Abstract
Background and objectives
Corticosteroids are commonly used in the treatment of hospitalized patients with COVID-19. The goals of the present study were to compare the efficacy and safety of different doses of dexamethasone in the treatment of patients with a diagnosis of moderate to severe COVID-19.
Methods
Hospitalized patients with a diagnosis of moderate to severe COVID-19 were assigned to intravenous low-dose (8 mg once daily), intermediate-dose (8 mg twice daily) or high-dose (8 mg thrice daily) dexamethasone for up to 10 days or until hospital discharge. Clinical response, 60-day survival and adverse effects were the main outcomes of the study.
Results
In the competing risk survival analysis, patients in the low-dose group had a higher clinical response than the high-dose group when considering death as a competing risk (HR = 2.03, 95% CI: 1.23–3.33, p = 0.03). Also, the survival was significantly longer in the low-dose group than the high-dose group (HR = 0.36, 95% CI = 0.15–0.83, p = 0.02). Leukocytosis and hyperglycemia were the most common side effects of dexamethasone. Although the incidence was not significantly different between the groups, some adverse effects were numerically higher in the intermediate-dose and high-dose groups than in the low-dose group.
Conclusions
Higher doses of dexamethasone not only failed to improve efficacy but also resulted in an increase in the number of adverse events and worsen survival in hospitalized patients with moderate to severe COVID-19 compared to the low-dose dexamethasone. (IRCT20100228003449N31).
Supplementary Information
The online version contains supplementary material available at 10.1007/s43440-021-00341-0.
Keywords: COVID-19, Dexamethasone, High-dose, Intermediate-dose, Low-dose
Background
Since late 2019, cumulative deaths of Coronavirus Disease 2019 (COVID-19) reached 5 million [1]. Although several worldwide and regional therapies were initially promising, most were abandoned due to minimal efficacy or safety concerns [2]. COVID-19 is a pending issue for governments and healthcare systems.
The symptoms were first recognized as cough, fever, dyspnea, sore throat, malaise, headache, and other influenza-like presentations [3]. However, acute respiratory distress syndrome (ARDS) and organ failure are common in severe and critical cases [4, 5].
The race for effective therapies of COVID-19 has started since the first days of the pandemic. However, few treatment options are currently available [6]. Medications like hydroxychloroquine, protease inhibitors and interferons were considered efficacious according to initial experiences but were later withdrawn from protocols due to lack of efficacy and risk of adverse effects [2, 7]. Two drugs with promising results in hospitalized patients are remdesivir and dexamethasone [8, 9].
Corticosteroid therapy was initially proposed for the treatment of SARS (severe acute respiratory syndrome) and MERS (Middle East Respiratory Syndrome) caused by coronaviruses like SARS-CoV-2 [10]. The rationale for corticosteroid therapy is to subside the cytokine storm in the progressive phase of COVID-19 [11].
Observational studies showed conflicting results about the efficacy of low-dose of corticosteroids in the management of COVID-19 [12, 13]. However, in RECOVERY trial, low-dose dexamethasone decreased mortality in hospitalized patients who required supplemental oxygen [8]. In another clinical trial, high-dose methylprednisolone implemented as pulse therapy decreased mortality in hospitalized patients with severe COVID-19 [14]. Considering pros and cons of corticosteroid therapy in viral infections, the optimal dose of corticosteroids in the treatment of COVID-19 is unknown. The goals of the present study were to compare the efficacy and safety of different doses of dexamethasone in the treatment of hospitalized patients with moderate to severe COVID-19.
Methods
Study design
This three-arm randomized clinical trial was conducted in Imam Khomeini Hospital Complex, a referral teaching center affiliated to Tehran University of Medical Sciences, Tehran, Iran. The first patient was recruited at 26 October 2020 and the last one finished the intervention at 25 January 2021. The Ethics Committee of Tehran University of Medical Sciences approved the study (reference number: IR.TUMS.MEDICINE.REC.1399.430). The trial was also registered (registration number: IRCT20100228003449N31).
Patients
Hospitalized adult patients (above 18 years old) with moderate to severe COVID-19 who required supplemental oxygen were enrolled. Reverse transcriptase-polymerase chain reaction (RT-PCR) of nasopharyngeal samples and a lung computed tomography (CT) scan were considered for all patients. Positive RT-PCR test or compatible lung involvement was considered for diagnosis of COVID-19. Symptoms like fever, cough, dyspnea, malaise, headache, weakness, myalgia, arthralgia, pharyngitis, anosmia, ageusia, gastrointestinal problems, chest discomfort along with the high respiratory rate, hypoxia, and hyperthermia would suggest the diagnosis of COVID-19 in the pandemic situation.
Severity of COVID-19 was defined according to the WHO interim guidance [15]. Moderate COVID-19 was considered when clinical signs of pneumonia (fever, cough, dyspnea, and high respiratory rate) were positive along with SpO2 between 90 and 93% on room air. Severe COVID-19 was described as clinical signs of pneumonia plus respiratory rate > 30 breaths/min or SpO2 < 90% on room air.
Exclusion criteria of the study were history of allergy to corticosteroids, uncontrolled diabetes mellitus with serum glucose above 250 mg/dL, active fungal or parasitic infections, closed-angle glaucoma, history of myopathy, history of corticosteroid-induced neuropsychiatric disorders, uncontrolled cardiovascular diseases like acute coronary syndrome, myocardial infarction, acute and massive thrombosis, uncontrolled hypertension (systolic blood pressure above 140 mmHg and diastolic blood pressure above 90 mmHg), acute viral hepatitis, pregnancy and lactation, history of corticosteroid therapy (for more than two weeks) and patients with critical COVID-19 (condition that would require the provision of life-sustaining therapies such as mechanical ventilation or vasopressor therapy).
Randomization and masking
Eligible patients who signed the consent form of the study were randomly assigned to low-dose, intermediate-dose, or high-dose dexamethasone group in 1:1:1 ratio. The randomization was performed using the permuted block method. Block sizes of 2 and 4 were selected. According to the list of random numbers created by Excel software, the statistician designed sequentially numbered opaque envelopes and then delivered to the clinical investigators. The statistician was unaware of the treatment group assignment. In addition, patients and clinical providers were blind regarding the randomization process. In terms of the treatment group assignment, patients but not physicians were blind.
Procedures
Patients in the low-dose, intermediate-dose, and high-dose groups received 8 mg once daily, 8 mg twice daily and 8 mg thrice daily dexamethasone as intravenous injection, respectively. Dexamethasone treatment was started within the first 24 h of admission and continued for up to 10 days or until hospital discharge. According to the hospital protocol, administration of antivirals, anticoagulants, antibiotics, analgesics, fluids, electrolytes, supplemental oxygen, vitamins, minerals, nutritional supports, and stress ulcer prophylaxis were the same for all patients.
All patients were assessed by a pulmonologist at the time of admission and at least once daily during the hospitalization course. In patients with mild hypoxemia (SpO2 between 90 and 92%), moderate hypoxemia (SpO2 between 85 and 89%) and severe hypoxemia (SpO2 less than 85%), nasal cannula, simple mask and mask with reservoir bag was utilized, respectively. If the goal (SpO2 ≥ 93%) was not achieved, non-invasive positive pressure or invasive ventilation was considered. In patients with PaO2/FiO2 of 200–300 and ≤ 200 mmHg, non-invasive positive pressure support and invasive mechanical ventilation was considered, respectively.
Demographic data, clinical symptoms, vital signs, laboratory data, baseline diseases, past medical history, past drug history, history of hospitalization due to COVID-19, medications, organ function, types of respiratory supports, need for ICU admission and mechanical ventilation, complications during the hospitalization course and dexamethasone-induced adverse effects were recorded. Each patient was followed weekly by telephone calls after hospital discharge for 60 days.
Thromboembolism prophylaxis was considered for all patients according to the hospital protocol. Either subcutaneous heparin 5000 international units three times daily or subcutaneous enoxaparin 40 mg daily was applied. In patients with body mass index ≥ 40 kg/m2, heparin and enoxaparin doses were increased to 7500 international units three times daily and 60 mg daily, respectively.
Outcomes’ measure
The primary outcome of the study was time to a clinical response that was described as improvement of at least two scores in the eight-category ordinal scale of the National Institute of Health (NIH). This scale is explained in eight categories as (1) discharge, with no limitations in usual activity (2) discharge, with some limitations in usual activity (3) hospital admission without the requirement of supplemental oxygen (4) hospital admission, requiring oxygen by mask or nasal cannula (5) hospital admission requiring non-invasive ventilation or high-flow oxygen (6) intubation and mechanical ventilation (7) mechanical ventilation and additional organ support like vasopressors, Renal Replacement Therapy (RRT) or Extracorporeal Membrane Oxygenation (ECMO) and (8) death.
The secondary outcomes of the study were time to 50% decrease in serum CRP level, time to respiratory rate ≤ 20 breaths per minute and time to peripheral oxygen saturation ≥ 93%. Other endpoints were hospital readmission, need for ICU admission and mechanical ventilation, duration of hospital and ICU stay, and 60-day survival.
Complications
Complications during hospitalization including adverse effects of dexamethasone were also recorded. Acute Kidney Injury (AKI), gastrointestinal (nausea, vomiting, gastrointestinal upset, bleeding), musculoskeletal (weakness, myopathy), hepatic (rise in serum aminotransferases and bilirubin), endocrine (hyperglycemia), hematologic (leukocytosis, lymphopenia, thrombocytosis or thrombocytopenia), cardiovascular (bradycardia, cardiac arrhythmia, heart failure, hypertension, myocardial infarction, tachycardia), psychiatric and neurologic (depression, emotional lability, euphoria, headache, insomnia, malaise) as well as secondary infections and drug allergy were closely monitored. A clinical pharmacist was responsible for daily patients’ monitoring regarding the adverse effects of dexamethasone. In the suspected events, patients were also assessed by relevant consultants.
Acute Kidney Injury (AKI) was described according to the KDIQO guideline [16]. Hepatic aminotransferase above three times the upper limit of normal or total bilirubin above 2 mg/dL was defined as acute hepatic injury [17]. Other side effects were leukocytosis (white blood cell count above 10,000 cell/mm3), lymphopenia (total lymphocyte count less than 1000 cell/mm3), thrombocytosis (platelet count above 400 × 109/L), hypertension (raise in blood pressure in a hypertensive patient or new-onset hypertension i.e. systolic blood pressure above 140 mmHg or diastolic blood pressure above 90 mmHg), hyperglycemia (blood glucose ≥ 180 mg/dL), heart failure (exacerbation of the existing heart failure or new onset heart failure), myocardial infarction according to the European Society of Cardiology (ESC) guideline [18], peripheral edema (edema in the legs or hands during hospitalization), arrhythmia, weakness (feeling of tiredness or exhausted), myopathy (generalized muscle weakness or discomfort, cramps and stiffness), myalgia (pain or discomfort in use of one or some muscles), agitation, anxiety, mood changes, sleep disturbance, delirium, thrombosis (according to the ESC guideline [19], oral candidiasis (oral thrush) and other secondary infections (according to microbial culture, signs and symptoms of infection or radiologic findings).
Sample size calculation
The study’s sample size was estimated according to the time to clinical response. The minimum range of factor to detect the difference was defined as Δ and the standard deviation was assumed as σ. In the sample size estimation, the ratio of ∆/σ considered equal 1, with 95% statistical power and 1% type 1 error rate and 10% attrition. The sample size was estimated as 48 patients in each group [20].
Statistical analysis
The numerical variables were reported as mean and standard deviation if they passed the Shapiro–Wilk test. Otherwise, the median and interquartile range (IQR) was used. Nominal variables were reported as frequencies and percentage. For comparing the numerical and nominal variables (including demographic and baseline characteristics of patients and outcomes), one-way ANOVA and Chi-square test was used, respectively.
Hazard ratio (HR) and 95% confidence interval (CI) were calculated by the competing risk survival model for primary and secondary outcomes. Kaplan–Meier plot was used to compare survival during 60-days follow-up between three groups and HR with 95% CI for clinical death was also calculated. The Cox proportional hazard model for survival was adjusted by age, gender and stage of the disease. The analysis of three groups was according to the intention-to-treat (ITT) method. A logistic regression model was designed for the detection of probable predictors of response to dexamethasone therapy. p-value of 0.05 was assumed as a significant difference in comparing groups. SPSS software (version 21.0) and Stata (version 14.0) was used for statistical analysis.
Results
Eligibility and baseline characteristics of participants
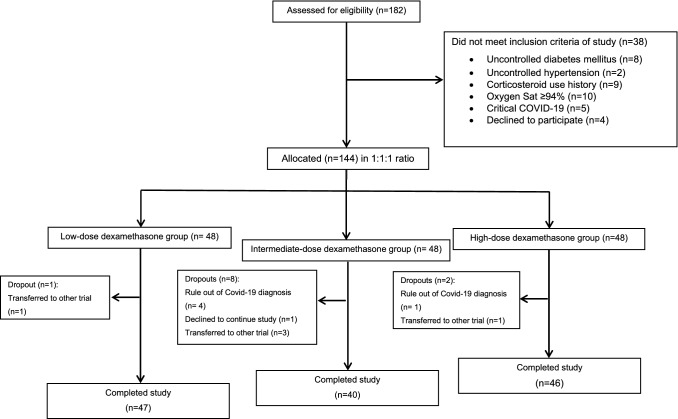
One-hundred forty-four patients met the inclusion criteria of the study. During the study period, 11 patients were excluded. The consort flow is shown in Fig. 1. The mean age of patients was 59 years in the low-dose and intermediate-dose groups and 56 years in the high-dose group. The diagnosis of COVID-19 was according to RT-PCR test and the compatible involvements in lung CT scan in 71.4% and 28.6% of patients, respectively. The percentage of male gender was 59.6%, 52.5% and 58.6% in the low-dose, intermediate-dose, and high-dose groups, respectively. Hypertension, diabetes mellitus, ischemic heart disease and hypothyroidism were the most common baseline diseases. Aspirin, angiotensin receptor blockers (ARB), beta-blockers and statins were frequent medications in past drug history of patients. There was no considerable difference between the groups regarding to these characteristics (Table 1).
Fig. 1.
Consort flow chart of the study. 144 out of 182 patients were allocated to the low-dose, intermediate-dose or high-dose group. Finally, 47, 40 and 46 patients in the low-dose, intermediate and high-dose groups completed the study, respectively
Table 1.
Baseline characteristics of participants
| Parameter | Low-dose group (n = 47) | Intermediate-dose group (n = 40) | High-dose group (n = 46) | p-value* |
|---|---|---|---|---|
| Sex, male | 28 (59.6) | 21 (52.5) | 31 (58.6) | 0.44 |
| Age (years) | 59 ± 14 | 59 ± 17 | 56 ± 16 | 0.11 |
| Obesity | 9 (19.1) | 8 (20.0) | 11 (23.9) | 0.56 |
| Smoker | 7 (14.9) | 3 (7.5) | 3 (6.5) | 0.35 |
| Alcoholic | 0 | 2 (5.0) | 1 (2.2) | 0.22 |
| Covid-19 history | 1 (2.1) | 1 (2.5) | 0 | 0.12 |
| History of hospitalization due to COVID-19 | 1 (2.1) | 1 (2.5) | 0 | 0.87 |
| Baseline diseases | ||||
| Hypertension | 21 (44.7) | 16 (40.0) | 11 (23.9) | 0.18 |
| Diabetes Mellitus | 13 (27.7) | 9 (22.5) | 8 (17.4) | 0.34 |
| Ischemic heart disease | 12 (25.5) | 5 (12.5) | 6 (13.0) | 0.12 |
| Hypothyroidism | 4 (8.5) | 3 (7.5) | 6 (13.0) | 0.90 |
| Respiratory disorders | 5 (10.6) | 1 (2.5) | 2 (4.3) | 0.21 |
| Cerebrovascular accident | 3 (6.4) | 3 (7.5) | 2 (4.3) | 0.19 |
| Dyslipidemia | 2 (4.3) | 2 (5.0) | 4 (8.7) | 0.15 |
| Neuropsychiatric disorders | 3 (6.4) | 5 (12.5) | 0 | 0.56 |
| Rheumatoid arthritis | 1 (2.1) | 0 | 1 (2.2) | 0.78 |
| Parkinson’s disease | 3 (6.4) | 0 | 2 (4.3) | 0.60 |
| Depression | 3 (6.4) | 1 (2.5) | 1 (2.2) | 0.39 |
| Malignancy | 2 (4.3) | 0 | 1 (2.2) | 0.23 |
| Renal disorders | 1 (2.1) | 0 | 1 (2.2) | 0.46 |
| Liver disorders | 1 (2.1) | 0 | 0 | 0.12 |
| Heart failure | 1 (2.1) | 1 (2.5) | 0 | 0.98 |
| Past drug history | ||||
| Aspirin | 17 (36.2) | 12 (30.0) | 8 (17.4) | 0.47 |
| ARB | 16 (34.0) | 10 (25.0) | 5 (10.9) | 0.23 |
| Statin | 11 (23.4) | 7 (17.5) | 10 (21.7) | 0.21 |
| Beta blocker | 11 (23.4) | 8 (20.0) | 8 (17.4) | 0.39 |
| Metformin | 10 (21.3) | 8 (20.0) | 5 (10.9) | 0.52 |
| Azithromycin | 5 (10.6) | 3 (7.5) | 9 (19.6) | 0.43 |
| Levothyroxine | 4 (8.5) | 3 (7.5) | 4 (8.7) | 0.50 |
| Sofosbuvir-ledipasvir | 3 (6.4) | 3 (7.5) | 5 (10.9) | 0.29 |
| Insulin | 4 (8.5) | 1 (2.5) | 0 | 0.57 |
| Doxycycline | 3 (6.4) | 1 (2.5) | 2 (4.3) | 0.39 |
| Hydroxychloroquine | 2 (4.3) | 1 (2.5) | 3 (6.5) | 0.38 |
| Immunosuppressants | 2 (4.3) | 2 (5.0) | 2 (4.3) | 0.13 |
| Supplements | 2 (4.3) | 1 (2.5) | 0 | 0.28 |
| Other antibiotics | 1 (2.1) | 2 (5.0) | 1 (2.2) | 0.46 |
| ACEI | 1 (2.1) | 1 (2.5) | 1 (2.2) | 0.55 |
Data are presented as n (%)
ACEI, Angiotensinogen Converting Enzyme Inhibitors, ARB, angiotensin receptor blockers
*p-value according to one-way ANOVA or Chi-square test
Hospitalization course
Cough and dyspnea were common complaints of patients at the time of hospital admission (Supplementary Table 1). Mean ± SD of SpO2 at the time of hospital admission was 87 ± 4%, 85 ± 3% and 85 ± 5% in the low-dose, intermediate-dose, and high-dose groups, respectively (p = 0.10). Other vital signs and the laboratory data at the time of hospital admission are summarized (Supplementary Table 2). Concomitant with dexamethasone, patients also received other medications (Supplementary Table 3).
Types of respiratory supports at admission and during the hospitalization are addressed (Supplementary Table 4). At admission, simple face mask face and mask with reservoir bag were common modalities for oxygen supplementation. However, during the hospitalization course some patients particularly in the intermediate-dose and high-dose groups became candidates for non-invasive or invasive ventilation. Estimated mean ± SD of PaO2/FiO2 ratio during the hospitalization course was 210 ± 93, 195 ± 75 and 168 ± 69 in the low-dose, intermediate-dose, and high-dose groups, respectively (p = 0.43). PaO2/FiO2 ratio of 300, 200 and 100 was considered as mild, moderate, and severe ARDS. There was no correlation between the severity of ARDS and response to the treatment.
Course of the disease was divided into 2 phases; before and after 7 days of onset of the symptoms. At admission, the onset of the symptoms for more than 7 days was noted by 76.6%, 62.5% and 78.3% of patients in the low-dose, intermediate-dose, and high-dose groups, respectively. Mean ± SD time from the onset of the symptoms to initiation of dexamethasone was 8.4 ± 1.8, 10.3 ± 2.6 and 9.7 ± 2.9 days in the low-dose, moderate-dose and high-dose groups, respectively (p = 0.07).
Median (IQR) duration of dexamethasone therapy in the low-dose, intermediate-dose and high-dose groups was 4 (3–6), 5 (3–10) and 5 (4–10) days, respectively (p = 0.14).
Primary and secondary outcomes
Time to clinical response was significantly different between the groups. Mean ± SD days to clinical response was 4.3 ± 1.9, 5.3 ± 2.0 and 6.1 ± 3.3 in the low-dose, intermediate-dose, and high-dose groups, respectively (p = 0.02). In all multivariate analysis, the high-dose group was assumed as a reference group and the low-dose and intermediate-dose groups were compared to this group. In competing risk survival analysis, patients in the low-dose group had more chance for a clinical response when considering death as the competing risk; for the low-dose group (HR = 2.03; 95% CI: 1.23–3.33, p = 0.03) and for the moderate-dose group (HR = 1.59; 95% CI: 0.92–2.76).
The same analysis was performed for time to decrease in serum CRP level and revealed that serum CRP level decreased faster in the intermediate-dose than the high-dose group; for the low-dose group (HR = 1.5; 95% CI: 0.75–3.00) and for the intermediate-dose group (HR = 2.13; 95% CI: 1.11–3.69, p = 0.04). In terms of time to respiratory rate ≤ 20, there was no significant difference between the groups; for the low-dose group (HR = 1.19; 95% CI: 0.73–1.91) and for the intermediate-dose group (HR = 1.08; 95% CI: 0.64–1.84). However, time to reach SpO2 ≥ 93% was significantly different between the groups; (HR = 2.66; 95% CI: 1.60- 4.41, p = 0.03) for the low-dose and (HR = 2.37; 95% CI: 1.47–3.82, p = 0.01) for the moderate-dose group. Other endpoints are shown in Table 2.
Table 2.
Primary and secondary outcomes
| Parameter | Low-dose group (n = 47) | Intermediate-dose group (n = 40) | High-dose group (n = 46) | p-value* |
|---|---|---|---|---|
| Time to clinical response (days) | 4.3 ± 1.9 | 5.3 ± 2.0 | 6.1 ± 3.3 | 0.025 |
| Time to 50% decrease of CRP level (days) | 4.6 ± 2.5 | 5.3 ± 2.4 | 6.0 ± 3.1 | 0.70 |
| Time to respiratory rate ≤ 20 breaths/min (days) | 3.9 ± 1.9 | 3.1 ± 1.8 | 3.8 ± 2.3 | 0.55 |
| Time to SpO2 ≥ 93% (days) | 4.2 ± 2.2 | 4.9 ± 2.7 | 4.9 ± 2.6 | 0.53 |
| Need for mechanical ventilation | 3 (6.4) | 5 (12.5) | 6 (13.0) | 0.51 |
| Duration of mechanical ventilation (days) | 3 ± 2 | 3 ± 2 | 4 ± 3 | 0.99 |
| Duration of hospital stay (days) | 5.7 ± 3.0 | 6.5 ± 3.4 | 7.0 ± 3.6 | 0.17 |
| Need for ICU admission | 5 (10.6) | 5 (12.5) | 9 (19.6) | 0.43 |
| Duration of ICU-stay (days) | 3.2 ± 1.5 | 3.4 ± 1.3 | 5.2 ± 3.4 | 0.17 |
| Hospital readmission | 1 (2.1) | 1 (2.5) | 1 (2.2) | 0.97 |
| 60-day mortality | 8 (17.0) | 12 (30.0) | 19 (41.3) | 0.06 |
Data are presented as n (%) or mean ± SD
*p-value according to one-way ANOVA or Chi-square test
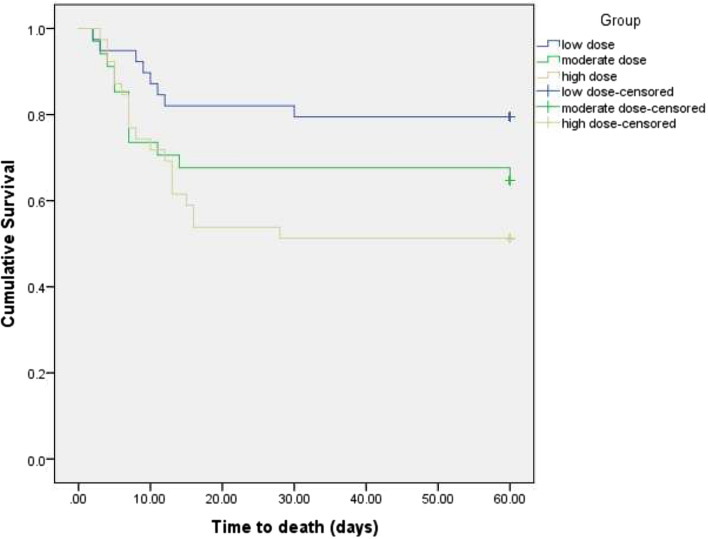
The 60-day mortality rate in the low-dose, intermediate-dose, and high-dose groups were 17%, 30% and 41.3%, respectively (p = 0.06). In Kaplan–Meier plot for survival time (Fig. 2) and in Cox proportional hazard model, survival was significantly longer in the low-dose than the high-dose group (HR = 0.36, 95% CI = 0.15–0.83, p = 0.02). However, this was not statistically different between the intermediate-dose and high-dose groups (HR = 0.7, 95% CI: 0.34–1.45). When the model was adjusted for age, gender and stage of the disease, the result became more significant for the low-dose group (HR = 0.30, 95% CI: 0.13–0.71, p = 0.006). The NNT (number needed to treat) was calculated, assuming the high-dose group as reference. The NNT was 4.1 for the low-dose and 8.8 for the intermediate-dose group.
Fig. 2.
Kaplan–Meier plot for survival time. In Kaplan–Meier plot, survival was significantly longer in the low-dose than the high-dose group p = 0.02). However, this was not statistically different between the intermediate-dose and high-dose groups (p = 0.34)
The probable predictors of response to dexamethasone therapy were included in a logistic regression model. Only SpO2 at admission and diabetes mellitus were found to be predictive variables (Table 3).
Table 3.
Probable predictors of response to dexamethasone therapy
| Variable | p-value* | Hazard ratio | 95% Confidence interval |
|---|---|---|---|
| Male sex over 70 years old | 0.31 | 1.52 | 0.67–3.45 |
| Baseline diseases | |||
| Hypertension | 0.046 | 2.50 | 1.01–6.16 |
| Diabetes mellitus | 0.02 | 0.33 | 0.13–0.82 |
| Signs and Symptoms at the time of hospital admission | |||
| Cough | 0.74 | 0.86 | 0.35–2.08 |
| Dyspnea | 0.09 | 0.45 | 0.18–1.14 |
| SpO2 | 0.02 | 1.10 | 1.01–1.20 |
| Type of oxygen support | 0.13 | 1.34 | 1.11–2.66 |
| Temperature | 0.49 | 0.80 | 0.43–1.48 |
| Other factors | |||
| Stage of disease (before or after 7 days of symptoms onset) | 0.70 | 0.84 | 0.34–2.05 |
| Medication during hospitalization | |||
| Statin | 0.22 | 0.59 | 0.25–1.38 |
| H-2 blockers | 0.52 | 1.58 | 0.38–6.56 |
| Remdesivir | 0.75 | 1.15 | 0.47–2.81 |
| Laboratory test disturbance at the time of hospital admission | |||
| Lymphopenia | 0.10 | 0.50 | 0.21–1.15 |
*p-value according to the logistic regression model
Adverse effects
Adverse effects of dexamethasone therapy in the intermediate-dose and high-dose groups were more frequent than the low-dose group. However, no significant difference between the groups was detected in this regard. Leukocytosis and hyperglycemia were the most common findings. The median (IQR) dose of NPH insulin was 20.5 (17.0–28.5), 20 (16–22), and 12 (8–14) IU in the high-dose, intermediate-dose, and low-dose groups, respectively (p = 0.22). The median (IQR) dose of regular insulin was 15 (8–18) IU in the low-dose, 18 (15–20) IU in the intermediate-dose, and 18 (12–30) IU in the high-dose groups. There was no statistical difference between the groups, considering doses of NPH (p = 0.29) and regular insulin (p = 0.30).
Although there was no significant difference between the groups, secondary infections were more common in the high-dose group than in other groups. Bacteremia, urinary tract infection and pneumonia due to Staphylococcus aureus, E. coli and Klebsiella pneumonia, respectively, were common infections. The details of these events are shown in Table 4.
Table 4.
Adverse events during the hospitalization course
| Parameter; n (%) | Low-dose group (n = 47) | Intermediate-dose group (n = 40) | High-dose group (n = 46) | p-value* |
|---|---|---|---|---|
| Acute Kidney Injury | 3 (6.4) | 0 | 1 (2.2) | 0.22 |
| Acute Hepatic Injury | 5 (10.6) | 3 (7.5) | 4 (8.7) | 0.90 |
| Leukocytosis | 18 (38.3) | 17 (42.5) | 22 (47.8) | 0.43 |
| Lymphopenia | 12 (25.5) | 12 (30.0) | 11 (23.9) | 0.81 |
| Thrombocytosis | 8 (17.0) | 4 (10.0) | 4 (8.7) | 0.50 |
| Arrhythmia | 6 (12.8) | 4 (10.0) | 11 (23.9) | 0.11 |
| Myocardial Infarction | 2 (4.3) | 0 | 1 (2.2) | 0.43 |
| Raise in blood pressure | 12 (25.5) | 15 (37.5) | 16 (34.8) | 0.33 |
| Peripheral Edema | 1 (2.1) | 0 | 3 (6.5) | 0.16 |
| Exacerbation of heart failure | 2 (4.3) | 0 | 1 (2.2) | 0.43 |
| Hyperglycemia | 14 (29.8) | 15 (37.5) | 22 (47.8) | 0.10 |
| Mood changes | 6 (12.8) | 8 (20.0) | 4 (8.7) | 0.32 |
| Anxiety | 4 (8.5) | 5 (12.5) | 1 (2.2) | 0.20 |
| Delirium | 1 (2.1) | 3 (7.5) | 0 | 0.11 |
| Agitation | 6 (12.8) | 5 (12.5) | 1 (2.2) | 0.16 |
| Sleep disturbances | 11 (23.4) | 8 (20.0) | 12 (26.1) | 0.72 |
| Myopathy | 1 (2.1) | 3 (7.5) | 2 (4.3) | 0.46 |
| Weakness | 5 (10.6) | 9 (22.5) | 6 (13.0) | 0.25 |
| Thrombosis | 0 | 0 | 1 (2.2) | 0.36 |
| Oral Candidiasis | 4 (8.5) | 9 (22.5) | 4 (8.7) | 0.08 |
| Secondary infections | 1 (2.1) | 1 (2.5) | 4 (8.7) | 0.20 |
Data are presented as n (%)
*p-value according to Chi-square test
Discussion
In this study, efficacy and safety of low-dose, intermediate-dose and high-dose of intravenous dexamethasone in the treatment of patients with a diagnosis of moderate to severe COVID-19 were compared. Time to the clinical response was significantly shorter in the low-dose group compared to the other groups. In the survival analysis, patients in the low-dose group had a significantly higher probability of survival than the high-dose group. In addition, some adverse effects including hyperglycemia and leukocytosis were more common in the high-dose group than the other groups.
The remarkable experiences with corticosteroid therapy in former coronavirus epidemics including MERS and SARS paved the way to be included in the treatment basket of COVID-19. Therapy with high-dose corticosteroid decreased oxygen requirement and improved lung radiologic abnormalities in patients with SARS [21]. In addition, survival and hospital stay improved in these patients [22].
One of the concerns regarding corticosteroid therapy in COVID-19 is a delay in the viral clearance. This phenomenon was reported in patients with MERS [23]. However, in patients with COVID-19, the delay in SARS-CoV-2 clearance following therapy with low-dose corticosteroid has not been reported [24]. Delay in the viral clearance may be due to other factors such as age and severity of the disease [25]. It seems that the effect of corticosteroid therapy on the viral clearance in COVID-19 is dose-dependent [24–27].
Corticosteroid therapy may worsen the therapy outcome in patients with COVID-19. In an observational study, although mortality was not significantly changed, more patients in the corticosteroid group progressed to the severe form of the disease. Also, corticosteroid therapy delayed the resolution of fever and viral clearance [13].
RECOVERY (Randomized Evaluation of COVID-19 Therapy) is a currently running trial looking into treatment options for coronavirus (COVID-19). In an arm of RECOVERY trial, the role of corticosteroid therapy in the treatment of COVID-19 has been examined. Low-dose dexamethasone (6 mg/daily for up to 10 days) significantly reduced the duration of hospital stay and 28-day mortality in hospitalized patients with COVID-19 [8].
Corticosteroids might decrease the mortality risk in patients with moderate to severe ARDS regardless of COVID-19 [28]. This effect has been also detected in COVID-19 patients with PaO2/FiO2 ratio < 200 at the time of hospital admission [29].
The appropriate routine, dose and duration of corticosteroid therapy for patients with COVID-19 have not been defined yet; albeit different corticosteroids with variable doses and durations were examined in patients with different disease severities.
Higher doses of corticosteroid therapy have been examined in a few studies. Corticosteroids administered at high doses improved survival and reduced the need for mechanical ventilation particularly in COVID-19 patients with severe pneumonia, respiratory failure, severe ARDS, at risk for hyper-inflammatory response and high serum levels of inflammatory biomarkers [14, 30–35]. However, most of the above-mentioned studies were retrospective. Randomized clinical trials are needed to confirm these effects. In an ongoing study (TACROVID trial), the efficacy and safety of methylprednisolone as pulse therapy (120 mg daily for three consecutive days) along with tacrolimus are targeted in patients with severe COVID-19 [36].
Low doses of corticosteroids have also been examined in patients with COVID-19. In an observational study, 40–80 mg daily methylprednisolone administered for about one week reduced mechanical ventilation requirement in patients with significant lung involvement. Secondary bacterial infections were recognized as complications of corticosteroid therapy used in this study [12]. Furthermore, in CoDEX trial, dexamethasone 20 mg daily for 5 days, proceeded by a dose of 10 mg daily for another 5 days or until discharge from ICU, resulted in prolonged survival and increased ventilator-free days in patients with COVID. In this trial, 31.1% and 28.4% of patients in the corticosteroid and control groups, respectively, required insulin therapy and, respectively, 21.9% and 29.1% developed secondary infections [37]. In the study of Fadel et al. administration of 0.5–1 mg/kg/day methylprednisolone for 3 days at the early stage of the infection decreased mortality rate and the necessity of hospitalization [38].
Safety is the main concern of corticosteroid therapy in patients with COVID-19. Importantly, in the CoDEX trial, the incidence of adverse effects including secondary infections and hyperglycemia were not significantly different in the treatment groups indicating that the therapy is considered safe [37].
Dexamethasone, hydrocortisone and methylprednisolone are the most studied corticosteroids in the treatment of patients with COVID-19. In Ko et al., study, the survival benefit of methylprednisolone and the equivalent dose of dexamethasone was compared in mechanically ventilated patients with COVID-19. In Ko et al., study, the survival benefit of methylprednisolone and the equivalent dose of dexamethasone was compared in mechanically ventilated patients with COVID-19. It was reported that the survival rate was significantly higher in patients who received methylprednisolone than those treated with dexamethasone [39]. In another study, methylprednisolone (2 mg/kg/day) was significantly superior to dexamethasone in terms of improvement of the clinical status of COVID-19 patients who required respiratory support [40]. In REMAP-CAP, a study that was terminated at the early stage due to the release of the RECOVERY trial results, two regimens of hydrocortisone were compared in patients with COVID-19 who required respiratory or cardiovascular support. In both regimens hydrocortisone improved organ support-free days [41].
One of the major concerns in patients with COVID-19 are secondary infections. Co-infection is uncommon (3.1%) but may be increased up to 4.7% during the hospitalization course [42].
Hyperglycemia is another dose-dependent adverse effect of corticosteroid therapy [43]. In our study, more patients in the high-dose dexamethasone group experienced hyperglycemia during the hospitalization course. In the RECOVERY trial, hyperglycemia, psychosis and gastrointestinal hemorrhage were detected [8].
In most studies, the time lapsed from the presentation of the symptoms and the start of corticosteroid therapy was not clearly defined [12, 37–41]. In our study, most patients received dexamethasone after 7 days from the onset of the symptoms.
Low-dose corticosteroid therapy is recommended as the standard of care for hospitalized patients with COVID-19 who require supplemental oxygen. In addition, remdesivir is recommended for hospitalized patients but not under mechanical ventilation [44, 45].
According to our hospital protocol, most patients received remdesivir as the antiviral regimen. Benefits of antiviral therapy in hospitalized patients with COVID-19 requiring supplemental oxygen are unclear. Considering design, population, stage and severity of the disease, the time of administration and concomitant medications, the main outcomes of the available studies are different. Results of a recently published large multi-center observational cohort study showed that remdesivir can improve survival if the treatment is implemented upon hospital admission [46]. However, in the DisCoVeRy trial, remdesivir did not show further clinical benefits in comparison to standard care [47].
In our study, the effect of concomitant treatments on dexamethasone response was evaluated using a logistic regression model. None of the treatments such as administration of statins, H-2 blockers, melatonin and remdesivir was a predictor. During the study period, 11 patients were excluded. Most patients (6 out of 11) stopped the protocol due to exclusion of COVID-19 diagnosis. At the same time, patients were free to choose other ongoing trials. Attrition or Myth 2 bias (dissimilar dropout rates between study arms) is a systematic error caused by unequal loss of participants from a randomized controlled trial. Enrolled patients might withdraw the protocol of the study due to unsatisfactory efficacy, adverse events, or death [48]. To resolve this type of error, intention-to-treat (ITT) analysis was applied.
Detection of long-term complications of COVID-19 or dexamethasone (hip necrosis, post-COVID syndrome, pulmonary embolism) was not possible due to a short, 60-day follow-up.
Conclusion
To the best of our knowledge, this was the first randomized clinical trial that compared efficacy and safety of different doses of dexamethasone in patients with moderate to severe COVID-19. A similar protocol in terms of supportive care, antiviral therapy, deep vein thrombosis, and stress ulcer prophylaxis was applied for all patients. Patients were followed for 60 days. Higher doses of dexamethasone not only failed to improve efficacy but also resulted in an increase in the number of adverse events and worsen survival in hospitalized patients with moderate to severe COVID-19 compared to the low-dose dexamethasone. Therefore, based on the results of the study the low-dose dexamethasone (8 mg/day) can be recommended for these patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the nurses and other staffs of Imam Khomeini Hospital Complex for their kind supports. No grant was received regarding this work.
Authors’ contributions
LA: patients’ assessment, editing the manuscript. NT: data gathering, Patients’ assessment. AN: assessment Inclusion criteria, data gathering. ED-M: interpretation of the data, drafting the manuscript. HK: primary investigator, editing the manuscript. MH: patients’ assessment. FG: patients’ assessment. SJ: patients’ assessment. HEK: patients’ assessment. MSY: data analysis.
Funding
The authors did not receive any funds for this work.
Availability of data and materials
Available per request.
Declarations
Conflict of interest
There is no conflict of interest for authors to declare.
Ethics approval
The Ethics Committee of Tehran University of Medical Sciences approved the study (reference number: IR.TUMS.MEDICINE.REC.1399.430).
Consent to participate
Inform consent was obtained from all participants or from a responsible first-degree family member.
Consent for publication
Patients signed informed consent regarding publishing their data.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Negar Toroghi and Ladan Abbasian both as first authors.
Contributor Information
Negar Toroghi, Email: negar.torjaf@yahoo.com.
Ladan Abbasian, Email: la.abbasian@gmail.com.
Anahid Nourian, Email: anahid.nouriam@gmail.com.
Effat Davoudi-Monfared, Email: edavudimonfared@gmail.com.
Hossein Khalili, Email: Khalilih@sina.tums.ac.ir, Email: khalilih@tums.ac.ir.
Malihe Hasannezhad, Email: malihehasannezhad@yahoo.com.
Fereshteh Ghiasvand, Email: ghiasvand_62@yahoo.com.
Sirous Jafari, Email: jafari_sirous@yahoo.com.
Hamid Emadi-Kouchak, Email: dr_emady@yahoo.com.
Mir Saeed Yekaninejad, Email: yekaninejad@yahoo.com.
References
- 1.World Health Organization, COVID-19 Weekly Epidemiological Update, 11 October 2021. 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---11-october-2021. Accessed 14 Oct 2021.
- 2.WHO Solidarity Trial Consortium Repurposed antiviral drugs for Covid-19—Interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24(1):154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Jiang B. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395(10228):e52. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RECOVERY Collaborative Group. Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin D, Chan-Tack K, Farley J, Sherwat A. FDA approval of remdesivir—a step in the right direction. N Engl J Med. 2020;383(27):2598–2600. doi: 10.1056/NEJMp2032369. [DOI] [PubMed] [Google Scholar]
- 10.Lee KH, Yoon S, Jeong GH, Kim JY, Han YJ, Hong SH, et al. Efficacy of corticosteroids in patients with SARS, MERS and COVID-19: a systematic review and meta-analysis. J Clin Med. 2020;9(8):2392. doi: 10.3390/jcm9082392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arabi YM, Chrousos GP, Meduri GU. The ten reasons why corticosteroid therapy reduces mortality in severe COVID-19. Intensive Care Med. 2020;46(11):2067–2070. doi: 10.1007/s00134-020-06223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Zhou X, Li T, Chan S, Yu Y, Ai JW, et al. Corticosteroid prevents COVID-19 progression within its therapeutic window: a multicentre, proof-of-concept, observational study. Emerg Microbes Infect. 2020;9(1):1869–1877. doi: 10.1080/22221751.2020.1807885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding C, Feng X, Chen Y, Yuan J, Yi P, Li Y, et al. Effect of Corticosteroid therapy on the duration of SARS-CoV-2 clearance in patients with mild COVID-19: a retrospective cohort study. Infect Dis Ther. 2020;9(4):943–952. doi: 10.1007/s40121-020-00337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6):2002808. doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. 2020. Clinical management of COVID-19: interim guidance, 27 May 2020. World Health Organization. https://apps.who.int/iris/handle/10665/332196.
- 16.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 17.Lescot T, Karvellas C, Beaussier M, Magder S. Acquired liver injury in the intensive care unit. Anesthesiology. 2012;117(4):898–904. doi: 10.1097/ALN.0b013e318266c6df. [DOI] [PubMed] [Google Scholar]
- 18.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, ESC Scientific Document Group et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 19.Mazzolai L, Aboyans V, Ageno W, Agnelli G, Alatri A, Bauersachs R, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J. 2018;39(47):4208–4218. doi: 10.1093/eurheartj/ehx003. [DOI] [PubMed] [Google Scholar]
- 20.Neter J, Kutner MH, Nachtsheim CJ, et al. Applied linear statistical models. 5th Edition, McGraw-Hill, Irwin, New York; 2005.
- 21.Ho JC, Ooi GC, Mok TY, Chan JW, Hung I, Lam B, et al. High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am J Respir Crit Care Med. 2003;168(12):1449–1456. doi: 10.1164/rccm.200306-766OC. [DOI] [PubMed] [Google Scholar]
- 22.Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Saudi Critical Care Trial Group et al. Corticosteroid therapy for critically ill patients with Middle East Respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 24.Fang X, Mei Q, Yang T, Li L, Wang Y, Tong F, et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spagnuolo V, Guffanti M, Galli L, Poli A, Querini PR, Ripa M, COVID-BioB study group et al. Viral clearance after early corticosteroid treatment in patients with moderate or severe covid-19. Sci Rep. 2020;10(1):21291. doi: 10.1038/s41598-020-78039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang R, Zhu C, Jian W, Xue L, Li C, Yan X, et al. Corticosteroid therapy is associated with the delay of SARS-CoV-2 clearance in COVID-19 patients. Eur J Pharmacol. 2020;889:173556. doi: 10.1016/j.ejphar.2020.173556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Hu Z, Song X. High-dose but not low-dose corticosteroids potentially delay viral shedding of patients with COVID-19. Clin Infect Dis. 2021;72(7):1297–1298. doi: 10.1093/cid/ciaa829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 29.Bartoletti M, Marconi L, Scudeller L, Pancaldi L, Tedeschi S, Giannella M, PREDICO Study Group et al. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicenter study. Clin Microbiol Infect. 2021;27(1):105–111. doi: 10.1016/j.cmi.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López Zúñiga MÁ, Moreno-Moral A, Ocaña-Granados A, Padilla-Moreno FA, Castillo-Fernández AM, Guillamón-Fernández D, et al. High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS ONE. 2021;16(1):e0243964. doi: 10.1371/journal.pone.0243964.PMID:33507958;PMCID:PMC7842890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vecchié A, Batticciotto A, Tangianu F, Bonaventura A, Pennella B, Abenante A, et al. High-dose dexamethasone treatment for COVID-19 severe acute respiratory distress syndrome: a retrospective study. Intern Emerg Med. 2021;16(7):1913–1919. doi: 10.1007/s11739-021-02800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papamanoli A, Yoo J, Grewal P, Predun W, Hotelling J, Jacob R, et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur J Clin Invest. 2021;51(2):e13458. doi: 10.1111/eci.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tromp K, van der Zee P, Rokx C, van Kampen J, Gommers D, Endeman H. Effect of methylprednisolone on inflammation and coagulation in patients with severe COVID-19: a retrospective cohort study. Biomark Insights. 2021;4(16):11772719211021647. doi: 10.1177/11772719211021647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Climente-Martí M, Ruiz-Millo O, López-Cruz I, Atienza-García Á, Martínez-Moragón E, Garijo-Gómez E, Doctor Peset COVID-19 Working Group et al. Impact of intermediate to high doses of methylprednisolone on mortality rate in patients with COVID-19 pneumonia-indu. Int J Clin Pract. 2021;75(9):14479. doi: 10.1111/ijcp.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinzón MA, Ortiz S, Holguín H, Betancur JF, Cardona Arango D, Laniado H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS ONE. 2021;16(5):e0252057. doi: 10.1371/journal.pone.0252057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solanich X, Antolí A, Padullés N, Fanlo-Maresma M, Iriarte A, Mitjavila F, et al. Pragmatic, open-label, single-center, randomized, phase II clinical trial to evaluate the efficacy and safety of methylprednisolone pulses and tacrolimus in patients with severe pneumonia secondary to COVID-19: the TACROVID trial protocol. Contemp Clin Trials Commun. 2021;21:100716. doi: 10.1016/j.conctc.2021.100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, COALITION COVID-19 Brazil III Investigators et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, Henry Ford COVID-19 Management Task Force et al. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020;71(16):2114–2120. doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko JJ, Wu C, Mehta N, Wald-Dickler N, Yang W, Qiao R. A Comparison of methylprednisolone and dexamethasone in intensive care patients with COVID-19. J Intensive Care Med. 2021;36(6):673–680. doi: 10.1177/0885066621994057. [DOI] [PubMed] [Google Scholar]
- 40.Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021;21(1):337. doi: 10.1186/s12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, COVID-19 Researchers Group et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamez-Pérez HE, Quintanilla-Flores DL, Rodríguez-Gutiérrez R, González-González JG, Tamez-Peña AL. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J Diabetes. 2015;6(8):1073–1081. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. 2021. https://www.covid19treatmentguidelines.nih.gov/. Accessed 14 Oct 2021. [PubMed]
- 45.- Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America 2021; Version 5.3.1. 2021. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 14 Oct 2021. [DOI] [PMC free article] [PubMed]
- 46.Mozaffari E, Chandak A, Zhang Z, Liang S, Thrun M, Gottlieb RL, et al. Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin Infect Dis. 2021;2021:ciab875. doi: 10.1093/cid/ciab875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D, DisCoVeRy Study Group et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell ML, Kenward MG, Fairclough DL, Horton NJ. Differential dropout and bias in randomised controlled trials: when it matters and when it may not. BMJ. 2013;21(346):e8668. doi: 10.1136/bmj.e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available per request.