Abstract
Objectives
Asthma is a chronic airway disorder associated with variable airflow limitations, which are triggered by different stimuli. The reversibility of airflow limitations reflects patients’ responses to the therapy with bronchodilators and improvements in airflow. This study aims to determine the treatment outcomes (improvements in forced expiratory volume in the first second (FEV1) and the number of asthma exacerbations) associated with the presence of airflow reversibility.
Methods
This retrospective cohort study included 154 adults (>18 years) who were diagnosed with asthma and had pulmonary function testing (PFT) at a tertiary care centre in KSA between January 1st, 2014 and May 31st, 2019. Smokers and patients with comorbidities or medications that could affect PFT were excluded from the analysis. Patients were classified as having a reversible airflow limitation when they exhibited a post-bronchodilator FEV1 increase of 12% and 200 mL. Exacerbations were defined as the need to use oral corticosteroids. Chi-square tests were used for comparative analyses.
Results
From our cohort, 42 patients exhibited reversibility. In contrast, 112 patients did not show any sign of reversibility. Asthmatics with baseline reversible airflow limitations experienced significant worsening of FEV1 during the follow-up period compared with those with no reversibility, showing a mean difference of 19.96 mL (p = 0.0206). There was no significant association between asthma reversibility and exacerbations (p = 0.23).
Conclusion
In our study, during the follow-up of patients with asthma, we found that the reversibility of airflow was associated with significantly worse FEV1, although this did not have a significant effect on exacerbations. Therefore, we recommend regular spirometry follow-ups, particularly for patients with significant airway reversibility.
Keywords: Asthma, Exacerbations, Forced expiratory volume 1, Pulmonary function test, Reversibility
الملخص
أهداف البحث
الربو هو اضطراب مزمن في مجرى الهواء يرتبط بحدود تدفق الهواء المتغيرة التي تسببها محفزات مختلفة. تعكس إمكانية عكس قيود تدفق الهواء استجابة المريض للعلاج بموسعات الشعب الهوائية والتحسن في تدفق الهواء. تهدف هذه الدراسة إلى تحديد نتائج العلاج (التحسينات في حجم الزفير القسري في الثانية الأولى وعدد حالات تفاقم الربو) المرتبطة بوجود انعكاس تدفق الهواء.
طرق البحث
تضمنت هذه الدراسة بأثر رجعي ١٥٤ بالغا (أكبر من ١٨ عاما) مصابين بالربو وخضعوا لاختبارات وظائف الرئة في مركز رعاية من الدرجة الثالثة في المملكة العربية السعودية بين ١ يناير ٢٠١٤ و٣١ مايو ٢٠١٩. تم استبعاد المدخنين والمرضى الذين يعانون من أمراض مصاحبة أو من يستعمل أدوية قد تؤثر على اختبارات وظائف الرئة. تم تصنيف المرضى على أنهم يعانون من قيود عكسية في تدفق الهواء عندما أظهروا زيادة حجم الزفير القسري في الثانية الأولى بعد توسيع القصبات بنسبة ١٢٪ و٢٠٠ مل. كما تم تعريف التفاقم على أنه الحاجة إلى استخدام الستيرويدات القشرية عن طريق الفم.
النتائج
أظهر ٤٢ مريضا قابلية للعكس. في المقابل، لم يظهر ١١٢ مريضا أي علامة على الانعكاسية. عانى مرضى الربو الذين يعانون من قيود تدفق الهواء القابلة للانعكاس من تدهور كبير في حجم الزفير القسري في الثانية الأولى أثناء المتابعة، مقارنة مع أولئك الذين ليس لديهم قابلية للانعكاسية، وبلغ متوسط الفرق ١٩.٩٦ مل. ولم يكن هناك ارتباط اعتباري بين الانعكاسية وقابلية حدوث تفاقم للحالة.
استنتاجات
في دراستنا، وأثناء متابعة مرضى الربو، وجدنا أن انعكاس تدفق الهواء كان مرتبطا بتدهور كبير في حجم الزفير القسري في الثانية الأولى، ولكن بدون تأثير كبير على التفاقم، نوصي بمتابعة قياس التنفس بانتظام، خاصة للمرضى الذين يعانون من انعكاسية مجرى الهواء.
الكلمات المفتاحية: الربو, التفاقم, حجم الزفير القسري في الثانية الأولى, اختبار وظائف الرئة, انعكاسية
Introduction
The World Health Organization (WHO) estimates that 235 million people are currently diagnosed with asthma, and it is considered the most common non-communicable disease among children.1 Additionally, asthma is a public health problem in all countries regardless of their level of development.1 For example, according to the Center for Disease Control and Prevention, asthma affects 25.7 million people in the United States alone.2 Similarly, KSA's Ministry of Health reports that the prevalence of asthma among the Saudi population (both adults and children) is approximately 15–25%.3 However, a national study among adults in KSA found that the prevalence of diagnosed asthma is 4.05% (95% confidence interval: 3.5–4.6%), which is relatively low.4
Asthma is a chronic respiratory disorder characterized by recurrent episodes of wheezing, shortness of breath, chest tightness, and/or coughing. These episodes vary in duration and intensity and are accompanied by limitations in expiratory airflow.5,6 Although these symptoms are typically associated with asthma, they are not considered definitive indications.5, 6, 7 Therefore, to achieve a definitive diagnosis of asthma, a patient's history should be used in conjunction with accurate diagnostic values such as peak expiratory flow, flow–volume relationships, and bronchodilator responses.5, 6, 7 To this end, spirometry is useful for measuring the reversibility of airflow limitations, which reflects the patient's response to bronchodilator treatments and improvements in airflow.7,8
For centuries, clinicians have attempted to classify asthma into different phenotypes, as some phenotypes exhibit a better response to treatment than others.9 In addition to allergic asthma, aspirin-exacerbated asthma, nonallergic asthma, infection-related asthma, and childhood pre-asthma phenotypes, one review article categorized other phenotypes as ‘biomarker-based’, ‘symptom-based’, and ‘trigger-induced’.9 Variability in airflow limitations after bronchodilator use may be useful in identifying a phenotype of asthma with a particular response to certain medications. However, few studies have investigated the association between airflow limitations and treatment responses. In the present study, we aimed to compare improvements in forced expiratory volume in the first second (FEV1) post treatments, as well as the number of asthma exacerbations between patients with and without reversible airflow limitations.
Materials and Methods
Population
A total of 154 adults diagnosed with asthma were included in this study. All patients diagnosed with asthma who underwent pulmonary function testing (PFT) at King Abdulaziz Medical City (Jeddah) between January 1st, 2014, and May 31st, 2019, were evaluated. Subjects were included in the study if they were at least 18 years old, had been diagnosed with asthma, underwent PFT during the study period, followed up for at least 6 months, and underwent another PFT at least 12 months after the initial PFT. Patients were diagnosed with asthma when they had typical clinical features that improved with bronchodilator treatment and/or inhaled steroid treatment. We categorized the sample of patients into two groups based on reversibility. Patients were classified as having reversible airflow limitation when they exhibited an FEV1 increase of 12% and 200 mL, 10–15 minutes after using a bronchodilator (200–400 μg Salbutamol).10 Patients who did not meet these criteria were not considered to have reversible airflow limitations. Moreover, baseline and follow-up PFTs were performed at least 12 months apart to accurately assess any changes in the variables.
Patients who were smokers, had been diagnosed with lung diseases that affect respiratory function, used medications that trigger bronchoconstriction (e.g. beta-blockers or angiotensin-converting enzyme inhibitors), had chest wall deformities, had neuromuscular disorders affecting the chest wall, had pulmonary congestion, or had significant anaemia (Hb < 10 mg/dL) were excluded from the sample. Patients with cardiovascular diseases such as aortic stenosis were also excluded, as instances of shortness of breath could affect the patient's performance in the pulmonary function test. Furthermore, heart failure imposes a significant burden on the lungs, which may manifest as restrictive patterns on PFT.
Study design and evaluation
This was a retrospective cohort study wherein participants were included using a consecutive sampling technique. Collected variables included demographic data such as age, gender, and body mass index (BMI). The following values were collected pre -and post-bronchodilator treatment at both the baseline and follow-up time points (with an interval of at least 12 months between the two): FEV1, forced vital capacity (FVC), FEV1/FVC ratio, percentage change in FEV1, and percentage change in FVC. The bronchodilator salbutamol (200 mg) was used in accordance with standard procedures. Other data collected included the presence of cardinal symptoms of asthma (coughing, wheezing, and dyspnoea), the frequency of exacerbations per year (defined based on the use of oral or inhaled corticosteroids due to worsening symptoms), and the frequency of visits to the emergency department due to asthma or asthmatic symptoms.
Statistical analysis
All analyses were performed using Version 23 (IBM Corp., Armonk, NY) of the Statistical Package for the Social Sciences (SPSS). For patient characteristics, continuous variables were reported as proportions and means. The outcomes were compared between patients with and without reversible airflow limitations using chi-square test, Student's t-test, and logistic regression analysis. Statistical significance was determined using a 95% confidence interval and a P-value of 0.05.
Results
Among the 154 patients in the sample, 42 exhibited reversibility, while 112 did not. The mean ages of patients with and without reversibility were 48.57 and 52.2 years old, respectively. Other baseline demographic and PFT characteristics are shown in Table 1, Table 2. The symptoms that were presented in asthmatic people with and without reversibility are illustrated in Table 3.
Table 1.
Baseline demographic characteristics for patients in the sample.
| Characteristics | Reversibility (n = 42) | No Reversibility (n = 112) |
|---|---|---|
| Mean age (years) | 48.57 [43.86–53.28] | 52.27 [49.33–55.2] |
| Male (%) | 12 (28.6%) [15.7%–44.6%] | 14 (12.5%) [7%–20%] |
| Diabetes Mellitus | 16 (38.1%) | 38 (33.9%) |
| Hypertension | 16 (38.1%) | 34 (30.4) |
| Renal Disease | 2 (4.8%) | 7 (6.25%) |
Table 2.
Pulmonary Function Test results for patients in the sample.
| Parameters | Reversibility |
No Reversibility |
||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| FEV 1 (L) | 1.78 [1.59–1.97] | 2.13 [1.91–2.34] | 1.90 [1.77–2.03] | 1.96 [1.83–2.09] |
| FEV 1 (%) Predicted | 62.9% [58.5–67.3] | 75.7% [71–80.4] | 75.2% [71.2–78.7] | 76.7% [73–80.4] |
| FVC (L) | 2.54 [2.29–2.79] | 2.79 [2.54–3.04] | 2.38 [2.24–2.53] | 2.40 [2.25–2.54] |
| FVC (%) Predicted | 72.48 [41–95] | 80.21 [59–99] | 76.73 [39–104] | 76.86 [40–104] |
| FEV1/FVC (%) | 0.70 [0.5–0.86] | 0.75 [0.54–0.91] | 0.79 [0.52–0.99] | 0.81 [0.47–1.30] |
FEV: Forced Expiratory volume. FVC: Forced Vital Capacity L: in Litre %: in Percentage.
Table 3.
Symptoms of asthmatics with and without reversibility.
| Symptoms | Reversibility (n = 42) | No Reversibility (n = 112) |
|---|---|---|
| Cough | 24 (57.1%) [0.42–0.73] | 66 (58.9%) [0.54–0.73] |
| Shortness of breath | 23 (54.8%) [0.39–0.71] | 54 (48.2%) [0.42–0.62] |
| Wheeze | 21 (50%) [0.35–0.67] | 37 (33%) [0.26–0.45] |
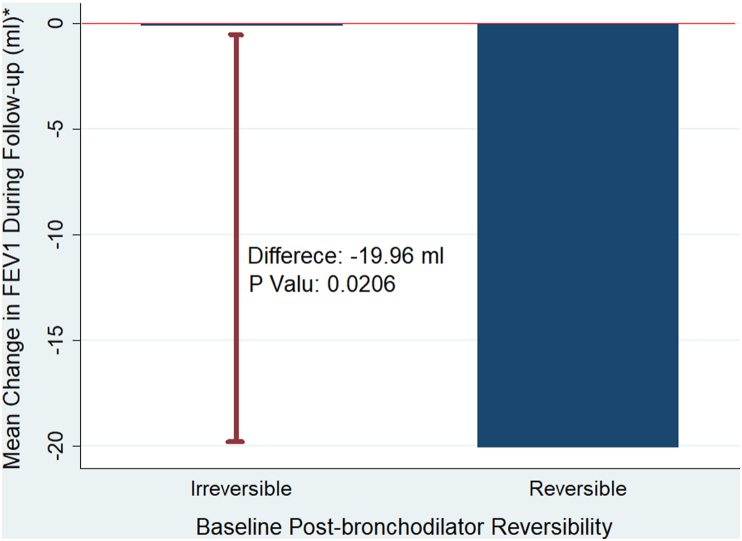
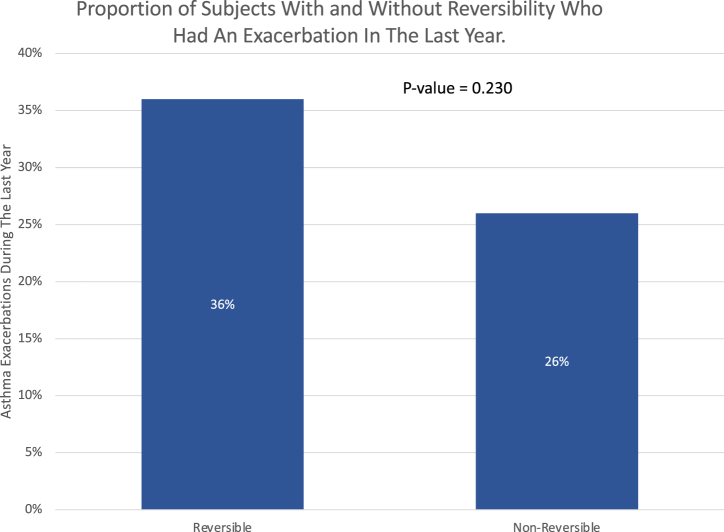
Patients with reversibility had significantly worse FEV1 than those without reversibility (20 ± 17.69 mL versus 0.11 ± 8.13 mL, p = 0.02) during the follow-up period (Figure 1). Additionally, patients with reversible airflow limitations experienced more frequent exacerbations than patients without reversibility (74% versus 36%). P = 0.23); however, the difference did not reach the threshold of statistical significance (Figure 2).
Figure 1.
This is a graphic illustration of the mean change in actual FEV1 in mL between patients with reversibility and those without. The blue column represents the magnitude of change in actual FEV1 among each group, and it shows that patients with irreversibility had almost no change in actual FEV1. The bold red line represents the difference in actual FEV1 between both groups. FEV1: forced expiratory volume in the first second.
Figure 2.
This graph illustrates a comparison of the number of asthma exacerbations over the previous year between asthmatics with and without reversibility.
In regards to the symptoms of asthma and their relation to exacerbations, 31 (37.80%) out of the 90 patients who displayed cough as a symptom had an exacerbation in the last year. In contrast, out of the 52 patients who did not have a cough, 10 (19.23%) had an exacerbation. This association was found to be statistically significant (P-value = 0.023). Additionally, out of the 58 patients who had wheezing, 24 (43.64%) had an exacerbation in the last year. On the other hand, only 17 patients (21.25%) out of the 80 who did not have wheezing experienced an exacerbation in the last year (P-value = 0.005).
Of the 123 patients (79.87%), who were prescribed short-acting beta agonists (SABA), 33 (26.83%) had significant reversibility. Additionally, 107 (69.48%) patients were prescribed long-acting beta agonists (LABA), but only 30 of them (28.04%) had significant reversibility. Only 27 (17.53%) patients were prescribed long-acting muscarinic antagonists (LAMA), 11 (40.74%) of which were asthmatics with significant reversibility; 143 patients (92.86%) were prescribed inhaled corticosteroids (ICS). In accordance with the guidelines issued by the Global Initiative for Asthma (GINA), ICS doses were classified into low, moderate, and high dose categories.6 Of the 143 patients, 43 (30.07%) were on low doses of ICS, only 9 (20.93%) of whom had reversibility. 61 (42.66%) patients were on moderate doses of ICS, 21 (34.43%) of which had reversibility. Finally, 39 patients (27.27%) were prescribed high doses of ICS and only 9 (23.08) of them had significant reversibility.
Discussion
The present study investigated the association between treatment outcomes and airflow reversibility in patients with asthma. Our findings indicate that asthmatic people with reversibility experienced worse lung function during the follow-up period compared to those without reversibility. One potential explanation is that these dynamic changes indicate more severe airway inflammation, which in turn predisposes patients to airway remodelling. In contrast to our findings, another study reported a significant correlation between the reversibility of FEV1 (defined as an increase in FEV1 in the 10 minutes after bronchodilator use) and improvements in FEV1 after a 3-month course of treatment.11 Furthermore, another study found that the mean baseline FEV1 was significantly lower in patients without reversibility than in those with reversibility.12 We compared our results to Graff et al., who defined no reversibility as FEV1/FVC<0.7 and FEV1<80% of predicted value in a study where 196 out of 1138 eligible patients met their irreversibility definition, and found that 72.27% of our patients had no reversibility as opposed to approximately 17% in their study. They reported that those with no reversibility were more often males, therefore, revealed a positive association between gender and reversibility.13
We also observed no difference in the rate of exacerbation between asthmatics with and without reversibility. In contrast, recent evidence suggests that there is a linear relationship between the degree of reversibility and the risk of exacerbations in people that have one or two exacerbations per year.14 Some researchers have hypothesized that patients with exacerbation-prone asthma (EPA), which is defined as having three or more exacerbations per year, have a distinct phenotype compared to other patients with similar FEV1 results. This phenotype may be characterized by increased airway closure due to remodelling via the hypertrophy of smooth muscle and thickening of the basement membrane.14 Furthermore, one study reported that the exacerbation rate was significantly correlated with decreases in FEV1.14,15
In addition, Graff et al. found a significant positive association between exacerbations and asthma without reversibility.13 Moreover, they hypothesized that smoking leads to more exacerbations and frequent hospitalization. This hypothesis is substantiated by data that indicate that higher doses of ICS and the use of oral corticosteroids in addition to sputum neutrophils were not associated with irreversibility and patients who never smoked.13,16 Since smokers were excluded from this study's sample, this may explain why a significant association between lack of reversibility and exacerbations was not found.13 Hence, encouraging patients with asthma to stop smoking is of utmost importance.16
Furthermore, we observed no relationship between reversibility and asthma-related visits to the emergency room (ER). This contrasts with the findings of studies which demonstrated that greater degrees of reversibility are associated with a higher likelihood of asthma-related ER visits and a higher risk of exacerbations in the future.17 Another study assessing dose-responsiveness to bronchodilation among children and adolescents found that those with poor bronchodilator responses had a two-fold increase in ER visits, as well as in the odds of a subsequent ER visit compared to those who had better responsiveness to bronchodilators.18
The presence of comorbidities such as diabetes, hypertension, and renal disease was not significantly associated with asthma exacerbations in our sample. However, contrary findings have been reported by other studies that documented association and pathophysiology of hypertension with asthma exacerbations. It was reported that exacerbations were not only controlled by type-2 inflammation, but also that factors of metabolic derangement and increased comorbidities play a role. Furthermore, the relationship is bidirectional, wherein chronic airway inflammation due to allergen exposure causes an increase in systemic blood pressure measurements.19, 20, 21 Moreover, some studies indicate that diabetes (similar to other metabolic changes) affects individuals with asthma rendering them more prone to exacerbations. This has been attributed to similarities between both conditions, as the pathogenesis involves the release of pro-inflammatory cytokines, which may facilitate airway inflammation and hyperresponsiveness.21,22 For asthmatic patients with renal diseases, our finding aligns with the accumulating body of evidence that suggests no significant relationship between renal diseases and asthma. Nonetheless, some studies have investigated surrogate markers related to renal diseases and their association with exacerbations. Interestingly, higher levels of uric acid, low serum calcium, and low magnesium levels were associated with a higher rate of asthma exacerbation, although no specific underlying pathologies were identified.23,24
The clinical triad of asthma, which includes coughing, wheezing, and dyspnoea, were individually analysed in relation to exacerbations. Our data suggest that a significantly higher proportion of patients who had a cough experienced an exacerbation compared to those who did not report the occurrence of cough. This association was also demonstrated to be statistically significant (P-value = 0.023). However, another study reported that there was no linear relationship between the degree of coughing and the exacerbation rate.25 Similar to coughing, wheezing as a symptom of asthma was found to have a statistically significant relationship with exacerbations in the last year (P-value = 0.005). In contrast, conflicting results were reported by another study, which found that worsening symptoms scores, coughing, and wheezing were not associated with exacerbation frequency.25 Furthermore, none of the symptoms in the clinical triad of asthma had a significant relationship with the presence of reversibility, or lack thereof.
Study subjects were heterogeneous in terms of their medication profile. Our findings indicated that only LAMA were associated with the presence of reversibility or an increased risk of exacerbation (P-value = 0.013). Conversely, a systematic review and meta-analysis, which investigated the addition of LAMA to inhaled corticosteroids maintenance therapy for the management of persistent uncontrolled asthma in patients 12 years and older that were taking inhaled corticosteroids alone, showed an association with lower exacerbation risk and improved spirometry measures.26 Additionally, another study showed that LAMA are preferably included in the treatment regimen of patients with known chronic obstructive pulmonary disease (COPD), as it effectively reduced exacerbations.25
Limitations
This study has some limitations including its small sample size, which is partly due to a lack of follow-up PFT among patients at the centre. Moreover, our study was retrospective and was conducted at a single centre, making it difficult to generalize the results. We recommend prospective multicentre studies with larger sample sizes to investigate the outcome in future studies.
Conclusion
Our findings suggest that asthmatic people with reversibility exhibit greater decreases in FEV1 during a 12-month follow-up period than those without reversibility. However, the presence of reversibility did not influence the risk of exacerbation in our population.
Recommendations
The greater FEV1 decline from the baseline seen in asthmatics with reversibility should be taken into consideration during monitoring and management. We recommend regular spirometry follow-ups to this end. This is particularly relevant for patients with significant airway reversibility. Future prospective multicentred studies with larger sample sizes are recommended to verify our findings.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Ethical approval was obtained from the King Abdullah International Medical Research Center's (KAIMRC) Institutional Review Board (Reference number: RYD-18-417780-164784; Date: 27 September 2018)
Authors’ contributions
ASA Conceptualized and designed the study; and supervised the project. SMA Conceptualized and designed the study; collected, and organized data; wrote the initial manuscript; and administrated the project and assignment of tasks. AKA Collected and organized data; wrote the initial manuscript; and administrated the project and assignment of tasks. RSA Provided statistical analysis and interpretation of the results; and wrote the results section. OMK Participated in data collection; and assisted in writing the methods and discussion sections. AAD Participated in data collection; assisted in writing the discussion section. All authors have critically reviewed and approved the final draft after revisions and are responsible for the content and similarity index of the manuscript.
Acknowledgment
We would like to acknowledge the research unit at King Saud bin Abdulaziz University for Health Sciences College of Medicine (Jeddah (KSAUHS, COM-J)) for their support. We would also like to acknowledge the medical records department at King Abdulaziz Medical City (KAMC) for their cooperation throughout the study period.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.World Health Organization (WHO) [cited 2021 May 30]. Asthma.https://www.who.int/news-room/fact-sheets/detail/asthma [Internet]. Available from: [Google Scholar]
- 2.CDC . Centers for Disease Control and Prevention; 2020. Asthma's effect on the nation.https://www.cdc.gov/asthma/asthmadata.htm [Internet] [cited 2020 Aug 30]. Available from: [Google Scholar]
- 3.- World Asthma Day [Internet]. [cited 2020 Aug 30]. https://www.moh.gov.sa/en/HealthAwareness/healthDay/2018/Pages/HealthDay-2018-05-02.aspx Health Days 2018. Available from: [Google Scholar]
- 4.Moradi-Lakeh M., El Bcheraoui C., Daoud F., Tuffaha M., Kravitz H., Al Saeedi M., et al. Prevalence of asthma in Saudi adults: findings from a national household survey, 2013. BMC Pulm Med. 2015 Dec;15(1):77. doi: 10.1186/s12890-015-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.diagnosis, monitoring and chronic asthma management | Guidance | NICE [Internet]. NICE; 2017. https://www.nice.org.uk/guidance/ng80 Overview | Asthma: [cited 2020 Aug 30]. Available from: [Google Scholar]
- 6.Global strategy for asthma management and prevention. 2020. https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf [Internet]. [cited 2020 Aug 30]. Available from: [Google Scholar]
- 7.Chhabra S.K. Clinical application of spirometry in asthma: why, when and how often? Lung India Off Organ Indian Chest Soc. 2015 Dec;32(6):635–637. doi: 10.4103/0970-2113.168139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quirt J., Hildebrand K.J., Mazza J., Noya F., Kim H. Asthma. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol. 2018;14(Suppl 2):50. doi: 10.1186/s13223-018-0279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hekking P.-P.W., Bel E.H. Developing and emerging clinical asthma phenotypes. J Allergy Clin Immunol Pract. 2014 Nov;2(6):671–680. doi: 10.1016/j.jaip.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Miller M.R. Standardisation of spirometry. Eur Respir J. 2005 Aug 1;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 11.Boskabady M.H., Fasihfar M., Maemoori G.A. Correlation between symptom score, wheeze, reversibility of pulmonary function tests and treatment response in asthma. Iran J Allergy, Asthma Immunol. 2003 Jun;2(2):61–67. [PubMed] [Google Scholar]
- 12.Boulet L.-P., Turcotte H., Turcot O., Chakir J. Airway inflammation in asthma with incomplete reversibility of airflow obstruction. Respir Med. 2003 Jun;97(6):739–744. doi: 10.1053/rmed.2003.1491. [DOI] [PubMed] [Google Scholar]
- 13.Graff S., Bricmont N., Moermans C., Henket M., Paulus V., Guissard F., et al. Clinical and biological factors associated with irreversible airway obstruction in adult asthma. Respir Med. 2020 Dec;175:106202. doi: 10.1016/j.rmed.2020.106202. [DOI] [PubMed] [Google Scholar]
- 14.Denlinger L.C., Phillips B.R., Ramratnam S., Ross K., Bhakta N.R., Cardet J.C., et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017 Feb;195(3):302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsunaga K., Hirano T., Oka A., Tanaka A., Kanai K., Kikuchi T., et al. Progression of irreversible airflow limitation in asthma: correlation with severe exacerbations. J Allergy Clin Immunol Pract. 2015 Sep;3(5):759–764.e1. doi: 10.1016/j.jaip.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Thomson N.C. Asthma and cigarette smoking. Eur Respir J. 2004 Nov 1;24(5):822–833. doi: 10.1183/09031936.04.00039004. [DOI] [PubMed] [Google Scholar]
- 17.Howrylak J.A., Fuhlbrigge A.L., Strunk R.C., Zeiger R.S., Weiss S.T., Raby B.A., et al. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J Allergy Clin Immunol. 2014 May;133(5):1289–1300. doi: 10.1016/j.jaci.2014.02.006. 1300.e1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunwell J.R., Nguyen K.M., Bruce A.C., Fitzpatrick A.M. Bronchodilator dose responsiveness in children and adolescents: clinical features and association with future asthma exacerbations. J Allergy Clin Immunol Pract. 2020 Mar;8(3):953–964. doi: 10.1016/j.jaip.2019.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hozawa S., Maeda S., Kikuchi A., Koinuma M. Exploratory research on asthma exacerbation risk factors using the Japanese claims database and machine learning: a retrospective cohort study. J Asthma Off J Assoc Care Asthma. 2021 Apr 29:1–19. doi: 10.1080/02770903.2021.1923740. [DOI] [PubMed] [Google Scholar]
- 20.Chaddha A., Broytman O., Teodorescu M. Effects of allergic airway inflammation and chronic intermittent hypoxia on systemic blood pressure. Am J Physiol Regul Integr Comp Physiol. 2020 Nov 1;319(5):R566–R574. doi: 10.1152/ajpregu.00325.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters M.C., Mauger D., Ross K.R., Phillips B., Gaston B., Cardet J.C., et al. Evidence for exacerbation-prone asthma and predictive biomarkers of exacerbation frequency. Am J Respir Crit Care Med. 2020 Oct 1;202(7):973–982. doi: 10.1164/rccm.201909-1813OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khateeb J., Fuchs E., Khamaisi M. Diabetes and lung disease: a neglected relationship. Rev Diabet Stud RDS. 2019 Feb 25;15:1–15. doi: 10.1900/RDS.2019.15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammad H.A., Abdulfttah M.T., Abdulazez A.O., Mahmoud A.M., Emam R.M. A study of electrolyte disturbances in patients with chronic stable asthma and with asthma attacks. Egypt J Chest Dis Tuberc. 2014 Jul;63(3):529–534. [Google Scholar]
- 24.Abdulnaby N.K., Sayed A.O., Shalaby N.M. Predictive value of serum uric acid in hospitalized adolescents and adults with acute asthma. Therapeut Clin Risk Manag. 2016;12:1701–1708. doi: 10.2147/TCRM.S116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morjaria J.B., Rigby A.S., Morice A.H. Asthma phenotypes: do cough and wheeze predict exacerbations in persistent asthma? Eur Respir J. 2017 Dec;50(6):1701366. doi: 10.1183/13993003.01366-2017. [DOI] [PubMed] [Google Scholar]
- 26.Sobieraj D.M., Baker W.L., Nguyen E., Weeda E.R., Coleman C.I., White C.M., et al. Association of inhaled corticosteroids and long-acting muscarinic antagonists with asthma Control in patients with uncontrolled, persistent asthma: a systematic review and meta-analysis. J Am Med Assoc. 2018 Apr 10;319(14):1473–1484. doi: 10.1001/jama.2018.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]