Abstract
Objective
The treatment of periodontitis with scaling and root planing has a good prognosis. However, periodontitis may also exacerbate. The mucoadhesive patch is one of the distribution systems of topical drugs, which is not irritable to the mucosa and eventually increases permeability. Based on phytochemical screening, mangosteen peel extract has an active ingredient with high anti-inflammatory and antibacterial properties. This study aims to understand the potential of a mucoadhesive patch loaded with mangosteen peel extract to inhibit alveolar bone damage in periodontitis.
Methods
This experimental laboratory research was conducted using 27 Wistar rats divided into three groups: the positive control group (0.7% tetracycline gel application), negative control group (mucoadhesive patch application), and experimental group (mucoadhesive patch loaded with mangosteen peel extract application). Rats were administered 1 × 109 colony-forming unit as much as 200 μL of Actinobacillus actinomycetemcomitans bacteria three times at 2-day intervals. Tetracycline gel and mucoadhesive patch application were administered for 1 h/day for 3 days. Three rats from each group were sacrificed on days 3, 5, and 7 after the application. The lower jaw was dissected for histopathological examination using haematoxylin and eosin staining to determine the number of osteoclasts and osteoblasts.
Results
The mucoadhesive patches loaded with mangosteen peel extract significantly reduced the number of osteoclasts and increased the number of osteoblasts in all groups (p < 0.05).
Conclusion
The mucoadhesive patches loaded with mangosteen peel extract can prevent alveolar bone damage in periodontitis by inhibiting the number of osteoclasts and increasing the number of osteoblasts.
Keywords: Mangosteen peel extract, Mucoadhesive patch, Osteoblast, Osteoclast, Periodontitis
الملخص
أهداف البحث
علاج التهاب دواعم السن عن طريق التحجيم وتخطيط الجذر يحمل تشخيصا جيدا. ومع ذلك، قد تحدث نوبات التهاب دواعم السن. الرقعة اللاصقة المخاطية هي أحد أنظمة توزيع الأدوية الموضعية التي لا تهيج الغشاء المخاطي وتزيد في النهاية من النفاذية. بناء على الفحص الكيميائي النباتي، يحتوي مستخلص قشر المانجوستين على مكون نشط يحتوي على خصائص عالية مضادة للالتهابات ومضادة للبكتيريا. الغرض من هذه الدراسة هو فهم إمكانات التصحيح المخاطي المحمل بمستخلص قشر المانجوستين لمنع تلف العظام السنخية في التهاب دواعم السن.
طرق البحث
تم إجراء هذا البحث المخبري التجريبي باستخدام ٢٧ فأرا من فئران ويستار مقسمة إلى ثلاث مجموعات: المجموعة الضابطة الإيجابية (٠.٧٪ هلام التتراسيكلين)، المجموعة الضابطة السلبية (اللصقة المخاطية)، والمجموعة التجريبية (رقعة مخاطية محملة بخلاصة قشر مانغوستين). أعطيت الفئران ١ X٩١٠ بقدر ٢٠٠ ميكرولتر من بكتيريا المشعشعة المصاحبة للورم الفطري ثلاث مرات لمدة يومين. أعطيت جل التتراسيكلين والرقعة اللاصقة المخاطية لمدة ١ ساعة/يوم لمدة ثلاثة أيام. قُتلت ثلاثة جرذان من كل مجموعة بشكل إنساني في اليوم الثالث والخامس والسابع بعد وضع الرقعة. تم قطع الفك السفلي من أجل الفحص المرضي للأنسجة باستخدام تلطيخ الهيماتوكسيلين إيوزين لتحديد عدد ناقضات العظم وبانيات العظم.
النتائج
تبين أن البقع اللاصقة المخاطية المحملة بمستخلص قشر المانجوستين تقلل بشكل كبير من عدد ناقضات العظم وتزيد من عدد بانيات العظم في جميع المجموعات.
الاستنتاجات
يمكن للبقع اللاصقة المخاطية المحملة بمستخلص قشر المانجوستين أن تمنع تلف العظام السنخية في التهاب دواعم السن عن طريق تثبيط عدد ناقضات العظم وزيادة عدد بانيات العظم.
الكلمات المفتاحية: التهاب دواعم السن, مستخلص قشر مانغوستين, رقعة لاصقة مخاطية, بانيات العظم, ناقضات العظم
Introduction
Periodontitis is an inflammation of the periodontal tissues, characterised by alveolar bone resorption,1 and is found in healthy individuals above the age of 30 years.2 In periodontitis, there is an increase in the number of Actinobacillus actinomycetemcomitans by up to 90%. A. actinomycetemcomitans are gram-negative bacteria that can produce exotoxins or endotoxins that function to provide invasive bacteria to periodontal tissues.3 Bacterial invasion into periodontal tissues can activate an immune response that can lead to tissue damage including alveolar bone resorption.4 The alveolar bone resorption is caused by an increase in osteoclast activity and a decrease in osteoblast activity due to an activated immune response by bacterial exotoxins or endotoxins.5,6 Currently, treatment of periodontitis is performed to protect teeth. Scaling and root planing (SRP) is one of the treatment modalities for periodontitis to eliminate biofilm and subgingival calculus.7 SRP can increase clinical attachment loss (CAL) between 0.55 and 1.29. Based on research, patients with periodontitis respond well to SRP and show good disease progression; however, exacerbations may occur when patients do not maintain oral hygiene.8 Modifications to treatment using SRP continue to be performed to improve treatment results, one of which is by using mucoadhesive patches.9, 10, 11
The mucoadhesive patch is a drug distribution system that has been widely used in the past few decades owing to its potential to optimise local drug distribution.12 Some of the benefits of using a mucoadhesive patch are low enzymatic activity, painless application, and increased therapeutic bioavailability.11 Mangosteen (Garcinia mangostana) is native to Southeast Asia, including Indonesia, Malaysia, and Thailand. Mangosteen skin, fruits, and peel have been widely used as drugs owing to their high therapeutic effect.13 Based on phytochemical screening, mangosteen peel extract contains saponins, tannins, polyphenols, flavonoids, and xanthones, which can inhibit the process of tissue damage.14,15 This study aimed to determine the potential of mucoadhesive patches loaded with mangosteen peel extract administration to inhibit the damage of alveolar bone in periodontitis by counting the number of osteoclasts and osteoblasts.
Materials and Methods
Mangosteen peel extract preparation
Mangosteen peel was obtained from Garcinia mangostana. Mangosteen peel was washed thoroughly with running water, cut, and dried in a 50 °C oven for 24 h. After drying, the mangosteen peel was crushed, blended, and filtered using a 2/9 sized filter. The mangosteen peel powder was then macerated in 96% ethanol using a magnetic stirrer for 2 days with mangosteen peel and ethanol at a ratio of 1:2.7
Creation of the mucoadhesive patches loaded with mangosteen peel extract
The first mixture was obtained by developing carboxymethyl cellulose sodium, which was sprinkled in 30 mL of water (ratio 1:20) left overnight and poured into a gel mass. Approximately 60.3 mL of hot water was gradually added to the gel base. The second mixture was prepared by dissolving ethanol with 0.5 mL mangosteen peel extract, followed by the addition of propylene glycol (2.5 gm) and stirring until it dissolves. The second mixture was added to the first mixture and stirred until it became homogeneous. The prepared mixture weighed as much as 70 gm when placed in a petri dish, and then dried in a memmert oven at 40 °C.16
Animals
The research conducted was an experimental laboratory study with a research design using a post-test-only control group design. The sample used in this study was male Wistar rats (Rattus novergicus), aged 5–6 months, weighing 250–300 gm, obtained from the Experimental Animal Laboratory of the Faculty of Veterinary Medicine, Airlangga University. The number of animals used in this study was determined by Lemeshow,17 with a total of 27 animals divided into nine groups.
Actinobacillus actinomycetemcomitans induced periodontitis
Wistar rats were adapted for 7 days before inducing periodontitis. The A. actinomycetemcomitans 1 × 109 colony-forming unit (CFU) was prepared according to the previous method.18 As much as 200 μL of A. actinomycetemcomitans 1 × 109 CFU was injected in the gingival sulcus of the mandibular anterior, three times a day for 2 days for the development of periodontitis.19
The treatment of periodontitis
Clinical periodontitis after the induction of A. actinomycetemcomitans was depicted as a swelling of the interdental incisive central mandibular area, and interdental resorption using histopathology examination.20,21 Wistar rats exhibiting signs of periodontitis were then anesthetised using an intraperitoneal injection of 0.1 mL/100 g of ketamine body weight. Anaesthesia was performed to facilitate the application of patches and to maintain mucoadhesive patches in the oral cavity of Wistar rats.
In the negative control group, mucoadhesive patches were applied; in the positive control group, 0.7% tetracycline gel was applied; and in the experimental group, the mucoadhesive patches loaded with mangosteen peel extract were applied. The mucoadhesive patch was maintained for 1 h in the periodontitis area and then removed. This procedure was performed once a day for 3 consecutive days.22,23
In the next 3, 5, and 7 days after treatment, each animal group was sacrificed using a lethal injection of ketamine (at least four times the anaesthetic dose or approximately 0.4 mL/100 gm body weight). Mandibular anterior biopsy was performed, and the animal was buried according to the ethics of experimental animals.24
Counting osteoblasts and osteoclasts
Haematoxylin-eosin (HE) staining was used to count the number of osteoblasts and osteoclasts. The single calibrated operator performed this procedure under a light microscope (Nikon H600L microscope; Nikon, Japan) at 400 × magnification in five fields of view.
Statistical analysis
The data obtained were then analysed using the Shapiro–Wilk normality test. The differences in osteoblasts and osteoclasts were analysed using Kruskal–Wallis and Mann–Whitney tests with p < 0.05 as a significant difference.
Results
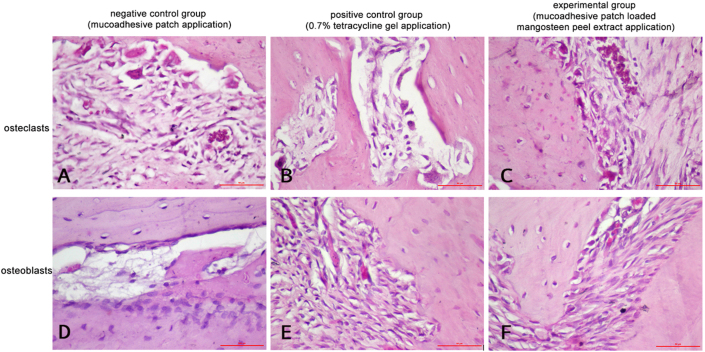
The number of osteoblasts and osteoclasts was calculated based on the histopathological features. The average number of osteoblasts and osteoclasts showed that there was an increase in the number of osteoblasts and a decrease in the number of osteoclasts in all study groups at 3, 5, and 7 days after treatment (Figure 1).
Figure 1.
The histopathology of osteoclasts (A, B, and C) and osteoblasts (C, D, and E) using haematoxylin and eosin (HE) staining with magnification 400 ×.
Table 1 shows a significant difference in osteoblast numbers in the negative control and experimental groups after 3, 5, and 7 days (p = 0.046, p = 0.046, and p = 0.044, respectively); however, does not show a significant difference in the negative control group with the positive control, or the positive control group with treatment after 3, 5, and 7 days (p = 0.178; p = 0.068; p = 0.072, respectively).
Table 1.
The number of osteoblasts.
| positive control group (0.7% tetracycline gel application) | negative control group (mucoadhesive patch application) | experimental group (mucoadhesive patch loaded mangosteen peel extract application) | p | |
|---|---|---|---|---|
| 3 days | 4.00 ± 1.00a | 5.33 ± 1.154 | 7.33 ± 1.54a | 0.046 |
| 5 days | 5.67 ± 1.15b | 7.67 ± 0.58 | 9.00 ± 1.00b | 0.046 |
| 7 days | 7.33 ± 1.80c | 9.67 ± 0.58 | 11.67 ± 2.08c | 0.044 |
a,b,c showed significant different with Mann–Whitney test.
Table 2 shows a significant difference in osteoclast numbers in the negative control group and the experimental group after 3 and 5 days (p = 0.043 and p = 0.043, respectively); however, does not show a significant difference in the negative control group with the positive control, or the positive control group with treatment after 3 and 5 days (p = 0.099; p = 0.068, respectively). The treatment after 7 days showed no differences in the negative control, positive control, or experimental groups (p > 0.05).
Table 2.
The number of osteoclasts.
| positive control group (0.7% tetracycline gel application) | negative control group (mucoadhesive patch application) | experimental group (mucoadhesive patch loaded mangosteen peel extract application) | p | |
|---|---|---|---|---|
| 3 days | 7.67 ± 0.58a | 6.57 ± 0.58 | 5.33 ± 0.58a | 0.043 |
| 5 days | 6.67 ± 0.58b | 5.33 ± 0.58 | 4.33 ± 1.15b | 0.043 |
| 7 days | 5.00 ± 1.00 | 4.33 ± 1.15 | 3.00 ± 1.00 | >0.05 |
a,b,c showed significant different with Mann–Whitney test.
Discussion
Based on the results of the study, the number of osteoblasts observed at 3, 5, and 7 days after treatment in all groups increased, while the number of osteoclasts observed in all groups decreased. This is consistent with the theory of the bone remodelling process, which involves the process of resorption by osteoclasts and the process of apposition by osteoblasts. In the resorption phase, osteoprotegerin (OPG), which is an inhibitor of receptor activator of nuclear factor-kappa-β ligand (RANKL), binds to receptor activator of nuclear factor-kappa-β (RANK). As it has a smaller amount of RANK than RANKL, it cannot inhibit RANKL from binding with RANK. When the remodelling process enters the reversal phase, which is a transition phase from the resorption to the formation phase, there is an increase in the amount of OPG; therefore, OPG can bind to RANKL and inhibit osteoclast differentiation and maturation thus, decreasing the number of osteoclasts and increasing the number of osteoblasts.20,25 The experimental group showed the highest number of osteoblasts and the lowest number of osteoclasts; therefore, it can be concluded that the mucoadhesive patch of mangosteen peel extract can inhibit alveolar bone damage in periodontitis-induced A. actinomycetemcomitans. This finding confirms the results of Mizuno et al. (2015), who showed that there was a decrease in the number of osteoclasts in rats with periodontitis after treatment. The decrease in the number of osteoclasts is caused by the inhibition of bacteria to invade tissue, causing the suppression of inflammatory mediators such as interleukin 1 (IL-1), interleukin 6 (IL-6), and tumour necrosis factor α (TNF-α).26
Mangosteen peel extract can inhibit alveolar bone resorption through xanthone, tannin, and flavonoids, which are antibacterial and anti-inflammatory. Xanthone destroys bacterial adhesin to inhibit the attachment of bacteria to the tissue,27 coagulating bacterial cells and eliminating bacterial virulence factors. Flavonoids interfere with bacterial metabolism by binding to proteins that can interfere with bacterial metabolism. This is supported by the use of mucoadhesive patches that can increase bioavailability and drug potential as by using mucoadhesive patches, the first-pass effect can be avoided so that the active ingredients contained in mangosteen peel extract can be well distributed to the target tissue.28 The negative control group showed the lowest number of osteoblasts and the highest number of osteoclasts. This is because, in the negative control group, the mucoadhesive patches of mangosteen peel extract or other antibacterial and anti-inflammatory ingredients were not applied; therefore, the inflammatory response continued. Lipopolysaccharides (LPS) from A. actinomycetemcomitans can bind to the toll-like receptor which is an LPS receptor and can activate various immune responses in the body. Active macrophages, neutrophils, and lymphocytes can stimulate the production of inflammatory mediators, such as IL-1, Il-6, TNF-α, and prostaglandin E2.21,29 IL-1 is a proinflammatory cytokine that has a high potential to induce bone demineralisation and synergise with TNF-α to stimulate bone resorption. IL-6, a proinflammatory cytokine can also stimulate alveolar bone resorption and can affect the expression of RANKL, RANK, and OPG.
There were significant differences between the negative control and experimental groups at 3 and 5 days after treatment. This is caused by the pharmacological effects of mangosteen peel extract and the drug distribution system using mucoadhesive patches so that it can inhibit bone damage in periodontitis induced by A. actinomycetemcomitans. On the seventh day after treatment, there were no significant differences between the negative control and experimental groups. This is because the observation has undergone a process of regeneration, and osteoclast differentiation decreases; thus, bone density increases.
The limitations of this study lie in the limited observations on osteoblasts and osteoclasts, where clinical parameters of periodontitis such as tooth mobility, CAL, probing, or pocket depth were not measured. However, the results of this study confirm that mucoadhesive patches loaded with mangosteen peel extract play a role in periodontitis, where osteoblasts and osteoclasts are the main indicators of periodontitis. To strengthen the results of this study, more research is needed to determine the effect of mucoadhesive patches loaded with mangosteen peel extract on other markers of periodontitis, anti-bacterial power in mucoadhesive patches, and application of mucoadhesive patches on the oral mucosa.
Conclusion
Within the limitations of this study, mucoadhesive patches loaded with mangosteen peel extract can prevent alveolar bone damage in periodontitis by inhibiting the number of osteoclasts and increasing the number of osteoblasts.
Source of funding
This work was supported by the Ministry of Higher Education, Republic of Indonesia 2020 in the schema Penelitian Dasar Unggulan Perguruan Tinggi (PDUPT) with grant number 1520/UN3/2019.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This study was performed in strict accordance with the Guide for the Care and Use of Laboratory Animals, National Health Research and Development Ethics Standard and Guidelines Council (2017), Minister of Health, Republic of Indonesia.
The protocol was approved by the Ethical Clearance of Health Experiment Committee, Faculty of Dental Medicine, Airlangga University, Surabaya, under registration number 541/HRECC.FODM/VII/2019 (date approval: 9 July 2019).
Authors’ contributions
RDR designed the study, supervision, visualisation and validation, funding acquisition, and revised the draft of the article. Y conducted the research, supervision, visualisation, and revised the article. S organised, analysed, and interpreted the data and revised the article. FMS conducted the research and collected the data. MAQJ conducted the research and collected the data. DML conducted the research and data collection. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity of the index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Hienz S.A., Paliwal S., Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res. 2015;2015:1–10. doi: 10.1155/2015/615486. http://www.hindawi.com/journals/jir/2015/615486/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billings M., Holtfreter B., Papapanou P.N., Mitnik G.L., Kocher T., Dye B.A. Age-dependent distribution of periodontitis in two countries: findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J Clin Periodontol. 2018;45(December 2017):S130–S148. doi: 10.1111/jcpe.12944. [DOI] [PubMed] [Google Scholar]
- 3.Ridwan R.D. The role of Actinobacillus actinomycetemcomitans fimbrial adhesin on MMP-8 activity in aggressive periodontitis pathogenesis. Dent J. 2012;45(4):181. [Google Scholar]
- 4.Luis Muñoz-Carrillo J., Elizabeth Hernández-Reyes V., Eduardo García-Huerta O., Chávez-Ruvalcaba F., Isabel Chávez-Ruvalcaba M., Mariana Chávez-Ruvalcaba K., et al. Periodontal disease - diagnostic and adjunctive non-surgical considerations. IntechOpen; 2020. Pathogenesis of periodontal disease; pp. 1–4.http://www.jicdro.org/text.asp?2015/7/1/11/153489 Available from: [Google Scholar]
- 5.Gama A., Navet B., Vargas J.W., Castaneda B., Lézot F. Bone resorption: an actor of dental and periodontal development? Front Physiol. 2015 Nov 5;6(NOV):1–7. doi: 10.3389/fphys.2015.00319. http://journal.frontiersin.org/Article/10.3389/fphys.2015.00319/abstract [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang X., Xie M., Xie Y., Mei F., Lu X., Li X., et al. The roles of osteocytes in alveolar bone destruction in periodontitis. J Transl Med. 2020;18:1–15. doi: 10.1186/s12967-020-02664-7. BioMed Central. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smiley C.J., Tracy S.L., Abt E., Michalowicz B.S., John M.T., Gunsolley J., et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015 Jul;146(7):525–535. doi: 10.1016/j.adaj.2015.01.026. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 8.Lertpimonchai A., Rattanasiri S., Arj-Ong Vallibhakara S., Attia J., Thakkinstian A. The association between oral hygiene and periodontitis: a systematic review and meta-analysis. Int Dent J. 2017 Dec;67(6):332–343. doi: 10.1111/idj.12317. https://linkinghub.elsevier.com/retrieve/pii/S0020653920318554 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshipura V., Yadalam U., Brahmavar B. Aggressive periodontitis: a review. J Int Clin Dent Res Organ. 2015;7(1):11. http://www.jicdro.org/text.asp?2015/7/1/11/153489 [Internet] Available from: [Google Scholar]
- 10.Sanz I., Alonso B., Carasol M., Herrera D., Sanz M. Nonsurgical treatment of periodontitis. J Evid Base Dent Pract. 2012 Sep;12(3):76–86. doi: 10.1016/S1532-3382(12)70019-2. Available from: [DOI] [PubMed] [Google Scholar]
- 11.Kaur A., Kaur G. Mucoadhesive buccal patches based on interpolymer complexes of chitosan–pectin for delivery of carvedilol. Saudi Pharm J. 2012 Jan;20(1):21–27. doi: 10.1016/j.jsps.2011.04.005. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koyi P.K., Khan A.B. Buccal patches: a review. Int J Pharm Sci Res. 2013;4(1):83–89. [Google Scholar]
- 13.Arundina I., Suardita K., Diyatri I., Meircurius Dwi C.S. Mangosteen skin (Gracinia mangostana L) as stem cell growth factor. J Int Dent Med Res. 2018;11(3) [Google Scholar]
- 14.Suhartati R., Apriyani F., Khusnul, Virgianti D.P., Fathurohman M. Antimicrobial activity test of mangosteen leaves ethanol extract ( Garcinia mangostana linn ) against Pseudomonas aeruginosa bacteria. J Phys Conf Ser. 2019 Jul;1179(1) https://iopscience.iop.org/article/10.1088/1742-6596/1179/1/012167 Available from: [Google Scholar]
- 15.Prasetya R.C., Haniastuti T., Purwanti N. Ekspresi COX-2 setelah pemberian ekstrak etanolik kulit manggis (Garcinia mangostana Linn) pada tikus wistar (COX-2 expression after mangosteen rind (Garcinia mangostana Linn) etanolic extract administration in wistar rats) Dent J. 2013 Dec 1;46(4):173. http://e-journal.unair.ac.id/index.php/MKG/article/view/753 Available from: [Google Scholar]
- 16.Shantiningsih R.R., Diba S.F. Efek aplikasi patch gingiva mukoadesif β-carotene akibat paparan radiografi panoramik. Maj Kedokt Gigi Indones. 2015 Dec 1;20(2):186. https://jurnal.ugm.ac.id/mkgi/article/view/9121 Available from: [Google Scholar]
- 17.Lameshow S., Hosmer D., Klar J., Lwanga S. John Willey; Chichester: 2000. Adequacy of sample size in Health study; p. 38. [Google Scholar]
- 18.Arundina I.R.A., Diyatri I., Surboyo M.D.C., Halimah A.N., Chusnurrafi F.I. The antibacterial effect of liquid smoke rice hull on porphyromonas gingivalis and its proliferative effects on osteoblast as periodontitis remedies: an invitro study. Int J Pharm Res. 2020;12(3):3466–3471. [Google Scholar]
- 19.Polak D., Wilensky A., Shapira L., Halabi A., Goldstein D., Weiss E.I., et al. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol. 2009 May;36(5):406–410. doi: 10.1111/j.1600-051X.2009.01393.x. Available from: [DOI] [PubMed] [Google Scholar]
- 20.Putu Swastini I.G.A.A., Bagus Mahadewa T.G., Widyadharma I.P.E. Alveolar bone osteoclast profile in the periodontitis wistar rats model with the snail slime (Achatina Fulica) application. Open Access Maced J Med Sci. 2019;7(10):1680–1684. doi: 10.3889/oamjms.2019.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budhy T.I., Arundina I., Surboyo M.D.C., Halimah A.N. The effects of rice husk liquid smoke in porphyromonas gingivalis-induced periodontitis. Eur J Dent. 2021 May 26 doi: 10.1055/s-0041-1727554. http://www.thieme-connect.de/DOI/DOI?10.1055/s-0041-1727554 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blagus T., Markelc B., Cemazar M., Kosjek T., Preat V., Miklavcic D., et al. In vivo real-time monitoring system of electroporation mediated control of transdermal and topical drug delivery. J Control Release. 2013 Dec;172(3):862–871. doi: 10.1016/j.jconrel.2013.09.030. Available from: [DOI] [PubMed] [Google Scholar]
- 23.Weinstain R., Segal E., Satchi-Fainaro R., Shabat D. Real-time monitoring of drug release. Chem Commun. 2010;46(4):553–555. doi: 10.1039/b919329d. http://xlink.rsc.org/?DOI=B919329D Available from: [DOI] [PubMed] [Google Scholar]
- 24.Santoso H.B. Struktur mikroskopis kartilago epifisialis tibia fetus mencit (Mus musculus l.) Dari induk dengan perlakuan kafein. Berk Penelit Hayati. 2006 Dec 31;12(1):69–74. http://berkalahayati.org/index.php/jurnal/article/view/401 Available from: [Google Scholar]
- 25.Wardhana A.S., Nirwana I., Budi H.S., Surboyo M.D.C. Role of hydroxyapatite and ellagic acid in the osteogenesis. Eur J Dent. 2020 Jul 20:1–6. doi: 10.1055/s-0040-1714039. http://www.thieme-connect.de/DOI/DOI?10.1055/s-0040-1714039 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizuno M., Miyazawa K., Tabuchi M., Tanaka M., Yoshizako M., Minamoto C., et al. Reveromycin A administration prevents alveolar bone loss in osteoprotegerin knockout mice with periodontal disease. Sci Rep. 2015;5(November):1–9. doi: 10.1038/srep16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widjaja J., Wahjuningrum D.A., Cahyani F. Antibacterial effect of xanthone from mangosteen pericarp extract (garcinia mangostana linn.) against porphyromonas gingivalis. J Int Dent Med Res. 2019;12(1):19–21. [Google Scholar]
- 28.Al-Bayaty F.H., Lim T.W. Generalized aggresive periodontitis associated with amelogenesis imperfecta and its multidisciplinary management options: case report and review of the literature. J Int Dent Med Res. 2018;11(2):459–464. [Google Scholar]
- 29.Surboyo M.D.C., Mahdani F.Y., Ernawati D.S., Sarasati A., Rezkita F. The macrophage responses during diabetic oral ulcer healing by liquid coconut shell smoke: an immunohistochemical analysis. Eur J Dent. 2020 Jul 24;14(3):410–414. doi: 10.1055/s-0040-1712776. http://www.thieme-connect.de/DOI/DOI?10.1055/s-0040-1712776 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]