Abstract
Background
There is little evidence about the factors that predict persistence/adherence in treatment-naïve patients with COPD in clinical practice. The aim of this study was to evaluate persistence and adherence levels among treatment-naïve patients diagnosed with COPD who had a prescribed inhaled medication, using data from real-world clinical practice.
Methods
Multicentric study with a 6 month-followed-up period. Patients were considered persistent if they collected all their inhaler refills. In a random sample of patients, we evaluated adherence using the Test of Adherence to Inhalers (TAI). We assessed Health Related Quality of Life (HRQL) with St George's Respiratory Questionnaire (SGRQ).
Results
Of the 114 patients included, 46 (40.4%) were defined as persistent. Patients who had awareness about COPD (adjusted RR 2.672, 95% CI 1.125–6.349) were more likely to be persistent; patients with multidose DPI were less likely to be persistent that those with single dose DPI (adjusted RR 0.341, 95% CI 0.133–0.877). Higher levels of SGRQ total were associated with a lower probability of persistence (adjusted RR 0.945, 95%CI 0.894–0.998). Patients who had had an appointment with their GP in the previous six months were more likely to be persistent (adjusted RR 3.107, 95% CI 1.022–9.466). Patients who had awareness about COPD and those with lower symptom SGQR score were more likely to be adherent (24/25, 96.0% vs 16/22, 72.7%, p = 0.025, and mean 29.1, sd 19.4 vs mean 41.4, sd 15.9, respectively, p = 0.026, respectively).
Conclusions
Less than 50% of patients were defined as persistent. Patients’ awareness of their disease and levels of HRQL were associated with high rate of persistence and adherence. In addition, frequent visits to general practitioner, increases the rate of persistence to treatment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-021-01754-6.
Keywords: Chronic obstructive pulmonary disease, Adherence, Persistence, Inhaler medication, Treatment-naïve patients
Introduction
A central element in chronic obstructive pulmonary disease (COPD) management is the use of bronchodilators [1]. Each type presents different advantages and disadvantages regarding use and previous data showed that between 4 and 94% of patients exhibited incorrect inhaler usage [2]. Adherence to treatment (the extent to which a person take medication with the agreed recommendations from a clinician) and persistence (the act of continuing the treatment for the prescribed duration) are two relevant factors of the effectiveness of a treatment. Addressing both adherence and persistence brings deeper understanding of a patient’s medication-taking behavior [3], and this is particularly important in respiratory medicine, where many patients in do not use their inhalers correctly.
Lack of adherence and persistence in patients diagnosed with COPD have been widely described in both controlled clinical studies and those in clinical practice. However, because randomized controlled trials usually include homogeneous patient populations, they do not represent the real-world clinical population. Although adherence levels observed in clinical trials may be around 70–90%, levels of adherence in clinical practice are far lower (40–60%) [4, 5].
Many factors influence persistence and adherence to therapy, including patient’s age, Health Related Quality of Life (HRQL), comorbidities, concomitant medications, knowledge about the disease and treatment, complexity of the treatment, lack of training and education on inhaler use, among others [6–9]. HRQL is an essential factor included in the definition of patient-reported outcomes (PROs), measurements of any health status aspect directly indicated by the patient, which are often associated with overall treatment efficacy [10]. Most of the studies aiming to assess the relationship between HRQL and adherence have been carried out in patients who have had inhaler treatment during more than 6 months. In a recent study, the type of inhaler, patient awareness about the disease and treatment, and higher Health Related Quality of Life (HRQL) were associated with good adherence [4]. A previous systematic review showed however, that an improved HRQL may be a trigger for non-adherence in patients with COPD, and thus, the relationship between medication adherence and HRQL may be dual [11]. Nevertheless, there is little evidence about the influence of HRQL as a predictor factor of persistence and adherence in treatment-naïve patients. In addition, the majority of the studies are based on anonymized claims data in which patients cannot be evaluated regarding relevant factors such as knowledge and perception of their disease and treatment [12].
The aim of this study was to evaluate persistence and adherence levels among treatment-naïve patients diagnosed with COPD who had a prescribed inhaled medication, using data from clinical practice.
Methods
Study design
Multi center prospective cohort study of treatment-naïve patients using COPD medications.
Population
A sample of consecutive treatment-naïve patients diagnosed with COPD were invited to participate in the study when they attended the pneumology department in the General Hospital of Elche, Hospital Sant Joan d’Alacant and General Hospital of Alicante, Spain, from March 2018 to January 2019. All the patients who were invited to participate in the study agreed to be included. Diagnosis was established after a spirometry test was carried out (ICD J40-J47). Only patients with no prior use of inhaler device were included. The exclusion criteria have previously been defined [4].
The majority of the population in Spain uses the National Health Care System as the main medical service in which prescription costs are mainly covered. In order to have access to patients’ medical records we only included patients who used this system and therefore, the insurance status is not a relevant variable in this study.
We defined the index date as the date of a patient’s first prescription claim for a COPD drug of interest and patients were followed-up for 6 months through the pharmaceutical electronic register. Given the relatively short follow-up period, the 114 patients used the same inhaler during the six subsequent refills.
Data collection
Outcome variables
Persistence was assessed through the evaluation of the six subsequent refills of the inclusion inhaler, (pharmaceutical electronic register). In order to calculate the drug use as a continuous variable, we assessed the total number of doses dispensed from therapy initiation divided by the prescribed daily dose. A patient was considered “persistent” if he/she had refilled the six prescriptions of the inhaler, and “non-persistent” if at least one of the prescriptions was missed. This binary variable (persistence yes/no) was the main outcome variable to assess persistence in our study.
In addition, we evaluated the different persistence profile for each patient to assess the different patient’s behavior. Hence, we evaluated patients according to the three components previously described [13]: initiation (the patient takes the first dose of a prescribed medication), implementation (from initiation until the last dose is taken) and discontinuation (the end of the therapy). We classified the patients into: late or non-initiation of the prescribed treatment (those patients who did not take the first dose of the prescribed medication and they initiated the treatment after the second prescription or later), sub-optimal implementation of the dosing regimen (those patients who initiated the treatment but they did not regularly collect their prescription) and early discontinuation of the treatment (those patients who omitted the next dose to be taken).
We also calculated persistence as a continuous variable (days of persistence), considering the number of days in the follow-up period, according to a previous study [14].
We assessed adherence in a random sample of patients at the end of the period of study. Adherence was assessed by phone through a self-reported method, the Test of Adherence to Inhalers’ (TAI) [15], which includes 12 questions classified into two main domains, the patient (items #1 to #10) and the health professional (items #11 and #12) domain. We used the 10-items TAI whose total score ranges from 10–50. Adherence is rated as good (score: 50), intermediate (score: 46–49), or poor (score: < 45). We classified patients in two groups, above and below the mean value of TAI score in the studied population. We also estimated adherence as a continuous variable (TAI score).
As we previously did [4], adherence and persistence were based on the primary inhaler, which was determined by the treating physician.
Independent variables
The following variables were assessed at the entry of the study before the initiation of the treatment.
We collected, through a standard questionnaire (Additional file 1): 1) sociodemographic variables: age, sex, educational level, and patients’ employment status (employed worker, self-employed worker, retired, unemployed; 2) clinical variables: smoking habit and physical activity. Physical activity was assessed through the following questions: Do you think you are active or sedentary?; Do you practice physical activity? (sometimes, almost always, always or never); Physical activity frequency (every day, once or twice/week, between 3 and 5 times/week or never). According to patients’ answers, we classified the patients into active or sedentary and the frequency of physical activity.
We also assessed whether patients received explanation about treatment and patients’ variables related with their awareness and perceptions on disease and its seriousness. In order to classify if a patient had awareness about COPD we included the following questions in the questionnaire: Do you know if you have COPD? (Yes, I suffer from this disease, No, I have another disease, Don’t know/no opinion); Do you know if COPD is a chronic disease? (Yes, No, Don’t know/no opinion); Do you know if COPD is a serious disease? (No, COPD is a mild disease, Yes, COPD is a serious/very serious disease, Don’t know/no opinion). A patient was classified as having awareness if he/she answered yes to all three questions.
b) We also collected other clinical variables through electronical medical records in the index data: patient’s COPD characteristics according to the Global Initiative for Obstructive Lung Disease (GOLD) [1]; number of visits to Emergency Services for breathing related problems in the last 12 months and frequency of medical appointments with the general practitioner. We also retrieved Body Mass Index (BMI) and treatment-related variables: type of inhaler device (single dose Dry Powder Inhalers (DPI), multidose DPI, Soft Mist Inhaler (SMI) and Metered dose inhalers (MDI)) and number of inhalers.
We addressed the following agents: inhaled corticosteroids (ICS), combination long-acting (LABA) beta2-agonists/ICS in one device, long-acting (LAMA) anticholinergics, combination LAMA/LABA in one device, and triple combination LAMA/LABA/ICS in one device. The classification of single-dose or multi-dose DPI depend on how the dry powder is stored, and it does not refer to the number of doses per inhalation o the combination of different agents [16].
Patients also completed the St George's Respiratory Questionnaire (SGRQ) [17]. The SGRQ is the most widely used qualified COPD-specific instrument that has 50 items comprising three domains: symptoms (eight items), activity (16 items), and impact (psychosocial) (26 items). Scores range from 0 (no impairment) to 100 (maximum impairment). A mean change score of 4 units is associated with slightly efficacious treatment, 8 units for moderately efficacious change and 12 units for very efficacious treatment [18]. According to a previous study in Spanish general population, overall mean SGRQ scale score was 8.4 (sd 11.3) [19]. In a previous study [4], the different mean SGRQ scale scores for patients with severe/very severe COPD vs patients with mild/moderate COPD were: symptoms: 44.2 (sd 22.2) vs 36.9 (sd 20.4); activity: 62.6 (sd 26.6) vs 48.7 (sd 22.3); impact: 42.8 (sd 16.4) vs 36.4 (sd 14.7), and total: 49.4 (sd 18.0) vs 40.4 (sd 14.9).
Sample size
According to previous studies [12], 10% of patients were defined as non-persistent. They defined non persistence as a treatment gap of > 90 days and > 180 days in sensitivity analysis (binary persistence yes/no). Given the shorter period of follow-up in our study (6 months), we defined non persistence as a treatment gap of > 30 days (if the patient failed to collect any of the 6 prescriptions). Therefore, we will need to include a total of 112 patients (5% precision and 95% confidence interval).
Statistical analysis
All data were computerised anonymously and checked to discard errors. Statistical precision was determined through the calculation of 95% confidence intervals using the appropriate method according to the type of measurement and the available data. All analyses carried out with the statistical programme Stata/SE (Stata Corp., College Station, Texas, USA).
We estimated the incidence of persistence according to relevant variables. The effect of potential variables predicting 6-month non persistence to therapy on a patient level was considered by means of a stratified analysis a Poisson regression (95% confidence intervals). For the multivariable regression models, a backward elimination methodology was used (the model included only predictors that reached statistical significance p < 0.005).
Results
Baseline patients’ characteristics
A total of 114 patients met the criteria for inclusion in the study (Table 1). Of the 114 patients, 63 (55.3) belong to the General Hospital of Elche, 30 (26.3%) to the Hospital Sant Joan d’Alacant, and 21 (18.4%) to the General Hospital of Alicante. The mean age was 65.9 years old (standard deviation (sd) 10.7), 77 (67.5%) were men and 22 (19.3%) patients did not have studies. More than half of patients were former or never smokers (63, 55.3%) and 94 (82.5%) had mild or moderate COPD (Table 2). Mean total SGQR score was 39.2 (sd 14.9); mean symptoms SGQR score was 36.5 (sd 19.5); mean activity SGQR score was 45.5 (sd 23.3), and mean impact SGQR score was 36.2 (sd 13.9). The majority of patients (38, 57.6%) usually had an appointment with their General Practitioner (GP) each 6 months or less. Of the 114 patients included, 58 (50.9%) had awareness about COPD seriousness; 53 (46.5%) had awareness about COPD chronicity. In addition, 55 (48.2%) had received explanation about their treatment.
Table 1.
Description of the study patients and the relationship between the sociodemographic and activity variables with persistence to inhalers
| Variables | Persistence | ||
|---|---|---|---|
| Yes (46, 40.4%) | No (68, 59.6%) | p value | |
| Sex | 0.977 | ||
| Men | 31 (67.4) | 46 (67.6) | |
| Women | 15 (32.6) | 22 (32.4) | |
| Age (mean, sd) | 66.28 (10.6) | 65.69 (10.8) | 0.774 |
| Education level | 0.843 | ||
| Without studies | 10 (23.3) | 12 (18.2) | |
| Primary studies | 19 (44.2) | 28 (42.4) | |
| Secondary studies | 10 (23.3) | 17 (25.8) | |
| University studies | 4 (9.3) | 9 (13.6) | |
| Employment situation | 0.573 | ||
| Employed worker | 13 (28.3) | 12 (17.6) | |
| Own-account worker | 4 (8.7) | 8 (11.8) | |
| Retired | 25 (54.3) | 40 (58.8) | |
| Unemployed | 4 (8.7) | 8 (11.8) | |
| BMI (median, range) | 26.9 (4.8) | 28.2 (5.3) | 0.213 |
| BMI | 0.238 | ||
| < 25 | 14 (30.4) | 23 (33.8) | |
| 25–30 | 23 (50.0) | 24 (35.3) | |
| > 30 | 9 (19.6) | 21 (30.9) | |
| Physical activity | 0.644 | ||
| Active | 32 (69.6) | 50 (73.5) | |
| Sedentary | 14 (30.4) | 18 (26.5) | |
| Frequency of physical activity (in active patients) | 0.699 | ||
| Every day | 16 (34.8) | 28 (41.2) | |
| 1–2/week | 14 (30.4) | 21 (30.9) | |
| 3–5/month | 7 (15.2) | 11 (16.2) | |
| Never | 9 (19.6) | 8 (11.8) | |
| Smoking behaviour | 0.032 | ||
| Current | 15 (32.6) | 36 (52.9) | |
| Former and never smoker | 31 (67.4) | 32 (47.1) | |
| Smoking pack/year (median, range) | 32.38 (27.99) | 33.78 (21.53) | 0.773 |
BMI body mass index, sd standard deviation
Table 2.
Description of the study patients and the relationship between the clinical variables with persistence to inhalers
| Variables | Persistence | ||
|---|---|---|---|
| Yes (46, 40.4%) | No (68, 59.6%) | p value | |
| Visits to GP | 0.040 | ||
| ≤ 6 months | 19 (73.1) | 19 (47.5) | |
| > 6 months | 7 (26.9) | 21 (52.5) | |
| Visits to the Emergency Service in the last 12 months | 0.361 | ||
| No | 36 (78.3) | 48 (70.6) | |
| Yes | 10 (21.7) | 20 (29.4) | |
| COPD | 0.535 | ||
| Mild + moderate | 35 (85.4) | 59 (89.4) | |
| Severe + very severe | 6 (14.6) | 7 (10.6) | |
| Knowledge about COPD severity and chronicity | 0.011 | ||
| No | 18 (39.1) | 43 (63.2) | |
| Yes | 28 (60.9) | 25 (36.8) | |
| To receive explanation about treatment | 0.223 | ||
| No | 27 (58.7) | 32 (47.1) | |
| Yes | 19 (41.3) | 36 (52.9) | |
| Number of inhalers | 0.110 | ||
| 1 | 32 (69.6) | 56 (82.4) | |
| 2/3 | 14 (30.4) | 12 (17.6) | |
| Health related quality of life (mean, sd) | |||
| Symptoms | 35.7 (27.2) | 46.7 (20.4) | 0.522 |
| Activity | 38.9 (27.2) | 46.7 (20.4) | 0.026 |
| Impact | 43.9 (27.2) | 46.7 (20.4) | 0.227 |
| Total | 36.0 (17.1) | 41.4 (13.4) | 0.023 |
| Inhaler device types | 0.021 | ||
| Single dose DPI | 21 (42.9) | 28 (57.1) | |
| Multidose DPI | 10 (25.6) | 29 (74.4) | |
| SMI | 8 (57.1) | 6 (42.9) | |
| MDI | 7 (58.3) | 5 (41.7) | |
DPI dry powder inhalers, SMI soft mist inhaler, MDI metered dose inhalers, GP general practitioner, COPD chronic obstructive pulmonary disease
Patients’ persistence profile
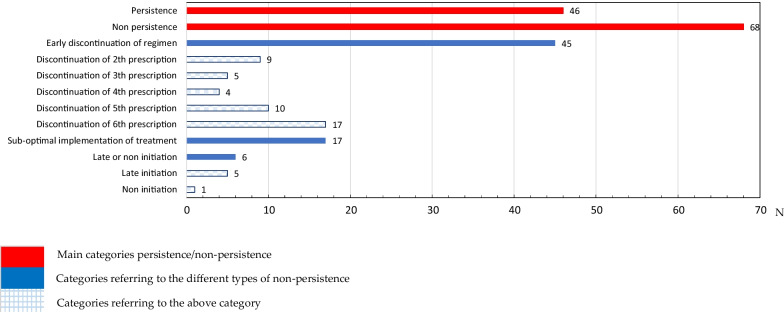
According to Fig. 1, patients’ persistence profiles were divided into different categories. Out of the 114 patients included in the study, 46 (40.4%) were defined as persistent. Of the 68 (59.6%) patients classified as non-persistent, 45/68 (66.2%) discontinued their treatment early, 17/68 (25.0%) had a sub-optimal implementation of the dosing regimen, and d) 6/68 (8.8%) started late or did not initiate their prescribed treatment (1 patient did not initiate the treatment). Of the 45 patients who discontinued their regimen early, most discontinued it before the 6th prescription (17/45, 37.8%) or before the 5th prescription (10/45, 22.2%).
Fig. 1.
Description of the number of patients included in the study according to their profile when collecting their prescription
Description of the variables associated with persistence
Those former and never smoker patients (31, 67.4%) were more likely to be persistent than those current smokers (15, 32.6%), p = 0.032 (Table 1). Those patients who had had an appointment with their GP in the previous six months were more likely to be persistent (19, 73.7% vs 7, 26.9%, p = 0.040). Those patients who had awareness about COPD seriousness and chronicity were more likely to be persistent (28, 60.8% vs 18, 39.1%), p = 0.011 (Table 2).
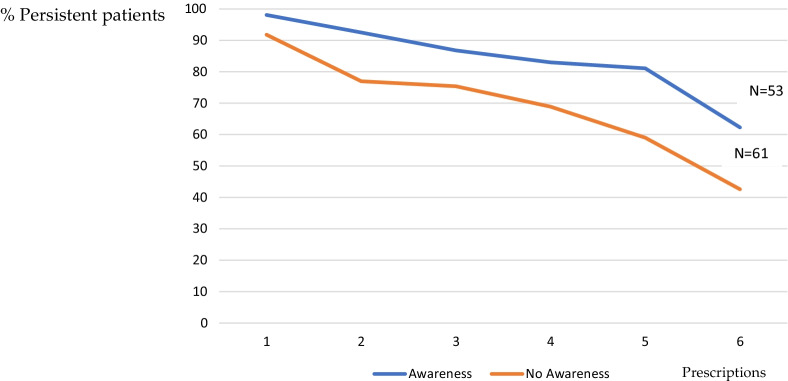
Figure 2 shows the different persistence rates between those patients who had awareness about COPD seriousness and chronicity and those who not along the period of study.
Fig. 2.
Percentage of persistent patients according to patients’ awareness about COPD severity and chronicity along the period of study
Of the 61 patients who did not have awareness about COPD seriousness and chronicity, 18 (39.1%) were classified as non-persistent, 26 (57.8%) early discontinued their treatment, 12 (70.6%) had a sub-optimal implementation of the dosing regimen, and 5 (83.3%) started late or did not initiate their prescribed treatment (p = 0.040).
Total SGRQ score was significantly higher in non-persistent patients than in those persistent patients (mean 41.4, sd 13.4 and mean 36.0, sd 17.1, respectively, p = 0.023). There were also differences according to activity SGQR score in non-persistent patients vs persistent patients (mean 46.7, sd 20.4 and mean 38.9, sd 27.2, respectively, p = 0.026) (Table 2).
According to inhaler device (Table 2), those patients using a single dose DPI (42.9%), SMI (57.1%) and MDI (58.3%) were more likely to be persistent than those using a multidose DPI (25.6%), p = 0.021. According to persistence categories, patients with multidose DPI were more likely to start late or did not initiate their prescribed treatment than patients with other inhaler device types (3, 7.7% vs 3, 4.0%) and to have a sub-optimal implementation of treatment (20, 51.3% vs 25, 33.3%), p = 0.021.
In multivariate analysis (adjusted by SGRQ total and activity, awareness about COPD seriousness and chronicity, visits to GP in the previous 6 months, smoking behaviour and type of inhaler), those patients who had awareness about COPD (adjusted RR 2.672, 95% CI 1.125–6.349, p = 0.026) and those patients who had had an appointment with their GP in the previous six months (adjusted RR 3.107, 95% CI 1.022–9.466, p = 0.046) were more likely to be persistent. Higher levels of SGRQ total were associated with a lower probability of persistence (adjusted RR 0.945, 95%CI 0.894–0.998, p = 0.041). Those patients with Multidose DPI were more likely to be no persistent that those with other inhaler devices (adjusted RR 0.341, 95% CI 0.133–0.877, p = 0.040) (Table 3).
Table 3.
Multivariate analysis assessing the relationship between different variables and probability of persistence
| Variables | RR | CI 95% | p value |
|---|---|---|---|
| Health related quality of life | |||
| Activity | 0.972 | 0.934–1.011 | 0.161 |
| Total | 0.945 | 0.894–0.998 | 0.041 |
| Awareness about COPD severity and chronicity | |||
| No | 1.00 | ||
| Yes | 2.672 | 1.125–6.349 | 0.026 |
| Visits to GP | |||
| > 6 months | 1.00 | ||
| ≤ 6 months | 3.107 | 1.022–9.446 | 0.046 |
| Smoking behaviour | |||
| Current | 1.00 | ||
| Former and never smoker | 2.515 | 1.010–6.260 | 0.048 |
| Inhaler device type | |||
| Other device | 1.00 | ||
| Multi DPI | 0.341 | 0.133–0.877 | 0.026 |
Mean days of persistence was 104.9 (sd 56.3). Mean days of persistence was lower in patients who did not have awareness about COPD (94.1, sd 58.7) in comparison with patients who related to have awareness about the disease (mean 116.6, sd 51.6), p = 0.035. Mean days of persistence were lower in patients using multidose DPI (89.3, sd 46.6) in comparison with patients using other inhaler devices, p = 0.036 (Additional file 1).
Adherence to treatment
Out of 114 patients, 47 (41.2%) were randomly selected to be evaluated about their adherence to inhaler device. There were no differences between those who answered the TAI test and those who not. Of the 47 patients, 27 (57.4%) were classified as good adherent, 13 (27.7%) as intermediate adherent and 7 (14.9%) as poor adherent. Mean (sd) followed-up period until the TAI questionnaire was carried out was 122 days (36.4) and there were not differences according to the different categories (good/intermediate/poor) (p = 0.308) (data not shown).
Mean TAI punctuation was 44.4 (sd. 8.2). Those patients with good/intermediate adherence were more likely to be classified as persistent (16/40, 40.0%) or to have an early discontinuation of regimen (18/40, 45.0%) than those with poor adherence (1/7, 14.3% and 2/7, 28.6%, respectively) (p = 0.031). Thus, patients who regularly took their medication (even if they stopped it earlier) were more likely to consider themselves as adherent patients than if they had a sub-optimal implementation of treatment or started it later.
Those patients who had awareness about COPD seriousness and chronicity were more likely to be adherent (24/25, 96.0%% vs 16/22, 72.7%), p = 0.025. Symptom SGQR score was significantly lower in patients with good adherence than in those with poor/intermediate adherence (mean 29.08, sd 19.43 and mean 41.35, sd 15.85, respectively, p = 0.026).
Discussion
In our study, only 40% of the treatment-naïve COPD patients had continued their treatment after 6 months of follow-up and 57% of the patients interviewed were classified as good adherent. In contrast with previous studies [8], we showed a correlation between persistence and adherence. Patients who did not have awareness about COPD, lower HRQL and patients with multidose DPI were more likely to be no persistent. In addition, patients who had awareness about COPD and those with lower symptom SGQR score were more likely to be adherent.
Our results showed a lower rate of persistence than previous studies. In a study in the United Kingdom, three quarters of newly diagnosed COPD patients continued their treatment after 6 months [20]. Nevertheless, the authors acknowledged the lack of an accurate diagnosis of COPD and a probable misclassification of drug exposure. In contrast, previous research found that only 12–27% of naïve-treatment patients taking drugs other than tiotropium persisted in treatment after 6 months [21].
Out of the 68 patients who were non-persistent, 45 (66.2%) had an early discontinuation of regimen. Hence, this pattern of early discontinuation indicates a lack of willingness to take the medication for more than a few months (40% of the patients discontinued the treatment before the fourth month). In addition, there was a relevant percentage of patients who showed a sub-optimal implementation of treatment and 5% of all patients either had a late initiation or did not initiate treatment. This has had an impact on COPD control and leads to the wasting of health-care resources burden [22]. Efforts should be made to improve this pattern when a patient initiates inhaler therapy, and health professionals should be aware of the factors that negatively influence persistence.
Fewer than 50% of patients admitted to having awareness about COPD seriousness and chronicity. This awareness regarding the disease was found to be the most powerful predictor of persistence and adherence to inhalation therapy, which has also been referred to by other authors [23, 24]. Subjects that were knowledgeable about COPD characteristics and those who had recently had an appointment with their GP showed a higher rate of persistence to treatment. Patients’ HRQL in baseline was also associated with persistence. COPD education strategies involving primary care centres need to be modified to achieve improved patient outcomes.
Persistent patients and those with good adherence showed in the baseline, higher levels of HRQL. Although evidence on the relationship between medication persistence/adherence in treatment-naïve patients is lacking, there are controversial data about the relationship between HRQL and adherence/persistence in experienced patients. Adherence can affect HRQL, but HRQL may also impact on medication adherence. A previous systematic review including studies in experienced patients showed a dual association between medication adherence and HRQL: the effect of medication adherence on HRQL might be a consequence of the effectiveness of the therapy, but an increased HRQL could be a trigger for non-adherence in subjects with COPD [11]. However, in another study which included patients with a chronic disease such as hypertension, when HRQL increased, the level of adherence to therapeutic recommendations also did [25]. HRQL can be affected by the existence of a chronic disease, its chronic nature and factors related with the negative impact of the disease on the patient’s physical, emotional and social wellbeing [26]. Therefore, HRQL can affect the person’s attitude towards the disease, as well as the attitude to treatment, which includes the appropriate level of adherence [27]. The inclusion of HRQL in clinical studies, as a relevant patient-reported outcome (PRO), provide important supporting evidence of factors related to persistence and adherence to treatment. The European Medicines Agency (EMA) and the US FDA consider patients’ perspective as an important aspect of drug approval [28, 29].
Device characteristics were also associated with different persistence rates. There is a growing availability of different types of medication and inhaler devices which constitute additional key factors in patients’ compliance with their medical therapy. More evidence is required to assist clinicians in prescribing the optimal medication, given that as many as 94% of patients made at least one error when they used the inhaler device [30]. In our study, although medication persistence was low for all the types of inhalers, patients in treatment with multidose DPI were less likely to be persistent. To our knowledge, this is the first study describing the relationship between inhaler devices and persistence in treatment-naïve patients with COPD which includes an analysis of patients’ persistence pattern. A previous retrospective cohort study did not show impact of inhaler device (multiple-dose versus single-dose inhaler) on COPD patients’ persistence [31]. However, the above study included some limitations related with the existence of an intensive monitoring and lung rehabilitation program in the study setting, and the lack of an ac-curacy diagnosis on the database (some asthma patients could have been classified as COPD patients).
This study has some limitations. First, and due to the longitudinal design, the observational period was limited to 6 months. Secondly, the sample size was small because of the need to evaluate patients’ awareness and perceptions about COPD and treatment. There is little evidence on persistence and adherence regarding treatment-naïve patients. We based the sample size estimation on a previous retrospective study with a 12-month followed-up period [11]. They defined non persistence as a treatment gap of > 90 days and > 180 days in sensitivity analysis (binary persistence yes/no). Given the shorter period of follow-up in our study (6 months), we defined non persistence as a treatment gap of > 30 days (if the patient failed to collect any of the 6 prescriptions). However, we included all the consecutive treatment-naïve patients who were diagnosed with COPD, excluding a selection bias, and increasing the external validity of the study (together with the inclusion of patients from different centres).
In addition, although we could only interview a random sample of patients about their adherence to treatment, patients who were interviewed had similar sociodemographic and clinical characteristics to those who were not.
We classified patients’ awareness about COPD considering three different questions to cover the description of patients’ awareness. The questionnaire applied to our study was previously used in a similar population in which the questionnaire was previously piloted to ensure patients understood the questions and the responses were not ambiguous [4]. As we previously did in that study, we decided to combinate the three questions related with awareness about COPD severity and chronicity, because there was a high concordance between them. In the present study, those patients who answered they knew they have COPD were more likely to know that COPD was a chronic disease (72.7%) and to know that COPD was a serious disease (77.3%). Therefore, we considered that this question included several aspects related with patients’ knowledge on COPD characteristics. In the literature, there are several questionnaires to assess patient’s awareness on COPD with different levels of accuracy [32], which have been carried out in different settings [33]. We decided to use the three questions because similar aspects had been previously asked to assess patients’ awareness on COPD [34]. We used these three questions with three options, ‘yes/no/don’t know’, which make them less time-consuming for patients, easier to score, and therefore suitable for use in this research. In addition, the results of this classification can be considered reliable since we showed not only differences between patients’ awareness about COPD seriousness and chronicity and persistence, but also a relationship between mean months of persistence and patients’ awareness.
Although previous evidence showed that GP’s emphasis on the importance of the medication could be a relevant facilitator of patients’ persistence [35], we did not consider it. Patients included in this study were recruited in the Pneumology Services when they were firstly prescribed an inhaler. Thus, the person who explained the diagnosis and the new treatment was the pneumologist, who is specialized in the respiratory system. Previous evidence stated that patients controlled by a pneumologist were less likely to perform the inhalation manoeuvers incorrectly in comparison with those controlled by a GP [36]. Thus, this factor would not have had a great impact on our results. We classified the patients according to the Global Initiative for Obstructive Lung Disease. However, we did not have access to spirometry data referring seven of the patients to classify the patients’ disease. We did not use the ABCD assessment tool that was updated in 2017. We started the study in 2018 but the protocol was approved by the different committees and authorities before the update of the guidelines. In multivariable analysis, we found no association between smoking behaviour and adherence to treatment. Previous evidence showed that smoking and highly educated young patients were associated with poor adherence to treatment when inhaler overuse was defined as poor adherence [37]. However, we did not include overuse as an aspect of adherence to treatment and the TAI questionnaire only detects underuse of inhalers.
The strength of this study lies in the fact that it is a prospective design in real-world practice with unselected population. Previous studies [38] have included extensive databases to evaluate aspects such as daily dosing frequency and adherence in treatment-naïve patients. However, this is the first study in which naïve-treatment patients’ awareness on both disease and treatment and impact of HRQL on persistence/adherence were evaluated.
Conclusions
In conclusion, given the low rate of persistence and adherence in treatment-naïve patients with COPD, it is essential to know which variables can predict this lack of compliance with their treatment. Raising patient awareness of the disease and improving cooperation between patients and clinicians should be implemented in practice before the choice of the inhaler to increase effectiveness of the treatment.
Supplementary Information
Additional file 1. Fig. S1: Questionnaire to collect patients’ sociodemographic and clinical variables at the entry of the study before the initiation of the treatment. Table S1: Description of the study patients and the relationship between the sociodemographic and activity variables with adherence and persistence to inhalers (as a continuous variables). Table S2: Description of the study patients and the relationship between the clinical variables with adherence and persistence to inhalers (as a continuous variables).
Acknowledgements
We would like to thank all the persons and institutions who have participated in this research: Dr. Luis Hernandez Blasco, from the Valencian Society of Pneumology, for his scientific and technical support, Inma Lassaletta, from the Pneumology Service of the University Hospital of Alicante. Medical staff of the Pneumology Service at the University Hospitals of Elche and San Juan. Amparo Talens, from the Pharmacy Service of the Hospital Sant Joan d´Alacant, and Francisca Ruiz Lozano from the Community Pharmacy ULM, in Alicante. Chiesi Spain, for allowing us to use the TAI test, and María Jesús Rambla Ramón for her support, documentation, and materials.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- GP
General practitioner
- HRQL
Health Related Quality of Life
- sd
Standard deviation
- SGRQ
St George's Respiratory Questionnaire
- TAI
Test of Adherence to Inhalers
- GOL
Global Initiative for Obstructive Lung Disease
- BMI
Body mass index
- DPI
Dry powder inhalers
- SMI
Soft mist inhaler
- MDI
Metered dose inhalers
- ICS
Inhaled corticosteroids
- LABA
Combination long-acting
- LAMA
Long-acting anticholinergics
- FDA
Food and Drug Administration
- EMEA
European Medicines Agency
- CI
Confidence interval
Authors' contributions
BL and EL-P conceived of and designed the study and obtained funding. BL, JG and EL-P acquired the data. BL prepared the data and BL, JG and EL-P interpreted statistical analyses. EL-P coordinated the data management, and BL wrote the study protocol. BL did the statistical analyses and drafted the data tables. BL, JG and EL-P co-wrote the manuscript. All authors critically revised the paper for important intellectual content and approved the final version. All authors read and approved the final manuscript.
Funding
This project received funding from the Valencian Government (GV/2017/193). Additionally, the study was co-supported by a grant from Sandoz, the generic pharmaceuticals division of Novartis. The funders were not involved in any aspect of the design of this study, preparation of results, or decision to submit for publication.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethic Committee (registration number 17/341) of the Hospital Clinic of San Juan de Alicante (Spain) and was classified as “EPA-OD” study (‘post-authorization studies with a different design to prospective follow-up’) by the Spanish Agency of Medicines and Health Products (AEMPS). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/19/2022
Following the original publication of this article, the authors flagged that the first and last names had been erronoeusly swapped for each of the authors; the names have since been corrected in the article
Change history
3/7/2022
A Correction to this paper has been published: 10.1186/s12890-022-01857-8
Contributor Information
Elsa López-Pintor, Email: elsa.lopez@umh.es.
Justo Grau, Email: justograu@yahoo.es.
Blanca Lumbreras, Email: blumbreras@umh.es.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management and prevention. 2018. https://goldcopd.org/wp-content/uploads/2018/02/WMS-GOLD-2018-Feb-Final-to-print-v2.pdf. Accessed 10 April 2021.
- 2.Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102:593–604. doi: 10.1016/j.rmed.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 4.López-Pintor E, Grau J, González I, et al. Impact of patients' perception of COPD and treatment on adherence and health-related quality of life in real-world: study in 53 community pharmacies. Respir Med. 2020;176:106280. doi: 10.1016/j.rmed.2020.106280. [DOI] [PubMed] [Google Scholar]
- 5.Breekveldt-Postma NS, Koerselman J, Erkens JA, Lammers JJ, Herings RMC. Enhanced persistence with tiotropium com-pared with other respiratory drugs in COPD. Respir Med. 2007;101:1398e405. doi: 10.1016/j.rmed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Chouaid C, Germain N, De Pouvourville G, et al. Patient preference for chronic obstructive pulmonary disease (COPD) treatment inhalers: a discrete choice experiment in France. Curr Med Res Opin. 2019;35:785–792. doi: 10.1080/03007995.2019.1574507. [DOI] [PubMed] [Google Scholar]
- 7.George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128:3198e204. doi: 10.1378/chest.128.5.3198. [DOI] [PubMed] [Google Scholar]
- 8.Mäkelä MJ, Backer V, Hedegaard M, et al. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:1481–1490. doi: 10.1016/j.rmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Sandler N, Holländer J, Långström D, et al. Evaluation of inhaler handling-errors, inhaler perception and preference with Spiromax, Easyhaler and Turbuhaler devices among healthy Finnish volunteers: a single site, single visit crossover study (Finhaler) BMJ Open Resp Res. 2016;3:e000119. doi: 10.1136/bmjresp-2015-000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs) Health Serv Insights. 2013;6:61–68. doi: 10.4137/HSI.S11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ágh T, Dömötör P, Bártfai Z, Inotai A, Fujsz E, Mészáros Á. Relationship between medication adherence and health-related quality of life in subjects with COPD: a systematic review. Respir Care. 2015;60:297–303. doi: 10.4187/respcare.03123. [DOI] [PubMed] [Google Scholar]
- 12.Mueller S, Wilke T, Bechtel B, Punekar YS, Mitzner K, Virchow JC. Non-persistence and non-adherence to long-acting COPD medication therapy: a retrospective cohort study based on a large German claims dataset. Respir Med. 2017;122:1–11. doi: 10.1016/j.rmed.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Vrijens B, De Geest S, Hughes DA et al; ABC Project Team. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691–705. 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed]
- 14.Dailey G, Kim MS, Lian JF. Patient compliance and persistence with antihyperglycemic drug regimens: evaluation of a medicaid patient population with type 2 diabetes mellitus. Clin Ther. 2020;23:1311–20. doi: 10.1016/s0149-2918(01)80110-7. [DOI] [PubMed] [Google Scholar]
- 15.Plaza V, Giner J, Curto E, Alonso-Ortiz MB, Orue MI, Vega JM, Cosío BG, Investigators of the RETAI study. Assessing adherence by combining the test of adherence to inhalers with pharmacy refill records. J Investig Allergol Clin Immunol. 2021;31:58–64. 10.18176/jiaci.0461. [DOI] [PubMed]
- 16.Atkins PJ. Dry powder inhalers: an overview. Respir Care. 2005;50:1304–1312. [PubMed] [Google Scholar]
- 17.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;Suppl. B:25–31. 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed]
- 18.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19:398–404. doi: 10.1183/09031936.02.00063702. [DOI] [PubMed] [Google Scholar]
- 19.Ferrer M, Villasante C, Alonso J, et al. Interpretation of quality of life scores from the St George's Respiratory Questionnaire. Eur Respir J. 2002;19:405–413. doi: 10.1183/09031936.02.00213202. [DOI] [PubMed] [Google Scholar]
- 20.Wurst KE, Punekar YS, Shukla A. Treatment evolution after COPD diagnosis in the UK primary care setting. PLoS ONE. 2014;9:e105296. doi: 10.1371/journal.pone.0105296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramer JA, Bradley-Kennedy C, Scalera A. Treatment persistence and compliance with medications for chronic obstructive pulmonary disease. Can Respir J. 2007;14:25–29. doi: 10.1155/2007/161652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:1481–1490. doi: 10.1016/j.rmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Kale MS, Federman AD, Krauskopf K, et al. The association of health literacy with illness and medication beliefs among patients with chronic obstructive pulmonary disease. PLoS ONE. 2015;10:e0123937. doi: 10.1371/journal.pone.0123937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128:3198–3204. doi: 10.1378/chest.128.5.3198. [DOI] [PubMed] [Google Scholar]
- 25.Uchmanowicz B, Chudiak A, Mazur G. The influence of quality of life on the level of adherence to therapeutic recommendations among elderly hypertensive patients. Patient Prefer Adherence. 2018;4:2593–2603. doi: 10.2147/PPA.S182172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brito DM, Araújo TL, Galvão MT, Moreira TM, Lopes MV. Quality of life and perception of illness among individuals with high blood pressure. Cad Saude Publica. 2008;4:933–940. doi: 10.1590/s0102-311x2008000400025. [DOI] [PubMed] [Google Scholar]
- 27.Megari K. Quality of life in chronic disease patients. Health Psychol Res. 2013;3:e27. doi: 10.4081/hpr.2013.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Ad-ministration, February 16, 2001. Value Health. 2003;6:522–531. doi: 10.1046/j.1524-4733.2003.65309.x. [DOI] [PubMed] [Google Scholar]
- 29.Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. European Medicines Agency; 2005. https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-healthrelated-quality-life-hrql-measures-evaluation_en.pdf. Accessed 1 Apr 2021.
- 30.Souza ML, Meneghini AC, Ferraz E, Vianna EO, Borges MC. Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol. 2009;35:824–831. doi: 10.1590/s1806-37132009000900002. [DOI] [PubMed] [Google Scholar]
- 31.van Boven JF, van Raaij JJ, van der Galiën R, et al. Impact of multiple-dose versus single-dose inhaler devices on COPD pa-tients' persistence with long-acting β2-agonists: a dispensing database analysis. NPJ Prim Care Respir Med. 2014;24:14069. doi: 10.1038/npjpcrm.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White R, Walker P, Roberts S, Kalisky S, White P. Bristol COPD Knowledge Questionnaire (BCKQ): testing what we teach patients about COPD. Chron Respir Dis. 2006;3:123–131. doi: 10.1191/1479972306cd117oa. [DOI] [PubMed] [Google Scholar]
- 33.Puente-Maestu L, Chancafe-Morgan J, Calle M, Rodríguez-Hermosa JL, Malo de Molina R, Ortega-González Á, Fuster A, Márquez-Martín E, Marcos PJ, Ramírez L, Ray S, Franks A; en nombre del grupo GemEPOC. Validation of the Spanish Ver-sion of the COPD-Q Questionnaire on COPD Knowledge. Arch Bronconeumol. 2016;1:12–6. 10.1016/j.arbres.2015.03.013. [DOI] [PubMed]
- 34.Hernandez P, Balter M, Bourbeau J, Hodder R. Living with chronic obstructive pulmonary disease: a survey of patients' knowledge and attitudes. Respir Med. 2009;7:1004–1012. doi: 10.1016/j.rmed.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Bereznicki BJ, Peterson GM, Jackson SL, Walters H, Hardley FL, Gavagna GA, Marshall DC. Describing drivers of and bar-riers to persistence with tiotropium in patients with chronic obstructive pulmonary disease: a mixed-methods approach. Int J Pharm Pract. 2015;2:154–157. doi: 10.1111/ijpp.12129. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Aymerich J, Escarrabill J, Marrades RM, Monsó E, Barreiro E, Antó JM; EFRAM Investigators. Differences in COPD care among doctors who control the disease: general practitioner vs. pneumologist. Respir Med. 2006;100:332–9. 10.1016/j.rmed.2005.04.021. [DOI] [PubMed]
- 37.Vanoverschelde A, van der Wel P, Putman B, Lahousse L. Determinants of poor inhaler technique and poor therapy adherence in obstructive lung diseases: a cross-sectional study in community pharmacies. BMJ Open Respir Res. 2021;8:e000823. doi: 10.1136/bmjresp-2020-000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toy EL, Beaulieu NU, McHale JM, Welland TR, Plauschinat CA, Swensen A, Duh MS. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105:435–441. doi: 10.1016/j.rmed.2010.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Fig. S1: Questionnaire to collect patients’ sociodemographic and clinical variables at the entry of the study before the initiation of the treatment. Table S1: Description of the study patients and the relationship between the sociodemographic and activity variables with adherence and persistence to inhalers (as a continuous variables). Table S2: Description of the study patients and the relationship between the clinical variables with adherence and persistence to inhalers (as a continuous variables).
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.