Abstract
Nasopharyngeal carcinoma (NPC) is a malignant epithelial tumor originating in the nasopharynx and has a high incidence in Southeast Asia and North Africa. To develop these comprehensive guidelines for the diagnosis and management of NPC, the Chinese Society of Clinical Oncology (CSCO) arranged a multi‐disciplinary team comprising of experts from all sub‐specialties of NPC to write, discuss, and revise the guidelines. Based on the findings of evidence‐based medicine in China and abroad, domestic experts have iteratively developed these guidelines to provide proper management of NPC. Overall, the guidelines describe the screening, clinical and pathological diagnosis, staging and risk assessment, therapies, and follow‐up of NPC, which aim to improve the management of NPC.
Keywords: Chinese Society of Clinical Oncology, CSCO, Nasopharyngeal carcinoma, Diagnosis, Staging, Risk, Radiotherapy, Chemotherapy, Surgery, Immunotherapy
Abbreviations
- 2D
two‐dimensional
- 3D
three‐dimensional
- AAA
anisotropic analytical algorithm
- AJCC
American Joint Committee on Cancer
- ASCO
American Society of Clinical Oncology
- CBCT
cone‐beam CT
- CCC
collapsed cone convolution
- CCRT
concurrent chemoradiotherapy
- CSCO
Chinese Society of Clinical Oncology
- CRT
conformal radiation therapy
- CT
computed tomography
- CTV
clinical target volume
- D2%
the dose specified for prescription is the 2% of volumn of PRV
- Dmean
mean dose
- EBER
Epstein‐Barr encoding region
- EBV
Epstein‐Barr virus
- EBNA1
Epstein‐Barr nuclear antigen 1
- ET_Bone
Eustachian tube bone
- GP
gemcitabine plus cisplatin
- GTV
gross tumor volume
- IAC
internal auditory canal
- IGRT
image‐guided radiotherapy
- IMRT
intensity‐modulated radiotherapy
- IQR
interquartile range
- MAC
maximum acceptance criteria
- MC
Monte Carlo
- MRI
magnetic resonance imaging
- NPC
nasopharyngeal carcinoma
- OAR
organs at risk
- OS
overall survival
- PCR
polymerase chain reaction
- PD‐1
programmed cell death 1
- PD‐L1
programmed cell death l ligand 1
- PET
positron emission tomography
- PF
cisplatin plus 5‐fluorouracil
- PFS
progression‐free survival
- PRV
planning organ at risk volume
- TNM
tumor‐node‐metastasis
- TPF
docetaxel, cisplatin and 5‐fluorouracil
- TPS
treatment planning system
- UICC
International Union Against Cancer Classification
- VCA
viral capsid antigen
- VMAT
volumetric‐modulated arc therapy
- WHO
World Health Organization.
1. BACKGROUND
Nasopharyngeal carcinoma (NPC) is an epithelial cancer arising from the nasopharynx epithelium [1]. In comparison with other tumors, NPC has a distinct geographical distribution of occurrence. Based on data from the International Agency for Research on Cancer, about 129,000 people were diagnosed with NPC in 2018, accounting for only 0.7% of all tumors diagnosed [2, 3]. Moreover, NPC is highly prevalent in East and Southeast Asia, particularly in South China [4]. In addition, the incidence of NPC is higher in males than in females, with a ratio of about 2.5:1 in China in 2015 [5].
The World Health Organization (WHO) subdivides NPC into three histological subtypes, namely, keratinizing squamous cell carcinoma, non‐keratinizing (differentiated or undifferentiated) carcinoma, and basaloid carcinoma. Undifferentiated carcinoma accounts for more than 95% of NPC in high‐incidence areas and is associated with better survival [1, 4]. NPC is believed to result from interactions among Epstein‐Barr virus (EBV) infection, genetics, and environmental factors, such as alcohol consumption and smoking [6].
The management of NPC has improved because of advances in radiotherapy technology, induction and concurrent chemotherapy, and accurate cancer staging systems [1, 4]. Here, the aim is to provide guidelines for the diagnosis and management of NPC via a process of iterative development among specialists based on their own experiences together with published evidence. The goals of these guidelines are to provide practical guidance to assist clinicians to better manage patients with NPC. In this guideline, the TNM staging descriptions are based on the 8th American Joint Committee on Cancer (AJCC) tumor‐node‐metastasis (TNM) classification for NPC [7].
The recent Chinese Society of Clinical Oncology (CSCO)‐American Society of Clinical Oncology (ASCO) guidelines took into account the conditions in different regions of the world for the development of guidelines [8]. In contrast, the discussed CSCO guidelines here are completely based on the actual situations in China to formulate diagnosis and treatment strategies for NPC. Additionally, the standards for establishing the CSCO guideline are completely based on the standards of CSCO. Finally, and equally important, the recent CSCO‐ASCO guidelines provided evidence‐based recommendations on chemoradiotherapy for patients with stage II‐IVa NPC. Here, the CSCO guidelines, by contrast, include the diagnosis, follow‐up, and treatment of NPC of different TNM stages.
2. DIAGNOSTIC PRINCIPLES FOR NASOPHARYNGEAL CARCINOMA
2.1. Imaging diagnosis
| Assessment | Grade I recommendations | Grade II recommendations | Grade III recommendations |
|---|---|---|---|
| Primary tumor | Nasopharyngeal MRI |
|
PET‐MR |
| Regional lymph node | Neck MRI |
|
|
| Distant metastasis |
|
Chest X‐ray Abdominal ultrasound |
|
Abbreviations: MRI, magnetic resonance imaging; CT, computed tomography; PET, positron emission tomography.
Notes
Magnetic resonance imaging (MRI) has replaced computed tomography (CT) as the first choice for the diagnosis, staging, efficacy evaluation, and follow‐up of NPC because of its advantages of high soft‐tissue resolution, multiparametric imaging, and non‐ionizing radiation. Compared with CT, MRI can better identify early‐stage NPC (stage I‐II) and has more sensitivity and specificity discriminative abilities for adjacent soft tissue infiltration, skull base invasion, cranial nerve infiltration, and retropharyngeal lymph node involvement [9, 10]. However, MRI involves a relatively long scanning time and is not suitable for patients who cannot tolerate long‐term examinations or have contraindications to MRI examinations (e.g., strong magnetic metal implants in the body, high fever, suffer from claustrophobia, and more). For such cases, CT scan is an alternative examination method. In addition, CT scans have thinner slice thickness and higher z‐axis resolution. Compared with MRI, CT makes it easier to recognize small suspicious metastatic lymph nodes [11]. Moreover, CT has a better display performance than MRI for bone destruction of the osteogenic skull base. For the above cases, nasopharyngeal MRI and CT examinations can be combined to improve the accuracy of diagnosing and staging NPC.
Fluorodeoxyglucose (18F) (18F‐FDG) positron emission tomography (PET)‐CT has high accuracy and sensitivity to identify NPC and can provide important clues for the diagnosis and treatment of cervical lymph node metastasis with unknown primary sites, especially for the biopsy of occult NPC. However, the soft tissue resolution of PET‐CT is worse than MRI, and the detected range of the primary nasopharyngeal lesion is often smaller than the actual lesion [12]. In addition, PET‐CT has disadvantages of high cost and the use of ionizing radiation. Therefore, PET‐CT is not recommended as the first choice for assessing the extent of primary tumors. For lymph node evaluations, PET‐CT has higher sensitivity and specificity than MRI, especially for detecting small lymph node metastasis [13, 14]. Additionally, because of its metabolic imaging, PET‐CT is superior to MRI in the differential diagnosis of nasopharyngeal primary tumor recurrence or tumor residuals and fibrosis after radiotherapy [15]. However, the accuracy of MRI remains slightly superior to that of PET‐CT for detecting and restaging primary tumor recurrence and tumor residuals [16]. Thus, it is recommended to combine PET‐CT with MRI for difficult diagnosis of recurrent or residual nasopharyngeal tumors [16]. In terms of follow‐up, more than 90% of recurrence or metastasis of NPC occurs within 5 years after the end of radical treatment, and patients with locoregionally advanced stages (T3‐4 or N2‐3) have a higher rate of recurrence or metastasis [17]. It is recommended to adopt a stratification management strategy for follow‐up, emphasizing lifelong follow‐up and providing close follow‐up to high‐risk patients within 5 years after the end of treatment [17].
18F‐FDG PET‐MRI can not only achieve the same or higher diagnostic sensitivity as PET‐CT but also improve the specificity of diagnosis, and effectively reduce the radiation dose of CT examination [18]. However, in clinical application, PET‐MRI machines have a low field strength (1.5 T), and the soft tissue resolution is lower than that of conventional 3.0 T MRI. PET examination involves ionizing radiation; therefore, it is not conducive to the administration of contrast agents for MRI local enhancement, thereby reducing the accuracy of the assessment of the extent of invasion of the primary tumor. The high price of PET‐MRI also limits its clinical applicability. Currently, whether PET‐MRI can replace PET‐CT and nasopharyngeal + neck MRI for pre‐treatment evaluation is still in the exploratory stage.
In the case of cervical lymph node swelling of unknown primary site, suspected lymph node metastasis in unconventional areas (such as the parotid gland, posterior occipital, and submental), and suspected small lymph node metastasis, it is necessary to clarify whether the primary tumor and lymph nodes in this area have metastasized and to carefully assess and exclude the presence of a second primary tumor.
For NPC patients with a very low risk of metastasis (stage N0‐1 and Epstein‐Barr virus (EBV) DNA < 4000 copies/mL), it is recommended to first perform abdominal ultrasound examination [19]. If distant metastasis is suspected, then an abdominal plain scan + enhanced MRI/CT examination is recommended.
The distant metastasis rate of NPC has been reported as 11%‐36%. The early detection of distant metastasis is very significant for accurate staging and the formulation of treatment strategies. 18F‐FDG PET‐CT has higher sensitivity and specificity to identify distant metastasis, compared to conventional imaging methods (chest X‐ray, ultrasound, and whole‐body bone scanning) [11, 19, 20]. For patients with a high risk of metastasis (such as stage N0‐1 and EBV DNA > 4000 copies/mL, stage N2‐3, or stage T3‐4), PET/CT has higher sensitivity for identifying distant metastasis than conventional imaging methods. It is recommended to perform PET‐CT examination routinely before treatment [19]. For patients with a continuous or progressive increase in EBV DNA after treatment, but no positive findings in conventional imaging examinations, PET‐CT examination is also recommended.
For patients with suspicious metastases in single distant organs or enlarged lymph nodes, atypical imaging findings, or without elevated plasma EBV DNA, it is recommended to perform imaging‐guided aspiration biopsy to obtain pathological evidence of metastasis and find or exclude the existence of a second primary tumor.
2.2. Pathological diagnosis
| Content | Grade I recommendations | Grade II recommendations | Grade III recommendations |
|---|---|---|---|
| Technique to obtain tissue or cytology analysis | Mass biopsy via nasopharyngoscopy: using forceps or puncture technique. |
|
|
| Pathological diagnosis | Nasopharyngeal tumors are diagnosed as NPC according to histopathological morphology. It can be divided into three subtypes: nasopharyngeal keratinizing squamous cell carcinoma, non‐keratinizing carcinoma (differentiated and undifferentiated), and basal‐like squamous cell carcinoma. The types of cervical mass puncture pathological diagnosis include metastatic non‐keratinizing carcinoma or metastatic undifferentiated carcinoma. | ||
| Molecular diagnosis |
Immunohistochemical/in situ hybridization detection: for cases of NPC whose pathological morphology cannot be accurately diagnosed, immunohistochemical (such as pan‐cytokeratin) or in situ hybridization (such as EBER) detection should be performed to assist the pathological diagnosis. Peripheral blood EBV antibody and EBV DNA: serum EBV antibody and plasma EBV DNA copy number may assist in the diagnosis of NPC. |
Plasma EBV DNA copy number can help in the diagnosis of distant metastasis or the recurrence of NPC after initial treatment. Its diagnostic accuracy in distant metastasis is higher than that of recurrence. |
Abbreviations: NPC, nasopharyngeal carcinoma; EBER, Epstein‐Barr encoding region; EBV, Epstein‐Barr virus.
Notes
In 1962, Liang et al. [21] first proposed the histopathological classification of NPC, dividing NPC into three categories: undifferentiated, poorly differentiated, and well‐differentiated carcinoma. Among them, undifferentiated carcinoma is a pleomorphic cell carcinoma; poorly differentiated carcinoma includes large round cell carcinoma, spindle cell carcinoma, and squamous cell carcinoma grade III (equivalent to poorly differentiated squamous cell carcinoma); and well‐differentiated carcinoma includes squamous cell carcinoma grade II, basal cell carcinoma, and columnar cell carcinoma (adenocarcinoma). Since then, China and the WHO have repeatedly revised the pathological classification of NPC. Currently, the third edition of the WHO staging (2003) is used internationally and classifies NPC into the following three categories: keratinizing squamous cell carcinoma, non‐keratinizing carcinoma, and basaloid squamous cell carcinoma. Among them, non‐keratinizing cancer accounts for the vast majority of NPC in China and can be further subdivided into differentiated and undifferentiated non‐keratinizing cancer [22]. A clear pathological classification is essential for accurate staging and to evaluate optimal treatment options [23]. However, the current pathological classification cannot effectively distinguish the prognosis of patients [22]. At present, the CSCO guidelines do not recommend determining subsequent individualized treatment strategies based on pathological test results [24]. For patients with NPC, the positive peripheral blood EBV antibody and EBV DNA copy number can assist in a diagnosis of NPC [25, 26]. A recent prospective randomized controlled trial found that the combination of two EBV antibodies, based on viral capsid antigen (VCA)‐IgA and Epstein‐Barr nuclear antigen 1 (EBNA1)‐IgA, could increase the early diagnostic rate of NPC by 21%‐79%, and reduce the risk of NPC‐related death by 88% [25]. Another prospective study found that the sensitivity and specificity of plasma EBV DNA copy numbers for the diagnosis of NPC were as high as 97.1% and 98.6%, respectively [26]. Please note that even if these molecular indicators are negative, the possibility of NPC cannot be ruled out [4]. Currently, real‐time fluorescent quantitative polymerase chain reaction (PCR) is mainly used for the quantitative detection of plasma/serum EBV DNA copy number, and the commonly amplified target gene is the BamHI‐W fragment. It should be noted that currently, there is no internationally recognized EBV DNA standardized testing process, and only the National Cancer Institute of the United States has made recommendations for EBV DNA standardized testing [27]. A recent retrospective study [28] found that the sensitivity, specificity, and accuracy of plasma EBV DNA copy number in the diagnosis of distant metastasis after initial treatment of NPC were 91.1%, 80.0%, and 92.8%, respectively (Note: The diagnostic accuracy of extrapulmonary metastasis is higher than that of lung metastasis). The sensitivity, specificity, and accuracy in the diagnosis of regional recurrence were 80.2%, 80.0%, and 85.9%, respectively, and were 68.8%, 80.0%, and 78.2%, respectively, in the diagnosis of local recurrence [28].
2.3. Clinical staging
Classification of the primary tumor based on the 8th AJCC/UICC TNM staging system
| Primary tumor (T) |
| TX: The primary tumor cannot be assessed |
| T0: No tumor identified, but there is EBV‐positive cervical lymph node(s) involvement. |
| Tis: Carcinoma in situ |
| T1: Tumor confined to the nasopharynx, or extension to the oropharynx and/or the nasal cavity without parapharyngeal involvement |
| T2: Tumor with extension to the parapharyngeal space, and/or adjacent soft tissue involvement (medial pterygoid, lateral pterygoid, and prevertebral muscles) |
| T3: Tumor with infiltration of bony structures at the skull base, cervical vertebra, pterygoid structures, and/or paranasal sinuses |
| T4: Tumor with intracranial extension, involvement of cranial nerves, the hypopharynx, orbit, parotid gland, and/or extensive soft tissue infiltration beyond the lateral surface of the lateral pterygoid muscle |
Abbreviations: AJCC, American Joint Committee on Cancer; T, tumor stage; N, nodal stage; M, metastatic stage; International Union Against Cancer Classification, UICC.
Lymph node classification based on the 8th AJCC/UICC TNM staging system
| Regional lymph node (N) |
| Nx: Regional lymph nodes cannot be assessed |
| N0: No regional lymph node metastasis |
| N1: Unilateral metastasis in cervical lymph node(s) and/or unilateral or bilateral metastasis in retropharyngeal lymph node(s), 6 cm or smaller in their greatest dimension, above the caudal border of the cricoid cartilage |
| N2: Bilateral metastasis in cervical lymph node(s), 6 cm or smaller in their greatest dimension, above the caudal border of the cricoid cartilage |
| N3: Unilateral or bilateral metastasis in cervical lymph node(s), larger than 6 cm in their greatest dimension, and/or extend below the caudal border of the cricoid cartilage |
Abbreviations: AJCC, American Joint Committee on Cancer; T, tumor stage; N, nodal stage; M, metastatic stage; International Union Against Cancer Classification, UICC.
Distant metastasis classification based on the 8th AJCC/UICC TNM staging system
| Distant metastasis (M) |
| M0: No distant metastasis |
| M1: Distant metastasis |
Abbreviations: AJCC, American Joint Committee on Cancer; T, tumor stage; N, nodal stage; M, metastatic stage; UICC, International Union Against Cancer Classification.
Overall classification based on the 8th edition of the AJCC/UICC TNM staging system
| TNM Stage | T stage | N stage | M stage |
|---|---|---|---|
| 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| II | T0‐1 | N1 | M0 |
| T2 | N0‐1 | M0 | |
| III | T0‐2 | N2 | M0 |
| T3 | N0‐2 | M0 | |
| IVa | T4 | N0‐2 | M0 |
| Any T | N3 | M0 | |
| IVb | Any T | Any N | M1 |
Abbreviations: AJCC, American Joint Committee on Cancer; T, tumor stage; N, nodal stage; M, metastatic stage; UICC, International Union Against Cancer Classification.
Notes
These guidelines use the 8th edition of the AJCC TNM staging system for the clinical staging of NPC [7]. Studies have shown that plasma EBV DNA combined with TNM staging can further improve the predictive power to accurately assess the prognosis of patients with NPC [29]. Medical institutions can combine the AJCC TNM staging and plasma EBV DNA copy number to determine the severity of a patient's disease.
3. RADIOTHERAPY FOR NASOPHARYNGEAL CARCINOMA
3.1. Basic principles of radiotherapy
| Content | Basic principle |
|---|---|
| Radiotherapy technology | It is recommended to use daily image‐guided intensity‐modulated radiotherapy, which can be used for sequential incremental radiotherapy or simultaneous push‐dose radiotherapy. |
| Prescription dose | The recommended prescription dose is 70 Gy (33‐35 fractions, 2.0‐2.12 Gy per fraction), completed within 7 weeks (1 fraction per day, 5 fractions per week). The dose can be adjusted according to the tumor volume and its response to radiotherapy/chemotherapy. |
Notes
Compared with traditional two‐dimensional (2D) or three‐dimensional (3D) radiotherapy, intensity‐modulated radiotherapy (IMRT) can produce a dose distribution that is highly suitable for the shape of the tumor target area. Thus, it can provide high‐dose radiation to NPC while protecting adjacent important structures.
Moreover, the benefits of IMRT in reducing adverse events, such as neurotoxicity, dry mouth, and dysphagia, have been demonstrated in three randomized controlled trials [30, 31, 32] and in meta‐analyses [33, 34]. A randomized controlled trial [30] and several meta‐analyses [34, 35, 36] also showed that IMRT improved the disease control rate and survival rate in patients with NPC.
The survival rate of patients with NPC has been improved significantly. However, long‐term survivors of NPC after radiotherapy often suffer severe adverse reactions [37]. The dose per fraction is one of the main factors affecting late adverse events. The Intergroup 0099 [38] and RTOG 0225 trials [39] adopted a radiotherapy scheme with 70 Gy/33‐35 fractions, 5 fractions per week, 2.0‐2.12 Gy/fraction, which showed good survival rates and manageable adverse events. Patients with residual lesions have poor prognoses [40, 41], therefore, one or two additional doses of radiotherapy of 2‐4 Gy can be considered for patients with residual lesions detected by MRI at the end of IMRT. For small primary lesions with good response, clinicians should consider slightly reducing the total dose (for example, to 66‐68 Gy). Hypofractionation should be avoided because of the risk of increased late toxicity, especially when radiotherapy is used in combination with chemotherapy. The Hong Kong NPC‐9902 [41] and NPC‐0501 trials [42] failed to prove that the clinical benefit of accelerated fractionation with 6 fractions per week was better than traditional fractionation with 5 fractions per week.
The most common acute adverse events in patients with NPC during radiotherapy include dermatitis and oral mucositis. Dermatitis mainly manifests as erythema, hyperpigmentation, alopecia, skin swelling, blisters, and ulceration of the epithelium. The common prevention and treatment measures are as follows: 1) During radiotherapy, patients should keep the local skin clean and dry. The exposed skin should not be scrubbed with rough towels or soap. The water temperature should not be too high when cleaning the face. Patients should wear a hat to avoid direct sunlight when going out. 2) When there is desquamation in the irradiated field, let it fall off by itself instead of tearing it by hand. 3) When a skin wet reaction occurs, effective ointment can be used on the skin of the irradiated field to promote repair of the injury. 4) The local skin of the irradiation field should be exposed and kept clean. Avoid using ethanol, iodine, or medical fabric. If infection occurs, antibiotics, per clinician recommendations, should be used in a timely manner. The common manifestations of radioactive mucositis are redness, swelling, pain, and ulceration of the oral mucosa. The occurrence and severity of radiation‐induced oral mucosal reactions increase with increasing cumulative doses. The parotid and salivary glands are within the irradiation range, therefore, the functions of the parotid and salivary glands are inhibited after radiotherapy, which reduces saliva secretion and leads to dry mouth. The common prevention and treatment measures for radioactive mucositis are as follows: 1) Always have drinking water bottles at hand for consumption and keeping the mouth moist. Drinking honeysuckle or Ophiopogon japonicus can also be considered. 2) A mouthwash can be prepared with ambroxol, dexamethasone, recombinant human epidermal growth factor, and compound vitamin B12. To prevent fungal infections, sodium bicarbonate solution can be used. 3) Use soft toothbrushes and fluoride toothpaste to brush teeth in the morning and evening. Gargle after meals and before sleeping. Perform mouth tapping exercises frequently to allow full gas exchange at the wrinkled walls of the oral mucosa, to destroy the microbiologic milieu of anaerobic bacteria, and to prevent secondary infection of the oral cavity. 4) Eat light, easy‐to‐digest liquid foods, and semi‐liquid foods. Eat high‐protein and vitamin‐rich foods at the same time. Avoid spicy foods. 5) If the pain is severe, non‐steroidal anti‐inflammatory drugs, weak opioids, or strong opioids can be used for symptomatic treatment according to the degree of pain.
When the oral ulcer is becoming severe or an infection occurs, antibiotics can be used. If the fungal infection is severe, antifungal drugs such as Diflucan (fluconazole) can be used. It is recommended to use throat swab culture and bacterial susceptibility tests to identify the infective bacteria. All treatments should be performed based on clinician consultation and recommendations.
3.2. Radiotherapy process
| Content | Basic principles |
|---|---|
| Position immobilization | Head, neck, and shoulder thermoplastic film + individualized Styrofoam head and neck cushion (recommended); head, neck, and shoulder thermoplastic film + head, neck and shoulder vacuum bag; head, neck, and shoulder thermoplastic film + water‐activated fixed pillow; head, neck, and shoulder thermoplastic film + standard resin headrest. |
| CT localization | The scanning position is the head‐first supine position. The scanning and reconstruction layer thickness is 3 mm. The scanning method is a 140 KV plain scan + 120 KV enhanced scan. Field of view includes the space sufficient to cover the widest part of the patient's shoulder. |
| MRI localization | The scanning position is the head‐first supine position. The scanning sequence is T1, T2, T1 enhancement, and T1 fat compression enhancement. The scanning layer thickness is 3 mm, layer spacing is 0 mm, and the scanning method is plain scanning + enhanced scanning. |
| Planning and design | IMRT reverse plan design is recommended for nasopharyngeal carcinoma radiotherapy plan. The fixed‐beam IMRT method is usually used, and the irradiation field is ≥ 5 beams, which are uniformly distributed in the same plane. Single arc or double arc VMAT can also be used. The weight or intensity of each subfield is adjusted through the inverse optimization process, such that the high‐dose distribution is highly conformed to the contour of the tumor target area in the three‐dimensional direction. |
| Plan validation | The content of IMRT dose verification should include point dose verification and dose distribution verification, and three‐dimensional dose verification, based on the patient's anatomy, is encouraged. The plan verification suggests that the actual gantry angle measurement and the multi‐angle synthetic dose verification method are optimized, and the absolute dose mode should be used to analyze the results. It is recommended to use global normalization to calculate the Gamma pass rate. Its tolerance limit: 3%/2 mm, 10% dose threshold, Gamma pass rate ≥ 95%; intervention limit: 3%/2 mm, 10% dose threshold, Gamma pass rate≥ 90%. |
| IGRT | Before treatment, at least 2D IGRT technology must be used to verify the patient's positioning. If possible, medical institutions can use kV or MV CBCT, MRI, and other imaging technologies to implement daily image guidance during high‐precision radiotherapy. |
Abbreviations: CBCT, cone‐beam CT; CT, computed tomography; IGRT, image‐guided radiotherapy; IMRT, intensity‐modulated radiotherapy; MRI, magnetic resonance imaging; VMAT, volumetric‐modulated arc therapy.
Notes
The recommended radiotherapy for NPC is IMRT, characterized by a highly conformable target dose and a steep edge dose, requiring higher accuracy of postural fixation [43]. At present, the main methods of position immobilization for patients with NPC are as follows: head, neck, and shoulder thermoplastic film + individualized styrofoam head and neck cushion; head, neck, and shoulder thermoplastic film + head, neck, and shoulder vacuum bag; head, neck, and shoulder thermoplastic film + water‐activated fixed pillow; and head, neck, and shoulder thermoplastic film + standard resin headrest. Among them, styrofoam fixation is more ideal in terms of fitness and accuracy, can achieve a high degree of individualized fitness, and has a good fixation effect on the head and neck [44, 45, 46]. In addition, an oral support articulator can be added to the above fixing methods. The use of oral support can reduce oral reactions, protect taste, reduce head and neck positioning errors, and better control the elevation of the mandible.
CT simulation is the most commonly used radiotherapy positioning technology in radiotherapy [47]. Positioning CT images is the basis of treatment plan design, and the electronic density information obtained through image CT value conversion can be used for accurate dose calculation of treatment plans. The positioning CT image has other functions, such as the establishment of a 3D coordinate system for the treatment plan, delineation of the target area, virtual simulation of the field, evaluation of the curative effect, and as a reference image for image‐guided radiotherapy. Compared with CT simulation, MR simulation has higher image resolution and can display soft tissues, such as the nerves and lymph nodes, more clearly. It also has better resolution and display ability for tumor infiltration [10]. Thus, MR simulation can be used as a supplementary method to CT simulation to help doctors better delineate the clinical target area [48]. When using MR simulation, all metal objects on the patient's body should be removed, and an MR‐specific posture fixation device should be used. It is suggested to choose a layer thickness of ≤3 mm when simulating the localization scanning which is beneficial to provide sufficient anatomical details for the target area and to outline organs at risk (OARs) [43].
Patients should maintain a consistent posture during fixed posture and simulated scanning. They should adopt the head‐first supine position, place hands naturally drooping on both sides of the body, and remove all items in the treatment area, such as dentures, hearing aids, wigs, earrings, and necklaces [43]. After the thermoplastic film is fixed, it is necessary to observe the fit with the human body contours, such as the forehead, nose bridge, chin, and shoulders to ensure that the patient's posture is correct [49, 50]. The specific data collected from the enhanced scan should be determined according to the patient's age, blood vessel condition, the type and concentration of the contrast agent, and the configuration of the machine. After the enhanced scan, the patient should be observed for 15 min and ensure the absence of discomforts after the injection of the contrast agent before leaving.
Compared with traditional 3D‐conformal radiation therapy (CRT), IMRT can optimize the weight of the beam in the irradiation field, such that the high‐dose distribution in the 3D volume is highly conformed to the contour of the tumor target area, thereby, reducing damage to the surrounding normal tissues. It is the preferred treatment technique in current NPC radiotherapy [51, 52]. The IMRT plan design for NPC mostly uses fixed‐beam IMRT or volumetric‐modulated arc therapy (VMAT) [53, 54]. To meet the clinical dosimetry requirements, fixed field intensity modulation should use more than five fields with uniform coplanar distribution; VMAT should use a single arc or double arc design. It is strongly recommended to design the intensity‐modulated reverse plan according to the dose distribution of the target area and the dose limit of the organ at risk. Using an optimization algorithm, the computer‐aided planning system calculates the weight of each subfield and the distribution of the ray intensity [55].
Currently, radiotherapy planning and dose calculation are mainly based on CT images. This is because the CT value can reflect the electron density of different tissues of the human body, which facilitates the corresponding correction for tissue inhomogeneity [56]. The range of dose calculation should generally cover the patient's outer contour, the body position fixation device, and the treatment bed [57]. Considering the calculation of accuracy and efficiency, it is recommended to use a 2.5‐3 mm calculation grid [58, 59, 60]. Accurate algorithms, such as anisotropic analytical algorithm (AAA), collapsed cone convolution (CCC), and Monte Carlo (MC), can be used to better calculate the final dose distribution to ensure the accuracy of IMRT [61].
In view of the high resolution of MRI for soft tissue and no additional X‐ray exposure risk, medical institutions can also generate virtual CT (synthetic CT) using MR‐CT image conversion to achieve an independent plan design and dose calculation based on MR images [62, 63, 64, 65].
Dose verification of IMRT plans is an important part of radiotherapy quality control and assurance. It can not only detect the accuracy of treatment planning system (TPS) dose calculation but also assure the integrity of treatment data transmission and the working status of accelerators. The content of dose verification usually includes point dose verification and dose distribution verification [66]. Medical institutions are also encouraged to carry out 3D dose verification based on the patient's anatomy.
The verification of the intensity modulation plan preferably uses the actual gantry angle measurement and the method of multi‐angle synthetic dose verification. The actual multi‐frame angle measurement is closer to the actual treatment situation. It can accurately reflect the influence of gravity on the accelerator frame, small handpiece, the multileaf collimator, and the attenuation of the treatment bed [67]. When comparing the measured results with the planned calculated dose distribution, it is recommended to use global normalization. The dose return point should be selected at the maximum dose point or other points in the high‐dose plateau area (at which the dose is higher than 90% of the maximum dose). Dose distribution comparison should use the absolute dose mode for comparison, and relative dose comparison or normalization of the dose in the relative dose mode should not be performed to avoid missing factors that may cause absolute dose deviation [67]. When using gamma analysis, the range of the gamma calculation should exclude low‐dose areas that have no clinical significance but could affect the results of the dose verification analysis. According to the recommendation of the AAPM TG218 report [67], when the tolerance limit of gamma analysis is 3%/2 mm under 10% dose threshold conditions, the pass rate should be ≥ 95%. If the pass rate of the gamma analysis is less than 90%, and the points that do not pass are widely distributed in the target area or OARs, the treatment plan cannot be executed.
Image‐guided radiotherapy (IGRT) uses various advanced imaging equipment to track the position and morphology of the tumor and its surrounding normal organs before and during treatment. Thus, it can minimize the placement error between fractional radiotherapy and achieve accurate irradiation [68, 69, 70, 71, 72, 73, 74]. Common IGRT techniques include 2D planar imaging, kilo‐voltage/mega‐voltage cone‐beam computed tomography (kV/MV CBCT), MR, and other 3D volume imaging techniques[75]. To ensure the accuracy of treatment, at least 2D IGRT technology must be used to verify the patient's positioning before treatment. It is recommended to use kV or MV CBCT to implement daily image guidance during high‐precision radiotherapy.
When CBCT is registered with the planned CT image, the registration range should include the tumor target area and the surrounding normal tissue structure. It is recommended to use bony registration algorithms to automatically register images and manually adjust the registration results according to bony landmarks (such as the upper cervical spine, skull base, and/or the mandible), cavities, and soft tissues. In this way, the superior‐inferior, anterior‐posterior, and left‐right direction offsets can be determined accurately [76].
In view of the high resolution of MR images of soft tissue and no additional X‐ray exposure risk, medical institutions are recommended to perform radiotherapy based on MRI guidance. Compared with kV/MV CBCT images, MR images can clearly show the morphology and contours of tumor target areas and surrounding normal tissues and organs. Virtual CT (synthetic CT) is generated through MRI‐CT image modal conversion, which can further realize MRI‐based independent plan design and dose calculation [62, 63, 64, 65], providing technical support for online adjustment of treatment conditions and enabling the implementation of adaptive radiotherapy.
3.3. Target delineation and the normal tissue limit
3.3.1. Target delineation and dose
| Basic principles | |
|---|---|
| GTV: Including primary GTV (GTVp) and lymph node GTV (GTVn) | Description |
| No induction chemotherapy | Tumor range (primary tumor + lymph node) is shown by clinical examination (physical examination + nasopharyngoscopy + imaging). |
| Induction chemotherapy | The tumor area (primary tumor + lymph node) after induction chemotherapy, and the range of bone and paranasal sinus infiltration, are determined according to the range before induction chemotherapy. |
| Primary CTV | |
| High‐risk CTVp1 (70 Gy) |
GTVp + 5 mm (including the whole nasopharynx). When adjacent to important OAR, the distance can be reduced to 1 mm. |
| Medium risk CTVp2 (60 Gy) |
GTVp + 10 mm When adjacent to an important OAR, the distance can be reduced to 2 mm. |
| Nasal cavity: posterior | At least 5 mm away from the posterior nostril. |
| Maxillary sinus: posterior | At least 5 mm away from the back wall. |
| Posterior ethmoid sinus | Including the vomer. |
| Skull base | Including the foramen ovale, foramen rotundum, foramen rupture, and apex. |
| Cavernous sinus | If the T stage is T3‐4 (including the affected side only). |
| Pterygoid fossa | All |
| Parapharyngeal space | All |
| Sphenoid sinus | T1‐2: Lower half; T3‐4: All. |
| Steep hill | If there is no invasion: First 1/3; if there is invasion: All. |
| Cervical lymph node CTV | |
| High‐risk CTVn1 (70 Gy) | CTVn1 + 5 mm (if there is capsule invasion, it should be considered as 10 mm). |
| Medium risk CTVn2 (60 Gy) | CTVn1 + 10 mm. |
| Retropharyngeal, II, III, Va area | Both sides should be included, and the ipsilateral area should be at least one area lower than the invaded area. |
| Area VIIb | The upper boundary of region II lymph nodes should be extended upwards to reach the bottom surface of the skull, covering the space of the posterior styloid process to include the lymph node of the posterior styloid process. |
| Area Ib |
The submandibular gland is involved, or tumors involve the anatomical structure of the drainage area of the lymph node with area Ib as the first station (the oral cavity and the anterior half of the nasal cavity). Invasion of area II lymph nodes with extracapsular invasion, or involvement of area II lymph nodes, with a maximum diameter of more than 2 cm, without extracapsular invasion. |
| Low‐risk CTVn3 (50 Gy) | |
| Areas IV and Vb to the clavicle |
If the ipsilateral cervical lymph nodes are not involved, areas IV and Vb may not be irradiated. Accordingly, if cervical lymph nodes are involved, ipsilateral areas IV and Vb need to be irradiated |
Abbreviations: GTV, gross tumor volume; CTV, clinical target volume; OAR, organs at risk.
Notes
The gross tumor volume (GTV) of NPC includes the primary tumor and cervical lymph nodes. Delineation is mainly based on clinical physical examination, electronic nasopharyngoscopy, and enhanced MRI examination of the nasopharynx and neck. To delineate the primary tumor GTV, we recommend using MRI and planned CT fusion. If possible, it is recommended to use an MRI‐compatible fixture to perform MRI scans in the treatment position. PET/CT has a certain guiding significance for the diagnosis of neck metastatic lymph nodes that do not meet the MRI diagnostic criteria [77]. There is no uniform standard for the delineation of the GTV after induction chemotherapy for NPC. However, based on published studies, it is recommended to delineate the tumor volume after induction chemotherapy. A phase III, multi‐center, randomized controlled clinical study involving 233 patients [78] and a phase II single‐arm clinical study involving 112 patients [79] indicated that in patients receiving induction chemotherapy, delineating the GTV according to the tumor volume after induction chemotherapy and the pre‐induction tumor area receiving at least a moderate dose (60‐64 Gy) irradiation did not affect the local area control rate and patients’ survival rate, compared with adopting the GTV before induction chemotherapy. Adopting the GTV after induction chemotherapy could improve the patient's quality of life (QoL) score significantly [78].
The clinical target volume (CTV) range of the primary tumor in the IMRT target area of NPC is mainly based on its local progression [80, 81], which can be divided into high‐, medium‐, and low‐risk areas. Currently, there is no fully unified CTV range. Therefore, taking the previously proposed international expert consensus as an example for reference [82], domestic centers can adjust the CTV range according to the actual situation. Overall, this recommendation has a good effect in clinical practice [83]. The CTV range of the cervical lymph nodes is mainly based on the law of lymph node metastasis: the cervical lymph nodes of patients NPC usually follow the same side sequence from top to bottom, and there are few jumping metastases [84]. For patients with negative cervical lymph nodes (including N0 and patients with only post‐pharyngeal lymph node metastasis), the preventive irradiation range is postpharyngeal, II, III, and Va areas [84, 85, 86, 87]. For patients with N1 disease, the preventive irradiation range on the negative side of the cervical lymph nodes is the posterior pharynx, II, III, and Va areas, and the positive side is the preventive irradiation of the whole neck [88, 89]. Generally, area Ia does not require preventive irradiation. Area Ib mainly needs preventive irradiation in the following high‐risk groups: submandibular gland involvement, disease involving the anatomical structure of the lymph node drainage area (oral and nasal front half) with area Ib as the first stop, lymph node invasion in area II accompanied by extracapsular invasion, or in cases where the maximum diameter of the lymph node in area II exceeds 2 cm [83, 90]. In general, the structure of the NPC target area is relatively complex, and the establishment of an automatic target area delineation system based on deep learning algorithms can improve the accuracy, consistency, and efficiency of target area delineation [91].
3.3.2. Normal tissue delineation and dose limitation
| Structure (TPS standard naming) | Delineation principles | Dose limitation |
|---|---|---|
| Brain stem | The boundary with surrounding tissues is clear; the upper boundary is the optic tract, which is drawn until the cerebellum disappears. | PRV D0.03 cm3 ≤ 54 Gy, MAC ≤ 60 Gy |
| Spinal cord | The spinal cord is delineated from the disappearance of the cerebellum to 2 cm below the lower edge of the clavicle head. | PRV D0.03 cm3 ≤ 45 Gy, MAC ≤ 50 Gy |
| Temporal lobe | From the upper boundary of the Sylvian fissure to the base of the middle cranial fossa; the posterior boundary is the petrous part of the temporal bone/tentorium/preoccipital notch, and the medial boundary is the cavernous sinus/sphenoid sinus/sella/Sylvian fissure, including the hippocampus, parahippocampal gyrus, and sulcus hippocampus, excluding the basal ganglia and insula. |
T1‐T2: PRV D0.03 cm3 ≤ 65 Gy T3‐T4: PRV D0.03 cm3 ≤ 70 Gy (MAC ≤ 72 Gy) |
| Optic nerve | Including the intraorbital segment and the optic canal segment. | PRV D0.03 cm3 ≤ 54 Gy, MAC ≤ 60 Gy |
| Chiasm | It is located above the pituitary gland and inside the middle cerebral artery, in a crisscross pattern, which can be seen in 1∼2 layers on a CT scan with a thickness of 3 mm. | PRV D0.03 cm3 ≤ 54 Gy, MAC ≤ 60 Gy |
| Pituitary gland | It is in the sella pituitary, which can be seen in 1∼2 layers on a CT scan with a thickness of 3 mm. | PRV D0.03 cm3 ≤ 60 Gy, MAC ≤ 65 Gy |
| Eye | Ensure that the retina is fully delineated. |
Dmean ≤ 35 Gy, or MAC of D0.03 cm3 ≤ 54 Gy |
| Lens | The boundary between the lens and the surrounding vitreous is clear. | D0.03 cm3 ≤ 6 Gy, MAC ≤ 15 Gy |
| Inner ear | The cochlea and IAC are delineated separately. | Dmean ≤ 45 Gy, MAC ≤ 55 Gy |
| Middle ear | Tympanic cavity and ET_Bone are delineated separately. |
Tympanum dmean ≤ 34 Gy Bony eustachian tube dmean ≤ 54 Gy |
| Parotid gland | The upper boundary is the zygoma, and the lower boundary is the styloid process. The delineation of the parotid gland includes the superficial and deep lobes of the parotid and paraparotid glands. | Dmean ≤ 26 Gy, or at least one parotid V30 Gy ≤ 50% |
| Submandibular gland | The boundary between the submandibular gland and surrounding tissues is clear. | Dmean ≤ 35 Gy |
| Oral cavity | Including tongue, gums, lip mucosa, buccal mucosa, and floor of the mouth. | Dmean ≤ 40 Gy, MAC ≤ 50 Gy |
| Temporomandibular joint | Including the joint head and joint socket, starting from the disappearance of the joint cavity, it is drawn to the upper level where the mandibular neck is curved in a C shape. | D2% ≤ 70 Gy, MAC ≤ 75 Gy |
| Mandible | The mandible should serve as an OAR and not be divided into left and right. | D2% ≤ 70 Gy, MAC ≤ 75 Gy |
| Thyroid | The boundary between the thyroid and surrounding tissue is clear. | V50 Gy ≤ 60%, or MAC of V60 Gy ≤ 10 cm2 |
| Pharyngeal const | The upper, middle, and hypopharyngeal constrictors are delineated separately. Pharyngeal const was delineated from the lower edge of the wing plate to the lower edge of the cricoid cartilage. The upper/middle boundary is the upper edge of the hyoid bone, and the middle/lower boundary is the lower edge of the hyoid bone. | Dmean ≤ 45 Gy, MAC ≤ 55 Gy |
| Larynx | The larynx supraglottic and larynx glottic are delineated separately. | Dmean ≤ 35 Gy, or D2% ≤ 50 Gy |
| Brachial plexus | It is difficult to identify on the image. It is delineated according to the anatomy. It originates from the intervertebral foramen of neck 5/6, 6/7, neck 7/thorax 1, thoracic 1/2, passed through the scalene muscle space, and travels above and behind the subclavian artery. | PRV D0.03 cm3≤ 66 Gy, MAC ≤ 70 Gy |
Abbreviations: CT, computed tomography; D2%, the dose specified for prescription is the 2% of the volume of PRV; Dmean, mean dose; ET_Bone, Eustachian tube bone; IAC, internal auditory canal; MAC, maximum acceptance criteria; OAR, organs at risk; PRV, planning organ at risk volume.
Notes
There is no fully unified standard reference for the scope and dose limit requirements of important OARs in NPC; therefore, two international expert consensuses previously reported could be used taken as examples for reference [92, 93]. To improve the degree of data standardization, the naming of OAR recommends using the standard naming of the “camel‐case body”, and the underline (_) followed by the L or R to distinguish the left and right sides when naming the bilateral organs [94]. The middle ear, inner ear, and temporomandibular joints are delineated using bone windows (1400‐1600/400‐600 HU or 3000‐4500/600‐800 HU), brain stem and temporal lobes are delineated using brain windows (80‐100/5‐50 HU), and the lateral boundary of the temporal lobe and other organs are delineated using soft tissue windows (300‐400/20‐120 HU). The recommendation of the delineation principle is mainly based on the anatomical definition of the OAR. Nerve tissues are recommended to be evaluated for a planning organ at risk volume (PRV) dose of 3 mm external expansion of the OAR. Except for the middle ear [95], the dose limits for other OARs are based on international expert consensus. Although the delineation of the target volume and OAR has international expert consensus for reference, clinicians have significant differences in opinions, and we should pay more attention to the impact of delineation differences in multi‐center clinical research [96]. Atlas‐based auto‐segmentation (ABAS) has been proven to improve the consistency of multi‐center delineation and OAR dose consistency [97]. Auto‐segmentation based on artificial intelligence shows a higher delineation accuracy [98, 99], and is obtained when applied to plan the optimization to obtain good results [100]. To improve the efficiency and consistency of OAR delineation, it is recommended to use ABAS or artificial intelligence‐based auto‐segmentation.
4. CHEMOTHERAPY FOR NASOPHARYNGEAL CARCINOMA
Types of chemotherapy
| Stage | Grade I recommendations | Grade II recommendations | Grade III recommendations |
|---|---|---|---|
| T1N0 | No chemotherapy [101] (evidence 2A) | ||
| T2N0 | Radiotherapy alone [101] (evidence 2B) | Concurrent chemoradiotherapy [102, 103] (with poor prognostic factors, such as large tumor volume or high EBV DNA copy number) (evidence 2A) | |
| T1‐2N1 | Concurrent chemoradiotherapy [102, 103] (evidence 2A) | Radiotherapy alone [101] (evidence 2A) | |
| T3N0 | Concurrent chemoradiotherapy [104, 105] (evidence 2A) |
Induction chemotherapy + concurrent chemoradiotherapy [106, 107, 108, 109, 110] (evidence 1B) Concurrent chemoradiotherapy + adjuvant chemotherapy [38, 111, 112] (evidence 1B) |
|
| III‐IVa (except T3N0) |
Induction chemotherapy + concurrent chemoradiotherapy [106, 107, 108, 109, 110] (evidence 1A) Induction chemotherapy + concurrent chemoradiotherapy + metronomic adjuvant chemotherapy (patients with a high risk of recurrence or metastasis) [113] (evidence 1A) |
Concurrent chemoradiotherapy + adjuvant chemotherapy [38, 111, 112] (evidence 1B) |
| Type of chemotherapy | Grade I recommendations | Grade II recommendations | Grade III recommendations |
|---|---|---|---|
| Induction chemotherapy |
Docetaxel + cisplatin + 5‐fluorouracil [106, 107] (evidence 1A) Gemcitabine + cisplatin [108] (evidence 1A) Docetaxel + cisplatin [109] (evidence 2A) |
Cisplatin + 5‐fluorouracil [110] (evidence 1B) Cisplatin + capecitabine [114] (evidence 1B) |
Grade I / II recommended induction chemotherapy + Cetuximab / Nimotuzumab [115] (evidence 2B) |
| Concurrent chemotherapy | Cisplatin [38, 104, 105, 111, 112] (evidence 1A) |
Nedaplatin [116] (evidence 1B) Oxaliplatin [117, 118] (evidence 1B) Carboplatin [119] (evidence 2A) |
Grade I / II recommended concurrent chemotherapy + Cetuximab / Nimotuzumab [120, 121] (evidence 2B) |
| Adjuvant chemotherapy |
Metronomic adjuvant capecitabine [113] (evidence 1A) |
Cisplatin + capecitabine [114] (evidence 1B) Gemcitabine + cisplatin [122] (evidence 2A) |
Capecitabine [4] (evidence 2B) Tegafur [4] (evidence 2B) Tegafur‐uracil [123] (evidence 2B) S‐1 [124] (evidence 2B) |
Notes
In the era of traditional 2D radiotherapy, the results of a randomized controlled trial reported by Chen et al. [102] revealed that compared with radiotherapy alone, concurrent chemoradiotherapy could significantly improve the 5‐year overall survival (OS) and progression‐free survival (PFS) of patients with stage II NPC. Compared with radiotherapy alone, concurrent chemoradiotherapy was associated with reduced distant metastasis rate but did not significantly improve the local control rate. However, it is worth noting that the study used the Chinese 1992 staging system. According to the 7th edition of the AJCC TNM classification criteria, 13% of patients would be reclassified as N2/III. The 10‐year long‐term results of the trial are consistent with the conclusions of the initial report, which suggested that the survival benefits of concurrent chemoradiotherapy are mainly reflected in patients with T2N1 disease [103]. In the era of IMRT, the role of concurrent chemotherapy in stage II NPC remains unclear. Recently, Huang et al. [125] reported a phase II randomized trial involving 84 patients with stage II NPC. The patients’ median follow‐up time in this trial was 75 months. The trial showed that the 5‐year OS and PFS for the concurrent chemoradiotherapy group and the IMRT group were 94% vs. 100% (P = 0.25) and 87% vs. 90% (P = 0.72), respectively. The concurrent chemoradiotherapy group was not superior to those in the IMRT group. Stage II NPC includes three subgroups (T2N0, T1N1, and T2N1). Among them, patients with N1 disease had a higher risk of distant metastasis. An ongoing large randomized controlled trial (CinicalTrials.gov, ID: NCT02633202) is evaluating the efficacy of IMRT combined with concurrent chemotherapy. It is expected to provide appropriate treatment recommendations for patients in this subgroup.
The Intergroup 0099 randomized controlled trial found that the survival outcomes of concurrent chemoradiotherapy and adjuvant chemotherapy were better than those of radiotherapy alone, thus, establishing the status of concurrent chemoradiotherapy as the standard treatment for locoregionally advanced (stage III‐IVa) NPC [38]. Subsequent randomized controlled trials from endemic areas also confirmed that the survival benefit of concurrent chemoradiotherapy, with or without adjuvant chemotherapy, was greater than that of radiotherapy alone in locoregionally advanced NPC (stage III‐IVa) [104, 105, 111, 112, 118]. A meta‐analysis of individual patient data, which included 19 randomized controlled trials, showed that concurrent chemoradiotherapy, with or without adjuvant chemotherapy, could remarkably improve OS (stage II‐IV) [126]. In contrast, adjuvant chemotherapy or induction chemotherapy plus radiotherapy could not significantly improve the survival rate. Therefore, concurrent chemoradiotherapy is regarded as the core treatment for locoregionally advanced NPC.
Notably, the Intergroup 0099 trial was conducted in the era of traditional radiotherapy. In the era of IMRT, it is controversial whether concurrent chemoradiotherapy plus adjuvant chemotherapy can bring additional benefits to patients with NPC. The preliminary results of a phase III randomized controlled trial [127] indicated that there was no significant difference in all outcome endpoints between the concurrent chemoradiotherapy group and the concurrent chemoradiotherapy plus adjuvant chemotherapy group in locoregionally advanced NPC (non‐metastatic stage III‐IV). The long‐term results [128] also confirmed these findings (5‐year OS rate: 80% vs. 83%, P = 0.35; 5‐year PFS rate: 71% vs. 75%, P = 0.72) (stage III‐IV). In another phase III trial [122], 104 high‐risk patients with NPC (stage IIB‐IV) with positive plasma EBV DNA after radiotherapy were randomly assigned to the observation group or the gemcitabine + cisplatin adjuvant chemotherapy group. This study was the first biomarker‐driven randomized controlled trial in NPC. The results displayed that adjuvant chemotherapy could not significantly improve OS and PFS (5‐year OS rate: 64% vs. 68%; P = 0.79; 5‐year PFS rate: 49% vs. 55%; P = 0.75).
The results of several meta‐analyses [129, 130, 131, 132] claimed that although concurrent chemoradiotherapy plus adjuvant chemotherapy has a potential benefit trend, the survival outcome of patients after concurrent chemoradiotherapy plus adjuvant chemotherapy has not been significantly improved (stage II‐IV). The tolerance of patients with NPC to adjuvant chemotherapy after radical radiotherapy is relatively poor. Only 50%‐76% of patients with NPC completed the prescribed course of adjuvant chemotherapy [38, 111, 112, 122, 127, 133, 134], which may explain why it is difficult for adjuvant chemotherapy to bring additional survival benefits to patients with NPC (stage II‐IV).
Compared with adjuvant chemotherapy, induction chemotherapy has many potential advantages such as early relief of patient symptoms, elimination of tiny metastatic lesions, and better compliance (stage IIB‐IV) [135]. In recent years, three large‐scale multi‐center randomized controlled trials from Guangzhou (China) have been published internationally [106‐108, 110, 136]. These studies used docetaxel, cisplatin and 5‐fluorouracil (TPF) [106, 107], cisplatin plus 5‐fluorouracil (PF) [110, 136], and gemcitabine plus cisplatin (GP) [108] as the induction chemotherapy regimens, respectively (stage III‐IV). Long‐term follow‐up confirmed that concurrent chemoradiotherapy (CCRT) plus TPF, PF or GP could significantly improve survival in locoregionally advanced NPC (stage III‐IVa) with no marked increase in late adverse events.
These studies confirmed the advantages of induction chemotherapy combined with concurrent chemoradiotherapy in terms of OS, PFS, and distant metastasis‐free survival. The combined analysis of individual patient data of four trials from endemic areas (stage III‐IV) [137] validated that induction chemotherapy plus concurrent chemoradiotherapy could significantly improve the OS (hazard ratio [HR] = 0.75; 95% confidence interval [CI] = 0.57‐0.99; 5‐year absolute benefit = 6%) and PFS (HR = 0.70; 95% CI = 0.56‐0.86; 5‐year absolute benefit = 9%), and the survival benefit was mainly from the reduction of the distant metastasis rate. A randomized controlled trial from Tunisia and France included 83 patients with locoregionally advanced NPC (stage III‐IVa) [138]. The results illustrated that TPF induction chemotherapy could markedly improve PFS and OS [138]. Therefore, in addition to concurrent chemoradiotherapy, induction chemotherapy also plays an important role in the treatment of locoregionally advanced NPC. It can improve the survival of patients with NPC, mainly via improving the control rate of distant metastasis.
However, it should be noted that most trials evaluating concurrent chemoradiotherapy plus induction chemotherapy are conducted in endemic areas. The applicability of induction chemotherapy in patients with NPC in non‐endemic areas requires further research. Additionally, because of the lack of data from prospective randomized trials that directly compared the two methods (induction chemotherapy plus concurrent chemoradiotherapy and concurrent chemoradiotherapy plus adjuvant chemotherapy), it is currently uncertain which chemotherapy sequence, induction‐concurrent or concurrent‐adjuvant, is more effective. Based on the above findings, inferential comparisons of clinical trials whose control group was concurrent chemoradiotherapy suggested that induction chemotherapy was superior to adjuvant chemotherapy in reducing distant metastasis rate. In the future, a head‐to‐head randomized trial comparing induction chemotherapy plus concurrent chemoradiotherapy and concurrent chemoradiotherapy plus adjuvant chemotherapy is needed.
Compared with other patients having locoregionally advanced NPC, patients with T3N0 disease have a relatively low risk of treatment failure [29]. Therefore, this subgroup has been excluded in some studies that added adjuvant chemotherapy or induction chemotherapy on the basis of concurrent chemoradiotherapy (stage III‐IV) [107, 108, 127, 136]. Considering the lack of data from randomized trials, experts recommend that patients with T3N0 NPC should carefully weigh the advantages and disadvantages of adjuvant chemotherapy or induction chemotherapy on the basis of concurrent chemoradiotherapy.
According to previous phase III clinical trials comparing the efficacy of concurrent chemoradiotherapy with or without adjuvant chemotherapy and radiotherapy alone (stage II‐IV) [38, 102, 105, 111], we recommend the use of cisplatin 100 mg/m2 once every three weeks or 40 mg/m2 once a week on the basis of radiotherapy. These trials confirmed that concurrent chemoradiotherapy is superior to radiotherapy alone in locoregionally advanced NPC. Three trials [38, 111, 112] used a chemotherapy regimen every three weeks (stage III‐IV); two trials [105, 133] used a weekly chemotherapy regimen (stage III‐IV); and one trial carried out by Chen et al. [102] used 7 cycles of 30 mg/m2 once a week (stage II). There have been head‐to‐head clinical trials comparing three‐week and weekly schedules. A phase II randomized controlled trial reported by Lee et al. [139] found that the efficacy and adverse events of the two regimens were not significantly different, and the weekly regimen seemed to be more conducive to improving the QoL of the patients (stage II‐IV). A large phase III randomized controlled trial involving 526 patients with locoregionally advanced NPC is underway (ChiCTR‐TRC‐12001979). The preliminary results revealed that there was no difference in the survival outcome of the two regimens. However, compared with the once every three weeks regimen (100 mg/m2 × 2), the weekly regimen (40 mg/m2 × 6) had higher rates of leukopenia (24.8% vs. 15.9%, P = 0.015) and thrombocytopenia (5.2% vs. 1.1%, P = 0.01) (stage II‐IV) [140]. The final results of this study would help to evaluate different dosing regimens comprehensively. It is worth noting that the cumulative dose of cisplatin in the once every three weeks regimen (200 mg/m2) is lower than that in the weekly regimen (240 mg/m2).
Taken together, the above summary of previous studies suggests that the cumulative dose of cisplatin is more important than the dosing regimen in terms of efficacy. In this regard, there is currently no evidence to guide the optimal dose intensity of concurrent cisplatin chemotherapy. However, exploratory analysis in some phase III clinical trials indicated that the cumulative dose of cisplatin should not be less than 200 mg/m2 to ensure efficacy (stage III‐IV) [141, 142, 143]. For patients with contraindications and cannot receive cisplatin chemotherapy, other concurrent chemotherapy drugs such as carboplatin (area under the curve [AUC] = 5‐6) (stage III‐IV) [119, 144], oxaliplatin (70 mg/m2, once a week) [21] and nedaplatin (100 mg/m2, once every three weeks) can be selected (stage II‐IV) [116].
A phase II randomized controlled trial published in 2009 [109] reported for the first time that the addition of two courses of docetaxel (75 mg/m2) plus cisplatin (75 mg/m2) induction chemotherapy before concurrent chemoradiotherapy could increase the 3‐year OS rate of patients with NPC from 68% to 94% (HR = 0.24; 95% CI = 0.08‐0.73) (stage II‐IV). Subsequently, two large phase III randomized controlled trials [106, 107, 108] assessed the TPF regimen (docetaxel 60 mg/m2, cisplatin 60 mg/m2, and 5‐fluorouracil 600 mg/m2 per day; continuous intravenous infusion for 120 hours; once every 3 weeks; a total of 3 courses) and GP regimen (gemcitabine 1000 mg/m2 on day 1 and day8, cisplatin 80 mg/m2; once every 3 weeks, a total of 3 courses) in patients with locoregionally advanced NPC (except T3‐4N0). In the TPF trials [106, 107], compared with the concurrent chemoradiotherapy group alone, the induction chemotherapy plus concurrent chemoradiotherapy group showed a significantly improved 5‐year OS (HR = 0.65; 95% CI = 0.43‐0.98), PFS (HR = 0.65; 95% CI = 0.43‐0.98), distant recurrence‐free survival (DFS) (HR = 0.60; 95% CI = 0.38‐0.95), and local recurrence‐free survival rates (HR = 0.58; 95% CI = 0.34‐0.99) (stage III‐IV). Although the doses of various drugs were reduced by 20% compared with another trial (75 mg/m2 of docetaxel, 75 mg/m2 of cisplatin, and 750 mg/m2 of 5‐fluorouracil per day, continuous intravenous infusion for 120 hours) [138], the rates of grade 3‐4 adverse events, such as neutropenia (35%), leukopenia (27%), and diarrhea (8%) remained high (stages T2b, T3, T4 and/or N1‐N3, M0). In another trial using GP induction chemotherapy [108], the patients’ 3‐year OS (HR = 0.43; 95% CI = 0.24‐0.77), PFS (HR = 0.51; 95% CI = 0.34‐0.77), and distant metastasis‐free survival rates(HR = 0.43; 95% CI = 0.25‐0.73) were improved compared with the concurrent chemoradiotherapy group (stage III‐IV). Patients tolerated the GP regimen relatively well, and the rates of grade 3‐4 neutropenia, leukopenia, and diarrhea were 21%, 11%, and 0.4%, respectively. Other recommended induction chemotherapy regimens include the PF regimen (cisplatin 80‐100 mg/m2, 5‐fluorouracil 800‐1000 mg/m2 per day, continuous intravenous infusion for 120 hours) and PX regimen (cisplatin 100 mg/m2, capecitabine 2000 mg/m2 per day, continuous administration for 14 days) (stage III‐IV) [110, 114, 136].
No randomized controlled study that directly compared different induction chemotherapy regimens has been published. Therefore, the induction chemotherapy regimen can be selected according to the patient's condition. Currently, some clinical trials are evaluating whether the use of lobaplatin or nedaplatin and other platinum drugs in induction chemotherapy could replace cisplatin, or capecitabine to replace 5‐fluorouracil, would improve the QoL of patients with NPC while ensuring non‐inferiority of efficacy (ChiCTR‐TRC‐13003285, NCT03503136).
The results of the Intergroup study determined the PF regimen (cisplatin 80 mg/m2 on day 1; 5‐fluorouracil 1000 mg/m2 on days 1‐4, continuous intravenous infusion for 96 hours, once every 4 weeks) as the standard regimen of adjuvant chemotherapy (stage III‐IV) [38]. If patients have contraindications and cannot tolerate cisplatin, carboplatin can be used instead of cisplatin. A single‐center non‐inferiority randomized trial compared the Intergroup regimen with adjuvant carboplatin (AUC 5, intravenous injection) + 5‐fluorouracil regimen (1000 mg/m2 daily continuous intravenous drip for 96 hours) (stage III‐IV) [119]. The results showed that 42% of patients using cisplatin completed the cycles of adjuvant chemotherapy, while 73% of patients using carboplatin completed adjuvant chemotherapy. The survival outcomes of the two groups were similar. The rates of nephrotoxicity, leukopenia, and anemia in the cisplatin group were higher, while the rate of thrombocytopenia in the carboplatin group was higher [119]. The team also conducted a multicenter randomized trial that compared carboplatin concurrent chemoradiation with carboplatin concurrent chemoradiation plus carboplatin and 5‐fluorouracil adjuvant chemotherapy in 175 patients with NPC (T2N0‐T4N2M0) (UICC/AJCC seventh edition) [145]. The results showed that the addition of carboplatin and 5‐fluorouracil adjuvant chemotherapy could significantly improve the patients’ 2‐year DFS.
As mentioned above, the main disadvantage of adjuvant chemotherapy is poor tolerability. Metronomic chemotherapy is an emerging antitumor modality. Unlike conventional chemotherapy which uses the maximum tolerated dose to treat tumors, the metronomic administration of chemotherapeutic agents such as 5‐fluorouracil by low‐dose and prolonged oral administration can maintain chemotherapeutic agents at a relatively low blood concentration for a long time. Metronomic chemotherapy can reduce adverse events while continuing antitumor therapy, especially suitable for the adjuvant treatment of patients after the end of chemoradiotherapy. A phase III trial validated that the addition of metronomic adjuvant capecitabine (650 mg/m2 twice daily) to radical chemoradiotherapy (concurrent chemoradiotherapy ± induction chemotherapy) significantly improved survival in patients with high‐risk locoregionally advanced (stage III‐IVa, excluding T3‐4N0 as well as T3N1) NPC [113]. Meanwhile, the regimen was safe, and the rates of serious adverse events were only 17%, which was tolerable by patients. Therefore, metronomic chemotherapy as adjuvant therapy after the end of radical chemoradiotherapy is recommended for patients at high risk of recurrence/metastasis.
5. TREATMENT OF RECURRENT AND METASTATIC NASOPHARYNGEAL CARCINOMA*
| Stratification | Grade I recommendations | Grade II recommendations | Grade III recommendations |
|---|---|---|---|
| First‐line treatment |
Cisplatin + gemcitabine + camrelizumab [146] (evidence 1A) Cisplatin + gemcitabine + toripalimab [147] (evidence 1A) Cisplatin + gemcitabine [148] (evidence 1A) Systemic chemotherapy + local radiotherapy † [149] (evidence 1A) |
Cisplatin / carboplatin + 5‐fluorouracil [150, 151] (evidence 1A) Cisplatin + docetaxel [152] (evidence 2A) Carboplatin + paclitaxel [153] (evidence 2A) Cisplatin + capecitabine [154] (evidence 2A) Cisplatin + albumin‐bound paclitaxel [155] (evidence 2A) |
Cisplatin + gemcitabine + Endostar [156] (evidence 2B) |
| Second‐ or further‐line treatment |
Monotherapy chemotherapy Capecitabine [157, 158] (evidence 2A) or docetaxel [159] (evidence 2A) or gemcitabine [160] (evidence 2A) (If the same drugs are not accepted in the first‐line treatment) Encourage patients to participate in clinical trials |
Gemcitabine + vinorelbine [161, 162] (evidence 2A) Irinotecan [163] (evidence 2A) (If the same drugs are not accepted in the first‐line treatment) |
Camrelizumab [164] (evidence 2B) Toripalimab [165] (evidence 2B) Nivolumab [166] (evidence 2B) Pembrolizumab [167] (evidence 2B) (Limited to PD‐L1 TPS ≥ 1%) (If PD‐1 / PD‐L1 inhibitors are not accepted in the first‐line treatment) |
| Third‐ or further‐line treatment |
Toripalimab [165] (evidence 2A) Camrelizumab [164, 168] (evidence 2A) (If PD‐1 / PD‐L1 inhibitors were not received in the past) Capecitabine [157, 158] (evidence 2A) or docetaxel [159] (evidence 2A) or gemcitabine [160] (evidence 2A) (If the same drugs were not received in the past) Encourage patients to participate in clinical trials |
Gemcitabine + vinorelbine [161, 162] (evidence 2A) Irinotecan [163] (evidence 2A) (If the same drugs are not accepted in the past) |
Nivolumab [166] (evidence 2B) Pembrolizumab [167] (evidence 2B) (Limited to PD‐L1 TPS ≥ 1%) (If PD‐1 / PD‐L1 inhibitors are not accepted in the first‐line treatment) |
Abbreviations: PD‐1, programmed cell death 1; PD‐L1, programmed cell death l ligand 1; TPS, treatment planning system.
*This recommendation is based on published papers only. Recurrent NPC, which can be treated by surgery or local radiotherapy should be referred to the part of the treatment of recurrent NPC.
†These treatments are limited to patients with newly diagnosed metastatic NPC who achieved partial or complete remission after 3 cycles of chemotherapy.
Notes
Recurrent or metastatic NPC is a group of heterogeneous diseases usually divided into: de novo metastasis, locoregional recurrence, and locoregional recurrence with distant metastasis [169]. Therefore, before deciding the treatment strategy, it is necessary to perform a comprehensive re‐staging assessment, including the nasopharynx, neck‐enhanced MRI, and whole‐body PET/CT or corresponding part‐enhanced CT scans, and/or whole‐body bone scans to identify local recurrences and the status of systemic metastases. For locoregionally recurrent NPC, patients can undergo salvage surgical treatment or re‐radiotherapy.
Currently, most recurrent NPC is not suitable for local treatment. For recurrent NPC and NPC with distant metastasis, the mainstream treatment option is still palliative systemic chemotherapy (Table 1 ). There is a lack of high‐quality clinical research in the field of systemic treatment of recurrent and metastatic NPC; therefore, previous chemotherapy regimens usually refer to platinum‐containing two‐drug or three‐drug regimens for head and neck squamous cell carcinomas [170, 171]. The most commonly used in the past has been platinum combined with 5‐fluorouracil (PF regimen) [150, 151]. For patients who cannot tolerate 5‐fluorouracil, capecitabine can be considered as an alternative [154].
TABLE 1.
First‐line chemotherapy for recurrent and metastatic nasopharyngeal carcinoma
| Chemotherapy regimen | Dose | Medication time | Duration and cycles |
|---|---|---|---|
| Cisplatin + gemcitabine + camrelizumab | Cisplatin 80 mg/m2 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| Gemcitabine 1000 mg/m2 | Day 1, 8 | 21 days as a cycle, 4‐6 cycles | |
| Camrelizumab 200 mg | Day 1 | 21 days as a cycle, continue to maintain until the disease progresses or the toxicity is intolerable | |
| Cisplatin + gemcitabine + toripalimab | Cisplatin 80 mg/m2 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| Gemcitabine 1000 mg/m2 | Day 1, 8 | 21 days as a cycle, 4‐6 cycles | |
| Toripalimab 240mg | Day 1 | 21 days as a cycle, continue to maintain until the disease progresses or the adverse events are intolerable | |
| Cisplatin + gemcitabine | Cisplatin 80 mg/m2 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| Gemcitabine 1000 mg/m2 | Day 1, 8 | ||
| Cisplatin + 5‐fluorouracil | Cisplatin 100 mg/m2 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| 5‐fluorouracil 1000 mg/m2 | Day 1‐4 | ||
| Cisplatin + docetaxel | Cisplatin 75 mg/m2 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| Docetaxel 75 mg/m2 | Day 1 | ||
| Cisplatin + docetaxel | Cisplatin 70 mg/m2 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| Docetaxel 35 mg/m2 | Day 1, 8 | ||
| Carboplatin + paclitaxel | Carboplatin AUC 5 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| Paclitaxel 175 mg/m2 | Day 1 | ||
| Cisplatin + albumin‐bound paclitaxel | Cisplatin 75 mg/m2 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| Albumin‐bound paclitaxel 100 mg/m2 | Days 1, 8, 15 | ||
| Cisplatin + albumin‐bound paclitaxel | Cisplatin 75 mg/m2 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| Albumin‐bound paclitaxel 140 mg/m2 | Day 1, 8 | ||
| Cisplatin + albumin‐bound paclitaxel | Cisplatin 75 mg/m2 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| Albumin‐bound paclitaxel 260 mg/m2 | Day 1 | ||
| Cisplatin + capecitabine | Cisplatin 80∼100 mg/m2 | Day 1 | 21 days as a cycle, 4‐6 cycles |
| Capecitabine 1000 mg/m2 | Days 1‐14 | Maintained until disease progression or intolerable adverse events | |
| Cisplatin + gemcitabine + Endostar | Cisplatin 80 mg/m2 | Day 1 | 21 days as a cycle, no more than 4 cycles |
| Gemcitabine 1000 mg/m2 | Day 1, 8 | 21 days as a cycle, no more than 4 cycles | |
| Endostar 15 mg | Day 1‐14 | 21 days as a cycle, no more than 4 cycles |
In 2016, a phase III randomized controlled study (GEM20110714) from the Sun Yat‐sen University Cancer Center confirmed that for first‐line treatment of recurrent metastatic NPC, GP regimen (gemcitabine 1 g/m2, on days 1 and 8; cisplatin 80 mg/m2; once every 3 weeks, up to 6 courses of treatment) demonstrated better efficacy compared with FP regimen (cisplatin 80 mg/m2; 5‐fluorouracil 1 g/m2, days 1‐4; once every 3 weeks, up to 6 courses of treatment) [148]. For the primary endpoint, the median PFS of the GP group was 7.0 months (interquartile range, IQR: 4.4‐9.9 months), and that of the FP group was 5.6 months (IQR: 3.0‐7.0 months), (HR = 0.55; 95% CI = 0.44‐0.68; P < 0.0001). In terms of secondary endpoints, the OS and objective response rate (ORR), the GP group was also better than the FP group (median OS, 29.1 months vs. 20.9 months; ORR, 64% vs. 42%). The adverse event spectrums of the GP and FP groups were different, but their overall safety was controllable. The GP regimen had a higher cost‐benefit ratio than the FP regimen [172]. This trial represented landmark significance and has since been used as the first‐line optimal plan for advanced NPC. Many subsequent studies evaluated the efficacy and safety of immunotherapy or anti‐angiogenesis therapy based on the GP regimen as standard first‐line chemotherapy. Among them, a phase I study reported the safety and anti‐tumor activity of GP combined with camrelizumab in the first‐line treatment of patients with recurrent and metastatic NPC. The ORR reached 91%, and the PFS rates at 6 and 12 months were 86% and 61%, respectively, which were worthy of further verification in a phase III study [164]. In addition, a phase II study [164] reported the safety and efficacy of the GP regimen combined with Endostar in the first‐line treatment of recurrent and metastatic NPC. The ORR of 28 patients reached 85.7%, the median PFS of these patients reached 19.4 months, and the 1‐year OS rate was 90.2%.
In 2021, the phase III CAPTAIN‐1st study (NCT03707509) from the Sun Yat‐sen University Cancer Center confirmed that camrelizumab (200 mg on day 1) combined with the GP regimen (gemcitabine 1 g/m2 on days 1 and 8; cisplatin 80 mg/m2; once every 3 weeks, 4‐6 courses) had better efficacy as the first‐line treatment of locoregionally recurrent or metastatic NPC compared with placebo combined with the GP regimen [146]. The median PFS assessed by the independent review committee as the primary endpoint was 10.8 (IQR: 8.5‐13.6) months in the camrelizumab group and 6.9 (IQR: 5.9‐7.9) months in the placebo group, respectively (HR = 0.51, 95% CI = 0.37‐0.69; P < 0.0001). In the duration of response (DoR) and ORR, the camrelizumab group was also better than the placebo group (median DoR, 9.9 months vs. 5.7 months; ORR, 88.1% vs. 80.6%). The median OS of both groups have not been completed yet. Preliminary data suggest that patients receiving camrelizumab combined with chemotherapy have a trend of improvement in survival (median OS, not reached vs. 22.6 months; HR = 0.67, 95% CI = 0.41‐1.11). The adverse event spectrums of the two groups were different, but the overall safety was controllable. Based on the results of the CAPTAIN‐1st study, the National Medical Products Administration (NMPA) has approved the combination of camrelizumab, cisplatin, and gemcitabine to be used as the first‐line treatment of locoregionally recurrent or metastatic NPC.
In the same year, the phase III JUPITER‐02 study (NCT03581786) from the Sun Yat‐sen University Cancer Center confirmed that in the first‐line treatment of locoregionally recurrent or metastatic NPC, toripalimab (240 mg on day 1) combined with the GP regimen (gemcitabine 1 g/m2 on days 1 and 8; cisplatin 80 mg/m2; once every 3 weeks, no more than 6 courses) had better efficacy than placebo combined with the GP regimen [147]. The median PFS assessed by the independent review committee as the primary endpoint was 11.7 months (95% CI: 11.0 to not estimable) in the toripalimab group and 8.0 (95% CI: 7.0‐9.5) months in the placebo group (HR 0.52, 95% CI 0.36‐0.74; P = 0.0003). The result indicated the significant differences between the two groups. In the secondary endpoint, including DoR and ORR, the toripalimab group was also better than the placebo group (median DoR, 10.0 months vs. 5.7 months; ORR, 77.4% vs. 66.4%). The adverse events of toripalimab combined with the GP regimen were manageable.
In addition, platinum combined with paclitaxel or docetaxel is also a common choice for first‐line chemotherapy. Although the platinum‐containing three‐drug regimen has a good objective effective rate and short‐term effect, it did not have significant OS benefit compared with platinum combined with paclitaxel, docetaxel or 5‐fluorouracil [152, 153, 170, 171, 173]. A phase I/II study indicated that albumin‐bound paclitaxel combined with cisplatin had a higher effective rate for recurrent and metastatic NPC, with reasonable safety [155]. The study found that there was no significant difference in the safety and efficacy of albumin‐bound paclitaxel used with single‐dosage (100 mg/m2 on days 1, 8, and 15, three weeks per course), two‐dosage (140 mg/m2, on days 1 and 8, three weeks per course), and three‐dosage (260 mg/m2 on day 1, three weeks per course). The combination of albumin‐bound paclitaxel and cisplatin is worthy of further large‐scale phase III studies.
In 2020, a small sample, phase III, randomized controlled trial reported the safety and efficacy of local radiotherapy in patients with newly diagnosed metastatic NPC [149]. The study found that for newly diagnosed patients with distant metastatic NPC who achieved partial or complete remission after 6 courses of the PF regimen (cisplatin 100 mg/m2; 5‐fluorouracil 1 g/m2, on days 1‐5; once every 3 weeks), the chemotherapy plus local regional radiotherapy group had a significantly longer OS (HR = 0.42; 95% CI = 0.23‐0.77; P = 0.004) and PFS (HR = 0.36; 95% CI = 0.23‐0.57) compared with the chemotherapy alone group. Moreover, there were no significant differences in acute hematological or gastrointestinal adverse events between the chemotherapy plus local regional radiotherapy group and the chemotherapy alone group. The safety of the PF regimen combined with local regional radiotherapy after chemotherapy was controllable. The final results of this study could help to further guide the application of local radiotherapy in distant metastatic NPC. However, in the era of standard first‐line chemotherapy of gemcitabine combined with cisplatin, the significance of local radiotherapy requires further study.
For patients with the first‐line platinum‐containing regimen failure, there is currently no standard salvage treatment plan, and the first‐line unused drugs are usually selected for single‐agent treatment. A number of studies have shown that capecitabine [157, 158], docetaxel [159], gemcitabine [160], vinorelbine combined with gemcitabine [161, 162], and irinotecan [163] have a certain efficacy on salvage treatment after the failure of platinum‐containing regimens. In recent years, multiple anti‐programmed cell death 1 (PD‐1) monoclonal antibodies have revealed certain salvage treatment values after the first‐line standard treatment for advanced NPC, with the single‐agent effective rates of 20%‐30% [163‐165, 167]. A multicenter phase II study (CAPTAIN) [168] reported the results of camrelizumab in the treatment of 156 patients with recurrent or metastatic NPC who experienced failure of first‐ and second‐line chemotherapy at the 2020 ESMO Conference. The results showed that the ORR was 28.2%, the median PFS was 3.7 months, and the median OS was 17.1 months. In January 2021, a phase II study (POLARIS‐02) [165] from the Sun Yat‐sen University Cancer Center showed that for patients (n = 190) with recurrent or metastatic NPC who received toripalimab monotherapy (51.6% as second‐line treatment, 48.4% as third‐ or further‐line treatment), their ORR was 20.5%, median PFS was 1.9 months, and median OS was 17.4 months. Based on the results of the POLARIS‐02 study, NMPA approved toripalimab for the treatment of patients with recurrent/metastatic NPC who had experienced failure of second‐ or further‐line systematic therapy. Currently, there are no phase III study results of anti‐PD‐1/programmed cell death‐ligand 1 (PD‐L1) antibodies in treating NPC. Therefore, it is a reasonable choice for patients with the first‐line platinum‐containing regimen failure to participate in a clinical study of anti‐PD‐1/PD‐L1 antibodies.
6. TREATMENT OF RECURRENT NASOPHARYNGEAL CARCINOMA*
| Stratification 1 | Stratification 2 | Grade I recommendations | Grade II recommendations | Grade III recommendations |
|---|---|---|---|---|
| Suitable for surgery | Local recurrence of nasopharynx | Surgery [174, 175, 176, 177, 178, 179, 180, 181, 182, 183] (evidence 1A) |
Re‐radiotherapy [174, 175, 176, 177, 178] (evidence 2A) Chemotherapy/ immunotherapy/ targeted therapy† (evidence 2A) |
|
| Neck recurrence | Surgery [174, 175, 184] (evidence 2A) | Radiotherapy [174, 175, 178] (evidence 2A) | ||
| Unsuitable for surgery | Suitable for radiotherapy | Radiotherapy with or without chemotherapy† [4, 177, 178, 185] (evidence 2A) | Chemotherapy/ immunotherapy/ targeted therapy† (evidence 2A) | |
| Unsuitable for radiotherapy | Chemotherapy/ immunotherapy/ targeted therapy† (evidence 2A) |
*This recommendation is based on published papers only.
†Refers to chemotherapy/immunotherapy/targeted therapy for metastatic NPC.
Definition of patients unsuitable for surgery: The patient's physical condition does not allow it, the surgery is refused for various reasons, or the tumor burden is too large to be removed.
Definition of patients unsuitable for radiotherapy: They are not expected to benefit from radiotherapy; taking into account factors such as age, Karnofsky performance status (KPS), gross tumor volume (GTV), recurrence T stage; they have regional lymph node metastasis; and they have grade ≥3 adverse events in the previous radiotherapy.
Notes
For patients with recurrent NPC, a comprehensive re‐staging assessment is needed to assess the recurrence or distant metastasis before treatment, including pathological biopsy of the nasopharynx, MRI of the nasopharyngeal and neck, and whole‐body PET‐CT.
For NPC patients whose tumor only recurs in the neck, neck lymph node dissection is an important part of radical treatment. Some patients may benefit from selective neck lymph node dissection [174, 175, 184]. Radiotherapy or lymph node dissection plus radiotherapy might also an alternative treatment approach [174, 175].
For NPC patients who only have local or regional recurrence of the primary tumor, surgery or re‐radiotherapy can be selected [176, 177, 178]. Re‐radiotherapy is an effective rescue treatment, especially for patients whose recurrence interval is more than 1 year [186]. The time interval of recurrence of the lesion, the location of the recurrent lesion, the relationship with adjacent organs, the dose of radiotherapy to the previous primary lesion, and the sensitivity of previous chemoradiotherapy all affect the choice of treatment. For patients with T1‐2 recurrent NPC, salvage surgery can be selected [186]. Among patients with T1‐2 recurrent NPC receiving salvage surgery, the 3‐year OS rate can reach 60%, while for patients with advanced T stage, positive surgical margins, and lymph node metastasis, salvage surgery cannot improve their prognosis significantly [187, 188]. A large multicenter randomized controlled study (ChiCTR‐TRC‐11001573) compared the efficacy and safety of endoscopic sinus surgery with those of IMRT in patients with surgically resectable recurrent NPC. The results indicated that the OS rate of patients in the surgery group was significantly higher than that in the radiotherapy group, and the risk of radiotherapy‐related complications in the surgery group was markedly reduced [179]. For patients with inoperable recurrent NPC, after comprehensively considering whether the patient was more than 50 years old, had KPS ≤70 points, whether the GTV was more than 30 cm3, whether it was recurrent T3‐4, whether the disease had regional lymph node metastasis, and whether there had been grade ≥3 adverse events in previous radiotherapy, patients were divided into high‐ and low‐risk groups [178, 185]. Patients in the low‐risk group had survival benefits from re‐radiotherapy and were suitable for re‐radiotherapy, while those in the high‐risk group did not benefit from radiotherapy, thus re‐radiotherapy was not recommended [178, 185]. For patients in the low‐risk group, after receiving re‐radiotherapy, they still had a chance for long‐time survival; whether radiotherapy should be combined with chemotherapy is still unclear [4, 176‐178, 185]. Re‐radiotherapy requires a full assessment of the intensity of the first course of radiotherapy, the time interval of recurrence of the lesion, the tolerance of normal tissues, the influence of the re‐radiotherapy dose on the therapeutic effect, and the possible short‐term and long‐term adverse events to the patients. Compared with IMRT, proton and heavy‐ion radiotherapy can further reduce the damage to normal tissues. Although no randomized controlled study has been published, a retrospective study suggested that proton and heavy‐ion radiotherapy have important application prospects in recurrent and metastatic NPC [4]. For patients who cannot receive local radical treatment again, they require palliative systemic treatment or best supportive treatment, just like patients with metastatic NPC.
7. FOLLOW‐UP VISITS
| Visits | Grade I recommendations | Grade II recommendations | Grade III recommendations |
|---|---|---|---|
| The first 3 years after treatment (every 3‐6 months) |
Symptom inquiry and physical examination; Nasopharyngoscopy; Detection of EBV DNA copy number in peripheral blood; Nasopharyngeal + neck MRI; Chest CT; Abdominal ultrasound or upper abdominal CT; Whole‐body bone scan; Thyroid function tests (every 6‐12 months). |
Nasopharyngeal and neck CT (for patients with contraindications to MRI examination); Chest X‐ray; PET‐CT (for patients with suspected distant metastasis or T4 or N3); Oral examination; Assessment of hearing, vision, swallowing, nutrition, and function. |
|
| The 4th and 5th years after treatment (every 6‐12 months) |
Symptom inquiry and physical examination; Nasopharyngoscopy; Detection of EBV DNA copy number in peripheral blood; Nasopharyngeal + neck MRI; Chest CT; Abdominal ultrasound or upper abdominal CT; Whole‐body bone scan; Thyroid function tests (every 6‐12 months). |
Nasopharyngeal and neck CT (for patients with contraindications to MRI examination); Chest X‐ray; PET‐CT (for patients with suspected distant metastasis or T4 or N3); Oral examination; Assessment of hearing, vision, swallowing, nutrition, and function. |
|
| More than 5 years after treatment (every 12 months) |
Symptom inquiry and physical examination; Nasopharyngoscopy; Detection of EBV DNA copy number in peripheral blood; Nasopharyngeal + neck MRI; Chest CT; Abdominal ultrasound or upper abdominal CT; Whole‐body bone scan; Thyroid function tests (every 6‐12 months). |
Nasopharyngeal and neck CT (for patients with contraindications to MRI examination); Chest X‐ray; PET‐CT (for patients with suspected distant metastasis or T4 or N3); Oral examination; Assessment of hearing, vision, swallowing, nutrition, and function. |
Abbreviations: EBV, Epstein‐Barr virus; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Notes
Post‐treatment follow‐up of NPC is very important. Its purpose is to evaluate the effect of treatment, to early detect recurrence and metastasis, to monitor and deal with treatment‐related complications, and to promote functional rehabilitation [189]. The first follow‐up of NPC is mainly the systematic and complete assessment of local and systemic lesions, which should start at 12‐16 weeks after the completion of chemoradiotherapy [108, 189]. Follow‐up of patients with NPC mainly includes two aspects. On the one hand, follow‐up can detect tumor failure events in a timely manner to allow rescue treatment to be administered as soon as possible to improve the patient's curative effect; on the other hand, follow‐up also assesses and deals with the late adverse events of patients after treatment, improving their QoL [187, 188]. However, as the frequency of patients’ follow‐up and the number of examination items increases, the required medical resources also increase accordingly. Therefore, it is necessary to formulate reasonable strategies to ensure timely detection of tumor recurrence events, without blindly increasing the frequency of follow‐up and examination items.
At present, the best follow‐up strategy for NPC has not been established. High‐quality randomized controlled clinical trial data are lacking, and evidence‐based medicine is scarce. Prospective follow‐up data are difficult to obtain; therefore, some experts have used big data platforms for long‐term follow‐up of NPC to explore the time limit, frequency, and follow‐up items [190, 191, 192].
In terms of the follow‐up time limit after treatment of NPC, a retrospective study showed that the risk of death within 5 years after treatment of patients with NPC mainly comes from tumor failure, and the risk of non‐tumor death is relatively small [192]. Therefore, patients with NPC should be followed up mainly for tumor recurrence and metastasis within 5 years after treatment. Currently, many studies have reported the 10‐year survival of patients with NPC after IMRT [193, 194, 195]. The results revealed that the disease risk of patients after treatment is mainly concentrated in the first 5 years after treatment. There are fewer failures afterward. Therefore, the follow‐up of patients with NPC should focus on the first 5 years after treatment.
There are little current data regarding the frequency of follow‐up for NPC. A real‐world study involving 7,043 patients with NPC described the dynamic changes in the risk of recurrence within 5 years after treatment. The study established a follow‐up strategy that balances the follow‐up effect and time cost, and constructed the basis for individualized follow‐up (Figure 1 ) [190]. Patients with NPC were grouped based on T category, N category, and EBV DNA according to a previous study in that it demonstrated better prognostic performance than the AJCC staging system [29]: subgroup 1 (T1N0), subgroup 2 (T2‐3N0 or T1‐3N1; T1‐3N2, EBV DNA ≤ 2000 copies/mL), subgroup 3 (T1‐3N2, EBV DNA > 2000 copies/mL; T4N0‐2), and subgroup 4 (any T and N3).
FIGURE 1.
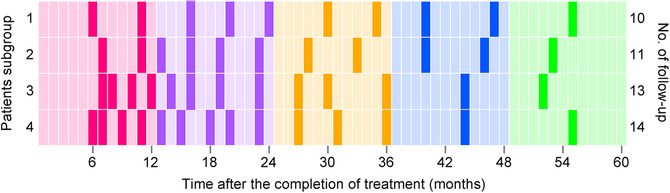
Individualized follow‐up strategy for patients with nasopharyngeal carcinoma. For patients in subgroup 1, the risk‐based follow‐up arrangement is a total of 10 follow‐up visits within 5 years (No. of follow‐up for the first 1 to 5 years, sequentially: 2, 3, 2, 2, and 1 per year, respectively); patients in subgroup 2 require a total of 11 follow‐up visits (No. of follow‐up for the first 1 to 5 years, sequentially: 2, 4, 2, 2, and 1 per year, respectively); subgroup 3 patients need a total of 13 follow‐up visits (No. of follow‐up for the first 1 to 5 years, sequentially: 4, 4, 3, 1, and 1 per year, respectively); subgroup 4 patients require a total of 14 follow‐up visits (No. of follow‐up for the first 1 to 5 years, sequentially: 4, 5, 3, 1, and 1 per year, respectively)
Figure 1 illustrates the individualized follow‐up strategy for patients with NPC. For patients in subgroup 1, the risk‐based follow‐up arrangement is a total of 10 follow‐up visits within 5 years (No. of follow‐up for the first 1 to 5 years, sequentially: 2, 3, 2, 2, and 1 per year, respectively); patients in subgroup 2 require a total of 11 follow‐up visits (No. of follow‐up for the first 1 to 5 years, sequentially: 2, 4, 2, 2, and 1 per year, respectively); subgroup 3 patients need a total of 13 follow‐up visits (No. of follow‐up for the first 1 to 5 years, sequentially: 4, 4, 3, 1, and 1 per year, respectively); subgroup 4 patients require a total of 14 follow‐up visits (No. of follow‐up for the first 1 to 5 years, sequentially: 4, 5, 3, 1, and 1 per year, respectively).
In terms of follow‐up methods for NPC, there is currently little evidence‐based medicine for formulating recommendations. For local recurrence and regional recurrence of NPC, current follow‐up methods include nasopharyngoscopy, nasopharyngeal and neck MRI, and EBV‐DNA detection [189, 196, 197]. In patients with locoregionally recurrent NPC, the proportion of patients with elevated plasma EBV‐DNA is about 50% [28]. Nasopharyngoscopy is more sensitive in recognizing recurrence in the nasopharyngeal mucosal surface but it cannot detect recurrence in the parapharyngeal, skull base, and intracranial lesions. MRI has good diagnostic sensitivity and specificity for recurrent NPC beyond the mucosal surface, and currently is a common clinical method used to review local and regional recurrence [198, 199]. A retrospective study suggested that patients with asymptomatic local early‐stage (stage T1‐2) disease after treatment might not need routine follow‐up with MRI, while patients with locoregionally advanced stage (stage T3‐4) disease are recommended to undergo MRI follow‐up once a year [191].
Distant metastasis has now become the main mode of treatment failure of NPC [52, 200, 201]; therefore, reexaminations of distant metastasis are the focus of follow‐up after treatment for patients with NPC. Examinations for distant metastasis mainly include PET/CT, chest and abdomen CT, whole‐body bone imaging, and EBV‐DNA detection [189, 196, 201]. The detection of EBV‐DNA is simple and easy to perform and has good diagnostic value for distant metastasis of NPC, representing a promising follow‐up method [28, 202‐204]. PET/CT has ideal specificity and sensitivity for the diagnosis of distant metastases. However, the current high cost of PET/CT limits its wide application in the follow‐up of NPC. Currently, chest and abdomen CT and whole‐body bone imaging are used commonly in the routine follow‐up of NPC. However, their clinical value is not yet clear, and further research is needed. Some studies have shown that under the guidance of EBV‐DNA detection, targeted imaging examinations might improve the economic benefit ratio of reexamination in patients with NPC [202, 205].
After IMRT in patients with NPC, there is a probability of about 3% of developing a second primary tumor, such as lung cancer, upper gastrointestinal tumor, hepatocellular carcinoma, colorectal carcinoma, and thyroid carcinoma [206]. Thus, follow‐up after treatment needs to pay attention to screening for common early second primary tumors. For patients with NPC after radiotherapy, it is recommended to examine thyroid function regularly to prevent hypothyroidism and to assess their dental function regularly [189, 196]. Radical radiotherapy might damage the important physiological functions of the head and neck organs. It is recommended that patients receive regular functional assessments, such as hearing, vision, swallowing, and nutrition, and actively receive rehabilitation [189, 196].
AUTHORS’ CONTRIBUTIONS
Conception and design of the guidelines: All authors. Manuscript writing: JM, LLT, YPC, MYC, FH, YS, XL and JLY. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no competing interests.
CONSENT FOR PUBLICATION
This paper was first published in Chinese by the Beijing: People's Medical Publishing House, entitled the “Guideline of Chinese Society of Clinical Oncology (CSCO): Nasopharyngeal Carcinoma. 2021” from the Committee on Guidelines of Chinese Society of Clinical Oncology. With appropriate permission, this English version is published in Cancer Communications.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
FUNDING
None to be declared.
ACKNOWLEDGMENTS
We thank Qing‐Jie Li, Cheng‐Guang Lin, Li Lin, Zhen‐Yu Qi, Cheng Xu, Lu‐Lu Zhang, Guan‐Qun Zhou (Department of Radiation Oncology, Sun Yat‐sen University Cancer Center), Rui You (Department of Nasopharyngeal Carcinoma, Sun Yat‐sen University Cancer Center), Shao‐Dong Hong (Medical Oncology Department, Sun Yat‐Sen University Cancer), Liang‐Ru Ke, Li‐Zhi Liu (Department of Medical Imaging, Sun Yat‐sen University Cancer Center), Jun‐Peng Lai, Jing‐Ping Yun (Department of Pathology, Sun Yat‐sen University Cancer Center), Jian‐Yong Shao (Department of Molecular Diagnostics, Sun Yat‐sen University Cancer Center), Xiao‐Hui Wang (Department of Liver Surgery, Sun Yat‐sen University Cancer Center), and De‐Sheng Xiao (Department of Pathology, Xiangya Hospital) for their assistance with guideline development.
Tang L‐L, Chen Yu‐P, Chen C‐B, Chen M‐Y, Chen N‐Y, Chen X‐Z, et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun. 2021;41:1195–1227. 10.1002/cac2.12218
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet (London, England). 2016;387(10022):1012‐24. 10.1016/s0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: cancer today. Lyon, France: International Agency for Research on Cancer 2018. https://gco.iarc.fr/today (accessed Dec 28, 2018). [Google Scholar]
- 4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet (London, England). 2019;394(10192):64‐80. 10.1016/s0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 5. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐32. 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 6. Tang LL, Chen WQ, Xue WQ, He YQ, Zheng RS, Zeng YX, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer letters. 2016;374(1):22‐30. 10.1016/j.canlet.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 7. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017. [Google Scholar]
- 8. Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH, et al. Chemotherapy in Combination With Radiotherapy for Definitive‐Intent Treatment of Stage II‐IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2021;39(7):840‐59. 10.1200/jco.20.03237. [DOI] [PubMed] [Google Scholar]
- 9. Sun XS, Liu SL, Luo MJ, Li XY, Chen QY, Guo SS, et al. The Association Between the Development of Radiation Therapy, Image Technology, and Chemotherapy, and the Survival of Patients With Nasopharyngeal Carcinoma: A Cohort Study From 1990 to 2012. International journal of radiation oncology, biology, physics. 2019;105(3):581‐90. 10.1016/j.ijrobp.2019.06.2549. [DOI] [PubMed] [Google Scholar]
- 10. Liao XB, Mao YP, Liu LZ, Tang LL, Sun Y, Wang Y, et al. How does magnetic resonance imaging influence staging according to AJCC staging system for nasopharyngeal carcinoma compared with computed tomography? International journal of radiation oncology, biology, physics. 2008;72(5):1368‐77. 10.1016/j.ijrobp.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 11. Chen WS, Li JJ, Hong L, Xing ZB, Wang F, Li CQ. Comparison of MRI, CT and 18F‐FDG PET/CT in the diagnosis of local and metastatic of nasopharyngeal carcinomas: an updated meta analysis of clinical studies. American journal of translational research. 2016;8(11):4532‐47. [PMC free article] [PubMed] [Google Scholar]
- 12. Lai V, Khong PL. Updates on MR imaging and ¹⁸F‐FDG PET/CT imaging in nasopharyngeal carcinoma. Oral oncology. 2014;50(6):539‐48. 10.1016/j.oraloncology.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 13. Chang JT, Chan SC, Yen TC, Liao CT, Lin CY, Lin KJ, et al. Nasopharyngeal carcinoma staging by (18)F‐fluorodeoxyglucose positron emission tomography. International journal of radiation oncology, biology, physics. 2005;62(2):501‐7. 10.1016/j.ijrobp.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 14. Peng H, Chen L, Tang LL, Li WF, Mao YP, Guo R, et al. Significant value of (18)F‐FDG‐PET/CT in diagnosing small cervical lymph node metastases in patients with nasopharyngeal carcinoma treated with intensity‐modulated radiotherapy. Chinese journal of cancer. 2017;36(1):95. 10.1186/s40880-017-0265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yen RF, Hung RL, Pan MH, Wang YH, Huang KM, Lui LT, et al. 18‐fluoro‐2‐deoxyglucose positron emission tomography in detecting residual/recurrent nasopharyngeal carcinomas and comparison with magnetic resonance imaging. Cancer. 2003;98(2):283‐7. 10.1002/cncr.11519. [DOI] [PubMed] [Google Scholar]
- 16. Comoretto M, Balestreri L, Borsatti E, Cimitan M, Franchin G, Lise M. Detection and restaging of residual and/or recurrent nasopharyngeal carcinoma after chemotherapy and radiation therapy: comparison of MR imaging and FDG PET/CT. Radiology. 2008;249(1):203‐11. 10.1148/radiol.2491071753. [DOI] [PubMed] [Google Scholar]
- 17. Zhou GQ, Lv JW, Tang LL, Mao YP, Guo R, Ma J, et al. Evaluation of the National Comprehensive Cancer Network and European Society for Medical Oncology Nasopharyngeal Carcinoma Surveillance Guidelines. Frontiers in oncology. 2020;10:119. 10.3389/fonc.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan SC, Yeh CH, Yen TC, Ng SH, Chang JT, Lin CY, et al. Clinical utility of simultaneous whole‐body (18)F‐FDG PET/MRI as a single‐step imaging modality in the staging of primary nasopharyngeal carcinoma. European journal of nuclear medicine and molecular imaging. 2018;45(8):1297‐308. 10.1007/s00259-018-3986-3. [DOI] [PubMed] [Google Scholar]
- 19. Tang LQ, Chen QY, Fan W, Liu H, Zhang L, Guo L, et al. Prospective study of tailoring whole‐body dual‐modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein‐Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(23):2861‐9. 10.1200/jco.2012.46.0816. [DOI] [PubMed] [Google Scholar]
- 20. Chua ML, Ong SC, Wee JT, Ng DC, Gao F, Tan TW, et al. Comparison of 4 modalities for distant metastasis staging in endemic nasopharyngeal carcinoma. Head & neck. 2009;31(3):346‐54. 10.1002/hed.20974. [DOI] [PubMed] [Google Scholar]
- 21. Liang PC, Ch'En CC, Chu CC, Hu YF, Chu HM, Tsung YS. The histopathologic classification, biologic characteristics and histogenesis of nasopharyngeal carcinomas. Chinese medical journal (Peking, China: 1932). 1962;81:629‐58. [PubMed] [Google Scholar]
- 22. Wang HY, Chang YL, To KF, Hwang JS, Mai HQ, Feng YF, et al. A new prognostic histopathologic classification of nasopharyngeal carcinoma. Chinese journal of cancer. 2016;35:41. 10.1186/s40880-016-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Helliwell TR, Giles TE. Pathological aspects of the assessment of head and neck cancers: United Kingdom National Multidisciplinary Guidelines. The Journal of laryngology and otology. 2016;130(S2):S59‐s65. 10.1017/s0022215116000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Comprehensive Cancer Network. NCCN clinical practice guidelines: head and neck cancers version 3, 2019. Ft. Washington, PA: NCCN, 2019. [Google Scholar]
- 25. Ji MF, Sheng W, Cheng WM, Ng MH, Wu BH, Yu X, et al. Incidence and mortality of nasopharyngeal carcinoma: interim analysis of a cluster randomized controlled screening trial (PRO‐NPC‐001) in southern China. Annals of oncology: official journal of the European Society for Medical Oncology. 2019;30(10):1630‐7. 10.1093/annonc/mdz231. [DOI] [PubMed] [Google Scholar]
- 26. Chan KCA, Woo JKS, King A, Zee BCY, Lam WKJ, Chan SL, et al. Analysis of Plasma Epstein‐Barr Virus DNA to Screen for Nasopharyngeal Cancer. The New England journal of medicine. 2017;377(6):513‐22. 10.1056/NEJMoa1701717. [DOI] [PubMed] [Google Scholar]
- 27. Kim KY, Le QT, Yom SS, Pinsky BA, Bratman SV, Ng RH, et al. Current State of PCR‐Based Epstein‐Barr Virus DNA Testing for Nasopharyngeal Cancer. Journal of the National Cancer Institute. 2017;109(4). 10.1093/jnci/djx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen FP, Huang XD, Lv JW, Wen DW, Zhou GQ, Lin L, et al. Prognostic potential of liquid biopsy tracking in the posttreatment surveillance of patients with nonmetastatic nasopharyngeal carcinoma. Cancer. 2020;126(10):2163‐73. 10.1002/cncr.32770. [DOI] [PubMed] [Google Scholar]
- 29. Guo R, Tang LL, Mao YP, Du XJ, Chen L, Zhang ZC, et al. Proposed modifications and incorporation of plasma Epstein‐Barr virus DNA improve the TNM staging system for Epstein‐Barr virus‐related nasopharyngeal carcinoma. Cancer. 2019;125(1):79‐89. 10.1002/cncr.31741. [DOI] [PubMed] [Google Scholar]
- 30. Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, et al. A prospective, randomized study comparing outcomes and toxicities of intensity‐modulated radiotherapy vs. conventional two‐dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;104(3):286‐93. 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 31. Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, et al. Prospective randomized study of intensity‐modulated radiotherapy on salivary gland function in early‐stage nasopharyngeal carcinoma patients. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(31):4873‐9. 10.1200/jco.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 32. Pow EH, Kwong DL, McMillan AS, Wong MC, Sham JS, Leung LH, et al. Xerostomia and quality of life after intensity‐modulated radiotherapy vs. conventional radiotherapy for early‐stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. International journal of radiation oncology, biology, physics. 2006;66(4):981‐91. 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 33. Liu F, Jin T, Liu L, Xiang Z, Yan R, Yang H. The role of concurrent chemotherapy for stage II nasopharyngeal carcinoma in the intensity‐modulated radiotherapy era: A systematic review and meta‐analysis. PloS one. 2018;13(3):e0194733. 10.1371/journal.pone.0194733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Du T, Xiao J, Qiu Z, Wu K. The effectiveness of intensity‐modulated radiation therapy versus 2D‐RT for the treatment of nasopharyngeal carcinoma: A systematic review and meta‐analysis. PloS one. 2019;14(7):e0219611. 10.1371/journal.pone.0219611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo MS, Huang GJ, Liu HB. Oncologic outcomes of IMRT versus CRT for nasopharyngeal carcinoma: A meta‐analysis. Medicine. 2019;98(24):e15951. 10.1097/md.0000000000015951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Co J, Mejia MB, Dizon JM. Evidence on effectiveness of intensity‐modulated radiotherapy versus 2‐dimensional radiotherapy in the treatment of nasopharyngeal carcinoma: Meta‐analysis and a systematic review of the literature. Head & neck. 2016;38 Suppl 1:E2130‐42. 10.1002/hed.23977. [DOI] [PubMed] [Google Scholar]
- 37. McDowell L, Corry J, Ringash J, Rischin D. Quality of Life, Toxicity and Unmet Needs in Nasopharyngeal Cancer Survivors. Frontiers in oncology. 2020;10:930. 10.3389/fonc.2020.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Al‐Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16(4):1310‐7. 10.1200/jco.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 39. Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity‐modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(22):3684‐90. 10.1200/jco.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lv JW, Zhou GQ, Li JX, Tang LL, Mao YP, Lin AH, et al. Magnetic Resonance Imaging‐Detected Tumor Residue after Intensity‐Modulated Radiation Therapy and its Association with Post‐Radiation Plasma Epstein‐Barr Virus Deoxyribonucleic Acid in Nasopharyngeal Carcinoma. Journal of Cancer. 2017;8(5):861‐9. 10.7150/jca.17957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He Y, Zhou Q, Shen L, Zhao Y, Lei M, Wei R, et al. A retrospective study of the prognostic value of MRI‐derived residual tumors at the end of intensity‐modulated radiotherapy in 358 patients with locally‐advanced nasopharyngeal carcinoma. Radiation oncology (London, England). 2015;10:89. 10.1186/s13014-015-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee AWM, Ngan RKC, Ng WT, Tung SY, Cheng AAC, Kwong DLW, et al. NPC‐0501 trial on the value of changing chemoradiotherapy sequence, replacing 5‐fluorouracil with capecitabine, and altering fractionation for patients with advanced nasopharyngeal carcinoma. Cancer. 2020;126(16):3674‐88. 10.1002/cncr.32972. [DOI] [PubMed] [Google Scholar]
- 43. Leech M, Coffey M, Mast M, Moura F, Osztavics A, Pasini D, et al. ESTRO ACROP guidelines for positioning, immobilisation and position verification of head and neck patients for radiation therapists. Technical innovations & patient support in radiation oncology. 2017;1:1‐7. 10.1016/j.tipsro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu S, Yao W, Hu J, He H, Zhang H, Chen C, et al. The research of accuracy immobilized using individualized polyurethane scaling agent compared to positioning foam with standard plastics pillow in the radiotherapy of nasopharyngeal carcinoma. Chin J Radiat Oncol. 2015,24(2):196‐9. 10.3760/cma.j.issn.1004-4221.2015.02.022. [in Chinese]. [DOI] [Google Scholar]
- 45. Lin CG, Xu SK, Yao WY, Wu YQ, Fang JL, Wu VWC. Comparison of set up accuracy among three common immobilisation systems for intensity modulated radiotherapy of nasopharyngeal carcinoma patients. Journal of medical radiation sciences. 2017;64(2):106‐13. 10.1002/jmrs.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu SK, Yao WY, Yan HL, Chen PF, Chen XG, He HL, et al. Setup errors in the applications of two immbolization techniques in neck irradiation. J Cancer Control Treat. 2019;32(6):528‐32. 10.3969/j.issn.1674-0904.2019.06.010. [in Chinese]. [DOI] [Google Scholar]
- 47. Baker GR. Localization: conventional and CT simulation. The British journal of radiology. 2006;79 Spec No 1:S36‐49. 10.1259/bjr/17748030. [DOI] [PubMed] [Google Scholar]
- 48. Korsager AS, Carl J, Riis Østergaard L. Comparison of manual and automatic MR‐CT registration for radiotherapy of prostate cancer. Journal of applied clinical medical physics. 2016;17(3):294‐303. 10.1120/jacmp.v17i3.6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bentel GC, Marks LB, Sherouse GW, Spencer DP. A customized head and neck support system. International journal of radiation oncology, biology, physics. 1995;32(1):245‐8. 10.1016/0360-3016(94)00412-e. [DOI] [PubMed] [Google Scholar]
- 50. Houweling AC, van der Meer S, van der Wal E, Terhaard CH, Raaijmakers CP. Improved immobilization using an individual head support in head and neck cancer patients. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2010;96(1):100‐3. 10.1016/j.radonc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 51. Xia P, Fu KK, Wong GW, Akazawa C, Verhey LJ. Comparison of treatment plans involving intensity‐modulated radiotherapy for nasopharyngeal carcinoma. International journal of radiation oncology, biology, physics. 2000;48(2):329‐37. 10.1016/s0360-3016(00)00585-x. [DOI] [PubMed] [Google Scholar]
- 52. Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, et al. Long‐term outcomes of intensity‐modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2014;110(3):398‐403. 10.1016/j.radonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 53. Verbakel WF, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity‐modulated arc therapy vs. conventional IMRT in head‐and‐neck cancer: a comparative planning and dosimetric study. International journal of radiation oncology, biology, physics. 2009;74(1):252‐9. 10.1016/j.ijrobp.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 54. Lu SH, Cheng JC, Kuo SH, Lee JJ, Chen LH, Wu JK, et al. Volumetric modulated arc therapy for nasopharyngeal carcinoma: a dosimetric comparison with TomoTherapy and step‐and‐shoot IMRT. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;104(3):324‐30. 10.1016/j.radonc.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 55. Cheng JC, Chao KS, Low D. Comparison of intensity modulated radiation therapy (IMRT) treatment techniques for nasopharyngeal carcinoma. International journal of cancer. 2001;96(2):126‐31. 10.1002/ijc.1004. [DOI] [PubMed] [Google Scholar]
- 56. Qi ZY, Huang SM, Deng XW. [Calibration of CT values used for radiation treatment planning and its impact factors]. Ai zheng = Aizheng = Chinese journal of cancer. 2006;25(1):110‐4. [PubMed] [Google Scholar]
- 57. Olch AJ, Gerig L, Li H, Mihaylov I, Morgan A. Dosimetric effects caused by couch tops and immobilization devices: report of AAPM Task Group 176. Medical physics. 2014;41(6):061501. 10.1118/1.4876299. [DOI] [PubMed] [Google Scholar]
- 58. Dempsey JF, Romeijn HE, Li JG, Low DA, Palta JR. A fourier analysis of the dose grid resolution required for accurate IMRT fluence map optimization. Medical physics. 2005;32(2):380‐8. 10.1118/1.1843354. [DOI] [PubMed] [Google Scholar]
- 59. Bedford JL, Childs PJ, Nordmark Hansen V, Mosleh‐Shirazi MA, Verhaegen F, Warrington AP. Commissioning and quality assurance of the Pinnacle(3) radiotherapy treatment planning system for external beam photons. The British journal of radiology. 2003;76(903):163‐76. 10.1259/bjr/42085182. [DOI] [PubMed] [Google Scholar]
- 60. Chung H, Jin H, Palta J, Suh TS, Kim S. Dose variations with varying calculation grid size in head and neck IMRT. Physics in medicine and biology. 2006;51(19):4841‐56. 10.1088/0031-9155/51/19/008. [DOI] [PubMed] [Google Scholar]
- 61. Hasenbalg F, Neuenschwander H, Mini R, Born EJ. Collapsed cone convolution and analytical anisotropic algorithm dose calculations compared to VMC++ Monte Carlo simulations in clinical cases. Physics in medicine and biology. 2007;52(13):3679‐91. 10.1088/0031-9155/52/13/002. [DOI] [PubMed] [Google Scholar]
- 62. Peng Y, Chen S, Qin A, Chen M, Gao X, Liu Y, et al. Magnetic resonance‐based synthetic computed tomography images generated using generative adversarial networks for nasopharyngeal carcinoma radiotherapy treatment planning. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2020;150:217‐24. 10.1016/j.radonc.2020.06.049. [DOI] [PubMed] [Google Scholar]
- 63. Qi M, Li Y, Wu A, Jia Q, Li B, Sun W, et al. Multi‐sequence MR image‐based synthetic CT generation using a generative adversarial network for head and neck MRI‐only radiotherapy. Medical physics. 2020;47(4):1880‐94. 10.1002/mp.14075. [DOI] [PubMed] [Google Scholar]
- 64. Dinkla AM, Florkow MC, Maspero M, Savenije MHF, Zijlstra F, Doornaert PAH, et al. Dosimetric evaluation of synthetic CT for head and neck radiotherapy generated by a patch‐based three‐dimensional convolutional neural network. Medical physics. 2019;46(9):4095‐104. 10.1002/mp.13663. [DOI] [PubMed] [Google Scholar]
- 65. Klages P, Benslimane I, Riyahi S, Jiang J, Hunt M, Deasy JO, et al. Patch‐based generative adversarial neural network models for head and neck MR‐only planning. Medical physics. 2020;47(2):626‐42. 10.1002/mp.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Low DA, Moran JM, Dempsey JF, Dong L, Oldham M. Dosimetry tools and techniques for IMRT. Medical physics. 2011;38(3):1313‐38. 10.1118/1.3514120. [DOI] [PubMed] [Google Scholar]
- 67. Miften M, Olch A, Mihailidis D, Moran J, Pawlicki T, Molineu A, et al. Tolerance limits and methodologies for IMRT measurement‐based verification QA: Recommendations of AAPM Task Group No. 218. Medical physics. 2018;45(4):e53‐e83. 10.1002/mp.12810. [DOI] [PubMed] [Google Scholar]
- 68. Hong TS, Tomé WA, Chappell RJ, Chinnaiyan P, Mehta MP, Harari PM. The impact of daily setup variations on head‐and‐neck intensity‐modulated radiation therapy. International journal of radiation oncology, biology, physics. 2005;61(3):779‐88. 10.1016/j.ijrobp.2004.07.696. [DOI] [PubMed] [Google Scholar]
- 69. Xing L, Lin Z, Donaldson SS, Le QT, Tate D, Goffinet DR, et al. Dosimetric effects of patient displacement and collimator and gantry angle misalignment on intensity modulated radiation therapy. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2000;56(1):97‐108. 10.1016/s0167-8140(00)00192-4. [DOI] [PubMed] [Google Scholar]
- 70. Han C, Chen YJ, Liu A, Schultheiss TE, Wong JY. Actual dose variation of parotid glands and spinal cord for nasopharyngeal cancer patients during radiotherapy. International journal of radiation oncology, biology, physics. 2008;70(4):1256‐62. 10.1016/j.ijrobp.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 71. Chen AM, Farwell DG, Luu Q, Donald PJ, Perks J, Purdy JA. Evaluation of the planning target volume in the treatment of head and neck cancer with intensity‐modulated radiotherapy: what is the appropriate expansion margin in the setting of daily image guidance? International journal of radiation oncology, biology, physics. 2011;81(4):943‐9. 10.1016/j.ijrobp.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 72. Den RB, Doemer A, Kubicek G, Bednarz G, Galvin JM, Keane WM, et al. Daily image guidance with cone‐beam computed tomography for head‐and‐neck cancer intensity‐modulated radiotherapy: a prospective study. International journal of radiation oncology, biology, physics. 2010;76(5):1353‐9. 10.1016/j.ijrobp.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 73. Shueng PW, Shen BJ, Wu LJ, Liao LJ, Hsiao CH, Lin YC, et al. Concurrent image‐guided intensity modulated radiotherapy and chemotherapy following neoadjuvant chemotherapy for locally advanced nasopharyngeal carcinoma. Radiation oncology (London, England). 2011;6:95. 10.1186/1748-717x-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Duma MN, Kampfer S, Schuster T, Aswathanarayana N, Fromm LS, Molls M, et al. Do we need daily image‐guided radiotherapy by megavoltage computed tomography in head and neck helical tomotherapy? The actual delivered dose to the spinal cord. International journal of radiation oncology, biology, physics. 2012;84(1):283‐8. 10.1016/j.ijrobp.2011.10.073. [DOI] [PubMed] [Google Scholar]
- 75. Nabavizadeh N, Elliott DA, Chen Y, Kusano AS, Mitin T, Thomas CR, Jr. , et al. Image Guided Radiation Therapy (IGRT) Practice Patterns and IGRT's Impact on Workflow and Treatment Planning: Results From a National Survey of American Society for Radiation Oncology Members. International journal of radiation oncology, biology, physics. 2016;94(4):850‐7. 10.1016/j.ijrobp.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 76. Zumsteg Z, DeMarco J, Lee SP, Steinberg ML, Lin CS, McBride W, et al. Image guidance during head‐and‐neck cancer radiation therapy: analysis of alignment trends with in‐room cone‐beam computed tomography scans. International journal of radiation oncology, biology, physics. 2012;83(2):712‐9. 10.1016/j.ijrobp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 77. Shen G, Xiao W, Han F, Fan W, Lin XP, Lu L, et al. Advantage of PET/CT in Target Delineation of MRI‐negative Cervical Lymph Nodes In Intensity‐Modulated Radiation Therapy Planning for Nasopharyngeal Carcinoma. Journal of Cancer. 2017;8(19):4117‐23. 10.7150/jca.21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yang H, Chen X, Lin S, Rong J, Yang M, Wen Q, et al. Treatment outcomes after reduction of the target volume of intensity‐modulated radiotherapy following induction chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: A prospective, multi‐center, randomized clinical trial. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2018;126(1):37‐42. 10.1016/j.radonc.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 79. Zhao C, Miao JJ, Hua YJ, Wang L, Han F, Lu LX, et al. Locoregional Control and Mild Late Toxicity After Reducing Target Volumes and Radiation Doses in Patients With Locoregionally Advanced Nasopharyngeal Carcinoma Treated With Induction Chemotherapy (IC) Followed by Concurrent Chemoradiotherapy: 10‐Year Results of a Phase 2 Study. International journal of radiation oncology, biology, physics. 2019;104(4):836‐44. 10.1016/j.ijrobp.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 80. Liang SB, Sun Y, Liu LZ, Chen Y, Chen L, Mao YP, et al. Extension of local disease in nasopharyngeal carcinoma detected by magnetic resonance imaging: improvement of clinical target volume delineation. International journal of radiation oncology, biology, physics. 2009;75(3):742‐50. 10.1016/j.ijrobp.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 81. Lin L, Lu Y, Wang XJ, Chen H, Yu S, Tian J, et al. Delineation of Neck Clinical Target Volume Specific to Nasopharyngeal Carcinoma Based on Lymph Node Distribution and the International Consensus Guidelines. International journal of radiation oncology, biology, physics. 2018;100(4):891‐902. 10.1016/j.ijrobp.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 82. Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC, AlHussain H, et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2018;126(1):25‐36. 10.1016/j.radonc.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 83. Zhang F, Cheng YK, Li WF, Guo R, Chen L, Sun Y, et al. Investigation of the feasibility of elective irradiation to neck level Ib using intensity‐modulated radiotherapy for patients with nasopharyngeal carcinoma: a retrospective analysis. BMC cancer. 2015;15:709. 10.1186/s12885-015-1669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tang L, Mao Y, Liu L, Liang S, Chen Y, Sun Y, et al. The volume to be irradiated during selective neck irradiation in nasopharyngeal carcinoma: analysis of the spread patterns in lymph nodes by magnetic resonance imaging. Cancer. 2009;115(3):680‐8. 10.1002/cncr.24049. [DOI] [PubMed] [Google Scholar]
- 85. Chen M, Tang LL, Sun Y, Mao YP, Li WF, Guo R, et al. Treatment outcomes and feasibility of partial neck irradiation for patients with nasopharyngeal carcinoma with only retropharyngeal lymph node metastasis after intensity‐modulated radiotherapy. Head & neck. 2014;36(4):468‐73. 10.1002/hed.23314. [DOI] [PubMed] [Google Scholar]
- 86. Gao Y, Zhu G, Lu J, Ying H, Kong L, Wu Y, et al. Is elective irradiation to the lower neck necessary for N0 nasopharyngeal carcinoma? International journal of radiation oncology, biology, physics. 2010;77(5):1397‐402. 10.1016/j.ijrobp.2009.06.062. [DOI] [PubMed] [Google Scholar]
- 87. Li JG, Yuan X, Zhang LL, Tang YQ, Liu L, Chen XD, et al. A randomized clinical trial comparing prophylactic upper versus whole‐neck irradiation in the treatment of patients with node‐negative nasopharyngeal carcinoma. Cancer. 2013;119(17):3170‐6. 10.1002/cncr.28201. [DOI] [PubMed] [Google Scholar]
- 88. Huang CL, Xu C, Zhang Y, Zhou GQ, Mao YP, Liu Q, et al. Feasibility of ipsilateral lower neck sparing irradiation for unilateral or bilateral neck node‐negative nasopharyngeal carcinoma: systemic review and meta‐analysis of 2, 521 patients. Radiation oncology (London, England). 2018;13(1):141. 10.1186/s13014-018-1087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tang LL, Tang XR, Li WF, Chen L, Tian L, Lin AH, et al. The feasibility of contralateral lower neck sparing intensity modulation radiated therapy for nasopharyngeal carcinoma patients with unilateral cervical lymph node involvement. Oral oncology. 2017;69:68‐73. 10.1016/j.oraloncology.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 90. Chen J, Ou D, He X, Hu C. Sparing level Ib lymph nodes by intensity‐modulated radiotherapy in the treatment of nasopharyngeal carcinoma. International journal of clinical oncology. 2014;19(6):998‐1004. 10.1007/s10147-013-0650-6. [DOI] [PubMed] [Google Scholar]
- 91. Lin L, Dou Q, Jin YM, Zhou GQ, Tang YQ, Chen WL, et al. Deep Learning for Automated Contouring of Primary Tumor Volumes by MRI for Nasopharyngeal Carcinoma. Radiology. 2019;291(3):677‐86. 10.1148/radiol.2019182012. [DOI] [PubMed] [Google Scholar]
- 92. Lee AW, Ng WT, Pan JJ, Chiang CL, Poh SS, Choi HC, et al. International Guideline on Dose Prioritization and Acceptance Criteria in Radiation Therapy Planning for Nasopharyngeal Carcinoma. International journal of radiation oncology, biology, physics. 2019;105(3):567‐80. 10.1016/j.ijrobp.2019.06.2540. [DOI] [PubMed] [Google Scholar]
- 93. Sun Y, Yu XL, Luo W, Lee AW, Wee JT, Lee N, et al. Recommendation for a contouring method and atlas of organs at risk in nasopharyngeal carcinoma patients receiving intensity‐modulated radiotherapy. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2014;110(3):390‐7. 10.1016/j.radonc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 94. Santanam L, Hurkmans C, Mutic S, van Vliet‐Vroegindeweij C, Brame S, Straube W, et al. Standardizing naming conventions in radiation oncology. International journal of radiation oncology, biology, physics. 2012;83(4):1344‐9. 10.1016/j.ijrobp.2011.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang SZ, Li J, Miyamoto CT, Chen F, Zhou LF, Zhang HY, et al. A study of middle ear function in the treatment of nasopharyngeal carcinoma with IMRT technique. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009;93(3):530‐3. 10.1016/j.radonc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 96. Peng YL, Chen L, Shen GZ, Li YN, Yao JJ, Xiao WW, et al. Interobserver variations in the delineation of target volumes and organs at risk and their impact on dose distribution in intensity‐modulated radiation therapy for nasopharyngeal carcinoma. Oral oncology. 2018;82:1‐7. 10.1016/j.oraloncology.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 97. Tao CJ, Yi JL, Chen NY, Ren W, Cheng J, Tung S, et al. Multi‐subject atlas‐based auto‐segmentation reduces interobserver variation and improves dosimetric parameter consistency for organs at risk in nasopharyngeal carcinoma: A multi‐institution clinical study. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2015;115(3):407‐11. 10.1016/j.radonc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 98. Brunenberg EJL, Steinseifer IK, van den Bosch S, Kaanders J, Brouwer CL, Gooding MJ, et al. External validation of deep learning‐based contouring of head and neck organs at risk. Physics and imaging in radiation oncology. 2020;15:8‐15. 10.1016/j.phro.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. van Dijk LV, Van den Bosch L, Aljabar P, Peressutti D, Both S, JHMS R, et al. Improving automatic delineation for head and neck organs at risk by Deep Learning Contouring. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2020;142:115‐23. 10.1016/j.radonc.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 100. van Rooij W, Dahele M, Ribeiro Brandao H, Delaney AR, Slotman BJ, Verbakel WF. Deep Learning‐Based Delineation of Head and Neck Organs at Risk: Geometric and Dosimetric Evaluation. International journal of radiation oncology, biology, physics. 2019;104(3):677‐84. 10.1016/j.ijrobp.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 101. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87‐108. 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 102. Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. Journal of the National Cancer Institute. 2011;103(23):1761‐70. 10.1093/jnci/djr432. [DOI] [PubMed] [Google Scholar]
- 103. Li XY, Chen QY, Sun XS, Liu SL, Yan JJ, Guo SS, et al. Ten‐year outcomes of survival and toxicity for a phase III randomised trial of concurrent chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma. European journal of cancer (Oxford, England: 1990). 2019;110:24‐31. 10.1016/j.ejca.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 104. Chan AT, Leung SF, Ngan RK, Teo PM, Lau WH, Kwan WH, et al. Overall survival after concurrent cisplatin‐radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. Journal of the National Cancer Institute. 2005;97(7):536‐9. 10.1093/jnci/dji084. [DOI] [PubMed] [Google Scholar]
- 105. Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy‐radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression‐free survival analysis of a phase III randomized trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(8):2038‐44. 10.1200/jco.2002.08.149. [DOI] [PubMed] [Google Scholar]
- 106. Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: Long‐term results of phase 3 randomized controlled trial. International journal of cancer. 2019;145(1):295‐305. 10.1002/ijc.32099. [DOI] [PubMed] [Google Scholar]
- 107. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. The Lancet Oncology. 2016;17(11):1509‐20. 10.1016/s1470-2045(16)30410-7. [DOI] [PubMed] [Google Scholar]
- 108. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. The New England journal of medicine. 2019;381(12):1124‐35. 10.1056/NEJMoa1905287. [DOI] [PubMed] [Google Scholar]
- 109. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin‐radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(2):242‐9. 10.1200/jco.2008.18.1545. [DOI] [PubMed] [Google Scholar]
- 110. Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long‐term results of a phase III multicentre randomised controlled trial. European journal of cancer (Oxford, England: 1990). 2019;119:87‐96. 10.1016/j.ejca.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 111. Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(27):6730‐8. 10.1200/jco.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 112. Lee AW, Tung SY, Chua DT, Ngan RK, Chappell R, Tung R, et al. Randomized trial of radiotherapy plus concurrent‐adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. Journal of the National Cancer Institute. 2010;102(15):1188‐98. 10.1093/jnci/djq258. [DOI] [PubMed] [Google Scholar]
- 113. Chen YP, Liu X, Zhou Q, Yang KY, Jin F, Zhu XD, et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open‐label, parallel‐group, randomised, controlled, phase 3 trial. Lancet (London, England). 2021;398(10297):303‐13. 10.1016/s0140-6736(21)01123-5. [DOI] [PubMed] [Google Scholar]
- 114. Lee AW, Ngan RK, Tung SY, Cheng A, Kwong DL, Lu TX, et al. Preliminary results of trial NPC‐0501 evaluating the therapeutic gain by changing from concurrent‐adjuvant to induction‐concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 2015;121(8):1328‐38. 10.1002/cncr.29208. [DOI] [PubMed] [Google Scholar]
- 115. Peng H, Tang LL, Liu X, Chen L, Li WF, Mao YP, et al. Anti‐epidermal growth factor receptor therapy concurrently with induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Cancer science. 2018;109(5):1609‐16. 10.1111/cas.13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tang LQ, Chen DP, Guo L, Mo HY, Huang Y, Guo SS, et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II‐IVB nasopharyngeal carcinoma: an open‐label, non‐inferiority, randomised phase 3 trial. The Lancet Oncology. 2018;19(4):461‐73. 10.1016/s1470-2045(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 117. Wu X, Huang PY, Peng PJ, Lu LX, Han F, Wu SX, et al. Long‐term follow‐up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24(8):2131‐6. 10.1093/annonc/mdt163. [DOI] [PubMed] [Google Scholar]
- 118. Zhang L, Zhao C, Peng PJ, Lu LX, Huang PY, Han F, et al. Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(33):8461‐8. 10.1200/jco.2004.00.3863. [DOI] [PubMed] [Google Scholar]
- 119. Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, Sumitsawan Y, Tharavichitkul E, Sukthomya V, et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non‐inferiority, open trial. European journal of cancer (Oxford, England: 1990). 2007;43(9):1399‐406. 10.1016/j.ejca.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 120. You R, Hua YJ, Liu YP, Yang Q, Zhang YN, Li JB, et al. Concurrent Chemoradiotherapy with or without Anti‐EGFR‐Targeted Treatment for Stage II‐IVb Nasopharyngeal Carcinoma: Retrospective Analysis with a Large Cohort and Long Follow‐up. Theranostics. 2017;7(8):2314‐24. 10.7150/thno.19710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. You R, Sun R, Hua YJ, Li CF, Li JB, Zou X, et al. Cetuximab or nimotuzumab plus intensity‐modulated radiotherapy versus cisplatin plus intensity‐modulated radiotherapy for stage II‐IVb nasopharyngeal carcinoma. International journal of cancer. 2017;141(6):1265‐76. 10.1002/ijc.30819. [DOI] [PubMed] [Google Scholar]
- 122. Chan ATC, Hui EP, Ngan RKC, Tung SY, Cheng ACK, Ng WT, et al. Analysis of Plasma Epstein‐Barr Virus DNA in Nasopharyngeal Cancer After Chemoradiation to Identify High‐Risk Patients for Adjuvant Chemotherapy: A Randomized Controlled Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2018:Jco2018777847. 10.1200/jco.2018.77.7847. [DOI] [PubMed] [Google Scholar]
- 123. Liu YC, Wang WY, Twu CW, Jiang RS, Liang KL, Wu CT, et al. Prognostic impact of adjuvant chemotherapy in high‐risk nasopharyngeal carcinoma patients. Oral oncology. 2017;64:15‐21. 10.1016/j.oraloncology.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 124. Zong J, Xu H, Chen B, Guo Q, Xu Y, Chen C, et al. Maintenance chemotherapy using S‐1 following definitive chemoradiotherapy in patients with N3 nasopharyngeal carcinoma. Radiation oncology (London, England). 2019;14(1):182. 10.1186/s13014-019-1387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Huang X, Chen X, Zhao C, Wang J, Wang K, Wang L, et al. Adding Concurrent Chemotherapy to Intensity‐Modulated Radiotherapy Does Not Improve Treatment Outcomes for Stage II Nasopharyngeal Carcinoma: A Phase 2 Multicenter Clinical Trial. Frontiers in oncology. 2020;10:1314. 10.3389/fonc.2020.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC‐NPC meta‐analysis. The Lancet Oncology. 2015;16(6):645‐55. 10.1016/s1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 127. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. The Lancet Oncology. 2012;13(2):163‐71. 10.1016/s1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- 128. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: Long‐term results of a phase 3 multicentre randomised controlled trial. European journal of cancer (Oxford, England: 1990). 2017;75:150‐8. 10.1016/j.ejca.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 129. Yan M, Kumachev A, Siu LL, Chan KK. Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: A Bayesian network meta‐analysis. European journal of cancer (Oxford, England: 1990). 2015;51(12):1570‐9. 10.1016/j.ejca.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 130. You R, Cao YS, Huang PY, Chen L, Yang Q, Liu YP, et al. The Changing Therapeutic Role of Chemo‐radiotherapy for Loco‐regionally Advanced Nasopharyngeal Carcinoma from Two/Three‐Dimensional Radiotherapy to Intensity‐Modulated Radiotherapy: A Network Meta‐Analysis. Theranostics. 2017;7(19):4825‐35. 10.7150/thno.21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chen YP, Wang ZX, Chen L, Liu X, Tang LL, Mao YP, et al. A Bayesian network meta‐analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26(1):205‐11. 10.1093/annonc/mdu507. [DOI] [PubMed] [Google Scholar]
- 132. Ribassin‐Majed L, Marguet S, Lee AWM, Ng WT, Ma J, Chan ATC, et al. What Is the Best Treatment of Locally Advanced Nasopharyngeal Carcinoma? An Individual Patient Data Network Meta‐Analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35(5):498‐505. 10.1200/jco.2016.67.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen Y, Liu MZ, Liang SB, Zong JF, Mao YP, Tang LL, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of china. International journal of radiation oncology, biology, physics. 2008;71(5):1356‐64. 10.1016/j.ijrobp.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 134. Lee AW, Tung SY, Chan AT, Chappell R, Fu YT, Lu TX, et al. Preliminary results of a randomized study (NPC‐9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. International journal of radiation oncology, biology, physics. 2006;66(1):142‐51. 10.1016/j.ijrobp.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 135. Chua DT, Ma J, Sham JS, Mai HQ, Choy DT, Hong MH, et al. Long‐term survival after cisplatin‐based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(6):1118‐24. 10.1200/jco.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 136. Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. European journal of cancer (Oxford, England: 1990). 2017;75:14‐23. 10.1016/j.ejca.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 137. Chen YP, Tang LL, Yang Q, Poh SS, Hui EP, Chan ATC, et al. Induction Chemotherapy plus Concurrent Chemoradiotherapy in Endemic Nasopharyngeal Carcinoma: Individual Patient Data Pooled Analysis of Four Randomized Trials. Clinical cancer research: an official journal of the American Association for Cancer Research. 2018;24(8):1824‐33. 10.1158/1078-0432.Ccr-17-2656. [DOI] [PubMed] [Google Scholar]
- 138. Frikha M, Auperin A, Tao Y, Elloumi F, Toumi N, Blanchard P, et al. A randomized trial of induction docetaxel‐cisplatin‐5FU followed by concomitant cisplatin‐RT versus concomitant cisplatin‐RT in nasopharyngeal carcinoma (GORTEC 2006‐02). Annals of oncology: official journal of the European Society for Medical Oncology. 2018;29(3):731‐6. 10.1093/annonc/mdx770. [DOI] [PubMed] [Google Scholar]
- 139. Lee JY, Sun JM, Oh DR, Lim SH, Goo J, Lee SH, et al. Comparison of weekly versus triweekly cisplatin delivered concurrently with radiation therapy in patients with locally advanced nasopharyngeal cancer: A multicenter randomized phase II trial (KCSG‐HN10‐02). Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2016;118(2):244‐50. 10.1016/j.radonc.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 140. Liang H, Xia W‐X, Lv X, Sun R, Zeng Q, Li S‐W, et al. Concurrent chemoradiotherapy with 3‐weekly versus weekly cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma: A phase 3 multicentre randomised controlled trial (ChiCTR‐TRC‐12001979). 2017;35(15_suppl):6006. 10.1200/JCO.2017.35.15_suppl.6006. [DOI] [Google Scholar]
- 141. Lee AW, Tung SY, Ngan RK, Chappell R, Chua DT, Lu TX, et al. Factors contributing to the efficacy of concurrent‐adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC‐9901 and NPC‐9902 Trials. European journal of cancer (Oxford, England: 1990). 2011;47(5):656‐66. 10.1016/j.ejca.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 142. Peng H, Chen L, Zhang Y, Li WF, Mao YP, Zhang F, et al. Prognostic Value of the Cumulative Cisplatin Dose During Concurrent Chemoradiotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma: A Secondary Analysis of a Prospective Phase III Clinical Trial. The oncologist. 2016;21(11):1369‐76. 10.1634/theoncologist.2016-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ng WT, Tung SY, Lee V, Ngan RKC, Choi HCW, Chan LLK, et al. Concurrent‐Adjuvant Chemoradiation Therapy for Stage III‐IVB Nasopharyngeal Carcinoma‐Exploration for Achieving Optimal 10‐Year Therapeutic Ratio. International journal of radiation oncology, biology, physics. 2018;101(5):1078‐86. 10.1016/j.ijrobp.2018.04.069. [DOI] [PubMed] [Google Scholar]
- 144. Huang PY, Zeng Q, Cao KJ, Guo X, Guo L, Mo HY, et al. Ten‐year outcomes of a randomised trial for locoregionally advanced nasopharyngeal carcinoma: A single‐institution experience from an endemic area. European journal of cancer (Oxford, England: 1990). 2015;51(13):1760‐70. 10.1016/j.ejca.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 145. Chitapanarux I, Kittichest R, Tungkasamit T, Asakit T, Chomprasert K, Chakrabandhu S, et al. Two‐year outcome of concurrent chemoradiation with carboplatin with or without adjuvant carboplatin/fluorouracil in nasopharyngeal cancer: A multicenter randomized trial. Current problems in cancer. 2021;45(1):100620. 10.1016/j.currproblcancer.2020.100620. [DOI] [PubMed] [Google Scholar]
- 146. Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first‐line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN‐1st): a multicentre, randomised, double‐blind, phase 3 trial. The Lancet Oncology. 2021. 10.1016/s1470-2045(21)00302-8. [DOI] [PubMed] [Google Scholar]
- 147. Mai HQ, Chen QY, Chen DP, et al. Toripalimab or placebo plus chemotherapy as first‐line treatment in advanced nasopharyngeal carcinoma: a multi‐center randomized phase 3 trial. Nat Med, 2021, Online First. [DOI] [PubMed] [Google Scholar]
- 148. Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open‐label, phase 3 trial. Lancet (London, England). 2016;388(10054):1883‐92. 10.1016/s0140-6736(16)31388-5. [DOI] [PubMed] [Google Scholar]
- 149. You R, Liu YP, Huang PY, Zou X, Sun R, He YX, et al. Efficacy and Safety of Locoregional Radiotherapy With Chemotherapy vs Chemotherapy Alone in De Novo Metastatic Nasopharyngeal Carcinoma: A Multicenter Phase 3 Randomized Clinical Trial. JAMA oncology. 2020;6(9):1345‐52. 10.1001/jamaoncol.2020.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Au E, Ang PT. A phase II trial of 5‐fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Annals of oncology: official journal of the European Society for Medical Oncology. 1994;5(1):87‐9. 10.1093/oxfordjournals.annonc.a058703. [DOI] [PubMed] [Google Scholar]
- 151. Yeo W, Leung TW, Leung SF, Teo PM, Chan AT, Lee WY, et al. Phase II study of the combination of carboplatin and 5‐fluorouracil in metastatic nasopharyngeal carcinoma. Cancer chemotherapy and pharmacology. 1996;38(5):466‐70. 10.1007/s002800050512. [DOI] [PubMed] [Google Scholar]
- 152. Ji JH, Yun T, Kim SB, Kang JH, Park JC, Cho IS, et al. A prospective multicentre phase II study of cisplatin and weekly docetaxel as first‐line treatment for recurrent or metastatic nasopharyngeal cancer (KCSG HN07‐01). European journal of cancer (Oxford, England: 1990). 2012;48(17):3198‐204. 10.1016/j.ejca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 153. Tan EH, Khoo KS, Wee J, Fong KW, Lee KS, Lee KM, et al. Phase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinoma. Annals of oncology: official journal of the European Society for Medical Oncology. 1999;10(2):235‐7. 10.1023/a:1008390929826. [DOI] [PubMed] [Google Scholar]
- 154. Li YH, Wang FH, Jiang WQ, Xiang XJ, Deng YM, Hu GQ, et al. Phase II study of capecitabine and cisplatin combination as first‐line chemotherapy in Chinese patients with metastatic nasopharyngeal carcinoma. Cancer chemotherapy and pharmacology. 2008;62(3):539‐44. 10.1007/s00280-007-0641-2. [DOI] [PubMed] [Google Scholar]
- 155. Huang Y, Liang W, Yang Y, Zhao L, Zhao H, Wu X, et al. Phase I/II dose‐finding study of nanoparticle albumin‐bound paclitaxel (nab®‐Paclitaxel) plus Cisplatin as Treatment for Metastatic Nasopharyngeal Carcinoma. BMC cancer. 2016;16:464. 10.1186/s12885-016-2517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Jin T, Li B, Chen XZ. A phase II trial of Endostar combined with gemcitabine and cisplatin chemotherapy in patients with metastatic nasopharyngeal carcinoma (NCT01612286). Oncology research. 2013;21(6):317‐23. 10.3727/096504014x13983417587401. [DOI] [PubMed] [Google Scholar]
- 157. Chua DT, Sham JS, Au GK. A phase II study of capecitabine in patients with recurrent and metastatic nasopharyngeal carcinoma pretreated with platinum‐based chemotherapy. Oral oncology. 2003;39(4):361‐6. 10.1016/s1368-8375(02)00120-3. [DOI] [PubMed] [Google Scholar]
- 158. Ciuleanu E, Irimie A, Ciuleanu TE, Popita V, Todor N, Ghilezan N. Capecitabine as salvage treatment in relapsed nasopharyngeal carcinoma: a phase II study. Journal of BUON: official journal of the Balkan Union of Oncology. 2008;13(1):37‐42. [PubMed] [Google Scholar]
- 159. Ngeow J, Lim WT, Leong SS, Ang MK, Toh CK, Gao F, et al. Docetaxel is effective in heavily pretreated patients with disseminated nasopharyngeal carcinoma. Annals of oncology: official journal of the European Society for Medical Oncology. 2011;22(3):718‐22. 10.1093/annonc/mdq425. [DOI] [PubMed] [Google Scholar]
- 160. Zhang L, Zhang Y, Huang PY, Xu F, Peng PJ, Guan ZZ. Phase II clinical study of gemcitabine in the treatment of patients with advanced nasopharyngeal carcinoma after the failure of platinum‐based chemotherapy. Cancer chemotherapy and pharmacology. 2008;61(1):33‐8. 10.1007/s00280-007-0441-8. [DOI] [PubMed] [Google Scholar]
- 161. Wang CC, Chang JY, Liu TW, Lin CY, Yu YC, Hong RL. Phase II study of gemcitabine plus vinorelbine in the treatment of cisplatin‐resistant nasopharyngeal carcinoma. Head & neck. 2006;28(1):74‐80. 10.1002/hed.20310. [DOI] [PubMed] [Google Scholar]
- 162. Chen C, Wang FH, Wang ZQ, An X, Luo HY, Zhang L, et al. Salvage gemcitabine‐vinorelbine chemotherapy in patients with metastatic nasopharyngeal carcinoma pretreated with platinum‐based chemotherapy. Oral oncology. 2012;48(11):1146‐51. 10.1016/j.oraloncology.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 163. Poon D, Chowbay B, Cheung YB, Leong SS, Tan EH. Phase II study of irinotecan (CPT‐11) as salvage therapy for advanced nasopharyngeal carcinoma. Cancer. 2005;103(3):576‐81. 10.1002/cncr.20802. [DOI] [PubMed] [Google Scholar]
- 164. Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, et al. Camrelizumab (SHR‐1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single‐arm, phase 1 trials. The Lancet Oncology. 2018;19(10):1338‐50. 10.1016/s1470-2045(18)30495-9. [DOI] [PubMed] [Google Scholar]
- 165. Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC, et al. Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS‐02). Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2021;39(7):704‐12. 10.1200/jco.20.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI‐9742). Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2018;36(14):1412‐8. 10.1200/jco.2017.77.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, Le Tourneau C, et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death‐Ligand 1‐Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE‐028 Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35(36):4050‐6. 10.1200/jco.2017.73.3675. [DOI] [PubMed] [Google Scholar]
- 168. Zhang L, Yang Y, Chen X, Li J, Pan J, He X, et al. 270MO A single‐arm, open‐label, multicenter phase II study of camrelizumab in patients with recurrent or metastatic (R/M) nasopharyngeal carcinoma (NPC) who had progressed on ≥2 lines of chemotherapy: CAPTAIN study. 2020;31.
- 169. Prawira A, Oosting SF, Chen TW, Delos Santos KA, Saluja R, Wang L, et al. Systemic therapies for recurrent or metastatic nasopharyngeal carcinoma: a systematic review. British journal of cancer. 2017;117(12):1743‐52. 10.1038/bjc.2017.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Jin Y, Shi YX, Cai XY, Xia XY, Cai YC, Cao Y, et al. Comparison of five cisplatin‐based regimens frequently used as the first‐line protocols in metastatic nasopharyngeal carcinoma. Journal of cancer research and clinical oncology. 2012;138(10):1717‐25. 10.1007/s00432-012-1219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ma SX, Zhou T, Huang Y, Yang YP, Zhan JH, Zhang YX, et al. The efficacy of first‐line chemotherapy in recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta‐analysis. Annals of translational medicine. 2018;6(11):201. 10.21037/atm.2018.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Chen X, Liang W, Wan N, Zhang L, Yang Y, Jiang J, et al. Cost‐effectiveness analysis of gemcitabine plus cisplatin versus fluorouracil plus cisplatin for first‐line treatment of recurrent or metastatic nasopharyngeal carcinoma. Oral oncology. 2019;94:80‐5. 10.1016/j.oraloncology.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 173. Chen C, Wang FH, An X, Luo HY, Wang ZQ, Liang Y, et al. Triplet combination with paclitaxel, cisplatin and 5‐FU is effective in metastatic and/or recurrent nasopharyngeal carcinoma. Cancer chemotherapy and pharmacology. 2013;71(2):371‐8. 10.1007/s00280-012-2020-x. [DOI] [PubMed] [Google Scholar]
- 174. Lang J, Hu C, Lu T, Pan J, Lin T. Chinese expert consensus on diagnosis and treatment of nasopharyngeal carcinoma: evidence from current practice and future perspectives. Cancer management and research. 2019;11:6365‐76. 10.2147/cmar.S197544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Lee AWM, Ng WT, Chan JYW, Corry J, Mäkitie A, Mendenhall WM, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer treatment reviews. 2019;79:101890. 10.1016/j.ctrv.2019.101890. [DOI] [PubMed] [Google Scholar]
- 176. You R, Zou X, Hua YJ, Han F, Li L, Zhao C, et al. Salvage endoscopic nasopharyngectomy is superior to intensity‐modulated radiation therapy for local recurrence of selected T1‐T3 nasopharyngeal carcinoma – A case‐matched comparison. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2015;115(3):399‐406. 10.1016/j.radonc.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 177. You R, Zou X, Wang SL, Jiang R, Tang LQ, Zhang WD, et al. New surgical staging system for patients with recurrent nasopharyngeal carcinoma based on the AJCC/UICC rTNM classification system. European journal of cancer (Oxford, England: 1990). 2015;51(13):1771‐9. 10.1016/j.ejca.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 178. Tian YM, Tian YH, Zeng L, Liu S, Guan Y, Lu TX, et al. Prognostic model for survival of local recurrent nasopharyngeal carcinoma with intensity‐modulated radiotherapy. British journal of cancer. 2014;110(2):297‐303. 10.1038/bjc.2013.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Liu YP, Wen YH, Tang J, Wei Y, You R, Zhu XL, et al. Endoscopic surgery compared with intensity‐modulated radiotherapy in resectable locally recurrent nasopharyngeal carcinoma: a multicentre, open‐label, randomised, controlled, phase 3 trial. The Lancet Oncology. 2021;22(3):381‐90. 10.1016/s1470-2045(20)30673-2. [DOI] [PubMed] [Google Scholar]
- 180. Chan JY, Tsang RK, Wei WI. Morbidities after maxillary swing nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head & neck. 2015;37(4):487‐92. 10.1002/hed.23633. [DOI] [PubMed] [Google Scholar]
- 181. Chen MY, Wang SL, Zhu YL, Shen GP, Qiu F, Luo DH, et al. Use of a posterior pedicle nasal septum and floor mucoperiosteum flap to resurface the nasopharynx after endoscopic nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head & neck. 2012;34(10):1383‐8. 10.1002/hed.21928. [DOI] [PubMed] [Google Scholar]
- 182. Zou X, Han F, Ma WJ, Deng MQ, Jiang R, Guo L, et al. Salvage endoscopic nasopharyngectomy and intensity‐modulated radiotherapy versus conventional radiotherapy in treating locally recurrent nasopharyngeal carcinoma. Head & neck. 2015;37(8):1108‐15. 10.1002/hed.23719. [DOI] [PubMed] [Google Scholar]
- 183. Yu KH, Leung SF, Tung SY, Zee B, Chua DT, Sze WM, et al. Survival outcome of patients with nasopharyngeal carcinoma with first local failure: a study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Head & neck. 2005;27(5):397‐405. 10.1002/hed.20161. [DOI] [PubMed] [Google Scholar]
- 184. Liu YP, Li H, You R, Li JB, Liu XK, Yang AK, et al. Surgery for isolated regional failure in nasopharyngeal carcinoma after radiation: Selective or comprehensive neck dissection. The Laryngoscope. 2019;129(2):387‐95. 10.1002/lary.27317. [DOI] [PubMed] [Google Scholar]
- 185. Li YQ, Tian YM, Tan SH, Liu MZ, Kusumawidjaja G, Ong EHW, et al. Prognostic Model for Stratification of Radioresistant Nasopharynx Carcinoma to Curative Salvage Radiotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2018;36(9):891‐9. 10.1200/jco.2017.75.5165. [DOI] [PubMed] [Google Scholar]
- 186. Qiu S, Lin S, Tham IW, Pan J, Lu J, Lu JJ. Intensity‐modulated radiation therapy in the salvage of locally recurrent nasopharyngeal carcinoma. International journal of radiation oncology, biology, physics. 2012;83(2):676‐83. 10.1016/j.ijrobp.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 187. To EW, Lai EC, Cheng JH, Pang PC, Williams MD, Teo PM. Nasopharyngectomy for recurrent nasopharyngeal carcinoma: a review of 31 patients and prognostic factors. The Laryngoscope. 2002;112(10):1877‐82. 10.1097/00005537-200210000-00033. [DOI] [PubMed] [Google Scholar]
- 188. Hao SP, Tsang NM, Chang KP, Hsu YS, Chen CK, Fang KH. Nasopharyngectomy for recurrent nasopharyngeal carcinoma: a review of 53 patients and prognostic factors. Acta oto‐laryngologica. 2008;128(4):473‐81. 10.1080/00016480701813806. [DOI] [PubMed] [Google Scholar]
- 189. Bossi P, Chan AT, Licitra L, Trama A, Orlandi E, Hui EP, et al. Nasopharyngeal carcinoma: ESMO‐EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow‐up(†). Annals of oncology: official journal of the European Society for Medical Oncology. 2021;32(4):452‐65. 10.1016/j.annonc.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 190. Zhou GQ, Wu CF, Deng B, Gao TS, Lv JW, Lin L, et al. An optimal posttreatment surveillance strategy for cancer survivors based on an individualized risk‐based approach. Nature communications. 2020;11(1):3872. 10.1038/s41467-020-17672-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zhou GQ, Wu CF, Zhang J, Mao YP, Tang LL, Chen L, et al. Cost‐Effectiveness Analysis of Routine Magnetic Resonance Imaging in the Follow‐Up of Patients With Nasopharyngeal Carcinoma After Intensity Modulated Radiation Therapy. International journal of radiation oncology, biology, physics. 2018;102(4):1382‐91. 10.1016/j.ijrobp.2018.01.117. [DOI] [PubMed] [Google Scholar]
- 192. Huang XD, Zhou GQ, Lv JW, Zhou HQ, Zhong CW, Wu CF, et al. Competing risk nomograms for nasopharyngeal carcinoma in the intensity‐modulated radiotherapy era: A big‐data, intelligence platform‐based analysis. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2018;129(2):389‐95. 10.1016/j.radonc.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 193. Zhang MX, Li J, Shen GP, Zou X, Xu JJ, Jiang R, et al. Intensity‐modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two‐dimensional radiotherapy: A 10‐year experience with a large cohort and long follow‐up. European journal of cancer (Oxford, England: 1990). 2015;51(17):2587‐95. 10.1016/j.ejca.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 194. Lee AWM, Tung SY, Ng WT, Lee V, Ngan RKC, Choi HCW, et al. A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10‐year outcomes for efficacy and toxicity. Cancer. 2017;123(21):4147‐57. 10.1002/cncr.30850. [DOI] [PubMed] [Google Scholar]
- 195. Wu LR, Liu YT, Jiang N, Fan YX, Wen J, Huang SF, et al. Ten‐year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity‐modulated radiotherapy: An analysis of 614 patients from a single center. Oral oncology. 2017;69:26‐32. 10.1016/j.oraloncology.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 196. Pfister DG, Spencer S, Adelstein D, et al. Nccn clinical practice guidelines in oncology: Head and neck cancers. Version 1.2021. Accessed nov 12, 2020. To view the most recent version, visit nccn.Org.
- 197. Li JX, Huang SM, Jiang XH, Ouyang B, Han F, Liu S, et al. Local failure patterns for patients with nasopharyngeal carcinoma after intensity‐modulated radiotherapy. Radiation oncology (London, England). 2014;9:87. 10.1186/1748-717x-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Glastonbury CM. Nasopharyngeal carcinoma: the role of magnetic resonance imaging in diagnosis, staging, treatment, and follow‐up. Topics in magnetic resonance imaging: TMRI. 2007;18(4):225‐35. 10.1097/RMR.0b013e3181572b3a. [DOI] [PubMed] [Google Scholar]
- 199. Yu E, O'Sullivan B, Kim J, Siu L, Bartlett E. Magnetic resonance imaging of nasopharyngeal carcinoma. Expert review of anticancer therapy. 2010;10(3):365‐75. 10.1586/era.10.9. [DOI] [PubMed] [Google Scholar]
- 200. Mao YP, Tang LL, Chen L, Sun Y, Qi ZY, Zhou GQ, et al. Prognostic factors and failure patterns in non‐metastatic nasopharyngeal carcinoma after intensity‐modulated radiotherapy. Chinese journal of cancer. 2016;35(1):103. 10.1186/s40880-016-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zhao W, Lei H, Zhu X, Li L, Qu S, Liang X. Investigation of long‐term survival outcomes and failure patterns of patients with nasopharyngeal carcinoma receiving intensity‐modulated radiotherapy: a retrospective analysis. Oncotarget. 2016;7(52):86914‐25. 10.18632/oncotarget.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hsu CL, Chan SC, Chang KP, Lin TL, Lin CY, Hsieh CH, et al. Clinical scenario of EBV DNA follow‐up in patients of treated localized nasopharyngeal carcinoma. Oral oncology. 2013;49(6):620‐5. 10.1016/j.oraloncology.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 203. Wang WY, Twu CW, Lin WY, Jiang RS, Liang KL, Chen KW, et al. Plasma Epstein‐Barr virus DNA screening followed by ¹⁸F‐fluoro‐2‐deoxy‐D‐glucose positron emission tomography in detecting posttreatment failures of nasopharyngeal carcinoma. Cancer. 2011;117(19):4452‐9. 10.1002/cncr.26069. [DOI] [PubMed] [Google Scholar]
- 204. Li WF, Zhang Y, Huang XB, Du XJ, Tang LL, Chen L, et al. Prognostic value of plasma Epstein‐Barr virus DNA level during posttreatment follow‐up in the patients with nasopharyngeal carcinoma having undergone intensity‐modulated radiotherapy. Chinese journal of cancer. 2017;36(1):87. 10.1186/s40880-017-0256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Hong RL, Lin CY, Ting LL, Ko JY, Hsu MM. Comparison of clinical and molecular surveillance in patients with advanced nasopharyngeal carcinoma after primary therapy: the potential role of quantitative analysis of circulating Epstein‐Barr virus DNA. Cancer. 2004;100(7):1429‐37. 10.1002/cncr.20129. [DOI] [PubMed] [Google Scholar]
- 206. Zhang LL, Li GH, Li YY, Qi ZY, Lin AH, Sun Y. Risk Assessment of Secondary Primary Malignancies in Nasopharyngeal Carcinoma: A Big‐Data Intelligence Platform‐Based Analysis of 6,377 Long‐term Survivors from an Endemic Area Treated with Intensity‐Modulated Radiation Therapy during 2003‐2013. Cancer research and treatment. 2019;51(3):982‐91. 10.4143/crt.2018.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


