Abstract
Age-related neurodegeneration characteristic of late-onset Alzheimer’s disease (LOAD) begins in middle age, well before symptoms. Translational models to identify modifiable risk factors are needed to understand etiology and identify therapeutic targets. Here we outline the evidence supporting the vervet monkey (Chlorocebus aethiops sabaeus) as a model of aging-related AD-like neuropathology and associated phenotypes including cognitive function, physical function, glucose handling, intestinal physiology, and CSF, blood, and neuroimaging biomarkers. This review provides the most comprehensive multi-system description of aging in vervets to date. This review synthesizes a large body of evidence that suggests that aging vervets exhibit a coordinated suite of traits consistent with early AD and provide a powerful, naturally occurring model for LOAD. Notably, relationships are identified between AD-like neuropathology and modifiable risk factors. Gaps in knowledge and key limitations are provided to shape future studies to illuminate mechanisms underlying divergent neurocognitive aging trajectories, and to develop interventions that increase resilience to aging-associated chronic disease, particularly LOAD.
Keywords: Alzheimer’s disease, Vervet, African green monkey, Cognitive Decline, Gait Speed
1. Introduction
The Problem.
Alzheimer’s disease (AD), the most common cause of dementia, affects 40 million people worldwide, and is expected to increase sharply due to the increasing proportion of aging adults (“2020 Alzheimer’s disease facts and figures,” 2020). The sporadic (i.e., late-onset AD or LOAD) form of AD accounts for approximately 95% of all cases, and age is the greatest risk factor for developing the disease. LOAD is characterized by a decades long preclinical phase, in which patients remain largely asymptomatic (Jack et al., 2018; Palmqvist et al., 2020; Ritchie, Ritchie, Yaffe, Skoog, & Scarmeas, 2015). There are no FDA-approved therapies to delay the symptom onset or progression of AD (“2020 Alzheimer’s disease facts and figures,” 2020). However, clinical trials have repeatedly found that early intervention is the most promising approach to alter disease course. Modifiable risk factors, including diet, obesity, hypertension, impaired glucose tolerance, psychosocial stress, and poor sleep, significantly increase later-life AD risk, and all are amenable to intervention (Dhana, Evans, Rajan, Bennett, & Morris, 2020; Edwards III, Gamez, Escobedo, Calderon, & Moreno-Gonzalez, 2019; Li et al., 2019; Winer et al., 2020; Yu et al., 2020). Due to the complexity of the disease and its extended time course, appropriate experimental models that closely recapitulate AD are needed to understand mechanisms, identify novel targets, and develop effective interventions.
Animal Models.
Several small animal models are available. However, they primarily model early-onset familial AD which accounts for only 5% of cases, and unfortunately, they have had limited predictive value for clinical trial outcomes. Most models are transgenic mice overexpressing mutant human genes, consistent with familial AD, which only partially recapitulate AD symptoms and neuropathologic features (Drummond & Wisniewski, 2017) and have not led to effective therapies to date. Therefore, non-rodent mammalian models that recapitulate risk factors for, and neuropathology of, LOAD are sorely needed. Nonhuman primates (NHPs) represent important models of unmanipulated cognitive aging that may be useful for studying characteristics of LOAD (Latimer et al., 2019; Phillips et al., 2014; Voytko & Tinkler, 2004). Advantages of NHP include their phylogenetic proximity and similarity to humans in brain structure and function, and their endocrine, social, and cognitive characteristics (Jasinska et al., 2013). Their relatively large size makes them favorable for imaging studies and cerebrospinal fluid collection. NHP beta-amyloid (Aβ) peptide, a hallmark pathology of AD, has 100% sequence homology with human Aβ and naturally accumulates in the brain with age (Drummond & Wisniewski, 2017; Podlisny, Tolan, & Selkoe, 1991). Here we review the utility and characteristics of one of these NHP models of age-related AD-like neuropathology and accompanying cognitive and physical decline, the vervet monkey (Chlorocebus aethiops sabaeus).
The Vervet Research Colony and Aging Vervet Cohort Study Population.
The studies in vervet monkeys described here were performed in the Vervet Research Colony (VRC) of Wake Forest School of Medicine. The VRC is a pedigreed, genomically sequenced, pathogen-free breeding colony of Caribbean-origin vervets (a.k.a. African green monkeys). Housing and husbandry practices encourage species-typical behavior. As of 2020 the population included about 300 animals, most of which live in 16 one-male multi-female breeding groups reflecting social composition of wild vervet groups, and 2 all-male groups. All animals were captive-born and mother-reared in species-typical social groups, are of known age, and range from newborn to natural end of life (0–29 years of age (yoa)). The 16 breeding groups are housed in enclosures with 1,000 sq. ft. of outdoor and 300 sq. ft. of indoor space. Food (LabDiet monkey chow) and water are provided ad libitum. VRC monkeys experience an array of environmental enrichment opportunities, including forage and sensory supplements. Infants and juveniles remain in the natal group with their mothers and female kin. Males are removed at adolescence and transferred to all-male or other breeding groups. Periodically new Caribbean-origin males are added, and adult males are rotated between breeding groups to mimic the natural process of male emigration/immigration, maintain genetic variability, minimize inbreeding depression, and promote long-term viability of the colony. The 2020 population includes descendants of the 57 original founders (29 females, 28 males), imported from St. Kitts and the West Indies between 1976 and 1985, with 24 of the original matrilines now in their 2nd-9th generation. Routine annual phenotyping includes body weight, body mass index, waist circumference, veterinary exam, complete blood count and chemistry, and plasma and serum are banked twice per year.
The husbandry practices at the VRC are ideal for studies of AD-like neuropathology and aging-related phenotypes. Members of the VRC do not undergo prolonged periods of social separation during data collection. Rather, VRC monkeys spend the vast majority of their time embedded within their social groups. Social separation is a reliable stressor for non-human primates (Novak, Hamel, Kelly, Dettmer, & Meyer, 2013; Shively et al., 2020; Taylor, Mustoe, Hochfelder, & French, 2015), and stress responses impact NHP learning and cognitive performance (Arnsten, 2015; Lyons, Lopez, Yang, & Schatzberg, 2000; McEwen & Sapolsky, 1995). Social isolation also has been linked to increased risk for neurodegenerative disorders, including LOAD in humans (National Academies of Sciences, Engineering & Medicine, 2020). Thus, minimizing stressors, particularly those associated with social separation, may be important for aging studies’ translational value given the effects of such stressors on NHP behavior and health (Shively et al., 2020; Snyder-Mackler et al., 2019; Wittig et al., 2016).
Ethical Considerations.
This research complied with protocols approved by the Wake Forest Institutional Animal Care Committee. All studies conducted at the VRC adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non-Human Primates.
2. The Evidence for an Age-Related Vervet Model of Late Onset Alzheimer’s Disease
Female vervets reach adulthood around 4 years of age, experience reproductive decline after 16 years, reach menopause around 24 years of age, and have a maximum lifespan of about 30 years (Atkins et al., 2014; Tacutu et al., 2018; Weigl, 2005). At the VRC, the oldest female lived 29.8 years, and the average lifespan for members of the aging cohort is approximately 24.9 years. To determine the degree to which vervets replicate the pathologic, physiological, and behavioral hallmarks of early AD, we extensively characterized nine middle-aged (mean=11.2 years) and nine elderly (mean=21.7 years) females (Latimer et al., 2019). A multisystem array of diagnostics was used to characterize phenotypes typically associated with AD pathophysiology including neuropathology, functional and structural neuroimaging, cerebrospinal fluid (CSF) biomarkers, and assessments of gait speed.
Aging-Related Neuropathologic Features (Figure 1).
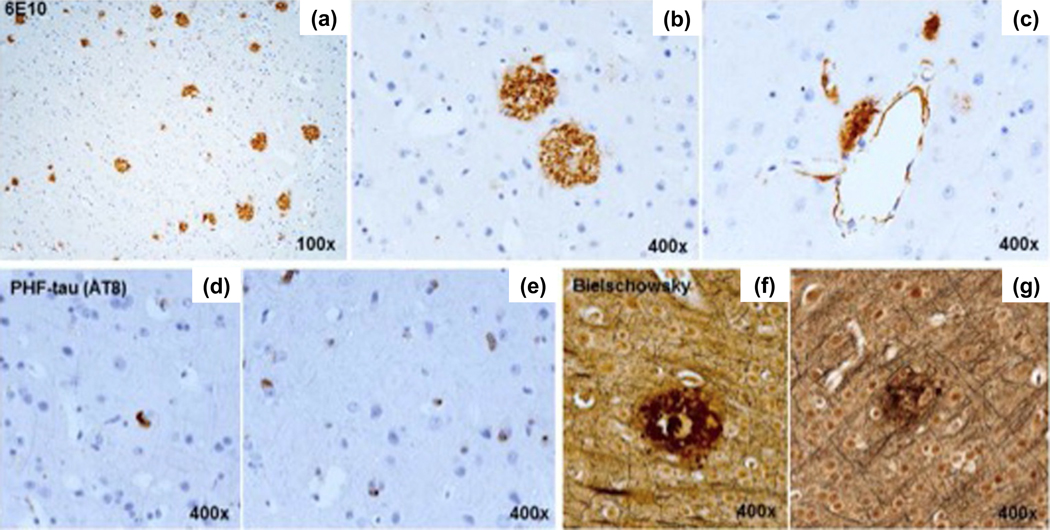
Figure 1.
(a, b) Aβ plaques were histologically similar to those in human AD. (c) Focal vascular wall Aβ was also noted. (d, e) Granular cytoplasmic PHF-tau aggregates were present; neurofibrillary tangles were rare. (f, g) Neuritic plaques were confirmed with Bielschowsky stain. Adapted from (Latimer et al., 2019).
To support direct translation to clinical studies, the brain collection protocol was adapted from the National Institute on Aging and Alzheimer’s Association (NIA-AA) guidelines for the collection of human brains for AD repositories (Montine et al., 2012). Aβ plaques were histologically similar to those in human AD, as has been previously reported (Kalinin et al., 2013; Lemere et al., 2004). Focal vascular wall Aβ was observed. Granular cytoplasmic paired helical filament (PHF)-tau immunoreactivity was common, but neurofibrillary tangles were rare. Neuritic plaques were confirmed with Bielschowsky stain. Amyloid plaque load ranked semi-quantitatively, by absent to severe extent, in multiple brain regions showed regional variation throughout the cerebral cortex. The temporal cortex, particularly the middle and superior temporal gyri, was most heavily affected, as illustrated in a heat map of coronal sections of the vervet brain (Figure 2a), similar to patterns observed in the early stages of human AD (Goethe et al., 2017). Compared to middle-aged vervets, old animals had greater plaque burden (Figure 2b). Tissue Aβ1–42 concentrations (guanidine-extracted) were higher in old than middle-aged vervets in the temporal and parietal lobes, and PHF-tau tissue concentrations (RIPA-extracted) were higher in old than middle-aged animals in the temporal lobe (Latimer et al., 2019).
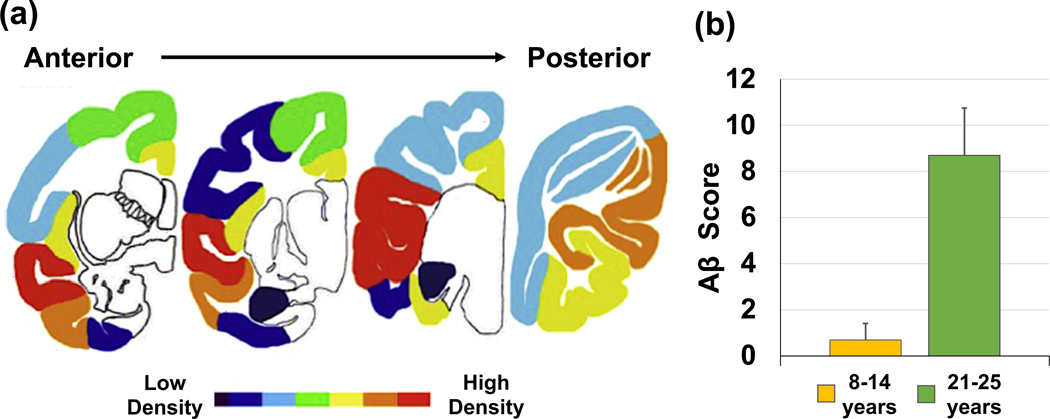
Figure 2.
(a) The density of Aβ pathology is illustrated by heat map. On average, Aβ plaque density was highest in the lateral temporal lobe and lowest in the medial temporal lobe regions. Aβ pathology was not found in deep cerebral nuclei, brainstem or cerebellum. Adapted from (Latimer et al., 2019). (b) Old vervets (21–25 years) had higher Aβ burdens than did middle-aged vervets (8–14 years). Aβ=β-amyloid. Adapted from (Latimer et al., 2019).
Functional and Structural Correlates of Neuropathology.
In humans with mild cognitive impairment (MCI), low cerebral metabolic rate of glucose utilization (CMRg) measured by 18F-flourodeoxyglucose (18F-FDG) positron emission topography (PET) in frontal, posterior cingulate cortex, and temporal lobe predicts conversion to AD (Villa, Lavitrano, Salvatore, & Combi, 2020) (Ma et al., 2018) (Kim et al., 2010; Ou et al., 2019). Structural magnetic resonance imaging (MRI) measures of temporal lobe and hippocampal volumes are smaller in age-associated memory impairment and MCI, and predict AD (Anstey & Maller, 2003; Varghese, Sheelakumari, James, & Mathuranath, 2013; Villa et al., 2020). CSF Aβ1–42, total tau, and tau phosphorylated at amino acid 181 (tau-P181) concentrations reflect changes in the intraparenchymal production, clearance, and aggregation rates of amyloid and tau proteins. Lower levels of CSF Aβ1–42 reflect increased brain amyloid aggregation, whereas elevated CSF tau-P181 and total tau are directly associated with increased tau pathology in the brain (Jack et al., 2013). Gait speed, a complex index of higher cognitive and motor integration, is associated with brain Aβ accumulation and is a risk factor for dementia including AD (Dumurgier et al., 2017; Latimer et al., 2019; Wennberg, Savica, Hagen, et al., 2017).
In the vervet model, FDG PET was used to determine functional metabolic differences in several brain regions as reflected in the CMRg. MRI was used to quantify volumetric differences in regions of interest. CSF Aβ1–42, total tau, and tau-P181 were assayed by Luminex-based INNO-BIA Alzbio3 assay, Aβ1–40 was measured by ELISA (Fujirebio, Malvern, PA), and gait speed was assessed by behavior observation as a measure of functional decline (Latimer et al., 2019) (see below for methods).
CSF Biomarkers.
Vervet CSF Aβ1–42 and Aβ1–40 levels correlated negatively with age and Aβ plaque density, as in humans. CSF Aβ1–42 was inversely related to guanidine-extracted Aβ1–42 in temporal cortex and showed a similar trend in parietal cortex, where CSF Aβ1–40 was negatively associated with guanidine-extracted Aβ1–42.
Gait Speed.
Slower gait speed was associated with increased age, lower CSF Aβ1–42 levels and greater concentrations of guanidine-extracted Aβ1–42 in temporal lobe.
MRI Volumes.
Older animals with greater Aβ plaque burden had reduced volumes of bilateral frontal, left prefrontal and putamen, a relationship also observed in humans (Whitwell et al., 2013). Greater PHF-tau tissue burden was associated with reduced volume bilaterally in insula and cerebellum, as well as the left temporal lobe. Higher CSF tau-P181 values were associated with reduced prefrontal and temporal volumes (Figure 3).
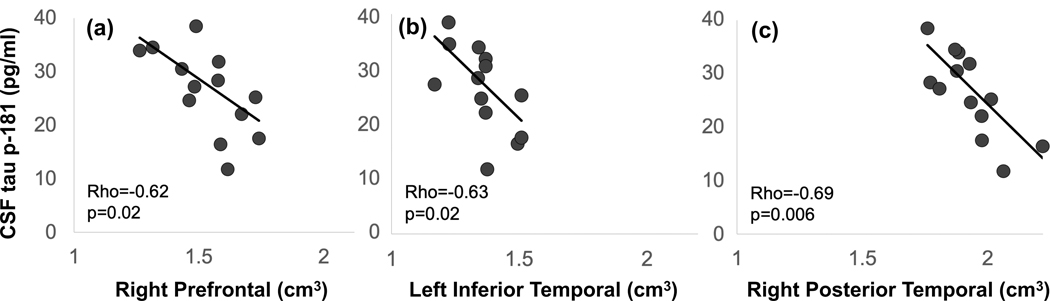
Figure 3.
Spearman rank order correlations between CSF tau-P181 and MRI volume for (a) right prefrontal, (b) left inferior temporal, and (c) right posterior temporal regions. Higher tau-p181 values were associated with reduced volumes. Abbreviations: CSF, cerebrospinal fluid; MRI, magnetic resonance imaging. Adapted from Latimer et al. (2019).
FDG PET CMRg.
CMRg was inversely associated with Aβ plaque density in bilateral hippocampus, parietal, occipital and cerebellar regions of interest, and left temporal lobe. CMRg in all brain regions was inversely associated with guanidine-extracted tau-P181 in the parietal lobe, and inversely associated with higher guanidine extracted Aβ in most regions (i.e., bilateral parietal, temporal visual and limbic, occipital, cingulate, hippocampus, amygdala, cerebellum, right prefrontal, frontal, and insula). CMRg in several brain areas (left hippocampus, caudate nucleus, putamen, cerebellum, occipital, and pons, and right cingulate, insula, and occipital) correlated positively with CSF Aβ1–42 (Figure 4).
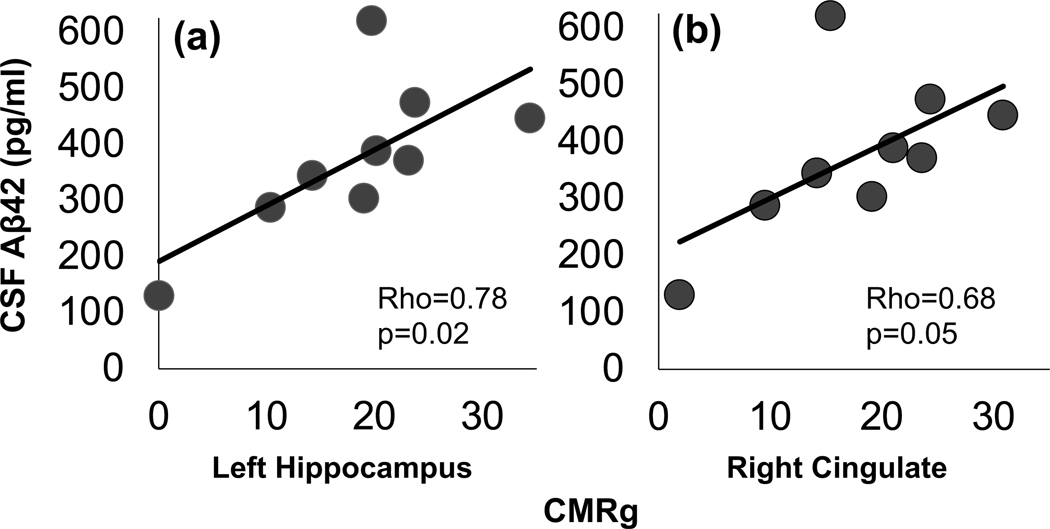
Figure 4.
Spearman rank order correlations between CSFAβ42 and cerebral metabolic rate of glucose utilization in (a) left hippocampus and (b) right cingulate. Abbreviations: CSF, cerebrospinal fluid; Aβ, β-amyloid. Adapted from (Latimer et al., 2019).
Summary.
These results suggest that aged vervets show human-like Aβ accumulation, that CSF and neuroimaging biomarkers are associated with neuropathologic features, and that behavioral changes accompanied AD pathologic changes. Overall, Latimer et al. found strong evidence that aged vervets recapitulated many of the characteristics of early AD and is thus a suitable model to study the early etiology of LOAD. Notwithstanding this strong body of evidence, these conclusions are based on investigations of elder females. Studying sex differences in aging patterns, as well as how sex may modulate the impact of environmental factors, is key to understanding disparities in disease risk (Dumurgier et al., 2017; Scheyer et al., 2018). While sex-specific investigations of females are needed because late-onset AD is more prevalent in women, and there may be sex differences in etiology, presentation, and outcomes (Mielke, Vemuri, & Rocca, 2014), future studies including elder males are needed to fully develop vervets as a model of early AD-like neuropathology.
Evidence of Age-Related Neuropathologic Changes from other Laboratories.
Cramer et al. (2018) also characterized AD-relevant phenotypes in vervets. Like Latimer et al., Cramer et al. found that Aβ plaque burden increased with age in cingulate, entorhinal, and frontal cortex, but not hippocampus or visual cortex, a pattern similar to that in humans (Thal, Rub, Orantes, & Braak, 2002). Aβ tissue levels, extracted with guanidine from prefrontal cortex also increased with age. Tau phosphorylated at Ser202 / Thr205, stained with AT8, was observed in 2 older animals throughout the entorhinal cortex and within the hippocampus. There was also evidence of Gallyas silver-stained neurofibrillary tangles, a pattern consistent with pathologic tau in early AD in human brains (Hansson et al., 2017). CSF total and phospho-tau increased with age. Like AD patients, old vervets had higher total tau/Aβ42 (Herukka, Hallikainen, Soininen, & Pirttilä, 2005).
Cramer et al. (2018) compared transcriptional profiles from visual cortex and dorsolateral prefrontal cortex of rhesus macaques (Macaca mulatta), vervets, and older humans with and without AD. Age-related changes in gene expression profiles in these two areas were similar to AD patients in aged vervets, but more similar to healthy aged humans in rhesus macaques (Cramer et al., 2018). The gene expression profile of AD patients and aged vervets was enriched with inflammatory cytokine and microglial genes, and in pathways associated with cell adhesion, migration, and morphogenesis (Podtelezhnikov et al., 2011), which are hypothesized to contribute to AD-like pathophysiologic processes (Hampel et al., 2020; Hashemiaghdam & Mroczek, 2020). Cramer et al studied 12 female and 7 male vervets (6 females and 5 females were 15 years of age and older), 14 female and 11 male rhesus (5 females and 1 male were aged 15 years or older), and their analysis controlled for sex. There were few sex X age effects on gene expression and no sex differences in other endpoints reported (Cramer et al., 2018). Thus, these similar molecular signatures within the vervet brain may provide opportunities to understand the biological mechanisms that contribute to neurodegenerative disease in humans.
Because of the similarities to humans in age-related AD-like neuropathologic features, vervets are being used to study the impact of Aβ oligomer administration, therapeutic interventions, and potential AD vaccines (Batista et al., 2018; Hara et al., 2016; Lemere et al., 2004).
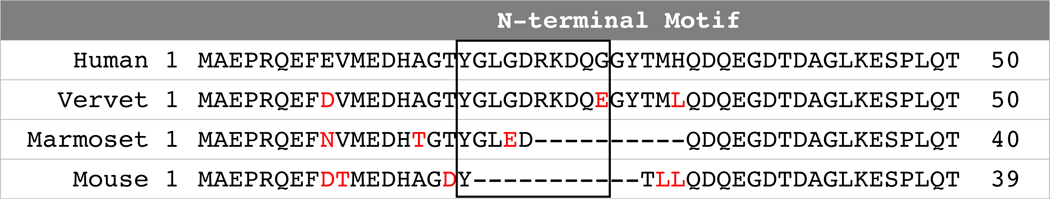
Tau.
Neurofibrillary tangles are composed of abnormal post-translationally modified tau, including hyperphosphorylation, and are the closest correlate of neurodegeneration and dementia in human AD. While neurofibrillary tangles are diagnostic of late stage AD, the series of post-translational modifications leading to this end point represent progressive disease stages and can be tracked (Arriagada, Growdon, Hedley-Whyte, & Hyman, 1992). The tau N-terminalis important in protein-protein interactions. Figure 5 shows that vervets share with humans the N-terminal tau motif also present in apes and other Cercopithecine monkeys, but not in rodents or marmosets. This characteristic represents an important potential advantage of the vervet model compared to rodents and marmosets (Sharma et al., 2019; Stefanoska et al., 2018). In a pilot study, we used capillary electrophoresis and a panel of anti-tau antibodies specific to epitopes common across all splice variants of human tau to assess tau in vervet brain extracts. Our pilot data have confirmed N-terminal motif expression as indicated by antibody immunoreactivity against amino acids 2–18 (based on amino acid numbering of the longest human isoform), and which is absent in mouse and marmoset tau (Orr unpublished data). Additionally, our pilot data suggest that adult vervets express all six tau protein isoforms expressed in human brain (Orr, unpublished data), a major strength over rodents and marmosets that do not express appreciable levels of 3R isoforms in adulthood (i.e., 1:1 ratio of 3R:4R), and a deficiency thought to contribute to the lack of naturally occurring pathologic tau in rodents (Liu & Gong, 2008; Sharma et al., 2019) In spite of these similarities, vervets, like most other NHPs, do not readily develop the extensive neurofibrillary tangles that are characteristic of AD, perhaps due to a shorter lifespan compared with humans, or to the ratio of tau isoforms (e.g. 3R-to-4R tau) which is central to differences in several tauopathies, including AD (Dujardin et al., 2018; Kaufman et al., 2016; Sanders et al., 2014). Nonetheless, these differences require further study to understand the mechanisms underlying apparent resistance to developing extensive neurofibrillary tangles. Such work may provide important insights into the mechanisms underlying NHPs’ resilience to AD symptoms and may lead to identification of novel therapeutic targets.
Figure 5.
Vervet tau is 98% identical to human tau and adults express all six human brain tau isoforms. Protein sequence alignment of vervet, marmoset, and mouse tau with the 1st 50 amino acids of the longest human tau isoform with sequences. Black box: primate-specific amino acid segment between residues 18–28, a region important for mediating protein-protein interactions. Red font: amino acids different than human tau.
Associations Between AD CSF Biomarkers and Impaired Glucose Metabolism.
CSF biomarkers also may be tied to metabolic perturbations. Like humans, some vervets develop prediabetes and type 2 diabetes with age (Kavanagh, Day, et al., 2019; Kavanagh et al., 2007), conditions which are associated with increased risk of LOAD (An et al., 2018; Craft, 2012; Ferreira, Fernandes, Vieira, & De Felice, 2018). Vervets therefore provide opportunities to explore the relationships between metabolic state and LOAD risk. Peripheral metabolic markers (fasting glucose and plasma lactate) and AD biomarkers (CSF Aβ1–40 and Aβ1–42) were recently evaluated in healthy, prediabetic, and diabetic monkeys aged 16–23 years (Kavanagh, Day, et al., 2019). Relative to healthy controls, prediabetic and diabetic animals showed decreased CSF Aβ1–40 and Aβ1–42, a signature consistent with Aβ accumulation in the brain (Tapiola et al., 2009). Significant correlations between CSF glucose and plasma lactate with CSF Aβ1–40 and Aβ1–42 also were observed, suggesting that glucose metabolism was associated with the production and accumulation of Aβ in the central nervous system. Taken together, these results suggest that Aβ accumulation in the brain may be directly related to glucose dysregulation (Figure 6a).
Figure 6.
The vervet model of AD-like neuropathology and related behavioral and physiological characteristics.(a) Potential relationships between metabolic state and AD biomarkers in the plasma and CSF (adapted from Kavanagh et al., 2019). (b) The measurement of walking speed. Marks were made with indelible markers at visible points on various structures throughout the home pens. Each landmark was given an identification (e.g. A, B…etc.), and distances between landmarks were measured. Every time a subject passed a landmark they were timed with a stopwatch until they passed another landmark, and time and landmarks were recorded. Walking speed was calculated as distance/time. (c) Older adult vervets had a lower Firmicutes:Bacteroidetes ratio than did younger adults (p=0.03) (adapted from Vemuri et al., 2020). (d) Two examples from the Wake Forest Maze Task of executive function; examples of an easy (left) maze and difficult (right) maze. (e) Coronal sections illustrating the density of Aβ pathology; illustrated by heat map (adapted from Latimer et al., 2019).
3. Aging Related Physical Decline
Human gait speed predicts disability, cardiovascular risk, and mortality, and slow gait is associated with increased risk for cognitive decline (Abellan van Kan et al., 2012; Best et al., 2016; Callisaya et al., 2015; Chou et al., 2019; Dumurgier et al., 2017; Handing et al., 2020; Hoogendijk et al., 2020; Montero-Odasso et al., 2020; Peel, Alapatt, Jones, & Hubbard, 2019; Rosso et al., 2017; Taniguchi, Yoshida, Fujiwara, Motohashi, & Shinkai, 2012; Tian et al., 2020; Verghese et al., 2002; Waite et al., 2005), and dementia, including all-cause AD (Dumurgier et al., 2017). Thus, diminished gait speed may provide a robust diagnostic for identifying at-risk phenotypes for targeted interventions. Despite considerable interest in this biomarker, the mechanisms underlying the relationships between diminishing gait and cognitive impairment are poorly understood.
NHP models, including vervets, provide key opportunities to more fully investigate mechanistic pathways mediating physical and cognitive declines with age. Ten years ago, we began to study gait speed and other aspects of physical function in vervets and macaques (M. fascicularis & M. radiata). We developed a measure of usual gait speed, defined as walking that is unprovoked either by conspecifics or desirable objects (e.g., food), as a translational metric for assessing functional capabilities, thereby enabling direct comparisons between findings in preclinical and clinical studies (Justice, Cesari, Seals, Shively, & Carter, 2016; Shively et al., 2012) (Figure 6b). We also assessed other locomotor behaviors, including all instances of locomotion, climbing, and leaping/jumping of NHPs in their home enclosures (Shively et al., 2012). While older and younger monkeys showed no differences in overall activity levels, aged monkeys climbed less, and tended to jump and leap less frequently than did younger animals. Older monkeys also had significantly slower gait speed, a difference of 20% compared to young monkeys. These findings were recapitulated in hamadryas baboons (Papio hamadryas) for which age-related declines in gait speeds were also reported (Huber, Gerow, & Nathanielsz, 2015), and similar observations of aging-related slow movement were reported in rhesus macaques (M. mulatta) (Walton, Branham, Gash, & Grondin, 2006). Taken together, these independent findings validate the use of movement and gait speed as markers of aging-related physical decline in both human and NHPs.
Physical declines in aging humans and vervets may reflect peripheral inflammatory and degenerative disease across diverse tissues. For example, older vervets have less muscle mass (sarcopenia) (Kavanagh et al., 2016; Santago et al., 2015), loss of muscle fiber and strength in association with gait speed reduction (Choi et al., 2013), and shoulder degeneration (Plate et al., 2013). Plate et al., also reported that old animals spent less time climbing and hanging, that their physical mobility was associated with the degree of shoulder degeneration, their muscles were less dense, and their cross-sectional muscle fiber area was lower (Plate et al., 2013). Vervets naturally develop knee osteoarthritis with aging that includes increased secretion of inflammatory cytokines from degenerated menisci (Stone et al., 2015). Understanding the degree to which these processes contribute to functional decline is imperative to determining the roles of central versus peripheral degeneration during aging.
Intestinal Physiology & Functional Decline.
Age-related changes in intestinal physiology – e.g., motility, permeability, and microbiome characteristics – may contribute to functional decline and AD risk (Kavanagh, Hsu, et al., 2019; Nagpal et al., 2018). In humans, microbiomes of AD patients showed lower richness (number of unique operational taxonomic units), diversity (alpha and beta), and a lower Firmicutes:Bacteroidetes ratio than healthy, age-matched individuals (Vogt et al., 2017). A significant positive correlation between the genus Bacteroidetes and CSF YKL-40, a marker of neuroinflammation, was also observed, potentially linking Bacteroidetes content to increased neuroinflammation (Vogt et al., 2017). Furthermore, circulating gut microbiota products (lipopolysaccharide, acetate, valerate) and systemic inflammatory biomarkers were associated with PET-determined brain amyloid load (Marizzoni et al., 2020). Vervets, like humans, exhibit increasing intestinal dysfunction with age (Mitchell et al., 2017). Compared to younger adults, old adults have a lower Firmicutes:Bacteroidetes ratio (Figure 6c), like elder humans, and a lower abundance of butyrate-producing microbes, consistent with less healthy profiles (Vemuri, Sherrill, Davis, & Kavanagh, 2020). Like humans, old vervets have reduced gait speed and sarcopenia. If they are sedentary, they also exhibit increased microbial translocation which is associated with inflammatory burden and skeletal muscle insulin insensitivity (Kavanagh et al., 2016; Mitchell et al., 2017; Wilson et al., 2018). These studies suggest that vervets may be a useful NHP model in which to determine gut microbiome influences on physical and cognitive decline and AD-like neuropathology (Askarova et al., 2020).
Neuropathologic Changes and Physical Decline.
Neuropathological features associated with AD – including Aβ peptide and pathologic tau accumulation – may interrupt neural networks in ways that manifest as slow gait (Wilson, Allcock, Mc Ardle, Taylor, & Rochester, 2019). In adults aged 50–69 years, slow gait was associated with PET measures of Aβ accumulation in multiple brain regions, particularly the temporal lobe (Wennberg, Savica, Hagen, et al., 2017). Pathologic tau also has been linked to gait disturbance (Muurling et al., 2020), but additional studies are needed to fully understand the connections between tauopathies and gait dysfunction (Wennberg, Savica, & Mielke, 2017). Like humans, slow gait in vervets was associated with lower CSF Aβ1–42 and higher brain concentrations of Aβ1–42 (Latimer et al., 2019). These findings suggest that slow gait in vervets reflects neuropathologic features, and thus may be a robust, inexpensive, and non-invasive metric of pathologic aging.
4. Aging Related Cognitive Decline
Cognitive impairments are hallmarks of neurodegenerative disorders. While episodic memory deficits during disease progression are the benchmark of AD diagnosis, more recent research suggests that working memory predicts subsequent episodic memory deficits (Memel, Woolverton, Bourassa, & Glisky, 2019), and that working memory and executive function in mild cognitive impairment may predict the transition to AD (Kirova, Bays, & Lagalwar, 2015). Thus, we focused on executive function and working memory.
Executive Function.
Executive function encompasses higher-order cognitive processes which reflect capabilities in working memory, mental flexibility, planning, self-control, and attention, and performance in this domain requires integration from several brain regions, including the frontal, parietal, and cerebellar lobes (Nowrangi, Lyketsos, Rao, & Munro, 2014). Ample evidence suggests that many of the psychological processes associated with executive function are shared across primates, although considerable intra- and interspecific variation exists (Rosati, 2017). Age-associated declines in executive function have been less well-studied, but interest in this area has increased considerably (Lacreuse, Raz, Schmidtke, Hopkins, & Herndon, 2020). Cramer et al. (2018) assessed aging effects on an object retrieval task, which requires attention, planning, and impulse control, all components of executive function, and reflects the integrity of the pre-frontal cortex (Jentsch, Taylor, Elsworth, Redmond Jr., & Roth, 1999; Jentsch, Taylor, Redmond Jr., et al., 1999; Wilkinson et al., 1997). In cognitively demanding trials in which vervets were required to navigate indirect access to a desired food object, older monkeys (24–26 yoa) succeeded in fewer trials than did younger monkeys (6–7 yoa).
Working Memory.
Working memory reflects an individual’s ability to retain and process information in the short-term. Age-related declines in this cognitive domain have been well-characterized for rhesus macaques and chimpanzees (Arnsten & Goldman-Rakic, 1985; Bachevalier et al., 1991; Bartus, Dean, & Fleming, 1978; Motley et al., 2018; Rapp & Amaral, 1989; Voytko, 1997) but not vervets.
We evaluated working memory and executive function in 30 female vervets embedded in their family groups in the VRC. This cohort included three age groups: middle-aged (8–15 yoa; n=10), older adults (16–20 yoa; n=6), and elderly adults (23–29 yoa; n=14). We chose to study this age range because of the growing evidence that the pathophysiology of AD begins in middle age (Ritchie et al., 2015), and menopause is a risk factor for AD (Mosconi et al., 2018; Scheyer et al., 2018).
The goal of this ongoing study is to simultaneously assess social, physical, and cognitive function, and brain structure and function longitudinally from middle to old age. To do so, assessments cannot require extensive time out of the social group, as that disrupts social relationships, or food restriction, because animals would either need to be removed from the group or the entire group would have to be food restricted. To circumvent these challenges, we developed a rapid test of executive function and then utilized a brief test of working memory.
Assessment of Executive Function: Wake Forest Maze Task (WFMT).
The WFMT requires attention, planning, working memory, mental flexibility, and impulse control and thus assesses executive function. It can be completed in 10 days, with 5 hours or less out of the social group (Watson, Shively, & Voytko, 1999). Briefly, the test consists of 38 mazes (half of which are mirror images of the other half to control for side preferences) of increasing complexity, using an NHP puzzle feeder apparatus (Primate Products) (Figure 6d). The task is to move the reward/treat through the maze to an opening for retrieval. The vervets are acclimated to the puzzle feeder for 30 min/day for 5 days and learn how to solve a simple maze to get the reward. They are then tested for 30 min/day for 5 days by presentation of the mazes sequentially, from simple to complex (Frye et al., 2021).
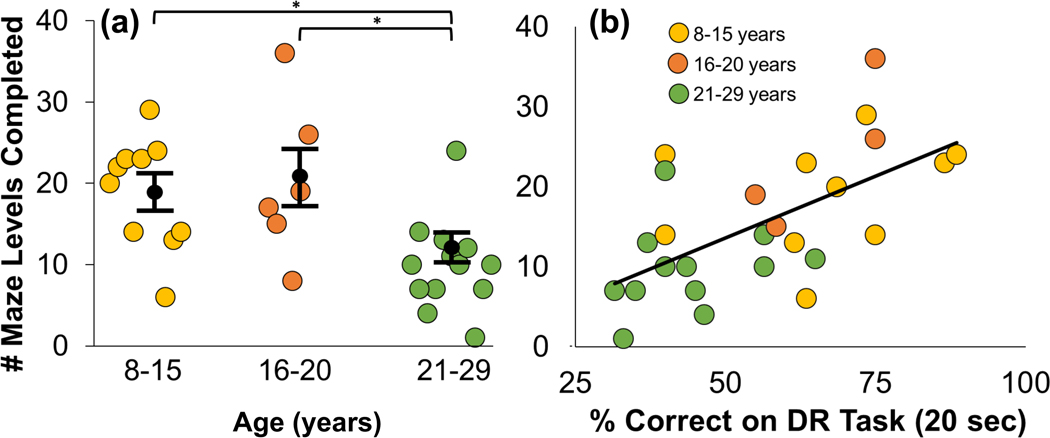
In our initial study, we observed that middle-aged female cynomolgus macaques (M. fascicularis) could solve more complex mazes than their older counterparts (Watson et al., 1999). In our adaption of the task to vervets (Frye et al., 2021), performance was scored as the highest and total numbers of levels completed, which reflects overall ability and spatial flexibility, respectively. Middle-aged (8–15 yoa) and older (16–20 yoa) vervets completed more levels overall than did the oldest group (21–29 yoa; Figure 7a), while age was unrelated to total time spent interacting with the maze task. These findings are consistent with comparable assessments of executive function in other NHPs (Lacreuse et al., 2020).
Figure 7.
Age-based performance on the Wake Forest Maze Task (WFMT) and Delayed Response (DR) task (a) Middle-aged (Tukey p=0.022) and older (Tukey p=0.023) monkeys completed more WFMT levels than did the oldest vervets (ANOVA: F(2,26)=5.917). (b) Pearson correlations between the total number of WFMT levels completed and percent correct in the DR task (20 second delay). Overall, performance in the WFMT agreed well with performance in the DR task (Rho=0.610; p=0.001) (Frye et al., 2021).
Assessment of Working Memory: The Delayed Response Task.
We adapted the delayed response (DR) task of working memory used in other laboratories with other NHP species (Baxter, Santistevan, Bliss-Moreau, & Morrison, 2018; Goldman, Rosvold, Vest, & Galkin, 1971; Goldman-Rakic, 1990; Jacobsen, 1935; James et al., 2007; Many Primates et al., 2019). We used a modified Wisconsin General Test apparatus, which consisted of three opaque boxes, separated from the focal monkey by a clear acrylic screen. During each trial, a food reward was randomly placed in one of the three opaque boxes, followed by a fixed delay, after which the monkey was allowed to select a one of the three boxes. Each testing session consisted of thirty individual trials. Performance was estimated as the proportion of trials that the focal monkey selected the baited box. Delays between baiting and selection increased throughout the protocol, ranging from 2.5 to 60 seconds. Similar to our findings in the WFMT of executive function, animals within the oldest age group (21–29 years) performed more poorly than their younger counterparts (Frye et al., 2021). These age differences were observed at the intermediate delays (15, 20, 30, and 40 seconds), whereas no age effects were observed during the easiest (2.5, 5, and 10 seconds) and most difficult delays (60 seconds), indicating ceiling and floor effects, respectively.
Relationships Between Maze Task and Delayed Response Performance.
Because working memory is a component of executive function (Diamond, 2013), we assessed the relationship between WFMT performance and DR performance. The total number of WFMT levels completed significantly correlated with accuracy in the DR task at multiple delays (Figure 7b: 20 second delay r=0.61, p=0.001). Notably, vervets were trained and tested in the WFMT over the course of 2 weeks (5 experimental hours total). An average of 21 days (range: 9–17 experimental hours) were needed to train and test individuals in the DR task (Frye et al., 2021).
5. Relationships Between Physical and Cognitive Decline: Gait Speed as a Biomarker of Cognitive Health
Assays of physical performance provide insights into brain health (Abellan van Kan et al., 2012; Best et al., 2016; Chou et al., 2019; Finkel, Ernsth-Bravell, & Pedersen, 2016; Grande et al., 2019; Handing et al., 2020; Inzitari et al., 2007; Justice et al., 2016; Okely & Deary, 2020; Rasmussen et al., 2019), so we examined the relationship between physical and cognitive declines in the Aging Vervet Cohort (AVC). We found positive associations between walking speeds and cognitive performance (Frye et al., 2021) (Figure 8a). Animals with slower gaits performed more poorly in cognitive assessments of executive function (i.e., the WFMT) and working memory (i.e., the DR task; Figure 8b). These findings suggest that aged vervet monkeys exhibit aging phenotypes similar to those of humans, and that slower gaits may reflect cognitive impairment. Gait speed assessment may provide an inexpensive means of screening for functional declines in large groups of NHPs.
Figure 8.
Gait speed predicted performance in the Wake Forest Maze Task (WFMT) and Delayed Response (DR) task. Individuals with faster gaits showed better cognitive performance in both the WFMT and DR tasks (a) Total WFMT levels completed (linear regression: t=3.94; p=0.001). (b) Number of correct trials in the DR task at the 20 second delay (linear regression: t=3.80; p=0.001) (Frye et al., 2021).
6. Conclusion
Summary.
The body of evidence presented here suggests that vervets exhibit a coordinated suite of traits that recapitulate early AD pathophysiologic and pathologic features in humans as illustrated in Figure 6. Elder vervets accumulate Aβ plaques and abnormally phosphorylated tau in brain regions similar to early AD in humans. Gait speed and CSF Aβ1–42 concentration correlate negatively with age and Aβ plaque density, and glucose hypometabolism and volumetric reductions accompany the accumulation of Aβ plaques. However, while characteristics of pathologic tau appear similar between vervets and early AD neuropathologic changes in humans, vervets do not develop the widespread neurofibrillary neurodegeneration characteristic of human AD. Decline in physical function, particularly when peripheral degenerative disorders of aging that are accompanied by increased inflammation are present, may be pathways through which peripheral aging promotes brain aging. Vervets, like humans, experience declines in executive function and working memory as they age, and slow gaits reflect cognitive decline. This comprehensive, integrated characterization of the vervet monkey provides the foundation for future studies of modifiable factors that increase risk or resilience as well as the mechanisms underlying AD etiology.
Gaps in Knowledge & Future Directions.
The vervet model may be useful to address several gaps in knowledge about factors that promote or protect from LOAD. For example, several lifestyle factors including sleep (Brzecka et al., 2018; Bubu et al., 2017; Hafycz & Naidoo, 2019; Mander, 2020; Mander, Winer, Jagust, & Walker, 2016; Phan & Malkani, 2019), social isolation (Evans, Martyr, Collins, Brayne, & Clare, 2019; Friedler, Crapser, & McCullough, 2015; National Academies of Sciences, Engineering & Medicine, 2020; Shankar, Hamer, McMunn, & Steptoe, 2013), impaired peripheral glucose metabolism (An et al., 2018; Craft, 2012; Ferreira et al., 2018), and gut dysbiosis (Askarova et al., 2020; Kowalski & Mulak, 2019) have been suggested to increase risk for developing AD. However, whether any of these factors are causal remains unclear. Experimental studies of all three are possible in vervets.
However, AD research using NHP models faces some challenges. One is the lack of validated commercially available biofluid (blood and CSF) and neuroimaging biomarkers (e.g., Aβ PET). In particular, the gold standard monoclonal antibody to human tau in the Fujirebio Lumipulse tau panel recognizes a tau epitope that differs in vervets and other NHPs, and thus is not useful to accurately measure total tau concentrations (Chen et al., 2018). Likewise, applicability of commonly used radioligands for Aβ PET such as [11C]PiB, is limited in NHP neuroimaging due to poor binding of the tracer to the abundant low-affinity Aβ binding sites in NHP brains (Oh et al., 2017; Van Dam & De Deyn, 2017). Third, there is a paucity of aged NHPs available for study, and for this reason, sample sizes in NHP aging studies often are small. Pooling of resources and standardization of techniques across institutions may increase statistical power and improve ability to detect small to moderate effects. Fourth, the majority of conclusions drawn from NHP models of aging are based on investigations of elder females (Machanda & Rosati, 2020). Studying sex differences in aging patterns, as well as how sex may modulate the impact of environmental factors, is key to understanding disparities in disease risk. Fifth, the Western diet, high in animal fats, protein and cholesterol, is associated with increased risk of AD (Baranowski, Marko, Fenech, Yang, & MacPherson, 2020). However, most NHP studies are conducted in monkeys consuming a chow diet, which is very low in fat and cholesterol, and high in vitamins, especially vitamin D, and does not resemble any human diet. Finally, normative physiologic data for NHPs across the life course are needed to distinguish healthy from pathological aging.
In addition to these gaps, a key question is the mechanism(s) underlying the apparent resistance of NHPs to development of the extensive neurofibrillary tangles that are characteristic of human AD, particularly given the high degree of similarity between human and vervet tau. Given the close correlation of progression of neurofibrillary tangle accumulation with cognitive impairment (Nelson, Braak, & Markesbery, 2009), determining the biologic mechanisms underlying resistance to neurofibrillary tangles may reveal therapeutic targets for interventions to slow or prevent the formation of this facet of AD.
Given that methodological and availability challenges are resolved, comprehensive, multisystem, longitudinal NHP studies, with postmortem neuropathologic validation, will be necessary to understand the similarities and differences in human and NHP brain aging, capitalize on the similarities to understand LOAD, and develop efficacious interventions.
Acknowledgments
We thank all the students, staff, and faculty at the Wake Forest School of Medicine who supported the vervet studies reviewed here.
Financial Support
This work was supported by: National Institutes of Health (NIH) K08 AG065426 (CSL), R01HL087103 (CAS), RF1AG058829 (CAS & SC), P30 AG049638 (SC), Intramural Grant, Department of Pathology, Wake Forest School of Medicine Intramural Grant, (CAS), Wake Forest Claude D. Pepper Older Americans Independence Center grant P30 AG21332 (SK), Vervet Research Colony (P40-OD010965) (MJ), the Wake Forest Clinical and Translational Science Institute (NCATS UL1TR001420), and the Nancy and Buster Alvord Endowment (CDK).
Footnotes
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors report no conflicts of interest.
Bibliography
- 2020 Alzheimer’s disease facts and figures. (2020). Alzheimers Dement. doi: 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- Abellan van Kan G, Rolland Y, Gillette-Guyonnet S, Gardette V, Annweiler C, Beauchet O, … Vellas B. (2012). Gait speed, body composition, and dementia. The EPIDOS-Toulouse cohort. J Gerontol A Biol Sci Med Sci, 67(4), 425–432. doi: 10.1093/gerona/glr177 [DOI] [PubMed] [Google Scholar]
- An Y, Varma VR, Varma S, Casanova R, Dammer E, Pletnikova O, … Thambisetty M. (2018). Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement, 14(3), 318–329. doi: 10.1016/j.jalz.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, & Maller JJ (2003). The role of volumetric MRI in understanding mild cognitive impairment and similar classifications. Aging Ment Health, 7(4), 238–250. doi: 10.1080/1360786031000120732 [DOI] [PubMed] [Google Scholar]
- Arnsten A, & Goldman-Rakic PS (1985). Catecholamines and cognitive decline in aged nonhuman primates. Annuals of the New York Academy of Science, 444, 218–234. doi: 10.1111/j.1749-6632.1985.tb37592.x [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2015). Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat Neurosci, 18(10), 1376–1385. doi: 10.1038/nn.4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, & Hyman BT (1992). Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology, 42(3 Pt 1), 631–639. doi: 10.1212/wnl.42.3.631 [DOI] [PubMed] [Google Scholar]
- Askarova S, Umbayev B, Masoud AR, Kaiyrlykyzy A, Safarova Y, Tsoy A, … Kushugulova A. (2020). The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s disease. Front Cell Infect Microbiol, 10, 104. doi: 10.3389/fcimb.2020.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins HM, Willson CJ, Silverstein M, J.M., Floyd E, Kaplan JR, & Appt SE (2014). Characterization of ovarian aging and reproductive senescence in vervet monkeys (Chlorocebus aethiops abaeus). Comparative Medicine, 64(1), 55–62. [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, & Cork LD (1991). Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging, 12, 99–111. [DOI] [PubMed] [Google Scholar]
- Baranowski BJ, Marko DM, Fenech RK, Yang AJT, & MacPherson REK (2020). Healthy brain, healthy life: a review of diet and exercise interventions to promote brain health and reduce Alzheimer’s disease risk. Appl Physiol Nutr Metab, 45(10), 1055–1065. doi: 10.1139/apnm-2019-0910 [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, & Fleming DL (1978). Aging in the rhesus monkey: Debilitating effects on short-term memory. Journal of Gerontology, 33, 858–871. [DOI] [PubMed] [Google Scholar]
- Batista AF, Forny-Germano L, Clarke JR, Lyra ESNM, Brito-Moreira J, Boehnke SE, … De Felice FG. (2018). The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J Pathol, 245(1), 85–100. doi: 10.1002/path.5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Santistevan AC, Bliss-Moreau E, & Morrison JH (2018). Timing of cyclic estradiol treatment differentially affects cognition in aged female rhesus monkeys. Behav Neurosci, 132(4), 213–223. doi: 10.1037/bne0000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Liu-Ambrose T, Boudreau RM, Ayonayon HN, Satterfield S, Simonsick EM, … Body Composition, S. (2016). An evaluation of the longitudinal, bidirectional associations between gait speed and cognition in older women and men. J Gerontol A Biol Sci Med Sci, 71(12), 1616–1623. doi: 10.1093/gerona/glw066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzecka A, Leszek J, Ashraf GM, Ejma M, Avila-Rodriguez MF, Yarla NS, … Aliev G. (2018). Sleep disorders associated with Alzheimer’s disease: A perspective. Front Neurosci, 12, 330. doi: 10.3389/fnins.2018.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastiao YV, Wen Y, … Anderson WM. (2017). Sleep, cognitive impairment, and Alzheimer’s disease: A systematic review and meta-analysis. Sleep, 40(1). doi: 10.1093/sleep/zsw032 [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard CL, Wood AG, Thrift AG, Wardill T, & Srikanth VK (2015). Longitudinal relationships between cognitive decline and gait slowing: The Tasmanian study of cognition and gait. J Gerontol A Biol Sci Med Sci, 70(10), 1226–1232. doi: 10.1093/gerona/glv066 [DOI] [PubMed] [Google Scholar]
- Chen JA, Fears SC, Jasinska AJ, Huang A, Al-Sharif NB, Scheibel KE, … Coppola G. (2018). Neurodegenerative disease biomarkers Abeta1–40, Abeta1–42, tau, and p-tau181 in the vervet monkey cerebrospinal fluid: Relation to normal aging, genetic influences, and cerebral amyloid angiopathy. Brain Behav, 8(2), e00903. doi: 10.1002/brb3.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Shively CA, Register TC, Feng X, Stehle J, High K, … Delbono O. (2013). Force-generation capacity of single vastus lateralis muscle fibers and physical function decline with age in African green vervet monkeys. J Gerontol A Biol Sci Med Sci, 68(3), 258–267. doi: 10.1093/gerona/gls143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MY, Nishita Y, Nakagawa T, Tange C, Tomida M, Shimokata H, … Arai H. (2019). Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr, 19(1), 186. doi: 10.1186/s12877-019-1199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S. (2012). Alzheimer disease: Insulin resistance and AD-extending the translational path. Nat Rev Neurol, 8(7), 360–362. doi: 10.1038/nrneurol.2012.112 [DOI] [PubMed] [Google Scholar]
- Cramer PE, Gentzel RC, Tanis KQ, Vardigan J, Wang Y, Connolly B, … Uslaner JM. (2018). Aging African green monkeys manifest transcriptional, pathological, and cognitive hallmarks of human Alzheimer’s disease. Neurobiol Aging, 64, 92–106. doi: 10.1016/j.neurobiolaging.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Dhana K, Evans DA, Rajan KB, Bennett DA, & Morris MC (2020). Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology, 95(4), e374–e383. doi: 10.1212/wnl.0000000000009816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. (2013). Executive functions. Annu Rev Psychol, 64, 135–168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond E, & Wisniewski T. (2017). Alzheimer’s disease: Experimental models and reality. Acta Neuropathol, 133(2), 155–175. doi: 10.1007/s00401-016-1662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin S, Begard S, Caillierez R, Lachaud C, Carrier S, Lieger S, … Buee L. (2018). Different tau species lead to heterogeneous tau pathology propagation and misfolding. Acta Neuropathol Commun, 6(1), 132. doi: 10.1186/s40478-018-0637-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumurgier J, Artaud F, Touraine C, Rouaud O, Tavernier B, Dufouil C, … Elbaz A. (2017). Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol A Biol Sci Med Sci, 72(5), 655–661. doi: 10.1093/gerona/glw110 [DOI] [PubMed] [Google Scholar]
- Edwards III GA, Gamez N, Escobedo G Jr., Calderon O, & Moreno-Gonzalez I. (2019). Modifiable risk factors for Alzheimer’s disease. Front Aging Neurosci, 11, 146. doi: 10.3389/fnagi.2019.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans IEM, Martyr A, Collins R, Brayne C, & Clare L. (2019). Social isolation and cognitive function in later life: A systematic review and meta-analysis. J Alzheimers Dis, 70(s1), S119–S144. doi: 10.3233/JAD-180501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LSS, Fernandes CS, Vieira MNN, & De Felice FG (2018). Insulin resistance in Alzheimer’s disease. Front Neurosci, 12, 830. doi: 10.3389/fnins.2018.00830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Ernsth-Bravell M, & Pedersen NL (2016). Temporal dynamics of motor functioning and cognitive aging. J Gerontol A Biol Sci Med Sci, 71(1), 109–116. doi: 10.1093/gerona/glv110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedler B, Crapser J, & McCullough L. (2015). One is the deadliest number: The detrimental effects of social isolation on cerebrovascular diseases and cognition. Acta Neuropathol, 129(4), 493–509. doi: 10.1007/s00401-014-1377-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye BM, Valure PM, Craft S, Baxter MG, Scott CS, Wise-Walden S, … Shively CA (2021). Temporal emergence of age-associated changes in cognitive and physical function in vervets (Chlorocebus aethiops sabaeus). Geroscience. doi: 10.1101/2020.09.28.313460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethe MJ, Barthel H, Sepulcre J, Dyrba M, Sabri O, & Teipel SJ (2017). In vivo staging of regional amyloid deposition. Neurology, 89, 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Vest B, & Galkin TW (1971). Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. Journal of Comparative and Physiological Psychology, 77(2), 212–220. doi: 10.1037/h0031649 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1990). Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res, 85, 325–335. doi: 10.1016/s0079-6123(08)62688-6 [DOI] [PubMed] [Google Scholar]
- Grande G, Haaksma ML, Rizzuto D, Melis RJF, Marengoni A, Onder G, … Vetrano DL (2019). Co-occurrence of cognitive impairment and physical frailty, and incidence of dementia: Systematic review and meta-analysis. Neurosci Biobehav Rev, 107, 96–103. doi: 10.1016/j.neubiorev.2019.09.001 [DOI] [PubMed] [Google Scholar]
- Hafycz JM, & Naidoo NN (2019). Sleep, aging, and cellular health: Aged-related changes in sleep and protein homeostasis converge in neurodegenerative diseases. Front Aging Neurosci, 11, 140. doi: 10.3389/fnagi.2019.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Caraci F, Cuello AC, Caruso G, Nistico R, Corbo M, … Lista S. (2020). A path toward precision medicine for neuroinflammatory mechanisms in Alzheimer’s disease. Front Immunol, 11, 456. doi: 10.3389/fimmu.2020.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handing E, Rapp S, Chen SH, Rejeski WJ, Wiberg M, Bandeen-Roche K, … Ip E. (2020). Heterogeneity in association between cognitive function and gait speed among older adults: An integrative data analysis study. The Journals of Gerontology: Series A, glaa211. doi: 10.1093/gerona/glaa211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Grothe MJ, Strandberg TO, Ohlsson T, Hagerstrom D, Jogi J, … Scholl M. (2017). Tau pathology distribution in Alzheimer’s disease corresponds differentially to cognition-relevant functional brain networks. Front Neurosci, 11, 167. doi: 10.3389/fnins.2017.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Ono F, Nakamura S, Matsumoto SE, Jin H, Hattori N, & Tabira T. (2016). An oral abeta vaccine using a recombinant adeno-associated virus vector in aged monkeys: Reduction in plaque amyloid and increase in abeta oligomers. J Alzheimers Dis, 54(3), 1047–1059. doi: 10.3233/jad-160514 [DOI] [PubMed] [Google Scholar]
- Hashemiaghdam A, & Mroczek M. (2020). Microglia heterogeneity and neurodegeneration: The emerging paradigm of the role of immunity in Alzheimer’s disease. J Neuroimmunol, 341, 577185. doi: 10.1016/j.jneuroim.2020.577185 [DOI] [PubMed] [Google Scholar]
- Herukka SK, Hallikainen M, Soininen H, & Pirttilä T. (2005). CSF Abeta42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment.Neurology, 64, 1294–1297. [DOI] [PubMed] [Google Scholar]
- Hoogendijk EO, Rijnhart JJM, Skoog J, Robitaille A, van den Hout A, Ferrucci L, … Muniz Terrera G. (2020). Gait speed as predictor of transition into cognitive impairment: Findings from three longitudinal studies on aging. Exp Gerontol, 129, 110783. doi: 10.1016/j.exger.2019.110783 [DOI] [PubMed] [Google Scholar]
- Huber HF, Gerow KG, & Nathanielsz PW (2015). Walking speed as an aging biomarker in baboons (Papio hamadryas). J Med Primatol, 44(6), 373–380. doi: 10.1111/jmp.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, Shorr R, … Rosano C. (2007). Gait speed predicts decline in attention and psychomotor speed in older adults: The health aging and body composition study. Neuroepidemiology, 29(3–4), 156–162. doi: 10.1159/000111577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, … Contributors. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement, 14(4), 535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, … Trojanowski JQ (2013). Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol, 12(2), 207–216. doi: 10.1016/s1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen CF (1935). Functions of frontal association areas in primates. Archives of Neurology and Psychiatry, 33, 358–369. [Google Scholar]
- James AS, Groman SM, Seu E, Jorgensen M, Fairbanks LA, & Jentsch JD (2007). Dimensions of impulsivity are associated with poor spatial working memory performance in monkeys. J Neurosci, 27(52), 14358–14364. doi: 10.1523/JNEUROSCI.4508-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Schmitt CA, Service SK, Cantor RM, Dewar K, Jentsch JD, … Freimer NB. (2013). Systems biology of the vervet monkey. ILAR Journal, 54(2), 122–143. doi: 10.1093/ilar/ilt049 % J ILAR Journal [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Elsworth JD, Redmond DE Jr., & Roth RH (1999). Altered frontal cortical dopaminergic transmission in monkeys after subchronic phencyclidine exposure: Involvement in frontostriatal cognitive deficits. Neuroscience, 90, 823e832. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Redmond DE Jr., Elsworth JD, Youngren KD, & Roth RH (1999). Dopamine D4 receptor antagonist reversal of subchronic phencyclidine-induced object retrieval/detour deficits in monkeys. Psychopharmacology (Berl), 142, 78e84. [DOI] [PubMed] [Google Scholar]
- Justice JN, Cesari M, Seals DR, Shively CA, & Carter CS (2016). Comparative approaches to understanding the relation between aging and physical function. J Gerontol A Biol Sci Med Sci, 71(10), 1243–1253. doi: 10.1093/gerona/glv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, Willard SL, Shively CA, Kaplan JR, Register TC, Jorgensen MJ, … Feinstein DL (2013). Development of amyloid burden in African green monkeys. Neurobiology of Aging, 34(10), 2361–2369. doi: 10.1016/j.neurobiolaging.2013.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman SK, Sanders DW, Thomas TL, Ruchinskas AJ, Vaquer-Alicea J, Sharma AM, … Diamond MI (2016). Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron, 92(4), 796–812. doi: 10.1016/j.neuron.2016.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Brown RN, Davis AT, Uberseder B, Floyd E, Pfisterer B, & Shively CA (2016). Microbial translocation and skeletal muscle in young and old vervet monkeys. Age (Dordr), 38(3), 58. doi: 10.1007/s11357-016-9924-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Day SM, Pait MC, Mortiz WR, Newgard CB, Ilkayeva O, … Macauley SL (2019). Type-2-Diabetes alters CSF but not plasma metabolomic and AD risk profiles in vervet monkeys. Front Neurosci, 13, 843. doi: 10.3389/fnins.2019.00843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson ME, Zhang L, … Wagner JD (2007). Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring), 15(7), 1666–1674. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Hsu FC, Davis AT, Kritchevsky SB, Rejeski WJ, & Kim S. (2019). Biomarkers of leaky gut are related to inflammation and reduced physical function in older adults with cardiometabolic disease and mobility limitations. Geroscience, 41(6), 923–933. doi: 10.1007/s11357-019-00112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Seo SW, Yoon DS, Chin J, Lee BH, Cheong HK, … Na DL (2010). Comparison of neuropsychological and FDG-PET findings between early- versus late-onset mild cognitive impairment: A five-year longitudinal study. Dement Geriatr Cogn Disord, 29(3), 213–223. doi: 10.1159/000278422 [DOI] [PubMed] [Google Scholar]
- Kirova AM, Bays RB, & Lagalwar S. (2015). Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed Res Int, 2015, 748212. doi: 10.1155/2015/748212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski K, & Mulak A. (2019). Brain-gut-microbiota axis in Alzheimer’s disease. J Neurogastroenterol Motil, 25(1), 48–60. doi: 10.5056/jnm18087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Raz N, Schmidtke D, Hopkins WD, & Herndon JG (2020). Age-related decline in executive function as a hallmark of cognitive ageing in primates: An overview of cognitive and neurobiological studies. Philos Trans R Soc Lond B Biol Sci, 375(1811), 20190618. doi: 10.1098/rstb.2019.0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer CS, Shively CA, Keene CD, Jorgensen MJ, Andrews RN, Register TC, … Craft S. (2019). A nonhuman primate model of early Alzheimer’s disease pathologic change: Implications for disease pathogenesis. Alzheimers Dement, 15(1), 93–105. doi: 10.1016/j.jalz.2018.06.3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, … Ervin FR (2004). Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol, 165(1), 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Zhang M, Xu W, Li JQ, Cao XP, Yu JT, & Tan L. (2019). Midlife modifiable risk factors for dementia: A systematic review and meta-analysis of 34 prospective cohort studies. Curr Alzheimer Res, 16(14), 1254–1268. doi: 10.2174/1567205017666200103111253 [DOI] [PubMed] [Google Scholar]
- Liu F, & Gong CX (2008). Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener, 3, 8. doi: 10.1186/1750-1326-3-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Lopez JM, Yang C, & Schatzberg AF (2000). Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. Journal of Neuroscience, 20(20), 7816–7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HR, Sheng LQ, Pan PL, Wang GD, Luo R, Shi HC, … Zhong JG (2018). Cerebral glucose metabolic prediction from amnestic mild cognitive impairment to Alzheimer’s dementia: a meta-analysis. Transl Neurodegener, 7, 9. doi: 10.1186/s40035-018-0114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanda ZP, & Rosati AG (2020). Shifting sociality during primate ageing. Philos Trans R Soc Lond B Biol Sci, 375(1811), 20190620. doi: 10.1098/rstb.2019.0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA (2020). Local sleep and Alzheimer’s disease pathophysiology. Front Neurosci, 14, 525970. doi: 10.3389/fnins.2020.525970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Winer JR, Jagust WJ, & Walker MP (2016). Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease?. Trends Neurosci, 39, 552–566 doi: 10.1016/j.tins.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Many Primates, Altschul DM, Beran MJ, Bohn M, Call J, DeTroy S, … Watzek J. (2019). Establishing an infrastructure for collaboration in primate cognition research. PLoS One, 14(10), e0223675. doi: 10.1371/journal.pone.0223675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marizzoni M, Cattaneo A, Mirabelli P, Festari C, Lopizzo N, Nicolosi V, … Frisoni GB. (2020). Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s disease. J Alzheimers Dis, 78(2), 683–697. doi: 10.3233/jad-200306 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Sapolsky RM (1995). Stress and cognitive function. Current Opinion in Neurobiology, 5, 205–216. doi: 10.1016/0959-4388(95)80028-X [DOI] [PubMed] [Google Scholar]
- Memel M, Woolverton CB, Bourassa K, & Glisky EL (2019). Working memory predicts subsequent episodic memory decline during healthy cognitive aging: evidence from a cross-lagged panel design. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, 26(5), 711–730. doi: 10.1080/13825585.2018.1521507 [DOI] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, & Rocca WA (2014). Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin Epidemiol, 6, 37–48. doi: 10.2147/CLEP.S37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EL, Davis AT, Brass K, Dendinger M, Barner R, Gharaibeh R, … Kavanagh K. (2017). Reduced intestinal motility, mucosal barrier function, and inflammation in aged monkeys. J Nutr Health Aging, 21(4), 354–361. doi: 10.1007/s12603-016-0725-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M, Speechley M, Muir-Hunter SW, Pieruccini-Faria F, Sarquis-Adamson Y, Hachinski V, … Cognition N. (2020). Dual decline in gait speed and cognition is associated with future dementia: Evidence for a phenotype. Age Ageing. doi: 10.1093/ageing/afaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, … Alzheimer’s A. (2012). National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol, 123(1), 1–11. doi: 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Rahman A, Diaz I, Wu X, Scheyer O, Hristov HW, … Brinton RD (2018). Increased Alzheimer’s risk during the menopause transition: A 3-year longitudinal brain imaging study. PLoS One, 13(12), e0207885. doi: 10.1371/journal.pone.0207885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley SE, Grossman YS, Janssen WGM, Baxter MG, Rapp PR, Dumitriu D, & Morrison JH (2018). Selective loss of thin spines in area 7a of the primate intraparietal sulcus predicts age-related working memory impairment. J Neurosci, 38(49), 10467–10478. doi: 10.1523/JNEUROSCI.1234-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muurling M, Rhodius-Meester HFM, Parkka J, van Gils M, Frederiksen KS, Bruun M, … de Boer C. (2020). Gait disturbances are associated with increased cognitive impairment and cerebrospinal fluid tau levels in a memory clinic cohort. J Alzheimers Dis, 76(3), 1061–1070. doi: 10.3233/JAD-200225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, … Yadav H. (2018). Gut microbiome and aging: Physiological and mechanistic insights. Nutr Healthy Aging, 4(4), 267–285. doi: 10.3233/NHA-170030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, & Medicine. (2020). Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Nelson PT, Braak H, & Markesbery WR (2009). Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J Neuropathol Exp Neurol, 68(1), 1–14. doi: 10.1097/NEN.0b013e3181919a48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Hamel AF, Kelly BJ, Dettmer AM, & Meyer JS (2013). Stress, the HPA axis, and nonhuman primate well-being: A review. Appl Anim Behav Sci, 143(2–4), 135–149. doi: 10.1016/j.applanim.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrangi MA, Lyketsos C, Rao V, & Munro CA (2014). Systematic review of neuroimaging correlates of executive functioning: Converging evidence from different clinical populations. J Neuropsychiatry Clin Neurosci, 26(2), 114–125. doi: 10.1176/appi.neuropsych [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Kim MH, Han SJ, Kang KJ, Ko IO, Kim Y, … Kim KM (2017). Preliminary PET Study of 18F-FC119S in Normal and Alzheimer’s Disease Models. Molecular Pharmaceutics, 14(9), 3114–3120. doi: 10.1021/acs.molpharmaceut.7b00351 [DOI] [PubMed] [Google Scholar]
- Okely JA, & Deary IJ (2020). Associations between declining physical and cognitive functions in the Lothian Birth Cohort 1936. J Gerontol A Biol Sci Med Sci, 75(7), 1393–1402. doi: 10.1093/gerona/glaa023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou YN, Xu W, Li JQ, Guo Y, Cui M, Chen KL, … Yu JT (2019). FDG-PET as an independent biomarker for Alzheimer’s biological diagnosis: A longitudinal study. Alzheimers Res Ther, 11(1), 57. doi: 10.1186/s13195-019-0512-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, … Hansson O. (2020). Discriminative accuracy of plasma phospho-tau217 for Alzheimer’s disease vs other neurodegenerative disorders. JAMA, 324(8), 772–778. doi:doi: 10.1001/jama.2020.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel NM, Alapatt LJ, Jones LV, & Hubbard RE (2019). The association between gait speed and cognitive status in community-dwelling older people: A systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci, 74(6), 943–948. doi: 10.1093/gerona/gly140 [DOI] [PubMed] [Google Scholar]
- Phan TX, & Malkani RG (2019). Sleep and circadian rhythm disruption and stress intersect in Alzheimer’s disease. Neurobiol Stress, 10, 100133. doi: 10.1016/j.ynstr.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, … Voytko ML (2014). Why primate models matter. Am J Primatol. doi: 10.1002/ajp.22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate JF, Bates CM, Mannava S, Smith TL, Jorgensen MJ, Register TC, … Tuohy CJ (2013). Age-related degenerative functional, radiographic, and histological changes of the shoulder in nonhuman primates. J Shoulder Elbow Surg, 22(8), 1019–1029. doi: 10.1016/j.jse.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny MB, Tolan DR, & Selkoe DJ (1991). Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer’s disease. Am J Pathol, 138(6), 1423–1435. [PMC free article] [PubMed] [Google Scholar]
- Podtelezhnikov AA, Tanis KQ, Nebozhyn M, Ray WJ, Stone DJ, & Loboda AP (2011). Molecular insights into the pathogenesis of Alzheimer’s disease and its relationship to normal aging. PLoS One, 6(12), e29610. doi: 10.1371/journal.pone.0029610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, & Amaral DG (1989). Evidence for task-dependent memory dysfunction in the aged monkey.J Neurosci, 9, 3568–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen LJH, Caspi A, Ambler A, Broadbent JM, Cohen HJ, d’Arbeloff T, … Moffitt TE (2019). Association of neurocognitive and physical function with gait speed in midlife. JAMA Netw Open, 2(10), e1913123. doi: 10.1001/jamanetworkopen.2019.13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K, Ritchie CW, Yaffe K, Skoog I, & Scarmeas N. (2015). Is late-onset Alzheimer’s disease really a disease of midlife? Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 1, 122–130. doi: 10.1016/j.trci.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG (2017). The evolution of primate executive function: From response control to strategic decision-making. In Evolution of Nervous Systems (pp. 423–437). [Google Scholar]
- Rosso AL, Verghese J, Metti AL, Boudreau RM, Aizenstein HJ, Kritchevsky SB, … Rosano C. (2017). Slowing gait and risk for cognitive impairment: The hippocampus as a shared neural substrate. Neurology, 89, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A, … Diamond MI (2014). Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron, 82(6), 1271–1288. doi: 10.1016/j.neuron.2014.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santago AC 2nd, Plate JF, Shively CA, Register TC, Smith TL, & Saul KR (2015). Age-related structural changes in upper extremity muscle tissue in a nonhuman primate model. J Shoulder Elbow Surg, 24(10), 1660–1668. doi: 10.1016/j.jse.2015.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer O, Rahman A, Hristov H, Berkowitz C, Isaacson RS, Diaz Brinton R, & Mosconi L. (2018). Female sex and Alzheimer’s risk: The menopause connection. J Prev Alzheimers Dis, 5(4), 225–230. doi: 10.14283/jpad.2018.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Hamer M, McMunn A, & Steptoe A. (2013). Social isolation and loneliness: Relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosomatic Medicine, 75(2), 161–170. doi: 10.1097/PSY.0b013e31827f09cd [DOI] [PubMed] [Google Scholar]
- Sharma G, Huo A, Kimura T, Shiozawa S, Kobayashi R, Sahara N, … Hisanaga SI (2019). Tau isoform expression and phosphorylation in marmoset brains. J Biol Chem, 294(30), 11433–11444. doi: 10.1074/jbc.RA119.008415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Appt SA, Chen H, Day SM, Frye BM, Shaltout HA, … Register, T. C. (2020). Mediterranean diet, stress resilience, and aging in nonhuman primates. Neurobiology of Stress 13. doi: 10.1016/j.ynstr.2020.100254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Willard SL, Register TC, Bennett AJ, Pierre PJ, Laudenslager ML, … Kritchevsky SB. (2012). Aging and physical mobility in group-housed Old World monkeys. Age (Dordr), 34(5), 1123–1131. doi: 10.1007/s11357-011-9350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Mackler N, Sanz J, Kohn JN, Voyles T, Pique-Regi R, Wilson ME, … Tung J. (2019). Social status alters chromatin accessibility and the gene regulatory response to glucocorticoid stimulation in rhesus macaques. Proc Natl Acad Sci U S A, 116(4), 1219–1228. doi: 10.1073/pnas.1811758115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanoska K, Volkerling A, Bertz J, Poljak A, Ke YD, Ittner LM, & Ittner A. (2018). An N-terminal motif unique to primate tau enables differential protein-protein interactions. J Biol Chem, 293(10), 3710–3719. doi: 10.1074/jbc.RA118.001784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AV, Vanderman KS, Willey JS, Long DL, Register TC, Shively CA, … Ferguson CM (2015). Osteoarthritic changes in vervet monkey knees correlate with meniscus degradation and increased matrix metalloproteinase and cytokine secretion. Osteoarthritis Cartilage, 23(10), 1780–1789. doi: 10.1016/j.joca.2015.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacutu R, Thornton D, Johnson E, Budovsky A, Barardo D, Craig T, … de Magalhaes JP (2018). Human Ageing Genomic Resources: New and updated databases. Nucleic Acids Res, 46(D1), D1083–D1090. doi: 10.1093/nar/gkx1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y, Yoshida H, Fujiwara Y, Motohashi Y, & Shinkai S. (2012). A prospective study of gait performance and subsequent cognitive decline in a general population of older Japanese. J Gerontol A Biol Sci Med Sci, 67(7), 796–803. doi: 10.1093/gerona/glr243 [DOI] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, & Pirttilä T. (2009). Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol, 66, 382–389. doi: 10.1001/archneurol.2008.596 [DOI] [PubMed] [Google Scholar]
- Taylor JH, Mustoe AC, Hochfelder B, & French JA (2015). Reunion behavior after social separation is associated with enhanced HPA recovery in young marmoset monkeys. Psychoneuroendocrinology, 57, 93–101. doi: 10.1016/j.psyneuen.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, & Braak H. (2002). Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology, 58, 1791–1800. [DOI] [PubMed] [Google Scholar]
- Tian Q, Resnick SM, Mielke MM, Yaffe K, Launer LJ, Jonsson PV, … Ferrucci L. (2020). Association of dual decline in memory and gait speed with risk for dementia among adults older than 60 years: A multicohort individual-level meta-analysis. JAMA Netw Open, 3(2), e1921636. doi: 10.1001/jamanetworkopen.2019.21636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam D, & De Deyn PP (2017). Non human primate models for Alzheimer’s disease-related research and drug discovery. Expert Opinion on Drug Discovery, 12(2), 187–200. doi: 10.1080/17460441.2017.1271320 [DOI] [PubMed] [Google Scholar]
- Varghese T, Sheelakumari R, James JS, & Mathuranath PS (2013). A review of neuroimaging biomarkers of Alzheimer’s disease. Neurology Asia, 18(3), 239–248. [PMC free article] [PubMed] [Google Scholar]
- Vemuri R, Sherrill C, Davis MA, & Kavanagh K. (2020). Age-related colonic mucosal microbiome community shifts in monkeys. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/glaa256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, & Buschke H. (2002). Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med, 347(22), 1761–1768. [DOI] [PubMed] [Google Scholar]
- Villa C, Lavitrano M, Salvatore E, & Combi R. (2020). Molecular and imaging biomarkers in Alzheimer’s disease: A focus on recent insights. J Pers Med, 10(3). doi: 10.3390/jpm10030061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, … Rey FE (2017). Gut microbiome alterations in Alzheimer’s disease. Sci Rep, 7(1), 13537. doi: 10.1038/s41598-017-13601-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML (1997). Functional and neurobiological similarities of aging in monkeys and humans. Age (Omaha), 20, 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, & Tinkler GP (2004). Cognitive function and its neural mechanisms in nonhuman primate models of aging, Alzheimer disease, and menopause. Front Biosci, 9, 1899–1914. [DOI] [PubMed] [Google Scholar]
- Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP,& Broe GA (2005). Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. Journal of the Neurological Sciences, 229, 89–93. doi: 10.1016/j.jns.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Walton A, Branham A, Gash DM, & Grondin R. (2006). Automated video analysis of age-related motor deficits in monkeys using EthoVision. Neurobiol Aging, 27(10), 1477–1483. doi: 10.1016/j.neurobiolaging.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Watson SL, Shively CA, & Voytko ML (1999). Can puzzle feeders be used as cognitive screening instruments? Differential performance of young and aged female monkeys on a puzzle feeder task. Am J Primatol, 49(2), 195–202. doi: [DOI] [PubMed] [Google Scholar]
- Weigl R. (2005). Longevity of mammals in captivity; from the living collections of the world. [Google Scholar]
- Wennberg AM, Savica R, Hagen CE, Roberts RO, Knopman DS, Hollman JH, … Mielke MM (2017). Cerebral amyloid deposition is associated with gait parameters in the Mayo Clinic Study of Aging. J Am Geriatr Soc, 65(4), 792–799. doi: 10.1111/jgs.14670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg AM, Savica R, & Mielke MM (2017). Association between various brain pathologies and gait disturbance. Dement Geriatr Cogn Disord, 43(3–4), 128–143. doi: 10.1159/000456541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Tosakulwong N, Weigand SD, Senjem ML, Lowe VJ, Gunter JL, … Jack CR Jr. (2013). Does amyloid deposition produce a specific atrophic signature in cognitively normal subjects? Neuroimage Clin, 2, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LS, Dias R, Thomas KL, Augood SJ, Everitt BJ, Robbins TW, & Roberts AC (1997). Contrasting effects of excitotoxic lesions of the prefrontal cortex on the behavioural response to D-amphetamine and presynaptic and postsynaptic measures of striatal dopamine function in monkeys. Neuroscience, 80, 717–730. [DOI] [PubMed] [Google Scholar]
- Wilson J, Allcock L, Mc Ardle R, Taylor JP, & Rochester L. (2019). The neural correlates of discrete gait characteristics in ageing: A structured review. Neurosci Biobehav Rev, 100, 344–369. doi: 10.1016/j.neubiorev.2018.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson QN, Wells M, Davis AT, Sherrill C, Tsilimigras MCB, Jones RB, … Kavanagh K. (2018). Greater microbial translocation and vulnerability to metabolic disease in healthy aged female monkeys. Sci Rep, 8(1), 11373. doi: 10.1038/s41598-018-29473-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JR, Mander BA, Kumar S, Reed M, Baker SL, Jagust WJ, & Walker MP (2020). Sleep disturbance forecasts β-amyloid accumulation across subsequent years. Curr Biol. doi: 10.1016/j.cub.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig RM, Crockford C, Weltring A, Langergraber KE, Deschner T, & Zuberbuhler K. (2016). Social support reduces stress hormone levels in wild chimpanzees across stressful events and everyday affiliations. Nat Commun, 7, 13361. doi: 10.1038/ncomms13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JT, Xu W, Tan CC, Andrieu S, Suckling J, Evangelou E, … Vellas B. (2020). Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. doi: 10.1136/jnnp-2019-321913 [DOI] [PMC free article] [PubMed] [Google Scholar]