Abstract
Immune responses are controlled by a combination of positive and negative cellular signals. Effector cells in the immune system express inhibitory receptors that serve to limit effector cell expansion and to protect the host from autoreactivity. gp49B is a receptor of unknown function that is expressed on activated mast cells and natural killer (NK) cells and whose cytoplasmic tail endows it with inhibitory potential. To gain insight into the function of gp49B in mice, we disrupted the gp49B gene by homologous recombination. gp49B0 mice were born at expected ratios, were healthy and fertile, and displayed normal long-term survival rates. gp49B0 mice showed no defect in NK or mast cell development. Furthermore, NK and mast cells from the gp49B0 mice showed activation properties in vitro similar to those of cells isolated from wild-type mice. Therefore, gp49B is not critical for the development, expansion, and maturation of mast cells and NK cells in vivo. The healthy status of gp49B0 mice makes them suitable for testing the role of gp49B in immune responses to infectious agents.
The regulation of target cell lysis by natural killer (NK) cells is provided in part by the expression of inhibitory receptors capable of blocking activation signals (9). In the mouse, NK cell activation is inhibited by the lectin-like receptors Ly49 and the CD94-NKG2 complex, which recognize class I molecules of the major histocompatibility complex (MHC), thereby protecting normal cells from NK lysis. In contrast, human NK cells use two types of MHC class I-specific inhibitory receptors, the lectin-like CD94-NKG2 and the killer cell immunoglobulin-like receptors (KIR). The closest relative of KIR in the mouse is a molecule called gp49 with two immunoglobulin (Ig) domains that share 33% sequence identity with those of KIR (1, 15). gp49 is expressed on a variety of cell types, including mast cells, NK cells, and 60% of bone marrow cells that also express the myeloid markers Mac1 and Gr-1 (1, 7, 13, 16, 17). Using a new monoclonal antibody (MAb) specific for gp49, Wang et al. showed that gp49 expression is induced on NK cells upon activation (16).
Two closely linked genes on mouse chromosome 10 encode the related proteins gp49A and gp49B (reference 8 and our unpublished results). These two receptors are almost identical in their extracellular regions but differ in their cytoplasmic domains (1). The cytoplasmic tail of gp49B contains two immunoreceptor tyrosine-based inhibition motifs that can deliver an inhibitory signal in mast and NK cells (7, 11, 13, 16). gp49A has a short cytoplasmic tail without any immunoreceptor tyrosine-based inhibition motifs and lacks the characteristic charged amino acid in the transmembrane region which is present in the activating forms of the KIR, CD94-NKG2, and Ly49 molecules (9). The ligand(s) for gp49A and gp49B remains unknown.
In an effort to determine the role of the potentially inhibitory gp49B receptor, we generated mice deficient in gp49B expression (gp49B0 mice).
MATERIALS AND METHODS
Cell lines and reagents.
Mouse NK cell populations and bone marrow-derived mast cells (BMMC) were generated as described previously (2, 3, 6). The human B-cell line 721.221 (14) was maintained as described previously (15). The anti-HLA-DR MAb L243 (American Type Culture Collection) used in cytotoxicity assays was used as purified IgG.
Generation of gp49B0 mice.
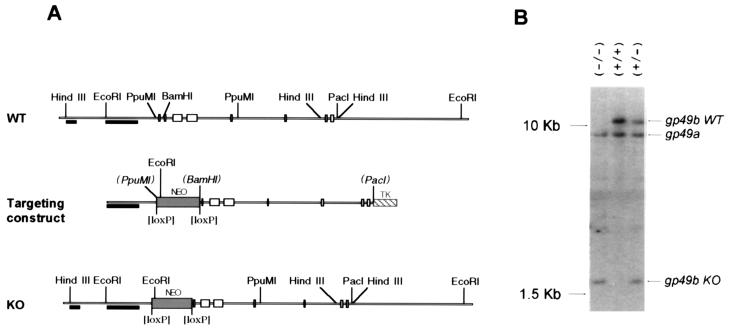
The strategy depicted in Fig. 1 was used to generate the targeting construct. A fragment of 220 bp containing the first exon of gp49B was replaced by the neo gene flanked by two loxP sites. The fragments between the EcoRI and PpuMI sites (1.5 kb) and between the BamHI and PacI sites (5.2 kb) were extracted from a bacterial artificial chromosome (BAC) genomic clone containing the gp49A and gp49B genes. These fragments were independently blunt-end cloned into the pSP72 vector. The neo gene from the pL2neo2 plasmid (a kind gift from Hua Gu, Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, National Institutes of Health) was cloned 3′ to the short fragment of gp49B, and the tk gene from the pHSV-tk plasmid (12) was cloned 3′ to the long fragment of gp49B. Finally, the short fragment of gp49B and the neo gene were cloned 5′ to the long gp49B fragment. Thirty micrograms of the targeting vector was linearized with SalI and electroporated into E14.1 embryonic stem (ES) cells. Clones resistant to G418 and ganciclovir were screened for homologous recombination by Southern blot hybridization after digestion of the DNA with HindIII and BamHI. The probe in this case was outside the targeting construct just 3′ of the first HindIII site, and in homologous recombination-positive ES cells a 2.5-kb band shifted to 9 kb, as expected. To exclude the possibility that the targeting construct inactivated the gp49A gene in addition to the gp49B gene, positive clones were further screened by Southern blotting after digestion with PacI and RsrII. Targeting of the gp49A gene would have resulted in a shift of a 23-kb band to 18 kb; no ES cells containing this targeting of gp49A were detected. Selected ES clones were injected into blastocysts to obtain chimeric mice. gp49B0 mice were identified by Southern blot hybridization after digestion of tail DNA with EcoRI. Mice used in these experiments were (C57BL/6 × O1a)F1.
FIG. 1.
Disruption of the gp49B gene. (A) The gp49B targeting construct was made by replacing the first exon between the PpuMI and BamHI sites with a neo gene flanked by loxP sites. Sites in parentheses were lost during construction of the targeting vector. The probe outside the targeting construct (short filled bar) was used to screen ES cell transfectants for homologous recombination, and a larger probe inside the construct (long filled bar) was used for screening mouse tail DNA. (B) Southern blot analysis of tail DNA from wild-type (+/+), gp49B+/− (+/−), and gp49B0 (−/−) mice. Insertion of the neo targeting construct into one gp49B allele results in the addition of an EcoRI site detected by the appearance of a 1.9-kb band and the loss of the 11-kb band. WT, wild type; KO, knockout.
Preparation of gp49-specific antisera.
An antiserum reactive with the Ig domains of gp49B was generated by immunizing rabbits with a gp49B-Ig fusion protein containing the extracellular portion of gp49B. This antiserum has been shown to react with both gp49A and gp49B (data not shown). Antisera specific for the gp49A or the gp49B cytoplasmic tail were generated by immunization with keyhole limpet hemocyanin-conjugated peptides CPRELSTPR and CIRTQEQNNS, which correspond to the unique C-terminal regions of gp49A and gp49B, respectively. The specificities of the C-terminal antisera were tested by Western blot analysis of lysates from 721.221 cells infected with recombinant vaccinia viruses encoding the cDNAs for either gp49B or a chimera of the cytoplasmic tail of gp49A linked to the extracellular portion of gp49B.
Flow cytometry.
All antibodies were obtained from PharMingen except the anti-gp49 antiserum described above and the fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit MAb (Jackson Immunoresearch). Samples were analyzed on a Becton Dickinson FACScan.
Immunoprecipitations, SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotting.
For the analysis of both NK cells and BMMC, 30 × 106 cells were surface biotinylated using ImmunoPure NHS-SS-Biotin (Pierce) and lysed in ice-cold lysis buffer (1% Brij 96, 20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride) for 20 min on ice. Lysates were cleared by centrifugation (21,000 × g, 4°C, 15 min). Cleared lysates were incubated with streptavidin-agarose beads (Upstate Biotechnology) for 2 h at 4°C. Beads were washed three times with ice-cold lysis buffer and boiled in reducing sodium dodecyl sulfate (SDS) sample buffer. Samples were then separated on a 10% polyacrylamide–Tris-glycine gel (Novex) and transferred to a polyvinylidene difluoride membrane (Immobilon P; Millipore). The membranes were blocked with 5% bovine serum albumin in TPBS (0.05% Tween 20 in phosphate-buffered saline) for 1 h at room temperature and then incubated for a second hour with rabbit antiserum specific for either gp49A or gp49B. Membranes were washed and incubated with peroxidase-coupled goat anti-rabbit antibody (Amersham) and, after a final washing, developed using SuperSignal West Pico (Pierce).
NK cell killing assays.
Target cells were grown to mid-log phase and 5 × 105 cells were labeled in 100 μl of CTL medium (Iscove's medium supplemented with 10% fetal calf serum, l-glutamine, and penicillin-streptomycin) with 100 μCi of 51Cr for 1 h at 37°C. Cells were washed twice in CTL medium and resuspended at 5 × 104 cells/ml in CTL medium. A total of 2,500 target cells were used in each sample well. Effector cells were resuspended in CTL medium supplemented with 100 U of recombinant interleukin-2 (IL-2) per ml, and in the case of the antibody-dependent cellular cytotoxicity experiment, 721.221 cells were preincubated with the anti-HLA-DR MAb L243 for 15 min at 25°C. Effector cells were mixed with labeled target cells in a V-bottom 96-well plate. Maximum release was determined by incubation in 1% Triton X-100. For spontaneous release, targets were incubated without effectors in CTL medium alone. All samples were done in triplicate. After a 1-min centrifugation at 200 × g, plates were incubated for 3 h at 37°C. Supernatant was harvested and 51Cr release was measured in a gamma counter. The percentage of specific release was calculated as follows: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Mast cell analysis.
Flow cytometric analysis of mast cells was performed on 3 × 105 BMMC as follows. All incubation steps were done on ice for 30 min in 100 μl of phosphate-buffered saline with 0.1% bovine serum albumin and 0.05% sodium azide. Unless specified, all antibody final concentrations were 10 μg/ml. FcɛRI expression was determined with biotinylated rat IgE (IR162). After washing, biotinylated rat IgE was revealed with streptavidin-phycoerythrin (diluted 1:2,500). Mast cell identity was confirmed using FITC-labeled anti-c-kit (PharMingen). Cellular degranulation was measured by the release of β-hexoseaminidase as described before (5), with minor modifications. Cells were incubated overnight with 500 ng of mouse anti-2,4-dinitrophenol (anti-DNP) IgE (Sigma) per ml per 106 cells, washed twice, and stimulated with DNP-albumin (Sigma) at various doses for 20 min.
RESULTS AND DISCUSSION
Generation of gp49B0 mice.
The gp49B gene in the ES cell line E14.1 was targeted with a construct in which a 220-bp fragment containing the first exon of gp49B was replaced by a neomycin (neo) gene flanked by two loxP sites (Fig. 1A). ES cells carrying this construct were isolated by double drug selection (with G418 and ganciclovir) and screened for homologous recombination by Southern blot analysis (see Materials and Methods). Two different restriction digests were performed to ensure that the construct inserted into the homologous region of the gp49B gene and that the neighboring gp49A gene remained intact (data not shown; see Materials and Methods). Selected ES clones were injected into blastocysts to obtain chimeric mice. gp49B mice were identified by Southern blot hybridization after digestion with EcoRI (Fig. 1B).
Surface expression of gp49 in wild-type and gp49B0 mice.
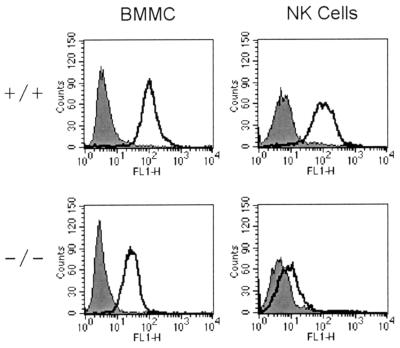
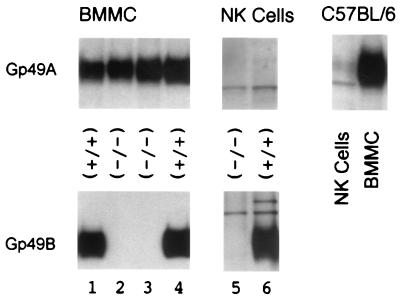
Expression of gp49 at the cell surface was measured by flow cytometry after binding of an antiserum raised against the extracellular domain of gp49B. This antiserum reacts with both gp49A and gp49B (C. Stebbins, unpublished data). BMMC and NK cells from gp49B0 mice show a reduction in surface expression of gp49 to 26% of the level in wild-type BMMC and 8% of the level in wild-type NK cells (Fig. 2). To test whether residual gp49 expression was due to gp49A, antisera specific for the unique sequences in the cytoplasmic tails of gp49A and gp49B were generated. Cell surface proteins were biotinylated, isolated with streptavidin-agarose, separated by SDS-PAGE, and transferred onto nitrocellulose. Membranes were probed with the specific anti-gp49A or anti-gp49B antisera. Figure 3 shows that BMMC and NK cells from gp49B0 mice lack gp49B and that, in agreement with cell surface staining (Fig. 2), NK cells express much less gp49A than BMMC do. Inactivation of the gp49B gene did not affect expression of gp49A (Fig. 3). To confirm this observation, gp49A expression was compared in NK cells and BMMC isolated from a C57BL/6 mouse. Direct comparison in Fig. 3 shows that NK cells express only a small fraction of the amount of gp49A expressed by BMMC. These results suggest that NK cells express predominantly gp49B and only a small amount of gp49A. A comparison of gp49A and gp49B expression in NK cells by reverse transcriptase PCR had not revealed this difference (16).
FIG. 2.
gp49 surface expression on BMMC and NK cells from wild-type (+/+) and gp49B0 (−/−) mice. BMMC and NK cells were stained with a rabbit polyclonal anti-gp49 antiserum that reacts with both gp49A and gp49B. FL1-H, green fluorescence.
FIG. 3.
Biochemical analysis of gp49 expression. Cell surface proteins were biotinylated and precipitated with streptavidin-agarose, separated by SDS-PAGE, blotted onto nitrocellulose, and probed with antiserum specific for gp49A or gp49B, as indicated. BMMC and NK cells from wild-type (+/+) and gp49B0 (−/−) mice were analyzed. Included is a comparison of the level of gp49A expression in BMMC and NK cells from a C57BL/6 mouse.
Development of NK and mast cells in gp49B0 mice appears to be normal.
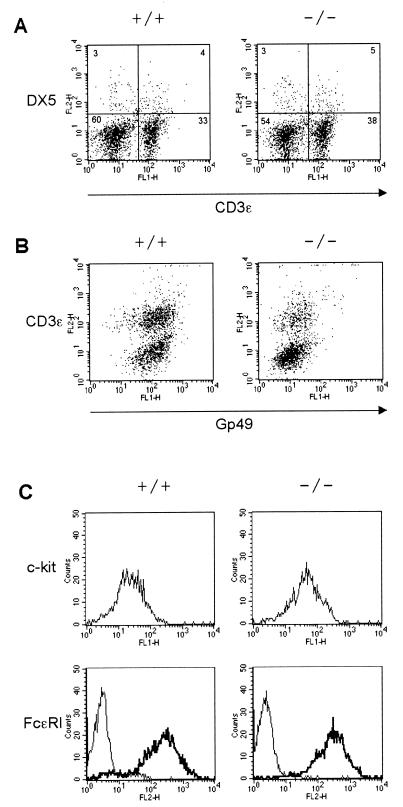
The inhibitory potential of gp49B suggests that this receptor may regulate NK or mast cell activation (7, 13). A phenotypic analysis of NK cells and BMMC was carried out in the gp49B0 mice to evaluate whether gp49B regulates the development of these two cell types. Splenocytes from gp49B0 mice were analyzed by double staining with CD3ɛ and DX5, a MAb specific for a pan-NK cell marker (Fig. 4A). Wild-type and gp49B0 splenocytes contained similar proportions of DX5+ cells (3%) and of CD3+ and CD3− cells. No significant difference was detected in the number of total splenocytes isolated from wild-type and gp49B0 mice (data not shown). Further analysis by flow cytometry of both fresh and IL-2-expanded NK cells revealed no difference in the expression of several other cell surface proteins, including Ly49A, Ly49C/I, c-kit, CD16, LFA-1α, and ICAM-1 (data not shown). Of interest, isolation and expansion of DX5+ splenocytes in IL-2 often yielded a population of CD3+ NK1.1+ cells. These results show that gp49 can also be expressed on this T-cell subset (Fig. 4B).
FIG. 4.
Analysis of NK and mast cells in wild-type and gp49B0 mice. (A) Unstimulated splenocytes prepared from wild-type (+/+) and gp49B0 (−/−) mice double-stained with FITC-conjugated anti-CD3ɛ and phycoerythrin (PE)-conjugated DX5. (B) IL-2-expanded DX5+ NK1.1+ cell populations from wild-type and gp49B0 mice double-stained with PE-conjugated anti-CD3ɛ and anti-gp49 antiserum followed by goat anti-rabbit IgG-FITC. (C) Expression of c-kit and FcɛRI in cultured BMMC from wild-type and gp49B0 mice. FcɛRI expression was determined by binding of biotinylated IgE followed by streptavidin-PE (thick line). The control for this experiment was streptavidin-PE alone (thin line). FL1-H, green fluorescence; FL2-H, red fluorescence.
Analysis of BMMC from wild-type and gp49B0 mice revealed no difference in expression of the cell surface marker c-kit (Fig. 4C). BMMC from both mice were equally able to bind IgE immune complexes (Fig. 4C). Histological analysis of several tissues rich in mast cells (ear, skin, tongue, and stomach) revealed no difference between the wild-type and gp49B0 mice in the number of mast cells resident in these tissues (data not shown).
NK and mast cells from gp49B0 mice are functionally normal.
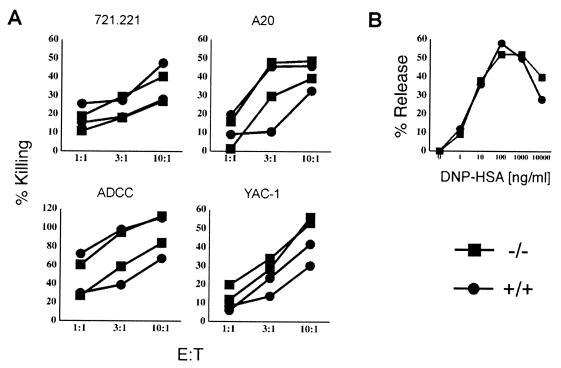
We analyzed the ability of NK cells to kill target cells and the ability of BMMC to degranulate upon FcɛRI cross-linking. Since gp49B can function as an inhibitory receptor, it was possible that NK cells from the gp49B0 mice would exhibit an increase in their ability to kill certain targets. On the other hand, it was also possible that gp49B0 mice would compensate for the loss of an inhibitory receptor by downmodulating their activation signals. Such adaptation has been reported for NK cells from mice deficient in MHC class I (4, 10). Two mouse cell lines, A20 and YAC-1, and the human cell line 721.221 were tested for their sensitivity to NK-mediated lysis. In addition, 721.221 cells coated with the mouse IgG MAb L243 were tested for sensitivity to antibody-dependent cellular cytotoxicity. Human cells are not expected to express a ligand for gp49 and are therefore suitable targets to evaluate the basal level of mouse NK cell activity. Figure 5A shows no detectable difference in the killing of target cells beyond those differences attributable to intermouse variability. Subtle changes in the lytic activity of NK cells from gp49B0 mice would have been difficult to detect due to this variability. The extent of degranulation by BMMC upon FcɛRI cross-linking was identical in the two strains of mice (Fig. 5B).
FIG. 5.
Functional analysis of NK and mast cells. (A) Killing of different target cells by NK cells. IL-2-expanded NK cells from wild-type (●) and gp49B0 (■) mice were used in a standard cytotoxicity assay at the indicated effector-to-target (E:T) ratios. The results are representative of several experiments that included at least four mice of each type. (B) FcɛRI-induced degranulation as measured by β-hexoseaminidase release. BMMC from wild-type and gp49B0 mice were incubated with 500 ng of DNP-specific mouse IgE per ml per 106 cells and stimulated with DNP-human serum albumin (DNP-HSA).
Conclusion.
gp49B0 mice developed normally, without any obvious phenotypic differences from wild-type mice. Therefore, gp49B expression is not essential for survival of mice in a clean environment. Analysis of activated NK and mast cells isolated from gp49B0 mice revealed that these cells were phenotypically and functionally similar to the same cells derived from wild-type mice. We conclude that gp49B is not required for the development or the in vitro activity of NK and mast cells. It is possible that gp49B0 mice have compensated for the lack of this receptor and that the in vivo function of gp49B is not readily observed in such mice. Alternatively, the main function of gp49B on NK and mast cells may be to control immune responses during certain infections or allergic reactions. Backcrossing of the gp49B0 mice to a congenic strain is required to test this possibility.
ACKNOWLEDGMENTS
S. Rojo and C. Stebbins contributed equally to this work.
Generation of gene-targeted ES cells and production of chimeric mice were performed by Ren-ju Hu in the laboratory of Hua Gu (Laboratory of Immunology, NIAID, NIH).
REFERENCES
- 1.Castells M C, Wu X, Arm J P, Austen K F, Katz H R. Cloning of the gp49B gene of the immunoglobulin superfamily and demonstration that one of its two products is an early-expressed mast cell surface protein originally described as gp49. J Biol Chem. 1994;269:8393–8401. [PubMed] [Google Scholar]
- 2.Dombrowicz D, Brini A T, Flamand V, Hicks E, Snouwaert J N, Kinet J P, Koller B H. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157:1645–1651. [PubMed] [Google Scholar]
- 3.Dombrowicz D, Flamand V, Brigman K K, Koller B H, Kinet J P. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 4.Dorfman J R, Zerrahn J, Coles M C, Raulet D H. The basis for self-tolerance of natural killer cells in beta2-microglobulin− and TAP-1− mice. J Immunol. 1997;159:5219–5225. [PubMed] [Google Scholar]
- 5.Hirasawa N, Scharenberg A, Yamamura H, Beaven M A, Kinet J P. A requirement for Syk in the activation of the microtubule-associated protein kinase/phospholipase A2 pathway by Fc epsilon R1 is not shared by a G protein-coupled receptor. J Biol Chem. 1995;270:10960–10967. doi: 10.1074/jbc.270.18.10960. [DOI] [PubMed] [Google Scholar]
- 6.Karlhofer F M, Yokoyama W M. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1.1 antigen. IL-2-activated NK cells possess additional specific stimulation pathways. J Immunol. 1991;146:3662–3673. [PubMed] [Google Scholar]
- 7.Katz H R, Austen K F. A newly recognized pathway for the negative regulation of mast cell-dependent hypersensitivity and inflammation mediated by an endogenous cell surface receptor of the gp49 family. J Immunol. 1997;158:5065–5070. [PubMed] [Google Scholar]
- 8.Kuroiwa A, Yamashita Y, Inui M, Yuasa T, Ono M, Nagabukuro A, Matsuda Y, Takai T. Association of tyrosine phosphatases SHP-1 and SHP-2, inositol 5-phosphatase SHIP with gp49B1, and chromosomal assignment of the gene. J Biol Chem. 1998;273:1070–1074. doi: 10.1074/jbc.273.2.1070. [DOI] [PubMed] [Google Scholar]
- 9.Lanier L L. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 10.Ljunggren H-G, Van Kaer L, Ploegh H L, Tonegawa S. Altered natural killer cell repertoire in Tap-1 mutant mice. Proc Natl Acad Sci USA. 1994;91:6520–6524. doi: 10.1073/pnas.91.14.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu-Kuo J M, Joyal D M, Austen K F, Katz H R. gp49B1 inhibits IgE-initiated mast cell activation through both immunoreceptor tyrosine-based inhibitory motifs, recruitment of src homology 2 domain-containing phosphatase-1, and suppression of early and late calcium mobilization. J Biol Chem. 1999;274:5791–5796. doi: 10.1074/jbc.274.9.5791. [DOI] [PubMed] [Google Scholar]
- 12.Mansour S L, Thomas K R, Capecchi M R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 13.Rojo S, Burshtyn D N, Long E O, Wagtmann N. Type I transmembrane receptor with inhibitory function in mouse mast cells and NK cells. J Immunol. 1997;158:9–12. [PubMed] [Google Scholar]
- 14.Shimizu Y, DeMars R. Production of human cells expressing individual transferred HLA-A-,-B,-C genes using an HLA-A,-B,-C null human cell line. J Immunol. 1989;142:3320–3328. [PubMed] [Google Scholar]
- 15.Wagtmann N, Rajagopalan S, Winter C C, Peruzzi M, Long E O. Killer cell inhibitory receptors specific for HLA-C and HLA-B identified by direct binding and by functional transfer. Immunity. 1995;3:801–809. doi: 10.1016/1074-7613(95)90069-1. [DOI] [PubMed] [Google Scholar]
- 16.Wang L L, Chu D T, Dokun A O, Yokoyama W M. Inducible expression of the gp49B inhibitory receptor on NK cells. J Immunol. 2000;164:5215–5220. doi: 10.4049/jimmunol.164.10.5215. [DOI] [PubMed] [Google Scholar]
- 17.Wang L L, Mehta I K, LeBlanc P A, Yokoyama W M. Mouse natural killer cells express gp49B1, a structural homologue of human killer inhibitory receptors. J Immunol. 1997;158:13–17. [PubMed] [Google Scholar]