Abstract
We describe a clinical candidate molecule from a new series of glutamate N-methyl-d-aspartate receptor subunit 2B–selective inhibitors that shows enhanced inhibition at extracellular acidic pH values relative to physiologic pH. This property should render these compounds more effective inhibitors of N-methyl-d-aspartate receptors at synapses responding to a high frequency of action potentials, since glutamate-containing vesicles are acidic within their lumen. In addition, acidification of penumbral regions around ischemic tissue should also enhance selective drug action for improved neuroprotection. The aryl piperazine we describe here shows strong neuroprotective actions with minimal side effects in preclinical studies. The clinical candidate molecule NP10679 has high oral bioavailability with good brain penetration and is suitable for both intravenous and oral dosing for therapeutic use in humans.
SIGNIFICANCE STATEMENT
This study identifies a new series of glutamate N-methyl-d-aspartate (NMDA) receptor subunit 2B–selective negative allosteric modulators with properties appropriate for clinical advancement. The compounds are more potent at acidic pH, associated with ischemic tissue, and this property should increase the therapeutic safety of this class by improving efficacy in affected tissue while sparing NMDA receptor block in healthy brain.
Introduction
Cerebral ischemia, stroke, subarachnoid hemorrhage (SAH), and traumatic brain injury (TBI) all produce substantial neuronal death that, if not fatal, can create lasting disabilities with significant societal impact. Few therapeutic options are currently available for stroke apart from dissolution of the vessel clot in a subset of patients (Turner et al., 2020) or clot retrieval when blockages occur in large arteries (Awad et al., 2020). SAH can be treated with Ca2+ channel blockers (Carlson et al., 2020); however, there remains considerable opportunity for improved therapies, as a significant fraction of patients progress to subsequent ischemic episodes and death (Longstreth et al., 1993). No pharmacological strategy for neuroprotection in TBI has been approved yet (Crupi et al., 2020).
Extracellular glutamate concentrations increase in injured central nervous system tissue in both animal models and human patients with acute injury (Supplemental Table 1 in Yuan et al., 2015). One consequence of increasing extracellular glutamate is overactivation of NMDA receptors (NMDARs) which can be neurotoxic (Olney, 1969; Choi et al., 1988). It logically follows that inhibition of NMDARs during insults that raise glutamate should be neuroprotective, and the efficacy of several NMDAR antagonists has been confirmed in animal models of injury (Supplemental Table 2 in Yuan et al., 2015). However, promising preclinical results have not yet translated to clinical success, as multiple clinical trials in stroke or TBI using NMDAR antagonists either failed to improve patient outcome or were associated with unacceptable side effects (Supplemental Table 3 in Yuan et al., 2015). Evaluation of these clinical data and trial designs identified multiple potential reasons for these failures (Morris et al., 1999; Albers et al., 2001; Sacco et al., 2001; Gladstone et al., 2002; Farin and Marshall, 2004). Moreover, the heterogeneous nature of acute brain injury requires careful management of the inclusion criteria, and improved image-based analysis of collateral blood flow and hypoperfusion with comprehensive postinjury analysis could diminish variability (Kidwell et al., 2001; Narayan et al., 2002; Guenego et al., 2020). Treatment of patients within hours by first responders is now possible and may prove essential (Saver, 2013; Shkirkova et al., 2018). Thrombectomy and clot retrieval have also created new opportunities to administer neuroprotectants directly to the site of the injury despite arterial blockage (Patel et al., 2020).
Since the discovery of GluN2B antagonists, numerous scaffolds of highly selective GluN2B NMDA receptor antagonists have been reported (reviewed by Layton et al., 2006; Mony et al., 2009; Koller and Urwyler, 2010; Ruppa et al., 2012). These include phenethanolamines such as ifenprodil (Williams, 1993), eliprodil (Carter et al., 1988), radiprodil (Michel et al., 2014; Auvin et al., 2020), traxoprodil (Chenard et al., 1995), Ro 25-6981 (Fischer et al., 1997), MK-0657 (Addy et al., 2009), plus additional scaffolds including propanolamines (Yuan et al., 2015), benzimidazoles (McCauley et al., 2004; Davies et al., 2012), cyclic benzamidines (Nguyen et al., 2007), amino cyclopentanes (Layton et al., 2011), piperidinyl pyrrolidinones (Marcin et al., 2018), and other compounds (McIntyre et al., 2009; Mosley et al., 2009; Brown et al., 2011; Buemi et al., 2014; Dey et al., 2018; Thum et al., 2018). Whereas crystallographic evaluation of ifenprodil at the GluN1/GluN2B heterodimer amino terminal domain interface (Karakas and Furukawa; 2014) is the likely pose for many GluN2B-selective inhibitors, recent structural data revealed a unique region within the binding cavity that can be occupied by compounds with a more compact scaffold (Kemp and Tasker, 2009; Stroebel et al., 2016). The propanolamine EU93-31 (Tahirovic et al., 2008), which exhibits pH dependence for inhibition of NMDA receptors (Yuan et al., 2015), occupies both portions of the binding pocket (Regan et al., 2019).
GluN2B-selective compounds have been tested in preclinical and clinical studies for use in cerebral stroke (Supplemental Table 3 in Yuan et al., 2015), TBI (Bullock et al., 1999; Merchant et al., 1999; Yurkewicz et al., 2005), Parkinson’s disease (Steece-Collier et al., 2000; Liverton et al., 2007; Nutt et al., 2008; Addy et al., 2009; Michel et al., 2014, 2015), depression (Preskorn et al., 2008; Li et al., 2010; Bristow et al., 2017), and pain (Taniguchi et al., 1997; Sang et al., 2003; Abe et al., 2005; Liverton et al., 2007; Mony et al., 2009; Labas et al., 2011; Swartjes et al., 2011). However, despite clear achievement of preclinical efficacy, no GluN2B-selective inhibitor has been approved for clinical use.
Here, we introduce a new scaffold for GluN2B-selective inhibitors and identify NP10679 with high pH sensitivity, efficacy in a murine model of transient ischemia, high oral bioavailability, brain penetration, and a preclinical profile that supports NP10679 as a clinical candidate.
Materials and Methods
Two-Electrode Voltage-Clamp Recordings from Xenopus laevis Oocytes
Stage V to VI Xenopus laevis unfertilized oocytes were purchased from Ecocyte (Austin, Texas) and injected with 5 ng of GluN1 and 10 ng of GluN2B ribonucleic acid made from cDNA. The cDNAs for human GluN1 and GluN2B, encoding National Center for Biotechnology Information reference sequences NM_007327.3 and NM_000834.3, respectively, were linearized, and ribonucleic acid made from cDNA was made as previously described (Traynelis et al., 1998). For activity measurements at GluN2A (NM_000833), GluN2C (NM_000835), and GluN2D (NM_000836) NMDA receptors cRNAs were prepared similarly. After injection, oocytes were incubated in Barth’s culture solution (88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM HEPES, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 10 U/ml Penicillin-Streptomycin, and 0.1 mg/ml gentamycin, at pH 7.4) at 18°C. Two-electrode voltage-clamp recordings were made at 22 to 23°C 2–7 days after injection using Warner OC725C amplifiers (VHOLD –40 mV). Briefly, oocytes were perfused in recording solution (90 mM NaCl, 1 mM KCl, 10 mM HEPES, 0.01 mM EDTA, and 0.5 mM BaCl2) adjusted to either pH 7.6 or 6.9 by addition of NaOH or HCl, respectively (pH 6.9 solutions were prepared by addition of HCl to pH 7.6 solutions to maintain an equal concentration of Na+ ions in both solutions). Drug concentration-response curves were obtained by application of increasing concentrations of the GluN2B-selective inhibitors in the presence of 100 μM glutamate and 30 μM glycine until steady-state conditions were obtained. Oocyte recordings were made from 4–10 oocytes per experiment (i.e., oocyte injection cycle) from ≥2 experiments. The concentration-response relationship for each oocyte was fit by eq. 1:
 |
where minimum is the residual response in saturating concentration of the experimental compounds (constrained to be ≥0), IC50 is the concentration of inhibitor that causes half-maximal inhibition, and nH is the Hill slope.
In Vivo Model of Transient Focal Ischemia
All protocols involving animals were approved by the Georgia State University Institutional Animal Care and Use Committee, an Association for Assessment and Accreditation of Laboratory Animal Care–accredited program, and was under the supervision of a licensed veterinarian. Mice were group-housed and provided nestlets and shelters with access to food pellets and water ad libitum under a 12-hour light/dark cycle. Mice were brought to a separate room and housed for at least 30 minutes prior to initiation of the surgery.
Mice (C57Bl6, >90 days old; Jackson Laboratories) were subjected to transient (60-minute) middle cerebral artery (MCA) occlusion (MCAo), and the infarct volume was measured 24 hours postreperfusion, similar as previously described (Yuan et al., 2015). Male mice were used for this experiment to reduce potential confound by progesterone variation through estrous cycles, which can have neuroprotective actions. Briefly, transient ischemia was induced in anesthetized (2% isoflurane/98% O2) mice by insertion of an intraluminal suture into the MCA for 60 minutes (Junge et al., 2003). The body temperature of each mouse was monitored with a rectal thermometer and maintained at 37°C through use of a homeothermic blanket. Changes in local cerebral blood flow were monitored with a laser Doppler flowmeter probe (Perimed) secured via glue to the skull 4–6 mm lateral and 2 mm posterior of bregma. An 11-mm 5-0 Dermalon or Look (SP185) black nylon nonabsorbable suture with the tip flame-rounded was introduced into the left internal carotid artery through the external carotid artery stump up to 10.5–11 mm of suture insertion. Only mice with a reduction in blood flow to <20% for 60 minutes and with recovery of blood flow to >90% after removal of the suture were included in the study. After the occlusion period, mice were placed back in their cages on a warming blanket (37°C) for several hours and monitored for righting reflex and ability to ambulate upon a gentle touch. At 24 hours postocclusion, mice were euthanized by isoflurane overdose, and the brain was quickly removed and cut into 2-mm sections and incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC) in phosphate-buffered saline (pH 7.4) at 37°C for 20 minutes and then placed at 4°C for imaging. The infarct area was then measured using National Institutes of Health IMAGE software (Scion Corporation, Beta 4.0.2 release). The lesioned area of each section was determined by digital threshold reductions in TTC staining to ≤20% lower intensity than that observed in the contralateral cortex. The infarct region was then manually outlined with a cursor, and the cubic volume of the infarct was determined for each slice and then summed across all four slices from each animal to obtain total infarct volume. A ratio of the contralateral to ipsilateral hemisphere volume was multiplied by the corresponding infarct section volume to correct for edema. Drug was administered by intraperitoneal injection 5 minutes prior to initiation of surgery (approximately 15 minutes prior to vessel occlusion). All drug doses were randomized, and investigator(s) were blinded throughout the study from the surgical procedure through analysis of stained sections to measure infarct volume. Based on historical variability and an anticipated effect size of 45%–50%, we estimated that n = 12 per group (four groups per study) was adequate to detect significant effects (α = 0.05) with sufficient power, (β = 0.90) (G*Power 3.1). The infarct volume after administration of a drug dose was compared with vehicle by one-way ANOVA and Dunnett’s tests (P < 0.05). A priori, mice were only removed from the study if the MCA was ruptured, resulting in a subarachnoid hemorrhage, or mice did not survive 24 hours postsurgery.
Measurement of Locomotor Activity and Rotarod Performance
Mice (C57Bl6, >90 days old; Jackson Laboratories) were placed in a closed (with light on) activity monitoring box for 1 hour to habituate prior to drug testing. After 1 hour, animals were removed and injected intraperitoneally with drug and then returned to the activity monitoring box, where total locomotor activity was monitored for 2 hours. The total number of light beam breaks in the cage (horizontal) was determined by computer, and results averaged for each drug. Results were analyzed by ANOVA and Dunnett’s post hoc test to compare horizontal activity of drug-treated groups to vehicle-treated controls. Male animals were used for these behavioral tests because only male mice were used in MCAo transient ischemia studies.
For Rotarod experiments, male C57BL/6 mice (>90 days old) were tested using a Rotamax 4/8 Rotarod (Columbus Instruments, Columbus, Ohio). Prior to training and testing, mice were brought to the testing room and allowed to acclimate for 2 hours prior to any further handling. Mice were placed on a rotating rod (5 rpm) that was 3.8 cm in diameter and 8 cm wide and elevated 30 cm from the floor of a chamber. After 10 seconds of rotation at a fixed velocity, the rotation was slowly accelerated from 5 to 35 rpm over a 5-minute period. The duration of time that the mouse could stay on the Rotarod, without hanging on for a full rotation or without falling, was recorded. Mice were trained four times at 25-minute intertrial intervals on each day for 2 days. On day 3, mice were randomly assigned to treatment groups and administered test drug or vehicle 25 minutes prior to testing (four trials with intertrial interval of 25 minutes) by intraperitoneal administration. Individuals performing the experiment were blinded to the identity of each treatment group. Results were analyzed by ANOVA and Dunnett’s post hoc test to compare duration of time on the rotating rod of drug-treated groups to vehicle-treated controls.
The locomotor and Rotarod studies were approved by the Georgia State University Institutional Animal Care and Use Committee, an Association for Assessment and Accreditation of Laboratory Animal Care–accredited institution, under the supervision of licensed veterinarians. Mice were group-housed and provided nestlets and shelters with access to food pellets and water ad libitum under a 12-hour light/dark cycle.
Pharmacokinetic Studies
Pharmacokinetic studies on NP10679 were outsourced to Anthem Biosciences (Bangalore, India) and were performed after obtaining the Institutional Animal Ethics Committee permission in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines. Evaluation of NP10679 properties was performed in male BALB/c mice (8–10 weeks old, 20–30 g). Briefly, mice were administered a 2 mg/kg or a 5 mg/kg dose (n = 3 each) by intraperitoneal injection (10 ml/kg dose volume), and blood samples were collected at 0.08, 0.25, 0.5, 1, 2, 4, 8, and 24 hours postdose in tubes containing sodium heparin on ice (study 4). In total, 100 μl of plasma was combined with 50 μl of internal standard (haloperidol, 10 μg/ml), and tubes were then spun at 4000g for 10 minutes (4°C), and plasma was transferred to clean tubes and stored at −80°C until analysis. The analyte NP10679 was quantified with an API 3200 Q-trap LC-MS/MS and compared with standards, and data were analyzed by WinNonlin 6.3 (Pharsight). In a separate study (study 3), BALB/c mice were administered either oral (10 mg/kg) or an intravenous (3 mg/kg) dose of NP10679 (injection volume of 10 ml/kg). Blood samples were collected on ice in sodium heparin tubes at 0.08, 0.25, 0.5, 1, 2, 4, 8, and 24 hours postdose. Samples were prepared and analyzed as described above, except here the internal standard was fluconazole (10 μg/ml). We also measured NP10679 in the brain compartment compared with plasma at 0.25 and 1 hour postdose with 3 mg/kg i.v. dosing in two separate studies with blood samples collected and prepared as described above. Here, brain samples were first washed in deionized water to remove blood, the weight was recorded, and then they were transferred into fresh water (1 ml), homogenized, and stored at −80°C until analysis. The ratio of compound in brain (grams) compared with plasma (milliliters) was then calculated. Study 1 used fluconazole as internal standard, whereas study 2A and 2B used haloperidol as the internal standard.
Formulation and Drug Dosing
For MCAo, locomotor, and Rotarod studies, NP10679, MK-801, and ifenprodil were formulated in 2% or 10% N′,N′-dimethylacetamide (DMA), 10% propylene glycol (PG), and 30% 2-hydroxypropyl-β-cyclodextrin in water (HPBCD), with a dose volume of 10 ml/kg, and administered intraperitoneally. Formulation for pharmacokinetic studies used either 2% or 10% N′,N′- dimethylacetamide/10% propylene glycol/30% 2-hydroxypropyl-β-cyclodextrin in water and a dose volume of 10 ml/kg (all routes of administration).
Liver Microsome Stability, Cytochrome P450 Inhibition, and Plasma Protein Binding
Metabolic stability was assessed using human and mouse liver microsomes (Xenotech). The final composition of the assay included 1 µM of test compounds or reference standards (imipramine and diclofenac sodium) prepared from DMSO stock, so that the final concentration of DMSO and acetonitrile was 0.2% and 0.8%, respectively. Compound was incubated with 0.5 mg/ml microsomal protein without (100 mM potassium phosphate buffer alone, pH 7.4) or with cofactors (5.0 mM glucose-6-phosphate, 0.06 U glucose-6-phosphate dehydrogenase, 2.0 mM MgCl2, 1.0 mM NADP+/NADPH). Test compounds and standards were incubated at 37°C with human and mouse liver microsomes; aliquots of the reaction mixture (100 µl) were removed at 0, 5, 15, 30, 60, and 120 minutes, and the reaction was stopped by the addition of 2.5 ml tertiary butyl methyl ether and shaken for 15 minutes. The samples were then spun at 4000 rpm for 15 minutes at 10°C, the organic phase was evaporated to dryness, and then samples were reconstituted with solvent for LC-MS/MS analysis. The percentage of the compound remaining after specified incubation period was calculated with respect to the peak areas of compound at time 0 minutes.
Inhibition of CYP2D6 and CYP3A4 was accomplished using recombinant human isoforms and a Vivid cytochrome P450 blue screening kit (Invitrogen) by incubating 2-fold serial dilutions (nine dilutions) of test compounds (highest concentrations were 1, 5, or 10 μM) with kit reagents and reaction buffer according to the manufacturer’s methods in a 96-well plate. Plates were then incubated at room temperature for 30 minutes before fluorescence was measured with a plate reader. For these studies, reference standards ketoconazole (CYP3A4) and quinidine (CYP2D6) were used as controls.
Plasma protein binding was performed with a rapid equilibrium dialysis device containing dialysis membrane with a molecular weight cutoff of 8000 Da (ThermoFisher). The plasma samples (pH 7.4) and test article (1 or 5 µM) or reference standards (Warfarin and Propranolol, 10 µM) were combined (DMSO final conc 0.1%) with 300 µl of plasma sample and added to the sample chamber, and 500 µl of buffer was added into the buffer chamber. The rapid equilibrium dialysis device was sealed with adhesive film and then incubated at 37°C with shaking at 300 rpm for 4 hours. After incubation, an aliquot of 50 μl was removed from each well (plasma and buffer side) and diluted with equal volume of opposite matrix (plasma or buffer alone) to nullify the matrix effect and then extracted for analysis by LC-MS/MS. The amount of free material was determined by:
 |
Lead Profiling Off-Target Screening
The in vitro effects of NP10679 on the human ether-à-go-go-related gene (hERG) potassium channel current (a surrogate for IKr , the rapidly activating, delayed rectifier cardiac potassium current) stably expressed in HEK mammalian cells were evaluated at room temperature using the QPatch HT (Sophion Bioscience A/S, Denmark), an automatic parallel patch-clamp system (ChanTest, Cleveland, OH). NP10679 was evaluated at 0.1, 0.3, 1, and 3 μM diluted in solution composed of (in mM) NaCl, 137; KCl, 4.0; CaCl2, 1.8; MgCl2, 1; HEPES, 10; glucose, 10; pH adjusted to 7.4 with NaCl. Each test concentration was tested in two or more cells (n ≥ 2). Duration of exposure to each test article concentration was 3 minutes. A positive control (0.5 μM E-4301) was used to confirm the sensitivity of the cells to an hERG inhibitor.
Off-target radioligand binding displacement studies of NP10679 were conducted at the National Institutes of Mental Health Psychoactive Drug Screening Program at the University of North Carolina at Chapel Hill (https://pdsp.unc.edu/). The NIMH PDSP is directed by Bryan L. Roth, MD, PhD, at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda, MD. (contract number HHSN-271-2008-00025-C; NIMH PDSP). Briefly, compounds were submitted to the NIMH PDSP program and screened at a single concentration (10 μM) of test article under equilibrium conditions for ability to displace specific radioligands from binding to their targets expressed in mammalian cell membranes in vitro. Each receptor target was assayed in quadruplicate, and the percent inhibition of radioligand binding at each target was determined at pH 7.4. If the percent inhibition was >50%, a full competition displacement binding study was conducted to determine an IC50, and from this, a binding Ki using the Cheng-Prusoff equation (Ki = IC50/[1 + (L/Kd)]), in which L is the radioligand concentration used in the competition binding assay and Kd is the radioligand equilibrium binding affinity determined in the above saturation binding assays.
The following targets (with radioligand in parentheses) were tested: serotonin receptor-1A ([3H]8-hydroxy-2-dipropylaminotetrain), serotonin receptor-1B ([3H]5-carboxamidotryptamine), serotonin receptor-1D ([3H]5-carboxamidotryptamine), serotonin receptor-1E ([3H]5HT), 5-Hydroxytryptamine2A ([3H]ketanserin), serotonin receptor-2B ([3H]lysergic acid diethylamide ), serotonin receptor-2C ([3H]mesulergine), serotonin receptor-3 ([3H]LY278584), serotonin receptor-5A ([3H]lysergic acid diethylamide), serotonin receptor-6 ([3H]lysergic acid diethylamide), serotonin receptor-7 ([3H]lysergic acid diethylamide), α adrenergic receptor-1A ([3H]prazosin), α adrenergic receptor-1B ([3H]Prazosin), α adrenergic receptor-1D ([3H]Prazosin), α adrenergic receptor-2A ([3H]rauwolscine), α adrenergic receptor-2B ([3H]rauwolscine), α adrenergic receptor-2C ([3H]rauwolscine), β adrenergic receptor-1 ([125I]pindolol), β adrenergic receptor-2 ([3H]CGP12177), β adrenergic receptor-3 ([3H]CGP12177), benzodiazepine rat brain receptor rat brain site ([3H]flunitrazepam), dopamine receptor-1 ([3H]SCH23390), dopamine receptor-2 ([3H]N-methylspiperone), dopamine receptor-3 ([3H]N-methylspiperone), dopamine receptor-4 ([3H]N-methylspiperone), dopamine receptor-5 ([3H]SCH23390), dopamine transporter ([3H]WIN35428), δ-opioid receptor ([3H]D-Ala(2),D-Leu(5) enkephalin), GABAA ([3H]muscimol), H1 ([3H]pyrilamine), histamine receptor-2 ([3H]tiotidine), histamine receptor-3 ([3H]α-methylhistamine), histamine receptor-4 ([3H]histamine), κ-opioid receptor ([3H]U69593), muscarinic receptor-1 ([3H]quinuclidinyl benzilate ), muscarinic receptor-2 ([3H]quinuclidinyl benzilate), muscarinic receptor-3 ([3H]quinuclidinyl benzilate), muscarinic receptor-4 ([3H]quinuclidinyl benzilate), muscarinic receptor-5 ([3H]quinuclidinyl benzilate), μ-opioid receptor ([3H]D-Ala(2),NMe-Phe(4), Gly-ol(5)enkephalin), norepinephrine transporter ([3H]nisoxetine), peripheral-type benzodiazepine receptor ([3H]PK11195), SERT ([3H]citalopram), σ1 ([3H]pentazocine(+)), and σ2 ([3H]1,3-di-o-tolylguanidine).
Some receptor targets were also tested in functional studies to establish whether NP10679 acted as an agonist or an antagonist of 5-HT2A receptor function at pH 7.4 (Porter et al., 1999; CEREP laboratories, France). To evaluate agonism, HEK293 cells transfected with human 5-HT2A receptors were incubated with increasing concentrations of NP10679 (duplicate wells/concentration) at 37°C for 30 minutes. Activation of the receptor was determined by changes in inositol phosphate-1 levels detected by an homogenous time resolved fluorescence method. Separate wells stimulated with 10 μM serotonin served as a positive control. To determine antagonism by NP10679, cells were incubated with increasing concentrations of the compound (duplicate wells/concentration) at 37°C for 30 minutes. Cells were stimulated with 100 nM serotonin. Activation of the receptor was determined by changes in inositol phosphate-1 levels detected by an homogenous time resolved fluorescence method. A control inhibitor, ketanserine, was run separately to confirm the accuracy and reliability of the assay data.
Similar studies were performed to evaluate agonism and antagonism in CHO cells transfected with human α1A-adrenergic receptors incubated with increasing concentrations of NP10679 (duplicate wells/concentration) at room temperature at pH 7.4 (Vicentic et al., 2002). Activation of the receptor was determined by changes in intracellular [Ca2+] by a fura-2 fluorimetry detection method. Separate wells were stimulated with 30 nM epinephrine as a positive control. To evaluate antagonism in CHO cells expressing human α1A-adrenergic receptors, wells were incubated with increasing concentrations of NP10679 (duplicate wells/concentration) at room temperature and then the cells were stimulated with 3 nM epinephrine. Activation of the receptor was determined by changes in intracellular [Ca2+] by a fura-2 fluorimetry detection method (CEREP, France).
To evaluate agonism and antagonism at human H1-histamine receptors, HEK293 cells transfected with H1 receptors were incubated with increasing concentrations of NP10679 (duplicate wells/concentration) at pH 7.4 at room temperature (Miller et al., 1999). Activation of the receptor was determined by changes in intracellular [Ca2+] by a fura-2 fluorimetry detection method. Separate wells were stimulated with 10 μM histamine as a positive control. To evaluate antagonism by NP10679, cells were incubated with increasing concentrations of the compound (duplicate wells/concentration) at room temperature, and cells were stimulated with 300 nM histamine. Activation of the receptor was determined by changes in intracellular [Ca2+] by a fura-2 fluorimetry detection method. A control inhibitor, pyrilamine, was run separately to confirm the accuracy and reliability of the assay data (CEREP, France).
Chemistry
Synthesis of EU-93-94 ((S)-N-(4-(3-(4-(3,4-dichlorophenyl)piperazin-1-yl)-2-hydroxypropoxy)phenyl)methanesulfonamide)
N-[4-[[(2S)-Oxiran-2-yl]methoxy]phenyl]methanesulfonamide (Tahirovic et al., 2008) (66 mg, 0.27 mmol) and 1-(3,4-dichlorophenyl) piperazine (62 mg, 0.27 mmol) were dissolved in ethanol (5 ml) and heated at reflux for 3 to 4 hours. The reaction mixture was then cooled to room temperature, and the solvent was evaporated in vacuo. The remaining residue was then purified via column chromatography on silica gel using 0%–30% 90%:10%:0.5% dichloromethane:methanol:NH3 in dichloromethane to yield N-[4-[(2S)-3-[4-(3,4-dichlorophenyl)piperazin-1-yl]-2-hydroxy-propoxy]phenyl]methanesulfonamide (110 mg, 0.23 mmol, 85.4% yield).
1H NMR (500 MHz, CDCl3): δ 7.23 (d d, J = 8.9, 0.7 Hz, 1H), 7.16 – 7.10 (m, 2H), 6.91 (dd, J = 2.9, 0.7 Hz, 1H), 6.87 – 6.82 (m, 2H), 6.70 (ddd, J = 9.0, 2.9, 0.7 Hz, 1H), 4.09 (ddt, J = 9.3, 5.2, 4.1 Hz, 1H), 3.98 – 3.87 (m, 2H), 3.20 – 3.07 (m, 4H), 2.86 (d, J = 0.7 Hz, 3H), 2.79 – 2.71 (m, 2H), 2.65 – 2.48 (m, 4H).
13C NMR (126 MHz, CDCl3): δ 156.77, 150.44, 132.70, 130.39, 130.03, 124.15, 122.31, 117.27, 115.37, 109.99, 70.51, 65.87, 60.49, 53.05, 48.65, 38.55.
HRMS calculated for C20H26N3O4Cl2F 474.10156; found 474.10258 [M + H].
Synthesis of NP10679 ((R)-6-(2-Hydroxy-3-(4-(4-(trifluoromethyl)phenyl)piperazin-1-yl)propoxy)-3,4-dihydroquinolin-2(1H)-one)
A suspension of (R)-6-(oxiran-2-ylmethoxy)-3,4-dihydroquinolin-2(1H)-one (100 g, 0.456 mol) and 1-(4-(trifluoromethyl)phenyl)piperazine (105 g, 0.456 mol) in ethanol (1 L) was stirred at 75°C for 21 hours with monitoring by HPLC. The reaction became a clear solution within 15 minutes at 75°C. The reaction mixture was cooled to 50°C, and the precipitated solid was filtered and washed with ethanol (200 ml). The collected solid was dried under vacuum to afford 175 g (85.3%) of the title compound. This material was analyzed by high performance liquid chromatography and found to be >99% pure, and with chiral high performance liquid chromotography to have > 98% enantiomeric purity. Recrystallization was performed by placing 260 g of NP10679 obtained from the previous steps in a 5-L round-bottom flask, to which was added a premixed solution of 1:1 methanol:acetone with constant stirring. The suspension was heated to 50°C with stirring until it became clear (approximately 30 minutes) and was then filtered through a 2-μM filter. The clear solution was cooled to 30°C over 15 minutes and added to water (13 L) under vigorous stirring over a 10-minute period. The precipitated solid NP10679 was stirred for 30 minutes at 30°C, filtered, washed with water (7.8 L) and dried in a vacuum tray drier at 70°C for 48 hours. The yield was 255 g of a white solid (98% yield).
1H NMR (400 MHz, DMSO-d6): δ 9.90 (brs , 1H), δ 7.50 (d, J = 16 Hz, 1H), 7.05 (d, J = 16 Hz, 1H), 6.85 – 6.70 (m, 3H), 4.90 (brd, 1H), 4.00 – 3.80 (m, 3H), 3.30 – 3.20 (m, 4H), 2.90 – 2.75 (m, 2H), 2.70 – 2.30 (m, 8H).
13C NMR (75 MHz, CDCl3): δ 171.82, 154.63, 153.09, 131.21, 130.04, 126.36, 124.94, 122.87, 121.22, 120.79, 120.36, 119.92, 116.30, 114.52, 113.95, 113.24, 70.70, 65.80, 60.48, 53.02, 47.98, 30.51, 25.58.
Mass spectrometry calculated for C23H26F3N3O3 500.47; found 500.30 [M+H].
Unless otherwise specified, all NMR spectra were obtained in deuterated chloroform (CDCl3) or deuterated dimethylsulfoxide (DMSO-d6) and referenced to the residual solvent peak; chemical shifts are reported in parts per million and coupling constants in hertz (Hz), s = singlet, d = doublet, t = triplet, dd = doublet of doublets, dt = doublet of triplets, ddd = doublet of doublets of doublets, etc. br = broad, m = multiplet.
Statistics
Values presented are means ± S.E.M. and are compared using Student’s paired or unpaired t test, or ANOVA followed by Dunnett’s test when appropriate. The number of observations was selected to yield a power of 0.9 for an α of 0.05.
Results
pH-Sensitive GluN2B-Selective Piperazines
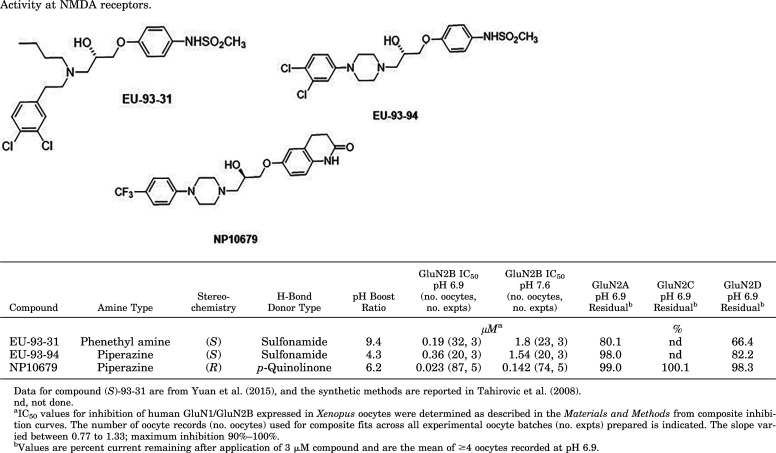
We synthesized analogs of the 93-series pH-sensitive inhibitor EU-93-31, which was previously described and supported neuroprotection in the MCAo model of transient ischemia (Yuan et al., 2015). We built upon analogs of EU-93-31 by converting the four-carbon N-coupled aliphatic chain of EU-93-31 to a piperazine linker core (Table 1). Compound EU-93-94 retained good pH sensitivity and was evaluated in vivo and, after a 3 mg/kg intravenous dose in rat, revealed a plasma half-life of 2.6 hours, which was considerably longer than that for EU-93-31 (0.92 hours; Yuan et al., 2015). Given this advantageous property of the piperazine scaffold, we evaluated the pH dependence of numerous analogs on human GluN1-1a/GluN2B receptors (hereafter GluN1/GluN2B) expressed in X. laevis oocytes by measuring the IC50 for each compound at pH 7.6 and pH 6.9, resulting in the selection of NP10679 for further evaluation (Table 1). NP10679 exhibits both a potent IC50 at pH 6.9 of 23 nM with a ratio of IC50 at pH 7.6 to that at pH 6.9 (referred to as pH boost) of 6.2-fold. Furthermore, the selectivity for inhibition of GluN2B receptors over GluN2A, GluN2C, or GluN2D receptors was maintained, as there was no considerable off-target inhibition at 3 μM for NP10679 (Fig. 1A; Table 1). We also determined the pH boost effect of other known GluN2B selective inhibitors—some tested clinically (radiprodil, traxoprodil) or well studied preclinically (ifenprodil, Ro 25-6981)—as reference standards (Fig. 1B). None of these four reference compounds possess high pH sensitivity for GluN2B inhibition, as all show <2-fold potency shifts in their IC50 values at pH 6.9.
TABLE 1.
Fig. 1.
Pharmacology of NP10679 at NMDA receptors in oocytes. (A) NP10679 concentration-response curves for inhibition of GluN2B NMDA receptors expressed in Xenopus oocytes at pH 6.9 (blue symbols) and pH 7.6 (black symbols). Each fitted line and associated symbols represent the result from one experiment (i.e., frog injection, five total). The symbols are means from 12–22 oocytes from each experiment. The NP10679 effect on GluN2A (circles), GluN2C (squares), and GluN2D (triangles) NMDA receptors is shown at the indicated concentrations; mean ± S.E.M., n = 4 for GluN2A, 2C, and 2D results. The inset shows a representative current trace for NP10679 inhibition of GluN2B receptors at 0.1 μM at the two indicated pH values. Max G/G is the receptor current obtained in 100 μM glutamate and 30 μM glycine. (B) The pH potency boost for NP10679 (blue bar, n = 5 experiments) is compared with the pH boost for known preclinical and clinical GluN2B-selective inhibitors (n = 3 experiments each). The pH Boost is the ratio of IC50 at pH 7.6/IC50 at pH 6.9.
In Vitro Drug Profiling of NP10679
Because this piperazine class of selective GluN2B inhibitors delivered relatively potent molecules with high pH dependence (Table 1), we chose to further evaluate compounds in this class for preclinical in vivo efficacy in a rodent model of cerebral ischemia for potential in vivo side effects, in vitro off-target binding profiles, and plasma pharmacokinetics after both intravenous and oral dosing in rodents. We selected NP10679 as an analog with a number of advantages, including good potency at pH 6.9 and a high degree of pH sensitivity. We proceeded to more fully characterize this compound as a prototype clinical candidate.
Metabolic stability was carried out using human and mouse liver microsomes with 1 µM NP10679 prepared from DMSO stock (DMSO 0.2% final). The compound and standards were incubated with human and mouse liver microsomes with or without cofactors, and the samples were extracted and analyzed using LC-MS/MS. NP10679 exhibited moderate to good stability in both human and mouse liver microsomes such that 72% of NP10679 remained in incubations with human microsomes and 54% remained in incubations with mouse liver microsomes in the presence of cofactors after a 1-hour incubation at 37°C. NP10679 at 1 µM also did not inhibit human recombinant cytochrome 450 enzyme isoforms CYP3A4 or CYP2D6. NP10679 bound to human, mouse, and dog plasma proteins at 97.7% (n = 2), 98.2% (n = 2), and 98.2% (n = 1), respectively.
NP10679 was also tested at 10 μM for binding to 41 neurotransmitter receptors, enzymes, and channels via displacement of a radioligand in multiple competitive receptor binding assays (Supplemental Figs. 4 and 5; Supplemental Table 1). Targets for which 10 μM NP10679 displaced >50% of the radioligand were followed up with full dose-effect displacement studies, which identified sub-micromolar Ki values for five of these targets, the 5-HT2A serotonin receptor (0.638 μM), the α1A (0.603 μM) and α1D (0.495 μM) adrenergic receptors, the H1 histamine receptor (0.040 μM), and the serotonin transporter SERT (0.135 μM) (Table 2). Three receptors (5-HT2A, α1A adrenergic, and H1 histamine) were also tested for functional agonism and antagonism; in all cases, the compound behaved as an antagonist (Supplemental Figs. 1–5; Table 2). Inhibition of the human delayed rectifier cardiac potassium current channel (hERG channel) was measured in mammalian HEK cells transfected with the hERG potassium channel cDNA via patch-clamp electrophysiology across four concentrations of NP10679, which revealed an IC50 for inhibition of 0.617 μM (Supplemental Fig. 6; Table 2).
TABLE 2.
Ki and functional inhibition at 12 off-target sites. The Ki values for 11 off targets that were first identified as hits (>50% radioligand displacement by 10 μM NP10679) in a screen of 41 targets are given. Ki values were determined from full binding displacement curves. The functional IC50 values for several targets were also determined. All assays were conducted at pH 7.4.
| Target | K i | Functional IC50 |
|---|---|---|
| μM | ||
| 5-HT1B | >10 | nd |
| 5-HT1D | 2.29 | nd |
| 5-HT2A | 0.638 | 1.71 |
| 5-HT2B | 1.92 | nd |
| α1A | 0.603 | 0.154 |
| α1B | 1.92 | nd |
| α1D | 0.495 | nd |
| α2C | 3.09 | nd |
| H1 | 0.040 | 0.073 |
| SERT | 0.135 | nd |
| σ2 | 1.98 | nd |
| hERG | nd | 0.617 |
α1A, α adrenergic receptor-1A; α1B, α adrenergic receptor-1B; α2C, α adrenergic receptor-2C; α1D, α adrenergic receptor-1D; 5-HT1B, serotonin receptor-1B; 5-HT2A, serotonin receptor-2A; 5-HT2B, serotonin receptor-2B; 5-HT1D, serotonin receptor-1D; nd, not done.
In Vivo Efficacy and Side Effect Studies
MCAo Neuroprotection and Pharmacokinetics
Prior generations of nonselective NMDAR inhibitors that blocked all NMDARs regardless of subunit composition produce both off- and on-target adverse effects, which complicated or aborted clinical development. The most prominent side effects reported included motor dysfunction, cognitive impairment, and psychotomimetic effects such as hallucinations and disorganized thought (Lees et al., 2000; Sacco et al., 2001; Diener et al., 2002; Rowland, 2005; Wood, 2005; Muir, 2006; Blagrove et al., 2009). Although GluN2B-selective NMDAR negative allosteric modulators appear to be tolerated better than competitive antagonists or channel blockers, they still exhibit some side effects (Chaperon et al., 2003; DeVry and Jentzsch 2003; Yurkewicz et al., 2005; Nicholson et al., 2007; Preskorn et al., 2008; Nutt et al., 2008). Our working hypothesis is that the pH sensitivity of the piperazine-containing compounds should increase the therapeutic ratio such that neuroprotection is achieved in ischemic penumbral regions surrounding the infarct (pH 6.9), with reduced inhibition of GluN2B-containing NMDARs in healthy brain tissue at pH 7.4.
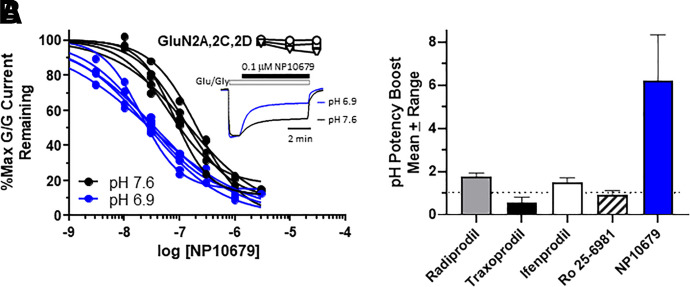
In MCAo experiments, all drug doses were randomized, and the investigator(s) were blinded throughout the study from surgery through analysis (see Materials and Methods). NP10679 was administered prior to transient ischemia induced by occlusion of the middle cerebral artery. Vehicle-treated mice exhibited substantial neuronal cell death with a 101 ± 8.7 mm3 infarct volume after 60 minutes of transient ischemia. By comparison, infarct volume was reduced in a dose-dependent manner by NP10679, with an ED50 of 1 mg/kg i.p. dose and a maximum infarct volume reduction of 52% (Fig. 2). Both the 5 mg/kg (56 ± 6.6 mm3) and 10 mg/kg (49 ± 3.0 mm3) NP10679 doses significantly reduced infarct volumes from vehicle control.
Fig. 2.
Neuroprotection by NP10679 reduces infarct volume in the MCAo model of transient ischemia in mice. (A) NP10679 reduces infarct volumes measured 24 hours post-MCAo with an ED50 of 1 mg/kg, i.p. NP10679 was administered 15 minutes prior to initiation of transient MCAo for 60 minutes. In all experiments, the drug doses were randomized and the investigators were blinded from surgical procedure through analysis of stained sections to determine infarct volume. The plot is pooled data across three independent experiments. Data are means ± S.E.M. for n = 9 (0.2 mg/kg), 13 (0.5 mg/kg), 21 (1 mg/kg), 12 (2 mg/kg), 12 (5 mg/kg), 24 (10 mg/kg), and 34 (Veh) mice. **P < 0.01 from vehicle (ANOVA, Dunnett’s). (B and C) Representative images of TTC-stained sections of vehicle (B) and drug-treated brain. (C). A representative image is shown for each dose, with each brain cut into four sections (top to bottom), and both sides of the section are shown (left and right images). The red portion of the image is TTC stain of undamaged tissue. Vehicle (Veh) is the formulation alone as described in Methods.
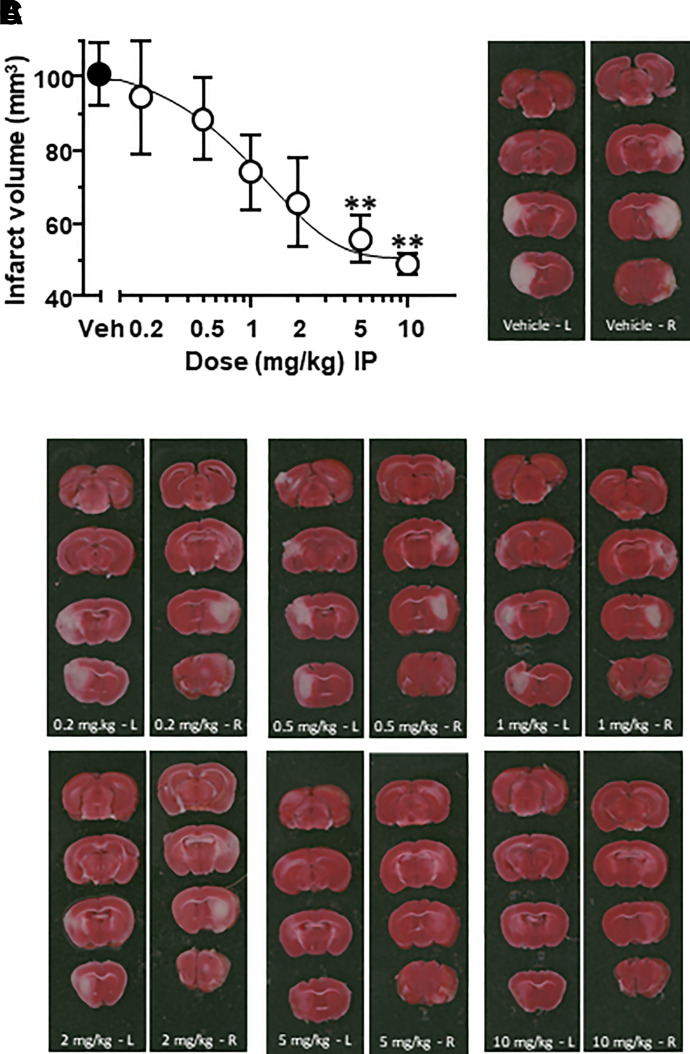
In pharmacokinetic studies, mice were dosed with a solution orally (10 mg/kg) and i.v. (3 mg/kg) to determine both oral bioavailability and plasma pharmacokinetics for NP10679 (Fig. 3A; Table 3). The plasma terminal half-life for the oral route was 7.06 hours, and for intravenous administration it was 8.56 hours, with a high volume of distribution of 1.59 L/kg and clearance of 2.44 ml/min per kilogram and high oral bioavailability (75.7%) (Table 3). In a separate study, mice were dosed i.p. with 2 and 5 mg/kg of NP10679 to provide drug disposition information in mice after the same dose and route of administration as used in the MCAo neuroprotection studies. Here, NP10679 displayed an expected dose dependence with peak levels of 581 and 1431 ng/ml of NP10679 in plasma 30 minutes postdosing, respectively, and with plasma half-lives of 7.5–9.9 hours (Table 3). Thus, a single intraperitoneal administration of NP10679 provided ample exposures to drive neuroprotection over a large fraction of the 24-hour postischemia period. In Fig. 3B, we present the free plasma levels (unbound drug) calculated at both 2 and 5 mg/kg i.p. doses, showing that free drug levels after the 5 mg/kg dose were above the IC50 for inhibition of GluN2B-containing NMDA receptors at pH 6.9 (Fig. 3B). Further, separate studies show that NP10679 exhibits high brain penetration as measured in two studies reporting a range of 1.3- to 2.6-fold higher levels found in the brain compartment compared with plasma levels in mice 1 hour after intravenous dosing (Table 3). Based on these brain:plasma ratios, we estimate that, after a 5 mg/kg i.p. dose used in MCAo studies, free drug concentration in brain may reach 60–134 nM, 51–103 nM, 33–66 nM, and 28–56 nM at 1, 2, 4, and 8 hours postdosing, respectively. Given the potency for NP10679 at pH 6.9 is 23 nM, we anticipate that occupancy of GluN2B receptors at pH 6.9 is sufficiently high to drive significant GluN2B inhibition.
Fig. 3.
Pharmacokinetics of NP10679 in mouse. (A) Total plasma levels of NP10679 after a 10 mg/kg oral dose (black symbols) and a 3 mg/kg i.v. dose (open symbols) in mice are plotted. (B) Free plasma levels of NP10679 after a 2 mg/kg (open symbols) and a 5 mg/kg i.p. (black symbols) dose in mice are given. For (A) and (B), data are means ± S.E.M. (n = 3 per data point). The gray dashed line indicates the IC50 of NP10679 for pH 6.9 inhibition of GluN2B receptors, the green dashed line is the functional IC50 at H1 histamine receptors, and the blue dashed line is the functional IC50 at hERG potassium channels.
TABLE 3.
Pharmacokinetic parameters determined in mice for NP10679.
| Oral Bioavailability | Intraperitoneal Dosing | Brain:Plasma Ratiosa | |||||
|---|---|---|---|---|---|---|---|
| Species | Mouse BALB/c | Mouse BALB/c | Mouse BALB/c | ||||
| Dose (mg/kg) | 10 | 3 | 2 | 5 | 3 | ||
| Route | Oral | i.v. | i.p. | i.p. | i.v. | ||
| Studyb | 3 | 3 | 4 | 4 | 1 | 2A | 2B |
| Plasma Cmax (ng/ml) | 3600 | 2350 | 581 | 1470 | 1740 | 600 | 817 |
| Plasma Tmax (h) | 1.0 | 0.08 | 0.5 | 0.5 | 1.0 | 1.0 | 1.0 |
| Brain conc. (ng/g) | — | — | — | — | 2300 | 1420 | 2100 |
| Brain:plasma ratio | — | — | — | — | 1.3 | 2.4 | 2.6 |
| Plasma AUClast (h*ng/ml) (0–24 h) | 45,000 | 17,800 | 4750 | 11,800 | — | — | — |
| Plasma AUCinf (h*ng/ml) | 49,500 | — | 5690 | 13,100 | — | — | — |
| Plasma AUCextrap (%) | 9.18 | — | 16.6 | 9.8 | — | — | — |
| Plasma T1/2 (h) | 7.06 | 8.56 | 9.9 | 7.5 | — | — | — |
| Plasma MRTlast | 7.16 | 7.08 | 7.5 | 6.7 | — | — | — |
| Vss (L/kg) | — | 1.59 | — | — | — | — | — |
| CL (ml/min per kilogram) | — | 2.44 | — | — | — | — | — |
| Bioavailability (F %) | 75.7 | — | — | — | — | — | — |
aBrain:plasma ratio studies reported only at 1 h.
bAll studies used 10% DMA/10% PG/30% HPBCD/50% sterile water formulation, except study 2B, which used 2% DMA/10% PG/30% HPBCD/58% sterile water. T1/2, elimination half-life; AUClast, area under the plasma concentration-time curve from time zero to last measurement; AUCinf, area under the plasma concentration-time curve from time zero to infinity; AUCextrap, area under the plasma concentration-time curve extrapolated from time t to infinity as a percentage of total AUC; MRTlast, mean residence time from time zero to last measurement; Vss, apparent volume of distribution at steady state; CL, apparent total body clearance of drug from plasma. A dash ( - ) indicates that parameter not determined.
Rotarod and Locomotor Activity
We subsequently tested whether NP10679 perturbed motor coordination or function. Mice were tested in a Rotarod challenge study after dosing with NP10679. Here, mice were trained on 2 consecutive days for ability to stay on the rotating and accelerating bar with four trials each day (intertrial interval of 25 minutes). Mice demonstrate improved performance from day 1 to day 2 and across intraday trials as shown in Fig. 4A. On day 3, mice were randomly assigned to treatment groups, dosed with vehicle or drug, and then tested four times beginning 25 minutes postdose, and the mean latency to fall was established for each trial (Fig. 4A). There was no significant impairment by NP10679 when dosed at 2 mg/kg or 5 mg/kg across all four trials. The 10 mg/kg NP10679 dose group had a reduced latency to fall in the fourth trial (87 ± 13 seconds) compared with vehicle (168 ± 14 seconds) that was significant (P < 0.01, ANOVA, Dunnett). However, no significant change from vehicle was observed in trials 1, 2, or 3 for this dose group. Reduced performance in trial 4 after 10 mg/kg NP10679 may be attributable to off-target inhibition of H1 histamine receptors (Table 2). By contrast, a 30 mg/kg dose of ifenprodil led to a significant reduction in the latency-to-fall score in all four trials tested, with a score in the fourth trial of 47 ± 12 seconds (mean ± S.E.M., n = 8; Fig. 4B). The higher dose for ifenprodil was selected because it is less potent than NP10679 for inhibition of GluN2B receptors, with the ifenprodil IC50 value of 170 nM for inhibition of GluN2B receptors (Kew et al., 1996; Mott et al., 1998) and 263 nM for neuroprotection in vitro (Chenard et al., 1991). Deficits observed in rotarod by ifenprodil may be related to α1-adrenergic receptor binding (110 nM) reported previously (Chenard et al., 1991, 1995).
Fig. 4.
Side effects measured in Rotarod challenge and locomotor activity. (A) Rotarod. Mice were trained on the Rotarod on 2 consecutive days (days 1 and 2), with four trials per day and an intertrial interval of 25 minutes. On day 3, mice were randomized to groups and administered vehicle (open circle), ifenprodil (open triangle, 30 mg/kg), or NP10679 at 2 mg/kg (light gray circle), 5 mg/kg (medium gray circle), or 10 mg/kg (black circle). The mean ± S.E.M. (n = 8) latency to fall for each trial was calculated for each group. *P < 0.01 from vehicle, ANOVA, Dunnett’s on day 3 within trials was determined. (B) Locomotor activity. Mice were habituated for 1 hour in a closed locomotor activity box and then removed and administered either vehicle (Veh, n = 6), MK-801 (MK at 0.3 mg/kg, n = 4) or NP10679 (20 mg/kg, n = 6 mice) by intraperitoneal injection and then placed back in the boxes, and the horizontal locomotor activity was counted for 2 hours. *P < 0.01, from vehicle (ANOVA, Dunnett’s). Total number of light beam breaks during the sample time are reported on the abscissa, which is representative of horizontal movement. Vehicle (Veh) is the formulation alone as described in Methods.
We also measured the ability of a single dose of NP10679 to alter the locomotor activity of mice in a closed, lighted chamber (Fig. 4B). After a 1-hour habituation period, mice were administered a 20 mg/kg dose of NP10679 or 0.3 mg/kg MK-801 and were returned to the closed lighted chamber, and horizontal activity was measured for 2 hours. NP10679 at this dose reduced horizontal activity, which was 1456 ± 194 beam breaks per 2 hours in drug compared with 2104 ± 219 beam breaks in vehicle (n = 6 each), but was not significant. Administration of 0.3 mg/kg MK-801 led to a large and significant increase (12,135 ± 3386; P < 0.01) in activity (n = 4).
Discussion
The most important finding of this study is the discovery of a new scaffold for GluN2B-selective antagonists that incorporates a piperazine into the chain linking two aromatic groups. This compound series shows strong sensitivity to extracellular pH, which should enhance therapeutic efficacy for treating acute injury while mitigating on-target side effects by virtue of enhanced potency in ischemic tissue that possesses an acidic extracellular pH compared with lower potency in healthy tissue at normal pH. Our in vivo preclinical data bear out this prediction, showing potent neuroprotection in a preclinical transient focal ischemia model, with limited behavioral effects on locomotor activity or Rotarod performance. Off-target profiling supports strong selectivity, with some activity at IC50 values for four biogenic amine receptors that were 3–74 times higher than the IC50 of NP10679 at GluN2B at pH 6.9 (23 nM), similar to what has been described for other pH-dependent GluN2B-selective inhibitors (Yuan et al., 2015). Similarly, there was no detectable inhibition of cytochrome P450 enzymes tested, and NP10679 has good metabolic stability in human and mouse liver microsomes and an acceptable plasma free fraction. In addition to these properties, NP10679 showed a pharmacokinetic profile that was superior to the straight chain analog EU93-31 (Yuan et al., 2015). In particular, NP10679 showed excellent brain penetration, a long plasma half-life, and high oral bioavailability (Table 3). This combination of properties suggests that clinical development of the piperazine series of GluN2B inhibitors such as NP10679 may provide an opportunity to advance this compound toward clinical trials for a number of unmet clinical needs involving acute neuronal injury. The utility of this class may be supported by the concept that conditions with high-frequency firing that produce metabolic changes in pH and local acidification, such as in inflammatory pain, may also be benefited.
One unique feature of this series of piperazine compounds is their pH sensitivity, which is higher than conventional well characterized GluN2B inhibitors that have been previously described (e.g., Fig. 1B). We specifically developed a structure activity relationship for this series with elevated pH sensitivity in mind to improve the safety profile of this series for use in acute injury. The idea builds on decades of work showing that extracellular pH acidifies during acute ischemic injury and in TBI for multiple reasons (Mutch and Hansen, 1984; Smith et al., 1986; Nedergaard et al., 1991; Katsura et al., 1992; Katsura and Siesjo, 1998). We do not, however, anticipate under normal excitatory synaptic transmission that NP10679 will appreciably engage the pH-sensitive mechanisms, since normal synaptic transmission does not produce detectable acidification. Rather, excitatory synaptic transmission typically produces a brief alkalinization (Tong et al., 2006; Makani and Chesler, 2007). In addition, reduced extracellular pH is not expected at extrasynaptic NMDA receptors in normal brain, suggesting NP10679 will be less effective in inhibiting these GluN2B-containing NMDA receptors under normal conditions. By contrast, the acidification that occurs during ischemia, driven both by elevated CO2-producing HCO3− and H+ and a shift to anaerobic metabolism with production of lactic acid, will broadly reduce pH throughout the extracellular space. We assume that these mechanisms, which are strong drivers of infarct and penumbral acidification during ischemia, will equally affect both synaptic and nonsynaptic GluN2B-NMDA receptors. Thus, piperazine-containing GluN2B inhibitors should be effective in a range of indications that may lead to local acidification in brain, including subarachnoid hemorrhage. Indeed, the pH-sensitive GluN2B inhibitor NP10075, which also contains a piperazine core, was recently shown to improve behavioral measures in a preclinical model of SAH (Wang et al., 2014).
The endogenous neurosteroid pregnenolone sulfate potentiates maximally activated GluN2A-containing NMDA receptors to a greater extent at pH 6.5 compared with pH 8.5, raising the possibility that neurosteroid modulators in general might exhibit pH-sensitive actions (Kostakis et al., 2011). Jang et al. (2004) show that although mutagenesis studies suggest pregnenolone sulfate (but not the inhibitory neurosteroid 3α5βS) shares structural determinants with proton inhibition on the GluN2B subunit, the potentiating actions of pregnenolone sulfate on these NMDA receptors are not dependent on extracellular pH. Multiple neurosteroid inhibitors, such as 3α-ol-5β-pregnan-20-one hemisuccinate, can inhibit NMDA receptors and reduce cell death in cultured neurons challenged with NMDA as well as in vivo after MCAo in rats (Weaver et al., 1997, 2000). The IC50 values for neurosteroid inhibition of NMDA receptors have not been reported at acidic pH, and thus it remains unclear whether this class of negative allosteric modulators exhibits pH dependence similar to NP10679. However, there is evidence that neurosteroid inhibition is use-dependent, which may be favorable under conditions observed during ischemia (Petrovic et al., 2005). Furthermore, negatively charged neurosteroid inhibitors (Weaver et al., 1997, 2000) may interact with charged amino acids that might alter their ionization state in acidic conditions, and thus the pH dependence remains an open question.
Whereas our in vivo side effect studies in Rotarod and locomotor activity after acute treatment with NP10679 showed limited effects at high doses, we have not tested NP10679 for cognitive side effects. Studies in rodents in the Morris water maze and operant-delayed match-to-position tasks with other selective GluN2B inhibitors have shown no adverse events (Guscott et al., 2003; Higgins et al., 2003; 2005) or have shown impairments in working memory in the water maze or Y maze (Zhang et al., 2013). However, when tested in nonhuman primates in cognitive tasks and learning paradigms after acute administration, two GluN2B inhibitors, traxoprodil and BMT-108908, produced cognitive impairments in a dose-dependent manner (Weed et al., 2016). In this study, effects were transient in nature and correlated with elevated GluN2B receptor occupancy. With respect to traxoprodil, which does not possess a significant pH sensitivity for NMDAR inhibition, we suggest the ∼6-fold pH-dependent shift in NP10679 potency may provide an additional advantage to further separate side effects from desired on-target ischemic tissue neuroprotection.
We believe the high potency, advantageous pharmacokinetics, pH sensitivity, demonstrated preclinical efficacy, and reduced side effects for piperazine analogs like NP10679 increase the likelihood that NMDA receptor inhibitors with these properties will be clinically viable for neurologic situations that are accompanied by acidification of the extracellular environment. If the pH sensitivity of the GluN2B-selective inhibitors can improve the therapeutic safety profile of these treatments in clinical trials for stroke that allow rapid delivery of drug to the site of action in patients stratified by image analysis (Hill et al., 2012; Saver 2013), the neuroprotective potential of NMDA receptor block may positively improve patient symptoms after acute injury.
Abbreviations
- GluN1
glutamate N-methyl-d-aspartate receptor subunit 1
- GluN2
glutamate N-methyl-d-aspartate receptor subunit 2
- H1
histamine receptor-1
- HEK
human embryonic kidney
- hERG
human ether-à-go-go
- HPLC
high-performance liquid chromatography
- HT2A
serotonin receptor-2A
- MCA
middle cerebral artery
- MCAo
middle cerebral artery occlusion
- NIMH
National Institutes of Mental Health
- NMDA
N-methyl-d-aspartate
- NMDAR
N-methyl-d-aspartate receptor
- PDSP
Psychoactive Drug Screening Program
- SAH
subarachnoid hemorrhage
- SERT
serotonin transporter
- TBI
traumatic brain injury
- TTC
2,3,5-triphenyltetrazolium chloride
Authorship Contributions
Participated in research design: Myers, Ruppa, Wilson, Tahirovic, Menaldino, Koszalka, Zazcek, Dingledine, Traynelis, Liotta.
Conducted experiments: Myers, Ruppa, Wilson, Tahirovic, Lyuboslavsky, Menaldino, Dentmon.
Performed data analysis: Myers, Ruppa, Wilson, Tahirovic, Lyuboslavsky, Menaldino.
Wrote or contributed to the writing of the manuscript: Myers, Ruppa, Wilson, Tahirovic, Lyuboslavsky, Menaldino, Dentmon, Koszalka, Zazcek, Dingledine, Traynelis, Liotta.
Footnotes
This work was supported by National Institutes of Health National Institute of Neurologic Disorders and Stroke [Grant NS111619] (S.F.T.), [Grant NS049666] (S.J.M.), and [Grant NS071657] (G.W.K.) and by the Georgia Research Alliance (R.J.D.) and the Advanced Technology Development Center (R.J.D.).
Several authors (Y.A.T., L.J.W., R.J.D., S.J.M., S.F.T., D.C.L.) are co-inventors of Emory- or NeurOp-owned (K.P.R., S.J.M., G.W.K.) patent technology, have an equity position (D.C.L., S.F.T., G.W.K., R.J.D., S.J.M., R.Z.), are a board member (D.C.L., R.J.D.), or past employees (R.Z., G.W.K., P.L., Y.A.T., K.R., L.J.W., Z.W.D., S.J.M.) for a company (NeurOp Inc.) that has licensed this technology. S.F.T. is a PI on research grants from Allergan, Biogen, and Janssen to Emory and is a member of the SAB for Sage Therapeutics, Eumentis Therapeutics, the GRIN2B Foundation, and the CureGRIN Foundation. S.F.T and R.J.D. have received licensing fees and royalties from Emory University.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Abe TMatsumura SKatano TMabuchi TTakagi KXu LYamamoto AHattori KYagi TWatanabe M, et al. (2005) Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci 22:1445–1454. [DOI] [PubMed] [Google Scholar]
- Addy C, Assaid C, Hreniuk D, Stroh M, Xu Y, Herring WJ, Ellenbogen A, Jinnah HA, Kirby L, Leibowitz MT, et al. (2009) Single-dose administration of MK-0657, an NR2B-selective NMDA antagonist, does not result in clinically meaningful improvement in motor function in patients with moderate Parkinson’s disease. J Clin Pharmacol 49:856–864. [DOI] [PubMed] [Google Scholar]
- Albers GW, Goldstein LB, Hall D, Lesko LM; Aptiganel Acute Stroke Investigators (2001) Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA 286:2673–2682. [DOI] [PubMed] [Google Scholar]
- Auvin S, Dozières-Puyravel B, Avbersek A, Sciberras D, Collier J, Leclercq K, Mares P, Kaminski RM, Muglia P (2020) Radiprodil, a NR2B negative allosteric modulator, from bench to bedside in infantile spasm syndrome. Ann Clin Transl Neurol 7:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad AW, Kilburg C, Ravindra VM, Scoville J, Joyce E, Grandhi R, Taussky P (2020) Predicting death after thrombectomy in the treatment of acute stroke. Front Surg 7:16 10.3389/fsurg.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagrove M, Morgan CJA, Curran HV, Bromley L, Brandner B (2009) The incidence of unpleasant dreams after sub-anaesthetic ketamine. Psychopharmacology (Berl) 203:109–120. [DOI] [PubMed] [Google Scholar]
- Bristow LJGulia JWeed MRSrikumar BNLi Y-WGraef JDNaidu PSSanmathi CAher JBastia T, et al. (2017) Preclinical characterization of (R)-3-((3S,4S)-3-fluoro-4-(4-hydroxyphenyl)piperidin-1-yl)-1-(4-methylbenzyl)pyrrolidin-2-one (BMS-986169), a novel, intravenous, glutamate N-methyl-d-aspartate 2B receptor negative allosteric modulator with potential in major depressive disorder. J Pharmacol Exp Ther 363:377–393. [DOI] [PubMed] [Google Scholar]
- Brown DGMaier DLSylvester MAHoerter TNMenhaji-Klotz ELasota CCHirata LTWilkins DEScott CWTrivedi S, et al. (2011) 2,6-Disubstituted pyrazines and related analogs as NR2B site antagonists of the NMDA receptor with anti-depressant activity. Bioorg Med Chem Lett 21:3399–3403. [DOI] [PubMed] [Google Scholar]
- Buemi MR, De Luca L, Ferro S, Gitto R (2014) Targeting GluN2B-containing N-methyl-D-aspartate receptors: design, synthesis, and binding affinity evaluation of novel 3-substituted indoles. Arch Pharm (Weinheim) 347:533–539. [DOI] [PubMed] [Google Scholar]
- Bullock MR, Merchant RE, Carmack CA, Doppenberg E, Shah AK, Wilner KD, Ko G, Williams SA (1999) An open-label study of CP-101,606 in subjects with a severe traumatic head injury or spontaneous intracerebral hemorrhage. Ann N Y Acad Sci 890:51–58. [DOI] [PubMed] [Google Scholar]
- Carlson APHänggi DWong GKEtminan NMayer SAAldrich FDiringer MNSchmutzhard EFaleck HJNg D, et al. ; NEWTON Investigators (2020) Single-dose intraventricular nimodipine microparticles versus oral nimodipine for aneurysmal subarachnoid hemorrhage. Stroke 51:1142–1149. [DOI] [PubMed] [Google Scholar]
- Carter CBenavides JLegendre PVincent JDNoel FThuret FLloyd KGArbilla SZivkovic BMacKenzie ET, et al. (1988) Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. II. Evidence for N-methyl-D-aspartate receptor antagonist properties. J Pharmacol Exp Ther 247:1222–1232. [PubMed] [Google Scholar]
- Chaperon F, Müller W, Auberson YP, Tricklebank MD, Neijt HC (2003) Substitution for PCP, disruption of prepulse inhibition and hyperactivity induced by N-methyl-D-aspartate receptor antagonists: preferential involvement of the NR2B rather than NR2A subunit. Behav Pharmacol 14:477–487. [DOI] [PubMed] [Google Scholar]
- Chenard BL, Shalaby IA, Koe BK, Ronau RT, Butler TW, Prochniak MA, Schmidt AW, Fox CB (1991) Separation of alpha 1 adrenergic and N-methyl-D-aspartate antagonist activity in a series of ifenprodil compounds. J Med Chem 34:3085–3090. [DOI] [PubMed] [Google Scholar]
- Chenard BLBordner JButler TWChambers LKCollins MADe Costa DLDucat MFDumont MLFox CBMena EE, et al. (1995) (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. J Med Chem 38:3138–3145. [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY, Peters S (1988) Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci 8:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crupi R, Cordaro M, Cuzzocrea S, Impellizzeri D (2020) Management of traumatic brain injury: from present to future. Antioxidants 9:E297 10.3390/antiox9040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DJCrowe MLucas NQuinn JMiller DDPritchard SGrose DBettini ECalcinaghi NVirginio C, et al. (2012) A novel series of benzimidazole NR2B-selective NMDA receptor antagonists. Bioorg Med Chem Lett 22:2620–2623. [DOI] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR (2003) Role of the NMDA receptor NR2B subunit in the discriminative stimulus effects of ketamine. Behav Pharmacol 14:229–235. [DOI] [PubMed] [Google Scholar]
- Dey S, Schepmann D, Wünsch B (2018) 2-Methyltetrahydro-3-benzazepin-1-ols - The missing link in SAR of GluN2B selective NMDA receptor antagonists. Bioorg Med Chem 26:501–508. [DOI] [PubMed] [Google Scholar]
- Diener HC, AlKhedr A, Busse O, Hacke W, Zingmark PH, Jonsson N, Basun H; Study group (2002) Treatment of acute ischaemic stroke with the low-affinity, use-dependent NMDA antagonist AR-R15896AR. A safety and tolerability study. J Neurol 249:561–568. [DOI] [PubMed] [Google Scholar]
- Farin A, Marshall LF (2004) Lessons from epidemiologic studies in clinical trials of traumatic brain injury. Acta Neurochir Suppl (Wien) 89:101–107. [DOI] [PubMed] [Google Scholar]
- Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA (1997) Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther 283:1285–1292. [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM; Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery (2002) Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke 33:2123–2136. [DOI] [PubMed] [Google Scholar]
- Guenego AFahed RAlbers GWKuraitis GSussman ESMartin BWMarcellus DGOlivot JMMarks MPLansberg MG, et al. (2020) Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur J Neurol 27:864–870. [DOI] [PubMed] [Google Scholar]
- Guscott MR, Clarke HF, Murray F, Grimwood S, Bristow LJ, Hutson PH (2003) The effect of (+/−)-CP-101,606, an NMDA receptor NR2B subunit selective antagonist, in the Morris watermaze. Eur J Pharmacol 476:193–199. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Ballard TM, Huwyler J, Kemp JA, Gill R (2003) Evaluation of the NR2B-selective NMDA receptor antagonist Ro 63-1908 on rodent behaviour: evidence for an involvement of NR2B NMDA receptors in response inhibition. Neuropharmacology 44:324–341. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Ballard TM, Enderlin M, Haman M, Kemp JA (2005) Evidence for improved performance in cognitive tasks following selective NR2B NMDA receptor antagonist pre-treatment in the rat. Psychopharmacology (Berl) 179:85–98. [DOI] [PubMed] [Google Scholar]
- Hill MDMartin RHMikulis DWong JHSilver FLTerbrugge KGMilot GClark WMMacdonald RLKelly ME, et al. ; ENACT trial investigators (2012) Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol 11:942–950. [DOI] [PubMed] [Google Scholar]
- Jang M-K, Mierke DF, Russek SJ, Farb DH (2004) A steroid modulatory domain on NR2B controls N-methyl-D-aspartate receptor proton sensitivity. Proc Natl Acad Sci USA 101:8198–8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, Chan PH, Traynelis SF (2003) The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc Natl Acad Sci USA 100:13019–13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E, Furukawa H (2014) Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 344:992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura K, Asplund B, Ekholm A, Siesjö BK (1992) Extra- and intracellular pH in the brain during ischaemia, related to tissue lactate content in normo- and hypercapnic rats. Eur J Neurosci 4:166–176. [DOI] [PubMed] [Google Scholar]
- Katsura K, Siesjo B (1998) Acid-base Metabolism in ischemia. In pH and Brain Function (Kalia, K. and Ransom, B. R., eds), New York, Wiley-Liss., p.563. [Google Scholar]
- Kemp JA, Tasker T (2009) inventors, Evotec Neurosciences Gmbh, assignee. Methods for treating disorders using NMDA NR2B-subtype selective antagonist. Patent WO 2009118187A1. 2009 Mar 26.
- Kew JNC, Trube G, Kemp JA (1996) A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. J Physiol 497:761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell CS, Liebeskind DS, Starkman S, Saver JL (2001) Trends in acute ischemic stroke trials through the 20th century. Stroke 32:1349–1359. [DOI] [PubMed] [Google Scholar]
- Koller M, Urwyler S (2010) Novel N-methyl-D-aspartate receptor antagonists: a review of compounds patented since 2006. Expert Opin Ther Pat 20:1683–1702. [DOI] [PubMed] [Google Scholar]
- Kostakis E, Jang M-K, Russek SJ, Gibbs TT, Farb DH (2011) A steroid modulatory domain in NR2A collaborates with NR1 exon-5 to control NMDAR modulation by pregnenolone sulfate and protons. J Neurochem 119:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labas RGilbert GNicole ODhilly MAbbas ATirel OBuisson AHenry JBarré LDebruyne D, et al. (2011) Synthesis, evaluation and metabolic studies of radiotracers containing a 4-(4-[18F]-fluorobenzyl)piperidin-1-yl moiety for the PET imaging of NR2B NMDA receptors. Eur J Med Chem 46:2295–2309. [DOI] [PubMed] [Google Scholar]
- Layton ME, Kelly MJ 3rd, Rodzinak KJ (2006) Recent advances in the development of NR2B subtype-selective NMDA receptor antagonists. Curr Top Med Chem 6:697–709. [DOI] [PubMed] [Google Scholar]
- Layton MEKelly MJ 3rdRodzinak KJSanderson PEYoung SDBednar RADilella AGMcDonald TPWang HMosser SD, et al. (2011) Discovery of 3-substituted aminocyclopentanes as potent and orally bioavailable NR2B subtype-selective NMDA antagonists. ACS Chem Neurosci 2:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees KR, Asplund K, Carolei A, Davis SM, Diener HC, Kaste M, Orgogozo JM, Whitehead J; GAIN International Investigators (2000) Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. Lancet 355:1949–1954. [DOI] [PubMed] [Google Scholar]
- Liverton NJBednar RABednar BButcher JWClaiborne CFClaremon DACunningham MDiLella AGGaul SLLibby BE, et al. (2007) Identification and characterization of 4-methylbenzyl 4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate, an orally bioavailable, brain penetrant NR2B selective N-methyl-D-aspartate receptor antagonist. J Med Chem 50:807–819. [DOI] [PubMed] [Google Scholar]
- Longstreth WT Jr, Nelson LM, Koepsell TD, van Belle G (1993) Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology 43:712–718. [DOI] [PubMed] [Google Scholar]
- Makani S, Chesler M (2007) Endogenous alkaline transients boost postsynaptic NMDA receptor responses in hippocampal CA1 pyramidal neurons. J Neurosci 27:7438–7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcin LRWarrier JThangathirupathy SShi JKarageorge GNPearce BCNg APark HKempson JLi J, et al. (2018) BMS-986163, a negative allosteric modulator of GluN2B with potential utility in major depressive disorder. ACS Med Chem Lett 9:472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley JATheberge CRRomano JJBillings SBAnderson KDClaremon DAFreidinger RMBednar RAMosser SDGaul SL, et al. (2004) NR2B-selective N-methyl-D-aspartate antagonists: synthesis and evaluation of 5-substituted benzimidazoles. J Med Chem 47:2089–2096. [DOI] [PubMed] [Google Scholar]
- McIntyre CJMcCauley JABednar BBednar RAButcher JWClaremon DACunningham MEFreidinger RMGaul SLHomnick CF, et al. (2009) Synthesis and evaluation of novel tricyclic benzo[4.5]cyclohepta[1.2]pyridine derivatives as NMDA/NR2B antagonists. Bioorg Med Chem Lett 19:5132–5135. [DOI] [PubMed] [Google Scholar]
- Merchant RE, Bullock MR, Carmack CA, Shah AK, Wilner KD, Ko G, Williams SA (1999) A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann N Y Acad Sci 890:42–50. [DOI] [PubMed] [Google Scholar]
- Michel A, Downey P, Nicolas JM, Scheller D (2014) Unprecedented therapeutic potential with a combination of A2A/NR2B receptor antagonists as observed in the 6-OHDA lesioned rat model of Parkinson’s disease. PLoS One 9:e114086 10.1371/journal.pone.0114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A, Downey P, Van Damme X, De Wolf C, Schwarting R, Scheller D (2015) Behavioural assessment of the A2a/NR2B combination in the unilateral 6-OHDA-lesioned rat model: a new method to examine the therapeutic potential of non-dopaminergic drugs. PLoS One 10:e0135949 10.1371/journal.pone.0135949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TR, Witte DG, Ireland LM, Kang CH, Roch JM, Masters JN, Esbenshade TA, Hancock AA (1999) Analysis of apparent noncompetitive responses to competitive H(1)-histamine receptor antagonists in fluorescent imaging plate reader-based calcium assays. J Biomol Screen 4:249–258. [DOI] [PubMed] [Google Scholar]
- Mony L, Kew JN, Gunthorpe MJ, Paoletti P (2009) Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol 157:1301–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GF, Bullock R, Marshall SB, Marmarou A, Maas A, Marshall LF; The Selfotel Investigators (1999) Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. J Neurosurg 91:737–743. [DOI] [PubMed] [Google Scholar]
- Mosley CAMyers SJMurray EESantangelo RTahirovic YAKurtkaya NMullasseril PYuan HLyuboslavsky PLe P, et al. (2009) Synthesis, structural activity-relationships, and biological evaluation of novel amide-based allosteric binding site antagonists in NR1A/NR2B N-methyl-D-aspartate receptors. Bioorg Med Chem 17:6463–6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R (1998) Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat Neurosci 1:659–667. [DOI] [PubMed] [Google Scholar]
- Muir KW (2006) Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol 6:53–60. [DOI] [PubMed] [Google Scholar]
- Mutch WA, Hansen AJ (1984) Extracellular pH changes during spreading depression and cerebral ischemia: mechanisms of brain pH regulation. J Cereb Blood Flow Metab 4:17–27. [DOI] [PubMed] [Google Scholar]
- Narayan RKMichel MEAnsell BBaethmann ABiegon ABracken MBBullock MRChoi SCClifton GLContant CF, et al. (2002) Clinical trials in head injury. J Neurotrauma 19:503–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA (1991) Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol 260:R581–R588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KL, Mansbach RS, Menniti FS, Balster RL (2007) The phencyclidine-like discriminative stimulus effects and reinforcing properties of the NR2B-selective N-methyl-D-aspartate antagonist CP-101 606 in rats and rhesus monkeys. Behav Pharmacol 18:731–743. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Gunzler SA, Kirchhoff T, Hogarth P, Weaver JL, Krams M, Jamerson B, Menniti FS, Landen JW (2008) Effects of a NR2B selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov Disord 23:1860–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KTClaiborne CFMcCauley JALibby BEClaremon DABednar RAMosser SDGaul SLConnolly TMCondra CL, et al. (2007) Cyclic benzamidines as orally efficacious NR2B-selective NMDA receptor antagonists. Bioorg Med Chem Lett 17:3997–4000. [DOI] [PubMed] [Google Scholar]
- Olney JW (1969) Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164:719–721. [DOI] [PubMed] [Google Scholar]
- Patel P, Yavagal D, Khandelwal P (2020) Hyperacute management of ischemic strokes: JACC focus seminar. J Am Coll Cardiol 75:1844–1856. [DOI] [PubMed] [Google Scholar]
- Petrovic M, Sedlacek M, Horak M, Chodounska H, Vyklický L Jr (2005) 20-oxo-5β-pregnan-3α-yl sulfate is a use-dependent NMDA receptor inhibitor. J Neurosci 25:8439–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RHP, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ (1999) Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol 128:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28:631–637. [DOI] [PubMed] [Google Scholar]
- Regan MC, Zhu Z, Yuan H, Myers SJ, Menaldino DS, Tahirovic YA, Liotta DC, Traynelis SF, Furukawa H (2019) Structural elements of a pH-sensitive inhibitor binding site in NMDA receptors. Nat Commun 10:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM (2005) Subanesthetic ketamine: how it alters physiology and behavior in humans. Aviat Space Environ Med 76(7, Suppl)C52–C58. [PubMed] [Google Scholar]
- Ruppa KB, King D, Olson RE (2012) NMDA antagonists of GluN2B subtype and modulators of GluN2A, GluN2C, and GluN2D subtypes—recent results and developments, in Annual Reports in Medicinal Chemistry, 47:89–103.Editor MC Desai. Elsevier, Oxford, UK. [Google Scholar]
- Sacco RL, DeRosa JT, Haley EC Jr, Levin B, Ordronneau P, Phillips SJ, Rundek T, Snipes RG, Thompson JL; Glycine Antagonist in Neuroprotection Americas Investigators (2001) Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: a randomized controlled trial. JAMA 285:1719–1728. [DOI] [PubMed] [Google Scholar]
- Sang CN, Weaver JJ, Jinga L, Wouden J, Saltarelli MD(2003) The NR2B subunit-selective NMDA receptor antagonist, CP-101,606, reduces spontaneous pain intensity in patients with central and peripheral neuropathic pain. Presented at the 33rd Annual Meeting of the Society for Neuroscience, New Orleans, LA, 2003; Abstract 814.9. [Google Scholar]
- Saver JL (2013) The 2012 Feinberg lecture: treatment swift and treatment sure. Stroke 44:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkirkova KSaver JLStarkman SWong GWeng JHamilton SLiebeskind DSEckstein MStratton SPratt F, et al. ; FAST-MAG Trial Coordinators and Investigators (2018) Frequency, predictors, and outcomes of prehospital and early postarrival neurological deterioration in acute stroke: exploratory analysis of the FAST-MAG randomized clinical trial. JAMA Neurol 75:1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, von Hanwehr R, Siesjö BK (1986) Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J Cereb Blood Flow Metab 6:574–583. [DOI] [PubMed] [Google Scholar]
- Steece-Collier K, Chambers LK, Jaw-Tsai SS, Menniti FS, Greenamyre JT (2000) Antiparkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-d-aspartate receptors. Exp Neurol 163:239–243. [DOI] [PubMed] [Google Scholar]
- Stroebel D, Buhl DL, Knafels JD, Chanda PK, Green M, Sciabola S, Mony L, Paoletti P, Pandit J (2016) A novel binding mode reveals two distinct classes of NMDA receptor GluN2B-selective antagonists. Mol Pharmacol 89:541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartjes M, Morariu A, Niesters M, Aarts L, Dahan A (2011) Nonselective and NR2B-selective N-methyl-D-aspartic acid receptor antagonists produce antinociception and long-term relief of allodynia in acute and neuropathic pain. Anesthesiology 115:165–174. [DOI] [PubMed] [Google Scholar]
- Tahirovic YAGeballe MGruszecka-Kowalik EMyers SJLyuboslavsky PLe PFrench AIrier HChoi WBEasterling K, et al. (2008) Enantiomeric propanolamines as selective N-methyl-D-aspartate 2B receptor antagonists. J Med Chem 51:5506–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Shinjo K, Mizutani M, Shimada K, Ishikawa T, Menniti FS, Nagahisa A (1997) Antinociceptive activity of CP-101,606, an NMDA receptor NR2B subunit antagonist. Br J Pharmacol 122:809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum S, Schepmann D, Kalinin DV, Ametamey SM, Wünsch B (2018) Replacement of the benzylpiperidine moiety with fluorinated phenylalkyl side chains for the development of GluN2B receptor ligands. ChemMedChem 13:2522–2529. [DOI] [PubMed] [Google Scholar]
- Tong C-K, Chen K, Chesler M (2006) Kinetics of activity-evoked pH transients and extracellular pH buffering in rat hippocampal slices. J Neurophysiol 95:3686–3697. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL (1998) Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J Neurosci 18:6163–6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AC, Schwamm LH, Etherton MR (2020) Acute ischemic stroke: improving access to intravenous tissue plasminogen activator. Expert Rev Cardiovasc Ther 18:277–287 10.1080/14779072.2020.1759422. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Robeva A, Rogge G, Uberti M, Minneman KP (2002) Biochemistry and pharmacology of epitope-tagged α(1)-adrenergic receptor subtypes. J Pharmacol Exp Ther 302:58–65. [DOI] [PubMed] [Google Scholar]
- Wang H, James ML, Venkatraman TN, Wilson LJ, Lyuboslavsky P, Myers SJ, Lascola CD, Laskowitz DT (2014) pH-sensitive NMDA inhibitors improve outcome in a murine model of SAH. Neurocrit Care 20:119–131. [DOI] [PubMed] [Google Scholar]
- Weaver CE Jr, Marek P, Park-Chung M, Tam SW, Farb DH (1997) Neuroprotective activity of a new class of steroidal inhibitors of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA 94:10450–10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CE, Land MB, Purdy RH, Richards KG, Gibbs TT, Farb DH (2000) Geometry and charge determine pharmacological effects of steroids on N-methyl-D-aspartate receptor-induced Ca(2+) accumulation and cell death. J Pharmacol Exp Ther 293:747–754. [PubMed] [Google Scholar]
- Weed MR, Bookbinder M, Polino J, Keavy D, Cardinal RN, Simmermacher-Mayer J, Cometa FL, King D, Thangathirupathy S, Macor JJE, Bristow LJ (2016) Negative allosteric modulators selective for the NR2B subtype of the NMDA receptor impair cognition in multiple domains. Neuropsychopharm 46:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K (1993) Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol 44:851–859. [PubMed] [Google Scholar]
- Wood PL (2005) The NMDA receptor complex: a long and winding road to therapeutics. IDrugs 8:229–235. [PubMed] [Google Scholar]
- Yuan HMyers SJWells GNicholson KLSwanger SALyuboslavsky PTahirovic YAMenaldino DSGanesh TWilson LJ, et al. (2015) Context-dependent GluN2B-selective inhibitors of NMDA receptor function are neuroprotective with minimal side effects. Neuron 85:1305–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkewicz L, Weaver J, Bullock MR, Marshall LF (2005) The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma 22:1428–1443. [DOI] [PubMed] [Google Scholar]
- Zhang X-H, Liu S-S, Yi F, Zhuo M, Li BM (2013) Delay-dependent impairment of spatial working memory with inhibition of NR2B-containing NMDA receptors in hippocampal CA1 region of rats. Mol Brain 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]