Abstract
Rituximab (RTX), an important therapeutic option for patients with rheumatic diseases, has been shown to reduce immune responses to various vaccines. We asked whether following SARS-CoV-2 vaccination, response rates in RTX treated patients are reduced and whether specific patient characteristics influence the responses. We recruited patients on chronic RTX therapy undergoing anti-SARS-CoV2 vaccination and measured the post-vaccination anti-spike IgG antibody levels. The median time from pre-vaccination RTX infusion to vaccination and from vaccination to the post-vaccination RTX infusion was 20.5 weeks and 7.2 weeks respectively. Only 36.5% of patients developed measurable titers of IgG anti-SARS-CoV-2 spike antibody after vaccination. Hypogammaglobulinemia (IgG and/or IgM) but not timing of vaccination, B cell numbers, or concomitant immune suppressive medications, correlated with sero-negativity (p = 0.004). Our results underscore the fact that even after B cell reconstitution, RTX induced chronic hypogammaglobulinemia significantly impairs the ability of the immune system to respond to SARS-CoV-2 vaccination.
Keywords: COVID-19, Vaccination, B cells, Rituximab, Autoimmune diseases
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been responsible for a global pandemic resulting in hundreds of millions of infections and millions of deaths worldwide. In an effort to curb transmission and morbidity, several vaccines against the SARS-CoV-2 virus have been developed using either novel liposomal mRNA-based delivery technology or an adenovirus-based delivery system. These vaccines elicited high levels of immunogenicity in immunocompetent individuals in the original vaccine trial [[1], [2], [3]]. Due to the severity of this health crisis, regulatory agencies, including the FDA, approved SARS-CoV-2 vaccines, initially under an emergency use authorization, thereby facilitating the largest ever global vaccination campaign. Consequently, there has been widespread vaccination of immunosuppressed patients with autoimmune rheumatic disease (AIRD) despite unclear efficacy data since trials excluded this population [[1], [2], [3]].
Rituximab (RTX) is a monoclonal antibody targeting CD20 that is an important therapeutic option in AIRD [4]. In recent years, there has been increasing evidence that patients treated with RTX are at increased risk of severe infections and poor vaccine responses, particularly in those with a history of hypogammaglobulinemia [5,6]. Recent studies have shown that use of RTX is associated with more severe SARS-CoV-2 infections, poor outcomes, and in some cases persistent infection and accelerated viral evolution highlighting the importance of effective vaccination in this population [7,8]. However, vaccination in this population can be challenging as data consistently shows reduced humoral and cellular immune responses to various vaccines after B-cell depletion with RTX [9,10].
In keeping with prior European vaccine recommendations, the ACR COVID-19 task force provided guidance with a moderate consensus that vaccination should be given as soon as possible in patients with AIRD, and in those who have received RTX, approximately 4 weeks before the next course of therapy [11]. Potential barriers to vaccination inside the recommended window include regional rules and regulations surrounding vaccine eligibility, vaccine hesitancy, and reluctance amongst providers to counsel for delays in vaccination in this high risk population [12]. Furthermore, the optimal timing of vaccination after RTX remains uncertain due to the lack of empirical efficacy data for SARS-CoV-2 vaccination.
The primary aim of this study was to assess the impact of vaccine timing, immunological status, demographics, and concomitant treatments on vaccine immunogenicity in AIRD patients treated with RTX. Secondary aims were to implement an educational intervention to physicians and patients and assess its effect on timing of COVID-19 vaccination in relation to RTX infusions.
2. Materials and methods
2.1. Study design
We performed a prospective single center study to optimize SARS-CoV-2 vaccination timing and to assess vaccine immunogenicity in AIRD patients who received RTX at an academic tertiary care hospital in Boston, MA, USA. We first educated faculty on the rationale for timing SARS-CoV-2 vaccines 24 weeks post-RTX. We measured timing of vaccine administration through telephone survey of patients. We then analyzed vaccine immunogenicity in relation to vaccine timing and other clinical and immunologic parameters. The project was approved by the BIDMC Institutional Review Board.
2.2. Study population
Adult patients (aged ≥18 years) were identified by cross-referencing names of ordering providers in the Division of Rheumatology at our institution with pharmacy infusion records for RTX administration from January 2020 through to February 2021. We included patients receiving RTX for an established AIRD, including but not limited to rheumatoid arthritis, antineutrophil cytoplasmic antibody associated vasculitis (AAV), IgG4-related disease, and connective tissue diseases (including systemic lupus erythematosus, mixed connective tissue disease, Sjogren's Syndrome, systemic sclerosis, and idiopathic inflammatory myositis). Diagnoses were determined clinically through provider notes. Exclusion criteria included pregnancy and, for the patient survey portion of the study, non-native English-speaking patients as we did not have survey administration in additional languages.
2.3. Data collection
Medical history, medications, indication for RTX by disease, date of last RTX infusion, and vaccine-related information, including, vaccine preferences, type of SARS-CoV-2 vaccine received, and dates of vaccine administration, were collected from a combination of medical records review and patient telephone survey. Post-vaccination serum IgG antibody levels against SARS-CoV-2 spike protein S1 receptor binding domain (RBD), absolute CD19 and CD20 counts within 2 months of vaccination, quantitative immunoglobulin levels within one year of vaccination, SARS-CoV-2 anti-nucleocapsid antibody, and documentation of prior SARS-CoV-2 infection were recorded when available. Hypogammaglobulinemia was defined as laboratory evidence of serum IgG <700 mg/dL or serum IgM <40 mg/dL within 1 year of vaccination date. Glucocorticoid dose was defined as low if less than 10 mg prednisone equivalent per day, moderate if between 10 and 19 mg per day, and high if greater than or equal to 20 mg per day. A prior SARS-CoV-2 infection was determined by either medical record review or by patient reported positive SARS-CoV-2 PCR and/or antinucleocapsid antibody tests. Study participants were contacted by phone after completion of vaccination to complete a survey regarding common side effects and potential adverse events.
2.4. Faculty educational intervention and survey
A one-hour educational program was designed targeting faculty and trainees within the division of Rheumatology at our institution. This included a formal presentation highlighting the severity of COVID-19 infections in RTX-treated patients and the rationale for establishing a SARS-CoV-2 vaccination plan with AIRD patients on RTX. Data on reduced humoral and cellular immune responses to various vaccines after B-cell depletion with RTX was reviewed along with the EULAR recommendations to give influenza and pneumococcal vaccination prior to initiation of RTX and at least 6 months after last dose and 4 weeks before next course of therapy [11]. Based on this data, a proposal recommending a similar delay of SARS-CoV-2 vaccination was discussed within the division and physicians were encouraged to have a discussion with their patients to make a vaccination plan. Faculty preference to either universally time all vaccines according to the proposed scheduled or to individualize the plan for each patient was elicited through email correspondence.
2.5. Patient educational intervention and survey
Patients were contacted by telephone from March through August of 2021. Study participants were surveyed on recent vaccine administration, and in those who had not yet received a vaccination, whether or not they were intending to obtain a COVID-19 vaccine. Vaccine administration was not part of the study.
2.6. Outcome measures
The primary outcome measure was the proportion of seropositive patients after vaccination with the BNT162b2 mRNA (manufactured by BioNtech/Pfizer), mRNA-1273 (manufactured by Moderna), and Ad26.COV2.SCovid-19 (manufactured by Janssen/Johnson & Johnson) in relation to time from most recent RTX dose. Major secondary outcomes include proportion of seropositive patients after vaccination in relation to age, additional immunological parameters at time of vaccination including B cell counts and immunoglobulin levels, and concomitant medication use. Finally, we assessed the effect of physician and patient education on vaccination timing.
2.7. Immunogenicity of the vaccine
Serum IgG antibody levels against SARS-CoV-2 spike protein S1 receptor binding domain (RBD) were measured using an antispike IgG enzyme immunoassay (Attelica IM COV2G (https://www.fda.gov/media/140699/download) or ADVIA Centaur COV2G via Quest Diagnostics Laboratory, (https://www.fda.gov/media/140704/download)). The sensitivity and specificity of these assays are >99%. An Index Value greater than or equal to 1.00 was considered as positive, according to the manufacturer's instruction.
2.8. Statistical analysis
Continuous variables are presented as median with IQR. Between group comparisons were done using the Wilcoxon rank sum test for continuous variables and the chi square test for categorical variables. We assessed the association of seropositivity post immunization with relevant variables using multivariate logistic regression. The analysis and the graphs were done using STATA®.
3. Results
3.1. Study population
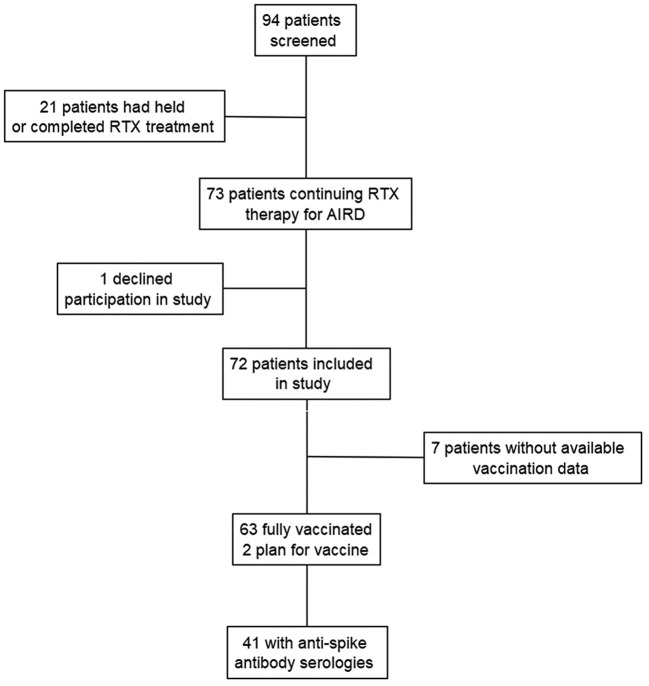
A total of 94 individuals were identified as having received RTX for AIRD between January 2020 and February 2021. Seventy-three patients were on continuous RTX infusions for treatment of AIRD. One individual declined participation in the study. Baseline patient characteristics of the 72 patients are listed in Table 1 and were typical of what would be expected in a cohort of patients with AIRD. Vaccination data was available for 65 patients, of which 63 were fully vaccinated. A total of 41 out of the 63 fully vaccinated patients had post-vaccination serum IgG anti-SARS-CoV2 Spike antibody levels measured (see Fig. 1 ). All anti-spike antibody titers were drawn between 2- and 26- weeks post vaccination (median [IQR] = 10.6 [4.9–13.9] weeks).
Table 1.
Patient demographics, clinical, and immunologic data of autoimmune rheumatic disease patients receiving rituximab.
| Patient characteristics | Total (n = 72) |
|---|---|
| Age (years), median (IQR) | 61.5 (53.6, 70) |
| Underlying disease: | |
| Rheumatoid arthritis, n (%) | 30 (42.2) |
| ANCA-associated vasculitis, n (%) | 18 (25.4) |
| Connective tissue disease⁎, n (%) | 21 (29.6) |
| IgG4-related disease, n (%) | 3 (4.2) |
| Rituximab Schedule | |
| Every 6 months, n (%) | 61 (85.9) |
| Every 12 months, n (%) | 10 (14.2) |
| Cumulative RTX (mg), median (IQR) | 7000 (4000, 11,000) |
| Concomitant DMARD (any), n (%) | 39 (54.9) |
| Mycophenolate mofetil, n (%) | 10 (14.1) |
| Methotrexate, n (%) | 12 (16.9) |
| Hydroxychloroquine, n (%) | 9 (12.7) |
| Steroids (prednisone equivalent) | |
| ≥ 20 mg/day, n (%) | 2 (2.8) |
| 10-19 mg/day, n (%) | 5 (7) |
| < 10 mg/day, n (%) | 31 (43.1) |
| No steroids, n (%) | 34 (47.2) |
| B cell counts: | |
| Absolute CD19 count (#/uL), median (IQR): (n = 28) | 14.77 (8.38, 21.57) |
| Absolute CD20 count (#/uL), median (IQR): (n = 28) | 9.02 (5.19, 14.67) |
| History of hypogammaglobulinemia n, (%) (n = 41) | 20 (48.8) |
| Immunoglobulin M hypogammaglobulinemia n, (%) (n = 41) | 17 (41.5) |
| Immunoglobulin G hypogammaglobulinemia n, (%) (n = 41) | 10 (24.4) |
| Immunoglobulin M (mg/dL), median (IQR) (n = 34) | 46 (19.5, 65) |
| Immunoglobulin A, median (IQR) (n = 35) | 176 (100, 255) |
| Immunoglobulin G, median (IQR) (n = 35) | 793 (665, 1048) |
| Documented history of COVID-19 infection, n (%) | 7 (9.9) |
| Influenza vaccine in 2020–2021 (yes/no) n (%) (n = 55) | 51 (92.7) |
| Patient group according to provider preference for SARS-CoV-2 vaccine: | |
| Universal recommendation for timing, n (%) | 41 (57.7) |
| Individualized approach to timing, n (%) | 30 (42.2) |
| Patient reported data on SARS-CoV-2 vaccine (n = 65) | |
| Refused, n (%) | 0 (0) |
| Planned, n (%) | 2 (3.1) |
| Received, n (%) | 63 (95.4) |
| Manufacturer of received vaccine (n = 63) | |
| BNT162b2 mRNA, n (%) | 31 (49.2) |
| mRNA-1273, n(%) | 26 (41.2) |
| Ad26.COV2.SCovid-19, n(%) | 6 (9.5) |
| Timing of SARS-CoV-2 vaccination post RTX (weeks), median (IQR) (n = 59) | 20.5 (13.3, 26) |
The connective tissue disease group is composed of systemic lupus erythematosus, inflammatory myopathies, anti-synthetase syndrome, overlap syndromes, and mixed connective tissue disease patients.
Fig. 1.
Flowchart of study enrollment and data collection. Patients with AIRD treated with RTX were included in the study. Patients who had RTX stopped or postponed indefinitely during study period were excluded. One patient declined participation in the study. Seventy-two patients were included for chart review and telephone survey. Sixty-three patients completed a full vaccine series and anti-spike antibody data was available for 41 of those patients.
The median age of the cohort was 61.5 years. RA was the most common indication for RTX followed by CTD and AAV. The majority of patients were maintained on every 6-month schedule of RTX infusion. About half of patients were on concomitant DMARDs, with Methotrexate (MTX), mycophenolate mofetil (MMF), and hydroxychloroquine (HCQ) being the most common. The majority of patients were not taking concomitant corticosteroids. In those taking steroids, majority were on less than 10 mg of prednisone equivalent per day. Seven patients had documented COVID-19 infections; however, 6 of these patients had infections prior to vaccination. Of the 41 patients with immunoglobulin data measured within a year of vaccination, nearly half had a history of either IgG or IgM hypogammaglobulinemia.
3.2. Rate and timing of vaccination
Providers specified an intent to recommend SARS-CoV-2 vaccination for all 72 (100%) AIRD patients on RTX. With respect to timing strategies, providers specified intent to universally recommend delayed vaccination for 57.7% patients and individualize the plan for the remainder of patients based on provider discretion.
Of the 65 patients with vaccination data, 63 (95.3%) completed the recommended series of one of the three available SARS-CoV-2 vaccines. The median time from pre-vaccination RTX infusion to first vaccine dose was 20.5 weeks and 24.3 weeks to second vaccine dose. The median time from second vaccine dose to the post-vaccination RTX infusion was 7.2 weeks. The median time in between the first and second doses of vaccines was 3.5 weeks. Vaccination after 20 weeks was more likely to occur if the provider universally recommended delayed vaccination rather than individualizing timing for each patient (p-value 0.02). Higher cumulative doses of RTX were associated with vaccination less than 20 weeks after pre-vaccination RTX infusion (p-value 0.04).
3.3. Immunogenicity of SARS-CoV-2 vaccines
Of the 41 individuals who had anti-spike antibody serologies measured, 15 (36.5%) had positive anti-spike antibody titers (Table 2 ). The interval from pre-vaccination RTX infusion to first and second dose of SARS-CoV-2 vaccine was not significant between seropositive and seronegative groups. There was no significant difference in seropositivity rates amongst the different vaccine types. Despite the wide variation in drawing anti-spike antibody titer in relation to vaccination, this did not seem to correlate with differences in seropositivity rates (p = 0.18) or anti-spike antibody titers (p = 0.28). Interestingly, individuals with positive titers had a longer average interval between vaccine doses, however, the effect size was small.
Table 2.
Patient demographics and immunological factors stratified by presence or absence of SARS-CoV-2 anti-spike antibody.
| Factor | Anti-Spike IgG antibody - negative | Anti-Spike IgG antibody - positive | p-value |
|---|---|---|---|
| N | 26 | 15 | |
| Age, median (IQR) | 67.5 (57, 73) | 58 (51, 66) | 0.080 |
| Indication for RTX (%) | |||
| RA | 9 (35) | 4 (27) | 0.7 |
| AAV | 8 (31) | 5 (33) | |
| CTD | 8 (31) | 4 (27) | |
| IgG4-RD | 1 (4) | 2 (13) | |
| CD19Abs, median (IQR) | 14.77 (7.22, 17.9) | 12.965 (6.44, 26.79) | 0.84 |
| CD20Abs, median (IQR) | 8.93 (5.2, 13.22) | 9.23 (3.86, 16.61) | 0.62 |
| IgG or IgM Hypogammaglobulinemia (%) | 13 (76) | 2 (20) | 0.004 |
| IgM, median (IQR) | 37 (18, 52) | 56 (22, 68) | 0.2 |
| IgA, median (IQR) | 162 (76, 240) | 338.5 (176, 451) | 0.008 |
| IgG, median (IQR) | 753.5 (533, 893.5) | 1052 (780, 1228) | 0.05 |
| Corticosteroids (prednisone equivalent) (%) | |||
| <10 mg/day | 22 (85) | 14 (93) | 0.37 |
| 10-19 mg/day | 3 (12) | 0 (0) | |
| >20 mg/day | 1 (4) | 1 (7) | |
| Concomitant csDMARDs use (%) | 13 (50) | 11 (73) | 0.14 |
| Mycophenolate mofetil | 5 (19) | 3 (20) | 0.95 |
| Hydroxychloroquine | 4 (15) | 3 (20) | 0.71 |
| Methotrexate | 2 (8) | 1 (7) | 0.9 |
| RTX cumulative dose (mg), median (IQR) | 6700 (3500, 10,000) | 7000 (4000, 10,000) | 0.9 |
| Covid Infection (%) | 0 (0) | 5 (36) (⁎) | 0.001 |
| Planned to time vaccination (%) | 25 (100) | 14 (93) | 0.19 |
| Time between first and second dose (weeks), median (IQR) | 3.1 (3, 4) | 4 (3, 4.1428571) | 0.07 |
| Time from pre-vaccination RTX to first vaccination (weeks), median (IQR) | 20.57 (18.71, 22.86) | 20.57 (13.29, 29.57) | 0.61 |
| Time from pre-vaccination RTX to second vaccination (weeks), median (IQR) | 24.42 (19.71, 26.86) | 23.57 (17, 34.86) | 0.8 |
| Time from second vaccine to next RTX infusion (weeks), median (IQR) | 7 (5.43, 12.57) | 6 (4.86, 11.86) | 0.49 |
| Covid vaccine received (%) | |||
| BNT162b2 mRNA | 15 (58) | 7 (47) | 0.58 |
| mRNA-1273 | 8 (31) | 7 (47) | |
| Ad26.COV2.SCovid-19 | 3 (12) | 1 (7) | |
RA = rheumatoid arthritis. AAV = ANCA-associated vasculitis. CTD = connective tissue disease. IgG4-RD=IgG4-related disease.
Of the 5 patients with documented prior SARS-CoV-2 infections, 4 acquired the infection prior to vaccination.
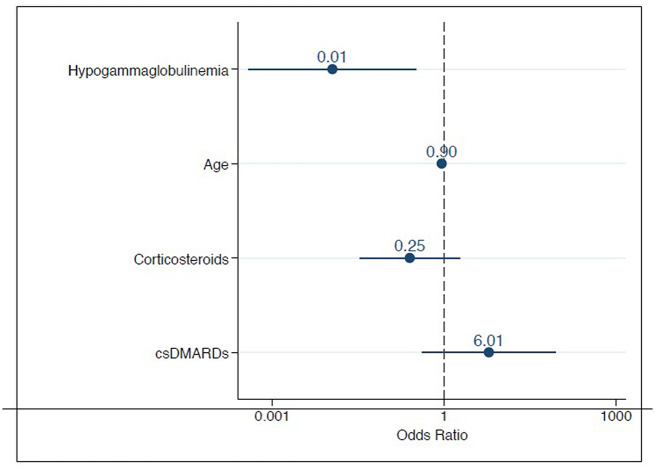
There was a trend toward patients being of younger age in the seropositive group by a median of 10 years (p-value = 0.08) vs. patients in the seronegative group. Underlying AIRD diagnosis did not significantly impact seropositivity. Absolute CD19 and CD20 cell counts were not significantly different between the seropositive and seronegative group, however, only two patients had undetectable B-cell counts within 2 months of vaccination. A history of hypogammaglobulinemia (IgG and IgM), was predictive of negative anti-spike antibody titer post vaccination (p-value = 0.004). This was further demonstrated to be predictive even when correcting for medications and age using logistic regression analysis (Fig. 2 ).
Fig. 2.
Multivariate logistic regression analysis of select factors correlated to post-immunization seropositivity. The odds ratios (OR) and confidence intervals for hypogammaglobulinemia, age, corticosteroid use, conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs) are shown.
Amongst the vaccinated individuals, 5 had either a documented or self-reported SARS-CoV-2 infection, however, 4 of the infections occurred prior to vaccination.
Concomitant medications in addition to RTX overall did not significantly impact the likelihood of seropositivity including steroid use (irrespective of dose) or other disease modifying antirheumatic drugs (DMARDs) such as MMF, MTX, or HCQ. Cumulative RTX dose at the time of vaccination was not statistically different between the two groups. In addition, there was no significant difference in seropositivity rates amongst the three vaccine types.
We further analyzed the effect of demographic and immunologic parameters to anti-spike antibody titers. Using linear regression, we found that not only seropositivity but also anti-spike antibody titers were significantly lower in hypogammaglobulinemic patients (p = 0.006) and did not correlate with age (p = 0.3), use of corticosteroids (p = 0.061) or DMARDs (p = 0.67).
3.4. Safety of SARS-CoV-2 vaccines
Fifty-one patients participated in the post-vaccination survey. Fourteen could not be reached via telephone, 6 were not contacted as they were non-English speaking, and one patient declined participation.
The most common patient reported side effects from the SARS-CoV-2 vaccines were pain at the injection site (52.4%) and fatigue (40.2%) (Table 3 ). There was no significant difference in rates of side effects between the three available vaccines received. One patient noted suicidal ideation after the BNT162b2 mRNA vaccine. There were no reported flares of rheumatic disease following vaccination and no serious or life-threatening side effects requiring hospitalization.
Table 3.
Patient reported side effects after SARS-CoV-2 vaccination.
| Reported symptom, n (%) | n = 82⁎ |
|---|---|
| Pain at injection site | 43 (52.4) |
| Fatigue | 33 (40.2) |
| Chills | 13 (15.8) |
| Headache | 13 (15.8) |
| Myalgias | 10 (12.2) |
| Subjective fever | 6 (7.3) |
| Arthralgias | 6 (7.3) |
| Nausea | 3 (3.6) |
| Swelling at injection site | 2 (2.4) |
| Rash at injection site | 2 (2.4) |
| Fever (documented >100.4o F) | 2 (2.4) |
| Other (Suicidal ideations) | 1 (1.2) |
| Lymph node swelling | 0 (0) |
| Hives | 0 (0) |
| Flare of Rheumatic disease | 0 (0) |
If vaccine given in two shot series, symptoms were counted separately for each dose administered.
4. Discussion
Herein, we report the results of a prospective single center real-world study on SARS-CoV-2 vaccination rates, timing, and immunogenicity in AIRD patients receiving RTX after a provider education intervention. The seropositivity rate of anti-spike IgG antibodies in our study (36.4%) is similar to recent studies involving patients actively on RTX (33–41%), and substantially lower than healthy controls in those cohorts [13,14]. Surprisingly, the interval time from RTX infusion to vaccination did not significantly differ between those with anti-spike antibody titers to those without. While others have demonstrated higher seropositivity rates with longer intervals from pre-vaccination RTX infusion, these differences were typically seen in those vaccinated at least 10 months from pre-vaccination RTX infusion [13,15,16]. Thus, it is likely that the number of patients vaccinated more than 10 months (40 weeks) post-RTX was insufficient to demonstrate a significant difference in seropositivity. The discordance between our results and these studies raises the possibility that the interval to optimize seropositivity during vaccination of AIRD patients post-RTX may be much longer than what is currently recommended by societal guidelines [11,[13], [14], [15], [16]].
Our data demonstrated a statistically significant association between IgG/IgM hypogammaglobulinemia and absence of seropositivity following vaccination. This was predictive even when correcting for age and concomitant medication use. Low IgM has been associated with a negative likelihood of seropositivity following SARS-CoV-2 vaccines in one recent study, but no such association was found with other immunoglobulin types [17]. Hypogammaglobulinemia is a known complication of ongoing RTX treatment, particularly in patients with AAV, prior CYC use, and concomitant glucocorticoid exposure [5,6]. The association between hypogammaglobulinemia and impaired humoral responses to other recommended vaccines in AIRD patients is well-established. More recently, hypogammaglobulinemia has been associated with increased rates of serious infection events [5,6]. Observational studies and expert panels highlight a role for screening immunoglobulins in AIRD on RTX [18,19]. Past observational studies involving our cohort as well as other North American cohorts [5,6,20] show that immunoglobulins are not consistently measured in AIRD on RTX. The association between hypogammaglobulinemia and impaired SARS-CoV-2 vaccine immunogenicity identified in our study further highlights the importance of educating providers on the rationale for screening immunoglobulins in AIRD patients on RTX. It also raises the questions of whether waiting for immunoglobulin recovery may be an effective strategy to improve vaccine efficacy.
While we did not find an association between B-cell reconstitution and vaccine in immunogenicity, due to the pragmatic nature of this study, less than half of patients had B-cell counts measured at the time of vaccination. Despite this, only 2 patients in our study had undetectable B-cell levels, which suggests that there are other factors contributing to low anti-spike IgG Ab seropositivity rate. Prior studies have shown that complete B-cell depletion negatively affects seropositivity rates [[13], [14], [15], [16], [17]]. B-cell reconstitution is highly variable and typically occurs within 6–9 months after RTX [21]. Although the presence of B-cell reconstitution is associated with higher rates of seropositivity following vaccination, the degree of B-cell reconstitution seems to be less impactful amongst those with reconstitution [[13], [14], [15], [16], [17]].
Following the education intervention, providers within our department indicated that they would recommend SARS-CoV-2 vaccination to each of their AIRD patients on RTX. Subsequent SARS-CoV-2 vaccination rates were high at 95.2%, (22.2% and 38.7% higher than regional and national rates according to data from the CDC (https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total). Vaccination rates were also remarkably higher than historical rates of other recommended vaccines amongst patients on RTX therapy [22,23]. Since lack of a physician's recommendation for vaccination has been shown to correlate with low vaccination rates in prior studies [24], faculty education on the role for timing vaccines and for recommending vaccination to patients should be prioritized.
The majority of patients received vaccination more than 20 weeks after their RTX infusion (with median delay of 20.5 weeks and 24.3 weeks for dose 1 and 2 respectively). The majority of patients also had the next planned RTX infusion >4 weeks following the second vaccine dose. Not surprisingly, vaccination >20 weeks after RTX infusion was associated with provider intent to universally recommend delayed vaccination. Interestingly, a higher cumulative RTX dose was associated with vaccination <20 weeks from RTX. We can speculate that this may be related to provider concerns for severe SARS-CoV-2 infections in these patients, however, this data was not available.
Although our data suggests that ongoing RTX therapy has a profound effect on seropositivity rates, there are several caveats that should be noted. First, it remains unclear whether seropositivity correlates to protective immunity from SARS-CoV-2 infection. Although humoral response is known to be an important surrogate for immunity as demonstrated in vitro and in vivo [25], T-cell responses also play an important role in cell mediated immunity. Recent studies assessing T cell response in patients to vaccination on B cell depleted therapy have shown that responses appear to remain intact, although likely impaired, in B-cell depleted and seronegative patients [26]. Therefore, although data on humoral responses suggest that delaying vaccination post-RTX confers improved vaccine immunogenicity, there is much to learn about the complete immune response and whether delaying vaccination is absolutely necessary given emerging evidence that T-cell responses can occur independently of antibody production.
The strength of our study was the relatively robust size of our cohort compared to similar cohorts published on this topic. In addition, this study represents a pragmatic effort from a single center to improve vaccination rates and timing for a high-risk population in the midst of the SARS-CoV-2 pandemic and highlights the difficulties that were likely faced by institutions and clinics worldwide. There are several limitations of our study including the observational nature of our study as we were unable to control what laboratory or clinical data was collected from patients. This led to several sample size limitations that may have underestimated the predictive effect of several parameters. In particular, our data may underrepresent the actual degree to which B-cell depletion affected seropositivity rates. Furthermore, we were unable to assess the role of T-cell responses to the SARS-Co-V-2 vaccine. Due to the limited time of follow-up after vaccination, we are also unable to comment on post-vaccination SARS-CoV-2 infection rates and severity, which is perhaps the most important patient-centered outcome. However, this is certainly a highly important area for further study as is the effect of a third vaccine on seropositivity rates in this population.
5. Conclusions
In our cohort, the implemented physician and patient education was associated with high vaccination rates and optimal timing according to the published guidelines. Despite that, the immunogenicity of vaccines was suboptimal; only approximately 1/3 of patients receiving RTX mounted the anticipated antibody responses. This lack of seroconversion following SARS-CoV-2 vaccination was associated with hypogammaglobulinemia, with older age being a potential independent factor. The timing between patients' most recent, pre-vaccination RTX infusion to SARS-CoV-2 vaccination did not affect seropositivity rates. In addition, and in contrast to findings from similar cohorts, we found that B-cell counts also did not predict rates of seropositivity despite the fact that most patients had reconsititution of B cell numbers. This finding underscores the fact that RTX has a profound effect on B cell function that does not normalize even after their numbers rebound. Lastly, concomitant medications, including conventional DMARDs and corticosteroid use, were not predictive of seropositivity following vaccination. However, as we and others have shown, past use of high dose corticosteroids do contribute to the development of chronic hypogammaglobulinemia in RTX treated patients. (new paragraph)Our results suggest that monitoring immunoglobulin levels is key in predicting immunogenicity to SARS-CoV-2 vaccination in patients on RTX. Even more importantly,waiting for immunoglobulin recovery to improve vaccine efficacy may be necessary, with the caveat that certain patients may develop chronic immunodeficiency post RTX. Additional prospective studies are ongoing to assess rates of infection and morbidity in the RTX treated AIRD population and how these correlate to anti-spike antibody seropositivity.
Footnotes
This work was not supported by any commercial interest. The authors declare no conflicts of interest.
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., C.S. Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., Stoop J., Tete S., Van Damme W., Leroux-Roels I., Berghmans P.J., Kimmel M., Van Damme P., de Hoon J., Smith W., Stephenson K.E., De Rosa S.C., Cohen K.W., McElrath M.J., Cormier E., Scheper G., Barouch D.H., Hendriks J., Struyf F., Douoguih M., Van Hoof J., Schuitemaker H. Interim results of a phase 1-2a trial of Ad26.COV2.S COVID-19 vaccine. N. Engl. J. Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas S.J., Moreira E.D., Jr., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G. Perez, Polack F.P., Zerbini C., Bailey R., Swanson K.A., Xu X., Roychoudhury S., Koury K., Bouguermouh S., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Tureci O., Nell H., Schaefer A., Unal S., Yang Q., Liberator P., Tresnan D.B., Mather S., Dormitzer P.R., Sahin U., Gruber W.C., Jansen K.U., C.C.T. Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone J.H., Merkel P.A., Spiera R., Seo P., Langford C.A., Hoffman G.S., Kallenberg C.G., Clair E.W. St, Turkiewicz A., Tchao N.K., Webber L., Ding L., Sejismundo L.P., Mieras K., Weitzenkamp D., Ikle D., Seyfert-Margolis V., Mueller M., Brunetta P., Allen N.B., Fervenza F.C., Geetha D., Keogh K.A., Kissin E.Y., Monach P.A., Peikert T., Stegeman C., Ytterberg S.R., Specks U., R.-I.R. Group Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Md Yusof M.Y., Vital E.M., McElvenny D.M., Hensor E.M.A., Das S., Dass S., Rawstron A.C., Buch M.H., Emery P., Savic S. Predicting severe infection and effects of Hypogammaglobulinemia during therapy with Rituximab in rheumatic and musculoskeletal diseases, arthritis. Rheumatol. 2019;71:1812–1823. doi: 10.1002/art.40937. [DOI] [PubMed] [Google Scholar]
- 6.Wade S.D., Kyttaris V.C. Rituximab-associated hypogammaglobulinemia in autoimmune rheumatic diseases: a single-center retrospective cohort study. Rheumatol. Int. 2021;41:1115–1124. doi: 10.1007/s00296-021-04847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark S.A., Clark L.E., Pan J., Coscia A., McKay L.G.A., Shankar S., Johnson R.I., Brusic V., Choudhary M.C., Regan J., Li J.Z., Griffiths A., J. Abraham, SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms. Cell. 2021;184:2605–2617 e2618. doi: 10.1016/j.cell.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparks J.A., Wallace Z.S., Seet A.M., Gianfrancesco M.A., Izadi Z., Hyrich K.L., Strangfeld A., Gossec L., Carmona L., Mateus E.F., Lawson-Tovey S., Trupin L., Rush S., Katz P., Schmajuk G., Jacobsohn L., Wise L., Gilbert E.L., Duarte-Garcia A., Valenzuela-Almada M.O., Pons-Estel G.J., Isnardi C.A., Berbotto G.A., Hsu T.Y., D’Silva K.M., Patel N.J., Kearsley-Fleet L., Schafer M., Ribeiro S.L.E., Al Emadi S., Tidblad L., Scire C.A., Raffeiner B., Thomas T., Flipo R.M., Avouac J., Seror R., Bernardes M., Cunha M.M., Hasseli R., Schulze-Koops H., Muller-Ladner U., Specker C., Souza V.A., Mota L., Gomides A.P.M., Dieude P., Nikiphorou E., Kronzer V.L., Singh N., Ugarte-Gil M.F., Wallace B., Akpabio A., Thomas R., Bhana S., Costello W., Grainger R., Hausmann J.S., Liew J.W., Sirotich E., Sufka P., Robinson P.C., Machado P.M., Yazdany J., Alliance C.-G.R. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann. Rheum. Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bingham C.O., 3rd, Looney R.J., Deodhar A., Halsey N., Greenwald M., Codding C., Trzaskoma B., Martin F., Agarwal S., Kelman A. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62:64–74. doi: 10.1002/art.25034. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg R.A., Jawad A.F., Boyer J., Maurer K., McDonald K., Prak E.T., Sullivan K.E. Rituximab-treated patients have a poor response to influenza vaccination. J. Clin. Immunol. 2013;33:388–396. doi: 10.1007/s10875-012-9813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furer V., Rondaan C., Heijstek M.W., Agmon-Levin N., van Assen S., Bijl M., Breedveld F.C., D’Amelio R., Dougados M., Kapetanovic M.C., van Laar J.M., de Thurah A., Landewe R.B., Molto A., Muller-Ladner U., Schreiber K., Smolar L., Walker J., Warnatz K., Wulffraat N.M., Elkayam O. Update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2019;79(2020):39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Fisk R.J. Barriers to vaccination for coronavirus disease 2019 (COVID-19) control: experience from the United States. Glob. Health J. 2021;5:51–55. doi: 10.1016/j.glohj.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., Zisapel M., Elalouf O., Kaufman I., Meidan R., Broyde A., Polachek A., Wollman J., Litinsky I., Meridor K., Nochomovitz H., Silberman A., Rosenberg D., Feld J., Haddad A., Gazzit T., Elias M., Higazi N., Kharouf F., Shefer G., Sharon O., Pel S., Nevo S., Elkayam O. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann. Rheum. Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 14.Mrak D., Tobudic S., Koblischke M., Graninger M., Radner H., Sieghart D., Hofer P., Perkmann T., Haslacher H., Thalhammer R., Winkler S., Bluml S., Stiasny K., Aberle J.H., Smolen J.S., Heinz L.X., Aletaha D., Bonelli M. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann. Rheum. Dis. 2021;80:1345–1350. doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- 15.Spiera R., Jinich S., Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann. Rheum. Dis. 2021;80:1357–1359. doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- 16.Deepak P., Kim W., Paley M.A., Yang M., Carvidi A.B., Demissie E.G., El-Qunni A.A., Haile A., Huang K., Kinnett B., Liebeskind M.J., Liu Z., McMorrow L.E., Paez D., Pawar N., Perantie D.C., Schriefer R.E., Sides S.E., Thapa M., Gergely M., Abushamma S., Akuse S., Klebert M., Mitchell L., Nix D., Graf J., Taylor K.E., Chahin S., Ciorba M.A., Katz P., Matloubian M., O’Halloran J.A., Presti R.M., Wu G.F., Whelan S.P.J., Buchser W.J., Gensler L.S., Nakamura M.C., Ellebedy A.H., Kim A.H.J. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann. Intern. Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly C.M., Koenig D., Ravi S.N., Azar A., Kant S., Dalal M., Duchen J., Seo P., Antiochos B., Paik J.J., Geetha D. Correspondence on “SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response” by Bonelli et al. Ann. Rheum. Dis. 2021;80 doi: 10.1136/annrheumdis-2021-220972. [DOI] [PubMed] [Google Scholar]
- 18.Reddy V., Martinez L., Isenberg D.A., Leandro M.J., Cambridge G. Pragmatic treatment of patients with systemic lupus erythematosus with Rituximab: long-term effects on serum immunoglobulins. Arthritis Care Res. 2017;69:857–866. doi: 10.1002/acr.22993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottenberg J.E., Ravaud P., Bardin T., Cacoub P., Cantagrel A., Combe B., Dougados M., Flipo R.M., Godeau B., Guillevin L., Le Loet X., Hachulla E., Schaeverbeke T., Sibilia J., Baron G., Mariette X., AutoImmunity, r. Rituximab, R. French society of, risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum. 2010;62:2625–2632. doi: 10.1002/art.27555. [DOI] [PubMed] [Google Scholar]
- 20.Barmettler S., Ong M.S., Farmer J.R., Choi H., Walter J. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw. Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anolik J.H., Friedberg J.W., Zheng B., Barnard J., Owen T., Cushing E., Kelly J., Milner E.C., Fisher R.I., Sanz I. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin. Immunol. 2007;122:139–145. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Feuchtenberger M., Kleinert S., Schwab S., Roll P., Scharbatke E.C., Ostermeier E., Voll R.E., Schafer A., Tony H.P. Vaccination survey in patients with rheumatoid arthritis: a cross-sectional study. Rheumatol. Int. 2012;32:1533–1539. doi: 10.1007/s00296-011-1808-z. [DOI] [PubMed] [Google Scholar]
- 23.Moulis G., Lapeyre-Mestre M., Mahevas M., Montastruc J.L., Sailler L. Need for an improved vaccination rate in primary immune thrombocytopenia patients exposed to rituximab or splenectomy. A nationwide population-based study in France. Am. J. Hematol. 2015;90:301–305. doi: 10.1002/ajh.23930. [DOI] [PubMed] [Google Scholar]
- 24.Assala M., Groh M., Blanche P., Vinter C., Cohen P., Le Guern V., Puechal X., Mouthon L., Le Jeunne C., Launay O., Kerneis S. Pneumococcal and influenza vaccination rates in patients treated with corticosteroids and/or immunosuppressive therapies for systemic autoimmune diseases: a cross-sectional study. Joint Bone Spine. 2017;84:365–366. doi: 10.1016/j.jbspin.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 26.Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., E.M. C, Mexhitaj I., Jacobs D., Babb A., Betts M.R., Prak E.T.L., Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]