Abstract
Background
Gonadotrophin‐releasing hormone (GnRH) antagonists can be used to prevent a luteinizing hormone (LH) surge during controlled ovarian hyperstimulation (COH) without the hypo‐oestrogenic side‐effects, flare‐up, or long down‐regulation period associated with agonists. The antagonists directly and rapidly inhibit gonadotrophin release within several hours through competitive binding to pituitary GnRH receptors. This property allows their use at any time during the follicular phase. Several different regimens have been described including multiple‐dose fixed (0.25 mg daily from day six to seven of stimulation), multiple‐dose flexible (0.25 mg daily when leading follicle is 14 to 15 mm), and single‐dose (single administration of 3 mg on day 7 to 8 of stimulation) protocols, with or without the addition of an oral contraceptive pill. Further, women receiving antagonists have been shown to have a lower incidence of ovarian hyperstimulation syndrome (OHSS). Assuming comparable clinical outcomes for the antagonist and agonist protocols, these benefits would justify a change from the standard long agonist protocol to antagonist regimens. This is an update of a Cochrane review first published in 2001, and previously updated in 2006 and 2011.
Objectives
To evaluate the effectiveness and safety of gonadotrophin‐releasing hormone (GnRH) antagonists compared with the standard long protocol of GnRH agonists for controlled ovarian hyperstimulation in assisted conception cycles.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group Trials Register (searched from inception to May 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, inception to 28 April 2015), Ovid MEDLINE (1966 to 28 April 2015), EMBASE (1980 to 28 April 2015), PsycINFO (1806 to 28 April 2015), CINAHL (to 28 April 2015) and trial registers to 28 April 2015, and handsearched bibliographies of relevant publications and reviews, and abstracts of major scientific meetings, for example the European Society of Human Reproduction and Embryology (ESHRE) and American Society for Reproductive Medicine (ASRM). We contacted the authors of eligible studies for missing or unpublished data. The evidence is current to 28 April 2015.
Selection criteria
Two review authors independently screened the relevant citations for randomised controlled trials (RCTs) comparing different GnRH agonist versus GnRH antagonist protocols in women undergoing in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI).
Data collection and analysis
Two review authors independently assessed trial eligibility and risk of bias, and extracted the data. The primary review outcomes were live birth and ovarian hyperstimulation syndrome (OHSS). Other adverse effects (miscarriage and cycle cancellation) were secondary outcomes. We combined data to calculate pooled odds ratios (ORs) and 95% confidence intervals (CIs). Statistical heterogeneity was assessed using the I2 statistic. We assessed the overall quality of the evidence for each comparison using GRADE methods.
Main results
We included 73 RCTs, with 12,212 participants, comparing GnRH antagonist to long‐course GnRH agonist protocols. The quality of the evidence was moderate: limitations were poor reporting of study methods.
Live birth
There was no evidence of a difference in live birth rate between GnRH antagonist and long course GnRH agonist (OR 1.02, 95% CI 0.85 to 1.23; 12 RCTs, n = 2303, I2= 27%, moderate quality evidence). The evidence suggested that if the chance of live birth following GnRH agonist is assumed to be 29%, the chance following GnRH antagonist would be between 25% and 33%.
OHSS
GnRH antagonist was associated with lower incidence of any grade of OHSS than GnRH agonist (OR 0.61, 95% C 0.51 to 0.72; 36 RCTs, n = 7944, I2 = 31%, moderate quality evidence). The evidence suggested that if the risk of OHSS following GnRH agonist is assumed to be 11%, the risk following GnRH antagonist would be between 6% and 9%.
Other adverse effects
There was no evidence of a difference in miscarriage rate per woman randomised between GnRH antagonist group and GnRH agonist group (OR 1.03, 95% CI 0.82 to 1.29; 34 RCTs, n = 7082, I2 = 0%, moderate quality evidence).
With respect to cycle cancellation, GnRH antagonist was associated with a lower incidence of cycle cancellation due to high risk of OHSS (OR 0.47, 95% CI 0.32 to 0.69; 19 RCTs, n = 4256, I2 = 0%). However cycle cancellation due to poor ovarian response was higher in women who received GnRH antagonist than those who were treated with GnRH agonist (OR 1.32, 95% CI 1.06 to 1.65; 25 RCTs, n = 5230, I2 = 68%; moderate quality evidence).
Authors' conclusions
There is moderate quality evidence that the use of GnRH antagonist compared with long‐course GnRH agonist protocols is associated with a substantial reduction in OHSS without reducing the likelihood of achieving live birth.
Keywords: Adult; Female; Humans; Reproductive Techniques, Assisted; Gonadotropin‐Releasing Hormone; Gonadotropin‐Releasing Hormone/agonists; Gonadotropin‐Releasing Hormone/antagonists & inhibitors; Live Birth; Ovarian Hyperstimulation Syndrome; Ovarian Hyperstimulation Syndrome/prevention & control; Ovulation Induction; Ovulation Induction/methods; Randomized Controlled Trials as Topic
Plain language summary
Gonadotrophin‐releasing hormone antagonists versus GnRH agonist in subfertile couples undergoing assisted reproductive technology
Review question
This updated Cochrane review evaluated the efficacy and safety of GnRH antagonists compared to the more widely‐used GnRH agonists (long‐course protocol).
Background:
Gonadotrophin‐releasing hormone (GnRH) agonist is commonly used to prevent cycle cancellation secondary to a premature luteinizing hormone (LH) surge, and thereby increase the chance of live birth in women undergoing assisted reproductive technology (ART), while reducing the risk of complications such as ovarian hyperstimulation syndrome (OHSS). Gonadotrophin‐releasing hormone (GnRH) antagonists are now being seriously considered as a potential means of achieving better treatment outcomes because the protocol is more flexible and antagonists may reduce OHSS more effectively than agonists. However, there is the need to evaluate the benefits as well as the safety of these GnRH antagonist regimens in comparison with the existing GnRH agonist regimens.
Study characteristics
We found 73 randomised controlled trials comparing GnRH antagonist with GnRH agonist in a total of 12,212 women undergoing ART. The evidence is current to May 2015
Key results
There was no evidence of a difference between the groups in live birth rates (i.e. rates at conclusion of a course of treatment). The evidence suggested that if the chance of live birth following GnRH agonist is assumed to be 29%, the chance following GnRH antagonist would be between 25% and 33%. However, the OHSS rates were much higher after GnRH agonist. The evidence suggested that if the risk of OHSS following GnRH agonist is assumed to be 11%, the risk following GnRH antagonist would be between 6% and 9%.
Quality of the evidence
The evidence was of moderate quality for both live birth and OHSS. The main limitations of the evidence were the possibility of publication bias for live birth (with small studies likely to report favourable outcomes for GnRH antagonist) and poor reporting of study methods for OHSS.
Summary of findings
Summary of findings for the main comparison. GnRH antagonist compared to long‐course GnRH agonist for assisted reproductive technology (ART).
| GnRH antagonist compared to long‐course GnRH agonist for assisted reproductive technology (ART) | ||||||
| Population: women undergoing assisted reproductive technology (ART) Settings: clinic for ART Intervention: GnRH antagonist Comparison: long‐course GnRH agonist | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long course GnRH agonist | GnRH antagonist | |||||
| Live birth rate per woman randomised | 286 per 1000 | 290 per 1000 (254 to 330) | OR 1.02 (0.85 to 1.23) | 2303 (12 studies) | ⊕⊕⊕⊝ moderate1 | |
| OHSS per woman randomised (any grade) | 114 per 1000 | 73 per 1000 (62 to 85) | OR 0.61 (0.51 to 0.72) | 7944 (36 studies) | ⊕⊕⊕⊝ moderate2 | |
| Ongoing pregnancy rate per woman randomised | 293 per 1000 | 276 per 1000 (256 to 295) | OR 0.92 (0.83 to 1.01) | 8311 (37 studies) | ⊕⊕⊕⊝ moderate2 | |
| Clinical pregnancy rate per woman randomised | 303 per 1000 | 283 per 1000 (267 to 303) | OR 0.91 (0.83 to 1) | 9959 (54 studies) | ⊕⊕⊕⊝ moderate2 | |
| Miscarriage rate per woman randomised | 48 per 1000 | 49 per 1000 (40 to 61) | OR 1.03 (0.82 to 1.29) | 7082 (34 studies) | ⊕⊕⊕⊝ moderate2 | |
| Cycle cancellation due to poor ovarian response | 64 per 1000 | 83 per 1000 (68 to 101) | OR 1.32 (1.06 to 1.65) | 5230 (25 studies) | ⊕⊝⊝⊝ moderate3,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Asymmetry of the funnel plot with small study effects in favour of GnRH antagonist 2 Most domains of the risk of bias were assessed as either 'unclear' or 'high' 3 Presence of significant heterogeneity among studies with inconsistency in the directions of effect estimates 4 Effect estimate with wide confidence interval
Background
Description of the condition
Controlled ovarian hyperstimulation (COH) coupled with in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) was one of the major advances in the treatment of subfertility in the second half of the 20th century. One aspect of COH‐IVF or ICSI that requires attention is the occurrence of a luteinizing hormone (LH) surge which may occur prematurely, before the leading follicle reaches the optimum diameter for triggering ovulation. Such premature LH surges prevent effective induction of multiple follicular maturation patterns for a significant number of women.
Gonadotrophin‐releasing hormone agonists (GnRH agonists) have played an important role in reducing the incidence of premature LH surges by reversibly blocking pituitary gonadotrophin secretion. As a result, the rates of cancellation of assisted conception cycles are decreased and pregnancy rates increased (Albano 1996; Hughes 1992). However, the use of GnRH agonists is not without disadvantages. Even though the standard long‐course GnRH agonist protocol proved to be the most efficacious protocol (Daya 2000) for the use of GnRH agonists, it requires two to three weeks for desensitisation, with relatively high costs due to an increased requirement for gonadotrophin injections, and the need for hormonal and ultrasonographic measurements (Olivennes 1994).
A common complication associated with ovarian stimulation with exogenous gonadotrophins is ovarian hyperstimulation syndrome (OHSS) (Mathur 2007). It usually occurs following a LH surge or after exposure to human chorionic gonadotrophin (hCG) (Mozes 1965). Most cases of OHSS are mild with few or no clinical consequences. However, severe cases occur occasionally with serious morbidity and mortalities (Delvigne 2002). Gonadotrophin‐releasing hormone analogues (GnRH agonists and antagonists) stabilise the luteal phase thereby preventing premature LH surges and reducing the risk of OHSS.
Description of the intervention
In 1999, gonadotrophin‐releasing hormone antagonists (GnRH antagonist) were introduced to the market to prevent LH surge, and it was assumed that GnRH antagonists might be a more patient‐friendly protocol than the mid‐luteal GnRH agonist protocol. GnRH antagonists cause immediate, reversible and dose‐related inhibition of gonadotrophin release by competitive blockade of the GnRH receptors in the pituitary gland, and therefore treatment can be restricted to those days when a premature LH surge is likely to occur (Duijkers 1998; Felberbaum 1995; Huirne 2007).
The first generation of GnRH antagonists were associated with allergic side‐effects due to an induced histamine release, which hampered the clinical development of these compounds. Third generation GnRH antagonists such as ganirelix (NV Organon, Oss, the Netherlands) and cetrorelix (ASTA‐Medica, Frankfurt am Main, Germany) have resolved these issues and are approved for clinical use (Olivennes 1998).
Two approaches have emerged in GnRH antagonist administration; the single‐dose protocol, in which one injection of GnRH antagonist (Cetrotide® 3 mg, Merck SeronoSA., Geneva, Switzerland ) is administered in the late phase of ovarian stimulation and the multiple‐dose regimen, in which 0.25 µg of cetrorelix or ganirelix is administered daily from stimulation day 6 onwards (fixed regimen). A flexible regimen based on the follicular size, has since been introduced to minimise the number and duration of GnRH antagonist injections (Huirne 2007).
In a natural ovulatory cycle, ovulation, the release of the dominant follicle from the ovary, usually occurs about 36 hours after LH surge. In women undergoing controlled ovarian stimulation (COS) during assisted reproductive technology (ART), certain agents are usually administered to mimic the natural LH surge. Ultrasound scan and blood oestrogen levels are used to determine the day on which to administer the triggering agents. Ovulation triggering agents include hCG and GnRH agonist. These agents have different modes of action and their use might, therefore, differentially influence the effectiveness of GnRH antagonists.
How the intervention might work
Applying GnRH antagonists for pituitary desensitisation during COH is expected to result in a dramatic reduction in the duration of GnRH analogue treatment and to reduce the amount of gonadotrophin needed for stimulation as compared with the long agonist protocol. Other potential benefits include a lower risk of developing severe ovarian hyperstimulation syndrome (OHSS) and avoidance of oestrogen deprivation symptoms (for example hot flushes, sleep disturbances, headaches) frequently observed in the pre‐stimulation phase of a long agonist protocol. Whether the previously mentioned benefits justify a change in routine treatment from the standard long‐course GnRH agonist protocol to the GnRH antagonist regimen depends on whether the clinical outcomes using these protocols are similar.
Why it is important to do this review
The first Cochrane review on this topic was published in 2001 and was updated in 2006 and 2011. As further RCTs have been published, this is a further update of the evidence on the comparative effectiveness of GnRH antagonists versus GnRH agonists in women undergoing COH‐IVF or ICSI, with respect to reducing the risk of OHSS and cycle cancellation while maintaining the live birth rate.
Objectives
To evaluate the effectiveness and safety of gonadotrophin‐releasing hormone (GnRH) antagonists compared with the standard long protocol of GnRH agonists for controlled ovarian hyperstimulation in assisted conception cycles.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCTs) with a parallel design were eligible for inclusion. Quasi‐randomised trials were not included (e.g. studies with evidence of inadequate sequence generation such as alternate days, patient numbers) as they are associated with a high risk of bias. If cross‐over studies, with cross‐over occurring between cycles, were available, we would have included only the first cycle, before the cross‐over.
Types of participants
Subfertile couples undergoing controlled ovarian hyperstimulation (COH) as part of an IVF or ICSI programme using GnRH antagonists or long‐course GnRH agonist protocols for the prevention of premature LH surges.
Types of interventions
Pituitary suppression with GnRH antagonists (for example cetrorelix, ganirelix) or long‐course GnRH agonists together with ovarian stimulation with recombinant or urinary human follicle stimulating hormone (hFSH) or human menopausal gonadotrophin (hMG), or both, or clomiphene citrate as part of an IVF or ICSI treatment cycles. Further, the use of oral contraceptive pill (OCP) pre‐treatment did not constitute an inclusion or exclusion criterion but rather was a variation in the protocols used.
Types of outcome measures
Primary outcomes
Live birth rate (LBR) per woman randomised, defined as delivery of a live fetus after 20 completed weeks of gestation.
Ovarian hyperstimulation syndrome (OHSS) rate per woman randomised, with grading as detected by clinical grading of OHSS, laboratory investigations (e.g. haematocrit, haemoglobin, renal function) or imaging techniques (ovarian and abdominal ultrasound, chest X‐ray), or both: all women, moderate or severe OHSS.
Secondary outcomes
Ongoing pregnancy rate (OPR) per woman randomised, defined as a pregnancy beyond 12 weeks' gestation.
Clinical pregnancy rate (CPR) per woman randomised, defined as the presence of a gestational sac ± fetal heart beat at transvaginal ultrasound.
-
Other adverse effects.
Miscarriage rate per woman randomised: miscarriage is defined as pregnancy loss before 20 weeks' gestation. Miscarriage rate per clinical pregnancy was analysed as a secondary analysis).
Cycle cancellation rate per woman randomised. Two types of cycle cancellation were assessed in separate analyses: cycle cancellation due to high risk of OHSS and cycle cancellation due to poor ovarian response.
Search methods for identification of studies
We searched for all published and unpublished RCTs of GnRH antagonist versus the long‐course GnRH agonist protocol in women undergoing COH‐IVF or ICSI using the following search strategy, without language restriction and in consultation with the Gynaecology and Fertility Group (CGF) (formerly known as Menstrual Disorders and Subfertility Group (MDSG)) Information Specialist. We performed the most recent searches on 28 April 2015.
Electronic searches
The following electronic databases, trial registers and websites were searched (from their inception).
Menstrual Disorders and Subfertility Group (MDSG) Specialised Register (updated search from 2010 to 28 April 2015) (Appendix 1).
Ovid Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library; Issue 4 2015) (updated search from 2010 to 28 April 2015) (Appendix 2).
Ovid MEDLINE (updated search from 2010 to 28 April 2015) (Appendix 3). The MEDLINE search was based on the Cochrane Highly Sensitive Search Strategy (HSSS) for identifying randomised trials in MEDLINE: sensitivity‐maximising version (Lefebvre 2011).
Ovid EMBASE (updated search 2010 up to 28 April 2015) (Appendix 4). The EMBASE search was combined with the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN) (http://sign.ac.uk/methodology/filters.html).
Ovid PsycINFO (updated search from 2010 to 28 April 2015) (Appendix 5). The PsycINFO search was combined with the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN) (http://sign.ac.uk/methodology/filters.html).
EBSCO CINAHL (Cumulative Index to Nursing and Allied Health Literature) (Appendix 6).
Trial registers for ongoing and registered trials: ISRCTN (http://www.isrctn.com/), ClinicalTrials.gov (https://clinicaltrials.gov/), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (www.who.int/trialsearch/Default.aspx).
DARE (Database of abstracts of reviews of effectiveness) in The Cochrane Library at http://onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html (for reference lists from relevant non‐Cochrane reviews
LILACS database http://lilacs.bvsalud.org/en/ (for trials from the Portuguese and Spanish speaking world)
Citation indexes on the ISI Web of Science (http://ipscience.thomsonreuters.com/product/web‐of‐science/).
LILACS (Latin American and Caribbean Health Sciences) (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F).
PubMed (www.ncbi.nlm.nih.gov/pubmed/). The PubMed search was combined with the random control filter for PubMed.
OpenGrey ‐ http://www.opengrey.eu/ for unpublished literature from Europe.
Searching other resources
We also searched the reference lists of all known primary studies, review articles, citation lists of relevant publications, abstracts of major scientific meetings (for example of the European Society of Human Reproduction and Embryology (ESHRE) and American Society for Reproductive Medicine (ASRM)). We contacted known experts and personal contacts regarding any unpublished materials.
In addition, we handsearched appropriate journals. The list of journals is in the CGF Module, which can be found in The Cochrane Library under BROWSE ‐ 'By Review Group' ‐ 'Cochrane Gynaecology and Fertility Group' ‐ then 'about this group' at the top of this page. We liaised with the CGF Information Specialist to avoid duplication of handsearching.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, we retrieved the full texts of all potentially eligible studies. Two review authors (RA and JB) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We contacted study investigators as required, to clarify study eligibility. We resolved disagreements as to study eligibility by discussion or by involving a third review author (MAY). We documented the selection process with a “PRISMA” flow chart (Figure 1) (Moher 2009)
1.
Study flow diagram.
Data extraction and management
We developed and piloted a standardised data extraction form for consistency and completeness. Three review authors (RA, JB or WSL) independently performed data extraction with discrepancies resolved by discussion. The data extraction forms included study demographics, patient characteristics and study risk of bias. We included this information in the review and presented it in the tables 'Characteristics of included studies' and 'Characteristics of excluded studies' according to the guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Where studies had multiple publications the review authors collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review, and such studies would have a single study ID with multiple references. We contacted trial authors to request additional information or data. We also received a response from the sponsoring pharmaceutical companies.
Assessment of risk of bias in included studies
Three authors (RA, JB and WSL) independently assessed the risk of bias of the included trials using The Cochrane 'Risk of bias' (RoB) tool (Higgins 2011b). The domains assessed were: (1) sequence generation (for example; was the method used for allocation sequence adequately described?); (2) allocation concealment (for example, was allocation adequately concealed?); (3) blinding of participants, personnel and outcome assessors (for example; was knowledge of the allocated intervention adequately prevented during the study?); (4) incomplete outcome data (for example, were incomplete outcome data adequately addressed?); (5) selective outcome reporting (for example, were reports of the study free of suggestion of selective outcome reporting?); and (6) other sources of bias (for example, was the study apparently free of other problems that could put it at a high risk of bias?). Other potential sources of bias included baseline imbalances, source of funding, early stopping for benefit, and appropriateness of cross‐over design. We resolved disagreements by discussion or by consulting a fourth review author. We described all judgements fully and presented the conclusions in the 'Risk of bias' table (see the Characteristics of included studies table), which was incorporated into the interpretation of review findings by means of sensitivity analyses (see below).
With respect to selective reporting, we sought published protocols and compared the outcomes between the protocol and the final published study, but the searches did not yield published protocols of any of the included studies. We took care to search for within‐trial selective reporting, such as non‐reporting of obvious outcomes, or reporting them in insufficient detail to allow for inclusion. Where identified studies failed to report the primary outcomes of live birth and OHSS but did report interim outcomes such as pregnancy, we undertook informal assessment as to whether the interim values (e.g. pregnancy rates) were similar to those reported in studies that also reported live birth.
Measures of treatment effect
We only reported dichotomous data (e.g. live birth rates) in this review and we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs). We reversed the direction of effect of individual studies, if required, to ensure consistency across trials. We presented 95% confidence intervals (CIs) for all outcomes. Where data to calculate ORs were not available, we utilised the most detailed numerical data available that facilitated similar analyses of included studies (e.g. test statistics, P values). We compared the magnitude and direction of effect reported by studies with how they were presented in the review, taking account of legitimate differences (Deeks 2011).
Unit of analysis issues
The primary analysis was per woman randomised. We also included per clinical pregnancy data for miscarriage. we contacted authors of studies that did not allow valid analysis of data (e.g. 'per cycle' data) and requested ‘per woman’ data. If no ‘per woman’ data was provided after contact, we did not include such studies in meta‐analyses.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible and made attempts to obtain missing data from the original trialists. Initially, we planned to undertake imputation of individual values for the primary outcomes if we were unable to obtain missing data from the original trialists but no data imputation was undertaken in the end and we analysed only the available data.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I2 statistic (Higgins 2003). We took an I2 statistic measurement greater than 50% to indicate substantial heterogeneity (Deeks 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the review authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. We used a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies) (Egger 1997).
Data synthesis
Where the studies were sufficiently similar, we combined the data using a fixed‐effect analysis (on the assumption that the underlying effect size was the same for all the trials in the analysis) comparing GnRH antagonist versus long course GnRH agonist.
An increase in the odds of a particular outcome that were likely to be beneficial (e.g. live birth) or detrimental (e.g. adverse effects), were displayed graphically in the meta‐analyses to the right of the centre‐line and a decrease in the odds of an outcome to the left of the centre‐line.
All analyses were performed using Review Manager software (RevMan) (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
Where there were sufficient data we performed subgroup analyses for the following variables, for live birth and pregnancy outcomes.
Triggering agent used for oocyte maturation (hCG, GnRH agonist, mixed (hCG/GnRH agonist) or unknown agent)
Minimal or standard level of stimulation
Where we detected substantial heterogeneity, we explored possible explanations in sensitivity analyses. We took any statistical heterogeneity into account when interpreting the results, especially where there was any variation in the direction of effect.
Sensitivity analysis
We conducted sensitivity analyses for LBR and OPR to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis (Moher 1999). These analyses included consideration of whether the review conclusions would have differed if:
a random‐effects model had been adopted;
the summary effect measure was risk ratio rather than odds ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro Guideline Development Tool (GRADEpro GDT 2015). This table evaluated the overall quality of the body of evidence for all review outcomes (live birth, OHSS, ongoing pregnancy, clinical pregnancy, miscarriage and cycle cancellation), using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). Judgements about evidence quality (high, unclear (moderate) or low) were justified, documented, and incorporated into reporting of results for each outcome (Table 1).
Results
Description of studies
See the table 'Characteristics of included studies'.
Results of the search
We retrieved 479 records after removal of duplicates, excluded 399 as ineligible, and assessed 80 full‐text articles. Of these, we excluded 51 and included 28 studies (29 reports). Seventy‐three randomised controlled studies (84 reports), involving 12,212 randomised women, met the inclusion criteria and were fully reviewed (Characteristics of included studies) (See Figure 1 for details of this process).
Included studies
Study characteristics
Twelve studies were multi‐centre (Albano 2000; Baart 2007; Barmat 2005; Euro Middle East 2001; Euro Orgalutran 2000; Fluker 2001; Heijnen 2007; Huirne 2006; Olivennes 2000; Qiao 2012; Rombauts 2006; Sauer 2004), 43 studies were single‐centre trials, while in the remaining studies it was unclear whether they were multi‐centre or single centre.
We considered sample size calculations to be appropriate when the authors of the studies pre‐calculated the number needed in each arm prior to starting the trial. This helps to prevent the occurrence of type II errors. Fifteen studies reported that they had performed a priori sample size calculations (Hwang 2004; Baart 2007; Cota 2012; Engmann 2008a; Heijnen 2007; Huirne 2006; Kim 2011; Kurzawa 2008; Lainas 2010; Sbracia 2009; Sunkara 2014; Tehraninejad 2010; Lin 2006; Depalo 2009; Tazegul 2008); 22 studies reported that they had not performed a sample size calculation; and it was not clear if sample size calculations had been performed in the remaining 36 studies.
Twenty‐three studies said that they had performed intention‐to‐treat analysis (Badrawi 2005; Choi 2012; Cota 2012; Depalo 2009; Engmann 2008a; Euro Middle East 2001; Euro Orgalutran 2000; Fluker 2001; Heijnen 2007; Hosseini 2010; Huirne 2006; Hwang 2004; Khalaf 2010; Kim 2004; Kim 2011; Lin 2006; Loutradis 2004; Marci 2005; Rombauts 2006; Sauer 2004; Serafini 2008; Sunkara 2014; Xavier 2005); 16 studies reported that the original analyses did not use the intention‐to‐treat principle (Albano 2000; Baart 2007; Bahceci 2005; Cheung 2005; El Sahwi 2005; Firouzabadi 2010; Hohmann 2003; Inza 2004; Kurzawa 2008; Kyono 2005; Lee 2005; Olivennes 2000; Sbracia 2009; Tazegul 2008; Tehraninejad 2010; Ye 2009); it was not reported clearly in the rest of the studies.
Participants
Seventy out of 73 studies reported that baseline characteristics were comparable between groups (Characteristics of included studies table) and three did not report information on this. In eighteen of the studies, age was the only reported characteristic compared.
Of the 73 included studies, 49 trials involved an unspecified population of infertile couples, while the remaining trials were performed in specific infertile populations. These populations were or included 'poor responders' (Al‐Karaki 2011; Cheung 2005; Inza 2004; Kim 2011; Kim 2012; Marci 2005; Mohamed 2006; Prapas 2013; Revelli 2014; Sbracia 2009; Sunkara 2014; Tazegul 2008; Toltager 2015) or had polycystic ovary syndrome (Bahceci 2005; Choi 2012; Engmann 2008a; Haydardedeoglu 2012; Hosseini 2010; Hwang 2004; Kim 2004; Kurzawa 2008; Lainas 2007; Lainas 2010; Moshin 2007; Tehraninejad 2010).
The number of randomised women ranged from 20 (Franco 2003) to 1099 (Toltager 2015), including both the GnRH agonist and antagonist groups.
Fifteen studies included 300 or more participants (Awata 2010; Euro Middle East 2001; Euro Orgalutran 2000; Tehraninejad 2011; Gizzo 2014; Haydardedeoglu 2012; Heijnen 2007; Martinez 2008; Prapas 2013; Revelli 2014; Rinaldi 2014; Rombauts 2006; Sbracia 2009; Toltager 2015). There were 30 studies with fewer than 100 participants (Anderson 2014; Barmat 2005; Celik 2011; Check 2004; Cheung 2005; Choi 2012; Cota 2012; Engmann 2008a; Ferrari 2006; Franco 2003; Friedler 2003; Hershko Klement 2015; Hoseini 2014; Hwang 2004; Inza 2004; Khalaf 2010; Kim 2004; Kurzawa 2008; Lainas 2007; Lavorato 2012; Lee 2005; Marci 2005; Mohamed 2006; Moraloglu 2008; Moshin 2007; Sauer 2004; Serafini 2008; Stenbaek 2015Tazegul 2008; Tehraninejad 2010).
Five studies were published before 2002. There were 28 studies published between 2002 and 2006, 18 studies published between 2007 and 2010 and 23 studies between 2011 and 2015
Intervention
All included studies compared GnRH antagonist with long‐course GnRH agonist protocols in women undergoing IVF or ICSI cycles.
We identified three types of antagonist protocols: (1) single, long‐acting administration (Hsieh 2008; Lee 2005; Moshin 2007; Olivennes 2000); (2) fixed, daily administration (Albano 2000; Cheung 2005; Euro Middle East 2001; Euro Orgalutran 2000; Firouzabadi 2010; Fluker 2001; Haydardedeoglu 2012; Hoseini 2014; Hsieh 2008; Huirne 2006; Hwang 2004; Martinez 2008; Moshin 2007; Sauer 2004); and (3) flexible daily administration (Baart 2007; Badrawi 2005; Bahceci 2005; Barmat 2005; Brelik 2004; Check 2004; Choi 2012; Depalo 2009; El Sahwi 2005; Engmann 2008a; Franco 2003; Hershko Klement 2015; Hohmann 2003; Karimzadeh 2010; Kim 2004; Kim 2011; Kurzawa 2008; Lainas 2007; Lainas 2010; Lee 2005; Lin 2006; Loutradis 2004; Marci 2005; Moraloglu 2008; Rombauts 2006; Sbracia 2009; Serafini 2008; Tazegul 2008; Tehraninejad 2010; Xavier 2005; Ye 2009). In the fixed daily protocol, in most of the studies, GnRH antagonist was begun on day six of FSH treatment regardless of follicle size. In the flexible daily protocol, GnRH antagonist was administered according to the lead follicle size and not the cycle date, nor the day of FSH administration. In 25 of the included studies, the type of antagonist protocol used was not reported.
In 44 included trials, the antagonist cetrorelix was administered (Albano 2000; Al‐Karaki 2011; Bahceci 2005; Brelik 2004; Cheung 2005; Cota 2012; Depalo 2009; El Sahwi 2005; Ferrari 2006; Ferrero 2010; Hershko Klement 2015; Hohmann 2003; Hoseini 2014; Hosseini 2010; Hsieh 2008; Huirne 2006; Hwang 2004; Khalaf 2010; Kim 2004; Kim 2011; Kim 2012; Kurzawa 2008; Kyono 2005; Lainas 2010; Lavorato 2012; Lee 2005; Lin 2006; Loutradis 2004; Marci 2005; Mohamed 2006; Moraloglu 2008; Moshin 2007; Olivennes 2000; Rabati 2012; Revelli 2014; Rinaldi 2014; Sauer 2004; Sbracia 2009; Serafini 2008; Sunkara 2014; Tehraninejad 2010; Tehraninejad 2011; Xavier 2005; Ye 2009). In 19 trials, the antagonist ganirelix was administered (Baart 2007; Badrawi 2005; Barmat 2005; Check 2004; Engmann 2008a; Euro Middle East 2001; Euro Orgalutran 2000; Firouzabadi 2010; Fluker 2001; Franco 2003; Gizzo 2014; Haydardedeoglu 2012; Karimzadeh 2010; Lainas 2007; Martinez 2008; Prapas 2013; Qiao 2012; Rombauts 2006; Stenbaek 2015). Three trials used both cetrorelix and ganirelix (Choi 2012; Papanikolaou 2012; Tazegul 2008) and in seven included trials the type of antagonist used was unclear (Anderson 2014; Awata 2010; Celik 2011; Friedler 2003; Heijnen 2007; Inza 2004; Toltager 2015).
Oral contraceptive pill pre‐treatment was used in 18 studies (Barmat 2005; Cheung 2005; Engmann 2008a; Haydardedeoglu 2012; Hershko Klement 2015; Hosseini 2010; Huirne 2006; Kim 2004; Kim 2011; Kim 2012; Kurzawa 2008; Kyono 2005; Lainas 2007; Lainas 2010; Moraloglu 2008; Rombauts 2006; Sauer 2004; Tehraninejad 2010). Further single trials used Diane (Hwang 2004), estradiol in the luteal phase (Franco 2003), and vaginal Nuvaring (Martinez 2008).
Women randomised to treatment with GnRH antagonist started ovarian stimulation on day two to three of the menstrual cycle. The GnRH antagonist was started on stimulation day six, by daily subcutaneous administration up to and including the day of human chorionic gonadotrophin (hCG) administration in the fixed protocol or depending on the dominant follicle size in the flexible protocol.The GnRH long agonist reference treatment was started in the mid‐luteal phase (cycle day 21 to 24) by either daily intranasal or subcutaneous administration.
Ovarian stimulation was started after two weeks if pituitary down‐regulation was established (serum estradiol level < 50 pg/ml). In both treatment groups, ovarian stimulation was started with a fixed daily dose of 150 IU or 225 IU recombinant follicle stimulating hormone (rFSH) or human menopausal gonadotrophin (hMG) for the first five stimulation days. Thereafter, the dose of FSH was adapted depending on the ovarian response, as monitored via ultrasonography (US). Triggering of ovulation was induced with hCG (10,000 IU) if at least three follicles that were more than 17 mm in diameter were observed by US.
Trigger used: the majority of the included studies used hCG trigger or did not state which trigger was used; one of the included studies used a combination of both hCG and GnRH agonist as trigger agents (Engmann 2008a).
Outcomes
Study participant follow up: the optimum follow up would be to report on the number of single, healthy babies going home with their parents (for example single, live, take‐home baby rate). If unavailable, other follow ups were assessed including the live birth rate (LBR) and ongoing pregnancy rate (OPR). None of the included trials described the single, live, take‐home baby rate or the take‐home baby rate. Twelve studies reported on the LBR (Albano 2000; Baart 2007; Barmat 2005; Heijnen 2007; Kim 2011; Kim 2012; Kurzawa 2008; Lin 2006; Marci 2005; Papanikolaou 2012; Rinaldi 2014; Ye 2009). Further, 36 trials reported the OPR and 55 studies reported the clinical pregnancy rate (CPR).
Thirty‐six studies reported OHSS incidence (Albano 2000; Badrawi 2005; Bahceci 2005; Barmat 2005; Engmann 2008a; Euro Middle East 2001; Euro Orgalutran 2000; Firouzabadi 2010; Fluker 2001; Haydardedeoglu 2012; Heijnen 2007; Hohmann 2003; Hosseini 2010; Hsieh 2008; Huirne 2006; Hwang 2004; Karimzadeh 2010; Kim 2012; Kurzawa 2008; Kyono 2005; Lainas 2007; Lainas 2010; Lee 2005; Lin 2006; Moraloglu 2008; Moshin 2007; Olivennes 2000; Papanikolaou 2012; Qiao 2012; Rabati 2012; Rombauts 2006; Serafini 2008; Tehraninejad 2010; Toltager 2015; Xavier 2005; Ye 2009).
Ten studies did not present data in a form that could be included in meta‐analysis (Anderson 2014; Awata 2010; Celik 2011; Choi 2012; Cota 2012; Ferrero 2010; Hoseini 2014; Khalaf 2010; Lavorato 2012; Stenbaek 2015).
Excluded studies
Forty seven studies were excluded for various reasons (see table Characteristics of excluded studies)
Risk of bias in included studies
For the risk of bias (ROB) of the included trials, please see Figure 2 and Figure 3.
2.
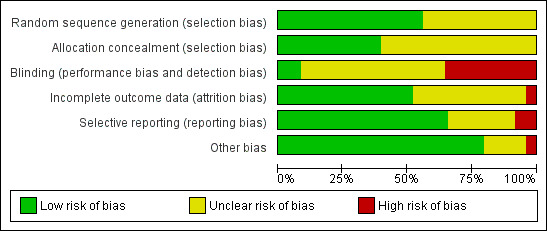
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation was done at the time of recruitment of participants.
All trials had a parallel design and proper randomisation was carried out by 39 studies by using: interactive voice response systems (Albano 2000; Euro Middle East 2001; Euro Orgalutran 2000; Rombauts 2006); stratified randomisation (Fluker 2001); computer‐generated random number tables with or without sealed envelopes for allocation concealment (Baart 2007; Badrawi 2005; Barmat 2005; Cota 2012; Depalo 2009; Engmann 2008a; Ferrari 2006; Firouzabadi 2010; Franco 2003; Tehraninejad 2011; Heijnen 2007; Hohmann 2003; Huirne 2006; Hwang 2004; Karimzadeh 2010; Kim 2011; Kim 2012; Kurzawa 2008; Lainas 2007; Lainas 2010; Lavorato 2012; Loutradis 2004; Martinez 2008; Moraloglu 2008; Papanikolaou 2012; Rinaldi 2014; Sauer 2004; Sbracia 2009; Tazegul 2008; Tehraninejad 2010; Ye 2009; Xavier 2005); or random number table (Bahceci 2005; Cheung 2005; Haydardedeoglu 2012 ).
Allocation concealment was properly performed by a nurse (Cota 2012; Lainas 2007; Papanikolaou 2012), by an interactive telephone system (Martinez 2008) or by a sealed opaque envelope (Haydardedeoglu 2012; Hershko Klement 2015; Prapas 2013; Revelli 2014; Rinaldi 2014).
The remaining trials did not report the methods of sequence generation or allocation concealment, or both.
Blinding
We examined blinding with regard to who was blinded in the trials. We looked for all levels of blinding and categorised them as follows: (i) double blind (neither the investigator nor the participants knew the allocation); (ii) single blind (only the investigator knew the allocation); (iii) no blinding (both the investigator and the participants knew the allocated treatment); (iv) unclear.
Since it was impossible to administer the different medications (that is long agonist and antagonist) according to one standard protocol without the use of a double dummy, almost all the studies were open‐label (that is no blinding). One study (Cheung 2005) blinded the clinicians and embryologists from the treatment allocation by using a nurse practitioner to administer the medications. The embryologist scoring the embryos, or the researcher, was blinded to the study groups in five trials (Baart 2007; Depalo 2009; El Sahwi 2005; Hwang 2004; Martinez 2008).
Twenty‐seven trials reported no blinding and we assessed them as being at high risk of bias (Albano 2000; Badrawi 2005; Bahceci 2005; Barmat 2005; Check 2004; Engmann 2008a; Euro Middle East 2001; Euro Orgalutran 2000; Firouzabadi 2010; Fluker 2001; Franco 2003; Friedler 2003; Heijnen 2007; Hohmann 2003; Kurzawa 2008; Kyono 2005; Lainas 2007; Lainas 2010; Loutradis 2004; Marci 2005; Olivennes 2000; Rombauts 2006; Sauer 2004; Tazegul 2008; Tehraninejad 2010; Xavier 2005; Ye 2009). The remaining trials did not clearly report if blinding was performed and we therefore assessed them as being at unclear risk of bias.
However, some of the outcome measures such as live birth were objectively assessed and non‐blinding of study outcome assessors was not likely to have affected their measurement.
Incomplete outcome data
We judged thirty‐seven of the included studies as being at low risk of bias in this domain, because they reported that there were no losses to follow up, proportions of withdrawals and reasons for withdrawals were balanced in both treatment groups, or women were analysed on the basis of intention‐to‐treat, where all women randomised were included in the final analysis whether or not they completed treatment. We judged the remaining studies either as unclear (where studies reported insufficient information with regard to attrition) or high risk of bias (where proportions of and reasons for withdrawals were not balanced between the two treatment groups and not all participants were included in the final analysis).
Selective reporting
Although the protocols of the included studies were not available for assessment, we scrutinised the methods section for pre‐specified outcome measures. Most of the included studies were rated as low risk of bias in this domain as they pre‐specified the outcomes on which data were reported in the methods section. The remaining studies were judged to be either at unclear risk, where there was insufficient information to make conclusive judgements, or low risk, where it was clear that they engaged in selective outcome reporting.
Other potential sources of bias
We found no potential sources of within‐study bias in most of the included studies as the baseline characteristics were similar between the treatment groups and were, therefore, rated to be at low risk of bias. The remaining studies were rated either as unclear risk, where there was insufficient information to arrive at a judgement, or high risk where there was evidence of significant differences in demographic characteristics between the treatment groups.
Effects of interventions
See: Table 1
The included studies enrolled a total of 12,212 randomised participants, although the sample size varied across the trials. We performed the analyses on the number of women randomised and not on the number of participants treated.
GnRH antagonist versus long course GnRH agonist
Primary outcomes
1.1 Live birth rate per woman randomised
1.1. Analysis.
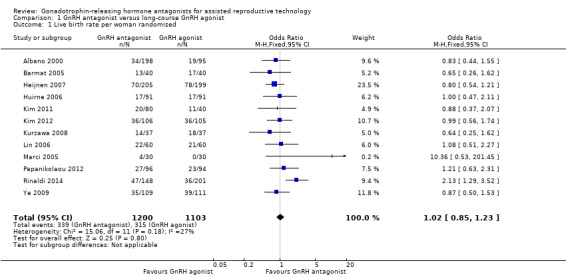
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 1 Live birth rate per woman randomised.
4.
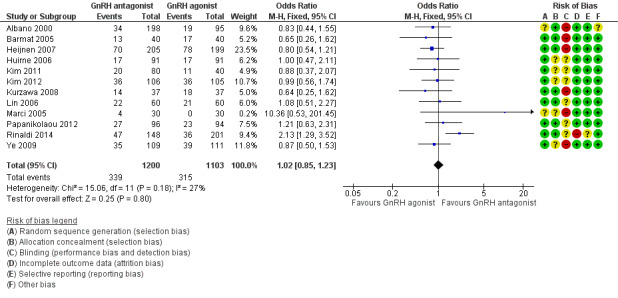
Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.1 Live birth rate per woman randomised.
Twelve trials reported live birth rates in 2303 women. There was no evidence of a difference following GnRH antagonist compared with GnRH agonist (OR 1.02, 95% CI 0.85 to 1.23; I2 = 27%, moderate quality evidence). The evidence suggested that if the chance of live birth following treatment with GnRH agonist is assumed to be 29%, the chance following treatment with GnRH antagonist would be between 25% and 33%. On sensitivity analysis, there was no change in the above conclusion using a random‐effects model (OR 1.01, 95% CI 0.80 to 1.27) or using risk ratio (RR) as a measure of effect estimate (RR 1.02, 95% CI 0.89 to 1.15). A funnel plot to explore the possibility of small study effect showed a tendency for estimates of the intervention effect to be more beneficial in smaller studies in the GnRH antagonist group (see Figure 5).
5.
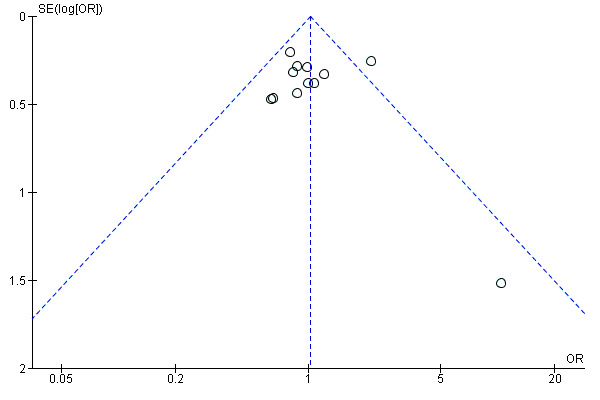
Funnel plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.1 Live birth rate per woman randomised.
1.2 Live birth rate per woman randomised ‐ Subgroup analysis: Minimal stimulation
1.2. Analysis.
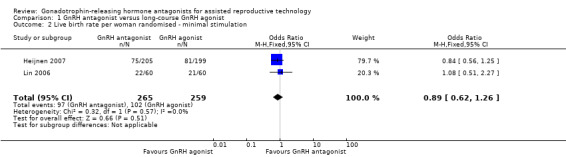
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 2 Live birth rate per woman randomised ‐ minimal stimulation.
6.
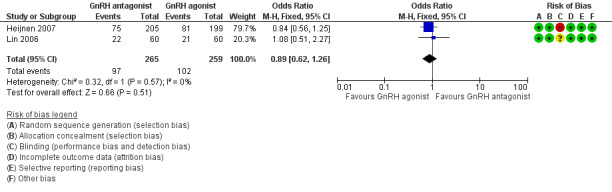
Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.2 Live birth rate per woman randomised ‐ minimal stimulation.
Two trials reported live birth rates in 524 women undergoing minimal stimulation IVF. There was no evidence of a difference following GnRH antagonist treatment compared with GnRH agonist treatment (OR 0.89, 95% CI 0.62 to 1.26; I2 = 0%).
1.3 Live birth rate per woman randomised ‐ Subgroup analysis: Grouped by trigger
1.3. Analysis.
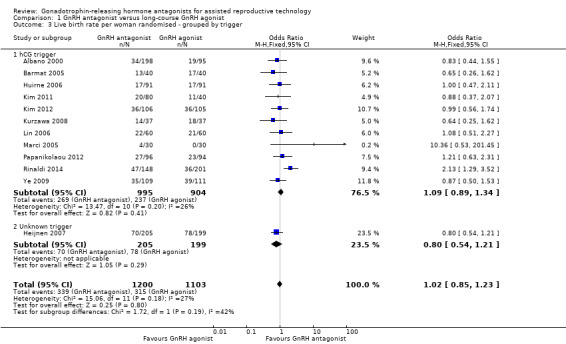
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 3 Live birth rate per woman randomised ‐ grouped by trigger.
7.
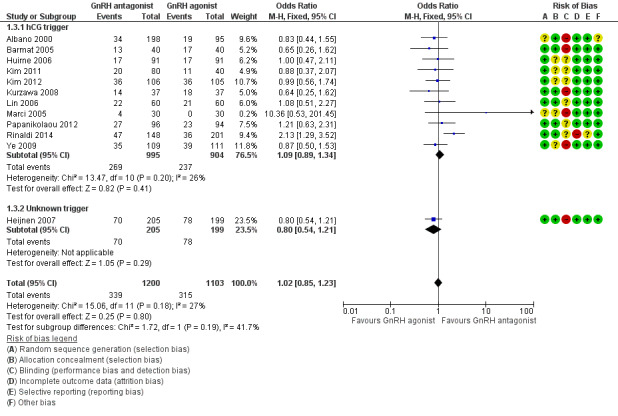
Forest plot of comparison: 1 GnRH antagonist versus long‐course GnRH agonist, outcome: 1.3 Live birth rate per woman randomised ‐ grouped by trigger.
1.3.1 hCG trigger
In a subgroup analysis, 11 trials reported live birth rates in 1899 women receiving hCG for ovarian maturation. There was no evidence of a difference following GnRH antagonist treatment compared with GnRH agonist treatment (OR 1.09, 95% CI 0.89 to 1.34; I2 = 26%).
1.3.2 Unknown trigger
One trial did not report the triggering agent used for ovarian maturation in 404 women. There was no evidence of a difference in live birth rate between GnRH antagonist and GnRH agonist treatment groups (OR 0.80, 95% CI 0.54 to 1.21).
1.4 Ovarian hyperstimulation per woman randomised
1.4. Analysis.
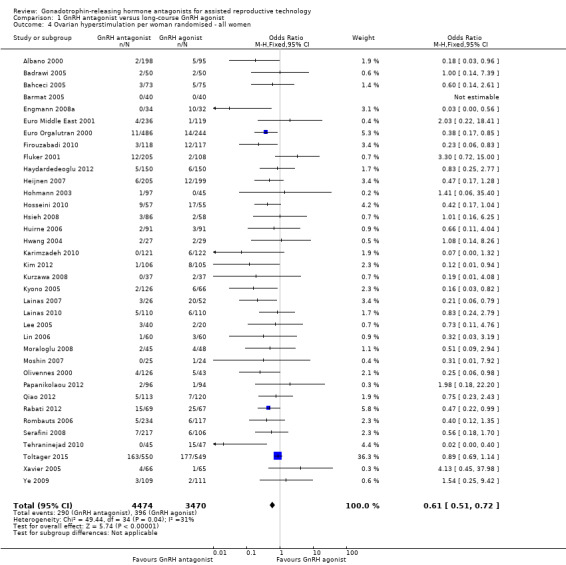
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 4 Ovarian hyperstimulation per woman randomised ‐ all women.
8.
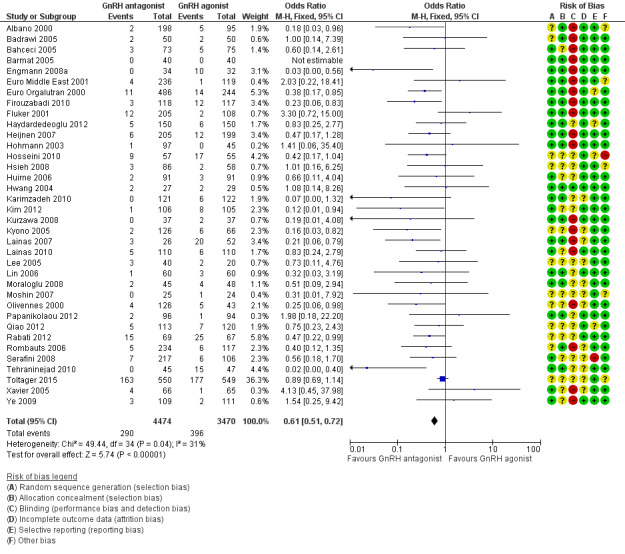
Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.4 Ovarian hyperstimulation per woman randomised ‐ all women.
Thirty‐six trials reported ovarian hyperstimulation rates in 7944 women. There was evidence of a lower OHSS rate in women who received GnRH antagonist compared with those were treated with GnRH agonist: 290/4474 (6%) versus 396/3470 (11%) (OR 0.61, 95% CI 0.51 to 0.72; I2 = 31%, moderate quality evidence). The evidence suggested that if the risk of OHSS following GnRH agonist is assumed to be 11%, the risk following GnRH antagonist would be between 6% and 9%.
1.5 Ovarian hyperstimulation per woman randomised ‐ Subgroup analysis: Moderate or severe OHSS
1.5. Analysis.
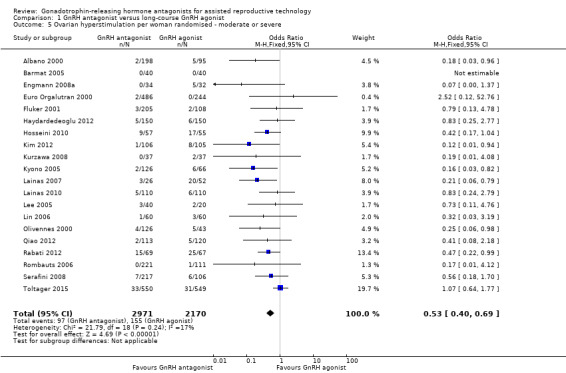
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe.
9.
Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe.
Twenty trials reported moderate or severe ovarian hyperstimulation rates in 5141 women. There was evidence of a lower rate of moderate or severe OHSS in GnRH antagonist compared with GnRH agonist groups: 97/2971 (3%) versus 155/2170 (7%) (OR 0.53, 95% CI 0.40 to 0.69; I2 = 17%).
Secondary outcomes
1.6 Ongoing pregnancy rate per woman randomised
1.6. Analysis.
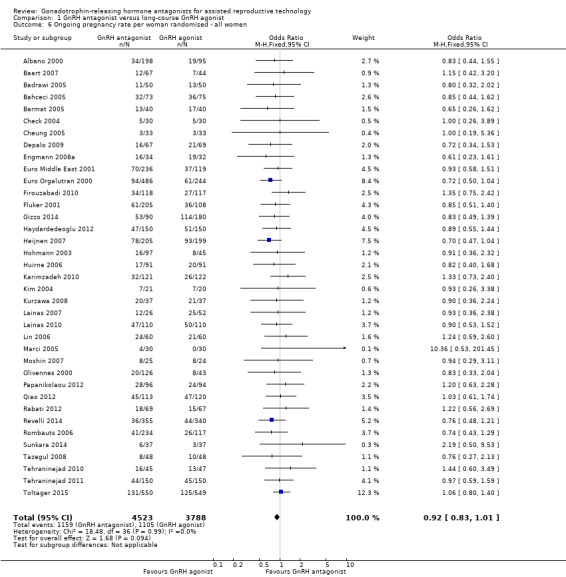
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 6 Ongoing pregnancy rate per woman randomised ‐ all women.
Thirty‐seven trials reported ongoing pregnancy rates in 8311 women. There was no evidence of a difference in ongoing pregnancy rate following treatment with GnRH antagonist compared with GnRH agonist (OR 0.92, 95% CI 0.83 to 1.01; I2 = 0%; moderate quality evidence). The evidence suggested that if the chance of ongoing pregnancy following GnRH agonist treatment is assumed to be 29%, the chance following GnRH antagonist treatment would be between 26% and 30%. There was no change in the conclusion on sensitivity analysis using either a random‐effects model (OR 0.91, 95% CI 0.83 to 1.01) or RR as a measure of treatment effect (RR 0.94, 95% CI 0.88 to 1.01).
1.7 Ongoing pregnancy rate per woman randomised ‐ Subgroup analysis: Minimal stimulation
1.7. Analysis.
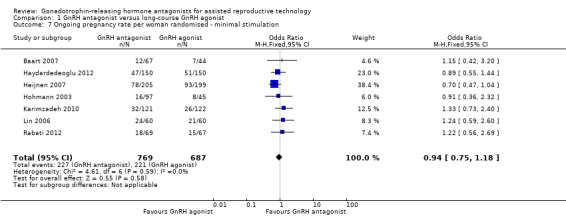
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 7 Ongoing pregnancy rate per woman randomised ‐ minimal stimulation.
Seven trials reported ongoing pregnancy rates in 1456 women undergoing minimal stimulation IVF. There was no evidence of a difference following GnRH antagonist treatment compared with GnRH agonist treatment (OR 0.94, 95% CI 0.75 to 1.18; I2 = 0%).
1.8 Ongoing pregnancy rate per woman randomised ‐ Subgroup analysis: Grouped by trigger
1.8. Analysis.
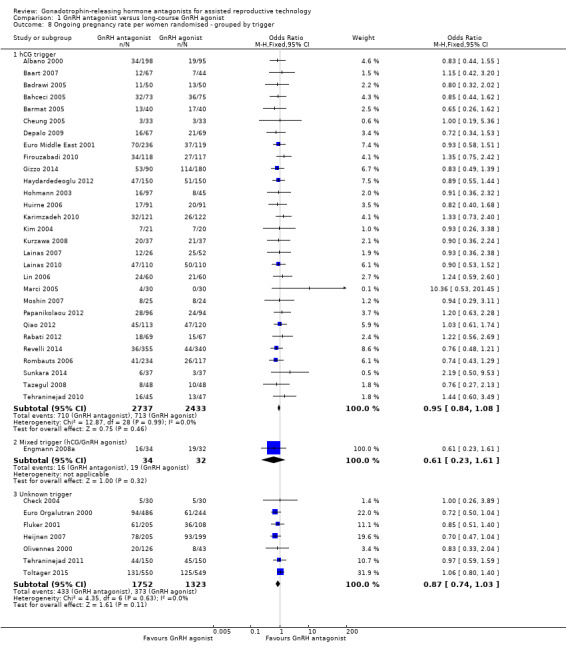
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 8 Ongoing pregnancy rate per women randomised ‐ grouped by trigger.
1.8.1 hCG trigger
In a subgroup analysis, 29 studies reported ongoing pregnancy rate in 5170 women in whom hCG was used to trigger oocyte maturation. There was no evidence of a difference in ongoing pregnancy rate between the two treatment groups (OR 0.95, 95% CI 0.84 to 1.08; I2 = 0%).
1.8.2 Mixed trigger
One study used hCG and GnRH agonist in GnRH antagonist and GnRH agonist groups respectively to trigger oocyte maturation in 66 women. There was no evidence of a difference in ongoing pregnancy rate between GnRH antagonist and GnRH agonist groups (OR 0.61, 95% CI 0.23 to 1.61).
1.8.3 Unknown trigger
In a subgroup analysis, seven trials reported ongoing pregnancy rates in 3075 women in whom the agent used in triggering oocyte maturation was unknown. There was no evidence of a difference following treatment with GnRH antagonist compared with GnRH agonist (OR 0.87, 95% CI 0.74 to 1.03; I2 = 0%).
1.9 Clinical pregnancy rate per woman randomised
1.9. Analysis.
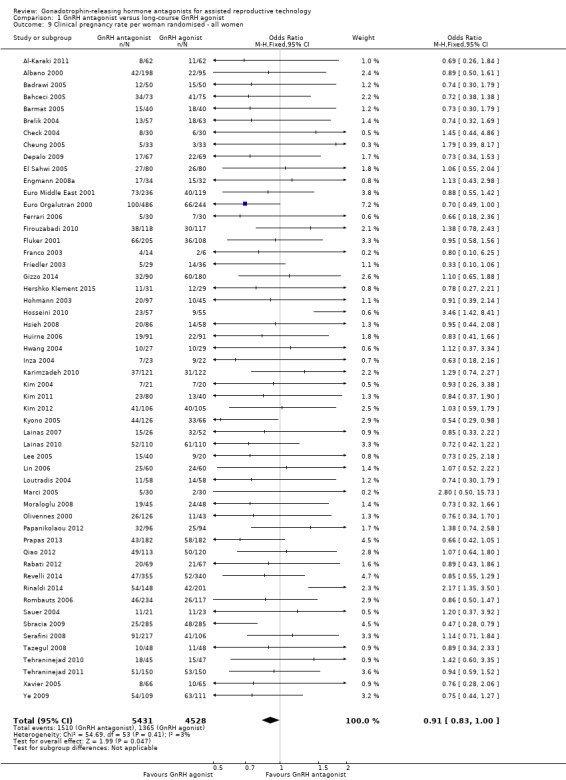
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 9 Clinical pregnancy rate per woman randomised ‐ all women.
Fifty‐four trials reported clinical pregnancy rates in 9959 women . There was evidence of a difference following GnRH antagonist treatment compared with GnRH agonist treatment with a smaller proportion of women reporting clinical pregnancies in the GnRH antagonist group: 1510/5431 (28%) versus 1365/4528 (30%) (OR 0.91, 95% CI 0.83 to 1.00; I2 = 1%, moderate quality evidence). The evidence suggested that if the chance of clinical pregnancy following GnRH agonist treatment is assumed to be 30%, the chance following GnRH antagonist treatment would be between 27% and 30%.
1.10 Clinical pregnancy rate per woman randomised ‐ Subgroup analysis: Minimal stimulation
1.10. Analysis.
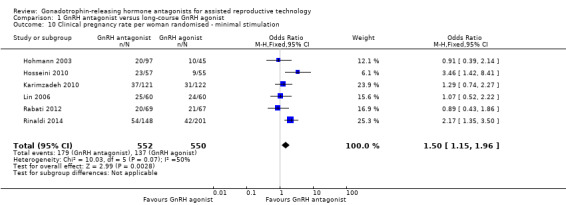
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 10 Clinical pregnancy rate per woman randomised ‐ minimal stimulation.
Six studies reported clinical pregnancy rate in 1102 women receiving minimal ovarian stimulation. There was evidence of a higher clinical pregnancy rate in the GnRH antagonist group compared to the GnRH agonist group: 179/552 (32%) versus 137/550 (25%) (OR 1.50, 95% CI 1.15 to 1.96; I2 = 50%).
1.11 Miscarriage rate per woman randomised
1.11. Analysis.
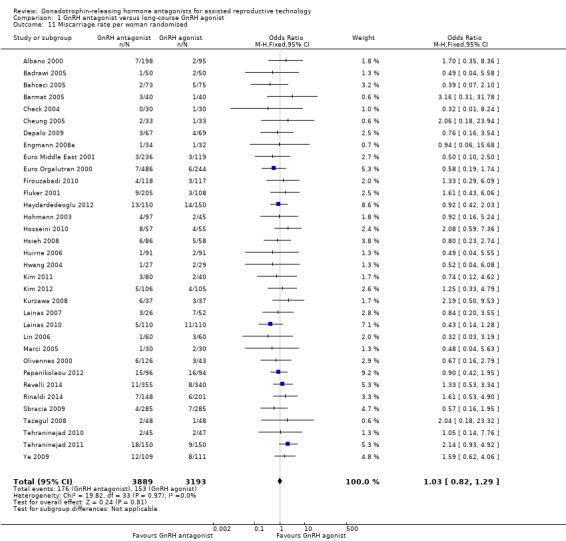
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 11 Miscarriage rate per woman randomised.
Thirty‐four trials reported miscarriage rates in 7082 women. There was no evidence of a difference following GnRH antagonist treatment compared with GnRH agonist treatment (OR 1.03, 95% CI 0.82 to 1.29; I2 = 0%; moderate quality evidence). The evidence suggested that if the risk of miscarriage following GnRH agonist treatment is assumed to be 5%, the risk following GnRH antagonist treatment would be between 4% and 6%.
1.12 Miscarriage rate per clinical pregnancy rate
1.12. Analysis.
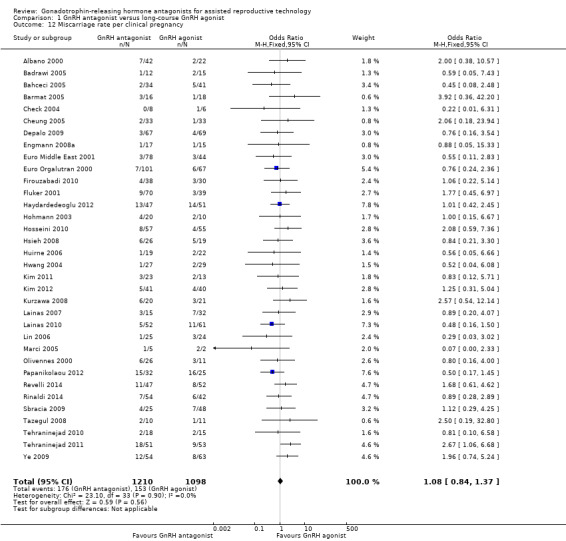
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 12 Miscarriage rate per clinical pregnancy.
Thirty‐four trials reported miscarriage rates per clinical pregnancy rates in 2308 women. There was no evidence of a difference following treatment with GnRH antagonist compared with GnRH agonist (OR 1.08, 95% CI 0.84 to 1.37; I2 = 0%)..
1.13 Cycle cancellation rate per woman randomised
1.13. Analysis.
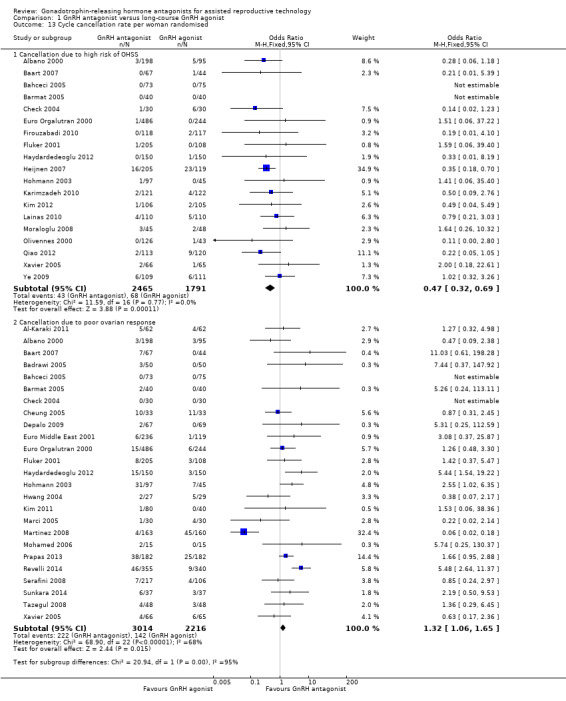
Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 13 Cycle cancellation rate per woman randomised.
1.13.1 Cancelled due to high risk of OHSS
Nineteen trials reported rates of cycle cancellation due to high risk of OHSS in 4256 women. There was evidence of a difference in cancellation rates with fewer cycles cancelled in the GnRH antagonist groups compared with the GnRH agonist groups (OR 0.47, 95% CI 0.32 to 0.69; I2 = 0%).
1.13.2 Cancellation due to poor ovarian response
Twenty‐five trials reported rates of cancellation due to poor ovarian response in 5230 women. There was evidence of a difference in cycle cancellation rates with more cycles cancelled in GnRH antagonist groups compared with GnRH agonist groups (OR 1.32, 95% CI 1.06 to 1.65; I2 = 68%, moderate quality evidence). The evidence suggested that if the risk of cycle cancellation following GnRH agonist treatment is assumed to be 6%, the risk following GnRH antagonist treatment would be between 7% and 10%. There was evidence of statistical heterogeneity among the trials that contributed data to the pooled effect estimate, with variations in the direction of effect estimates of individual trials. On sensitivity analysis using a random‐effects model, there was no evidence of a difference in cancellation rate between the two treatment groups (OR 1.38, 95% CI 0.82 to 2.31). Thus there is some degree of uncertainty with respect to this outcome, as it is sensitive to the choice of statistical model.
Discussion
Summary of main results
The previous version of this systematic review included 45 studies, while this updated version includes 73 RCTs and 12,212 randomised women. To our knowledge this systematic review and meta‐analysis represents the most recent and largest amount of evidence comparing the use of GnRH antagonist with long‐course GnRH agonist protocols in IVF or ICSI treatment cycles.
In this updated version of the review we focused on the effectiveness and safety of GnRH antagonist compared to GnRH agonist cycles in ART. Regarding effectiveness, there was no evidence of differences in live birth rate and ongoing pregnancy rate between GnRH agonist and GnRH antagonist LH peak suppression protocols.
With regard to safety, GnRH antagonists substantially reduced the incidence of OHSS. For the overall population from assembled studies, the evidence suggested that, if the risk of OHSS following GnRH agonist is assumed to be 11%, the risk following GnRH antagonist would be between 6% and 9%. In addition, there was evidence of a lower rate of moderate or severe OHSS in women who received the GnRH antagonist protocol compared with those who were treated with the GnRH agonist long protocol. However there was no evidence of a difference in miscarriage rates per woman randomised between the two treatment protocols. There was no clear picture with respect to cycle cancellation between the two treatment groups. While fewer cycles were cancelled in the GnRH antagonist group due to high risk of OHSS, there is some degree of uncertainty with cancellation due to poor ovarian response, as this outcome was sensitive to the choice of statistical model. In summary, there is moderate quality evidence that the use of GnRH antagonist compared with long‐course GnRH agonist protocols is associated with a substantial reduction in OHSS without reducing the likelihood of achieving live birth or ongoing pregnancy.
Previous versions of this systematic review showed substantially lower clinical and ongoing pregnancy rates for the GnRH antagonist protocol. Two earlier meta‐analyses of studies, comparing fixed and flexible GnRH antagonist protocols directly, demonstrated a trend towards higher pregnancy rates when using the fixed protocol, possibly explained by better LH control (Al‐Inany 2005; Kolibianakis 2006). The improved performance of antagonist cycles in the present update cannot be explained by the relative use of fixed protocols however, as relatively few new fixed protocols were included.
Several studies have suggested that LH instability decreases the probability of pregnancy in antagonist cycles (Bosch 2003; Kolibianakis 2003; Seow 2010; Shoham 2002). LH instability is defined as any fluctuation in LH level, either a LH surge or rise in LH concentration, in the course of ovarian hyperstimulation. A decrease in the relative incidence of LH instability in the current review can possibly have improved pregnancy outcomes in antagonist cycles, although the mechanism for such change is still unclear. Further studies are needed to investigate the possible role of LH‐instability in the improvement of pregnancy outcomes of GnRH antagonist cycles.
Increased favourable pregnancy outcomes with GnRH antagonist treatment may also be the result of an improved learning curve with the relatively new GnRH antagonist over the last 15 years. Extensive experience with GnRH antagonist protocols in large studies, leading to more favourable study outcomes, may have positively influenced pregnancy outcomes of GnRH antagonist cycles. Finally, changes in the use of OCP pretreatment (Griesinger 2008), scheduling of hCG for final oocyte maturation (Kolibianakis 2004; Tremellen 2010; Orvieto 2008) or patient selection (Sbracia 2009) may all have contributed to the optimisation of the use of antagonist cycles in ART. However, the improvement in pregnancy outcomes could also be due to the effects of potential bias in the included studies. For example, the forest plot (Figure 5) suggests a tendency for publication of studies with more favourable outcomes with the possibility of existence of unpublished studies with less favourable outcomes.
Previous work on the role of OCP pretreatment in direct comparison studies has indicated that OCP pretreatment leads to a longer duration of stimulation, higher oocyte yield, but reduced ongoing pregnancy rate (Smulders 2010). Also a trend towards lower pregnancy rates when using OCP pretreatment has been observed in a separate meta‐analysis (Griesinger 2008). As such, it has been recommended that OCP pretreatment does not seem to be the regimen of choice for GnRH antagonist cycles. In the previous versions of this review, however, a subgroup analysis of studies that used OCP pretreatment revealed no substantial difference between the agonist and antagonist groups for ongoing or clinical pregnancy rates. The percentage of women receiving OCP pretreatment in the 2011 update was comparable with the preceding version in 2006.
Overall completeness and applicability of evidence
Overall, the data demonstrate that GnRH antagonist is useful in women undergoing IVF or ICSI because it substantially reduces the occurrence of OHSS without reducing the chances of achieving live a live birth.
A long‐course GnRH agonist protocol with maximum ovarian stimulation has been the standard protocol for many decades. However, it is relatively complex and expensive, requires long treatment cycles and intensive monitoring, and leads to an abnormal hormonal environment in women. There is now an eager desire to shift to more patient‐friendly, mild ovarian‐stimulation regimens in which GnRH antagonist may be a suitable solution because there is evidence to suggest that its use is associated with comparable pregnancy outcomes.
A good number of the included studies did not report live birth and OHSS: 12 of the included studies reported data on live birth while only 36 reported data on OHSS. One study used single embryo transfer in the antagonist arm and double embryo transfer in the agonist arm. Some of the outcomes of interest were reported by some of the included studies in such a way that they could not be included in meta‐analyses. For example, some of the denominators were reported as 'per oocyte' or 'per embryo' transferred, where the numbers of oocytes or embryos transferred were not equal to the number of women randomised. In some of the included studies, some outcomes were not properly defined making it difficult to categorise such outcomes, for example, 'pregnancy rate' which could either be 'ongoing' or 'clinical' pregnancy. We included a small number of studies because they met the inclusion criteria, although they did not report data on any of the outcomes of interest. With respect to the triggering agent used for oocyte maturation, the majority of the studies either used hCG or did not report the triggering agent used. Thus no comparison could be made between the triggering agents such as hCG versus GnRH agonist.
Quality of the evidence
The evidence was of moderate quality using GRADE ratings for live birth, OHSS, ongoing pregnancy, clinical pregnancy, miscarriage and cycle cancellation due to poor ovarian response. The main limitations in the evidence were poor reporting of study methods. For example, a majority of the included studies either did not report the processes involved in random sequence generation and allocation concealment or reported vague and insufficient information on the processes, thereby making it difficult to make conclusive judgements on these domains of risk of bias. Poor reporting also affected the assessment of other domains of risk of bias with most of them being rated as 'unclear'. For live birth, there was evidence suggestive of the possibility of reporting (publication) bias with small studies more likely to report favourable outcomes for GnRH antagonist.
Potential biases in the review process
Although comprehensive searches were undertaken to ensure that all eligible studies were identified, it is not impossible that some potentially eligible studies could have been left out.
Agreements and disagreements with other studies or reviews
A systematic review and meta‐analysis, Youssef 2012, compared the effectiveness and safety of various protocols including GnRH antagonist versus long‐course GnRH agonist protocol. There was no evidence of a difference between women who received GnRH antagonist and those who were treated with long‐course GnRH agonist protocol in three RCTs, either in clinical pregnancy rate (OR 0.72, 95% CI 0.49 to 1.05, five RCTs) or cycle cancellation rate (OR 1.25, 95% CI 0.76 to 2.05).
There is a systematic review and meta‐analysis that included 22 RCTs (n = 3176) to compare GnRH antagonists and GnRH agonists (Kolibianakis 2006). The reported outcome measure, clinical pregnancy or ongoing pregnancy, was converted to live births in 12 studies using the published data. No evidence of a difference was detected in the probability of a live birth between the two GnRH analogues (OR 0.86, 95% CI 0.72 to 1.02). The result remained stable in a subgroup analysis that ordered the studies by type of population studied, gonadotrophin type used for stimulation, type of agonist protocol used, type of agonist used, type of antagonist protocol used, type of antagonist used, presence of allocation concealment, presence of co‐intervention and the way the information on live births was retrieved.
A systematic review and meta‐analysis, Franco 2006, evaluated the efficacy of gonadotrophin antagonist versus GnRH agonist in poor ovarian responders in IVF and ICSI cycles. The review included six RCTs that compared GnRH antagonist to long or short GnRH agonist. There was no difference between GnRH antagonist and GnRH agonist (long and flare‐up protocols) with respect to cycle cancellation rate, number of mature oocytes and clinical pregnancy rate per cycle initiated, per oocyte retrieval and per embryo transfer. When the meta‐analysis was applied to the two trials that had used GnRH antagonist versus long protocols of GnRH agonist, a significantly higher number of retrieved oocytes was observed in the GnRH antagonist protocols (MD 1.12, 95% CI 0.18 to 2.05; P = 0.018).
In another systematic review and meta‐analysis of four RCTs (n = 874) ongoing pregnancy rate was the main outcome (Griesinger 2008). There was no evidence of a statistically significant difference between women with and without OCP pre‐treatment (OR 0.74, 95% CI 0.53 to 1.03). Duration of gonadotrophin stimulation (1.41 days, 95% CI 1.13 to 1.68) and gonadotrophin consumption (542 IU, 95% CI 127 to 956) were significantly increased after OCP pre‐treatment. No significant differences were observed regarding the number of retrieved oocytes.
Authors' conclusions
Implications for practice.
The GnRH antagonist protocol is a short and simple protocol with evidence suggesting a comparable live birth rate and a substantial reduction in the incidence of ovarian hyperstimulation syndrome when compared to GnRH agonist long protocol in women undergoing ART.
Implications for research.
In view of the shortcomings noted in the included studies, especially with regard to the methods of reporting of trial procedures, more properly designed studies in accordance with the CONSORT statement are need to further evaluate the effectiveness and safety of the GnRH antagonist protocol (Schulz 2010). For example, it would be desirable to have trials with low risk of bias with primary outcomes of live birth and OHSS. In addition, further studies are needed to assess this treatment regimen in poor and high responders. We attempted to subgroup treatment regimens by the ovulation triggering agent but no data were available for a proper analysis, as the majority of the included studies either used hCG or did not specify their triggering agents. This is a potential area to be explored by future research. It is also important to understand why pregnancy outcomes have become progressively more favourable with the use of GnRH antagonists. One possible explanation for this could be a decrease in LH instability. This area should be further investigated. Although not a focus of the current update, the potential effects of OCP pretreatment should be further investigated.
Patient satisfaction surveys should also be undertaken to evaluate their impression about GnRH antagonist treatment regimens.
What's new
| Date | Event | Description |
|---|---|---|
| 31 August 2016 | Review declared as stable | Further evidence is unlikely to change the conclusions of this review. |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 9 May 2016 | Amended | Correction to reinstate data removed in error (Marci 2005) |
| 3 February 2016 | New citation required and conclusions have changed | The addition of new studies did not change the conclusions of this review. |
| 3 February 2016 | New search has been performed | This review has been updated, and 28 new studies added. |
| 7 July 2011 | Amended | Minor amendments to new citation version published May 2011 |
| 13 April 2011 | New search has been performed |
|
| 13 April 2011 | New citation required and conclusions have changed | The conclusion has changed |
| 13 June 2008 | Amended | Converted to new review format. |
| 19 May 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank all members of the Gynaecology and Fertility Group for their valuable support in this review. The authors also wish to acknowledge the following people for their contributions to the previous versions of the review: Monique D. Sterrenburg, Janine G Smit, Ahmed M Abou‐Setta, and Mohamed Aboulghar.
Appendices
Appendix 1. MDSG specialised register
MDSG Search string for HA412 Procite platform
Keywords CONTAINS "GnRH antagonist" or "GnRh antagonists" or "Antagon" or "ceterolix" or "cetrolix" or "cetrorelix" or "cetrotide" or "Ganirelix" or "Luteinising hormone releasing hormone" or "Lutenising hormone releasing hormone" or "LHRH antagonists" or Title CONTAINS "GnRH antagonist" or "GnRh antagonists" or "Antagon" or "ceterolix" or "cetrolix" or "cetrorelix" or "cetrotide" or "Ganirelix" or "Luteinising hormone releasing hormone" or "Lutenising hormone releasing hormone" or "LHRH antagonists"
AND
Keywords CONTAINS "GnRH a", "GnRH agonist" or "GnRH agonist short protocol" or "GnRH agonist vs antagonist" or "GnRH agonists" or "GnRHa" or "GnRHa‐gonadotropin" or"Gonadotrophin releasing agonist" or "buserelin" or "Buserelin Acetate" or "buserelin naferelin" or "busereline" or "Goserelin" or "goserelin acetate" or "Gosereline " or "Leuprolide" or "leuprolide acetate" or "leuprolide depot" or "leuprorelin" or "leuprolin" or "leuprorelin acetate" or "Nafarelin" or "Nafarelin Study Group" or "triptoielin" or "triptoreline" or "triptoreline pamoat" or "triptorelyn" or "triptrolein" or "Lupron" or "Zoladex" or "deslorelin" or "decapeptyl" or "decapeptyl‐daily" or "decapeptyl‐depot" or Title CONTAINS "GnRH a", "GnRH agonist" or "GnRH agonist short protocol" or "GnRH agonist vs antagonist" or "GnRH agonists" or "GnRHa" or "GnRHa‐gonadotropin" or"Gonadotrophin releasing agonist" or "buserelin" or "Buserelin Acetate" or "buserelin naferelin" or "busereline" or "Goserelin" or "goserelin acetate" or "Gosereline " or "Leuprolide" or "leuprolide acetate" or "leuprolide depot" or "leuprorelin" or "leuprolin" or "leuprorelin acetate" or "Nafarelin" or "Nafarelin Study Group" or "triptoielin" or "triptoreline" or "triptoreline pamoat" or "triptorelyn" or "triptrolein" or "Lupron" or "Zoladex" or "deslorelin" or "decapeptyl" or "decapeptyl‐daily" or "decapeptyl‐depot"
Appendix 2. Ovid Cochrane Central Register of Controlled Trials (CENTRAL)
From inception to April 2015 1 Hormone Antagonists/ (305) 2 gonadotropin releasing hormone antagonist$.tw. (87) 3 gonadotrophin releasing hormone antagonist$.tw. (29) 4 GnRH antagonist$.tw. (555) 5 Gn‐RH antagonist$.tw. (0) 6 (Cetrorelix or Cetrotide$).tw. (139) 7 Ganirelix.tw. (81) 8 (Abarelix or Plenaxis).tw. (11) 9 Antagon.tw. (10) 10 Degarelix.tw. (29) 11 or/1‐10 (855) 12 exp gonadotropin‐releasing hormone/ or exp buserelin/ or exp goserelin/ or exp leuprolide/ or exp nafarelin/ or exp triptorelin/ (1885) 13 gonadotropin releasing hormone agonist$.tw. (359) 14 gonadotrophin releasing hormone agonist$.tw. (146) 15 GnRH agonist$.tw. (796) 16 Gn‐RH agonist$.tw. (4) 17 (buserelin or goserelin).tw. (668) 18 (leuprolide or nafarelin).tw. (536) 19 triptorelin.tw. (196) 20 (Lupron or Eligard).tw. (37) 21 (Suprefact or Suprecor).tw. (9) 22 Synarel.tw. (3) 23 Supprelin.tw. (0) 24 Zoladex.tw. (227) 25 deslorelin.tw. (9) 26 Suprelorin.tw. (0) 27 Ovuplant.tw. (0) 28 (decapeptyl or trelstar).tw. (58) 29 (profact or receptal).tw. (4) 30 suprecur.tw. (0) 31 tiloryth.tw. (0) 32 (GNRH‐a or GNRH a).tw. (1393) 33 or/12‐32 (3280) 34 11 and 33 (520)
Appendix 3. Ovid MEDLINE(R)
From inception to April 2015 1 Hormone Antagonists/ (4693) 2 gonadotropin releasing hormone antagonist$.tw. (480) 3 gonadotrophin releasing hormone antagonist$.tw. (125) 4 GnRH antagonist$.tw. (2080) 5 Gn‐RH antagonist$.tw. (7) 6 (Cetrorelix or Cetrotide$).tw. (448) 7 Ganirelix.tw. (136) 8 (Abarelix or Plenaxis).tw. (53) 9 Antagon.tw. (17) 10 Degarelix.tw. (114) 11 or/1‐10 (6692) 12 exp gonadotropin‐releasing hormone/ or exp buserelin/ or exp goserelin/ or exp leuprolide/ or exp nafarelin/ or exp triptorelin/ (29123) 13 gonadotropin releasing hormone agonist$.tw. (1745) 14 gonadotrophin releasing hormone agonist$.tw. (467) 15 GnRH agonist$.tw. (3564) 16 Gn‐RH agonist$.tw. (52) 17 (buserelin or goserelin).tw. (2026) 18 (leuprolide or nafarelin).tw. (1812) 19 triptorelin.tw. (563) 20 (Lupron or Eligard).tw. (163) 21 (Suprefact or Suprecor).tw. (24) 22 Synarel.tw. (12) 23 Supprelin.tw. (2) 24 Zoladex.tw. (373) 25 deslorelin.tw. (204) 26 Suprelorin.tw. (16) 27 Ovuplant.tw. (11) 28 (decapeptyl or trelstar).tw. (208) 29 (profact or receptal).tw. (28) 30 suprecur.tw. (5) 31 tiloryth.tw. (0) 32 (GNRH‐a or GNRH a).tw. (937) 33 or/12‐32 (31184) 34 11 and 33 (2358) 35 randomized controlled trial.pt. (392594) 36 controlled clinical trial.pt. (89288) 37 randomized.ab. (317546) 38 placebo.tw. (165796) 39 clinical trials as topic.sh. (172358) 40 randomly.ab. (229154) 41 trial.ti. (136960) 42 (crossover or cross‐over or cross over).tw. (63745) 43 or/35‐42 (975714) 44 (animals not (humans and animals)).sh. (3933883) 45 43 not 44 (898609) 46 34 and 45 (500)
Appendix 4. Ovid EMBASE
From inception to April 2015 1 Hormone Antagonist/ (1475) 2 Gonadorelin Antagonist/ (4507) 3 gonadotropin releasing hormone antagonist$.tw. (533) 4 Gnrh Antagonist$.tw. (2984) 5 Luteinizing Hormone Releasing Hormone Antagonist$.tw. (88) 6 Lhrh Antagonist$.tw. (354) 7 Cetrorelix.tw. (670) 8 cetrorelix/ or ganirelix/ (2218) 9 ganirelix.tw. (306) 10 Cetrotide.tw. (636) 11 Antagon.tw. (118) 12 Orgalutr?n.tw. (397) 13 Degarelix.tw. (226) 14 or/1‐13 (7917) 15 Gonadorelin Agonist/ (11188) 16 GnRH agonist$.tw. (4929) 17 gonadotropin releasing hormone agonist$.tw. (2015) 18 Lhrh Agonist$.tw. (1323) 19 Luteinizing Hormone Releasing Hormone Agonist$.tw. (562) 20 TRIPTORELIN/ (4176) 21 Triptorelin.tw. (824) 22 (Arvekap or Decapeptyl or Detryptorelin or Trelstar or Tryptorelin).tw. (1772) 23 BUSERELIN/ (4084) 24 Buserelin.tw. (1473) 25 (Bigonist or Busereline or Receptal or Superfact or Suprefact).tw. (1135) 26 (GNRH‐a or GNRH a).tw. (1127) 27 or/15‐26 (19742) 28 14 and 27 (2960) 29 Clinical Trial/ (843206) 30 Randomized Controlled Trial/ (368416) 31 exp randomization/ (66003) 32 Single Blind Procedure/ (20039) 33 Double Blind Procedure/ (119722) 34 Crossover Procedure/ (42461) 35 Placebo/ (254717) 36 Randomi?ed controlled trial$.tw. (114462) 37 Rct.tw. (16650) 38 random allocation.tw. (1399) 39 randomly allocated.tw. (22089) 40 allocated randomly.tw. (2010) 41 (allocated adj2 random).tw. (721) 42 Single blind$.tw. (15600) 43 Double blind$.tw. (149516) 44 ((treble or triple) adj blind$).tw. (439) 45 placebo$.tw. (212288) 46 prospective study/ (286502) 47 or/29‐46 (1449548) 48 case study/ (31233) 49 case report.tw. (279055) 50 abstract report/ or letter/ (920165) 51 or/48‐50 (1224269) 52 47 not 51 (1410589) 53 28 and 52 (929)
Appendix 5. Ovid PsycINFO
PsycINFO <1806 to April 2015> 1 gonadotrop?in releasing hormone antagonist$.tw. (10) 2 GnRH antagonist$.tw. (20) 3 (Cetrorelix or Cetrotide$).tw. (5) 4 (Ganirelix or Degarelix).tw. (3) 5 or/1‐4 (29) 6 gonadotrop?in releasing hormone agonist$.tw. (58) 7 GnRH agonist$.tw. (57) 8 (buserelin or goserelin).tw. (27) 9 (leuprolide or nafarelin).tw. (73) 10 triptorelin.tw. (24) 11 (Lupron or Eligard).tw. (15) 12 Zoladex.tw. (4) 13 deslorelin.tw. (5) 14 (decapeptyl or trelstar).tw. (2) 15 (GnRH‐a or GNRH a).tw. (8) 16 or/6‐15 (195) 17 5 and 16 (7) 18 random.tw. (43340) 19 control.tw. (336514) 20 double‐blind.tw. (18756) 21 clinical trials/ (8577) 22 placebo/ (4049) 23 exp Treatment/ (612193) 24 or/18‐23 (938572) 25 17 and 24 (3)
Appendix 6. EBSCO CINAHL
CINAHL search strategy for HA412 28.04.15
| # | Query | Results |
| S34 | S21 AND S33 | 56 |
| S33 | S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 | 956,465 |
| S32 | TX allocat* random* | 4,252 |
| S31 | (MH "Quantitative Studies") | 13,346 |
| S30 | (MH "Placebos") | 9,191 |
| S29 | TX placebo* | 33,691 |
| S28 | TX random* allocat* | 4,252 |
| S27 | (MH "Random Assignment") | 39,039 |
| S26 | TX randomi* control* trial* | 86,342 |
| S25 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 765,037 |
| S24 | TX clinic* n1 trial* | 171,259 |
| S23 | PT Clinical trial | 77,774 |
| S22 | (MH "Clinical Trials+") | 186,608 |
| S21 | S9 AND S20 | 92 |
| S20 | S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 | 805 |
| S19 | (MM "Goserelin") | 93 |
| S18 | (MM "Leuprolide") | 124 |
| S17 | TX (GNRH‐a or GNRH a) | 129 |
| S16 | TX (decapeptyl or trelstar) | 8 |
| S15 | TX triptorelin or TX Zoladex | 61 |
| S14 | TX (leuprolide or nafarelin) | 286 |
| S13 | TX (buserelin or goserelin) | 246 |
| S12 | TX GnRH agonist* | 160 |
| S11 | TX gonadotrophin releasing hormone agonist* | 37 |
| S10 | TX gonadotropin releasing hormone agonist* | 190 |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 | 159 |
| S8 | TX Degarelix | 35 |
| S7 | TX Antagon | 2 |
| S6 | TX (Abarelix or Plenaxis) | 8 |
| S5 | TX Ganirelix | 13 |
| S4 | TX (Cetrorelix or Cetrotide) | 14 |
| S3 | TX GnRH antagonist* | 86 |
| S2 | TX gonadotrophin releasing hormone antagonist* | 16 |
| S1 | TX gonadotropin releasing hormone antagonist* | 47 |
Data and analyses
Comparison 1. GnRH antagonist versus long‐course GnRH agonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman randomised | 12 | 2303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| 2 Live birth rate per woman randomised ‐ minimal stimulation | 2 | 524 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.26] |
| 3 Live birth rate per woman randomised ‐ grouped by trigger | 12 | 2303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| 3.1 hCG trigger | 11 | 1899 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.89, 1.34] |
| 3.2 Unknown trigger | 1 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.21] |
| 4 Ovarian hyperstimulation per woman randomised ‐ all women | 36 | 7944 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.51, 0.72] |
| 5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe | 20 | 5141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.40, 0.69] |
| 6 Ongoing pregnancy rate per woman randomised ‐ all women | 37 | 8311 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.01] |
| 7 Ongoing pregnancy rate per woman randomised ‐ minimal stimulation | 7 | 1456 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.18] |
| 8 Ongoing pregnancy rate per women randomised ‐ grouped by trigger | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 hCG trigger | 29 | 5170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.08] |
| 8.2 Mixed trigger (hCG/GnRH agonist) | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.61] |
| 8.3 Unknown trigger | 7 | 3075 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 9 Clinical pregnancy rate per woman randomised ‐ all women | 54 | 9959 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 10 Clinical pregnancy rate per woman randomised ‐ minimal stimulation | 6 | 1102 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.15, 1.96] |
| 11 Miscarriage rate per woman randomised | 34 | 7082 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 12 Miscarriage rate per clinical pregnancy | 34 | 2308 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.37] |
| 13 Cycle cancellation rate per woman randomised | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Cancellation due to high risk of OHSS | 19 | 4256 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.69] |
| 13.2 Cancellation due to poor ovarian response | 25 | 5230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.06, 1.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Karaki 2011.
| Methods | RCT, parallel design | |
| Participants | 124 poor responders undergoing IVF | |
| Interventions |
GnRH antagonist (n = 62): 450 IU HP‐FSH (Urofollitropin) + cetrorelix 0.25 mg daily added to the ovarian stimulation when the largest follicle measures ≥ 14 mm (flexible protocol) GnRH agonist (n = 62): 450 IU HP‐FSH (Urofollitropin) + triptoreline 0.05 mg daily (half the standard dose) initiated in the mid‐luteal phase prior to the treatment cycle (minidose long protocol) Mean number of embryos transferred: antagonist: 2.1 ± 1.2 versus agonist: 2.3 ± 1.3 Follow‐up: Up to clinical pregnancy |
|
| Outcomes | Clinical pregnancy rate, cycle cancellation rate, duration of stimulation, total gonadotrophins requirement, estradiol level on day of hCG, mean numbers of mature oocytes retrieved, mean numbers of embryos formed, mean numbers of embryos transferred | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not reported but unlikely to influence measurement of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data provided regarding withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available to identify outcomes of interest. Live birth rate not reported |
| Other bias | Low risk | None identified |
Albano 2000.
| Methods | RCT, open‐label parallel design, multi‐centre (7 centres), multi‐national | |
| Participants | 293 Infertile couples undergoing ovarian stimulation for IVF‐ET with or without ICSI Inclusion criteria: with no more than three previous IVF‐ET attempts with all causes of infertility (except polycystic ovary and moderate or severe endometriosis) Baseline characteristics: age 31.9 ± 3.7 versus 31.6 ± 3.8. Duration of infertility: not stated. FSH: not stated. BMI not stated |
|
| Interventions |
Group I (n = 198): hMG (menogon, humegon, pergonal) was started at 2 or 3 ampoules for four days and the dose was adjusted according to response + multiple‐dose regimen of 0.25 mg of GnRH antagonist (cetrorelix) was administered SC starting from day 6 of hMG treatment to 115 participants up to and including day of hCG administration (Fixed) Group II, GnRH agonist (n = 95): mid‐luteal GnRH analogue (Buserlin 150 µg four times daily intranasally) + hMG (menogon, humegon,pergonal) was started at 2 or 3 ampoules for four days and the dose was adjusted according to response Luteal phase support: daily vaginal progesterone or hCG injections |
|
| Outcomes | Premature LH surge defined as (LH > 10 IU/L) and progesterone level > 1 ng/L. Stimulation length, no. of hMG ampoules. E2 on hCG, no of oocytes retrieved, clinical pregnancy/OPU, clinical pregnancy/ET. Miscarriage Ectopic. Moderate or severe OHSS. Clinical pregnancy was defined as fetal heart beat on ultrasonography. Ongoing pregnancy was defined as pregnancy ongoing after 12 weeks of amenorrhoea | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported as a randomised trial with no further details of how randomisation was performed |
| Allocation concealment (selection bias) | Low risk | Concealed; central telephone, 2:1 randomisation ratio |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include most expected outcomes |
| Other bias | Unclear risk | Supported by pharmaceutical company, the study appears to be free from other sources of bias |
Anderson 2014.
| Methods | RCT | |
| Participants | 41 women Inclusion criteria: 21 to 40 years, anti‐Mullerian hormone > 1 ng/ml Exclusion criteria: no details Setting and timing: no details of setting. USA. No details of timing Baseline characteristics: no details |
|
| Interventions |
GnRH antagonist (no details) (n = 21) when follicle size reached 12 mm during the COS cycle Agonist (Leuprolide acetate) (n = 20) starting on day 18 of the oral contraceptive pill cycle Fixed protocol of human derived gonadotrophins at a 3:1 ratio (225/75 IU) for the first four days of stimulation followed by a flexible protocol to improve response hCG given when at least three follicles reached 17 mm diameter All women underwent a cycle using an oral contraceptive pill before starting the controlled ovarian stimulation cycle for IVF |
|
| Outcomes | E2, LH, oocytes retrieved, mature oocytes, fertilisation rate, implantation rate, pregnancy rate | |
| Notes | The table included in the abstract does not match the abstract. No data could be included in the meta‐analyses Sample size calculation ‐ unclear ITT analysis ‐ unclear Funding ‐ Ferring Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomised' no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details, unlikely to be blinded but this should not influence fertility outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Conference abstract, unclear if this is the full sample size or reporting preliminary data |
| Selective reporting (reporting bias) | High risk | No raw data reported. The table does not match the abstract. No full paper identified |
| Other bias | Unclear risk | Conference abstract only. No details of demographics |
Awata 2010.
| Methods | RCT | |
| Participants | 413 women (564 cycles) Inclusion criteria: women < 40 years undergoing COH but no other details Exclusion criteria: not stated Baseline characteristics: not stated Setting and timing: no details |
|
| Interventions | Agonist long protocol ‐ no details Agonist short protocol ‐ no details Antagonist protocol ‐ no details |
|
| Outcomes | Pregnancy rate, miscarriage rate | |
| Notes | Data are only reported as the number of antral follicles and not by the allocated treatment. The denominator for the pregnancy rate does not match either the total number of women or the number of cycles. Data could not be included in a meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘randomly received’ no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | High risk | Conference abstract only. No details as to number allocated to each group. Pregnancy and miscarriage rates reported only |
| Other bias | Unclear risk | Conference abstract only |
Baart 2007.
| Methods | RCT, two‐centre trial | |
| Participants | 111 infertile women undergoing IVF/ICSI Inclusion criteria: were not at an a priori increased risk for chromosomally abnormal embryos. < 38 years of age, regular indication for IVF and with a partner with a sperm count > 5 million progressively motile sperm per millilitre, regular menstrual cycles (ranging from 25 to 35 days), BMI 19 ‐ 29 kg m2, no known chromosomal abnormalities, no relevant systemic disease or uterine and ovarian abnormalities, no history of recurrent miscarriage, and no previous IVF cycles not resulting in an embryo transfer Baseline characteristics:female age (years) 34.1 (28 – 37) vs 33.2 (22 – 37), basal FSH 8.1 (4.4 – 13.8) vs 7.6 (5.5 – 18.4), Inhibin B level on cycle day 3 or 4: 86 (2 – 1056) vs 88 (15 – 593) |
|
| Interventions |
Group I (n = 67):150 IU FSH on 2nd day of the cycles (fixed) + 0.25 mg SC of GnRH antagonist co‐treatment (ganirelix (Orgalutran)) administered when at least one follicle measuring > 14 mm (flexible protocol) GnRH agonist (n = 44): 225 IU rFSH (fixed) + long GnRH agonist, 0.1 mg triptorelin(Long GnRH agonist protocol) Oocyte maturation triggering: 10,000 IU SC hCG (Pregnyl) when one follicle > 18 mm plus 2 follicles > 15 mm Oocyte retrieval: 35 hours later, followed by IVF/ICSI. Maximum embryos transferred: 2 Follow up: OPR was confirmed by vaginal ultrasound scan at 12 weeks of gestation |
|
| Outcomes | Primary outcome measures: ovarian response, as assessed by the number of oocytes obtained and the proportion of chromosomally abnormal embryos per participant. This was expressed as the ratio of abnormal embryos on the number of embryos diagnosed per participant. Secondary outcome measures: proportion of fertilised oocytes, the proportion of embryos with normal morphology and the proportion of embryos biopsied and diagnosed | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule in a ratio of 4:6 (conventional group: mild group) |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes. After the participant agreed to participate, the next available numbered envelope on entry into the study was opened by the treating physician during the preparatory IVF consultation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Embryologist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | High risk | Funding was provided by university and non‐governmental organisation Early stopping due to benefit occurred. "The proportion of chromosomally abnormal embryos per patient was found to be significantly reduced after mild ovarian stimulation (P ¼ 0.02, which is below the Pocock critical bound of 0.0354 for a single interim analysis after 61% (111 of 181) of women had been included (Pocock, 1977)) and the study was terminated." |
Badrawi 2005.
| Methods | RCT, single‐centre, open‐label, parallel design | |
| Participants | 100 infertile women undergoing ICSI Inclusion criteria: primary infertility patients, 18 to 39 years old, with regular menstrual cycle and FSH levels < 10 IU/L done at cycle day 3 and ultrasound examination showed normal uterus Exclusion criteria:women with severe endometriosis (American Fertility Society stage III and IV), and azoospermic males were excluded from the study Baseline characteristics: age 30.8 ± 4.8 vs 30.28 ± 5.9 years |
|
| Interventions |
GnRH antagonist (n = 50): 225 IU Menogon hMG (menogon, humegon, pergonal) was started at 2 or 3 ampoules for four days (adjusted) + 0.25 mg of GnRH antagonist SC ganirelix started from day 6 of hMG treatment/lead follicle measures 14 mm (Flexible multiple‐dose GnRH antagonist)
GnRH agonist (n = 50): mid‐luteal GnRH analogue, buserelin 150 ug four times daily intranasally (Suprefact) + 225 IU hMG (menogon, humegon, pergonal) was started at 2 or 3 ampoules for four days and the dose was adjusted according to response (Long GnRH agonist) Oocyte maturation triggering: hCG 10,000 IU (Choriomon) was administered deeply IM when the leading follicle reached 20 mm in mean diameter with at least three follicles > 18 mm Oocyte retrieval: 34 ‐ 36 hours Embryo transfer: 2 ‐ 3 days after OPU Luteal phase support: Cyclogest (Shire Pharmaceuticals Ltd., Andover, UK) vaginal pessaries, 400 mg twice a day continued for two weeks. B‐hCG was done two weeks following embryo transfer and if negative Cyclogest was stopped. If, however, pregnancy test (B‐hCG) was positive, Cyclogyst was continued until 12 weeks' gestation |
|
| Outcomes | Female partner age Infertility duration years Baseline FSH mIU/ml Day 3 LH mIU/ml Day 14 E2 pg/ml E2 pg/ml on day of hCG hMG ampoule Stimulation duration Number of follicles Size of follicles (mm) Endometrial thickness Number of oocytes retrieved Number of MII oocytes Number of oocytes fertilised Fertilisation rate Embryos No of transferred embryos Pregnancy rate/ET Abortion rate OHSS |
|
| Notes | Number of participants at randomisation: 100 (ganirelix 50/Superfact 50) Number of participants at stimulation: 100 (ganirelix 50/Superfact 50) Number of participants at OPU: 95 (ganirelix 47/Superfact 50) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation stated to be using sealed envelopes without any further details |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes data, LBR was not reported |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias. |
Bahceci 2005.
| Methods | RCT, single‐centre, open‐label, parallel design, randomisation: 1:1 (cetrorelix: leuprolide acetate) ratio | |
| Participants | 148 women with PCOS, no previous ART or had hyperprolactinaemia or thyroid abnormalities | |
| Interventions |
GnRH antagonist (n = 75): antagonist protocol: cetrorelix 0.25 mg/d subcutaneously started when the leading follicle reached 14 mm. hCG 10,000 IU administered when at least two follicles reached 18 mm (Flexible) GnRH agonist (n = 70): agonist protocol: L.A. 0.5 mg, on day 14 of the cycle. Daily administration of gonadotrophins, 2 or 3 ampoules initiated on the third day of the anteceding menstrual period (Long GnRH agonist protocol) Oocyte maturation triggering: hCG 10,000 IU administered when at least two follicles reached 18 mm |
|
| Outcomes | Days of analogue treatment Number of women who reached the day of hCG (%) Number of hMG ampoules Days of hMG treatment Number of follicles on the day of hCG injection Number of women with oocyte retrieval Number of women with ovum retrieval Number of women with one or more fertilised oocytes Number of COC Number of 2 pronuclear oocytes Number of embryo transfers Number of clinical pregnancies | |
| Notes | Number of participants at randomisation: 148 (cetrorelix: 75/leuprolide acetate: 73) Number of participants at stimulation: 129 (cetrorelix: 59/leuprolide acetate: 70) Number of participants at OPU: 129 (cetrorelix: 59/leuprolide acetate: 70) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes data, LBR not reported |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Barmat 2005.
| Methods | RCT, multi‐centre (4 US centres), open‐label, parallel design | |
| Participants | 80 women undergoing IVF/ICSI Inclusion criteria: < 39 years of age, day 3 FSH level of < 10, E2 level of < 60 pg/mL, AFC > 5 with a menstrual cycle range of 26 ‐ 34 days, and no more than one previous failed IVF or IVF/ICSI cycle. BMI 19 ‐ 32 kg/m2 no hydrosalpinx present by hysterosalpingogram, laparoscopy, or ultrasound within the past year Male factor infertility cases could be included (ICSI and/or frozen sperm) with the exception of nonobstructive azoospermia. Only one study cycle was allowed Exclusion criteria: history of previous poor response (< 4 follicles and/or an E2 level of < 500 pg/mL on the day of hCG), had taken infertility medications (clomiphene and/or gonadotrophins) within the past month, or had failed to consent to taking OCs, GnRH‐analogues, or gonadotrophins. |
|
| Interventions |
GnRH antagonist (n = 40): OC (Desogen; Organon USA) on cycle days 2 to 4 for 14 to 28 days + 300 IU/day rFSH SC (adjusted) + 250 µg ganirelix was initiated when a lead follicle obtained a mean diameter of 12 to 14 mm (flexible) GnRH agonist (n= 40): leuprolide (GnRH‐agonist group), 0.5 mg per day during the mid‐luteal phase with approximately a 5‐day overlap with the OCs. Once adequate pituitary desensitisation was achieved the dose of GnRH agonist was reduced to 0.25 mg per day + 300 IU/day rFSH SC (adjusted) Oocyte maturation triggering: at follicular diameter 16 ‐ 18 mm, 5000 to 10,000 IU of hCG (Pregnyl). In cases at risk of ovarian hyperstimulation syndrome, the physician could give a dose of 5000 IU of hCG Oocyte retrieval: 35 to 36 hours later Embryo transfer: at 3 or 5 days Luteal phase support: progesterone, one centre treated women with P, 25 mg IM, on the day of retrieval, followed by P, 50 mg IM daily, with some women being supplemented with hCG 2,500 IU on days 3 and 6 after retrieval. The other centres prescribed luteal support with a daily dose of P (50 mg IM). |
|
| Outcomes | Participants to oocyte retrieval (n = 77) Days from OCP to oocyte retrieval Days on OC Stimulation day 1 E2 (pg/mL) Recombinant FSH (IU) Days of recombinant FSH Stimulation day of ganirelix start Days of leuprolide or ganirelix LH day hCG (IU/L) E2 day hCG (pg/mL) P4 day hCG (pg/mL) No of follicles Follicle sizes Number of oocytes retrieved Number of mature oocytes Number of 2 pronuclear embryos Number of embryos transferred Percentage of women with cryopreservation Embryos cryopreserved/participant with cryopreservation Number of pregnancies/embryos transferred (%) Number of pregnancies/cycle started (%) Number of ongoing pregnancies/embryos transferred (%) Number of ongoing pregnancies/cycle started (%) Number of implanted embryos (%) Number of ongoing twin gestations (%) Delivered pregnancies | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dark sealed envelopes (true), randomisation: 1:1 (ganirelix acetate: leuprolide acetate) ratio |
| Allocation concealment (selection bias) | Low risk | Dark sealed envelopes (true) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported clearly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Brelik 2004.
| Methods | RCT, single‐centre, parallel design, randomisation: 1:1 (cetrorelix: triptorelin) ratio | |
| Participants |
120 infertile women undergoing IVF/ICSI Inclusion/Exclusion criteria: Infertile women undergoing IVF regardless of the indications or pre‐stimulatory LH levels |
|
| Interventions |
GnRH antagonist (n = 57): in the antagonist arm, rFSH from day 2 of the cycle, and when the leading follicle reached 13 mm cetrorelix 0.25 mg (Cetrotide; Serono) was started. The LH levels were checked on the day, when the leading follicle reached 13 mm (LH1) and 21 mm (LH2) (Flexible) GnRH agonist (n = 63): in the agonist arm, triptorelin 3,75 (Diphereline SR 3,75mg; Beaufour Ipsen) was administered on day 20 of the preceding cycle and 14 days later if E2 < 30 pg/mL, stimulation with rFSH (Gonal‐F; Serono) was initiated |
|
| Outcomes | Number of FSH ampoules used Number of oocytes retrieved Fertilisation rate (%) of all retrieved oocytes Early cleavage rate (%) Grade A embryos rate (%) Number of embryos transferred Clinical pregnancy rate (%) LH levels | |
| Notes | Number of participants at randomisation: 120 (cetrorelix: 57/ triptorelin: 63) Number of participants at stimulation: 120 (cetrorelix: 57/ triptorelin: 63) Number of participants at OPU: 120 (cetrorelix: 57/ triptorelin: 63) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported clearly |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported clearly |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Celik 2011.
| Methods | Two‐arm parallel RCT | |
| Participants | 60 infertile women undergoing IVF at IVF centre; no further details were reported about participants | |
| Interventions | GnRH antagonist: no details were reported GnRH agonist: no details were given |
|
| Outcomes | Only pregnancy rate was reported but type of pregnancy was not defined | |
| Notes | Unclear definition of pregnancy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was reported on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was reported on blinding of participants and/or personnel; however, non blinding of outcome assessment may not affect some outcomes of interest as they are objectively assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information was reported on incomplete outcome data/attrition |
| Selective reporting (reporting bias) | Unclear risk | Methods section not detailed enough to make conclusive judgement |
| Other bias | Low risk | None identified |
Check 2004.
| Methods | RCT, single‐centre, open‐label, parallel design, randomisation: 1:1 (ganirelix: leuprolide) ratio | |
| Participants | Couples requiring IVF or intracytoplasmic sperm injection (ICSI) | |
| Interventions | Agonist regimen: leuprolide acetate 0.5 mg qd for 10 days from mid‐luteal phase Antagonist regimen: 250 μg ganirelix when dominant follicle is at least 14 mm and estradiol is at least 1000 pg/ml (flexible) | |
| Outcomes | Clinical pregnancy, viable pregnancy, implantation rate | |
| Notes | Number of participants at randomisation: 60 (ganirelix: 30/leuprolide: 30) Number of participants at stimulation: 54 (ganirelix: 24/leuprolide: 30) Number of participants at OPU: 54 (ganirelix: 24/leuprolide: 30) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis was not used and drop‐out rate was above 10% with reasons provided |
| Selective reporting (reporting bias) | Low risk | Protocol not available but materials match results |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
Cheung 2005.
| Methods | RCT, single‐centre, parallel design | |
| Participants |
66 infertile women undergoing IVF/ICSI Inclusion/ Exclusion criteria: Poor responders were classified as patients who had exhibited a poor ovarian response with < 3 mature follicles on a long GnRH agonist protocol in their previous IVF cycles, or those with repeated high basal levels of FSH > 10 IU/l Women with polycystic ovaries were excluded from the study |
|
| Interventions |
GnRH antagonist (n = 33): OCP (Nordette) 30 µg of ethinyl estradiol and 150 µg of levonorgestrel for 21 days + 300 IU daily rFSH (Gonal‐F) + 0.25 mg SC cetrorelix (Cetrotide) (fixed), multi‐dose GnRH antagonist protocol starting on day 6 of the stimulation (fixed)
GnRH agonist (n = 33): long GnRH agonist protocol, buserelin acetate nasal spray (Suprecur) daily dose of 600 µg starting at the mid‐luteal phase of the preceding cycle + 300 IU daily rFSH (Gonal‐F) (long GnRH agonist)
Oocyte maturation triggering: 10,000 IU of IM hCG (Profasi) when the leading follicles reached 18 ‐ 20 mm together with at least three mature follicles > 16 mm Oocyte retrieval: 36 hrs later. ICSI was performed only in cases with severe male factor or previous fertilisation failure Maximum number of embryo transfer: depending on the number of embryos available, up to three embryos were transferred on day 3 after oocyte retrieval Luteal phase support: IM hCG (Profasi) 2000 IU given every 3 days for four doses starting on the day of oocyte retrieval A clinical pregnancy was established when there was a gestational sac seen on ultrasonography |
|
| Outcomes | The main outcome measures were duration of stimulation, consumption of gonadotrophins, cycle cancellation rate, and the number of mature follicles recruited and total oocytes retrieved. The hormone levels throughout the cycle, laboratory outcomes and clinical pregnancy rates were also reviewed | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table (true), randomisation: 1:1 (cetrorelix: buserelin acetate) ratio |
| Allocation concealment (selection bias) | Low risk | Performed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Choi 2012.
| Methods | Three‐arm randomised controlled trial. Single centre | |
| Participants | 61 infertile women (67 cycles) Inclusion criteria: PCOS (ESHRE/ASRM criteria). No other details Exclusion criteria: no details Setting and timing ‐ Seoul, Korea. April 2009 to November 2010 Baseline characteristics: age ‐ antagonist 32.9 ± 2.9 years versus agonist 34.4 ± 3.8 years |
|
| Interventions |
IVM/IVF with FSH and hCG priming protocol (n = 11 women; 14 cycles) Antagonist multidose flexible protocol (n = 36 women; 39 cycles). Stimulation started on day 2 or 3 and 0.25 mg cetrorelix acetate or 0.25 mg ganirelix acetate administered when follicles > 12 mm or serum E2 > 200 pg/ml Agonist long protocol (n = 14 women; 14 cycles) 0.5 mg buserelin acetate or 0.1 mg leuprolide acetate given from mid‐luteal phase of previous cycle to day of hCG administration 250 micrograms hCG given when lead follicle > 18 mm diameter Oocyte retrieval 36 hours after hCG Dose of gonadotrophin antagonist 1656.7 ± 669.77 versus agonist 2700.0 ± 824.3 |
|
| Outcomes | OHSS, clinical pregnancy, miscarriage, live birth rate: all reported as 'per cycle' and thus could not be pooled | |
| Notes | Sample size calculation ‐ no ITT analysis ‐ yes Funding ‐ not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomized' no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details. Unlikely that participants and researchers were blinded but unlikely to affect fertility outcome. Blinding of outcome assessors is unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported on all women analysed |
| Selective reporting (reporting bias) | Low risk | Outcomes reported including live birth rate |
| Other bias | Unclear risk | Groups appeared balanced at baseline. Data is per cycle and not per woman randomised and could not be included in the meta‐analysis |
Cota 2012.
| Methods | Randomised controlled trial | |
| Participants | 64 women undergoing ICSI (first cycle) Inclusion criteria: 37 years or less, first IVF/ICSI cycle, BMI < 30 kg/m2, regular menses, both ovaries present Exclusion criteria: PCOS, severe endometriosis, ovarian cysts assessed by transvaginal ultrasound, basal FSH 10 IU/ml or greater Baseline characteristics: age ‐ antagonist group 32.5 ± 3.0 years versus agonist group 33.2 ± 3.0 years Setting and timing: Center for Human Reproduction, Brazil |
|
| Interventions |
GnRH antagonist (n = 32) cetrorelix. Ovarian stimulation using 150 to 225 IU recombinant FSH and 75 IU/day recombinant LH for five days starting on day 3. Follicular development monitored on Day 8. rFSH adapted according to ovarian response and rLH increased to 150 IU/day when one or more follicles reached 10 mm or more in diameter. Cetrorelix 0.25 mg/day SC started when at least one follicle was 14 mm or greater in diameter. Administered until day of hCG injection GnRH agonist (n = 32) leuprolide acetate 1 mg/day starting in luteal phase of previous cycle for 14 days. Ovarian stimulation using 150 to 225 IU rFSH and 75 IU/day rLH for 7 days. rFSH adapted according to ovarian response and rLH increased to 150 IU/day when one or more follicles reached 10 mm or more in diameter. Administered until day of hCG injection Oocyte maturation: 250 micrograms recombinant hCG given SC when at least two follicles were 17 mm or greater in diameter Oocyte retrieval: 34 ‐ 36 hours after hCG injection Total dose gonadotrophins: antagonist group 1877.3 ± 817 IU versus agonist group 2185.5 IU |
|
| Outcomes | Oocyte morphology. Implantation rate, pregnancy rate (not defined) | |
| Notes | Sample size calculation: yes ITT analysis ‐ yes Funding: no details Data cannot be included in a meta‐analysis as it is unclear if the pregnancy rate refers to a biochemical or clinical or ongoing pregnancy. Another trial Lavorato 2012 reports on 32 women from the same authoring group and centre with the outcomes of DNA fragmentation and apoptosis in granulosa cells. No pregnancy data reported in this paper either |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Double randomisation using computer‐generated number table then lots |
| Allocation concealment (selection bias) | Low risk | One nurse, blinded to subject identities, performed all the lot draws |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No evidence of blinding of participants. Blinding of outcome assessors unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised appear to be analysed |
| Selective reporting (reporting bias) | Unclear risk | Authors report pregnancy rate but it is not defined, therefore unclear if it is biochemical, clinical or ongoing and cannot be included in a meta‐analysis. Other data is reported per oocyte of which there were 300 per group |
| Other bias | Low risk | Groups appeared balanced at baseline |
Depalo 2009.
| Methods | RCT, single‐centre study | |
| Participants | 136 consecutive patients undergoing ICSI Inclusion criteria: age 24 ‐ 42 years, baseline FSH level < 10 IU/ml, absence of uterine or ovarian abnormalities or severe endometriosis or polycystic ovary syndrome, no more than three previous IVF attempts and, no oral contraceptive pills taken before the stimulation cycle. Male factor infertility cases, such as number of spermatozoa < 5.0 million/ml and < 30% motility were included. Criteria for cycle cancellation were as follows: 53 follicles with diameter 14 mm after 8– 10 days of stimulation Baseline characteristics: age 34.4 ± 4 vs 34 ± 3.9 yrs. Basal FSH (mIU/ml) 6.4 ± 2.4 vs 5.7 ± 2. Basal E2 (pg/ml) 21.71 ± 3 vs 16.7 ± 9.4. BMI (kg/m2) 23.7 ± 4.1 vs 22.7 ± 3.4 |
|
| Interventions |
GnRH antagonist (n = 67): a daily dose of cetrorelix 0.25 mg (Cetrotide) was administered when a leading follicle reached a diameter of 12 – 14 mm + rFSH (Gonal F) starting on cycle day 2 – 3 at a dose of 225 UI/daily, for the first five days (adjusted) (Flexible protocol) GnRH agonist (n= 69): 0.1 mg triptorelin (Decapeptyl 0.1 mg) were administered subcutaneously daily, starting in the late luteal phase (day 21) of the previous cycle + rFSH starting on cycle day 2 – 3 at a dose of 225 UI/daily, for the first five days (adjusted) Final oocyte maturation: was achieved with 6500 UI of hCG (Ovitrelle) when two or more follicles reached a diameter 18 mm Oocyte retrieval: 35 – 36 hrs after hCG administration Embryo transfer: 3 embryos on day 2 Luteal phase support: was supplemented with progesterone in oil, 50 mg/ day (Prontogest) starting the day after oocyte retrieval and continuing until 12 weeks' gestation if pregnancy was achieved |
|
| Outcomes | Oocyte and embryo grading, implantation rate, clinical pregnancy and ongoing rates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list at initiation of stimulation, to receive GnRH antagonist or agonist, by using a free internet software for randomisation (Graphpad Software QuickCalcs) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The embryologist scoring the embryos was blinded to the study groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported in a pre‐specified manner |
| Other bias | Low risk | Baseline characteristics balanced in both groups |
El Sahwi 2005.
| Methods | RCT, single‐centre, parallel design, randomisation: 1:1 | |
| Participants | 160 patients scheduled for ICSI | |
| Interventions | Agonist group were treated with buserelin/hMG stimulation (long luteal protocol) while the antagonist group were treated with cetrorelix/hMG stimulation (flexible protocol). | |
| Outcomes | The treatment period Number of hMG ampoules used Number of abandoned cycles Number of oocytes retrieved Fertilization rate Implantation rate Clinical pregnancy rate The occurrence of hyperstimulation syndrome (OHSS) The convenience and compliance of participants | |
| Notes | Number of participants at randomisation: 160 (cetrorelix: 80/buserelin: 80) Number of participants at stimulation: 160 (cetrorelix: 80/buserelin: 80) Number of participants at OPU: 142 (cetrorelix: 74/buserelin: 68) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as randomisation using opaque envelopes without any further details |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcomes data, LBR/OPR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Engmann 2008a.
| Methods | RCT, single‐centre | |
| Participants | 60 women (high responders), aged 20 – 39 years , normal basal FSH concentration ≤ 10.0 IU/L), and undergoing their first cycle of IVF with either PCOS or PCOM (defined according to the Rotterdam consensus guidelines (Rotterdam 2004)) or undergoing a subsequent cycle with a history of high response in a previous IVF cycle. Participants were recruited for the trial for only one cycle Exclusion criteria: women with hypogonadotrophic hypogonadism were excluded Baseline characteristics: age (yrs) 32.0 ± 3.7 vs 33.1 ± 3.6, BMI (kg/m2) 28.3 ± 7.1 vs 30.7 ± 6.4. Baseline serum FSH (IU/L) 5.4 ± 1.8 vs 5.3 ± 1.2 |
|
| Interventions |
Study group: OCPs for 21 days + 112 ‐ 225 IU/day rFSH + ganirelix acetate when the dominant follicle ≥ 14 mm (Flexible multiple‐dose protocol) Control group: OCP for 25 days overlapping with 1 mg leuprolide acetate (Lupron), then reduced to 0.5 mg once down‐regulation was achieved (Low‐dose long GnRH agonist protocol) + 112 ‐ 225 IU/day rFSH Oocyte maturation triggering: when 2 ‐ 3 leading follicles were > 18 mm in diameter Study group: GnRH agonist, 1 mg approximately 12 hours after the last dose of ganirelix Control group: 3300 to 10,000 IU hCG (Profasi) Oocyte retrieval: 35 hours later, followed by IVF/ ICSI Maximum number of embryos transferred: 3 Luteal phase support: study group: 50 mg IM P in oil daily + 0.1 mg transdermal E2 patches every other day; control group: 50 mg IM P in oil daily Follow up: an ultrasound scan was carried out five to six weeks after oocyte retrieval to determine the viability of the pregnancy. A second ultrasound was performed at 12 weeks’ gestation to confirm any ongoing pregnancy (positive heart beat) |
|
| Outcomes | OHSS, implantation rate, number of oocytes retrieved, proportion of mature oocytes retrieved, fertilisation rate, mid‐luteal phase mean ovarian volume (MOV), clinical and ongoing pregnancy rates, and luteal phase serum E2 and P levels | |
| Notes | The diagnosis of OHSS was based on the criteria by Golan et al | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. To ensure similar distribution of previous high response in the two groups, separate randomisation schedules were drawn up for women undergoing their first cycle and for women with a previous high response by the use of stratified randomised blocks |
| Allocation concealment (selection bias) | Low risk | Research nurse used a series of consecutively numbered sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Euro Middle East 2001.
| Methods | RCT, multi‐centre (12 centres, 9 countries), Europe and Middle East, multi‐national, open‐label, parallel | |
| Participants | 321 infertile couples undergoing ovarian stimulation for IVF‐ET with or without ICSI with all causes of infertility Inclusion criteria: healthy female partners of infertile couples, age at time of screening ≥ 18 but < 39 years, a body mass index (BMI) between 18 and 29 kg/m², a regular menstrual cycle, and willing to give written informed consent Age: not stated Duration of infertility: ganirelix 4.3 years, triptorelin 4.1 years FSH: ganirelix 5.8 IU/ml, triptorelin 2.8 IU/ml BMI: not stated |
|
| Interventions |
GnRH antagonist (n = 215): 150 IU rFSH (Puregon) (adjusted) + multiple‐dose regimen of 0.25 mg of GnRH antagonist (ganirelix) was administered SC starting from day 6 of stimulation (fixed) GnRH agonist (n=106): mid‐luteal GnRH analogue (triptorelin 0.1 mg SC ) + 150 IU rFSH (Puregon) (adjusted) Oocyte maturation triggering: hCG (Pregnyl) 10,000 IU in 1 ml saline when at least three follicles ≥ 17 mm Oocyte retrieval: About 30 ‐ 36 hours later followed by IVF or ICSI Maximum embryos transferred: 3 Luteal phase support: done according to the centre routine practice Follow up: until ongoing pregnancy |
|
| Outcomes | Premature LH surge: (defined as (LH > 10 IU/L) and progesterone level > 1 ng/L) ganirelix group 1 versus triptorelin group 0 Stimulation length: ganirelix group 9 versus triptorelin group 26 rFSH: ganirelix group 1350 IU versus triptorelin group 1800 IU E2 on hCG: ganirelix group 1090 pg/ml versus triptorelin group 1370 pg/ml Number of oocytes retrieved: ganirelix group 7.9 ± 5.1 versus triptorelin group 9.6 ± 6.8 Number of embryos obtained: ganirelix group 4.0 ± 3.0 versus triptorelin group 4.7 ± 3.0 Number of embryos transferred: not mentioned Implantation rate: ganirelix group 22.9 versus triptorelin group 22.9 Clinical preg/cycle: ganirelix group 32.3 versus triptorelin group 37.8 Clinical preg/ET: ganirelix group 35.8 versus triptorelin group 41.7 Ongoing pregnancy rate: ganirelix group 31.4 versus triptorelin group 33.9 Cancellation: ganirelix group 22 versus triptorelin group 15 Miscarriage: ganirelix group 10.3 versus triptorelin group 11.4 Ectopic: ganirelix group 2 versus triptorelin group 0 OHSS: ganirelix group 4 versus triptorelin group 1 Severe OHSS: only one case ganirelix group Local reaction: ganirelix group 11.9 versus triptorelin group 24.1 |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive response voice system, stratified randomisation, 2:1 randomisation ratio |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
Euro Orgalutran 2000.
| Methods | RCT, multi‐centre (20 centres), open‐label, parallel design | |
| Participants | 730 Infertile couples undergoing ovarian stimulation for IVF‐ET with or without ICSI with all causes of infertility Baseline characteristics: age ganirelix 31.9 ± 3.6, buserelin 31.9 ± 3.8, duration of infertility: ganirelix 4.5 ± 2.7, buserelin 4.4 ± 2.7, FSH: ganirelix 7.7, buserelin 8.4, BMI ganirelix 23 ± 2.9, buserelin 23 ± 2.7 |
|
| Interventions |
GnRH antagonist (n= 486): rFSH (Puregon) was started at fixed daily dose of 150 IU for five days and the dose was adjusted according to response + multiple‐dose regimen of 0.25 mg of GnRH antagonist (ganirelix) was administered SC starting from day 6 of hMG treatment (fixed) GnRH agonist (n= 244): mid‐luteal GnRH analogue (buserelin 0.6 or 1.2 mg four times daily intranasally) + rFSH (Puregon) was started at fixed daily dose of 150 IU for 5 days and the dose was adjusted according to response IVF was done in 357 cases, ICSI was done in 291 cases and 10 cases had both IVF and ICSI Luteal phase support: was done according to the centre's routine practice |
|
| Outcomes | Premature LH surge defined as (LH > 10 IU/L) and progesterone level > 1 ng/L Stimulation length Number of hMG ampoules E2 on hCG No of oocytes retrieved No of embryos obtained No of embryos transferred Implantation rate Clinical pregnancy/OPU Clinical pregnancy/ET Miscarriage Ectopic OHSS Moderate or severe OHSS | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive response voice system (true), 2:1 randomisation ratio |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Ferrari 2006.
| Methods | RCT, parallel design | |
| Participants | 60 women undergoing IVF treatment Inclusion criteria: healthy female partners of infertile couples, regular menstrual cycles of 26 ‐ 34 days, BMI between 20 and 25 kg/m2, age between 20 and 39 years at the time of screening, baseline serum FSH level < 10 IU/l, and baseline serum E2 level ≤ 45 pg/ml on cycle day 3 Baseline characteristics: Age (years) 34.0 ± 4.3 vs. 34.6 ± 4.3, BMI (kg/m2) 22.9 ± 0.8 vs. 23.4 ± 0.7, baseline serum FSH (IU/l) 6.63 ± 3.25 vs. 6.73 ± 2.09, baseline serum LH (IU/l) 4.60 ± 2.21 vs. 4.53 ± 1.92 |
|
| Interventions |
GnRH antagonist (n = 30): 225 IU daily SC rFSH (Gonal F) from cycle day 3 (adjusted) + 0.25 mg SC daily cetrorelix acetate (Cetrotide) when there was a 14 mm dominant follicle until and including the day of hCG administration GnRH agonist (n = 30): 0.5 mg/day SC leuprorelin acetate (Enantone die) beginning at the mid‐luteal phase + 225 IU SC rFSH (Gonal‐F) starting 14 days after pituitary down‐regulation (adjusted). (long protocol) Oocyte maturation triggering: 10,000 IU of hCG (Gonasi HP) Oocyte retrieval: 36 hours after hCG administration Mean number of embryos transferred: antagonist: 2.10 ± 1.50 vs. agonist: 2.67 ± 0.96 Luteal phase support: 100 mg intravaginal micronised progesterone (Esolut) twice a day starting one day before embryo transfer Follow‐up: Clinical monitoring was carried out daily by transvaginal pelvic ultrasound (6.5 MHz) and E2 assay up till clinical pregnancy |
|
| Outcomes | Clinical pregnancy rate, serum E2 levels on day 8 of follicular stimulation, serum E2 levels on day of hCG administration, serum LH levels on hCG day, number of mature follicles, number of retrieved oocytes, number of transferred embryos, follicular fluid (FF) insulin‐like growth factor‐I (IGF‐I) level, FF vascular endothelial growth factor (VEGF) level, FF E2 and androstenedione levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The patients were assigned randomly (computer‐generated randomization list; SPSS Inc., Chicago, IL, USA) to two different GnRH analogue regimens GnRH‐a (Group A, 30 patients) and Gn‐RH‐ant (Group B, 30 patients)” |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported but unlikely to affect measurement of outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number randomised = 60, number analysed = 60 |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes reported as pre‐specified however live birth rate not reported |
| Other bias | Low risk | None identified |
Ferrero 2010.
| Methods | Randomised controlled trial, single centre | |
| Participants | 144 women Inclusion criteria: no specific details but included women who had had moderate or severe OHSS or who had been at risk of OHSS during their first IVF/ICSI cycle with a mid‐luteal long agonist protocol Exclusion criteria: no details Setting and timing: Italy; no details of timing Baseline characteristics: not stated |
|
| Interventions |
Antagonist ‐ cetrorelix 0.25 mg/day starting on day 3 of the menstrual cycle Agonist ‐ triptorelin 0.1 mg/day starting on day 21 of the menstrual cycle Ovarian stimulation was achieved with rFSH initiated on day 3 of the cycle at a maximum dose of 150 IU and dose adjusted depending on ovarian response Luteal phase support ‐ with micronised progesterone vaginal gel (no other details) Follow‐up ‐ followed‐up to live birth |
|
| Outcomes | Live birth, clinical pregnancy, cancellation rate, oocytes retrieved | |
| Notes | As no denominators for groups are given, the data cannot be included in a meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open label but blinding unlikely to effect fertility outcome. No details of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although the abstract states that there were 144 women randomised, there are no other denominators to indicate how many women were allocated to each group. No details on withdrawals or losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract only. Outcomes are not pre‐specified. OHSS not reported |
| Other bias | Unclear risk | Conference abstract only. Groups were reported as being similar at baseline |
Firouzabadi 2010.
| Methods | RCT, single‐centre | |
| Participants | 235 infertile women undergoing IVF/ICSI Inclusion criteria: first cycle of the ART, age < 35 years, and basal FSH < 10 IU/L Exclusion criteria: previous IVF or ICSI, hyperprolactinaemia, hyperthyroidism, hypothyroidism, uterine abnormality, severe endometriosis, or solitary ovary Baseline characteristics:age (years) 28.71 ± 2.8 vs 28.36 ± 3.1, BMI (kg/m2) 28.1 ± 3.4 vs 27.54 ± 4.3, basal FSH (IU/L) 5.77 ±1.2 vs 5.54 ± 1.1 |
|
| Interventions |
GnRH antagonist (n =118): 225 IU rFSH on 2nd day of the cycles (adjusted) + HMG + 0.25 mg SC of ganirelix took place on the 6th day of the stimulation (fixed protocol) GnRH agonist (n = 117): 150‐225 IU rFSH (adjusted) + long GnRH agonist, 500 μg buserelin per day (Suprefact) (SC), during menstrual cycle 21 and onwards, once down‐regulation was achieved, the dose of buserelin was reduced to 250 µg (low‐dose GnRH agonist protocol) Oocyte maturation triggering: hCG 10,000 IU (Profasi) was administered intramuscularly (IM) when at least two follicles were 18 mm Oocyte retrieval: 36 hours later, followed by IVF/ICSI Maximum of embryo transferred: 3 Luteal phase support: 800 mg daily cyclogest suppository (Aburaihan, Iran) was started on the day of oocyte collection to provide luteal phase support, and it continued until the fetal heart activity was documented by ultrasonography Follow up: the serum hCG level on day 16 after the oocyte recovery was tested to determine chemical pregnancy, if any; a vaginal ultrasonography has been carried out on day 35 following the oocyte recovery for documentation of fetal heart activity and confirmation of a clinical pregnancy |
|
| Outcomes |
Primary outcome measures: clinical pregnancy rate per cycle and ongoing pregnancy, which later were defined as pregnancy proceeding beyond the 12th gestational week Secondary outcome measures: OHSS, defined by ≥ 15 follicles with a mean diameter ≥ 14 mm per each ovary at the end of the follicular phase of stimulation and/or E2 levels on the day of hCG administration > 3000 pg/mL and/or presence of ascites after hCG administration in ultrasonography |
|
| Notes |
Drop out: GnRH antagonist group: 6, GnRH agonist: 9 8 cycles of ET cancelled (due to poor quality of embryos in GnRH antagonist group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedules |
| Allocation concealment (selection bias) | Low risk | Sealed in envelopes and handed to participants |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Fluker 2001.
| Methods | RCT, multi‐centre (11 centres, United States and Canada), open‐label, parallel design | |
| Participants | 313 infertile couples undergoing ovarian stimulation for IVF‐ET with or without ICSI with all causes of infertility Baseline characteristics: age: ganirelix 33.0 ± 3.4 vs leuprolide 32.8 ± 4.0 Duration of infertility: ganirelix 4.1 ± 3.0 vs leuprolide 3.8 ± 2.6. FSH: ganirelix 7.9 IU/ml vs leuprolide 3.3 IU/ml. BMI: ganirelix 23.0 ± 3.0 vs leuprolide 23.0 ± 3.0 |
|
| Interventions |
GnRH antagonist (n = 205): A multiple‐dose regimen of 0.25 mg of GnRH antagonist (ganirelix) was administered SC starting from day 6 of rFSH treatment up to and including day of hCG administration (Fixed) + rFSH (Follistim) was started at fixed daily dose of 150 IU for five days and the dose was adjusted according to response GnRH agonist (n= 108): mid‐luteal GnRH analogue (leuprolide 1.0 mg SC) to 99 participants of the control group + rFSH (Follistim) was started at fixed daily dose of 150 IU for five days and the dose was adjusted according to response Luteal phase support was done according to the centre's routine practice |
|
| Outcomes | Premature LH surge defined as (LH > 10 IU/L) and progesterone level > 1 ng/L
ganirelix group versus leuprolide group Stimulation length ganirelix group versus leuprolide group rFSH: ganirelix group IU versus leuprolide group IU E2 on hCG ganirelix group pg/ml versus leuprolide group pg/ml Number of oocytes retrieved ganirelix group ± versus leuprolide group ± Number of embryos obtained ganirelix group ± versus leuprolide group ± Number of embryos transferred Implantation rate ganirelix group versus leuprolide group Clinical pregnancy/cycle ganirelix group versus leuprolide group Clinical pregnancy/ET ganirelix group versus leuprolide group Ongoing pregnancy rate ganirelix group versus leuprolide group Cancellation ganirelix group versus leuprolide group Miscarriage ganirelix group versus leuprolide group Ectopic ganirelix group versus leuprolide group OHSS ganirelix group 4 versus leuprolide group 1 Severe OHSS: ganirelix 3 cases, leuprolide 2 Local reaction ganirelix group 11.9 versus leuprolide group 24.1 |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive response voice system (true), 2:1 randomisation ratio, stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Franco 2003.
| Methods | RCT, open‐label, parallel design, single‐centre | |
| Participants | 20 participants without specific ovulatory dysfunction aged ≤ 37 who would be submitted to ovarian stimulation | |
| Interventions |
Group A (n = 14): 4 mg/day of estradiol valerate was started and continued for 14 days + rFSH (Puregon) was started one day after the end of estradiol valerate in a fixed dose of 150 ‐ 300 UI + ganirelix (Organolutran, Organon) was taken in a dose of 0.25 mg/day when the follicular diameter was ≥ 15 mm, and continued until the day of the hCG administration (Flexible) Group B (n = 6): In the 21st day of the menstrual cycle, a dose of 200 µg of nafarelin acetate was taken through nasal twice a day. After 14 days of administration of the agonist, with the blockage established (menstruation), the administration of recombinant FSH was started in a fixed dose of 150 ‐ 300 IU for a period of five days. Oocyte maturation triggering: 5 –10.000 IU uhCG at least two follicles ≥ 17 mm Maximum number of embryos transferred: 2 ‐ 3 Luteal phase support: not reported |
|
| Outcomes | No of oocytes retrieved Fertilisation rate Implantation rate Pregnancy rate | |
| Notes | Number of participants at randomisation: 20 (ganirelix: 14/ nafarelin: 6) Number of participants at stimulation: N/A Number of participants at OPU: N/A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list, randomisation: 2:1 (ganirelix:nafarelin) ratio |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Friedler 2003.
| Methods | RCT, open‐label, parallel design, single‐centre | |
| Participants | < 40 years old undergoing IVF due to male or tubal infertility | |
| Interventions | All participants received vaginal micronised progesterone (300 mg/day) as luteal supplementation. LP characteristics were compared between the two groups and between the conception and non conception cycles. Estradiol (E2), progesterone and LH levels were measured on the day of hCG administration (day 0), days +5, +8, +11 and +16. (Unclear) | |
| Outcomes | E2 and progesterone levels Clinical pregnancy rate Implantation rates | |
| Notes | Power calculation: no power calculation
Number of participants at randomisation: unclear
Number of participants at stimulation: unclear
Number of participants at OPU: unclear Type of antagonist protocol: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Protocol is not available but the methods and results match |
| Selective reporting (reporting bias) | Low risk | Intention‐to‐treat analysis was not used but the drop‐out rate was less than 10% |
| Other bias | Low risk | Baseline characteristics are similar |
Gizzo 2014.
| Methods | Superiority, randomised open‐label, single centre RCT | |
| Participants | 360 idiopathic subfertile participants Inclusion criteria: subfertile women aged between 18 and 50 years with BMI between 18 and 30 Exclusion criteria: women with previous ovarian surgery, women with a previous diagnosis of endometriosis, women treated for benign endouterine disease (such as endometrial polyps, submucous myomas, intrauterine synechiae and/or uterine septus) in the six months before IVF cycle, history of abnormalities in thyroid pattern or alteration in basal serum prolactin value and E2 peak at ovulation induction < 13 nmol/l, history of smoking; untreated uterine myomas; absence of major systemic disease such as diabetes, multiple sclerosis, adrenal diseases, karyotype abnormalities, mutations of the cystic fibrosis gene, acquired or inherited thrombophilia and immunological disorders; previous or actual neoplasia; previous chemo and/or radio treatment for neoplasia; severe qualitative and quantitative alteration in partner's semen (according to World Health Organization guidelines); absence of retrieved oocytes at pick‐up and absence of at least one fertilised oocyte Baseline characteristics: age (years) 37.97 ± 3.73 vs. 35.80 ± 4.35 |
|
| Interventions |
GnRH antagonist (n = 90): rFSH (Gonal‐F) with a starting dose (maintained for the first five days) of 100, 225 and 300 UI per day in estimated high, normo and poor responders, respectively, administered in third day after spontaneous menstruation (pending the basal E2 < 0.3 nmol/l) (adjusted) + daily dose of
0.25 mg/0.5 ml ganirelix starting from the TVS detection of at least one follicle > 14 mm in diameter and continued until hCG administration (short protocol) GnRH agonist (n =180): rFSH (Gonal‐F) with a starting dose (maintained for the first five days) of 100, 225 and 300 UI per day in estimated high, normo and poor responders, respectively, administered at achievement of hypothalamic suppression + daily dose (0.1 mg) of triptorelin acetate (long protocol) Oocyte maturation triggering: 250 mg rhCG SC Luteal phase support: low‐dose PG (200 mg vaginal capsule twice daily) or high‐dose PG (200 mg vaginal capsule three times daily plus 100 mg intramuscular daily) or high‐dose PG (200 mg vaginal capsule three times daily plus 100 mg intramuscular daily) in association with valerate E2 (2 mg vaginal tablet twice daily) Follow‐up: Up to ongoing pregnancy |
|
| Outcomes | Ongoing pregnancy, clinical pregnancy, mean value of E₂max at ovulation induction, mean value of endometrial thickness at pick‐up day, quality of embryos | |
| Notes | Three‐arm study, excluded short GnRH agonist protocol (n = 90) "After oocyte fertilization, according to the casual envelopes produced by software randomization, in each Group (A, B and C) patients were randomly assigned (with a randomization of 1:1) to one of three different subgroups (A1, A2 and A3; B1, B2 and B3 and C1, C2 and C3) in relation to the different pharmacological LPS starting from the first day after pick‐up
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study randomised participants into subgroups using appropriate random sequence generation but randomisation into large groups A, B, C not clearly stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported for the randomisation of participants into large groups A, B, C |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study is an open label trial. “As limitations we report:… the impossibility to blind patients and clinicians to the treatment…” “All serum sample were analysed by a single laboratory as well all the endometrial thickness measurements were performed by two skilled sonographers blinded to patients’ treatment” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised = 360, number analysed = unclear |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in a pre‐specified manner. However, live birth rate not reported |
| Other bias | Low risk | None identified |
Haydardedeoglu 2012.
| Methods | Single centre RCT | |
| Participants | 300 women with PCOS Inclusion criteria: women with PCOS younger than 35 years old and older than 23 years old: where diagnosis of PCOS was based on the Rotterdam 2004 Criteria, so patients with oligomenorrhoea (an irregular cycle duration greater than 45 days or less than 6 menstrual periods per year) and/or anovulation who also had at least one of the characteristics of hyperandrogenism (a hirsutism score of greater than 7 according to Ferriman and Gallwey (Ferriman 1961), and/or an elevated serum testosterone level which is over 0.8 ng/dl and measured in an Immulite One autoanalyser (Bio Diagnostic Products Corp., USA) using the chemiluminescent method) were diagnosed with PCOS after all the other causes of hyperandrogenism were excluded. Couples in their first IVF/ICSI cycles, women with PCOS whose body mass index was lower than 30 kg/m² and higher than 20 kg/m2 Exclusion criteria: women with PCOS whose ovaries did not appear polycystic (where having polycystic ovaries identified by ultrasonography was defined as the presence of 12 or more follicles in each ovary measuring 2 – 9 mm in diameter, and/or increased ovarian volume (> 10 ml)) Patients treated with hormonal medications and other oral anti‐diabetics within the previous three months Baseline characteristics: age (years) 27.57 ± 3.54 vs. 27.70 ± 3.59, BMI (kg/m2) 25.74 ± 4.37 vs. 24.97 ± 4.36, duration of infertility (years) 6.24 ± 3.64 vs. 5.85 ± 3.42, FSH (IU/ml) 4.77 ± 1.80 vs. 5.03 ± 1.36, LH (IU/ml) 5.94 ± 4.17 vs. 5.60 ± 3.49, E2 (pg/ml) 42.82 ± 33.62 vs. 38.83 ± 25.02, IVF/ICSI indications: PCOS + male factor (%) 40.7 (61/150) vs. 38.7 (58/150), PCOS only (%) 54.7 (82/150) vs. 53.3 (80/150), PCOS tubal factor (%) 4.7 (7/150) vs. 8 (12/150). |
|
| Interventions |
GnRH antagonist (n = 150): OCPs (30 μg of ethinyl estradiol (E2) and 3 mg of drosprinone (Yasmin)) for 21 days starting on cycle day 3 prior to the treatment cycle + 150 IU rFSH (Puregon) initiated on day 3 of menstruation after discontinuation of OCPs + 0.25 mg daily SC ganirelix initiated on day 6 of gonadotrophin stimulation until day of hCG administration. (OCP + GnRH fixed antagonist protocol) GnRH agonist (n = 150): OCPs (30 μg of ethinyl estradiol (E2) and 3 mg of drosprinone (Yasmin)) for 21 days starting on cycle day 3 prior to the treatment cycle + 1 mg daily leuprolide acetate (Lucrin) beginning on day 21 of the preceding menstruation (last three tablets of OCP). Dose reduced to 0.5 mg after ovarian suppression was achieved, until the day of hCG + 150 IU rFSH (Puregon) if there were no cysts ≥ 2 cm and the E2 was < 50 pg/ml. (OCP + long GnRH agonist protocol) Oocyte maturation triggering: 10,000 IU hCG when at least three follicles had a maximum diameter of > 17 mm Oocyte retrieval: 35 – 36 h after hCG injection, ICSI was performed after two hours of incubation and embryos were transferred on day 3 Maximum embryos transferred: 3 Luteal phase support: 90 mg/day intravaginal progesterone (Crinone 8% gel) starting after embryo transfer until the 8th gestational week Follow‐up: Up to ongoing pregnancy |
|
| Outcomes | Ongoing pregnancy rate, miscarriage rate, OHSS rate, cycle cancellation rate, day 5 E2 level, E2 level at the day of hCG, progesterone level at the day of hCG, endometrium at the day of hCG, duration of COH, total gonadotrophin used, total cost of COH, MII oocyte number, germinal vesicle number, fertilisation rate, grade 1 embryo number, grade 2 embryo number, total transferred embryos, positive hCG rate, biochemical pregnancy rate, implantation rate, cryopreservation rate, multiple pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The 300 eligible patients were randomized into two groups by an allocation sequence generated from a random numbers table and assigned using consecutively numbered opaque, sealed envelopes on the day of initiation of OCP. Study subjects were randomized in blocks of 30; i.e. of every 30 subjects randomized, Fifteen were allocated to the GnRH agonist and Fifteen were allocated to the GnRH antagonist arm in a random manner.” |
| Allocation concealment (selection bias) | Low risk | “…assigned using consecutively numbered opaque, sealed envelopes on the day of initiation of OCP.” |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported but unlikely to affect measurement of outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number randomised = 300, number analysed = 300 |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the pre‐specified manner, except that clinical pregnancy listed as outcome measure at protocol but not reported in study. Live birth rate not reported |
| Other bias | Low risk | None identified |
Heijnen 2007.
| Methods | RCT, two centres, parallel‐group randomised, open‐label, non‐inferiority effectiveness trial | |
| Participants | 404 infertile women undergoing IVF/ICSI Inclusion criteria: no previous IVF treatment or had borne a healthy child after previous IVF treatment, were aged younger than 38 years, and had a menstrual cycle length of 25–35 days and a body‐mass index of 18–28 kg/m² Baseline characteristics: age of women (years) 32.9 (3.1) vs 32.8 (3.2), BMI (kg/m²) 23.0 (2.6) vs 23.2 (2.5), Duration of infertility (years) 3.6 (1.9) vs 3.6 (2.1) |
|
| Interventions |
GnRH antagonist (n = 205): mild ovarian stimulation with gonadotrophin‐releasing hormone [GnRH] antagonist co treatment combined with single embryo transfer (mild protocol) GnRH agonist (n = 199): (stimulation with a GnRH agonist long protocol and transfer of two embryos (long GnRH agonist protocol) |
|
| Outcomes | Primary outcome measures: pregnancy and term live‐birth within one year of randomisation; total costs per couple and child up to six weeks after expected delivery; and participant’s discomfort | |
| Notes |
Supernumerary high‐quality embryos: cryopreserved and thawed for transfer in a subsequent unstimulated cycle before the start of a new IVF treatment cycle. These frozen‐thawed embryo‐transfer cycles were treated as a part of the previous IVF cycle. In both groups either one or two cryopreserved embryos were transferred, according to the participant’s preference Fund: ZonMw (Netherlands), programme Doelmatigheidsonderzoek |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random blocks of size four and six were stratified by centre |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes and made available at each centre; envelopes were sequentially allocated to consecutive participants and opened by treating physicians at IVF planning consultations |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free of other source of bias |
Hershko Klement 2015.
| Methods | Pilot randomised controlled trial, single centre | |
| Participants | 60 women Inclusion criteria: indication for IVF/ICSI Exclusion criteria: ovulatory factor (WHO class I‐III), age ≥ 37 years, more than three previous unsuccessful IVF cycles, TESA/TESE cycles, surrogacy cycles and refusal to participate Setting and timing: IVF unit, Israel. August 2012 to July 2013 Baseline characteristics: age ‐ agonist group 28.6 ± 4.3; antagonist group 29.8 ± 4.3 |
|
| Interventions |
Antagonist ‐ programmed to start on a Friday, antagonist (Cetrotide) ‐ no details of dose) was used in a flexible way. Programming achieved with oral estradiol valerate 2 mg twice daily during early follicular phase from day 2 of a spontaneous menstrual cycle until the first Friday following Agonist ‐ Long luteal agonist protocol (triptorelin‐ no details of dose) Mean number of embryos transferred: antagonist: 1.4 ± 0.7 vs agonist 1.2 ± 0.6 Number of ampoules used: antagonist 24.9 ± 8.1 vs agonist 25.5 ± 13.3 Controlled ovarian hyperstimulation initiated with rFSH alone or in combination with hMG. Adjustments made dependant on individual response |
|
| Outcomes | Clinical pregnancy is the only prespecified outcome. Also reported on fertilisation rate, embryo quality, number of embryos transferred, amount of FSH, follicular size | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no other details |
| Allocation concealment (selection bias) | Low risk | Allocation using sealed envelopes handled by an administrator not involved in the study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open label study but blinding unlikely to effect fertility outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 60 women were randomised and nine did not complete treatment (15%). Three chose another fertility unit, three decided to stop fertility treatment, two were screened out during routine laboratory testing and one had a spontaneous extrauterine pregnancy that required surgical intervention and then postponed fertility treatment. The groups that these women were allocated to was not specified 27/31 women in the antagonist group were analysed and 24/29 in the agonist group |
| Selective reporting (reporting bias) | Unclear risk | Clinical pregnancy rate was the only prespecified outcome although there are more outcomes reported in the results section. OHSS was not reported |
| Other bias | Low risk | Groups were balanced at baseline |
Hohmann 2003.
| Methods | RCT, university‐affiliated IVF centre, open‐label, parallel design, randomisation: 2:1 (cetrorelix:triptoreline) ratio | |
| Participants |
142 infertile women undergoing IVF/ICSI Inclusion criteria: age between 20 to 38 yrs; BMI 19 to 29 kg/m2; history of regular menstrual cycles, ranging from 25 to 35 days; no relevant systemic disease, severe endometriosis, or uterine and ovarian abnormalities; no more than three previous IVF cycles; and no previous IVF cycle with a poor response or ovarian hyperstimulation syndrome |
|
| Interventions |
Group A: GnRH agonist triptoreline (Decapeptyl) 1 mg/d, SC starting one week before the expected menses (usually cycle day 21) + fixed daily dose of 150 IU rFSH SC (Gonal‐F) Groups B and C: GnRH antagonist cetrorelix (Cetrotide) 0.25 mg/d, SC commencing when the largest follicle had reached a diameter of 14 mm + rFSH was initiated on cycle day 2 (group B) or 5 (group C). (Flexiblle) Oocyte maturation triggering: when the leading follicle had reached a diameter of 18 mm or more and at least three follicles had reached a diameter of 15 mm or more, 10,000 IU hCG (Pregnyl) was administered Embryo transfer: 35 hrs before the planned time of oocyte retrieval followed by IVF with or without ICSI Maximum number of embryos transferred: 2 embryos were transferred at 3 ‐ 5 days Luteal support: intravaginal progesterone (P; Progestan, Organon; 200 mg, three times daily) was given from the day of oocyte retrieval until a urine pregnancy test was performed 17 days later |
|
| Outcomes | Included patients (n = 142) Age (yr) Body mass index (kg/m2) FSH day 2/3 (IU/litre) Inhibin Bday 2/3 (ng/litre) Participants undergoing oocyte retrieval (n 104) n (% per started cycle) Cycle day start cetrorelix Day hCG FSH (IU/litre) LH (IU/litre) E2 (n mol/litre) P (n mol/litre) Number of follicles (10 mm) day hCG Number of follicles (15 mm) day hCG Number of oocytes retrieved Number of embryos Fertilisation rate per subject (%) Number of pregnancies (%) Number of ongoing pregnancies (%) Number of twin pregnancies (%) | |
| Notes | Number of participants at randomisation: 169 (cetrorelix: NA/ triptoreline: NA) Number of participants at stimulation: 142 (cetrorelix: 45/ triptoreline: 97) Number of participants at OPU: 104 (cetrorelix: 38/ triptoreline: 66) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Schedule assigned via numbered sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Hoseini 2014.
| Methods | Randomised controlled trial | |
| Participants | 50 infertile couples undergoing IVF/ICSI Inclusion criteria: male factor, tubal factor or unexplained subfertility, no ovulatory dysfunction, age 40 years or less, normal basal FSH and LH (< 10 mIU/ml) Exclusion criteria: no details Baseline characteristics: age ‐ antagonist 33.8 ± 5.6 years versus agonist 30.4 ± 5.5 years Setting and timing: infertility centre, Iran June 2012 to November 2013 |
|
| Interventions |
Antagonist Fixed multi‐dose protocol (n = 24) cetrorelix acetate 0.25 mg/day on day 6 until day of hCG injection Agonist long protocol (n = 26) with OCP pre‐treatment starting on day 2 or day 3 of previous cycle. Buserelin acetate 500 micrograms started on day 21 until pituitary down‐regulation. Reduced to 250 micrograms per day when follicle > 10 mm diameter, E2 > 50 pg/ml until the day of hCG injection Ovarian stimulation started on day 3 using rFSH 150 to 225 IU hCG 5000 IU given IM when at least three follicles had a mean diameter of 17 mm Ooycte retrieval 34 to 36 hours after hCG injection |
|
| Outcomes | Gene expression. No pregnancy outcomes reported for inclusion in a meta‐analysis | |
| Notes | Sample size calculation ‐ no ITT analysis ‐ no Funding ‐ Deputy Ministry for Research, Tehran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details for participants or outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details were reported to make a conclusive judgement |
| Selective reporting (reporting bias) | High risk | No pregnancy outcomes reported. Unable to include any data in a meta‐analysis |
| Other bias | Low risk | Groups appear balanced at baseline |
Hosseini 2010.
| Methods | Randomised controlled trial. Single centre | |
| Participants | 112 infertile women with PCOS (Rotterdam criteria) Inclusion criteria: PCOS, < 35 years, normal BMI (< 27 kg/m2), normal prolactin, normal thyroid levels, normal semen analysis for male partner Setting and Timing: Department of Infertility, Tehran, Iran. During 2006 Baseline characteristics: age‐ antagonist 27.8 ± 3.4 years versus agonist 29.3 ± 4.2 years |
|
| Interventions | All participants had folic acid 1 mg/day before the induction cycle, low‐dose OCP on day 3 of previous cycle and doxycycline 100 mg twice daily for the first 10 days of the previous cycle Antagonist (n = 57) ovarian stimulation with Gonal F 150 IU commenced on day 3. When follicular diameter ≥ 14 mm cetrorelix 0.25 mg/day SC given for three days. HMG given after seventh day of stimulation. Agonist (n = 55) buserelin 0.5 mg SC started on day 21 of previous cycle. Ovarian stimulation with Gonal F 150 IU commenced on day 3 of next cycle and replaced with HMG after the seventh day of stimulation Oocycte maturation triggered when at least two follicles ≥ 17 mm and hCG 10,000 IU given IM Oocyte retrieval 36 to 38 hours after hCG. Luteal phase support ‐ progesterone suppository cyclogest 800 mg daily |
|
| Outcomes | Clinical pregnancy, chemical pregnancy, number of oocytes retrieved, number and days of gonadotrophins, miscarriage, multiple pregnancy, OHSS and severe OHSS | |
| Notes | Sample size calculation ‐ no ITT analysis ‐ yes Funding ‐ none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomized' |
| Allocation concealment (selection bias) | Unclear risk | 'Sequential' no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details. Unlikely to have been blinded but blinding unlikely to affect fertility outcomes. Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Women randomised were analysed. No losses reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. However, did not report on live birth or ongoing pregnancy as an outcome |
| Other bias | High risk | Women in the agonist group were slightly older at baseline |
Hsieh 2008.
| Methods | RCT phase III, open‐label, single‐centre study | |
| Participants | 251 infertile women undergoing IVF/ICSI Inclusion criteria: age at least 18 years but not older than 39 years; and body weight of 40 – 70 kg Baseline characteristics: age (yr) 33.9 ± 4.4 vs 32.3 ± 2.1 vs 31.6 ± 2.4 vs 30.9 ± 2.5 vs 32.1 ± 2.7; BMI (kg/m2) 20.6 ± 1.4 vs 19.0 ± 1.0 vs 19.5 ± 1.1 vs 20.7 ± 2.1 vs 21.1 ± 1.8; Baseline FSH (IU/L) 4.0 ± 1.8 vs 3.7 ± 1.6 vs 3.9 ± 1.3 vs 3.8 ± 1.4 vs 3.6 ± 1.8 |
|
| Interventions |
Down‐regulation Group 1 (n = 86): cetrorelix 0.25 mg/day, cetrorelix was administered from menstrual day 8 until the day of hCG administration. (Fixed) Group 2 (n = 28): cetrorelix 0.2 mg/day Group 3 (n = 30): cetrorelix 0.15 mg/day Group 4 (n = 58): leuprolide acetate 0.5 mg/day, administered on days 21–23 of the previous menstrual cycle Group 5 (n = 49): leuprolide acetate depot 1.88 mg. Single dose leuprolide acetate depot subcutaneous COH: 150 – 225 IU/day rFSH (Gonal‐F) in women < 34 years old, 225 ‐ 300 IU rFSH in women > 34 years Final oocyte maturation triggering: 5000 IU hCG were given when at least three mature ≥ 18 mm follicles were obtained Oocytes retrievals: 36 hrs later Maximum embryo transfer: six embryos were transferred at 72 hrs after IVF/ICSI injection Luteal phase support: hCG (2000 IU/day) on days 1, 4 and 7 post‐ET and progesterone (400 mg/day; Utrogeston) from day 1 post‐ET Follow up: clinical pregnancy was determined by visualisation of a gestational sac, and fetal viability by ultrasound four weeks post‐ET |
|
| Outcomes | Gn dosage, and serum concentration of LH and E2 on the day of hCG administration, retrieved oocyte and embryo numbers, development of OHSS, embryo quality, and pregnancy rate (PR), implantation rate (IR) and abortion rate (AR) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Unclear risk | No source of other bias identified |
Huirne 2006.
| Methods | RCT, multi‐centre (eight European IVF centres), phase IIIb study | |
| Participants | 182 infertile women undergoing IVF/ICSI Inclusion criteria: participants needed to have a regular IVF/ICSI indication, a male partner with viable sperm in the ejaculate (testicular biopsy or epididymal sperm was not allowed), aged between 18 and 39 years Exclusion criteria: people with any previous assisted reproduction treatment cycles with less than three oocytes or three or more consecutive ART cycles without a clinical pregnancy or with any contraindication to ART, gonadotrophins or OC pills. People with a significant systemic disease Baseline characteristics: age (years) 32.8 ± 3.8 vs 32.2 ± 4.2. BMI (kg/m2) 23.7 ± 4.0 vs 22.6 ± 3.5. FSH (IU/l) on cycle day 2 or 3 7.2 ± 2.2 vs 7.4 ± 3.3 0. Estradiol (pmol/l) on cycle day 2 or 3 138 ± 55, 148 ± 103 |
|
| Interventions |
GnRH antagonist (n = 91 ): daily OCPs (30 μg ethinyl E2 and 150 μg levonorgestrel) for 21 – 28 days + r‐hFSH 150 – 225 IU (Gonal‐F) according to the study centre’s standard practice (adjusted)+ daily cetrorelix 0.25 mg subcutaneously started on stimulation day 6 and continued up to and including the day of r‐hCG administration. (Fixed) Group 2 (n = 91): daily buserelin, 500 μg, subcutaneously at the mid‐luteal phase of a natural cycle for at least 10 days until down‐regulation was achieved, after which the dose was reduced to 200 μg/day + r‐hFSH 150 – 225 IU (Gonal‐F), according to the study centre’s standard practice (adjusted) Final oocyte triggering: r‐hCG 250 μg (= 6500 IU) (Ovitrelle) was injected as soon as the largest follicle reached a mean diameter ≥ 18 mm and at least two other follicles of a mean diameter ≥ 16 mm Oocyte retrieval: 34 – 38 hrs after r‐hCG administration under ultrasound guidance, followed by a standard IVF or ICSI procedure Maximum number of embryo transfer: no more than two to three embryos were replaced either 2 – 3 days or 5 – 6 (blastocyst transfer) days Luteal phase support: intravaginal natural progesterone (three times daily 200 mg Progestan®, Organon, Oss, The Netherlands) was started as luteal support. This was continued up to a negative pregnancy test or during the first three weeks of pregnancy |
|
| Outcomes | Number of oocytes retrieved in IVF/ICSI patients Pregnancy was defined as continuing increase in serum hCG. In that case, four and 10 weeks after embryo transfer, ultrasound was performed to assess the number of fetal sacs and heart activity. Clinical pregnancy was defined as the presence of a fetal sac, with or without heart activity. Ongoing pregnancy as a positive heart activity at a gestational age of 12 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated concealed randomisation list. Randomisation was performed by centre |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Hwang 2004.
| Methods | RCT, single‐centre Part II trial | |
| Participants | 60 PCOS infertile women undergoing IVF/ICSI Inclusion criteria: PCOS included: (i) chronic anovulation manifested by the symptoms of oligomenorrhoea (0.40 days per cycle), amenorrhoea or irregular menstrual cycle and confirmed by a basal body temperature chart or serum progesterone determination; (ii) ultrasonographic evidence of polycystic ovaries an enlarged ovary with > .10 peripherally located follicles of 3 – 8 mm diameter around a dense central stroma; and (iii) at least one of the two hormonal abnormalities (a) normal FSH concentration (3 – 10 mIU/ml) and elevated LH concentration ( .10 mIU/ml) or LH /FSH ratio .2; and (b) hyperandrogaenemia (serum testosterone concentrations .0.8 ng/ml). A diagnosis of congenital adrenal hyperplasia, Cushing’s syndrome, androgen‐producing tumours, hyperprolactinaemia and thyroid dysfunction were all excluded Exclusion criteria: Women older than 38 years or with serum FSH levels .12 mIU/ml. Baseline characteristics: age (years) 31.4 ± 3.5 vs 31.7 ± 3.7. Duration of infertility (years) 4.4 ± 1.9 vs 4.4 ± 1.6. BMI (kg/m2) 23.2 ± 2.8 vs 23.4 ± 2.9. Baseline FSH 5.8 ± 1.2 vs 5.4 ± 1.7 |
|
| Interventions |
GnRH antagonist: Diane‐35/day from day 5 of the cycle for 21 days + cetrorelix acetate was then initiated with a single dose of 0.25 mg administered SC + from day 4 to day 9, cetrorelix acetate was reduced to 0.125 mg/day + 150 IU of hMG (Pergonal) every day. The dose of cetrorelix acetate was increased to 0.25 mg/day from day 10 until the day before hCG (Pregnyl; NY Organon) injection, and the dose of HMG (Fixed)
GnRH agonist: GnRH agonist long protocol. A GnRH agonist, buserelin acetate (Supremon), 500mg/day was administered from day 3 of induced or spontaneous menstruation. After 14 days of buserelin injection, buserelin was continued until the day of hCG injection, while the dosage was decreased to 250 mg/day at the beginning of hMG administration + 150 IU/day hMG was prescribed for six days beginning from the day of ensuing pituitary down‐regulation Oocyte maturation triggering: hCG, 10,000 IU, was administered IM when at least two follicles reached 18 mm in diameter with adequate E2 response Oocyte retrieval: was performed 36 hrs later Embryo transfer: was performed three days after oocyte recovery Luteal phase support: 600 mg of vaginally administered micronised progesterone (Utrogestan) daily starting from the day after oocyte retrieval Follow up: clinical pregnancy was defined as a visible fetal heart beat on ultrasonography at seven weeks of gestation |
|
| Outcomes |
The primary outcome measures: fertilisation, pregnancy and implantation rates The secondary outcome measures: serum LH and testosterone status upon starting and during HMG administration, and the total days of injection |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation numbers with a block size of 10 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The laboratory staff were blinded to the stimulation protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias. |
Inza 2004.
| Methods | RCT, single‐centre, parallel design | |
| Participants | Patients < 40, with FSH levels on day 3 < 12 IU/ml | |
| Interventions | GnRH agonist long protocol versus GnRH antagonist protocol (type of antagonist protocol: N/A) (unknown) | |
| Outcomes | Number and quality of retrieved oocytes Amount of gonadotrophins used Days of stimulation Final estradiol levels Fertilisation rate Number and quality of embryos transferred Pregnancy rate Implantation rate | |
| Notes | Number of participants at randomisation: 45 (antagonist: 23/agonist: 22) Number of participants at stimulation: 45 (antagonist: 23/ agonist: 22) Number of participants at OPU: 45 (antagonist: 23/ agonist: 22) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Karimzadeh 2010.
| Methods | RCT, single‐centre trial | |
| Participants | 243 women who were candidates for ART Inclusion criteria: age 18 – 35 years, presence of a regular and proven ovulatory menstruation cycle with a length of 26 to 35 days, basal FSH < 10 IU/L, BMI of 18 – 30 kg/m2 and first IVF attempt. Indication for IVF were unexplained infertility, male factor, tubal factor, early stage endometriosis and cervical factor Baseline characteristics: age (years) 30.0 ± 2.3 vs 29.4 ± 2.4, BMI (kg/m2) 25.9 ± 2.3 vs 25.3 ± 1.9. Basal FSH (IU/L) 6.5 ± 1.2 vs 5.9 ± 1 |
|
| Interventions |
GnRH antagonist (n = 121): clomiphene citrate 100 mg from cycle day three through seven + rFSH 75 IU daily from cycle day 5 + 0.25 mg GnRH antagonist (ganirelix) daily was started with dominant follicle ≥ 12 mm and in this day 75 IU human menopausal gonadotrophin (hMG) (Menogon) increased to the initial gonadotrophin. (mild Flexible GnRH antagonist protocol) GnRH agonist (n = 122): buserelin (Suprefact) 500 µg SC everyday for menstrual cycle 21, once down‐regulation had been achieved, then the dose of buserelin would be reduced to 250 lg + 150 – 225 IU rFSH (Gonal F) SC Oocyte maturation triggering: Human chorionic gonadotrophin 10,000 IU (Pregnyl) was given when one to three follicles reached 18 mm. Oocyte retrieval: 34 to 36 hrs after hCG and IVF or ICSI was performed ET: on day 2 or 3, under ultrasound guidance Luteal support: progesterone in oil 100 mg daily IM was started on the day of oocyte retrieval and continued until the documentation of fetal heart activity on ultrasound Follow up: pregnancy was confirmed by measuring serum ß‐hCG levels 12 days after ET. Clinical pregnancy was considered as the presence of gestational sac with fetal heart activity by TVS that was performed three weeks after positive ß‐hCG |
|
| Outcomes |
Primary outcome measures: clinical pregnancy rate per cycle and ongoing pregnancy; later were defined as pregnancy proceeding beyond the 12th gestational week Secondary outcome: OHSS, defined by ≥ 15 follicles with a mean diameter ≥ 14 mm per each ovary at the end of the follicular phase of stimulation, and/or E2 levels on the day of hCG administration 3,000 pg/mL and/or presence of ascites after hCG administration in ultrasonography |
|
| Notes | In control group (GnRH agonist/gonadotrophin) six participants were excluded, 13 participants did not come back, and follow up in three participants lost. In study group (CC/gonadotrophin/antagonist) two participants were excluded, 12 participants did not come back, and follow up in seven participants lost | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data, however the study did not address live birth rate |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Khalaf 2010.
| Methods | Randomised controlled trial. Single centre | |
| Participants | 50 women Inclusion criteria: ≤ 35 years, regular menstrual cycles, normal basal FSH (≤ 10 IU/L), LH (≤ 10 IU/L) and estradiol ≤ 50 pg/ml), BMI 18 to 24 kg/m2 Exclusion criteria: clinical evidence of endometriosis, PCOS or OHSS during stimulation Setting and timing: Assisted Reproduction Unit, Caen, France. Timing not specified Baseline characteristics: age ‐ antagonist 31 ± 0.7 years versus agonist 31 ± 0.8 years |
|
| Interventions |
Antagonist ‐ (n = 22) cetrorelix 0.25 mg daily (multiple dose protocol) starting day when dominant follicle ≥ 14 mm and continued up to day of hCG administration Agonist ‐ (n = 28) triptorelin 0.1 mg/day from first day of menstrual cycle up to day of hCG administration rFSH 225 IU/day on second day of menstrual cycle hCG (5000 IU) when at least three follicles 18 mm diameter |
|
| Outcomes | Aromatase activity in granulosa lutein cells | |
| Notes | Sample size calculation ‐ no ITT analysis ‐ yes Funding ‐ Prgramme Hospitalier Recherche Clinique |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomly assigned' 'ballot'; no further details reported |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 'without blinding'. Lack of blinding is unlikely to affect fertility outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were analysed |
| Selective reporting (reporting bias) | High risk | There were no pregnancy outcomes pre‐specified |
| Other bias | Low risk | Groups appear balanced at baseline |
Kim 2004.
| Methods | RCT, single‐centre, parallel design | |
| Participants | 41 women undergoing IVF/ICSI Inclusion/exclusion criteria: not stated |
|
| Interventions |
GnRH antagonist: OCP+ cetrorelix 0.125 mg/day, was administered on days 1 and 2 of COH with rFSH, and cetrorelix 0.25 mg/day was restarted when the leading follicle reached a mean diameter of 13 mm and continued to the day of hCG injection. (Flexible) GnRH agonist: no details were available for the agonist group, except that they were down‐regulated with triptorelin (triptorelin 0.1 mg/day) |
|
| Outcomes | Number of retrieved oocytes Number of MII oocytes Number of embryos transferred Fertilisation rate Ongoing pregnancy rate | |
| Notes | Number of participants at randomisation: 41 (cetrorelix: 21/ triptorelin: 20) Number of participants at stimulation: 41 (cetrorelix: 21/ triptorelin: 20) Number of participants at OPU: 41 (cetrorelix: 21/ triptorelin: 20) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcomes data, however LBR did not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
Kim 2011.
| Methods | Parallel group study Number of women randomised: 120 Number of withdrawals: none Number of women analysed: 120 Duration of study: one cycle | |
| Participants | Country: authors are from South Korea 120 poor responders (repeated day 3 levels of FHS > 8,5 mIU/mL, and/or antral follicle count ≤ 5) Inclusion criteria: not clearly stated Exclusion criteria: PCOS (Rotterdam criteria) Mean age: Group 1: 36.7 ± 3.1 years, Group 2: 35.9 ± 2.8 years, Group 3: 36.4 ± 3.3 years Setting: University‐based infertility clinic, Seoul, South Korea |
|
| Interventions | Pretreatment was ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg for 21 days in the cycle preceding controlled ovarian stimulation Group 1: GnRH antagonist multiple dose protocol after OCP pretreatment (n = 40) ovarian stimulation commenced five days after OCP discontinued using rFSH 225 IU/day (dose‐adjusted every three to four days). Cetrotide 0.25 mg started when lead follicle was 14 mm diameter and continued until day of hCG injection versus Group 2 GnRH antagonist multiple‐dose protocol without OCP pretreatment (n = 40) ovarian stimulation commenced on cycle day three using rFSH 225 IU/day (dose adjusted every three to four days). Cerotide 0.25 mg started when lead follicle was 14 mm diameter and continued until day of hCG injection. versus Group 3 GnRH agonist luteal low‐dose long protocol without OCP pretreatment (n = 40). Daily injection of decapeptyl 0.1 mg started from mid‐luteal phase and continued until menses followed by a dose reduction to 0.05 mg daily and continued until day of hCG injection Dose of rFSH: multidose protocol with OCP 2925.0 ± 423.9 IU versus multidose protocol without OCP 2905.0 ± 421.8 IU versus agonist 3273.6 ± 438.3 IU |
|
| Outcomes | Primary ‐ number of mature oocytes retrieved Secondary ‐ total amount and days of rFSH, number of fertilised oocytes and grade I and II embryos, implantation rate, clinical pregnancy rate per cycle and live birth rate per cycle, miscarriage rate |
|
| Notes | Power calculation ‐ yes ITT analysis ‐ yes Funding ‐ not reported Earlier publications were Kim 2005 and Kim 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'computer‐generated lists' |
| Allocation concealment (selection bias) | Unclear risk | 'The sequence of allocation to the three groups was provided to the investigating physicians...' |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Trial not blinded but unlikely to affect outcome of pregnancy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In groups 1 and 3 there were no losses, withdrawals or cancellations. In group 2, one cycle was cancelled before embryo transfer |
| Selective reporting (reporting bias) | Low risk | All outcomes listed were reported: although multiple pregnancy is reported it is not listed a priori |
| Other bias | Low risk | Groups were balanced at baseline |
Kim 2012.
| Methods | RCT, single centre trial | |
| Participants | 211 infertile women with PCOS undergoing IVF Inclusion criteria: infertile women with PCOS (PCOS diagnosis was based on the revised PCOS diagnostic criteria of the 2003 Rotterdam consensus). Good health with normal cardiac, hepatic and renal functions, and had experienced spontaneous onset of puberty and normal sexual development Exclusion criteria: women who has taken any hormonal therapy within the preceding three months Baseline characteristics: age (years) 32.5 ± 4.5 vs. 32.2 ± 4.2, BMI (kg/m2) 22.9 ± 3.1 vs. 22.7 ± 2.9, infertility duration (years) 3.3 ± 1.6 vs. 3.1 ± 1.3, number of nullipara 64 (60.4) vs. 66 (62.9), AFC 27.7 ± 4.1 vs. 26.5 ± 3.9, fasting glucose (mg/dL) 97.4 ± 20.1 vs. 96.4 ± 18.4, two‐hour glucose after 75 g glucose load (mg/dL) 132.5 ± 27.8 vs. 128.5 ± 24.6, basal FSH (IU/L) 4.2 ± 1.3 vs. 4.3 ± 1.0, basal LH (IU/L) 7.5 ± 1.7 vs. 7.2 ± 1.6 |
|
| Interventions |
GnRH antagonist (n = 106): OCP + 50 to 150 IU of rhFSH (Gonal‐F) five days after discontinuation of OCP (adjusted) + 0.125 mg/day cetrorelix (Cetrotide) in the morning of stimulation days 1 and 2. When the mean diameter of lead follicle reached 13 mm, cetrorelix at a dose of 0.25 mg/day was started again and continued daily up to the day of r‐hCG injection. (Multiple dose protocol) GnRH agonist (n = 105): OCP + 50 to 150 IU of r‐hFSH (Gonal‐F) (adjusted) + 0.1 mg/day triptorelin (Decapeptyl) from day 18 of OCP pretreatment cycle. When pituitary desensitisation was achieved, ovarian stimulation was started and the dose of triptorelin was reduced to 0.05 mg daily and continued up to day of r‐hCG administration. (Long protocol) Oocyte maturation triggering: 250 μg r‐hCG SC when one or more follicles reached a mean diameter of 17 mm Oocyte retrieval: 36 hours after r‐hCG injection, followed by IVF or ICSI on the third day after oocyte retrieval Maximum embryos transferred: 4 Luteal phase support: 90 mg vaginal progesterone gel (Crinone gel 8%) once daily from the day of oocyte retrieval Follow‐up: Pregnancies were confirmed by rising serum β‐hCG concentrations and transvaginal ultrasonographic evidence of a gestational sac. The serum level of β‐hCG was measured 11 days after ET |
|
| Outcomes | Live birth rate, miscarriage rate, clinical pregnancy rate, incidence of severe OHSS, cycle cancellation rate, progesterone levels, estradiol levels and endometrial thickness on the day of hCG injection, total amount and days of r‐hFSH administered, the numbers of retrieved, mature, fertilised oocytes and good quality embryos, numbers of embryos transferred and cryopreserved, embryo implantation rate, multiple pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects, aged 25 to 39 years, were randomized into either the GnRH antagonist MDP‐EL (antagonist group, n = 106) or the GnRH agonist LP (agonist group, n = 105) by the use of sealed envelopes and a computer‐generated list." |
| Allocation concealment (selection bias) | Unclear risk | Study did not report whether envelope was sequentially‐numbered, opaque and safe‐guarded. “…by the use of sealed envelopes. The sequence of allocation to the two groups was provided to the investigating physicians and randomization was performed as planned according to the randomization list order.” |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not reported but it is unlikely to influence measurement of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number randomised = 211, number analysed = 208 (missing data balanced across the groups, and reasons similar) “One cycle (0.9%) in the antagonist group and 2 cycles (1.9%) in the agonist group were cancelled after oocyte retrieval due to a high risk of OHSS. There was no significant difference in cycle cancellation rate between the two groups” |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as it is in the pre‐specified manner |
| Other bias | Low risk | None detected |
Kurzawa 2008.
| Methods | RCT, single‐centre trial | |
| Participants | 74 PCOS meeting Rotterdam criteria undergoing ICSI Inclusion criteria: male factor subfertility, several unsuccessful intrauterine inseminations, previous ineffective IVF (none or < 30% of fertilisations), age ≤ 35 years, BMI < 26 kg/m2, basal FSH < 12 mIU/ml, negative HBV and HCV virus infection and HIV Exclusion criteria: ≥ 2 miscarriages, ≥ 3 unsuccessful IVF/ICSI cycles, anatomical abnormalities of the uterus on laparoscopy or hysteroscopy and existence of ovarian cysts Baseline characteristics:age (years) 31.33 ± 3.91 vs 30.36 ± 3.40, BMI (kg/m2) 23.1 ± 1.3 vs 22.3 ± 1.6 |
|
| Interventions |
GnRH antagonist (n = 37): OCP (Cilest) + 150 IU rFSH on 2nd day of the cycle (adjusted) + 0.25 mg SC of cetrorelix acetate (Cetrotide) administered when follicles reached a diameter of 14 mm (flexible protocol) GnRH agonist (n = 37): OCP (Cilest) + 150 IU rFSH (adjusted) + long GnRH agonist triptorelin (Diphereline SR 3.75 ) (DepotGnRH agonist protocol) Oocyte maturation triggering: 10,000 IU hCG or SC injection of 250 μg hCG when the dominant follicle reached ≥ 18 mm with the following two ≥ 16 mm and estradiol level between 1000 and 4000 pg/mL Oocyte retrieval: 36 hours later, followed by ICSI Maximum of embryo transferred: 3 Luteal phase support: oral 30 mg/day of dydrogesterone (Duphaston), and intravaginal 150 mg/day of progesterone Follow up: Pregnancy was checked by pregnancy test in serum 14 days after ET and confirmed by vaginal ultrasound scan at 12 weeks of gestation |
|
| Outcomes | Primary endpoints Embryological: Matured oocyte (M2) rate, defined as proportion of metaphase II to total number of retrieved oocytes Fertilisation rate, defined as proportion of two pronuclei oocytes to number of injected oocytes Quality of zygotes on the first day of culture Quality of embryos on the third day of culture Secondary endpoints Clinical: Delivery per attempt, defined as a live birth after 32 weeks of gestation Clinical pregnancy per attempt, defined as an ongoing pregnancy at 12 weeks of gestation Implantation rate; defined as gestational sacs per number of transferred embryos Multiple pregnancy per viable pregnancy Miscarriage per intrauterine pregnancy, defined as a miscarriage of an ongoing pregnancy after 12 weeks of gestation Occurrence of severe OHSS Number of days of gonadotrophin treatment Gonadotrophin consumption Correlation between serum LH level and IVF outcome | |
| Notes | Financial support—grant number KBN 2 P05E 034 28 from State Committee for Scientific Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random letters (A for GnRH antagonists protocol or B for GnRH agonists protocol) |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Kyono 2005.
| Methods | RCT, open‐label, parallel design, single‐centre | |
| Participants | Women under the age of 40 yrs with previous cycles < 3 times, and BMI < 27 kg/m2 underwent COH for ART | |
| Interventions | Participants were treated with GnRH agonist long protocol, GnRH antagonist protocol, and GnRH antagonist with hCG 200 IU protocol following contraceptive pills for two to three weeks as pretreatment. (Unknown) | |
| Outcomes | Total amount of FSH dosage, Blood E2 level at hCG injection, the number of oocytes, small follicle (< 10 mm) counts at OPU, day 3 embryo high quality rate, clinical pregnancy rate, and severe OHSS rate | |
| Notes | Number of participants at randomisation: 192 (cetrorelix: 126/ buserelin: 66) Number of participants at stimulation: N/A Number of participants at OPU: N/A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Lainas 2007.
| Methods | RCT, single‐centre | |
| Participants | 78 infertile women with PCOS undergoing IVF/ICSI Inclusion criteria: PCOS (presence of oligo‐ovulation/anovulation and polycystic ovaries), age 18 – 39 years, less than three previous IVF/ICSI attempts, no endometriotic cyst present as assessed by transvaginal, ultrasound examination, and basal hormonal levels of FSH in the early follicular phase of 10 IU l21 Exclusion criteria: women with known previous poor ovarian response Baseline characteristics: age (years) 32.0 (14) vs 30.5 (16), BMI (kg m2) 23.2 (20.9) vs 23.6 (18.9), FSH (IU l21) 6.3 (1.7) vs 5.8 (2.6) |
|
| Interventions |
GnRH antagonist (n = 26): OCP + 150 IU rFSH on 2nd day of the cycle (adjusted) + 0.25 mg SC of ganirelix (Orgalutran) administered on day 2 of menses/day 1 of stimulation (flexible protocol) GnRH agonist (n = 52): OCP + 150 IU rFSH (adjusted) + long GnRH agonist, 0.1 mg triptorelin three days before discontinuation of the OCP, once down‐regulation was achieved, the dose of GnRH agonist was decreased on that day to 0.05 mg/day (low‐dose GnRH agonist protocol) Oocyte maturation triggering: 3 follicles > 17 mm, 10,000 IU of hCG was administered Oocyte retrieval: 35 ‐ 36 hours later, followed by IVF/ ICSI Maximum embryos transferred: 3 Luteal phase support: 600 mg of micronised progesterone was initiated two days after oocyte retrieval Follow up: OPR was confirmed by vaginal ultrasound scan at 12 weeks of gestation |
|
| Outcomes | Primary outcome measure: E2 levels on day 5 of stimulation Secondary outcome measures: follicular development, LH and progesterone levels | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list in a 1:2 ratio |
| Allocation concealment (selection bias) | Low risk | By a study nurse, the responsible physicians (investigators) were not involved in the randomisation process |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither participants nor doctors were blinded to the treatment assigned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Lainas 2010.
| Methods | RCT, single‐centre | |
| Participants | 220 PCOS women undergoing ICSI Inclusion criteria: PCOS (presence of oligo‐ovulation/anovulation) and polycystic ovaries, age 18 – 39 years, no endometriotic cyst present, as assessed by transvaginal ultrasound examination, basal FSH 10 IU/ml Exclusion criteria: women with known previous poor ovarian response Baseline characteristics:age (years) 32 (29 – 35) vs 31 (28 – 35), BMI (kg/m2) 23.2 (20.9 – 25.8) vs 24.6 (20.9 – 29.3), FSH (IU/l) 6.0 (4.3 – 6.9) vs 6.2 (4.8 – 7.5), LH (IU/l) vs 5.9 (3.4 – 7.6) 5.3 (4.0 – 7.5) |
|
| Interventions |
GnRH antagonist (n = 110): OCP + 150 IU FSH on 2nd day of the cycle (adjusted) + 0.25 mg SC of cetrorelix acetate (Cetrotide) administered when at least one of the following criteria were fulfilled, the presence of at least one follicle measuring > 14 mm, serum E2 levels > 600 pg/ml; and serum LH levels > 10 IU/l (flexible protocol) GnRH agonist (n = 110): OCP (Cilest) + 150 IU rFSH (adjusted) + long GnRH agonist, 0.1 mg triptorelin three days before discontinuation of the OCP, once down‐regulation was achieved, the dose of GnRH agonist was decreased on that day to 0.05 mg/day (low‐dose GnRH agonist protocol) Oocyte maturation triggering: 3 follicles > 17 mm, 5000 IU of hCG was administered Oocyte retrieval: 35 ‐ 36 hours later, followed by IVF/ ICSI Maximum of embryo transferred: 3 Luteal phase support: 600 mg of micronised progesterone was initiated two days after oocyte retrieval Follow up: OPR was confirmed by vaginal ultrasound scan at 12 weeks of gestation |
|
| Outcomes | The primary outcome measure: ongoing pregnancy rate per participant randomised. Ongoing pregnancy and clinical pregnancy were defined as the presence of gestational sac with fetal heart beat detection at 12 weeks and at 6 – 7 weeks of gestation, respectively Secondary outcome measures: OHSS incidence, duration of rFSH stimulation, total dose of rFSH, E2 and progesterone concentration on the day of hCG administration, cycle cancellation rate, number of cumulus‐oocyte complexes (COCs) retrieved, number of metaphase II oocytes and fertilisation rates | |
| Notes | OHSS classification: a modified classification system based on combined criteria previously reported (Golan 1989; Navot 1992; Rizk 1999) was used in the current study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list, in a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither participants nor doctors were blinded to the treatment assigned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Lavorato 2012.
| Methods | Two‐arm parallel RCT | |
| Participants | 32 infertile women undergoing ICSI cycle Inclusion criteria: women aged 37 years or less and in their first IVF/ICSI cycle, BMI < 30 kg/m2, regular menses and the presence of two normal ovaries Baseline characteristics: age (years) antagonist: 32.1 ± 3.1; agonist: 33.7 ± 2.7, P = 0.12; BMI: GnRH antagonist 23.4 ± 2.7, GnRH agonist 23.7 ± 3.1, P = 0.75 |
|
| Interventions |
GnRH antagonist (n = 16): on the third day of the menstrual cycle, ovarian stimulation was started with a fixed dose of 150 – 225 IU rFSH and 75 IU/day rLH for five days. On the eighth day of the menstrual cycle (sixth day of ovarian stimulation), follicular development was monitored by transvaginal ultrasound. The dose of rFSH was adapted according to the ovarian response, and supplementation with rLH was increased to 150 IU/day when one or more follicles measuring 10 mm in diameter were found. The GnRH antagonist, at a dose of 0.25 mg/day SC was started when at least one follicle greater or equal to 14 mm was observed on ultrasound GnRH agonist (n = 16) First, pituitary down‐regulation was started during the luteal phase of the previous menstrual cycle with the GnRH agonist at a dose of 1 mg/day for 14 days. Then, ovarian stimulation was started with a fixed dose of 150–225 IU recombinant FSH (rFSH/Gonal F1; Serono, SP, Brazil) with 75 IU/day rLH (Luveris1; Serono, SP, Brazil) for seven days. On the eighth day of ovarian stimulation, follicular development was monitored by transvaginal ultrasound. The dose of rFSH was adapted according to the ovarian response, and rLH supplementation was increased to 150 IU/day when one or more follicles measuring greater than or equal to 10 mm in diameter were found Additional support: for both groups, 250 mg r‐hCG (Ovidrel1; Serono, SP, Brazil) was administered SC when at least two follicles reached a diameter of 17 mm during final oocyte maturation. Oocyte retrieval was performed by transvaginal aspiration under ultrasound guidance 34 – 36 h after r‐hCG injection |
|
| Outcomes | None of the reported outcomes (DNA fragmentation, apoptosis) were relevant to the review | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | The method used in allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was reported on blinding of participants and personnel, including outcome assessors. However, none of the reported outcomes were relevant to the review |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None of the reported outcomes were relevant to the review |
| Selective reporting (reporting bias) | Unclear risk | None of the reported outcomes were relevant to the review |
| Other bias | Low risk | Groups were balanced at baseline |
Lee 2005.
| Methods | RCT, single‐centre, university hospital, tertiary medical centre, parallel design | |
| Participants | 61 infertile women undergoing IVF/ICSI Inclusion criteria: were no more than 39 years of age, a history of regular menstruation cycles (menstrual cycle length 26 – 33 days), BMI 18 – 29 kg/m2, no history of poor ovarian response or reserve (less than three oocytes in a previous IVF cycle), baseline FSH levels < 11 IU/L, normal results for serum liver and renal function testing, presence of two ovaries, and no pill or hormone pretreatment within three months prior to stimulation cycle Exclusion criteria: ovarian‐factor or uterine‐factor infertility, or those suffering ovarian cysts, as determined by the use of ultrasound at the commencement of a stimulation cycle |
|
| Interventions | COS with either a multiple‐dose (MD) or a single‐dose (SD) protocol for GnRH antagonist (cetrorelix) administration (Flexible), or with a long protocol (LP) for GnRH agonist (buserelin) administration, followed by oocyte retrieval, IVF/ICSI, and embryo transfer | |
| Outcomes | Amount of hMG administered (ampoules) Period of hMG stimulation (days) Serum E2 level on day of hCG administration (pg/mL) Numbers of follicles (size 10 mm) on day of hCG administration Thickness of endometrium (mm) on day of hCG administration Number of oocytes retrieved Fertilisation rate (%) Number of zygotes Number of transferred embryos Number of frozen embryos Implantation rated Pregnancy rated | |
| Notes | Number of participants at randomisation: 61 (cetrorelix: 41/ buserelin: 20) Number of participants at stimulation: 60 (cetrorelix: 40/ buserelin: 20) Number of participants at OPU: 60 (cetrorelix: 40/ buserelin: 20) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported clearly |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported clearly |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcomes data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | University grant; the study appears to be free from other sources of bias |
Lin 2006.
| Methods | RCT, single‐centre trial | |
| Participants | 120 infertile women undergoing ICSI Inclusion criteria: age 20 – 38 years with regular cycles, normal basal FSH < 10 mIU/ml, LH < 10 mIU/ml and E2 < 60 pg/ml), BMI 18.5 ‐ 24.9 kg/m2, male‐factor infertility Exclusion criteria: endometriosis, anovulation, PCOS and hydrosalpinx Baseline characteristics: age (years) 31.3 ± 4.4 vs 30.7 ± 4.4, weight (kg) 53.5 ± 8.2 vs 55.2 ± 8.2, day 3 FSH level (mIU/ml) 5.12 ± 1.76 vs 5.28 ± 1.44, Day 3 LH level (mIU/ml) 4.75 ± 2.19 vs 4.31 ± 2.39 |
|
| Interventions |
GnRH antagonist (n = 60): 100 mg/day CC cd 3 to 7 + 2 ‐ 4 ampoules hMG was given on days 4, 6, 8 and 9 + 2.5 mg cetrorelix acetate (Cetrotide 1) when the leading follicle had reached 14 mm (Flexible) GnRH agonist (n = 60): 0.5 mg/day buserelin GnRH agonist long protocol + 2 – 4 ampoules of hMG (Pergonal) or FSH (Metrodin) Oocyte maturation triggering: 10,000 IU hCG (Pregnyl 1), when at least two follicles had reached 18 mm Oocyte retrieval: 34 – 36 hrs, followed by ICSI Maximum embryo transfer: not stated Luteal phase support: 600 mg/day vaginally of micronised progesterone (Utrogestan) starting from the day after oocyte retrieval Follow up: up to live birth |
|
| Outcomes |
Primary outcome measure: amount of gonadotrophin used Secondary outcome measures: endometrial thickness, number of oocytes and MII oocytes recovered, as well as rates of fertilisation and pregnancy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed in envelopes and the physicians were not aware of the allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Loutradis 2004.
| Methods | RCT, university‐affiliated IVF centre, open‐label, parallel design | |
| Participants | Aged between 20 and 38 years, no low response in a previous treatment cycle, no uterine or ovarian anomalies, and history of regular menstrual cycles ranging from 25 to 35 days | |
| Interventions |
GnRH antagonist: started ovarian stimulation on day 3 of the cycle with the administration of 225 IU of rFSH. Group B was treated with the GnRH antagonist cetrorelix (0.25mg/day, SC; Cetrotide) (Flexible), commencing when the largest follicle had reached a diameter of 14 mm, and simultaneous augmentation of 75 IU of FSH was initiated up to and including the day of hCG administration GnRH agonist: was treated with the GnRH agonist triptoreline (1 mg/day SC; Decapeptyl) starting one week before the expected menses. After down‐regulation was achieved (serum E2 < 50 pg), ovarian stimulation was commenced with a fixed daily dose of 225 IU of rFSH. Oocyte maturation triggering: when the leading follicle had reached a diameter of 18 mm in group A and 20 mm in group B and at least two follicles had reached 15 mm or more, rFSH was discontinued and a single 10,000 IU hCG dose (Pregnyl) was administered |
|
| Outcomes | Peak E2 (pg/mL) Total dosage of gonadotrophins (IU) Duration of gonadotrophin administration (in days) Number of oocytes retrieved Total number of good‐quality embryos Number of ETs Clinical pregnancy rates Implantation rates Number of cryopreserved embryos | |
| Notes | Number of participants at randomisation: 116 (cetrorelix: 58/ triptoreline: 58) Number of participants at stimulation: 116 (cetrorelix: 58/ triptoreline: 58) Number of participants at OPU: 116 (cetrorelix: 58/ triptoreline: 58) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table, randomisation: 1:1 (cetrorelix:triptoreline) ratio |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Marci 2005.
| Methods | RCT, single‐centre, open‐label, parallel design | |
| Participants | 60 infertile women (poor responders) undergoing IVF/ICSI Inclusion criteria: estradiol concentrations < 600 pg/ml concentration on the day of hCG administration and a poor response (number of oocytes retrieved < 3) after a previous standard long protocol using analogues for down‐regulation and recombinant gonadotrophin at a dose of 225 IU for stimulation (rFSH, Gonal‐F) |
|
| Interventions |
GnRH antagonist (n = 30): 375 IU rFSH (Gonal‐F) from cycle day 2 + GnRH antagonist cetrorelix 0.25 mg per day was then administered from when the two lead follicles had reached 14 mm diameter, irrespective of the day of the cycle until the day of hCG injection. (Flexible) GnRH agonist (n = 30): by analogues from day 23 of the cycle (Enantone 3.75 mg) + 375 IU daily , SC, rFSH, (Gonal‐F) from day 3 of the next cycle at a dose of 375 IU. In group B (n = 30), ovarian stimulation started at day 2 with rFSH at a dose of 375 IU (Gonal‐F) Oocyte maturation triggering: hCG (Profasi; Serono) 10,000 IU was administered IM 24 hrs after the last rFSH injection when at least two follicles had reached a diameter of 17 mm Oocyte retrieval: 36 hrs after hCG administration followed by IVF/ICSI Embryo transfers: were performed 48 hrs after oocyte retrieval Luteal phase: 2 × 200 mg/day of micronised vaginal progesterone (Prometrium) Follow up: serum hCG concentrations were measured 14 days after embryo transfer. Clinical pregnancies were confirmed 28 ‐ 35 days after embryo transfer by the presence of a gestational sac under ultrasound |
|
| Outcomes | Age (years) Initiated cycles Stopped cycles Cycles with oocyte retrieval Stimulation duration (days) Number of ampoules Follicles > 15 mm Oocytes retrieved Oocytes fertilised Cycles with transfers Embryos transferred Endometrial thickness (mm) Clinical pregnancies |
|
| Notes | Number of participants at randomisation: 60 (cetrorelix: 30/ enantone: 30) Number of participants at stimulation: 60 (cetrorelix: 30/ enantone: 30) Number of participants at OPU: 55 (cetrorelix: 29/ enantone: 26) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Martinez 2008.
| Methods | RCT, single‐centre, donor‐recipient cycle | |
| Participants |
323 Donors: < 35 years, baseline FSH < 10 U, BMI < 30 kg/m2, no history of hereditary disease Baseline characteristics: Age (years) 27.2 + 4.7 vs 26.5 + 4.7, BMI (kg/m2) 23.0 + 3.5 vs 23.1 + 3.0, baseline FSH (IU/ml) 7.0 + 2.3 vs 6.5 + 2.1 Setting and timing: private hospital, Spain. January 2005 to November 2006 |
|
| Interventions |
GnRH antagonist: vaginal contraceptive (Nuvaring) + rFSH 150–200 U/day + ganirelix (Orgalutran) 0.25 mg/day, from day 6 of stimulation (Fixed) GnRH agonist: vaginal contraceptive (Nuvaring) + leuprorelin acetate, 3.75 mg + 2 – 3 ampoules per day of hMG Oocyte maturation triggering: 10,000 U of hCG when at least three follicles > 20 mm in diameter Oocyte retrieval: 35 ‐ 36 hours later, followed by IVF/ ICSI Follow up: 10 – 14 days after puncture |
|
| Outcomes | Clinical pregnancy rate (confirmed by the presence of a gestational sac in the ultrasound examination carried out 4 – 5 weeks after transfer) The implantation rate was calculated by dividing the number of gestational sacs by the number of embryos transferred Other secondary results were the total number of OCCs retrieved, the number of days and total dose of gonadotrophin stimulation, and plasma estradiol levels on the day of hCG administration |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Low risk | Telephone call |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Researchers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias Study funding not reported |
Mohamed 2006.
| Methods | RCT | |
| Participants | 30 women Inclusion criteria: known to be low responders (developed fewer than six follicles > 12 mm in previous IVF cycles under the standard mid‐luteal phase down‐regulation protocol) Exclusion criteria: abnormally high FSH > 13 IU/l Setting and timing: centre for assisted conception, UK. timing not specified Baseline characteristics: age agonist group 37 (95% CI 35 to 39) vs antagonist group 36 (95% CI 33.5 to 38) |
|
| Interventions |
Agonist group ‐ 500 micrograms buserelin SC daily starting day 1 of menstrual cycle Antagonist group ‐ cetrorelix 0.25 mg SC daily started on cycle day 8 and continued until day of hCG injection Ovarian stimulation started on cycle day 3 with 225 to 375 IU gonadotrophin daily based on highest dose reached in previous IVF cycle Both buserelin and gonadotrophin continued until day of hCG 10,000 IU injection Gonadotrophin increased by 75 IU/day if fewer than three follicles measuring 12 mm or more were found on cycle day 9 Luteal support ‐ progesterone used for luteal support 400 mg twice daily Number of ampoules ‐ agonist 44.5 (95% CI 38.5 to 54) vs antagonist group 35.5 (95% CI 33 to 41) |
|
| Outcomes | Serum LH and E2 levels, cycle cancellation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" but no other details |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used but no details as to whether opaque or given out sequentially |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details but blinding unlikely. The lack of blinding is unlikely to effect the fertility outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two women not analysed from 30 women randomised due to cycle cancellation. Both were in the antagonist group |
| Selective reporting (reporting bias) | Unclear risk | No pregnancy outcomes of relevance were reported. The primary outcomes relate to serum LH and E2 levels Cycle cancellation was reported but was not pre‐specified as an outcome |
| Other bias | Low risk | No other bias identified, groups were balanced at baseline |
Moraloglu 2008.
| Methods | RCT, single‐centre | |
| Participants | 93 women undergoing IVF/ICSI between May 2005 and August 2006, Age: 25 – 38 years
Exclusion criteria: history of previous poor response (< 4 follicles and/or serum estradiol (E2) level < 500 pg/ml on the day of hCG), ≥ previous IVF cycles, PCOS or azoospermia, were aged over 38 years, had a BMI ≥ 30 kg/m2 and basal FSH measurement ≥ 10 IU/ml, and those with relevant systemic disease, severe endometriosis or uterine and ovarian abnormalities Baseline characteristics: age: 30.91 ± 5.52 vs 30.25 ± 4.94. BMI: 29.36 ± 4.45 vs 26.58 ± 3.32. Basal E2: 6.63 ± 1.33 vs 6.32 ± 1.77. AFC: 5.02 ± 2.56 vs 8.02 ± 2.95 |
|
| Interventions |
GnRH antagonist (n = 45): OCP (Desogesteral + 225 IU FSH + 0.25 mg SC of cetrorelix acetate (Cetrotide) administered when follicles reached a diameter of ≥ 14 mm (flexible protocol) GnRH agonist (n = 48): OCP (Desogesteral + 225 IU FSH + long GnRH agonist leuprolide acetate 1 mg/day SC (Lupron) one week before the expected menses with approximately a five‐day overlap with the OCP. The dose of GnRH agonist was then reduced to 0.5 mg/day, (Low‐dose GnRH agonist protocol) Oocyte maturation triggering: hCG 5000 IU (Profai) SC > 3 follicles of 18 mm in diameter Oocyte retrieval: 36 hours later, followed by IVF/ ICSI Maximum embryos transferred: 3 Luteal phase support: intravaginal progesterone gel (Crinone 8%) and was started no later than the day of ET until a urine pregnancy test was performed 12 days later Follow up: An ultrasound scan was carried out five to six weeks after oocyte retrieval to determine the viability of the pregnancy. A second ultrasound was performed at 12 weeks’ gestation to confirm any ongoing pregnancy (positive heart beat) |
|
| Outcomes | Antral follicle numbers Total stimulation duration, days Total gonadotrophin consumption, IU/l Serum E2 value on day of hCG Cycles cancelled for premature LH surge (%) Cycles cancelled for fertilisation failure (%) Number of oocytes per retrieval Number of mature oocytes (M2) Number of fertilised oocytes, fertilisation rate (%) Total embryos obtained Number of embryos transferred Clinical pregnancy per cycle initiated (%) Number of cycles with OHSS (%) Clinical pregnancy per transfer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcomes data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias. Not reported |
Moshin 2007.
| Methods | RCT, single‐centre trial | |
| Participants | 49 PCOS infertile women undergoing IVF/ICSI | |
| Interventions |
GnRH antagonist (n = 25): 225 IU FSH on 2nd day of the cycle (fixed) + 0.25 mg SC of GnRH antagonist cetrorelix (Cetrotide, Serono International, Switzerland) started on the sixth day of stimulation (fixed protocol) GnRH agonist (n = 24): 225 IU rFSH (fixed) + long GnRH agonist, 3.75 mg of triptorelin (Dipherelin) in the mild‐luteal phase of the preceding cycle(long depot GnRH agonist protocol) Oocyte maturation triggering: 10,000 IU SC hCG (Pregnyl) when one follicle 18 ‐ 20 mm Oocyte retrieval: 35 hours later, followed by IVF/ ICSI |
|
| Outcomes | Duration of stimulation, number of ampoules of FSH, fertilisation rate, implantation rate, ongoing pregnancy rate, OHSS incidence | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcomes data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
Olivennes 2000.
| Methods | RCT, multi‐centre (9 centres ), open‐label, parallel design | |
| Participants | 169 infertile couples undergoing ovarian stimulation for IVF‐ET with or without ICSI Inclusion criteria: with no more than three previous IVF‐ET attempts with all causes of infertility (except polycystic ovary and moderate or severe endometriosis) Baseline characteristics: age cetrorelix 31.4 ± 3.7, decapeptyl 31.8 ± 3.8. Duration of infertility: cetrorelix 59.3 ± 35, decapeptyl 55.3 ± 38.1. FSH: cetrorelix 6.3 ± 2, decapeptyl 6.3 ±1.9. BMI cetrorelix 22.4, decapeptyl 22.8 |
|
| Interventions | A single dose of 3 mg of GnRH antagonist (cetrorelix) (Depot) was administered SC to 115 participants On day 7 of hMG mid‐luteal GnRH analogue (decapeptyl 3.75) Ovarian suppression was confirmed by E2 > 50 pg/ml / FSH and LH < 10 IU/L, P < 1 µg/ml Then hMG (menogon) was started at 2 or 3 ampoules for four days and the dose was adjusted according to response Luteal phase support using daily vaginal progesterone ICSI was done in 12 women in the cetrorelix group and five women in the decapeptyl group. |
|
| Outcomes | Premature LH surge defined as (LH > 10 IU/L) and progesterone level > 1 ng/L
Stimulation length
Number of hMG ampoules
E2 on hCG
Number of oocytes retrieved
Number of embryos obtained
Number of embryos transferred
Clinical pregnancy/OPU
Clinical pregnancy/ET
Miscarriage
Ectopic
OHSS
Moderate or severe OHSS Clinical pregnancy was defined as fetal heart beat on ultrasonography Ongoing pregnancy was defined as pregnancy ongoing after 12 weeks of amenorrhoea |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcomes data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Papanikolaou 2012.
| Methods | RCT, single centre | |
| Participants | 190 women Inclusion criteria: < 39 years, FSH < 12 mIU/mL, could start gonadotrophin at a dose fixed for five days and with a range of 100 to 300 IU, < 3 previous IVF cycles Exclusion criteria: known endocrine disorders, stage III/IV endometriosis, cases where blood was drawn and analysed in another laboratory, ovarian simulation cancellation if progesterone was > 1.5 ng/ml and estradiol was > 80 pg/ml on the day of initiation of gonadotrophins Setting and Timing: setting unclear, Greece. August 2007 to December 2009 Baseline characteristics: age agonist 32.8 ± 0.3 SE vs antagonist 32.2 ± 0.3 SE |
|
| Interventions |
Antagonist ‐ gonadotrophins administered from day 2 of the cycle if the hormone levels were basal and co‐treatment with ganirelix 0.25 mg or cetrorelix 0.25 mg started on day 6 of stimulation Agonist ‐ long protocol started on day 21 of preceding cycle with intranasal buserelin (600 mg /day). Gonadotrophins were administered after two to three weeks of down‐regulation and once hormonal levels were basal Gonadotrophin dose 150 ‐ 300 IU for all participants and remained fixed for five days. After this period the dose could be adjusted and individualised based on follicular growth, and serum estradiol levels Oocycte maturation induced with 250 micrograms of re hCG when at least 3 follicles of 17‐18 mm were present Oocycte retrieval 36 hours after hCG |
|
| Outcomes | Live birth, ongoing pregnancy, miscarriage, clinical pregnancy rates. OHSS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated list" |
| Allocation concealment (selection bias) | Low risk | "allocation to treatment arms was performed by a consulting nurse who had no intervention in the patients’ treatment" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Both participants and treating physicians were aware of the exact protocol followed. Lack of blinding is unlikely to affect the fertility outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were analysed using ITT principle for those with available data. There were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All reported outcomes were pre‐specified |
| Other bias | Low risk | No other evidence of bias |
Prapas 2013.
| Methods | Randomised controlled trial. Single centre | |
| Participants | 364 women Inclusion criteria: poor responders (exhibited a poor ovarian response with zero to four oocytes retrieved at a previous IVF cycle) Exclusion criteria: known severe endometriosis (stage III/IV), FSH ≥ 14 mIU/mL Setting and timing: IVF centre, Thessaloniki, Greece. January 2007 to December 2011 Baseline characteristics: age antagonist 36.2 ± 4.4 years, agonist 36.2 ± 4.5 years |
|
| Interventions |
Antagonist ‐ rFSH (puregon, NV Organon) 450 IU/day was commenced on day 2 of menstruation and the antagonist ganirelix (Orgalutran) 0.25 mg was added in the afternoon of the sixth day of stimulation Agonist ‐ triptorelin SC 0.1 mg/day commencing on the afternoon of the 21st day of the cycle prior to stimulation. Triptorelin SC 0.05 mg/day plus rFSH (puregon, NV Organon) 450 IU/day was commenced on day 2 of menstruation or later when estradiol was ≤ 50 pg/mL All women were given a contraceptive pill (Gynofen; Shering Hellas) for the cycle prior to stimulation (day 2 of the cycle to day 21). All women monitored by TVS and serum estradiol on day 2 and after four days of stimulation Daily dose of rFSH adjusted according to ovarian response based on estradiol levels and the number and size of follicles hCG 10,000 IU administered when one or more follicles present with a mean diameter on ultrasound ≥ 17 mm and serum estradiol ≥ 500 pg/mL Oocyte retrieval 34 to 36 hrs after hCG Luteal phase support with progesterone 200 mg three times daily Number of embryos transferred: antagonist 1.92 ± 0.8 vs agonist 2.08 ± 0.8 |
|
| Outcomes | Oocyte retrieval rate, fertilisation rate, number of embryos transferred, cancellation rate, implantation rate, clinical pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "assigned at random" . ‘No further details were reported |
| Allocation concealment (selection bias) | Low risk | "using sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Woman was blinded to allocation, no further details were reported on other personnel. However non blinding of outcome assessors not likely to affect some of the outcome measures as they were objectively assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Antagonist group 168/182 completed. Agonist group 162/182 completed. No reasons given for withdrawals or losses to follow up |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes listed were not pre‐specified. No live birth or OHSS data were reported in this trial |
| Other bias | Low risk | No other source of bias identified. Groups balanced at baseline |
Qiao 2012.
| Methods | RCT. Multi‐centre (number of sites not specified) | |
| Participants | 233 women Inclusion criteria: Chinese women, aged ≥ 18 and ≤ 35 years, BMI 18 to 29 kg/m2, normal menstrual cycle, access to ejaculatory sperm and for whom COS and IVF/ICSI were indicated Exclusion criteria: not specified. Setting and timing: reproductive centres, China. July 2007 to December 2008 Baseline characteristics: age ‐ antagonist 29.3 ± 2.8 years vs agonist 29.1 ± 3.0 years |
|
| Interventions |
Antagonist ‐ ganirelix 0.25 mg/day administered from stimulation day 6 up to and including the day of hCG 10,000 IU injection when three follicles ≥ 17 mm Agonist ‐ long protocol triptorelin 0.05 mg/day started during the luteal phase (days 21 to 24) up to and including the day of hCG 10,000 IU injection when three follicles ≥ 17 mm Starting dose of rFSH was 150 IU and dose could be adjusted from day 6 onwards depending on the ovarian response Luteal phase support IM progesterone (min. dose 60 mg) |
|
| Outcomes | rFSH required, number of oocytes retrieved, embryo quality, ongoing pregnancy rate, fertilisation rate In addition data reported for cancellation rate, OHSS and multiple pregnancy rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open label, no blinding of participants or researchers. Lack of blinding is unlikely to affect fertility outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. States that intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | Unclear risk | Only ongoing pregnancy was specified as a secondary outcome. The authors reported on multiple pregnancy and clinical pregnancy rate and OHSS in the results. Live birth was not reported at all |
| Other bias | Low risk | No other source of bias identified. Groups were balanced at baseline |
Rabati 2012.
| Methods | RCT | |
| Participants |
136 women undergoing ART Inclusion criteria: undergoing ARTs for the first time, serum FSH level ≤ 10 IU/ litre on day 3 of menstrual cycle, male or female factor of infertility Exclusion criteria: previous history of IVF or ICSI, hyperprolactinaemia, thyroid dysfunction, uterine abnormality, severe endometriosis (diagnosed by laparoscopy), secondary infertility Setting and timing: infertility centre, Iran. Timing not specified Baseline characteristics: age ‐antagonist 28.36 ± 3.4 years vs agonist 28.65 ± 3.9 years |
|
| Interventions |
Antagonist ‐ ovarian stimulation commenced on day 2 of the cycle with rFSH (Gonal F, Serono, Switzerland) 75 IU daily SC On day 6 of stimulation 0.25 mg cetrorelix when the follicle reached 14 mm diameter Agonist ‐ 500 μg SC buserelin daily (Suprefact, Aventis, Germany) commenced on 21st day of previous menstrual cycle and continued until baseline evaluation of serum level of E2 on day 2 of menstruation. If serum E2 < 50 pg/ml then buserelin reduced to 250 μg daily and ovarian stimulation commenced with SC rFSH (Gonal F, Serono, Switzerland) 75 IU daily Based on ovarian response detected by ultrasonography every two to three days, gonadotrophin dose was adjusted Administration of buserelin or cetrorelix continued until the time of the hCG (10,000 IU) injection (IM) when there were at least three follicles with a mean diameter of 18 mm Oocyte retrieval after 36 hours Luteal support with cyclogest suppository 800 mg Number of ampoules: antagonist 17.04 ± 6.04 vs agonist 20.14 ± 9.51 Number of embryos transferred: antagonist 2.66 ± 0.9 vs agonist 2.71 ± 0.86 |
|
| Outcomes | Clinical pregnancy rate, ongoing pregnancy rate, moderate or severe OHSS | |
| Notes | Sample size calculation: not reported ITT analysis: Yes Funder: Isfahan University of Medical Sciences |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised", no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not reported but unlikely to be blinding as protocols varied and no placebo was used. Lack of blinding unlikely to affect fertility outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified were reported. Live birth was not reported |
| Other bias | Low risk | No evidence of other bias. Groups were balanced at baseline |
Revelli 2014.
| Methods | RCT, parallel design. | |
| Participants | 695 women with clinical, endocrine and ultrasound characteristics suggesting a low ovarian reserve and a poor responsiveness to COH Inclusion criteria: women undergoing IVF classified as “expected poor responders”: circulating day 3 FSH between 10 and 20 IU/l in the presence of estradiol (E2) serum level < 80 pg/ml; circulating AMH between 0.14 and 1.0 ng/ml; AFC between 4 and 10 Exclusion criteria: Women with basal FSH > 20 IU/l, undetectable AMH levels, AFC < 3 and aged more than 43 years Baseline characteristics: age (years) 38.5 ± 3.4 vs. 37.5 ± 3.6, BMI (kg/m2) 22.8 ± 3.8 vs. 23.1 ± 4.3, basal FSH (IU/L) 12.4 ± 4.4 vs. 13.7 ± 2.9, AMH (ng/ml) 0.71 ± 0.44 vs. 0.68 ± 0.35, AFC 5.3 ± 2.7 vs. 6.2 ± 2.8 |
|
| Interventions |
GnRH antagonist (n = 355): 100 mg/day CC (Serophene) orally for five days (from cycle day 2 to 6) + 150 IU/day SC Gn (Meropur) from cycle day 5 + 0.25 mg/d SC. GnRH‐antagonist cetrorelix (Cetrotide) from cycle day 8 until the day of hCG administration. (“mild” CC/Gn/GnRH‐antagonist protocol) GnRH agonist (n = 340): 0.8 mg/d intranasal GnRH agonist (Suprefact) starting from run‐in cycle day 21 for 14 days, at the beginning of Gn administration, the dose was reduced to 0.4 mg/d and continued during ovarian stimulation + 300 IU exogenous Gn (Meropur) after confirmation of pituitary block, dosage increased up to a maximum of 450 IU/d after one week. (Long protocol with GnRH‐agonist plus Gn at high doses) Oocyte maturation triggering: 10,000 IU SC hCG (Gonasi HP) when the leading follicle reached 18 mm, with appropriate serum E2 levels Oocyte retrieval: 36 hours after hCG injection, either IVF or ICSI was performed according to the clinical indication. After two days of in vitro culture, embryos transferred Maximum embryos transferred: 2 Luteal phase support: 180 mg/d natural progesterone (Crinone 8) for 15 days from the day of ET Follow‐up: pregnancy was assessed by serum hCG assay after 15 days from ET and then confirmed by transvaginal ultrasound after two further weeks |
|
| Outcomes | Ongoing pregnancy rate, miscarriage rate, clinical pregnancy rate, cycle cancellation rate, endometrial thickness at OPU, cycles without retrieved oocytes, MII oocytes/OPU, number of oocytes retrieved, totally administered Gn dose, length of the ovarian stimulation, fertilisation rate (FR), implantation rate (IR), number of transferred embryos, hCG positive tests, number of gestational sacs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed using a computerized algorithm without any restriction” |
| Allocation concealment (selection bias) | Low risk | “Allocation concealment was obtained using sequentially numbered dark envelopes: until they were opened at the time of allocation” |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | “…both physicians and patients were blinded to the study” however blinding of outcome assessment not described but unlikely to influence measurement of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number randomised = 695, number analysed = 640 (55 Lost to follow‐up due to cancelled cycle (OPU not performed)); analysis was not based on ITT |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the pre‐specified manner. Live birth rate not reported |
| Other bias | Low risk | None identified |
Rinaldi 2014.
| Methods | Prospective RCT, two centres | |
| Participants | 349 women Inclusion criteria: age 27 to 38 years, undergoing IVF, infertility due to tubal factor, moderate endometriosis, male factor or idiopathic subfertility. Basal FSH and LH < 12 mIU/mL, E2 < 50 pg/mL, prolactin < 30 ng/mL, regular menstrual and ovulatory cycles, normal uterine cavity, BMI 20 to 26 kg/m2. Previous cycle cancelled due to high risk of OHSS Exclusion criteria: no details Baseline characteristics: age antagonist 35.2 ± 4.7 years versus agonist 35.1 ± 4.9 years Setting and timing: assisted reproduction centre. January 2008 to December 2012 |
|
| Interventions |
Antagonist: mild/minimal stimulation protocol 75 IU/day of rFSH and 0.25 mg per day cetrorelix administered when the lead follicle was 14 mm diameter (n = 148) Agonist: triptorelin 0.1 mg per day SC from mid‐luteal phase of the previous cycle for a minimal of 14 days followed by 150 IU FSH and adjusted as necessary based on follicular size and E2 level Oocyte maturation triggered by 10,000 IU hCG Luteal phase support commenced on day of oocyte retrieval using progesterone 50 mg/ml IM daily |
|
| Outcomes | Live birth, clinical pregnancy, and miscarriage. Also reported on oocytes retrieved, fertilisation rate, embryo cleavage rate, embryos transferred, clinical pregnancy, implantation rate, live birth rate, miscarriage rate, OHSS | |
| Notes | Sample size calculation ‐ yes ITT analysis ‐ yes Funding ‐ no details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized" "using computer generated random assignment" |
| Allocation concealment (selection bias) | Low risk | "sealed and numbered envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "open label" unlikely to influence fertility outcomes. No details as to whether outcome assessors were blinded; however, non‐blinding of outcome assessors not likely to affect some of the outcome measures as they were objectively assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The proportion of women who did not undergo embryo transfer differed between the two treatment groups; reasons for not having embryo transfer were not reported and not all participants randomised were included in the final data analysis |
| Selective reporting (reporting bias) | Unclear risk | Only prespecified clinical pregnancy as an outcome but reported on other pregnancy outcomes including live birth and miscarriage rate. Data are reported per embryo transferred and not per woman randomised. However, numbers of embryos transferred were same as numbers of women |
| Other bias | Low risk | Groups balanced at baseline |
Rombauts 2006.
| Methods | RCT, 10 IVF centres, open‐label, parallel design | |
| Participants | 351 infertile women undergoing IVF/ICSI Inclusion criteria: healthy females of infertile couples, age at time of screening between 18 and 39 years, BMI between 18 and 29 kg/m2, body weight < 90 kg, a normal menstrual cycle with a range of 24 ‐ 35 days and an intra‐individual variation of ± 3 days, and willingness to give written informed consent Exclusion criteria: included contraindications for the use of gonadotrophins, endocrine abnormalities (e.g. polycystic ovary syndrome), more than three unsuccessful controlled ovarian stimulation cycles, a history of low or no ovarian response during FSH/HMG treatment, and clinically relevant abnormal laboratory values (including hormones) or medical examination findings |
|
| Interventions |
GnRH antagonist: treatment with the GnRH antagonist ganirelix (0.25 mg, Orgalutran) was started on day 5/6 of rFSH treatment. If no follicles 14 mm were observed by ultrasonography on that day, the start of ganirelix was delayed. Injections containing 0.25 mg ganirelix per 0.5 ml were administered SC in the thigh, once daily in the morning, until and including the day of hCG administration. (Flexible) GnRH agonist: in the OC‐scheduled group, women started taking a combined OC pill (30 µg ethinyl oestradiol/150 µg desogestrel) Marvelon® (NV Organon) on day 1 of the menstrual cycle. They took it daily for between 14 and 28 days, depending on the planned start of rFSH treatment Women in the nafarelin group started pretreatment with the GnRH agonist nafarelin (Synarel®; Pharmacia, Australia) on day 21 ‐ 24 of the preceding cycle. Nafarelin was administered intranasally at a daily dose of 0.8 mg until and including the day of hCG administration In all three groups ovarian stimulation was performed with rFSH (follitropin beta, Puregon®; NV Organon, The Netherlands), which was administered SC once daily in the morning at a fixed dose of 200 IU during the first 5 ‐ 6 days. After this period the dosage of rFSH could be adjusted depending on the ovarian response as assessed by ultrasound. Treatment was continued until (and including) the day of hCG administration. In the OC‐scheduled ganirelix group, stimulation with rFSH was started two days after discontinuation of the OC (irrespective of whether or not menses had started), in the non‐scheduled group on day 2 ‐ 3 of the menstrual cycle and in the nafarelin group after 2 ‐ 4 weeks of nafarelin treatment [as soon as pituitary down‐regulation had been achieved (i.e. serum estradiol 50 pg/ml or 200 pmol/l); if this stage was not achieved after four weeks of nafarelin treatment, the subject discontinued]. hCG, 10,000 IU in 1 ml saline (Pregnyl®, NV Organon, the Netherlands), was administered, either SC or IM, when at least three follicles 17 mm or at least one follicle 20 mm were observed on ultrasound. In case of risk of OHSS, the hCG dose was reduced to 5000 IU. Oocyte retrieval was performed 30 ‐ 36 hrs after hCG administration, followed by IVF or ICSI. No more than three embryos were transferred 2 ‐ 3 days after oocyte retrieval. Progesterone for luteal support was given daily (doses and administration form as per usual protocol of the participating centre), starting at the latest on the day of embryo transfer, for two weeks or up to menses The study was approved by the Ethics Committee of each participating centre. All women gave written informed consent. The study was performed according to the principles of the Declaration of Helsinki, and the ICH/Good Clinical Practice guidelines. The study was monitored by uniformly trained Clinical Research Associates of Organon with assistance of a contract research organisation for the clinics in Perth and Adelaide |
|
| Outcomes | Prior to the start of treatment, a physical and gynaecological examination was performed to exclude any abnormality. Blood samples were taken for routine biochemistry, haematology, and hormonal parameters. A pregnancy test (urinary hCG) was performed. Blood samples for hormone assessments were taken just before the first rFSH injection (treatment day 1) and at least once every two days from day 5/6 of rFSH treatment (in the antagonist groups just before ganirelix injection) up to and including the day of hCG. Serum FSH, LH, estradiol, and progesterone values were determined by means of the automated Wallac AutoDelfia Fluoroimmunoassay system (PerkinElmer Inc., Wellesley MA, USA) at a central laboratory (ABL BV, Assen, The Netherlands). The maximum intra‐assay and inter‐assay coefficients of variation were 3.3% for FSH, 3.4% for LH, 4.9% for estradiol, and 4.3% for progesterone. To measure follicular development, ultrasonography was performed at least once every two days from day 5/6 of rFSH treatment up to and including the day of hCG. Other parameters assessed were treatment failure (defined as the number of women who did not have an hCG injection or who received an hCG injection because of premature luteinisation), number of LH rises (LH = 10 IU/l), number of oocytes retrieved, number of good quality embryos (grade 1 (defined as excellent: no fragmentation) and grade 2 (defined as good: 1 ‐ 20% fragmentation)), fertilisation rate, implantation rate, and ongoing pregnancy rate (assessed by ultrasound = 12 ‐ 16 weeks after embryo transfer) |
|
| Notes | Number of participants at randomisation: 351 (ganirelix: 234/ nafarelin: 117) Number of participants at stimulation: 332 (ganirelix: 221/ nafarelin: 111) Number of participants at OPU: 313 (ganirelix: 212/ nafarelin: 101) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Low risk | Interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcomes data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Sauer 2004.
| Methods | RCT, open‐label, multi‐centre study. Phase III trial | |
| Participants | 74 infertile women (aged 18 ‐ 39 years) undergoing ICSI
Inclusion criteria: regular menstrual cycles, BMi < 35 kg/m2. Both ovaries present, no clinical signs of pelvic or uterine abnormalities, normal cervical cytology, wash‐out period completed for any previous IVF drug protocols and FSH concentrations in the normal range. All women were also required to be willing and able to comply with the study protocol Baseline characteristics: mean age (± SD) of the ITT population was 32.6 ± 4.0 years. The age range was broad (22 ‐ 39 years) and there were no significant differences between the three treatment groups. Mean BMI was 24.2 ± 4.5 g/m2. again with no significant differences between groups. Fifty‐one of the 73 women in the ITT population (69.99%) were White and the proportion of White women did not differ between treatment groups |
|
| Interventions |
GnRH antagonist: OCP pretreatment for 14 – 18 days, followed by cetrorelix (3 mg), starting on day 7 + rFSH 225 IU, starting on day 5 after OCP/dose adjustments after day 6
GnRH agonist: leuprorelin (0.5 mg/ day reduced to 0.25 mg/day after down‐regulation was achieved), long luteal, overlapping with OCP pretreatment for seven days + rFSH 225 IU, starting on day 5 after OCP/dose adjustments after day 6 GnRH antagonist II: OCP + cetrorelix and r‐FSH together with mid‐cycle r‐LH Oocyte maturation: hCG rhCG 250 μg when at least one follicle was ≥ 18 mm and at least two follicles were ≥ 16 mm and E2 within acceptable range Embryo transfer: no more than three embryos were to be replaced: two if transferred at blastocyst stage Luteal phase support: Micronised progesterone according to centres’ practice |
|
| Outcomes | The primary efficacy end‐point: the number of metaphase II oocytes retrieved per patient Secondary efficacy: end‐points were the duration and total dose of r‐hFSH therapy, the total number of follicles > 14 mm on the day of r‐hCG administration, oocyte and embryo quality and development, the number of participants with at least one embryo considered viable for cryopreservation, oestradiol concentration per follicle > 10 mm, total number of oocytes, implantation rates per‐embryos transferred and pregnancy rates (biochemical and clinical) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, internet‐based system. Randomisation 1:1:1 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Sbracia 2009.
| Methods | RCT, single‐centre | |
| Participants | 564 low responders, undergoing their first IVF cycle were eligible for the study Inclusion criteria: age 40 years or older and no previous IVF cycle Exclusion criteria: PCOS, FSH > 10 IU/ mL, a previous IVF cycle, and age 45 years or older Baseline characteristics: maternal age, years 42.3 1.4 vs 42.1 1.5, BMI 25.1 2.6 vs 24.8 2.4, basal FSH levels, IU/L 7.0 2.5 vs 6.9 2.4 |
|
| Interventions |
Group A (n= 285): 300 IU/day r‐hFSH (Gonal‐F) + 0.25 GnRH antagonist cetrorelix (Cetrotide) when the leading follicle ≃ 14 mm or the E2 plasma levels were 600 pg/mL (flexible multiple‐dose protocol) Group C (n= 285): buserelin 0.4 mg/day long GnRH agonist + 225 IU/day rhFSH (Gonal‐F) (GnRH agonist protocol) Oocyte maturation triggering: 10,000 IU of IM hCG when plasma E2 between 800 and 3500 pg/mL and at least three follicles > 16 mm in mean diameter Oocyte retrieval: 36 hours later, followed by ICSI Maximum number of embryos transferred: 3 Luteal phase support: 50 mg daily of P (Prontogest) IM from the day of replacement Follow up: pregnancies were confirmed by a rising titre of serum b‐hCG 12 days after ET and ultrasound demonstration of the gestation sac four weeks after the transfer |
|
| Outcomes | Primary outcomes: clinical pregnancy rate per cycle started and per transfer Secondary outcomes: days of stimulation, E2 at the day of hCG, amount of FSH administered, number of oocytes yielded, number of embryos transferred, implantation rate, and abortion rate | |
| Notes | Drop out: four women in the cetrorelix group and two in the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation number sequence at the time that their cycle was scheduled |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data, however, LBR, OPR were not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | Groups balanced at baseline |
Serafini 2008.
| Methods | RCT, single‐centre, parallel design | |
| Participants | 323 women of reproductive age Inclusion criteria: indication for IVF/ICSI, age 21 to 39 years, presence of two functional ovaries, normal uterus based on hysterosalpingography or hysteroscopic evaluation, fewer than three previous IVF/ICSI attempts, early follicular phase serum FSH 15 IU/L or less and E2 60 pg/ml or less, no history of low response, BMI 25 kg/m2 or less, no untreated endocrinological disease, no treatment with gonadotrophin for three or more months before study, male partner sperm 1% or greater strict morphology Exclusion criteria: not stated Baseline characteristics: age Group A 33.5 ± 0.4 years versus Group B 34.4 ± 0.4 years versus Group C 33.4 ± 0.3 years Setting and timing: Centre for Reproductive Medicine, Brazil. July 2002 to August 2005 |
|
| Interventions | All protocols included an initial r‐ hFSH (Gonal‐F) dose ranging from 150 to 300 IU daily dependant on age, and the participants were grouped as follows: Group A (antagonist): r‐hFSH beginning on day 2 or 3 was continued in the full dose until follicles reached 13 to 14 mm or reached day 6 of stimulation, when the r‐hFSH dose was lowered to 75 IU and the participants began with 200 IU hCG along with cetrorelix 0.25 mg (flexible) continued until day of hCG injection 10,000 IU Group B (antagonist II): r‐hFSH beginning on day 2 or 3 was continued in the full dose until two or more codominant follicles reached 18 mm diameter. 0.25 mg cetrorelix daily SC began either when two codominant follicles reached 13 to 14 mm diameter or participant reached day 6 of stimulation. Continued until day of hCG injection Group C (agonist): Leuprolide 0.5 mg SC daily administered in mid‐luteal phase of previous menstrual cycle after which rFSH was administered. Continued until day of hCG injection 10,000 IU rFSH was adjusted based on number and size of follicles Oocyte retrieval ‐ 35 to 36 hours after hCG injection Luteal phase support ‐ daily IM progesterone in oil 25 mg and vaginal administration of one full applicator of 8% Crinone gel at bedtime beginning on the day after oocyte retrieval Dose of hFSH ‐ Group A 1674.7 ± 59.4 IU versus Group B 2197.9 ± 83.1 IU versus Group C 21,567 ± 80.8 IU |
|
| Outcomes | Number of mature oocytes retrieved
Number of normally fertilised oocytes
Number of cycles with high‐quality embryos
Number of embryo transfers
Implantation rate
Pregnancy rate
Incidence of severe ovarian hyperstimulation syndrome (OHSS) Dose of hFSH E2 Number and quality of embryos |
|
| Notes | Sample size calculation ‐ yes ITT analysis ‐ yes Funding ‐ no details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | "A research nurse handed each patient a unique identification envelope in sequential chronological order". Unclear if sealed and opaque |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding unlikely. No details on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 23 subjects withdrew consent. 4/110 from group A; 11/107 from group B, 8/106 from group C. No reasons given. Group A had four cancellations (three due to poor ovarian response and one for no embryo transfer), group B had 10 cancellations (two conceived, four poor ovarian response, one stopped ovarian stimulation and three had no embryo transfer); Group C had six cancellations (four poor ovarian response and two had no embryo transfer) Group A had 102/110 analysed, Group B 86/107 and Group C 92/106 |
| Selective reporting (reporting bias) | High risk | Live birth rate was not addressed by the study |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Stenbaek 2015.
| Methods | RCT | |
| Participants | 83 women undergoing IVF/ICSI Inclusion criteria: not reported Exclusion criteria: prior IVF, uterine anomalies, testicular sperm aspiration needed, allergy to medication, reduced liver or kidney function, aged over 40 years, current or prior anti‐depressant medication Baseline characteristics: median age antagonist 31.2 years versus agonist 36.4 years Setting and timing: Copenhagen, Denmark. 2010 to 2012 |
|
| Interventions |
Antagonist (n = 42) rSH given for ovarian stimulation (150 to 225 IU depending on age) starting on day 2 to 3 of cycle. After five days women received ganirelix 0.25 mg daily Agonist (n = 41) nasal nafarelin acetate 200 mg 3 times daily starting on cycle day 21. After 14 days rFSH (150 to 225 IU depending on age). Nafarelin continued until day of oocyte pickup Ovulation induction hCG 6500 IU SC when three largest follicles were 17 mm or larger Oocyte retrieval 36 to 38 hours after hCG injection |
|
| Outcomes | Personality inventory, profile of mood states, perceived stress scale, symptom checklist (revised), major depression inventory, E2. No pregnancy outcomes reported and therefore no data that could be included in a meta‐analysis | |
| Notes | Sample size calculation: No ITT analysis: unclear Funding: Danish Research Council for Independent Research and MSD Add on to large Danish trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further details were reported |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details for participants, researchers or outcome assessors although non‐blinding of outcome assessors not likely to affect some of the outcome measures as they were objectively assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were any losses or if all women were analysed |
| Selective reporting (reporting bias) | Unclear risk | No pregnancy outcomes were reported and therefore no data could be included in a meta‐analysis |
| Other bias | High risk | Women in agonist group were significantly older than women in antagonist group at baseline |
Sunkara 2014.
| Methods | RCT. Two centres | |
| Participants | 111 women Inclusion criteria: poor responder (previous IVF cycle with stimulation using daily gonadotrophin ≥ 300 IU and who had ≤ 3 oocytes retrieved or had cycle cancelled due to ≤ 3 mature follicles developing) Exclusion criteria: > 40 years, single ovary Setting and timing: assisted conception unit, London, UK. March 2007 to May 2012 Baseline characteristics: age ‐ antagonist 37.4 ± 3.4 years versus agonist 36.7 ± 2.6 years |
|
| Interventions |
Antagonist ‐ Gonadotrophin injections 450 IU/day after ultrasound confirmation of quiescence of the ovaries, presence of thin endometrium (≤ 5 mm) and recording of antral follicles on day 2 or 3 cetrorelix (Cetrotide; Merck‐Serono) 0.25 mg daily when the lead follicle reached a diameter of 14 mm. Both gonadotrophin and cetrorelix injections continued until administration of hCG (n = 37). Agonist ‐ Pituitary down‐regulation with nafarelin nasal spray 400 µg twice daily (Synarel; Pharmacia) commenced in the mid‐luteal phase and continued for two weeks. After confirmation of down‐regulation by ultrasound and recording of antral follicles, ovarian stimulation was commenced with gonadotrophin injections 450 IU/day and reduced dose of nafarelin 200 µg twice daily until hCG injection. hCG administered when three antral follicles reached ≥ 17 mm diameter (n = 37). There was a third group that received a short agonist protocol. This arm is not described further here as it is not a comparison for this review (n = 37) Oocyte retrieval performed 34 to 38 hours after hCG Luteal phase support with progesterone pessaries 400 mg once or twice daily commencing on the day of oocyte retrieval and continued to negative pregnancy test or 8 weeks' gestation Gonadotrophin dose: antagonist 4740.0 ± 1131.9 versus antagonist 5540.32 ± 1216.1 Embryos transferred: antagonist 1.8 ± 0.6 versus agonist 1.7 ± 0.5 |
|
| Outcomes | Oocytes retrieved, dose of gonadotrophin, cycle cancellation, fertilisation rate, embryo transferred, clinical pregnancy rate, ongoing pregnancy rate | |
| Notes | Sample size calculation ‐ yes ITT analysis ‐ yes Funding ‐ assisted conception unit |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "internet based block randomization"; no further details were reported |
| Allocation concealment (selection bias) | Unclear risk | "allocated by a third party" "distant"; no further details were reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "doctor performing oocyte retrieval and the embryologist involved were blinded to the treatment allocation"; no information was available on blinding of participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For the long agonist regimen 31/37 women received the allocated intervention (five decided not to have further IVF treatment and there was one spontaneous pregnancy). For the antagonist regimen 30/37 women received the allocated intervention (six decided not to have further IVF treatment and there was one spontaneous pregnancy) ITT analysis was used and 37 women were analysed in each group |
| Selective reporting (reporting bias) | High risk | Clinical pregnancy rate is not given per group and live birth and OHSS are not reported at all |
| Other bias | Low risk | No other source of bias identified. Groups balanced at baseline |
Tazegul 2008.
| Methods | RCT, single‐centre | |
| Participants | 96 poor responders who underwent ICSI‐ET cycles Inclusion criteria: baseline follicle stimulating hormone (FSH) < 13 m IU/ml, estradiol level on the day of human chorionic gonadotrophin (hCG) injection < 500 pg/ ml and a poor response (failure in obtaining of at least three follicles > 16 mm in diameter and the number of mature oocytes retrieved less than four) after a previous ovarian stimulation cycle Exclusion criteria were: presence of a clinically significant systemic disease; diabetes mellitus; polycystic ovaries or any other endocrine disorder; submucosal polyp, myoma or uterine septum which were detected on hysteroscopy or hysterosalpingography. Intracytoplasmic sperm injection and assisted hatching were performed in all cycles. Baseline characteristics: Age (years) 38.3 ± 4.23 vs 37.9 ± 74.87. Baseline FSH (IU/mL) 6.31 ± 2.19 vs 6.27 ± 2.82 |
|
| Interventions |
GnRH antagonist (n= 48): 300 IU r‐FSH and hMG starting on the second day of menstruation for 6 days (adjusted) + 0.25 mg of cetrorelix (Cetrotide) or 0.25 mg ganirelix (Orgalutran) were administered subcutaneously per day when the leading follicle reached 14 mm in diameter until the hCG injection. (Flexible) GnRH agonist (n= 48): 1 mg/ day leuprolide acetate (Lucrin) started on the 21st day prior to menstruation for pituitary desensitization. When exogenous gonadotrophins were started on day 2 of menstruation, the dose of leuprolide acetate was decreased to 0.5 mg/day + 300 IU rFSH and hMG starting on the second day of menstruation for 6 days (adjusted) Oocyte maturation triggering: When the leading follicle reached 18 mm in diameter or at least two follicles were >17 mm in diameter, a total of 10,000 units of hCG were administered intramuscularly. Oocyte retrieval: was performed 35–37 hrs later Embryos transfer: day 2–3 luteal phase support: micronized vaginal progesterone, 600 mg/day, until the tenth week of gestation in cases where a pregnancy was achieved Follow up: clinical pregnancy was confirmed 28–35 days after embryo transfer by a gestational sac under ultrasound. Ongoing pregnancy was defined as fetal heart beat at 10–12 weeks of gestation. Early pregnancy loss was defined as the proportion of patients with initially positive hCG in whom pregnancy failed to develop before 12 weeks of gestation. |
|
| Outcomes | Clinical and ongoing pregnancy per randomised patient, the duration of stimulation, consumption of gonadotrophins, cycle cancellation rate, the number of oocytes retrieved and embryos transferred The hormone levels throughout the cycle | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based program. |
| Allocation concealment (selection bias) | Unclear risk | No details were reported to make a conclusive judgement |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcomes data, however LBR did not addressed by the study |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Tehraninejad 2010.
| Methods | RCT, single‐centre trial | |
| Participants | 95 PCOs infertile women undergoing IVF/ICSI treatment Inclusion criteria: age < 35 years basal FSH < 10 IU/L and undergoing their first cycle of ART Exclusion criteria: secondary infertility, previous IVF or ICSI, thyroid dysfunction, hyper prolactinemia, uterine abnormality and solitary ovary Baseline characteristics: age (years) 28.99 ± 6.1 vs 30.43 ± 5/08. Duration of infertility (years) 7.82 ± 4.70 vs 8.6 ± 4.61, BMI (kg/m2) 28.99 ± 6.12 vs 30.43 ± 5/08. Baseline FSH (IU/L) 5.4 ± 1.80 vs 5.3 ± 1.22 |
|
| Interventions |
GnRH antagonist (n= 45): OCP for 21 days in the previous cycles + 150 – 225 IU hMG (Merional) IM based on the patient's age and BMI + 0.25 GnRH antagonist cetrorelix (Cetrotide) when the leading follicle ≃ 14 mm (flexible multiple‐dose protocol) GnRH agonist (n= 47): OCP (30 g ethinyl estradiol plus 0.3 mg levonorgestrel) for 21 days + 500 µg buserelin per day (Superfact) SC, commenced on day 19 – 20 of OCP cycle. Once the down‐regulation was achieved, the dose of buserelin was reduced to 250 µg daily + 150 – 225 IU hMG (Merional) IM once daily depending on patient's age and BMI (GnRH agonist protocol) Oocyte maturation triggering: when at least two leading follicles were 18 mm in diameter, serum E2 levels were measured. If E2 level was measured to be less than 3000 pg/ml, participants in both groups would receive 10,000 IU hCG (Profasi) IM. In the control group, if E2 level was > 3000 pg/ml, hMG administration was stopped while Superfact injection was continued. Daily measurement of E2 level was performed and hCG was administered when E2 level fell below 3000 pg/ml (Coasting). In the study group, if E2 > 3000 pg/ml, Superfact 500 mg SQ was administered for final oocyte maturation Oocyte retrieval: 34 ‐ 36 hours later, followed by IVF/ICSI Maximum number of embryos transferred: 3 Luteal phase support: 800 mg vaginal micronised progesterone (Cyclogest) and 4 mg oral estradiol valerate daily started the evening after oocyte retrieval and continued until a negative pregnancy test or a 10‐week gestation Follow up: the serum hCG level on day 16 after oocyte recovery was tested to determine chemical pregnancy, if any, vaginal ultra sonography would be carried out on day 35 of oocyte retrieval for documentation of fetal heart activity and confirming a clinical pregnancy |
|
| Outcomes |
The primary outcome measures: incidence of moderate and severe OHSS The secondary endpoints: fertilisation and pregnancy rate Additional outcomes: number of oocytes retrieved, number of good quality embryos transferred, E2 level on the day of hCG administration, number of HMG ampoules used and the total days of treatment |
|
| Notes | The diagnosis of OHSS was based on the criteria by Golan 1989 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomised schedules |
| Allocation concealment (selection bias) | Low risk | Sealed in envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported clearly |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Tehraninejad 2011.
| Methods | Two‐arm parallel trial | |
| Participants | 300 normo‐responder women undergoing IVF in infertility clinic | |
| Interventions | GnRH antagonist: no details reported GnRH agonist: no further details reported |
|
| Outcomes | Ongoing pregnancy rate and clinical pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were allocated to two groups according to a sequence of computer generated random numbers (0 or 1).” |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not reported but unlikely to influence measurement of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number randomised = 300, number analysed = 300 |
| Selective reporting (reporting bias) | Unclear risk | Methods section not detailed enough to make conclusive judgement on reporting bias |
| Other bias | Unclear risk | Insufficient details to make a conclusive judgement |
Toltager 2015.
| Methods | Two‐arm parallel RCT | |
| Participants | 1099 women undergoing first IVF/ICSI cycles, less than 40 years of age including both low and high responders | |
| Interventions |
GnRH‐antagonist (550 women, mean age 32.1, BMI 23.1); no further details were given about treatment GnRH‐agonist (549 women, mean age 32.0, BMI 22.7); no further details were reported about treatment Fixed rFSH dose of 150 IU or 225 IU depending on the age (less than or equal to 36 years or greater than 36 years) with dose adjustment at stimulation day 6 |
|
| Outcomes | Ongoing pregnancy rates OHSS rates (mild, moderate and severe) |
|
| Notes | This is a conference abstract with limited information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information was provided on sequence generation; it was only stated that randomisation was done in ratio 1:1 |
| Allocation concealment (selection bias) | Unclear risk | No information was given on allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided on blinding of participants and/or personnel, including outcome assessors; however, non‐blinding of outcome assessors not likely to affect some of the outcome measures as they were objectively assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information was reported on attrition, withdrawals or exclusions and number of women analysed in each treatment group at the end of study was not reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures were pre‐specified |
| Other bias | Unclear risk | It was unclear if the numbers of participants were balanced at randomisation as the numbers of participants in the treatment groups were not reported |
Xavier 2005.
| Methods | RCT, single‐centre, open‐label design | |
| Participants | 131 infertile women undergoing IVF/ICSI Inclusion/Exclusion criteria: women were considered eligible if they were scheduled for controlled ovarian stimulation and IVF with or without ICSI. Women older than 40 years or with day 3 FSH = 10 IU/L, or with more than three previous IVF/ICSI cycles were excluded from the study |
|
| Interventions | GnRH antagonist protocol: rFSH treatment was begun on day 3 of the menstrual cycle. The starting dose for the first five days varied between 150 and 450 IU, depending on age and previous experience and was administered daily by SC injection. Thereafter, the dose was adjusted on the basis of ultrasonographic and analytic findings. On day 6 of rFSH treatment, cetrorelix was started if the ovarian response was adequate (at least one follicle = 13 mm or serum estradiol levels = 400 pg/mL). If the ovarian response was not adequate, cetrorelix administration was postponed until ultrasonographic or analytic criteria were achieved (Flexible). 250 microgram was administered daily by SC injection. When at least three follicles = 17 mm were observed, rFSH and cetrorelix administration was interrupted and hCG (10,000 IU IM) was administered for the timed oocyte retrieval 35 h later. Vaginal micronised progesterone was started 24 h after oocyte retrieval for luteal support in a standard dose of 600 mg daily for 14 days. Serum hCG was to be measured approximately two weeks after embryo transfer. Any pregnancy was confirmed by vaginal ultrasound scan at six weeks' gestation GnRH agonist protocol: on cycle days 21 ‐ 23, 0.6 mg of buserelin acetate was started, by daily SC injection until menses had begun and adequate suppression was achieved (serum estradiol level = 50 pg/mL), at which time treatment with rFSH was started. The starting dose for the first five days varied between 150 and 450 IU, depending on age and previous experience of the participant and was administered daily by SC injection. Thereafter, the dose was adjusted on the basis of ultrasonographic and analytic findings and the cycle management was the same in both groups | |
| Outcomes | The main outcome measures for assessing efficacy and safety of both protocols were: the clinical pregnancy rate per cycle and per transfer (gestational sac visualised on ultrasound at six weeks' gestation), number of oocytes collected, number of days of stimulation, number of days of analogue administration and the number of detected cases of moderate and severe OHSS. Other variables assessed were the total amount of rFSH used, serum estradiol level on the day of hCG administration, number of follicles = 15 mm on the day of the oocyte retrieval, endometrial thickness on the day of the oocyte retrieval, fertilisation rate, quality of the embryos transferred and the number of cancelled cycles. The quality of embryos used for transfer were classified using the following grading system: (A) no fragmentation; (B) 1% ‐ 20% fragmentation; (C) 21% ‐ 50% fragmentation; (D) = 51% fragmentation. The OHS classification utilised in this study was the one proposed by Golan 1989 | |
| Notes | Number of participants at randomisation: 131 (cetrorelix: 66/buserelin: 65) Number of participants at stimulation: 112 (cetrorelix: 53/buserelin: 59) Number of participants at OPU: 112 (cetrorelix: 53/buserelin: 59) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table, randomisation: 1:1 (Cetrorelix:Buserelin) ratio |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Ye 2009.
| Methods | RCT, single‐centre | |
| Participants | 220 IVF/ICSI cycles were included, age 25 to 35 years old, BMI 18 – 25 kg/m2; the number of previous IVF cycles < 3, and no previous poor response to ovarian stimulation (poor ovarian response was characterised by cancellation of the cycle due to either poor follicular development or ≤ 4 cumulus oocyte‐ complexes collected at oocyte retrieval); normal ovulatory cycles (25 to 35 days), both ovaries present and normal uterus; no hormone therapy within the past three months; and no current or past diseases affecting ovaries, gonadotrophin, sex steroid secretion, clearance or excretion Baseline characteristics: age (range) 30.3 ± 2.8 (24 – 35) vs 30.2 ± 2.8 (25 – 35), BMI (range) 20.7 ± 1.9 (16.9 – 24.9) vs 21.0 ± 1.8 (17.7 – 25), Basal FSH (IU/L) 6.2 ± 1.6 vs 6.5 ± 1.3 | |
| Interventions |
Study group: E2 pre‐treatment oral estradiol valerate 4 mg preceding the IVF cycle from day 21 until day 2 of next cycle + 225 IU of rFSH (Gonal‐F, Serono) from day 3 + 0.25 GnRH antagonist cetrorelix (Cetrotide) was injected daily when the leading follicles reached 12 – 14 mm in diameter (flexible)
Control group: triptorelin (Decapeptyl) 0.1 mg SC preceding the IVF cycle from day 21, when pituitary down‐regulation was achieved, the triptorelin dose was reduced to 0.05 mg/d + 225 IU of rFSH (Gonal‐F) Final oocyte maturation triggering: 10,000 IU hCG (Profasi) were given when at least three mature ≥ 18 mm follicles were obtained Oocytes retrievals: 36 hrs later Embryo transfer: 2 to 3 embryos were transferred at 72 hrs after IVF/ ICSI injection Luteal phase support: IM progesterone 80 mg/day starting on the day of oocyte retrieval until the day of pregnancy test. If a pregnancy occurred, progesterone administration was extended up to 10 to 12 weeks of pregnancy |
|
| Outcomes | Number of oocytes collected, MII oocytes, fertilisation, implantation, live birth and early pregnancy rate, and hormone profiles (LH, P, E2) | |
| Notes | The early pregnancy loss was defined as spontaneous abortion before 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
AFC: Antral Follicle Count
AMH: anti‐mullerian hormone
CC: clomiphene citrate
COS: controlled ovarian stimulation
ET: embryo transfer
FSH: follicle‐stimulating hormone
hCG: human chorionic gonadotrophin
IM: intramuscularly
IVF‐ET: in vitro fertilisation embryo transfer
MII = metaphase II
SC: subcutaneously
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ashrafi 2004 | No available data for inclusion |
| Bonduelle 2010 | Retrospective analysis |
| Cattani 2000 | No available data for inclusion |
| Causio 2004 | Quasi‐randomised study |
| Crosignani 2007 | RCT in IUI cycles |
| D'Amato 2004 | Quasi‐randomised trials, were randomly assigned to all women on the basis of the day of the week of their first appointment |
| Davar 2012 | Microdose GnRH agonist protocol used and number of women in each group not specified |
| De Klerk 2007 | Overlap with Eijkemans 2006; Heijnen 2007; Polinder 2008 |
| Dudley 2010 | Cross‐over study. Data not provided separately before and after cross‐over |
| Eijkemans 2006 | Overlap with Heijnen 2007 |
| Engmann 2008b | RCT, participant randomised on the day of hCG to receive, evaluate the effect of using vaginal micronised E2 administration, in addition to progesterone supplementation as luteal support, on clinical pregnancy rates in patients undergoing their first cycle of IVF/intracytoplasmic sperm injection (ICSI) treatment |
| Evangelio 2011 | Irrelevant interventions. Study objective was “to determine whether the addition of recombinant LH in women with low response predictors, improved response to ovarian stimulation and clinical outcome in cycles of IVF/ICSI with a GnRH antagonist protocol.” Study did not compare with long GnRH agonist protocol arm |
| Fabregues 2012 | Administration of GnRH analogues in the luteal phase of ART index cycle. Type GnRH agonist protocol not reported. No data/numbers provided to extract although the outcome reported fulfils the outcomes of interest |
| Ficicioglu 2010 | Retrospective study |
| Freitas 2004 | No available data for inclusion |
| Ghosh 2003 | Marked heterogeneity between the two study groups |
| Gordts 2011 | Study used a GnRH agonist short protocol for pituitary suppression |
| Guivarc’h‐Levêque 2010 | RCT, quasi‐randomised, as odd or even days of the consultation delivery of treatment |
| Ibrahim 2011 | Study used microdose flare‐up GnRH agonist protocol for pituitary suppression |
| Jindal 2013 | Numbers of participants randomised at baseline to each treatment group were not reported |
| Karimzadeh 2011 | Study used microdose GnRH agonist flare‐up (microdose protocol) for pituitary suppression |
| Kdous 2009 | Retrospective study |
| Kim 2010 | Retrospective study |
| Lee 2008 | Prospective observational/comparative study |
| Lin 1999 | No available data for inclusion. Surrogate outcome. Failure to contact authors |
| Londra 2003 | Not reported to be an RCT |
| Maldonado 2011 | Study used short GnRH agonist (triptoreline) protocol in alternate days for pituitary suppression |
| Maldonado 2013 | Study used GnRH agonist short regimen for pituitary suppression |
| Malhotra 2013 | Study used microdose flare‐up GnRH agonist protocol for pituitary suppression. No data (numbers) given for cycle cancellation rate although it was mentioned as being "similar in both groups". Clinical pregnancy rates were expressed as ‘per cycle’ in percentage |
| Mohsen 2013 | Protocol used microdose flare‐up |
| Orvieto 2007 | Prospective observational study |
| Orvieto 2008 | Retrospective trial |
| Ozdogan 2012 | Study used GnRH agonist microdose flare‐up protocol for pituitary suppression |
| Pabuccu 2005 | No available data for inclusion |
| Perino 2002 | No available data for inclusion. Failure to contact authors |
| Pinto 2009 | Prospective observational study |
| Polinder 2008 | Overlap with Heijnen 2007 |
| Prapas 2005 | RCT, some women were used twice as donors |
| Saini 2010 | Study did not specify the number of women in each group. It is impossible to separate out, from each group, the number of women from the number of cycles to obtain the right unit of measurement (per woman) for the outcomes of interest |
| Shamma 2003 | Donor oocyte cycles |
| Tanaka 2014b | Numbers of women randomised to each treatment group was not reported |
| Tiras 2013 | Probably not RCT, described as controlled clinical study. Clinical pregnancy rate expressed as ‘per embryo transfer’. Unit of measurement for miscarriage rate not specified. Other outcomes reported do not fulfil the review’s outcomes of interest |
| Verpoest 2013 | The number of women randomised in each group was not stated |
| Vlaisavljevic 2003 | Inadequate randomisation (quasi‐randomised trial) |
| Wang 2008 | Study used microdose GnRH agonist protocol for pituitary suppression |
| Willman 2005 | No available data for inclusion |
| Zikopoulos 2005 | IUI treatment cycles instead of IVF/ICSI |
Characteristics of studies awaiting assessment [ordered by study ID]
Toftager 2016.
| Methods | open‐label, RCT a web‐based concealed randomization code |
| Participants | 1099 infertile women referred for their first IVF/ICSI at two public fertility clinics All less than 40 years of age and with no uterine malformation including women with poor ovarian reserve, polycystic ovary syndrome and irregular cycles. |
| Interventions | women allocated to either short GnRH antagonist or longGnRHagonist protocol in a 1:1 ratio and enrolled over a 5‐year period |
| Outcomes | difference in severe OHSS, rates of mild and moderate OHSS, positive plasma (p)‐hCG, on‐going pregnancy and live birth |
| Notes | A total of 49 women withdrewtheir consent, thus 1050 subjects were allocated to the GnRH antagonist (n ¼ 534) and agonist protocol (n ¼ 516), respectively. |
Differences between protocol and review
In the 2016 update of this review, outcomes have been subgrouped with respect to the type of triggering agent and level of stimulation (minimal or standard). The protocol had the following subgroups.
GnRH antagonist regimen (fixed or flexible).
GnRH antagonist type (cetrorelix or ganirelix).
GnRH antagonist plus pre‐treatment with oral contraceptive pill (OCP).
Patient characteristics (polycystic ovary syndrome (PCOS); poor responders).
Patients undergoing mild ovarian stimulation.
Miscarriage rate per woman randomised has been introduced as one of the secondary outcomes, with miscarriage rate per clinical pregnancy (a secondary outcome in the protocol) retained as a secondary analysis.
Contributions of authors
For the 2016 update Reuben Olugbenga Ayeleke and Julie Brown screened the searches and selected studies for inclusion. Reuben Olugbenga Ayeleke, Julie Brown and Wai Sun Lam extracted and entered data; Reuben Olugbenga Ayeleke contributed to the modification and updating of the review text; Hesham Al‐Inany, Frank J Broekmans and Mohamed Abdel Fattah Mahmoud Youssef contributed to the discussion and interpretation of results.
For the 2011 update
Hesham Al‐Inany: took the lead in writing the protocol, review, and update, performing initial searches of databases for trials, was involved in selecting trials for inclusion, performed independent data extraction and quality assessment of the included trials, and was responsible for statistical analysis and interpretation of the data.
Mohamed Abdel Fattah Mahmoud Youssef: performed updated searches of databases for new trials, was involved in selecting trials for inclusion, performed independent data extraction and quality assessment of the included trials, and was responsible for statistical analysis and interpretation of the data.
Mohamed Aboulghar: commented on drafts of the protocol and review.
Frank JM Broekmans: contributed to discussion and commented on review.
Monique D Sterrenburg: contributed to discussion and commented on review.
Janine G Smit: contributed to data analysis checks and discussion, and commented on review.
Ahmed Abou‐Setta: was involved in selecting trials for inclusion, performed independent data extraction and quality assessment of the included trials, and contributed to discussion and interpretation of results.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Previous author Professor Dr Mohamed Aboulghar was an investigator in one of the included trials, the European Middle East Orgalutran trial Euro Middle East 2001.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Albano 2000 {published data only}
- Albano C, Felberbaum R, Smitz J, Riethmuller‐Winzen H, Engel J, Diedrich K, et al. Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone‐releasing hormone (LHRH)‐antagonist cetrorelix and the LHRH‐agonist buserlin. Human Reproduction 2000;15(3):526‐31. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Felberbaum RE, Devroey P, Albano C, Riethmuller‐Winzen H, Schuler A, et al. Significant reduction of the incidence of ovarian hyperstimulation syndrome (OHSS) by using the LHRH antagonist cetrorelix (Cetrotide) in controlled ovarian stimulation for assisted reproduction. Archives of Gynecology and Obstetrics 2000;264(1):29‐32. [DOI] [PubMed] [Google Scholar]
Al‐Karaki 2011 {published data only}
- Al‐Karaki R, Irzouqi R, Khalifa F, Taher M, Sarraf M. Effectiveness of flexible GnRH antagonist protocol versus minidose long GnRH agonist protocol in poor‐responder patients undergoing IVF. Human Reproduction 2011;26(1):i47. [Google Scholar]
Anderson 2014 {published data only}
- Anderson S, Pereira NE, Brasile DR, Orris JJ, Davies EB, Glassner MJ. Comparison of antagonist to agonist in controlled ovarian stimulation (COS) cycles using human‐derived gonadotropin on in vitro fertilization (IVF) outcomes. A prospective randomized controlled study. Fertility and sterility. 2014; Vol. 102 (3; Suppl 1):e224.
Awata 2010 {published data only}
- Awata S, Tanaka A, Nagayoshi M. Selection of an optimal controlled ovarian hyperstimulation method in relation to the number of antral follicles in patients less than 40 years old. Fertility and Sterility 2010;94 suppl 1(4):S164 Abstract no. P‐244. [Google Scholar]
Baart 2007 {published data only}
- Baart EB, Martini E, Eijkemans MJ, Opstal D, Beckers NGM, Verhoeff A, et al. Milder ovarian stimulation for in‐vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Human Reproduction 2007;22(4):980‐8. [DOI] [PubMed] [Google Scholar]
Badrawi 2005 {published data only}
- Badrawi A, Al‐Inany H, Hussein M, Zaki S, Ramzy AM. Agonist versus antagonist in ICSI cycles: a randomized trial and cost effectiveness analysis. Middle East Fertility Society Journal 2005;10(1):49‐54. [Google Scholar]
Bahceci 2005 {published data only}
- Bahceci M, Ulug U, Ben‐Shlomo I, Erden HF, Akman MA. Use of a GnRH antagonist in controlled ovarian hyperstimulation for assisted conception in women with polycystic ovary disease: a randomized, prospective, pilot study. Journal of Reproductive Medicine 2005;50(2):84‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Barmat 2005 {published data only}
- Barmat LI, Chantilis SJ, Hurst BS, Dickey RP. A randomized prospective trial comparing gonadotropin‐releasing hormone (GnRH) antagonist/recombinant follicle‐stimulating hormone (r FSH) versus GnRH‐agonist/r FSH in women pretreated with oral contraceptives before in vitro fertilization. Fertility and Sterility 2005;83(2):321‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brelik 2004 {published data only}
- Brelik P, Kurzawa R, Baczkowski T, Sienkiewicz R, Glabowski W. Assessment of the predictive value of LH levels in IVF cycles stimulated with GnRH antagonists and agonists. Human Reproduction 2004;19:i62. [Google Scholar]
Celik 2011 {published data only}
- Celik N, Celik O, Aktan E, Ozerol E, Celik E, Bozkurt K, et al. Plasma urocortin levels in women undergoing long agonist and antagonist protocols for IVF. Human Reproduction 2011;26(1):i203. [Google Scholar]
Check 2004 {published data only}
- Check ML, Check JH, Choel JK, Davies E, Kiefer D. Effect of antagonists vs agonists on in vitro fertilization outcome. Clinical and Experimental Obstetrics & Gynecology 2004;31(4):257‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Cheung 2005 {published data only}
- Cheung LP, Lam PM, Lok IH, Chiu TT, Yeung SY, Tjer CC, et al. GnRH antagonist versus long GnRH agonist protocol in poor responders undergoing IVF: a randomized controlled trial. Human Reproduction (Oxford, England) 2005;20(3):616‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Choi 2012 {published data only}
- Choi MH, Kim HO, Cha SW, Koong MKK, Kim JY, Park CW. IVF comparison of ART outcomes in infertile PCOS women; in vitro maturation (IVM) vs. GnRH agonist vs. GnRH antagonist cycles. Clinical Experimental Reproductive Medicine December 2012;39(4):S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MH, Lee SH, Kim HO, Cha SH, Kim JY, Yang KM, et al. Comparison of assisted reproductive technology outcomes in infertile women with polycystic ovary syndrome: In vitro maturation, GnRH agonist, and GnRH antagonist cycles. Clinical & Experimental Reproductive Medicine 2012;39(4):166‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cota 2012 {published data only}
- Cota AM, Oliveira JB, Petersen CG, Mauri AL, Massaro FC, Silva LF, et al. GnRH agonist versus GnRH antagonist in assisted reproduction cycles: oocyte morphology. Reproductive Biology and Endocrinology 2012;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorato HL, Oliveira JB, Petersen CG, Vagnini L, Mauri AL, Cavagna M, et al. GnRH agonist versus GnRH antagonist in IVF/ICSI cycles with recombinant LH supplementation: DNA fragmentation and apoptosis granulosa cells. European Journal of Obstetrics & Gynecology and Reproductive Biology 2012;165:61‐5. [DOI] [PubMed] [Google Scholar]
Depalo 2009 {published data only}
- Depalo R, Lorusso F, Palmisano M, Bassi E, Totaro I, Vacca M, et al. Follicular growth and oocyte maturation in GnRH agonist and antagonist protocols for in vitro fertilisation and embryo transfer. Gynecological Endocrinology 2009;25(5):328‐34. [DOI] [PubMed] [Google Scholar]
El Sahwi 2005 {published data only}
- Sahwi S. GnRH agonists versus GnRH antagonists in controlled ovarian stimulation in ICSI trials. Book of Abstracts, 8th International Symposium on GnRH Analogues in Cancer and Human Reproduction. Salzburg, Austria. 2005:A65.
Engmann 2008a {published data only}
- Engmann L, DiLuigi A, Schmidt D, Nulsen D, Nulsen J, Benadiva C. The use of gonadotropin‐releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high‐risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospsective randomised controlled study. Fertility and Sterility 2008;89(1):84‐91. [DOI] [PubMed] [Google Scholar]
Euro Middle East 2001 {published data only}
- European and Middle East Orgalutran Study Group. Comparable clinical outcome using the GnRH antagonist ganirelix or a long protocol of the GnRH agonist triptorelin for the prevention of premature LH surges in women undergoing ovarian stimulation. Human Reproduction (Oxford, England) 2001;16(4):644‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sonntag B, Kiesel L, Nieschlag E, Behre HM. Association of inhibin B serum levels with parameters of follicular response in a randomized controlled trial comparing GnRH agonist versus antagonist protocols for ovarian hyperstimulation. Journal of Assisted Reproduction and Genetics 2004;21(7):249‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Euro Orgalutran 2000 {published data only}
- The European Orgalutran Study Group, Borm G, Mannaerts B. Treatment with the gonadotrophin‐releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone is effective, safe and convenient: results of a controlled, randomized, multicentre trial. Human Reproduction (Oxford, England) 15;7:1490‐8. [DOI] [PubMed] [Google Scholar]
Ferrari 2006 {published data only}
- Ferrari B, Pezzuto A, Barusi L, Coppola F. Follicular fluid vascular endothelial growth factor concentrations are increased during GnRH antagonist/FSH ovarian stimulation cycles. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;124(1):70‐6. [DOI] [PubMed] [Google Scholar]
- Ferrari B, Pezzuto A, Barusi L, Coppola F. Gonadotrophin‐releasing hormone antagonists increase follicular fluid insulin‐like growth factor‐I and vascular endothelial growth factor during ovarian stimulation cycles. Gynecological Endocrinology June 2006;22(6):289‐96. [DOI: 10.1080/09513590600777602] [DOI] [PubMed] [Google Scholar]
Ferrero 2010 {published data only}
- Ferrero S, Abbamonte L H, Privamera MR, Levi S, Venturini PL, Anserini P. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients at high risk of ovarian hyperstimulation syndrome: A prospective randomized controlled trial. Fertility and Sterility 2010;94 suppl 1(4):S28 Abstract no. O‐92. [Google Scholar]
Firouzabadi 2010 {published data only}
- Firouzabadi RD, Ahmadi S, Oskouian H, Davar R. Comparing GnRH agonist long protocol and GnRH antagonist protocol in outcome the first cycle of ART. Archives of Gynecology and Obstetrics 2010;281(1):81‐5. [PUBMED: PMID: 19357861 ] [DOI] [PubMed] [Google Scholar]
Fluker 2001 {published and unpublished data}
- Fluker M, Grifo J, Leader A, Levy M, Meldrum D, Muasher SJ, et al. for The North American Ganirelix Study Group. Efficacy and safety of ganirelix acetate versus leuprolide acetate in women undergoing controlled ovarian hyperstimulation. Fertility and Sterility 2001;75(1):38‐45. [DOI] [PubMed] [Google Scholar]
Franco 2003 {published data only}
- Franco Jr JG, Baruffi RLR, Petersen CG, Mauri AL, Felipe V, Contart P. Comparison of ovarian stimulation with recombinant FSH After 2nd phase protocols with GnRH Analogs (I‐estradiol + ganirelix versus II‐nafarelin) [Comparacao da estimulacao ovariana com FSH recombinante apos protocolo de 2A fase com analogos do GNRH (I ‐estradiol + ganirelix versus II‐nafarelin)]. Jornal Brasileiro de Reproducao Assistida 2003;7(1):26‐32. [Google Scholar]
Friedler 2003 {published data only}
- Friedler S, Gilboa S, Schachter M, Raziel R, Strassburger D, Kasterstein E, et al. Luteal phase characteristics following GnRH antagonist or agonist treatment: a randomized comparative study. Human Reproduction (Oxford, England) 2003;18 Suppl 1:26. [Google Scholar]
Gizzo 2014 {published data only}
- Gizzo S, Andrisani A, Esposito F, Noventa M, Gangi S, Angioni S, et al. Which luteal phase support is better for each IVF stimulation protocol to achieve the highest pregnancy rate? A superiority randomized clinical trial. Gynecological Endocrinology 2014;30(12):902‐8. [DOI: 10.3109/09513590.2014.964638] [DOI] [PubMed] [Google Scholar]
Haydardedeoglu 2012 {published data only}
- Haydardedeoglu B, Kilicdag EB, Parlakgumus AH, Zeyneloglu HB. IVF/ICSI outcomes of the OCP plus GnRH agonist protocol versus the OCP plus GnRH antagonist fixed protocol in women with PCOS: a randomized trial. Archives of Gynecology and Obstetrics September 2012;286(3):763‐769. [DOI] [PubMed] [Google Scholar]
Heijnen 2007 {published data only}
- Heijnen EM, Eijkemans MJ, Klerk C, Polinder S, Beckers NG, Klinkert ER, et al. A mild treatment strategy for in‐vitro fertilisation: a randomised non‐inferiority trial. The Lancet 2007;369:743‐9. [DOI] [PubMed] [Google Scholar]
Hershko Klement 2015 {published data only}
- Hershko Klement A, Berkovitz A, Wiser A, Gonen O, Amichay K, Cohen I, et al. GnRH‐antagonist programming versus GnRH agonist protocol: a randomized trial. European Journal of Obstetrics Gynecology and Reproductive Biology 2015;185:170‐3. [DOI] [PubMed] [Google Scholar]
- Hershko Klement A, Berkovitz A, Wiser A, Gonen O, Amichay K, Shulman A. Follicular estrogen for GnRH‐antagonist protocol programming: a prospective randomized clinical trial. Fertility and Sterility. 2013; Vol. 1:S523.
Hohmann 2003 {published data only}
- Hohmann FP, Macklon NS, Fauser BC. A randomized comparison of two ovarian stimulation protocols with gonadotropin‐releasing hormone (GnRH) antagonist cotreatment for in vitro fertilization commencing recombinant follicle‐stimulating hormone on cycle day 2 or 5 with the standard long GnRH agonist protocol. The Journal of Clinical Endocrinology and Metabolism 2003;88(1):166‐73. [DOI] [PubMed] [Google Scholar]
Hoseini 2014 {published data only}
- Hoseini FS, Mugahi SM, Akbari‐Asbagh F, Eftekhari‐Yazdi P, Aflatoonian B, Aghaee‐Bakhtiari SH, et al. A randomized controlled trial of gonadotropin‐releasing hormone antagonist in Iranian infertile couples: oocyte gene expression. Journal of Pharmaceutical Sciences 2014;22:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosseini 2010 {published data only}
- Hosseini MA, Aleyasin A, Saeedi H, Mahdavi A. Comparison of gonadotropin‐releasing hormone agonists and antagonists in assisted reproduction cycles of polycystic ovarian syndrome patients. Journal of Obstetrics & Gynaecology Research 2010;36(3):605‐10. [DOI] [PubMed] [Google Scholar]
Hsieh 2008 {published data only}
- Hsieh YY, Chang CC, Tsai HD. Comparisons of different dosages of gonadotropin‐releasing hormone (GnRH) antagonist, short‐acting form and single, half‐dose, long‐acting form of GnRH agonist during controlled ovarian hyperstimulation and in vitro fertilization. Taiwan Journal of Obstetrics and Gynecology 2008;47:66‐74. [DOI] [PubMed] [Google Scholar]
Huirne 2006 {published data only}
- Huirne JA, Loenen AC, Donnez J, Pirard C, Homburg R, Schats R, et al. Effect of an oral contraceptive pill on follicular development in IVF/ICSI patients receiving a GnRH antagonist: a randomized study. Reproductive Biomedicine Online 2006;13(2):235‐45. [DOI] [PubMed] [Google Scholar]
Hwang 2004 {published data only}
- Hwang JL, Seow K, Lin Y, Huang L, Hsieh B, Tsai Y, et al. Ovarian stimulation by concomitant administration of cetrorelix acetate and HMG following Diane‐35 pre‐treatment for patients with polycystic ovary syndrome: a prospective randomized study. Human Reproduction 2004;19(9):1993‐2000. [DOI] [PubMed] [Google Scholar]
Inza 2004 {published data only}
- Inza R, Thillo G, Lombardi E, Bisioli C, Diradourian M, Kenny A. Reproductive performance in second IVF cycles treated with the use of either GnRH antagonists (‐antag) vs GnRH agonists (‐ag) after failure with long protocols with GnRH agonists: a prospective randomized trial. Fertility and Sterility 2004;82 Suppl:233‐4. [Google Scholar]
Karimzadeh 2010 {published data only}
- Karimzadeh MA, Ahmadi S, Oskouian H, Rahmani E. Comparison of mild stimulation and conventional stimulation in ART outcome. Archives of Gynecology and Obstetrics 2010;281(4):741‐6. [DOI] [PubMed] [Google Scholar]
Khalaf 2010 {published data only}
- Khalaf M, Mittre H, Levallet J, Hanoux V, Denoual C, Herlicoviez M, et al. GnRH agonist and GnRH antagonist protocols in ovarian stimulation: differential regulation pathway of aromatase expression in human granulosa cells. Reproductive Biomedicine Online 2010;21(1):56‐65. [DOI] [PubMed] [Google Scholar]
Kim 2004 {published data only}
- Kim C‐H, Lee Y‐J, Hong S‐H, Nah H‐Y, Kim S‐H, Chae H‐D, et al. Efficacy of a GnRH antagonist during early and late controlled ovarian hyperstimulation period in women with polycystic ovary syndrome undergoing IVF‐ET. Human Reproduction (Oxford, England) 2004;19:i104‐5. [Google Scholar]
Kim 2011 {published data only}
- Kim C‐H, Jeon G‐H, Cheon Y‐P, Jeon I, Kim S‐H, Chae H‐D, et al. Comparison of GnRH antagonist protocol with or without oral contraceptive pill pretreatment and GnRH agonist low‐dose long protocol in low responders undergoing IVF/intracytoplasmic sperm injection. Fertility and Sterility 2009;92(5):1758‐60. [DOI] [PubMed] [Google Scholar]
- Kim C‐H, You R‐M, Kang H‐K, Ahn J‐W, Jeon I, Lee J‐W, et al. GnRH antagonist multiple dose protocol with oral contraceptive pill pre‐treatment in poor responders undergoing IVF/ICSI. Clinical and Experimental Reproductive Medicine 2011;38(4):228‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lee HA, Lee JW, Lee YJ, Nah HY, Hong SH, et al. The efficacy of oral contraceptive pretreatment in controlled ovarian hyperstimulation using a GnRH antagonist for low responders [poster]. Abstracts of the 21st Annual Meeting of the ESHRE. Copenhagen, Denmark, 2005. Human Reproduction. 2005; Vol. 20 Suppl 1:i105‐6.
Kim 2012 {published data only}
- Kim C‐H, Moon J‐W, Kang H‐J, Ahn J‐W, Kim S‐H, Chae H‐D, et al. Effectiveness of GnRH antagonist multiple dose protocol applied during early and late follicular phase compared with GnRH agonist long protocol in non‐obese and obese patients with polycystic ovary syndrome undergoing IVF/ICSI. Clinical and Experimental Reproductive Medicine 2012;39(1):22‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurzawa 2008 {published data only}
- Kurzawa R, Ciepiela P, Baczkowski T, Safranow K, Brelik P. Comparison of embryological and clinical outcome in GnRH antagonist vs. GnRH agonist protocols for in vitro fertilization in PCOS non‐obese patients. A prospective randomized study. Journal for Assisted Reproduction and Genetics 2008;25(8):365‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyono 2005 {published data only}
- Kyono K, Fuchinoue K, Nakajo Y, Yagi A, Sasaki K. A prospective randomized study of three ovulation induction protocols for IVF: GnRH agonist versus antagonist with and without low dose hCG. Fertility and Sterility 2004;82 Suppl:31. [Google Scholar]
- Kyono K, Nakajo Y, Sasaki S, Kumagai S, Suzuki S. A prospective randomized study of three different controlled ovarian hyperstimulation (COH) protocols. Fertility and Sterility 2005;84 Suppl 1:299. [Google Scholar]
Lainas 2007 {published data only}
- Lainas TG, Petsas GK, Zorovilis IZ, Lliadis GS, Lainas GT, Gazlaris HE, et al. Initiation of GnRH antagonist on day 1 of stimulation as compared to the long agonist protocol in PCOS patients: a randomised controlled trial: effect on hormonal levels and follicular development. Human Reproduction 2007;22(6):1540‐6. [DOI] [PubMed] [Google Scholar]
Lainas 2010 {published data only}
- Basly M, Achour R, Ben Jemaa S, Chnitir M, Messaoudi L, Chibani M, et al. Flexible Gnrh antagonist protocol versus Gnrh agonist long protocol in patients with polycystic ovary syndrome treated for IVF: a prospective randomised controlled trial (RCT). Internet Journal of Gynecology & Obstetrics 2012;16(3):1. [Google Scholar]
- Lainas TG, Sfontouris IA, Zorzovilis IZ, Petsas GK, Lainas GT, Alexopoulou E, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: a prospective randomised controlled trial (RCT). Human Reproduction 2010;25(3):683‐9. [DOI] [PubMed] [Google Scholar]
Lavorato 2012 {unpublished data only}
- Lavorato HL, Oliveira JB, Petersen CG, Vagnini L, Maur AL, Cavagna M, et al. GnRH agonist versus GnRH antagonist in IVF/ICSI cycles with recombinant LH supplementation: DNA fragmentation and apoptosis in granulosa cells. European Journal of Obstetrics & Gynecology and Reproductive Biology 2012;165(1):61‐5. [DOI] [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee TH, Wu MH, Chen HF, Chen MJ, Ho HN, Yang YS. Ovarian response and follicular development for single‐dose and multiple‐dose protocols for gonadotropin‐releasing hormone antagonist administration. Fertility and Sterility 2005;83(6):1700‐7. [DOI] [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin YH, Hwang JL, Seow KM, Huang LW, Hsieh BC, Tzeng CR. Comparison of outcome of clomiphene citrate/human menopausal gonadotropin/cetrorelix protocol and buserelin long protocol ‐ a randomized study. Gynecological Endocrinology 2006;22(6):297‐302. [DOI] [PubMed] [Google Scholar]
Loutradis 2004 {published data only}
- Loutradis D, Stefanidis K, Drakakis P, Milingos S, Antsaklis A, Michalas S. A modified gonadotropin‐releasing hormone (GnRH) antagonist protocol failed to increase clinical pregnancy rates in comparison with the long GnRH protocol. Fertility and Sterility 2004;82(5):1446‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marci 2005 {published data only}
- Marci R, Caserta D, Dolo V, Tatone C, Pavan A, Moscarini M. GnRH antagonist in IVF poor‐responder patients: results of a randomized trial. Reproductive Biomedicine Online 2005;11(2):189‐93. [DOI] [PubMed] [Google Scholar]
- Marci R, Caserta D, Farina M, Dessole S, Germond M, Tatone C, et al. The use of GnRH antagonist in ovarian stimulation for IVF cycles can achieve good pregnancy rates in poor responder patients. Human Reproduction (Oxford, England) 2002;17:115‐6. [Google Scholar]
Martinez 2008 {published data only}
- Martínez F, Clua E, Parera N, Rodríguez I, Boada M, Coroleu B. Prospective, randomized, comparative study of leuprorelin + human menopausal gonadotropins versus ganirelix + recombinant follicle‐stimulating hormone in oocyte donors and pregnancy rates among the corresponding recipients. Gynecological Endocrinology 2008;24(4):188‐93. [DOI] [PubMed] [Google Scholar]
Mohamed 2006 {published data only}
- Mohamed KA, Davies WAR, Lashen H. Effect of gonadotropin‐releasing hormone agonist and antagonist on steroidogenesis of low responders undergoing in vitro fertilization. Gynecological Endocrinology 2006;22(2):57‐62. [DOI] [PubMed] [Google Scholar]
Moraloglu 2008 {published data only}
- Moraloglu O, Kilic S, Karayalçin R, Yuksel B, Tasdemir N, Ugur M. Comparison of GnRH agonists and antagonists in normo‐responder IVF/ICSI in Turkish female patients. Advances in Therapy 2008;25(3):266‐73. [DOI] [PubMed] [Google Scholar]
Moshin 2007 {published data only}
- Moshin V, Croitor M, Hotineanu A. GnRH antagonist versus long GnRH agonists protocol in PCOS patients undergoing IVF treatment. Abstracts of the 23rd Annual Meeting of the ESHRE, Lyon, France 2007;22 Suppl 1:i121. [Google Scholar]
Olivennes 2000 {published data only}
- Olivennes F, Belaisch‐Allart, Emperare J, Dechaud H, S Alvarez S, Moreau L. Prospective randomized, controlled study of in vitro fertilization‐embryo transfer with a single dose of a luteinizing hormone‐releasing hormone (LH‐RH) antagonist (cetrorelix) or a depot formula of an LH‐RH agonist (triptorelin). Fertility and Sterility 2000;73(2):314‐20. [DOI] [PubMed] [Google Scholar]
Papanikolaou 2012 {published data only}
- Papanikolaou EG, Pados G, Grimbizis G, Bili E, Kyriazi L, Polyzos NP, et al. GnRH‐agonist versus GnRH‐antagonist IVF cycles: is the reproductive outcome affected by the incidence of progesterone elevation on the day of HCG triggering? A randomized prospective study. Human Reproduction 2012;27(6):1822‐8. [DOI] [PubMed] [Google Scholar]
Prapas 2013 {published data only}
- Prapas Y, Petousis S, Dagklis T, Panagiotidis Y, Papatheodorou A, Assunta I, et al. GnRH antagonist versus long GnRH agonist protocol in poor IVF responders: a randomized clinical trial. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2013;166(1):43‐6. [DOI] [PubMed] [Google Scholar]
Qiao 2012 {published data only}
- Qiao J, Lu G, Zhang HW, Chen H, Ma C, Olofsson JI, et al. A randomized controlled trial of the GnRH antagonist ganirelix in Chinese normal responders: high efficacy and pregnancy rates. Gynecological Endocrinology 2012;28(10):800‐4. [DOI] [PubMed] [Google Scholar]
Rabati 2012 {published data only}
- Rabati BK, Zeidi SN. Investigation of pregnancy outcome and ovarian hyper stimulation syndrome prevention in agonist and antagonist gonadotropin‐releasing hormone protocol. Journal of Research in Medical Sciences 2012;17(11):1063‐6. [PMC free article] [PubMed] [Google Scholar]
Revelli 2014 {published data only}
- Revelli A, Chiadò A, Dalmasso P, Stabile V, Evangelista F, Basso G, et al. “Mild” vs. “long” protocol for controlled ovarian hyperstimulation in patients with expected poor ovarian responsiveness undergoing in vitro fertilization (IVF): a large prospective randomized trial. Journal of Assisted Reproduction and Genetics July 2014;31(7):809‐15. [DOI: 10.1007/s10815-014-0227-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rinaldi 2014 {published data only}
- Rinaldi L, Lisi F, Selman H. Mild/minimal stimulation protocol for ovarian stimulation of patients at high risk of developing ovarian hyperstimulation syndrome. Journal of Endocrinological Investigation 2014;37:65‐70. [DOI] [PubMed] [Google Scholar]
Rombauts 2006 {published data only}
- Rombauts L, Healy D, Norman RJ. A comparative randomized trial to assess the impact of oral contraceptive pretreatment on follicular growth and hormone profiles in GnRH antagonist‐treated patients. Human Reproduction (Oxford, England) 2006;21(1):95‐103. [DOI] [PubMed] [Google Scholar]
Sauer 2004 {published data only}
- Sauer MV, Thornton MH, Schoolcraft W, Frishman GN. Comparative efficacy and safety of cetrorelix with or without mid‐cycle recombinant LH and leuprolide acetate for inhibition of premature LH surges in assisted reproduction. Reproductive Biomedicine Online 2004;9(5):487‐93. [DOI] [PubMed] [Google Scholar]
Sbracia 2009 {published data only}
- Sbracia M, Colabianchi J, Giallonardo A, Giannini P, Piscitelli C, Morgia F, et al. Cetrorelix protocol versus gonadotropin‐releasing hormone analog suppression long protocol for superovulation in intracytoplasmic sperm injection patients older than 40. Fertility and Sterility 2009;91(5):1842‐7. [DOI] [PubMed] [Google Scholar]
Serafini 2008 {published data only}
- Serafini P, Yadid I, Alegretti J, Panzan M, Cosloversusky M, Motta E. A prospective, randomized trial of three ovulation induction protocols for IVF including a novel approach with low‐dose HCG and GnRH antagonist in the mid‐late follicular phase. Human Reproduction (Oxford, England) 2003;18:1. [Google Scholar]
- Serafini P, Yadid I, Motta ELA, Alegretti JR, Fioavanti J, Coslovsky M. Ovarian stimulation with daily late follicular phase administration of low dose human chorionic gonadotropin for in vitro fertilization: a prospective randomized trial. Fertility and Sterility 2006;86(4):830‐8. [DOI] [PubMed] [Google Scholar]
Stenbaek 2015 {published data only}
- Stenbaek DS, Toftager M, Hjordt LV, Jensen PS, Holst KK, Bryndorf T, et al. Mental distress and personality in women undergoing GnRH agonist versus GnRH antagonist protocols for assisted reproductive technology. Human Reproduction 2015;30(1):103‐10. [DOI] [PubMed] [Google Scholar]
Sunkara 2014 {published data only}
- Sunkara S, Coomarasamy A, Faris R, Braude P, Khalaf Y. Effectiveness of the GnRH agonist long, GnRH agonist short and GnRH antagonist regimens in poor responders undergoing IVF treatment: a three arm randomised controlled trial. Human Reproduction. 2013; Vol. 28:i348.
- Sunkara SK, Coomarasamy A, Faris R, Braude P, Khalaf Y. Long gonadotropin‐releasing hormone agonist versus short agonist versus antagonist regimens in poor responders undergoing in vitro fertilization: A randomized controlled trial. Fertility and Sterility 2014;101(1):147‐53. [DOI] [PubMed] [Google Scholar]
Tazegul 2008 {published data only}
- Tazegül A, Görkemli H, Ozdemir S, Aktan TM. Comparison of multiple dose GnRH antagonist and minidose long agonist protocols in poor responders undergoing in vitro fertilization: a randomized controlled trial. Archives of Gynecology and Obstetrics 2008;278(5):467‐72. [DOI] [PubMed] [Google Scholar]
Tehraninejad 2010 {published data only}
- Tehraninejad ES, Nasiri R, Rashidi B, Haghollahi F, Ataie M. Comparison of GnRH antagonist with long GnRH agonist protocol after OCP pretreatment in PCOs patients. Archives of Gynecology and Obstetrics 2010;282(3):319‐25. [DOI] [PubMed] [Google Scholar]
Tehraninejad 2011 {published data only}
- Nezamabadi AG, Tehraninejad ES, Rashidi B. GnRH Antagonist versus Agonist in Normoresponders Undergoing ICSI: A Randomized Clinical Trial in Iran. International Journal of Fertility and Sterility 2013;7(1):106. [PMC free article] [PubMed] [Google Scholar]
- Tehraninejad E, Nezamabadi AG, Rashidi B, Sohrabi M, Bagheri M, Haghollahi F, et al. GnRH antagonist versus agonist in normoresponders undergoing ICSI: a randomized clinical trial in Iran. Iranian Journal of Reproductive Medicine 2011;9(3):171‐6. [IRCT138902283950N1]] [PMC free article] [PubMed] [Google Scholar]
Toltager 2015 {published data only}
- Toltager M, Bogstad J, Lossl, K, et al. Pregnancy rates and risk of ovarian hyperstimulation syndrome (OHSS) in a fixed GnRH‐antagonist versus GnRH‐agonist protocol: randomised controlled trial including 1099 first IVF/ICSI CYCLES. Abstract of the 31st annual meeting of ESHRE I Lisbon, Portigal. 2015:i94.
Xavier 2005 {published data only}
- Xavier P, Gamboa C, Calejo L, Silva J, Stevenson D, Nunes A, et al. A randomised study of GnRH antagonist (cetrorelix) versus agonist (busereline) for controlled ovarian stimulation: effect on safety and efficacy. Eurpean Journal of Obstetrics, Gynecology, and Reproductive Biology 2005;120(2):185‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ye 2009 {published data only}
- Ye H, Huang G, Zeng P, Pei L. IVF/ICSI outcomes between cycles with luteal estradiol (E2) pre‐treatment before GnRH antagonist protocol and standard long GnRH agonist protocol: a prospective and randomized study. Journal of Assisted Reproduction and Genetics 2009;2(3):105‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ashrafi 2004 {published data only}
- Ashrafi M, Mohammadzadeh A, Ezabadi Z, Baghestani AR. A comparative study of GnRH‐ant and GnRH‐ag protocols on IVF‐ICSI in PCOD patients. Book of Abstracts, 18th World Congress on Fertility and Sterility (IFFS 2004), Montreal Canada. 2004.
Bonduelle 2010 {published data only}
- Bonduelle M, Oberyé J, Mannaerts B, Devroey P. Large prospective, pregnancy and infant follow‐up trial assures the health of 1000 fetuses conceived after treatment with the GnRH antagonist ganirelix during controlled ovarian stimulation. Human Reproduction 2010;25(6):1433‐40. [DOI] [PubMed] [Google Scholar]
Cattani 2000 {published data only}
- Cattani R, Cela V, Cristello F, Matteucci C, Valentino V, Artini P. Efficacy and safety of gonadotrophin releasing hormone (GnRH) antagonist administration in infertile women during controlled ovarian hyperstimulation. Gynecological Endocrinology. 2000; Vol. 14, issue Suppl 2:212.
Causio 2004 {published data only}
- Causio F, Sarcina E, Leonetti T. GnRH agonist versus GnRH antagonist in an IVF program: luteal phase hormonal characteristics. Human Reproduction 2004;19:i103. [Google Scholar]
Crosignani 2007 {published data only}
- Crosignani PG, Somigliana E. Effect of GnRH antagonists in FSH mildly stimulated intrauterine insemination cycles: a multi centre randomized trial. Human Reproduction 2007;22(2):500‐5. [DOI] [PubMed] [Google Scholar]
D'Amato 2004 {published data only}
- D'Amato G, Caroppo E, Pasquadibisceglie A, Carone D, Vitti A, Vizziello GM. A novel protocol of ovulation induction with delayed gonadotropin‐releasing hormone antagonist administration combined with high‐dose recombinant follicle‐stimulating hormone and clomiphene citrate for poor responders and women over 35 years. Fertility and Sterility 2004;81(6):1572‐7. [DOI] [PubMed] [Google Scholar]
Davar 2012 {published data only}
- Davar R, Rahsepar M, Rahmani E. A comparative study of luteal estradiol pre‐treatment in GnRH antagonist protocols and in micro dose flare protocols for poor responding patients. Fertlity and Sterility 2012;6(1):107. [DOI] [PubMed] [Google Scholar]
De Klerk 2007 {published data only}
- Klerk C, Macklon NS, Heijnen EMEW, Eijkemans MJC, Fauser BCJM, Passchier J, Hunfeld JAM. The psychological impact of IVF failure after two or more cycles of IVF with a mild versus standard treatment strategy. Human Reproduction 2007;22(9):2554‐8. [DOI] [PubMed] [Google Scholar]
Dudley 2010 {published data only}
- Dudley PS, Thyer AC, Davis LB, Klein NA, Criniti AR, Soules MR. A new IVF stimulation protocol improves live birth rate in women with diminished ovarian reserve (DOR). Fertility and Sterility September 2010;94(4):S85‐S86. [DOI: 10.1016/j.fertnstert.2010.07.329] [DOI] [Google Scholar]
Eijkemans 2006 {published data only}
- Eijkemans MJ, Heijnen EM, Klerk C, Habbema JD, Fauser BC. Comparison of different treatment strategies in IVF with cumulative live birth over a given period of time as the primary end‐point: methodological considerations on a randomized controlled non‐inferiority trial. Human Reproduction (Oxford, England) 2006;21(2):344‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Engmann 2008b {published data only}
- Engmann L, DiLuigi A, Schmidt D, Benadiva C, Maier D, Nulsen J. The effect of luteal phase vaginal estradiol supplementation on the success of in vitro fertilization treatment: a prospective randomized study. Fertility and Sterility 2008;89(3):554‐61. [DOI] [PubMed] [Google Scholar]
Evangelio 2011 {published data only}
- Evangelio PM, Buendicho IE, Yeste AMM, Gustem RB, Egea IB, Hernandez NC. Randomized prospective study on the effect of the addition of business cycles exogenous LH IVF/ICSI GnRH antagonist predictors in patients with low response [Estudio prospectivo aleatorizado sobre el efecto de la adicion de actividad LH exogena en ciclos FIV/ICSI con antagonistas de GnRH en pacientes con factores predictivos de baja respuesta]. Revista Iberoamericana de Fertilidad 2011;28(3):219‐27. [Google Scholar]
Fabregues 2012 {published data only}
- Fabregues F, Iraola A, Casals G, Peralta S, Creus M, Balasch J. Evaluation of GnRH agonists and antagonists in tertiary prevention of OHSS. Clinical, neurohormonal and vasoactive effects in the luteal phase in high risk patients. Human Reproduction 2012;27(2):ii26‐ii28. [Google Scholar]
Ficicioglu 2010 {published data only}
- Ficicioglu C, Kumbak B, Akcin O. Comparison of follicular fluid and serum cytokine concentrations in women undergoing assisted reproductive treatment with GnRH agonist long and antagonist protocols. Gynecological Endocrinology 2010;26(3):181‐6. [DOI] [PubMed] [Google Scholar]
Freitas 2004 {published data only}
- Freitas GC, Cavagna M, Dzik A, Soares JB, Szterenfeld C, Izzo VM. Gonadotropin‐releasing hormone (GnRH)‐agonist versus GnRH‐antagonist in ovarian stimulation for assisted reproductive techniques: results of a prospective randomized trial. Fertility and Sterility 2004;82 Suppl:231‐2. [Google Scholar]
Ghosh 2003 {published data only}
- Ghosh M, Shirazee HH, Vijay PK, Chakravarty BN. Comparative evaluation of CC/FSH with single dose antagonist versus conventional long protocol gonadotrophin stimulation for IVF in women with several attempts of failed IUI for unexplained infertility. Human Reproduction (Oxford, England) 2003;18 Suppl 1:114‐5. [P‐333] [Google Scholar]
Gordts 2011 {published data only}
- Gordts S, Puttemans P, Campo R, Valkenburg M, Gordts S. A prospective randomized study comparing a GnRH‐antagonist versus a GnRH‐agonist short protocol for ovarian stimulation in patients referred for IVF. Fertility and Sterility September 2011;96(3):S176. [PMC free article] [PubMed] [Google Scholar]
Guivarc’h‐Levêque 2010 {published data only}
- Guivarc’h‐Levêque A, Arvis P, Bouchet JL, Broux PL, Moy L, Priou G, et al. Efficiency of antagonist IVF cycle programming by estrogens [Efficacité de la programmation des cycles FIV en antagonistes par les estrogenes]. Gynécologie Obstétrique & Fertilité 2010;38:18‐22. [DOI] [PubMed] [Google Scholar]
Ibrahim 2011 {published data only}
- Ibrahim ZM, Mohamed Youssef HY, Elbialy MM, Farrag MM. Micro‐dose flare‐up gonadotrophin‐releasing hormone (GnRH) agonist vs. flexible gonadotrophin‐releasing hormone (GnRH) antagonist protocol in patient with poor ovarian reserve. Middle East Fertility Society Journal 2011;16:272‐277. [DOI: 10.1016/j.mefs.2011.06.003] [DOI] [Google Scholar]
Jindal 2013 {published data only}
- Jindal A, Singh R. A prospective randomised controlled study comparing a low‐cost antagonist protocol using oral ovulation inducing agents in IVF‐ICSI cycles with a standard agonist long protocol. Fertility and Sterility October 2013;100(3):S273. [Google Scholar]
Karimzadeh 2011 {published data only}
- Karimzadeh MA, Mashayekhy M, Mohammadian F, Moghaddam FM. Comparison of mild and microdose GnRH agonist flare protocols on IVF outcome in poor responders. Archives of Gynecology and Obstetrics 2011;283:1159‐64. [DOI: 10.1007/s00404-010-1828-z] [DOI] [PubMed] [Google Scholar]
Kdous 2009 {published data only}
- Kdous M, Chaker A, Bouyahia M, Zhioua F, Zhioua A. Increased risk of early pregnancy loss and lower live birth rate with GnRH antagonists vs. long GNRH agonist protocol in PCOS women undergoing controlled ovarian hyperstimulation. La Tunisie Medicale 2009;87(12):834‐42. [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. A comparative study on the outcomes of in vitro fertilization between women with polycystic ovary syndrome and those with sonographic polycystic ovary‐only in GnRH antagonist cycles. Archives of Gynecology and Obstetrics 2010;282(2):199‐205. [PUBMED: 20182736 [PubMed ‐ as supplied by publisher]] [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee JR, Kim SH, Kim SM, Jee BC, Ku SY, Suh CS, et al. Follicular fluid anti‐Müllerian hormone and inhibin B concentrations: comparison between gonadotropin‐releasing hormone (GnRH) agonist and GnRH antagonist cycles. Fertility and Sterility 2008;89(4):860‐7. [DOI] [PubMed] [Google Scholar]
Lin 1999 {published data only}
- Lin Y, Kahn JA, Hillensjo T. Is there a difference in the function of granulosa‐luteal cells in patients undergoing in‐vitro fertilization either with gonadotrophin‐releasing hormone agonist or gonadotrophin‐releasing hormone antagonist?. Human Reproduction (Oxford, England) 1999;14(4):885‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Londra 2003 {published data only}
- Londra L, Inza R, Lombardi E, Marconi G, Young E, Kenny A. GnRh antagonist versus GnRh agonist in good prognosis IVF patients. Human Reproduction 2003;18 Suppl 1:114. [Google Scholar]
Maldonado 2011 {published data only}
- Maldonado LG, Setti AS, Franco JG, Braga DPAF, Figueira RCS, Iaconelli A, et al. Pituitary suppression with GnRH agonist on alternate days: can costs, effectiveness and comfort be brought together?. Human Reproduction 2011;26(1):i151. [Google Scholar]
Maldonado 2013 {published data only}
- Maldonado LGL, Franco JG, Setti AS, Iaconelli A, Borges E. Cost‐effectiveness comparison between pituitary down‐regulation with a gonadotropin‐releasing hormone agonist short regimen on alternate days and an antagonist protocol for assisted fertilization treatments. Fertility and Sterility May 2013;99(6):1615‐1622. [DOI: 10.1016/j.fertnstert.2013.01.095] [DOI] [PubMed] [Google Scholar]
Malhotra 2013 {published data only}
- Malhotra N, Singh N. Microdose GnRH agonist flare‐up versus flexible GnRH antagonist protocol in poor responders undergoing in‐vitro fertilization (IVF) cycles: a randomized controlled trial. Fertility and Sterility 2013;100(3):S106. [DOI: 10.1016/j.fertnstert.2013.07.1695] [DOI] [Google Scholar]
Mohsen 2013 {published data only}
- Mohsen IA, Din RE. Minimal stimulation protocol using letrozole versus microdose flare up GnRH agonist protocol in women with poor ovarian response undergoing ICSI. Gynecological Endocrinology 2013;29(2):105‐8. [DOI] [PubMed] [Google Scholar]
Orvieto 2007 {published data only}
- Orvieto R, Volodarsky M, Hod E, Homburg R, Rabinson J, Zohav E, et al. Controlled ovarian hyperstimulation using multi‐dose gonadotropin‐releasing hormone (GnRH) antagonist results in less systemic inflammation than the GnRH‐agonist long protocol. Gynecological Endocrinology 2007;23(8):494‐6. [DOI] [PubMed] [Google Scholar]
Orvieto 2008 {published data only}
- Orvieto R, Meltzer S, Rabinson J. GnRH agonist versus GnRH antagonist in ovarian stimulation: the role of endometrial receptivity. Fertility and Sterility 2008;90(4):1294‐6. [DOI] [PubMed] [Google Scholar]
Ozdogan 2012 {published data only}
- Ozdogan S, Ozdegirmenci O, Dilbaz S, Demir B, Cinar O, Dilbaz B, et al. A randomized prospective study of a gonadotropin‐releasing hormone antagonist versus agonist microdose flare‐up protocol in poor responder patients. Human Reproduction 2012;27(2):ii302. [CENTRAL: CN‐01026190] [Google Scholar]
Pabuccu 2005 {published data only}
- Pabuccu R, Onalan G, Selam B, Ceyhan T, Akar M, Onalan R. Comparison of GnRH agonist and antagonist protocols among patients with mild‐moderate endometriosis and endometrioma: a novel clinical approach. Fertility and Sterility 2005;84 Suppl 1:197‐8. [Google Scholar]
Perino 2002 {published data only}
- Perino M, Abate A, Brigandi A, Magnoli D, Abate F. Comparison between the GnRH antagonist ganirelix and the GnRH agonist buserelin: a prospective randomized controlled study. Human Reproduction (Oxford, England) 2002;17:33. [O‐096] [Google Scholar]
Pinto 2009 {published data only}
- Pinto F, Oliveira C, Cardoso MF, Teixeira‐da‐Silva J, Silva J, Sousa M, et al. Impact of GnRH ovarian stimulation protocols on intra‐cytoplasmic sperm injection outcomes. Reproductive Biology and Endocrinology 2009;7(5):1‐10. [DOI: 10.1007/s00404-010-1429-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Polinder 2008 {published data only}
- Polinder S, Heijnen EM, Macklon NS, Habbema JD, Fauser BJ, Eijkemans MJ. Cost‐effectiveness of a mild compared with a standard strategy for IVF: a randomized comparison using cumulative term live birth as the primary endpoint. Human Reproduction 2008;23(2):316‐23. [DOI] [PubMed] [Google Scholar]
Prapas 2005 {published data only}
- Prapas N, Prapas Y, Panagiotidis Y, Prapa S, Vanderzwalmen P, Schoysman R, et al. GnRH agonist versus GnRH antagonist in oocyte donation cycles: a prospective randomized study. Human Reproduction (Oxford, England) 2005;20(6):1516‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saini 2010 {published data only}
- Saini P, Saini A. New protocol of ovulation induction with GnRH antagonist compared with GnRH analogue long protocol in IVFET/ICSI cycles. Abstracts of the 26th Annual Meeting of ESHRE, Rome, Italy, 27 June – 30 June, 2010 June 2010;26:i317. [Google Scholar]
Shamma 2003 {published data only}
- Shamma F, Grossman M, Abuzeid M, al Hosn L, Ayers J, Fakih M. Comparison of daily ganirelix administration to a long protocol agonist for controlled ovarian hyperstimulation in oocyte donors: the results of a prospective randomized controlled trial. Fertility and Sterility 2003;80 Suppl 3:37‐8. [Google Scholar]
Tanaka 2014b {published data only}
- Tanaka A, Nagayoshi M, Tanaka I. Selection of an optimal controlled ovarian hyperstimulation method in relation to the number of antral follicles in patients less than 40 years old. Fertility and Sterility September 2014;102(3):e223. [DOI: 10.1016/j.fertnstert.2014.07.756] [DOI] [Google Scholar]
Tiras 2013 {published data only}
- Tiras B, Leylek OA, Halicigil C, Saltik A, Kavci N. Does single dose GNRH agonist administration after oocyte pick‐up in antagonist cycles improve endometrial receptivity and pregnancy rates?. Fertility and Sterility October 2013;100(3):S59‐S60. [Google Scholar]
Verpoest 2013 {published data only}
- Verpoest W, Vloeberghs V, Staessen C, Devos A, Rycke M, Bonduelle M, et al. The effect of the type of ovarian stimulation protocol on PGD results: a prospective randomised trial. Human Reproduction 2013;28(1):i44. [Google Scholar]
Vlaisavljevic 2003 {published data only}
- Vlaisavljevic V, Reljic M, Lovrec VG, Kovacic B. Comparable effectiveness using flexible single‐dose GnRH antagonist (cetrorelix) and single‐dose long GnRH agonist (goserelin) protocol for IVF cycles‐‐a prospective, randomized study. Reproductive Biomedicine Online 2003;7(3):301‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang B, Sun HX, Hu YL, Chen H, Zhang NY. Application of GnRH‐antagonist to IVF‐ET for patients with poor ovarian response [GnRH拮抗剂用于卵巢反应不良患者的体外受精胚胎移植]. National Journal of Andrology May 2008;14(5):423‐6. [PubMed] [Google Scholar]
Willman 2005 {published data only}
- Willman SP, Kliman HJ. Comparison of the luteal phase after pituitary suppression with GnRH‐agonist versus GnRH antagonist in controlled ovarian stimulation. Fertility and Sterility 2005;84 Suppl:308. [Google Scholar]
Zikopoulos 2005 {published data only}
- Zikopoulos K, Kaponis A, Adonakis G, Sotiriadis A, Kalantaridou S, Georgiou I, et al. A prospective randomized study comparing gonadotropin‐releasing hormone agonists or gonadotropin‐releasing hormone antagonists in couples with unexplained infertility and/or mild oligozoospermia. Fertility and Sterility 2005;83(5):1354‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Toftager 2016 {published data only}
- Toftager M, Bogstad J, Bryndorf T, Løssl K, Roskær J, Holland T, et al. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Human Reproduction 2016;0(0. Advance Access published April 8. Accessed 26 April 2016):1–12. [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Inany 2005
- Al‐Inany H, Aboulghar MA, Mansour RT, Serour GI. Optimizing GnRH antagonist administration: meta‐analysis of fixed versus flexible protocol. Reproductive biomedicine online 2005;10(5):567‐70. [PUBMED: 15949209] [DOI] [PubMed] [Google Scholar]
Albano 1996
- Albano C, Smitz J, Camus M, Riethmuller‐Winzen H, Siebert‐Weiger M, Diedrich K, et al. Hormonal profile during the follicular phase in cycles stimulated with a combination of human menopausal gonadotrophin and gonadotrophin‐releasing hormone antagonist (cetrorelix). Human Reproduction (Oxford, England) 1996;11(10):2114‐8. [DOI] [PubMed] [Google Scholar]
Bosch 2003
- Bosch E, Valencia I, Escudero E, Crespo J, Simon C, Remohi J, et al. Premature luteinization during gonadotropin‐releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertility and Sterility 2003;80(6):1444‐9. [DOI] [PubMed] [Google Scholar]
Brinsden 1995
- Brinsden PR, Wada I, Tan SL, et al. Diagnosis, prevention and management of ovarian hyperstimulation syndrome. Br J Obstet Gynaecol 1995;102:767–772. [DOI] [PubMed] [Google Scholar]
Daya 2000
- Daya S. Gonadotropin releasing hormone agonist protocols for pituitary desensitization in in vitro fertilization and gamete intrafallopian transfer cycles. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001299] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Delvigne 2002
- Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Human Reproduction Update 2002;8:559‐77. [DOI] [PubMed] [Google Scholar]
Duijkers 1998
- Duijkers IJM, Klipping C, Willemsen WNP, Krone D, Schneider E, Niebch G, et al. Single and multiple dose pharmacokinetics and pharmacodynamics of the gonadotrophin‐releasing hormone antagonist cetrorelix in healthy female volunteers. Human Reproduction 1998;13(9):2392‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felberbaum 1995
- Felberbaum RE, Reissmann T, Kuper W, Bauer O, al Hasani S, Diedrich C, et al. Preserved pituitary response under ovarian stimulation with hMG and GnRH‐antagonists (cetrorelix) in women with tubal infertility. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1995;61(2):151‐5. [DOI] [PubMed] [Google Scholar]
Ferriman 1961
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. Journal of Clinical Endocrinology 1961;21:1440–1447. [DOI] [PubMed] [Google Scholar]
Golan 1989
- Golan A. Ron‐El R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstet. Gynecol. Surv 1989;44:430‐40. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org. GRADEpro Guideline Develoopment Tool. McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org, 2015.
Griesinger 2008
- Griesinger G, Venetis CA, Marx T, Diedrich K, Tarlatzis BC, Kolibianakis EM. Oral contraceptive pill pretreatment in ovarian stimulation with GnRH antagonists for IVF: a systematic review and meta‐analysis. Fertility and Sterility 2008;90(4):1055. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 1992
- Hughes EG, Fedorkow DM, Daya S, Sagle MA, Koppel P, Collins JA. The routine use of gonadotropin‐releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta‐analysis of randomized trials. Fertility and Sterility 1992;58:888‐96. [DOI] [PubMed] [Google Scholar]
Huirne 2007
- Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to agonists for use in IVF?. Human Reproduction 2007;11:2805‐13. [DOI] [PubMed] [Google Scholar]
Kolibianakis 2003
- Kolibianakis EM, Albano C, Camus M, Tournaye H, Steirteghem AC, Devroey P. Initiation of gonadotropin‐releasing hormone antagonist on day 1 as compared to day 6 of stimulation: effect on hormonal levels and follicular development in in vitro fertilization cycles.. Journal of Clinical Endocrinology & Metabolism 2003;88:5632‐7. [DOI] [PubMed] [Google Scholar]
Kolibianakis 2004
- Kolibianakis EM, Zikopoulos K, Smitz J, Camus M, Tournaye H, Steirteghemand AC, et al. Elevated progesterone at initiation of stimulation is associated with a lower ongoing pregnancy rate after IVF using GnRH antagonists. Human Reproduction 2004;19(7):1525‐9. [DOI] [PubMed] [Google Scholar]
Kolibianakis 2006
- Kolibianakis EM, Collins J, Tarlatzis BC, Devroey P, Diedrich K, Griesinger G. Among patients treated for IVF with gonadotrophins and GnRH analogues, is the probability of live birth dependent on the type of analogue used? A systematic review and meta‐analysis. Human reproduction update 2006;12(6):651‐71. [PUBMED: 16920869] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Mathur 2007
- Mathur R, Kailasam C, Jenkins J. Review of the evidence base strategies to prevent ovarian hyperstimulation syndrome. Human Fertility 2007;10:75‐85. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. The Lancet 1999;352(9128):609‐18. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mozes 1965
- Mozes M, Bogokowsky H, Antebi E, Lunenfeld B, Rabau E, Serr DM, et al. Thromboembolic phenomena after ovarian stimulation with human gonadotrophins. Lancet 1965;2:1213‐5. [DOI] [PubMed] [Google Scholar]
Navot 1992
- Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertility and Sterility 1992;58:249‐61. [DOI] [PubMed] [Google Scholar]
Olivennes 1994
- Olivennes F, Fanchin R, Bouchard P, Ziegler D, Taieb J, Selva J. The single or dual administration of the gonadotrophin‐releasing hormone antagonist cetrorelix in an in‐vitro fertilisation‐embryo transfer program. Fertility and Sterility 1994;62(3):468‐76. [DOI] [PubMed] [Google Scholar]
Olivennes 1998
- Olivennes F, Alvarez S, Bouchard P, Fanchin R, Salat‐Baroux J, Frydman R. The use of GnRH antagonist (cetrorelix) in a single dose protocol in IVF‐embryo transfer: a dose finding study of 3 versus 2mg. Human Reproduction (Oxford,England) 1998;13(9):2411‐4. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rizk 1999
- Rizk B, Aboulghar MA. Classification, pathophysiology and management of ovarian hyperstimulation syndrome. Brinsden P (ed) In‐Vitro Fertilization and Assisted Reproduction. New York, London: The Parthenon Publishing Group, 1999:131‐55. [Google Scholar]
Rotterdam 2004
- Rotterdam ESHRE/ASRM‐Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19(1):41‐7. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine 2010;152:Epub 24 March. [DOI] [PubMed] [Google Scholar]
Seow 2010
- Seow KM, Lin YH, Hsieh BC, Huang LW, Huang SC, Chen CY, et al. Characteristics of progesterone changes in women with subtle progesterone rise in recombinant follicle‐stimulating hormone and gonadotropin‐releasing hormone antagonist cycle. Gynecologic and Obstetric Investigation 2010;70:64–8. [DOI] [PubMed] [Google Scholar]
Shoham 2002
- Shoham Z. The clinical therapeutic window for luteinizing hormone in controlled ovarian stimulation. Fertility and Sterility 2002;77:1170‐7. [DOI] [PubMed] [Google Scholar]
Tremellen 2010
- Tremellen KP, Lane M. Avoidance of weekend oocyte retrievals during GnRH antagonist treatment by simple advancement or delay of hCG administration does not adversely affect IVF live birth outcomes. Humanan Reproduction 2010;25(5):1219‐24. [DOI] [PubMed] [Google Scholar]
Youssef 2012
- Youssef M, Aboulfoutouh L, Veen F, Wely M. Could mild/minimal ovarian stimulation‐IVF displace conventional IVF in poor responder women undergoing IVF/ICSI treatment cycles: systematic review & meta‐analysis. Human Reproduction 2012;27. [Google Scholar]
References to other published versions of this review
Al‐Inany 2006
- Al‐Inany H, Aboulghar M. Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001750] [DOI] [PubMed] [Google Scholar]
Al‐Inany 2009
- Al‐Inany HG, Abou‐Setta AM, Aboulghar MA. Gonadotrophin‐releasing hormone antagonists for assisted conception. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001750.pub2] [DOI] [PubMed] [Google Scholar]
Al‐Inany 2011
- Al‐Inany HG, Youssef MAFM, Aboulghar M, Broekmans FJ, Sterrenburg MD, Smit JG, et al. Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]


