Abbreviations
- ALK
anaplastic lymphoma kinase
- ARMS
amplification refractory mutation system
- EGFR
epidermal growth factor receptor
- MLR
monocyte‐to‐lymphocyte ratio
- NGS
Next‐Generation Sequencing
- NLR
neutrophil‐to‐lymphocyte ratio
- NSCLC
non‐small cell lung cancer
- OS
overall survival
- PD‐1
programmed cell death‐1
- PDL1
programmed cell death‐ligand 1
- PFS
progression‐free survival
- PLR
Platelet‐to‐lymphocyte ratio
- STK11
serine threonine kinase 11
- TP53
tumor protein p53
Dear Editor,
The RAS gene is one of the most frequent oncogenes in human cancers, with significantly different mutation frequencies. The RAS family contains three isoforms: KRAS, HRAS and NRAS, with the KRAS mutations being more common than the other two. The KRAS mutation rate varies in non‐small cell lung cancer (NSCLC) patients of different races: 27% in Caucasians [1] and approximately 10% in Asians [2, 3]. Recently, the U.S. Food and Drug Administration (FDA) approved sotorasib (AMG510) and adagrasib (MRTX849) for the treatment of metastatic NSCLC harboring KRAS G12C mutations. In the face of novel treatment choices for KRAS‐mutated NSCLC, it is indispensable to learn more about the systemic treatment of these patients. Clinical studies have shown that Caucasian patients with KRAS‐mutated NSCLC had poor outcomes following first‐line chemotherapy [4, 5]. However, studies on treatment outcomes of Asian patients with KRAS‐mutated NSCLC are lacking. As the overwhelming majority of cases are diagnosed with lung adenocarcinoma, the standard first‐line treatment for metastatic disease is pemetrexed‐based doublet chemotherapy in combination with bevacizumab and/or immunotherapy in China. Herein, we investigated the clinical characteristics of KRAS mutation subtypes, co‐occurring genomic alterations, and efficacy of first‐line pemetrexed‐platinum chemotherapy in Chinese KRAS‐mutated NSCLC patients.
The data of 5180 patients with NSCLC (either de novo or relapsed) who underwent genetic testing at Shandong Cancer Hospital between January 2016 and October 2020 were reviewed (Supplementary Figure S1). Data on clinical features, mutation subtypes, and co‐mutations of KRAS‐mutated lung cancer were collected. A paired study was also performed to evaluate the effect of chemotherapy in advanced‐stage patients with KRAS mutations. The prognostic factors of advanced‐stage KRAS‐mutated patients were analyzed. Patient selection and assessment protocols are detailed in the Supplementary Material.
Of the 5180 NSCLC patients screened, 477 had RAS mutations, and 471 had KRAS mutations. Among the 1239 patients who underwent amplification refractory mutation system (ARMS)‐PCR testing, 103 had KRAS mutations (8.3%), and two had NRAS mutations (Supplementary Figure S2). In the other 3941 patients who underwent next‐generation sequencing (NGS) testing, 368 had KRAS mutations (9.3%), and one of them had KRAS and NRAS co‐mutations. In addition, three patients had NRAS mutations, and one had an HRAS mutation.
The characteristics of the 1239 patients who underwent ARMS‐PCR testing are shown in Supplementary Table S1. Compared with patients with epidermal growth factor receptor (EGFR) mutations, those with KRAS mutations had a higher proportion of smoking history (n = 64, 62.1%), males (n = 84, 81.6%), and were older (63.0 ± 9.2 years). The relationship between gene mutation profiles and brain metastasis at the time of diagnosis was analyzed, and we observed that T stage, N stage, pathological type, and EGFR mutation were predictive of brain metastasis, while KRAS mutation was not a risk factor (P = 0.819) (Supplementary Table S2).
The RAS mutation subtypes in 372 patients who underwent NGS testing were analyzed. The three most common RAS mutation sites were KRAS G12C (n = 106, 28.5%), G12D (n = 79, 21.1%), and G12V (n = 75, 20.2%) (Supplementary Table S3 and Figure 1A). Notably, 2.7% (n = 10) of RAS‐mutated patients had two RAS mutation sites. KRAS‐mutated NSCLC is reported to have a high frequency of co‐mutations in cancer‐associated pathways [6]. Co‐mutation information was available for 215 patients, and tumor protein p53 (TP53) (n = 107, 49.8%) and serine threonine kinase 11 (STK11) (n = 40, 18.6%) were the most common mutated genes in RAS‐mutated NSCLC (Figure 1B).
FIGURE 1.
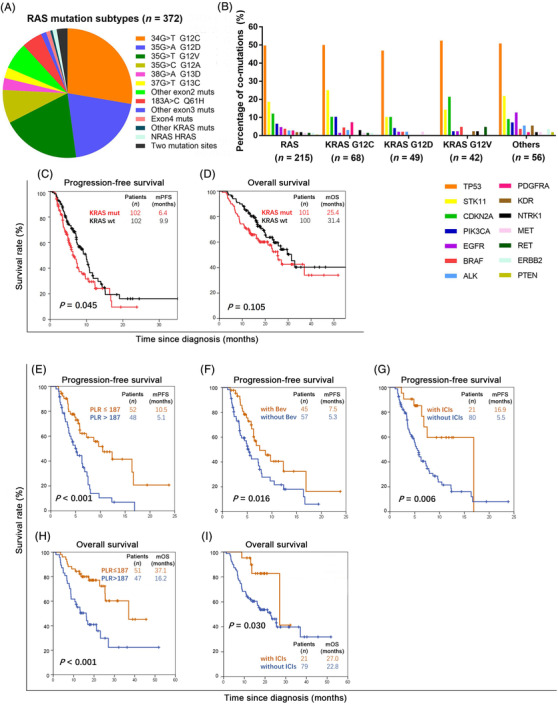
Clinical characteristics and chemotherapy outcomes in patients with KRAS‐mutated NSCLC. A. RAS mutation subtypes of 372 NSCLC patients who underwent NGS. B. Co‐mutations in patients with RAS‐mutated NSCLC. C. PFS of KRAS‐mutated and KRAS‐wild‐type patients. D. OS of KRAS‐mutated and KRAS‐wild‐type patients. E. PFS curve of KRAS‐mutated patients based on PLR. F. PFS curve of KRAS‐mutated patients based on the administration of bevacizumab combination. G. PFS curve of KRAS‐mutated patients based on the administration of immunotherapy combination. H. OS curve of KRAS‐mutated patients based on PLR. I. OS curve of KRAS‐mutated patients based on the administration of immunotherapy combination.
Abbreviations: NSCLC, non‐small cell lung cancer; NGS, next‐generation sequencing; KRAS, Kirsten rat sarcoma viral oncogene homolog; NRAS, Neuroblastoma rat sarcoma viral oncogene homolog; HRAS, Harvey rat sarcoma viral oncogene homolog; RAS, rat sarcoma gene; PFS, progression‐free survival; OS, overall survival; mut, mutation; wt, wild‐type; PLR, platelet‐to‐lymphocyte ratio; bev, bevacizumab; ICIs, immune checkpoint inhibitors
Next, KRAS‐mutated and KRAS‐wild‐type patients who had received pemetrexed‐based doublet chemotherapy as first‐line therapy were selected. Patients in the two groups were matched for the shown clinical features (Supplementary Table S4). After a median follow‐up period of 19.7 (95% confidence interval [CI] 17.5–21.8) months, the median progression‐free survival (PFS) in the KRAS‐mutated group and KRAS wild‐type group was 6.4 (95% CI 5.1–7.7) and 9.9 (95% CI 7.7–12.1) months, respectively, and the difference was significant (P = 0.045) (Figure 1C). KRAS‐mutated patients tended to have shorter median overall survival (OS), but the difference was not significant (25.4 vs. 31.4 months, P = 0.105) (Figure 1D).
The prognostic factors of KRAS‐mutated patients who had received pemetrexed‐platinum chemotherapy were further analyzed using univariate and multivariate analyses (Supplementary Table S5 and S6). The platelet‐to‐lymphocyte ratio (PLR) (hazard ratio [HR] = 2.38, P = 0.019), bevacizumab combination (HR = 0.52, P = 0.019), and immunotherapy combination (HR = 0.30, P = 0.004) were independent predictive factors for PFS (Figure 1E–G). KRAS mutation type was not an independent predictive factor for PFS; however, patients with KRAS G12C mutation had the shortest median PFS (4.5 months, 95% CI 0–9.1 months) (Supplementary Figure S3A). PLR (HR = 2.55, P = 0.030) and immunotherapy combination (HR = 0.31, P = 0.025) were independent influencing factors of OS (Figure 1H and I).
In this study, the KRAS mutation rate was 8.8% in patients who underwent ARMS‐PCR testing and 9.3% in those who underwent NGS testing, which is in accordance with a previous study [2]. Only seven patients had NRAS or HRAS mutations. KRAS‐mutated cancer patients tended to be male and elderly, 62.1% had a history of smoking while Caucasian patients comprised of a higher proportion of females (58%) and history of smoking (93%) [1]. Whether the KRAS mutation subtype is associated with survival remains controversial [7, 8]. Although the KRAS G12C group was associated with shortest PFS in this study, no survival difference in different KRAS mutation subtypes was found.
The therapeutic response and survival of lung cancer patients without EGFR or anaplastic lymphoma kinase (ALK) driver gene alterations receiving first‐line chemotherapy are difficult to predict. Recently, He et al. [9] showed that molecular signatures could only provide limited information. In this study, NSCLC patients were classified into four subtypes based on genomic alteration characteristics, however, no survival difference between the subtypes were observed. Our findings showed that NSCLC patients with KRAS mutations had worse PFS after initial pemetrexed‐based platinum doublets than those without driver genes, and the survival difference seems mainly from those receiving chemotherapy alone and those with bevacizumab combination (Supplementary Figure S3B and S3C). When comparing the PFS of those with immunotherapy combination in the two groups (Supplementary Figure S3D), the survival curves almost overlapped. In addition, immunotherapy combination was an independent influencing factor for both PFS and OS in KRAS‐mutated NSCLC patients. Therefore, a combination of chemotherapy and immunotherapy could be recommended to improve the efficacy of these patients. PLR, neutrophil‐to‐lymphocyte ratio (NLR), and monocyte‐to‐lymphocyte ratio (MLR) are factors that reflect systemic inflammation, and studies have shown their relationship with poor prognosis in NSCLC [10]. Here, we also found that these factors were predictive in KRAS‐mutated NSCLC, and PLR was an independent influencing factor for both PFS and OS.
The limitation of this study was that its retrospective nature, and the drugs used as anti‐programmed cell death‐1 (PD‐1) or programmed cell death‐ligand 1 (PD‐L1) immunotherapy were diverse. In addition, the ARMS‐PCR test was performed in 23.9% (n = 1239) of NSCLC patients screened. Although paired analyses were performed to ensure comparability between the clinical characteristics of the KRAS‐mutated and KRAS‐wild‐type groups, there could remain unavoidable risk of selection bias.
In summary, the KRAS mutation rate in Chinese NSCLC patients seemed lower than that in Caucasians, and the proportion of KRAS G12C mutation was no more than 30%. Compared with KRAS wild‐type patients, KRAS‐mutated patients had worse PFS after pemetrexed‐platinum chemotherapy. Lastly, a combination of chemotherapy and immunotherapy could improve the survival of patients with KRAS‐mutated lung adenocarcinoma.
FUNDING
This work was financially supported by grants from the National Key Research and Development Project (2018YFC1313200), the Shandong Natural Science Foundation (ZR2019LZL019), the Jinan Scientific and Technology Development Project (201805005 and 201907122), Shandong Medical and Health Science and Technology Development Plan (202009030748), and the Youth Training Program for High‐level Projects of Jinan Central Hospital (202105002).
AUTHORS' CONTRIBUTIONS
YWZ and LGX designed the research. YWZ, QHL, and XRS acquired the data and performed patients’ selection process. YWZ and QHL analyzed the data and wrote the manuscript. XLL and HXZ reviewed the medical images. XRS and LGX revised the manuscript. All authors critically reviewed the manuscript and approved the contents.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and approved by Shandong Cancer Hospital Shandong Cancer Hospital and Institute (No. SDTHEC2020010012).
Supporting information
Supporting Information
Figure S1
Figure S2
Figure S3
ACKNOWLEDGMENTS
The authors are thankful to all the patients included and their clinicians in charge.
Contributor Information
Xiaorong Sun, Email: 251400067@qq.com.
Ligang Xing, Email: xinglg@medmail.com.cn.
REFERENCES
- 1. El OB, Behera M, Kim S, Berry LD, Sica G, Pillai RN, et al. Characteristics and outcomes of patients with metastatic KRAS‐mutant lung adenocarcinomas: the lung cancer mutation consortium experience. J Thorac Oncol. 2019;14(5):876‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu SY, Sun H, Zhou JY, Jie GL, Xie Z, Shao Y, et al. Clinical characteristics and prognostic value of the KRAS G12C mutation in Chinese non‐small cell lung cancer patients. Biomark Res. 2020;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Y, Li H, Zhu J, Zhang Y, Liu X, Li R, et al. The prevalence and concurrent pathogenic mutations of KRAS (G12C) in northeast chinese non‐small‐cell lung cancer patients. Cancer Manag Res. 2021;13:2447‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ricciuti B, Brambilla M, Cortellini A, De Giglio A, Ficorella C, Sidoni A, et al. Clinical outcomes to pemetrexed‐based versus non‐pemetrexed‐based platinum doublets in patients with KRAS‐mutant advanced non‐squamous non‐small cell lung cancer. Clin Transl Oncol. 2020;22(5):708‐716. [DOI] [PubMed] [Google Scholar]
- 5. Ghimessy AK, Gellert A, Schlegl E, Hegedus B, Raso E, Barbai T, et al. KRAS mutations predict response and outcome in advanced lung adenocarcinoma patients receiving first‐line bevacizumab and platinum‐based chemotherapy. Cancers (Basel). 2019;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scheffler M, Ihle MA, Hein R, Merkelbach‐Bruse S, Scheel AH, Siemanowski J, et al. K‐ras mutation subtypes in NSCLC and associated co‐occuring mutations in other oncogenic pathways. J Thorac Oncol. 2019;14(4):606‐616. [DOI] [PubMed] [Google Scholar]
- 7. Arbour KC, Rizvi H, Plodkowski AJ, Hellmann MD, Knezevic A, Heller G, et al. Treatment outcomes and clinical characteristics of patients with KRAS‐G12C‐mutant non‐small cell lung cancer. Clin Cancer Res. 2021;27(8):2209‐2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finn SP, Addeo A, Dafni U, Thunnissen E, Bubendorf L, Madsen LB, et al. Prognostic impact of KRAS G12C mutation in patients with NSCLC: results from the ETOP Lungscape Project. J Thorac Oncol. 2021. [DOI] [PubMed] [Google Scholar]
- 9. He Y, Song L, Wang H, Chen P, Liu Y, Sun H, et al. Mutational profile evaluates response and survival to first‐line chemotherapy in lung cancer. Adv Sci (Weinh). 2021;8(4):2003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Zeng WH, Zhou YQ, Ji YY, Li WZ, Zhang LY, et al. Neutrophil‐to‐lymphocyte ratio predicted long‐term chemotherapy benefits in stage IIIB‐IV non‐squamous non‐small cell lung cancer patients without sensitive mutations. Onco Targets Ther. 2019;12:8779‐8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Figure S1
Figure S2
Figure S3


