Abstract
The Rho family of GTPases plays a major role in the organization of the actin cytoskeleton. These G proteins are activated by guanine nucleotide exchange factors that stimulate the exchange of bound GDP for GTP. In their GTP-bound state, these G proteins interact with downstream effectors. Vav2 is an exchange factor for Rho family GTPases. It is a ubiquitously expressed homologue of Vav1, and like Vav1, it has previously been shown to be activated by tyrosine phosphorylation. Because Vav1 becomes tyrosine phosphorylated and activated following integrin engagement in hematopoietic cells, we investigated the tyrosine phosphorylation of Vav2 in response to integrin-mediated adhesion in fibroblasts and epithelial cells. However, no tyrosine phosphorylation of Vav2 was detected in response to integrin engagement. In contrast, treating cells with either epidermal growth factor or platelet-derived growth factor stimulated tyrosine phosphorylation of Vav2. We have examined the effects of overexpressing either wild-type or amino-terminally truncated (constitutively active) forms of Vav2 as fusion proteins with green fluorescent protein. Overexpression of either wild-type or constitutively active Vav2 resulted in prominent membrane ruffles and enhanced stress fibers. These cells revealed elevated rates of cell migration that were inhibited by expression of dominant negative forms of Rac1 and Cdc42. Using a binding assay to measure the activity of Rac1, Cdc42, and RhoA, we found that overexpression of Vav2 resulted in increased activity of each of these G proteins. Expression of a carboxy-terminal fragment of Vav2 decreased the elevation of Rac1 activity induced by epidermal growth factor, consistent with Vav2 mediating activation of Rac1 downstream from growth factor receptors.
The Rho family of GTP-binding proteins regulates many important cellular processes, such as cell migration, the organization of the cytoskeleton, cell-matrix and cell-cell adhesion, cell cycle progression, and gene expression (22, 44, 50). At present, 14 members of the Rho family have been identified in mammalian cells (55), but most attention has been directed towards three widely expressed members, RhoA, Rac1, and Cdc42. These three proteins have both unique and overlapping functions. In terms of cell migration and cytoskeletal organization, they regulate distinct processes: Cdc42 controls the assembly of filopodia (28, 33), Rac1 stimulates the formation of lamellipodia and membrane ruffles (42), and RhoA regulates the assembly of stress fibers (41). Like other G proteins, Rho family members act as molecular switches and are active with GTP bound and inactive with GDP bound. The switch between these states is regulated by guanine nucleotide exchange factors (GEFs), which exchange GDP for GTP, and by GTPase activating proteins (GAPs), which stimulate the endogenous GTPase activity of these proteins, resulting in hydrolysis of the bound GTP (22, 50). In turn, the activities of these GEFs and GAPs are regulated by various signaling pathways that are initiated by ligand binding to cell surface receptors. Both soluble agents, such as growth factors (24, 35, 41, 42), and extracellular matrix (ECM) proteins (5, 14, 38, 40) have been shown to activate Rho family members. Some of the steps in these signaling pathways remain to be elucidated. Specifically, many of the GEFs that mediate activation of Cdc42, Rac1, and RhoA downstream from surface receptors have not been identified.
One of the best characterized GEFs is Vav1, which is restricted in its distribution to hematopoietic cells (8, 9). Vav1 has been shown to act on Rac1, Cdc42, and RhoA in vitro (16, 23, 36, 46). The exchange factor activity of Vav1 is regulated by tyrosine phosphorylation (16, 23), and numerous studies have revealed that Vav1 is rapidly tyrosine phosphorylated in response to diverse stimuli (reviewed in reference 9). For example, Vav1 becomes tyrosine phosphorylated following ligation of T- and B-cell receptors and in response to many different cytokines and growth factors binding to their receptors (8, 9). In several situations, engagement of specific integrins has also been shown to stimulate Vav1 tyrosine phosphorylation. This has been observed following antibody ligation of β2 integrins in human neutrophils (54), following antibody ligation of β1 integrins in myeloid cells (20), and in platelets as they adhere to fibrinogen via the integrin αIIbβ3 or to collagen or fibronectin via β1 integrins (12). Pursuing the pathway from αIIbβ3 engagement to Vav1 activation, Miranti and coworkers were able to reconstitute Vav1 activation in CHO cells by coexpressing αIIbβ3, Vav1, and the tyrosine kinase Syk (31). Previous work had identified Syk as a tyrosine kinase that becomes rapidly activated in response to αIIbβ3 engagement in platelets, independent of the activation of the focal adhesion kinase (FAK) (15, 18), a tyrosine kinase that is prominently activated following integrin-mediated adhesion (21, 29). In another study, expression of Vav1 in CHO cells resulted in its tyrosine phosphorylation in response to β1 integrin-mediated adhesion. This was accompanied by increased stress fibers and lamellipodia, consistent with Vav1-mediated activation of RhoA and Rac1 (53).
Due to its hematopoietic cell-specific expression, Vav1 cannot be responsible for integrin-mediated activation of Rho family G proteins in other cell types. The discovery of a more ubiquitously distributed isoform, Vav2 (25, 45), provided a candidate GEF that may act downstream from integrins in nonhematopoietic cells. The objective of this study was to determine whether Vav2 was tyrosine phosphorylated and consequently activated in response to β1 integrin engagement and to determine which Rho family members are activated by Vav2. We found that unlike Vav1, Vav2 is not tyrosine phosphorylated following integrin-mediated cell adhesion to ECM. However, we have found that it is tyrosine phosphorylated in response to the growth factors epidermal growth factor (EGF) and platelet-derived growth factor (PDGF). Moreover, expression of a dominant negative Vav2 construct was found to diminish the elevation in Rac1 activity induced by EGF, suggesting that Vav2 contributes to Rac1 activation in response to growth factor stimulation of cells. Examination of actin cytoskeletal structures in cells transfected with an activated form of Vav2 revealed extensive lamellipodia and membrane ruffles but also prominent stress fibers. The observed effects on the cytoskeleton prompted investigation of a role for Vav2 in cell motility. Cells expressing activated Vav2 exhibited enhanced motility that could be blocked by dominant negative forms of Rac1 or Cdc42. These findings indicated that Vav2 may act on Rac1, Cdc42, and RhoA in vivo. An assay to specifically detect GTP-bound versions of these proteins confirmed that activated Vav2 increases the intracellular levels of active Rac1, Cdc42, and RhoA.
MATERIALS AND METHODS
Mammalian expression vectors.
Full-length human Vav2 cDNA was the generous gift of David Kwiatkowski (25). To generate Vav2 constructs that were fused with green fluorescent protein (GFP), Vav2 DNA constructs were engineered to contain an EcoRI restriction site at the 5′ end and a HindIII restriction site at the 3′ end to allow for directed subcloning into the multiple cloning site in the mammalian expression vector, pEGFP-N1 (Clontech, Palo Alto, Calif.). To ligate the N-terminally truncated Vav2 (amino acids [aa] 184 to 878) to the pEGFP vector, a HindIII-EcoRI fragment was amplified from human Vav2 cDNA with oligonucleotides 5′ AAG GTG AAG CTT CAG CGC GCC ATG ATT AGA TAC 3′ and 5′ CTG AAT TCG CTG GAT GCC CTC CTC 3′ by PCR using Pfu Turbo polymerase (Stratagene, La Jolla, Calif.). A C-terminal Vav2 construct (aa 594 to 878) was amplified using oligonucleotides 5′ CCC AAG CTT GGC GCC ATG CAG AAT TAC CAT 3′ and 5′ CTG AAT TCG CTG GAT GCC CTC CTC 3′. All constructs were designed with a Kozak sequence and ATG to be in frame with GFP on the pEGFP-N1 vector. All PCR-generated fragments were sequenced to confirm that no errors were introduced during PCR.
Antibodies.
To generate polyclonal antibodies against Vav2, rabbits were immunized with recombinant Vav2 proteins fused to glutathione-S-transferase (GST). To this end, human Vav2 cDNA was used as the template for a PCR that generated the C-terminal of Vav2, consisting of the Src homology 3 (SH3)-SH2-SH3 domains (aa 574 to 876). The primers 5′ CCC TGG AAT TCC ATC TCT CCT GCA GAT CTG 3′ and 5′ TCA CTG AAT TCC CTC CTC TTC TAG GTA CGT TGA AGG AAA 3′ were used. The PCR product (925 bp) was designed to contain EcoRI restriction sites at both the 3′ and 5′ end to allow for subcloning into the bacterial expression vector, pGEX-4T3 (Amersham Pharmacia Biotechnology, Piscataway, N.J.). The resulting GST-Vav2 (SH3-SH2-SH3) fusion protein was expressed in Escherichia coli DH5α. After overnight induction with isopropyl-β-d-thiogalactopyranoside (IPTG) at 25°C, bacteria were resuspended in Tris-buffered saline (TBS) with 1% Triton X-100 and lysed by sonication. Fusion proteins were purified by chromatography on glutathione-Sepharose (Amersham Pharmacia Biotechnology) from clarified bacterial lysates and concentrated with Centricon concentrators (Amicon Inc., Beverly, Mass.). Aliquots (0.5 to 1 mg) of fusion protein were used to immunize rabbits. After a series of immunizations, a high-titer antiserum was obtained that interacted with Vav2. This rabbit antiserum was used at 1:20,000 for immunoblotting.
Cell culture.
NIH 3T3 and BALB/c3T3 fibroblasts were cultured at 37°C with 10% CO2 in Dulbecco's modified Eagle's medium (DMEM) with high glucose, supplemented with 10% bovine calf serum. CHOK1 and HEK293 cells were cultured at 37°C with 10 and 5% CO2, respectively, in DMEM supplemented with 10% fetal bovine serum. All media contained penicillin G (100 U/ml), streptomycin sulfate (100 μg/ml), and amphotericin B (25 μg/ml). Culture media for CHO cells contained additional 1 mM MEM nonessential amino acids (Life Technologies, Grand Island, N.Y.).
Transient transfection.
Lipofectamine Plus was obtained from Life Technologies and used for transfection of NIH 3T3, BALB/c3T3, HEK293, and CHO cells essentially according to the manufacturer's instructions. Briefly, 106 cells were plated on 100-mm-diameter tissue culture dishes 18 to 24 h prior to transfection. Four micrograms of the various Vav2 DNA constructs or vector alone and 20 μl of Plus reagent as well as 30 μl of Lipofectamine were added to each plate in 1.5 ml of DMEM. After 2 h, cells were washed once with DMEM and cultured in their regular medium. Cells were used for various assays at 24 to 48 h posttransfection.
Migration assay.
To assess the migratory behavior of cells transfected with various Vav2 DNA constructs, we performed migration assays using a Transwell cell culture chamber containing polycarbonate membrane inserts with 8-μm pores (Corning Costar Corp., Cambridge, Mass.). The undersides of the porous membranes were coated with fibronectin at 10 μg/ml in Dulbecco's phosphate-buffered saline (PBS) for 1 h at 37°C and then blocked with 2% bovine serum albumin in PBS for 0.5 h at room temperature. After blocking, membranes were rinsed with PBS for 5 min and 500 μl of DMEM was added to the lower chamber. Transfected cells (105) were plated into each chamber in DMEM and allowed to migrate through the pores for 2 h. Cells that migrated through the membrane were detected by staining for actin, by GFP fluorescence or, in the case of cells expressing myc-tagged dominant negative constructs of Rac1 and Cdc42, by immunostaining for myc.
Immunoprecipitation and immunoblotting.
HEK293 or NIH 3T3 cells were washed with PBS and lysed in modified RIPA buffer (25 mM Tris [pH 7.4], 150 mM NaCl, 10 mM MgCl2, 2 mM EGTA, 0.02% sodium dodecyl sulfate [SDS], 0.2% deoxycholate, 1% NP-40) for 5 min on ice. Lysates were clarified by centrifugation at 12,000 × g for 10 min. Protein concentrations were measured using the Coomassie protein assay reagent, with bovine serum albumin as a standard, following the manufacturer's instructions (Pierce, Rockford, Ill.). Vav2 was immunoprecipitated from cell lysates (1 mg of protein), using 2 μl of Vav2 rabbit antiserum. Immunoprecipitates were collected by incubating with protein A-Sepharose (Sigma, St. Louis, Mo.) for 1 h at 4°C. The immunoprecipitates were washed three times with modified RIPA buffer and bound proteins were eluted by boiling in SDS-polyacrylamide gel electrophoresis sample buffer. Immunoprecipitates and cell lysates were analyzed on SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. Blotting was performed as described previously. Peroxidase-conjugated secondary antibodies were from Chemicon (Temecula, Calif.). Blots were developed using SuperSignal Substrate for Western blotting (Pierce) and then exposed to Kodak Scientific Image film.
Activity assays for Rac1, Cdc42, and RhoA.
The assay of Rac1 activity was performed as previously described (4) with some modifications. Cells were first rinsed once with 20 mM HEPES, pH 7.4, and 150 mM NaCl and then lysed in RIPA buffer with 500 mM NaCl and protease inhibitors. GTP-bound Rac1 (i.e., activated Rac1) was affinity precipitated from cell lysates (350 to 500 μg of protein) using an immobilized GST fusion construct of the Rac1 binding domain of murine p65Pak (the p21Rac binding domain [PBD]) that binds to Rac1-GTP but not to Rac1-GDP (3). The GST-PBD construct was kindly provided by R. A. Cerione and S. Bagrodia (Cornell University, Ithaca, N.Y.). Rac1 that sedimented with the GST-PBD beads was separated using SDS-polyacrylamide gel electrophoresis transferred to polyvinylidene difluoride membrane and blotted with an antibody against Rac1 (Transduction Labs, San Diego, Calif.). Cdc42-GTP binds to the same PBD construct (3) and so the same assay was used to measure Cdc42 activity, except that the blots were probed with an antibody against Cdc42 (Transduction Labs). Essentially the same assay was used to measure RhoA-GTP, except for these assays, the RhoA binding domain (RBD) of Rhotekin (40) was used as a GST construct (kindly provided by L. Petch, University of North Carolina at Chapel Hill). RhoA that sedimented with the GST-RBD beads was detected with an antibody against RhoA (Transduction Labs).
Quantitation of bands on immunoblots was performed using Metamorph software (Universal Imaging, Westchester, Pa.). Films were scanned with a ScanJet 6100C film scanner, and the images were imported into Metamorph for quantitation. For each pulldown assay, the level of GTPase sedimented was normalized relative to the amount of the GTPase in the cell lysate. This was done to avoid errors arising from different levels of expression of the GTPases in different samples.
For some experiments (see Fig. 8), quantitation of Rac1 activity was performed using phosphorimager analysis of immunoblots. For these experiments, blots were developed using enhanced chemifluorescence (Amersham Life Sciences, Little Chalfont, Buckinghamshire, United Kingdom). Blots were scanned with a Storm phosphorimager under blue fluorescence. Quantitation of band intensities was performed using ImageQuant. Statistical significance was calculated using the Student two-sample t test. P values of <0.05 were considered significant.
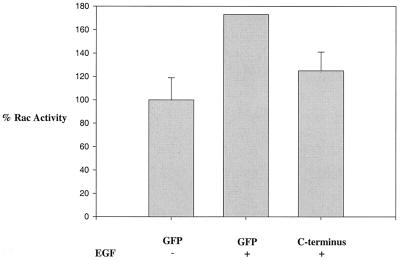
FIG. 8.
Expression of the carboxy terminus of Vav2 decreases EGF-induced Rac1 activation. HEK293 cells were either untreated (−) or stimulated for 20 min with EGF (100 ng/ml), and the level of active Rac1 was measured using sedimentation with GST-PBD beads, blotting for Rac1, and phosphorimager analysis. The level of Rac1 activity in unstimulated cells was set at 100%. Comparison of EGF-treated cells with unstimulated cells indicated an increase in Rac1 activity of approximately 1.8-fold (P = 0.01). Expression of the Vav2 C-terminal construct compared with expression of GFP decreased the level of Rac1 activity in EGF-stimulated cells by approximately 30% (P = 0.03). The data were compiled from four separate experiments. Error bars, standard error of the mean.
Immunofluorescence.
Cells were processed for immunofluorescence microscopy as previously described (30). Briefly, cells were fixed in 3.7% formaldehyde in PBS for 10 min, rinsed in TBS at pH 7.6 for 3 min, and then permeabilized for 8 min in TBS containing 0.5% Triton X-100. Expression of the various Vav2-GFP constructs was determined by GFP fluorescence. Actin was visualized using phalloidin labeled with Texas red (Molecular Probes, Eugene, Oreg.). Microscopy was performed on a Zeiss Axiophot microscope. Images were acquired with a MicroMAX 5 MHz cooled charge-coupled device camera (Princeton Instrument, Trenton, N.J.) and processed using Metamorph Image software (Universal Imaging).
RESULTS
Vav2 is tyrosine phosphorylated in response to growth factors but not in response to extracellular matrix.
Integrin-mediated cell adhesion results in tyrosine phosphorylation of several signaling molecules, such as FAK, paxillin, and p130Cas (2, 7, 13, 47). To determine if Vav2 becomes tyrosine phosphorylated during integrin-mediated adhesion, Vav2 was immunoprecipitated from serum-starved HEK293 cells that were either held in suspension or allowed to attach and spread on fibronectin for times of 10, 20, 30, or 60 min. The immunoprecipitated Vav2 was analyzed for the presence of phosphotyrosine by immunoblotting (Fig. 1a). Low levels of phosphotyrosine were detected in Vav2 from cells in suspension and those adhering to fibronectin at all time points, but no increase in Vav2 tyrosine phosphorylation was detected in response to adhesion. Similarly, Vav2 tyrosine phosphorylation was not observed in response to adhesion to collagen (data not shown). In addition, no stimulation of Vav2 tyrosine phosphorylation was observed when NIH 3T3 cells were plated on fibronectin (data not shown).
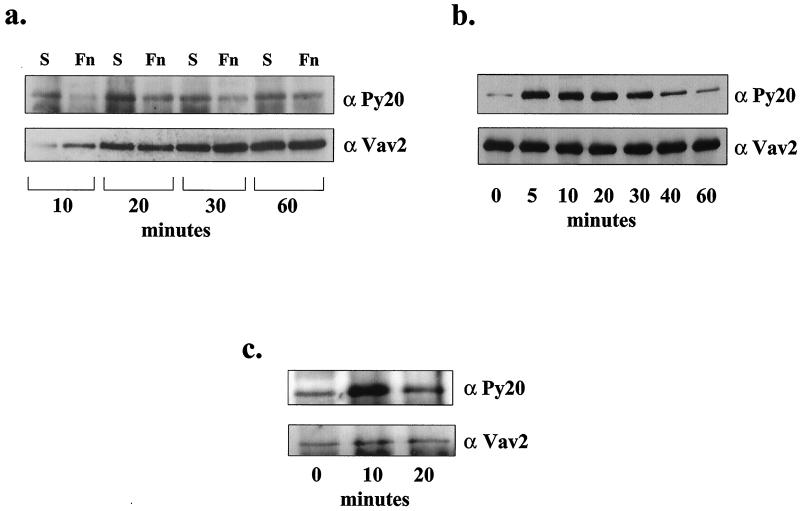
FIG. 1.
Vav2 is tyrosine phosphorylated in response to growth factors but not in response to adhesion to ECM. (a) Upper panel: antiphosphotyrosine blot of endogenous Vav2 immunoprecipitated from HEK293 cells held in suspension (S) or plated on fibronectin (Fn) for 10, 20, 30, or 60 min. Bottom panel: the blot was stripped and reprobed for Vav2 with an anti-Vav2 antibody to compare the amounts of Vav2 immunoprecipitated for each time point. (b) Upper panel: antiphosphotyrosine blot of Vav2 immunoprecipitated from serum-starved HEK293 cells that were treated with EGF (100 ng/ml) for 0, 5, 10, 20, 30, 40, or 60 min. Bottom panel: the amount of Vav2 protein immunoprecipitated was compared by stripping the blot and reprobing with anti-Vav2 antibody. (c) Vav2 is tyrosine phosphorylated in response to PDGF-BB. NIH 3T3 cells were treated for 0, 10, and 20 min with PDGF-BB (50 ng/ml). Vav2 was immunoprecipitated and blotted for phosphotyrosine (upper panel), and Vav2 (lower panel).
Exploring other factors that might stimulate the tyrosine phosphorylation of Vav2, serum-starved, adherent HEK293 cells were treated with EGF (100 ng/ml) for 0, 5, 10, 20, 30, 40, or 60 min, and Vav2 was immunoprecipitated and analyzed by blotting with antiphosphotyrosine (Fig. 1b). Under these conditions, Vav2 revealed robust tyrosine phosphorylation, which had already peaked by 5 min of stimulation with EGF. Densitometric analysis of the phosphotyrosine blot revealed that the level of phosphotyrosine in Vav2 was elevated approximately sevenfold at the 5-min time point. At 60 min of EGF stimulation, the level of phosphotyrosine in Vav2 had decreased to twofold above the unstimulated level. To examine whether other growth factors also stimulated Vav2 tyrosine phosphorylation, NIH 3T3 cells were treated with PDGF-BB (50 ng/ml), and Vav2 was immunoprecipitated and analyzed for phosphotyrosine by blotting (Fig. 1c). Again, tyrosine phosphorylation was observed in response to this growth factor (fourfold elevation in phosphotyrosine at 10 min of PDGF treatment), but the phosphorylation was more transient than that induced by EGF, and by 20 min it had decreased to just twofold above the background level. Previous work has demonstrated that the exchange factor activity of Vav2 (like that of Vav1) is regulated by tyrosine phosphorylation (46), suggesting that Vav2's exchange factor activity is downstream of soluble growth factors but not downstream of β1 integrin engagement. We also examined whether the tyrosine phosphorylation of Vav2 induced by growth factors was affected by cell adhesion to ECM. We observed that EGF treatment of either suspended or adherent cells both resulted in elevated tyrosine phosphorylation of Vav2 (data not shown).
Wild-type and N-terminally truncated Vav2 induce membrane ruffling and lamellipodia in fibroblasts.
In order to study the activity of Vav2 in cells, we generated a set of Vav2 constructs (Fig. 2), each one fused to GFP at the carboxy terminus so that the expression of the constructs could be monitored in live cells. These constructs included wild-type Vav2, a construct in which the amino-terminal calponin homology (CH) domain and acidic domain (AD) are deleted (Δ184N Vav2), and a construct consisting of the carboxy-terminal SH2 domain flanked by the two SH3 domains (SH3-SH2-SH3). Previous work has shown that deletion of the amino-terminal CH domain and AD results in a form of Vav2 that is constitutively active in vitro with respect to exchange factor activity and that expression of such mutants is a potent inducer of transformation in NIH 3T3 cells (1, 45, 46).
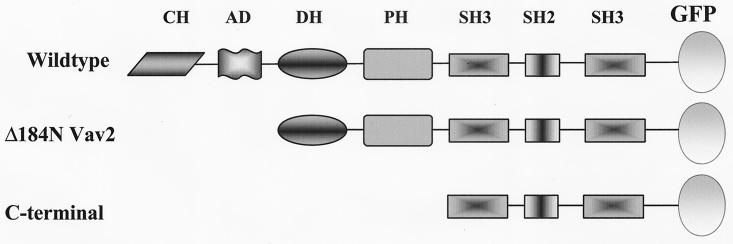
FIG. 2.
Schematic representation of Vav2 fusion proteins used in this paper. Each construct was fused at the C terminus to GFP. The domains indicated are the CH, AD, Dbl homology (DH), pleckstrin homology (PH), SH3, and SH2 domains. Δ184N is a constitutively active, amino-terminally truncated version of Vav2 that lacks the CH domain and AD. The carboxy-terminal mutant contains only the SH2 domain flanked by the two SH3 domains.
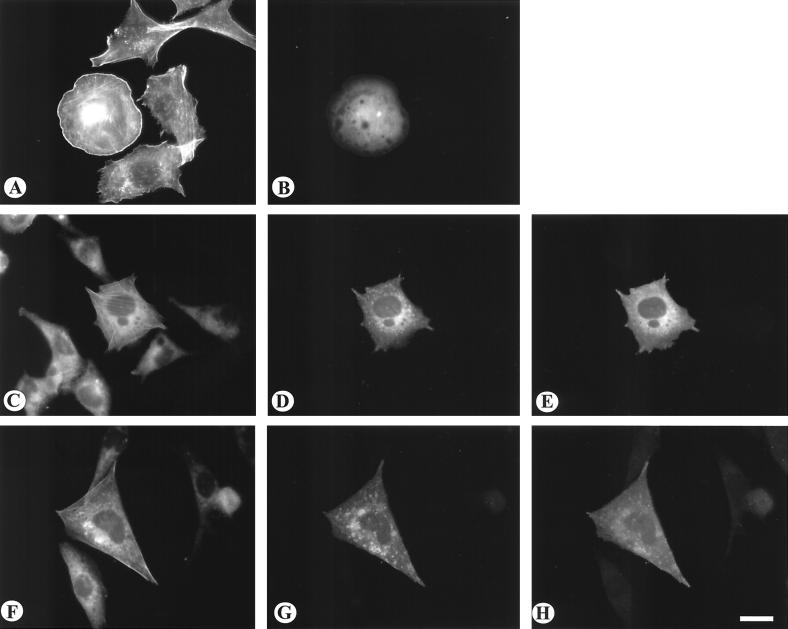
To examine the effect of these GFP-tagged Vav2 constructs on cell morphology, cytoskeletal organization, and cell migration, the constructs were expressed in a variety of cell types using transient transfection. Expression of either wild-type Vav2 or Δ184N resulted in a reorganization of actin in NIH 3T3 and BALB/c3T3 cells (Fig. 3). (These cell types were used because of their well-spread morphology, prominent stress fibers, and ability to develop easily visualized membrane ruffles.) Both constructs induced extensive membrane ruffling and lamellipodial extension. In general, Δ184N Vav2 was more potent and often generated broad lamellipodia that extended around much of the cell margin (Fig. 3 E to H). Many cells expressing wild-type Vav2 or Δ184N Vav2 revealed stress fibers that were more prominent than the stress fibers of untransfected cells or cells transfected with GFP alone (Fig. 3A and B). No significant effects on morphology or actin organization were observed in cells in which the SH3-SH2-SH3 construct was expressed (data not shown). We examined the state of tyrosine phosphorylation of the expressed wild-type and Δ184N Vav2 constructs by immunoprecipitating these from cell lysates using an antibody against GFP. Immunoblotting these immunoprecipitates revealed that both the wild-type and Δ184N Vav2-GFP constructs were tyrosine phosphorylated (data not shown).
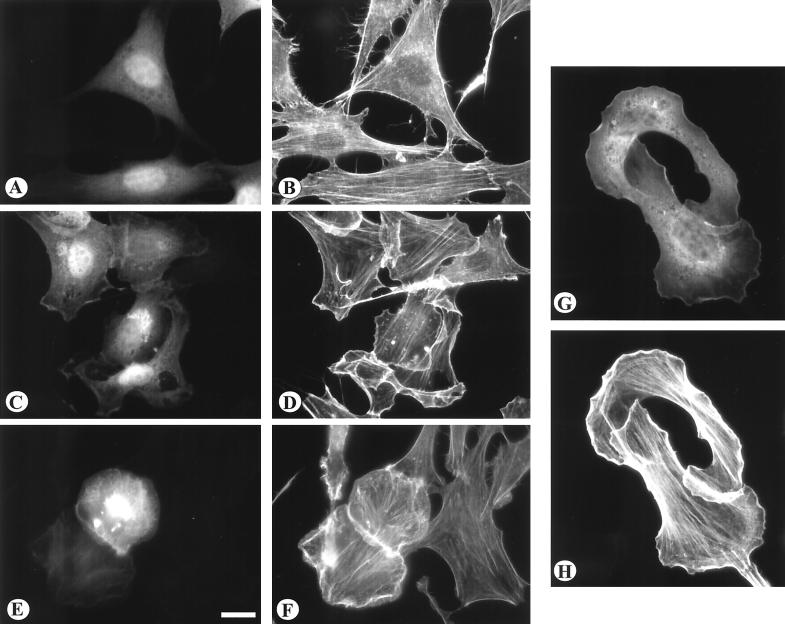
FIG. 3.
The effects of Vav2 transient expression on the actin cytoskeleton. NIH 3T3 cells (A to F) were transiently transfected with GFP alone (A and B), with wild-type Vav2 as a GFP construct (C and D), or with the Δ184N Vav2-GFP (E and F). BALB/c3T3 cells (G and H) were transfected with Δ184N Vav2 as a GFP fusion protein. Transfected cells were visualized for GFP (A, C, E, and G). The distribution of actin was visualized by staining with phalloidin conjugated with Texas red (B, D, F, and H). Note that expression of both wild-type and Δ184N Vav2 induced prominent lamellipodia and membrane ruffles. Bar = 20μm.
The above analysis was performed on transfected cells that were maintained in the presence of serum. We wished to examine the effects of these Vav2 constructs on cells in the absence of either serum or growth factors. For these experiments, NIH 3T3 cells were transfected and after 24 h were transferred to serum-free conditions for an additional 24 h before fixing and staining for actin. Nontransfected, serum-starved cells showed decreased stress fibers and no membrane ruffling activity (Fig. 4). However, cells overexpressing either wild-type Vav2 (Fig. 4A and B) or Δ184N Vav2 (Fig. 4C and D) continued to reveal prominent stress fibers and membrane ruffles under serum-free conditions.
FIG. 4.
The effects of Vav2 transient expression on actin organization in serum-starved cells. NIH 3T3 cells were transiently transfected with either wild-type Vav2-GFP (A and B) or Δ184N Vav2-GFP (C, D). After 24 hours in the presence of serum, the cells were starved for a further 24 hours before fixation and staining for actin. The transfected cells are shown in A and C, the organization of actin is shown in B and D. Bar = 20 μm.
Cell migration is induced by Vav2 and can be blocked by N17Rac and N17Cdc42.
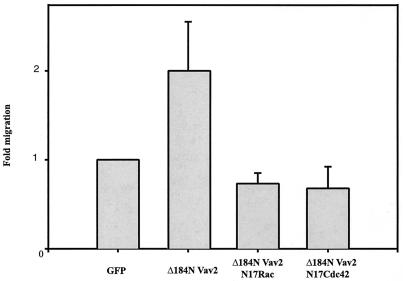
The prominent membrane ruffling displayed by cells in which wild-type Vav2 or Δ184N Vav2 was expressed indicated that these cells might display enhanced migratory behavior. To explore the effects of Vav2 on cell migration, we used a Transwell assay, in which the lower surface of a porous polycarbonate membrane was coated with fibronectin and the number of cells migrating through the membrane after 2 h under serum-free conditions was determined by counting. For these experiments, the Δ184N Vav2 construct was used because of its higher potency. Migration was assayed using CHO cells because of their high transfection efficiency and because their migratory activity is easily determined using Transwell filters. Expression of Δ184N Vav2 in CHO cells resulted in an approximately twofold increase in the number of cells migrating through the Transwell filter compared with untransfected cells or cells expressing GFP only (Fig. 5). To explore whether this stimulation of cell migration involved Rac1 or Cdc42, cells were cotransfected with Δ184N Vav2 and dominant negative versions of Rac1 (N17Rac1) or Cdc42 (N17Cdc42). Coexpression of either N17Rac1 or N17Cdc42 blocked the increase in migration induced by Δ184N Vav2 (Fig. 5). In addition, cotransfection of N17Rac1 or N17Cdc42 blocked the morphological phenotype of prominent membrane ruffles and lamellipodia in CHO cells expressing Δ184N Vav2 (Fig. 6). Coexpression of the dominant negative constructs did not alter the level of expression of Δ184N Vav2 (data not shown).
FIG. 5.
Constitutively activated Vav2 induced an increase in cell migration that was inhibited by dominant negative forms of Rac1 and Cdc42. Transwell assays were performed on CHO cells transiently transfected with GFP alone, Δ184N Vav2-GFP alone, or Δ184N Vav2-GFP cotransfected with myc-tagged N17Rac1 or N17Cdc42. Cells were plated on the Transwell membrane in serum-free medium in the absence of ECM and growth factors. The underside of the Transwell membrane was coated with fibronectin (10 μg/ml). Cells migrating through the membrane in a 2-h period were counted. The bar graph represents three separate experiments (error bars, standard error of the mean). For the inhibition of migration by N17Rac1, P = 0.015. For the inhibition of migration by N17Cdc42, P = 0.005.
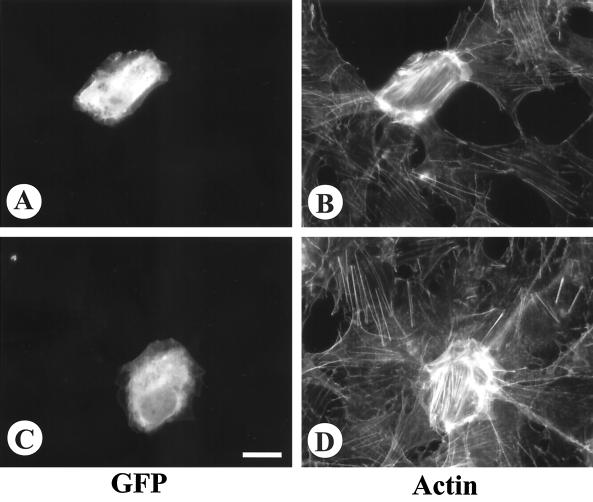
FIG. 6.
Effect of coexpressing dominant negative Cdc42 and Rac1 on the morphology of cells expressing constitutively active Vav2. CHO cells were transfected with Δ184N Vav2-GFP either alone (A and B) or cotransfected with myc-tagged N17Cdc42 (C to E) or myc-tagged N17Rac1 (F to H). Actin was visualized by staining with rhodamine-phalloidin (A, C, and F). Cells expressing Δ184N Vav2-GFP were visualized by GFP fluorescence (B, D, and G). Cells expressing the myc-tagged constructs were visualized by immunofluorescence (E and H). Bar = 20 μm.
Vav2 increases Rac1, Cdc42, and RhoA activity.
The above results suggest that Rac1, Cdc42, and RhoA are involved in the Vav2-induced phenotype of increased migration and in the morphological effects generated by Vav2 overexpression or activation. However, the use of dominant negative Rho family members is indirect. These dominant negative constructs work by competing with the endogenous G proteins for binding to exchange factors (17, 50). Many GEFs activate more than one Rho family member (10, 49, 51); therefore, dominant negative forms of these G proteins may block the activity of a related but distinct family member. Indeed, this probably accounts for the inhibition of membrane ruffling by dominant negative N17Cdc42 noted above. Consequently, we wished to measure directly the activity of Rac1, Cdc42, and RhoA in cells transfected with our Vav2 constructs. Because of their high transfection efficiency, CHO cells were used for these experiments. Measuring the amount of GTP bound to Rho family proteins has been difficult because of high intrinsic GTPase rates and because antibodies against these proteins are generally not good for immunoprecipitation. We have employed affinity precipitation assays to measure the amount of these proteins that have GTP bound (4, 6, 40, 43). To measure the amounts of active Rac1 and Cdc42, we used a GST construct of PAK3, which selectively binds to the GTP-bound but not GDP-bound forms of Rac1 and Cdc42 (3, 4). To measure the level of active RhoA, we used a GST construct of Rhotekin, which selectively binds to the GTP-bound form of RhoA but not the GDP-bound form (40). Glutathione-Sepharose beads complexed to the described GST fusion proteins were used to affinity precipitate GTP-bound Rac1, Cdc42, or RhoA. The amount of active G protein was detected by blotting with antibodies to Rac1, Cdc42, and RhoA.
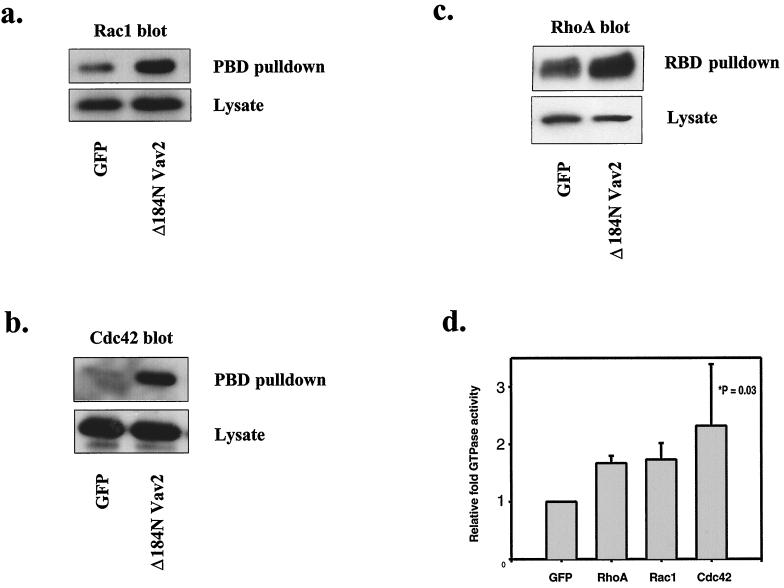
Expression of Δ184N Vav2 in CHO cells resulted in elevated levels of active Rac1, Cdc42, and RhoA compared with control cells expressing GFP alone (Fig. 7). With RhoA and Rac1, the level of increase was about 1.7-fold, whereas with Cdc42, the increase in several experiments was greater, although more variable (Fig. 7d). These experiments involved transient transfection of Δ184N Vav2, and only about 30% of the cells on a dish were transfected. Consequently, the level of active RhoA and Rac1 in the cells expressing Δ184N Vav2 would have been elevated approximately 2.5-fold above the level in untransfected cells. For Cdc42, the level of activation would have been still higher. We also investigated the effect of transfecting wild-type Vav2 on Rac1 and RhoA activity and found that overexpression of Vav2 increased the level of activity of both of these proteins, but to a lesser extent than Δ184N Vav2 (data not shown). All the above experiments were conducted with cells grown in the presence of serum. We were concerned that factors present in serum, such as lysophosphatidic acid, would activate RhoA (41) and that greater effects on RhoA activity might be seen in the absence of serum. Consequently, we also examined the effect of Δ184N Vav2 expression on RhoA activity in serum-starved cells. However, this revealed essentially equivalent levels of RhoA activation in response to Δ184N Vav2 expression in the absence of serum as in the presence of serum (data not shown).
FIG. 7.
Vav2 expression elevates the activity of Rac1, Cdc42, and RhoA in cells. (a to c) Blots with anti-Rac1, Cdc42, and RhoA are shown, respectively. In each case, the top panels show immunoblots of the protein that was sedimented by either the GST-PBD beads (i.e., Rac1-GTP or Cdc42-GTP) or the GST-RBD beads (i.e., RhoA-GTP) after incubation with lysates from cells transfected with GFP or Δ184N Vav2. The bottom panels show blots of the corresponding cell lysates. (d) Graphical representation of relative activity of RhoA, Rac1, and Cdc42 in cells transfected with Δ184N Vav2. The activity level of RhoA, Rac1, and Cdc42 in cells transfected with GFP was set at 1. Each bar represents the mean of three separate experiments (error bars, standard error of the mean). For the increase in RhoA and Rac activity, P < 0.001. For the increase in Cdc42 activity, P = 0.03.
Previous studies have demonstrated that growth factors such as EGF and PDGF stimulate Rac activity (24, 35, 42). We have shown here that these growth factors also result in the tyrosine phosphorylation of Vav2 (Figure 1b and c), and previously tyrosine phosphorylation of Vav2 has been shown to activate it as an exchange factor (46). To determine whether Vav2 was required for growth factor-mediated activation of Rac1, we expressed the SH3-SH2-SH3 carboxy-terminal construct as a putative dominant negative form of Vav2. HEK293 cells were transfected with GFP alone or with the SH3-SH2-SH3 Vav2 C-terminal construct. The cells were stimulated with EGF for 20 min, and the level of active Rac1 was measured (Fig. 8). For these experiments, quantitation was performed by phosphorimager analysis of the immunoblots. Stimulation of the cells with EGF resulted in an approximately 1.8-fold elevation in Rac1 activity. Expression of the C-terminal Vav2 construct consistently reduced the level of EGF-stimulated Rac1 activity to about 30% of the level achieved by EGF stimulation of cells expressing GFP (P = 0.03). Because only a fraction of the cells were transfected with the Vav2 C terminus, these results suggest that expression of this Vav2 construct blocked the elevation in Rac1 activity induced by EGF. This supports the idea that EGF stimulates a rise in Rac1 activity, at least in part through activation of endogenous Vav2.
DISCUSSION
At the outset of this work, we were interested in determining whether the GEF Vav2 acts downstream of β1 integrin engagement in nonhematopoietic cells. β1 integrins mediate the adhesion of cells to many ECM proteins (27). During the process of adhesion and spreading on ECM substrates, cells extend filopodia and lamellipodia, and over a longer time course they develop stress fibers. These cytoskeletal rearrangements are regulated by Cdc42, Rac1, and RhoA, respectively, three low-molecular-weight G proteins that belong to the Ras superfamily (22, 50). The activity of these proteins can be stimulated by many soluble factors, and recent work has shown that β1 integrin-mediated adhesion itself stimulates rapid activation of Cdc42 and Rac1 (14, 38) and a slower activation of RhoA (5, 40). The pathway from β1 integrins to the activation of these Rho family proteins has not been determined, but it is likely to involve activation of GEFs or inhibition of GAPs.
Previous work has demonstrated a link between multiple integrins and Vav1, a Rho family GEF restricted to hematopoietic cells. Vav1 becomes activated in response to tyrosine phosphorylation (16, 23). Platelet adhesion to fibrinogen via the integrin αIIbβ3, or to collagen or fibronectin via β1 integrins leads to a rapid tyrosine phosphorylation of Vav1 (12). Expression of αIIbβ3 and Vav1 in CHO cells, together with the hematopoietic tyrosine kinase, Syk, led to activation of Vav1 when these cells adhered to fibrinogen via the expressed αIIbβ3 (31). In addition, these cells developed a pronounced Rac1 phenotype of extensive lamellipodia and ruffling membranes. This work led us to investigate whether the widely distributed Vav family member Vav2 might function as a GEF downstream from β1 integrin engagement in nonhematopoietic cells. Vav2 shares extensive homology with Vav1, including the same domain structure (9, 45). Tyrosine phosphorylation has been shown to activate Vav2 as it does Vav1 (46); therefore, we used tyrosine phosphorylation as an indicator of Vav2 activity. Contrary to our expectations, we were unable to detect elevated phosphotyrosine in Vav2 in cells plated on fibronectin or collagen, both of which are ligands for β1 integrins. These results indicated that Vav2 is unlikely to act as a Rho family GEF downstream of integrins in the fibroblastic and epithelial cells that we have examined. We began to explore other agents that might promote Vav2 tyrosine phosphorylation and hence activation. We found that the growth factors EGF and PDGF resulted in a rapid but transient tyrosine phosphorylation of this exchange factor. Similarly, Moores and colleagues have also found that Vav2 is tyrosine phosphorylated in response to EGF and PDGF, but not tyrosine phosphorylated in response to integrin-mediated adhesion (31a). After completion of our work, the association of tyrosine phosphorylated Vav2 with the EGF and PDGF receptors was described (37).
We have been interested in the targets of Vav2 activity. Initial studies measuring GEF activity in vitro indicated that Vav2 differed from Vav1 with respect to its targets in the Rho family (46). Vav1 has been shown to act on Rac1, Cdc42, and RhoA (16, 23), whereas Vav2 was noted to act on RhoA and RhoG, but not on Rac1 or Cdc42 (46). A different result, however, has been obtained from Abe et al., who have found that Vav2 has exchange factor activity for Rac1, Cdc42, and RhoA in vitro (1). To explore the activity of Vav2 in cells, we have expressed a GFP-tagged construct of wild-type Vav2, as well as a GFP-tagged, amino-terminally truncated version of the protein. Amino-terminal truncation of many GEFs renders them constitutively active and oncogenic (10, 51), and this is also true with Vav1 and Vav2 (1, 9, 46). We have examined both the actin cytoskeletal organizations of cells overexpressing these Vav2 constructs and measured the level of active Rac1, Cdc42, and RhoA. Morphologically, cells expressing Vav2 or its oncogenic, truncated form revealed both a Rac1 and a RhoA phenotype. The cells displayed extensive lamellipodia and ruffling membranes but also had prominent stress fibers. In addition, these cells showed enhanced cell migration. We found that the increased migration was inhibited by coexpression of dominant negative forms of Rac1 or Cdc42. These dominant negative constructs also inhibited the prominent membrane ruffling induced in cells expressing the constitutively active form of Vav2.
In order to measure the level of active Rac1, Cdc42, and RhoA, we have used affinity precipitation assays in which only the GTP-bound forms of these proteins are sedimented by binding to immobilized effector fusion proteins (4, 6, 40, 43). Expression of these Vav2 constructs, particularly the amino-terminally truncated form, resulted in elevated levels of Rac1, Cdc42, and RhoA. These results are consistent with the mixed Rac1 and RhoA phenotypes, i.e., with cells exhibiting prominent ruffling membranes and also stress fibers. In general, we did not observe an obvious Cdc42 morphological phenotype, but the development of filopodia is often obscured by Rac1 activation, as filopodia become engulfed by lamellipodia and membrane ruffles. Our results indicate that Vav2 activates Rac1, Cdc42, and RhoA. Although this appears to differ from the results of Schuebel et al. (46), who noted Vav2 activating RhoA and RhoG in vitro but not Rac1 or Cdc42, it should be noted that RhoG has been implicated in downstream activation of both Rac1 and Cdc42 (19). At present, we cannot say whether the elevation of Rac1 and Cdc42 activity in cells overexpressing Vav2 is due to a direct activation of these proteins by Vav2 or is indirect and mediated by Vav2 acting on RhoG.
The tyrosine phosphorylation of Vav2 in response to stimulating cells with EGF or PDGF suggests that it contributes to Rac1 activity induced by these growth factors. Consistent with this possibility, we found that expression of a carboxy-terminal fragment of Vav2, which is predicted to compete with Vav2 for interactions mediated via its SH3 and SH2 domains, decreased the level of active Rac1 in cells stimulated with EGF (Fig. 8). Similarly, in preliminary studies, we have noted that expression of this C-terminal construct of Vav2 decreases Rac1 activity in cells stimulated with PDGF (data not shown). Previous work, however, has implicated Sos1 (32) and Sos1 complexed with Eps8 and E3b1/Abi-1 (48) in the elevation of Rac1-GTP levels downstream from receptor tyrosine kinases. It seems likely that there are multiple pathways that lead from receptor tyrosine kinases to Rac1 activation. In future work, it will be important to compare the relative contributions of these and other GEFs to the activation of Rho family proteins following cell stimulation by growth factors.
Although our data demonstrate a role for Vav2 in growth factor signaling to the cytoskeleton, the morphological phenotype of cells expressing activated Vav2 differs from that of growth factor-stimulated cells. Cells overexpressing either full-length or activated Vav2 display both extensive membrane ruffles and increased stress fibers, indicative of a combination of Rac1 and RhoA activation. In contrast, growth factors, such as EGF and PDGF, rapidly induce membrane ruffling, but this is accompanied by a loss of stress fibers and focal adhesions (39, 42, 52). In some situations, this initial induction of membrane ruffles in response to growth factors is followed by a slower development of stress fibers (42). It is possible that the function of Vav2 downstream from growth factor receptors is to mediate the second phase of actin rearrangements in which both membrane ruffles and stress fibers coexist. However, the time course of Vav2 tyrosine phosphorylation in response to growth factors is rapid and would be expected to result in an immediate stimulation of both Rac1 and RhoA activity. An alternative explanation is that, coincident with the initial activation of Vav2 by growth factors, a Rho GAP is simultaneously activated and that this antagonizes RhoA activation. Previous studies have indeed provided evidence for activation of p190RhoGAP in response to EGF stimulation (11). One scenario that we can envisage is a biphasic response to growth factor stimulation, in which transient activation of p190RhoGAP is coupled with a more sustained activation of Vav2. This would result first in a decrease in RhoA activity that would be followed by an increase with time. Such a response may be important in cell migration, a process that is triggered by growth factors like PDGF and EGF. Evidence has been presented that too-strong adhesion, such as that provided by focal adhesions, can antagonize cell migration (26). The disassembly of stress fibers and focal adhesions induced by growth factors has been suggested to remove a brake that would otherwise retard migration. However, recent work has established that, while too much RhoA activity inhibits migration, some RhoA activity is necessary (34). Consequently, the activation of Vav2 by EGF and PDGF may contribute to cell migration not only by activating Rac1 and Cdc42, but also by activating RhoA.
Vav2 contains many domains involved in binding other components. It seems likely that its interactions with other components will be important in regulating its activity in various situations. A goal for the future will be to identify these interactions and to determine how these interactions affect Vav2 activity and contribute to regulating complex events such as cell migration.
ACKNOWLEDGMENTS
We are most grateful to Joan Brugge and Sheri Moores for sharing their data with us prior to their publication. David Kwiatkowski kindly provided us with Vav2 cDNA. Many of our laboratory colleagues have contributed advice and encouragement. We especially thank Bill Arthur, Nikki Noren, Leslie Petch, Sarita Sastry, Amy Shaub, and Becky Worthylake. B.L. thanks Simone Schoenwaelder and Magda Chrzanowska-Wodnicka for sustained encouragement. We thank Michele Alexandre for technical assistance.
This work was supported by NIH grant GM29860.
REFERENCES
- 1.Abe K, Rossman K L, Liu B, Ritola K D, Chiang D, Campbell S L, Burridge K, Der C J. Vav2 is an activator of Cdc42, Rac1 and RhoA. J Biol Chem. 2000;275:10141–10149. doi: 10.1074/jbc.275.14.10141. [DOI] [PubMed] [Google Scholar]
- 2.Aplin A E, Howe A, Alahari S K, Juliano R L. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules and selectins. Pharmacol Rev. 1998;50:197–262. [PubMed] [Google Scholar]
- 3.Bagrodia S, Taylor S J, Creasy C L, Chernoff J, Cerione R A. Identification of a mouse p21Cdc42/Rac activated kinase. J Biol Chem. 1995;270:22731–22737. doi: 10.1074/jbc.270.39.22731. . (Erratum, 271:1250, 1996.) [DOI] [PubMed] [Google Scholar]
- 4.Bagrodia S, Taylor S J, Jordon K A, Van'Aelst L, Cerione R A. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- 5.Barry S T, Flinn H M, Humphries M J, Critchley D R, Ridley A J. Requirement for Rho in integrin signalling. Cell Adhes Commun. 1997;4:387–398. doi: 10.3109/15419069709004456. [DOI] [PubMed] [Google Scholar]
- 6.Benard V, Bohl B P, Bokoch G M. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 7.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility and signaling. Annu Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 8.Bustelo X R. The VAV family of signal transduction molecules. Crit Rev Oncog. 1996;7:65–88. doi: 10.1615/critrevoncog.v7.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 9.Bustelo X R. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerione R A, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- 11.Chang J H, Gill S, Settleman J, Parsons S J. c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J Cell Biol. 1995;130:355–368. doi: 10.1083/jcb.130.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cichowski K, Brugge J S, Brass L F. Thrombin receptor activation and integrin engagement stimulate tyrosine phosphorylation of the proto-oncogene product, p95Vav, in platelets. J Biol Chem. 1996;271:7544–7550. doi: 10.1074/jbc.271.13.7544. [DOI] [PubMed] [Google Scholar]
- 13.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 14.Clark E A, King W G, Brugge J S, Symons M, Hynes R O. Integrin-mediated signals regulated by members of the Rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark E A, Shattil S J, Ginsberg M H, Bolen J, Brugge J S. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin alpha IIb beta 3. J Biol Chem. 1994;269:28859–28864. [PubMed] [Google Scholar]
- 16.Crespo P, Schuebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 17.Feig L A. Tools of the trade: use of dominant inhibitory mutants of Ras-family GTPases. Nat Cell Biol. 1999;1:E25–E27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Zoller K E, Ginsberg M H, Brugge J S, Shattil S J. Regulation of the pp72syk protein tyrosine kinase by platelet integrin alpha IIb beta 3. EMBO J. 1997;16:6414–6425. doi: 10.1093/emboj/16.21.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauthier-Rouviere C, Vignal E, Meriane M, Roux P, Montcourier P, Fort P. RhoG GTPase controls a pathway that independently activates Rac1 and Cdc42Hs. Mol Biol Cell. 1998;9:1379–1394. doi: 10.1091/mbc.9.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotoh A, Takahira H, Geahlen R L, Broxmeyer H E. Cross-linking of integrins induces tyrosine phosphorylation of the proto-oncogene product Vav and the protein tyrosine kinase Syk in human factor-dependent myeloid cells. Cell Growth Differ. 1997;8:721–729. [PubMed] [Google Scholar]
- 21.Guan J L, Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- 22.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 23.Han J W, Das B, Wei W, Van Aelst L, Mosteller R D, Khosravi-Far R, Westwick J K, Der C J, Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkins P T, Eguinoa A, Qiu R G, Stokoe D, Cooke F T, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M, Stephens L. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 25.Henske E P, Short M P, Jozwiak S, Bovey C M, Ramlakhan S, Haines J L, Kwiatkowski D J. Identification of VAV2 on 9q34 and its exclusion as the tuberous sclerosis gene TSC1. Ann Hum Genet. 1995;59:25–37. doi: 10.1111/j.1469-1809.1995.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 26.Huttenlocher A, Sandborg R R, Horwitz A F. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 27.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 28.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipfert L, Haimovich B, Schaller M D, Cobb B S, Parsons J T, Brugge J S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu B P, Chrzanowska-Wodnicka M, Burridge K. Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via the GTP-binding protein Rho. Cell Adhes Commun. 1998;5:249–255. doi: 10.3109/15419069809040295. [DOI] [PubMed] [Google Scholar]
- 31.Miranti C K, Leng L, Mascherger P, Brugge J S, Shattil S J. Identification of a novel integrin signaling pathway involving the kinase Syk and the guanine nucleotide exchange factor Vav1. Curr Biol. 1998;8:1289–1299. doi: 10.1016/s0960-9822(07)00559-3. [DOI] [PubMed] [Google Scholar]
- 31a.Moores S L, Selfors L M, Fredericks J, Breit T, Fujikawa K, Alt F W, Brugge J S, Swat W. Vav family proteins couple to diverse cell surface receptors. Mol Cell Biol. 2000;20:6364–6373. doi: 10.1128/mcb.20.17.6364-6373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nimnual A S, Yatsula B A, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 33.Nobes C D, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 34.Nobes C D, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nobes C D, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- 36.Olson M F, Pasteris N G, Gorski J L, Hall A. Faciogenital dysplasia protein (FGD1) and VAV, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- 37.Pandey A, Podtelejnikov A V, Blagoev B, Bustelo X R, Mann M, Lodish H F. Analysis of receptor signaling pathways by mass spectrometry: identification of Vav-2 as a substrate of the epidermal and platelet-derived growth factor receptors. Proc Natl Acad Sci USA. 2000;97:179–184. doi: 10.1073/pnas.97.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price L S, Leng J, Schwartz M A, Bokoch G M. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rankin S, Rozengurt E. Platelet-derived growth factor modulation of focal adhesion kinase (p125FAK) and paxillin tyrosine phosphorylation in Swiss 3T3 cells. Bell-shaped dose response and cross-talk with bombesin. J Biol Chem. 1994;269:704–710. [PubMed] [Google Scholar]
- 40.Ren X D, Kiosses W B, Schwartz M A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridley A J, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 42.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 43.Sander E E, van Delft S, ten Klooster J P, Reid T, van der Kammen R A, Michiels F, Collard J G. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoenwaelder S M, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 45.Schuebel K E, Bustelo X R, Nielsen D A, Song B, Barbacid M, Goldman D, Lee I J. Isolation and characterization of murine vav2, a member of the vav family of proto-oncogenes. Oncogene. 1996;13:363–371. [PubMed] [Google Scholar]
- 46.Schuebel K E, Movilla N, Rosa J L, Bustelo X R. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–6621. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz M A, Schaller M D, Ginsberg M H. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 48.Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind J S, Bjarnegard M, Betsholtz C, Di Fiore P P. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- 49.Stam J C, Collard J G. The DH protein family, exchange factors for Rho-like GTPases. Prog Mol Subcell Biol. 1999;22:51–83. doi: 10.1007/978-3-642-58591-3_4. [DOI] [PubMed] [Google Scholar]
- 50.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 51.Whitehead I P, Campbell S, Rossman K L, Der C J. Dbl family proteins. Biochim Biophys Acta. 1997;1332:F1–F23. doi: 10.1016/s0304-419x(96)00040-6. [DOI] [PubMed] [Google Scholar]
- 52.Xie H, Pallero M A, Gupta K, Chang P, Ware M F, Witke W, Kwiatkowski D J, Lauffenburger D A, Murphy-Ullrich J E, Wells A. EGF receptor regulation of cell motility—EGF induces disassembly of focal adhesions independently of the motility-associated PLC-gamma signaling pathway. J Cell Sci. 1998;111:615–624. doi: 10.1242/jcs.111.5.615. [DOI] [PubMed] [Google Scholar]
- 53.Yron I, Deckert M, Reff M E, Munshi A, Schwartz M A, Altman A. Integrin-dependent tyrosine phosphorylation and growth regulation by Vav. Cell Adhes Commun. 1999;7:1–11. doi: 10.3109/15419069909034388. [DOI] [PubMed] [Google Scholar]
- 54.Zheng L, Sjolander A, Eckerdal J, Andersson T. Antibody-induced engagement of beta 2 integrins on adherent human neutrophils triggers activation of p21ras through tyrosine phosphorylation of the protooncogene product Vav. Proc Natl Acad Sci USA. 1996;93:8431–8436. doi: 10.1073/pnas.93.16.8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zohn I M, Campbell S L, Khosravi-Far R, Rossman K L, Der C J. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene. 1998;17:1415–1438. doi: 10.1038/sj.onc.1202181. [DOI] [PubMed] [Google Scholar]