Abstract
Bladder cancer (BC) is one of the most prevalent cancers around the world and, if not treated well, has high morbidity and mortality. Many studies have indicated that there may be various roles for the aryl hydrocarbon receptor (AHR) in the immune system. The aim of this study was to determine the frequency of Foxp3+ regulatory T (Treg) and T helper 17 cells (Th17) in BC tissue in comparison with controls and determine the relationship between AHR, Foxp3+ Treg and Th17 cells in BC. A total of 40 patients with BC were enrolled in this study. The control group was selected from non‐tumoural parts of bladder tissues from the patients who have undergone cystoscopy. The percentage of regulatory T cells (Foxp3+/CD4+) and Th17 (IL‐17+/CD4+), as well as AHR+ cells in BC tissues and controls, were determined by immunohistochemistry. The results of this study showed that the number of Foxp3+ Treg and Th17 is significantly higher in bladder tumour tissues in comparison with non‐tumoural tissues. Also, the percentage of AHR+ lymphocytes and AHR+ cells was increased significantly in bladder tumour tissues rather than non‐tumoural tissues. This study also found a relation between AHR and Foxp3+/CD4+ T lymphocytes ratio cells in BC. The percentage of Foxp3+ Tregs and AHR+ cells were significantly correlated with the grade and stage of BC. An increase in the percentage of Foxp3+ Treg and Th17 cells may play an important role in tumour immunity; and determining the relationship between AHR and differentiation of Th17/Foxp3+Treg in BC can lead to a potential cancer therapeutic possibility.
Keywords: aryl hydrocarbon receptor, bladder cancer, Foxp3+ regulatory T, T helper 17
1. INTRODUCTION
Bladder cancer (BC) is a term used to describe the growth of malignant cells that has initiated in the urinary bladder. It is common, with around 38 000 men and 15 000 women diagnosed every year in the United States. 1 Approximately 430 000 new cases were diagnosed and about 165 000 deaths occurred worldwide in 2012. 2 BC is one of the most prevalent cancers around the world and, if not treated satisfactorily, has a high morbidity and mortality. BC has a direct relation with smoking, exposure to chemicals in the workplace, some medications and herbal supplements, exposure to arsenic in drinking water, insufficient fluid intake, race and ethnicity, genetics, age and gender in that men are three to four times more likely to develop this cancer than women. 2 The most common type of BC is urothelial carcinoma. 3 The immune system has a dual function in carcinogenesis, consisting of both destruction of cancer cells and facilitation of growth and spread through the mechanism of immune escape. 4 Studies have further shown that a variety of T‐cell populations infiltrate in tumour tissues. 5 , 6 CD4+ cells or T helper (Th) cells can mediate diversified roles in tumour‐immune system interactions and can orchestrate several important functions in tumorigenesis. 7 Regulatory T cells (Tregs), formerly known as suppressor T cells, comprise a subpopulation of T cells which modulate the immune system. Tregs are usually characterized by expression of CD4, high levels of CD25, and the transcription factor forkhead box P3 (FOXP3). It is well established that Foxp3+ Tregs play crucial roles in maintaining self‐tolerance and immune suppression due to several functions such as cell‐cell contact or production of interleukin 10 (IL‐10) and transforming growth factor‐β (TGF‐β). 8 , 9 Th17 cells have been identified as a distinct Th cell subpopulation and a novel Th lineage‐mediating immune response in both animal models and humans. 10 The aryl hydrocarbon receptor (AHR) is a transcription factor that regulates gene expression. AHR activity is increased in tumours compared with surrounding non‐malignant and stromal cells, suggesting that AHR might influence tumour development. The published articles indicate that the development of Foxp3+Tregs and Th17 cells is closely related and AHR can have a potential role in their proliferation. 11 , 12 Since the role of AHR in BC and its association with the prevalence of Th17 and Foxp3+Tregs in this cancer has not yet been investigated and also because of the contradictions about the role of Th17 lymphocytes in tumour immunity, in this study we aimed to elucidate the relation of AHR with Foxp3+Tregs and Th17 cells’ infiltration in patients with BC.
2. MATERIAL AND METHODS
2.1. Patient sampling
The appropriate Institutional Ethics Committees of the Shahrekord University of Medical Sciences approved the human studies. Written informed consent was obtained from all study participants. Clinical information such as age, gender, grade and stage was collected from medical files. The initial diagnosis of the probability of bladder cancer was checked via studying radiology images by a urologist, and then after obtaining the biopsy samples (containing tumoural and non‐tumoural tissues) by cystoscopy, they were transferred to the pathology laboratory and examined by a pathologist. Non‐tumoural tissues were distinguished from tumoural tissues based on their morphology. Also, in the tumour tissue the levels of malignancy such as low‐grade and high‐grade were determined and reported by the pathologist. The histological grade and stage of BC were classified based on the WHO grading system and TNM classification system. 13 The eligibility of patients for this study was based on the availability of tumour tissues in the sections from routine diagnostic cystoscopy and the quality of formalin‐fixed paraffin‐embedded tissue blocks.
2.2. Histopathological examination and immunohistochemistry
Sections of transurethral resection (TUR) of the bladder were first fixed in 10% buffered formalin and then embedded in paraffin. The paraffin blocks were cut with microtome into 4‐μm thick sections and placed on poly‐L‐lysine‐coated slides. Paraffin sections were dried and heated in a 68°C oven overnight, and then, the sections were deparaffinized by using three changes of xylene and were hydrated using a series of alcohols (100%, 100%, 80% and 70%). For antigen retrieval, the sections were flooded with citrate buffer (10 mmol/L Sodium Citrate, 0.05% Tween 20, pH 6.0) and exposed to pressure and heat by autoclaving for 20 minutes. Then in order to block nonspecific background staining, the sections were incubated with protein block (ab94665, Abcam, UK) for 2 hours. After that Rabbit anti‐human CD4 antibody (ab133616, Abcam, UK) at a 1:400 dilution, Rabbit anti‐human FOXP3 antibody (ab99963, Abcam, UK) at a 1:400 dilution, Rabbit anti‐human IL‐17 antibody (ab79056, Abcam, UK) at a 1:600 dilution and Mouse anti‐AHR antibody (sc‐133088, Santa Cruz, UK) at a 1:200 dilution were applied. Afterwards, the sections were incubated overnight in a proper humidified chamber at 4°C. On the next day, 3% H2O2 in TBS for 15 minutes was used to block endogenous peroxidase activity. Later, biotinylated goat anti‐rabbit and mouse IgG (ab93697, Abcam, UK) was applied and the sections were incubated for 1 hour at room temperature. Then, the sections were incubated for 10 minutes at room temperature with Streptavidin Peroxidase HRP Plus. Then, the sections were incubated with a chromogen called 3‐diaminobenzidine tetrahydrochloride (DAB) (ab94665, Abcam, UK) for 10 minutes. Then, sections were placed for 1 minute with Meyer's hematoxylin and washed with cool water and then mounted. Human kidney tissue was used as a positive control for AHR, and human Hodgkin's lymphoma tissue was used as a positive control for CD4, Foxp3 and IL17. Also, sections without primary antibody were used as a negative control. The percentage of CD4+ lymphocytes, IL‐17+ lymphocytes, Foxp3+ lymphocytes, AHR+ lymphocytes and AHR+ cells were calculated by counting positive cells in the entire area of tissue section at 10 high‐quality fields with the maximum number of positive cells, and the mean percentage of positive cells in 10 fields were reported as the final results. Lymphocytes were identified from other cells based on their morphology by pathologist and histologist. For CD4, IL‐17 and FOXP3, only positive lymphocytes were counted. For AHR, the percentage of AHR+ lymphocytes and AHR+ cells (including lymphocytes and other positive cells) were reported.
2.3. Statistical analysis
All data were evaluated by SPSS19.0 (SPSS Inc, Chicago, IL, USA) and GraphPad Prism software version 8 (GraphPad Software, La Jolla, CA, USA). The t test was used to determine the comparisons between cancer and control groups. Spearman test, one‐way ANOVA and unpaired t test were used for correlation analysis to assess the relationship between variables. P‐value less than 0.05 was assumed statistically significant.
3. RESULTS
3.1. Study population
A total of 40 patients with BC (30 males, 10 females), diagnosed by biopsy obtained during cystoscopy at Kashani Hospital in Shahrekord, were enrolled in the present study. None of the patients in this research received radiotherapy, chemotherapy or other medical interventions before the surgery, and also, patients with concurrent malignancies, autoimmune diseases or immune deficiencies were excluded from this study. The control group was selected from the non‐tumoural parts of bladder tissues from the patients who were undergone cystoscopy for BC screening. The non‐tumoural tissues were distinguished from tumoural tissues based on the tissue's morphology. The mean age was 65.63 ± 14.42 Table 1. Data on age are expressed mean ± SD, and other data on Table 1 are reported as percentages.
TABLE 1.
Demographic characterization of patients
| Characteristics | Value, N (%) |
|---|---|
| Age (y) | 65.63 ± 14.42 |
| Gender | |
| Male | 30 (75) |
| Female | 10 (25) |
| Histological grade | |
| Low | 25 (62.5) |
| High | 15 (37.5) |
| Stage | |
| pTa | 30 (75.00) |
| pT1 | 8 (20.00) |
| pT2 | 2 (5.00) |
3.2. Increased percentage of T CD4+ lymphocytes, IL‐17+ lymphocytes, Foxp3+ lymphocytes, ratio of Th17/CD4+ and ratio of Foxp3+Treg/CD4+ in tumour tissues in comparison with non‐tumoural tissue
Immunohistochemical staining of CD4 and IL‐17 and Foxp3 in tumour samples and controls is also displayed in Figure 1. Based on the results of this study, there is a significant increase in the percentage of T CD4+ lymphocytes (Figure 2A, P < 0.0001), IL‐17+ lymphocytes (Figure 2B, P < 0.0001) and Foxp3+ lymphocytes (Figure 2C, P < 0.0001) in tumour tissues than non‐tumoural tissue. Also, the ratio of IL‐17+/CD4+ lymphocytes (Figure 2D, P < 0.0001) and Foxp3+/CD4+ lymphocytes (Figure 2E, P < 0.0001) in tumour tissues was significantly higher in comparison with non‐tumoural tissues, which confirms a noticeable increase in Th17 and Foxp3+ Treg populations in tumour microenvironment.
FIGURE 1.

Immunohistochemical staining of (A and B) CD4+ T lymphocytes in bladder tumour tissue and non‐tumoural tissue. (C and D) IL‐17+ lymphocytes in bladder tumour tissue and non‐tumoural tissue. (E and F) Foxp3+ lymphocytes in bladder tumour tissue and non‐tumoural tissue. G and H) AHR+ cells in bladder tumour tissue and non‐tumoural tissue (A magnification of 40×). The arrows show the target lymphocytes in the bladder tissue
FIGURE 2.
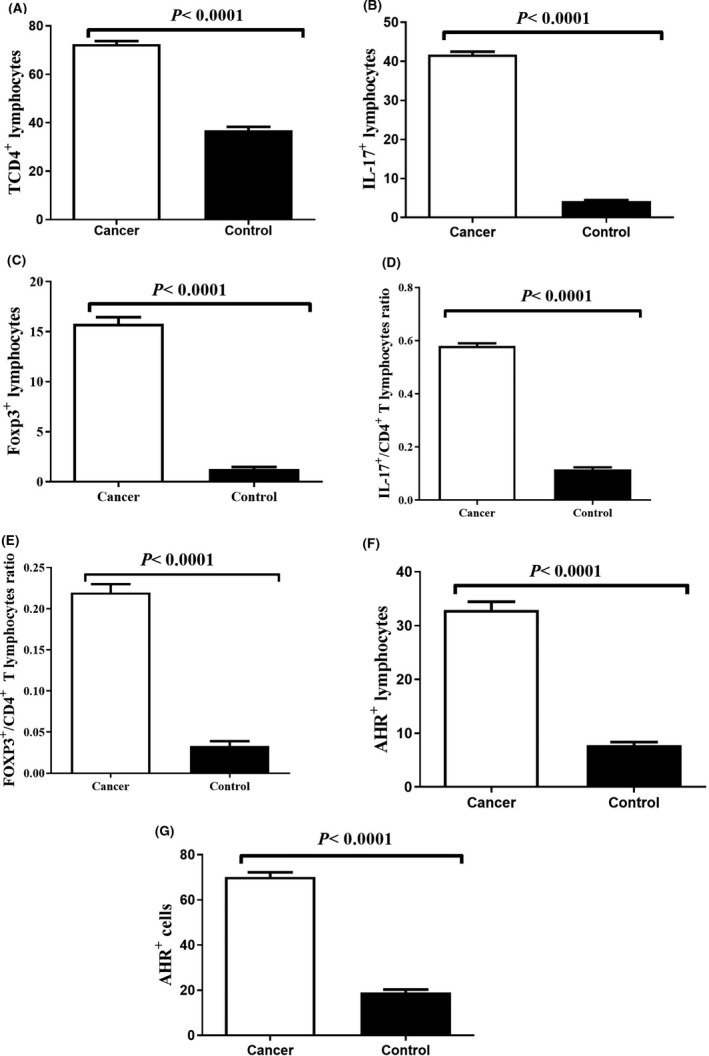
Frequency of (A) T CD4+ lymphocytes, (B) IL‐17+ lymphocytes, (C) Foxp3+ lymphocytes, (D) ratio of IL‐17+/CD4+ T lymphocytes, (E) ratio of Foxp3+/CD4+ T lymphocytes, (F) AHR+ lymphocytes, and (G) AHR+ cells in tumour tissues in comparison with non‐tumoural tissue. P‐value < 0.05 was considered statistically significant using t test
3.3. Immunohistochemical staining of AHR+ lymphocyte and AHR+ cells showed a considerable increase in tumour tissues in comparison with non‐tumoural tissue
The percentage of AHR+ lymphocytes and AHR+ cells (lymphocytes and other cells) in tumour tissues and non‐tumoural tissues were also calculated in this study by immunostaining Figure 1G,H. We observed a significant increase in the percentage of AHR+ lymphocytes and AHR+ cells in the tumoural tissues in comparison with non‐tumoural tissues (Figure 2F,G, P < 0.0001).
3.4. Correlation between the percentage of AHR+ lymphocyte and AHR+ cells with the percentage of IL‐17+ lymphocytes, the percentage of Foxp3+ lymphocytes and the ratio of IL‐17+/CD4+ and Foxp3+/CD4+T lymphocytes
Our data indicated a statistically significant correlation between the percentage of AHR+ cells and the ratio of Foxp3+ Treg/CD4+ lymphocytes in BC (Figure 3D , r = 0.417, P = 0.007). The results showed no significant correlation between other variables.
FIGURE 3.
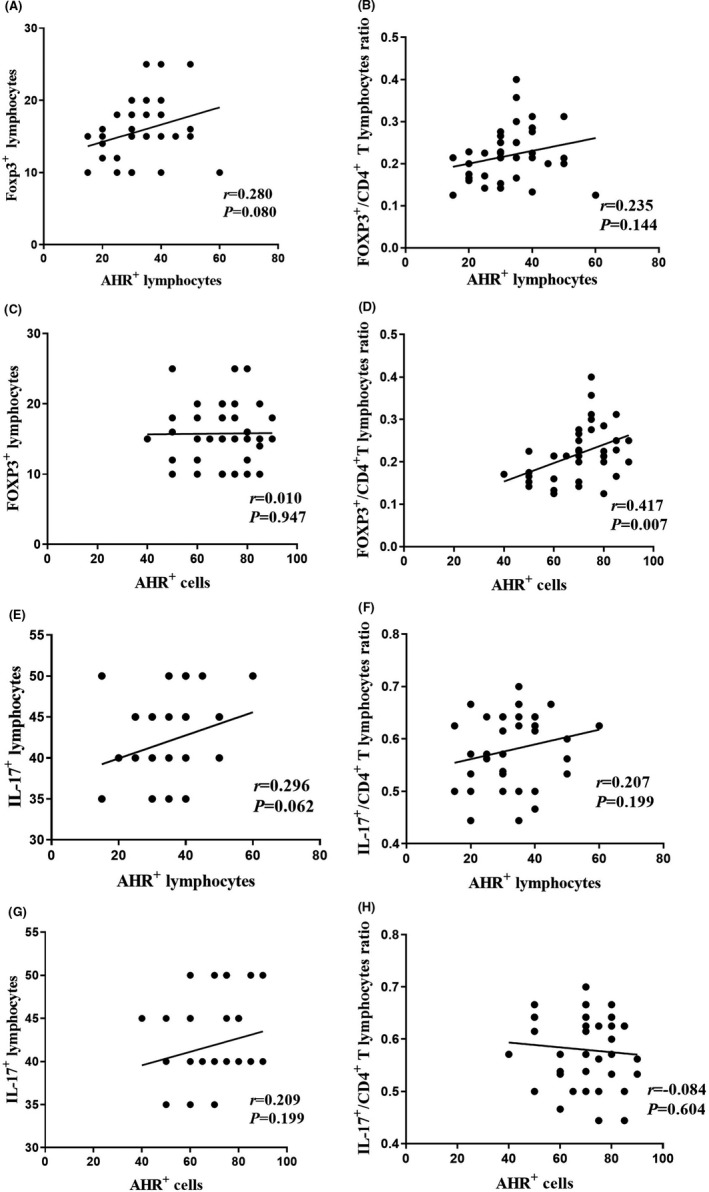
Correlation between AHR+ lymphocytes and AHR+ cells with Foxp3+ lymphocytes, ratio of Foxp3+/CD4+ T lymphocytes, IL‐17+ lymphocytes and ratio of IL‐17+/CD4+ T lymphocytes in tumour tissues (graphs A‐H). P value < 0.05 was considered statistically significant Spearman correlation test
3.5. Correlation between the grade of BC with the percentage of T CD4+ lymphocytes, IL‐17+ lymphocytes, the ratio of IL‐17+/CD4+ T lymphocytes, the percentage of Foxp3+ lymphocytes, the ratio of Foxp3+/CD4+ T lymphocytes and the percentage of AHR+ lymphocyte and AHR+ cells
Although the correlation between the grade of BC with the percentage of CD4+ T lymphocytes (Figure 4A, P = 0.6165), IL‐17+ lymphocytes (Figure 4B, P = 0.3504) and the ratio of IL‐17+/CD4+ T lymphocytes (Figure 4C, P = 0.6067) was not statistically significant, statistical tests also showed a significant correlation between the grade of BC and the percentage of Foxp3+ lymphocytes (Figure 4D, P < 0.0001), the ratio of Foxp3+/CD4+ T lymphocytes (Figure 4E, P = 0.0001), the percentage of AHR+ lymphocytes (Figure 4F, P = 0.0268) and the percentage of AHR+ cells (Figure 4G, P < 0.0001).
FIGURE 4.
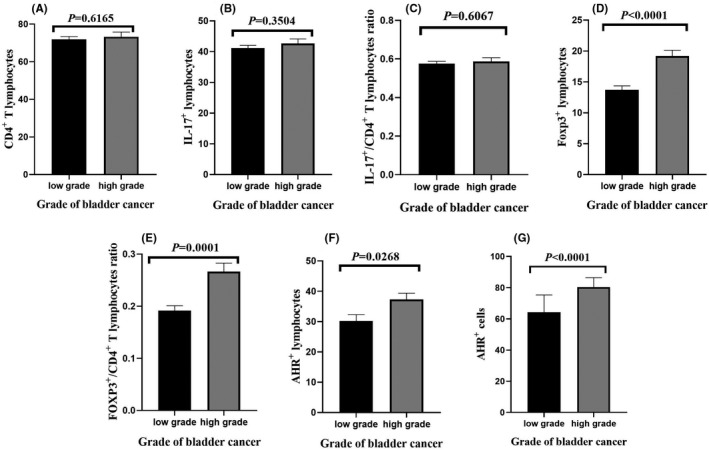
Correlation between grade of bladder cancer with (A) number of T CD4+ lymphocytes, (B) IL‐17+ lymphocytes, (C) IL‐17+/CD4+ T lymphocyte ratio, (D) Foxp3+ lymphocytes, (E) Foxp3+/CD4+ T lymphocytes ratio, (F) AHR+ lymphocyte and (G) AHR+ cells. P‐value < 0.05 was considered statistically significant using t test
3.6. Correlation between the stage of BC with the percentage of T CD4+ lymphocytes, the percentage of IL‐17+ lymphocytes, the ratio of IL‐17+/CD4+ T lymphocytes, the percentage of Foxp3+ lymphocytes, the ratio of Foxp3+/CD4+ T lymphocytes and the percentage of AHR+ lymphocyte and AHR+ cells
Based on the results of this study, there is a significant correlation between the stage of BC and the percentage of Foxp3+ lymphocytes (Figure 5D, P = 0.0035), the ratio of Foxp3+/CD4+ T lymphocytes (Figure 5E, P = 0.0026) and the percentage of AHR+ cells (Figure 5G, P = 0.0458). The correlation between the stage of BC and the percentage of CD4+ T lymphocytes (Figure 5A, P = 0.8686), the percentage of IL‐17+ lymphocytes (Figure 5B, P = 0.2148), the ratio of IL‐17+/CD4+ T lymphocytes (Figure 5C, P = 0.1558) and the percentage of AHR+ lymphocytes (Figure 5F, P = 0.2903) was not statistically significant.
FIGURE 5.
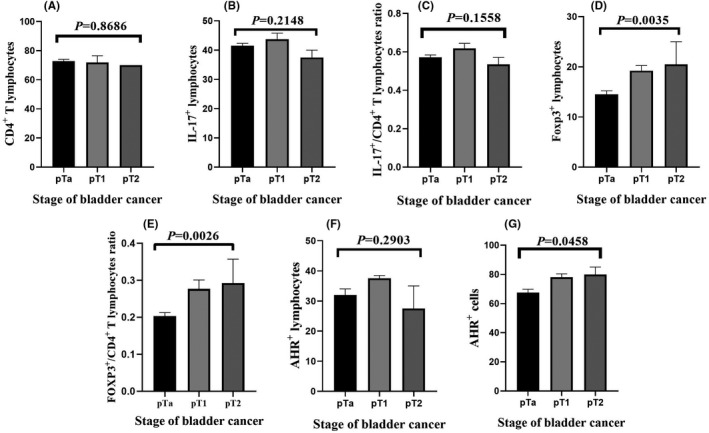
Correlation between stage of bladder cancer with (A) number of T CD4+ lymphocytes, (B) IL‐17+ lymphocytes, (C) IL‐17+/CD4+ T lymphocyte ratio, (D) Foxp3+ lymphocytes, (E) Foxp3+/CD4+ T lymphocytes ratio, (F) AHR+ lymphocyte and (G) AHR+ cells. P‐value < 0.05 was considered statistically significant using one‐way ANOVA test
3.7. Correlation between Th17 and Foxp3+ Treg cells
The results of statistical tests showed that the ratio of IL‐17+/CD4+ T lymphocytes and the ratio of Foxp3+/CD4+ T lymphocytes in the bladder tumour tissue of the patient group had a positive but non‐significant correlation (Figure 6, P = 0.285).
FIGURE 6.
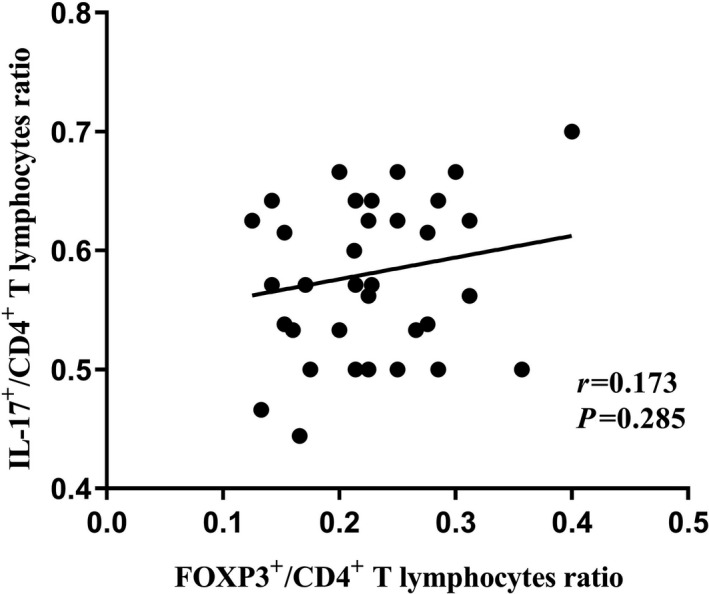
Correlation between the ratio of IL‐17+/CD4+ T lymphocytes and Foxp3+/CD4+ T lymphocytes in BC tumour tissue. P‐value < 0.05 was considered statistically significant using Spearman correlation test
4. DISCUSSION
There has been an explosion of scientific studies on the role of immune cells especially T cells in tumourigenesis. Several studies have suggested a meaningful relationship between the frequency of Tregs and tumour progression. Studies on gastric cancer, oesophageal cancer and renal cell carcinoma showed an obvious increase in the population of Tregs in peripheral blood and tumour‐infiltrating lymphocytes. 14 The contribution of Tregs in some tumours such as BC is not fully investigated. Our results show an obvious increase in the percentage of Foxp3+ lymphocytes and the ratio of CD4+/Foxp3+ T lymphocytes in tumour tissues compared with non‐tumoural tissues. Our data also showed a statistically significant correlation between the percentage of Foxp3+ lymphocytes and the ratio of Foxp3+/CD4+ T lymphocytes with BC grade and stage, which confirms the suppression role of Foxp3+ Tregs in anti‐tumour immune responses. Other investigations in BC indicated a significant increase in CD4+ Foxp3+ Tregs number in BC patients with lymph node invasion compared with samples with no lymph node invasion and a positive correlation between Treg cells with BC stage which suggests the inhibitory role of Tregs in anti‐tumour responses. 15 Another study that confirms our results was done on breast cancer which showed a significantly higher population of Foxp3+ Tregs in invasive breast carcinomas than in normal breast. This research indicates that Foxp3+ Tregs can be potential therapeutic targets for breast cancer treatment. 16 Our results also showed an increase in the percentage of Foxp3+ lymphocytes (including CD4+ Foxp3+ T lymphocytes and CD8+ Foxp3+ T lymphocytes). Recent studies have also identified CD8+ Foxp3+ Tregs with regulatory roles in cancers. Chen et al indicated that CD8+ Foxp3+ T cells significantly increased in non‐small‐cell lung cancer patients. 17
Treg infiltration can favour tumour progression due to suppression of anti‐tumour immune responses. Thus, suppression of immune regulatory factors such as Tregs could induce more effective immune responses against tumour cells. Efforts are being made to target tumour infiltrating Tregs as a new approach to cancer chemotherapy. Targeting Tregs markers such as CD25 or Foxp3 and blockage of immune checkpoints like CTLA‐4 (Cytotoxic T‐lymphocyte Associated Protein‐4) and PD‐1 (Programmed cell Death‐1) can result in depletion of tumour‐infiltrating Tregs and may lead to remarkable clinical effects. 18 Applying PD‐1 blockade in clinical trials for metastatic urothelial carcinoma showed significant response rate and minimum side effects. 19 Although a recent study by Sharma et al 20 on depleting Tregs via using anti‐CTLA‐4 in BC did not report clinically meaningful anti‐tumour responses, Maeda et al 21 reported that CCR4 blockade successfully leads to Treg suppression and increased survival of canine models with BC with minimum side effects. Blocking CCR8 (expressed on Tregs) can also effectively inhibit the progression of muscle‐invasive bladder cancer in human by suppressing tumour‐infiltrating Tregs. 22
Th17 cells can have controversial roles in cancer biology. 23 Our results show a considerable increase in the percentage of IL‐17+ lymphocytes and the ratio of IL‐17+/CD4+ T lymphocytes in tumour tissues in comparison with non‐tumoural tissues. Our data did not show a statistically significant correlation between the percentage of IL‐17+ lymphocytes and the ratio of IL‐17+/CD4+ T lymphocytes with the grade and stage of BC. It was probably because of heterogeneity of patient samples. Due to the limited availability of patient samples and the random selection of samples, the frequency of some the stages and grades was lower in the study population. Although the percentage of IL‐17+ lymphocytes and the ratio of IL‐17+/CD4+ T lymphocytes showed a minor increase in high grades rather than low grades which may suggest that Th17 cells can have pro‐tumour activities in BC. Our results are in agreement with the recent studies that confirmed Th17 cells can have pro‐tumour activities by several mechanisms such as promoting angiogenesis, inducing neutrophil influx, recruitment and suppressive functions of MDSCs and release of anti‐apoptotic factors. 23 Our findings are also in consistent with another report on BC that showed a significant increase in Th17 population in BC patients comparing controls. A higher Il‐17A, Il‐23 and Il‐6 levels were also found in BC patients. 24 An earlier research by Chi et al 25 also indicated increased frequencies of Treg and Th17 cells in PBMC and tumour tissue of BC patients. No significant correlation was found between Th17 cells and grade or stage of BC. 25 Iida et al 26 also reported that CD4+ Th17 cells’ infiltration in the tumour area generate IL‐17, which leads to tumour progression via angiogenesis and neutrophil infiltration in gastric cancer. Also, deeper tumour invasion and more positive lymph node involvement were reported in patients with higher IL‐17 expression. 26 In support of our finding, Numasaki et al 27 showed that IL‐17 enhances the net angiogenic activity by targeting CXCR‐2 signalling, leading to progression of human non‐small‐cell lung cancer. In contrast to our findings, Kryczek et al 28 reported that Th17 cells may have protective roles in tumour immunity in ovarian cancer. It also states that IL‐17 can promote effector CD8+ T cells and NK cells’ migration to tumour microenvironment by inducing the expression of CXCL9 and CXCL10.
As AHR is well known for its potential roles in toxicology, it is now well established that this molecule also has important roles in cancer. AHR is overexpressed in some malignancies like the ovarian cancer. 29 AHR has emerging roles in development of Th17 and Foxp3+ Treg. 30 AHR is expressed in immune cells such as granulocytes, NK cells, macrophages, dendritic cells and lymphocytes. 31 So, we evaluated the percentage of not only AHR+ lymphocytes but also the total AHR+ cells. Our results showed a significant increase in the percentage of AHR+ lymphocytes and total AHR+ cells in BC tissues in comparison with non‐tumoural tissues. There was also a statistically significant correlation between the grade of BC and the percentage of AHR+ lymphocytes and AHR+ cells and also a significant correlation between the stage of BC and the percentage of AHR+ cells. This proves the certain role of AHR in BC. We established that there is a positive significant correlation between the percentage of AHR+ cells and the ratio of Foxp3+/CD4+T lymphocytes in BC. AHR activation by IDO1 via Kynurenine formation correlates with grade, stage and progression of BC. 32 A study by Yu et al 33 showed a statistically significant increase in AHR expression in human non‐muscle‐invasive BC in comparison with controls and muscle‐invasive BC using IHC and Western blot. It also indicates a higher rate of muscle‐invasive BC in AHR‐deficient mice than the wild type which confirms the various roles of AHR in tumour progression. In agreement with our results, Mezrich et al 34 demonstrated that AHR is correlated positively with the frequency of Foxp3+ Tregs. Gandhi et al also reported that the activation of AHR can induce human type 1 regulatory T cell‐like and Foxp3+ Tregs. 11 However, we did not find any significant correlation between the percentage of AHR+ lymphocytes and AHR+ cells with the percentage of Foxp3+ lymphocytes and IL‐17+ lymphocytes and the ratio of IL‐17+/CD4+ T lymphocytes in BC and control samples which is probably because of low sample numbers and limited sample size. More studies are needed to elucidate the correlation between AHR and IL‐17+ lymphocytes in BC. Also, the number of markers that are used in the IHC method is limited. Therefore, we suggest that a combination of CD4, CD25 and CD127 cell surface markers can be used for identification of Tregs by the flow cytometric method.
In conclusion, increase in the percentage of Foxp3+ Treg and Th17 cells may play an important role in tumour progression by suppressing the anti‐tumour immune responses. Also, determining the relation between AHR and differentiation of Th17 and Foxp3+ Treg in BC can lead to potential cancer medical solutions. Since it has been shown that Foxp3+ Tregs improve tumour progression by suppressing the immune system, focusing on the molecules that influence their differentiation, including AHR, may lead to the introduction of new therapeutic strategies for cancer therapy.
CONFLICT OF INTEREST
This manuscript was approved by all authors. No competing interests declared.
ETHICAL APPROVAL
This study was approved by the ethical board of Shahrekord University of medical sciences with number: IR.Skums.REC.1397.38.
ACKNOWLEDGEMENTS
This study was financially supported by research deputy of Shahrekord University of Medial Sciences with grant number 2725. The authors of this paper are thankful to the staffs of Students Research Committee, Shahrekord University of Medical Sciences, Shahrekord, Iran and the staffs of Cellular & Molecular Research Center, Shahrekord University of Medical Sciences. The authors are also grateful to the authorities of the pathology unit of Shahrekord Kashani Hospital for their valuable cooperation.
Fattahi S, Karimi M, Ghatreh‐Samani M, et al. Correlation between aryl hydrocarbon receptor and IL‐17+ and Foxp3+ T‐cell infiltration in bladder cancer. Int J Exp Path. 2021;102:249–259. 10.1111/iep.12392
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7‐30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2. Cumberbatch MGK, Jubber I, Black PC, et al. Epidemiology of bladder cancer: A systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74(6):784‐795. 10.1016/j.eururo.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 3. Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6):4‐34. [DOI] [PubMed] [Google Scholar]
- 4. Arum CJ, Anderssen E, Viset T, et al. Cancer immunoediting from immunosurveillance to tumor escape in microvillus‐formed niche: A study of syngeneic orthotopic rat bladder cancer model in comparison with human bladder cancer. Neoplasia. 2010;12(6):434‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125(23):5591–5596. [DOI] [PubMed] [Google Scholar]
- 6. Gajewski TF, Schreiber H, Fu Y‐X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189(5):753‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hara M, Kingsley CI, Niimi M, et al. IL‐10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166(6):3789‐3796. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura K, Kitani A, Strober W. Cell contact–dependent immunosuppression by CD4+ CD25+ regulatory T cells is mediated by cell surface–bound transforming growth factor β. J Exp Med. 2001;194(5):629‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13(6):668‐677. 10.1016/j.autrev.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 11. Gandhi R, Kumar D, Burns EJ, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell–like and Foxp3+ regulatory T cells. Nat Immunol. 2010;11(9):846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links T H 17‐cell‐mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. [DOI] [PubMed] [Google Scholar]
- 13. Colombel M, Soloway M, Akaza H, et al. Epidemiology, staging, grading, and risk stratification of bladder cancer. Eur Urol Suppl. 2008;7(10):618‐626. [Google Scholar]
- 14. Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor‐infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9(12):4404‐4408. [PubMed] [Google Scholar]
- 15. Ariafar A, Vahidi Y, Fakhimi M, Asadollahpour A, Erfani N, Faghih Z. Prognostic significance of CD4‐positive regulatory T cells in tumor draining lymph nodes from patients with bladder cancer. Heliyon. 2020;6(12):e05556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high‐risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373‐5380. [DOI] [PubMed] [Google Scholar]
- 17. Chen C, Chen D, Zhang Y, et al. Changes of CD4+ CD25+ FOXP3+ and CD8+ CD28− regulatory T cells in non‐small cell lung cancer patients undergoing surgery. Int Immunopharmacol. 2014;18(2):255‐261. [DOI] [PubMed] [Google Scholar]
- 18. Finotello F, Trajanoski Z. New strategies for cancer immunotherapy: Targeting regulatory T cells. Genome Med. 2017;9(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou TC, Sankin AI, Porcelli SA, Perlin DS, Schoenberg MP, Zang X. A review of the PD‐1/PD‐L1 checkpoint in bladder cancer: From mediator of immune escape to target for treatment. Urol Oncol. 2017;35(1):14‐20. [DOI] [PubMed] [Google Scholar]
- 20. Sharma A, Subudhi SK, Blando J, et al. Anti‐CTLA‐4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers. Clin Cancer Res. 2019;25(4):1233‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maeda S, Murakami K, Inoue A, Yonezawa T, Matsuki N. CCR4 blockade depletes regulatory T cells and prolongs survival in a canine model of bladder cancer. Cancer Immunol Res. 2019;7(9):1175‐1187. [DOI] [PubMed] [Google Scholar]
- 22. Wang T, Zhou Q, Zeng H, et al. CCR8 blockade primes anti‐tumor immunity through intratumoral regulatory T cells destabilization in muscle‐invasive bladder cancer. Cancer Immunol Immunother. 2020;69(9):1855–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hemdan NY. Anti‐cancer versus cancer‐promoting effects of the interleukin‐17‐producing T helper cells. Immunol Lett. 2013;149(1–2):123‐133. [DOI] [PubMed] [Google Scholar]
- 24. Chugh S, Anand V, Swaroop L, Sharma M, Seth A, Sharma A. Involvement of Th17 cells in patients of urothelial carcinoma of bladder. Hum Immunol. 2013;74(10):1258‐1262. [DOI] [PubMed] [Google Scholar]
- 25. Chi L, Lu H, Li G, et al. Involvement of T helper type 17 and regulatory T cell activity in tumour immunology of bladder carcinoma. Clin Exp Immunol. 2010;161(3):480‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iida T, Iwahashi M, Katsuda M, et al. Tumor‐infiltrating CD4+ Th17 cells produce IL‐17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncol Rep. 2011;25(5):1271‐1277. [DOI] [PubMed] [Google Scholar]
- 27. Numasaki M, Watanabe M, Suzuki T, et al. IL‐17 enhances the net angiogenic activity and in vivo growth of human non‐small cell lung cancer in SCID mice through promoting CXCR‐2‐dependent angiogenesis. J Immunol. 2005;175(9):6177‐6189. [DOI] [PubMed] [Google Scholar]
- 28. Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eltom SE, Gasmelseed AA, Saoudi‐Guentri D. The aryl hydrocarbon receptor is over‐expressed and constitutively activated in advanced breast carcinoma. Cancer Research. 2006;66(8):408‐408. [Google Scholar]
- 30. Zhou L. AHR function in lymphocytes: Emerging concepts. Trends Immunol. 2016;37(1):17‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ikuta T, Kurosumi M, Yatsuoka T, Nishimura Y. Tissue distribution of aryl hydrocarbon receptor in the intestine: Implication of putative roles in tumor suppression. Exp Cell Res. 2016;343(2):126‐134. [DOI] [PubMed] [Google Scholar]
- 32. Matheus LHG, Dalmazzo SV, Brito RBO, et al. 1‐Methyl‐D‐tryptophan activates aryl hydrocarbon receptor, a pathway associated with bladder cancer progression. BMC Cancer. 2020;20(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu J, Lu Y, Muto S, Ide H, Horie S. The dual function of aryl hydrocarbon receptor in bladder carcinogenesis. Anticancer Res. 2020;40(3):1345‐1357. [DOI] [PubMed] [Google Scholar]
- 34. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190‐3198. [DOI] [PMC free article] [PubMed] [Google Scholar]


