Abstract
Background
The purpose of this study was to retrospectively analyze clinical characteristics and prognostic risk factors of urosepsis patients admitted to two intensive care units in Shanghai, China.
Methods
Clinical data from patients diagnosed with urosepsis were retrospectively retrieved and analyzed from ICU in two regional medical centers from January 2015 to December 2019.
Results
Two hundred two patients were included in the subsequent analysis eventually, with an average age of 72.02 ± 9.66 years, 79.21% of the patients were female and the mortality rate of 15.84%.The proportion of patients with chronic underlying diseases such as diabetes and hypertension was relatively high (56.44, 49.50%, respectively), and the incidence of shock was also high (41.58%) correspondingly. The most common pathogen isolated was Escherichia coli (79.20%), of which the extended-spectrumβ-lactamases (ESBLs)(+) accounted for 42.57%. In multivariate analysis, the strongest predictors for death were mechanical ventilation (OR 7.260, 95% CI 2.200–23.963; P = 0.001),chronic kidney disease (CKD) (OR 5.140, 95% CI 1.596–16.550; P = 0.006), APACHE II score (OR 1.321, 95% CI 1.184–1.473; P < 0.001) and lactate (OR 1.258, 95% CI 1.037–1.527; P = 0.020). Both APACHE II score and lactate had the ideal predictive value, with the area under the ROC curve (AUC) of 0.858 and 0.805 respectively.
Conclusion
The patients with urosepsis were characterized by a higher proportion of female, older age, more percentage of comorbidities in this region, and patients with ESBLs (+) Escherichia coli infection were more prone to shock. Mechanical ventilation, comorbidity with CKD, APACHE II score and lactate were independent risk factors for death in urosepsis patient, but lactate level and APACHE II score had better predictive value for prognosis.
Keywords: Urosepsis, Urinary tract infections, Clinical characteristics, Prognosis
Background
Sepsis is one of the most common causes of death among hospitalized patients in intensive care units (ICU) [1]. A recent study showed there were 48.9 million sepsis cases and 11 million sepsis-related deaths worldwide in 2017, making sepsis a global public health problem [2, 3]. Two retrospective studies from China showed that the sepsis mortality exceeded 30% by 2020 [4, 5], making it a heavy burden on the health care system in China.
Sepsis is life-threatening and associated with physiological, pathological and biological abnormalities caused by a dysregulated host response to infections [6]. The common sites of sepsis infection include the lungs, urinary system, abdominal cavity, skin and soft tissue. The different sites of infection may be closely related to the prognosis of patients [7]. Current literature reports show that there are certain differences in the incidence of urosepsis in different countries and regions [5, 8, 9], which may be related to inconsistent diagnostic criteria of urosepsis in recent years.
Even though some studies have summarized the epidemiological characteristics, etiology and prognostic risk factors of urosepsis patients, the susceptibility factors and etiology of patients with urosepsis are different worldwide [10–12]. At present, there are few studies focusing on the prognosis of patients with urosepsis in the ICU, especially no reports from Shanghai, China. The purpose of this study was to retrospectively analyze clinical data and predict the potential risk factors for urosepsis patients admitted to ICUs of two regional medical centers in Shanghai over the past 5 years to help clinicians improve their understanding and clinical prognosis, ultimately contributing to the clinical treatment of patients with urinary system infections.
Methods
Study design
A retrospective study was performed in two regional medical centers of Shanghai. Patients with clinical and microbiological diagnoses of septic urinary tract infections admitted to the Department of Critical Care Medicine at Pudong Hospital and Gongli Hospital from January 2015 to December 2019 were enrolled. All patients received standard treatment for sepsis, including antibiotics and fluid resuscitation, as well as mechanical ventilation, vasoactive drugs, and renal replacement therapy, which may be required. The indications of operation were determined by the urologist. They were all monitored in the ICU until their condition improved and they were transferred out of the ICU or died. The study were approved by the Ethics Committee of Shanghai Pudong Hospital of Fudan University and Ethics Committee of Gongli Hospital Affiliated to Naval Medical University, and complied with the Principles of the Declaration of Helsinki. As this study was a retrospective study and medical intervention was not required, the informed consent for this study was waived by the Ethics Committee of Shanghai Pudong Hospital of Fudan University and Ethics Committee of Gongli Hospital Affiliated to Naval Medical University.
Inclusion and exclusion criteria
Inclusion criteria included the following:1) sepsis caused by a urinary tract infection; 2) Sepsis diagnosed according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis 3).
Exclusion criteria included the following: 1) Concurrent infection at other sites; 2) Malignant tumor patients; 3) Uremia patients undergoing dialysis; 4) Incomplete clinical data.
Data collection
Data were collected from electronic medical records of two hospitals. Clinical retrospective data was retrieved including age, gender, primary diseases in the urinary system, underlying diseases, comorbidities, laboratory tests, interventions such as the use of mechanical ventilation and Continuous renal replacement therapy (CRRT), etiological results, length of stay (LOS) in ICU and hospital, and hospital mortality. Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores were also assessed. These clinical data were at their worst condition state within the first 24 h after diagnosis of urosepsis.
Definitions
Urosepsis is defined as sepsis caused by a urinary tract infection. Sepsis was diagnosed according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis 3) [6] :Sepsis should be defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. For clinical operationalization, organ dysfunction can be represented by an increase in the sepsis-related SOFA score of 2 points or more. Septic shock is defined as persistence of hypotension after adequate fluid resuscitation in patients with sepsis, requiring a vasopressor to maintain a mean arterial pressure of 65 mmHg or greater and serum lactate level greater than 2 mmol/L (> 18 mg/dL).
Statistical analysis
The SPSS 23.0 statistical software package (IBM, Chicago, IL, USA) was used for statistical analysis analysis. The continuous variables were expressed as mean standard deviation and analyzed using a t-test. The categorical variables were expressed as numbers (percentage) and compared with a chi-square test or Fischer exact test. Variables with a P-value < 0.1 in univariate logistic regression analysis were included in multivariate logistic regression analysis. The backward selection method was used to determine the variables included in the final model. Results were expressed as odds ratios (OR) and 95% confidence intervals (95% CI). The discriminatory power of risk factors was determined from the area under the receiver operating characteristic (AUROC) curve with corresponding 95% CIs. Two-sided P-values< 0.05 were considered as statistically significant.
Results
Demographic and clinical data
A total of 216 hospitalized patients diagnosed with urosepsis were retrieved from the electronic medical records, 14 of whom were excluded from the study and 202 patients were included in the subsequent analysis eventually (Fig. 1). The average age of the enrolled 202 cases was 72.02 ± 9.66 years of age (28–90 years of age), the percentage of patients that were over ≥65 years of age was 76.24% (154/202). A total of 79.21% of the patients were female and a total of 32 of the 202 patients died within 28 days, with the mortality rate being 15.84%. The top two comorbidities observed in these patients were diabetes (56.44%) and hypertension (49.50%). All patients were divided into a survival group and death group. The demographic and clinical characteristics of these patients were summarized in Table 1. There were no differences in age, gender, surgical treatment, comorbidities, long-term indwelling catheter history, white blood count (WBC), hemoglobin (Hb), platelets, C-reactive protein (CRP) and total bilirubin (TBiL) D-dimer between the two groups. The rate of shock in the survival group was significantly lower than in the death group (30.59% vs 62.50%, P = 0.001). The APACHE II and SOFA scores were also significantly lower than in the death group (16.75 ± 4.18 vs 24.00 ± 5.59, 5.49 ± 3.36 vs 10.22 ± 3.37; all P < 0.001). The proportion of comorbidities with chronic kidney disease in the survival group was 16.47%, which was far lower than the 43.75% observed in the death group (P = 0.003). In terms of laboratory tests, WBC, procalcitonin (PCT), Serum creatinine (SCr) and lactate in the survival group were lower than in the death group (all P < 0.05), while Hb, albumin and oxygenation index were significantly higher than in the death group (all P < 0.05). Within 24 h of ICU admission, the death group needed more mechanical ventilation (P = 0.002), but there was no difference in the proportion of CRRT between two groups(P = 0.279). Compared with the death group, the survival group had longer hospital LOS (P < 0.001), but the ICU LOS was similar.
Fig. 1.
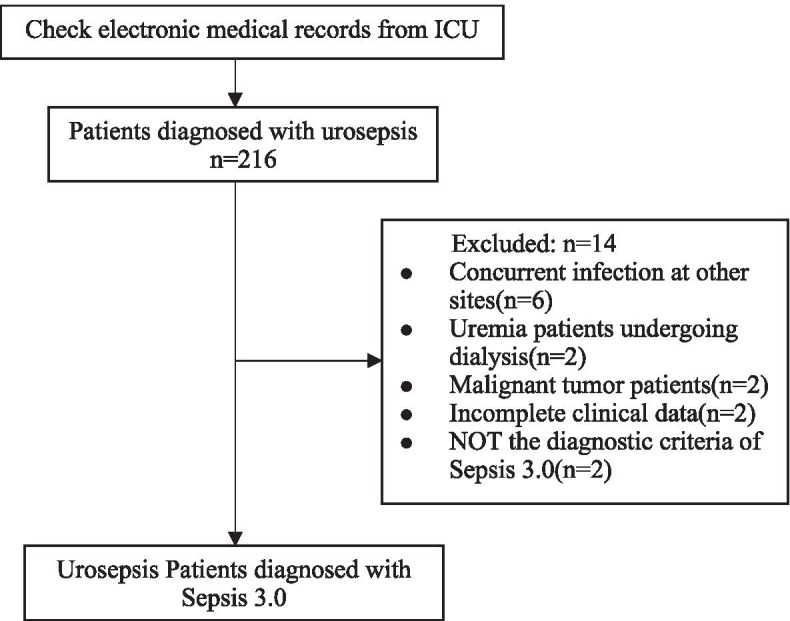
The flow diagram description for inclusion in the study population
Table 1.
Demographic and clinical data of the patients included in this study
| Total (n = 202) |
Survival group (n = 170) |
Death group (n = 32) |
χ2/t | P-value | |
|---|---|---|---|---|---|
| Age (years) | 72.02 ± 9.66 | 71.27 ± 9.68 | 76.00 ± 8.61 | 2.577 | 0.011 |
| Female (%) | 160 (79.21) | 138 (81.18) | 22 (68.75) | 2.525 | 0.112 |
| Shock(%) | 74 (36.63) | 52 (30.59) | 20 (62.50) | 11.956 | 0.001 |
| Surgical treatment(%) | 170 (84.16) | 146 (85.88) | 24 (75.00) | 2.392 | 0.122 |
| APACHE II score | 17.90 ± 5.16 | 16.75 ± 4.18 | 24.00 ± 5.59 | 8.487 | < 0.001 |
| SOFA score | 6.24 ± 3.78 | 5.49 ± 3.36 | 10.22 ± 3.37 | 7.284 | < 0.001 |
| Comorbidities | |||||
| Diabetes(%) | 114 (56.44) | 92 (54.12) | 22 (68.75) | 2.345 | 0.126 |
| Hypertension(%) | 100 (49.50) | 82 (48.24) | 18 (56.25) | 0.692 | 0.405 |
| COPD(%) | 20 (9.90) | 18 (10.59) | 2 (6.25) | 0.568 | 0.451 |
| Chronic heart disease(%) | 58 (28.71) | 52 (30.59) | 6 (18.75) | 1.844 | 0.174 |
| Chronic kidney disease(%) | 42 (20.79) | 28 (16.47) | 14 (43.75) | 12.169 | < 0.001 |
| Cerebrovascular disease(%) | 52 (25.74) | 40 (23.53) | 12 (37.50) | 2.750 | 0.097 |
| Long-term indwelling catheter (%) | 26 (12.87) | 20 (11.76) | 6 (18.75) | 1.172 | 0.279 |
| Laboratory findings | |||||
| WBC(× 109/L) | 17.52 ± 8.79 | 16.94 ± 8.73 | 20.58 ± 8.60 | 2.170 | 0.031 |
| Hb(g/L) | 107.49 ± 13.09 | 108.33 + 12.70 | 103.06 ± 14.43 | 2.107 | 0.036 |
| Platelet(×109/L) | 140.55 ± 69.36 | 144.72 ± 67.92 | 118.38 ± 73.78 | 1.985 | 0.048 |
| CRP (mg/L) | 107.85 ± 61.32 | 107.25 ± 62.68 | 111.05 ± 54.32 | 0.321 | 0.748 |
| PCT (ng/mL) | 49.17 ± 58.05 | 43.85 ± 53.72 | 77.43 ± 71.67 | 3.065 | 0.002 |
| SCr (μmol/L) | 165.90 ± 148.94 | 148.86 ± 140.46 | 256.44 ± 161.96 | 3.877 | < 0.001 |
| TBil (μmol/L) | 12.64 ± 10.13 | 12.10 ± 8.14 | 15.47 ± 17.14 | 1.734 | 0.084 |
| Albumin (g/L) | 30.25 ± 3.08 | 30.56 ± 2.92 | 28.60 ± 3.41 | 3.388 | 0.001 |
| D-dimer (mg/L) | 6.36 ± 6.80 | 6.20 ± 7.23 | 7.25 ± 3.80 | 0.799 | 0.425 |
| Oxygenation index (mmHg) | 274.39 ± 79.71 | 281.87 ± 77.96 | 234.63 ± 78.25 | 3.143 | 0.002 |
| Lactate (mmol/L) | 3.66 ± 2.33 | 3.25 ± 1.98 | 5.83 ± 2.84 | 6.294 | < 0.001 |
| Mechanical ventilation(%) | 37 (18.3) | 25 (14.71) | 12 (37.50) | 9.352 | 0.002 |
| CRRT(%) | 26 (12.87) | 20 (11.76) | 6 (18.75) | 1.172 | 0.279 |
| ICU LOS (days) | 9.07 ± 2.66 | 9.17 ± 2.76 | 8.56 ± 2.00 | 1.176 | 0.241 |
| Hospital LOS (days) | 13.14 ± 4.40 | 13.77 ± 4.41 | 9.84 ± 2.44 | 4.882 | < 0.001 |
APACHE II Acute physiology and chronic health evaluation II, SOFA Sequential organ failure assessment, COPD Chronic obstructive pulmonary disorder, WBC White blood count, Hb Hemoglobin, CRP C-reactive protein, PCT Procalcitonin, SCr Serum creatinine, TBiL Total bilirubin; CRRT Continuous renal replacement therapy, LOS length of stay
Risk factors and predictive value for prognosis
To analyze the risk factors associated with death in urosepsis patients with, univariate analysis was performed for thirteen variables, including age, shock, APACHE II score, SOFA score, comorbidity of chronic kidney disease and mechanical ventilation, laboratory tests such as WBC, Hb, PCT, SCr, Albumin, oxygenation index and lactate. Then, multivariate logistic regression analysis was performed for statistically significant results. The data analysis showed that the strongest predictors for death in urosepsis patients were mechanical ventilation (OR 7.260, 95% CI 2.200–23.963; P = 0.001), chronic kidney disease (CKD) (OR 5.140, 95% CI 1.596–16.550; P = 0.006), APACHE II score (OR 1.321, 95% CI 1.184–1.473; P < 0.001) and lactate (OR 1.258, 95% CI 1.037–1.527; P = 0.020) (Tables 2 and 3). Then predictive value of these three variables in predicting mortality risk of urosepsis patients was analyzed using the ROC curve. The results showed that both the APACHE II score and lactate had the ideal predictive value, with the area under the ROC curve (AUC) of 0.858 and 0.805 respectively, while comorbidity of chronic kidney disease and mechanical ventilation was 0.636 and 0.614(Table 4, Fig. 2).
Table 2.
Univariate analysis of variables associated with urosepsis prognosis
| OR | 95% CI | P-value | |
|---|---|---|---|
| Age | 1.058 | 1.012–1.107 | 0.013 |
| Shock | 3.782 | 1.722–8.304 | 0.001 |
| APACHE II score | 1.307 | 1.196–1.428 | < 0.001 |
| SOFA score | 1.398 | 1.243–1.572 | < 0.001 |
| Chronic kidney disease(%) | 3.944 | 1.759–8.845 | 0.001 |
| WBC | 1.046 | 1.003–1.091 | 0.034 |
| Hb | 0.969 | 0.940–0998 | 0.039 |
| PCT (ng/mL) | 1.008 | 1.003–1.014 | 0.004 |
| SCr (μmol/L) | 1.004 | 1.001–1.006 | 0.003 |
| Albumin (g/L) | 0.805 | 0.704–0.919 | 0.001 |
| Oxygenation index (mmHg) | 0.991 | 0.985–0.997 | 0.003 |
| Lactate (mmol/L) | 1.501 | 1.268–1.777 | < 0.001 |
| Mechanical ventilation | 3.480 | 1.514–7.997 | 0.003 |
CI Confidence interval, APACHE II Acute physiology and chronic health evaluation II, SOFA Sequential organ failure assessment, WBC White blood count, PCT Procalcitonin, SCr Serum creatinine
Table 3.
Multivariate analysis of variables associated with urosepsis prognosis
| OR | 95% CI | P-value | |
|---|---|---|---|
| Mechanical ventilation | 7.260 | 2.200–23.963 | 0.001 |
| APACHE II score | 1.321 | 1.184–1.473 | < 0.001 |
| Chronic kidney disease | 5.140 | 1.596–16.550 | 0.006 |
| Lactate | 1.258 | 1.037–1.527 | 0.020 |
CI Confidence interval, APACHE II Acute physiology and chronic health evaluation II
Table 4.
ROC curve analysis of prediction for prognosis in patients with urosepsis
| Variables | AUC | 95% CI | P-value |
|---|---|---|---|
| Mechanical ventilation | 0.614 | 0.500–0.728 | 0.041 |
| APACHE II score | 0.858 | 0.799–0.918 | 0.000 |
| Chronic kidney disease | 0.636 | 0.523–0.750 | 0.014 |
| Lactate | 0.805 | 0.717–0.892 | 0.000 |
CI Confidence interval, APACHE II Acute physiology and chronic health evaluation II
Fig. 2.
ROC curve analysis of prediction for prognosis in patients with urosepsis. ROC receiver operating characteristic, APACHE II acute physiology and chronic health evaluation II
Distribution of pathogens
Pathogenic bacteria were derived from blood and urine cultures of urosepsis patients (Table 5) and a total of 202 strains were identified. Among these strains, 186 (92.08%) were gram-negative bacilli, 4 were (1.98%) gram-positive cocci and 12 (5.94%) were fungi. The most common species of Gram-negative bacteria were Escherichia coli (160 strains, 79.20%), of which extended-spectrumβ-lactamases (ESBLs)(+) accounted for 42.57% (86 strains), ESBLs(−) accounted for 36.63% (74 strains), and others included Klebsiella pneumoniae (10 strains, 4.95%), Proteus mirabilis (8 strains, 3.96%), Proteus Penthes (4 strains, 1.98%), Klebsiella aerogenes (2 strain, 0.99%) and Pseudomonas putida (2 strain, 0.99%). We compared all pathogen groups and their associated with shock. There were a total of 118 cases and 84 cases in the non-shock group and the shock group, respectively. The results showed that the proportion of Escherichia coli ESBLs (+) and Klebsiella pneumoniae isolated in the shock group were significantly higher than in the non-shock group (57.14% vs 32.20%, P<0.001; 9.52% vs 1.69%, P = 0.041), the proportion of Escherichia coli ESBLs(−) was obviously lower in the shock versus non-shock group (11.90% vs 54.24%, P<0.001), and other groups comparisons were not statistically significant (all P>0.05) (Table 5).
Table 5.
Distribution of pathogens isolated from urosepsis patients experiencing uroseptic shock
| Total (n = 202) | Urosepsis (n = 118) |
Uroseptic shock(n = 84) | χ2 | P-value | |
|---|---|---|---|---|---|
| Gram-negative bacteria(%) | 186 (92.08) | 112 (94.92) | 74 (88.10) | 3.129 | 0.077 |
| Escherichia coli ESBLs(−) | 74 (36.63) | 64 (54.24%) | 10 (11.90%) | 37.881 | <0.001 |
| Escherichia coli ESBLs(+) | 86 (42.57) | 38 (32.20%) | 48 (57.14%) | 12.483 | <0.001 |
| Klebsiella pneumoniae | 10 (4.95) | 2 (1.69%) | 8 (9.52%) | 4.186 | 0.041 |
| Proteus mirabilis | 8 (3.96) | 4 (3.39%) | 4 (4.76%) | 0.016 | 0.889 |
| Proteus penneri | 4 (1.98) | 2 (1.69%) | 2 (2.38%) | 0.000 | 1.000 |
| Klebsiella aerogenes | 2 (0.99) | 0 (0.00%) | 2 (2.38%) | NA | NA |
| Pseudomonas putida | 2 (0.99) | 2 (1.69%) | 0 (0.00%) | NA | NA |
| Gram-positive bacteria(%) | 4 (1.98) | 2 (1.69%) | 2 (2.38%) | 0.000 | 1.000 |
| Staphylococcus aureus | 4 (1.98) | 2 (1.69%) | 2 (2.38%) | 0.000 | 1.000 |
| Fungus(%) | 12 (5.94) | 4 (3.39%) | 8 (9.52%) | 2.298 | 0.130 |
| Candida albicans | 8 (3.96) | 2 (1.69%) | 6 (7.14%) | 2.531 | 0.112 |
| Candida pseudotropicalis | 2 (0.99) | 0 (0.00%) | 2 (2.38%) | NA | NA |
| Candida glabrata | 2 (0.99) | 2 (1.69%) | 0 (0.00%) | NA | NA |
ESBLs Extended-spectrumβ-lactamases
Discussion
In the current study, a retrospective analysis was performed using clinical data of urosepsis patients admitted to two ICUs over the past 5 years using sepsis 3.0 diagnostic criterion and this was also the first clinical analysis performed in this region to our knowledge. Our study indicated that the majority of urosepsis patients admitted to the ICU were elderly and nearly 80% of the patients were women. The age and proportion of women were obviously higher than what were observed in the study reported by Xin-Hua Qiang et al. [11], who found the average age to be 43.6 ± 12.5 years old and the percentage of females to be 53%. A study performed by Ying Jiang et al. [10] reported that the average age of urosepsis patients was 59.85 years old and 50% were women from Mainland China. In addition, the mortality rate was found to be 15.84% in this study. A possible explanation for this high mortality rate may be related to the fact that their hospitals are large provincial and municipal medical centers [10, 11], while our hospital is a regional medical center. The patients in this study were older, with a higher proportion of patients having chronic underlying diseases, such as diabetes and hypertension and there was also a greater incidence of shock. A study from a regional medical center in Taiwan showed that compared with patients younger than 65, elderly urosepsis patients over the age of 80 have a significantly higher risk for shock (OR 1.99, P = 0.004), but men show an increased risk of shock (OR 1.54, P = 0.022 )[12]. In addition, our study revealed that 12.87% of patients showed a history of long-term indwelling catheterization, which may be related to up to 25.74% of patients with previous cerebrovascular disease. While the length of stay in the ICU was similar in both groups, the length of hospital stay in the survival group was longer, which is similar to the results of a multicenter study from mainland China [5].
Multivariate logistic regression analysis revealed that the OR of chronic kidney disease was 5.140, suggesting that urosepsis patients complicated with chronic kidney disease have a high risk of death. This was also supported by a study performed by Yi-wenn Yvonne Huang et al [13]. Many epidemiological studies revealed significant associations between chronic kidney disease with poor outcomes in sepsis patients that may be related to the accumulation of inflammatory cytokines attenuating immune function caused by decreased renal clearance, platelet dysfunction and thrombocytopenia in patients with chronic kidney disease [14]. Even though our analysis showed that the proportion of diabetes and hypertension in comorbidities was higher and the ratio of chronic heart failure reached 28.71%, none showed an increase in the risk of death. This result differs than what was reported by Sinapidis D et al. [15] and Chen PY et al. [16], which may be associated with the demographic characteristics of this study population. As the subjects of this study were urosepsis patients, the proportion of patients requiring mechanical ventilation was not high. Our study showed that the OR of mechanical ventilation was 7.260, indicating that these patients had more severe systemic organ function damage, leading to a worse prognosis.
Hyperlactatemia (usually ≥4 mmol/L) represents low perfusion in sepsis patients tissues, which has been included in the 2016 SSC guidelines for the early identification of sepsis and septic shock [6], also closely correlated with worse prognosis of sepsis patients [17]. Although some evidence has suggested that the APACHE II score may provide inaccurate information in the certain patients, such as unconscious patients may score too high, however, it is undeniable that it is still the most widely used score to evaluate critically ill patients so far, and it has a good prognostic evaluation value for patients with sepsis [18, 19]. Our results revealed that compared with mechanical ventilation and chronic kidney disease, both the lactate level and the APACHE II score had better predictive value for the prognosis of patients with urosepsis, which indicated that lactate level and the APACHE II score may provide a better risk assessment and may be useful as a prognostic marker for urosepsis patients.
In urosepsis, the most commonly isolated pathogen is Escherichia coli, followed by other types of Enterobacteriaceae. In contrast to pulmonary or abdominal sepsis, most urosepsis cases are caused by a single microorganism [20]. Our retrospective analysis showed that the vast majority of isolated pathogens were gram-negative bacteria. Escherichia coli accounted for 86.02% of all gram-negative bacteria and 79.2% of all bacteria, both were significantly higher than the 75 and 58%, respectively reported by Xin-Hua Qiang et al. [11], and the 64.62 and 48.28%, respectively reported by Ying Jiang et al. [10]. In addition, our data showed that more than half of the 160 Escherichia coli strains isolated were ESBLs (+). Such a high resistance trend is similar to the results from a multicenter study performed in Europe in 2015 [21]. A study from Canada showed that in 176 Escherichia coli strains isolated from blood cultures of urosepsis patients, the ratio of ESBLs (+) and ESBLs (−) was 1:2 [13]. In a study performed by Ying Jiang et al. [10], 34 of the 42 Escherichia coli strains were ESBLs (+), and the ratio of ESBL (+) and ESBL (−) reached 4:1. Even though some of these studies contained small sample sizes, they still indicated that different geographical and demographic differences, economic differences and even prescription behavior may lead to different distributions of drug-resistant bacteria, thus affecting prognosis [11, 22–24]. In addition, subgroup analysis showed that the proportion of ESBLs (+) of Escherichia coli in the uroseptic shock group was significantly higher than that the non-shock group(P<0.001).
Besides, this study further showed the proportion of Enterobacteriaceae was 90.10%, which was significantly higher than what was reported by Xin-Hua Qiang et al. [11] (66.67%) and Ying Jiang et al. [10] (67.82%), and also higher than what was reported by the European multicenter study performed in 2015 [21], showing that Enterobacteriaceae accounted for 43% of all the isolated pathogens. This is beneficial for the early and rapid anti-infection treatment of patients with sepsis to regularly monitor pathogenic distribution and drug resistance mechanisms of common infections in the region. For the initial antibiotic treatment of urosepsis patients in areas that have high incidence of ESBLs (+), the generally recommended antibiotics are carbapenems, but clinicians need to be alert to the production of carbapenemase strains [25].
Upper urinary tract obstruction is an important cause of urosepsis and is the most common cause of urinary tract stones. Surgical interventions such as shock wave lithotripsy and percutaneous nephrolithotripsy (PCNL) are the main treatments of upper urinary tract stones [26]. PCNL may cause excessive pressure in the renal collecting system during an operation and a large number of bacteria or toxins will enter the blood and thus lead to sepsis [27]. Our study also found that up to 84.16% of patients had a history of surgery. Unfortunately, since this was a retrospective study, we were still unable to determine which patients were secondary to urogenic sepsis after surgery. The above issues need to be further analyzed and confirmed by future research.
The treatment of urosepsis requires interdisciplinary, including emergency unit, urological specialties, and intensive care medicine [28, 29]. At present, most clinical studies of urosepsis are written from the perspective of urologists, but there are few clinical research papers conducted from the perspective of ICU physicians. The advantage of this study is that we have explored the clinical characteristics and prognostic factors of patients with urosepsis in our region from the perspective of the ICU to identify patients with high risk of death and provide early intervention. To our knowledge, this is the first retrospective study of urosepsis patients in ICU in Shanghai, China, with the larger sample size compared with other clinical studies to date. However, this study still faces some limitations that should be pointed out. First, as a retrospective study, some unmeasured confounders might influence the final results. But we conducted the study in the 2 large medical centers with adequate sample size to minimize the underlying effects. Second, since most patients had a history of emergency surgeries and patients without surgery were also admitted to ICU from the emergency department, community-acquired infections may have contributed to the differences observed in our study versus some others. Third, a small number of patients may be associated with the spread of bacterial toxins during surgery, but we cannot accurately distinguish these patients. We plan to conduct prospective studies involving more large regional medical centers to further confirm these findings.
Conclusion
The patients with urosepsis were characterized by a higher proportion of female, older age, and more percentage of comorbidities in this region. Escherichia coli was more common in etiology, and patients with ESBLs (+) Escherichia coli were more prone to shock. Mechanical ventilation, comorbidity with chronic kidney disease, APACHE II and lactate were independent risk factors for death in urosepsis patient. Lactate level and the APACHE II score had better predictive value for prognosis.
Acknowledgements
We acknowledge all the people who helped us.
Abbreviations
- ICU
Intensive care units
- PCNL
Percutaneous nephrolithotripsy
- APACHE II
Acute physiology and chronic health evaluation II
- SOFA
Sequential organ failure assessment
- COPD
Chronic obstructive pulmonary disorder
- WBC
White blood count
- Hb
Hemoglobin
- CRP
C-reactive protein
- PCT
Procalcitonin
- SCr
Serum creatinine
- TBiL
Total bilirubin
- ESBLs
Extended-spectrumβ-lactamases
Authors’ contributions
YS and QF contributed to the study design. YS, WL, QF, BY, GY contributed to the data collection and statistical analysis. YS drafted the manuscript and QF modified it. All authors have approved the final draft of the manuscript.
Funding
This work was supported by Research Grant for Health Science and Technology of Pudong Municipal Commission of Health committee of Shanghai (Grant NO.PW2019A-25), the fund of Key Discipline Construction Project of Pudong Health Bureau of Shanghai (Grant NO. PWZxk2017–20) and Project of Key Medical Specialty and Treatment Center of Pudong Hospital of Fudan University (Grant NO. Tszb2021–04).
Availability of data and materials
In order to protect patient privacy, the datasets used and analyzed are not publicly available, but they are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study were approved by the Ethics Committee of Shanghai Pudong Hospital of Fudan University and Ethics Committee of Gongli Hospital Affiliated to Naval Medical University, and complied with the Principles of the Declaration of Helsinki. As this study was a retrospective study and medical intervention was not required, the informed consent for this study was waived by the Ethics Committee of Shanghai Pudong Hospital of Fudan University and Ethics Committee of Gongli Hospital Affiliated to Naval Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Sheng and Wen-long Zheng contributed equally to this work.
Contributor Information
Ying Sheng, Email: sweetheart1999@126.com.
Wen-long Zheng, Email: zwl58013002@126.com.
Qi-fang Shi, Email: shiqifangdr123@163.com.
Bing-yu Zhang, Email: pyzz78@sina.com.
Guang-yao Yang, Email: qq503349231@126.com.
References
- 1.Rello J, Valenzuela-Sánchez F, Ruiz-Rodriguez M, Moyano S. Sepsis: a review of advances in management. Adv Ther. 2017;34(11):2393–2411. doi: 10.1007/s12325-017-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing Sepsis as a Global Health priority-a WHO resolution. N Engl J Med. 2017;377(5):414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 4.Zhao J, He Y, Xu P, Liu J, Ye S, Cao Y. Serum ammonia levels on admission for predicting sepsis patient mortality at D28 in the emergency department: A 2-center retrospective study. Medicine (Baltimore) 2020;99(11):e19477. doi: 10.1097/MD.0000000000019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie J, Wang H, Kang Y, Zhou L, Liu Z, Qin B, Ma X, Cao X, Chen D, Lu W, et al. The epidemiology of Sepsis in Chinese ICUs: a National Cross-Sectional Survey. Crit Care Med. 2020;48(3):e209–e218. doi: 10.1097/CCM.0000000000004155. [DOI] [PubMed] [Google Scholar]
- 6.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nachtigall I, Tafelski S, Rothbart A, Kaufner L, Schmidt M, Tamarkin A, Kartachov M, Zebedies D, Trefzer T, Wernecke KD, et al. Gender- related outcome difference is related to course of sepsis on mixed ICUs: a prospective, observational clinical study. Crit Care. 2011;15(3):R151. doi: 10.1186/cc10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogura H, Gando S, Saitoh D, Takeyama N, Kushimoto S, Fujishima S, Mayumi T, Araki T, Ikeda H, Kotani J, et al. Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother. 2014;20(3):157–162. doi: 10.1016/j.jiac.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Álvaro-Meca A, Jiménez-Sousa MA, Micheloud D, Sánchez-Lopez A, Heredia-Rodríguez M, Tamayo E. Resino S;epidemiological trends of sepsis in the twenty-first century (2000-2013): an analysis of incidence, mortality, and associated costs in Spain. Popul Health Metrics. 2018;16(1):4. doi: 10.1186/s12963-018-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y, Li J, Zhang Y, Hu X, Zhang X, Shang X, et al. Clinical situations of bacteriology and prognosis in patients with Urosepsis. Biomed Res Int. 2019;2019:3080827. doi: 10.1155/2019/3080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiang XH, Yu TO, Li YN, Zhou LX. Prognosis risk of Urosepsis in critical care medicine: a prospective observational study. Biomed Res Int. 2016;2016:9028924. doi: 10.1155/2016/9028924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiao CY, Chen TH, Lee YC, Hsiao MC, Hung PH, Wang MC. Risk factors for uroseptic shock in hospitalized patients aged over 80 years with urinary tract infection. Ann Transl Med. 2020;8:477. doi: 10.21037/atm.2020.03.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YY, Alleyne A, Leung V, Chapman M. Urosepsis due to extended-Spectrum β- lactamase-producing Escherichia coli: a retrospective, single-Centre review of risk factors and clinical outcomes. Can J Hosp Pharm. 2018;71(2):119–127. [PMC free article] [PubMed] [Google Scholar]
- 14.Doi K. Role of kidney injury in sepsis. J Intensive Care. 2016;4:17. doi: 10.1186/s40560-016-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinapidis D, Kosmas V, Vittoros V, Koutelidakis IM, Pantazi A, Stefos A, Katsaros KE, Akinosoglou K, Bristianou M, Toutouzas K, et al. Progression into sepsis: an individualized process varying by the interaction of comorbidities with the underlying infection. BMC Infect Dis. 2018;18(1):242. doi: 10.1186/s12879-018-3156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen PY, Luo CW, Chen MH, Yang ML, Kuan YH. Epidemiological characteristics of postoperative sepsis. Open Med (Wars) 2019;14:928–938. doi: 10.1515/med-2019-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang HE, Park DW. Lactate as a biomarker for Sepsis prognosis? Infect Chemother. 2016;48(3):252–253. doi: 10.3947/ic.2016.48.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Shen Y, Li Z, Fei A, Wang H, Ge Q, Pan S. Prognostic significance of APACHE II score and plasma suPAR in Chinese patients with sepsis:a prospective observational study. BMC Anesthesiol. 2016;16(1):46. doi: 10.1186/s12871-016-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giamarellos-Bourboulis EJ, Norrby-Teglund A, Mylona V, Savva A, Tsangaris I, Dimopoulou I, Mouktaroudi M, Raftogiannis M, Georgitsi M, Linnér A, et al. Risk assessment in sepsis: a new prognostication rule by APACHE II score and serum soluble urokinase plasminogen activator receptor. Crit Care. 2012;16(4):R149. doi: 10.1186/cc11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonkat G, Cai T, Veeratterapillay R, Bruyère F, Bartoletti R, Pilatz A, Köves B, Geerlings SE, Pradere B, Pickard R, et al. Management of Urosepsis in 2018. Eur Urol Focus. 2019;5(1):5–9. doi: 10.1016/j.euf.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Tandoğdu Z, Bartoletti R, Cai T, Çek M, Grabe M, Kulchavenya E, Köves B, Menon V, Naber K, Perepanova T, et al. Antimicrobial resistance in urosepsis: outcomes from the multinational, multicenter global prevalence of infections in urology (GPIU) study 2003-2013. World J Urol. 2016;34(8):1193–1200. doi: 10.1007/s00345-015-1722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu J, Huang Y, Lv X. Crisis of antimicrobial resistance in China: now and the future. Front Microbiol. 2019;10:2240. doi: 10.3389/fmicb.2019.02240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brolund A, Lagerqvist N, Byfors S, Struelens MJ, Monnet DL, Albiger B, Kohlenberg A, European Antimicrobial Resistance Genes Surveillance Network EURGen-Net Capacity Survey Group Worsening epidemiological situation of carbapenemase-producing Enterobacteriaceae in Europe, assessment by national experts from 37 countries, July 2018. Euro Surveill. 2019;24(9):1900123. doi: 10.2807/1560-7917.ES.2019.24.9.1900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David S, Reuter S, Harris SR, Glasner C, Feltwell T, Argimon S, Abudahab K, Goater R, Giani T, Errico G, et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol. 2019;4(11):1919–1929. doi: 10.1038/s41564-019-0492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassetti M, Peghin M, Pecori D. The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2016;29(6):583–594. doi: 10.1097/QCO.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi K, Cho SY, Ng AC, Usawachintachit M, Tan YK, Deng YL, Shen CH, Gyawali P, Alenezi H, Basiri A, et al. The urological Association of Asia clinical guideline for urinary stone disease. Int J Urol. 2019;26(7):688–709. doi: 10.1111/iju.13957. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez J, Smith A, Geavlete P, Shah H, Kural AR, de Sio M, Amón Sesmero JH, Hoznek A, de la Rosette J. CROES PCNL study group. Urinary tract infections and post-operative fever in percutaneous nephrolithotomy. World J Urol. 2013;31(5):1135–1140. doi: 10.1007/s00345-012-0836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagenlehner FM, Weidner W, Naber KG. Optimal management of urosepsis from the urological perspective. Int J Antimicrob Agents. 2007;30(5):390–397. doi: 10.1016/j.ijantimicag.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Marx G, Reinhart K. Urosepsis: from the intensive care viewpoint. Int J Antimicrob Agents. 2008;31(Suppl 1):S79–S84. doi: 10.1016/j.ijantimicag.2007.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
In order to protect patient privacy, the datasets used and analyzed are not publicly available, but they are available from the corresponding author on reasonable request.



