Abstract
Gastropod molluscs such as nudibranchs are important members of deep-sea benthic ecosystems. However, data on the trophic ecology and feeding specialization of these animals are limited to date. The method of fatty acid trophic markers (FATM) was applied to determine the dietary preferences of nudibranchs off the Kuril Islands. Fatty acid (FA) compositions of Dendronotus sp., Tritonia tetraquetra, and Colga pacifica collected from deep waters were analyzed and compared with those of Aeolidia papillosa and Coryphella verrucosa from the offshore zone. The high level of FATM such as 22:5n-6 and C20 monounsaturated FAs indicated that Dendronotus sp. preys on sea anemones and/or anthoathecates hydroids similarly to that of shallow-water species A. papillosa and C. verrucosa. The high percentage of tetracosapolyenoic acids and the ratio 24:6n-3/24:5n-6 indicated that T. tetraquetra preys on soft corals such as Gersemia and/or Acanella at a depth of 250 m, but soft corals of the family Primnoidae may be the main item in the diet of T. tetraquetra at a depth of 500 m. The high content of Δ 7,13-22:2 and 22:6n-3 shows that C. pacifica can feed on bryozoans. In C. pacifica, 22:5n-6 may be synthesized intrinsically by the mollusks, whereas odd-chain and branched saturated FAs originate from associated bacteria.
Keywords: Nudibranchia, Food webs, Cold-water species, Dietary fatty acids, Lipids
Introduction
Nudibranchs are a group of marine soft-bodied gastropod mollusks (Gastropoda: Nudibranchia Cuvier, 1817). The greatest diversity of nudibranchs is observed in warm shallow waters, although nudibranchs occur worldwide, from Arctic to Antarctic regions, with some species discovered at a depth near 2,500 m (Ekimova et al., 2015; Bertsch, 2020). Identification of food sources of nudibranchs is important for understanding their ecology and description of trophic interactions in marine benthic ecosystems (Ekimova et al., 2019b). Nudibranchs are mostly carnivorous, but detritus and microalgae may comprise some part of their diet (Ekimova, Deart & Schepetov, 2019a; Ekimova et al., 2019b). Nudibranch can feed on soft corals, reef-building corals, sponges, bryozoans, tunicates, barnacles, sea anemones, jellyfish, ophiuroids, colonial hydroids, and other nudibranchs (Barnes & Bullough, 1996; McDonald & Nybakken, 1997; McDonald & Nybakken, 1999; Goodheart et al., 2017). Many nudibranch species exhibit high dietary specialization (Hoover et al., 2012; Goodheart et al., 2017; Ekimova et al., 2019b; Imbs & Grigorchuk, 2019; Mikhlina et al., 2018; Mikhlina, Ekimova & Vortsepneva, 2020). In contrast to shallow-water species, data on feeding regimes of deep-sea nudibranch species still remain limited (Chimienti et al., 2020).
Fatty acids (FAs) have been used as biochemical markers to trace predator–prey relationships in marine ecosystems for more than 40 years (Budge, Iverson & Koopman, 2006; Kelly & Scheibling, 2012; Braeckman et al., 2015; Calado & Leal, 2015). The method of FA trophic markers (FATM) was already successfully applied to determine possible origins of food in several nudibranch species from tropical shallow waters (Zhukova, 2014) and the deep-sea nudibranchs Tritonia tetraquetra (Pallas, 1788), Dendronotus sp., and D. robustus A.E. Verril, 1870 collected in the Kurile Islands region (Imbs, 2016; Imbs & Chernyshev, 2019; Imbs & Grigorchuk, 2019). FATM showed that Dendronotus sp. and T. tetraquetra prey on different species of cold-water soft corals, while D. robustus may consume hydrocorals and bryozoans (Imbs & Grigorchuk, 2019). The difference in food sources between these two species belonging to the same genus (Dendronotus) and inhabiting the same waters was detected by using FATM. The detection of large amounts of dietary FAs in T. tetraquetra (Imbs, 2016; Imbs & Chernyshev, 2019) showed that the FATM method could be successfully apply for the study of trophic ecology of cold-water nudibranchs.
Waters around the Kuril Islands, with their significant depth differences, are one of the world’s most productive marine ecosystem (Shuntov, Ivanov & Dulepova, 2019). Nudibranchs are a common animal group of this area and, therefore, play an important role on trophic dynamics in the ecosystem studied. To expand our knowledge on trophic ecology of deep-sea mollusks, FA composition of total lipids of three nudibranch species (Colga pacifica (Bergh, 1894), Tritonia tetraquetra, and Dendronotus sp.) collected from deep waters (up to 500 m) were analyzed and compared with those of two nudibranch species (Aeolidia papillosa (Linnaeus, 1761) and Coryphella verrucosa (M. Sars, 1829)) from the offshore zone (about 20 m) of the Kurile Islands. Dietary preferences of these five species were studied using the method of FATM. A possible influence of depth on nudibranch feeding specialization was discussed.
Materials and Methods
Sample collection
Sampling was conducted aboard the R/V Akademik Oparin near Simushir Island (Kuril Islands, Sea of Okhotsk, 47°08′N, 152°14′E) in July 2019. In total, 18 nudibranchs were sampled. Three specimens of C. verrucosa and 2 specimens of A. papillosa were collected at a depth of 20 m by SCUBA and referred as a shallow-water group. Three specimens of C. pacifica, 3 specimens of Dendronotus sp., and 5 specimens of T. tetraquetra were collected at the depth of 250–500 m by dredging and referred as a deep-sea group. The nudibranch Dendronotus sp. was different from the one already reported (Imbs & Grigorchuk, 2019). Each specimen sampled was photographed (Fig. 1); one piece of foot tissue was fixed in 96% EtOH for molecular analysis to confirm the identification of nudibranchs according to Ekimova et al. (2019b); another piece of foot tissue was frozen at −80 °C for lipid analysis. Unfortunately, two frozen samples (one for A. papillosa and one for T. tetraquetra) were lost in transit.
Figure 1. External views of studied nudibranchs.
(A and B) Aeolidia papillosa (dorsal and ventral view, respectively); (C and D) Colga pacifica (dorsal and lateral view, respectively); (E), Coryphella verrucosa; (F) Dendronotus sp.; (G and H) Tritonia tetraquetra (dorsal and ventral view, respectively). Scale bar 10 mm. Photos by A. Maiorova.
Morphological analysis
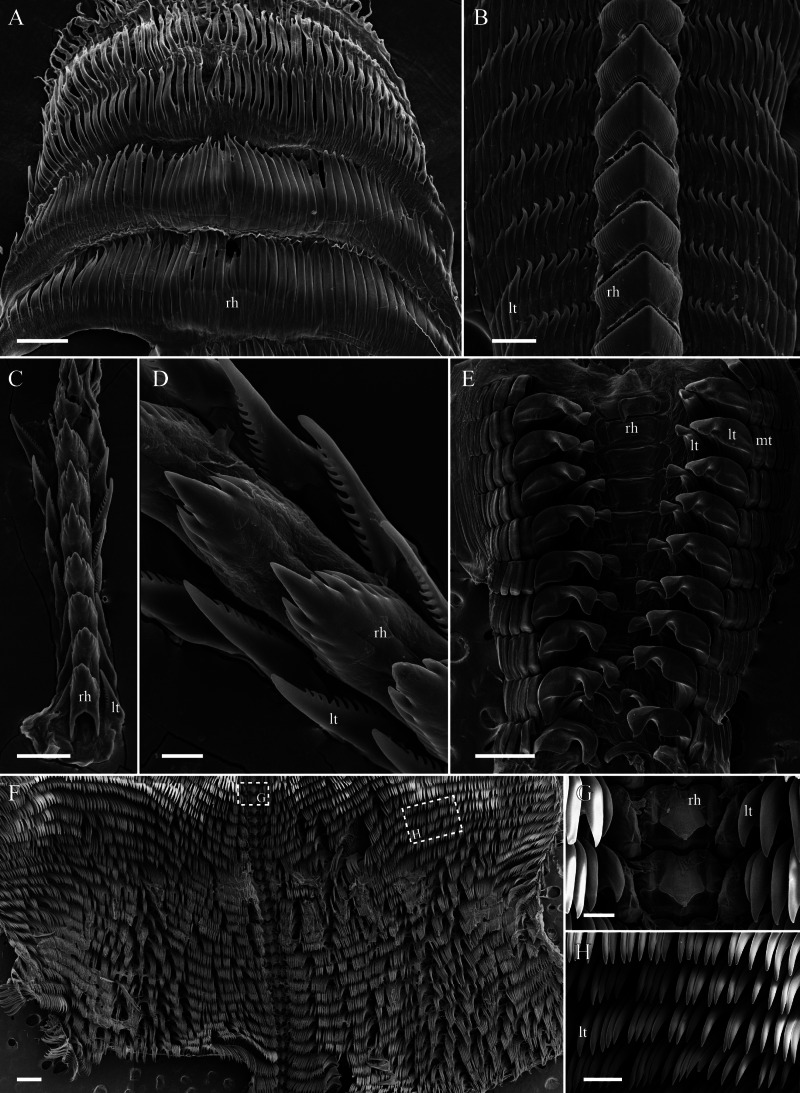
To study the radular morphology of each species, the buccal mass was extracted and soaked in proteinase K solution for 2 h at 60 °C. When connective and muscle tissues were dissolved, samples were rinsed in distilled water, air-dried, mounted on an aluminium stub, and sputter-coated with gold for visualization under a JEOL JSM 6380 scanning electron microscope. The radular morphology of each species is shown in Fig. 2.
Figure 2. Radular morphology of studied nudibranch species.
(A) Aeolidia papillosa, posterior portion of uniserial radula. (B) Dendronotus sp., middle portion of polycerial radula. (C) Coryphella verrucosa, triserial radula. (D) Coryphella verrucosa, rachidian and lateral teeth. (E) Colga pacifica. (F) Tritonia tetraquetra, posterior portion of polyserial radula. (G) Tritonia tetraquetra, rachidian teeth and innermost lateral teeth. (H) Tritonia tetraquetra, middle lateral teeth. Abbreviations: rh, rachidian tooth; lt, lateral tooth; mt, marginal teeth. Scale bars: A–C, G – 100 µm; D – 30 µm; E, F – 500 µm; H – 200 µm.
Lipid preparation and fatty acid analysis
Lipids were extracted from the specimens as described by Bligh & Dyer (1959). FA methyl ethers (FAME) were prepared using the method of Carreau & Dubacq (1978) and were purified by preparative thin-layer chromatography in benzene. The 4,4-dimethyloxazoline (DMOX) derivatives of FA were prepared according to the method of Svetashev (2011). A gas chromatography analysis of FAME was conducted with a GC-2010 chromatograph (Shimadzu, Kyoto, Japan) with a flame ionization detector. A Supelcowax 10 (Supelco, Bellefonte, USA) capillary column (30 m × 0.25 mm ID, film thickness 25 µm) was held for 2 min at 170 °C, then heated with a 2 °C min−1 ramp to 240 °C that was held for 5 min. A sample volume of 1 µL (about 1 mg mL−1) was injected. The injector (250 °C) and detector (260 °C) temperatures were constant. Helium was used as the carrier gas at a linear velocity of 30 cm s−1. FAME peaks were analyzed by comparing their retention time with those of the standards (a mixture of PUFA methyl esters No. 3 from menhaden oil, Sigma-Aldrich Co., USA). The concentrations of individual FAs were calculated from the integrated area (% of total FAs). Identification of FAs was confirmed by gas chromatography–mass spectrometry (GC–MS) of their methyl esters and DMOX derivatives on a GCMS-2010 Ultra instrument (Shimadzu, Kyoto, Japan) (electron impact at 70 eV) and a MDN-5s (Supelco, Bellefonte, USA) capillary column (30 m × 0.25 mm ID). Carrier gas was He at 30 cm s−1. The G–MS analysis of FAME was performed at 160 °C with a 2 °C min−1 ramp to 240 °C that was held for 20 min. The injector and detector temperatures were 250 °C. GC–MS of DMOX derivatives was performed at 210 °C with a 3 °C min−1 ramp to 270 °C that was held for 40 min. The injector and detector temperatures were 270 °C. Spectra were compared with the NIST library and the online FA mass spectra archive website (Christie, 2021).
Statistical analysis
Differences in FA composition (only for species with 3 replicates) were investigated using PERMANOVA (Anderson, Gorley & Clarke, 2008; Clarke & Gorley, 2015). The PERMANOVA analysis was based on Bray–Curtis similarity matrices, using 9,999 random permutations of raw data. After the PERMANOVA routines, pairwise Monte Carlo tests were performed between all pairs of species. PERMDISP routines was performed to test homogeneity of multivariate dispersions. A nMDS ordination plot was used to visualize the similarity relationship among individuals and groups of individuals. The FAs that characterized and discriminated these groups were identified by SIMPER. The tests mentioned above were carried out using Primer 7+ software (PRIMER-e, New Zealand). Significance of differences in mean contents of FA between the nudibranch species was tested by one-way analysis of variance (ANOVA). Raw data were used after being tested for the homogeneity of variances (Levene’s test) and normality of data distribution (Shapiro–Wilk test). Significant differences between levels were examined post hoc with Tukey–Kramer HSD multiple comparisons test. To represent differences between the nudibranch species, the variables (square roots of FA contents) were included in principal components analyses (PCA). These statistical analyses were performed using STATISTICA 5.1 (StatSoft, Inc., USA). Cluster analysis was performed using Ward’s method (Minimum variance method) and the pvclust() function in the pvclust package provides p-values for hierarchical clustering based on multiscale bootstrap resampling (Suzuki & Shimodaira, 2006) available in the R-Studio software (R-Tools Technology, Canada). A statistical probability of p < 0.05 was considered significant. Values are represented as mean ± standard deviation.
Results
The full FA composition of total lipids in the five nudibranch species from different depths is summarized in Supplement Table S1. The average contents of the major 20 FAs are shown in Table 1. The main saturated FA (SFA) was 16:0, and the major monounsaturated FAs (MUFAs) were 20:1n-9 and 20:1n-7. Lipids of all nudibranchs contained branched and odd-chain SFAs; the highest levels of these acids were detected in some specimens of A. papillosa and C. pacifica (up to 11 and 17% of total FAs, respectively).
Table 1. Fatty acid composition (% of total FAs) of nudibranch mollusks.
The species were collected at different depths near Simushir Island (Kuril Islands, Sea of Okhotsk). SFAs, saturated FAs; MUFAs, monounsaturated FAs; PUFAs, polyunsaturated FAs, n-3/n-6, the n-6 PUFAs/ n-3 PUFAs ratio. Values are means ± SD; asterisks indicate significant differences (p < 0.05) between the groups of shallow-water species (C. verrucosa and A. papillosa) and deep-sea species (C. pacifica, T. tetraquetra, and Dendronotus sp).
| Fatty acids | Species names and sampling depths | Comparison of shallow-water and deep-sea groups by ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Shallow-water group | Deep-sea group | ||||||
|
Coryphella verrucosa, 0–20 m, n= 3 |
Aeolidia papillosa, 0–20 m, n= 2 |
Colga pacifica, 285–304 m, n= 3 |
Tritonia tetraquetra, 210–516 m, n= 5 |
Dendronotus sp., 210–516 m, n= 3 |
F 1,14 | p | |
| 14:0* | 4.2 ± 2.4 | 0.8 ± 0.0 | 1.3 ± 0.6 | 0.4 ± 0.2 | 0.9 ± 0.4 | 7.612 | 0.015 |
| 16:0 | 11.2 ± 2.9 | 7.7 ± 0.9 | 9.3 ± 1.3 | 14.7 ± 2.2 | 12.6 ± 1.7 | 2.456 | 0.139 |
| 16:1n-7* | 2.8 ± 1.0 | 0.7 ± 0.1 | 1.4 ± 0.2 | 0.6 ± 0.1 | 0.8 ± 0.2 | 7.436 | 0.016 |
| 18:0 | 2.7 ± 1.8 | 5.1 ± 0.0 | 3.7 ± 0.4 | 5.5 ± 1.0 | 8.2 ± 1.0 | 3.798 | 0.072 |
| 18:1n-9 | 3.5 ± 1.7 | 1.5 ± 0.1 | 1.3 ± 0.1 | 2.8 ± 0.6 | 2.5 ± 0.6 | 0.495 | 0.493 |
| 18:3n-3 | 1.1 ± 0.2 | 3.5 ± 0.6 | 3.5 ± 1.1 | 0.5 ± 0.1 | 0.8 ± 0.2 | 0.679 | 0.424 |
| 20:1n-11* | 2.8 ± 0.1 | 1.3 ± 0.0 | 1.1 ± 0.1 | 0.6 ± 0.1 | 1.1 ± 0.5 | 6.982 | 0.019 |
| 20:1n-9 | 9.4 ± 2.6 | 1.8 ± 0.1 | 1.9 ± 0.5 | 1.5 ± 0.1 | 4.0 ± 1.1 | 3.053 | 0.102 |
| 20:1n-7* | 5.9 ± 2.3 | 4.3 ± 0.0 | 1.9 ± 0.1 | 2.6 ± 0.2 | 3.9 ± 1.3 | 5.417 | 0.035 |
| Δ5,11-20:2 | 1.3 ± 0.9 | 2.5 ± 0.1 | 0.6 ± 0.2 | 1.6 ± 0.5 | 5.5 ± 0.3 | 0.389 | 0.543 |
| 20:4n-6* | 3.2 ± 0.9 | 3.8 ± 0.2 | 4.3 ± 3.0 | 15.1 ± 4.1 | 8.3 ± 2.5 | 6.290 | 0.025 |
| 20:5n-3* | 25.8 ± 12.4 | 15.5 ± 2.8 | 7.4 ± 3.1 | 13.7 ± 2.8 | 18.4 ± 0.5 | 4.647 | 0.049 |
| Δ7,13-22:2* | 0.6 ± 0.2 | 2.6 ± 0.4 | 10.8 ± 4.7 | 8.8 ± 2.1 | 3.2 ± 1.1 | 11.629 | 0.004 |
| Δ7,15-22:2 | 0.3 ± 0.2 | 1.8 ± 0.4 | 0.7 ± 0.1 | 2.5 ± 0.4 | 1.1 ± 0.4 | 1.249 | 0.282 |
| 22:4n-6* | 3.1 ± 1.8 | 4.1 ± 0.1 | 0.9 ± 0.5 | 0.6 ± 0.1 | 1.6 ± 1.5 | 7.726 | 0.015 |
| 22:5n-6 | 0.1 ± 0.1 | 3.5 ± 0.1 | 9.8 ± 5.9 | 0.6 ± 0.2 | 2.8 ± 1.0 | 0.504 | 0.489 |
| 22:5n-3* | 2.5 ± 1.1 | 8.5 ± 1.9 | 1.4 ± 0.6 | 0.9 ± 0.2 | 2.0 ± 0.5 | 11.292 | 0.005 |
| 22:6n-3 | 8.5 ± 2.1 | 8.3 ± 0.0 | 12.0 ± 3.8 | 0.6 ± 0.1 | 11.0 ± 0.7 | 0.274 | 0.609 |
| 24:5n-6 | 0.0 ± 0.0 | 0.4 ± 0.1 | 0.5 ± 0.4 | 4.8 ± 2.8 | 0.0 ± 0.0 | 2.548 | 0.133 |
| 24:6n-3 | 0.9 ± 0.1 | 0.4 ± 0.0 | 0.7 ± 0.5 | 12.9 ± 2.6 | 0.3 ± 0.1 | 2.933 | 0.109 |
| SFAs | 21.5 ± 2.2 | 22.5 ± 0.9 | 25.3 ± 2.8 | 23.8 ± 2.1 | 25.4 ± 2.1 | 5.951 | 0.029 |
| MUFAs | 27.1 ± 13 | 14.5 ± 0.9 | 12.3 ± 1.4 | 9.7 ± 0.5 | 15.8 ± 1.3 | 7.895 | 0.014 |
| PUFAs | 46.0 ± 16.0 | 44.3 ± 4.4 | 40.5 ± 5.1 | 51.7 ± 3.0 | 44.6 ± 1.5 | 0.104 | 0.752 |
| n − 3/n − 6 | 5.8 ± 1.7 | 2.9 ± 0.3 | 1.1 ± 0.6 | 1.4 ± 0.8 | 2.5 ± 0.3 | 18.998 | 0.001 |
Acids 20:4n-6, 20:5n-3, and 22:6n-3 dominated polyunsaturated FAs (PUFAs) of the nudibranchs studied except for T. tetraquetra. The lowest level of 22:6n-3 (HSD test, p = 0.0004) and considerable amounts (F4,11 = 22.2735, p < 0.0001) of very-long-chain tetracosapolyenoic acids (TPA), 24:5n-6 and 24:6n-3, were found in T. tetraquetra. The ratio 24:6n-3/24:5n-6 in Tritonia specimens from a depth of 450–516 m (7.0 ± 2.2) was higher than that in Tritonia specimens from a depth of 210–247 m (1.3 ± 0.1). The level of 20:5n-3 was significantly lower (HSD test, p = 0.012) in the deep-sea C. pacifica than that in the shallow-water C. verrucosa. Unusually high percentages of 22:5n-6 were detected in two specimens of C. pacifica (9.6 and 18.6% of total FAs). Individuals of A. papillosa contained the highest level of 22:5n-3 (up to 9.9% of total FAs). Several non-methylene-interrupted FAs (NMI FAs) were present in total FAs of all mollusk species. The highest level (HSD test, p = 0.0007) of Δ5,11-20:2 in T. tetraquetra specimens distinguished them from other nudibranchs. All species (except for C. verrucosa) contained noticeable amounts of Δ7,13-22:2.
The PERMANOVA results (Table 2) were corroborated by the nMDS plot (Fig. S1) and revealed significant differences (p < 0.001) between species. The pairwise comparison has shown significant (p < 0.05) differences for all pairs of species (Table 2), with the exception of the pair Dendronotus sp. and C. verrucosa. To detail the impact of each FA in similarity and dissimilarity among all nudibranch studied, the FA composition data were analyzed by SIMPER. Table S2 shows the first five FAs that contribute more than 7% in the similarity or dissimilarity. The high level of arachidonic acid (20:4n-6) in T. tetraquetra specimens distinguished them from other nudibranchs studied. The level of 20:4n-6 in Tritonia specimens from a depth of 450–516 m (10.4–13.6%) was lower than that in Tritonia specimens from a depth of 210–247 m (18.5–20.0%) (Table S1).
Table 2. Results of PERMANOVA pair-wise test of the fatty acid composition of nudibranch mollusks.
| Groups | t | p (MC) |
|---|---|---|
| CV, CP* | 2.1514 | 0.0296 |
| CV, TS* | 2.6979 | 0.0133 |
| CV, DS | 1.6093 | 0.1118 |
| CP, TS* | 3.4079 | 0.0086 |
| CP, DS* | 2.6239 | 0.0199 |
| TS, DS* | 4.1244 | 0.0024 |
Notes.
p (MC) are p values obtained by Monte-Carlo sampling. CV, C. verrucosa; CP, C. pacifica; DS, Dendronotus sp.; TT, T. tetraquetra. Asterisks indicates significant differences (p <0.05).
Analyses of the FA composition data by ANOVA identified certain FAs that were mainly responsible for the difference between species from deep and shallow waters (Table 1). Compared to the group of shallow-water species, deep-sea ones contained significantly higher (p < 0.05) levels of 20:4n-6 and Δ7,15-22:2, but significantly lower (p < 0.05) levels of 14:0, 16:1n-7, 20:1n-11, 20:1n-7, 20:5n-3, 22:4n-6, and 22:5n-3. No differences (p > 0.05) were found for other FAs listed in Table 1.
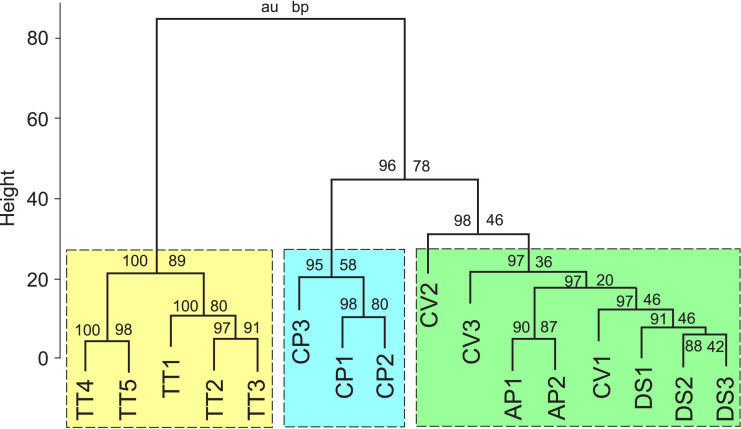
Results of a cluster analysis of the FA composition data (Table 1) for the five nudibranch species are shown in Fig. 3. All studied specimens were subdivided into three groups: the first and second groups consisted of deep-sea specimens of T. tetraquetra and C. pacifica, respectively, and the third group combined specimens of the deep-sea Dendronotus sp. with the shallow-water species A. papillosa and C. verrucosa.
Figure 3. Results of a cluster analysis of the FA composition data for the five nudibranch species.
The numerals on the branches represent are bootstrap probability (BP) value of a cluster and approximately unbiased (AU) probability values. TT, Tritonia tetraquetra; CP, Colga pacifica; CV, Coryphella verrucosa; DS, Dendronotus sp.; AP, Aeolidia papillosa.
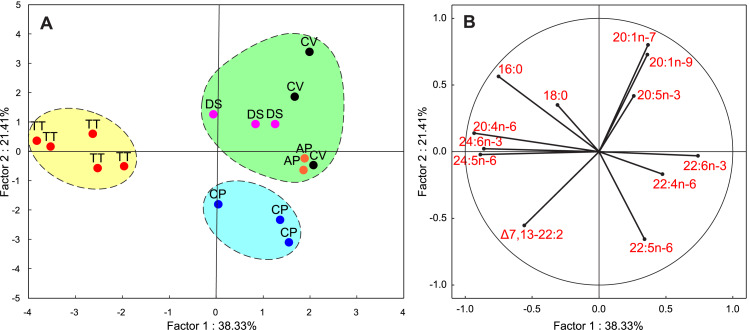
The FAs listed in Table 1 were used as variables for PCA. In this analysis, the first two PCA components explained 50% of the variance of the FA composition data. Figure 4A shows that T. tetraquetra is clearly separated from all other nudibranch species along the first PCA component, linking positively with 20:4n-6, 24:5n-6, and 24:6n-3, and negatively with 22:4n-6 and 22:6n-3 (Fig. 4B). The second PCA component separates C. pacifica from the group of Dendronotus sp., A. papillosa, and C. verrucosa (Fig. 2A). Figure 2B shows that the level of SFAs (16:0 and 18:0), MUFAs (20:1n-9 and 20:1n-7), and 20:5n-3 vs. the level of 22:5n-6 and NMI FAs is significant for this separation. The level of 22:5n-6 was significantly higher (F1,14 = 6.555, p = 0.023) in the group of Dendronotus sp., A. papillosa, and C. verrucosa than that in T. tetraquetra and C. pacifica. The PCA results (Fig. 4) agree with the results of cluster analysis (Fig. 3) and show a significant difference in FA profiles between deep-sea T. tetraquetra, C. pacifica, and the two shallow-water species. Both statistical methods confirm that the FA profiles of the deep-sea Dendronotus sp. and the shallow-water species are similar.
Figure 4. Results of a principal component analysis (PCA) of the FA composition data for the five nudibranch species.
(A) The plot of the first two principal components; variables were the major fatty acids (see Table 1). Ellipses were drawn manually to outline three groups according to results of the cluster analysis (see Fig. 3). (B) The projectiosn of 12 variables is shown. TT, Tritonia tetraquetra; CP, Colga pacifica; CV, Coryphella verrucosa; DS, Dendronotus sp.; AP, Aeolidia papillosa.
Discussion
The representatives of nudibranchs of genera Aeolidia, Coryphella, and Dendronotus prey on various groups of Cnidaria (Hall & Todd, 1986). The nudibranch A. papillosa is known to prey on sea anemones, grabbing their soft tissues by highly denticulated uniserial radula (Fig. 2A), and consume their nematocyst (stinging capsular organelles) to protect against other predators (Hall & Todd, 1986; Vorobyeva, Malakhov & Ekimova, 2021). The considerable levels of 22:5n-6 and C20-22 MUFAs are characteristic for the FA composition of shallow- and deep-water sea anemones (Kiyashko et al., 2014; Revel et al., 2016). Obviously, the noticeable amounts of 22:5n-6 and C20 MUFAs that we found in A. papillosa most likely originate from sea anemone lipids consumed by this nudibranch species.
The nudibranchs A. papillosa and C. verrucosa occur in the same shallow-water community, but C. verrucosa demonstrates non-specified feeding mode (Mikhlina, Vortsepneva & Tzetlin, 2015), and its radula (Figs. 2C, 2D) does not take part in biting the prey. This species is known as a non-specialized cnidarian feeder preying on scyphoid jellyfish (Hernroth & Grondahl, 1985; Ostman, 1997), soft corals (Sebens, 1983; Allmon & Sebens, 1988), and hydroids of orders Anthoathecata (the genera Tubularia, Clava, and Hydractinia) and Leptothecata (the genus Obelia) (Kuzirian, 1979). C24 PUFAs are proposed as biomarkers for marine food web studies (Drazen et al., 2008; Blanchet-Aurigny et al., 2015). Very-long-chain C24 PUFAs are FATM of jellyfish and soft corals (Svetashev & Vysotskii, 1998; Imbs et al., 2010; Imbs, 2016; Svetashev, 2019). Trace amounts of C24 PUFAs in C. verrucosa indicate that this species from the Kuril Islands probably preys on anthoathecates hydroids, which may be a source of the increasing levels of 22:5n-6 and C20 MUFAs in this nudibranch species. C. verrucosa is characterized by the least intraspecific similarity in the composition of fatty acids. It may also indicate a wide range of food supplies for this species.
The radula morphology in Dendronotus sp. (Fig. 2B) is very similar to that of Dendronotus lacteus and D. rufus and has a large number of knife-like lateral teeth that nudibranchs may use for biting off soft tissues of polyps (Ekimova et al., 2019b). There is some evidence that D. lacteus and D. rufus feed on hydroids of family Sertulariidae (order Leptothecata), scyphistomaes, and anemones (Ekimova et al., 2019b). Considering the close similarity between the FATM profiles of A. papillosa, C. verrucosa, and Dendronotus sp., we assume that the increased 22:5n-6 and C20 MUFAs levels recorded in the deep-sea nudibranch Dendronotus sp. from the Sea of Okhotsk likely indicate its preying on sea anemones and/or anthoathecates hydroids, similarly to shallow-water species A. papillosa and C. verrucosa. A dietary resemblance and smoothing of lipid profiles by dietary FAs may be a possible reason of the resemblance in FATM between evolutionary distant species.
Several species of the genus Tritonia are known to be obligate predators feeding on soft corals (Allmon & Sebens, 1988; Goddard, 2006). Recently, an analysis of the FA composition of the nudibranch T. tetraquetra preying on soft corals (the Sea of Okhotsk) has shown an intensive transfer of a soft coral FATM (24:5n-6 and 24:6n-3) from prey to predator (Imbs, 2016). The ratio 24:6n-3/24:5n-6 was compared between T. tetraquetra (1.1 ± 0.2) and several soft coral species. As a result, the soft corals Gersemia rubiformis and Acanella sp. were suggested as the probable food sources of T. tetraquetra (Imbs, 2016; Imbs & Chernyshev, 2019). No significant differences in the ratio 24:6n-3/24:5n-6 were earlier found between T. tetraquetra specimens collected at different depths.
In the present study, the high levels of 24:6n-3 and 24:5n-6, which are observed in T. tetraquetra from Simushir Island, confirm preying on soft corals. Based on the ratio 24:6n-3/24:5n-6, we can assume that T. tetraquetra at a depth of 250 m mainly feed on the Gersemia and/or Acanella soft corals. The increase in the ratio 24:6n-3/24:5n-6 accompanying by the decrease in the 20:4n-6 level the in T. tetraquetra with increasing depth indicates a change in the taxonomic group of soft corals consumed. Among deep-sea soft corals that occur in the Sea of Okhotsk, the very high ratio 24:6n-3/24:5n-6 = 95 ÷310 and the lowest level of 20:4n-6 (1.7 ± 0.3%) is characteristic of soft corals within the family Primnoidae (Imbs, 2016), which most likely make a considerable contribution in diet of T. tetraquetra at a depth of 500 m. Our field observations show that T. tetraquetra is often found in communities of various groups of soft corals (Octocorallia) (Fig. S3), which apparently dominate food sources of this nudibranch.
Species of genus Colga can feed on members of phylum Bryozoa (Grischenko & Martynov, 1997; Behrens, 2004). At least 18 species of bryozoans were earlier found in a digestive tract of C. pacifica (Martynov & Baranets, 2002). A noticeable level of Δ7,13-22:2 and 22:6n-3 has been detected in total FAs of the bryozoan Dendrobeania flustroides from the Sea of Okhotsk (Demidkova, 2010). The high content of these two FAs in C. pacifica confirms that this deep-sea species likely feeds on bryozoans. The low intraspecific similarity in the FA composition revealed for C. pacifica may indicate a lack of food specialization towards any particular species of bryozoan.
Other characteristic FAs of C. pacifica such as 22:5n-6 and odd-chain/branched SFAs may originate from own biosynthesis and associated microorganisms, respectively. The unexpectedly high content of 22:5n-6 found in C. pacifica may be a result of high activity of C2 elongase and Δ4 desaturase that convert 20:4n-6 into 22:5n-6. Such activity has been hypothesized in the hydrocoral Millepora to explain the extremely high levels of 22:5n-6 and 22:6n-3 (Imbs, Dang & Nguyen, 2019; Imbs et al., 2021). The relatively low level of 20:5n-3 in C. pacifica can be due to either conversion of 20:5n-3 to 22:6n-3 or a deficiency on dietary 20:5n-3 in deep waters (Kiyashko et al., 2014). Odd-chain and branched SFAs in marine invertebrates indicate the presence of associated bacteria (Kharlamenko & Kiyashko, 2018). Various bacteria have been found in visceral organs of nudibranchs (Zhukova & Eliseikina, 2012). An abundant bacterial community may be a cause of the highest level of “bacterial” SFAs in C. pacifica.
Conclusions
FA profiles of five nudibranch mollusk species belonging to families Polyceridae, Tritoniidae, Dendronotidae, Coryphellidae, and Aeolidiidae were determined. The feeding specialization of deep-sea and shallow-water species were compared on the base of FATM present in their body tissues. Different species originating from different depths, but with similar food sources, showed similar FATM profiles. Species composition of soft corals consumed by T. tetraquetra appear to change with increasing depth. Deep-sea nudibranchs of genus Colga are most promising objects for future studies, as the proportion between dietary and self-synthesize PUFAs that they feature should be assessed. Future studies employing molecular barcodes to identify nudibranchs gut content can confirm our assumptions on the feeding regimes of the deep-sea species here reported, as FATM provide indirect evidence of trophic interactions and often impair the identification of prey at genus or species level.
Supplemental Information
Values show the contribution of individual FAs to the percent similarity or dissimilarity of the group. Total similarity or dissimilarity between groups is given in bold. TT, Tritonia tetraquetra; CP, Colga pacifica; CV, Coryphella verrucosa; DS, Dendronotus sp.; AP, Aeolidia papillosa.
AP, Aeolidia papillosa; CV, C. verrucosa; CP, C. pacifica; DS, Dendronotus sp.; TS1–TS3, T. tetraquetra specimens from a depth of 450–516 m; TS4 and TS5, T. tetraquetra specimens from a depth of 210–247 m.
A, the mollusk on the Primnoa corals, the Sea of Japan, depth 676 m; B, the mollusk on the Primnoa corals, the Sea of Japan, depth 676 m; C, the mollusk near Heteropolypus rylovi and Corallimorphus pilatus, the Bering Sea, depth 381 m. The mollusks are pointed by cycles.
Funding Statement
This work was supported by the Ministry of Science and Higher Education, Russian Federation (grant No. 13.1902.21.0012 for ID, contract No. 075-15-2020-796). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Anatolii Komisarenko performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Vladimir Mordukhovich conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Irina Ekimova performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Andrey Imbs conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary File.
References
- Allmon & Sebens (1988).Allmon RA, Sebens KP. Feeding biology and ecological impact of an introduced nudibranch, Tritonia plebeia, New England, USA. Marine Biology. 1988;99(3):375–385. doi: 10.1007/BF02112130. [DOI] [Google Scholar]
- Anderson, Gorley & Clarke (2008).Anderson MJ, Gorley RN, Clarke KR. PERMANOVA for PRIMER: guide to software and statistical methods. PRIMER-E Ltd; UK: 2008. [Google Scholar]
- Barnes & Bullough (1996).Barnes DKA, Bullough LW. Some observations on the diet and distribution of nudibranchs at Signy Island, Antarctica. Journal of Molluscan Studies. 1996;62:281–287. doi: 10.1093/mollus/62.3.281. [DOI] [Google Scholar]
- Behrens (2004).Behrens DW. Pacific Coast Nudibranchs, Supplement II. New species to the Pacific Coast and new information on the oldies. California Academy of Sciences. 2004;55:11–54. [Google Scholar]
- Bertsch (2020).Bertsch H. A history of eastern Pacific marine heterobranch research. The Nautilus. 2020;134(2):71–88. [Google Scholar]
- Blanchet-Aurigny et al. (2015).Blanchet-Aurigny A, Dubois SF, Quere C, Guillou M, Pernet F. Trophic niche of two co-occurring ophiuroid species in impacted coastal systems, derived from fatty acid and stable isotope analyses. Marine Ecology Progress Series. 2015;525:127–141. doi: 10.3354/meps11169. [DOI] [Google Scholar]
- Bligh & Dyer (1959).Bligh EG, Dyer WJA. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Braeckman et al. (2015).Braeckman U, Provoost P, Sabbe K, Soetaert K, Middelburg JJ, Vincx M, Vanaverbeke J. Temporal dynamics in a shallow coastal benthic food web: insights from fatty acid biomarkers and their stable isotopes. Marine Environmental Research. 2015;108:55–68. doi: 10.1016/j.marenvres.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Budge, Iverson & Koopman (2006).Budge SM, Iverson SJ, Koopman HN. Studying trophic ecology in marine ecosystems using fatty acids: a primer on analysis and interpretation. Marine Mammal Science. 2006;22(4):759–801. doi: 10.1111/j.1748-7692.2006.00079.x. [DOI] [Google Scholar]
- Calado & Leal (2015).Calado R, Leal MC. Trophic ecology of benthic marine invertebrates with bi-phasic life cycles: what are we still missing? Advances in Marine Biology. 2015;71:1–70. doi: 10.1016/bs.amb.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Carreau & Dubacq (1978).Carreau JP, Dubacq JP. Adaptation of macro-scale method to the micro-scale for FA methyl transesterification of biological lipid extracts. Journal of Chromatography A. 1978;151:384–390. doi: 10.1016/S0021-9673(00)88356-9. [DOI] [Google Scholar]
- Chimienti et al. (2020).Chimienti G, Angeletti L, Furfaro G, Canese S, Taviani M. Habitat, morphology and trophism of Tritonia callogorgiae sp. nov. a large nudibranch inhabiting Callogorgia verticillata forests in the Mediterranean Sea. Deep Sea Research Part I: Oceanographic Research Papers. 2020;165:103364. doi: 10.1016/j.dsr.2020.103364. [DOI] [Google Scholar]
- Christie (2021).Christie WW. Methyl esters of FA. Archive of mass spectra. The lipid web. https://www.lipidhome.co.uk/ms/methesters/me-arch/index.htm. [22 June 2021];2021
- Clarke & Gorley (2015).Clarke KR, Gorley RN. PRIMER v7: user manual/tutorial. PRIMER-E Ltd; UK: 2015. [Google Scholar]
- Demidkova (2010).Demidkova DA. The composition of fatty acids and aldehydes of the marine bryozoans Berenicea meandrina and Dendrobeania flustroides (Bryozoa: Gymnolaemata) Russian Journal of Marine Biology. 2010;36:300–304. doi: 10.1134/S1063074010040085. [DOI] [Google Scholar]
- Drazen et al. (2008).Drazen JC, Popp BN, Choy CA, Clemente T, De Forest L, Smith KL. Bypassing the abyssal benthic food web: Macrourid diet in the eastern North Pacific inferred from stomach content and stable isotopes analyses. Limnology and Oceanography. 2008;53(6):2644–2654. doi: 10.4319/lo.2008.53.6.2644. [DOI] [Google Scholar]
- Ekimova, Deart & Schepetov (2019a).Ekimova I, Deart Y, Schepetov D. Living with a giant parchment tube worm: a description of a new nudibranch species (Gastropoda: Heterobranchia) associated with the annelid Chaetopterus. Marine Biodiversity. 2019a;49(1):289–300. doi: 10.1007/s12526-017-0795-z. [DOI] [Google Scholar]
- Ekimova et al. (2015).Ekimova I, Korshunova T, Schepetov D, Neretina T, Sanamyan N, Martynov A. Integrative systematics of northern and Arctic nudibranchs of the genus Dendronotus (Mollusca, Gastropoda), with descriptions of three new species. Zoological Journal of the Linnean Society. 2015;173(4):841–886. doi: 10.1111/zoj.12214. [DOI] [Google Scholar]
- Ekimova et al. (2019b).Ekimova I, Valdes A, Chichvarkhin A, Antokhina T, Lindsay T, Schepetov D. Diet-driven ecological radiation and allopatric speciation result in high species diversity in a temperate-cold water marine genus Dendronotus (Gastropoda: Nudibranchia) Molecular Phylogenetics and Evolution. 2019b;141:106609. doi: 10.1016/j.ympev.2019.106609. [DOI] [PubMed] [Google Scholar]
- Goddard (2006).Goddard JHR. Stealthy slugs and communicating corals: polyp withdrawal by an aggregating soft coral in response to injured neighbors. Canadian Journal of Zoology. 2006;84:66–71. doi: 10.1139/Z05-178. [DOI] [Google Scholar]
- Goodheart et al. (2017).Goodheart JA, Bazinet AL, Valdes A, Collins AG, Cummings MP. Prey preference follows phylogeny: evolutionary dietary patterns within the marine gastropod group Cladobranchia (Gastropoda: Heterobranchia: Nudibranchia) BMC Evolutionary Biology. 2017;17:221. doi: 10.1186/s12862-017-1066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grischenko & Martynov (1997).Grischenko AV, Martynov AV. Bryozoa as food items for the genera Colga and Triopha (Nudibranchia, Doridacea). Russian and international conference on Bryozoa; 1997. http://www.bryozoa.net/russabst.html. [Google Scholar]
- Hall & Todd (1986).Hall SJ, Todd CD. Growth and reproduction in the aeolid nudibranch Aeolidia papillosa (L) Journal of Molluscan Studies. 1986;52:193–205. doi: 10.1093/mollus/52.3.193. [DOI] [Google Scholar]
- Hernroth & Grondahl (1985).Hernroth L, Grondahl F. On the biology of Aurelia aurita (L) 2. Major factors regulating the occurrence of ephyrae and young medusae in the gullmar fjord, Western Sweden. Bulletin of Marine Science. 1985;37(2):567–576. [Google Scholar]
- Hoover et al. (2012).Hoover RA, Armour R, Dow I, Purcell JE. Nudibranch predation and dietary preference for the polyps of Aurelia labiata (Cnidaria: Scyphozoa) Hydrobiologia. 2012;690:199–213. doi: 10.1007/s10750-012-1044-x. [DOI] [Google Scholar]
- Imbs (2016).Imbs AB. High level of tetracosapolyenoic fatty acids in the cold-water mollusk Tochuina tetraquetra is a result of the nudibranch feeding on soft corals. Polar Biology. 2016;39(8):1511–1514. doi: 10.1007/s00300-015-1865-y. [DOI] [Google Scholar]
- Imbs & Chernyshev (2019).Imbs AB, Chernyshev AV. Tracing of lipid markers of soft corals in a polar lipidome of the nudibranch mollusk Tritonia tetraquetra from the Sea of Okhotsk. Polar Biology. 2019;42(2):245–256. doi: 10.1007/s00300-018-2418-y. [DOI] [Google Scholar]
- Imbs, Dang & Nguyen (2019).Imbs AB, Dang LPT, Nguyen KB. Comparative lipidomic analysis of phospholipids of hydrocorals and corals from tropical and cold-water regions. PLOS ONE. 2019;14(4):e0215759. doi: 10.1371/journal.pone.0215759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbs et al. (2021).Imbs AB, Ermolenko EV, Grigorchuk VP, Dang LPT. Seasonal variation in the lipidome of two species of Millepora hydrocorals from Vietnam coastal waters (the South China Sea) Coral Reefs. 2021;40:719–734. doi: 10.1007/s00338-021-02073-2. [DOI] [Google Scholar]
- Imbs & Grigorchuk (2019).Imbs AB, Grigorchuk VP. Lipidomic study of the influence of dietary fatty acids on structural lipids of cold-water nudibranch mollusks. Scientific Reports. 2019;9:20013. doi: 10.1038/s41598-019-56746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbs et al. (2010).Imbs AB, Yakovleva IM, Latyshev NA, Pham LQ. Biosynthesis of polyunsaturated fatty acids in zooxanthellae and polyps of corals. Russian Journal of Marine Biology. 2010;36(6):452–457. doi: 10.1134/S1063074010060076. [DOI] [Google Scholar]
- Kelly & Scheibling (2012).Kelly JR, Scheibling RE. Fatty acids as dietary tracers in benthic food webs. Marine Ecology Progress Series. 2012;446:1–22. doi: 10.3354/meps09559. [DOI] [Google Scholar]
- Kharlamenko & Kiyashko (2018).Kharlamenko VI, Kiyashko SI. Fatty acid and stable isotope compositions in shallow-water bivalve mollusks and their food. Russian Journal of Marine Biology. 2018;44(2):100–111. doi: 10.1134/S1063074018020050. [DOI] [Google Scholar]
- Kiyashko et al. (2014).Kiyashko SI, Kharlamenko VI, Sanamyan K, Alalykina IL, Wurzberg L. Trophic structure of the abyssal benthic community in the Sea of Japan inferred from stable isotope and fatty acid analyses. Marine Ecology Progress Series. 2014;500:121–137. doi: 10.3354/meps10663. [DOI] [Google Scholar]
- Kuzirian (1979).Kuzirian AM. Taxonomy and biology of 4 New England coryphellid nudibranchs (gastropoda-opisthobranchia) Journal of Molluscan Studies. 1979;45:239–261. [Google Scholar]
- Martynov & Baranets (2002).Martynov AV, Baranets ON. A revision of the genus Colga Bergh (Opisthobranchia, Polyceridae), with description of a new species from the North Pacific. Ruthenica. 2002;12(1):23–43. [Google Scholar]
- McDonald & Nybakken (1997).McDonald G, Nybakken J. Introduction and the suborder Arminacea. 2. vol. 40. Veliger; 1997. A worldwide review of the food of nudibranch mollusks 1; pp. 157–159. [Google Scholar]
- McDonald & Nybakken (1999).McDonald G, Nybakken J. The suborder Dendronotacea. vol. 42. Veliger; 1999. A worldwide review of the food of nudibranch mollusks. Part II; pp. 62–66. [Google Scholar]
- Mikhlina, Ekimova & Vortsepneva (2020).Mikhlina A, Ekimova I, Vortsepneva E. Functional morphology and post-larval development of the buccal complex in Eubranchus rupium (Nudibranchia: Aeolidida: Fionidae) Zoology. 2020;143:125850. doi: 10.1016/j.zool.2020.125850. [DOI] [PubMed] [Google Scholar]
- Mikhlina et al. (2018).Mikhlina AL, Tzetlin AB, Ekimova IA, Vortsepneva EV. Drilling in the dorid species Vayssierea cf. elegans (Gastropoda: Nudibranchia): Functional and comparative morphological aspects. Journal of Morphology. 2018;280(1):119–132. doi: 10.1002/jmor.20922. [DOI] [PubMed] [Google Scholar]
- Mikhlina, Vortsepneva & Tzetlin (2015).Mikhlina AL, Vortsepneva EV, Tzetlin AB. Functional morphology of the buccal complex of Flabellina verrucosa (Gastropoda: Opisthobranchia) Invertebrate Zoology. 2015;12:175–196. doi: 10.15298/invertzool.12.2.04. [DOI] [Google Scholar]
- Ostman (1997).Ostman C. Abundance, feeding behaviour and nematocysts of scyphopolyps (Cnidaria) and nematocysts in their predator, the nudibranch Coryphella verrucosa (Mollusca) Hydrobiologia. 1997;355:21–28. doi: 10.1023/A:1003065726381. [DOI] [Google Scholar]
- Revel et al. (2016).Revel J, Massi L, Mehiri M, Boutoute M, Mayzaud P, Capron L, Sabourault C. Differential distribution of lipids in epidermis, gastrodermis and hosted Symbiodinium in the sea anemone Anemonia viridis. Comparative Biochemistry and Physiology A. 2016;191:140–151. doi: 10.1016/j.cbpa.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Sebens (1983).Sebens KP. The larval and juvenile ecology of the temperate octocoral Alcyonium siderium Verrill 2. Fecundity, survival, and juvenile growth. Journal of Experimental Marine Biology and Ecology. 1983;72(3):263–285. doi: 10.1016/0022-0981(83)90111-9. [DOI] [Google Scholar]
- Shuntov, Ivanov & Dulepova (2019).Shuntov VP, Ivanov OA, Dulepova EP. Biological resources in the Sea of Okhotsk large marine ecosystem: their status and commercial use. Deep Sea Research. 2019;163:33–45. doi: 10.1016/j.dsr2.2019.01.006. [DOI] [Google Scholar]
- Suzuki & Shimodaira (2006).Suzuki R, Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22(12):1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- Svetashev (2011).Svetashev VI. Mild method for preparation of 4, 4-dimethyloxazoline derivatives of polyunsaturated fatty acids for GC–MS. Lipids. 2011;46(5):463–467. doi: 10.1007/s11745-011-3550-4. [DOI] [PubMed] [Google Scholar]
- Svetashev (2019).Svetashev VI. Fatty acids of the medusae Aurelia aurita (Linnaeus, 1758) and Rhopilema esculentum (Kishinouye, 1891): the presence of families of polyenoic acids with 24 and 26 carbon atoms. Russian Journal of Marine Biology. 2019;45(2):113–117. doi: 10.1134/S1063074019020123. [DOI] [Google Scholar]
- Svetashev & Vysotskii (1998).Svetashev VI, Vysotskii MV. Fatty acids of Heliopora coerulea and chemotaxonomic significance of tetracosapolyenoic acids in coelenterates. Comparative biochemistry and Physiology B. 1998;119:73–75. doi: 10.1016/S0305-0491(97)00231-9. [DOI] [Google Scholar]
- Vorobyeva, Malakhov & Ekimova (2021).Vorobyeva OA, Malakhov VV, Ekimova IA. General and fine structure of Aeolidia papillosa cnidosacs (Gastropoda: Nudibranchia) Journal of Morphology. 2021;282:754–768. doi: 10.1002/jmor.21346. [DOI] [PubMed] [Google Scholar]
- Zhukova (2014).Zhukova NV. Lipids and fatty acids of nudibranch mollusks: potential sources of bioactive compounds. Marine Drugs. 2014;12(8):4578–4592. doi: 10.3390/md12084578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukova & Eliseikina (2012).Zhukova NV, Eliseikina MG. Symbiotic bacteria in the nudibranch mollusk Dendrodoris nigra: FA composition and ultrastructure analysis. Marine Biology. 2012;159:1783–1794. doi: 10.1007/S00227-012-1969-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values show the contribution of individual FAs to the percent similarity or dissimilarity of the group. Total similarity or dissimilarity between groups is given in bold. TT, Tritonia tetraquetra; CP, Colga pacifica; CV, Coryphella verrucosa; DS, Dendronotus sp.; AP, Aeolidia papillosa.
AP, Aeolidia papillosa; CV, C. verrucosa; CP, C. pacifica; DS, Dendronotus sp.; TS1–TS3, T. tetraquetra specimens from a depth of 450–516 m; TS4 and TS5, T. tetraquetra specimens from a depth of 210–247 m.
A, the mollusk on the Primnoa corals, the Sea of Japan, depth 676 m; B, the mollusk on the Primnoa corals, the Sea of Japan, depth 676 m; C, the mollusk near Heteropolypus rylovi and Corallimorphus pilatus, the Bering Sea, depth 381 m. The mollusks are pointed by cycles.
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary File.