Highlights
-
•
The hybrid USMW technology effects are due to the synergy between both.
-
•
The USMW-assisted drying and frying preserve the quality of foods.
-
•
The USMW-assisted extraction leads to high yields in short times.
-
•
The USMW-assisted enzymatic hydrolysis produces bioactive peptides.
Keywords: Hybrid technology, Ultrasound, Microwave, Food quality, Food bioactivity
Abstract
With the growing of consumer’s demand for products ready to eat that can be elaborated with greener technologies without affecting to their organoleptic characteristics, the application of ultrasound combined with microwaves has been widely studied on food preservation treatments (drying, frying), extraction of high-value added compounds and enzymatic hydrolysis of proteins. This review presents a complete picture of current knowledge on the ultrasound combined with microwaves including the mechanisms, influencing factors, advantages and drawbacks, emphasising in several synergistic effects observed in different processes of strong importance in the food industry. Recent research has shown that this hybrid technology could not only minimise the disadvantages of power US for drying and frying but also improve the product quality and the efficiency of both cooking processes by lowering the energy consumption. Regarding extraction, current studies have corroborated that the combined method presents higher yields in less time, in comparison with those in the respective ultrasound and microwave separately. Additionally, recent results have indicated that the bioactive compounds extracted by this combined technology exhibit promising antitumor activities as well as antioxidant and hepatoprotective effects. Remarkably, this hybrid technology has been shown as a good pre-treatment since the structural changes that are produced in the molecules facilitate the subsequent action of enzymes. However, the combination of these techniques still requires a proper design to develop and optimized conditions are required to make a scale process, and it may lead to a major step concerning a sustainable development and utilization of bioactive compounds from natural products in real life.
1. Introduction
Today food processing applies different unit operations to the transformation of raw material to reach food products safe for human consumption with good sensorial, nutritional and bioactive properties [1]. Traditional food processing methods depends on expending heat to diminish the growth of microorganisms but flavor, color, texture and nutritional and bioactive properties are not always preserved for thermally sensitive foods [1]. This has generated a need in research and development for the optimization of the traditional processing or application of novel non-thermal emerging and innovative technologies [2].
The high-intensity (10 to 1000 W/cm2) or low-frequency ultrasound (20 to 100 kHz) is an emerging and green processing technology that modify physical, biochemical, and mechanical properties of food products by acoustic cavitation increasing the temperature and pressure, among other mechanisms [3], [4]. In fact, high-intensity ultrasound (US) also has significant applications in numerous unit operations of extraction, freezing, drying, emulsification, defoaming, tenderization, concentration and thawing [3], [4], [5], [6]. Moreover, US has been used to improve the functional properties and bioactivity of polysaccharides and proteins [7], [8], [9]. However, the US is not effective against microbial grown and enzymatic inactivation [10] and could produce negative effect associated with hydroxyl radical generation such off-flavours, change in physical parameters and degradation of components [11].
Microwave (MW) processing techniques that use electromagnetic field with frequency from 300 MHz to 300 GHz [12] and have been extensively used in the food industry in the field of food processing such as drying, heating or cooking, pasteurization and preservation of foods, due to its significant reduction in cooking time and energy consumption [13], [14]. In the last years, the advantage of MW-assisted food processing, including extraction, have increased its popularity as alternative to conventional processing to enhance the product quality and process. Thus, the application of MW has been extended to the processes such freeze drying, convective drying, freezing, vacuum drying, vacuum frying and osmotic dehydration [15].
The application of hybrid USMW food processing technique with concomitant synergistic effect based on theoretical and experimental knowledge should allow the removing of defects of each individual technique [16]. Several food processing procedures using USMW technology have been applied to improve the product quality, including the increase of their bioactivity, and the process efficiency. However, to the best of our knowledge, there is not a compilation of studies as these here reviewed about the application of hybrid USMW in the food technology. Thus, this review is focused on drying, frying, bioactive extraction and enzymatic protein hydrolysis by the combination of US and MW, paying attention to the effect on quality and bioactivity/biofunctional properties of foods and on the feasibility and process profitability.
2. Combined ultrasound and microwave technologies in food processing
2.1. Ultrasound/microwave-assisted drying
The current consumer's interest in foods having good organoleptic characteristics is not only nutritious but also vehicles of certain components and/or ingredients with bioactivity is well known. Moreover, new products ready to eat with improved organoleptic characteristics are being one eligible option. In this context, dehydration has emerged as one of the most attractive food processing. By dehydration, vegetables and fruits, among others, can be easily produced and stored and transported at relatively low cost. In addition, the resulting products have different characteristics than the initial ones, diversifying the market.
To date, most dehydrated products are obtained by convective drying, usually preceded by different pre-treatments. Three phases happen during drying. The first one is the induction period, where water transfer mechanisms are combined. In the second stage, the maximum drying rate occurs. In the last phase, known as falling drying rate period, the amount of removed water from inside to the surface is lower than the evaporated water from the surrounding surface. Although by convection products with a high shelf life are obtained, their quality, in most cases, is far from what should be offered to the current consumer, due to the chemical, physical and physical–chemical modifications that originate [17].
To improve the final quality of dried products, one of the alternatives that has attracted great interest in recent years is the application of emerging technologies such as US. Numerous articles have been published on this technology, which can be applied in liquid systems, during pre-drying process treatment: in-line application (blanching, osmotic dehydration) and in the drying process itself: in-situ application [18]. Regarding the usefulness of US for the drying of foods, there are studies on the kinetics of moisture loss during its intensification by US [19], [20], [21]. US produce a number of effects such as microagitation, microscopic channel creation (“sponge effect”), and cavitation that facilitate the removal of water from inside the food. The synergistic effect of the US and temperature on US-assisted convective drying allows dehydrations to be carried out at a lower temperature and shorter time due to cavitation, streaming and “sponge” effects, aspect of paramount importance for thermolabile bioactive compounds as well as sensorial attributes [22], [23], [24] and energy consumption [25], [26]. More recently, the final quality of the dehydrated product and its shelf life has been investigated. No significant negative effects of US on the quality have been stated. Most of the investigations have confirmed the production of foods with satisfactory microbiological counts, colour, texture after rehydration, vitamin content and limited Maillard reaction products [21], [27]. Despite the several examples of US application in food processing, drying assisted by US is still in the experimental stage so far, probably attributed to the scarce evidence on US apparatus that would be suitable for industry. The airborne application of US for drying intensification is constrained by the extremely problematic energy transmission of US at high power in the air. To partly solve these complications, ultrasonic direct-contact systems have been used, although their application at industrial level is intricate. In the design of new transducers, for the moment, the most important advances have been performed by PUSONICS Company (https://www.pusonics.es/) [28].
2.1.1. Effect on drying
With the aim to minimise the disadvantages of power US for drying, it has also been combined with other physical procedures. Thus, hybrid drying technologies have been investigated by combining US with microwaves (MW) [29], [30].
The application of added energy sources intends to add their separate singularities and generate synergies. MW radiation involves an important thermal effect, which gives rise to temperature increase of the particle being dried and a better homogeneity in the distribution of temperature. Different models of hybrid drying by these technologies have been developed [31].
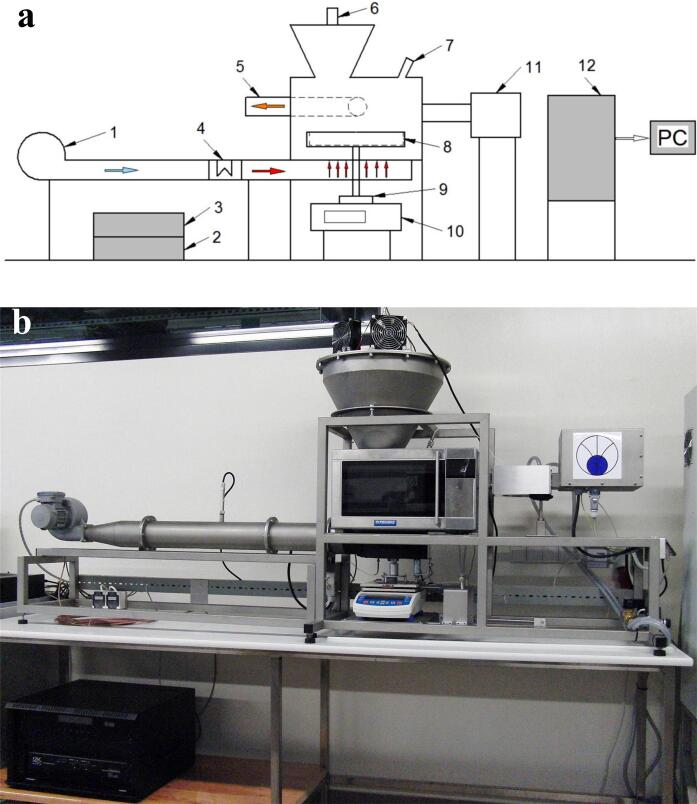
A dryer proposal permitting to use US for drying improvement was described by Kowalski and Mierzwa [32] and Kowalski and Pawlowski [33]. The device includes a dryer that permits drying by using convective and microwaves, separately as well as in different combinations (hybrid processes). The in-situ system is equipped with an Airborne Ultrasound System (AUS) developed by Pusonics S.L. (Spain). Ultrasound is applied to the material being dried through the air (air-borne) (Fig. 1).
Fig. 1.
a) Scheme of the hybrid dryer: 1. Fan, 2. Airborne Ultrasound System (AUS), 3. Ultrasound feeder, 4. Electric heater, 5. Air outlet, 6. Ultrasound transducer AUS, 7. Pyrometer, 8. Rotating sample pan, 9. Drive sample pan, 10. Balance, 11. Microwave generator, 12. Control cupboard. b) Photo of the hybrid system. Szadzińska et al. [34].
The modifications of the material temperature during drying have been assessed together with the efficacy of hybrid procedures on the basis of the drying kinetics curves. Some articles afford opposite conclusions, probably due to the different substrates. For green pepper, the drying rate was improved [34], whereas for carrots the rate of the process was not affected [35]. The effect of the microwave energy (100 W) in an airborne Ultrasound-assisted drying system (100–200 W, 55 °C) of raspberries was a decrease in the drying time of 79% with the assistance of MW and 59% without it [29], with a lower energy consumption resulting in energy saving of 14–23% for conventional-US, 54% for conventional-MW, and 59% for conventional-MWUS as compared to conventional, and an increase up to 4-folder in the average drying rate with the application of both US and MW in conventional treatment [36].
Kroenke et al. [37], described the changes in drying kinetics of carrots when US and MW intensified convective drying and they found similar drying rates in this in-situ USMW hybrid combination as compared to MW alone, and, even, more energy consumption was observed in the former. These results were probably ascribed to the low permeability of the substrate and a quite large air flow rate. US can have lower effectiveness in material with limited porosity and elevated rigidity, which is the case of carrot.
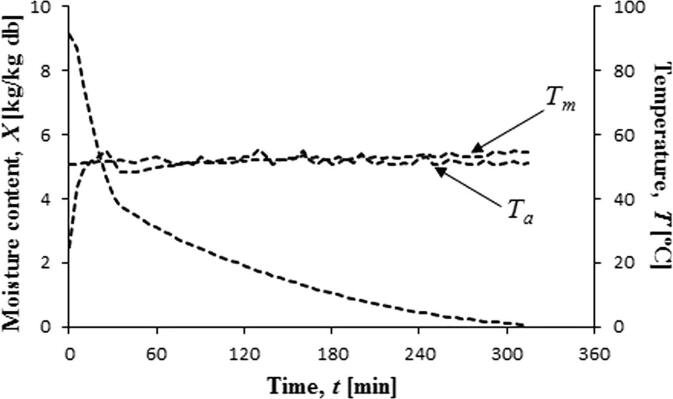
Szadzinska et al. [38] studied the convective drying of strawberries enhanced with MW (100 W) followed by US (200 W) (Fig. 2). In the intensification of convective drying by MW and US the total energy consumption increased by only 0.5 kWh and significantly increased the efficacy of heat and mass transfer in these fruits since the former produces a “heating effect“ and the latter gives rise to a ”vibration effect“, and both together accelerate the drying rate by a ”synergistic effect“. This result was later confirmed by Kroehnke et al. [37] who indicated that the vibration effect was showed as the most influential factor, but “heating” and “synergistic” effects are also significant which may be recognise by its low efficiency as the thermal energy increases.
Fig. 2.
Convective-microwave drying of strawberries assisted with ultrasound. Szadzinska et al. [38].
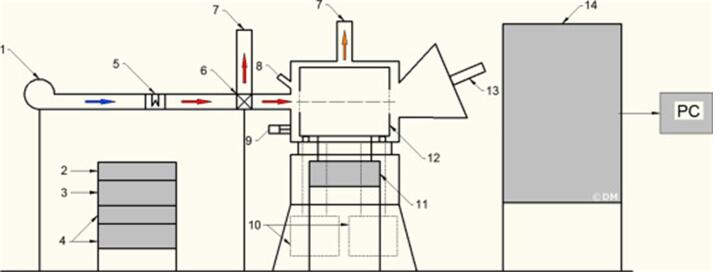
Using the same US transductor aforementioned, a similar hybrid apparatus was also developed (Fig. 3), but, in this case, the sample holder was bigger (maximum mass 2 kg vs 0.5 kg) and during operation the samples were intensively mixed [31]. In the model of Konopacka et al. [39] similar construction of a hybrid (convective-microwave) dryer was presented, but with differences in the direction of US and size of the drying chamber. The device can operate as a rotary (batch load: 1 kg of carrot cubes) and a tray dryer (batch load: 0.25 kg of carrot cubes), the latter being the best to reduce the drying rate. In carrots, these authors presented that the quantity of water evaporated during the initial stage of the treatment was about 60% superior for US-assisted drying comparing convective drying. In general, positive effects are described when US are assisted with MW treatments. The US airborne difficulties can be improved by MW and raw material properties should be taken into account for process effectiveness.
Fig. 3.
Scheme of drum hybrid dryer: 1. Blower, 2. AUS controller, 3. AUS amplifier, 4. Microwave feeders, 5. Heater, 6. Pneumatic valve, 7. Air outlet, 8. Pyrometer, 9. Drum drive, 10. Microwave generators, 11. Balance, 12. Rotatable drum, 13. AUS transducer, 14. Control unit. Musielak et al. [31].
2.1.2. Effect on quality of dried products
Regarding the effect on quality, a positive effect of US on colour modification was observed in convective and microwave-convective drying of carrot [35] and green pepper [38]. However, the change in the variation of colour (ΔE) in carrots was higher in the samples treated with the combination conventional-MW-US as compared to only MW but lower when the comparison was with US but a power values of US upper than 125 W [37]. In strawberries, the highest value of total colour change was found for the samples dried in the treatment conventional-MW-US, and this was attributed to the continuous application of hot air together with MW and US which seriously reinforced the ‘‘heating effect” and impaired the natural dyes [38].
Kroehnke et al. [37] also found that the data of aw of carrots subjected to conventional-MW-US were similar to those of MW and lower than those of conventional and US treatments. In all treatments the values of aw guaranteed the stability of the dried products against microorganisms and enzymes (aw < 0.6). Also, an enhancement of the rate and ratio of rehydration were attained applying US in the case of MW-convective drying of green pepper [34].
Natural colorants, such as carotenoids, have been also investigated. Kroehnke et al. [35] found that US application beneficed the retention of carotenoids in carrots dried convectively and by MW. In a later article, Kroehnke et al. [37] observed a different behaviour depending on the type of carotenoids. Thus, α-carotene and β-carotene retention was better in the hybrid combination conventional-MW-US as compared to MW, but in the case of lutein was worse; in all cases, the values of carotenoids retention were lower in comparison to those of samples of carrots treated only by US (Table 1). On the other hand, Konopacka et al. [35] stated that US application had no influence on the final content of anthocyanin in the case of blueberries.
Table 1.
Retention of carotenoids (%) in conventional, ultrasound (US) and microwaves (MW) treated carrot samples. Modified from Kroenke et al. (2018).
| Fresh sample (mg/kg d.m) | CV | CVUS 75 W | CVUS 125 W | CVUS 200 W | CVMW 100 W | CVUS 75 W MW 100 W | CVUS 125 W MW 100 W | CVUS 200 W MW 100 W | |
|---|---|---|---|---|---|---|---|---|---|
| Component | Retention of component (%) | ||||||||
| α-carotene β-carotene lutein Total amount |
144 476 17 637 |
87c 68 a 94 a 73b |
98 d 97c 95 a 98c |
89c 86 e 87 ac 86 d |
78b 76b 47 a 75b |
54 a 60 d 93 a 60 a |
59 a 67 a 25b 63 a |
73b 78b 19b 75b |
57 a 65 a 41b 62 a |
a, b, c, d, e – means in row followed by different letters are significantly different at p < 0.05.
It has been exposed by Kroehnke et al., [35] that the antioxidant capacity of carrots could be enhanced by US application, both for convective and MW-convective drying processes. Though, the results attained for polyphenols was not related to this improvement. Polyphenols were affected in a lower degree in the hybrid conventional-MW-US treatments as compared to separated MW and US with the exception of the highest values of US power. This fact could be due to the increase of temperature that diminishes the effect of US on the inactivation of enzymes by US. A similar trend was observed in the antioxidant capacity [37]. Combination of US-MW assisted technology to drying process can affect colour variation of food, probably by a reinforced “heating effect”. However, the different natural pigments, polyphenols content and their antioxidant activity can be less affected with this combination of assisted technology.
2.2. Ultrasound/Microwave-assisted vacuum frying
Deep-frying is the most common cooking process which is still being used to prepare a variety of food products in industrial and domestic scales. Compared to other cooking processes, frying produces some distinctive flavour characteristics, in addition to other undesirable and unacceptable attributes. Researchers have taken various technical approaches for reducing the oil content of fried products without compromising product quality.
Although Vacuum frying (VF) technology produces better quality products with less oil content, there are certain disadvantages, such as backward heating method, low efficiency especially with low-temperature frying and relatively high oil uptake in products [40]. Face to this situation, Microwave assisted vacuum frying (MVF) resulted a more efficient method including better quality parameters [41]. Furthermore, the application of US and combined US and MW in the VF may hand in promising results in the effectiveness improvement including the energy efficiency (short the frying and effective moisture diffusivity), low frying temperature and reducing oil uptake [42].
2.2.1. Effect on frying kinetic
Then, application of US and MW in vacuum VF (USMWVF) is recommended to achieve higher moisture evaporation rate and higher drying efficiency [43]. The schematic diagram of the in-situ USMWVF equipment design by Aorun Microwave Industry is showed in the Fig. 4, which has a frying vessel of 15 L oil elaborated with polytetrafluoroethylene (PTEF), which does not absorb microwave energy and is stable at temperature of 150 °C or higher [43], [44].
Fig. 4.
Schematic diagram of ultrasound and microwave-assisted vacuum frying (USMVF) instrument. 1. Oil tank, 2. Microwave source and heating system, 3. Ultrasound source and vacuum pressure balance system, 4. Vacuum chamber, 5. Frying chamber, &. Circulation pump, 7. Electric cabin door system, 8. Bending and centrifugation system, 9. Controller and operation panel. Adapted from Devi et al., [48] and Zhang et al. [97].
Kinetic dehydration was studied in fried edamame samples using USMWVF process. Different power degrees of US (0, 150, 300, 600 W) and temperature (80, 90, and 100 °C) were utilized in a constant microwave power and frequency of 1000 W and 28 kHz. The USMWVF process at every frying temperature increased the dehydration kinetic and the effective moisture diffusivity (De) when compared to the non-USMWVF process.
Kinetics of oil uptake was also studied in edamame samples [45]. A first order model fitted properly the values of oil uptake during VF, MWVF, and USMWVF. For all the studied conditions of the model, the specific rate increased with the application of MW and US and frying temperature, however the equilibrium oil content decreased. Furthermore, USMWVF edamame samples were evaluated under storage temperatures (0, 10 and 25 °C). Moisture and oil content, water activity, and vitamin C and chlorophyll retention were analyzed for 6 months. Vitamin C and chlorophyll content retention was 72% and 80%, at the storage temperature of 10 °C and, 54% and 73% at 25 °C respectively [45]. The MW and US applied in the VF process showed a synergistic effect in promoting the quality parameters, mainly by the shorter frying time, applied in different kinds of products, such as pumpkin chips [46], apple slides [47] or mushroom chips [44]. Therefore, the UMWVF process is more effective than MWVF and can be preferably used in frying foods [44]. This process increases moisture evaporation rate and drying efficiency and reduces the oil uptake, improvement sensorial and nutritional attributes during storage.
2.2.2. Effect on quality of fried products
Several studies demonstrate positive effects on qualitive attributes of fried products after processes (600 W to 1000 W of MW power; 150 W to 600 W of US power at 28 kHz, 15–16 min of frying). It was found that USMWVF accounts for the highest moisture loss rate in pumpkin chip samples [46], apple slides [47]. Similar results were presented with the increase of microwave power and frying temperature in mushroom chips [48]. Lower water activities were also determined in fried edamame samples [49].
On the other hand, lower oil uptake rate was also found in pumpkin chips [46] and mushroom chips [48]. In the last case, USMVF could reduce oil content by 16–20% compared to VF and MWVF. On the contrary, in apple slides [47] the ultimate oil uptake in USMWVF products was similar to that in MWVF products. Moreover, texture of chips under USMVF chips was better in comparison with only VF or MWVF process. In relation to the above behavior, microstructure observations revealed that the surface structures of the fried samples are preserved better by US treatment, showing the smoothest and less distorted cells, which also improvement crispness of pumpkin chips [46]. Similar increasing of textural crispness in apple slides was also observed by USMWVF application [47].US-enhanced frying preserves the color of original samples [48] or in some cases improves them. This is the case of apple slides [47] which the USMWVF treatment produced more desirable yellow color including less Maillard reaction compared to MWVF technology [47]. A color improvement was also reported by Islam, Zhang and Fan [49] in fried edamame samples.
USMWVF minimizes the loss of nutrients and bioactive compounds. The contents of protein, total phenolic compound, and total flavonoid compound in the fried mushroom chips were preserved to a greater extent by the treatment when US was applied [48]. Vitamin C and chlorophyll retention of fried edamame was highest in USMWVF process [49]. These studies demonstrates than USMWVF maintains sensorial and nutritional attributes, due to high moisture loss rate and preservation of surface structures. The faster water activity reduction and the lowest oil uptake also improve the sensorial and nutritional attributes during storage.
2.3. Ultrasound/Microwave-assisted extraction (USMW-AE)
In the recent years, the combination of USMW-AE technology has shown to be more effective for extracting high-value compounds from different matrices in comparison to the use of both technologies alone [50]. Mechanical effects produced by US lead to higher penetration of solvent into the cellular material, thus enhancing the contact surface area therefore, improving mass transfer by disrupting cell walls [50], [51] through chain detexturation mechanism in the following order: local erosion, shear forces, sonoporation, fragmentation, capillary effect and detexturation [52].
Meanwhile, MW heat the sample quickly, which can facilitate the transfer of the target compounds into the solvent, resulting in enhancement of extraction efficiency [53]. Consequently, combination of these technologies can involve several synergistic advantages including shorter extraction time, reduced solvent requirements, improved energy efficiency and lower costs [54], [55], [56], [57]. Moreover, this combined technology is quite flexible since the US and MW irradiations not only can be in-situ or in-line but also can be implemented continuously or intermittently. In the intermittent way, the US or MW irradiation stops for an interval during the extraction process, and this on/off cycle would be repeated many times. The on/off cycles in both irradiations can be synchronized or independent. The intermittent way is usually implemented when too high energy input into the extraction system, especially when no refluxing system is installed in the extraction system where too much evaporation of solvent can be occurred, in particular for low boiling point solvents such as methanol, ethanol and so on. The intermittent way could help to reduce the evaporation and control the temperature of the solvent [58].
The irradiation in-line could be completed using two individual techniques (US and MW), each of which occurs an irradiation. The in-situ irradiations would introduce more energy and resultant heat to the extraction solvent in a same duration, while it requires specially built system to incorporate the both irradiations in a same reactor/container. The Fig. 5 shows a schematic of a commercially available combined MW and US assisted extraction system.
Fig. 5.
Ultrasound in combination with microwave processor (a) commercially available and (b) schematic diagram of ultrasound in combination with microwave bath type system. Adapted from Ojha et al. [98].
2.3.1. Effect on extraction efficiency
With the aim of understanding the effects of process conditions of USMW-AE on the efficiency of the sequential and simultaneous extraction, some authors optimised the extraction parameters by response surface methodology in order to obtain the upper yield of high-value at the lowest resource spending [51], [59], [60]. In the case of in-line USMW-AE, a significant effect of time, power and/or temperature over the quantity of the anthraquinones extracted from stems and leaves of Heterophyllaea pustulata Hook f. (Rubiaceae). Thus, the combination US-AE with benzene at 50 °C during 60 min, followed by MW-AE using ethyl acetate as extraction solvent at 900 W constant power for 15 min, gave the highest yields in comparison with US-AE and Soxhlet extraction. Moreover, the energy consumption of USMW-AE was about 3.8 × 106 kJ/g anthraquinone, which was less than that of Soxhlet (6.2 × 106 kJ/g anthraquinone) [61]. Many studies have employed in-line USMW-AE techniques which could potentially reduce or even prevent from the degradation of target extract. [61], [62]. Bagherian et al. [62] investigated the effect of USMW as a pretreatment step for 30 min, and after adding HCl, pectin extract from grapefruit was heated in the microwave with a power of 450 W for 10 min. The results showed the yield of pectin by USMW-AE method (31.88%) was higher than that by MW-AE (27.81%), US-AE (17.92%) and conventional method (19.26%). Additionally, Liew et al. [63] studied the optimal conditions for in-line USMW-AE on pomelo peel using citric acid and made a comparison with MWUS-AE, MW-AE and US-AE. Under the optimised conditions, USMW-AE obtained the highest yield followed by MWUS-AE, then MW-AE, and finally US-AE.
On the other hand, most studies of USMW-AE technology involved in-situ USMW-AE irradiation. Thus, Jha et al. [64] obtained the maximum yield (1.75 mg/g GAE) of phenolic compounds extracted by USMW-AE simultaneously from husk of black rice at 10.02 min sonication time, 49.46 °C of sonication temperature, 1:40:79 (w/v) solute solvent ratio, 67.34% ethanol concentration, 10.02 min sonication time and 31.11 s microwave time working with constant frequency of 2450 MHz. Furthermore, yield was significantly affected initially linearly with increase in microwave time at constant ethanol concentration. However, a marginal increase was observed when ethanol concentration, microwave time and sonication time increased, keeping solute solvent ratio constant. Likewise, Garcia-Vaquero et al. [65] reported that time of extraction significantly influenced the yields of fucose-sulphated polysaccharides from brown macroalgae reaching the best concentrations (3.5 g/100 g vegetal extract) using simultaneously 100% of ultrasonic amplitude and 1000 W of microwave power during 5 min. Thereby, Lou et al. [66] observed that the simultaneous application of MW and US reduced the extraction time as much as 30 s from burdock leaves employing ionic liquids as a green solvent. Remarkably, Trujillo-Mayol et al. [67] and Qv et al. [68] highlighted the combined methods to be efficient than the conventional and non-conventional methods of extraction separately. In fact, Qv et al. [68] revealed that the powers and operating times in the combined method were lower than those in the respective US-AE and MW-AE optimised separately. Thus, the highest yield of lipid yield from green algae Dunaliella tertiolecta was reached applying 320 and 280 W of US and MW power for 4 min and 120 s, respectively, and liquid/solid ratio 100 mL/g. The same behavior was found by Sun et al. [57] and Lu et al. [69] who extracted by US-AE, MW-AE, USMW-AE and conventionally polysaccharides from Camptotheca acuminate fruit and oligosaccharides from lotus seeds, respectively. Trujillo-Mayol et al. [67] demonstrated that USMW-AE was more efficient, requiring 86% less time to approach the maximum recovery of total phenolics from avocado peel using ethanol–water (80:20 v/v). In terms of efficiency, USMW-AE not only required less energy (42.4 kWh) to obtain 100 g of dry extract but also the request for raw material for combined extraction was lower in comparison to MW-AE or US-AE (400 vs ≥ 602.5 g dry peel/100 g of extract).
2.3.2. Effect on bioactive compounds and bioactivity
In the previous section, the attention has been paid mainly on the effects of the parameters of USMW-AE that make influence on efficiency since, as indicated, it is an excellent technology in order to improve the yield of the extracted compounds. In addition, several studies have shown that extracts obtained by USMW-AE present a higher bioactivity as compared to those recovered by US-AE or MW-AE alone.
Zhou et al. [70] optimised USMW-AE conditions to significantly improve the oil quality of single-cell oil from Mortierella isabellina NTG1-121. This study suggested that USMW-AE could be an alternative to other technologies since the oil was rich in oleic acid (43.98%), linoleic acid (11.56%) and gamma linolenic acid (11.38%), which is comparable to the fatty-acid composition of several edible oils. In this way, Chen et al. [71] also optimised extraction parameters to maximize the yield and purity of polysaccharides possessing potential antitumor activities from the Inonotus obliquus fungus. Their results indicated that the USMW-AE had great potential and efficiency compared with traditional hot water extraction with no significant changes in antitumor activities. Furthermore, Alonso-Carrillo et al. [72] studied the extraction of phenolic compounds from Satureja macrostema using USMW-AE and reflux methods. Satureja macrostema is an aromatic herb containing polyphenolic compounds including flavonoids, which have been reported to have antioxidant and hepatoprotective effects. Their results showed significantly higher total phenolic content and lower median inhibition concentration values compared to reflux extraction, resulting in a higher radical scavenging ability, which can be attributed to the lower temperatures used in microwave-ultrasound assisted extraction and the higher stability of the extracted compounds.
Combined US-MW technology has been proposed as pretreatment or in-line process to produce changes in food protein structure and improve both their degree of hydrolysis (DH) as well as the release of peptides with enhanced bioactivity and techno-functional properties after enzymolysis [73], [74], [75], [76].
The influence of USMW-AE on the antioxidant capacity of the macroalgal extracts from A. nodosum was studied by Garcia-Vaquero et al. [65]. The highest levels of antioxidant capacity measured by ferric reducing antioxidant power (FRAP) (140.37 µM trolox/mg) were obtained by applying simultaneously 250 W MW power and 50% of US amplitude during 5 min, while the highest activities (75.86%) again 2,2-diphenyl-1-picrylhydrazyl (DPPH) were achieved in extracts obtained at 1000 W of MW power, 20% sonication amplitude for 5 min.
Regarding the antibacterial activity of avocado peel extracts obtained by USMW-AE displayed the upper activity against L. monocytogenes (≥500 µg/mL). Unlike US-AE, 2-fold higher concentration of USMW-AE extracts was required to be effective to inhibit E. coli and S. aureus. However, the same minimum inhibitory concentration (125 µg/mL) was observed for B. cereus in all extracts regardless technology used [67]. In this study, the Andrographolide from Sinta extracted sequentially applying sonication during 10 min and 10 min irradiation with a microwave power of 280 W, was of intermediate susceptibility to Escherichia coli but resistant to both Bacillus clausii and Klebsiella spp [77]. In other words, only the Sinta obtained using USMW-AE contained bioactive compounds with antibacterial activity for the Escherichia coli.
According to the antiproliferative activities, Gharibzahedi et al. [78] demonstrated that pectins extracted from fig skin using USMW-AE inhibited significantly the growth of human cancer cell lines (HepG2 and A549, in a dose-dependent behavior (100–1000 µg/mL). These results were associated with the immunological activity of carboxyl and acetyl groups in their structure. In this way, Rubi et al. [77] also reported a cytotoxic activity of the Sinta extract obtained from USMW-AE using nauplii shrimps. Other plausible explanation could be related to its free radicals scavenging activities and ferric reducing capacity at concentrations higher than 500 µg/mL due to the high number of uronic acids (mainly GalA). Albeit, in-line USMW-AE was more efficient than others conventional and non-conventional extraction methods, the highest yields of the compounds with enhanced bioactivity were achieved using sequential application of both technologies at low energy and time conditions.
2.4. Ultrasound and microwave-assisted enzymatic protein hydrolysis
Bioactive peptides are a hot topic of research since their positive health promoting effects including antimicrobial, antioxidant, anticarcinogenic, hypocholesterolemic, antihypertensive and immunomodulatory activities [79]. In addition, bioactive peptides play several roles in the food formulation such sweetener, color preservative, acidity regulator, anti-caking agent, emulsifier, flavor enhancer, thickener, foam formation, water and oil retention capacity to improve food quality [80]. Bioactive peptides are small protein molecules having<20 units of amino acids and they are commonly produced from different food protein or by-products by enzymatic hydrolysis using microbial endo- or exo-proteases such as flavourzyme, alcalase, trypsin, neutrase, protamex, neutrase, pepsin, pepsin-pancreatin, papain or enzyme combinations [81].
Nodaways, the food industry is focused in the development of innovative food processing safe, friendly with the environment, rapid, efficient that allows to obtain better quality products with good nutritional, organoleptic and functional properties. Thus, the high-intensity (10 to 1000 W/cm2) and low-frequency ultrasound (20 to 100 kHz) has been successfully applied to improve numerous unit operations [6] and modify functional properties of proteins such as solubility, gelling, foaming and emulsifying properties [82]. In addition, US has been proposed as an innovative processing in the development of functional food ingredients including the production of protein hydrolysate and bioactive peptides from animal and plant foods by enzymatic hydrolysis [83].
Structural changes of proteins induced by cavitation, which are associated with disruption electrostatic interactions, increases in free sulfhydryl groups, surface hydrophobic, and among other changes that facilitate the release bioactive peptides during the enzyme-catalyzed hydrolytic process [83]. An increase of Angiotensin Converting Enzyme (ACE) inhibitory activity (32.1 %) was showed in a hydrolysate obtained from wheat germ protein pretreated with US at 600 W for 10 min [84]. In other study, US pretreatment of corn gluten meal proteins (250 W, 20 kHz for 15 min) and hydrolysis catalyzed by neutrase (10 min) indicated that the highest ACE inhibitory activity [6]. In other study, the application of US (400 W for 30 min) changes the compact quaternary and tertiary structures of heat denatured soy protein and allow to produce antioxidant peptides by controlled enzymatic hydrolysis (45 °C for 4 h) with neutrase [85]. Similarly, the pretreatment of oat protein with US (750 W for 20 min) followed by 60 min of enzymolysis increase the ACE inhibitory activities of the peptides compared to the unpretreated samples [83]. Recently, dual-frequency US (20/40 kHz, 50 W/L at 53.5 °C for 10 min) pretreatment following for the rice protein hydrolysis catalyzed by neutrase increased the ACE inhibitory activity, which were accomplished for the changes in the soluble and hydrophobic, free sulfhydryl and surface hydrophobicity of the proteins [86]. However, the US-pretreatment effects are influenced for the ultrasonic systems, thus ultrasonic probe (191.1 W/cm2, 10 min) resulted in a significant increase in ACE-inhibitory activity of hydrolysate of wheat germ protein compared with ultrasonic bath operating at single or dual fixed and sweep frequencies [76]. Moreover, optimal conditions to improve the release bioactive peptides as well as the calorimetry and chemical dosimetry of the different ultrasonic system have not been characterized in order to extrapolate the US conditions. On the other hand, the MW-assisted enzymatic hydrolysis at 235.5 W for 19.5 min using alcalase has been applied to increase the ACE inhibitory activity by 14.2% compared to the conventional enzymatic hydrolysis [87]. In other study, protein hydrolysates from by-product trout by MW pretreatment followed by conventional enzymatic hydrolysis and MW-assisted enzymatic hydrolysis showed antioxidant capacity and reducing power after simulated gastrointestinal digestion [88].
2.4.1. Degree of hydrolysis
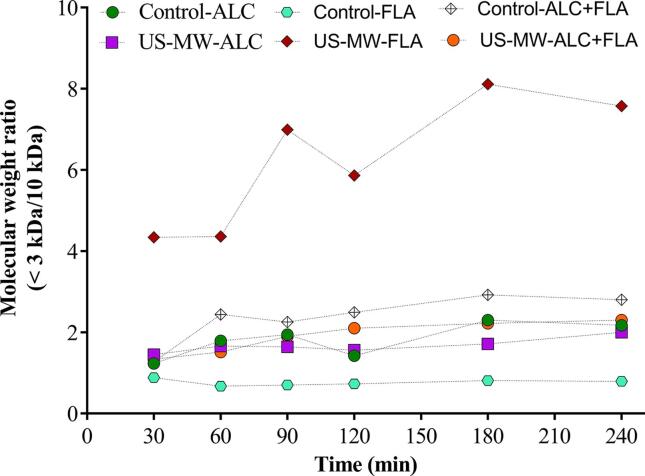
An increase of the DH (5.98 ± 0.48 to 28.53 ± 0.65) catalyzed by enzymes can be reached both USMW treatment as well as USMW-assisted process compared with untreated or control samples for different food protein sources (Table 2). The changes of DH are influenced by the conditions of US and MW (potency, time and temperature) as well as type of used enzymes and enzymatic reaction conditions. The use of the mixture of endopeptidase (alcalase, ALC) and endo- exo-peptidase (flavourzyme, FLAV) allow to obtain a better DH in a USMW-assisted enzymatic hydrolysis of sweet potato protein after 180 min at 50 °C, pH 8.0 (Table 2). The DH increases is associated with structural modification of food protein, which favoring the interaction with enzyme although in the case of USMW-assisted hydrolysis it is possible changes in the structure of enzymes that has not been measured. Moreover, DH is not reflected by molecular weight distribution, in fact FLAV showed a strongly increasing of molecular weight ratio (<3 kDa/10 kDa) compared to ALC (Fig. 6), but no a significant increase of DH (Table 2). This behavior could be explained by the size and type or amino acid of the peptides that affect the reaction rate with o-phthalaldehyde (OPA) commonly used to measure DH of proteins [89]. In this way, the pretreatment of Chinese sturgeon protein with US-MW produced after alcalase-catalyzed hydrolysis, a significant difference in fraction hydrolysates between 180 and 1000 Da compared to untreated control sample [76], which are related with the content of bioactive peptides in the hydrolysates. Therefore, the conditions US and MW as well as type of proteases to use should be found for each source of protein to optimize the DH.
Table 2.
Increase of degree of enzymatic hydrolysis of food protein pre-treated by combined ultrasound (US) and microwave (MW).
| Food protein source | US-MW pretreatment | Enzymatic hydrolysis | Increase of degree of hydrolysis (%) a | Reference |
|---|---|---|---|---|
| Milk protein concentrates (5% w/w) Chinese sturgeon (Acipenser sinensis) protein Golden threadfin bream (Nemipterus virgatus) myofibrillar protein Sweet potato protein |
US for 10 min (20 kHz, power not reported) and MW for 10 min at 800 W US 40 kHz-50 W and MW power 50 W at 55 °C for 8 min US100-MW100 b US200–MW100 b US300–MW100 b US400–MW100 b US 40 kHz-MW10-800 at 50 °C for 30, 60 and 180 min |
Digestive enzymes (pepsin at 37° C for 2 h, pH 2.0, and trypsin at 37 °C for 2 h, pH 7.5) Alcalase (3.5 %, pH 8.5, 55 °C for 20 min) Pepsin (0.25 mg/mL, pH 2.0, 37 °C) Alcalase (0.25 mg/mL, pH 9.0, 60 °C) Pepsin (0.25 mg/mL, pH 2.0, 37 °C) Alcalase (0.25 mg/mL, pH 9.0, 60 °C) Pepsin (0.25 mg/mL, pH 2.0, 37 °C) Alcalase (0.25 mg/mL, pH 9.0, 60 °C) Pepsin (0.25 mg/mL, pH 2.0, 37 °C) Alcalase (0.25 mg/mL, pH 9.0, 60 °C) Alcalase (4%, pH 8.0, 50 °C) 30 min 90 min 180 min Flavourzyme (4%, pH 8.0, 50 °C) 30 min 90 min 180 min Alcalase + Flavourzyme (4%, pH 8.0, 50 °C) 30 min 90 min 180 min |
12.97 ± 1.37 8.96 ± 1.70 5.98 ± 0.48 8.05 ± 0.18 9.99 ± 1.53 10.22 ± 1.16 12.06 ± 0.50 12.43 ± 0.40 7.64 ± 1.25 7.84 ± 1.02 18.18 ± 0.32 20.06 ± 0.04 17.10 ± 0.39 13.44 ± 0.65 14.98 ± 0.78 24.69 ± 0.62 20.96 ± 0.65 25.23 ± 0.20 28.53 ± 0.65 |
[72], [75], [73], [74] |
Control without pretreatment was used for the comparison; b Number is the US and MW power (Watts) and pretreatment was carried out at 51° C for 8 min.
Fig. 6.
Ratio of molecular weight (<3 kDa/10 kDa) of sweet potato protein hydrolysates at different times by US-MW-assisted enzymatic hydrolysis. ALC = Alcalase, FLA = Flavourzyme and ALC + FLA = Alcalase + Flavourzyme. Adapted from Habinshuti et al. [75].
2.4.2. Antioxidant capacity
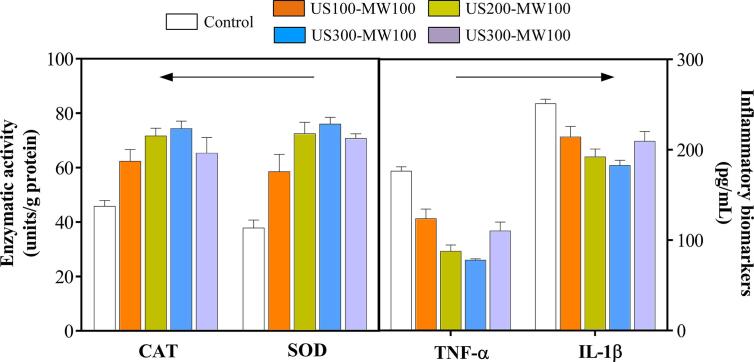
The modification of food protein structure to bioactive peptide production can be categorized in USMW pretreatment of protein and simultaneously application on both protein (substrate) and enzymes. Moreover, US has been done using probe ultrasonic system or ultrasonic-microwave cooperative extractor/reactor, different treatment conditions: power, frequency and temperatures, as well as digestive enzyme or different proteases or their combination (Table 3). However, the use of experimental design to optimize the USMW condition is scarce in order to maximize the bioactive peptides production by enzymolysis. In the case of the production of peptide antioxidants from milk protein concentrate previously treated by a combination of US and MW, the US power has not been reported and only one treatment condition has been applied, which did not improve the free radical scavenging activity against DPPH and FRAP measured by IC50 of filtrates (<5 kDa) of the hydrolysates obtained by digestive enzymes pepsin and trypsin at 37 °C for 2 h (Table 3). However, when enzymatic hydrolysis was carried out using bacterial alcalase (3.5% w/w) at 55 °C for 20 min after apply US (40 kHz-50 W) and MW (50 W) for 8 min to the Chinese sturgeon protein, the antioxidant capacity against DPPH and 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) free radical was improved as reflected by a lower IC50 compare to the hydrolysates obtained without pretreatment (Table 3). All above, depicts the DH in last samples was lower than milk protein concentrate hydrolysates, which reflect that antioxidant capacity of hydrolysates is related with protein source and obtained peptides profiles. In other study, US (100–400 W)-MW (100 W)-assisted enzymatic hydrolysis at 51° C for 8 min with alcalase or pepsin produced peptide with higher antioxidant capacity (IC50 from 2.1 to 5.1 mg/mL) than enzymatic reaction using untreated myofibrillar protein (IC50 ∼ 10 mg/mL) measured by DPPH assay. The peptides with a maximum antioxidant capacity were obtained by US 300 W and MW 100 W-assisted alcalase hydrolysis even better than pepsin hydrolysis (Table 3) despite both are endopeptidases with a very broad substrate, which indicate that slight modifications in the food protein structure or enzymes by USMW could produce peptides with differential antioxidant capacity. Peptides from sweet potato protein by USMW-assisted (US 40 kHz-MW10-800 at 50 °C for 180 min) enzymatic hydrolysis have also showed antioxidant capacity against biological free radical such as hydroxyl and peroxyl. Peptides obtained with alcalase an flavourzyme exhibited the highest hydroxyl scavenging capacity compared to the those obtained with the mixture of proteases. Thus, an increase in hydroxyl scavenging capacity the about 57% was obtained for the alcalase and 53% for the flavourzyme respect to the control without protein treatment (Table 3). However, flavourzyme produced a significative increase of ORAC value compared to the control (∼35%) (Table 3). Moreover, peptides (6 mg/mL) produced by enzymatic hydrolysis of Golden threadfin bream myofibrillar protein treated USMW (US 100–400 W and MW 100 W) induced antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD) in RAW 264.7 cells stimulated by hydrogen peroxide without adverse effect on cell viability (Fig. 7). In addition, a reduction of cytokines TNF-α and IL-1β levels was showed for the peptides prepared from protein by USMW-assisted enzymatic hydrolysis (Fig. 7). The nature of source protein influence on the obtention of bioactive peptide after treatment USMW together the type of proteases, therefore, the USWM condition could also produce the oxidation of some amino acids with reducing power such cysteine.
Table 3.
Antioxidant activity and ACE inhibition activity of peptides obtained by enzymatic hydrolysis of food proteins pre-treated by combined ultrasound (US) and microwave (MW).
| Food protein source [reference] |
US-MW pretreatment |
Antioxidant capacitya |
ACE inhibition activityc |
||
|---|---|---|---|---|---|
| DPPHa | ABTSa | FRAPa | |||
| Milk protein concentrates[72] Chinese sturgeon (Acipenser sinensis) protein[75] Golden threadfin bream (Nemipterus virgatus) myofibrillar protein[73] | US for 10 min (20 kHz, power not reported) and MW for 10 min at 800 WUS 40 kHz-50 W and MW power 50 W at 55 °C for 8 minUS100-MW100 b Pepsin Alcalase US200–MW100 b Pepsin Alcalase US300–MW100 b Pepsin Alcalase US400–MW100 b Pepsin Alcalase | 0.77 (0.62) 3.01 ± 0.04 (3.78 ± 0.04) 5.1 ± 0.3 4.9 ± 0.6 4.5 ± 0.3 2.8 ± 0.1 4.1 ± 0.8 2.3 ± 0.2 4.7 ± 0.8 4.1 ± 0.8 | 1.85 ± 0.03 (2.69 ± 0.04) | 0.32 (0.30) | 6.7 ± 0.7 6.2 ± 0.5 4.9 ± 0.3 5.2 ± 0.6 4.6 ± 0.4 3.5 ± 0.1 7.5 ± 0.5 5.3 ± 0.4 |
| Sweet potato protein [74] | US 40 kHz-MW10-800 at 50 °C for 180 min ALC FLA ALC + FLA |
OHd 67.11 ± 0.38 (42.69 ± 1.27) 57.04 ± 0.82 (37.10 ± 0.6) 60.06 ± 0.09 (52.18 ± 0.09) |
ORAC (µg TE/mL) e 110.32 ± 1.42 (102.59 ± 5.24) 121.80 ± 12.34 (89.97 ± 4.31) 106.32 ± 3.59 (96.74 ± 3.36) |
IC50 in mg/mL (control);
Control hydrolysates by pepsin = 10.60 ± 0.6 and alcalase = 10.1 ± 0.4;
Control 16.9 ± 0.3;
% of hydroxyl radical scavenging (control) and
Oxyradical Antioxidant Capacity and TE is Trolox Equivalent.
Fig. 7.
Effects of myofibrillar protein peptide (6 mg/mL) on the activities of catalase (CAT), superoxide dismutase (SOD) and inflammatory biomarkers TNF-α and IL-1β in RAW 264.7 cells stimulated by H2O2. Adapted from Li et al. [74].
2.4.3. ACE inhibition activity
Peptides produced by enzymatic hydrolysis after treated of golden threadfin bream myofibrillar protein USMW (US 100–400 W and MW 100 W) showed higher ACE inhibition activity than untreated control (IC50 = 3.5 ± 0.1–7.5 ± 0.5 versus IC50 = 16.9 ± 0.3) (Table 2). Similar to the antioxidant activity, ACE inhibition activity was higher for peptides obtained for the alcalase than those produced by hydrolysis catalyzed by pepsin (Table 2). In fact, the ACE inhibition activity of peptides showed a correlation with their antioxidant capacity measured by DPPH assay (Pearson r = 0.752; p < 0.05). A higher ACE inhibition activity of peptides was obtained at US = 300 W and MW = 100 W, which was accomplished for a modification of the protein secondary structure α-helix (47.88 ± 0.49 to 31.23 ± 0.13), β-sheet (22.57 ± 0.61 to 8.61 ± 0.20), β-turn (19.25 ± 1.27 to 33.27 ± 2.05), and random coil (10.3 ± 1.2 to 26.89 ± 1.75) [74]. The treatment of protein could be a good alternative to improve the biological activity of bioactive peptides, which associated with secondary and tertiary structure of protein. However, the optimal conditions of USWV should be stablished for each protein source.
3. Concluding remarks
It is very well known the benefit of the application of US or MW for food processing and to improve the functionality of food components. Both separated technologies are being used within the concept of “Green Food Manufacturing” to try to obtain products and ingredients with excellent quality. In spite of the fact that ultrasound have numerous applications, their industrial use has been scarcely developed, mainly in some specific cases. Though a huge effort is being done to save the involved obstacles, one of the main approaches is the combination, in sequence or simultaneous, with other technologies such as microwaves. Microwave heating and ultrasonic irradiation are among the easiest, cheap, and valued tools, this hybrid combination being extensive and fruitfully studied in applied chemistry but with less application in food technology. In addition to save energy, they promote faster and more selective modifications. Microwaves guarantee fast reactions via selective or volumetric heating, whereas ultrasound improve the mass transfer between phases, mixing and permitting an enhanced homogeneity in the distribution of temperature, one of the main problems associated to microwaves. Recent advances proof that such a combination is promising and safe, although the benefits depend on the type of involved process. There are not too many papers published in this field, however an increasing interest is raising in the last years.
Although in this review we have tried to compile the most recent studies (last ten years) on the application of the in-situ or in-line hybrid combination of US and MW on food processing preservation (drying, frying), extraction of bioactives and enzymatic hydrolysis of proteins, it is relevant to consider that this technique arises one decade ago for analytical purposes [90]. Since the beginning to date, several research groups have studied the possibilities of this hybrid technologies, paying attention to the developing of different apparatus for applications in chemistry, organic and inorganic synthesis and processing [91], [92], [93], [94], [95], [96]. These studies, among others, have been the basis for a better understanding of the different mechanisms involved, the effects and the applications of the hybrid combination ultrasound and microwaves.
Regarding drying, an interesting intensification by US and MW of convective drying of fruits and vegetable has been used in different investigations with the objective to increase the drying rate, but the results obtained so far are controversial, since different apparatus, conditions and substrates are used. Even, some researchers have reported an increase in the energy consumption. Recent papers have observed that the main effects are due to the heating and the synergy of both procedures, although vibration was found to be most important factor. Parameters such as colour, aw, rehydration capacity, carotenoids, polyphenols and antioxidant activity have been studied, and, for the moment, the data have not shown a clear conclusion on the usefulness of the hybrid combination of MW and US for food drying.
In the case of frying, the application of US and MW in vacuum frying gives rise to a better drying efficiency and lower oil uptake as compared to vacuum frying and microwave vacuum frying. In addition, the quality of the final product is also improved with lower values of aw, better texture, preservation of microstructure, crispness, color, higher retention of nutrients and bioactives (protein, polyphenols, flavonoids, vitamin C, chlorophyll). In general, the studies observed a synergistic effect between the involved technologies, which can lead us toward a practical application of USMW for food frying.
The use of hybrid US and MW irradiation in plant molecule extraction is really noteworthy. This combination is often superior to ultrasound- and MW-only assisted extractions (US-AE and MW-AE, respectively) and leads to higher yields in shorter reaction times. The mechanic effect of ultrasound provokes a better penetration of the solvent in the material to be extracted due to the disruption of cells increasing the mass transfer; the heating originated by microwaves also improve the extraction efficiency. In practical terms this technology is easy to perform using different modes of action: simultaneous or in sequence and can be implemented in a continuous or intermittent mode. The latter is more adequate to avoid or reduce an excessive evaporation of the solvent. Although the use of one or other system depend on the substrate and the objective of the operation, it is possible to say that under simultaneous operation the yields are higher, and the extraction time shorter. In addition to this, the quality and bioactivity of the different foods/components extracted by this hybrid technology was improved as compared to the equivalent traditional processes. As examples it is possible to indicate the extraction of oil, polysaccharides, phenolic compounds, flavonoids, antibacterial compounds. Definitely, the combination of US and MW for extraction is perhaps the most promising option to transfer to the industry.
Considering the application of US and MW to the enzymatic production of protein hydrolysates and bioactive peptides, their usefulness in separate modes has been pointed out. The hybrid combination of both has been shown as a good pre-treatment since the structural changes that are produced in the molecules facilitate the subsequent action of enzymes. Thus, it has been observed an increase in the degree of hydrolysis in different sources of proteins and peptides with improved antioxidant activity and ACE inhibition. Therefore, this application of ultrasound and microwaves combination can constitute an important advance in the obtainment of bioactive compounds derived from proteins.
In sum, we have in front a real challenge in the Food Science and Technology area. Although the combination of these such as different sources of energy is a real synergic hybrid technology which already allows a control in pilot plant and industrial scale, more studies are needed to try to improve their application. It is very important to consider in the design of the devices that microwaves heat very quick the inside of the medium, and, as it is very well known, the effectivity of ultrasound can diminish at temperature higher than 60 °C. Undoubtedly, the excellent results obtained in some applications deserve more investigation to study if the observed benefits can be still further.
CRediT authorship contribution statement
Nerea Muñoz-Almagro: Conceptualization, Writing – original draft. Eduardo Morales-Soriano: Conceptualization, Writing – original draft. Mar Villamiel: Conceptualization, Methodology, Software, Writing – review & editing. Luis Condezo-Hoyos: Conceptualization, Funding acquisition, Methodology, Software, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence.
Acknowledgments
This research was funded by Fondo Nacional de Desarrollo Científico y Tecnológico y de Innovación Tecnológica (FONDECYT, Peru), Project 168-2020 Obtención de pectina antioxidante mediante la aplicación de la tecnología verde de ultrasonido de frecuencia intermedia.
Contributor Information
Mar Villamiel, Email: m.villamiel@csic.es.
Luis Condezo-Hoyos, Email: lcondezo@lamolina.edu.pe.
References
- 1.Augusto P.E.D. Challenges, trends and opportunities in food processing. Curr. Opin. Food Sci. 2020;35:72–78. doi: 10.1016/j.cofs.2020.03.005. [DOI] [Google Scholar]
- 2.Zhang Z.-H., Wang L.-H., Zeng X.-A., Han Z., Brennan C.S. Non-thermal technologies and its current and future application in the food industry: a review. Int. J. Food Sci. Technol. 2019;54(1):1–13. doi: 10.1111/ijfs.2019.54.issue-110.1111/ijfs.13903. [DOI] [Google Scholar]
- 3.Li W., Gamlath C.J., Pathak R., Martin G.J.O., Ashokkumar M. Ultrasound – The Physical and Chemical Effects Integral to Food Processing. Innov. Food Process. Technol. 2021:329–358. doi: 10.1016/b978-0-08-100596-5.22679-6. [DOI] [Google Scholar]
- 4.Khadhraoui B., Fabiano-Tixier A.-S., Robinet P., Imbert R., Chemat F. Ultrasound technology for food processing, preservation. and extraction. 2019 doi: 10.1016/b978-0-12-815353-6.00002-1. [DOI] [Google Scholar]
- 5.Chen F., Zhang M., Yang C.-H. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020;63:104953. doi: 10.1016/j.ultsonch.2019.104953. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava N., Mor R.S., Kumar K., Sharanagat V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021;70:105293. doi: 10.1016/j.ultsonch.2020.105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X., Majzoobi M., Farahnaky A. Ultrasound-assisted modification of functional properties and biological activity of biopolymers: A review. Ultrason. Sonochem. 2020;65:105057. doi: 10.1016/j.ultsonch.2020.105057. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan J.J., Park M., Beevers J., Greenwood R.W., Norton I.T. Applications of ultrasound for the functional modification of proteins and nanoemulsion formation: A review. Food Hydrocolloids. 2017;71:299–310. doi: 10.1016/j.foodhyd.2016.12.037. [DOI] [Google Scholar]
- 9.Téllez-Morales J.A., Hernández-Santo B., Rodríguez-Miranda J. Effect of ultrasound on the techno-functional properties of food components/ingredients: A review. Ultrason. Sonochem. 2020;61:104787. doi: 10.1016/j.ultsonch.2019.104787. [DOI] [PubMed] [Google Scholar]
- 10.Huang G., Chen S., Dai C., Sun L., Sun W., Tang Y., Xiong F., He R., Ma H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017;37:144–149. doi: 10.1016/j.ultsonch.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Chemat F., Zill-e-Huma, Khan M.K. Khan, Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011;18(4):813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Regier M., Knoerzer K., Schubert H. Introducing microwave-assisted processing of food: Fundamentals of the technology, Second Edi. Elsevier Ltd. 2017 doi: 10.1016/B978-0-08-100528-6.00001-2. [DOI] [Google Scholar]
- 13.Guo Q., Sun D.W., Cheng J.H., Han Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017;67:236–247. doi: 10.1016/j.tifs.2017.07.007. [DOI] [Google Scholar]
- 14.Orsat V., Raghavan G.S.V., Krishnaswamy K. Microwave technology for food processing: An overview of current and future applications, Second Edi. Elsevier Ltd. 2017 doi: 10.1016/B978-0-08-100528-6.00005-X. [DOI] [Google Scholar]
- 15.Chizoba Ekezie F.G., Sun D.W., Han Z., Cheng J.H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci. Technol. 2017;67:58–69. doi: 10.1016/j.tifs.2017.05.014. [DOI] [Google Scholar]
- 16.Chen F., Zhang X., Zhang Q., Du X., Yang L., Zu Y., Yang F. Simultaneous synergistic microwave–ultrasonic extraction and hydrolysis for preparation of trans-resveratrol in tree peony seed oil-extracted residues using imidazolium-based ionic liquid. Ind. Crops Prod. 2016;94:266–280. doi: 10.1016/j.indcrop.2016.08.048. [DOI] [Google Scholar]
- 17.Traffano-Schiffo M.V., Castro-Giráldez M., Fito P.J., Balaguer N. Thermodynamic model of meat drying by infrarred thermography. J. Food Eng. 2014;128:103–110. doi: 10.1016/j.jfoodeng.2013.12.024. [DOI] [Google Scholar]
- 18.M. Villamiel, A. Montilla, J.V. García-Pérez, J.A. Cárcel, J. Benedito, eds., Ultrasound in Food Processing: Recent Advances, John Wiley & Sons, Ltd, Chichester, UK, 2017. https://doi.org/10.1002/9781118964156.
- 19.Cárcel J.A., Garcia-Perez J.V., Riera E., Mulet A. Improvement of Convective Drying of Carrot by Applying Power Ultrasound—Influence of Mass Load Density. Drying Technol. 2011;29(2):174–182. doi: 10.1080/07373937.2010.483032. [DOI] [Google Scholar]
- 20.Garcia-Perez Jose V., Carcel Juan A., Riera Enrique, Rosselló Carmen, Mulet Antonio. Intensification of Low-Temperature Drying by Using Ultrasound. Drying Technol. 2012;30(11-12):1199–1208. doi: 10.1080/07373937.2012.675533. [DOI] [Google Scholar]
- 21.Gamboa-Santos J., Montilla A., Cárcel J.A., Villamiel M., Garcia-Perez J.V. Air-borne ultrasound application in the convective drying of strawberry. J. Food Eng. 2014;128:132–139. doi: 10.1016/j.jfoodeng.2013.12.021. [DOI] [Google Scholar]
- 22.García-Pérez José V., Ozuna César, Ortuño Carmen, Cárcel Juan A., Mulet Antonio. Modeling Ultrasonically Assisted Convective Drying of Eggplant. Drying Technol. 2011;29(13):1499–1509. doi: 10.1080/07373937.2011.576321. [DOI] [Google Scholar]
- 23.C. Ozuna, T. Gómez Álvarez-Arenas, E. Riera, J.A. Cárcel, J. V Garcia-Perez, Influence of material structure on air-borne ultrasonic application in drying, Ultrasonics Sonochemistry. 21 (2014) 1235–1243. https://doi.org/10.1016/j.ultsonch.2013.12.015. [DOI] [PubMed]
- 24.Misra N.N., Martynenko Alex, Chemat Farid, Paniwnyk Larysa, Barba Francisco J., Jambrak Anet Režek. Thermodynamics, transport phenomena, and electrochemistry of external field-assisted nonthermal food technologies. Crit Rev Food Sci Nutr. 2018;58(11):1832–1863. doi: 10.1080/10408398.2017.1287660. [DOI] [PubMed] [Google Scholar]
- 25.Chemat F., Abert-Vian M., Fabiano-Tixier A.S., Strube J., Uhlenbrock L., Gunjevic V., Cravotto G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019;118:248–263. doi: 10.1016/j.trac.2019.05.037. [DOI] [Google Scholar]
- 26.Chemat Farid, Abert Vian Maryline, Fabiano-Tixier Anne-Sylvie, Nutrizio Marinela, Režek Jambrak Anet, Munekata Paulo E.S., Lorenzo Jose M., Barba Francisco J., Binello Arianna, Cravotto Giancarlo. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020;22(8):2325–2353. doi: 10.1039/C9GC03878G. [DOI] [Google Scholar]
- 27.Soria Ana Cristina, Corzo-Martínez Marta, Montilla Antonia, Riera Enrique, Gamboa-Santos Juliana, Villamiel Mar. Chemical and physicochemical quality parameters in carrots dehydrated by power ultrasound. J. Agric. Food Chem. 2010;58(13):7715–7722. doi: 10.1021/jf100762e. [DOI] [PubMed] [Google Scholar]
- 28.J.V. Villamiel, M., Riera, E., García-Pérez, The use of ultrasound for drying, degassing and defoaming of foods, in: Innovative Food Processing Technologies: A Comprehensive Review, Elsevier, Chichester, UK, 2020: p. In press. https://doi.org/10.1002/9781118964156.
- 29.Kowalski S.J., Pawłowski A., Szadzińska J., Łechtańska J., Stasiak M. High power airborne ultrasound assist in combined drying of raspberries. Innovative Food Sci. Emerg. Technol. 2016;34:225–233. doi: 10.1016/j.ifset.2016.02.006. [DOI] [Google Scholar]
- 30.Li Kun, Zhang Min, Mujumdar Arun S., Chitrakar Bimal. Recent developments in physical field-based drying techniques for fruits and vegetables. Drying Technol. 2019;37(15):1954–1973. doi: 10.1080/07373937.2018.1546733. [DOI] [Google Scholar]
- 31.Musielak G., Mierzwa D., Kroehnke J. Food drying enhancement by ultrasound – A review. Trends Food Sci. Technol. 2016;56:126–141. doi: 10.1016/j.tifs.2016.08.003. [DOI] [Google Scholar]
- 32.Kowalski S.J., Mierzwa D. US-Assisted Convective Drying of Biological Materials. Drying Technol. 2015;33(13):1601–1613. doi: 10.1080/07373937.2015.1026985. [DOI] [Google Scholar]
- 33.Kowalski S.J., Pawłowski A. Intensification of apple drying due to ultrasound enhancement. J. Food Eng. 2015;156:1–9. doi: 10.1016/j.jfoodeng.2015.01.023. [DOI] [Google Scholar]
- 34.Szadzińska J., Łechtańska J., Kowalski S.J., Stasiak M. The effect of high power airborne ultrasound and microwaves on convective drying effectiveness and quality of green pepper. Ultrason. Sonochem. 2017;34:531–539. doi: 10.1016/j.ultsonch.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 35.S. Kroehnke, J., Radziejewska-Kubzdela, E., Musielak, G., Stasiak, Ultrasonic-assisted and microwave -assisted convective drying of Carrot: Drying kinetics and quality analysis, In Proceedings of the 5th European Drying Conference (Eurodrying 2015). (2015) 195–201. https://doi.org/10.1016/j.ultsonch.2018.05.040.
- 36.Szadzińska Justyna, Łechtańska Joanna, Pashminehazar Reihaneh, Kharaghani Abdolreza, Tsotsas Evangelos. Microwave- and ultrasound-assisted convective drying of raspberries: Drying kinetics and microstructural changes. Drying Technol. 2019;37(1):1–12. doi: 10.1080/07373937.2018.1433199. [DOI] [Google Scholar]
- 37.Kroehnke Joanna, Szadzińska Justyna, Stasiak Marcin, Radziejewska-Kubzdela Elżbieta, Biegańska-Marecik Róża, Musielak Grzegorz. Ultrasound- and microwave-assisted convective drying of carrots – Process kinetics and product’s quality analysis. Ultrason. Sonochem. 2018;48:249–258. doi: 10.1016/j.ultsonch.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 38.Szadzińska J., Kowalski S.J., Stasiak M. Microwave and ultrasound enhancement of convective drying of strawberries: Experimental and modeling efficiency. Int. J. Heat Mass Transf. 2016;103:1065–1074. doi: 10.1016/j.ijheatmasstransfer.2016.08.001. [DOI] [Google Scholar]
- 39.K. Konopacka, D., Parosa, R., Piecko, J., Polubok, A., Siucinska, Ultrasound & microwave hybrid drying device for colored fruit preservation Product quality and energy efficiency, In Proceedings of the 8th Asia-Pacific Drying Conference (ADC 2015) (Kuala-Lumpur, Malaysia). (2015) 252–258. https://doi.org/10.1016/j.ultsonch.2018.05.040.
- 40.Albertos I., Martin-Diana A.B., Sanz M.A., Barat J.M., Diez A.M., Jaime I., Rico D. Effect of high pressure processing or freezing technologies as pretreatment in vacuum fried carrot snacks. Innovative Food Sci. Emerg. Technol. 2016;33:115–122. doi: 10.1016/j.ifset.2015.11.004. [DOI] [Google Scholar]
- 41.Su Ya, Zhang Min, Zhang Weiming, Adhikari Benu, Yang Zaixing. Application of novel microwave-assisted vacuum frying to reduce the oil uptake and improve the quality of potato chips. LWT - Food Sci. Technol. 2016;73:490–497. doi: 10.1016/j.lwt.2016.06.047. [DOI] [Google Scholar]
- 42.Su Ya, Zhang Min, Adhikari Benu, Mujumdar Arun S., Zhang Weiming. Improving the energy efficiency and the quality of fried products using a novel vacuum frying assisted by combined ultrasound and microwave technology. Innovative Food Sci. Emerg. Technol. 2018;50:148–159. doi: 10.1016/j.ifset.2018.10.011. [DOI] [Google Scholar]
- 43.Zhang Xiaotian, Zhang Min, Adhikari Benu. Recent developments in frying technologies applied to fresh foods. Trends Food Sci. Technol. 2020;98:68–81. doi: 10.1016/j.tifs.2020.02.007. [DOI] [Google Scholar]
- 44.Devi Shoma, Zhang Min, Mujumdar Arun S. Influence of ultrasound and microwave-assisted vacuum frying on quality parameters of fried product and the stability of frying oil. Drying Technol. 2021;39(5):655–668. doi: 10.1080/07373937.2019.1702995. [DOI] [Google Scholar]
- 45.Islam Mojaharul, Zhang Min, Mujumdar Arun S. Low temperature vacuum frying of edamame assisted by ultrasound and microwave: Effects on the kinetics of oil and product storage properties. Drying Technol. 2021;39(5):608–619. doi: 10.1080/07373937.2019.1700272. [DOI] [Google Scholar]
- 46.Huang Meng-sha, Zhang Min, Bhandari Bhesh. Synergistic effects of ultrasound and microwave on the pumpkin slices qualities during ultrasound-assisted microwave vacuum frying. J. Food Process Eng. 2018;41(6):e12835. doi: 10.1111/jfpe.2018.41.issue-610.1111/jfpe.12835. [DOI] [Google Scholar]
- 47.Al Faruq Abdulla, Zhang Min, Adhikari Benu. A novel vacuum frying technology of apple slices combined with ultrasound and microwave. Ultrason. Sonochem. 2019;52:522–529. doi: 10.1016/j.ultsonch.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Devi Shoma, Zhang Min, Law Chung Lim. Effect of ultrasound and microwave assisted vacuum frying on mushroom (Agaricus bisporus) chips quality. Food Biosci. 2018;25:111–117. doi: 10.1016/j.fbio.2018.08.004. [DOI] [Google Scholar]
- 49.Islam Mojaharul, Zhang Min, Fan Dongcui. Ultrasonically enhanced low-temperature microwave-assisted vacuum frying of edamame: Effects on dehydration kinetics and improved quality attributes. Drying Technol. 2019;37(16):2087–2104. doi: 10.1080/07373937.2018.1558234. [DOI] [Google Scholar]
- 50.Wen Le, Zhang Zhihang, Sun Da-Wen, Sivagnanam Saravana Periaswamy, Tiwari Brijesh K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020;60(11):1826–1841. doi: 10.1080/10408398.2019.1602823. [DOI] [PubMed] [Google Scholar]
- 51.Chemat F., Rombaut N., Sicaire A.G., Meullemiestre A., Fabiano-Tixier A.S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 52.Khadhraoui B., Turk M., Fabiano-Tixier A.S., Petitcolas E., Robinet P., Imbert R., Maâtaoui M.E., Chemat F. Histo-cytochemistry and scanning electron microscopy for studying spatial and temporal extraction of metabolites induced by ultrasound. Towards chain detexturation mechanism. Ultrason. Sonochem. 2018;42:482–492. doi: 10.1016/j.ultsonch.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 53.Vinatoru M., Mason T.J., Calinescu I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials, TrAC - Trends Anal. Chem. 2017;97:159–178. doi: 10.1016/j.trac.2017.09.002. [DOI] [Google Scholar]
- 54.Lefsih Khalef, Giacomazza Daniela, Dahmoune Farid, Mangione Maria Rosalia, Bulone Donatella, San Biagio Pier Luigi, Passantino Rosa, Costa Maria Assunta, Guarrasi Valeria, Madani Khodir. Pectin from Opuntia ficus indica: Optimization of microwave-assisted extraction and preliminary characterization. Food Chem. 2017;221:91–99. doi: 10.1016/j.foodchem.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 55.Moorthy I.G., Maran J.P., Ilakya S., Anitha S.L., Sabarima S.P., Priya B. Ultrasonics Sonochemistry Ultrasound assisted extraction of pectin from waste Artocarpus heterophyllus fruit peel. Ultrason. - Sonochem. 2017;34:525–530. doi: 10.1016/j.ultsonch.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 56.Xu S.Y., Liu J.P., Huang X., Du L.P., Shi F.L., Dong R., Huang X.T., Zheng K., Liu Y., Cheong K.L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT - Food Sci. Technol. 2018;90:577–582. doi: 10.1016/j.lwt.2018.01.007. [DOI] [Google Scholar]
- 57.Sun H., Li C., Ni Y., Yao L., Jiang H., Ren X., Fu Y., Zhao C. Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohydr. Polym. 2019;206:557–564. doi: 10.1016/j.carbpol.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 58.Marić M., Grassino A.N., Zhu Z., Barba F.J., Brnčić M., Rimac Brnčić S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018;76:28–37. doi: 10.1016/j.tifs.2018.03.022. [DOI] [Google Scholar]
- 59.Chemat F., Rombaut N., Meullemiestre A., Turk M., Perino S., Fabiano-Tixier A.-S., Abert-Vian M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017;41:357–377. doi: 10.1016/j.ifset.2017.04.016. [DOI] [Google Scholar]
- 60.Wu D., Gao T., Yang H., Du Y., Li C., Wei L., Zhou T., Lu J., Bi H. Simultaneous microwave/ultrasonic-assisted enzymatic extraction of antioxidant ingredients from Nitraria tangutorun Bobr. juice by-products. Ind. Crops Prod. 2015;66:229–238. doi: 10.1016/j.indcrop.2014.12.054. [DOI] [Google Scholar]
- 61.Barrera Vázquez M.F., Comini L.R., Martini R.E., Núñez Montoya S.C., Bottini S., Cabrera J.L. Comparisons between conventional, ultrasound-assisted and microwave-assisted methods for extraction of anthraquinones from Heterophyllaea pustulata Hook f. (Rubiaceae) Ultrason. Sonochem. 2014;21(2):478–484. doi: 10.1016/j.ultsonch.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 62.Bagherian Homa, Zokaee Ashtiani Farzin, Fouladitajar Amir, Mohtashamy Mahdy. Chemical Engineering and Processing : Process Intensification Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process Intensif. 2011;50(11-12):1237–1243. doi: 10.1016/j.cep.2011.08.002. [DOI] [Google Scholar]
- 63.Liew S.Q., Ngoh G.C., Yusoff R., Teoh W.H. Sequential ultrasound-microwave assisted acid extraction (UMAE) of pectin from pomelo peels. Int. J. Biol. Macromol. 2016;93:426–435. doi: 10.1016/j.ijbiomac.2016.08.065. [DOI] [PubMed] [Google Scholar]
- 64.Jha Pankaj, Das Arup Jyoti, Deka Sankar Chandra. Optimization of ultrasound and microwave assisted extractions of polyphenols from black rice (Oryza sativa cv. Poireton) husk. J. Food Sci. Technol. 2017;54(12):3847–3858. doi: 10.1007/s13197-017-2832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia-Vaquero M., Ummat V., Tiwari B., Rajauria G. Exploring ultrasound, microwave and ultrasound-microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar. Drugs. 2020;18:1–15. doi: 10.3390/md18030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lou Z., Wang H., Zhu S., Chen S., Zhang M., Wang Z. Ionic liquids based simultaneous ultrasonic and microwave assisted extraction of phenolic compounds from burdock leaves. Anal. Chim. Acta. 2012;716:28–33. doi: 10.1016/j.aca.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Trujillo-Mayol I., Céspedes-Acuña C., Silva F.L., Alarcón-Enos J. Improvement of the polyphenol extraction from avocado peel by assisted ultrasound and microwaves. J. Food Process Eng. 2019;42:1–11. doi: 10.1111/jfpe.13197. [DOI] [Google Scholar]
- 68.Qv Xiao-Ying, Zhou Qin-Fan, Jiang Jian-Guo. Ultrasound-enhanced and microwave-assisted extraction of lipid from Dunaliella tertiolecta and fatty acid profile analysis. J. Sep. Sci. 2014;37(20):2991–2999. doi: 10.1002/jssc.v37.2010.1002/jssc.201400458. [DOI] [PubMed] [Google Scholar]
- 69.Lu X., Zheng Z., Li H., Cao R., Zheng Y., Yu H., Xiao J., Miao S., Zheng B. Optimization of ultrasonic-microwave assisted extraction of oligosaccharides from lotus (Nelumbo nucifera Gaertn.) seeds. Ind. Crops Prod. 2017;107:546–557. doi: 10.1016/j.indcrop.2017.05.060. [DOI] [Google Scholar]
- 70.Zhou Cuixia, Sun Dengyue, Sun Xin, Zhu Chuanhe, Wang Qun. Combining Ultrasound and Microwave to Improve the Yield and Quality of Single-Cell Oil from Mortierella isabellina NTG1−121, JAOCS. J. Am. Oil Chem. Soc. 2018;95(12):1535–1547. doi: 10.1002/aocs.2018.95.issue-1210.1002/aocs.12134. [DOI] [Google Scholar]
- 71.Chen Yiyong, Gu Xiaohong, Huang Sheng-quan, Li Jinwei, Wang Xin, Tang Jian. Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti-tumor activities. Int. J. Biol. Macromol. 2010;46(4):429–435. doi: 10.1016/j.ijbiomac.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Alonso-Carrillo Nancy, Aguilar-Santamaría Ma. de los Ángeles, Vernon-Carter E. Jaime, Jiménez-Alvarado Rubén, Cruz-Sosa Francisco, Román-Guerrero Angélica. Extraction of phenolic compounds from Satureja macrostema using microwave-ultrasound assisted and reflux methods and evaluation of their antioxidant activity and cytotoxicity. Ind. Crops Prod. 2017;103:213–221. doi: 10.1016/j.indcrop.2017.04.002. [DOI] [Google Scholar]
- 73.Uluko H., Zhang S., Liu L., Tsakama M., Lu J., Lv J. Effects of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J. Funct. Foods. 2015;18:1138–1146. doi: 10.1016/j.jff.2014.11.024. [DOI] [Google Scholar]
- 74.Li Zhiyu, Wang Jianyi, Zheng Baodong, Guo Zebin. Impact of combined ultrasound-microwave treatment on structural and functional properties of golden threadfin bream (Nemipterus virgatus) myofibrillar proteins and hydrolysates. Ultrason. Sonochem. 2020;65:105063. doi: 10.1016/j.ultsonch.2020.105063. [DOI] [PubMed] [Google Scholar]
- 75.Habinshuti Ildephonse, Mu Tai-Hua, Zhang Miao. Ultrasound microwave-assisted enzymatic production and characterisation of antioxidant peptides from sweet potato protein. Ultrason. Sonochem. 2020;69:105262. doi: 10.1016/j.ultsonch.2020.105262. [DOI] [PubMed] [Google Scholar]
- 76.Noman Anwar, Qixing Jiang, Xu Yanshun, Abed Sherif M., Obadi Mohammed, Ali Abdelmoneim H., AL‐Bukhaiti Wedad Q., Xia Wenshui. Effects of ultrasonic, microwave, and combined ultrasonic-microwave pretreatments on the enzymatic hydrolysis process and protein hydrolysate properties obtained from Chinese sturgeon (Acipenser sinensis) J. Food Biochem. 2020;44(8) doi: 10.1111/jfbc.v44.810.1111/jfbc.13292. [DOI] [PubMed] [Google Scholar]
- 77.Rubi R.V.C., Olay J.G., Calugay P.E., Diaz M.V., Dimayuga K.F.L., Gagui F.M.G., Tare K.D. Ultrasound-microwave assisted extraction (Umae) of andrographolide from sinta (andrographis paniculata) with its bioactivity assessment. J. Environ. Sci. Manage. 2020;23:1–7. [Google Scholar]
- 78.Gharibzahedi Seyed Mohammad Taghi, Smith Brennan, Guo Ya. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019;222:114992. doi: 10.1016/j.carbpol.2019.114992. [DOI] [PubMed] [Google Scholar]
- 79.Görgüç Ahmet, Gençdağ Esra, Yılmaz Fatih Mehmet. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments – A review. Food Res. Int. 2020;136:109504. doi: 10.1016/j.foodres.2020.109504. [DOI] [PubMed] [Google Scholar]
- 80.Faustino M., Veiga M., Sousa P., Costa E.M., Silva S., Pintado M. Agro-food byproducts as a new source of natural food additives. Molecules. 2019;24:1–23. doi: 10.3390/molecules24061056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.L.Y. Chew, G.T. Toh, A. Ismail, Application of proteases for the production of bioactive peptides, Elsevier Inc., 2018. https://doi.org/10.1016/B978-0-12-813280-7.00015-3.
- 82.Hu Weiwei, Chen Shiguo, Wu Dongmei, Zheng Jiaqi, Ye Xingqian. Ultrasonic-assisted citrus pectin modification in the bicarbonate-activated hydrogen peroxide system: Chemical and microstructural analysis. Ultrason. Sonochem. 2019;58:104576. doi: 10.1016/j.ultsonch.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 83.Ozuna C., Paniagua-Martínez I., Castaño-Tostado E., Ozimek L., Amaya-Llano S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015;77:685–696. doi: 10.1016/j.foodres.2015.10.015. [DOI] [Google Scholar]
- 84.Huang L., Liu B., Ma H., Zhang X. Combined effect of ultrasound and enzymatic treatments on production of ACE inhibitory peptides from wheat germ protein. J. Food Process. Preserv. 2014;38(4):1632–1640. doi: 10.1111/jfpp.12125. [DOI] [Google Scholar]
- 85.Xu Jing, Zhao Qingshan, Qu Yanyan, Ye Fei. Antioxidant activity and anti-exercise-fatigue effect of highly denatured soybean meal hydrolysate prepared using neutrase. J. Food Sci. Technol. 2015;52(4):1982–1992. doi: 10.1007/s13197-013-1220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Xue, Wang Lingling, Zhang Fengying, Ma Haile. Effects of multi-mode S-type ultrasound pretreatment on the preparation of ACE inhibitory peptide from rice protein. Food Chem. 2020;331:127216. doi: 10.1016/j.foodchem.2020.127216. [DOI] [PubMed] [Google Scholar]
- 87.Li M., Xia S., Zhang Y., Li X. Optimization of ACE inhibitory peptides from black soybean by microwave-assisted enzymatic method and study on its stability. Lwt. 2018;98:358–365. doi: 10.1016/j.lwt.2018.08.045. [DOI] [Google Scholar]
- 88.Ketnawa S., Wickramathilaka M., Liceaga A.M. Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: Purification and identification. Food Chem. 2018;254:36–46. doi: 10.1016/j.foodchem.2018.01.133. [DOI] [PubMed] [Google Scholar]
- 89.Rutherfurd S.M. Methodology for determining degree of hydrolysis of proteins in hydrolysates: A Review. J. AOAC Int. 2010;93:1515–1522. doi: 10.1093/jaoac/93.5.1515. [DOI] [PubMed] [Google Scholar]
- 90.Lagha A., Chemat S., Bartels P.V., Chemat F. Microwave - Ultrasound combined reactor suitable for atmospheric sample preparation procedure of biological and chemical products. Analusis. 1999;27(5):452–457. doi: 10.1051/analusis:1999124. [DOI] [Google Scholar]
- 91.Peng Y., Song G. Simultaneous microwave and ultrasound irradiation: A rapid synthesis of hydrazides. Green Chem. 2001;3:302–304. doi: 10.1039/b108878p. [DOI] [Google Scholar]
- 92.Leonelli Cristina, Mason Timothy J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process Intensif. 2010;49(9):885–900. doi: 10.1016/j.cep.2010.05.006. [DOI] [Google Scholar]
- 93.Cravotto Giancarlo, Boffa Luisa, Mantegna Stefano, Perego Patrizia, Avogadro Milvio, Cintas Pedro. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008;15(5):898–902. doi: 10.1016/j.ultsonch.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 94.Cravotto Giancarlo, Beggiato Marina, Penoni Andrea, Palmisano Giovanni, Tollari Stefano, Lévêque Jean-Marc, Bonrath Werner. High-intensity ultrasound and microwave, alone or combined, promote Pd/C-catalyzed aryl-aryl couplings. Tetrahedron Lett. 2005;46(13):2267–2271. doi: 10.1016/j.tetlet.2005.02.015. [DOI] [Google Scholar]
- 95.Cintas P., Tagliapietra S., Caporaso M., Tabasso S., Cravotto G. Enabling technologies built on a sonochemical platform: Challenges and opportunities. Ultrason. Sonochem. 2015;25:8–16. doi: 10.1016/j.ultsonch.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Vinatoru I. M; Calinescu, Microwave and Ultrasounds together – A challenge. IEEE Trans. Software Eng. 2019;24:233–244. doi: 10.1145/1390630.1390641. [DOI] [Google Scholar]
- 97.Zhang Jian, Zhang Yuqin, Wang Yan, Xing Lujuan, Zhang Wangang. Influences of ultrasonic-assisted frying on the flavor characteristics of fried meatballs. Innovative Food Sci. Emerg. Technol. 2020;62:102365. doi: 10.1016/j.ifset.2020.102365. [DOI] [Google Scholar]
- 98.Ojha K. Shikha, Aznar Ramón, O'Donnell Colm, Tiwari Brijesh K. Ultrasound technology for the extraction of biologically active molecules from plant, animal and marine sources. TrAC - Trends Anal. Chem. 2020;122:115663. doi: 10.1016/j.trac.2019.115663. [DOI] [Google Scholar]