Summary:
Classic galactosemia (CG) is a rare inborn error of metabolism that results from profound deficiency of galactose-1-P uridylyltransferase (GALT). Despite early detection and rapid and lifelong dietary restriction of galactose, which is the current standard of care, most patients grow to experience a broad range of complications that can include motor difficulties. The goal of this study was to characterize hand fine motor control deficit among children and adults with classic galactosemia (CG). Specifically, we used Neuroglyphics software to collect digital Archimedes spiral drawings on a touch screen from 57 volunteers with CG (cases) and 80 controls. Hand fine motor control was scored as root mean square (RMS) of spirals drawn relative to an idealized template. Presence of tremor was defined as a peak in periodicity of changes in drawing speed or direction in the 4-8 Hz range. We observed a highly significant difference (p<0.001) in RMS scores between cases and controls, with almost 51% of cases showing at least 1 of 4 spirals scoring outside the 95th percentile for controls. The corresponding prevalence for controls was 10%. Similarly, more than 35% of cases, and almost 14% of controls, showed at least 1 of 4 spirals with a tremor amplitude above the 95th % cutoff for controls. Our results both confirm and extend what is known about hand fine motor control deficit among children and adults with CG and establish digital assessment as a useful approach to quantify this outcome.
Keywords: galactosemia, hand fine motor control, Archimedes spiral, Neuroglyphics, modifiers
Introduction:
Classic galactosemia (CG) is a rare inborn error of metabolism that impacts close to 1/50,000 infants born in the US (Pyhtila et al 2015). With the benefit of early detection by newborn screening and early and lifelong dietary restriction of galactose, which is the current standard of care, most affected infants are now spared the potentially lethal acute sequelae of disease. However, most grow to experience long-term complications that can include speech, cognitive, behavioral, and motor deficits in patients of both sexes, and primary ovarian insufficiency in girls and women (Berry 2014). The mechanisms underlying these deficits remain unclear and there is currently no known intervention that prevents or reverses them.
Here we focus on a specific subset of motor outcomes in CG: hand fine motor control. Prior studies have addressed motor deficit in CG using a combination of self-report surveys, interviews, medical records review, and video-recordings evaluated by experts (Kuiper et al 2019, Rubio-Agusti et al 2013, Rubio-Gozalbo et al 2019, Waggoner et al 1990). For example, of 37 British or Dutch patients assessed, Kuiper and colleagues (Kuiper et al 2019) reported that 48.6% met criteria for either a fine or gross-motor disorder, or both, and of these, 16% showed tremor. Of body regions, the upper extremities were most frequently affected. In that cohort, sex, age, time of diagnosis, strictness of dietary compliance, speech, fertility in women, special education, and employment status were not significantly associated with presence of a motor disorder.
In a separate, larger study based on medical records review of patients with CG, Rubio-Gozalbo and colleagues (Rubio-Gozalbo et al 2019) reported that 52% of 323 patients experienced neurological or motor complications, with tremor being the most frequent (almost 31%); these complications were manifest at all ages and equally between sexes.
In the study reported here, we used sets of digital Archimedes spirals collected from each of 57 cases and 80 controls and scored by objective measures to quantify hand fine motor control. That we used a digital approach (Haubenberger et al 2011) that did not rely on self or observer subjective interpretation is important and contrasts with other studies reported previously for CG. That we collected and analyzed repeated measures (up to 4 spirals) from each participant is also important because isolated episodes of sub-clinical kinetic hand tremor are common in the general population (Bhatia et al 2018, Raethjen et al 2000).
This study had 2 goals. First, we wanted to test whether an objective, digital approach would be able to detect and quantify hand fine motor control deficits among children and adults with CG. If yes, we wanted to test how the prevalence detected by this method would compare to prior reports in other cohorts. Second, given the varied prevalence of tremor as an ostensible cause of motor control deficit described in prior studies of CG (Kuiper et al 2019, Rubio-Agusti et al 2013, Rubio-Gozalbo et al 2019, Waggoner et al 1990), we also wanted to ask whether tremor appeared to be a major contributor to hand fine motor control deficit in our cohort. In addressing these questions, our study both confirms and substantially extends what is understood about hand fine motor control deficit in CG and establishes digital assessment as an appropriate research tool to follow this outcome in patients.
Materials and Methods:
Study subjects:
A total of 137 volunteers participated in this study: 57 with a confirmed diagnosis of CG (cases) and 80 without (controls). All study subjects were 6 to 65 years old at the time of testing, and all were enrolled following appropriate informed consent in Emory IRB00024933 (PI: JL Fridovich-Keil). All cases and most controls, who were the unaffected family members of cases, were recruited and tested at the Galactosemia Foundation Conference in Denver, CO in 2018. Some additional unrelated teen and young adult controls from Atlanta, GA were also recruited for the study to ensure adequate representation of controls in specific age and gender categories that were otherwise under-represented. Demographic and other summary statistics for the study cohort are presented in Table 1.
Table 1.
Summary Statistics of Cases and Controls
| Cases (n=57) | Controls (n=80) | ||
|---|---|---|---|
| Age (all 6-65 years old) | |||
| mean +/− SD | 20.8 +/− 12.36 | 24.93 +/− 15.14 | |
|
| |||
| Sex | |||
| female | 44% (n=25) | 59% (n=47) | |
| male | 56% (n=32) | 41% (n=33) | |
|
| |||
| Race and ethnicity | % of known | % of known | |
| White | 0.92 (n=47) | 0.81 (n=48) | |
| Black | 0.00 (n=0) | 0.02 (n=1) | |
| Asian | 0.00 (n=0) | 0.08 (n=5) | |
| Mixed or Other | 0.08 (n=4) | 0.08 (n=5) | |
| Unknown | 0.11 of total (n=6) | 0.26 of total (n=21) | |
| Hispanic | 0.10 (n=5) | 0.03 (n=2) | |
| not Hispanic | 0.90 (n=46) | 0.97 (n=57) | |
| Unknown | 0.11 of total (n=6) | 0.26 of total (n=21) | |
|
| |||
| Handedness (%, n) | |||
| right | 0.93 (n=53) | 0.93 (n=74) | |
| left | 0.035 (n=2) | 0.05 (n=4) | |
| ambidextrous | 0.00 (n=0) | 0.01 (n=1) | |
| unknown | 0.035 (n=2) | 0.01 (n=1) | |
|
| |||
| GALT residual activity (% wild-type as defined in a yeast model system) | |||
| > 0.4% | 0.17 (n=10) | na | |
| < 0.4% | 0.54 (n=31) | na | |
| unknown | 0.28 (n=16) | na | |
|
| |||
| Self-reported history of speech difficulties | |||
| yes | 0.47 (n=27) | na | |
| no | 0.44 (n=25) | na | |
| unknown | 0.09 (n=5) | na | |
|
| |||
| Rigor of dietary restriction of galactose by age (where known) | |||
| birth to 1 year (n=47) | (n=5) dairy only (n=12) dairy + 1 other (n=32) dairy + >1 other |
na | |
| 2 to 5 years (n=49) | (n=7) dairy only (n=13) dairy + 1 other (n=29) dairy + >1 other |
na | |
| 6+ years (n=43) | (n=16) dairy only (n=11) dairy + 1 other (n=16) dairy + >1 other |
na | |
Collection of digital Archimedes spirals from adults and children using the Neuroglyphics software system:
All spirals were drawn on a Surface Pro 3 touchscreen running Neuroglyphics software (http://www.neuroglyphics.org/Default.aspx) (Haubenberger et al 2016) that recorded pen (stylus) position in 3 dimensions with a sampling rate of 200 Hz and spatial resolution of 2,000 values per 1 cm displacement. Each volunteer was seated individually at a table and shown demonstrations of how to draw spirals on the digital surface using both the right and left hands, starting in the middle of the tablet field and spiraling outward by tracing the imagined mid-point between two spiral outlines visible on the screen (Supplemental Figure 1, panel A). Spirals drawn with the right hand were drawn in the clockwise direction and spirals drawn with the left hand were drawn in the counterclockwise direction. Each participant was asked to draw their best possible spiral, drawing as slowly or as quickly as they wanted, mindful that while drawing their elbow and hand must not touch the tablet or table. Parents were allowed to sit or stand next to their child during testing, but not help with the task. Once each volunteer confirmed they understood the task, they were given the stylus and recorded drawing 2 successive spirals with each hand, for a total of 4 spirals each. For a very small number of participants, 1 or more spirals were not scorable, so our data set of scored spirals included 219 spirals from cases, and 316 from controls.
Data analyses:
To normalize for variable start and end points among spirals (Haubenberger et al 2016), we clipped each spiral to 3.5 turns (fixed-space) before scoring for deviation from an idealized spiral in the same field by calculation of a root mean square (RMS) value. We also differentiated the raw data file from each spiral using Fast Fourier Transformation (FFT) to calculate stylus tip velocity (mm/msec) and frequency (Hz). For this study, amplitude of kinetic hand tremor while drawing was defined as the area under the velocity spectrum curve in the range of 4-8 Hz (Hess and Pullman 2012). RMS and tremor amplitude scores for cases and controls were natural log (ln) transformed to accommodate the multiple outliers among cases. Because the data still were not normally distributed, we compared populations using a non-parametric Wilcoxon Rank-Sum test.
Demographic covariates:
We used ANOVA to compare linear models with and without adjustment for available demographic variables of sex, race, and ethnicity to test whether any of these might improve the prediction of Ln(RMS) or Ln(tremor amplitude) among cases or controls; none did (p>0.05 in all comparisons). Age was a significant predictor (p = 0.035) among both cases and controls to the extent that in both groups children showed higher RMS scores than teens or adults. Further, because our cohort included both related and unrelated individuals, we tested whether relatedness might be a significant covariate; it was not (p=0.8394).
Other potential covariates:
We also tested other variables for possible association with hand fine motor control deficit in our cohort using a linear mixed-effects model with the response variable being Ln(RMS) or Ln(tremor amplitude), the random effect being participant ID, and the fixed effect being the covariate of interest. Covariates tested included: handedness, predicted residual GALT activity, history of speech problems, age at initiation of dietary restriction of galactose, severity of acute neonatal symptoms scored on a scale from 0 (no acute symptoms) to 15 (multiple moderate or severe symptoms), composite score from the ABAS-3 (Adaptive Behaviors Assessment System) (von Buttlar et al 2021), and rigor of dietary galactose restriction in infancy (0-1 years), early childhood (1-5 years), or when age 6 and above (Frederick et al 2017). Because many of these variables were not normally distributed in our cohort we again used non-parametric analyses that did not assume normality. Finally, RMS and tremor amplitude scores were not averaged for these analyses, but were considered individually, and the random effect of the participant ID was used to account for the within-subject correlation of repeated measures.
Results:
Detection of hand fine motor control deficit in both children and adults with classic galactosemia (CG) by analysis of digital Archimedes spirals
Our first goal was to test whether direct assessment using digital Archimedes spirals drawn by children and adults with CG would detect motor control deficits (Kuiper et al 2019, Rubio-Agusti et al 2013, Rubio-Gozalbo et al 2019, Waggoner et al 1990). If yes, we also wanted to ask how the prevalence of deficits detected in our cohort would compare with prior studies. As described in Materials and Methods, we calculated root mean square (RMS) as a measure of hand fine motor control. To be clear, an ideal spiral would have an RMS score of 0, and the further from ideal a drawn spiral, the higher the RMS score would be.
Our data showed a clear and statistically significant difference in RMS scores between cases and controls regardless of whether the RMS scores were averaged across all spirals collected from each volunteer (p<0.000001), just the dominant-hand spirals (p<0.00001), just the non-dominant-hand spirals (p<0.00001), or all individual spirals (p<0.001) (Figure 1). Using these RMS scores we then estimated the prevalence of hand fine motor control deficit among participants in our study by calculating the number of cases with spirals whose Ln(RMS) score fell above the 95th percentile cutoff defined for controls. We used median to measure the center and percentiles of each distribution rather than mean and standard deviation in order to minimize any artifacts from the non-normality of our data. We found that 29 of 57 cases (almost 51%) had at least 1 spiral with an Ln(RMS) score above the 95th % cutoff; 24 (42%) had at least 2 spirals above the 95th % cutoff; 16 (28%) had at least 3 spirals above the 95th % cutoff; and 8 (14%) had all 4 spirals above the 95th % cutoff for controls (Figure 1, panel B). Only 8 of 80 controls (10%) had at least 1 spiral with an Ln(RMS) score above the 95th % cutoff; 5 (6%) had at least 2 spirals above the 95th % cutoff; 3 (almost 4%) had at least 3 spirals above the 95th % cutoff; and none had all 4 spirals above the 95th % cutoff (Figure 1, panel B).
Figure 1: Hand fine motor control deficit in children and adults with classic galactosemia quantified by RMS score of digital Archimedes spirals.
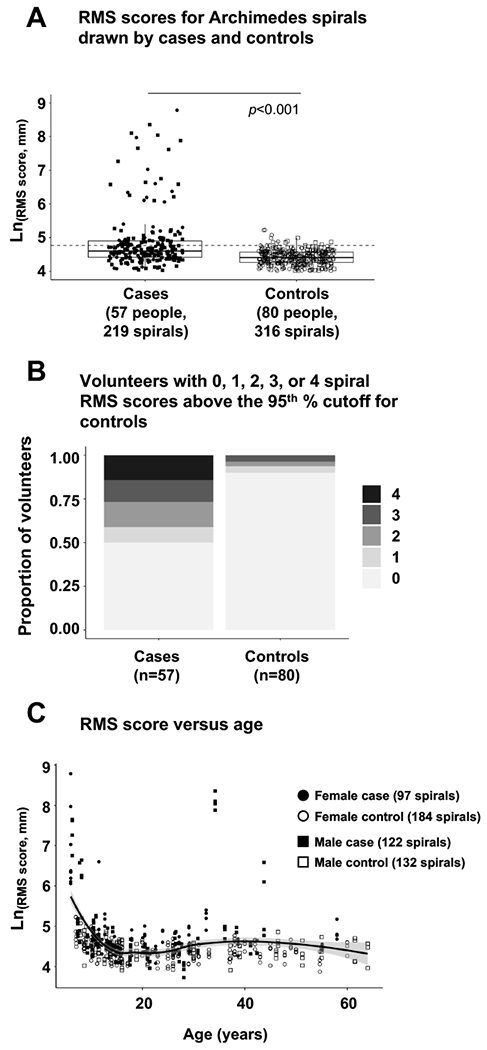
(Panel A) Box and whisker plots of Ln(RMS score) for individual spirals drawn by cases and controls. Each box illustrates the median (heavy line) and upper and lower quartile boundaries for the population presented. Whiskers illustrate the highest and lowest individual data points that are no more than 1.5 times the interquartile range (IQR), and any points falling outside this limit represent suspected outliers. Filled symbols represent individuals with classic galactosemia (cases), open symbols represent individuals without galactosemia (controls). Circles represent females; squares represent males. The dotted horizontal line marks the 95th % cutoff for controls. (Panel B) Proportion of cases and controls presenting 0, 1, 2, 3, or 4 spirals with RMS scores above the 95th % cutoff defined for controls. (Panel C) Individual spiral RMS scores for cases and controls as a function of age. The solid line presents a best-fit curve to the combined data, and the shaded area represents the 95% range for all subjects (cases and controls).
Testing demographic factors for possible association with deficit in hand fine motor control
To test whether markers of hand fine motor control deficit associated with demographic factors among volunteers in our cohort we applied linear mixed-effects models as described in Materials and Methods. Sex (p=0.334), race (p=0.807), and ethnicity (p=0.206) all failed to show even nominally significant association with RMS score. In contrast, age was nominally associated with RMS score (p=0.035) for both cases and controls, but only to the extent that younger children showed higher scores than did teens or adults (Figure 1, panel C). Older adults did not show greater prevalence of deficit than younger adults.
To test whether the relationship between age and RMS or tremor amplitude might be different for cases and controls we calculated median RMS and tremor amplitude scores from individual spirals drawn by each of the following groups: cases ages 6-10, cases older than 10, controls ages 6-10, and controls older than 10 (Supplemental Table 1). Cases ages 6-10 showed RMS scores that were, on average, 50.45% higher than cases older than 10, and tremor amplitude scores that were, on average, 28.59% higher than cases older than 10. Controls ages 6-10 showed RMS scores that were, on average, 28.59% higher than controls older than 10, and tremor amplitude scores that were, on average, 17.49% higher than controls older than 10.
Testing other candidate covariates for possible association with hand fine motor control deficit among cases
Finally, we used linear mixed-effects models to test whether other possible covariates associated with RMS scores among cases. The factors tested included: handedness (p=0.878, n=55), age at dietary restriction of galactose (p=0.1327, n=43), severity of acute symptoms in the neonatal period (p=0.5211, n=44), history of speech difficulties (p=0.222, n=52), ABAS-3 composite score (p=0.1722, n=19), predicted residual GALT activity (p=0.822, n=41), and rigor of dietary galactose exposure (p=0.879, n=47 for birth to <1year, n=49 for 1-5 years, n=43 for age at least 6 years). To the limits of our data, none showed even nominal association. Similarly, tremor amplitude failed to show even nominally significant association with the factors tested.
Detection of kinetic hand tremor in both children and adults with classic galactosemia (CG) by analysis of digital Archimedes spirals
Our second goal was to test for presence and prevalence of kinetic hand tremor among cases and controls. As described in Materials and Methods, we quantified kinetic hand tremor as tremor amplitude calculated from the area under the velocity spectrum curve in the range of 4-8 Hz (Hess and Pullman 2012). As with RMS, we saw a clear and statistically significant difference in tremor amplitude scores between cases and controls (p<0.001) (Figure 2, panel A).
Figure 2: Hand kinetic tremor quantified by tremor amplitude of digital Archimedes spirals drawn by cases and controls.
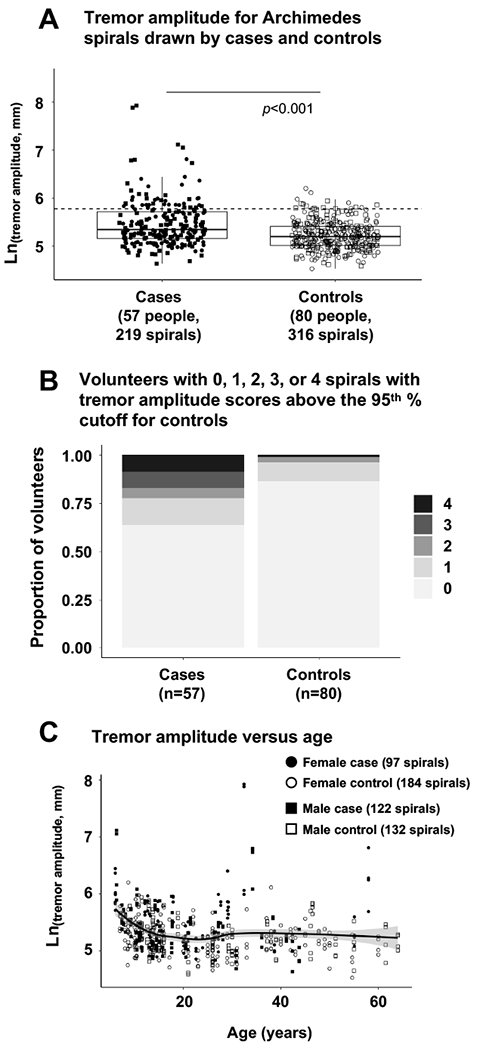
(Panel A) Box plots of Ln(tremor amplitude) for individual spirals drawn by cases and controls. Each box illustrates the median (heavy line) and upper and lower quartile boundaries for the population presented. Whiskers illustrate the highest and lowest individual data points that are no more than 1.5 times the interquartile range (IQR); any points falling outside this limit represent suspected outliers. Filled symbols represent individuals with classic galactosemia (cases); open symbols represent individuals without galactosemia (controls). Circles represent females; squares represent males. The dotted horizontal line marks the 95% cutoff for controls. (Panel B) Proportion of cases and controls presenting 0, 1, 2, 3, or 4 spirals with tremor amplitude scores above the 95th percentile cutoff defined for controls. (Panel C) Individual spiral RMS scores for cases and controls as a function of age. The solid line presents a best-fit curve to the combined data, and the shaded area represents the 95% range for all subjects (cases and controls).
To determine the prevalence of kinetic hand tremor among volunteers in our study we calculated the number of cases with spirals whose Ln(tremor amplitude) score fell above the 95th % cutoff as defined for controls. As above, we used median to measure the center and percentiles of each distribution. We found that 20 of 57 cases (35%) had at least 1 spiral with an Ln(tremor amplitude) score above the 95th % cutoff; 13 (23%) had at least 2 spirals above the 95th % cutoff; 10 (almost 18%) had at least 3 spirals above the 95th % cutoff; and 5 (almost 9%) had all 4 spirals above the 95th % cutoff for controls (Figure 2, panel B). As with RMS score, and for both cases and controls, children showed higher tremor amplitude scores than did teens or adults (Figure 1, panel C).
Relationship between RMS score and tremor amplitude for cases and controls
Finally, we explored the possible relationship between RMS score and tremor amplitude quantified for spirals drawn by cases and controls. As illustrated in Figure 3, the majority of points clustered with both Ln(RMS) and Ln(tremor amplitude) scores limited to a small range. A substantial number of cases, but not controls, fell notably outside the cluster and showed a loose correlation between exceptionally high RMS score and exceptionally high tremor amplitude. However, these data were not sufficient to establish with certainty whether tremor is a major cause of hand fine motor control deficit in CG.
Figure 3: Relationship between RMS score and tremor amplitude of digital Archimedes spirals drawn by cases and controls.
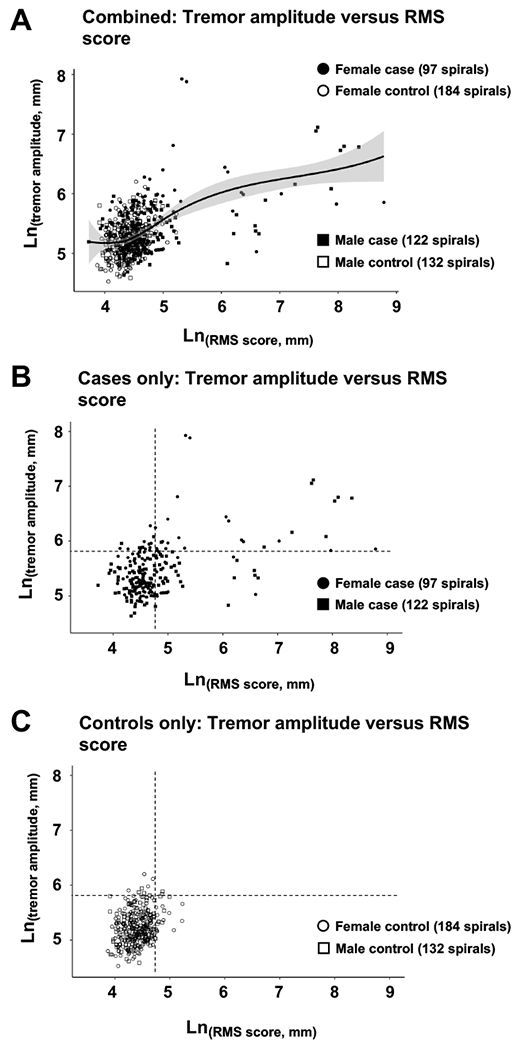
(Panel A) Individual spiral tremor amplitude scores as a function of RMS score for both cases and controls. The solid line presents a best-fit curve to the combined data, and the shaded area represents the 95% range for all subjects. (Panel B) Individual spiral tremor amplitude scores as a function of RMS score for cases only. The dashed lines represent the 95th % cutoffs for Ln(RMS score) and Ln(tremor amplitude) as defined for controls. (Panel C) Individual spiral tremor amplitude scores as a function of RMS score for controls only. The dashed lines represent the 95th % cutoffs for Ln(RMS score) and Ln(tremor amplitude) as defined for controls.
Discussion:
The results reported here are important for 2 reasons. First, we have both confirmed and extended from prior studies detailing the prevalence and characteristics of hand fine motor control deficits among children and adults with classic galactosemia. Our results therefore help to define the natural history of this long-term outcome among patients. Second, our results establish digital analysis of Archimedes spirals as a valuable approach to quantify both hand fine motor control and kinetic hand tremor in this patient population.
Results in context:
Prior studies of motor deficit in patients with classic galactosemia have relied on a combination of self-report surveys, interviews, medical records review, and video-recordings evaluated by experts (Kuiper et al 2019, Rubio-Agusti et al 2013, Rubio-Gozalbo et al 2019, Waggoner et al 1990). Those studies have reported prevalence of fine and/or gross motor deficits in 48% (Kuiper et al 2019) to 52% (Rubio-Gozalbo et al 2019) of patents with tremor present in only a subset. While not fully comparable, those numbers are consistent with the results reported here: 51% cases showing at least one spiral RMS score above the 95th % cutoff for controls, and 35% showing at least one spiral tremor amplitude score above the 95th % cutoff for controls.
Also, like prior studies, we found no association between evidence of hand fine motor control deficit and a selection of possible covariates tested, including demographic and other factors. Of particular interest, the deficits we noted were present in both children and adults, and we saw no increase in prevalence or severity of this outcome in subjects of increasing age. While true longitudinal studies with repeated measures of individual volunteers will be required to address the question of possible outcome progression, the cross-sectional data reported here and in prior studies are at least consistent with the idea that motor control deficits in classic galactosemia are not progressive in most patients. It is also interesting to note that while children in both case and control groups showed more difficulties with hand fine motor control than did teens or adults, the disparity was greater for cases. Whether this disparity is clinically meaningful, and perhaps secondary to other aspects of developmental delay common among children with CG, remains unknown.
Utility of digital assessment:
That our data were collected and analyzed using a simple, scalable digital approach that identified and scored hand fine motor control deficits and kinetic hand tremors at a comparable prevalence to more traditional approaches opens the door to using digital data capture and analysis for future studies involving very large numbers of volunteers, or repeat testing of volunteers, such as over time, or before and after a candidate treatment.
Limitations:
While a major step forward, this study also had notable limitations. For example, despite the test administrator’s best efforts, it is possible that some participants may have misunderstood the instructions and so may have compromised their spirals in some way; for example, by drawing too fast or too slow. If tremor or other causes of hand fine motor control deficit are intermittent, it is also possible that, despite collecting and analyzing 4 separate spirals from almost every person, the spirals collected from some participants may not have been representative. Cohort size and composition were also limitations: while 57 cases may be large for a rare disorder, we cannot rule out that the volunteers in our study may not have been representative of the full patient population. For example, most of the volunteers in this study self-identified as white, and all were 6-65 years old, so we cannot reliably extrapolate to more diverse or younger or older groups.
The Neuroglyphics digital system running on a Surface Pro tablet also conferred limitations. For example, irregularities visible only at the beginning of a spiral drawing would likely have been missed as the 1st 4 quadrants were generally cropped prior to analysis. Extremely unrecognizable spirals (e.g., Supplemental Figure 1, panel C) may also have been handled poorly by the scoring system (Haubenberger et al 2016, Haubenberger et al 2011). Further, if the pen tip left the surface of the tablet during drawing it was not scored, so that a tremor or other problem with motor control that occurred during that time might have been missed. In addition, our approach tested only hand, and to some extent arm, fine motor control. Whether these results give insight into gross motor deficits or fine motor deficits involving other body parts remains unknown. Finally, whether the hand fine motor control deficits described here represent clinically significant challenges that negatively impact quality of life of the participants was not tested here and so remains unknown.
Conclusion:
Despite limitations, the implication of the results presented here is simple and important: hand fine motor control deficit in classic galactosemia, with or without kinetic tremor, is a robust outcome that is amenable to direct and objective assessment even for large cohorts. This realization should facilitate future studies of intervention and outcomes in classic galactosemia.
Supplementary Material
Supplemental Figure 1: Examples of spirals drawn in Neuroglyphics. In all spirals, dots indicate start and stop points, and rectangles show where each drawing was cropped leaving 3.5 spiral turns for analysis. (Panel A) Spiral showing both low RMS and low tremor amplitude scores. (Panel B) Spiral showing moderate RMS score with a high tremor amplitude. (Panel C) Spiral showing a high RMS score but only moderate tremor amplitude.
1-sentence take-home message:
Both children and adults with classic galactosemia experience increased prevalence of deficits in hand fine motor control that can be easily detected and quantified by objective analysis of digital Archimedes spirals.
Acknowledgments
We are extremely grateful to the many individuals and families who participated in this study, to Dr. Camilo Toro who created and maintains the Neuroglyphics program, and to the Galactosemia Foundation for enabling us to collect data at their 2018 conference. We also thank Ms. Martine Williams and Ms. Annie McNeill for helping with some of the data collection.
Funding:
This work was supported by National Institutes of Health grant R01 DK107900 (to JLFK).
Footnotes
One color diagram in supplemental data.
Conflicts of interest: None of the authors have any conflicts of interest to disclose.
Ethics approval: All human subjects work reported here was approved by the Emory University Institutional Review Board as part of protocol 00024933 (PI: JL Fridovich-Keil).
Patient consent statement: All volunteers participated following appropriate informed consent (and assent, for pediatric volunteers) in Emory IRB protocol 00024933.
IACUC: This study did not involve animal subjects.
References
- Bhatia KP, Bain P, Bajaj N et al. (2018) Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 33: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick AB, Cutler DJ, Fridovich-Keil JL (2017) Rigor of non-dairy galactose restriction in early childhood, measured by retrospective survey, does not associate with severity of five long-term outcomes quantified in 231 children and adults with classic galactosemia. J Inherit Metab Dis 40: 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubenberger D, Abbruzzese G, Bain PG et al. (2016) Transducer-based evaluation of tremor. Mov Disord 31: 1327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubenberger D, Kalowitz D, Nahab FB et al. (2011) Validation of Digital Spiral Analysis as Outcome Parameter for Clinical Trials in Essential Tremor. Movement Disorders 26: 2073–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CW, Pullman SL (2012) Tremor: clinical phenomenology and assessment techniques. Tremor Other Hyperkinet Mov (N Y) 2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper A, Grunewald S, Murphy E et al. (2019) Movement disorders and nonmotor neuropsychological symptoms in children and adults with classic galactosemia. J Inher Metab Dis 42: 451–458. [DOI] [PubMed] [Google Scholar]
- Pyhtila BM, Shaw KA, Neumann SE, Fridovich-Keil JL (2015) Newborn screening for galactosemia in the United States: looking back, looking around, and looking ahead. JIMD Rep 15: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raethjen J, Pawlas F, Lindemann M, Wenzelburger R, Deuschl G (2000) Determinants of physiologic tremor in a large normal population. Clinical Neurophysiology 111: 1825–1837. [DOI] [PubMed] [Google Scholar]
- Rubio-Agusti I, Carecchio M, Bhatia KP et al. (2013) Movement disorders in adult patients with classical galactosemia. Mov Disord 28: 804–10. [DOI] [PubMed] [Google Scholar]
- Rubio-Gozalbo ME, Haskovic M, Bosch AM et al. (2019) The natural history of classic galactosemia: lessons from the GalNet registry. Orphanet J Rare Dis 14: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Buttlar AM, Zabel TA, Pritchard AE, Cannon AD (2021) Concordance of the Adaptive Behavior Assessment System, second and third editions. J Intellect Disabil Res 65: 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner DD, Buist NR, Donnell GN (1990) Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis 13: 802–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Examples of spirals drawn in Neuroglyphics. In all spirals, dots indicate start and stop points, and rectangles show where each drawing was cropped leaving 3.5 spiral turns for analysis. (Panel A) Spiral showing both low RMS and low tremor amplitude scores. (Panel B) Spiral showing moderate RMS score with a high tremor amplitude. (Panel C) Spiral showing a high RMS score but only moderate tremor amplitude.


