Abstract
V(D)J recombination is the mechanism by which antigen receptor genes are assembled. The site-specific cleavage mediated by RAG1 and RAG2 proteins generates two types of double-strand DNA breaks: blunt signal ends and covalently sealed hairpin coding ends. Although these DNA breaks are mainly resolved into coding joints and signal joints, they can participate in a nonstandard joining process, forming hybrid and open/shut joints that link coding ends to signal ends. In addition, the broken DNA molecules excised from different receptor gene loci could potentially be joined to generate interlocus joints. The interlocus recombination process may contribute to the translocation between antigen receptor genes and oncogenes, leading to malignant transformation of lymphocytes. To investigate the underlying mechanisms of these nonstandard recombination events, we took advantage of recombination-inducible cell lines derived from scid homozygous (s/s) and scid heterozygous (s/+) mice by transforming B-cell precursors with a temperature-sensitive Abelson murine leukemia virus mutant (ts-Ab-MLV). We can manipulate the level of recombination cleavage and end resolution by altering the cell culture temperature. By analyzing various recombination products in scid and s/+ ts-Ab-MLV transformants, we report in this study that scid cells make higher levels of interlocus and hybrid joints than their normal counterparts. These joints arise concurrently with the formation of intralocus joints, as well as with the appearance of opened coding ends. The junctions of these joining products exhibit excessive nucleotide deletions, a characteristic of scid coding joints. These data suggest that an inability of scid cells to promptly resolve their recombination ends exposes the ends to a random joining process, which can conceivably lead to chromosomal translocations.
Developing lymphocytes have the unique ability to generate diverse antigen receptor molecules, immunoglobulins, and T-cell receptors. The assembly of these receptor genes is achieved through site-specific recombination events from separately encoded gene segments, variable (V), diversity (D), and joining (J) regions, in a process known as V(D)J recombination (6). Each gene segment is flanked by conserved recombination signal sequences (RSS) with a spacer of 12 or 23 nucleotides (12-RSS or 23-RSS, respectively). V(D)J recombination, catalyzed by enzymes encoded by recombination-activating genes 1 and 2 (RAG1 and RAG2), takes place at the junctions between RSS and coding gene segments (5, 19). Cleavage occurs coordinately at 12-RSS and 23-RSS, in accordance with what is known as the 12/23 rule. This site-specific cleavage generates two types of broken DNA molecules: blunt-opened signal ends and covalently sealed coding ends (22, 39).
The joining of these ends to form signal joints (SJ) and coding joints (CJ) is mediated by nonhomologous end joining machinery, which is believed to be the principal pathway to repair double-stranded breaks (DSBs) in vertebrate cells (29, 30). Identification of these proteins has been facilitated by analyses of mutant cells with defects in both V(D)J recombination and DSB repair. The first instance of such defects came from the characterization of the severe combined immunodeficient (scid) mouse (10, 20). The scid mutation was mapped to the gene encoding the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) (3, 7). Other proteins involved in both V(D)J recombination and DSB repair, such as Ku70/80, XRCC4, and ligase IV have also been identified and characterized (9, 18, 24, 38, 45, 49). These gene products have been shown to play an important role in the formation of both SJ and CJ, whereas DNA-PKcs seems to be essential only for CJ formation (40, 49).
V(D)J recombination may lead to other outcomes, such as joining of coding ends to signal ends to form hybrid joints (HJ) and rejoining of the original pair of coding and signal ends to form open/shut joints (17, 36, 37). In addition, the joining of two ends residing on different chromosomes can produce interlocus joints (IJ) (31, 51). Generation of HJ and open/shut joints fails to promote lymphocyte differentiation and possibly prevents the locus from further rearrangements if the rejoined RSS suffer nucleotide loss (35). Recombination between two chromosomes causes chromosomal instabilities, which were found to be correlated with an increased risk of lymphoid malignancies in patients with Hodgkin's disease receiving chemotherapy (31, 32). Thus, these so-called nonstandard V(D)J recombination events, in theory, should be strictly prohibited in normal lymphocyte development. Indeed, interlocus recombination products were rarely detectable in normal cells (33, 42, 51). The underlying mechanism for the rarity of interlocus recombination is not known. Interestingly, scid thymocytes appear to have a higher level of IJ than their normal counterpart, especially after their exposure to ionizing radiation (43). Although this finding suggests that functional DNA-PKcs may somehow prevent interlocus recombination, the mechanism of its molecular action is not clear.
Recently, by using recombination-inducible cell lines derived from transformation of normal B-cell precursors with temperature-sensitive (ts) Abelson murine leukemia virus (Ab-MLV), Bailey and Rosenberg detected interlocus recombination products during an activation of V(D)J recombination (4). This recombination event, however, is much restrained and at a level 1,000-fold less than that of intralocus recombination, a finding which resembles the finding with normal lymphocytes (52). To directly test the role of DNA-PKcs in nonstandard V(D)J recombination, we developed recombination-inducible ts-Ab-MLV cell lines from both scid homozygous (s/s) and scid heterozygous (s/+) mice that bear the bcl-2 transgene (13). These cell lines exhibit temperature-dependent characteristics similar to those reported by Chen et al. (14, 15), such as G1 cell cycle arrest, up-regulation of RAG1 and RAG2 and initiation of recombination at light chain loci when they are incubated at the nonpermissive temperature (13; unpublished observation). Due to the presence of the bcl-2 transgene, these transformed cells delay or prevent an apoptotic response and have more time to resolve their recombination intermediates. Therefore, the nonstandard recombination joints made in normal and scid transformants could be directly compared during the same course of recombination induction. Our studies reveal that scid transformants produce higher levels of both IJ and HJ than their s/+ counterparts, which express functional DNA-PKcs. These findings are discussed in conjunction with a model for coding end resolution in both cell types.
MATERIALS AND METHODS
Cell culture and DNA preparation.
As described previously, temperature-sensitive pre-B-cell lines FL2-1 and A-1 were derived from s/s and s/+ bcl-2 transgenic mice, respectively, by transformation of fetal B-cell precursors with ts-Ab-MLV (13). Cells were kept at the permissive temperature, 33°C. To induce V(D)J recombination, cell cultures were shifted to the nonpermissive temperature, 39°C, for 48 h. Some of the cells were then returned to 33°C for 24 or 48 h. Both scid and s/+ ts-Ab-MLV transformants grown under different culture conditions were harvested for preparation of genomic DNA, as described previously (11). DNA was dissolved in water at a concentration of 100,000 cell genome equivalents per μl. Alternatively, DNA was prepared in an agarose plug following the procedure described before (13).
PCR amplification of ends and joints.
Recombination signal ends were detected by ligation-mediated PCR (LMPCR), a procedure previously reported by Roth et al (48). The linker-ligated Vλ1/2 signal ends (Vλ1-SE) and Jλ1-SE were amplified by a PCR using linker-specific primer YC25 (5′-GCTATGTACTACCCGGGAATTCGTG-3′) (48) and the primer specific to the 3′ region of Vλ1 (YC24: 5′-CAATGATTCTATGTTGTGCC-3′) and YC25 together with the primer complementary to the 5′ region of Jλ1 (YC23: 5′-GCTGCATACATCACAGATGC-3′), respectively. The Jκ1-SE was amplified using the same primers described previously (13). For semiquantitative comparison of LMPCR products between s/+ and s/s ts-Ab-MLV transformants (s/+-ts and s/s-ts cells, respectively), serial dilutions of ligated DNA molecules were amplified to ensure the linearity of PCR amplification.
VλJλ CJ were amplified with primers complementary to Vλ1/2 and Jλ1 coding regions (YC15 [5′-AGAAGCTTGTGACTCAGGAATCTGCA-3′] and YC16 [5′-CAGGATCCTAGGACAGTCAGTTTGGT-3′]). VλVκ interlocus CJ (which obey the 12/23 rule) were amplified with YC15 and the degenerated Vκ primer (MB46 [12]). VλJκ interlocus CJ (which violate the 12/23 rule) were amplified with primers complementary to the Vλ1/2 (YC15) and Jκ2 coding regions (YC37: 5′-TCCCTCCTTAACACCTGATCTGAG-3′). Vλ1Jκ interlocus SJ were amplified with primers complementary to the 3′ region of Vλ1-RSS (YC-24) and the 5′ region of Jκ1-RSS (MB224 [13]). VλJλ HJ were amplified with YC15 and the primer complementary to the 5′ region of Jλ1 (YC-36: 5′-TTCAGTGATGTCACCACCTTCC-3′). DNA amplification was carried out in 30-μl PCR mixtures containing 10 mM Tris-HCL, pH 8.3, 50 mM KCL, 2 mM MgCl2, 10 μg of gelatin/μl, a 2 μM concentration of each primer, a 0.2 mM concentration of each deoxynucleoside triphosphate, and 0.5 U of Taq polymerase (Promega). The DNA mixture was first denatured at 95°C for 5 min; this was followed by 20 to 28 cycles of PCR amplification (20 cycles for actin, 25 cycles for VλJλ-CJ, and 28 cycles for VλVκ-CJ, VλJκ-SJ, VλJκ-CJ, and VλJλ-HJ). Each cycle consisted of 1 min at 94°C, 45 s at either 60 (actin, SJ) or 63°C (VλJλ-CJ, VλJκ-CJ, VλVκ-CJ, VλJκ-SJ, and VλJλ-HJ), and then 90 s at 72°C. Finally, the PCR products were extended for 10 min at 72°C. Serial dilutions of DNA samples were included to determine the linearity of the PCR.
Southern blotting.
One-third of each PCR mixture was run on a 1.2% agarose gel and transferred onto a GeneScreen Plus hybridization transfer membrane (NEN). Blots were then hybridized with the following probes: (i) Vλ1 insert to reveal various Vλ1-associated PCR products, such as CJ, HJ, and IJ; (ii) Jκ insert to confirm the Vλ1Jκ IJ; and (iii) plasmid actin to reveal actin PCR products (12). Probes were labeled with [32P]dCTP using the Prime-It II kit (Stratagene). The autoradiograph representing actin and CJ PCR products was produced with only a 1- to 2-h exposure at −80°C, whereas a longer exposure (8 to 12 h) was required to reveal the bands for HJ and IJ. The intensities of PCR bands were analyzed with a PhosphorImager and quantified using Image Quant software (Molecular Dynamics). The relative amount of each rearranged product was normalized against the control actin, i.e., expressed as counts per minute for the sample/counts per minute for actin.
Cloning and sequencing.
Primary PCR products were subjected to a second round of PCR using the appropriate internal primers, and the resulting PCR products were separated by electrophoresis and purified using a QIAEX II gel extraction kit (Qiagen). The purified PCR products were cloned into a TOPO TA cloning vector (Invitrogen) and sequenced by an automated DNA sequencer (ABI 737). The sequence of each clone was compared to the germ line Vλ, Jλ, and Jκ regions from the GenBank database by Blast similarity. The lack of a corresponding region was characterized as a deletion.
RESULTS
Recombination initiation at both κ and λ gene loci.
We have previously demonstrated that incubating cells at the nonpermissive temperature induces the production of recombination intermediates in s/s-ts and s/+-ts cells (13). This system enables the fate of recombination intermediates to be directly examined, i.e., joining products made in situ. It has been reported that ts-Ab-MLV transformants can be induced to rearrange both κ- and λ-chain genes (44). Thus, we were particularly interested in determining whether the newly generated recombination intermediates in scid cells are more vulnerable to undergoing nonstandard recombination events, such as making IJ and HJ. A comparison of these joining products between s/s-ts and s/+-ts cells should be made in cells that have similar levels of recombination cleavage. Yet different cell lines may have different levels of recombination activity at different gene loci, which would ultimately affect the amounts of various joining products. To assess the recombination activity at κ and λ gene loci in s/s-ts and s/+-ts cells, we analyzed the Jκ signal ends (Jκ-SE) and Vλ1 and Jλ1 signal ends (Vλ-SE and Jλ-SE).
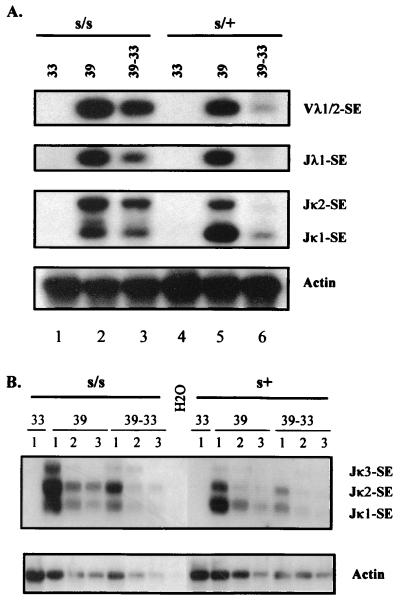
It is apparent from Fig. 1A that at the nonpermissive temperature of 39°C the levels of signal ends generated from the three gene loci in s/s-ts and s/+-ts cells are comparable. Semiquantitative PCR analysis shows a relatively linear amplification of signal ends (Fig. 1B). Upon shifting cells from 39 to 33°C for 24 h, the amount of signal ends was significantly reduced in s/+-ts cells and to a lesser extent in s/s-ts cells (Fig. 1A, lanes 3 and 6). This reflects a partial defect of scid cells in the resolution of signal ends as reported previously (8, 11, 40). Nonetheless, comparable levels of recombination cleavage were initiated simultaneously at both κ and λ gene loci in the s/s-ts and s/+-ts cells. From this analysis, we infer that similar levels of coding ends should be produced in the two cell types, though these ends are rapidly resolved in s/+-ts cells but are persistent in s/s-ts cells (11, 13). Therefore, various joining products of the newly generated coding ends can be directly compared between s/s-ts and s/+-ts cells.
FIG. 1.
Simultaneous induction of V(D)J recombination cleavage at both κ and λ gene loci. (A) Analysis of recombination signal ends at three gene loci, Vλ1/2, Jλ1, and Jκ1/2. DNA samples were isolated from ts-Ab-MLV transformants of s/+ and s/s cells that had been subjected to various culture conditions: 33°C (33), 39°C for 3 days (39), or 39°C for 2 days followed by 1 day at 33°C (39–33). Recombination signal ends cleaved at Vλ1, Jλ1, and Jκ gene loci (Vλ1-SE, Jλ1-SE, and Jκ-SE, respectively) were amplified by LMPCR and revealed by Southern blot analysis using Vλ1, Jλ1, and Jκ1 probes, respectively. Amplification of the actin gene was used as a control for the input DNA. (B) Semiquantitative analysis of Jκ signal ends (Jκ-SE). Serial dilutions of ligated DNA molecules were subjected to PCR for amplifying Jκ signal ends. 1, 2, and 3, undiluted, threefold diluted, and ninefold diluted input DNA, respectively.
Interlocus rearrangement in s/s-ts cells.
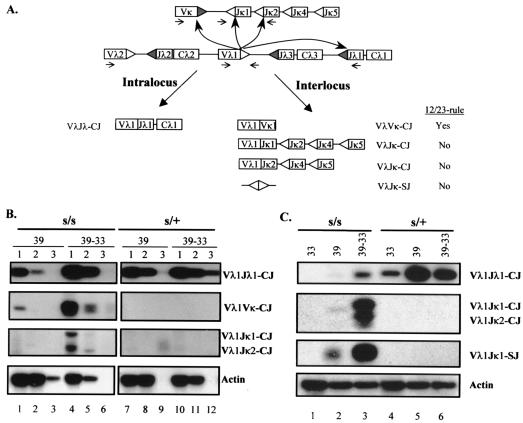
Given that recombination cleavage can be initiated at κ and λ gene loci (Fig. 1), recombination between these two loci can potentially occur. Figure 2A is a schematic diagram illustrating the detection of interlocus as well as intralocus rearrangements. Two types of IJ could be formed at the Vλ1 locus: a Vλ1Vκ joint that follows the recombination 12/23 rule and a Vλ1/2Jκ joint that violates the 12/23 rule. These joining products along with Vλ1Jλ1 joints can be amplified by a PCR with appropriate primers (as shown in Fig. 2A) and revealed by hybridization with a Vλ1 probe.
FIG. 2.
Analysis of L-chain interlocus rearrangements in s/s and s/+ ts-Ab-MLV transformants. (A) Diagrammatic representation of intralocus and interlocus L-chain gene rearrangements. The 12-RSS and 23-RSS are indicated by shaded and white triangles, respectively. Large curved arrows illustrate the direction of rearrangements; small arrows represent primers. The intralocus rearrangement at the λ1 gene locus gives rise to Vλ1/2Jλ1 CJ. Three types of interlocus recombination products are analyzed: the Vλ1/2Vκ CJ that follow the 12/23 rule, Vλ1/2Jκ1 (and Vλ1/2Jκ2) CJ, and Vλ1Jκ1 signal joints that violate the 12/23 rule. (B) Semiquantitative analysis of cis and trans CJ on diluted DNA. The amplified PCR products were probed with a Vλ1 probe. For details, see the Fig. 1 legend. (C) PCR and Southern blot analysis of standard Vλ1Jλ1 CJ, interlocus Vλ1/2Jκ1/2 CJ, and signal joints (SJ).
A semiquantitative analysis of VλVκ and VλJκ joints is presented in Fig. 2B (see Table 2, experiment 3). Although s/+-ts cells produce substantial amounts of Vλ1Jλ1 coding joints, they fail to make detectable Vλ1/2Vκ or Vλ1/2Jκ1/2 CJ (Fig. 2B, lanes 7 to 12). The s/+-ts cells maintained at 33°C show some background level of rearrangement, as evidenced by the presence of VλJλ joints (Fig. 2C, lane 4). This cis rearrangement was greatly increased when the cells were cultured at 39°C, while no trans rearrangement was found (Fig. 2C, lanes 4 to 6). Thus, the frequency of interlocus recombination is extremely low in s/+-ts cells. This finding is consistent with the previous report by Bailey and Rosenberg in which the VλJκ joints (trans rearrangement) were estimated to form at a frequency about 1,000-fold less than that of VλJλ joints (cis rearrangement) (4).
TABLE 2.
Sequence analysis of Vλ1/2Jκ1 CJ in s/s-ts cells
| Clone | Vλ1/2 coding sequence | Jκ1 coding sequence | No. of nucleotides deleted from germ line sequence for:
|
|
|---|---|---|---|---|
| Vλ1/2 | Jκ1 | |||
| Germ line | TACAGCAACCATT | GTGGACGTTCGGTGGAGGCACC | ||
| SL15 | TACAGCAACCA | 3-GACGTTCGGTGGAGGCACC | 2 | 3 |
| SL07 | TACAGCAAa | 5 | 33 | |
| SL17 | TA | 11 | 87 | |
| SL03 | 13 | 87 | ||
| SL13 | 25 | 58 | ||
| SL06 | 49 | 54 | ||
| SL02 | 75 | 90 | ||
| SL14 | 35 | 105 | ||
| SL20 | 50 | 95 | ||
| SL12 | 75 | 134 | ||
| SL04 | 94 | 260 | ||
The underlined nucleotides represent the homology between the Vλ1 and Jκ1 regions.
In contrast, both VλVκ and Vλ1/2Jκ1/2 CJ were readily detectable in the s/s-ts cells that were cultured at 39°C followed by a 48-h incubation at 33°C (Fig. 2B). The level of VλVκ joints appears somewhat higher than that of Vλ1Jκ joints. This difference may reflect the fact that the interlocus recombination between 12-RSS and 23-RSS is more favorable than the one between two 23-RSS. Alternatively, different levels of the two types of IJ could also be attributed to an artifact in PCR amplification. Essentially all Vκ coding segments that are joined to the Vλ1/2 gene segment could be amplified since a degenerate Vκ primer was used in this PCR. On the other hand, the usage of the Jκ2 primer allows amplification of only a fraction of VλJκ IJ, i.e., VλJκ1 and VλJκ2. Nevertheless, the presence of Vλ1Jκ1/2 joints indicates that interlocus recombination in s/s-ts cells does not always obey the 12/23 rule, i.e., the joints can form without the synaptic formation between the 12-RSS on one locus and the 23-RSS on the other locus. It is more likely that the recombination ends generated from different chromosomes are joined nonspecifically.
The VλJκ CJ could even be detected in the s/s-ts cells that made small amounts of intralocus joints when the cells were returned to 33°C for only 24 h (Fig. 2C, lane 3). In addition to VλJκ CJ, their reciprocal SJ were readily detectable in s/s-ts but not s/+-ts cells (Fig. 2C). The Vλ1Jκ1 SJ contain nucleotide modifications, as they are resistant to BsiHKAI (an isoschizomer of HgiAI; New England Biolabs), a restriction enzyme that recognizes a perfect junction of SJ (data not shown). Thus, the formation of Vλ1/2-to-Jκ1 interlocus signal joints results from an aberrant recombination event that mimics CJ formation in scid cells.
As summarized in Table 1, scid cells make a higher level of IJ than their s/+ counterparts. It is even more striking if the ratios of IJ to intralocus joints for the s/+-ts cells and s/s-ts cells are compared (Table 1). This finding was also confirmed in seven s/+-ts clones and eight s/s-ts clones (unpublished observation). Therefore, recombination activation leads to the production of IJ in s/s-ts cells but much less so in s/+-ts cells.
TABLE 1.
Quantitation of relative levels of intralocus and interlocus joining products
| Expt | Culture temp conditionsa | Intensity (cpm for sample/cpm for actin)b for:
|
|||||
|---|---|---|---|---|---|---|---|
| s/+ cells
|
s/s cells
|
||||||
| CJ | IJ | IJ/CJc | CJ | IJ | IJ/CJ | ||
| 1 | 33 | 0.3 | <0.01 | 0.02 | <0.01 | 0.003 | —d |
| 39 | 6.8 | <0.01 | <0.01 | <0.01 | 0.004 | — | |
| 39–33 | 6.6 | <0.01 | <0.01 | 1.3 | 0.1 | 0.08 | |
| 2 | 33 | 1.4 | 0.02 | 0.01 | <0.01 | <0.01 | — |
| 39 | 3.5 | 0.02 | <0.01 | 0.14 | 0.01 | 0.07 | |
| 39–33 | 3.9 | 0.02 | <0.01 | 2.8 | 0.7 | 0.25 | |
| 3e | 39–33 | 2.9 ± 0.7 | <0.01f | <0.01 | 2.5 ± 0.2 | 0.89 ± 0.21f | 0.36 |
| <0.01g | <0.01 | 0.22 ± 0.05g | 0.09 | ||||
Cells were cultured at 33°C (33), 39°C for 2 days (39), or 2 days at 39°C followed by an incubation at 33°C for 1 (experiments 1 and 2) or 2 days (experiment 3) (39-33).
Values shown correspond to the amount of joining products relative to the amount for control actin. The intensity of hybridizing bands was quantitated with a PhosphorImager.
The ratio is derived by dividing the value for an IJ with the value for an CJ. For example, 0.36 is the ratio of 0.89 (VλVκ) to 2.5 (VλJλ).
—, both CJ and IJ values are too small to be significant for ratio calculation.
The quantitation is derived from Fig. 2B. Values are average intensities of the PCR products ± standard error on two different dilutions for each DNA sample (i.e., undiluted and threefold-diluted DNA).
VλVκ.
VλJκ.
To examine the quality of IJ, the PCR products of Vλ1/2Jκ1 CJ made in s/s-ts cells were cloned and sequenced, as shown in Table 2. Several independent clones were analyzed. All but one show a loss of nucleotides, ranging from 10 to 260 nucleotides. Thus, the formation of scid trans CJ is error prone, similar to the abnormality found in their cis CJ (13).
Formation of HJ in s/s-ts cells.
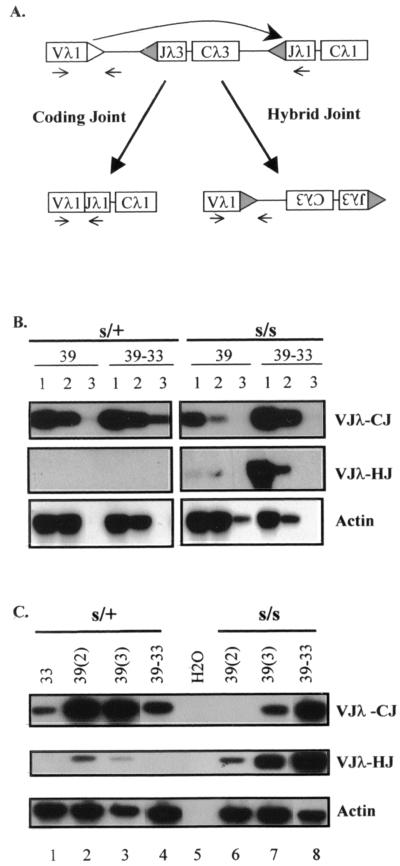
To evaluate HJ formation in s/s-ts and s/+-ts cells, we chose the Vλ1 and Jλ1 gene loci due to the simplicity of the Vλ1-to-Jλ1 rearrangement. Figure 3A illustrates the strategy for amplification of Vλ1Jλ1 HJ, as well as corresponding Vλ1Jλ1 CJ. The HJ amplified in this scheme should be formed via inversional recombination.
FIG. 3.
Analysis of hybrid joints in s/s and s/+ ts-Ab-MLV transformants. (A) Diagram illustrating the formation and the detection of CJ and HJ products. The λ gene segments (rectangular boxes) are flanked by RSS (triangles). Upon recombination cleavage, the Vλ1 coding ends can be resolved into Vλ1Jλ1 CJ or joined to the Jλ1 RSS as HJ (large arrows). Small arrows represent PCR primers used to amplify these joining products. (B) Semiquantitative analysis of (HJ). DNA samples were prepared from both s/s-ts and s/+-ts cells under similar culture conditions, as described in the Fig. 1 legend. (C) Comparison of HJ and intralocus CJ between s/+-ts (s/+) and s/s-ts (s/s) cells. DNA samples were prepared from cells cultured at 33°C (33), at 39°C for 2 days [39(2)], at 39°C for 3 days [39(3)], or at 39°C for 3 days followed by 2 days at 33°C (39-33).
A semiquantitative analysis of HJ and cis CJ clearly indicates that s/s-ts cells make more HJ than the s/+-ts cells (Fig. 3B), very similar to the finding in the IJ analysis (Fig. 2). Occasionally, s/+-ts cells do make a small amount of HJ. However, the culture conditions required to make HJ are different in these two cell types. In s/+-ts cells, the HJ appeared 2 days after recombination induction at 39°C but decreased 1 day later and completely disappeared after a temperature shift from 39 to 33°C (Fig. 3C, lanes 2 to 4). This reduction may reflect a secondary recombination event, in which the cleavage might be made at the junction of the newly formed HJ since the RSS in these HJ is still intact (see below). In contrast, the level of HJ in s/s-ts cells was elevated upon longer exposure at 39°C and increased more drastically upon the temperature shift to 33°C (Fig. 3C, lanes 6 to 8). Thus, the HJ made by these two cell types differ in quantity as well as in the time course of their production. These findings indicate that there are probably two different mechanisms underlying the formation of HJ. One, exhibited by s/+-ts cells, appears to be correlated with the up-regulation of RAG expression, whereas the other, evident in s/s-ts cells, seems to be correlated with the down-regulation of RAG expression.
Quantitative analyses of HJ are summarized in Table 3. It is clear that the s/s-ts cells make more HJ than the s/+-ts cells, as estimated by the absolute number of HJ as well as the ratio of HJ to CJ. Therefore, in s/+-ts cells, the newly generated Vλ1 coding ends were joined primarily to Jλ1 (and possibly Jλ3) coding ends. In s/s-ts cells, however, the Vλ1 coding ends can joined to the Jλ1 signal ends in addition to making intralocus joints and IJ (Fig. 2 and 3). These data further support the idea that three different recombination products, cis CJ, trans IJ, and HJ, may be formed through a common pathway.
TABLE 3.
Quantitative analysis of Vλ1Jλ1 HJ
| Expt | Culture temp conditionsa | Intensity (cpm for sample/cpm for actin)b for:
|
|||||
|---|---|---|---|---|---|---|---|
| s/+ cells
|
s/s cells
|
||||||
| CJ | HJ | HJ/CJc | CJ | HJ | HJ/CJ | ||
| 1 | 33 | 0.3 | 0.01 | 0.029 | <0.01 | <0.01 | —d |
| 39 | 6.8 | 0.04 | 0.005 | <0.01 | <0.01 | — | |
| 39-33 | 6.6 | 0.01 | 0.001 | 1.3 | 0.2 | 0.160 | |
| 2 | 33 | 1.4 | 0.02 | 0.014 | 0.01 | <0.01 | — |
| 39 | 3.5 | 0.02 | 0.004 | 0.14 | 0.18 | 1.3 | |
| 39-33 | 3.9 | 0.03 | 0.008 | 2.8 | 1.2 | 0.43 | |
| 3e | 39-33 | 2.9 ± 0.7 | <0.01 | <0.01 | 2.5 | 1.1 ± 0.48 | 0.44 |
As defined for Table 1.
See Table 1, footnote b, for details.
The ratio is derived by dividing the value for an HJ by the value for a CJ.
—, both CJ and HJ values are too small to be significant for ratio calculation.
For details of the quantitation for this experiment, see Table 1, footnote e.
The HJ recovered from both s/+-ts and s/s-ts cells under different culture conditions were cloned for sequence analyses. As shown in Table 4, multiple independent clones with unique sequences could be identified in both cell types. The HJ made in s/+-ts cells remain relatively intact, missing only 2 nucleotides from the Vλ1 coding segment but none from the Jλ1 RSS. On the other hand, all HJ recovered from s/s-ts cells had lost numerous nucleotides. With the exception of the two junctions that bear an intact Jλ1 signal sequence, the majority of the clones have extensive deletions from both signal and coding gene segments. Taken together, these findings indicate that the HJ made in s/+-ts cells are relatively intact whereas the formation of HJ in scid cells is error prone, similar to the finding for intralocus and interlocus CJ (Tables 2 and 3). Again, the differences in the levels of integrity of HJ observed in s/s-ts and s/+-ts cells further argue for different mechanisms underlying HJ formation in these two cell types.
TABLE 4.
Sequence analysis of Vλ1/2Jλ1 HJ made in s/+-ts and s/s-ts cellsa
| Cloneb | Vλ1/2 coding sequence | N/Pc | Jλ1 RSS | No. of ntf deleted from germ line sequence for:
|
|
|---|---|---|---|---|---|
| Vλ1 | Jλ1 | ||||
| Germ line | TACAGCAACCATTd | CACTGTG + 12 nt + GCAAAAAA | |||
| TACAGCACCCATTTe | |||||
| s/+-39 | |||||
| 1-2 (2) | TACAGCAACCATT | CACTGTG + 12 nt + GCAAAAAA | 0 | 0 | |
| 1-3 (3) | TACAGCAACCA | CACTGTG + 12 nt + GCAAAAAA | 2 | 0 | |
| 1-5 | TACAGCAACCA | CACTGTG + 12 nt + GCAAAAAA | 2 | 0 | |
| 2-1 | TACAGCAACCA | CACTGTG + 12 nt + GCAAAAAA | 2 | 0 | |
| 2-2 (4) | TACAGCAACCATT | CACTGTG + 12 nt + GCAAAAAA | 0 | 0 | |
| 2-8 (2) | TACAGCACCCATTT | G | CACTGTG + 12 nt + GCAAAAAA | 0 | 0 |
| s/s-39 | |||||
| 3-6 | TAC | GCAAAAAA | 10 | 19 | |
| 3-2 (2) | 3 nt + GCAAAAAA | 24 | 4 | ||
| 3-3 (5) | 12 nt + GCAAAAAA | 40 | 7 | ||
| s/s-39-33 | |||||
| 4-5 | 8 nt + GCAAAAAA | 48 | 11 | ||
| 4-6 (4) | 31 | 37 | |||
| 5-3 (3) | CACTGTG + 12 nt + GCAAAAAA | 34 | 0 | ||
| 5-9 | CACTGTG + 12 nt + GCAAAAAA | 82 | 0 | ||
| 5-2 (3) | 18 | 26 | |||
| 5-6 | 32 | 26 | |||
| 5-4 (2) | 67 | 30 | |||
| 5-14 | 37 | 34 | |||
| 5-8 | 33 | 38 | |||
| 5-5 (6) | 33 | 50 | |||
PCR products, amplified from s/+ or s/s cells cultured at either 39°C for 3 days (s/+-39 and s/s-39) or 39°C for 3 days followed by 2 days at 33°C (s/s-39-33), were cloned and sequenced.
Some sequences are compiled from two different experiments. The number before the hyphen designates the individual PCR, and the number after the hyphen represents individual repeats of cloning. The number in parentheses indicates the number of clones for the particular sequence.
N and P nucleotide addition.
Vλ1.
Vλ2.
nt, nucleotides.
DISCUSSION
High level of interlocus and hybrid recombination in s/s-ts cells.
Our recombination-inducible cell lines provide a model system to further elucidate the mechanisms involved in the formation of CJ, IJ, and HJ from newly generated recombination intermediates. The Vλ1 locus was of particular interest due to its easy experimental assessment in various rearranging events. As shown in Fig. 2 and 3, the Vλ1 coding ends made in situ could potentially be resolved into at least three different joining products: conventional Vλ1Jλ1 CJ, Vλ1Vκ and Vλ1Jκ IJ, and Vλ1Jλ1 HJ. If we assume that these three joining products constitute the possible resolution outcomes for Vλ1 coding ends, using experiment 2 of Tables 1 and Table 3 as an example, the distributions of CJ, IJ, and HJ are 59, 15, and 26% in s/s-ts cells and 98.6, 0.6, and 0.8% in s/+-ts cells, respectively. Therefore, s/s-ts cells produce higher levels of IJ and 4J compared to s/+-ts cells.
Although interlocus recombination has been demonstrated in many immunoglobulin and T-cell receptor gene loci, it is an extremely rare event in normal cells (4, 33). The molecular basis for this disfavored status remains elusive. Recently, by using extrachromosomal V(D)J recombination substrates, Han et al. have demonstrated that intermolecular CJ formation is prohibited at the joining step (26). On the other hand, by increasing the local concentrations of two separate recombination substrates, Tevelev and Schatz have observed a higher level of intermolecular joining products (50). The formation of these products appears to follow the 12/23 rule, i.e., requiring synaptic pairing and cleavage between the 12-RSS on one molecule and the 23-RSS on the other molecule (50). Thus, two individual recombination substrates may have to be held in a synaptic complex or kept in close vicinity for their cleavage and joining. Alternatively, if the recombination ends are not promptly joined or are not constrained in a synaptic complex, they could nonspecifically join to each other, regardless of which recombination loci they are generated from. This speculation is in agreement with our finding of VλJκ CJ in s/s-ts cells. The formation of these IJ is not governed by the 12/23 rule but rather depends on a joining process that operates after the cells return from the nonpermissive to the permissive temperature (Fig. 2B and C). This culture condition allows an opening of hairpin coding ends but a slow joining of the opened ends, as reported in our recent study (11). Thus, similar to what is found for the formation of intralocus CJ, the amount of interlocus CJ seems to be determined by the level of opened coding ends (Fig. 2 and 3) (11). Our finding suggests that interlocus recombination found in scid cells is likely to result from nonspecific joining of opened coding ends rather than paired excision of different recombination alleles.
Similar to the finding from analysis of IJ, the level of HJ was found to be higher in s/s-ts than in s/+-ts cells (Fig. 3). In addition, the junctions of the HJ were also different in the two cell types, i.e., intact for s/+-ts cells and aberrant for s/s-ts cells (Table 3). Our present finding differs from earlier studies reported by Roth's group in which comparable levels of HJ were detected among normal, scid, and DNA-PKcs-deficient Slip mice, as well as in Ku80−/− and XRCC4−/− mutant cells (8, 9, 25, 27). Although many of these HJ bear intact coding and signal sequences, some did show aberrant junctions (25, 27), which was also reported before (40). Two pathways have been postulated to mediate the formation of HJ: one mediated by RAG proteins and another via nonspecific disruption of the synaptic complexes followed by end joining (27). The stability of the synaptic complexes, which may ultimately be influenced by cellular environment and the structure of recombination loci, could conceivably impose the selection of a particular pathway. It is possible that culture conditions that induce s/s-ts cells to make various joining products favor the second pathway of HJ formation, i.e., disruption of synaptic complexes and nonspecific joining of recombination intermediates. Thus, lack of functional DNA-PKcs yields cells with a high level of unresolved coding ends and signal ends. These ends could, in turn, be diverted to a random joining process, generating nonstandard recombination products.
Role of DNA-PKcs in modulating the postcleavage complex.
Several lines of evidence suggest that recombination intermediates are held in a synaptic complex that contains RAG1 and RAG2 proteins (2, 19, 23, 28, 34, 47). This complex is then targeted for processing and joining, which are dependent on the DNA-PK complex (2, 54). The resolution carried out in the synaptic complex should be much more efficient and specific than the random ligation of free ends. The observation that s/+-ts cells rapidly form cis CJ and rarely make trans CJ or trans SJ is consistent with the synaptic model. On the other hand, the slow kinetics of CJ formation and high levels of IJ in s/s-ts cells are more compatible with random association of free ends. Thus, functional DNA-PKcs may somehow confine the processing and joining within the postcleavage complex to ensure appropriate end resolution, as postulated before (53, 54).
There are two different postcleavage states present in scid cells defective in DNA-PKcs, as revealed by the temperature-dependent resolution of coding ends. At the nonpermissive temperature, the ends present in the postcleavage synaptic complex are relatively inaccessible to enzymatic nicking and joining, as the majority of the coding ends remain in a covalently sealed hairpin structure (13). The occasional detection of various joining products (cis and trans CJ and HJ in s/s-ts cells (Tables 1 and 4) at 39°C may reflect an incomplete blockade of the complex to nicking and joining machinery. Upon a return to the permissive temperature, however, a rapid conversion of hairpin ends to opened ends and the appearance of CJ (11, 13) suggest an increased accessibility of recombination ends to processing and joining machinery. This condition is likely to result from a nonspecific disassembly of the synaptic complex. Consequently, these loose ends would be accessible to one another to form CJ as well as to engage in inappropriate interactions leading to the production of IJ and HJ.
Although these recombination-inducible cell lines offer a model system to delineate mechanistic processes of recombination cleavage and recombination resolution, they contain factors, such as a v-abl oncogene and a bcl-2 transgene, that are not present in developing lymphocytes but that can influence the recombination outcomes. It has been reported that the activity of v-abl tyrosine kinase can modulate RAG expression (15, 46), which can ultimately affect recombination cleavage and possibly resolution (19). We do not know the counterparts of the v-abl-mediated signaling in nontransformed lymphocytes, nor can we exclude the possibility that the recombination products detected in our scid ts cell lines may result from v-abl-mediated artifacts. However, the temperature-dependent resolution of recombination coding ends in these s/s-ts cells bears some similarities to the recombination events in scid thymocytes that have been exposed to ionizing radiation, such as concurrent up-regulation of intralocus and interlocus recombination (43). Thus, temperature changes and ionizing radiation may lead to a similar action in processing recombination intermediates even though the scid ts cells and the irradiated scid thymocytes would use different signaling pathways to regulate their recombination machinery.
The presence of bcl-2 can rescue the cells that fail to resolve the recombination ends or that have undergone abnormal recombination and thereby allows the detection of aberrant recombination products. In addition, due to constitutive expression of bcl-2 transgenes, these ts-Ab-MLV cell lines afford the ability to induce vigorous V(D)J recombination activity, which may perturb the balance between recombination cleavage and recombination joining. The normal DSB repair machinery may be overwhelmed by the overproduction of recombination intermediates. As a result, some recombination intermediates made in DNA-PKcs-proficient cells may “escape” from the synaptic complex and become prone to nucleotide deletions and abnormal joining. It is possible that the rare IJ recovered from our s/+-ts cells (Table 1, experiment 2) could be formed by this scheme. Therefore, even in DNA-PKcs-proficient cells, the joining products could occasionally be “scid-like” and generated by a DNA-PKcs-independent pathway, especially when their recombination system is overwhelmed by overproduction of recombination intermediates or possibly other DSBs.
Higher frequencies of interlocus recombination have been reported in patients who are associated with an increased risk of developing cancer, such as ataxia telangiectasia patients, patients with Hodgkin's disease who have undergone chemotherapy, and agricultural workers who have been exposed to pesticides (1, 31, 32, 41). The recent findings of immunoglobulin H translocation in pro-B-cell lymphoma in scid/p53−/− (52), Ku80−/−/p53−/− (16), and XRCC4−/−/p53−/− mice (21) provide the direct evidence for the oncogenic potential of V(D)J recombination in DSB repair-deficient cells. Our data further points out that recombination intermediates, if not joined or kept in a synaptic complex, could potentially be misjoined to any available ends, resulting in translocation and oncogenic transformation.
ACKNOWLEDGMENTS
We thank M. Anderson for his initial detection of HJ in scid-ts cells, S. Bingham for his expertise in DNA sequencing, and E. Birge for his patience in editing the manuscript. We also thank M. J. Bosma, M. Gellert, E. Grant, S. M. Lewis, N. Rosenberg, and D. B. Roth for their insight and critical review of the manuscript.
This work was supported by National Institute of Health grant CA73857 (to Y.C.).
REFERENCES
- 1.Abdallah J M, Lombardi D P, Kirsch I R. Genetic instability in patients with Hodgkin's disease undergoing chemotherapy. J Clin Investig. 1995;96:2744–2747. doi: 10.1172/JCI118343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal A, Schatz D G. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell. 1997;89:43–53. doi: 10.1016/s0092-8674(00)80181-6. [DOI] [PubMed] [Google Scholar]
- 3.Araki R, Fujimori A, Hamatani K, Mita K, Saito T, Mori M, Fukumura R, Morimyo M, Muto M, Itoh M, Tatsumi K, Abe M. Nonsense mutation at Tyr-4046 in the DNA-dependent protein kinase catalytic subunit of severe combined immune deficiency mice. Proc Natl Acad Sci USA. 1997;94:2438–2443. doi: 10.1073/pnas.94.6.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey S N, Rosenberg N. Assessing the pathogenic potential of the V(D)J recombinase by interlocus immunoglobulin light-chain gene rearrangement. Mol Cell Biol. 1997;17:887–894. doi: 10.1128/mcb.17.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailin T, Mo X, Sadofsky M J. A RAG1 and RAG2 tetramer complex is active in cleavage in V(D)J recombination. Mol Cell Biol. 1999;19:4664–4671. doi: 10.1128/mcb.19.7.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackwell T, Alt F. Molecular characterization of the lymphoid V(D)J recombination activity. J Biol Chem. 1989;264:10327–10330. [PubMed] [Google Scholar]
- 7.Blunt T, Finnie N J, Taccioli G E, Smith G C, Demengeot J, Gottlieb T M, Mizuta R, Varghese A J, Alt F W, Jeggo P A, Jackson S P. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 8.Bogue M A, Jhappan C, Roth D B. Analysis of variable (diversity) joining recombination in DNA-dependent protein kinase (DNA-PK)-deficient mice reveals DNA-PK-independent pathways for both signal and coding joint formation. Proc Natl Acad Sci USA. 1998;95:15559–15564. doi: 10.1073/pnas.95.26.15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogue M A, Wang C, Zhu C, Roth D B. V(D)J recombination in Ku86-deficient mice: distinct effects on coding, signal, and hybrid joint formation. Immunity. 1997;7:37–47. doi: 10.1016/s1074-7613(00)80508-7. [DOI] [PubMed] [Google Scholar]
- 10.Bosma G C, Custer R P, Bosma M J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 11.Brown M L, Chang Y. Metabolism of recombination coding ends in scid cells. J Immunol. 2000;164:4135–4142. doi: 10.4049/jimmunol.164.8.4135. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y, Bosma G C, Bosma M J. Development of B cells in scid mice with immunoglobulin transgenes: implications for the control of V(D)J recombination. Immunity. 1995;2:607–616. doi: 10.1016/1074-7613(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 13.Chang Y, Brown M L. Formation of coding joints in V(D)J recombination-inducible severe combined immune deficient pre-B cell lines. Proc Natl Acad Sci USA. 1999;96:191–196. doi: 10.1073/pnas.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y Y, Rosenberg N. Lymphoid cells transformed by Abelson virus require the v-abl protein-tyrosine kinase only during early G1. Proc Natl Acad Sci USA. 1992;89:6683–6687. doi: 10.1073/pnas.89.15.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y Y, Wang L C, Huang M S, Rosenberg N. An active v-abl protein tyrosine kinase blocks immunoglobulin light-chain gene rearrangement. Genes Dev. 1994;8:688–697. doi: 10.1101/gad.8.6.688. [DOI] [PubMed] [Google Scholar]
- 16.Difilippantonio M J, Zhu J, Chen H T, Meffre E, Nussenzweig M C, Max E E, Ried T, Nussenzweig A. DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature. 2000;404:510–514. doi: 10.1038/35006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fish S M, Bosma M J. Abnormal deletions in the T-cell receptor δ locus of mouse thymocytes. Mol Cell Biol. 1994;14:4455–4464. doi: 10.1128/mcb.14.7.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank K M, Sekiguchi J M, Seidl K J, Swat W, Rathbun G A, Cheng H-L, Davidson L, Kangaloo L, Alt F W. Late embryonic lethality and impaired VDJ recombination in mice lacking DNA ligase IV. Nature. 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 19.Fugmann S D, Lee A I, Shockett P E, Villey I J, Schatz D G. The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu Rev Immunnol. 2000;18:495–527. doi: 10.1146/annurev.immunol.18.1.495. [DOI] [PubMed] [Google Scholar]
- 20.Fulop G M, Phillips R A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347:479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Ferguson D O, Xie W, Manls J P, Sekiguchi J, Frank K M, Chaudhuri J, Horner J, DePinho R A, Alt F W. Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature. 2000;404:897–900. doi: 10.1038/35009138. [DOI] [PubMed] [Google Scholar]
- 22.Gellert M. V(D)J recombination gets a break. Trends Genet. 1992;8:408–412. doi: 10.1016/0168-9525(92)90322-u. [DOI] [PubMed] [Google Scholar]
- 23.Grawunder U, Lieber M R. A complex of RAG-1 and RAG-2 proteins persists on DNA after single-strand cleavage at V(D)J recombination signal sequences. Nucleic Acids Res. 1997;25:1375–1382. doi: 10.1093/nar/25.7.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Y, Seidl K J, Rathbun G A, Zhu C, Manis J P, van der Stoep N, Davidson L, Cheng H-L, Sekiguchi J M, Frank K, Stanhope-Baker P, Schlissel M S, Roth D B, Alt F W. Growth retardation and leaky SCID phenotype of Ku 70-deficient mice. Immunity. 1997;7:653–665. doi: 10.1016/s1074-7613(00)80386-6. [DOI] [PubMed] [Google Scholar]
- 25.Han J-O, Erskine L A, Purugganan M M, Stamato T D, Roth D B. V(D)J recombination intermediates and non-standard products in XRCC4-deficient cells. Nucleic Acids Res. 1998;26:3769–3775. doi: 10.1093/nar/26.16.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J-O, Steen S B, Roth D. Intermolecular V(D)J recombination is prohibited specifically at the joining step. Mol Cell. 1999;3:331–338. doi: 10.1016/s1097-2765(00)80460-8. [DOI] [PubMed] [Google Scholar]
- 27.Han J-O, Steen S B, Roth D B. Ku86 is not required for protection of signal ends or for formation of nonstandard V(D)J recombination products. Mol Cell Biol. 1997;17:2226–2234. doi: 10.1128/mcb.17.4.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiom K, Gellert M. A stable RAG1-RAG2-DNA complex that is active in V(D)J cleavage. Cell. 1997;88:65–72. doi: 10.1016/s0092-8674(00)81859-0. [DOI] [PubMed] [Google Scholar]
- 29.Jeggo P. DNA-PK: at the cross-roads of biochemistry and genetics. Mutat Res. 1997;384:1–14. doi: 10.1016/s0921-8777(97)00009-8. [DOI] [PubMed] [Google Scholar]
- 30.Kanaar R, Hoeijmakers J H J, van Gent D C. Molecular mechanisms of DNA double strand break repair. Trends Cell Biol. 1999;8:483–489. doi: 10.1016/s0962-8924(98)01383-x. [DOI] [PubMed] [Google Scholar]
- 31.Kirsch I R. Trans-rearrangements and the risk of lymphoid malignancy. Ann Oncol. 1997;8:S45–S48. [PubMed] [Google Scholar]
- 32.Kirsch I R, Lista F. Lymphocyte-specific genomic instability and risk of lymphoid malignancy. Semin Immunol. 1997;9:207–215. doi: 10.1006/smim.1997.0071. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi Y, Tycko B, Soreng A L, Sklar J. Transrearrangement between antigen receptor genes in normal human lymphoid tissues and in ataxia telangiectasia. J Immunol. 1991;147:3201–3209. [PubMed] [Google Scholar]
- 34.Leu T M, Eastman Q M, Schatz D G. Coding joint formation in a cell-free V(D)J recombination system. Immunity. 1997;7:303–314. doi: 10.1016/s1074-7613(00)80532-4. [DOI] [PubMed] [Google Scholar]
- 35.Lewis S M. The mechanism of V(D)J joining: lessons from molecular, immunological, and comparative analyses. Adv Immunol. 1994;56:27–150. doi: 10.1016/s0065-2776(08)60450-2. [DOI] [PubMed] [Google Scholar]
- 36.Lewis S M, Hesse J E. Cutting and closing without recombination in V(D)J joining. EMBO J. 1991;10:3631–3639. doi: 10.1002/j.1460-2075.1991.tb04929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis S M, Hesse J E, Mizuuchi K, Gellert M. Novel strand exchanges in V(D)J recombination. Cell. 1988;55:1099–1107. doi: 10.1016/0092-8674(88)90254-1. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Alt F W. Identification of the XRCC4 gene: complementation of the DSBR and V(D)J recombination defects of XR-1 cells. Curr Top Microbiol Immunol. 1996;217:143–150. doi: 10.1007/978-3-642-50140-1_10. [DOI] [PubMed] [Google Scholar]
- 39.Lieber M. The mechanism of V(D)J recombination: a balance of diversity, specificity, and stability. Cell. 1992;70:873–876. doi: 10.1016/0092-8674(92)90237-7. [DOI] [PubMed] [Google Scholar]
- 40.Lieber M R, Hessie J E, Lewis S, Bosma G C, Rosenberg N, Mizuuchi K, Bosma M J, Gellert M. The defect in murine severe combined immune deficiency: joining of signal segments but not coding segments in V(D)J recombination. Cell. 1988;55:7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- 41.Lipkowitz S, Stern M-H, Kirsch I R. Interlocus V-J recombination measures genomic instability in agriculture workers at risk for lymphoid malignancies. Proc Natl Acad Sci USA. 1992;89:5301–5305. doi: 10.1073/pnas.89.12.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipkowitz S, Stern M H, Kirsch I R. Hybrid T cell receptor genes formed by interlocus recombination in normal and ataxia-telangiectasis lymphocytes. J Exp Med. 1990;172:409–418. doi: 10.1084/jem.172.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lista F, Bertness V, Guidos C J, Danska J S, Kirsch I R. The absolute number of trans-rearrangements between the TCRG and TCRB loci is predictive of lymphoma risk: a severe combined immune deficiency (scid) murine model. Cancer Res. 1997;57:4408–4413. [PubMed] [Google Scholar]
- 44.Liu D, Jenab J, Rosenberg N. κ and λ rearrangement occurs simultaneously in transformed pre-B cells. J Immunol. 1997;159:6061–6069. [PubMed] [Google Scholar]
- 45.Nussenzweig A, Chen C, da Costa Soares C, Sanchez M, Sokol K, Nussenzweig M C, Li G C. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature. 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 46.Ramsden D A, Gellert M. Formation and resolution of double-strand break intermediates in V(D)J rearrangement. Genes Dev. 1995;9:2409–2420. doi: 10.1101/gad.9.19.2409. [DOI] [PubMed] [Google Scholar]
- 47.Ramsden D A, Paull T T, Gellert M. Cell-free V(D)J recombination. Nature. 1997;388:488–491. doi: 10.1038/41351. [DOI] [PubMed] [Google Scholar]
- 48.Roth D B, Zhu C, Gellert M. Characterization of broken DNA molecules associated with V(D)J recombination. Proc Natl Acad Sci USA. 1993;90:10788–10792. doi: 10.1073/pnas.90.22.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taccioli G E, Rathbu G, Oltz E, Stamato T, Jeggo P A, Alt F W. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993;260:207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- 50.Tevelev A, Schatz D G. Intermolecular V(D)J recombination. J Biol Chem. 2000;275:8341–8348. doi: 10.1074/jbc.275.12.8341. [DOI] [PubMed] [Google Scholar]
- 51.Tycko B, Palmer J, Sklar J. T-cell receptor gene trans-rearrangements: chimeric γδ genes in normal lymphoid tissues. Science. 1989;245:1242–1246. doi: 10.1126/science.2551037. [DOI] [PubMed] [Google Scholar]
- 52.Vanasse G J, Halbrook J, Thomas S, Burgess A, Hoekstra M F, Disteche C M, Willerford D M. Genetic pathway to recurrent chromosome translocation in murine lymphoma involves V(D)J recombinase. J Clin Investig. 1999;103:1669–1675. doi: 10.1172/JCI6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu C, Bogue M A, Lim D-S, Hasty P, Roth D B. Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 54.Zhu C, Roth D B. Characterization of coding ends in thymocytes of scid mice: implications for the mechanism of V(D)J recombination. Immunity. 1995;2:101–112. doi: 10.1016/1074-7613(95)90082-9. [DOI] [PubMed] [Google Scholar]