefit all patients to the same degree. Several clinicopathological factors may be associated with a proportionally greater degree of response to immunotherapy. The standard factors of age, performance status, and disease burden may also play an important role in patient selection, especially given the emerging role of consolidative RT for oligometastatic NSCLC.
Additionally, PD‐L1 testing is currently the most accepted biomarker of treatment response for immunotherapy alone [20, 21, 22]. It is often hypothesized that RT may benefit patients with low PD‐L1 to a greater degree because the response to immunotherapy alone in high expressors of PD‐L1 may be considerably higher than that of cases with low PD‐L1 expression levels [17, 23]. As a result, because immunotherapy alone seems to provide a higher degree of local effects for the former, RT may be more often required to control disease in the latter.
The tumor mutational burden (TMB) may be an additional important biomarker of immunotherapy response, and a correlation between this marker and response rates to anti‐PD‐1 or anti‐PD‐L1 therapy has been demonstrated across several tumor types [24, 25, 26, 27]. TMB is defined as the total number of mutations, including both base substitutions and short insertions/deletions, per coding area of the tumor genome. However, TMB is not as widely utilized or validated as compared to PD‐L1 status, and it remains currently unclear whether this should be utilized as a robust marker for patient selection.
Lastly, another emerging area of further research is whether a given patient's immune function may relate to the benefit from iRT. Adequate immune function (especially lymphocytic function) is required to exert the downstream effects of iRT, and data suggest that patients without adequate immune function (e.g. lymphopenia) are at lower risk of deriving a benefit from iRT [28, 29]. However, the data have not been well validated by larger and more robust datasets.
In summary, enhancing the efficacy of iRT may be done by proper patient selection. The clinicopathological factors mentioned above may assist in performing more careful patient selection for iRT, thereby improving its efficacy and patients’ outcomes.
4. USING RADIOTHERAPY TO OVERCOME IMMUNOTHERAPY RESISTANCE: UPDATES AND CHALLENGES
RT may play an important role in advanced NSCLC based on four major pieces of evidence. First, RT has been associated with improved survival in patients with oligometastatic NSCLC [30, 31]. Second, RT can increase the release and presentation of antigens, thereby enhancing dendritic cell (DC) function, augmenting T cell sensitization, and promoting antitumor immune responses [32, 33, 34, 35]. Third, RT can regulate the tumor microenvironment and increase the infiltration of cytotoxic CD8+ T lymphocytes, which play a key role in the antitumor immune response [36]. Finally, RT reduces immunotherapy resistance by reshaping the tumor microenvironment [37]. Traditional theory suggests that ionizing RT mainly damages DNA to kill tumor cells [38, 39]. However, an alternative concept is that radiotherapy can control distant metastatic lesions outside the irradiated field in addition to local control, which was coined the “abscopal effect” and was initially proposed in 1953 [14]. The theoretical basis for this effect is that the tumor cells killed by RT serve as an in situ tumor vaccine by releasing tumor‐associated antigens (TAAs). These are then captured by dendritic cells (DCs), which then activate CD8+ T cells that home in to tumors, activate systemic immunogenicity, induce abscopal effects, and control tumor proliferation [40, 41, 42] (Figure 1).
FIGURE 1.
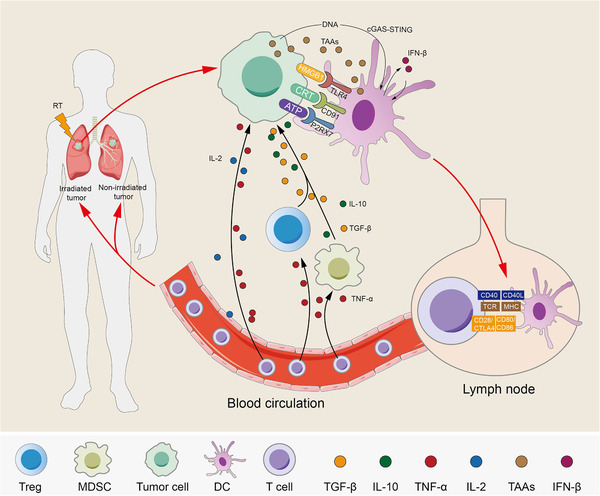
Radiotherapy‐induced effects on tumor cells. Radiotherapy (RT) induces immunogenic death of tumor cells which increases the release of tumor‐associated antigens (TAAs) and damage‐associated molecular patterns such as high‐mobility group box 1 (HMGB1) and adenosine triphosphate (ATP), and enhances the surface expression of calreticulin (CRT). Secretion promotes the activation and maturation of dendritic cells (DCs) through their corresponding receptors. DCs that sense cancer cell‐derived DNA induce interferon‐β (IFN‐β) production through the cyclic GMP‐AMP synthase (cGAS)‐ stimulator of interferon genes (STING) pathway. In turn, IFN‐β promotes the activation and maturation of DCs. DCs take up tumor‐associated antigens (TAAs) and migrate to draining lymph nodes and then present the TAAs on major histocompatibility complex class I (MHCI) to T cells through the T‐cell receptor (TCR), which requires the costimulatory molecules CD80/86‐CD28/cytotoxic T lymphocyte‐associated protein 4 (CTLA4) and CD40L‐CD40. Otherwise, these are not sufficient to cause T cell activation and proliferation in the absence of costimulatory signals. Activated T cells are transported to irradiated lesions and distant nonirradiated lesions through the blood circulation. At the same time, tumor cell immunogenic death leads to the release of cytokines, the immune‐promoting factors tumor necrosis factor‐α (TNF‐α) and interleukin‐2 (IL‐2) recruit activated T cells to kill tumor cells through upregulated MHCI, and the immunosuppressive factors such as TGF‐β and IL‐10 recruit immunosuppressive cells such as regulatory T cells (Tregs) and myeloid‐derived suppressor cells (MDSCs) to inhibit immune effects. However, activated T cells cause the apoptosis of Tregs and MDSCs through cytokines such as TNF‐α. Abbreviations: Radiotherapy, RT; tumor‐associated antigens, TAAs; high‐mobility group box 1, HMGB1; adenosine triphosphate, ATP; calreticulin, CRT; dendritic cells, DCs; interferon‐β, IFN‐β; cyclic GMP‐AMP synthase, cGAS; stimulator of interferon genes, STING; tumor‐associated antigens, TAAs; major histocompatibility complex class I, MHC1; T‐cell receptor, TCR; Cytotoxic T lymphocyte‐associated protein 4, CTLA4; tumor necrosis factor‐α, TNF‐α; interleukin‐2, IL‐2; Myeloid‐derived suppressor cells, MDSCs; P2X7 receptor, P2RX7; transforming growth factor‐β, TGF‐β; regulatory T cells, Tregs
Conventionally fractionated radiotherapy has historically been the preferred approach to treat NSCLC, especially in the definitive setting [43]. However, with the improvement of radiotherapeutic technologies, image guidance, and radiation physics, hypofractionated radiotherapy has become widely administered in patients with tumors of appropriate size and location, such as tumors of up to 5‐7 cm in size and not overlapping the mediastinal organs such as the trachea or esophagus. Hypofractionation, especially with stereotactic RT, may allow reduced dose exposure to uninvolved areas of the cardiopulmonary system and thus better preserve the absolute lymphocyte count in efforts to induce a stronger abscopal response [29].
Preclinical studies have confirmed that the abscopal effect can occur in immunocompetent settings, but not in immunodeficient conditions [44, 45]. Such studies have revealed that antitumor immunity is the key factor affecting the efficacy of radiotherapy. For example, in a syngeneic mouse model of fibrosarcoma, the radiotherapy dose needed to control tumors in immunocompetent mice was lower than that needed for immunodeficient mice [44]. Similarly, in another investigation, mouse melanoma B16 tumors implanted into immunocompetent hosts responded to high doses of radiation but tumors grown in immunocompromised hosts did not respond to radiotherapy and were more susceptible to metastasis [46].
Distinct immune therapies might differentially affect primary and abscopal tumor responses. For example, vaccination is an emerging field of research that has brought promising results for the future of immunotherapy. It is feasible to boost RT‐induced immune responses and to achieve immunosuppression by inhibiting immunosuppressive molecules, such as PD‐1, with activated whole tumor cell vaccines [47]. Additionally, recent studies have reported that elevated levels of novel oxidative phosphorylation (OXPHOS) in tumors are an important factor associated with immunotherapy efficacy [48, 49]. Furthermore, anti‐PD‐1 antibodies or radiotherapy can increase OXPHOS levels [49, 50, 51]. Therefore, combination treatment with radiotherapy and OXPHOS inhibitors could also be an effective strategy against PD‐1 resistance in NSCLC [52].
Preclinical studies have demonstrated that high‐dose radiation may cause the accumulation of endogenous cytosolic DNA, resulting in the activation of the cyclic GMP‐AMP synthase (cGAS)‐stimulator of interferon genes (STING) pathway [53, 54]. DCs in the tumor microenvironment can take up double‐stranded DNA (dsDNA) from dying tumor cells after radiotherapy, although the detailed mechanisms remain debatable, subsequently activating the cGAS‐STING interferonβ (IFNβ) pathway in DCs and evoking an immune response [53, 55, 56]. However, following radiotherapy, cancer cells also produce adequate cytosolic dsDNA which comes from damaged mitochondria, binds endogenous cGAS, activates the downstream STING‐IFNβ pathway, and promotes tumor immunity [57, 58]. Additionally, an intact cGAS‐STING pathway in irradiated tumor cells is indispensable for irradiation‐provoked abscopal effects. Although anti‐PD‐1/PD‐L1 immunotherapy depends on adequate T cell function in the tumor microenvironment, Fu et al. [59] demonstrated that, in the context of a mismatch repair‐deficient (dMMR) background, a competent cGAS‐STING‐IFNβ pathway within tumor cells is required for tumor suppression.
In brief, better knowledge of the immune mechanisms of radiotherapy may result in more efficient and effective use of RT, and additionally provide information for the design of iRT studies, opening up new avenues for cancer therapy.
5. LOW‐DOSE RT TO BOOST THE EFFICACY OF iRT: UPDATES AND CHALLENGES
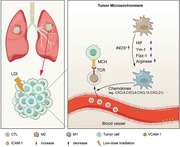
A major deterrent to the efficacy of immunotherapy is that the tumor microenvironment is suboptimally conducive to T cell engraftment. Vascular barriers, lack of appropriate cytokines, and stromal immunosuppressive factors may play important roles in inhibiting T cell infiltration and exerting an antitumor effect [60, 61]. High‐dose RT (conventionally fractionated or hypofractionated (including SBRT)) helps to induce the production and release of cytokines and chemokines by killing tumor cells, thereby creating an inflammatory microenvironment in the context of immunogenic cell death to promote T cell infiltration [62, 63, 64]. However, high‐dose RT cannot largely address potent immunosuppressive factors such as the inhibitory tumor stroma. As a result, there has been a recently posited theory that low‐dose irradiation (LDI) may address these limitations. There is no standard definition of LDI but it most commonly involves intentional delivery of 0.5‐2 Gy per fraction up to 1‐10 Gy total dose [65, 66, 67, 68], which is canonically thought to be non‐tumoricidal.
Radiobiological data of LDI also support additional synergistic effects of LDI. Studies using dynamic microscopic imaging, a technique that allows experiments to be visualized in real‐time, have confirmed that X‐rays ranging from 0.1 Gy can also kill some tumor cells owing to a phenomenon called radiotherapy hypersensitivity [69, 70, 71]. In some clinical studies, LDI used in combination with chemotherapy and administered at a dose to induce radiation hypersensitivity has achieved surprising rates of tumor control [72, 73, 74]. Therefore, the combination of LDI with other treatments, such as chemotherapy or immunotherapy, may be a new therapeutic approach for patients.
Recent studies in mouse models have shown that a single fraction of LDI can reshape the tumor microenvironment, including the polarization of M1 macrophages [75, 76]. Inducible nitric oxide synthase‐positive (iNOS+) M1 macrophages can produce chemokines to recruit effector T cells and cause normalization of tumor vessels and the inflammatory response, inducing T cell infiltration [77, 78, 79], as shown in Figure 2. The clinical benefit conferred by LDI‐mediated remodeling of macrophages was verified in a retrospective study of pancreatic cancer patients who had previously received LDI as neoadjuvant therapy. In these patients, LDI could significantly increase the ratio of iNOS+ macrophages to CD8+ T cells, and reduce the average diameter of tumor blood vessels [77]. By contrast, LDI can actually attenuate inflammatory lesions, as observed in patients with benign inflammation or degenerative diseases caused by autoimmune T cells [80]. Therefore, further clinical studies comparing the effects of LDI on the tumor microenvironment are required to confirm the findings in mouse models and establish an optimal range of LDI doses that can be used to reshape macrophages and improve T cell infiltration.
FIGURE 2.
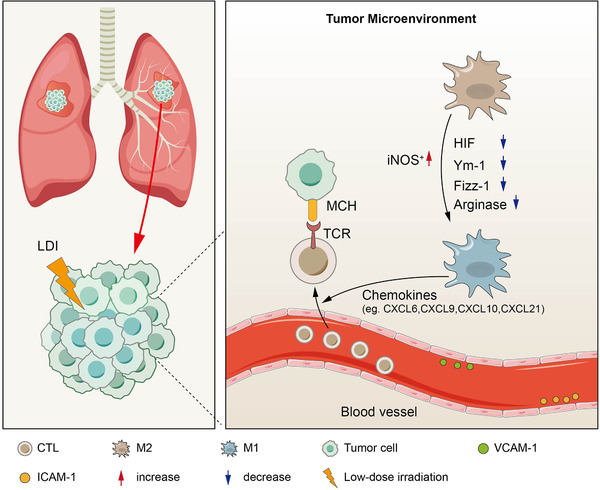
Low‐dose irradiation remodels the tumor microenvironment. Two main mechanisms exist by which radiation enhances tumor‐infiltrating lymphocytes (TILs). One is increased expression of chemokines that enhance immune cell migration and invasion, and the other relates to changes in the vascular endothelium that enhance immune cell extravasation. Low‐dose irradiation (LDI) induces M1 macrophage polarization by regulating the corresponding molecules, such as inducible nitric oxide synthase‐positive (iNOS+), Hypoxia‐inducible factor‐1 (HIF‐1), chitinase‐like‐3 (Ym‐1), Found in the inflammatory zone‐1 (Fizz‐1), Arginase, and iNOS+ M1 macrophages, which produce chemokines to recruit effector T cells and cause T cell infiltration. LDI increases vascular cell adhesion molecule 1 (VCAM‐1) and intercellular adhesion molecule‐1 (ICAM‐1) expression in human vascular endothelial cells, causing normalization of tumor vessels. Abbreviations: low‐dose irradiation, LDI; tumor‐infiltrating lymphocytes, TILs; inducible nitric oxide synthase‐positive, iNOS+; Hypoxia‐inducible factor‐1, HIF‐1; chitinase‐like‐3, Ym‐1; found in inflammatory zone‐1, Fizz‐1; M1 macrophages, M1; M2 macrophages, M2; vascular cell adhesion molecule 1, VCAM‐1; intercellular adhesion molecule‐1, ICAM‐1; major histocompatibility complex, MHC; T‐cell receptor, TCR; cytotoxic T‐lymphocyte, CTL; C‐X‐C motif chemokine, CXCL
Although it has been proven that in situ vaccine effects and abscopal effects are triggered by relatively high doses of hypofractionated radiotherapy, no direct evidence has indicated that LDI can trigger the same effects. However, LDI can remodel the tumor microenvironment and facilitate T cell homing in patients lacking tumor‐infiltrating CD8+ T cells [77, 81]. LDI may be important in the preparation phase of inducing T cell homing in combination with ICI therapy, as described above. Comparing the efficacy of different LDI schemes in combination with ICIs must be investigated in future clinical trials. Such strategies could be used as a palliative option for patients who are refractory to other treatments, including ICIs, which can reshape the tumor microenvironment to induce new antitumor responses. Eventually, a combination of high‐dose SBRT used to trigger an in situ vaccine effect in a few metastatic lesions, together with LDI used to target other metastatic lesions to promote T cell attack, could maximize the abscopal effect.
Although LDI cannot kill tumor cells, it can activate immune cells and regulate the tumor microenvironment to improve the efficacy of immunotherapy. A recently completed clinical trial in which SBRT in combination with ipilimumab was used to treat advanced metastatic lesions found that tumors exposed to LDI (due to proximity to the target tumor) were more likely to respond than lesions distant from the targeted tumor [82]. Based on this finding, our team has developed a new treatment paradigm combining high‐dose radiotherapy and LDI to promote the efficacy of systemic immunotherapy [66]. In this model, high‐dose radiation aims to increase antigen release and presentation and promote immune cell activation, while LDI aims to promote immune cell infiltration into the stroma and tumor bed of distant tumors. The results of a nonrandomized phase II trial using both LDI and high‐dose RT in conjunction with immunotherapy showed that the areas of disease exposed to LDI more often responded locally than those not subjected to LDI [65].
Taken together, LDI offers an emerging approach to address the known mechanistic limitations of higher‐dose RT as part of an iRT paradigm. Much more extensive investigation is required, but nevertheless, this approach remains an important method to base future study.
6. FURTHER CHALLENGES
Although iRT shows promising efficacy for clinical application, the treatment efficacy still needs to be further optimized. Additionally, exploring the optimized dose and fractionation of radiotherapy, sequencing of therapies, and further exploring the mechanistic interaction between radiotherapy and immunotherapy may provide more effective combined therapeutic options in the future.
7. CONCLUSIONS
This review assessed and discussed several strategies to enhance the efficacy of combining RT and immunotherapy for advanced NSCLC. These include better elucidation on clinical and pathologic biomarkers that may improve patient selection, along with an increased mechanistic understanding of using RT to overcome immunotherapy resistance, as well as low‐dose RT to enhance immune infiltration into tumors. Taken together, given the current state of this rapidly expanding realm, these futuristic strategies may be reflected upon to further enhance the efficacy of immunotherapy for a wider group of patients than currently exists.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
All authors conceived, directed, and revised this manuscript.
ACKNOWLEDGMENTS
The study was supported by funds from The National Key Research and Development Projects of China (2018YFC1312201), Radiation Oncology Innovate Unit, Chinese Academy of Medical Sciences (2019RU071), Academic Promotion Program of Shandong First Medical University (2019ZL002), Foundation of National Natural Science Foundation of China (81972863, 81627901 and 82030082) and Science Foundation of Shandong (ZR2020 LZL016).
Shang S, Liu J, Verma V, Wu M, Welsh J, Yu J, et al. Combined treatment of non‐small cell lung cancer using radiotherapy and immunotherapy: challenges and updates. Cancer Commun. 2021;41:1086–1099. 10.1002/cac2.12226
Shijie Shang and Jie Liu contributed equally to this work.
Contributor Information
Jinming Yu, Email: sdyujinming@163.com.
Dawei Chen, Email: dave0505@yeah.net.
REFERENCES
- 1. Weiner LM. Cancer immunotherapy–the endgame begins. N Engl J Med. 2008;358(25):2664–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated Analysis of KEYNOTE‐024: Pembrolizumab Versus Platinum‐Based Chemotherapy for Advanced Non‐Small‐Cell Lung Cancer With PD‐L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019;37(7):537–46. [DOI] [PubMed] [Google Scholar]
- 3. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. [DOI] [PubMed] [Google Scholar]
- 4. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab Plus Chemotherapy for First‐Line Treatment of Nonsquamous NSCLC: Results From the Randomized Phase 3 IMpower132 Trial. J Thorac Oncol. 2021;16(4):653–64. [DOI] [PubMed] [Google Scholar]
- 5. Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem Immune‐Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non‐Small Cell Lung Cancer. JAMA Oncol. 2020;6(12):1952–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five‐Year Overall Survival for Patients With Advanced Non‒Small‐Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE‐001 Study. J Clin Oncol. 2019;37(28):2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell. 2020;37(4):443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti‐angiogenesis in cancer treatment. Mol Cancer. 2019;18(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kon E, Benhar I. Immune checkpoint inhibitor combinations: Current efforts and important aspects for success. Drug Resist Updat. 2019;45:13–29. [DOI] [PubMed] [Google Scholar]
- 10. Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA‐4 blockade. Nat Med. 2018;24(12):1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hui R, Özgüroğlu M, Villegas A, Daniel D, Vicente D, Murakami S, et al. Patient‐reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non‐small‐cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(12):1670–80. [DOI] [PubMed] [Google Scholar]
- 12. Faivre‐Finn C, Vicente D, Kurata T, Planchard D, Paz‐Ares L, Vansteenkiste JF, et al. Four‐Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC‐an Update From the PACIFIC Trial. J Thorac Oncol. 2021;16(5):860–67. [DOI] [PubMed] [Google Scholar]
- 13. Jabbour SK, Lee KH, Frost N, Breder V, Kowalski DM, Pollock T, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non‐Small Cell Lung Cancer: The Phase 2 KEYNOTE‐799 Nonrandomized Trial. JAMA Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26(305):234–41. [DOI] [PubMed] [Google Scholar]
- 15. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non‐Small Cell Lung Cancer: Results of the PEMBRO‐RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5(9):1276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Theelen W, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts J, et al. Pembrolizumab with or without radiotherapy for metastatic non‐small‐cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9(5):467–75. [DOI] [PubMed] [Google Scholar]
- 18. Welsh J, Menon H, Chen D, Verma V, Tang C, Altan M, et al. Pembrolizumab with or without radiation therapy for metastatic non‐small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non‐Small‐Cell Lung Cancer. N Engl J Med. 2017;377(20):1919–29. [DOI] [PubMed] [Google Scholar]
- 20. Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD‐1/PD‐L1 expression and tumour mutational burden: a systematic review‐based approach. Ann Oncol. 2019;30(8):1232–43. [DOI] [PubMed] [Google Scholar]
- 21. Schoenfeld AJ, Rizvi H, Bandlamudi C, Sauter JL, Travis WD, Rekhtman N, et al. Clinical and molecular correlates of PD‐L1 expression in patients with lung adenocarcinomas. Ann Oncol. 2020;31(5):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor‐based immunotherapy. Lancet Oncol. 2016;17(12):e542–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kordbacheh T, Honeychurch J, Blackhall F, Faivre‐Finn C, Illidge T. Radiotherapy and anti‐PD‐1/PD‐L1 combinations in lung cancer: building better translational research platforms. Ann Oncol. 2018;29(2):301–10. [DOI] [PubMed] [Google Scholar]
- 24. Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD‐1 Inhibition. N Engl J Med. 2017;377(25):2500–01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science. 2015;348(6230):124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu S, Stein JE, Rimm DL, Wang DW, Bell JM, Johnson DB, et al. Comparison of Biomarker Modalities for Predicting Response to PD‐1/PD‐L1 Checkpoint Blockade: A Systematic Review and Meta‐analysis. JAMA Oncol. 2019;5(8):1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen D, Patel RR, Verma V, Ramapriyan R, Barsoumian HB, Cortez MA, et al. Interaction between lymphopenia, radiotherapy technique, dosimetry, and survival outcomes in lung cancer patients receiving combined immunotherapy and radiotherapy. Radiother Oncol. 2020;150:114–20. [DOI] [PubMed] [Google Scholar]
- 29. Chen D, Verma V, Patel RR, Barsoumian HB, Cortez MA, Welsh JW. Absolute Lymphocyte Count Predicts Abscopal Responses and Outcomes in Patients Receiving Combined Immunotherapy and Radiation Therapy: Analysis of 3 Phase 1/2 Trials. Int J Radiat Oncol Biol Phys. 2020;108(1):196–203. [DOI] [PubMed] [Google Scholar]
- 30. Gomez DR, Tang C, Zhang J, Blumenschein GR, Jr. , Hernandez M, Lee JJ, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non‐Small‐Cell Lung Cancer: Long‐Term Results of a Multi‐Institutional, Phase II, Randomized Study. J Clin Oncol. 2019;37(18):1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative Radiotherapy for Limited Metastatic Non‐Small‐Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018;4(1):e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Twyman‐Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non‐redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dovedi SJ, Adlard AL, Lipowska‐Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD‐L1 blockade. Cancer Res. 2014;74(19):5458–68. [DOI] [PubMed] [Google Scholar]
- 35. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti‐PD‐L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Galluzzi L, Zitvogel L, Kroemer G. Immunological Mechanisms Underneath the Efficacy of Cancer Therapy. Cancer Immunol Res. 2016;4(11):895–902. [DOI] [PubMed] [Google Scholar]
- 37. Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1(9):1325–32. [DOI] [PubMed] [Google Scholar]
- 38. Ross GM. Induction of cell death by radiotherapy. Endocr Relat Cancer. 1999;6(1):41–4. [DOI] [PubMed] [Google Scholar]
- 39. Santivasi WL, Xia F. Ionizing radiation‐induced DNA damage, response, and repair. Antioxid Redox Signal. 2014;21(2):251–9. [DOI] [PubMed] [Google Scholar]
- 40. Hu ZI, McArthur HL, Ho AY. The Abscopal Effect of Radiation Therapy: What Is It and How Can We Use It in Breast Cancer? Curr Breast Cancer Rep. 2017;9(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356(1):82–90. [DOI] [PubMed] [Google Scholar]
- 42. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14(6):365–79. [DOI] [PubMed] [Google Scholar]
- 43. Koukourakis MI, Koukouraki S, Giatromanolaki A, Archimandritis SC, Skarlatos J, Beroukas K, et al. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non‐small‐cell lung cancer and head and neck cancer. J Clin Oncol. 1999;17(11):3512–21. [DOI] [PubMed] [Google Scholar]
- 44. Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63(5):1229–35. [PubMed] [Google Scholar]
- 45. Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci. 2014;15(1):927–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rückert M, Deloch L, Frey B, Schlücker E, Fietkau R, Gaipl US. Combinations of Radiotherapy with Vaccination and Immune Checkpoint Inhibition Differently Affect Primary and Abscopal Tumor Growth and the Tumor Microenvironment. Cancers (Basel). 2021;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pustylnikov S, Costabile F, Beghi S, Facciabene A. Targeting mitochondria in cancer: current concepts and immunotherapy approaches. Transl Res. 2018;202:35–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S, et al. Mitochondrial activation chemicals synergize with surface receptor PD‐1 blockade for T cell‐dependent antitumor activity. Proc Natl Acad Sci U S A. 2017;114(5):E761–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ashton TM, McKenna WG, Kunz‐Schughart LA, Higgins GS. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin Cancer Res. 2018;24(11):2482–90. [DOI] [PubMed] [Google Scholar]
- 51. Le M, McNeill FE, Seymour CB, Rusin A, Diamond K, Rainbow AJ, et al. Modulation of oxidative phosphorylation (OXPHOS) by radiation‐ induced biophotons. Environ Res. 2018;163:80–87. [DOI] [PubMed] [Google Scholar]
- 52. Chen D, Barsoumian HB, Fischer G, Yang L, Verma V, Younes AI, et al. Combination treatment with radiotherapy and a novel oxidative phosphorylation inhibitor overcomes PD‐1 resistance and enhances antitumor immunity. J Immunother Cancer. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vanpouille‐Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy‐induced tumour immunogenicity. Nat Commun. 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING‐Dependent Cytosolic DNA Sensing Promotes Radiation‐Induced Type I Interferon‐Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kwon J, Bakhoum SF. The Cytosolic DNA‐Sensing cGAS‐STING Pathway in Cancer. Cancer Discov. 2020;10(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING‐dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schadt L, Sparano C, Schweiger NA, Silina K, Cecconi V, Lucchiari G, et al. Cancer‐Cell‐Intrinsic cGAS Expression Mediates Tumor Immunogenicity. Cell Rep. 2019;29(5):1236–48.e7. [DOI] [PubMed] [Google Scholar]
- 58. Chen J, Cao Y, Markelc B, Kaeppler J, Vermeer JA, Muschel RJ. Type I IFN protects cancer cells from CD8+ T cell‐mediated cytotoxicity after radiation. J Clin Invest. 2019;129(10):4224–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu C, Guan J, Lu S, Jin Q, Rousseau B, Lu T, et al. DNA Sensing in Mismatch Repair‐Deficient Tumor Cells Is Essential for Anti‐tumor Immunity. Cancer Cell. 2021;39(1):96–108.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498–509. [DOI] [PubMed] [Google Scholar]
- 62. McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20(4):203–17. [DOI] [PubMed] [Google Scholar]
- 63. Darragh LB, Oweida AJ, Karam SD. Overcoming Resistance to Combination Radiation‐Immunotherapy: A Focus on Contributing Pathways Within the Tumor Microenvironment. Front Immunol. 2018;9:3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Donlon NE, Power R, Hayes C, Reynolds JV, Lysaght J. Radiotherapy, immunotherapy, and the tumour microenvironment: Turning an immunosuppressive milieu into a therapeutic opportunity. Cancer Lett. 2021;502:84–96. [DOI] [PubMed] [Google Scholar]
- 65. Patel RR, He K, Barsoumian HB, Chang JY, Tang C, Verma V, et al. High‐dose irradiation in combination with non‐ablative low‐dose radiation to treat metastatic disease after progression on immunotherapy: Results of a phase II trial. Radiother Oncol. 2021;162:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Menon H, Chen D, Ramapriyan R, Verma V, Barsoumian HB, Cushman TR, et al. Influence of low‐dose radiation on abscopal responses in patients receiving high‐dose radiation and immunotherapy. J Immunother Cancer. 2019;7(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yin L, Xue J, Li R, Zhou L, Deng L, Chen L, et al. Effect of Low‐Dose Radiation Therapy on Abscopal Responses to Hypofractionated Radiation Therapy and Anti‐PD1 in Mice and Patients With Non‐Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2020;108(1):212–24. [DOI] [PubMed] [Google Scholar]
- 68. Barsoumian HB, Ramapriyan R, Younes AI, Caetano MS, Menon H, Comeaux NI, et al. Low‐dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low‐dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001;49(2):379–89. [DOI] [PubMed] [Google Scholar]
- 70. Short S, Mayes C, Woodcock M, Johns H, Joiner MC. Low dose hypersensitivity in the T98G human glioblastoma cell line. Int J Radiat Biol. 1999;75(7):847–55. [DOI] [PubMed] [Google Scholar]
- 71. Marples B, Lambin P, Skov KA, Joiner MC. Low dose hyper‐radiosensitivity and increased radioresistance in mammalian cells. Int J Radiat Biol. 1997;71(6):721–35. [DOI] [PubMed] [Google Scholar]
- 72. Mantini G, Valentini V, Meduri B, Margaritora S, Balducci M, Micciché F, et al. Low‐dose radiotherapy as a chemo‐potentiator of a chemotherapy regimen with pemetrexed for recurrent non‐small‐cell lung cancer: a prospective phase II study. Radiother Oncol. 2012;105(2):161–6. [DOI] [PubMed] [Google Scholar]
- 73. Regine WF, Hanna N, Garofalo MC, Doyle A, Arnold S, Kataria R, et al. Low‐dose radiotherapy as a chemopotentiator of gemcitabine in tumors of the pancreas or small bowel: a phase I study exploring a new treatment paradigm. Int J Radiat Oncol Biol Phys. 2007;68(1):172–7. [DOI] [PubMed] [Google Scholar]
- 74. Balducci M, Chiesa S, Diletto B, D'Agostino GR, Mangiola A, Manfrida S, et al. Low‐dose fractionated radiotherapy and concomitant chemotherapy in glioblastoma multiforme with poor prognosis: a feasibility study. Neuro Oncol. 2012;14(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Deloch L, Fuchs J, Rückert M, Fietkau R, Frey B, Gaipl US. Low‐Dose Irradiation Differentially Impacts Macrophage Phenotype in Dependence of Fibroblast‐Like Synoviocytes and Radiation Dose. J Immunol Res. 2019;2019:3161750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nadella V, Singh S, Jain A, Jain M, Vasquez KM, Sharma A, et al. Low dose radiation primed iNOS + M1macrophages modulate angiogenic programming of tumor derived endothelium. Mol Carcinog. 2018;57(11):1664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low‐dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. [DOI] [PubMed] [Google Scholar]
- 78. Prakash H, Klug F, Nadella V, Mazumdar V, Schmitz‐Winnenthal H, Umansky L. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor‐associated macrophages: lesson from insulinoma. Carcinogenesis. 2016;37(3):301–13. [DOI] [PubMed] [Google Scholar]
- 79. De Palma M, Coukos G, Hanahan D. A new twist on radiation oncology: low‐dose irradiation elicits immunostimulatory macrophages that unlock barriers to tumor immunotherapy. Cancer Cell. 2013;24(5):559–61. [DOI] [PubMed] [Google Scholar]
- 80. Rödel F, Frey B, Gaipl U, Keilholz L, Fournier C, Manda K, et al. Modulation of inflammatory immune reactions by low‐dose ionizing radiation: molecular mechanisms and clinical application. Curr Med Chem. 2012;19(12):1741–50. [DOI] [PubMed] [Google Scholar]
- 81. Liu J, Zhou J, Wu M, Hu C, Yang J, Li D, et al. Low‐Dose Total Body Irradiation Can Enhance Systemic Immune Related Response Induced by Hypo‐Fractionated Radiation. Front Immunol. 2019;10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Welsh JW, Tang C, de Groot P, Naing A, Hess KR, Heymach JV, et al. Phase II Trial of Ipilimumab with Stereotactic Radiation Therapy for Metastatic Disease: Outcomes, Toxicities, and Low‐Dose Radiation‐Related Abscopal Responses. Cancer Immunol Res. 2019;7(12):1903–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


