Abstract
A subset of rats that self-administer 3,4-methylenedioxypyrovalerone (MDPV) develop unusually high levels of drug taking. A history of responding maintained by cocaine, but not food, prevents the development of this high-responder phenotype; however, it is unclear how histories of noncontingent cocaine exposure or self-administering drugs from other pharmacological classes would affect its development. In the current studies, 5 groups of male Sprague-Dawley rats were used to determine whether histories of responding maintained by drugs from different pharmacological classes (e.g., MDPV, cocaine, fentanyl, nicotine, or ketamine) would differentially impact the development of the high-responder phenotype when MDPV was available for self-administration. Two additional groups were used to determine whether noncontingent exposure to cocaine would prevent the development of the high-responder phenotype when MDPV was available for self-administration, and whether noncontingent exposure to MDPV would facilitate the development of the high-responder phenotype when cocaine was available for self-administration. Consistent with previous reports, a history of response-contingent cocaine, and to a lesser extent noncontingent cocaine, prevented the MDPV high-responder phenotype; however, when responding was initially maintained by fentanyl, nicotine, or ketamine, the MDPV high-responder phenotype developed in ∼45% of rats. By manipulating behavioral and pharmacological histories prior to evaluating MDPV self-administration, the current studies provide additional evidence that a history of response-contingent (or noncontingent) cocaine can prevent the transition from well regulated to aberrant drug-taking when responding is maintained by MDPV. Although the mechanism(s) that underlies this novel high-responder phenotype are unknown, elucidation may provide insight into individual differences relating to substance use disorder.
SIGNIFICANCE STATEMENT
A subset of outbred Sprague-Dawley rats self-administer high levels of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Understanding the behavioral and/or pharmacological factors that can prevent the development of dysregulated MDPV self-administration may provide insight into individual differences in vulnerability to develop a substance use disorder.
Introduction
Synthetic cathinones are a family of novel psychoactive substances colloquially known as bath salts, legal highs, research chemicals, or plant food. These compounds function as either monoamine uptake inhibitors [e.g., 3,4-methylenedioxypyrovalerone (MDPV), α-pyrrolidinopentiophenone (α-PVP)] or releasers (e.g., 3,4-methylenedioxy-N-methylcathinone, 4-methylmethcathinone). Although they vary in selectivity for the dopamine, norepinephrine, and serotonin transporters, their effects are similar to prototypical illicit stimulants (e.g., cocaine, methamphetamine, or 3,4-methylenedioxymethamphetamine). Synthetic cathinone users often report repeated bouts of drug administration (i.e., binges) over hours/days, using more drug than intended, high amounts of drug craving, and euphoric effects stronger than those of cocaine (e.g., Winstock et al., 2011; Lenz et al., 2013; Stoica and Felthous, 2013). Synthetic cathinone use also results in a large number of emergency room visits and calls to poison control centers due to acute toxicity, including hypertension, tachycardia, anxiety, and paranoia (e.g., Winstock et al., 2011; Lenz et al., 2013; Stoica and Felthous, 2013). Although anecdotal, these data suggest that the abuse-related and toxic effects of synthetic cathinones (or bath salts preparations) might be greater than for drugs such as cocaine or methamphetamine.
Preclinical studies in rats and monkeys using behavioral economics and/or progressive ratio (PR) schedules of reinforcement to directly compare the reinforcing effectiveness of synthetic cathinones, such as MDPV and α-PVP, to traditional stimulant drugs of abuse suggest that MDPV and related synthetic cathinones function as more effective reinforcers than either cocaine or methamphetamine (Aarde et al., 2013; Watterson et al., 2014; Gannon et al., 2017; 2018a; Huskinson et al., 2017; Collins et al., 2019; Doyle et al., 2021b; but see de Moura et al., 2021). Structure-activity relationships suggest that the reinforcing effectiveness of MDPV and α-PVP is positively correlated with their selectivity for the dopamine relative to serotonin transporter (Gannon et al., 2018a).
In addition to functioning as highly effective reinforcers, pyrrolidine-containing synthetic cathinones, including MDPV, often maintain unusually high levels of responding during acquisition of self-administration, sometimes referred to as spiking or “binge-like” patterns of acquisition (Aarde et al., 2015; Javadi-Paydar et al., 2018). Although not explicitly described as such, similar observations have been made in our laboratory and others (Watterson et al., 2014; Gannon et al., 2017, 2018b; Doyle et al., 2021b), suggesting that MDPV and related synthetic cathinones are associated with unusually high rates of drug intake, a phenomena that might be related to binge-like patterns of consumption of drugs, alcohol, sugar, or high-fat foods (e.g., Avena, Rada, and Hoebel, 2008; Corwin, Avena, and Boggiano, 2011; Carnicella, Ron, and Barak, 2014). Not only are these high levels of intake apparent when rats are learning the drug-taking response, but in a subset of male and female rats, these unusually high levels of drug intake persist across time and over a range of doses (Gannon et al., 2017, 2018b; Doyle et al., 2021b). This upward shift in the fixed ratio (FR) 5 dose-response curve suggests that these animals are not simply more sensitive to the reinforcing effects of MDPV (i.e., not due to a leftward shift in the dose-response curve), but rather that their drug-taking behavior has transitioned from well regulated to aberrant, akin to what is observed in humans as they transition from recreational to more problematic patterns of drug taking. In addition to high levels of drug intake, these high-responder rats also engage in high levels of responding during signaled periods of drug unavailability (Gannon et al., 2017, 2018b, 2021; Doyle et al., 2021b) and will work harder to obtain drug infusions under PR schedules of reinforcement (Gannon et al., 2017, but see Doyle et al., 2021b), two aspects thought to be related to substance use disorders in humans (e.g., Deroche-Gamonet et al., 2004).
Although the high level of drug intake is the most striking behavioral feature of this novel phenotype, the criterion we have used to operationally define high- (and low-) responders is the proportion of total active lever responses made during the 5-second postinfusion timeout, with rats making ≥20% of their total responses during this period classified as high-responders (Gannon et al., 2017, 2018b; Doyle et al., 2021b). Since timeout responding is significantly and positively correlated with drug intake and remains elevated in high-responders regardless of the unit dose of MDPV available, this single criterion can be applied to responding maintained by both small and large unit doses, despite the fact that they maintain relatively high and low rates of responding, respectively. Relatedly, although the self-administration of other drugs such as cocaine and oxycodone is not typically associated with responding during postinfusion timeouts, when these drugs are substituted for MDPV, the core features of this phenotype (e.g., high levels of drug intake and high levels of responding when drug is not available; Gannon et al., 2017; 2018a; 2021) persist, suggesting that the high-responder phenotype first identified with MDPV self-administration may provide a novel model for better understanding individual differences in drug taking as they relate to substance use disorders in humans.
Interestingly, although this high-responder phenotype is both reliable [i.e., observed in 78 of 184 (42%) rats across five studies, including this one; Gannon et al., 2017; 2018b; Doyle et al., 2021a; 2021b] and enduring, evidence from two of these studies (Gannon et al., 2017; Doyle et al., 2021b) suggests that a history of cocaine self-administration can prevent or delay the development of high levels of MDPV intake. Although the inability of a history of responding maintained by food delivery to alter the development of this high-responder phenotype for MDPV self-administration (Doyle et al., 2021b) suggests that simply providing a history of operant responding is insufficient to prevent the development of the high-responder phenotype, the mechanism(s) by which cocaine interferes with the transition from regulated to dysregulated or aberrant patterns of MDPV self-administration remain unclear.
As such, the present study sought to better define the behavioral and pharmacologic determinants of the MDPV high-responder phenotype by evaluating MDPV self-administration in rats that were first allowed to self-administer drugs with diverse pharmacological mechanisms and nonoverlapping discriminative stimulus effects as well as rats that were provided noncontingent histories of cocaine and MDPV exposure to match those achieved through response-contingent self-administration. Thus, the present study had three primary goals: 1) to directly compare the proportion of rats that develop the MDPV high-responder phenotype after acquiring responding maintained by MDPV, cocaine, fentanyl, ketamine, and nicotine; 2) to use a yoked, noncontingent cocaine exposure paradigm to determine whether reinforcement (i.e., response-contingent cocaine) and pharmacologic (i.e., noncontingent cocaine) histories would both disrupt the development of high levels of MDPV self-administration; and 3) to use a yoked, noncontingent MDPV exposure paradigm to determine whether reinforcement (i.e., response-contingent MDPV) and pharmacologic (i.e., noncontingent MDPV) histories would both establish high levels of cocaine self-administration.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (n = 112; 275–300g upon arrival; Envigo, Indianapolis, IN) were singly housed in a temperature- and humidity-controlled environment with a 14-hour light/dark cycle (lights on at 6:00 AM). Rats had ad libitum access to food and water except during experimental sessions, or when mild food restriction (15 g/day) was briefly (3–8 days) used in a subset of rats (n = 6) to promote acquisition of drug-taking. All procedures were conducted in accordance with Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and the guide for care and use of laboratory animals (National Research Council, 2011).
Surgical Procedure
Briefly, rats were anesthetized with 2%–3% isoflurane and prepared with chronic indwelling catheters in the left femoral vein and a vascular access button exteriorized in the scapular region (Doyle et al., 2021a; 2021b). To prevent infection, 60,000U/rat penicillin G was administered once, subcutaneously, after surgery. Rats were allowed to recover for 5–7 days before beginning experimental sessions. Rats were flushed daily with 0.5 ml heparinized saline (100U/ml) during the recovery period and after self-administration sessions, and with 0.2 ml saline before each session.
Apparatus
Operant sessions were conducted in standard operant chambers (Med Associates Inc., St. Albans, VT), located within ventilated, sound- and light-attenuated cubicles. Chambers contained two levers located on one wall with a set of 3 LEDs (green, yellow, and red) above each lever; a white house light was located at the top of the opposite wall. Variable speed syringe drivers were used to administer all infusions through Tygon tubing that was connected to a fluid swivel and spring tether held in place by a counterbalance arm.
Experiment 1
Acquisition
To determine the effects of different drug self-administration histories on the development of the high-responder phenotype, 80 rats (n = 16/drug history group) responded for either MDPV (0.032 mg/kg/inf), cocaine (0.32 mg/kg/inf), fentanyl (0.0032 mg/kg/inf), nicotine (0.032 mg/kg/inf), or ketamine (1.0 mg/kg/inf) under an FR1 schedule of reinforcement during at least 10 daily 90-minute sessions. Doses were selected based on each drug’s relative reinforcing potency/relative position on the descending limb of FR dose-response curves (i.e., half log beyond the peak; Collins and Woods, 2007; Caine et al., 2014; Wade et al., 2015; Doyle et al., 2021b). Illumination of the yellow LED above the active lever (counterbalanced across rats) signaled drug availability. When the FR response requirement was met, an infusion (∼1-second in duration) was delivered, and the infusion-paired stimuli (house light and 3 LEDs above the active lever) were illuminated. A 5-second timeout (TO) was initiated coincident with the start of the infusion during which the infusion-paired stimuli were illuminated, and no additional infusions could be earned. For rats that met acquisition criteria (i.e., ≥10 reinforcers and ≥80% responses on the active lever for two consecutive sessions) by the 10th session, the FR requirement was subsequently increased to an FR5. For rats that responded for fentanyl, nicotine, or ketamine, the FR was first increased to an FR3 for at least 3 sessions and until ≥10 reinforcers were earned prior to increasing the response requirement to an FR5. Rats that failed to meet acquisition criteria within 10 sessions (cocaine: n = 5; fentanyl: n = 1; nicotine: n = 2; ketamine: n = 2) remained on FR1 until acquisition criteria were met. Those that did not meet the criterion for number of infusions by the 10th session (n = 6) were mildly food-restricted (15 g food/day) beginning after the 10th session and/or received noncontingent infusions (at 1, 3, 5, 10, and 15 minutes after the start of the session) for up to 4 sessions (beginning between sessions 11 and 14), until acquisition criteria were met (3–8 sessions). Catheter patency was assessed using 5 mg/kg methohexital as needed (e.g., an increase in pressure when flushing, or whether a rat exhibited extinction-like levels of responding). All catheters remained patent throughout the duration of these studies.
Fixed Ratio 5 Responding
After acquiring responding under an FR1, rats then responded for their original reinforcer under FR5:TO 5-second schedule of reinforcement for at least 10 sessions and until meeting stability criteria (i.e., ±20% of the mean for 3 consecutive sessions; no increasing or decreasing trend), or a maximum of 15 sessions. After this, all groups except the MDPV history group (which went on to self-administer 0.32 mg/kg/inf cocaine) self-administered MDPV (0.032 mg/kg/inf) for at least 10 sessions and until reaching the stability criteria defined above, or a maximum of 15 sessions. As previously described, rats were classified as high- or low-responders based on whether they made ≥20% or <20%, respectively, of their total active lever responses during the 5-second postinfusion TO periods (Gannon et al., 2017, 2018b; Doyle et al., 2021b).
Experiment 2
Noncontingent Histories
To differentiate between the pharmacological and behavioral effects of MDPV and cocaine self-administration, 32 rats (n = 16/group) received noncontingent drug infusions of either MDPV (0.032 mg/kg/inf) or cocaine (0.32 mg/kg/inf) through a yoked-infusion paradigm. Each yoked rat was paired with one of the rats that self-administered MDPV or cocaine in experiment 1. The yoked rats were placed into operant chambers, where responding on levers had no scheduled consequence, but unsignaled MDPV or cocaine infusions were delivered when the “primary” rat from experiment 1 earned a drug infusion. The yoked procedure continued for the total duration of the original drug self-administration (i.e., ≥10 sessions of the primary rat responding under an FR1:TO 5-second and 10–15 sessions of responding under an FR5:TO 5-second schedule).
Self-Administration
After ∼25 sessions of noncontingent drug administration, yoked rats began self-administration under an FR1:TO 5-second schedule of reinforcement. Rats that received yoked MDPV infusions were allowed to respond for cocaine (0.32 mg/kg/inf), whereas rats that received yoked cocaine infusions were allowed to respond for MDPV (0.032 mg/kg/inf). After 10 sessions of self-administration, rats that did not meet acquisition criteria (n = 1 for yoked MDPV history) were mildly food-restricted (15 g of food/day) until acquisition criteria were met. Once acquisition criteria were met, the response requirement was increased to an FR5 for at least 10 sessions and until stability criteria were met, or a maximum of 15 sessions. Rats were then classified as high- or low-responders based on percent TO responding (Gannon et al., 2017, 2018b; Doyle et al., 2021b).
Statistical Analyses
Number of infusions earned when responding under FR1 or FR5 are both represented as the mean ± S.E.M. The mean number of infusions earned during the final 3 sessions of responding under FR1 and FR5 conditions for the original reinforcer (i.e., MDPV, cocaine, nicotine, fentanyl, or ketamine), were compared by a two-way (drug and FR) repeated measures (FR) ANOVA. The number of sessions to reach acquisition criteria (in rats meeting the criteria within 10 sessions) in rats with no prior drug history were analyzed by a one-way ANOVA with Tukey’s post hoc analyses. A two-way ANOVA (drug and no prior drug history vs. noncontingent history) was used to compare the sessions to reach acquisition criteria in rats that acquired on MDPV or cocaine, with Sidak’s multiple comparisons test. A two-way (day and phenotype) repeated measures (day) ANOVA was used to compare the number of infusions earned by high- and low-responders self-administering MDPV in all groups, except cocaine, which was excluded from analysis because there was only 1 high-responder.
Drugs
Racemic MDPV HCl was synthesized by Agnieszka Sulima and Kenner Rice (Bethesda, MD). Fentanyl and cocaine HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Nicotine and ketamine were purchased from Sigma-Aldrich (St. Louis, MO) and Henry Schein (Dublin, OH), respectively. Doses of MDPV, cocaine, fentanyl, and ketamine were based on salt weights, whereas doses of nicotine were based on the free base weight of nicotine. All drugs were dissolved in sterile 0.9% saline. For nicotine solutions, the pH was adjusted to ∼7 by adding 1N NaOH dropwise. Drugs were administered intravenously in a volume of 0.1 ml/kg body weight.
Results
Experiment 1: Acquisition of Responding
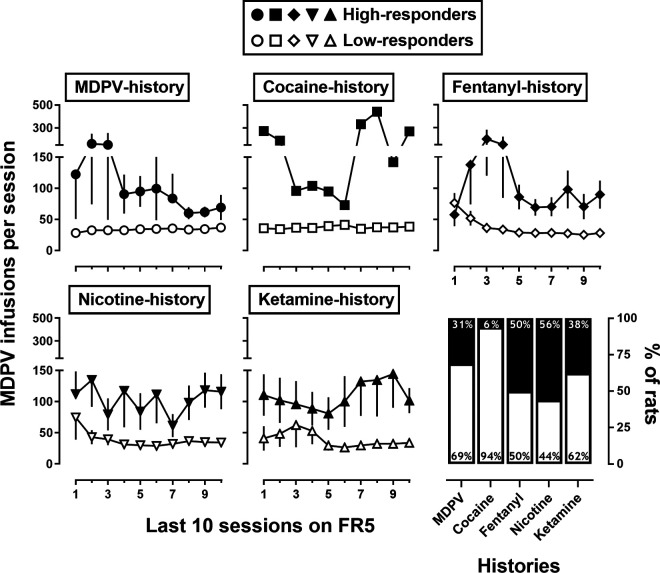
A majority of rats met the acquisition criteria within the first 10 sessions (Fig. 1; Table 1). A one-way ANOVA indicated there was a main effect of drug [F(4,64) = 5.6; P = 0.0009] on the number of sessions to meet acquisition criteria, where acquisition of ketamine self-administration required a greater number of sessions than when either MDPV or cocaine was available for self-administration (Table 1; Tukey’s post hoc; p < 0.05 for both). There were no significant differences in the number of sessions to meet acquisition criteria for fentanyl or nicotine self-administration. Generally, when the FR requirement was increased from FR1 to FR5, rats increased their responding ∼5-fold to earn a comparable number of infusions, and there were no significant differences in number of infusions earned [F(1,105) = 0.07; P = 0.79; Fig. 1]. After self-administering their initial drug under the FR5 schedule of reinforcement for 10–15 sessions, 5 of 16 (31%) rats that self-administered MDPV met the high-responder criterion (i.e., ≥20% of their total active lever responses made during the postinfusion TO periods; Gannon et al., 2017, 2018b; Doyle et al., 2021b), as did 3 of 16 (19%) rats that self-administered fentanyl, and 1 of 16 (6%) that self-administered cocaine (data not shown). In contrast, none of the rats that self-administered nicotine or ketamine met the criterion for being classified as a high-responder.
Fig. 1.
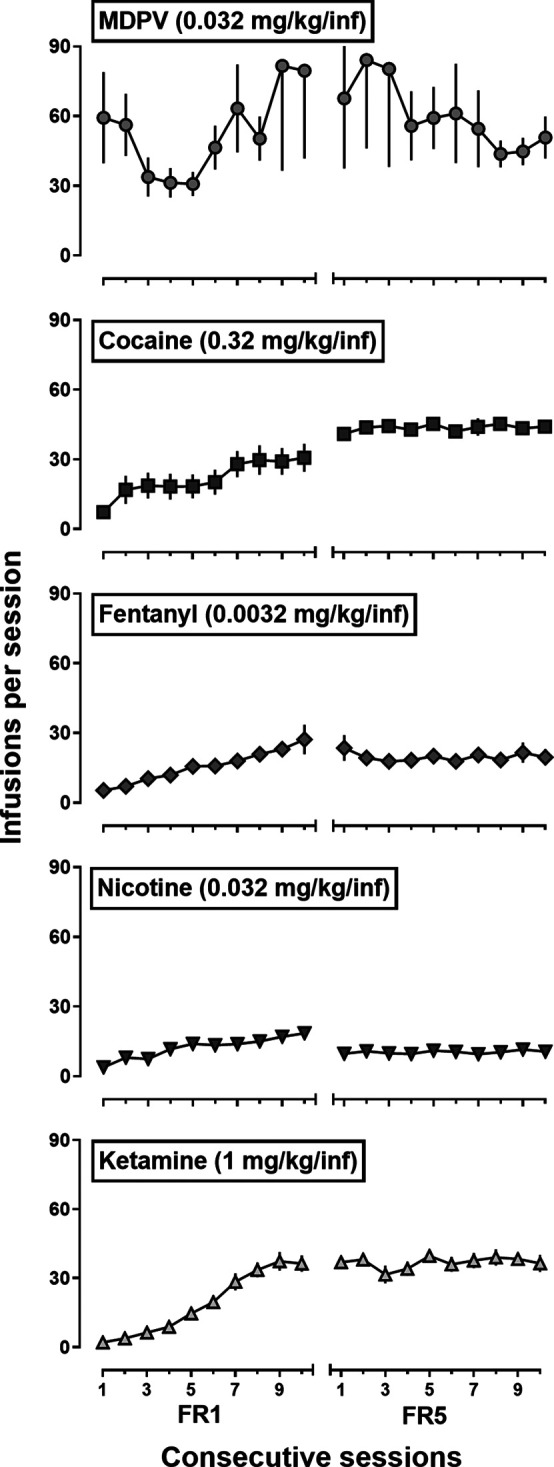
Number of infusions earned during 90-minute self-administration sessions by adult male Sprague-Dawley rats (n = 16/drug) responding for 0.032 mg/kg/infusion MDPV (circles), 0.32 mg/kg/infusion cocaine (squares), 0.0032 mg/kg/infusion fentanyl (diamonds), 0.032 mg/kg/infusion nicotine (downward triangles), or 1 mg/kg/infusion ketamine (upward triangles) during the initial 10 sessions on a FR1:TO 5-second schedule of reinforcement and final 10 sessions of infusions earned under a FR5:TO 5-second schedule of reinforcement. Symbols represent mean (±1 S.E.M.) number of infusions earned across 20 sessions of self-administration. Abscissa: session number (day) where the left sessions 1–10 were responding under FR1 and right sessions 1–10 were responding under FR5. Ordinates: mean number of infusions earned. A two-way repeated measures ANOVA found no significant difference between the mean number of infusions earned during the final three sessions of responding under FR1 compared with FR5 [F(1,105) = 0.07; P = 0.79].
TABLE 1.
Mean sessions to meet acquisition criteria (two consecutive sessions of earning ≥10 infusions and ≥80% of responses made on the active lever), number of rats meeting acquisition criteria within 10 sessions, mean number of infusions earned the last three sessions of acquisition (FR1), and mean number of infusions earned under an FR5 schedule of reinforcement
| History | Sessions to acquire mean + S.E.M. |
Rats acquired n acquired / total n (%) |
Infusions on FR1 mean + S.E.M. |
Infusions on FR5 mean + SEM |
|---|---|---|---|---|
| MDPV group | 4.5 + 0.5 | 16/16 (100) | 69.6 + 29.8 | 41.1 + 4.2 |
| MDPV high-responders | 4.6 + 0.7 | 5/5 (100) | 165.4 + 115.2 | 60.1 + 10.4 |
| MDPV low-responders | 4.5 + 0.7 | 11/11 (100) | 35.4 + 2.8 | 33.2 + 2.1 |
| Cocaine | 4.9 + 0.6 | 10/16 (62.5) | 29.8 + 6.0 | 42.7 + 2.3 |
| Fentanyl | 6.0 + 0.6 | 15/16 (93.8) | 22.7 + 4.2 | 21.1 + 3.0 |
| Nicotine | 6.7 + 0.6 | 14/16 (87.5) | 15.7 + 1.8 | 10.9 + 0.8 |
| Ketamine | 7.9 + 0.5 a,b | 14/16 (87.5) | 31.8 + 3.3 | 41.5 + 2.3 |
| Noncontingent cocaine MDPV self-administration |
2.2 + 0.1 a | 16/16 (100) | 43.9 + 2.6 | 77.0 + 23.7 |
|
Noncontingent cocaine
MDPV high-responders |
2.3 + 0.3 | 3/3 (100) | 46.8 + 9.6 | 242.7 + 73.3 |
|
Noncontingent cocaine
MDPV low-responders |
2.2 + 0.1 | 13/13 (100) | 43.4 + 2.6 | 38.8 + 7.9 |
| Noncontingent MDPV Cocaine self-administration |
2.7 + 0.3 b | 15/16 (93.8) | 48.0 + 4.9 | 44.3 + 1.9 |
a P < 0.05; significantly different from MDPV group
b P < 0.05; significantly different from cocaine
Experiment 1: Substitution to MDPV Self-Administration
To determine whether self-administration history could prevent/delay the development of the high-responder phenotype, rats that initially self-administered cocaine, fentanyl, nicotine, or ketamine (drug history groups) were allowed to self-administer 0.032 mg/kg/infusion MDPV under FR5 for 10–15 sessions (Fig. 2). The number of MDPV infusions earned by each of these groups is shown in Fig. 2, as is the percent of rats that met the high-responder criterion during the final 3 sessions of MDPV self-administration (i.e., sessions 8–10 or 13–15 of MDPV self-administration, when stability criteria were reached). Two-way repeated measures ANOVAs found main effects of phenotype for all groups, except cocaine, [MDPV: F(1,14) = 7.8, P = 0.01; fentanyl-history: F(1,14) = 6.9, P = 0.02; nicotine-history: F(1,14) = 9.0, P = 0.01; ketamine-history: F(1,14) = 12.4, P = 0.003], where high-responders earned more infusions than low-responders. Approximately 45% of rats with a history of responding maintained by fentanyl (50%), nicotine (56%), or ketamine (38%) developed the high-responder phenotype for MDPV; in contrast, only 1 of 16 (6%) rats with a history of responding maintained by cocaine developed the high-responder phenotype for MDPV (Fig. 2). Low-responder rats earned a similar number of MDPV infusions across reinforcement histories (mean: 33–41 infusions), and high-responder rats earned 2–3 times as many infusions as low-responder rats across reinforcement histories (Fig. 2). Percent TO responding averaged less than 10% for low-responder rats and ∼40% for high-responder rats (data not shown).
Fig. 2.
Number of infusions of 0.032 mg/kg/infusion MDPV self-administered per session by rats (n = 16/drug) with a history of responding for MDPV (circles), cocaine (squares), fentanyl (diamonds), nicotine (downward triangles), or ketamine (upward triangles). Open symbols indicate rats classified as low-responders (<20% of total active lever responses made during 5-second postinfusion timeout), where filled symbols indicate rats classified as high-responders (≥20% of total active lever responses made during 5-second postinfusion timeout). Symbols represent mean (±1 S.E.M.) number of infusions. Abscissa: session number for the final 10 sessions of responding for MDPV. Ordinates: mean number of infusions earned. Bottom right panel: Percent of rats classified as high- or low-responders when responding for MDPV, separated by prior self-administration history. Open bars represent percent of low-responders, where filled bars represent percent of high-responders. Abscissa: self-administration history. Ordinates: percent of rats classified as high- or low-responders. A two-way repeated measures ANOVA compared number of infusions earned by high- and low-responders for each drug history (except cocaine, due to n = 1 high-responder) and found significantly different levels of drug intake by phenotype (see text for details).
Experiment 2: Noncontingent MDPV/Cocaine Histories
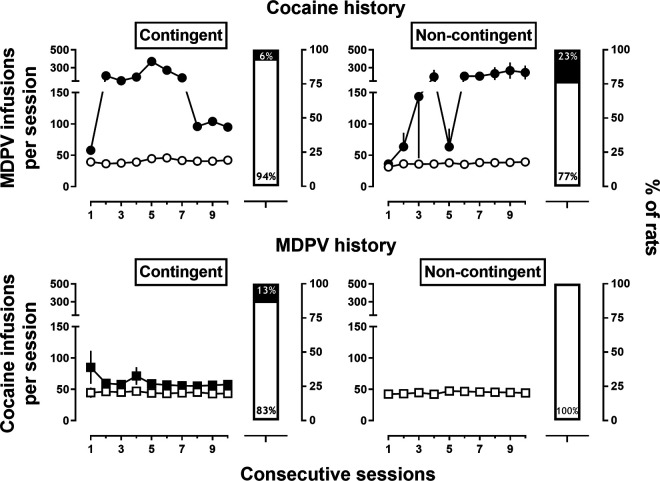
Two groups of rats (n = 16/group) received noncontingent, yoked infusions of MDPV (0.032mg/kg/inf) or cocaine (0.32 mg/kg/inf) to determine the role of response contingencies in the development of the high-responder phenotype. Rats with a noncontingent drug history acquired self-administration significantly more rapidly than experimentally naive rats (P < 0.005 for both), with nearly all rats meeting acquisition criteria within 3 sessions (Table 1). After 10–15 sessions of cocaine self-administration under an FR5 schedule of reinforcement, none of the MDPV-yoked rats developed the high-responder phenotype. In contrast, 2 of the 5 high-responders from response-contingent MDPV history group maintained the high-responder phenotype after 10 sessions of cocaine availability (Fig. 3). Three of 16 (19%) cocaine-yoked rats developed the high-responder phenotype when they were subsequently allowed to self-administer MDPV (Fig. 3). These rats displayed a similar, if not more extreme, high-responder phenotype as rats with other drug histories, earning ∼240 infusions on average during the last 3 sessions (Fig. 3) compared with the 60–130 infusions earned on average by high-responders with other drug histories (Fig. 2).
Fig. 3.
Top row: Number of infusions of 0.32 mg/kg/infusion of cocaine self-administered by rats with a contingent/self-administered (left) or noncontingent/yoked (right) history of MDPV. Bottom row: Number of infusions of 0.032 mg/kg/infusion MDPV earned by rats with a contingent/self-administered (left) or noncontingent/yoked (right) history of cocaine. All: Symbols represent mean (±1 S.E.M.) number of infusions earned under a FR5 schedule of reinforcement, whereas bars represent percent or rats classified as high- or low-responders. Open symbols/bars represent low-responders and filled symbols/bars represent high-responders. Abscissa: session number for the final 10 sessions of responding for MDPV. Left ordinates (symbols): mean number of infusions earned. Right ordinates (bars): percent of rats classified as high- or low-responders.
Discussion
Prior studies have reported that a subset of rats develop unusually high and persistent levels of drug intake when allowed to self-administer MDPV or structurally-related synthetic cathinones (Gannon et al., 2017, 2018b; Doyle et al., 2021b). In addition to being observed across multiple studies and laboratories, because this unusual pattern of drug-taking is enduring and readily transfers to other stimulants (e.g., cocaine, methamphetamine; Gannon et al., 2017), we believe that this high-responder phenotype represents a novel model to investigate the binge-like and/or compulsive patterns of stimulant use often reported by humans. Accordingly, the present study sought to better define the behavioral and pharmacologic determinants of the high-responder phenotype by evaluating MDPV self-administration in rats with histories of reinforcement by drugs with diverse pharmacological mechanisms and nonoverlapping discriminative stimulus effects as well as rats that were provided pharmacologic histories of noncontingent cocaine and MDPV exposure to match those achieved through response-contingent self-administration. In addition to replicating prior findings (Gannon et al., 2017; Doyle et al., 2021b) by demonstrating that a subset of rats responding for MDPV (∼30%), but not cocaine, develop a high-responder phenotype characterized by persistently high levels of drug-taking combined with high levels of responding during postinfusion TO periods, the present studies also showed that: 1) unlike when responding was previously maintained by cocaine, histories of responding maintained by fentanyl, nicotine, or ketamine failed to prevent the development of the MDPV high-responder phenotype; 2) when provided a history of noncontingent cocaine exposure, an intermediate proportion of rats went on to develop the MDPV high-responder phenotype; and 3) when provided a history of noncontingent MDPV exposure, rats failed to develop the high-responder phenotype for cocaine self-administration. Together, these findings suggest that both behavioral and pharmacological histories play a role in the development of the high-responder phenotype, and highlight the importance of response-contingent drug administration in both establishing (MDPV history) and preventing (cocaine history) the aberrant patterns of responding and high levels of drug intake that may be related to the development of substance use disorders in humans.
Previous studies suggested that reinforcement histories with cocaine, but not food, can prevent the development of the MDPV high-responder phenotype (Gannon et al., 2017; Doyle et al., 2021b). The current study replicated and extended this basic finding by determining whether histories of reinforcement by drugs from other pharmacological classes would also interact with the development of the MDPV high-responder phenotype. Although 1 of the 16 (6%) rats with a history of cocaine self-administration developed the high-responder phenotype when MDPV was available for self-administration, rats that were provided histories of fentanyl (50%), nicotine (56%), or ketamine (38%) self-administration went on to develop the MDPV high-responder phenotype at proportions similar to that observed in rats that had only ever self-administered MDPV (31%). These findings suggest a history of cocaine self-administration is unique in its capacity to prevent the development of this high-responder phenotype; however, further studies will be required to determine whether the self-administration of other stimulant drugs (e.g., methamphetamine) is also capable of preventing the high-responder phenotype. In addition, because these studies only assessed the impact of relatively short/limited access (i.e., 90-minute daily sessions) to cocaine reinforcement, it will be important to determine whether cocaine would retain its protective effects if rats were provided histories of cocaine reinforcement under long/extended access or intermittent access conditions, which have been shown to result in escalation of drug-taking, increases in compulsive-like behaviors (Ahmed and Koob, 1998; Vanderschuren and Everitt, 2004), and changes reinforcing effectiveness, cue-induced reinstatement, and dopamine transporter sensitivity (Zimmer et al., 2012; Calipari et al., 2013; Allain et al., 2018; Garcia et al., 2020) that are thought to contribute to substance use disorder-related phenotypes.
To determine whether the contingency between the response and the drug effect was important to the protective effects of cocaine (and the faciliatory effects of MDPV), the current study also incorporated two groups of rats that received noncontingent infusions of cocaine (or MDPV) through a yoked procedure that matched drug exposures (i.e., the timing and number of infusions) to rats that were actively self-administering cocaine (or MDPV). Importantly, when compared with rats that were actively self-administering cocaine (6%) or other drugs (44%; collapsed across MDPV, fentanyl, ketamine, and nicotine), a history of noncontingent cocaine exposure appeared to provide an intermediate protection (19%) against development of the high-responder phenotype. When taken together with the finding that a history of noncontingent MDPV exposure failed to facilitate the development of the high-responder phenotype for cocaine, these findings suggest that the capacity of cocaine to interfere with the development of the high-responder phenotype for MDPV is dependent on both behavioral and pharmacological aspects of these histories. Although the relative importance of the contingencies of reinforcement is likely related to associations that are formed between the response and the drug effects, aspects of the pharmacologic properties of cocaine (and MDPV) that contribute to the protective (and faciliatory) effects are less clear.
Given that MDPV is much more selective than cocaine at inhibiting uptake at DAT relative to SERT (∼750-fold vs. 1.2-fold; Gannon et al., 2018a), it is possible that actions at SERT, or lack thereof, are important for the protective and faciliatory effects of cocaine and MDPV, respectively. Supporting this notion, other DAT-preferring, pyrrolidine-containing cathinones (e.g., 3,4-methylenedioxy-apyrrolidinobutiophenone α-PVP) are also associated with the development of this high-responder phenotype (Gannon et al., 2018b). However, similar phenotypes are not observed in rats that self-administer methylphenidate (Collins et al., 2012), another DAT-selective uptake inhibitor (Markowitz and Patrick, 2008), suggesting that other factors are more important in establishing the high-responder phenotype. Further supporting this, genetic deletion of SERT did not alter the reinforcing effects of cocaine in mice (Thomsen et al., 2009); however, additional studies evaluating MDPV and cocaine self-administration in SERT-knockout mice are needed to rule out a role for SERT in the protective effects of cocaine. Another possibility is that, because the discriminative stimulus effects of cocaine and MDPV overlap (e.g., Gatch et al., 2013; Collins et al., 2016; Seaman et al., 2021), rats are unable to differentiate between conditions in which cocaine or MDPV are available for self-administration. This would be particularly important if potentially aversive effects of cocaine, perhaps related to inhibition of cardiovascular sodium channels (Matthews and Collins, 1983), result in rats first learning to self-administer cocaine in a more regulated and/or cautious manner before gaining access to MDPV. Indeed, bolus doses of MDPV as large as 5.6 mg/kg intravenously in rats (Hambuchen et al., 2017) and 140 mg/kg i.p. in mice (Muskiewicz et al., 2020) have been administered without producing convulsions. Conversely, cocaine-induced convulsions can be observed after bolus doses of ∼9 mg/kg intravenously in rats (Mets et al., 1998) and 56 mg/kg i.p. in mice (Ko et al., 2007). When compared with doses of MDPV and cocaine to maintain half-maximal responding under a PR schedule in rats (0.017 and 0.17 mg/kg/inf, respectively; Gannon et al., 2018a), or cocaine-like discriminative stimulus effects in mice (0.1 and 3 mg/kg, i.p., respectively; Gannon et al., 2016), the potency ratio for MDPV to produce abuse-related and toxic/aversive effects is at least 6- to 70-fold greater than that for cocaine. Thus, the relatively narrow window between the reinforcing/abuse-related effects and the convulsant/aversive effects for cocaine relative to MDPV could be one factor that moderates the level of intake for cocaine, but not MDPV. Although one might hypothesize that the unpredictable nature of noncontingent cocaine infusions would result in their being more aversive than response-contingent cocaine infusions, the contextual associations learned under such scenarios may be insufficient to impact subsequent MDPV self-administration.
Although there are insufficient data to directly compare the onset of action for MDPV and cocaine, a recent study in rats self-administering cocaine (Canchy et al., 2021) suggests that response latencies can be used as a behavioral readout of pharmacokinetic properties of cocaine. Indeed, they reported that upon completing a response requirement, rats withhold responding until sometime after the drug effect is perceived; however, if a cocaine infusion was omitted, rats began responding sooner. The time to peak effect for cocaine (increases in synaptic dopamine levels) is estimated to be tens of seconds (Wise and Kiyatkin, 2011; Canchy et al., 2021), and although little is known about the time to peak effect for MDPV, it is unlikely to be less than 5 seconds, the duration of the TO in the present studies. Nevertheless, if one assumes that the TO responding associated with MDPV self-administration is due to a slower time to peak effect, then one would also expect that substituting MDPV for cocaine would result in more TO responding (i.e., MDPV would be perceived as a delayed/omitted reward), whereas substituting cocaine for MDPV would result in less TO responding (i.e., cocaine would be perceived as a more immediate reward). Given that neither of these outcomes were observed, we do not believe that pharmacokinetics, at least related to the time to peak effect, is a causal factor in facilitating or preventing the high-responder phenotype.
Consistent with patterns of responding maintained by drug reinforcers under simple FR schedules with relatively short postinfusion TOs, very little TO responding was observed when responding was maintained by cocaine, nicotine, or ketamine; however, when fentanyl was available, a subset of rats (3 of 16) made ∼26% of their active lever responses during TOs and earned nearly twice as many infusions than the other rats (∼32 vs. ∼17 infusions per session). Although this suggests that the high-responder phenotype may not be unique to synthetic cathinones, the importance of TO responding to the overall phenotype is not entirely clear. Others have also reported fairly large variability in fentanyl intake in rats whose responding was maintained by intravenous or vapor fentanyl (e.g., Stevenson et al., 2020; Bakhti-Suroosh et al., 2021; McConnell et al., 2021), suggesting that fentanyl may facilitate a high-responder phenotype similar to that seen with MDPV self-administration. Taken together with recent findings suggesting that the high-responder phenotype persists when oxycodone is substituted for MDPV (Gannon et al., 2021), the current findings highlight the need to further explore interactions between this high-responder phenotype and opioid self-administration. Additionally, studies evaluating whether a history of cocaine self-administration can similarly prevent the development of this fentanyl high-responder phenotype could provide insight into the mechanism(s) that underlie the development of these high-responder phenotypes as well as their prevention by cocaine.
In summary, the current study builds on previous work demonstrating that subsets of rats that self-administer MDPV and structurally-related synthetic cathinones develop high levels of drug-taking and responding during periods of drug unavailability (i.e., drug seeking), both of which are key features of substance use disorders in humans. In addition, the present study provided further evidence that a history of cocaine administration can prevent the development of the MDPV high-responder phenotype, and advanced our understanding by demonstrating that these effects (as well as the faciliatory effects of MDPV) are dependent upon the response-contingent administration of these drugs. Although the precise mechanisms that underly the development (and prevention) of this high-responder phenotype remain unclear, that they were not influenced by histories of responding maintained by drugs from other pharmacologic classes suggests that the transition from well regulated and aberrant drug-taking depends on interactions between behavioral/reinforcement and pharmacological histories. Moreover, given that our findings were generated using outbred Sprague-Dawley rats, it is possible that this high-responder phenotype could reflect a genetic predisposition for the development of high levels of drug-taking. Further studies that explore individual differences will be important to better understand interactions among the genetic, behavioral, and pharmacological determinants of vulnerability of individuals to develop a substance use disorder.
Acknowledgments
The authors would also like to thank Rachel DeSantis, Melson Mesmin, Karen Jimenez, and Juan Morales for their technical assistance in the completion of these studies.
Abbreviations
- α-PVP
α-pyrrolidinopentiophenone
- FR
fixed ratio
- DAT
dopamine transporter
- MDPV
3,4-methylenedioxypyrovalerone
- PR
progressive ratio
- SERT
serotonin transporter
- TO
timeout
Authorship Contributions
Participated in research design: Doyle and Collins.
Conducted experiments: Doyle.
Contributed new reagents or analytical tools: Sulima and Rice.
Performed data analysis: Doyle.
Wrote or contributed to the writing of the manuscript: Doyle and Collins.
Footnotes
This work was supported by the National Institutes of Health National Institute of Drug Abuse [R01 DA039146; R36 DA050955], the jointly sponsored National Institutes of Health Predoctoral Training Program in the Neurosciences [T32 NS082145], and the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism.
No author has an actual or perceived conflict of interest with the contents of this article.
Part of this work was presented as a poster or oral presentation at the following conferences: Doyle MR, DeSantis RE, Sulima A, Rice KC, Collins GT (2019) Influence of drug histories on the development of high levels of MDPV self-administration. College on Problems of Drug Dependence Annual Meeting; San Antonio, TX. Doyle MR, DeSantis RE, Sulima A, Rice KC, Collins GT (2019) Effects of response-contingent and noncontingent drug history on the development of high levels of MDPV self-administration. Experiment Biology/ASPET Annual Meeting; Orlando, FL. Doyle MR, DeSantis RE, Sulima A, Rice KC, Collins GT (2019) Individual differences in high levels of MDPV self-administration: interactions with nicotine. Behavior, Biology and Chemistry: Translational Research in Addiction Annual Meeting; San Antonio, TX.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA (2013) The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA (2015) Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 232:1867–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300. [DOI] [PubMed] [Google Scholar]
- Allain F, Bouayad-Gervais K, Samaha AN (2018) High and escalating levels of cocaine intake are dissociable from subsequent incentive motivation for the drug in rats. Psychopharmacology (Berl) 235:317–328. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG (2008) Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 32:20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhti-Suroosh A, Towers EB, Lynch WJ (2021) A buprenorphine-validated rat model of opioid use disorder optimized to study sex differences in vulnerability to relapse. Psychopharmacology (Berl) 238:1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK (2014) Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp Clin Psychopharmacol 22:9–22. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR (2013) Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology 38:2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canchy L, Girardeau P, Durand A, Vouillac-Mendoza C, Ahmed SH(2021) Pharmacokinetics trumps pharmacodynamics during cocaine choice: a reconciliation with the dopamine hypothesis of addiction. Neuropsychopharmacology 46:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S (2014) Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 48:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Narasimhan D, Cunningham AR, Zaks ME, Nichols J, Ko MC, Sunahara RK, Woods JH (2012) Long-lasting effects of a PEGylated mutant cocaine esterase (CocE) on the reinforcing and discriminative stimulus effects of cocaine in rats. Neuropsychopharmacology 37:1092–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Abbott M, Galindo K, Rush EL, Rice KC, France CP (2016) Discriminative stimulus effects of binary drug mixtures: studies with cocaine, MDPV, and caffeine. J Pharmacol Exp Ther 359:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Sulima A, Rice KC, France CP (2019) Self-administration of the synthetic cathinones 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinopentiophenone (α-PVP) in rhesus monkeys. Psychopharmacology (Berl) 236:3677–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH (2007) Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J Pharmacol Exp Ther 323:599–605. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Avena NM, Boggiano MM (2011) Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav 104:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura FB, Sherwood A, Prisinzano TE, Paronis CA, Bergman J, Kohut SJ (2021) Reinforcing effects of synthetic cathinones in rhesus monkeys: Dose-response and behavioral economic analyses. Pharmacol Biochem Behav 202:173112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV(2004) Evidence for addiction-like behavior in the rat. Science 305:1014–1017. [DOI] [PubMed] [Google Scholar]
- Doyle MR, Sulima A, Rice KC, Collins GT (2021a) Interactions between reinforcement history and drug-primed reinstatement: Studies with MDPV and mixtures of MDPV and caffeine. Addict Biol 26:e12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MR, Sulima A, Rice KC, Collins GT (2021b) MDPV self-administration in female rats: influence of reinforcement history. Psychopharmacology (Berl) 238:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE (2016) Stereoselective effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone in mice: Drug discrimination, locomotor activity, and thermoregulation. J Pharmacol Exp Ther 356:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Baumann MH, Walther D, Jimenez-Morigosa C, Sulima A, Rice KC, Collins GT (2018a) The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 43:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, Collins GT (2018b) Relative reinforcing effects of second-generation synthetic cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology 134 (Pt A):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT (2017) Individual differences in the relative reinforcing effects of 3, 4-methylenedioxypyrovalerone under fixed and progressive ratio schedules of reinforcement in rats. J Pharmacol Exp Ther 361:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Rice KC, Murnane KS (2021) MDPV “high-responder” rats also self-administer more oxycodone than their “low-responder” counterparts under a fixed ratio schedule of reinforcement. Psychopharmacology (Berl) 238:1183–1192. [DOI] [PubMed] [Google Scholar]
- Garcia AF, Webb IG, Yager LM, Seo MB, Ferguson SM (2020) Intermittent but not continuous access to cocaine produces individual variability in addiction susceptibility in rats. Psychopharmacology (Berl) 237:2929–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ (2013) Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol 24:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambuchen MD, Hendrickson HP, Gunnell MG, McClenahan SJ, Ewing LE, Gibson DM, Berquist MD, Owens SM (2017) The pharmacokinetics of racemic MDPV and its (R) and (S) enantiomers in female and male rats. Drug Alcohol Depend 179:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, Freeman KB (2017) Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology (Berl) 234:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi-Paydar M, Harvey EL, Grant Y, Vandewater SA, Creehan KM, Nguyen JD, Dickerson TJ, Taffe MA (2018) Binge-like acquisition of α-pyrrolidinopentiophenone (α-PVP) self-administration in female rats. Psychopharmacology (Berl) 235:2447–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Bowen LD, Narasimhan D, Berlin AA, Lukacs NW, Sunahara RK, Cooper ZD, Woods JH (2007) Cocaine esterase: interactions with cocaine and immune responses in mice. J Pharmacol Exp Ther 320:926–933. [DOI] [PubMed] [Google Scholar]
- Lenz J, Brown J, Flagg S, Oh R, Batts K, Ditzler T, Johnson J (2013) Cristalius: a case in designer drugs. Mil Med 178:e893–e895. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Patrick KS (2008) Differential pharmacokinetics and pharmacodynamics of methylphenidate enantiomers: does chirality matter? J Clin Psychopharmacol 28(3, Suppl 2)S54–S61. [DOI] [PubMed] [Google Scholar]
- Matthews JC, Collins A (1983) Interactions of cocaine and cocaine congeners with sodium channels. Biochem Pharmacol 32:455–460. [DOI] [PubMed] [Google Scholar]
- McConnell SA, Brandner AJ, Blank BA, Kearns DN, Koob GF, Vendruscolo LF, Tunstall BJ (2021) Demand for fentanyl becomes inelastic following extended access to fentanyl vapor self-administration. Neuropharmacology 182:108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mets BWinger GCabrera CSeo SJamdar SYang GZhao KBriscoe RJAlmonte RWoods JH, et al. (1998) A catalytic antibody against cocaine prevents cocaine’s reinforcing and toxic effects in rats. Proc Natl Acad Sci USA 95:10176–10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskiewicz DE, Resendiz-Gutierrez F, Issa O, Hall FS (2020) Synthetic psychoactive cathinones: hypothermia and reduced lethality compared to methamphetamine and methylenedioxymethamphetamine. Pharmacol Biochem Behav 191:172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the care and use of laboratory animals, Ed. 8th. National Academy Press, Washington, DC. [Google Scholar]
- Seaman RW, Doyle MR, Sulima A, Rice KC, Collins GT (2021) Discriminative stimulus effects of 3,4-methylenedioxypyrovalerone (MDPV) and structurally related synthetic cathinones. Behav Pharmacol DOI: 10.1097/FBP.0000000000000624 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson GWGiuvelis DCormier JCone KAtherton PKrivitsky RWarner ESt Laurent BDutra JBidlack JM, et al. (2020) Behavioral pharmacology of the mixed-action delta-selective opioid receptor agonist BBI-11008: studies on acute, inflammatory and neuropathic pain, respiration, and drug self-administration. Psychopharmacology (Berl) 237:1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica MV, Felthous AR (2013) Acute psychosis induced by bath salts: A case report with clinical and forensic implications. J Forensic Sci 58:530–533. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB (2009) Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci 29:1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ (2004) Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305:1017–1019. [DOI] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF (2015) Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol 19:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J (2011) Mephedrone: use, subjective effects and health risks. Addiction 106:1991–1996. [DOI] [PubMed] [Google Scholar]
- Wise RA, Kiyatkin EA(2011) Differentiating the rapid actions of cocaine. Nat Rev Neurosci 12:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DCS (2012) The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37:1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]