Abstract
Licorice is a medicinal and food plant widely used to treat diseases and produce food additives, because of its unique chemical constituents like polysaccharides, flavones, and saponins. Glycyrrhiza Polysaccharides (GPS-1) are water-soluble neutral polysaccharides extracted from licorice. Currently, GPS-1 is administrated to chickens by gavage every d for 14 d to observe the impact of GPS-1 on the Newcastle disease vaccine. To determine the immunity of these chickens to NDV, blood serum levels of hemagglutinin-inhibition (HI) antibody, and immunoglobulins IgA and IgG were measured. Meanwhile, the expression levels of cytokines IL-2, IL-4, IL-17, and IFN-γ were measured to evaluate the degree of immune booster activity. The chickens’ spleen and peripheral blood lymphocytes displayed a significant increase in the proportion of CD4+ and CD8+ T cells after booster treatments with GPS-1. The results indicated that GPS-1 had a significant, dose-dependent, immune-boosting effect which could enhance NDV vaccine immunity in chickens.
Key words: Glycyrrhiza polysaccharides, Newcastle disease, immune response, immune booster
INTRODUCTION
The Newcastle disease virus (NDV), one of the most dangerous viruses for the industry of poultry husbandry, belongs to the family Paramyxoviridae in the order Mononegavirales. It primarily invades the respiratory and digestive tracts, resulting in decreased egg laying, significant weight loss, and other harm to the birds (Homme and Easterday, 1970; Qiu et al., 2016). NDV can lead to respiratory and digestive distress including elevated body temperature, dyspnea, coughing, greenish diarrhea and thin-shelled eggs, which causes severe economic losses in the poultry industry (Liu et al., 2019). At present, ND vaccine is widely used in clinic to reduce the hazard of NDV, but the disadvantages of vaccine, such as producing early immune response and short-lasting humoral antibodies, have limited its application (Guo et al., 2021). Thus, novel immune boosters are in urgent demand to strengthen the protective abilities of NDV vaccines.
At present, immune boosters are modern tools for increasing vaccine efficacy, which can effectively reduce the number of immunizations and the amount of antigen used. (Shi et al., 2018; Kimani et al., 2021). Immune boosters may not only assist antigens in stimulating the body to produce a strong immune response, resulting in high titers of antibodies, but also maintain antibody levels on the mucosal surface and in the blood for a considerably extended time frame (Coffman et al., 2010). Immune boosters can play many different roles, like promoting the synthesis and secretion of antibodies, increasing the quantity and activity of immune cells like lymphocytes, enhancing the number of CD4+ T cells and CD8+ T cells and promoting expression of immune-related cytokines (Schneerson et al., 1980). Therefore, the rational use of immune boosters can effectively compensate for the deficiencies of vaccines.
Polysaccharides are a kind of carbohydrate polymers comprised of more than 10 monosaccharides, which are obtained from plants, animals, and microorganisms, with various bioactivities such as immunomodulatory, antioxidant, antitumor, and anticoagulant therapeutic agents (Cor et al., 2018). Polysaccharides, as one of typical immune boosters, can significantly enhance the body's abilities to produce specific immune responses. Polysaccharides such as lentinan, epimedium polysaccharide, astragalus polysaccharide, and angelica polysaccharide have immune-enhancing effects like splenic and peripheral blood lymphocyte promotion, and increasing the activities of peritoneal macrophages and natural killer cells in mice (Xue et al., 2008). Additionally, comparing to the chemical medicines, polysaccharides have relatively low toxicity in animals and are widely used in animal husbandry (Wang et al., 2018a). Thus, polysaccharide has a large potential in the poultry industry.
Licorice, a traditional medicinal and food plant, has a wide and long history of application in both diet and pharmacology (Shin et al., 2008). In recent years, more and more active ingredients such as polysaccharides, flavones, and saponins were obtained from licorice, showing some activities in drug development and food industry (Hayama et al., 2015). Among them, Glycyrrhiza polysaccharides (GPS) as functional compounds have attracted more and more researchers in both pharmaceutical and alimentary field (Chu et al., 2012). GPS are consist of different types of monosaccharides like glucose, galactose, and mannose (Pan et al., 2020), and have various medicinal uses such as antitumor agent, antibacterial, antiviral, antioxidant, and protective agent to the liver and kidney (Ayeka et al., 2016; Wu et al., 2017; Lian et al., 2018; Zhang et al., 2018; Hao et al., 2020; Mutaillifu et al., 2020; Pan et al., 2020). GPS can boost immune function, stimulate the antigen presenting cells like macrophages and dendritic cells, as well as help secrete cytokines and induce immune cells to mature. Therefore, GPS may be used as a potential immune booster.
In our previous work, we extracted and purified one water-soluble polysaccharide (named GPS-1) from licorice and studied its immunological activities (Wu et al., 2017). It was found that GPS-1 can increase the expression of cytokines IL-2, IL-10, and IFN- γ in chicken dendritic cells and stimulate the proliferation of chicken lymphocytes. In the current study, further experiments were designed and performed in vivo to explore the immune-modulating effects of GPS-1 on young chickens shortly after NDV vaccination. The indicators of humoral and cellular immunity in chickens, including HI antibody levels, immunoglobulin levels, expression of cytokines IL-2, IL-4, IL-17 and IFN-γ, and typing of T lymphocytes were used to evaluate the efficacy of GPS-1 as an immune booster.
MATERIALS AND METHODS
Extraction of Glycyrrhiza Polysaccharides
In accordance with our standard laboratory extraction method of GPS-1 from Chinese licorice, the root of Glycyrrhiza uralensis was defatted with 95% ethanol solution for 4 h and dried at room temperature to remove the fat-soluble constituents (Wu et al., 2017). The treated root was powdered and extracted twice in boiling water under reflux. The extracts were diluted and centrifuged to give the supernatants, which were combined and lyophilized, then precipitated by 90% alcohol (v/v) until the final alcohol content was 80% (v/v). The precipitate was collected and dissolved in water, then treated by trichloroacetic acid to precipitate proteins and yield the total deproteinated Glycyrrhiza polysaccharides. The total deproteinated polysaccharides were then dissolved in water again and dialyzed by running tap water for 2 d and distilled water for 3 d, then lyophilized to yield a white, powdery precipitate. The white powder was dissolved in deionized water to give 20 mg/mL and separated by DEAE-52 cellulose gel column (Solarbio, Beijing, China) chromatography with sodium chloride solution (gradient: 0–0.1–1M) at a flow rate of 0.5 mL/min, and 15 mL per tube. The polysaccharide content of each eluate was analyzed by the phenol-sulfuric acid method to create elution curves (Mutaillifu et al., 2020). The eluates with polysaccharides were selected, combined, and concentrated by lyophilization, then adjusted to 20 mg/mL and purified via Sephadex G-100 column l (Sigma-Aldrich Chemical Company, Shanghai, China) chromatography with deionized water at 0.5 mL/min. The concentration of polysaccharides in each eluate (10 mL/tube) was determined, and eluates with the highest polysaccharide content were combined and lyophilized to obtain the isolated Glycyrrhiza uralensis polysaccharide GPS-1 in powder form.
The GPS-1 administered by gavage was dissolved in standard saline solution at 3 different concentrations (high, medium, and low): GPS-H (600 mg/kg), GPS-M (450 mg/kg), and GPS-L (300 mg/kg) (Wang et al., 2013; Ayeka et al., 2017; Aipire et al., 2020). The 3 purified GPS-1 concentrations were sterilized at 100°C for 30 min and stored at 4°C.
Vaccinations and GPS-1 Treatment
One-day-old, male Hy-Line brown chickens with no immunity to NDV were kept in observation for 10 d before 100 of these chickens were randomly divided into 5 groups of 20 chickens per group. One group was randomly appointed as the control, and no vaccine or other immune treatment was given. The remaining 4 groups were immunized by Newcastle disease virus (ND-Ⅳ LaSota [Jiangsu Academy of Agricultural Sciences]) vaccine with eye drops and nose drops. The date of immunization was recorded as d 0. The ND-immunized groups were housed separately from the control group to avoid interference. The immunized groups were then randomly divided into the Glycyrrhiza polysaccharides high concentration group (GPS-H), Glycyrrhiza polysaccharides medium concentration group (GPS-M), Glycyrrhiza polysaccharides low concentration group (GPS-L), and the simple immunization group that received no immune booster (ND group). GPS-1 was administered by gavage at varying doses to the groups GPS-H, GPS-M, and GPS-L on the 1st d after immunization, while saline was administered to the control and ND groups. These gavage treatments were administered once a d for 14 d, as seen below in Table 1.
Table 1.
Experimental grouping and oral medication of 100 chickens.
| Group | Antigen | Oral administration |
|---|---|---|
| Control | None | Normal saline |
| ND | Inactivated NDV antigen | Normal saline |
| GPS-L | Inactivated NDV antigen | GPS-L (300 mg/kg) |
| GPS-M | Inactivated NDV antigen | GPS-M (450 mg/kg) |
| GPS-H | Inactivated NDV antigen | GPS-H (600 mg/kg) |
Average Daily Weight Gain and Organ Index
To calculate the average daily weight gain rate, all chickens were weighed and numbered at the time of NDV vaccination. On d 7, 14, 21, and 28 after immunization, 5 chickens per group were randomly selected and weighed. The average daily gain rate was calculated by [(W2-W1)/N]/(W1/N) × 100%, where W2 represents the current weight of each selected chicken in grams, W1 represents the previous weight of each selected chicken (g), and N represents the number of feeding days.
On the 7th and 28th d after vaccination, 5 chickens from each group were randomly selected, weighed, and euthanized. The spleen, thymus, and bursa of Fabricius were collected and weighed to calculate the organ index, which is equal to the organ weight (g)/body weight (kg).
Hemagglutination Inhibition (HI) Assay
Serum was collected from 5 chickens randomly sampled from each group of chickens on d 7, 14, 21, and 28 after immunization. The hemagglutination inhibition titer was determined using a suspension prepared from 1% chicken red blood cells. The maximum dilution of the serum with complete inhibition of agglutination was recorded as the antibody titer of the serum. The titer of serum ND-HI antibody was evaluated by a standard HI assay (Wang et al., 2018b; Elaish et al., 2019).
Determination of Anti-ND IgG and IgA
The titers of antibodies IgA and IgG against ND-Ⅳ LaSota antigen in serum were determined by ELISA technique (Wang et al., 2010). Five chickens from each group were randomly selected for serum collection on d 7, 14, 21, and 28 after immunization. The serum collection method was as follows: diluted Newcastle disease antigen solution was used to coat a 96-well plate, soaking overnight at 4°C, then the supernatant was discarded. The 96-well plate was then sealed with PBS containing 1% BSA. Afterward, diluted serum was added to each well, incubated for 30 min at 37°C, treated with rabbit antichicken HRP-conjugated (Bioss) IgG for another 30 min at 37°C. After treatment, each well was washed twice with PBS and stained with TMB color development solution for 7 min at 37°C in darkness, ending with the development termination solution. Absorbance was measured by a plate reader (Thermo Fisher) at 450 nm to provide the OD value.
Isolation of Spleen Lymphocytes and Peripheral Blood Lymphocytes
Five chickens were randomly selected from each group 14 d after immunization. Their spleens were removed under aseptic conditions, then rinsed 3 times with sterile PBS and placed in a flat dish. They were cut up, ground down and filtered by a tissue sieve to produce a ground spleen suspension. The suspension was slowly added to the upper layer of the lymphocyte separation solution (Solarbio, Beijing, China) at a ratio of 1:1 and centrifuged for 20 min at 3,000 rpm. After centrifugation, the white misty spleen lymphocytes at the interface were carefully aspirated, rinsed twice with PBS and centrifuged for 15 min at 2,500 rpm (Hou et al., 2010; Chimeno Zoth et al., 2012).
On the 14th d after immunization, 2 mL of blood was taken under sterile conditions and anticoagulated with heparin (Solarbio, Beijing, China), diluted 1:1 with PBS and then carefully added this diluted blood to the upper layer of the lymphocyte separation solution (Solarbio, Beijing, China) at a ratio of 1:1 and centrifuged at 2,500 rpm/min for 20 min. Afterward, the peripheral blood lymphocytes at the middle boundary were harvested, washed twice with PBS and centrifuged at 1,500 rpm for 15 min (Lam and Vasconcelos, 1994; Gu et al., 2020).
CD3+CD4+ and CD3+CD8+ T Cell Activation
After resuspension, both spleen and peripheral blood lymphocytes from each group were adjusted to 1 × 107 cells/mL. Each group's samples were prepared as a 1 mL cell suspension and a 1 μL mixture consisting of antichicken CD3-FITC, antichicken CD4-PE and antichicken CD8-APC antibodies (Southern Biotech, Birmingham, AL), incubated for 30 min at 4°C in darkness. After centrifugation at 1,000 rpm for 5 min, cells in each tube were collected and analyzed by cell flow cytometry (BD Accuri C6) (Scherer et al., 2018; Fu et al., 2019; Lindenwald et al., 2019).
ELISA Analysis of Cytokines in Serum
On d 14 after immunization, 5 chickens in each group were randomly selected to collect serum from. The contents of IL-2, IL-4, IL-17, and IFN-γ in serum were determined according to the ELISA (LiankeBio, Hangzhou, China) protocol.
Histopathological Analysis
The spleen, thymus, and bursa of Fabricius of 5 of the immunized chickens were collected on d 28 after vaccination. They were fixed with 4% paraformaldehyde and hematoxylin and eosin (HE) stained.
Immunofluorescence of Tissue Sections
The spleens of immunized chickens were collected 28 d after immunization. The immunofluorescence samples of spleen tissue were prepared by incubating spleen sections with CD4+ or CD8+ antibodies (Southern Biotech) at 4°C in darkness overnight. Then, secondary antibodies (HRP-conjugated goat antirabbit) were added and treated the samples for 1 h at 4°C in darkness. FITC (green), CY3 (red), and DAPI (blue) were used to stain the CD8+ T cells, CD4+ T cells and nuclei, respectively. The sections were rinsed, then observed and imaged by confocal microscopy.
Statistical Analysis
The data were presented as the mean ± standard error of the mean (SEM). A One-way analysis of variance (ANOVA) followed by an LSD mean separation test was used to evaluate the significance of differences among groups. Calculated probability (P) values less than 0.05 were considered statistically significant. Figures were exported using GraphPad Prism software. * means P < 0.05. ** means P < 0.01 and ***means P < 0.001. All these outcomes are compared with the unvaccinated group (control). Likewise, # means P < 0.05; ## means P < 0.01; ### means P < 0.001. All of these are compared with the simple vaccination group (ND).
RESULTS
Average Daily Gain and Organ Index
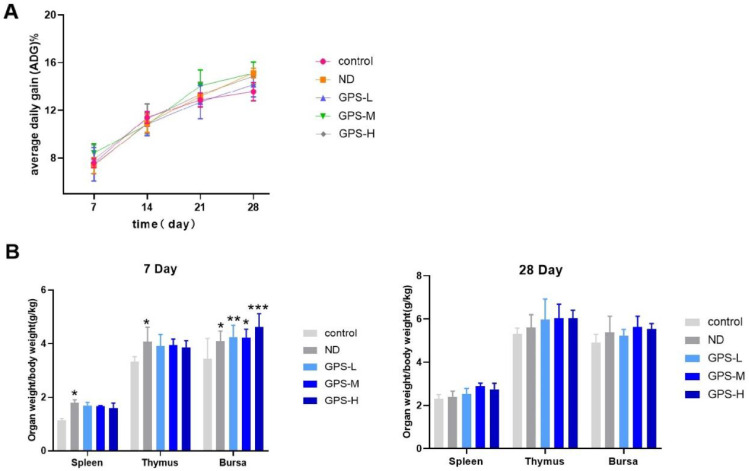
On the 7th, 14th, 21st, and 28th d after immunization, there were no significant differences in the rate of average daily weight gain between the control, ND, GPS-L, GPS-M, and GPS-H groups, which indicates that feeding GPS-1 for 14 consecutive d has no effect on the chicken's body weight and other production performance (Figure 1A).
Figure 1.
Average daily gain and organ index
(A) Effects of GPS-H (600 mg/kg), GPS-M (450 mg/kg), and GPS-L (300 mg/kg) on average daily gain of chickens (n = 5). (B) Organ index of spleen, thymus, and bursa of Fabricius on the 7th and 28th d after the first vaccination (n = 5).
On the 7th and 28th d after immunization, the spleen, thymus, and bursa of Fabricius were collected from 5 chickens from each group, and the organ index of each immune organ was calculated separately. As shown in Figure 1B, on the 7th d, the bursa of Fabricius organ indices for the ND group and GPS-1 groups (GPS-L, GPS-M, GPS-H) were notably higher than those in the control group. The thymus and spleen organ indices of the ND group were significantly higher than those in the control group. However, compared with both the control and ND groups, the thymus and spleen organ indices in the GPS-L, GPS-M and GPS-H groups did not improve. On the 28th d, there was no significant difference in organ indices between any groups.
Antibody Responses
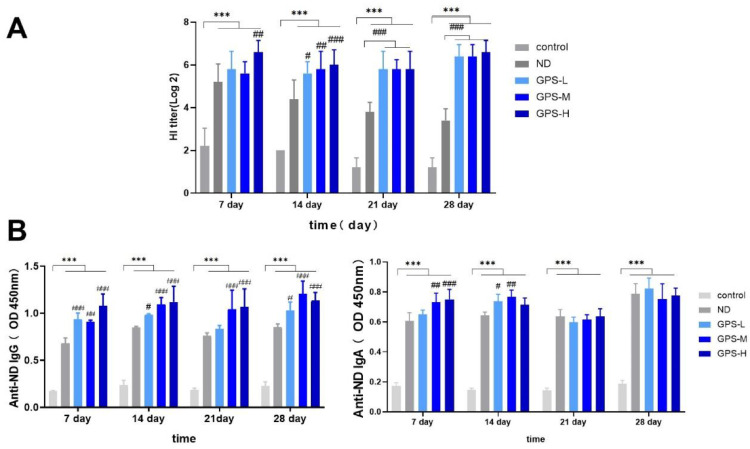
The day before immunization, serum was collected from each chicken to detect the maternal antibody levels. The potency of the maternal antibodies was measured at 2.06 (Log2). Subsequently, serum was collected on the 7th, 14th, 21st, and 28th d after immunization, and the ND-HI antibody titer is shown in the Figure 2A. The result implies that the NDV vaccine was effective in eliciting an immune response. On the 7th, 14th, 21st, and 28th d after immunization, the ND-HI antibody titers of the ND vaccinated group were much higher than that of the control group (P < 0.001). The effects of different concentrations of GPS-1 as a supplement to the ND vaccine were observed: compared with the ND group, all concentrations of GPS-1 could effectively increase ND-HI antibody titers on the 14th, 21st, and 28th d after immunization. Notably, only the high concentration of GPS-1 (600 mg/kg) given to the GPS-H group increased ND-HI antibody titers on the 7th d (P < 0.01). Thus, NDV vaccine can effectively provide specific immune protection for chickens, and GPS-1 can significantly increase ND-HI antibody levels after NDV vaccination and maintain high levels of ND-HI antibody titers. GPS-1 can have a good synergistic effect with NDV above 300 mg/kg, and the best effect can be obtained at 600 mg/kg.
Figure 2.
Antibody responses
*P < 0.05, **P < 0.01, and ***P < 0.001, all relative to the unvaccinated group (control). #P < 0.05, ##P < 0.01, ###P < 0.001, all compared with the simple vaccination group (ND).
(A) Serum HI antibody titers (Log2) from each group on the 7th, 14th, 21st, and 28th d after vaccination (n = 5). (B) Antigen-specific IgG and IgA titers in serum from different groups chickens on the 7th, 14th, 21st, and 28th d after vaccination (n = 5).
The expression levels of Newcastle disease-specific IgG and IgA antibodies in the serum were measured on the 7th, 14th, 21st, and 28th d after vaccination. As shown in Figure 2B, the specific IgG expression levels of the ND group and GPS-1 groups (GPS-L, GPS-M, GPS-H) were appreciably elevated in a dose-dependent manner compared with the control group. The results of specific IgG expression levels were consistent with that of ND-HI antibody levels (Figure 2A). The serum IgA expression levels can be observed in Figure 2B. On the 7th, 14th, 21st, and 28th d after immunization, the serum IgA expression levels of the ND group and all GPS-1 groups (GPS-L, GPS-M, and GPS-H) increased considerably compared to the control group (P < 0.001). In contrast with the NDV vaccination group, medium and high concentrations of GPS-1 booster increased the serum IgA levels on the 7th d. Similarly, on the 14th d, the serum IgA levels of the GPS-L and GPS-M groups were both significantly higher than that of the ND group. However, on the 21st and 28th d after the vaccination, there was no significance difference between the ND group and GPS-1 groups in the expression of serum IgA. This may be related to the easily degradable nature of IgA, which usually requires continuous stimulation to obtain higher levels of IgA for a longer period of times.
Determination of CD3+CD4+ and CD3+CD8+ T Cell Activation
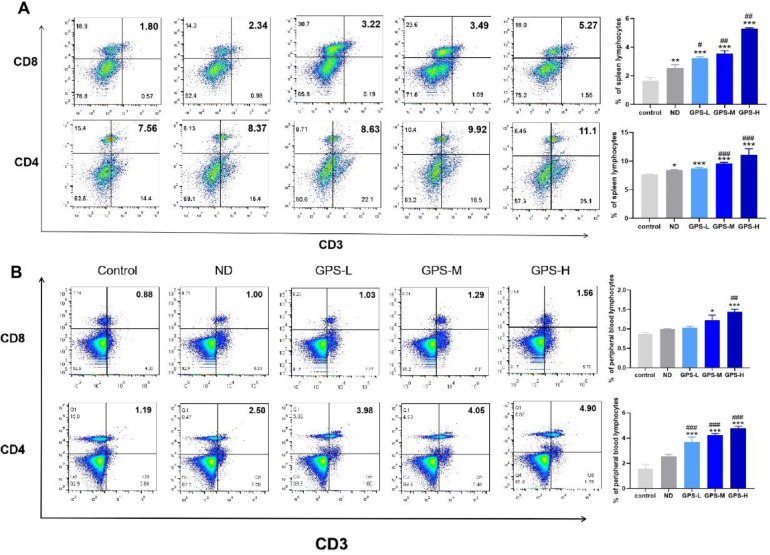
On the 14th d after immunization, the percentages of CD3+CD8+ T cells and CD3+CD4+ T cells in spleen lymphocytes were determined by flow cytometry. As shown in Figure 3A, the use of the NDV vaccine could help to increase the percentages of CD3+CD8+ T cells and CD3+CD4+ T cells in spleen lymphocytes. Moreover, compared with the ND group, all 3 concentrations of GPS-1 could increase the percentage of CD3+CD8+ T cells in spleen lymphocytes, while only medium and high concentrations of GPS-1 could raise in the percentage of CD3+CD4+ T cells in spleen lymphocytes.
Figure 3.
CD3+CD4+ and CD3+CD8+ T cell activation
*P < 0.05, **P < 0.01, and ***P < 0.001, all relative to the unvaccinated group (control). #P < 0.05, ##P < 0.01, ###P < 0.001, all compared with the simple vaccination group (ND).
(A) The percentages of CD3+CD4+ and CD3+CD8+ T spleen lymphocytes isolated from chickens treated with 600 mg /kg, 450 mg/kg, and 300 mg/kg of GPS-1 at 14 d after NDV vaccination (n = 5), and the representative flow cytometry plots of CD3+CD4+ and CD3+CD8+ T lymphocytes.
(B) The percentages of CD3+CD4+ and CD3+CD8+ T cells isolated from peripheral blood lymphocytes collected from chickens treated with 600 mg /kg, 450 mg/kg, and 300 mg/kg of GPS-1 at 14 d after NDV vaccination (n = 5), and the representative flow cytometry plots of CD3+CD4+ and CD3+CD8+ T lymphocytes.
On the 14th d after immunization, the percentages of CD3+CD8+ T cells and CD3+CD4+ T cells in peripheral blood lymphocytes were examined by flow cytometry. Figure 3B shows that compared with the control group, oral administration of medium and high concentrations of GPS-1 after 14 d of NDV inoculation can effectively enhance the proportion of CD3+CD8+T cells in peripheral blood lymphocytes. Compared with the ND group, the GPS-H group induced more CD3+CD8+T cell activation, which was statistically significant (P < 0.01). Secondly, NDV vaccination significantly increased the proportion of CD3+CD4+ T cells in peripheral blood lymphocytes, and progressively higher concentrations of GPS-1 further stimulate the activation of CD3+CD4+ T cells. In the peripheral blood lymphocytes, high concentrations of GPS-1 had a proliferative and activating effect on CD3+CD8+ T cells, while all concentrations of GPS-1 were shown to increase the proliferation and activation of CD3+CD4+ T cells where appears to be a concentration-dependent manner.
Cytokines in Serum
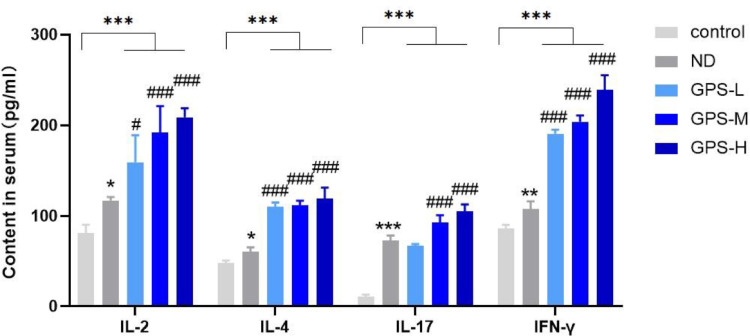
The serum expression levels of IL-2, IL-4, IL-17, and IFN-γ were measured using ELISA kits on the 14th d after NDV vaccination. As shown in Figure 4, NDV vaccination increased the secretion of IL-2, IL-4, IL-17, and IFN-γ cytokines (P < 0.001). Different concentrations of GPS-1 also increased the expression levels of IL-2, IL-4, and IFN-γ to different degrees, while only medium and high concentrations of GPS-1 promoted the secretion of IL-17 cytokines. The specific variability is shown in Figure 4.
Figure 4.
The expression of cytokines in serum.
*P < 0.05, **P < 0.01, and ***P < 0.001, all relative to the unvaccinated group (control). #P < 0.05, ##P < 0.01, ###P < 0.001, all compared with the simple vaccination group (ND).
The expression levels of cytokine IL-2, IL-4, IL-17, and IFN-γ in serum among Control, ND, GPS-L, GPS-M, and GPS-H groups of chickens on the 14th d after NDV vaccination (n = 5).
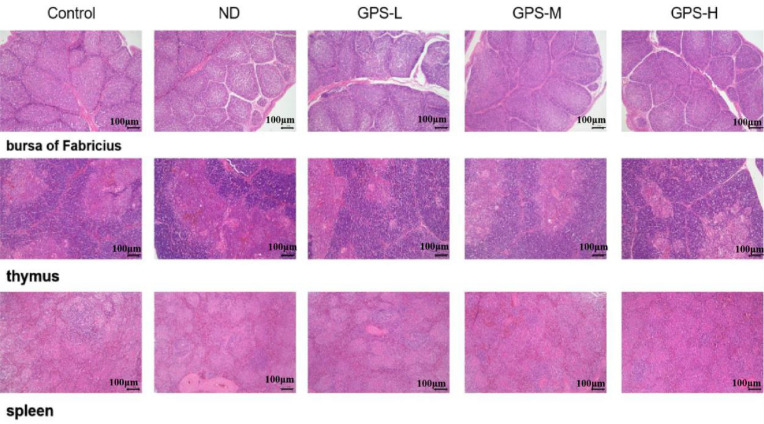
Histology Analysis
Figure 5 shows the HE stained sections of 3 important immune organs: the bursa of Fabricius (upper), thymus (middle), and spleen (lower) from each group on the 28th d after NDV vaccination. Compared with the control group, the ND group and the GPS-1 groups (GPS-L, GPS-M, and GPS-H) showed no significant toxic or inflammatory infiltrates in the bursa of Fabricius, thymus, and spleen, which confirmed that 600 mg/kg is a safe oral dose of GPS-1 for young chickens. This result was consistent with the results of the average daily weight gain rate (Figure 1A).
Figure 5.
Results of HE histological analysis (100 ×, 100 μm scale bar)
Histological analysis of bursa of Fabricius, thymus, and spleen from different groups on the 28th d after NDV immunization.
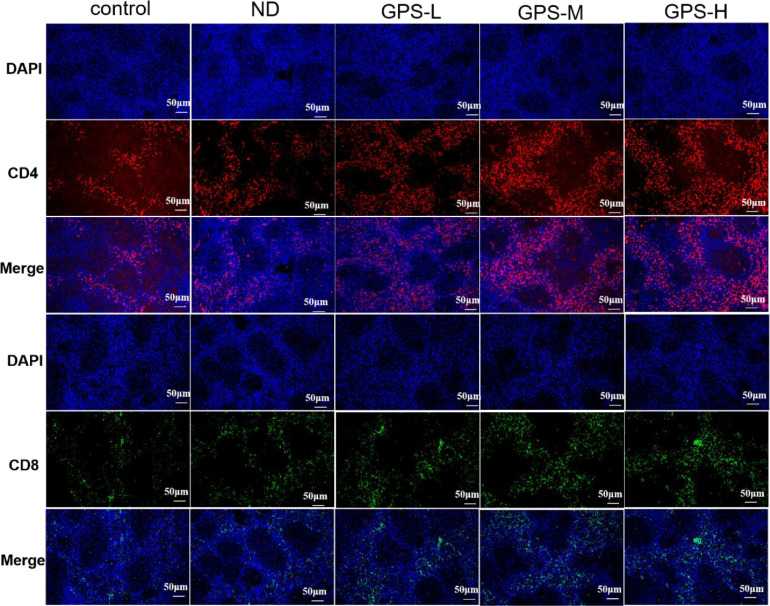
Immunofluorescence (IF) Analysis of Spleen
As a peripheral immune organ of the chicken, the spleen plays an important role in the humoral and cellular immune responses. The GPS-1 induced lymphocytic immune response of the spleen was visualized by immunofluorescence analysis and depicted in Figure 6. On the 28th d after immunization, the expressions of CD4+ and CD8+ in the spleens of the chickens from vaccination groups increased relative to the control. Moreover, all concentrations of GPS-1 could enhance the effects of NDV vaccine on the expressions of CD4+ and CD8+ in chickens’ spleens. However, the expressions of CD4+ and CD8+ raised less dramatically when the concentration of GPS-1 administered after vaccination was below 600 mg/kg (GPS-H dose).
Figure 6.
Results of spleen immunofluorescence analysis.
Immunofluorescence analysis of spleen CD4 (red) or CD8 (green) and DAPI (blue) in different treatment groups on the 28th d after NDV immunization.
DISCUSSION
Polysaccharides are often used as immune boosters to treat symptoms of immune deficiency in cancer or radiation therapies (Pelley and Strickland, 2000) and are also given to animals by oral feeding or injection to their immunity (Hristov et al., 1999). In this experiment, we immunized chickens by administering oral GPS-1 along with ND vaccine injection. Currently, vaccination is the most effective way to prevent and control NDV outbreaks in poultry industry (Ding et al., 2019). Despite decades of research and development, the ND vaccine still has some shortcomings. Incomplete or improper immunization often results in the disease and death of poultry after infection with virulent ND, and the humoral antibodies produced by NDV attenuated vaccine are low-level and short-lasting (Dimitrov et al., 2017). In this experiment, GPS may be helpful to solve the defect of ND vaccine.
To obtain credible results, some visual indicators were measured to show the efficacy of the ND vaccine and the immune boosting effect of GPS-1 in chickens. So, ND-HI levels were used to determine the effectiveness of the NDV vaccine. Because hemagglutinin (HA) is the main glycoprotein on the surface of ND virus and the main target antigen of humoral responses (Zhang et al., 2014). The results of the present investigation indicate that the different concentrations of GPS-1 tested could dose-dependently increase the expression of NDV antibodies (Figure 2A). This result most directly indicated that GPS-1 could effectively help the ND vaccine to produce higher efficacy.
GPS-1 can widely promote humoral immunity in chickens. Both IgG and IgA are secreted by B lymphocytes as part of humoral immunity. IgG is a basic indicator widely distributed in the body which reflects the virally induced humoral immunity level, and is extremely sensitive to viruses (Lin et al., 2008). IgA is a type of globulin mainly distributes in mucosa, which can help to improve the humoral immunity of chickens by preventing the attack of ND virus to their respiratory and digestive tracts. In this experiment, GPS-1 feedings of different concentrations were able to elevate the level of IgG and IgA throughout the 14-d administration period (Figure 2B). It was well established that polysaccharides could enhance the secretion of B lymphocytes, which was consistent with the results of the present investigation. However, after the GPS-1 administration period, the concentration of IgG in serum remained high among all vaccination groups, while IgA showed no statistically significant differences among any vaccination groups, with or without GPS-1. It was previously established that IgG levels could be maintained in organs and the humoral immune response for a longer period, while IgA could disappear rapidly without the stimulation of immune boosters, and our results were in accordance with this phenomenon.
Similarly, GPS-1 has been shown to be effective in modulating T-cell immunity, the results suggested that GPS-1 boosts immunity by activating lymphocytes. The activation of helper T cells (CD4+ T) and cytotoxic T cells (CD8+ T) are crucial for cellular immune responses and immune regulation (Liu et al., 2009; Cao et al., 2010). CD4+ is mainly expressed in helper T (Th) cells and is the receptor of Th cell TCR necessary to recognize antigens (Zhu et al., 2010). CD8+ T cells, also known as killer T cells, play an important role in recognizing and presenting antigens in specific immune responses (Clark et al., 2016). CD8+ T cells can differentiate and eliminate abnormal cells, such as those infected by pathogens (Meng et al., 2016). Numerous sources have reported that CD4+ T cells can promote CD8+ T cell expression (Abb et al., 1977; Aarvak et al., 1999; Ahrends and Borst, 2018). In this experiment, GPS-1 increased the proportion of both CD4+ T cells and CD8+ T cells in the spleen and peripheral blood lymphocytes. This was consistent with the results reported in the literature. CD4+ T cells could be categorized into Th1, Th2, and Th17 cell types according to the cytokines they secrete Th1 cells mainly secrete IL-2, IFN- γ, and TNF-α, and their function is to participate in the regulation of cellular immunity. Th2 cells can assist in the activation of B cells and mainly secrete IL-4 and IL-6 (Doan et al., 2020). Th17 is a newly discovered T cell subset capable of secreting IL-17, which can promote mucous glands to secrete a large amount of mucus and enhance mucosal immunity (Gaffen et al., 2014). Based on the flow cytometry analysis of spleen lymphocytes and peripheral blood lymphocytes after NDV vaccination, GPS-1 up-regulated the CD4+ T cells and CD8+ T cells in the spleen and peripheral blood (Figure 3), which led to an increase of cytokines. The levels of IL-2 and IFN-γ in serum were increased on the 28th d (Figure 4), which demonstrated the improvement of activity of CD8+ T cells. Similarly, the increase of IL-4 and IL-17 reflected the activation of CD4+ T cells. Present results indicated that GPS-1 enhanced the CD8+ mediated cytotoxic immune response, while the immunofluorescence data in Figure 6 illustrated the evidence that GPS-1 also expanded the range of responses activated by CD4+ (Hashiguchi et al., 2000).
This experiment verified GPS-1 as an immune booster capable of enhancing the antibody potency of the NDV vaccine (Figure 2A) and explored the reasons for this result. GPS-1 effectively enhanced the efficacy of the NDV vaccine in young chickens by promoting the activation of CD4+ and CD8+ T lymphocytes in the spleen and peripheral blood, which in turn promoted Th1 cytokines IL-2 and IFN-γ, Th2 cytokine IL-4, and the expression of cytokine IL-17 in Th17 cells. Elevation of these indicators suggested that GPS-1 could be used as an immune booster to improve the effectiveness of the NDV vaccine.
CONCLUSIONS
This study revealed the synergic activities of GPS-1 and the NDV vaccine in chickens. Being used in conjunction with NDV vaccination, GPS-1 could elevate the percentage of CD4+ and CD8+ cells in the spleen and peripheral blood lymphocytes. The resulting immune response displayed a dose-dependent increase in IL-2, IL-4, IL-17, and IFN-γ. This cytokine response induced chickens to produce more NDV antibodies, specifically IgG, and IgA. Moreover, even the highest concentration of GPS-1 in this experiment (600 mg/kg) showed no observable adverse effect to the young chickens. Our result suggested that GPS-1 may be valuable in the prevention of Newcastle disease and may thereby reduce the loss of poultry and marketable eggs.
Acknowledgments
ACKNOWLEDGMENTS
This research was financially supported by the National Natural Science Foundation of China (NSFC, Grant No. 31872514), the Open Project Program of Beijing Key Laboratory of Traditional Chinese Veterinary Medicine at Beijing University of Agriculture (No. kf-tcvm202101), Yunnan Provincial Science and Technology Department-Applied Basic Research Joint Special Funds of Yunnan University of Chinese Medicine [2018FF001 (-020), 2019FF002(-012)] and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We would like to extend our gratitude to all our distinguished colleagues who supported us in the Institute of Traditional Chinese Veterinary Medicine of Nanjing Agricultural University.
Credit Authorship Contribution Statement: Yi Wu: Conceptualization, Funding acquisition, Project ad-ministration, Supervision. Yu Wu: Data curation, Writing-Original draft preparation, Visualization. Nannan Li: Data curation, Visualization. Tao Zhang: Methodology and Editing. Yanyun Che: Writing-Reviewing, Methodology. Kun Duan: Writing-Reviewing and Editing. Audrey D Nguyễn: Writing-Reviewing and Editing. Yuedi Wang: Data curation. Hui Zhou: Data validation. Xin Wan: Data validation. Hongjun Lei: Data validation. Cristabelle De Souza: Writing-Reviewing and Editing. Kun Li: Methodology. Jiaguo Liu: Methodology. Deyun Wang: Methodology
Ethics Statement: 100 one-day-old male Hy-Line Brown chickens with no immunity to NDV were purchased from Nanjing Tegeili Agricultural Cooperative and raised in the Experimental Animal Center of Nanjing Agricultural University (NO. PZ2020101). They were kept in specific pathogen free (SPF) conditions at 37°C, with a daily cycle of 12 h of light and 12 h of darkness, and provided with normal drinking water and adequate food. All animal handling and care were performed following the Guide for the Care and Use of Laboratory Animals at Nanjing Agricultural University.
DISCLOSURES
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
References
- Aarvak T., Chabaud M., Kallberg E., Miossec P., Natvig J.B. Change in the Th1/Th2 phenotype of memory T-cell clones from rheumatoid arthritis synovium. Scand. J. Immunol. 1999;50:1–9. doi: 10.1046/j.1365-3083.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- Abb J., Kolb H.J., Rodt H.V., Grosse-Wilde H., Rieder I., Thierfelder S. In vitro and in vivo immune response to specific antigens in canine marrow graft recipients. Z Immunitatsforsch Immunobiol. 1977;153:152–161. [PubMed] [Google Scholar]
- Ahrends T., Borst J. The opposing roles of CD4(+) T cells in anti-tumour immunity. Immunology. 2018;154:582–592. doi: 10.1111/imm.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aipire A., Yuan P., Aimaier A., Cai S., Mahabati M., Lu J., Ying T., Zhang B., Li J. Preparation, characterization, and immuno-enhancing activity of polysaccharides from glycyrrhiza uralensis. Biomolecules. 2020;10:e8294. doi: 10.3390/biom10010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayeka P.A., Bian Y., Githaiga P.M., Zhao Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complement Altern. Med. 2017;17:536. doi: 10.1186/s12906-017-2030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayeka P.A., Bian Y., Mwitari P.G., Chu X., Zhang Y., Uzayisenga R., Otachi E.O. Immunomodulatory and anticancer potential of Gan cao (Glycyrrhiza uralensis Fisch.) polysaccharides by CT-26 colon carcinoma cell growth inhibition and cytokine IL-7 upregulation in vitro. BMC Complement. Altern. Med. 2016;16:206. doi: 10.1186/s12906-016-1171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Li X.Q., Wang X., Fan H.T., Zhang X.N., Hou Y., Liu S.B., Mei Q.B. A novel polysaccharide, isolated from Angelica sinensis (Oliv.) Diels induces the apoptosis of cervical cancer HeLa cells through an intrinsic apoptotic pathway. Phytomedicine. 2010;17:598–605. doi: 10.1016/j.phymed.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Chimeno Zoth S., Carballeda J.M., Gomez E., Gravisaco M.J., Carrillo E., Berinstein A. Modulation of innate immunity in chickens induced by in vivo administration of baculovirus. Vet. Immunol. Immunopathol. 2012;145:241–247. doi: 10.1016/j.vetimm.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Chu X., Ci X., Wei M., Yang X., Cao Q., Guan M., Li H., Deng Y., Feng H., Deng X. Licochalcone a inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo. J. Agric. Food Chem. 2012;60:3947–3954. doi: 10.1021/jf2051587. [DOI] [PubMed] [Google Scholar]
- Clark G.J., Kupresanin F., Fromm P.D., Ju X., Muusers L., Silveira P.A., Elgundi Z., Gasiorowski R.E., Papadimitrious M.S., Bryant C., Lee K.M., Clarke C.J., Young J.W., Chan A., Harman A., Botting R., Cabezon R., Benitez-Ribas D., Brooks A.E., Dunbar P.R., Hart D.N. New insights into the phenotype of human dendritic cell populations. Clin. Transl. Immunol. 2016;5:e61. doi: 10.1038/cti.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cor D., Knez Z., Knez Hrncic M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of ganoderma lucidum terpenoids and polysaccharides: a review. Molecules. 2018;23:649. doi: 10.3390/molecules23030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov K.M., Afonso C.L., Yu Q., Miller P.J. Newcastle disease vaccines-a solved problem or a continuous challenge? Vet. Microbiol. 2017;206:126–136. doi: 10.1016/j.vetmic.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Chen P., Bao X., Li A., Jiang Y., Hu Y., Ge J., Zhao Y., Wang B., Liu J., Chen H. Recombinant duck enteritis viruses expressing the Newcastle disease virus (NDV) F gene protects chickens from lethal NDV challenge. Vet. Microbiol. 2019;232:146–150. doi: 10.1016/j.vetmic.2019.04.022. [DOI] [PubMed] [Google Scholar]
- Doan T.D., Wang H.Y., Ke G.M., Cheng L.T. N-terminus of flagellin fused to an antigen improves vaccine efficacy against Pasteurella multocida infection in chickens. Vaccines (Basel) 2020;8:283. doi: 10.3390/vaccines8020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaish M., Xia M., Ngunjiri J.M., Ghorbani A., Jang H., Kc M., Abundo M.C., Dhakal S., Gourapura R., Jiang X., Lee C.W. Protective immunity against influenza virus challenge by norovirus P particle-M2e and HA2-AtCYN vaccines in chickens. Vaccine. 2019;37:6454–6462. doi: 10.1016/j.vaccine.2019.08.082. [DOI] [PubMed] [Google Scholar]
- Fu L., Wang X., Zhai J., Qi W., Jing L., Ge Y., Gao X., Liu C., Lv X., Zheng S. Changes in apoptosis, proliferation and T lymphocyte subtype on thymic cells of SPF chickens infected with reticuloendotheliosis virus. Mol. Immunol. 2019;111:87–94. doi: 10.1016/j.molimm.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Gaffen S.L., Jain R., Garg A.V., Cua D.J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P., Wusiman A., Zhang Y., Cai G., Xu S., Zhu S., Liu Z., Hu Y., Liu J., Wang D. Polyethylenimine-coated PLGA nanoparticles-encapsulated Angelica sinensis polysaccharide as an adjuvant for H9N2 vaccine to improve immune responses in chickens compared to Alum and oil-based adjuvants. Vet. Microbiol. 2020;251 doi: 10.1016/j.vetmic.2020.108894. [DOI] [PubMed] [Google Scholar]
- Guo L.X., Nie F.R., Huang A.Q., Wang R.N., Li M.Y., Deng H.Y., Zhou Y.Z., Zhou X.M., Huang Y.K., Zhou J., Ji Y.D. Transcriptomic analysis of chicken immune response to infection of different doses of Newcastle disease vaccine. Gene. 2021;766 doi: 10.1016/j.gene.2020.145077. [DOI] [PubMed] [Google Scholar]
- Hao B., Wang X., Ma X., Jin Y., Fan W., Laba C., Wujin C., Wang Y., Liang J. Preparation of complex microcapsules of soluble polysaccharide from Glycyrrhiza uralensis and its application in wound repair and scar inhibition. Int. J. Biol. Macromol. 2020;156:906–917. doi: 10.1016/j.ijbiomac.2020.03.121. [DOI] [PubMed] [Google Scholar]
- Hashiguchi M., Hachimura S., Ametani A., Kaminogawa S. Th2 polarization enhanced by oral administration of higher doses of antigen. Cytotechnology. 2000;33:237–245. doi: 10.1023/A:1008102304740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama K., Takahashi M., Suzuki M., Ezawa K., Yamazaki M., Matsukawa T., Kishi A., Sato N., Abe S. [Anti-Candida activity of aroma candy and its protective activity against murine oral candidiasis] Med. Mycol. J. 2015;56:J23–J29. doi: 10.3314/mmj.56.J23. [DOI] [PubMed] [Google Scholar]
- Homme, P. J., and B. C. Easterday 1970. Avian influenza virus infections.
- Hou Y., Hou Y., Yanyan L., Qin G., Li J. Extraction and purification of a lectin from red kidney bean and preliminary immune function studies of the lectin and four Chinese herbal polysaccharides. J. Biomed. Biotechnol. 2010;2010 doi: 10.1155/2010/217342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov A.N., McAllister T.A., Cheng K.J. Effect of diet, digesta processing, freezing and extraction procedure on same polysaccharide-degrading activities of ruminal contents. Can. J. Anim. Sci. 1999;79:73–81. [Google Scholar]
- Kimani F.W., Ajit J., Galluppi A., Manna S., Howitz W.J., Tang S., Esser-Kahn A.P. Receptor-ligand kinetics influence themechanism of action of covalently linked TLR ligands. ACS Chem. Biol. 2021;16:380–388. doi: 10.1021/acschembio.0c00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K.M., Vasconcelos A.C. Newcastle disease virus-induced apoptosis in chicken peripheral blood lymphocytes. Vet. Immunol. Immunopathol. 1994;44:45–56. doi: 10.1016/0165-2427(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Lian K.X., Zhu X.Q., Chen J., Liu G., Gu X.L. Selenylation modification: enhancement of the antioxidant activity of a Glycyrrhiza uralensis polysaccharide. Glycoconj. J. 2018;35:243–253. doi: 10.1007/s10719-018-9817-8. [DOI] [PubMed] [Google Scholar]
- Lin F., Huang J.X., Chen C., Liu J., Cui Y.L. [Preparation of hen egg yolk immunoglobulin and partial plasma high abundant protein depletion by prepared chicken immunoglobulin coupled on GoldMag carrier] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008;24:706–709. [PubMed] [Google Scholar]
- Lindenwald R., Pendl H., Scholtes H., Schuberth H.J., Rautenschlein S. Flow-cytometric analysis of circulating leukocyte populations in turkeys: Establishment of a whole blood analysis approach and investigations on possible influencing factors. Vet. Immunol. Immunopathol. 2019;210:46–54. doi: 10.1016/j.vetimm.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Liu T., Song Y., Yang Y., Bu Y., Cheng J., Zhang G., Xue J. Hemagglutinin-Neuraminidase and fusion genes are determinants of NDV thermostability. Vet. Microbiol. 2019;228:53–60. doi: 10.1016/j.vetmic.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Liu Y., Jiao F., Qiu Y., Li W., Lao F., Zhou G., Sun B., Xing G., Dong J., Zhao Y., Chai Z., Chen C. The effect of Gd@C82(OH)22 nanoparticles on the release of Th1/Th2 cytokines and induction of TNF-alpha mediated cellular immunity. Biomaterials. 2009;30:3934–3945. doi: 10.1016/j.biomaterials.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Meng C., Zhi X., Li C., Li C., Chen Z., Qiu X., Ding C., Ma L., Lu H., Chen D., Liu G., Cui D. Graphene oxides decorated with carnosine as an adjuvant to modulate innate immune and improve adaptive immunity in vivo. ACS Nano. 2016;10:2203–2213. doi: 10.1021/acsnano.5b06750. [DOI] [PubMed] [Google Scholar]
- Mutaillifu P., Bobakulov K., Abuduwaili A., Huojiaaihemaiti H., Nuerxiati R., Aisa H.A., Yili A. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macromol. 2020;144:751–759. doi: 10.1016/j.ijbiomac.2019.11.245. [DOI] [PubMed] [Google Scholar]
- Pan L.C., Zhu Y.M., Zhu Z.Y., Xue W., Liu C.Y., Sun H.Q., Yue Y. Chemical structure and effects of antioxidation and against alpha-glucosidase of natural polysaccharide from Glycyrrhiza inflata Batalin. Int. J. Biol. Macromol. 2020;155:560–571. doi: 10.1016/j.ijbiomac.2020.03.192. [DOI] [PubMed] [Google Scholar]
- Pelley R.P., Strickland F.M. Plants, polysaccharides, and the treatment and prevention of neoplasia. Crit. Rev. Oncog. 2000;11:189–225. [PubMed] [Google Scholar]
- Qiu X., Fu Q., Meng C., Yu S., Zhan Y., Dong L., Ren T., Sun Y., Tan L., Song C., Han X., Ding C. Kinetic analysis of RNA editing of Newcastle disease virus P gene in the early period of infection. Acta Virol. 2016;60:71–77. doi: 10.4149/av_2016_01_71. [DOI] [PubMed] [Google Scholar]
- Scherer S., Huhle D., Gobel T.W. Identification of chicken GITR and GITR ligand, proof of their mutual interaction, and analysis of chicken GITR tissue distribution by a novel antibody that reveals expression on activated T Cells and erythrocytes. Immunohorizons. 2018;2:324–337. doi: 10.4049/immunohorizons.1800065. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J.B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Kou Y., Xiao J., Zhang L., Gao F., Kong W., Su W., Jiang C., Zhang Y. Comparison of immunogenicity, efficacy and transcriptome changes of inactivated rabies virus vaccine with different adjuvants. Vaccine. 2018;36:5020–5029. doi: 10.1016/j.vaccine.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Shin S., Jang J.Y., Choi B.I., Baek I.J., Yon J.M., Hwang B.Y., Park D., Jeon J.H., Nam S.Y., Yun Y.W., Kim Y.B. Licorice extract does not impair the male reproductive function of rats. Exp. Anim. Tokyo. 2008;57:11–17. doi: 10.1538/expanim.57.11. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhao Z., Zhang C., Liu Y., Ding K., Li Y., Cheng X., Chen P. [Influence of fusion protein of IBDV VP2 and chicken interleukin-2 on immune response in chicken] Sheng Wu Gong Cheng Xue Bao. 2010;26:476–482. [PubMed] [Google Scholar]
- Wang F., Pang J.D., Huang L.L., Wang R., Li D., Sun K., Wang L.T., Zhang L.M. Nanoscale polysaccharide derivative as an AEG-1 siRNA carrier for effective osteosarcoma therapy. Int. J. Nanomed. 2018;13:857–875. doi: 10.2147/IJN.S147747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Meng X., Yang R., Qin T., Li Y., Zhang L., Fei C., Zhen W., Zhang K., Wang X., Hu Y., Xue F. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int. J. Biol. Macromol. 2013;59:178–183. doi: 10.1016/j.ijbiomac.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Wang M., Wei Y., Pu J., Bing G., Sun Y., Sun H., Wei F., Liu J. Cross- immunity of a H9N2 live attenuated influenza vaccine against H5N2 highly pathogenic avian influenza virus in chickens. Vet. Microbiol. 2018;220:57–66. doi: 10.1016/j.vetmic.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Wu Y., Yi L., Li E., Li Y., Lu Y., Wang P., Zhou H., Liu J., Hu Y., Wang D. Optimization of Glycyrrhiza polysaccharide liposome by response surface methodology and its immune activities. Int. J. Biol. Macromol. 2017;102:68–75. doi: 10.1016/j.ijbiomac.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Xue J., Xu Y., Jin L., Liu G., Sun Y., Li S., Zhang J. Effects of traditional Chinese medicine on immune responses in abalone, Haliotis discus hannai Ino. Fish Shellfish Immunol. 2008;24:752–758. doi: 10.1016/j.fsi.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Zhang W., Wang L., Liu Y., Chen X., Li J., Yang T., An W., Ma X., Pan R., Ma G. Comparison of PLA microparticles and alum as adjuvants for H5N1 influenza split vaccine: adjuvanticity evaluation and preliminary action mode analysis. Pharm. Res. 2014;31:1015–1031. doi: 10.1007/s11095-013-1224-z. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhao S., Song X., Jia J., Zhang Z., Zhou H., Fu H., Cui H., Hu S., Fang M., Liu X., Bian Y. Inhibition effect of glycyrrhiza polysaccharide (GCP) on tumor growth through regulation of the gut microbiota composition. J. Pharmacol. Sci. 2018;137:324–332. doi: 10.1016/j.jphs.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Zhu J., Yamane H., Paul W.E. Differentiation of effector CD4 T cell populations (*) Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]