Abstract
Background
The effects of COVID-19 lockdown measures on maternal and fetal health remain unclear. We examined the associations of COVID-19 lockdown with gestational length and preterm birth (PTB) in a Chinese population.
Methods
We obtained medical records of 595,396 singleton live infants born between 2015 and 2020 in 5 cities in Guangdong Province, South China. The exposed group (N = 101,900) included women who experienced the COVID-19 Level I lockdown (1/23–2/24/2020) during pregnancy, while the unexposed group (N = 493,496) included women who were pregnant during the same calendar months in 2015–2019. Cumulative exposure was calculated based on days exposed to different levels of emergency responses with different weighting. Generalized linear regression models were applied to estimate the associations of lockdown exposure with gestational length and risk of PTB (< 37 weeks).
Results
The exposed group had a shorter mean gestational length than the unexposed group (38.66 vs 38.74 weeks: adjusted β = − 0.06 week [95%CI, − 0.07, − 0.05 week]). The exposed group also had a higher risk of PTB (5.7% vs 5.3%; adjusted OR = 1.08 [95%CI, 1.05, 1.11]). These associations seemed to be stronger when exposure occurred before or during the 23rd gestational week (GW) than during or after the 24th GW. Similarly, higher cumulative lockdown exposure was associated with a shorter gestational length and a higher risk of PTB.
Conclusions
The COVID-19 lockdown measures were associated with a slightly shorter gestational length and a moderately higher risk of PTB. Early and middle pregnancy periods may be a more susceptible exposure window.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-021-04268-5.
Keywords: COVID-19, Lockdown, Gestational length, Preterm, China
Background
The ongoing COVID-19 pandemic has spread throughout the world and affected billions of people [1, 2]. Various measures have been implemented around the world to control the pandemic, including restricting large social movements and gatherings, closing international and interstate borders, controlling travel, and implementing partial or full lockdown of cities and regions [3–5]. These measures have effectively controlled the spread of COVID-19 and reduced the anthropogenic emissions of air pollution [6], which have resulted in substantial health benefits [7]. However, these measures have also caused huge economic loss, unemployment, shortage of medical resources, and psychological stress [8–11], which may lead to adverse health outcomes [12, 13].
Pregnant women and fetuses may be susceptible populations to the effects of lockdown and restriction measures. A few studies have reported that the COVID-19 lockdown measures may increase the risk of adverse birth outcomes such as stillbirth and cesarean delivery [12, 13]. Preterm birth (PTB) is one of the most important adverse birth outcomes and a major cause of death in children under 5 years of age [14]. Several studies have examined the associations of COVID-19 lockdown measures with the risk of PTB, but the results were inconsistent [12, 13, 15–18]. A study in London reported an increase in the incidence of PTB during the COVID-19 pandemic period over the pre-pandemic period.12 Another study conducted in Nepal also observed a greater risk of PTB during the COVID-19 lockdown than before lockdown [12]. In contrast, studies conducted in Denmark and the Netherlands observed a substantial reduction in the risk of PTB during the COVID-19 periods than before lockdown [16, 17]. The other two studies conducted in China and Botswana did not find any significant association between the COVID-19 lockdown and the risk of PTB [13, 18]. The inconsistent findings across these studies may be attributable to differences in study design, sample size, demographic characteristics of study subjects, and socioeconomic developments of societies [19, 20].
Although the studies have preliminarily estimated the associations between COVID-19 lockdown and PTB, several research issues or gaps need to be addressed. First, the susceptibility of pregnant women to environmental factors largely depends on the stage of pregnancy [21, 22]. Previous studies estimated the overall rate of PTB in pregnant women exposed to COVID-19 lockdown measures [12, 13, 15, 16, 18, 23–25], but did not consider their pregnancy stage when lockdown occurred. This may lead to an underestimation of PTB risk during the lockdown if pregnant women with a gestational age > 36 weeks were also included. Second, lockdown intensity usually varied over time [20]. However, none of the previous studies considered the change in intensity of lockdown exposures. Third, previous studies have suggested a seasonal variation in the incidence of PTB [26, 27]. The seasonal effects should be considered in selecting the control periods for the COVID-19 lockdown. However, some previous studies applied the annual or multiple years’ average incidence of PTB as the reference [13, 16, 18], which might lead to biased findings. Fourth, the follow-up time (2–4 months) in previous studies was not long enough to capture the birth outcomes of pregnant women who experienced the lockdown in their early pregnancy [12, 13, 15, 16, 18].
To fill these research gaps, we comprehensively elucidated the association of the COVID-19 lockdown on gestational length and PTB risk in South China by quantifying the timing and intensity of exposure, considering seasonal effects, and allowing sufficient follow-up time. This study could provide in-depth insights to inform management practices regarding pregnancy and childbirth during and after lockdown.
Methods
Study design, settings, and subjects
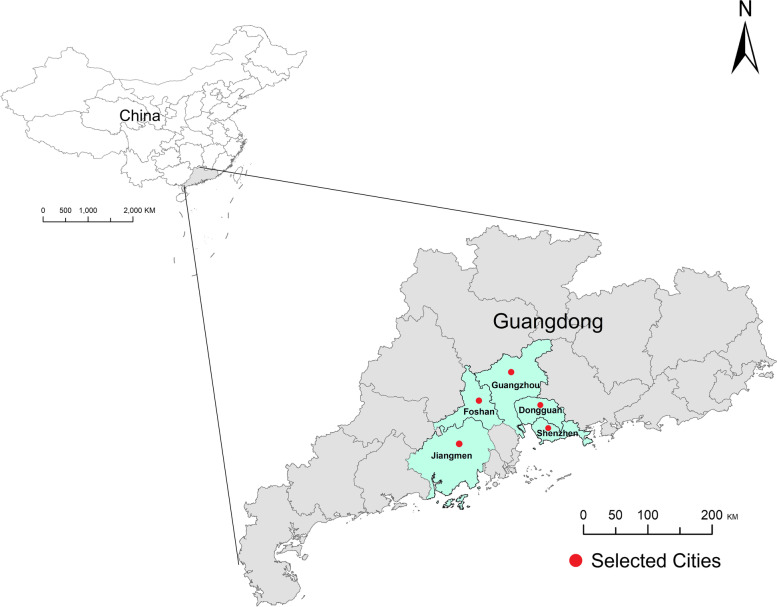
This study was a hospital-based retrospective study comparing the risk of PTB between the COVID-19 lockdown period in 2020 and the same periods in 2015–2019. We selected all hospitals in Foshan (n = 62) and several other hospitals in Guangzhou (n = 1), Shenzhen (n = 1), Dongguan (n = 2), and Jiangmen (n = 1) in Guangdong Province, South China, as study settings (Fig. 1 - Map). All hospital birth data from 1/1/2015 to 12/31/2020 were collected (n = 749,059). Birth records with multiple births (n = 27,659), stillbirths (n = 726), or missing information on key variables (n = 2883) were excluded. Moreover, 122,395 births were excluded because their pregnancy did not overlap with the COVID-19 lockdown in 2020 or the same calendar months in 2015–2019. Finally, 595,396 mother-newborn pairs were included. None of these women had a positive SARS-CoV-2 test result (Fig. S1 - Flowchart).
Fig. 1.
Geographic locations of the 5 study cities in Guangdong Province, South China
Data collection
The following information on each birth was extracted from the hospital information system or birth record system: infant sex, date of birth, delivery type (vaginal or cesarean), gestational weeks (GW) at birth, maternal age, parity, pregnancy complications such as hypertensive disorders of pregnancy (HDP) and gestational diabetes mellitus (GDM), and major adverse pregnancy outcomes such as miscarriage and stillbirth. Geographic information system covariates (geographic map) come from the Data Center for Resources and Environmental Sciences (https://www.resdc.cn). We carefully checked the accuracy and quality of source data. Implausible values and outliers were either corrected or recoded as missing.
Exposure assessment
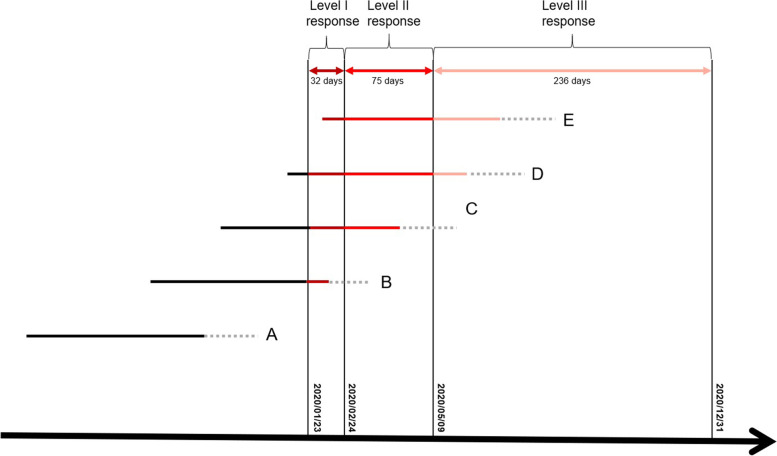
The National Emergency Response Plan for Public Emergencies by the China State Council defined 4 levels of emergency response: Level I (extremely serious), Level II (serious), Level III (relatively serious), and Level IV (common) [28]. After the outbreak of COVID-19, the Guangdong Provincial Government announced a Level I response on 1/23/2020 and later degraded the response level to Level II and Level III on 2/24/2020 and 5/9/2020, respectively. The Level III response was maintained after 5/9/2020. During the Level I response, offices, shops, colleges, schools, childcare facilities, and all other non-essential institutions were shut down. Residents’ social activities and gatherings were rigorously restricted. Most of the workforce adapted to a new work-from-home mode due to traffic and mobility restrictions. Fewer restriction measures were implemented during the Level II and Level III responses. During the Level II response, crowded areas were temporarily closed and disinfected before reopening. During the Level III response, people’s lives gradually returned to normal. All shopping malls, supermarkets, hotels, restaurants, and other living areas were reopened with routine precautionary measures such as wearing masks and practicing social distancing (Table S1).
We defined the period with a Level I response (1/23-2/24/2020) as Level I lockdown. Women who were pregnant during the Level I lockdown period were defined as the exposed group (N = 101,900). Women who were pregnant during the same calendar months in 2015–2019 were defined as the unexposed group (N = 493,496). This served to control for the seasonal effect, as our data indicated a significant variation in PTB rate across calendar months of conception (Fig. S2).
To further explore the potential susceptible exposure window, we divided the exposed group into 11 subgroups according to their GW on 1/23/2020. We determined the day of conception based on the gestational length and date of birth. For example, pregnant women were recorded as the first group when the date of conception crossed with the Level I lockdown, and the second group were recorded as women with GWs less than 4 weeks on 1/23/2020. (Fig. S3). The gestational age of all women over 41 GWs was consistently recorded as the group of 41st GW. Similarly, the unexposed group was divided into correspondingly matching subgroups. With each pair of subgroups (exposed vs unexposed), we estimated the associations of lockdown exposure with gestational length and PTB.
Restriction measures during the Level II and Level III responses may also have adverse effects on PTB risk. Therefore, we quantitatively estimated individual cumulative exposure dose to lockdown by assigning different weightings to days with different levels of emergency responses: 1/22/2020 or earlier (no response, weighting = 0), 1/23–2/24/2020 (Level I, weighting = 3), 2/25–5/9/2020 (Level II, weighting = 2), and 5/10–12/31/2020 (Level III, weighting = 1). Moreover, to account for the potential effect modification by the timing of exposure, we only estimated the cumulative exposure dose in their first 22 GWs, a conventional cut-off value of the shortest GW for a newborn to survive with current medical technology (Fig. 2) [29]. The distribution of the lockdown exposure dose in the exposed group is shown in Fig. S4.
Fig. 2.
Approach to calculating individual cumulative exposure dose to lockdown in the first 22 GWs.
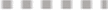 : Weeks after 22 GWs. Note: A, B, C, D and E represent subgroups of pregnant women with different GWs during the Level I lockdown; We assigned a weighting value of 3 to the days with Level I response, 2 to the days with Level II response, 1 to the days with Level III response, and 0 to days before lockdown (no exposure)
: Weeks after 22 GWs. Note: A, B, C, D and E represent subgroups of pregnant women with different GWs during the Level I lockdown; We assigned a weighting value of 3 to the days with Level I response, 2 to the days with Level II response, 1 to the days with Level III response, and 0 to days before lockdown (no exposure)
Outcome measures
According to the World Health Organization, PTB was defined as gestational length ≤ 37 completed weeks [30]. Moderate PTB (MPTB) was defined as the gestational length between 32 and 36 completed weeks. Very PTB (VPTB) was defined as gestational length < 32 completed weeks. The VPTB included extremely PTB (gestational length < 28 completed weeks).
Potential confounders
The following variables were considered as potential confounders: maternal age, marital status, parity, residential district, delivery type, and infant sex. These variables were selected based on biological plausibility, literature review, and availability of information.
Potential mediators
To facilitate interpretation of our findings regarding the association between COVID lockdown and PTB, we considered pregnancy complications (HDP and GDM) and changes in air pollution around lockdown beyond regular seasonal variation as two potential mediators. HDP included gestational hypertension, preeclampsia/eclampsia, chronic hypertension, and chronic hypertension with superimposed preeclampsia [31, 32] (Table S2). Daily air pollutants (PM10, PM2.5, NO2, SO2, and CO) data in the selected cities during 2015–2020 were collected from the National Urban Air Quality Real-time Publishing Platform (http://106.37.208.233:20035/). The average air pollutant concentrations during, after the Level I lockdown in 2020, and during the same calendar months in 2015–2019 were calculated.
Statistical analysis
A Chi-square test was used to assess the differences in socio-demographic and pregnancy characteristics between the exposed and unexposed groups. A generalized linear model (GLM) was applied to estimate the associations of Level I lockdown exposure with gestational length (linear regression) and PTB risk (binary logistic regression), after adjusting for potential confounders. A multinomial logistic regression model was used when PTB was further divided into MPTB and VPTB, with term birth as the reference. An interaction test was conducted to examine the potential modification effects of infant sex by comparing the association coefficients between male and female infants [33].
Similarly, GLM and multinomial logistic regression models were employed to examine the association of cumulative exposure dose with gestational length or PTB. The cumulative exposure dose in the exposed group was divided into four groups by quartiles (Q1, Q2, Q3, and Q4). The association of each quartile of cumulative exposure (vs unexposed) with gestational length or PTB was estimated. A trend test was conducted by assuming the values of quartiles as a continuous variable.
All analyses were performed using R3.6.1 (R Development Core Team 2019, https://www.r-project.org). The map in Fig. 1 was performed using packages ggplot2 in the R3.6.1. All the tests were two-sided and a P < 0.05 was statistically significant.
Results
General characteristics of study participants
Out of the 595,396 women included, 101,900 (17.1%) were in the exposed group and the other 493,496 (82.9%) were in the unexposed group (Table 1). The exposed group had higher proportions of participants older than 30 years (52.8% vs 49.4%), with GDM (15.4% vs 12.3%), multiparity (21.8% vs 15.9%), and natural delivery (62.2% vs 60.3%), but a lower proportion of HDP (2.3% vs 2.7%) than the unexposed group.
Table 1.
General characteristics of study participants
|
Unexposed group (n = 493,496) No. of participants (%) |
Exposed group (n = 101,900) No. of participants0 (%) |
χ2 | P | |
| Maternal age (years) | ||||
| < 24 | 50,255 (10.2) | 8412 (8.3) | 660.24 | < 0.001 |
| 24–26 | 81,222 (16.5) | 15,864 (15.6) | ||
| 27–29 | 118,040 (23.9) | 23,723 (23.3) | ||
| 30–32 | 102,817 (20.8) | 23,585 (23.1) | ||
| 33–35 | 72,330 (14.7) | 16,094 (15.8) | ||
| > 35 | 68,832 (13.9) | 14,222 (13.9) | ||
| Residential city | ||||
| Guangzhou | 19,850 (4.0) | 2970 (2.9) | 1193.80 | < 0.001 |
| Dongguan | 34,579 (7.0) | 5641 (5.5) | ||
| Jiangmen | 18,107 (3.7) | 3303 (3.3) | ||
| Shenzhen | 75,334 (15.3) | 13,280 (13.0) | ||
| Foshan | 345,626 (70.0) | 76,706 (75.3) | ||
| Infant sex | < 0.01 | 0.950 | ||
| Male | 263,153 (53.3) | 54,349 (53.3) | ||
| Female | 230,343 (46.7) | 47,551 (46.7) | ||
| Pregnancy complications (N = 173,064)a | ||||
| Hypertensive disorders of pregnancy (HDP) | ||||
| No | 143,933 (97.3) | 24,369 (96.7) | 96.57 | < 0.001 |
| Yes | 3937 (2.7) | 825 (2.3) | ||
| Gestational hypertension | 971 (0.7) | 252 (1.0) | ||
| Pre-eclampsia / Eclampsia | 2712 (1.8) | 473 (1.9) | ||
| Chronic hypertension | 141 (0.1) | 44 (0.2) | ||
| Chronic hypertension with superimposed pre-eclampsia | 113 (0.1) | 56 (0.2) | ||
| Gestational diabetes mellitus (GDM) | ||||
| No | 129,653 (87.7) | 21,313 (84.6) | 183.64 | < 0.001 |
| Yes | 18,217 (12.3) | 3881 (15.4) | ||
| Preterm birth | ||||
| No | 467,865 (94.8) | 96,307 (94.5) | 15.58 | < 0.001 |
| Yes | 25,631 (5.2) | 5593 (5.5) | ||
| Very premature (< 32 GWs) | 2121 (0.4) | 443 (0.4) | ||
| Moderate/late premature (32–36 GWs) | 23,510 (4.8) | 5150 (5.1) | ||
| Stillbirth (N = 595,904) | ||||
| No | 493,496 (99.92) | 101,900 (99.90) | 3.36 | 0.067 |
| Yes | 405 (0.08) | 103 (0.10) | ||
| Marital status | ||||
| Married | 488,376 (99.0) | 100,631 (98.8) | 472.03 | < 0.001 |
| Unmarried | 4263 (0.8) | 732 (0.7) | ||
| Other | 857 (0.2) | 537 (0.5) | ||
| Parity | ||||
| 0 (Primiparas) | 415,074 (84.1) | 79,686 (78.2) | 2121.60 | < 0.001 |
| 1 (Multiparas) | 63,158 (12.8) | 17,603 (17.3) | ||
| 2–4 (Multiparas) | 15,264 (3.1) | 4611 (4.5) | ||
| Delivery type | ||||
| Natural delivery | 297,591 (60.3) | 63,394 (62.2) | 1871.40 | < 0.001 |
| Operative vaginal delivery | 16,735 (3.4) | 1055 (1.0) | ||
| Cesarean delivery | 179,027 (36.3) | 37,298 (36.6) | ||
| Other | 143 (< 0.1) | 153 (0.2) | ||
| Mean ± SD | Mean ± SD | t | P | |
| Maternal age (years) | 29.78 ± 5.09 | 30.07 ± 4.94 | 17.11 | < 0.001 |
| Gestational length (week Mean ± SD) | 38.74 ± 1.46 | 38.66 ± 1.46 | 16.22 | < 0.001 |
a Data that were not available in hospitals in Foshan, because the information were not recorded in the birth certification system
Associations of COVID-19 lockdown exposure with gestational length
The exposed group had a shorter gestational length than the unexposed group (38.66 ± 1.46 weeks vs 38.74 ± 1.46 weeks). The Level I response (vs no exposure) was significantly associated with a 0.06 (95%CI: 0.05, 0.07) week decrease in gestational length in the total study sample after adjusting for confounders (Table 2). Subgroup analyses showed significant associations between lockdown exposure and decreased gestational length only among pregnant women whose gestational ages were < 24 GWs or 28th-31st GWs on the first day of lockdown (1/23/2020). The mean difference varied between − 0.11 and − 0.04 weeks.
Table 2.
Associations of exposure to the COVID-19 lockdown with gestational length
| No. of participants | Gestational length (week, Mean ± SD) | Mean difference in gestational length (week) | ||||
| Unexposed group | Exposed group b | Unexposed group | Exposed group b | Crude β (95% CI) | Adjusted β (95% CI) * | |
| Gestational week at the beginning of the Level I lockdown | ||||||
| All | 493,496 | 101,900 | 38.74 ± 1.46 | 38.66 ± 1.46 | −0.08 (−0.09, −0.07) | −0.06 (−0.07, −0.05) |
| Conception during the lockdown | 64,645 | 11,317 | 38.72 ± 1.52 | 38.64 ± 1.49 | −0.08 (− 0.11, − 0.05) | −0.04 (− 0.07, − 0.01) |
| Prior to 4th | 53,300 | 10,937 | 38.71 ± 1.50 | 38.64 ± 1.50 | −0.07 (− 0.10, − 0.04) | − 0.10 (− 0.14, − 0.07) |
| 4th -7th | 50,973 | 10,494 | 38.67 ± 1.52 | 38.52 ± 1.54 | − 0.14 (− 0.17, − 0.11) | − 0.13 (− 0.16, − 0.09) |
| 8th -11th | 48,926 | 10,237 | 38.70 ± 1.50 | 38.58 ± 1.54 | −0.12 (− 0.15, − 0.08) | −0.10 (− 0.13, − 0.07) |
| 12th -15th | 46,255 | 9844 | 38.73 ± 1.51 | 38.61 ± 1.55 | −0.11 (− 0.15, − 0.08) | −0.11 (− 0.14, − 0.07) |
| 16th -19th | 45,913 | 9539 | 38.74 ± 1.48 | 38.63 ± 1.52 | −0.11 (− 0.14, − 0.08) | −0.10 (− 0.13, − 0.06) |
| 20th -23rd | 41,017 | 8830 | 38.74 ± 1.49 | 38.64 ± 1.52 | −0.10 (− 0.14, − 0.07) | −0.10 (− 0.13, − 0.06) |
| 24th -27th | 40,358 | 8750 | 38.68 ± 1.49 | 38.66 ± 1.44 | −0.02 (− 0.06, 0.01) | −0.01 (− 0.04, 0.03) |
| 28th -31st | 38,146 | 8101 | 38.72 ± 1.39 | 38.63 ± 1.36 | −0.09 (− 0.12, − 0.06) | −0.07 (− 0.10, − 0.04) |
| 32nd -36th | 47,382 | 10,213 | 38.74 ± 1.21 | 38.73 ± 1.18 | −0.01 (− 0.03, 0.02) | 0.02 (− 0.01, 0.04) |
| 37th - 41st | 16,581 | 3638 | 39.40 ± 0.92 | 39.41 ± 0.93 | 0.01 (−0.02, 0.04) | 0.03 (0.01, 0.07) |
| Exposure dose (Mean ± SD) | Gestational length (week, Mean ± SD) | Mean difference in gestational length (week) | ||||
| Unexposed group | Exposed group | Unexposed group | Exposed group | Crude β (95% CI) | Adjusted β (95% CI) * | |
| Cumulative exposure dose in the first 22 weeks during the Level I to the Level III lockdown a | ||||||
| Per 100 unit increase in all participants | 0 ± 0 | 195.08 ± 82.21 | 38.74 ± 1.45 | 38.61 ± 1.52 | −0.06 (−0.07, −0.05) | −0.05 (− 0.06, − 0.04) |
| Categories of cumulative exposure dose | ||||||
| Unexposed group | 0 ± 0 | – | 38.74 ± 1.45 | – | Reference | Reference |
| Q1 (< 132) | – | 73.40 ± 38.11 | – | 38.64 ± 1.52 | −0.10 (− 0.13, − 0.08) | −0.09 (− 0.11, − 0.07) |
| Q2 (132–225) | – | 178.66 ± 27.17 | – | 38.59 ± 1.54 | −0.15 (− 0.17, − 0.12) | −0.13 (− 0.16, − 0.11) |
| Q3 (226–263) | – | 247.18 ± 10.58 | – | 38.58 ± 1.51 | −0.16 (− 0.18, − 0.13) | −0.14 (− 0.16, − 0.11) |
| Q4 (≥264) | – | 278.80 ± 8.59 | – | 38.62 ± 1.51 | − 0.12 (− 0.14, − 0.10)* | −0.09 (− 0.11, − 0.07) |
| P for trend test | < 0.001 | |||||
In calculating the cumulative exposure dose to lockdown, we assigned a weighting of 3 to days with Level I response, 2 to days with Level II response, 1 to days with Level III response, and 0 to other days
* Adjusted for maternal age, marital status, parity, residential city, delivery type and infant sex
a The exposed group refers to the pregnant women who have experienced the COVID-19 lockdown in their first 22 GWs. The other participants were defined as the unexposed group. The individual cumulative exposure dose was calculated by combining the weightings with the overlap between their pregnancy period ≤22 GWs and the three levels of responses. Q1-Q4 were defined as the cumulative exposure dose of the exposed group classified by quartiles, and the unexposed group was used as reference
b Pregnant women who have experienced the COVID-19 lockdown (from 1/23/2020 to 2/24/2020) during any period of their pregnancy were defined as the exposed group. We further divided the exposed group into subgroups according to their gestational weeks (GW) on 1/23/2020, the beginning of lockdown
-: Not applicable
We observed a negative association between cumulative lockdown exposure dose and gestational length (Table 2). Each 100 unit increase in the cumulative exposure dose during the first 22 GWs was associated with a 0.05 (95%CI: 0.04, 0.06) week decrease in gestational length, after adjusting for confounders. In addition, compared to the unexposed group, the Q1, Q2, Q3 and Q4 quantiles of cumulative exposure were associated with 0.09 (0.07, 0.11), 0.13 (0.11, 0.16), 0.14 (0.11, 0.16), and 0.09 (0.07, 0.11) weeks decrease in gestational length, respectively.
Associations of COVID-19 lockdown exposure with PTB
A higher PTB rate (5.7% vs 5.3%) and MPTB rate (5.2% vs 4.9%) were observed in the exposed group compared to the unexposed group in the total sample. Significant increases in PTB risk (adjusted OR = 1.08, 95%CI: 1.05, 1.11) and MPTB risk (adjusted OR = 1.09, 95%CI: 1.05, 1.12) were also observed after adjusting for confounders (Table 3). However, the association between lockdown and VPTB was not statistically significant (adjusted OR = 1.04, 95%CI: 0.94, 1.16). Subgroup analyses showed significant associations of lockdown exposure with increases in PTB and MPTB only among pregnant women < 24 GWs on the first day of lockdown. The OR values varied between 1.10 and 1.20 for PTB and MPTB.
Table 3.
Associations of exposure to the COVID-19 lockdown with preterm birth
| Unexposed group (n, %) | Exposed group (n, %) a | OR for PTB (95%CI) | ||||||||||||
| Term birth | PTB | Term birth | PTB | MPTB + VPTB | MPTB | VPTB | ||||||||
| MPTB + VPTB | MPTB | VPTB | MPTB + VPTB | MPTB | VPTB | Crude OR | Adjusted OR * | Crude OR | Adjusted OR * | Crude OR | Adjusted OR * | |||
| Gestational week at the beginning of the Level I lockdown | ||||||||||||||
| All | 451,284 (94.7) | 25,631 (5.3) | 23,510 (4.9) | 2121 (0.4) | 92,669 (94.3) | 5593 (5.7) | 5150 (5.2) | 443 (0.5) | 1.06 (1.03, 1.09) | 1.08 (1.05, 1.11) | 1.07 (1.03, 1.10) | 1.09 (1.05, 1.12) | 1.02 (0.92, 1.13) | 1.04 (0.94, 1.16) |
| Conception during the lockdown | 61,117 (94.5) | 3528 (5.5) | 3171 (4.9) | 357 (0.6) | 10,682 (94.4) | 635 (5.6) | 573 (5.1) | 62 (0.5) | 1.03 (0.94, 1.12) | 1.09 (0.99, 1.20) | 1.03 (0.94, 1.13) | 1.08 (0.98, 1.19) | 0.99 (0.76, 1.30) | 1.20 (0.90, 1.61) |
| Prior to 4th | 50,272 (94.3) | 3028 (5.7) | 2746 (5.2) | 282 (0.5) | 10,295 (94.1) | 642 (5.9) | 591 (5.4) | 51 (0.5) | 1.04 (0.95, 1.13) | 1.18 (1.08, 1.29) | 1.05 (0.96, 1.15) | 1.20 (1.09, 1.32) | 0.88 (0.65, 1.19) | 0.97 (0.71, 1.32) |
| 4th -7th | 48,023 (94.3) | 2950 (5.7) | 2671 (5.2) | 279 (0.5) | 9821 (93.6) | 673 (6.4) | 613 (5.8) | 60 (0.6) | 1.12 (1.02, 1.22) | 1.12 (1.03, 1.22) | 1.12 (1.03, 1.23) | 1.13 (1.03, 1.24) | 1.05 (0.79, 1.39) | 1.07 (0.81, 1.42) |
| 8th -11th | 46,128 (94.3) | 2798 (5.7) | 2544 (5.2) | 254 (0.5) | 9581 (93.6) | 656 (6.4) | 597 (5.8) | 59 (0.6) | 1.13 (1.03, 1.23) | 1.14 (1.04, 1.24) | 1.13 (1.03, 1.24) | 1.14 (1.04, 1.25) | 1.12 (0.84, 1.49) | 1.13 (0.85, 1.51) |
| 12th -15th | 43,652 (94.4) | 2603 (5.6) | 2356 (5.1) | 247 (0.5) | 9236 (93.9) | 608 (6.1) | 546 (5.5) | 62 (0.6) | 1.10 (1.01, 1.21) | 1.11 (1.02, 1.22) | 1.10 (1.00, 1.21) | 1.10 (1.00, 1.21) | 1.19 (0.90, 1.57) | 1.21 (0.91, 1.59) |
| 16th -19th | 43,439 (94.6) | 2474 (5.4) | 2252 (4.9) | 222 (0.5) | 8966 (94.0) | 573 (6.0) | 524 (5.5) | 49 (0.5) | 1.12 (1.02, 1.23) | 1.14 (1.04, 1.25) | 1.13 (1.02, 1.24) | 1.14 (1.04, 1.26) | 1.07 (0.78, 1.46) | 1.11 (0.82, 1.52) |
| 20th -23rd | 38,806 (94.6) | 2211 (5.4) | 2011 (4.9) | 200 (0.5) | 8281 (93.8) | 549 (6.2) | 502 (5.7) | 47 (0.5) | 1.16 (1.06, 1.28) | 1.19 (1.08, 1.31) | 1.17 (1.06, 1.29) | 1.19 (1.08, 1.32) | 1.10 (0.80, 1.51) | 1.14 (0.83, 1.57) |
| 24th -27th | 38,052 (94.3) | 2306 (5.7) | 2112 (5.2) | 194 (0.5) | 8291 (94.8) | 459 (5.2) | 420 (4.8) | 39 (0.4) | 0.91 (0.82, 1.01) | 0.93 (0.84, 1.03) | 0.91 (0.82, 1.02) | 0.93 (0.83, 1.03) | 0.92 (0.65, 1.30) | 0.99 (0.70, 1.40) |
| 28th -31st | 36,145 (94.8) | 2001 (5.2) | 1915 (5.0) | 86 (0.2) | 7663 (94.6) | 438 (5.4) | 424 (5.2) | 14 (0.2) | 1.03 (0.93, 1.15) | 1.05 (0.95, 1.17) | 1.04 (0.94, 1.16) | 1.06 (0.95, 1.19) | 0.77 (0.44, 1.35) | 0.80 (0.45, 1.42) |
| 32nd -36th | 45,650 (96.3) | 1732 (3.7) | 1732 (3.7) | NA | 9853 (96.5) | 360 (3.5) | 360 (3.5) | NA | 0.96 (0.86, 1.08) | 0.97 (0.86, 1.09) | 0.96 (0.86, 1.08) | 0.97 (0.86, 1.09) | NA | NA |
|
Exposure dose in unexposed group (Mean ± SD) |
Exposure dose in exposed group (Mean ± SD) |
OR for PTB (95%CI) | ||||||||||||
| Term + PTB | Term | PTB | MPTB + VPTB | MPTB | VPTB | |||||||||
| MPTB + VPTB | MPTB | VPTB | Crude OR | Adjusted OR* | Crude OR | Adjusted OR* | Crude OR | Adjusted OR* | ||||||
| Cumulative exposure dose in the first 22 weeks during Level I to Level 3 lockdown b | ||||||||||||||
| Per 100 unit increase | 0 ± 0 | 195.14 ± 82.19 | 194.15 ± 82.57 | 194.23 ± 82.60 | 193.35 ± 82.38 | 1.06 (1.04, 1.08) | 1.07 (1.05, 1.09) | 1.05 (1.04 1.07) | 1.07 (1.05, 1.08) | 1.10 (1.05, 1.16) | 1.12 (1.06, 1.18) | |||
| Categories of cumulative exposure dose | ||||||||||||||
| Unexposed group | 0 ± 0 | – | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Q1 (< 132) | – | 73.52 ± 38.09 | 71.59 ± 38.28 | 71.49 ± 38.32 | 72.57 ± 38.07 | 1.14 (1.07, 1.22) | 1.16 (1.08, 1.23) | 1.13 (1.05, 1.21) | 1.14 (1.07, 1.22) | 1.27 (1.03, 1.67) | 1.31 (1.06, 1.62) | |||
| Q2 (132–225) | – | 178.64 ± 27.17 | 179.01 ± 27.26 | 179.14 ± 27.26 | 177.76 ± 27.29 | 1.21 (1.13, 1.29) | 1.22 (1.14, 1.30) | 1.19 (1.11, 1.27) | 1.20 (1.12, 1.28) | 1.39 (1.14, 1.70) | 1.43 (1.17, 1.74) | |||
| Q3 (226–263) | – | 247.16 ± 10.59 | 247.52 ± 10.28 | 247.50 ± 10.26 | 247.91 ± 10.58 | 1.11 (1.04 1.19) | 1.14 (1.07, 1.22) | 1.10 (1.03, 1.18) | 1.13 (1.05, 1.21) | 1.25 (1.01, 1.54) | 1.27 (1.03, 1.58) | |||
| Q4 (≥264) | – | 278.81 ± 8.57 | 278.69 ± 8.77 | 278.55 ± 8.76 | 280.23 ± 8.79 | 1.14 (1.06, 1.21) | 1.19 (1.11, 1.27) | 1.13 (1.05, 1.21) | 1.18 (1.10, 1.26) | 1.21 (0.97, 1.49) | 1.25 (1.01, 1.55) | |||
| P for trend test | < 0.001 | < 0.001 | < 0.001 | |||||||||||
In calculating the cumulative exposure dose to lockdown, we assigned a weighting of 3 to days with Level I response, 2 to days with Level II response, 1 to days with Level III response, and 0 to other days
PTB preterm birth, MPTB moderate preterm birth, VPTB very preterm birth
N/A: There is no VPTB case in the subgroup
*: Adjusted for maternal age, marital status, parity, residential city, delivery type and infant sex
a Pregnant women who have experienced the COVID-19 lockdown (from 1/23/2020 to 2/24/2020) during any period of their pregnancy were defined as the exposed group. We further divided the exposed group into subgroups. According to their gestational weeks (GW) on 1/23/2020, the beginning of lockdown
b: The exposed group refers to the pregnant women who have experienced the COVID-19 lockdown in their first 22 GWs. The rest of included participants were defined as the unexposed group. The individual cumulative exposure dose was calculated by combining the weightings with the overlap between their pregnancy period ≤22 GWs and the three levels of responses. Q1-Q4 were defined as the cumulative exposure dose of the exposed group classified by quartiles, and the unexposed group were used as reference
-: Not applicable
We also observed a positive association between cumulative exposure dose to lockdown and PTB risk (Table 3 and Table S3). Each 100 unit increase in the lockdown exposure during the first 22 GWs was significantly associated with 1.07 (95%CI: 1.05, 1.09), 1.07 (1.05, 1.08), and 1.12 (1.06, 1.18) times higher risks in PTB, MPTB, and VPTB, respectively. The adjusted ORs of PTB for the Q1, Q2, Q3 and Q4 quartiles of cumulative exposure (vs no exposure) were 1.16 (1.08, 1.23), 1.22 (1.14, 1.30), 1.14 (1.07, 1.22), and 1.19 (1.11, 1.27), respectively.
Effect modification by infant sex in the associations of lockdown exposure with gestational length and PTB
Subgroup analyses showed similar associations of Level I lockdown with gestational length [adjusted β = − 0.06 (95%CI: − 0.08, − 0.05) week vs adjusted β = − 0.06 (− 0.08, 0.05) week] or risk of PTB [adjusted OR = 1.09 (95%CI: 1.04, 1.14) vs adjusted OR = 1.08 (1.03, 1.13)] in male infants and in female infants (Table 4). There was no significant sex interaction (P > 0.05) in these associations.
Table 4.
Modification effects of infant sex on the associations of COVID-19 lockdown exposure with gestational length and PTB risk
| No. of participants | Gestational length (week, Mean ± SD) |
Mean difference in gestational length (week) Adjusted β (95% CI) * |
P for modification effects of infant sex | ||||||||
| Male | Female | Male | Female | Male | Female | ||||||
| Unexposed group | Exposed group a | Unexposed group | Exposed group a | Unexposed group | Exposed group a | Unexposed group | Exposed group a | ||||
| Gestational week during the Level I lockdown | |||||||||||
| All | 263,153 | 54,349 | 230,343 | 47,551 | 38.66 ± 1.48 | 38.58 ± 1.48 | 38.82 ± 1.43 | 38.74 ± 1.44 | −0.06 (−0.08, −0.05) | −0.06 (−0.08, −0.05) | > 0.05 |
| No. of participants | PTB rate (N, %) |
PTB risk Adjusted OR (95%CI) * |
P for modification effects of infant sex | ||||||||
| Male | Female | Male | Female | Male | Female | ||||||
| Unexposed group | Exposed group a | Unexposed group | Exposed group a | Unexposed group | Exposed group a | Unexposed group | Exposed group a | ||||
| Gestational week during the Level I lockdown | |||||||||||
| All | 254,522 | 52,471 | 222,393 | 45,791 | 14,873 (5.8) | 3269 (6.2) | 10,758 (4.8) | 2324 (5.1) | 1.09 (1.04, 1.13) | 1.08 (1.03, 1.13) | > 0.05 |
PTB preterm birth
* Adjusted for maternal age, marital status, residential city, delivery type and parity
a Pregnant women who have experienced the COVID-19 lockdown (from 1/23/2020 to 2/24/2020) during their any period of pregnancy were defined as the exposed group
Discussion
This study comprehensively examined the associations of the COVID-19 lockdown with gestational length and risk of PTB using a large database from South China. We found that the lockdown exposure was significantly associated with a slightly shorter gestational length and a moderately higher risk of PTB. These associations were greater among women who were in early or middle pregnancy during the Level I lockdown period. There were also significant exposure-response associations of higher cumulative exposures to lockdown with a shorter gestational length and an increased risk of PTB.
Our finding of a positive association between the COVID-19 lockdown and risk of PTB was consistent with some previous studies. For example, a study from California found a modest increase in PTB rates (OR = 1.11; 95% CI, 1.03–1.20) among pregnant women at 28 + 0 and 31 + 6 weeks during the COVID-19 pandemic compared with 2016–2019 [34]. Another study from Italy also reported a slight increase in very preterm rate during the COVID-19 lockdown period in 2020 (0.79%) compared with the same period in 2019 (0.55%) [35]. Several reasons possibly explained the increased risk of PTB. First, the lack of medical resources during the COVID-19 pandemic and lockdown measures might interrupt the timely antenatal care for pregnant women [13, 36, 37]. Secondly, fear and panic about the pandemic could make pregnant women reluctant to seek help from medical institutions, and further impacted the timely detection and diagnosis of pregnancy complications [13, 38]. For example, we observed a higher rate of GDM in the exposed group than the unexposed group. This suggested a potential mediation role of GDM, as GDM is a critical risk factor of PTB [14]. In addition, pregnant women have always been considered a susceptible population to mental disorders [39]. The lockdown and restriction measures could increase psychological problems in pregnant women through concomitant financial problems and increased stress [38, 40], particularly if they were socioeconomically disadvantaged [41]. The closure of entertainment venues also reduced the outlets for negative feelings [42]. A previous study observed a more pronounced increase in depression and anxiety in pregnant women during the COVID-19 pandemic than in the general population [43]. Lastly, the nutritional status of pregnant women was also of concern. During the lockdown period, the decreased supply of fresh foods could lead to an inadequate intake of vegetables and high-fiber foods. Meanwhile, the intake of high-carbohydrate foods might have increased because they were relatively easier to obtain and store [13]. It was reported that the overweight and obesity rates increased during the lockdown period due to unbalanced diets and less exercise [44]. This suggested that maternal stress and obesity during the lockdown might influence the risk of PTB [13, 43].
We further observed that women in early and middle pregnancy during the Level I lockdown had a greater risk of PTB, which also contributed to the health effects of the COVID-19 lockdown. Zhang et al. reported that women in the first and second trimesters of pregnancy during the lockdown had more severe psychological disorders [22]. A simple explanation could be that these mothers continued to experience Level II and III lockdown after the Level I lockdown, which may have led to more cumulative effects on their fetal health. This was supported by our observed positive association between PTB risk and cumulative exposure to the lockdown of all levels in the first 24 GWs. An alternative explanation could be that early and middle pregnancy is a critical period for fetal development because the majority of fetal organs and tissues retain plasticity at that time [45]. As a result, lockdown-induced poor diet, depression, and anxiety problems in early and middle pregnancy may substantially interrupt fetal development [46–48].
It should be noted that several other previous studies reported different results with our findings. For example, studies in Denmark and the Netherlands have both provided evidence of a significant reduction in the number of extremely preterm and a decline in the incidence of moderate-to-late preterm birth following the implementation of national COVID-19 mitigation measures compared with years prior to the COVID-19 pandemic [16, 17]. A large sample study in China also found a statistically significant decrease in the incidence of moderate-to-late preterm birth during the COVID-19 mitigation measures compared with the same periods during 2014–2019 [19]. However, studies in the US states of Pennsylvania and Massachusetts found no change in the lockdown-related PTB during the COVID-19 pandemic [49, 50]. Although the mechanisms underlying these negative or null associations were unclear, several socio-environmental and behavioral modifiers were proposed [11, 51]. First, the lockdown measures increased company and support from partners and family, which could reduce the existing psychological stress in pregnant women. Second, working from home increased their rest time at home and decreased work-related stress. Third, the reduced anthropogenic emissions improved the air quality, which could benefit maternal and fetal health. Fourth, precautionary behavioral changes were promoted during the lockdown, including social distancing, enhanced hand hygiene, and the use of face masks. These behavioral changes could potentially reduce the chances of other common viral infections in addition to COVID-19 during pregnancy. Finally, lockdown measures also reduced daily commuting, road traffic incidents, and consumption of cigarettes, coffee, alcohol, prescription drugs, and street drugs due to limited accessibility [11, 51].
Those inconsistent associations of lockdown exposure with maternal and fetal health reported in previous studies [11, 16, 17] may have a few other explanations. First, some studies [16] had small sample sizes and potentially inadequate statistical power to detect an association between lockdown exposure and PTB. Second, the seemingly decreased risk of adverse pregnancy outcomes related to lockdown might be partially related to the reduced number of ultrasound scans and screening, which increased the possibility of under-diagnoses of early pregnancy loss, miscarriages, or stillbirths. Third, the health effects of lockdown may last for several months, but previous studies did not track participants long enough to assess the total effects of lockdown, which could have led to underestimations. In this study, we used the data of pregnant women who experienced the Level I lockdown until the end of 2020 and were able to obtain birth outcomes of all exposed women by covering the entire pregnancy. Fourth, air quality improvement during the lockdown was proposed as a major contributor to the reduced risk of PTB. In this study, we also found a substantial reduction in air pollution during the lockdown (Table S4), which was consistent with previous studies [6, 52]. Fifth, seasonal effects and pregnancy stages were not considered in most previous studies, which could lead to biased results. To evaluate this potential bias, we estimated the difference in PTB rates between new births during the Level I lockdown and all previous births during the entire year (rather than matching the calendar months) from 2015 to 2019. We did not find a significant association between lockdown and PTB risk (Table S5). Finally, although the lockdown measures may increase company and support from partners and family, the potential increase in family conflicts and domestic abuse should also be considered [53]. These findings suggest that the health effects of COVID-19 lockdown were comprehensively affected by socio-environmental changes and behavioral modifications and that improvement in one factor could not make up for the overall disadvantage [16, 34].
Limitations
Several limitations need to be addressed. First, as the COVID-19 pandemic and associated lockdown measures occurred unanticipatedly, we can only extract general demographic information, pregnancy complications and pregnancy outcomes from hospital information systems or birth record systems. We were unable to gather information on the management of high-risk preterm pregnancies, such as the frequency and form of examination of these patients, gynecological examination, serology, bacteriological findings of cervical and vaginal swabs, ultrasound examination of cervical length and some others. In addition, we may miss some other gestation-length-related outcomes such as early pregnancy losses, miscarriages, and stillbirths. These competing outcomes of PTB may potentially downplay the impact of lockdown on pregnancy and PTB [19]. Previous studies reported an increased rate of stillbirth related to the COVID-19 lockdown [12, 15]. Our supplemental analyses also showed a higher stillbirth risk in the exposed group than in the unexposed group (Table S6). Second, some studies have shown that smoking, alcohol consumption and reduced physical activity during pregnancy can lead to higher rates of PTB [54–56]. But we can’t get the individual behavior of these pregnant women from the hospital system, so their potential mediation roles were not evaluated in our analyses. Third, this study was conducted in only five cities in South China, which limited the generalization of our findings. However, we chose representative hospitals in each city. For example, Shenzhen Maternal and Child Health Hospital is the largest local hospital which provided medical serves to people across the city. Fourth, due to the coexistence of the COVID-19 pandemic and lockdown status, we could not separate their induvial impacts on the outcomes. In addition, many measures are usually implemented at the same time, we could not also determine their impact on the risk of PTB.
Conclusions
Within a large dataset of birth records from South China, we found that COVID-19 lockdown was associated with a slightly shorter gestational length and a moderately higher risk of PTB. Early and middle pregnancy might be a more susceptible exposure window. The COVID-19 control measures were implemented in many countries to reduce the spread of infections and related morbidities. Meanwhile, the incidence of PTB remains high globally, and options for the prevention of PTB are very limited [57]. Our findings suggest more attention and efforts are needed to support pregnant women during the lockdown, particularly for those with previous PTB as they are more susceptible [58]. Health professionals should make appropriate and timely treatment decisions for pregnant women during the lockdown.
Supplementary Information
Additional file 1 : Table S1. The prevention and control measures of three level response in Guangdong province. Table S2. Definitions of pregnancy complications selected in this study. Table S3. Proportions of study participants in the exposed and unexposed groups. Table S4. The differences in air pollution between lockdown and pre-lockdown periods in the study area. Table S5. Associations between exposure to COVID-19 lockdown at birth and preterm birth. Table S6. Associations of exposure to COVID-19 lockdown with stillbirth. Figure S1. Selection process of study subjects. Figure S2. Rate of preterm birth in each calendar month during 2015-2019 (before the COVID-19 pandemic). Figure S3. Division of participants into subgroups according to their GW on January 23rd, 2020. Figure S4. Distribution of the cumulative exposure dose in the first 22 GWs in the exposed group during lockdown.
Acknowledgments
The authors thank the participants who participated in the study.
Abbreviations
- COVID-19
Coronavirus disease 2019
- PTB
Preterm birth
- MPTB
Moderate preterm birth
- VPTB
Very preterm birth
- GW
Gestational week
- HDP
Hypertensive disorders of pregnancy
- GDM
Gestational diabetes mellitus.
Authors’ contributions
TL, WM, and XW designed the study; TL, MD, RQ, JW, JF, and YY analyzed the data, interpreted the results, and drafted the manuscript; RQ, JF, YY, HZ, SZ, YL, YP, HC, JJ, QL, XL, GC, YC, ZH, GH, SC, JH, and JX cleaned and interpreted the data; TL, BW, ER, WM, and XW edited and revised the manuscript; All authors approved the final draft of the manuscript. TL and WM verified the underlying data. WM and XW are the study guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
The study was funded by the National Natural Science Foundation of China (81874276, 42175181); Natural Science Foundation of Guangdong Province (2019A1515011264); Science and Technology Planning Project of Guangdong Province (2018B020207006); Science and Technology Program of Guangzhou (202102080565); Chinese Postdoctoral Science Foundation (2020T130020ZX); and Foshan Key Technology Project for COVID-19 (2020001000376). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Availability of data and materials
The statistical code and meta data during the current study are not publicly available due to the qualitative nature of the data, but are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Guangdong Provincial Center for Disease Control and Prevention (No. W96-027E-2020004). Written informed consent was obtained from all participants. All the procedures were performed following the ethical standards of the institutional research committee and the 1964 Helsinki declaration and its later amendments.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Moran Dong, Rui Qian, Jiaqi Wang, Jingjie Fan and Yufeng Ye contributed equally to this work.
References
- 1.Johns Hopkins University of Medicine . COVID-19 case tracker. Follow global cases and trends. 2020. [Google Scholar]
- 2.Jia X, Chen J, Li L, Jia N, Jiangtulu B, Xue T, et al. Modeling the prevalence of asymptomatic COVID-19 infections in the Chinese mainland. Innovation. 2020;1(2):100026. doi: 10.1016/j.xinn.2020.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen M, Peng Z, Xiao Y, Zhang L. Modeling the epidemic trend of the 2019 novel coronavirus outbreak in China. Innovation. 2020;1(3):100048. doi: 10.1016/j.xinn.2020.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin G, Zhang T, Zhang Y, Wang Q. Statewide stay-at-home directives on the spread of COVID-19 in metropolitan and nonmetropolitan counties in the United States. J Rural Health. 2021;37(1):222–223. doi: 10.1111/jrh.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao H, Zhang L, Marley G, Tang W. Differentiating COVID-19 response strategies. Innovation. 2020;1(1):100003. doi: 10.1016/j.xinn.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Urrego D, Rodríguez-Urrego L. Air quality during the COVID-19: PM(2.5) analysis in the 50 most polluted capital cities in the world. Environ Pollut. 2020;266(Pt 1):115042. doi: 10.1016/j.envpol.2020.115042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen LA, Chien LC, Li Y, Lin G. Nonuniform impacts of COVID-19 lockdown on air quality over the United States. Sci Total Environ. 2020;745:141105. doi: 10.1016/j.scitotenv.2020.141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabir M, Afzal MS, Khan A, Ahmed H. COVID-19 pandemic and economic cost; impact on forcibly displaced people. Travel Med Infect Dis. 2020;35:101661. doi: 10.1016/j.tmaid.2020.101661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Hu X, Xie J. Spatial inequalities of COVID-19 mortality rate in relation to socioeconomic and environmental factors across England. Sci Total Environ. 2021;758:143595. doi: 10.1016/j.scitotenv.2020.143595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philip RK, Purtill H, Reidy E, Daly M, Imcha M, McGrath D, et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: a ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob Health. 2020;5(9):e003075. doi: 10.1136/bmjgh-2020-003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kc A, Gurung R, Kinney MV, Sunny AK, Moinuddin M, Basnet O, et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health. 2020;8(10):e1273–e1281. doi: 10.1016/S2214-109X(20)30345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Yin H, Jin Z, Zhang H, Leng B, Luo Y, et al. Impact of Wuhan lockdown on the indications of cesarean delivery and newborn weights during the epidemic period of COVID-19. PLoS One. 2020;15(8):e0237420. doi: 10.1371/journal.pone.0237420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. Jama. 2020;324(7):705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedermann G, Hedley PL, Bækvad-Hansen M, Hjalgrim H, Rostgaard K, Poorisrisak P, et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):93–95. doi: 10.1136/archdischild-2020-319990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. 2020;5(11):e604–e611. doi: 10.1016/S2468-2667(20)30223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caniglia EC, Magosi LE, Zash R, Diseko M, Mayondi G, Mabuta J, et al. Modest reduction in adverse birth outcomes following the COVID-19 lockdown. Am J Obstet Gynecol. 2021;224(6):615.e1–615.e12. doi: 10.1016/j.ajog.2020.12.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bian Z, Qu X, Ying H, Liu X. Are COVID-19 mitigation measures reducing preterm birth rate in China? BMJ Glob Health. 2021;6(8):e006359. doi: 10.1136/bmjgh-2021-006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldenberg RL, McClure EM. Have coronavirus disease 2019 (COVID-19) community lockdowns reduced preterm birth rates? Obstet Gynecol. 2021;137(3):399–402. doi: 10.1097/AOG.0000000000004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira G, Belanger K, Ebisu K, Bell ML. Fine particulate matter and risk of preterm birth in Connecticut in 2000-2006: a longitudinal study. Am J Epidemiol. 2014;179(1):67–74. doi: 10.1093/aje/kwt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Ma ZF. Psychological responses and lifestyle changes among pregnant women with respect to the early stages of COVID-19 pandemic. Int J Soc Psychiatry. 2021;67(4):344–350. doi: 10.1177/0020764020952116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonnell S, McNamee E, Lindow SW, O'Connell MP. The impact of the Covid-19 pandemic on maternity services: a review of maternal and neonatal outcomes before, during and after the pandemic. Eur J Obstet Gynecol Reprod Biol. 2020;255:172–176. doi: 10.1016/j.ejogrb.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnaez J, Ochoa-Sangrador C, Caserío S, Gutiérrez EP, Jiménez MDP, Castañón L, et al. Lack of changes in preterm delivery and stillbirths during COVID-19 lockdown in a European region. Eur J Pediatr. 2021;180(6):1997–2002. doi: 10.1007/s00431-021-03984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matheson A, McGannon CJ, Malhotra A, Palmer KR, Stewart AE, Wallace EM, et al. Prematurity rates during the coronavirus disease 2019 (COVID-19) pandemic lockdown in Melbourne, Australia. Obstet Gynecol. 2021;137(3):405–407. doi: 10.1097/AOG.0000000000004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He JR, Liu Y, Xia XY, Ma WJ, Lin HL, Kan HD, et al. Ambient temperature and the risk of preterm birth in Guangzhou, China (2001-2011) Environ Health Perspect. 2016;124(7):1100–1106. doi: 10.1289/ehp.1509778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo T, Wang Y, Zhang H, Zhang Y, Zhao J, Wang Y, et al. The association between ambient temperature and the risk of preterm birth in China. Sci Total Environ. 2018;613-614:439–446. doi: 10.1016/j.scitotenv.2017.09.104. [DOI] [PubMed] [Google Scholar]
- 28.Ministry of Emergency Management P . National emergency response plan for public emergencies. 2006. [Google Scholar]
- 29.Chang HH, Warren JL, Darrow LA, Reich BJ, Waller LA. Assessment of critical exposure and outcome windows in time-to-event analysis with application to air pollution and preterm birth study. Biostatistics. 2015;16(3):509–521. doi: 10.1093/biostatistics/kxu060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization . Preterm birth. 2018. [Google Scholar]
- 31.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American College of Obstetricians and Gynecologists and Task Force on Hypertension in Pregnancy Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–s22. [PubMed] [Google Scholar]
- 33.Schenker N, Gentleman JF. On judging the significance of differences by examining the overlap between confidence intervals. Am Stat. 2001;55(3):182–186. [Google Scholar]
- 34.Main EK, Chang SC, Carpenter AM, Wise PH, Stevenson DK, Shaw GM, et al. Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am J Obstet Gynecol. 2021;224(2):239–241. doi: 10.1016/j.ajog.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Curtis M, Villani L, Polo A. Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(4):456. doi: 10.1136/archdischild-2020-320682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.China Daily . Lessons from COVID-19 outbreak. 2020. [Google Scholar]
- 37.Hoay Khoo VP, Morsillo J, Zhang L. Achieving positive mental health and wellbeing on the COVID-19 frontline. Innovation. 2020;1(2):100024. doi: 10.1016/j.xinn.2020.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbett GA, Milne SJ, Hehir MP, Lindow SW, O'Connell MP. Health anxiety and behavioural changes of pregnant women during the COVID-19 pandemic. Eur J Obstet Gynecol Reprod Biol. 2020;249:96–97. doi: 10.1016/j.ejogrb.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization . Maternal mental health. 2019. [Google Scholar]
- 40.Thapa SB, Mainali A, Schwank SE, Acharya G. Maternal mental health in the time of the COVID-19 pandemic. Acta Obstet Gynecol Scand. 2020;99(7):817–818. doi: 10.1111/aogs.13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher J, Cabral de Mello M, Patel V, Rahman A, Tran T, Holton S, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ. 2012;90(2):139g–149g. doi: 10.2471/BLT.11.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ministry of culture and tourism of the People’s Republic of China . Novel coronavirus pneumonia epidemic prevention and control work has been actively deployed in various province. 2020. [Google Scholar]
- 43.López-Morales H, Del Valle MV, Canet-Juric L, Andrés ML, Galli JI, Poó F, et al. Mental health of pregnant women during the COVID-19 pandemic: a longitudinal study. Psychiatry Res. 2021;295:113567. doi: 10.1016/j.psychres.2020.113567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sidor A, Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients. 2020;12(6):1657 [DOI] [PMC free article] [PubMed]
- 45.Langley-Evans SC. Nutrition in early life and the programming of adult disease: a review. J Hum Nutr Diet. 2015;28(Suppl 1):1–14. doi: 10.1111/jhn.12212. [DOI] [PubMed] [Google Scholar]
- 46.van de Loo KFE, Vlenterie R, Nikkels SJ, Merkus P, Roukema J, Verhaak CM, et al. Depression and anxiety during pregnancy: the influence of maternal characteristics. Birth. 2018;45(4):478–489. doi: 10.1111/birt.12343. [DOI] [PubMed] [Google Scholar]
- 47.Madzorera I, Isanaka S, Wang M, Msamanga GI, Urassa W, Hertzmark E, et al. Maternal dietary diversity and dietary quality scores in relation to adverse birth outcomes in Tanzanian women. Am J Clin Nutr. 2020;112(3):695–706. doi: 10.1093/ajcn/nqaa172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fatima M, Srivastav S, Mondal AC. Prenatal stress and depression associated neuronal development in neonates. Int J Dev Neurosci. 2017;60:1–7. doi: 10.1016/j.ijdevneu.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Handley SC, Mullin AM, Elovitz MA, Gerson KD, Montoya-Williams D, Lorch SA, et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 pandemic, March-June 2020. Jama. 2021;325(1):87–89. doi: 10.1001/jama.2020.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood R, Sinnott C, Goldfarb I, Clapp M, McElrath T, Little S. Preterm birth during the coronavirus disease 2019 (COVID-19) pandemic in a large hospital system in the United States. Obstet Gynecol. 2021;137(3):403–404. doi: 10.1097/AOG.0000000000004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lian X, Huang J, Huang R, Liu C, Wang L, Zhang T. Impact of city lockdown on the air quality of COVID-19-hit of Wuhan city. Sci Total Environ. 2020;742:140556. doi: 10.1016/j.scitotenv.2020.140556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Yang J. Risk and imbalance: a narrative analysis of daily life of marriage and divorce among urban youth during the COVID-19 epidemic. J Humanit. 2020;10:13–19. [Google Scholar]
- 54.Kyrklund-Blomberg NB, Cnattingius S. Preterm birth and maternal smoking: risks related to gestational age and onset of delivery. Am J Obstet Gynecol. 1998;179(4):1051–1055. doi: 10.1016/s0002-9378(98)70214-5. [DOI] [PubMed] [Google Scholar]
- 55.Marbury MC, Linn S, Monson R, Schoenbaum S, Stubblefield PG, Ryan KJ. The association of alcohol consumption with outcome of pregnancy. Am J Public Health. 1983;73(10):1165–1168. doi: 10.2105/ajph.73.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masten Y, Song H, Esperat CR, McMurry LJ. A maternity care home model of enhanced prenatal care to reduce preterm birth rate and NICU use. Birth. 2021. https://doi.org/10.1111/birt.12579. [DOI] [PubMed]
- 57.Matei A, Saccone G, Vogel JP, Armson AB. Primary and secondary prevention of preterm birth: a review of systematic reviews and ongoing randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2019;236:224–239. doi: 10.1016/j.ejogrb.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 58.Koullali B, Oudijk MA, Nijman TA, Mol BW, Pajkrt E. Risk assessment and management to prevent preterm birth. Semin Fetal Neonatal Med. 2016;21(2):80–88. doi: 10.1016/j.siny.2016.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 : Table S1. The prevention and control measures of three level response in Guangdong province. Table S2. Definitions of pregnancy complications selected in this study. Table S3. Proportions of study participants in the exposed and unexposed groups. Table S4. The differences in air pollution between lockdown and pre-lockdown periods in the study area. Table S5. Associations between exposure to COVID-19 lockdown at birth and preterm birth. Table S6. Associations of exposure to COVID-19 lockdown with stillbirth. Figure S1. Selection process of study subjects. Figure S2. Rate of preterm birth in each calendar month during 2015-2019 (before the COVID-19 pandemic). Figure S3. Division of participants into subgroups according to their GW on January 23rd, 2020. Figure S4. Distribution of the cumulative exposure dose in the first 22 GWs in the exposed group during lockdown.
Data Availability Statement
The statistical code and meta data during the current study are not publicly available due to the qualitative nature of the data, but are available from the corresponding author upon request.