Abstract
Background
Current treatment options for human epidermal growth factor receptor 2 (HER2)‐overexpressing gastric cancer at third‐line have shown limited clinical benefit. Further, there is no specific treatment for HER2 immunohistochemistry (IHC) 2+ and fluorescence in‐situ hybridization‐negative patients. Here, we report the efficacy and safety of a novel anti‐HER2 antibody RC48 for patients with HER2‐overexpressing, advanced gastric or gastroesophageal junction cancer.
Methods
Patients with HER2‐overexpressing (IHC 2+ or 3+), locally advanced or metastatic gastric or gastroesophageal junction cancer who were under at least second‐line therapy were eligible and received RC48 2.5 mg/kg alone every 2 weeks. The primary endpoint was the objective response rate (ORR) assessed by an independent review committee. Secondary endpoints included progression‐free survival (PFS), overall survival (OS), duration of response, time to progression, disease control rate, and safety.
Results
Of 179 patients screened, 125 were eligible and received RC48 treatment. The ORR was 24.8% (95% confidence interval [CI]: 17.5%‐33.3%). The median PFS and OS were 4.1 months (95% CI: 3.7‐4.9 months) and 7.9 months (95% CI: 6.7‐9.9 months), respectively. The most frequently reported adverse events were decreased white blood cell count (53.6%), asthenia (53.6%), hair loss (53.6%), decreased neutrophil count (52.0%), anemia (49.6%), and increased aspartate aminotransferase level (43.2%). Serious adverse events (SAEs) occurred in 45 (36.0%) patients, and RC48‐related SAEs were mainly decreased neutrophil count (3.2%). Seven patients had adverse events that led to death were not RC48‐related.
Conclusions
RC48 showed promising activity with manageable safety, suggesting potential application in patients with HER2‐overexpressing, advanced gastric or gastroesophageal junction cancer who have previously received at least two lines of chemotherapy.
Keywords: antibody‐drug conjugate, gastric cancer, HER2‐overexpressing, phase II clinical trial, RC48, third‐line therapy
RC48 is safe and efficacious for HER2‐overexpressing gastric cancer patients. Patients received trastuzumab showed similar efficacy with trastuzumab‐naïve patients. HER2 status doesn't have significant effect on clinical outcomes.
ABBREVIATIONS
- ADC
antibody‐drug conjugate
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CI
confidence interval
- CR
complete response
- DCR
disease control rate
- DOR
duration of response
- ECOG
Eastern Cooperative Oncology Group
- FISH
fluorescence in‐situ hybridization
- HER2
human epidermal growth factor receptor 2
- IHC
immunohistochemistry
- IRC
independent review committee
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- SAE
Serious adverse event
- SD
stable disease
- T‐DM1
trastuzumab‐emtansine
- TTP
time to progression
1. BACKGROUND
Gastric cancer (GC) or gastroesophageal junction adenocarcinoma (GEJA) is the fifth commonest cancer and the fourth leading cause of cancer deaths worldwide [1], which is especially prevalent in China, with an incidence of 29.3/100,000 and mortality of 21.2/100,000 [2]. In about 20% of GC patients, human epidermal growth factor receptor 2 (HER2) is overexpressed (immunohistochemistry [IHC] score of 3+ or IHC 2+ and positive by fluorescence in‐situ hybridization [FISH]) [3].
Based on the phase III ToGA trial results, trastuzumab is the only drug approved to be added to the first‐line chemotherapy for HER2‐overexpressing advanced GC [4, 5]. However, there is no alternative anti‐HER2 drug approved for second‐line treatment. Other molecular agents targeting HER2, such as lapatinib, have shown limited efficacy on previously treated HER2‐positive advanced GC, and the antibody‐drug conjugate (ADC) trastuzumab‐emtansine (T‐DM1) was not superior to second‐line chemotherapy [6, 7]. Presently, third‐line treatment options for advanced GC patients include chemotherapy (irinotecan, paclitaxel, and TAS‐102), immunotherapy (nivolumab and pembrolizumab), and targeted therapy (apatinib) [4, 8]. However, the objective response rate (ORR) was generally low (2.84%‐11.6%) [9, 10, 11, 12]. In contrast, trastuzumab deruxtecan, also known as DS‐8201, is an ADC that has shown clinical benefit as third‐line therapy for patients with HER2‐positive advanced GC and approved by the Food and Drug Administration (FDA) in the United States and the Ministry of Health, Labour and Welfare (MHLW) in Japan. Although trastuzumab deruxtecan may lead to a relatively high rate of interstitial lung disease (10%), the safety profile is still manageable [13].
RC48 is a novel recombinant human anti‐HER2 monoclonal antibody conjugated with a microtubule inhibitor (monomethyl auristatin E) via a cleavable linker. Preclinical study results showed that RC48 had a strong anti‐tumor effect on many tumor cell lines and animal models with HER2 expression [14, 15, 16]. In addition, in vitro studies had shown that RC48 could trigger antibody‐dependent cell‐mediated cytotoxicity on HER2‐overexpressing cancer cells and suppress tumor growth [14]. In previous studies, RC48 also showed a good safety profile and promising activity towards solid tumors, especially GC, and showed equivalent efficacy for HER2‐low expression (defined as HER2 IHC 2+&FISH‐) and HER2‐high expression (defined as HER2 IHC 2+&FISH+ or HER2 IHC 3+) tumors [17, 18, 19].
To address the unmet need of patients with HER2‐overexpressing advanced GC who have previously received two chemotherapy regimens but progressed, a single‐arm, phase II study was designed to evaluate the efficacy of RC48.
2. PARTICIPANTS AND METHODS
2.1. Study design and participants
This study is a single‐arm, open‐labeled, phase II trial carried out at 31 academic hospitals or cancer centers in China. Eligible patients were at least 18 years old with a life expectancy of more than 12 weeks; histologically confirmed, locally advanced or metastatic GC or GEJA; an Eastern Cooperative Oncology Group (ECOG) performance status of 0‐1; at least one unresectable, measurable tumor lesion according to the Response Evaluation Criteria In Solid Tumors (RECIST version 1.1); adaptable function of the heart, liver, and kidney; and documented histologically confirmed HER2 overexpression (defined as IHC 3+ or 2+). Main exclusion criteria included carcinomatous meningitis or untreated central nervous system metastases. Patients who had received the following treatments within 4 weeks before enrolment were not eligible: chemotherapy (6 weeks of nitrosourea and mitomycin C, 2 weeks of oral 5‐fluorouracil); radiotherapy (2 weeks of palliative local radiotherapy for bone metastases); targeted treatment; immunotherapy; traditional Chinese medicine treatment (if the instructions clearly mentioned anti‐tumor effect); T‐DM1 or participated in clinical studies of ADCs; any clinical investigational drug; a major surgery; any live vaccine (or had any vaccine planned to be received during the study). The study protocol was approved by a relevant institutional review board or ethics committee at each study center. This study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent before enrolment.
2.2. Procedures
All eligible patients received 2.5 mg/kg of RC48 (RemeGen, Yantai, Shandong, China) alone by intravenous infusion during 30‐90 min (60 min is recommended) every two weeks. Computed tomography (CT) or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis was performed at baseline (within 28 days before RC48 treatment initiation) and every six weeks afterwards for the evaluation of tumor response by an independent review committee (IRC) using RECIST version 1.1. Patients remained on the study until withdrawal for progressive disease (PD), intolerable adverse events (AEs), death, withdrawal of consent, loss to follow‐up, or for other reasons at the discretion of the investigator. Dose interruptions and reductions were permitted to manage AEs (Supplementary Table S1).
Safety assessments were performed at each study visit. All AEs were monitored and graded according to the Common Terminology Criteria for Adverse Events version 4.03. The last safety monitoring was performed on day 28 after the last treatment.
2.3. Outcomes
The primary endpoint of this study was the proportion of patients achieving an IRC confirmed objective response, defined as a complete response (CR) or partial response (PR), according to RECIST version 1.1. The ORR was based on the best overall response. The secondary endpoints were ORR assessed by investigators, progression‐free survival (PFS; the period from the first study dose until disease progression or death, whichever occurred first), OS (the time from the first dose until death or loss to follow‐up), duration of response (DOR; the time from the first documentation of objective response to the first documentation of disease progression or death), time to progression (TTP; the time from the date of the first dose to the first documentation of disease progression assessed by IRC or death from tumor progression), disease control rate (DCR; the proportion of patients with CR, PR, or stable disease [SD]), and safety. Toxicity was assessed by using National Cancer Institute‐Common Toxicity Criteria for Adverse Events version 4.03.
2.4. Statistical analyses
The sample size was estimated based on an assumed ORR of 20% among patients who failed at least two systemic treatments. This study was designed to have at least 85% power at a one‐sided significance level of 2.5% to reject the null hypothesis of a proportion of patients with an ORR of 10% or less in this population. Considering an assumed drop‐out rate of 10%, we planned to recruit 125 patients. The proportion of patients achieving an objective response or disease control was calculated during the study, and 95% confidence intervals (CIs) were calculated by using the Clopper‐Pearson method. The Kaplan‐Meier method was used to calculate PFS and OS and plot survival curves. The median survival was calculated according to the curve, and the 95% CI was estimated via the Greenwood formula [20]. Fisher's exact test was used to compare ORR between subgroups. OS and PFS in each subgroup were compared using log‐rank tests. All statistical analyses were two‐sided, and P < 0.05 was considered statistically significant. SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
3. RESULTS
3.1. Patients
Between July 10, 2018, and December 6, 2019, 179 patients were assessed, of whom 125 patients were found eligible for this study and treated with at least one dose of RC48. As of November 20, 2020, one patient (0.8%) continued to receive RC48 treatment. The most common reasons for treatment termination were disease progression (76 patients, 60.8%) and AEs (16 patients, 12.8%; Figure 1).
FIGURE 1.
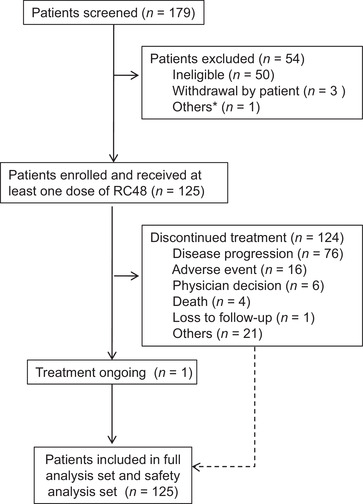
Flow diagram of study enrollment, treatment, and outcomes. *One patient agree to quit the trial due to the rapid progression of the tumor, short expected survival, and limited potential benefit from RC48
As of November 20, 2020, 125 patients were included in the full analysis set, and the 12‐month follow‐up data were collected. Their baseline characteristics are shown in Table 1. The median treatment duration was 12.0 weeks (range, 2.0‐38.0 weeks), and the median follow‐up was 7.6 months (range, 0.8‐23.7 months). Among the 125 patients, 97 (77.6%) had GC, and 28 (22.4%) had GEJA; 59 (47.2%) had received three or more lines of treatment in the past. Most of the patients were in poor condition, and only 29 (23.2%) had an ECOG performance status of 0 (Table 1).
TABLE 1.
Baseline demographic and clinical characteristics of the 125 enrolled patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer
| Characteristics | Whole cohort |
|---|---|
| Age [years; median (range)] | 58 (24‐70) |
| Sex [cases (%)] | |
| Female | 34 (27.2) |
| Male | 91 (72.8) |
| ECOG performance status [cases (%)] | |
| 0 | 29 (23.2) |
| 1 | 96 (76.8) |
| Time from diagnosis to enrollment [months; median (range)] | 17.1 (3.8‐94.5) |
| Histological type [cases (%)] | |
| Adenocarcinoma | 120 (96.0) |
| Adenosquamous cell carcinoma | 1 (0.8) |
| Others | 2 (1.6)* |
| Unknown | 2 (1.6) |
| Primary site [cases (%)] | |
| Stomach | 97 (77.6) |
| Gastroesophageal junction | 28 (22.4) |
| Burden of target tumor lesion [cases (%)] | |
| <5 cm | 38 (30.4%) |
| ≥5 cm to <10 cm | 43 (34.4%) |
| ≥10 cm | 38 (30.4%) |
| Undetermined | 6 (4.7)** |
| Metastatic site [cases (%)] | |
| Lymph node | 95 (76.0) |
| Liver | 70 (56.0) |
| Lung | 57 (45.6) |
| Bone | 24 (19.2) |
| Adrenal gland | 14 (11.2) |
| Pleura | 6 (4.8) |
| Kidney | 3 (2.4) |
| Colorectum or esophagus | 5 (4.0) |
| Others | 65 (52.0) |
| Number of metastatic sites [cases (%)] | |
| <2 | 16 (12.8) |
| ≥2 | 109 (87.2) |
| Number of previous lines of therapy [cases (%)] | |
| 2 | 66 (52.8) |
| ≥3 | 59 (47.2) |
| Previous trastuzumab treatment [cases (%)] | |
| Yes | 72 (57.6) |
| No | 53 (42.4) |
| Previous taxane treatment [cases (%)] | |
| Yes | 107 (85.6) |
| No | 18 (14.4) |
| Clinical stage [cases (%)] | |
| IIB | 1 (0.8) |
| IV | 124 (99.2) |
| HER2 status [cases (%)] | |
| IHC 3+ | 64 (51.2) |
| IHC 2+&FISH+ | 12 (9.6) |
| IHC 2+&FISH‐ | 6 (4.8) |
| IHC 2+&FISH undetermined | 43 (34.4) |
One patient with poorly differentiated adenocarcinoma mixed with signet ring cell carcinoma. One patient with dysplasia and canceration of the cardiac gland.
There were six patients evaluated as having no target lesson by IRC at baseline.
ECOG Eastern Cooperative Oncology Group, HER2 human epidermal growth factor receptor 2, IHC immunohistochemistry, FISH fluorescence in situ hybridization.
3.2. Efficacy
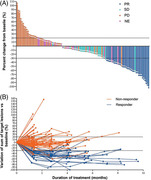
All 125 patients were treated with RC48 at 2.5 mg/kg alone. The overall ORR assessed by the IRC was 24.8% (95% CI: 17.5%‐33.3%). The median DOR was 4.7 months (95% CI: 3.4‐6.9 months; Supplementary Figure S1), and the DCR was 42.4% (95% CI: 33.6%‐51.6%; Table 2, Figure 2).
TABLE 2.
Summary of responses of the 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer to RC48
| Variables | Whole cohort |
|---|---|
| Best overall response [cases (%)] | |
| Complete response | 0 (0.0) |
| Partial response | 31 (24.8) |
| Unconfirmed partial response* | 2 (1.6) |
| Stable disease ≥ 12 weeks | 22 (17.6) |
| Stable disease < 12 weeks** | 18 (14.4) |
| Progressive disease | 36 (28.8) |
| NE*** | 16 (12.8) |
| ORR [estimate (95% CI)] | 24.8 (17.5‐33.3) |
| Disease control rate [estimate (95% CI)] | 42.4 (33.6‐51.6) |
| ORR assessed by investigator [estimate (95% CI)] | 24.0 (16.8‐32.5) |
| Progression‐free survival | |
| Disease progression or death [cases (%)] | 107 (85.6) |
| Median (95% CI) (months) | 4.1 (3.7‐4.9) |
| Range (months) | 0‐19.4 |
| Overall survival | |
| Death or loss to follow‐up [cases (%)] | 97 (77.6) |
| Median (95% CI) (months) | 7.9 (6.7‐9.9) |
| Range (months) | 0.8‐23.7 |
| Duration of response | |
| Response to RC48 [cases (%)] | 31 (24.8) |
| Median (95% CI) (months) | 4.7 (3.4‐6.9) |
| Range (months) | 2.2‐15.3 |
| Time to progression | |
| Disease progression [cases (%)] | 87 (69.6) |
| Median (95% CI) (months) | 4.2 (3.9‐5.4) |
| Range (months) | 0‐19.4 |
Tumor response was assessed by an IRC except for the ORR assessed by investigators. ORR includes complete response and partial response. Disease control rates include complete response, partial response, and stable disease ≥ 12 weeks.
Two patients were evaluated as having PR at the first assessment by both investigators and IRC but discontinued the treatment before the second assessment.
Ten patients were evaluated as having PD at first assessment by investigators and were assessed as having SD by the IRC. Due to early discontinuation of treatment, SD duration could not be assessed. Eight patients were evaluated as having SD at first assessment by both investigators and IRC but discontinued the treatment before the third assessment because of adverse events or the investigator/sponsor considered that it was not suitable to continue the trial considering the maximum benefits for the subject.
Six patients were evaluated as having no target lesson by IRC; 7 discontinued the treatment before the second assessment because the investigator/sponsor considered that it was not suitable to continue the trial considering the maximum benefits for the subject; 2 died before the second assessment, and 1 was not evaluable by imaging examination by the IRC.
Abbreviations: PR partial response, SD stable disease, PD progression disease, NE not evaluable, ORR objective response rate, CI confidence interval, IRC independent review committee, HER2 human epidermal growth factor receptor 2.
FIGURE 2.
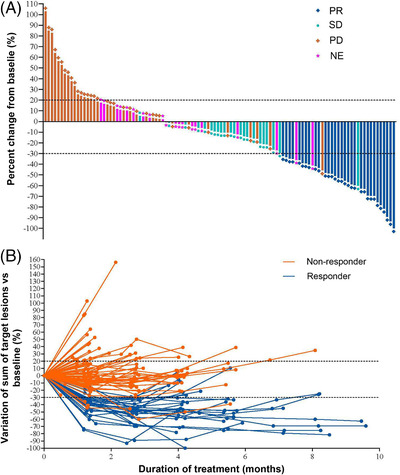
Treatment responses of 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48. A, Waterfall plot of best objective response assessed by IRC. B, Spider plot of best objective response assessed by IRC. Dots indicate the IRC assessment time points. Dashed lines at ‐30% or 20% indicate the minimum change in tumor size for a PR or PD respectively by RECIST 1.1. Non‐responders indicate the patients with SD or PD. Responders indicate the patients with PR. Abbreviations: IRC independent review committee, PR partial response, SD stable disease, PD progression disease, NE not evaluable, RECIST Response Evaluation Criteria In Solid Tumors, HER2 human epidermal growth factor receptor 2
PFS and TTP were calculated based on the IRC assessment. As of November 20, 2020, 107 patients (85.6%) reported disease progression or died. The median PFS was 4.1 months (95% CI: 3.7‐4.9 months), and the 6‐month PFS rate was 26.2% (Table 2, Figure 3A). TTP events such as disease progression or death due to progression were seen in 87 patients (69.6%), with a median TTP of 4.2 months (95% CI: 3.9‐5.4 months; Table 2). Nighty‐seven patients (77.6%) died, with a median OS of 7.9 months (95% CI: 6.7‐9.9 months) and a 1‐year survival rate of 33.3% (95% CI: 25.0%‐41.9%; Table 2, Figure 3B).
FIGURE 3.
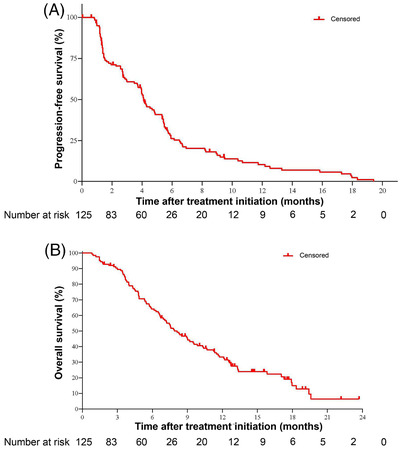
Kaplan‐Meier curves of progression‐free survival and overall survival for the 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48. A, The estimated progression‐free survival in all patients was 4.1 months (95% CI, 3.7‐4.9 months). B, The estimated overall survival in all patients was 7.9 months (95% CI, 6.7‐9.9 months). Abbreviations: HER2 human epidermal growth factor receptor 2, CI confidence interval
Subgroup analysis was conducted according to age, ECOG performance status, primary site, tumor burden, number of metastatic sites, HER2 status, previous trastuzumab treatment, previous taxane treatment, and number of previous lines of therapy. The patients over 65 years old showed significantly prolonged PFS than younger patients (P = 0.044), whereas no other subgroups showed significant benefits in their ORR, PFS, or OS results (Supplementary Tables S2‐S3 and Supplementary Figure S2).
3.3. Safety
Adverse events are summarized in Table 3. All patients reported at least one AE. The most common AEs included decreased white blood cell (WBC) count (53.6%), asthenia (53.6%), hair loss (53.6%), decreased neutrophil count (52.0%), anemia (49.6%), and increased aspartate aminotransferase levels (AST; 43.2%). Grade 3‐5 RC48‐related AEs (Supplementary Table S4) were observed in 40 patients (32.0%), of which the most common were decreased neutrophil count (14.4%), decreased WBC count (14.4%), and anemia (5.6%).
TABLE 3.
Adverse events in the 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48
| Event | All grade [cases (%)] | Grade 3‐5* [cases (%)] |
|---|---|---|
| Any AEs | 125 (100) | 71 (56.8) |
| Decreased WBC count | 67 (53.6) | 18 (14.4) |
| Asthenia | 67 (53.6) | 3 (2.4) |
| Hair loss | 67 (53.6) | 0 |
| Decreased neutrophil count | 65 (52.0) | 18 (14.4) |
| Anemia | 62 (49.6) | 16 (12.8) |
| Increased AST | 54 (43.2) | 2 (1.6) |
| Decreased appetite | 48 (38.4) | 1 (0.8) |
| Weight loss | 48 (38.4) | 1 (0.8) |
| Nausea | 46 (36.8) | 1 (0.8) |
| Hypoesthesia | 41 (32.8) | 4 (3.2) |
| Increased ALT | 40 (32.0) | 1 (0.8) |
| Vomiting | 40 (32.0) | 0 |
| Constipation | 37 (29.6) | 0 |
| Abdominal distention | 34 (27.2) | 1 (0.8) |
| Abdominal pain | 32 (25.6) | 1 (0.8) |
| Diarrhea | 24 (19.2) | 1 (0.8) |
| Decreased platelet count | 24 (19.2) | 2 (1.6) |
| Hyponatremia | 24 (19.2) | 8 (6.4) |
| Pruritus | 24 (19.2) | 0 |
| Hypoalbuminemia | 23 (18.4) | 2 (1.6) |
| Hypokalemia | 23 (18.4) | 7 (5.6) |
| Limb pain | 21 (16.8) | 2 (1.6) |
| Pyrexia | 20 (16.0) | 0 |
| Insomnia | 19 (15.2) | 0 |
| Increased lactate dehydrogenase | 19 (15.2) | 0 |
| Increased gamma‐glutamyltransferase | 17 (13.6) | 4 (3.2) |
| Back pain | 16 (12.8) | 1 (0.8) |
| Epigastric pain | 16 (12.8) | 0 |
| Increased ALP | 16 (12.8) | 3 (2.4) |
| Joint pain | 15 (12.0) | 1 (0.8) |
| Decreased lymphocyte count | 14 (11.2) | 6 (4.8) |
| Hyperglycemia | 13 (10.4) | 3 (2.4) |
This table shows all‐grade adverse events occurring in at least 10% of patients from the initiation to day 28 after the last treatment.
Grade 5 adverse events occurred in 7 patients which were not RC48‐related.
Abbreviations: AE adverse event, WBC white blood cell, AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase, HER2 human epidermal growth factor receptor 2.
Indeed, in this study, hematological abnormalities were the commonest AEs. The rates of decreased WBC count and neutrophil count, and anemia were all over 40%. Abnormal liver function was also common, mainly manifested by increased AST (43.2%) and alanine aminotransferase levels (32.0%) and were predominantly grade 1 or 2 (Table 3). Grade 3 and 4 abnormal blood electrolyte levels were also reported, including in 8 patients (6.4%) with hyponatremia and 7 (5.6%) with hypokalemia. Serious adverse events (SAEs) were observed in 45 patients (36.0%), including intestinal obstruction in 7 patients (5.6%), upper gastrointestinal bleeding in 5 patients (4.0%), decreased neutrophil count in 4 patients (3.2%), and liver function abnormality in 3 patients (2.4%). Regarding intestinal obstruction, the commonest SAE, 4 cases were of grade 2, 2 were of grade 3, and the grade was not reported in 1 case. RC48‐related SAEs were observed in 20 patients (16.0%) and manifested mainly as decreased neutrophil count (3.2%). AEs that resulted in dose interruption, drug suspension, or discontinuation were observed in 13 (10.2%), 63 (50.4%), and 18 (14.4%) patients, respectively. Seven patients (4.7%) died during the study, which were not related to RC48 (Supplementary Table S5).
4. DISCUSSION
In the present study, we assessed the efficacy and safety of RC48 as a third‐line or later‐line therapy in Chinese patients with HER2‐overexpressing GC or GEJA. The clinical response to RC48 was encouraging. The study reached its primary endpoint, with 31 (24.8%, 95% CI: 17.5%‐33.3%) of 125 patients achieving an objective response, which was consistent with the prior phase I study [17, 18]. Additionally, on November 20, 2020, the median PFS was 4.1 months (95% CI: 3.7‐4.9 months), and the 1‐year OS rate was 33.3% (95% CI: 25.0%‐41.9%).
In our trial, patients were recruited according to their documented HER2 status. This study included not only conventional HER2‐overexpressing (IHC 3+ or IHC 2+&FISH+) patients but also HER2 IHC 2+&FISH‐ patients, which is normally defined as having low HER2 expression. Based on the previous studies, ADC drugs might have an anti‐tumor effect on non‐targeted antigen‐positive cells due to the bystander effect and have potential in treating HER2+ heterogeneous tumors, even for tumor cells with low HER2 expression [17, 18, 21–23].
In the present study, there were no significant differences in ORR, PFS, and OS between patients with HER2 IHC 2+ and IHC 3+ tumors. However, the ORR of HER2 IHC 2+&FISH‐ patients was lower than that of conventional HER2‐positive patients [16.7% (95% CI, 0.9%‐63.5%) vs. 26.3% (95% CI, 17.2%‐37.9%)]. One of the reasons for this finding could be the small sample size of patients with HER2 IHC 2+&FISH‐. According to the protocol, only HER2 IHC results were required. Due to the lack of FISH information, only 6 patients were confirmed to have HER2 IHC 2+&FISH‐ tumor, and 1 of them exhibited PR. The clinical benefit of patients with HER2 IHC 2+&FISH‐ tumors should be further investigated in future studies.
In this study, 53 patients (42.4%) had not received trastuzumab previously. This is because some of the patients could not afford it as trastuzumab was not covered by insurance until the end of 2017. Another reason is that some of the patients had HER2 IHC 2+&FISH‐ tumors, for which trastuzumab treatment is not recommended [24]. Subgroup analysis showed no significant differences in ORR, PFS, and OS between trastuzumab‐treated and trastuzumab‐naïve patients, which was in line with the results of patients with HER2‐positive locally advanced or metastatic GC or GEJA treated with trastuzumab emtansine [7].
Regarding the RC48 safety profile, the rate of SAE in this study was 36.0%, which was consistent with the rates (32%‐43%) reported by other studies on third‐line advanced GC [9, 25]. However, the commonest SAE (intestinal obstruction) was occurred in 7 patients, among which 5 were not considered to be related to RC48: 1 patient had medical history of intestinal obstruction; 1 had peritoneal carcinomatosis which may lead to intestinal obstruction; 2 had disease progressed rapidly during the treatment which may lead to intestinal obstruction; and 1 had gastroesophageal reflux disease at the time of enrollment which indicated the progression of GC and risk of intestinal obstruction. Grade 3 and 4 AEs were reported by 69 patients (55.2%); the most common of these AEs was myelosuppression, which occurred in 18 patients (14.4%) and was considered drug‐related. Among the 18 patients with grade 3‐4 myelosuppression AEs, only 1 had grade 4 febrile neutropenia and sepsis. Although myelosuppression is the main toxicity of RC48, the same as other chemotherapy drugs widely used in clinical practice, it can be clinically managed. In this study, 16 patients (12.8%) had grade 3 anemia, which could be due to the disease. Studies have shown that 27%‐40% of advanced GC patients would have anemia, of which 5.5% were RC48‐related grade 3 and 4 anemia [26, 27]. Interstitial lung disease or pneumonitis is an AE of special interest that occurred in trastuzumab deruxtecan at a rate of 9% with a death rate of 2.6% [28]. However it was not observed with RC48.
While promising, these data are limited by the non‐randomized single‐arm design of the study and a relatively small sample size. The efficacy and safety of RC48 and those of the current third‐line treatment of patients with advanced GC could not be compared in this study. A randomized controlled trial is ongoing (NCT04714190) to compare RC48 with standard treatment (taxan) in participants with HER2‐overexpressing locally advanced or metastatic GC.
5. CONCLUSIONS
This study met its primary endpoint. RC48 demonstrated promising activity with a manageable safety profile in patients with HER2‐overexpressing advanced GC who were refractory or intolerant to at least two lines of standard chemotherapy. Further investigations will help to validate the potential anti‐tumor activity and safety of RC48 in various settings, including combination with PD‐1 inhibitor.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The clinical trial protocol was approved by the institutional review board of each center. Each subject provided written informed consent before enrollment.
CONSENT FOR PUBLICATION
Not applicable.
DATA SHARING
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHORS' CONTRIBUTIONS
LS and YB designed the trial, wrote the protocol, and coordinated the study. ZP, TL, JW, AW, YH, LY, XZ, NF, SL, ZL, KG, JL, JX, QF, RX, LZ, EL, YS, GY, CB, YL, JZ, JY, XL, NX, CG, YS, DM, GD, SL, and TD enrolled patients. JF managed, analyzed, and interpreted the data. ZP, TL, and JW drafted the manuscript. All authors reviewed and revised the paper, and approved the submitted version.
CONFLICT OF INTEREST STATEMENT
JF is employee and shareholder of RemeGen, Ltd. All the other authors declare no conflict of interest.
Supporting information
Figure S1 and S2
Supplementary Figure S1. A swimmer plot shows treatment responses among 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48. Each bar represents the period of time from the first dose to the last dose of RC48. Arrows denote ongoing treatment on November 20, 2020. Abbreviations: PR partial response, PD, progressive disease, DOR duration of response, HER2 human epidermal growth factor receptor 2.
Supplementary Figure S2. Subgroup analysis of 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48 according to their baseline characteristics. A, Kaplan‐Meier estimates of progression‐free survival according to HER2 status. B, Kaplan‐Meier estimates of overall survival according to HER2 status. Crosses denote censored observations. C, Objective response rates and 95% CIs in key subgroups based on baseline characteristics. Abbreviations: ECOG Eastern Cooperative Oncology Group, IHC immunohistochemistry, ORR objective response rate, CI confidence interval, HER2 human epidermal growth factor receptor 2.
Supplementary Table S1. Dose modification of RC48 in patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer
Supplementary Table S2. Subgroup analysis of objective response rate, progression‐free survival, and overall survival of the 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48.
Supplementary Table S3. Subgroup analysis of 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48 according to their HER2 status
Supplementary Table S4. RC48‐related adverse events in 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48
Supplementary Table S5. Serious adverse events and adverse events leading to discontinuation, dose interruption, and suspension of RC48 in 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer
ACKNOWLEDGMENTS
This study was funded by RemeGen Co., Ltd. This work was also supported by the National Natural Science Foundation of China (No. 91959205), the Ministry of Science and Technology of China (No. 2017YFC1308900, No. 2018ZX09201‐015), and Beijing Municipal Health Commission (No. 2020‐1‐1022). We thank the patients and their families and the participating study teams for making this study possible; members of the independent review committee for their role in this trial.
Peng Z, Liu T, Wei J, Wang A, He Y, Yang L, et al. Efficacy and safety of a novel anti‐HER2 therapeutic antibody RC48 in patients with HER2‐overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single‐arm phase II study. Cancer Commun. 2021;41:1173–1182. 10.1002/cac2.12214
ClinicalTrials.gov ID: NCT03556345
Contributor Information
Yi Ba, Email: bayi@tjmuch.com.
Lin Shen, Email: linshenpku@163.com.
REFERENCES
- 1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;0:1–41. [DOI] [PubMed] [Google Scholar]
- 2. Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 2019;41(1):19–28. [DOI] [PubMed] [Google Scholar]
- 3. Van Cutsem E, Bang YJ, Feng‐yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. NCCN Clinical Practice Guidelines in Oncology: Gastric cancer (version 2.2020). https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- 5. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376(9742):687–97. [DOI] [PubMed] [Google Scholar]
- 6. Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second‐line treatment of HER2‐amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. J Clin Oncol. 2014;32:2039–49. [DOI] [PubMed] [Google Scholar]
- 7. Thuss‐Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. Trastuzumab Emtansine Versus Taxane Use for Previously Treated HER2‐positive Locally Advanced or Metastatic Gastric or Gastro‐Oesophageal Junction Adenocarcinoma (GATSBY): An International Randomised, Open‐Label, Adaptive, Phase 2/3 Study. Lancet Oncol. 2017;18(5):640–53. [DOI] [PubMed] [Google Scholar]
- 8. Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical Guidelines for the Diagnosis and Treatment of Gastric Cancer. Cancer Commun (Lond). 2019;39(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2018;19:1437–48. [DOI] [PubMed] [Google Scholar]
- 10. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Ken Kato, et al. Nivolumab in patients with advanced gastric or gastro‐oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO‐4538‐12, ATTRACTION‐2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;390:2461–71. [DOI] [PubMed] [Google Scholar]
- 11. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE‐059 trial. JAMA Oncol. 2018;4(5):e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Qin S, Xu JM, Xiong JP, Wu CP, Bai YX, et al. Randomized, double‐blind, placebo‐controlled phase III trial of apatinib in patients with chemotherapy‐refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–54. [DOI] [PubMed] [Google Scholar]
- 13. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab Deruxtecan in Previously Treated HER2‐Positive Gastric Cancer. N Engl J Med. 2020;382(25):2419–30. [DOI] [PubMed] [Google Scholar]
- 14. Li HW, Yu C, Jiang J, Huang CJ, Yao XJ, Xu QY, et al. An anti‐HER2 antibody conjugated with monomethyl auristatin E is highly effective in HER2‐positive human gastric cancer. Cancer Biol Ther. 2016;17(4):346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang J, Dong LH, Wang L, Wang L, Zhang J, Chen F, et al. HER2‐targeted antibody drug conjugates for ovarian cancer therapy. Eur J Pharm Sci. 2016;93:274–86. [DOI] [PubMed] [Google Scholar]
- 16. Yao X, Jiang J, Wang X, Huang CJ, Li D, Xie K, et al: A novel humanized anti‐HER2 antibody conjugated with MMAE exerts potent anti‐tumor activity. Breast Cancer Res Treat. 2015;153:123–33. [DOI] [PubMed] [Google Scholar]
- 17. Xu Y, Wang Y, Gong J, Zhang X, Peng Z, Sheng X, et al. Open‐label, Multicenter, Phase I study of the recombinant humanized anti‐HER2 monoclonal antibody‐MMAE conjugate RC48‐ADC in patients with HER2‐positive advanced solid tumors. Gastric Cancer. 2021;24(4):913–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu BH, Wang JY, Zhang QY, Liu YJ, Feng JF, Wang WX, et al. An open‐label, multicenter, phase Ib study to evaluate RC48‐ADC in patients with HER2‐positive metastatic breast cancer. J Clin Oncol. 2018;36(15_suppl):1028. [Google Scholar]
- 19. Sheng XN, Yan XQ, Wang L, Shi YX, Yao X, Luo H, et al. Open‐label, Multicenter, Phase II Study of RC48‐ADC, a HER2‐Targeting Antibody‐Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin Cancer Res. 2021;27:43–51. [DOI] [PubMed] [Google Scholar]
- 20. Pokhrel A, Dyba T, Hakulinen T. A Greenwood formula for standard error of the age‐standardised relative survival ratio, Eur J Cancer. 2008;44(3):441‐7. [DOI] [PubMed] [Google Scholar]
- 21. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumour Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing, N Engl J Med. 2012;366(10):883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sahin U, Hartmann F, Senter P, Pohl C, Engert A, Diehl V, et al. Specific activation of the prodrug mitomycin phosphate by a bispecific anti‐CD30/anti‐alkaline phosphatase monoclonal antibody. Cancer Res. 1990;50(21):6944–8. [PubMed] [Google Scholar]
- 23. Xu ZY, Guo DD, Jiang ZL, Tong RS, Jiang PD, Bai L, et al. Novel HER2‐Targeting Antibody‐Drug Conjugates of Trastuzumab Beyond T‐DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS‐8201a) and (Vic‐)Trastuzumab Duocarmazine (SYD985). Eur J Med Chem. 2019;183:111682. [DOI] [PubMed] [Google Scholar]
- 24. HERCEPTIN® (trastuzumab) Drug instructions . Initial U.S. Approval: 1998. Genentech, Incorporated.
- 25. Pavlakis N, Sjoquist KM, Martin AJ, Tsobanis E, Yip S, Kang YK, et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo‐Controlled Phase II Trial. J Clin Oncol. 2016;34(23):2728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen JG, Cheong JH, Hyung WJ, Kim J, Choi SH, Noh SH. Pretreatment anemia is associated with poorer survival in patients with stage I and II gastric cancer. J Surg Oncol. 2005; 91(2): 126–30. [DOI] [PubMed] [Google Scholar]
- 27. Liu XC, Qiu HB, Huang YY, Xu DZ, Li W, Li YF, et al. Impact of preoperative anemia on outcomes in patients undergoing curative resection for gastric cancer: a single‐institution retrospective analysis of 2163 Chinese patients. Cancer Med. 2018;7(2):360–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ENHERTU® (fam‐trastuzumab deruxtecan‐nxki) for injection, for intravenous use Drug instructions . Initial U.S. Approval: 2019. Daiichi Sankyo, Incorporated.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 and S2
Supplementary Figure S1. A swimmer plot shows treatment responses among 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48. Each bar represents the period of time from the first dose to the last dose of RC48. Arrows denote ongoing treatment on November 20, 2020. Abbreviations: PR partial response, PD, progressive disease, DOR duration of response, HER2 human epidermal growth factor receptor 2.
Supplementary Figure S2. Subgroup analysis of 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48 according to their baseline characteristics. A, Kaplan‐Meier estimates of progression‐free survival according to HER2 status. B, Kaplan‐Meier estimates of overall survival according to HER2 status. Crosses denote censored observations. C, Objective response rates and 95% CIs in key subgroups based on baseline characteristics. Abbreviations: ECOG Eastern Cooperative Oncology Group, IHC immunohistochemistry, ORR objective response rate, CI confidence interval, HER2 human epidermal growth factor receptor 2.
Supplementary Table S1. Dose modification of RC48 in patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer
Supplementary Table S2. Subgroup analysis of objective response rate, progression‐free survival, and overall survival of the 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48.
Supplementary Table S3. Subgroup analysis of 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48 according to their HER2 status
Supplementary Table S4. RC48‐related adverse events in 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer treated with RC48
Supplementary Table S5. Serious adverse events and adverse events leading to discontinuation, dose interruption, and suspension of RC48 in 125 Chinese patients with HER2‐overexpressing, locally advanced or metastatic gastric cancer


