Abstract
The main purpose of this study was to investigate the presence of retinal autofluorescence findings in COVID-19 patients. Observational study conducted in São Paulo in 2020. Demographic, medical history, and concomitant events, as well as medications used, hospitalization details, and laboratory test results, were obtained. Patients underwent eye examination and multimodal imaging, including color, red-free, autofluorescence fundus photography and optical coherence tomography. Eighteen patients had autofluorescence findings (6 females; average age 54 years, range 31 to 86 years; 26 eyes). Hyper-autofluorescence findings were present in 6 patients, Hypo-autofluorescence in 14 patients, and 6 patients had mixed pattern lesions. Retinal autofluorescence abnormalities were present in COVID-19 patients and may be secondary to primary or secondary changes caused by the SARS-CoV-2.
Keywords: Coronavirus, SARS-CoV-2 disease, Eye, Optical coherence tomography, Autofluorescence
Introduction
Early clinical evidence suggests that cases of COVID-19 are frequently characterized by increased inflammation, renin-angiotensin-aldosterone system (RAS) imbalance, and a particular form of vasculopathy, thrombotic microangiopathy, and intravascular coagulopathy [1].
The retina could be affected either by direct tissue damage from SARS-CoV-2 and its immunogenicity or by thrombotic complications [2, 3]. Primary or secondary retinal abnormalities mostly related to vascular structures have been reported on multimodal imaging studies [4–6].
Fundus autofluorescence (FAF) imaging provides a topographic mapping of lipofuscin distribution in the retinal pigment epithelium (RPE) cell monolayer, and other fluorophores occur with the outer retina and the sub-neurosensory space [7]. This study aims to investigate FAF findings in COVID-19 patients.
Methods
The study was approved by the institutional and national ethics research committees (Research Ethics Committee of Federal University of Sao Paulo UNIFESP #30725020.8.0000.5505 and INVITARE Pesquisa Clínica Auditoria e Consultoria Institutional Review Board Ethics Committee number 3.975.953). All patients or their representatives agreed to participate.
We conducted a observational study evaluating outpatients with confirmed COVID-19 diagnosis based on positive antibody tests (immunoglobulin G and immunoglobulin M titers) or PCR (using nasal/oral swabs). Patients with previous ophthalmological history and patients for whom fundus exam was impossible were excluded.
Demographic and clinical information covering medical history, concomitant medical events and medications, hospitalization details, and laboratory tests were obtained. Ophthalmic examination included measurement of best-corrected visual acuity (BCVA), Goldman applanation tonometry (IOP), and both anterior and posterior biomicroscopy. Binocular indirect fundus examination and color, red-free, and autofluorescence fundus photography were performed (Topcon DRI-OCT Triton Swept-source OCT, and California Optos®). Optical coherence tomography (OCT) imaging included: Angio Retina 3.0 mm2; HD Angio Retina 6.0 mm2; Enhanced HD Line; Cross Line; Raster; Radial Lines; Ganglion cell complex (GCC) (Optovue RTVue-XR Avanti®).
The data were analyzed using the STATA 14.0 program (StataCorp LP, College Station, TX, USA). Frequency tables were used for descriptive analyses.
Results
In late 2020, as part of the eye examination of a group of 106 patients, 18 patients with FAF changes were identified. The average time between diagnosis and the first eye exam was 44 days (±22 days), we’ve considered this time frame to start with symptoms onset. None of the patients had a previous ophthalmologic history, specially concerning previous ocular inflammation. All patients were evaluated at a convalescence period and disease severity ranged from mild to severe. We have considered severe cases patients whom required mechanical ventilatory support, moderate cases the ones whom required hospitalization but non-invasive ventilation and mild cases the ones without hospitalization. Of the 18 patients, 12 required previous hospital admission and were examined after hospital discharge. Table 1 presents data regarding epidemiology and clinical examination.
Table 1.
Patients demography (n = 18)
| Median ± SD | |
|---|---|
| Age | 54 ± 15 years |
| Female | 6 (33%) |
| BCVA OD | 0.15 ± 0.25 (20/28) |
| BCVA OS | 0.09 ± 0.17 (20/24) |
| Days between symptoms onset and evaluation | 44. (±24 days) |
| Type 2 diabetes | 2 (11%) |
| High blood pressure | 6 (33%) |
| Diabetes and high blood pressure concomitantly | 2 (11%) |
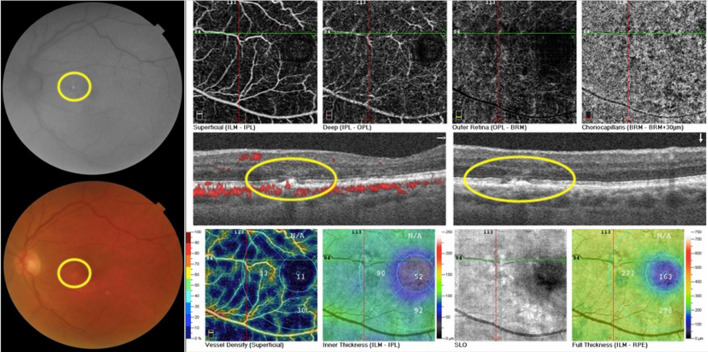
Among the 18 patients, 10 had findings only in one eye and 8 in both eyes. Most of these were depicted at posterior pole (16 eyes) and 4 other eyes had alterations contiguous to the optic nerve. Hyper-autofluorescence (HyperFAF) (Fig. 1) was present in 6 eyes of 5 patients (27.8%–5/18), and one eye presented with uniquely HyperFAF. OCT of those areas was associated with the outer retina findings, mainly in the interdigitation and ellipsoid zones (Fig. 1). Hypo-autofluorescence (HypoFAF) (Fig. 2) was present in 18 eyes of 14 patients (77.8%–14/18). OCT of those areas was associated with outer retina cell loss and RPE elevation. One eye also presented with subretinal fluid.
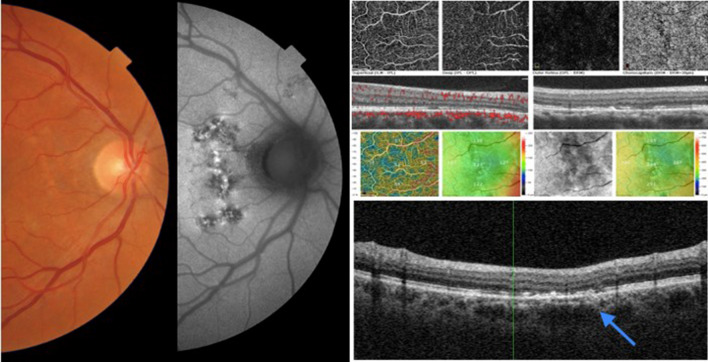
Fig. 1.
Composite of the left eye of patient 7. Composite depicts a hypopigmented area in the papillomacular bundle more evident as a hyper-autofluorescent lesion on FAF. OCT B-scan of the lesion displays RPE irregularity with adjacent cell loss in the ellipsoid zone
Fig. 2.
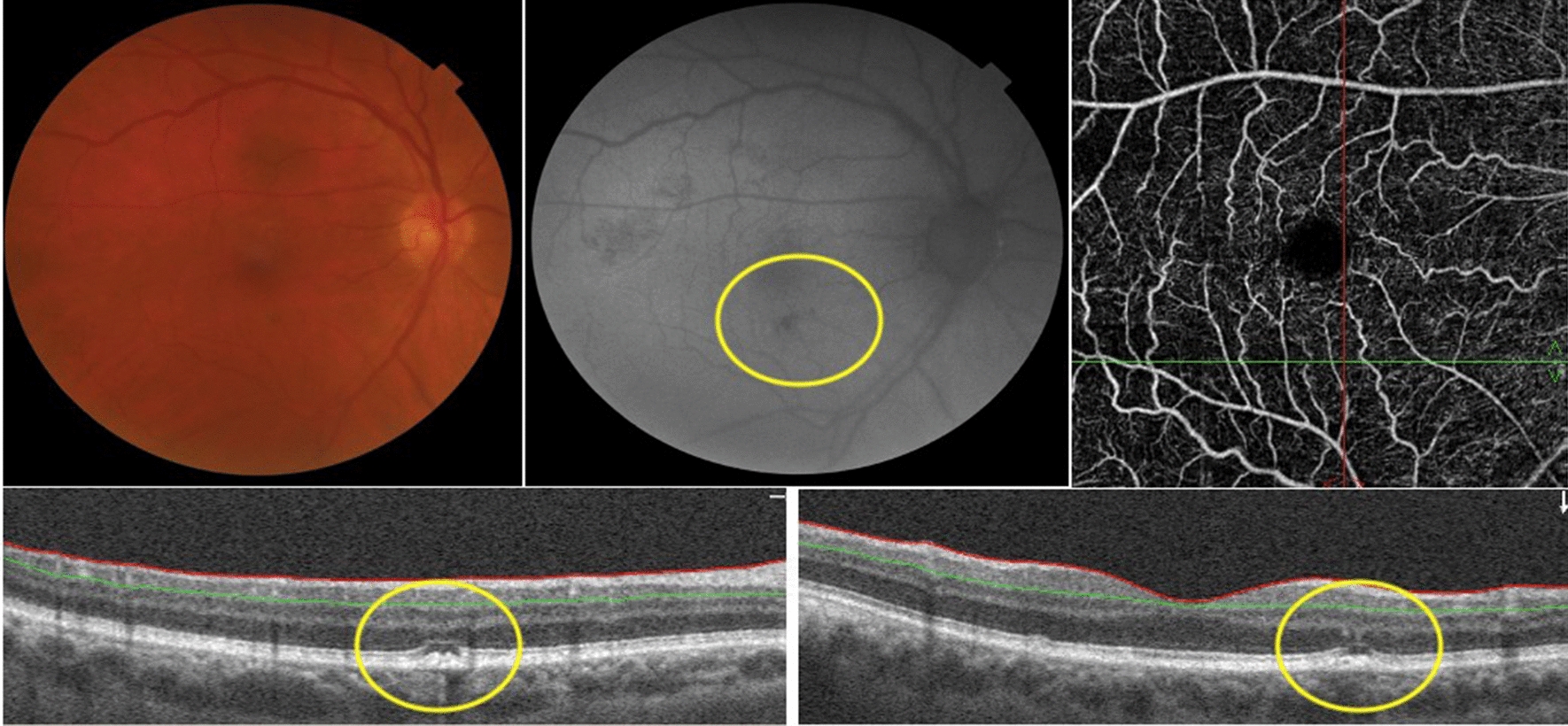
Composite of the right eye of patient 7. Image shows a hypopigmented area in the papillomacular bundle more evident as a hypo-autofluorescent lesion on FAF. OCT B-scan of the lesion displays RPE irregularity with adjacent cell loss in the ellipsoid zone
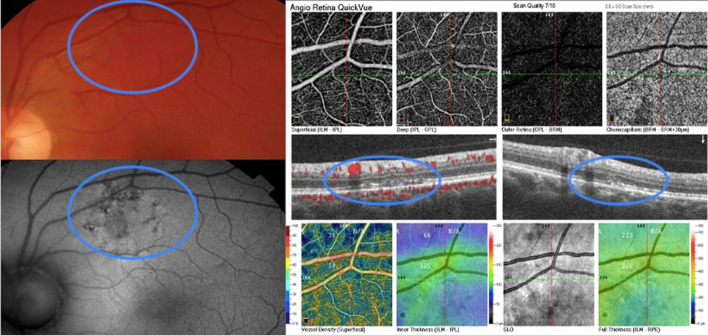
Seven eyes of 6 patients (33.3%–6/18) showed mixed patterns of hyper-autofluorescence and hypo-autofluorescence. These findings were predominantly seen adjacent to vascular structures, especially veins, in different retinal areas (Figs. 3 and 4). OCT findings of retinal thinning were associated with a disturbance of the ellipsoid zone, photoreceptors outer segments, and interdigitation zone (Figs. 3 and 4).
Fig. 3.
Composite of the left eye of patient 6. FAF shows a retinal alteration more prominent at FAF with a mixed pattern of hyper-hypoFAF. OCT B-scan of the area displays disruption of the interdigitation zone, outer segment layer, and ellipsoid zone
Fig. 4.
Composite of the right eye of patient 10. This composite shows a retinal alteration more prominent at FAF with a mixed pattern of hyper-hypoFAF. OCT B-scan displays retinal thinning due to loss of external retina layers and outer nuclear layer
Table 2 presents the correlation between autofluorescence patterns, OCT findings and patient data.
Table 2.
Correlation between autofluorescence patterns, OCT findings and patient data
| Age (Years) |
Comorbidities | FAF patterns | OCT Findings | Hospitalization | IOT | Anticoagulation | Antibiotic | D-dimer | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Right eye | Left eye | Right eye | Left eye | ||||||||
| 1 | 51 | None | HypoFAF | HypoFAF | RPE irregularity | RPE elevation | N | N | N | N | N |
| 2 | 82 | None | Hypo-hyper-FAF | Isolated areas of HypoFAF and HyperFAF | Choroidal irregularity with area of RPE disruption and subretinal fluid | Area of cellular loss at the level of the ellipsoid zone | Y | N | – | – | – |
| 3 | 51 | None | HypoFAF | None | Cystoid spaces in the interdigitation zone | None | N | N | N | N | N |
| 4 | 63 | High blood pressure and diabetes | None | HypoFAF | None | Cellular loss at ellipsoid zone | Y | Y | Y | N | 1.20 ng/mL |
| 5 | 53 | None | Isolated areas of HyperFAF and isolated areas of HypoFAF | Isolated areas of HyperFAF and isolated areas of HypoFAF | Disruption of the interdigitation zone | Disruption of the interdigitation zone with local retinal thinning | Y | N | N | Y | 0.45 ng/mL |
| 6 | 86 | None | Hypo-hyper-FAF | Hypo-hyper-FAF | Disruption of the interdigitation zone, outer segment layer and ellipsoid zone | Disruption of the interdigitation zone, outer segment layer and ellipsoid zone | Y | N | N | Y | 1.63 ng/mL |
| 7 | 51 | High blood pressure and diabetes | HypoFAF | HyperFAF | RPE irregularity with subretinal liquid and disruption of the ellipsoid zone | RPE irregularity with adjacent cell loss at the ellipsoid zone | Y | N | N | N | 0.53ng/mL |
| 8 | 69 | None | Hypo-hyper-FAF | HypoFAF | RPE elevation | RPE irregularity | Y | N | N | Y | N |
| 9 | 66 | High blood pressure | HypoFAF | None | External limiting membrane irregularity | None | Y | N | Y | Y | 0.61 ng/mL |
| 10 | 49 | None | Hypo-hyper-FAF | HypoFAF | Retinal thinning and loss of external retina and outer nuclear layers | RPE elevation and outer retinal layer loss | No | No | No | No | – |
| 11 | 57 | High blood pressure | None | Hypo-hyper-FAF | None | Retinal thinning due to loss of external retinal and outer nuclear layers | Y | N | N | Y | 0.71 ng/mL |
| 12 | 62 | High blood pressure | HypoFAF | None | Retinal Pigmented Epithelial Detachment and adjacent loss of ellipsoid zone | None | Y | N | Y | N | – |
| 13 | 48 | None | Isolated areas of HyperFAF and HypoFAF | None | RPE elevation | None | Y | N | – | – | 0.85 ng/mL |
| 14 | 31 | None | None | HypoFAF | None | Disruption of the interdigitation and outer segment layers and adjacent scarring | -- | -- | N | N | N |
| 15 | 35 | None | HypoFAF | None | None | None | N | N | N | Y | N |
| 16 | 58 | High blood pressure | HypoFAF, with surrounding area of HyperFAF | None | Disruption of external retina with adjacent scarring | None | Y | N | Y | Y | 0.93 ng/mL |
| 17 | 34 | None | HypoFAF | HypoFAF | RPE and ellipsoid irregularity | RPE irregularity and elevation | Y | N | N | N | 1.80 ng/mL |
| 18 | 41 | None | Hypo-hyper-FAF | None | Choroidal elevetaion | None | N | N | N | N | N |
Discussion
FAF is a non-invasive diagnostic tool that documents the metabolic status of lipofuscin levels throughout the eye’s posterior pole. It can be a helpful marker of outer retinal health helpful in monitoring various ophthalmic conditions, including uveitis or photoreceptor diseases. HyperFAF patterns are often related to active retinal pigmentary epithelium inflammation, and HypoFAF patterns are found in chronic and scar lesions.
The FAF patterns reported here appeared similar to those previously described in other diseases, such as syphilis [8], tuberculosis [9], inflammatory maculopathies [10], and even age-related macular degeneration [11] and may implicate pathogenic mechanisms. Our small sample prevented us the use of multiple logistic regressions to assess whether comorbidities, treatment performed, or changes in laboratory tests were related to the ophthalmological findings. Since the descriptive nature of the study aiming to report the FAF findings in patients its valuable to emphasize the multiple confusing factors, specially the heterogeneity of the population involved.
Fundus autofluorescence results from the interaction between natural fluorophores and the adjacent tissues, and variety of clinical COVID-19 presentations [12–15] can explain the broad spectrum of findings [12]. Schmitz-Valckenberg et al. have previously reported that inflammatory diseases may present different pattern of FAF over time [16], and according to the affected area, it can appear hypo-autofluorescent early and mixed later on.
Previous publications have reported retinal findings in COVID19 patients [5, 12–15, 17, 18]. FAF alterations have been presented among case reports [17–19] and the frequency has increased since the beginning of the pandemic. To our knowledge this is the biggest number of cases congregated and, in face of a new and still poorly understood disease, a more detailed analysis of the RPE-choriocapillaris complex may contribute to the better understand of COVID-19 pathophysiology in the eye ant it’s presumed effect, bring new light in it’s pathophysiology. The high prevalence of a hyper-hypo-autofluorescence pattern near vascular structures suggests that vessels may be preferentially affected, which agrees with other studies suggesting a vascular component to the SARS-CoV-2 pathogenesis [20, 21].
Conclusions
Autofluorescence may be an useful resource to detect lesions otherwise missed. The presence of hyper-autofluorescence speaks in favor of acuter lesions and towards a somewhat neglected RPE-choriocapillaris complex disfunction. Further investigation is mandatory to better understand the pathophysiology and presumed long term implications.
Acknowledgements
Not applicable.
Authors’ contributions
PMM, AAAM, AMCB wrote the manuscript. PM, AM, AB, WMM, VS were responsible for data collection. PMM, ACR, RBR, MF analyzed and interpreted the data. PS, MF, PA, HS and RBJ were major contributors in writing and reviewing the manuscript. All authors read and approved the final manuscript.
Funding
Vision Institute - IPEPO, FINEP, CAPES, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [403482/2020-8].
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the institutional and national ethics research committees under the following parameters: CAAE: 30725020.8.0000.5505, approval number 4.100.149, available at https://plataformabrasil.saude.gov.br.
Consent for publication
All patients or their representatives agreed to participate through written agreement according to the consent included as supplementary material.
Competing interests
Marinho, Marcos, Branco, Mourad, Sakamoto, Romano, Farah, Rosen, Schor, Abraao, Nascimento, Belfort Jr declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Paula M. Marinho, Alléxya A. A. Marcos and Ana M. C. Branco contributed equally to the realization of this manuscript and should be considered as the first authors
References
- 1.Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clinica Chimica Acta. 2020;507:167–73. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa ÍF, Bonifácio LP, Bellissimo-Rodrigues F, Rocha EM, Jorge R, Bollela VR, et al. Ocular findings among patients surviving COVID-19. Sci Rep. 2021;11:11085. doi: 10.1038/s41598-021-90482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R. Retinal findings in patients with COVID-19. Lancet. 2020;395:1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marinho PM, Nascimento H, Marcos AAA, Romano AC, Rosen RB, Belfort R. Reply to Editorial: Interpretation of OCT and fundus findings in COVID-19 patients in recent Lancet publication. Eye. 2020; http://www.nature.com/articles/s41433-020-01283-2. Accessed 15 Jul 2021. [DOI] [PMC free article] [PubMed]
- 7.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto Y, Spaide RF. Autofluorescence imaging of acute syphilitic posterior placoid chorioretinitis. Retin Cases Brief Rep. 2007;1:123–7. doi: 10.1097/01.iae.0000242759.80833.39. [DOI] [PubMed] [Google Scholar]
- 9.Samy A, Lightman S, Ismetova F, Talat L, Tomkins-Netzer O. Role of autofluorescence in inflammatory/infective diseases of the retina and choroid. J Ophthalmol. 2014;2014:1–9. doi: 10.1155/2014/418193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh S, Lee A, Forooghian F, Bergstrom C, Yan J, Lee C. Fundus autofluorescence features in the inflammatory maculopathies. Clin Ophthalmol. 2014;8:2001. doi: 10.2147/OPTH.S68446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yung M, Klufas MA, Sarraf D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retin Vitr. 2016;2:12. doi: 10.1186/s40942-016-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira LA, Soares LCM, Nascimento PA, Cirillo LRN, Sakuma HT, da Veiga GL, et al. Retinal findings in hospitalised patients with severe COVID-19. Br J Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2020-317576. [DOI] [PubMed] [Google Scholar]
- 13.Gascon P, Briantais A, Bertrand E, Ramtohul P, Comet A, Beylerian M, et al. Covid-19-associated retinopathy: a case report. Ocul Immunol Inflamm. 2020;28:1293–7. doi: 10.1080/09273948.2020.1825751. [DOI] [PubMed] [Google Scholar]
- 14.Zago Filho LA, Lima LH, Melo GB, Zett C, Farah ME. Vitritis and outer retinal abnormalities in a patient with COVID-19. Ocular Immunol Inflamm. 2020;28:1298–300. doi: 10.1080/09273948.2020.1821898. [DOI] [PubMed] [Google Scholar]
- 15.Gaba WH, Ahmed D, Al Nuaimi RK, Dhanhani AA, Eatamadi H. Bilateral central retinal vein occlusion in a 40-year-old man with severe coronavirus disease 2019 (COVID-19) pneumonia. Am J Case Rep. 2020;21. https://www.amjcaserep.com/abstract/index/idArt/927691. Accessed 15 July 2021. [DOI] [PMC free article] [PubMed]
- 16.R. S. Chorioretinal Inflammatoy Disorders. In: Holz FG, Schmitz-Valckenberg S, Spaide RF, Bird AC, editors. Atlas of fundus autofluorescence imaging: with 1 table. Berlin Heidelberg: Springer; 2007:207–239.
- 17.Duff SM, Wilde M, Khurshid G. Branch retinal vein occlusion in a COVID-19 positive patient. Cureus. 2021; https://www.cureus.com/articles/51955-branch-retinal-vein-occlusion-in-a-covid-19-positive-patient. Accessed 15 July 2021. [DOI] [PMC free article] [PubMed]
- 18.Olguín-Manríquez F, Cernichiaro-Espinosa L, Olguín-Manríquez A, Manríquez-Arias R, Flores-Villalobos EO, Kawakami-Campos PA. Unilateral acute posterior multifocal placoid pigment epitheliopathy in a convalescent COVID-19 patient. Int J Retin Vitr. 2021;7:41. doi: 10.1186/s40942-021-00312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Souza EC, de Campos VE, Duker JS. Atypical unilateral multifocal choroiditis in a COVID-19 positive patient. Am J Ophthalmol Case Rep. 2021;22:101034. doi: 10.1016/j.ajoc.2021.101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqi HK, Libby P, Ridker PM. COVID-19—a vascular disease. Trends in Cardiovasc Med. 2021;31:1–5. doi: 10.1016/j.tcm.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–40. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.