Abstract
Regulators of G protein signaling (RGS) proteins modulate signaling by G protein–coupled receptors. Using a knock-in transgenic mouse model with a mutation in Gαo that does not bind RGS proteins (RGS-insensitive), we determined the effect of RGS proteins on presynaptic μ opioid receptor (MOR)-mediated inhibition of GABA release in the ventrolateral periaqueductal gray (vlPAG). The MOR agonists [d-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) and met-enkephalin (ME) inhibited evoked inhibitory postsynaptic currents (eIPSCs) in the RGS-insensitive mice compared with wild-type (WT) littermates, respectively. Fentanyl inhibited eIPSCs similarly in both WT and RGS-insensitive mice. There were no differences in opioid agonist inhibition of spontaneous GABA release between the genotypes. To further probe the mechanism underlying these differences between opioid inhibition of evoked and spontaneous GABA release, specific myristoylated Gα peptide inhibitors for Gαo1 and Gαi1-3 that block receptor–G protein interactions were used to test the preference of agonists for MOR-Gα complexes. The Gαo1 inhibitor reduced DAMGO inhibition of eIPSCs, but Gαi1-3 inhibitors had no effect. Both Gαo1 and Gαi1-3 inhibitors separately reduced fentanyl inhibition of eIPSCs but had no effects on ME inhibition. Gαi1-3 inhibitors blocked the inhibitory effects of ME and fentanyl on miniature postsynaptic current (mIPSC) frequency, but both Gαo1 and Gαi1-3 inhibitors were needed to block the effects of DAMGO. Finally, baclofen-mediated inhibition of GABA release is unaffected in the RGS-insensitive mice and in the presence of Gαo1 and Gαi1-3 inhibitor peptides, suggesting that GABAB receptor coupling to G proteins in vlPAG presynaptic terminals is different than MOR coupling.
SIGNIFICANCE STATEMENT
Presynaptic μ opioid receptors (MORs) in the ventrolateral periaqueductal gray are critical for opioid analgesia and are negatively regulated by RGS proteins. These data in RGS-insensitive mice provide evidence that MOR agonists differ in preference for Gαo versus Gαi and regulation by RGS proteins in presynaptic terminals, providing a mechanism for functional selectivity between agonists. The results further define important differences in MOR and GABAB receptor coupling to G proteins that could be exploited for new pain therapies.
Introduction
Regulators of G protein signaling (RGS) proteins accelerate the hydrolysis of GTP to GDP, terminating G protein signaling. These proteins comprise a large family of proteins that differ in structure and function and are expressed in various tissues with overlapping distributions (Traynor and Neubig, 2005). RGS proteins bind to active G proteins to regulate both temporal and spatial signaling to downstream effectors (Hollinger and Hepler, 2002; Neubig, 2015). In addition, RGS proteins recognize specific Gα proteins (Masuho et al., 2020), highlighting the importance of understanding RGS-Gα interactions within discrete neural circuits. Specific RGS protein knockout mouse models have been generated to probe regulation of G protein–coupled receptor (GPCR) signaling by RGS proteins, but there is evidence of strong compensation by redundant RGS proteins in various knockout lines (Grillet et al., 2005). To circumvent this issue, we use a mutant mouse line that has a knock-in mutation in the Gαo subunit (G184S) that does not bind to any RGS proteins (RGS-insensitive) (Goldenstein et al., 2009).
μ Opioid receptors (MORs) are GPCRs that typically couple to inhibitory G proteins, including Gαo and Gαi subunits (Gaibelet et al., 1999). However, opioid analgesia is dependent on MOR coupling to Gαo but not Gαi (Lamberts et al., 2011, 2013). In addition, different MOR agonists preferentially bind MORs coupled to specific G protein subunits (Massotte et al., 2002; Clark et al., 2006). This differential coupling constitutes one determinant of functional selectivity of opioid agonists, and differential activation of G proteins by MOR agonists could have important impacts in understanding opioid-mediated behaviors. The ventrolateral periaqueductal gray (vlPAG) is a supraspinal site important for opioid-induced analgesia. MORs expressed postsynaptically on a subpopulation of vlPAG neurons are coupled to GIRK channels that hyperpolarize the cells (Chieng and Christie, 1994; Ingram et al., 2007, 2008). We observed in our prior studies that MOR coupling to GIRK channels was reduced in the RGS-insensitive mice, indicating that RGS proteins support signaling to some effectors (McPherson et al., 2018) in addition to their well-known negative regulation via GTPase accelerating activity (Clark et al., 2003, 2008; Lamberts et al., 2013). High-efficacy synthetic agonists [d-Ala(2), N-Me-Phe(4),Gly(5)-ol]-enkephalin (DAMGO) and fentanyl were less effective in the RGS-insensitive mice, but the GIRK currents induced by the peptide agonist met-enkephalin (ME) were unaffected (McPherson et al., 2018). These effects were further confirmed using selective peptide inhibitors of Gαo and Gαi subunits showing that ME-induced GIRK currents could be inhibited only with the Gαi peptide inhibitor. Taken together, these results support the idea that different opioid agonists recruit or prefer receptors bound to specific G proteins, similar to observations in cell lines (Milligan et al., 1990a; Moon, et al., 2001; Clark and Traynor, 2006). However, the loss of MOR coupling to GIRK channels in the RGS-insensitive mice does not explain the enhanced analgesia observed in these mice (Lamberts et al., 2013) so we have continued to examine presynaptic MOR signaling in the vlPAG.
MORs expressed on presynaptic terminals are coupled to phospholipase A2 resulting in inhibition of neurotransmitter release (Vaughan et al., 1997; Ingram et al., 1998). RGS proteins negatively regulate presynaptic MORs that inhibit GABA release (Lamberts et al., 2013), but the G proteins that are involved in presynaptic MOR signaling have not been identified previously. In these studies, we have examined several MOR agonists for their ability to activate MOR signaling via Gαo or Gαi, using the RGS-insensitive mice to further define MOR signaling in GABAergic terminals within the vlPAG. Based on observations that RGS-insensitive mice display enhanced antinociception, we hypothesized that MOR inhibition of presynaptic GABA release is enhanced in these mice. Furthermore, we expected to find differences between agonists in the presence of the selective Gαo and Gαi peptide inhibitors.
Materials and Methods
These studies used male and female heterozygous (Het) (RGS-insensitive Het) mice for a mutation in the Gαo protein (G184S) that is insensitive to RGS protein binding (Goldenstein et al., 2009) and wild-type (WT) 129S1/SvImJ littermates. Homozygous knock-in mice die in utero, so WT mice were compared with Het mice. WT mice were used in the studies assessing the effect of G protein peptide inhibitors. Mice were group-housed with unlimited access to food and water. Lights were maintained on a 12-hour light/dark cycle (lights on at 7:00 AM). Mice were sacrificed, and cellular recordings were conducted during the light phase of this cycle. The Institutional Animal Care and Use Committee at Oregon Health & Science University approved all experimental procedures. Experiments were conducted in accordance with the United States National Research Council Guide for the Care and Use of Laboratory Animals.
Electrophysiological Recordings
Mice (postnatal day >25) were anesthetized with isoflurane, brains were removed, and brain slices containing the vlPAG were cut with a vibratome (180–220 µm thick) in sucrose cutting buffer containing the following (in mM): 75 NaCl, 2.5 KCl, 0.1 CaCl2, 6 MgSO4, 1.2 NaH2PO4, 25 NaHCO3, 2.5 dextrose, and 50 sucrose. They were then placed in a holding chamber with artificial cerebral spinal fluid containing the following (in mM): 126 NaCl, 21.4 NaHCO3, 11.1 dextrose, 2.5 KCl, 2.6 CaCl2, 1.2 MgCl2, and 1.2 NaH2PO4, pH 7.35, equilibrated with 95% O2/5% CO2 until moved into a recording chamber. In experiments using myristoylated Gαo and Gαi peptide inhibitors, slices were incubated for at least 30 minutes in artificial cerebral spinal fluid plus inhibitors (1–10 µM) before recording. Recordings were made with electrodes pulled to 2–4 MOhm resistance with an internal solution consisting of the following (in mM): 140 CsCl, 10 HEPES, 10 KCl, 1 MgCl2, 1 EGTA, 0.3 CaCl2, 4 MgATP, and 3 NaGTP, pH 7.4. Neurons were voltage-clamped at −70 mV. Junction potentials of 5 mV were corrected at the beginning of the experiments. Access resistance was monitored throughout the experiments. Data were collected with Axopatch 200B microelectrode amplifier (Molecular Devices) at 5 kHz and low-pass filtered at 2 kHz. Currents were digitized with InstruTECH ITC-18 (HEKA), collected via AxoGraph data acquisition software, and analyzed using AxoGraph (Axograph Scientific). The Het mice tend to be smaller, so experimenters were not blind to genotype; however, data analysis was done blind to genotype. In experiments using Gαo and Gαi inhibitor peptides, all mice were WT, but the analyses of peak drug effects were measured blind to slice treatment.
Reagents
DAMGO, ME acetate salt hydrate, and fentanyl citrate salt (fentanyl) were obtained from Sigma-Aldrich, and (R,S)-baclofen and CGP 55845 hydrochloride were purchased from Abcam. Myristoylated Gα peptide inhibitors were synthesized by GenScript (Piscataway, NJ) as follows: Gαo1 (MGIANNLRGCGLY), Gαi1/2 (MGIKNNLKDCGLP), and Gαi3 (MGIKNNLKECGLT) according to sequences for mini-gene vectors designed by the Hamm laboratory (Vanderbilt University Medical Center, Nashville, TN) (Gilchrist et al., 2002). We were unable to obtain the peptide for Gαo2 at sufficient purity (<60%) to use in slice experiments. The Gαi inhibitors were combined as a cocktail.
Statistical Analyses
All data are expressed as mean and S.D. Data were analyzed with Prism 9 (GraphPad Software). Each electrophysiological recording from a single neuron is treated as an individual observation because the vlPAG contains heterogenous cell populations; however, all datasets contain recordings from at least three separate animals. Drug effects were reversed by specific antagonists, and peak drug effects were measured as an increase in current from the average of baseline and washout or the presence of antagonists. Differences between groups were assessed using Student’s t test or ANOVA when appropriate (significance is denoted as *p < 0.05, **p < 0.01, ****p < 0.0001).
Results
Opioid Inhibition of Evoked GABA Release
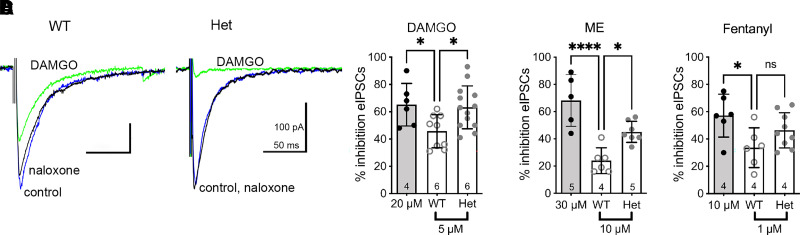
To test the hypothesis that RGS proteins affect opioid signaling in presynaptic terminals, we compared the ability of several opioid agonists to inhibit evoked GABAergic inhibitory postsynaptic currents (eIPSCs) in vlPAG neurons of WT and RGS-insensitive Het mice. The studies used concentrations of opioid agonists that were consistent with our previous study examining opioid activation of GIRK channels in the vlPAG (McPherson et al., 2018). For comparison, we also tested maximal concentrations of each agonist so we could assess the efficacy of each agonist at inhibiting presynaptic GABA release (Fig. 1). The maximal % inhibition was the same for all three opioid drugs. Het mice had similar effects to WT mice at the maximal concentrations (mean ± S.D.; DAMGO: 66 ± 15%, n = 6; ME: -68 ± 19%, n = 5; fentanyl: 57 ± 16%, n = 4). Interestingly, differences between WT and RGS-insensitive Het mice appeared at submaximal concentrations of these MOR agonists. The selective MOR agonist DAMGO (5 µM) inhibited eIPSCs 37% more in neurons from the RGS-insensitive Het mice (95% CI = 54–73; Fig. 1, A and B). The nonselective agonist ME (10 µM) inhibited the eIPSCs 88% more in cells from the RGS-insensitive Het mice (95% CI = 38–52; Fig. 1C). These results are consistent with our prior report showing an increase in morphine and ME inhibition of eIPSCs in RGS-insensitive Het mice (Lamberts et al., 2013). In contrast, fentanyl (1 µM) inhibited GABAergic eIPSCs similarly in neurons from both WT and RGS-insensitive Het mice (Fig. 1D).
Fig. 1.
Opioid agonist inhibition of evoked IPSCs is differentially affected in RGS-insensitive mice. (A) Representative traces depicting inhibition of eIPSCs by DAMGO (5 µM) in WT and RGS-insensitive (Het) mice. The inhibition is reversed by naloxone. (B) Combined experiments of % inhibition (±S.D.) by a maximal DAMGO concentration (20 µM; gray bar) and a submaximal concentration (5 µM) in WT compared with Het mice (one-way ANOVA; F(2, 25) = 4.9, P = 0.02; Dunnett’s, *P < 0.05). (C) Combined experiments of % inhibition (±S.D.) by a maximal ME concentration (30 µM; gray bar) and a submaximal concentration (10 µM) in WT compared with Het mice (one-way ANOVA; F(2, 15)= 17.7, P = 0.0001; Dunnett’s, *P < 0.05; ****P < 0.0001). (D) Combined experiments of % inhibition (±S.D.) by a maximal fentanyl concentration (10 µM; gray bar) and a submaximal concentration (1 µM) in WT compared with Het mice (one-way ANOVA; F(2, 18)= 4.1, P = 0.03; Dunnett’s, *P < 0.05). Symbols in bars denote recordings, and numbers denote number of animals used in each group.
In a subset of experiments, a paired pulse protocol (2 stimuli, 50–100 milliseconds apart) was used to examine the probability of GABA release from presynaptic terminals in WT and RGS-insensitive Het mice. Paired-pulse ratios (PP ratio = P2/P1) for eIPSCs in slices from Het mice (0.6 ± 0.2; n = 8) were lower than those in WT mice (1.1 ± 0.1; n = 12; t(18) = 2.2, *P = 0.04). A lower PP ratio indicates a higher release probability in the RGS-insensitive Het mice. This change in release probability could be due to changes in endogenous opioid tone, so we tested whether tone could be measured in slices from either the WT or RGS-insensitive Het mice using naloxone (5–10 µM). Spontaneous IPSCs (in the absence of tetrodotoxin) were similar in the absence and presence of naloxone for both genotypes (% change ± S.D. in naloxone for WT: 103 ± 20%; one-sample t test, t5 = 0.4, P = 0.7, and Het: 95 ± 19%; one-sample t test, t6 = 0.8, P = 0.5), indicating a lack of endogenous opioid tone in either genotype.
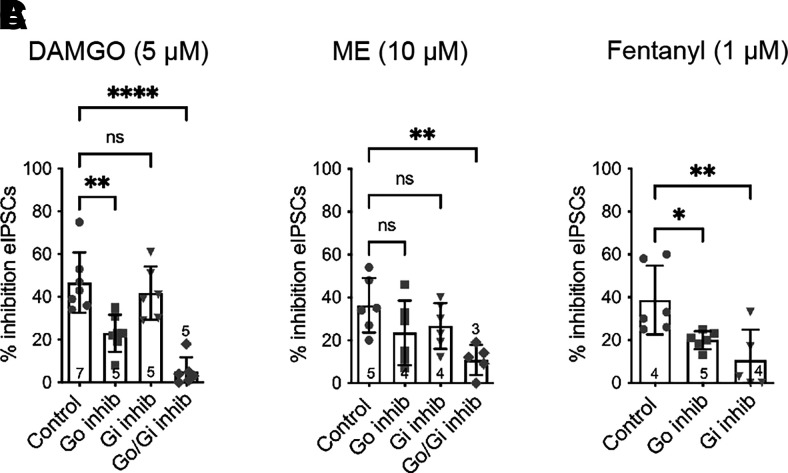
To determine whether the difference between the agonists in the RGS-insensitive mice was due to a preference for Gαo versus Gαi subunits coupling to MORs in presynaptic terminals, selective peptide inhibitors of each subunit binding sites were tested. Inhibitor peptides corresponding to the carboxy terminal amino acids of the Gα subunit compete for binding to the receptor, inhibiting activation of the G proteins (Gilchrist et al., 2002). The effect of DAMGO was reduced in the presence of the Gαo inhibitor, but the Gαi inhibitor had no effect (Fig. 2A). Adding all of the peptide inhibitors together essentially abolished DAMGO-mediated inhibition of eIPSCs. Neither the Gαo nor Gαi peptide inhibitors significantly reduced ME inhibition (Fig. 2B); however, the combined inhibitors also significantly reduced ME inhibition of eIPSCs. Finally, both Gαo and Gαi inhibitor peptides superfused alone reduced fentanyl inhibition (Fig. 2C). These data suggest that DAMGO preferentially activates MOR-Gαo in presynaptic terminals in the vlPAG, but ME and fentanyl are less selective.
Fig. 2.
MOR agonists differentially activate Gα subunits to inhibit evoked GABA release. (A) DAMGO (5 µM)-mediated inhibition of eIPSCs in the absence (control) and presence of Gαo peptide inhibitor and Gαi peptide inhibitors (one-way ANOVA, F(3, 22) = 19.1, P = 0.0001; Dunnett’s, **P < 0.001, ****P < 0.0001). (B) ME (10 µM)-mediated inhibition of eIPSCs in absence and presence of inhibitors (one-way ANOVA, F(3, 19) = 4.2; P = 0.02; Dunnett’s, **P < 0.001). (C) Fentanyl (1 µM)-mediated inhibition of eIPSCs in absence and presence of inhibitors (one-way ANOVA, F(2, 14) = 7.3, P = 0.007, Dunnett’s, *P < 0.05, ** P < 0.001). Symbols in bars denote recordings, and numbers denote number of animals used in each bar. inhib, inhibitor; ns, not significant.
Male and female mice were used throughout the studies. DAMGO recordings from WT mice had approximately equal numbers of recordings from male and female mice with similar inhibition in both sexes [males: 49 ± 10% (S.D.); n = 8 vs. females: 41 ± 10% (S.D.), n = 7; t(13) = 1.4, P = 0.19]. In addition, no noted differences were observed with the other agonists. The lack of sex differences is consistent with our prior study (McPherson et al., 2018).
Opioid Inhibition of Spontaneous GABA Release
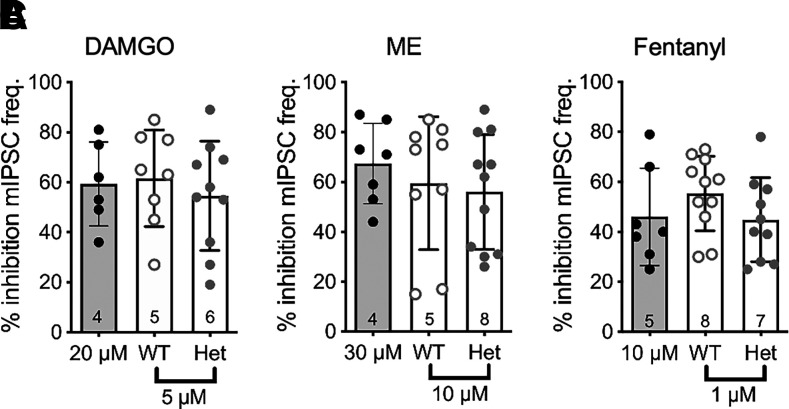
The change in PP ratio indicated that the RGS-insensitive Het mice have altered evoked GABA release, so we were interested in whether spontaneous release (in the presence of tetrodotoxin ) was altered in the knock-in mouse line. Interevent intervals of mIPSCs measured in the presence of tetrodotoxin (500 nM) were similar in WT [0.33 ± 0.22 seconds (S.D.), n = 27] and Het mice [0.24 ± 0.15 seconds (S.D.), n = 27; t(52) = 1.6, P = 0.1].
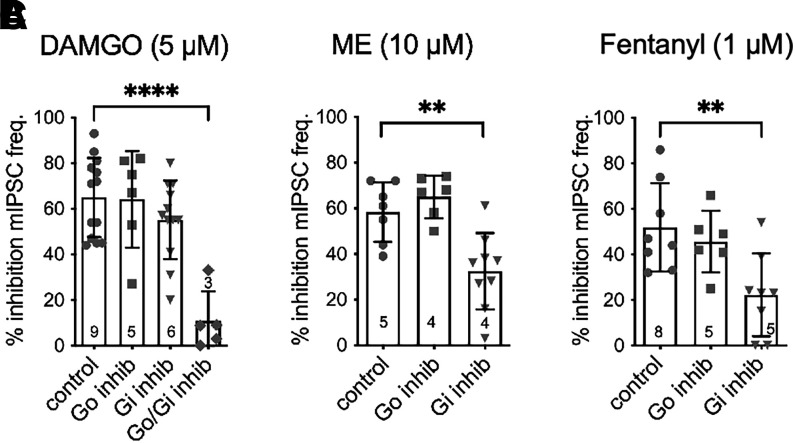
All three opioid agonists inhibited spontaneous mIPSC frequency to similar degrees in both the WT and Het mice (Fig. 3). The mIPSC amplitude as well as rise and decay kinetics (unpublished data) were also not different between agonists, indicating that the opioid modulation of mIPSC frequency was due to presynaptic modulation of release. There were no sex differences in the amount of inhibition induced by any of the three opioid agonists (unpublished data). These results suggest that either RGS proteins have little impact on opioid modulation of spontaneous release in presynaptic terminals or that inhibition of spontaneous release is not dependent on Gαo since the RGS-insensitive knock-in mutation is specific for Gαo. To test whether MOR-Gαi coupling is involved in opioid inhibition of spontaneous GABA release in WT mice, we examined the effects of the specific Gαo and Gαi peptide inhibitors. Neither of the inhibitors superfused alone reduced inhibition of mIPSC frequency by DAMGO (Fig. 4A), but the inhibitors applied to slices together reduced DAMGO-mediated inhibition by 83% (95% CI = −5 to 27) compared with control. In contrast, inhibition by ME and fentanyl was reduced in the presence of the Gαi inhibitors but unaffected in the presence of the Gαo inhibitor peptide (Fig. 4, B and C). These results are consistent with findings in the RGS-insensitive Het mice that have a mutation specifically in Gαo that perturbs RGS protein binding and subsequent GTP hydrolysis and indicate that MOR-Gαi coupling is important for the inhibition of spontaneous GABA release.
Fig. 3.
Opioid inhibition of GABAergic mIPSCs is not altered in RGS-insensitive mice. (A) Combined experiments of % inhibition (±S.D.) by a maximal DAMGO concentration (20 µM; gray bar) and a submaximal concentration (5 µM) in WT compared with Het mice (one-way ANOVA; F(2, 21) = 0.3, P = 0.8). (B) Combined experiments of % inhibition (±S.D.) by a maximal ME concentration (ME 30 µM; gray bar) and a submaximal concentration (10 µM) in WT compared with Het mice (one-way ANOVA, F(2, 24) = 0.5, P = 0.6). (C) Combined experiments of % inhibition (±S.D.) by a maximal fentanyl concentration (10 µM; gray bar) and a submaximal concentration (1 µM) in WT compared with Het mice (one-way ANOVA; F(2, 25)= 1.2, P = 0.3). Symbols in bars denote recordings, and numbers denote number of animals used in each bar. freq., frequency.
Fig. 4.
MOR-Gαi coupling is more important for inhibition of spontaneous GABA release. (A) Inhibition of mIPSCs by DAMGO (5 µM) is unaffected by Gαo and Gαi inhibitors alone (one-way ANOVA; F(3, 32) = 12.6, P = 0.0001; Dunnett’s, ****P = 0.0001). (B) Inhibition by ME is reduced in the presence of Gαi inhibitors but not by the Gαo inhibitor (F(2, 19) = 11.8, P = 0.001, Dunnett’s, **P < 0.01). (C) Inhibition by fentanyl is reduced in the presence of Gαi inhibitors but not by the Gαo inhibitor (F(2, 19) = 6.2, P = 0.01, Dunnett’s, **P < 0.01). Symbols in bars denote recordings, and numbers denote number of animals used in each bar. inhib, inhibitor.
GABAB-Mediated Inhibition of GABA Release Is Unaffected by Gαo or GαI Peptide Inhibitors
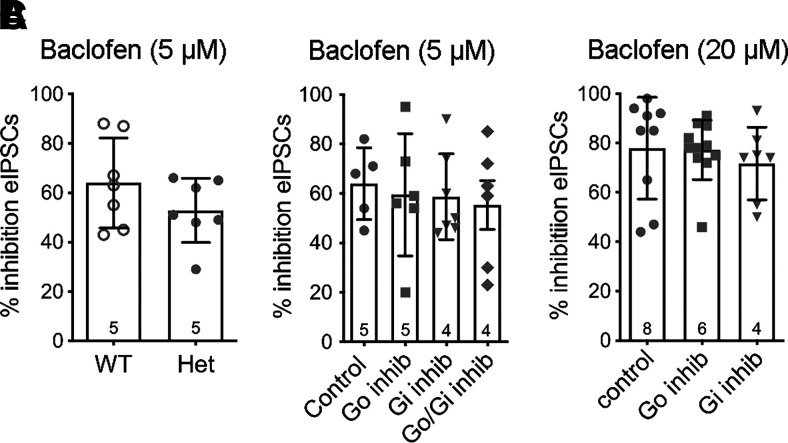
Our previous study found no difference in the amount of inhibition of evoked GABA release induced by a maximal concentration of the GABAB agonist baclofen (20 µM) between slices from WT and RGS-insensitive Het mice (McPherson et al., 2018). Because RGS proteins have less influence on high-efficacy agonists, especially at maximal concentrations (Clark et al., 2008), we repeated the studies using a lower concentration of baclofen (5 µM). This concentration of baclofen also inhibited evoked GABA release to a similar extent in slices from the two genotypes (Fig. 5A). Consistent with these results, incubation of slices in the Gαo and Gαi inhibitors did not alter the responses to either concentration of baclofen (5 µM or 20 µM; Fig. 5, B and C, respectively). Baclofen was typically tested on the same cells before or after an opioid response that was affected by either the Gαo or Gαi inhibitor, indicating that these peptide inhibitors were effective in blocking binding of the Gα subunits in a given experiment and providing positive controls.
Fig. 5.
Baclofen-mediated inhibition of evoked GABA release is not affected in slices from RGS-insensitive mice or by Gαo/i peptide inhibitors. (A) Baclofen (5 µM) inhibition is similar in WT and RGS-insensitive (Het) mice (t(12) = 1.3, P = 0.2). (B) Baclofen (5 µM)-mediated inhibition is not altered in the presence of peptide inhibitors (F(3, 20) = 0.2, P = 0.9). (C) Baclofen (20 µM)-mediated inhibition is not altered by the peptide inhibitors (F(2, 24) = 0.3, P = 0.7). Symbols in bars denote recordings, and numbers denote number of animals used in each bar. inhib, inhibitor.
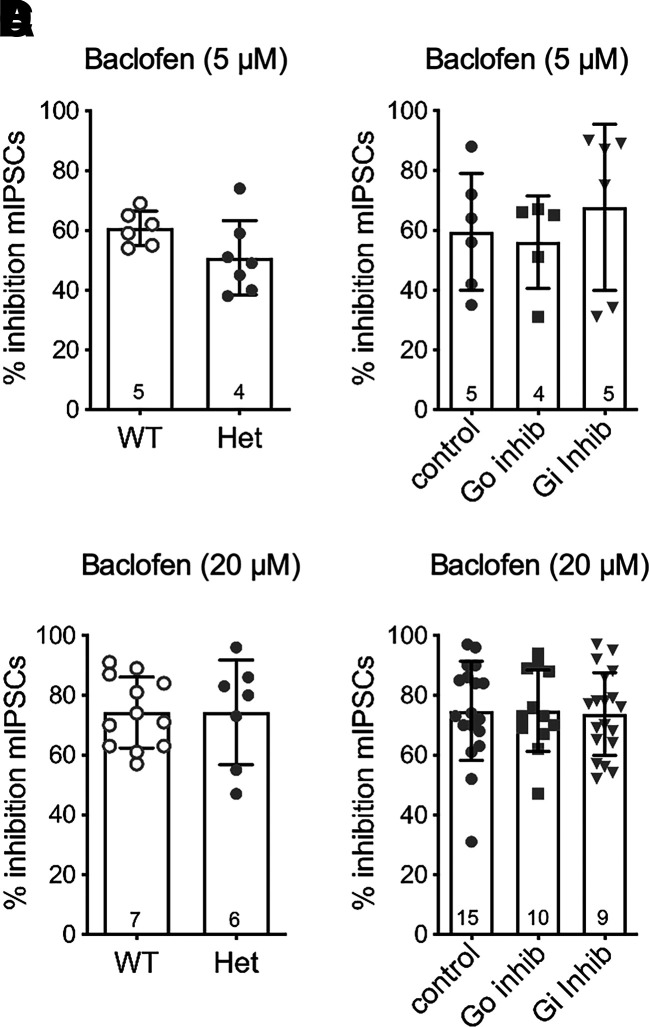
Both concentrations of baclofen were also tested for inhibition of spontaneous release of GABA (Fig. 6). The data show that baclofen inhibition of mIPSC frequency is similar in both the WT and RGS-insensitive Het mice, and the inhibition is unaffected by the Gαo and Gαi peptide inhibitors. Similar results were obtained at both 5 and 20 μM concentrations of baclofen.
Fig. 6.
Baclofen-mediated inhibition of spontaneous GABA release in slices is not affected in RGS-insensitive mice or in the presence of Gαo/i peptide inhibitors. (A) Baclofen (5 µM) inhibition is similar in WT and RGS-insensitive (Het) mice (t(11) = 1.8, P = 0.1). (B) Baclofen (5 µM)-mediated inhibition is not altered in the peptide inhibitors (F(2, 14) = 0.4, P = 0.7). (C) Baclofen (20 µM) inhibition is similar in WT and RGS-insensitive (Het) mice (t(17) = 0.005, P = 1.0). (D) Baclofen (20 µM)-mediated inhibition is not altered in the peptide inhibitors (F(2, 46) = 0.03, P = 1.0). Symbols in bars denote recordings, and numbers denote number of animals used in each bar. inhib, inhibitor.
Discussion
These studies used a transgenic knock-in mutant mouse model with a mutation in Gαo (G148S) that blocks RGS protein binding (Goldenstein et al., 2009). The advantage of this model is that it is unbiased with regard to RGS protein subtypes because compensatory expression of RGS proteins can obscure RGS regulation in knockout mice (Grillet et al., 2005). Opioid analgesia is reduced in Gαo knockout mice (Lamberts et al., 2011) providing evidence that MOR couples to Gαo in analgesia pathways. Consistent with the knockout data, RGS-insensitive Het mice display enhanced supraspinal morphine analgesia (Lamberts et al., 2013). Since MOR inhibition of GABA release in the vlPAG is important for opioid analgesia (Moreau and Fields, 1986; Vaughan and Christie, 1997; Budai and Fields, 1998; Bobeck et al., 2014), we expected that inhibition of GABA release by opioid agonists would be increased in the RGS-insensitive Het mice. Importantly, we observed differences between MOR agonists in the RGS-insensitive Het mice suggesting that RGS regulation plays a role in functional selectivity of MOR agonists. In addition, MORs activate different Gα subunits to inhibit evoked and spontaneous GABA release. Finally, GABAB-mediated inhibition of GABA release is not altered in the Het mice and is unaffected by peptide inhibitors of either Gαo or Gαi subunits.
We first examined opioid inhibition of evoked GABA release in the vlPAG. DAMGO and ME but not fentanyl inhibited GABA release more in the RGS-insensitive Het mice. Since submaximal concentrations were used for each of the agonists, the lack of increase with fentanyl in the recordings from RGS-insensitive Het mice was not attributed to a ceiling effect. These data are consistent with recent data showing that inhibition of RGS4 in the PAG enhanced morphine but not fentanyl antinociception (Morgan et al., 2020). There is evidence that RGS protein GTPase-accelerating activity is more evident with low- compared with high-efficacy MOR agonists (Clark et al., 2008); however, the maximal inhibition by all agonists was comparable. Thus, the differences between agonists in inhibiting GABA release in the two genotypes are likely due to a different mechanism, such as the ability of fentanyl-bound MORs to couple to Gαi.
MORs activate pertussis-toxin (PTX)-sensitive Gαo and Gαi subunits (Williams, et al., 2013). Analgesia induced by morphine (Parenti et al., 1986; Lutfy et al., 1991; Shah et al., 1994) and DAMGO (Sanchez-Blazquez and Garzon, 1988) is reduced in the presence of PTX. To probe the signaling of specific G proteins in vlPAG presynaptic terminals further, we used myristoylated peptide inhibitors of Gαo and Gαi subunits. Incubation of slices with the Gαo peptide inhibitor reduced the inhibition by DAMGO and fentanyl but not ME. Gαo inhibition of Ca2+ channels is more potent than Gαi (Hescheler et al., 1987), and there are differences in coupling between Gα subunits and effectors (McKenzie and Milligan, 1990; Milligan et al., 1990a,b; Moon et al., 2001). Thus, it is reasonable that inhibition by DAMGO and fentanyl was reduced by the Gαo peptide inhibitor since evoked release is dependent on voltage-gated Ca2+ channels (Hubbard et al., 1968). Incubation of slices with Gαi peptide inhibitors reduced fentanyl but not DAMGO or ME inhibition of eIPSCs. These results indicate that coupling to Gαi subunits is equally effective at inhibiting eIPSCs in the vlPAG, and that DAMGO and fentanyl form different MOR complexes in presynaptic terminals. Combining the peptide inhibitors reduced the effects of both DAMGO and ME compared with incubating slices in either inhibitor alone. Together with the knowledge that only small differences exist in the potency of DAMGO to stimulate different Gαi versus Gαo subtypes (Clark et al., 2006), these results indicate there is redundancy of Gi/o proteins for activation by MOR. It is interesting to note that the endogenous peptide ME is less sensitive to both peptide inhibitors given alone compared with DAMGO and fentanyl, suggesting that ME-bound MORs couple equally well to Gαo1 and Gαi1-3 subunits. The data highlight the importance of G protein subunit expression and levels as a factor in MOR coupling to effectors (Connor and Christie, 1999).
A surprising finding in these studies was the difference in G protein subunits involved in MOR inhibition of spontaneous GABA release. Inhibition by all three opioid agonists was similar in both the WT and RGS-insensitive Het mice, and the Gαo peptide inhibitor did not affect opioid inhibition of mIPSC frequency. Instead, the Gαi1-3 peptide inhibitors applied alone decreased inhibition by ME and fentanyl without affecting DAMGO-mediated inhibition. However, DAMGO inhibition was reduced in the presence of all inhibitors. This pattern supports the results with DAMGO on evoked release and further suggests that DAMGO preferentially couples to MOR-Gαo subunits (Laugwitz et al., 1993; Chakrabarti et al., 1995; Clark et al., 2006). The data presented here indicate that opioid inhibition of spontaneous release is mediated by Gαi subunits, explaining why opioid inhibition of spontaneous GABA release was unaffected in the RGS-insensitive Het mice. Thus, these studies are not able to determine whether RGS proteins regulate MOR inhibition of spontaneous release. The molecular mechanisms involved in MOR regulation of spontaneous release are not completely understood, but there are data to support direct G protein βγ subunit regulation of release machinery (Zurawski et al., 2016, 2019).
GABAB receptors also readily inhibit evoked and spontaneous GABA release in the vlPAG (Vaughan et al., 1997). In the RGS-insensitive Het mice, baclofen inhibited both evoked and spontaneous GABA release similarly to WT mice. Since the Gαo and Gαi peptide inhibitors were ineffective at blocking baclofen inhibition even when applied together, we are not able to make a statement regarding the ability of RGS proteins to modulate GABAB signaling. The results are interesting considering data that GABAB coupling to voltage-gated Ca2+ channels is abolished by PTX (Connor and Christie, 1998). However, GABAB-Gi protein coupling has different structural features compared with other GPCR classes. Agonists at this receptor do not induce outward movement of transmembrane domain 6 to provide a cavity for the binding of the C terminus of the G proteins (Shen et al., 2021). Consequently, the peptide inhibitors used in this study designed to mimic the Gα-C-terminal interaction with the receptor core may not bind to the GABAB receptor to block G protein binding. Alternatively, Gαz is a G protein with 60% sequence homology to the Gi family (Tsu et al., 1997), is densely expressed in the vlPAG, and couples to MOR (Garzon et al., 1998, 2005; Gaspari, et al., 2018). The Gαz residues that bind to MOR have not been identified, so it is possible the peptide inhibitors would not block Gαz coupling to MOR or GABAB receptors, especially given substitution of tyrosine in the place of the PTX-sensitive cysteine in the Gαz C terminus.
The descending pain modulatory pathway is sexually dimorphic (Loyd et al., 2006, 2008, 2014), and MOR agonists are more efficacious in males than females (Fullerton et al., 2018). There were no sex differences in either genotype in opioid agonist inhibition of evoked and spontaneous GABA release. Thus, sex differences in opioid signaling are not explained by RGS-mediated regulation of signaling, at least via Gαo subunits. This is consistent with the lack of sex differences in MOR coupling to GIRK channels in the WT and RGS-insensitive Het mice (McPherson,et al., 2018).
Our results showing enhanced MOR inhibition of presynaptic GABA release by several opioid agonists in the RGS-insensitive mice in addition to morphine, which we examined in our previous paper (Lamberts et al., 2013), provide a mechanism for the increase in opioid antinociception on the supraspinal hot-plate test observed in RGS-insensitive mice (Lamberts et al., 2013). There is substantial evidence that opioid inhibition of GABA release in the vlPAG activates descending pain modulatory circuits that produce analgesia (Cheng et al., 1986; Moreau and Fields, 1986). We previously reported that postsynaptic MOR coupling to GIRK channels is reduced in RGS-insensitive mice (McPherson et al., 2018), possibly through loss of a scaffolding function of RGS proteins (Zhong et al., 2003). Although it is tempting to argue that postsynaptic MORs in the vlPAG do not play a role in opioid-induced antinociception, an equally valid interpretation is that MOR coupling to GIRK channels opposes supraspinal antinociceptive circuits, and removal of this MOR signaling supports opioid analgesia in the RGS-insensitive Het mice (Lamberts et al., 2013). Indeed, blocking both GIRK channels and presynaptic MOR signaling decreases morphine antinociception (Morgan et al., 2020). Inhibition of RGS4 in the vlPAG enhances opioid-induced antinociception, suggesting that RGS4 may play an important role in regulating presynaptic MOR signaling through Gαo. However, RGS gene expression in the PAG includes RGS4, RGS7, RGS8, RGS10, RGS17, and RGS20 (https://alleninstitute.org/legal/citation-policy/ Allen Brain Atlas), and these RGS proteins bind preferentially to different G proteins (Masuho et al., 2020). Thus, additional RGS proteins may also regulate opioid analgesia through MOR coupling in the PAG.
Acknowledgments
The authors thank Katherine Suchland, Samantha Rios, and Drs. Laura Kozell and Janet Lowe for technical support.
Abbreviations
- CI
confidence interval
- DAMGO
[d-Ala2, N-MePhe4, Gly-ol]-enkephalin
- eIPSC
evoked inhibitory postsynaptic current
- GIRK
G protein–coupled inwardly rectifying potassium channel
- GPCR
G protein–coupled receptor
- ME
met-enkephalin
- MOR
μ opioid receptor
- Het
heterozygous
- PAG
periaqueductal gray
- PP
paired-pulse
- RGS
regulators of G protein signaling
- vlPAG
ventrolateral PAG
- WT
wild type
Authorship Contributions
Participated in research design: Traynor, Ingram.
Conducted experiments: Bouchet, McPherson, Li, Ingram.
Contributed new reagents or analytic tools: Traynor.
Performed data analysis: Bouchet, McPherson, Ingram.
Wrote or contributed to the writing of the manuscript: Bouchet, McPherson, Traynor, Ingram.
Footnotes
This work was supported by National Institutes of Health National Institute on Drug Abuse [Grant R01DA035316 JRT and F31 DA052114 CAB].
The authors have no conflicts of interest.
References
- Bobeck EN, Chen Q, Morgan MM, Ingram SL (2014) Contribution of adenylyl cyclase modulation of pre- and postsynaptic GABA neurotransmission to morphine antinociception and tolerance. Neuropsychopharmacology 39:2142–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budai D, Fields HL (1998) Endogenous opioid peptides acting at mu-opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons. J Neurophysiol 79:677–687. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Prather PL, Yu L, Law PY, Loh HH (1995) Expression of the mu-opioid receptor in CHO cells: ability of mu-opioid ligands to promote alpha-azidoanilido[32P]GTP labeling of multiple G protein alpha subunits. J Neurochem 64:2534–2543. [DOI] [PubMed] [Google Scholar]
- Cheng ZF, Fields HL, Heinricher MM (1986) Morphine microinjected into the periaqueductal gray has differential effects on 3 classes of medullary neurons. Brain Res 375:57–65. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ (1994) Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br J Pharmacol 113:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MJ, Furman CA, Gilson TD, Traynor JR (2006) Comparison of the relative efficacy and potency of mu-opioid agonists to activate Galpha(i/o) proteins containing a pertussis toxin-insensitive mutation. J Pharmacol Exp Ther 317:858–864. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Harrison C, Zhong H, Neubig RR, Traynor JR (2003) Endogenous RGS protein action modulates mu-opioid signaling through Galphao. Effects on adenylyl cyclase, extracellular signal-regulated kinases, and intracellular calcium pathways. J Biol Chem 278:9418–9425. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Linderman JJ, Traynor JR (2008) Endogenous regulators of G protein signaling differentially modulate full and partial mu-opioid agonists at adenylyl cyclase as predicted by a collision coupling model. Mol Pharmacol 73:1538–1548. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Traynor JR (2006) Mediation of adenylyl cyclase sensitization by PTX-insensitive GalphaoA, Galphai1, Galphai2 or Galphai3. J Neurochem 99:1494–1504. [DOI] [PubMed] [Google Scholar]
- Connor M, Christie MD (1999) Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol 26:493–499. [DOI] [PubMed] [Google Scholar]
- Connor M, Christie MJ (1998) Modulation of Ca2+ channel currents of acutely dissociated rat periaqueductal grey neurons. J Physiol 509:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton EF, Doyle HH, Murphy AZ (2018) Impact of sex on pain and opioid analgesia: a review. Curr Opin Behav Sci 23:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaibelet G, Meilhoc E, Riond J, Saves I, Exner T, Liaubet L, Nürnberg B, Masson JM, Emorine LJ (1999) Nonselective coupling of the human mu-opioid receptor to multiple inhibitory G-protein isoforms. Eur J Biochem 261:517–523. [DOI] [PubMed] [Google Scholar]
- Garzón J, Castro M, Sánchez-Blázquez P (1998) Influence of Gz and Gi2 transducer proteins in the affinity of opioid agonists to mu receptors. Eur J Neurosci 10:2557–2564. [DOI] [PubMed] [Google Scholar]
- Garzón J, Rodríguez-Muñoz M, López-Fando A, Sánchez-Blázquez P (2005) The RGSZ2 protein exists in a complex with mu-opioid receptors and regulates the desensitizing capacity of Gz proteins. Neuropsychopharmacology 30:1632–1648. [DOI] [PubMed] [Google Scholar]
- Gaspari S, Purushothaman I, Cogliani V, Sakloth F, Neve RL, Howland D, Ring RH, Ross EM, Shen L, Zachariou V (2018) Suppression of RGSz1 function optimizes the actions of opioid analgesics by mechanisms that involve the Wnt/β-catenin pathway. Proc Natl Acad Sci USA 115:E2085–E2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A, Li A, Hamm HE (2002) G alpha COOH-terminal minigene vectors dissect heterotrimeric G protein signaling. Sci STKE 2002:pl1. [DOI] [PubMed] [Google Scholar]
- Goldenstein BLNelson BWXu KLuger EJPribula JAWald JMO’Shea LAWeinshenker DCharbeneau RAHuang X, et al. (2009) Regulator of G protein signaling protein suppression of Galphao protein-mediated alpha2A adrenergic receptor inhibition of mouse hippocampal CA3 epileptiform activity. Mol Pharmacol 75:1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet N, Pattyn A, Contet C, Kieffer BL, Goridis C, Brunet JF (2005) Generation and characterization of Rgs4 mutant mice. Mol Cell Biol 25:4221–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J, Rosenthal W, Trautwein W, Schultz G (1987) The GTP-binding protein, Go, regulates neuronal calcium channels. Nature 325:445–447. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR (2002) Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev 54:527–559. [DOI] [PubMed] [Google Scholar]
- Hubbard JI, Jones SF, Landau EM (1968) On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol 196:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, Morgan MM (2007) Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology 32:600–606. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Macey TA, Fossum EN, Morgan MM (2008) Tolerance to repeated morphine administration is associated with increased potency of opioid agonists. Neuropsychopharmacology 33:2494–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ (1998) Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci 18:10269–10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts JT, Jutkiewicz EM, Mortensen RM, Traynor JR (2011) μ-Opioid receptor coupling to Gα(o) plays an important role in opioid antinociception. Neuropsychopharmacology 36:2041–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts JT, Smith CE, Li MH, Ingram SL, Neubig RR, Traynor JR (2013) Differential control of opioid antinociception to thermal stimuli in a knock-in mouse expressing regulator of G-protein signaling-insensitive Gαo protein. J Neurosci 33:4369–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugwitz KL, Offermanns S, Spicher K, Schultz G (1993) mu and delta opioid receptors differentially couple to G protein subtypes in membranes of human neuroblastoma SH-SY5Y cells. Neuron 10:233–242. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Morgan MM, Murphy AZ (2008) Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci 27:1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ (2006) Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol 496:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ (2014) The neuroanatomy of sexual dimorphism in opioid analgesia. Exp Neurol 259:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Chang SC, Candido J, Jang Y, Sierra V, Yoburn BC (1991) Modification of morphine-induced analgesia and toxicity by pertussis toxin. Brain Res 544:191–195. [DOI] [PubMed] [Google Scholar]
- Massotte D, Brillet K, Kieffer B, Milligan G (2002) Agonists activate Gi1 alpha or Gi2 alpha fused to the human mu opioid receptor differently. J Neurochem 81:1372–1382. [DOI] [PubMed] [Google Scholar]
- Masuho I, Balaji S, Muntean BS, Skamangas NK, Chavali S, Tesmer JJG, Babu MM, Martemyanov KA (2020) A global map of G protein signaling regulation by RGS proteins. Cell 183:503–521.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie FR, Milligan G (1990) Delta-opioid-receptor-mediated inhibition of adenylate cyclase is transduced specifically by the guanine-nucleotide-binding protein Gi2. Biochem J 267:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson KB, Leff ER, Li MH, Meurice C, Tai S, Traynor JR, Ingram SL (2018) Regulators of G-protein signaling (RGS) proteins promote receptor coupling to G-protein-coupledinwardly rectifying potassium (GIRK) channels. J Neurosci 38:8737–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, McKenzie FR, McClue SJ, Mitchell FM, Mullaney I (1990a) Guanine nucleotide binding proteins in neuroblastoma x glioma hybrid, NG108-15, cells. Regulation of expression and function. Int J Biochem 22:701–707. [DOI] [PubMed] [Google Scholar]
- Milligan G, Mitchell FM, Mullaney I, McClue SJ, McKenzie FR (1990b) The role and specificity of guanine nucleotide binding proteins in receptor-effector coupling. Symp Soc Exp Biol 44:157–172. [PubMed] [Google Scholar]
- Moon HE, Cavalli A, Bahia DS, Hoffmann M, Massotte D, Milligan G (2001) The human delta opioid receptor activates G(i1)alpha more efficiently than G(o1)alpha. J Neurochem 76:1805–1813. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Fields HL (1986) Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res 397:37–46. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Tran A, Wescom RL, Bobeck EN (2020) Differences in antinociceptive signalling mechanisms following morphine and fentanyl microinjections into the rat periaqueductal gray. Eur J Pain 24:617–624. [DOI] [PubMed] [Google Scholar]
- Neubig RR (2015) RGS-insensitive G proteins as in vivo probes of RGS function. Prog Mol Biol Transl Sci 133:13–30. [DOI] [PubMed] [Google Scholar]
- Parenti M, Tirone F, Giagnoni G, Pecora N, Parolaro D (1986) Pertussis toxin inhibits the antinociceptive action of morphine in the rat. Eur J Pharmacol 124:357–359. [DOI] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Garzón J (1988) Pertussis toxin differentially reduces the efficacy of opioids to produce supraspinal analgesia in the mouse. Eur J Pharmacol 152:357–361. [DOI] [PubMed] [Google Scholar]
- Shah S, Duttaroy A, Davis T, Yoburn BC (1994) Spinal and supraspinal effects of pertussis toxin on opioid analgesia. Pharmacol Biochem Behav 49:773–776. [DOI] [PubMed] [Google Scholar]
- Shen CMao CXu CJin NZhang HShen D-DShen QWang XHou TChen Z, et al. (2021) Structural basis of GABAB receptor-gi protein coupling. Nature 594:594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor JR, Neubig RR (2005) Regulators of G protein signaling & drugs of abuse. Mol Interv 5:30–41. [DOI] [PubMed] [Google Scholar]
- Tsu RC, Ho MK, Yung LY, Joshi S, Wong YH (1997) Role of amino- and carboxyl-terminal regions of G(alphaZ) in the recognition of Gi-coupled receptors. Mol Pharmacol 52:38–45. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ (1997) Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol 498:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ (1997) How opioids inhibit GABA-mediated neurotransmission. Nature 390:611–614. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65:223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Wade SM, Woolf PJ, Linderman JJ, Traynor JR, Neubig RR (2003) A spatial focusing model for G protein signals. Regulator of G protein signaling (RGS) protien-mediated kinetic scaffolding. J Biol Chem 278:7278–7284. [DOI] [PubMed] [Google Scholar]
- Zurawski Z, Rodriguez S, Hyde K, Alford S, Hamm HE (2016) Gβγ binds to the extreme C terminus of SNAP25 to mediate the action of Gi/o-coupled G protein-coupled receptors. Mol Pharmacol 89:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski ZThompson Gray ADBrady LJPage BChurch EHarris NADohn MRYim YYHyde KMortlock DP, et al. (2019) Disabling the Gβγ-SNARE interaction disrupts GPCR-mediated presynaptic inhibition, leading to physiological and behavioral phenotypes. Sci Signal 12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]