Abstract
Background: The anastomosis leak in colon resections is a crucial post-operative complication with significant morbidity and mortality. Methods: Forty (40) Wistar rats were allocated in two groups. In SHAM group only anastomosis was performed. In ILEUS group anastomosis was performed following one day of ileus. Animals in both groups were subdivided in two groups according to the day they were sacrificed, 4th or 8th post-operative day. A number of variables between the groups were estimated. Results: Body weight loss was higher following obstructive ileus on both days. Adhesion score in 4th and 8th post-operative day was higher in ILEUS1, ILEUS2 groups compared to SHAM1, SHAM2 groups respectively (p<0.001 for both). Neovascularization decreased following obstructive ileus compared to control on the 4th day (ILEUS1 vs. SHAM1, p=0.038). Bursting pressure was lower in ILEUS2 group than SHAM2 group (p<0.001). The number of fibroblasts decreased following obstructive ileus compared to control on the 4th and 8th day (ILEUS1 vs. SHAM1, p=0.001, ILEUS2 vs SHAM2, p=0.016). Hydroxyproline concentration was decreased in ILEUS2 group compared to SHAM2 group (p<0.001). Conclusions: The balance of collagenolysis and collagenogenesis plays a decisive role in the healing of anastomoses following bowel obstruction. Under those circumstances, anastomosis’ bursting pressure is reduced owning to decreased neovascularization, reduced fibroblast presence and lower hydroxyproline concertation. In our study, local inflammation, neocollagen concentration and collagenase activity were not associated with this adverse effect. However, further research should delineate the mechanisms of healing of colonic anastomoses and identify those factors that can improve our outcomes.
Keywords: Anastomosis, ileus, leak, bursting pressure, collagen.
INTRODUCTION
Colonic surgery is attracting increasing interest due to its particular anatomical and pathophysiological characteristics and the increased incidence of colonic malignancy. Colorectal cancer is the third most common cancer in the world, and surgery is the mainstay of treatment. It is estimated that 1 out of 20 people over 75 will develop colon cancer1,2. A prerequisite for the success of surgery and the reduction of post-operative complications is the creation of safe anastomosis following surgical resection3,4. The healing of anastomoses represents a complex process of repairing tissue damage, the end result of which depends on several local and systemic factors. The healing process is the subject of many clinical and experimental studies, as it is an important prognostic factor of postoperative morbidity and mortality in colorectal cancer interventions5-7.
Anastomosis leakage in colon resections, especially for cancer, remains a very serious post-operative complication with significant morbidity and mortality. Despite advances in surgical techniques, suture materials, adequate preoperative preparation, and perioperative chemoprophylaxis, the rate of leakage from anastomosis has remained high (3-30%) over the last few decades4,8-10. In particular, it ranges from more than 17% in selective to 30% in urgent interventions. It is also known that more than 30% of patients with colorectal cancer first present with obstructive ileus symptomatology and 10% of them need urgent surgical treatment, which is accompanied by an increased incidence of anastomosis leakage11-13.
The healing of anastomosis after colon resection following obstructive ileus is difficult. On the one hand, the large proximal dilation of the intestine causes wall ischemia, on the other hand, the large difference in the diameters of the two parts that are anastomosed leads to an increased likelihood of anastomosis dehiscence. Additionally, ischemia of the intestinal wall, hypovolemia and electrolyte disturbances can inhibit anastomotic healing. The creation of protective ileostomy or colostomy proximal of the anastomosis, although it does not appear to affect healing, seems to reduce postoperative morbidity and mortality by protecting against possible anastomotic dehiscence14.
The aim of the current experimental study was to evaluate the healing of colonic anastomosis following large bowel obstruction. Anastomosis healing was evaluated by measuring anastomosis ruptures and the gravity of adhesions by measuring the bursting pressure of intact anastomoses, quantifying hydroxyproline and collagenase and evaluating histological parameters of the healing process in the area of anastomosis.
MATERIALS AND METHODS
Experimental animals
An experimental animal protocol was designed to minimize discomfort and pain to the animals. For this research, forty male rats of the Wistar species were used, each weighing 200-300 g. The Ethical Committee of the Department of Veterinary Services of the Prefecture of Thessaloniki approved this research (S.N.: 13/11872/11-09-08) and all necessary approved protocols for laboratory animal care were applied. The rats were under a 12-hour cycle of light and dark conditions for seven to ten days prior to the operation. Each animal was individually housed and had unlimited access to both food and water before and after the operation. No antibiotics were administrated. The sacrifice was performed using intracardial administration of KCL solution of 10% concentration.
Experimental groups
The animals were divided randomly into 2 groups of 20 rats each.
ILEUS GROUP (n = 20 animals): Anastomosis was performed following one day of obstructive ileus:
ILEUS1 (10 animals): the sacrifice took place on the 4th post-op day
ILEUS2 (10 animals): the sacrifice took place on the 8th post-op day
SHAM GROUP (n = 20 animals): Only anastomosis was performed. SHAM1 (10 animals): the sacrifice took place on the 4th post-op day SHAM2 (10 animals): the sacrifice took place on the 8th post-op day
SHAM1 (10 animals): the sacrifice took place on the 4th post-op day
SHAM2 (10 animals): the sacrifice took place on the 8th post-op day
Anaesthesia and operative technique
The surgery was initiated using a 3cm incision at the midline. The anaesthesia was administered intraperitoneally using thiopental at a 40mg per kilogram concentration. A 1cm part of the colon was resected at a distance of 5cm from the rectum followed by an end-to-end anastomosis. Eight polypropylene sutures were used for the anastomosis, with 6-0 at a single layer. The obstructive ileus was initially “created” using 3-0 silk suture at a 5cm distance from the rectum. 24 hours later, a 1cm part of the large intestine around the ligation area was excised and an end-to-end anastomosis was performed. The abdominal wall was closed using 3-0 silk sutures.
Body Weight
All animals were weighed at the start of the experiment and before sacrifice. Measurement was done with precision scales according to a code assigned to each animal after the anaesthesia was administered.
Macroscopic examination
On the sacrifice day, the animals were subjected to laparotomy after proper administration of anaesthesia. During the operation, the anastomotic parts of the intestine were dissected and examined macroscopically. The presence of abscess near the anastomosis or peritonitis, the presence of adhesions and anastomotic integrity were evaluated using the van der Ham scale15. Score 0 represented no adhesion formation, score 1 a minimum number of adhesions, score 2 moderate adhesion between the anastomosis and, for example, an intestine loop and score 3 represented severe adhesion formation plus the presence of abscess.
Bursting pressure
Bursting pressure was evaluated ex vivo. A 2.5cm part of the colon containing the anastomoses was removed alongside the attached adhesions. A catheter was inserted into the distal part of the removed colon after the proximal part was closed with a silk 3-0 suture. The catheter was secured at the bursting pressure measuring device. The catheter was fixed to the bursting pressure apparatus, as described in previous research16-18. Through this, the lumen of the bowel was filled with normal saline solution at a 1ml/min rate. The pressure, measured in mmHg, at which a leakage of normal saline or rupture occurred was defined as the bursting pressure. The exact point of a possible leak or rupture during the measurement of bursting pressure was noted, due to the fact that in some rats that point was the site of the anastomosis and in others was at another point.
Histological assessment
After the bursting pressure measurement, the resected part of the intestine was separated from the mesentery and the fat then cleaned with normal saline. The segment of the colon containing the anastomosis was resected, with half-cm parts on each side. This was divided into two parts in the vertical level. One part of the segmented colon was placed in a formaldehyde solution of 4% concentration for histology examination and then stained with eosin-haematoxylin stain. Under a light microscope, the anastomosis was evaluated histologically using the 0-4 Erlich-Hunt grading scale with Phillips et al.’s modification19. The parameters included: the infiltration of inflammatory cells, the formation of new blood vessels, fibroblast activity and the deposition of collagen20. These parameters were evaluated individually using a numbered scale: 0 (-) = no evidence; 1 (+) = occasional evidence; 2 (++) = light scattering; 3 (+++) = abundant evidence; and 4 (++++) = confluent fibres or cells.
Hydroxyproline
The evaluation of collagen deposition in the anastomosis was performed by evaluating the hydroxyproline percentage. For that purpose, the second part of the resected colon with the anastomosis was weighed and kept at -20°C after being cut into two segments vertically. The hydroxyproline quantity in the tissues was measured with modified methods from other experiments21,22. The samples were first lyophilized, then the tissue specimens were homogenized using a polytron homogenizer in distilled water. The collagen that was soluble to acid was taken from the tissue of the specimens using incubation overnight with an acetic acid solution of 0.5mol/l at 4°C. 70 μL of standard/test sample was hydrolysed in 30 μL NaOH 10.125 mol/L for 25 min at 120℃ by autoclaving. After hydrolysation, the sample was blended with a chloramines-T reagent buffer (0.056mol/L) at room temperature. The oxidation processed lasted for 25 minutes. The chromophore growth was followed by adding Ehrlich’s reagent. The absorption was assessed at 550nm using a Biotek μQuant™ spectrophotometer. The presence of hydroxyproline in unknown tissue extracts was determined from the standard curve after absorbance values were plotted against the concentration of standard hydroxyproline. The outcomes were evaluated in μg per tissue gram21.
Type Ⅰ Collagenase
The density of type I collagenase was also measured in another part of the anastomotic area using an ELISA kit (USCNLIFEm E0212r). A plate was precoated with specific antibodies for type I collagenase (polyclonal, conjugated to biotin). The control sample was then added. Avidin, which was also conjugated to hydroxyproline, was also added to the plate and hatched. The development of chromophore occurred after adding TMB substrate solution. The reaction was terminated using a sulphuric acid solution. Using spectrophotometry, the change in colour was assessed at 450nm (by Stat Fax-210™ spectrophotometer (Awareness Technology Inc.)) The density of type I collagenase in random tissue specimens was extracted from the standard curve. The results were measured in μg per gram of wet tissue23.
Statistical analysis
The data extracted from the experiment were summarized using statistical descriptive indices of central tendency and dispersion. Data appear as mean value +/- standard deviation as they were normally distributed after the Shapiro-Wilk test for normality. ANOVA was performed to compare all the study groups. For the post hoc pairwise comparisons the independent t-test were used with the Bonferoni correction Paired before-and-after observations on the same subjects were analyzed using the paired t-test. Percentages were compared using the Fisher’s Exact Test. The level of statistical significance was set at p value <0.05 for the comparisons between the groups. All the statistical analyses were performed using the IBM SPSS Statistics (Version 22) enhanced with the module Exact Tests.
RESULTS
Bodyweight
The mean body weight was calculated both preoperatively and on the day of sacrifice (4th and 8th postoperative days). The difference between the mean values was evaluated. According to the statistical analysis, the mean weight between the subgroups did not present a statistically significant difference at the beginning of the experiment (p=0.707). However, the mean body weight differed between groups at the end of the experiment (p<0.001), as the weight in group ILEUS2 was significantly lower than in group ILEUS1 (p=0.003). Bodyweights at the beginning and end of the experiment within the same group did not differ significantly between group SHAM1 (p=0.168) and SHAM2 (p=0.09) but did differ in groups ILEUS1 (p<0.001) and ILEUS2 (p<0.001). Figure 1 presents the bodyweights at the beginning and end of the experiment.
Figure 1. Comparative bar chart of body weight.
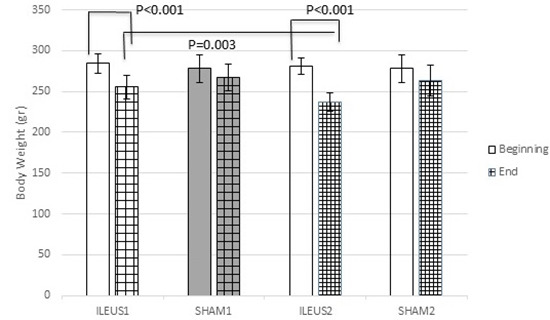
Statistically significant difference in body weight in both 4th and 8th post-operative day was noted in ILEUS group. In addition, body weight in ILEUS2 subgroup was statistically significant lower compared to ILEUS1 subgroup after the intervention.
Bodyweight decreased in all experimental groups from the day of the experiment to the day of sacrifice. Bodyweight changes differed significantly among the subgroups (p < 0.001). In particular, significant differences in bodyweight change were found between subgroups SHAM1 and ILEUS1 (p < 0.001), SHAM2 and ILEUS2 (p < 0.001), and ILEUS1 and ILEUS2 (p=0.001). Figure 2 presents the bodyweight changes.
Figure 2. Comparative bar chart of body weight changes.
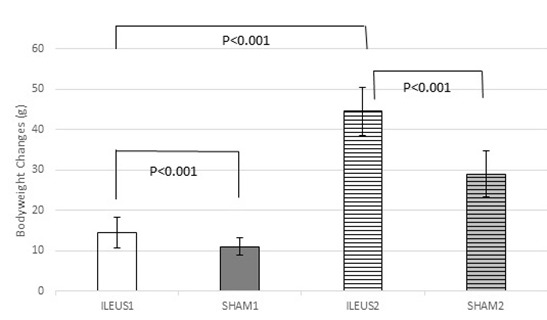
In ILEUS subgroup, there was a statistically significant increase in body weight changes from the fourth to the eighth day. In addition, weight changes in SHAM2 subgroup was decreased in comparison to ILEUS2 subgroup and SHAM1 in comparison to ILEUS1.
Anastomotic dehiscence
All animals survived the experiment. Sacrifice occurred at the 4th and 8th postoperative days, according to the protocol of the experiment. The colon segment that was removed was examined macroscopically to determine whether the anastomosis was intact. The frequency of rupture of the anastomosis was evaluated on both the 4th and 8th postoperative days. On the 4th postoperative day in the SHAM1 group, no rupture occurred, while in the ILEUS1 group there were 2 animals with anastomotic dehiscence (20%). However, there was no statistically significant difference in the rupture percentages (p=0.474). At the 8th postoperative day, no anastomotic leak occurred in the SHAM2 group, while 3 animals presented with anastomotic leak in the ILEUS2 group (30%). However, there was no statistically significant difference in the dehiscence percentages (p=0.211). The burst of the anastomosis resulted in perianastomotic abscess and peritonitis. In addition, no difference in dehiscence percentages was noted between the SHAM1 and SHAM2 groups and the ILEUS1 and ILEUS2 groups (p=1).
Adhesions
The evaluation of adhesions was done during the macroscopic examination of the anastomosis, according to the van der Hamm scale. According to this scale, on the 4th postoperative day, 90% of the rats showed no adhesions in subgroup SHAM1, while 10% had grade 1 adhesions. In contrast, in subgroup ILEUS1, all rats exhibited adhesions, with 80% being grade 1 and 20% grade 3. At the 8th postoperative day, 90% of the SHAM2 subgroup rats showed no adhesions, while 10% had grade 1 adhesions. In the subgroup ILEUS2, all rats showed adhesions, with 80% of them grade 2 and 20% grade 3. The adhesion formation scores differed significantly between groups (p < 0.001; Figure 3).
Figure 3. Comparative bar chart of adhesion formation (mean).
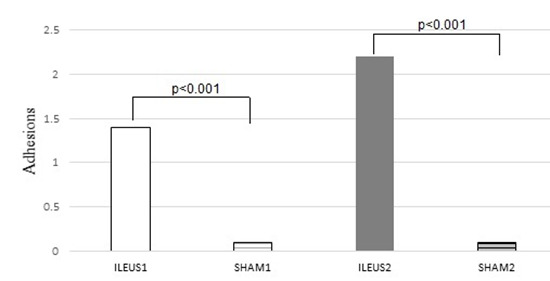
The adhesion formation score was statistically significantly higher in both ILEUS subgroups compared to SHAM.
On both the 4th and 8th postoperative days, adhesion scores were significantly higher in the ILEUS1 and ILEUS2 groups compared to the SHAM1 and SHAM2 groups (p<0.001 for both). Also, while the adhesion formation score didn’t differ in the SHAM group between the 4th and 8th days, in the ILEUS group, adhesions increased significantly from the 4th to the 8th day (p=0.015).
Bursting Pressure
The bursting pressure at the ruptured anastomosis was set at 0mm Hg. There was a significant difference in bursting pressure among groups (p < 0.001). On the 4th postoperative day, the ILEUS1 group presented lower mean bursting pressure values compared to SHAM1, but the difference was not significant (p=1). However, on the 8th day, the bursting pressure in the ILEUS2 group was significantly lower than in SHAM2 (p<0.001; Figure 4). Comparing the bursting pressures on the 4th and 8th days within each group revealed that it increased for both groups, but the difference was significant only in the SHAM group (p<0.001), and not for the ILEUS group (p=0.202).
Figure 4. Comparative bar chart presenting bursting pressures (mmHg) (mean ± standard deviation).
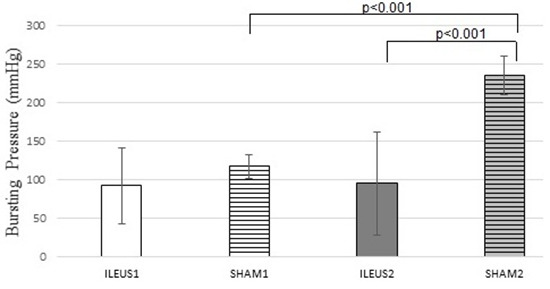
A statistically significant increase in the bursting pressure was noted on the eighth postoperative day in the SHAM2 subgroup compared to the ILEUS2 and SHAM1 subgroups.
Leakage Site
During the evaluation of bursting pressure, the examined parts of the colon ruptured at the anastomotic site or elsewhere. On the 4th day, the rupture rate at the anastomotic site was greater in the ILEUS1 group (90%) compared to the SHAM1 group (50%); however, the difference was not statistically significant (p=0.141). On the 8th day, the results were analogous to the previous ones, with the ILEUS2 group presenting with a greater incidence of rupturing at the anastomotic site (80%) compared with the SHAM2 group (0%), but the difference was statistically significant (p=0.005). Comparing the leakage site on the 4th and 8th days within each group, it was observed that while leakage at the anastomotic site decreased from the 4th to the 8th day in both groups, this difference was significant for the SHAM group (p=0.033), but not for the ILEUS group (p=1).
Histological Examination
The segments from the anastomotic region were assessed microscopically after haematoxylin and eosin staining. The classification was semiquantitative/ categorical and was based on the Erlich-Hunt scale, as amended by Philips19. The analysis was done in a blind fashion by an experienced pathologist. In each group, the evaluated parameters were leukocytosis, neovascularization, fibroblasts and neo-collagen generation.
Leukocytosis
Statistical analysis didn’t reveal any significant differences among groups (p=0.999). Leukocytosis increased following obstructive ileus, compared to the control group, both on the 4th and 8th postoperative days, but none of the differences were significant. Comparing leukocytosis on the 4th and 8th days within each group revealed that a significant decrease from the 4th to the 8th day occurred in both groups (ILEUS1 vs. ILEUS2 with p < 0.001 and SHAM1 vs. SHAM2 with p < 0.001; Figure 5).
Figure 5. Comparative bar chart presenting the average inflammatory cell infiltration, according to the scale of Ehrlich and Hunt, as modified by Philips et al. (mean ± standard deviation).
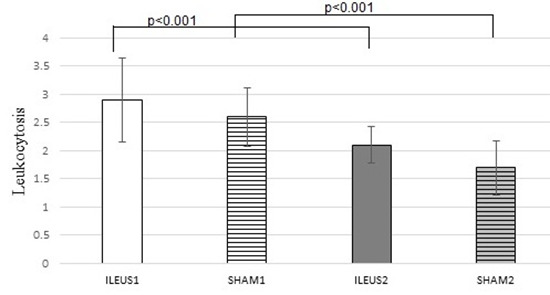
Increased leucocytosis in ILEUS1 subgroup compared to ILEUS2 subgroup was noted. In addition, increased leucocytosis was noted in SHAM1 group compared to SHAM2 group.
Neovascularization
Statistical analysis revealed significant differences among groups (p < 0.001). Neovascularization significantly decreased following obstructive ileus, compared to the control, on the 4th day (ILEUS1 vs. SHAM1 with p=0.038), but not on the 8th day (ILEUS2 vs. SHAM2 with p=0.559). Comparing neovascularization on the 4th and 8th days within each group revealed that a significant increase from the 4th to the 8th day occurred in both groups (ILEUS1 vs. ILEUS2 with p<0.001 and SHAM1 vs. SHAM2 with p<0.001; Figure 6).
Figure 6. Comparative bar chart presenting the average new vessel formation (neoangiogenesis), according to the scale of Ehrlich and Hunt, as modified by Philips et al. (0–4) (mean ± standard deviation).
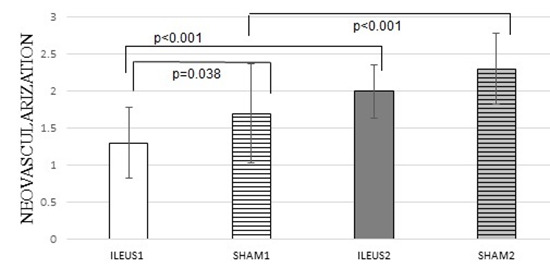
The average neoangiogenesis was statistically significantly increased in ILEUS2 subgroup compared to ILEUS1 subgroup. In addition, increased neovascularization was noted in SHAM2 group compared to SHAM1 group. Furthermore, augmented neoangiogenesis was revealed in SHAM1 group compared to ILEUS1 group.
Fibroblasts
Statistical analysis revealed significant differences among groups (p < 0.001). Fibroblasts significantly decreased following obstructive ileus, compared to the control, both on the 4th and on the 8th days (ILEUS1 vs. SHAM1 with p=0.001 and ILEUS2 vs. SHAM2 with p=0.016). Comparing fibroblasts on the 4th and 8th days within each group revealed that a significant increase from the 4th to the 8th day occurred in both groups (ILEUS1 vs. ILEUS2 with p < 0.001 and SHAM1 vs. SHAM2 with p=0.001; Figure 7).
Figure 7. Comparative bar chart presenting the average fibroblast activity, according to the scale of Ehrlich and Hunt, as modified by Philips et al. (0–4) (mean ± standard deviation).

The average fibroblast activity was statistically significantly higher in ILEUS2 subgroup compared to ILEUS1 subgroup. In addition, increased fibroblast activity was noted in SHAM2 group compared to SHAM1 group. Furthermore, augmented fibroblast activity was revealed in SHAM1 group compared to ILEUS1 group SHAM2 group compared to ILEUS2 group.
Neocollagen
Statistical analysis revealed significant differences among groups (p < 0.001). Neocollagen was not affected by obstructive ileus on the 4th day and slightly decreased, but not significantly, on the 8th day compared to the control (p=0.287). Comparing neocollagen on the 4th and 8th days within each group showed that a significant increase from the 4th to the 8th day occurred in both groups (ILEUS1 vs. ILEUS2 with p<0.001 and SHAM1 vs. SHAM2 with p<0.001; Figure 8).
Figure 8. Comparative bar chart presenting the average collagen deposition, according to the scale of Ehrlich and Hunt, as modified by Philips et al. (0–4) (mean ± standard deviation).
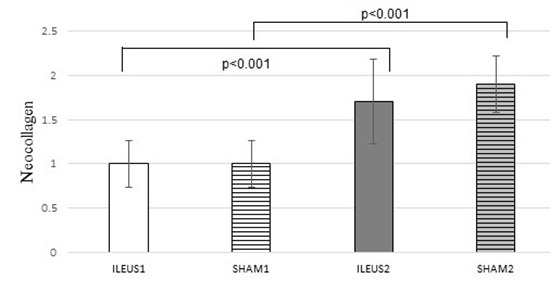
The average collagen deposition was statistically significantly higher in each subgroup in collagen deposition from the fourth to the eighth day.
Hydroxyproline
Hydroxyproline concentration differed significantly between groups (p<0.001). Specifically, it was similar on the 4th day in the ILEUS1 and SHAM1 groups (p=1), but significantly decreased on the 8th day following obstructive ileus, compared to the control (ILEUS2 vs. SHAM2 with p<0.001; Figure 9). Comparing hydroxyproline on the 4th and 8th days within each group showed that a significant increase from the 4th to the 8th day occurred in the control group, but not after obstructive ileus (SHAM1 vs. SHAM2 with p<0.001 and ILEUS1 vs. ILEUS2 with p=0.664).
Figure 9. Comparative bar chart of hydroxyproline tissue contents (μg/g tissue) at the anastomotic site (mean ± standard deviation).
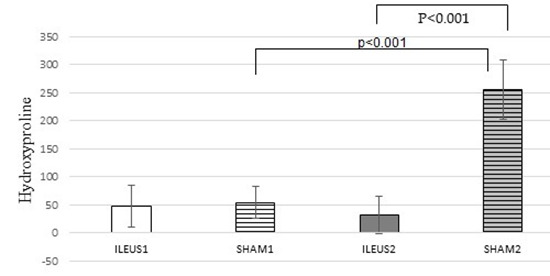
In SHAM subgroup, there was a statistically significant increase in hydroxyproline tissue content from the fourth to the eighth day. In addition, hydroxyproline tissue contents at the anastomotic site in SHAM2 subgroup was increased in comparison to ILEUS2 subgroup.
Type I Collagenase
Collagenase concentration did not differ significantly between groups (p=0.221). Specifically, while it decreased on the 4th day in the ILEUS1 group, compared to the SHAM1 group, the difference was not significant (p=0.928);
furthermore, it increased on the 8th day following obstructive ileus, compared to the control, but the difference was not significant (p=0.1). Comparing collagenase on the 4th and 8th days within each group revealed that while an increase from the 4th to the 8th day occurred in both the control group and after obstructive ileus, no significant difference was observed (Figure 10).
Figure 10. Comparative bar chart of collagenase I tissue contents (μg/g tissue) at the anastomotic site (mean ± Standard deviation).
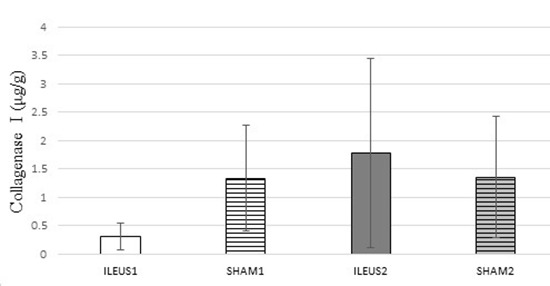
No statistically significant difference was noted.
DISCUSSION8,9,24-109
The healing of anastomosis is a complex biological procedure of restoring tissue damage. The final result depends on both regional and systemic factors. Insufficiency of the mechanisms of the anastomosis healing process results in anastomotic dehiscence, which increases postoperative morbidity and mortality8,9. This risk is particularly high in the case of emergency surgical treatment of obstructive colorectal ileus due to neoplasm, during which the section of the intestine carrying the neoplasm is excised, followed by primary anastomosis24,25.
It is well known that a high proportion of colorectal cancer (8-30%) initially manifests with obstructive ileus symptomatology26,27. The ileus causes disturbances in the intestinal wall perfusion above the obstruction, resulting in poor blood supply to the anastomosis and increased risk of leaking28,29.The prognosis of patients undergoing urgent surgery is worse than those undergoing elective surgery30-32. It is generally believed that excision and anastomosis at one time in the occluded colon is feasible for most patients, except those who are hemodynamically unstable or present with peritonitis24,30. Recent animal studies on minimizing the risk of anastomotic leak provided favourable results. Omiganan, an anti-microbial peptide, enhanced the safety of gastrointestinal anastomoses in rats with intraperitoneal sepsis108, while selective inhibition of hydroxylases proved beneficial105. However, this increases the likelihood of anastomotic dehiscence and, consequently, the development of intra-abdominal abscesses and wound infection33,34.
Despite the improvement in surgical techniques, colorectal surgery is accompanied by significant post-operative morbidity and mortality. The presence of anastomotic leakage or rupture is the most common complication and the most important cause of post-operative morbidity and mortality after colectomy. A rupture is defined as the complete or partial separation of the anastomosis labia, while a leak refers to the presence of intraluminal intestinal contents in the extraluminal area33. The frequency of leakage varies from 0.2 to 4% for colon anastomoses and from 3 to 21% for rectal anastomoses33. This postoperative complication leads to prolonged hospital stays, increased hospitalization costs and increased mortality (from 2.7% to 10-16%)35.
Escape from anastomosis is a multifactorial process that involves local and / or general factors, as mentioned above. All these factors have a common feature: they affect the composition of the new or the degradation of the old collagen, which is a key point in the healing process. In the early phase of healing, anastomosis has increased destruction of old collagen fibres and a reduced composition of new ones. This period is very important because the integrity and strength of the anastomosis depends on the complementary strength of the sutures, as the fibrin layer is very thin and cannot keep the flaps united36. In the first 3 to 5 days, collagen degradation is prominent 2.5cm on both sides of the anastomosis, compared to its composition. After 5-7 days, there is an increase in inflammatory response, neovascularization, fibroblasts and collagen fibres. More specifically, in the first week, the synthesis of type III collagen and fibronectin predominates, followed by the synthesis of type I collagen37,38. Experimental studies have shown a significant reduction in collagen concentrations in the anastomosis area during the early postoperative period39,40. Thus, anastomotic leakage usually occurs between 5 and 7 postoperative days (remodeling phase).
In cases of obstructive ileus, the observed pathophysiological disorders are: 1) changes in intestinal mobility, 2) intestinal content concentration prior to obstruction, 3) distension of the central part of the intestine by the addition of gases and liquids, 4) multiplication of microorganisms and bacterial translocation, 5) changes in blood flow, 6) homeostasis disturbances and 7) systematic disorders24,41. Excessive growth of bacteria in the occluded part of the intestine is also due to the loss of self-purification capacity. The effect of all this is the concentration of a significant amount of intestinal, fluid and air content in the part of the bowel before the obstruction42. That also results in increased pressure before occlusion. While normal intraluminal pressure ranges between 2 and 4 mm Hg, during occlusion it may reach up to 30 mm Hg. This increased intraluminal pressure causes both the coincidence of veins and an increase in hydrostatic pressure in the blood capillaries, with consequent increased fluid output to the intestinal lumen and to the peritoneal cavity12,43. Rapid growth of pathogenic microbial flora (mainly bacteroides) in the supernatant of the obstruction results in increased production of NH4, H2 and CO2. The concentration of these intestinal gasses causes further bowel dilatation44. Compression of the intestinal wall veins, which leads to increased diffusion of fluids from the capillaries to the intercellular space, is followed by an increase in edema of the intestinal wall and consequently the obstruction of arterioles. This results in ischemia, which is more pronounced initially in the intestinal mucosa. The prolongation of ischemia leads to necrosis, perforation of the intestine, excretion of intestinal contents in the peritoneal cavity and peritonitis44,45. The ileus also disrupts the intestinal barrier, resulting in the entry of microbes and endotoxins into systemic circulation via the portal vein. Within 6-12 hours of the occlusion, microorganisms are found within the peritoneal cavity (bacterial translocation), and systemic endotoxemia begins on the 4th day. Endotoxemia may lead to the development of Systemic Inflammatory Reaction Syndrome (SIRS) or Multidrug Disorder Syndrome (MODS)46,47.
It is known that 8-30% of patients with colorectal cancer first appear with obstructive ileus symptomatology and 10% of these require urgent surgical treatment. Obstruction is usually found in the left colon, as its diameter is shorter and its content denser29,42. The ileus causes the reported local and systemic disorders, which inhibit the anastomosis healing process and lead to an increased frequency of dehiscence of the anastomosis. More specifically, the ileus causes the following disorders in the healing of anastomoses: disruption of anastomosis circulation and disruption of bowel wall. Mucosal haemorrhage, especially of the submucosal layer, determines perfusion of the anastomosis. It is known that the capillary network of the colon is less developed than that of the small intestine, which also explains its greater susceptibility to decreased blood flow13,22. In the obstructive ileus, the increased intraluminal pressure causes ischemia of the intestinal wall. In particular, the mucosa is edematous and hypo-perfused, and extensive swelling develops in the submucosal layer. Also, as the ischaemia is prolonged, ulcerations in the intestinal mucosa develop due to destruction of the epithelial cells. Ischemic lesions at this stage are usually reversible. Over the time, however, the lesions extend to the musculoskeletal and the entire thickness of the wall and are irreversible, which can lead to perforation of the intestine41,43. After the cause of the obstructive ileus has been removed, the ischemic bowel is reperfused, and there is a possibility of ischemia-reperfusion injuries43,44. This results in severe tissue alterations in the intestinal epithelium and capillary endothelium due to the release of oxygen free radicals. Ischemic lesions, destruction of the parietal capillaries and secretion of vasoconstrictor substances result in disorder of the anastomosis infusion and inhibit its healing45,48. Experimental studies have shown that ileus increases the activity of metalloproteinases, which reduce the deposition of new collagen in the anastomosis and increase the degradation of the old. Their greater concentration and consequent greater collagen degradation is observed near the staple line. The end result is a delay in the healing of anastomosis and an increased chance of leakage42. Newer studies demonstrated that selective matrix metalloproteinases (MMP)’ inhibition increased anastomotic breaking strength106 and the the application of protective local agents, such as albumin/glutaraldehyde, in colonic anastomoses leads to better outcomes109.
Patients with colorectal cancer, particularly those with an obstructive ileus condition, show a decrease in body weight49,50. It has also been found that subnutrition and hypoproteinaemia inhibit healing of anastomoses, as they reduce collagen synthesis51. In an experimental study of obstructive ileus and tacrolimus, Raptis et al.23 observed greater reduction in the body weights of the experimental animals in the ileus group than in the other group. In our study, both on the 4th and 8th postoperative days, the presence of obstructive ileus led to significant body weight loss compared to the control.
The rate of anastomotic leakage is the most important indicator for the inadequacy of the mechanisms for healing bowel anastomoses, ranging from 3 to 30% 52-55. In occlusive ileus, the risk of anastomotic leakage is greater28,56. Raptis et al.23 found that leaks were more common in the ileus group than in the control group. Similarly, in our study, the frequency of rupture from the anastomosis increased in obstructive ileus conditions. Specifically, on the 4th postoperative day, anastomosis leakage was observed in 20% with obstructive ileus, but the difference with the control was not statistically significant. Similarly, on the 8th postoperative day, anastomotic leakage presented in 30% of rats with ileus, but this difference was also not statistically significant.
Adhesions occur when the organs of the peritoneal cavity and/or the peritoneal wall are traumatized. These are fibrous bands that connect the organs of the peritoneal cavity, either with each other or with the peritoneal peritoneum, and can contribute to microleaks from the anastomosis site, while improving perfusion56,57. Adhesion formation is determined by the severity of intestinal trauma, the activation of fibroblasts, the action of metalloproteinases and the adequacy of the fibrinolytic mechanism58-60. The quantitative evaluation of adhesions in our study was done according to the van der Hamm scale in all subgroups 61. Hoffman et al.57 also reported that adhesions occur more frequently in obstructive ileus. In the current experimental study, both in the 4th and 8th postoperative days, the obstructive ileus subgroup showed significantly more adhesions compared to the control group.
The adequacy of colonic anastomosis healing is assessed by measuring the anastomosis bursting pressure, which is the most reliable method of mechanical power assessment. The bursting pressure is measured by the resistance of the intestinal wall to increased intraluminal pressure62. During the early post-operative period (between day 3 and day 5), there is a dramatic reduction in the collagen concentration of up to 40%, due to increased collagenolysis, closer to the anastomosis site. This explains why intestinal disruption in these cases occurs in the area of reperfusion59,63. The occurrence of rupture outside of anastomosis occurs in labile areas or in focal necrosis sites. With the progressive increase in collagen synthesis from day 5, the strength of anastomosis increases, resulting in the seventh postoperative day to gain 50% of its measured potency. After the 14th postoperative day, the sutures no longer contribute to the strength of the anastomosis and rupture usually occurs away from the anastomosis39. Measurement of anastomosis bursting pressure is the most reliable method of assessing mechanical strength in the first postoperative week and is affected by the amount of collagen deposited37,53,64,65. Obstructive ileus also affects the mechanical strength of anastomoses27,65. Raptis et al.23 found a decrease in mean bursting pressure in the ileus group relative to the control group. In our study, on the 4th postoperative day, while the obstructive ileus subgroup showed lower mean bursting pressure compared to the control, the difference was not significant. However, on the 8th postoperative day, mean bursting pressure in the ileus subgroup was statistically significantly lower compared to control subgroup.
An essential element for evaluating the effects of bursting pressure and assessing the adequacy of healing mechanisms is the point of the bowel part where a rupture occurs in the performance of the corresponding test10,66. Ruptures in the area of the anastomosis occur in less-powerful mechanical anastomoses or anastomoses found in early healing (3rd to 5th day). In contrast, ruptures away from anastomosis occur in very strong mechanical anastomoses or anastomoses, where the healing process is at an advanced stage, particularly in the remodeling phase67,68. Raptis et al.23 found that the rupture rate on anastomosis was higher in the ileus group relative to the control group. In our study, on the fourth postoperative day, the highest incidence of anastomosis rupture was observed after obstructive ileus conditions compared to the control group, but this difference was not statistically significant. However, on the 8th postoperative day, a statistically significantly highest incidence of anastomosis rupture was observed after obstructive ileus conditions compared to the control group.
Healing consists of chained biological reactions that lead to collagen deposition at the anastomosis69-71. The most important stage of the healing process is the inflammation phase, in which a fibrin clot is formed. That clot bridges the trauma gap. Inflammatory cells (neutrophils, macrophages) also accumulate in the area. Neutrophils remove foreign bodies and necrotized tissues by releasing proteolytic factors like collagenase. Also, macrophages produce growth factors, which are responsible for the migration of fibroblasts and epithelial and endothelial cells to the wound site, resulting in the formation of granulomatous tissue69,70,72. The extension or inhibition of this phase may cause disruption of the anastomosis healing mechanisms. Although the process of healing the anastomoses of the colon is similar to that of skin, there are several differences particular to the gastrointestinal tract73-75. First, the intestinal mucosal epithelium has greater regenerative capacity, leading to faster wound repair. Anastomoses of the digestive tract acquire their maximum strength in 14-21 days, while skin sutures can take as long as the 120th day70,74,76. During the first 3-5 days, collagenolysis is predominant in the area of anastomosis. This period is the most critical, as the sheared ends of the bowel are held together only by sutures or clips. The strength of the anastomosis gradually increases until the 12th-14th days77. Also, smooth muscle cells and fibroblasts are responsible for the production of collagen in the intestine, as opposed to the skin, where these cells do not play such a role. Between the 3rd and 5th days, an abundance of undifferentiated mesenchymal cells accompanies the invasion of the capillaries in the muscle layer. These cells are transformed into smooth muscle cells and phagocytes77,78. The formation of smooth muscle cells at the wound edges is responsible for the reshaping of the lost smooth muscle tissue. Additional factors that differentiate the intestine from the skin and other tissues are its multi-layered architecture, its high content of micro-organisms, the effect of serum on the formation of a barrier on the staple line and its particular vascularization74.
The healing of the anastomoses of the colon involves succession and complex processes70,76,79. It evolves immediately after trauma for the structural and functional repair of the colon tissues. All processes involve the epithelial and fibrous connective tissue with its three components—cells, matrix and fibres—and aim to synthesize new connective tissue and heal the anastomosis73,79-82. The mechanisms of healing are distinguished in four overlapping phases:80-88 A) haemostasis phase, B) inflammatory phase, C) production phase and D) remodeling phase. Haemostasis is distinguished in the vascular phase where there is a contraction of the bleeding vessels, the platelet phase, where platelets accumulate and the platelet or temporal thrombus is formed, and the coagulation phase89. The inflammation phase follows the haemostasis phase, beginning 24 hours after the wound and lasting for 3-4 days. The accumulation of platelets is followed by the infiltration of the area by white blood cells, which arrive after their dissection from the vascular wall90. The transudation is neutrophilic polymorphonuclear, mononuclear macrophages and lymphocytes. Immune system cells significantly contribute to the regulation of the anastomosis healing process, through the secretion of molecular signals, such as cytokines, lymphokines and growth factors91. The activity of polymorphonuclear cells involves the phagocytosis of bacteria (by opsonization), release of collagenase and elastase and the degradation of elastin, the basal membrane of the vessels. In addition, they produce IL-1 and TNF-α, which activate fibroblasts. In the current study, while leukocytosis increased following obstructive ileus, none of the differences observed were significant compared to the control.
The proliferation phase begins after the 3rd day and ends on the 12th day of healing. It involves the synthesis of new collagen, angiogenesis and the replacement of temporary fibrous tissue from granular tissue. Granular tissue is a loose connective tissue of fibrin, fibronectin, collagen fibres, glycosaminoglycans and hyaluronic acid38,92. In addition, it contains macrophages, fibroblasts and neoplastic vessels. Its presence lasts from day 3 to day 21 and is determined by various substances produced in the inflammatory phase37,93. In the productive phase of healing, which begins 6-7 days after anastomosis and is completed in 12-16 days, neovascularization also plays an important role. In particular, endothelial cells from the adjacent capillaries invade the anastomosis and form tubular formations surrounded by collagen fibres and the primary capillaries. This process is regulated by the action of promoters and inhibiters. Promoter agents include FGF, TGF-α and VEGF. Turlu et al. showed that TGF-β1 represents a potential therapeutic agent for the prevention of anastomotic leakage by increasing collagen synthesis and collagen deposition104. Positive effects also have local factors, such as hypoxia, low pH and high levels of lactic acid89. It has also been found that the ileus causes disturbances in the intestinal wall perfusion. Raptis et al.23 found a significant decrease in neovascularization in the ileus group compared to the control group. In our study, on the 4th postoperative day, the obstructive ileus group showed lower neovascularization compared to the control and this difference was statistically significant. However, on the 8th postoperative day, while neovascularization was reduced in ileus subgroup, the decrease was not significant compared to the control.
During the production phase, the area of anastomosis is predominantly occupied by endothelial cells and fibroblasts. The most important processes, both in this phase and in the remodeling phase, are collagenolysis and collagenogenesis, the balance of which plays a decisive role in the healing of anastomoses. A significant increase in collagenase was found in the gastrointestinal tract following anastomosis in the colon75,94. An important factor directly affecting collagenase activity is the presence of bacteria in the anastomosis. Bacteria are sources of proteolytic enzymes that produce collagenase and can cause increased collagen breakdown at the site of anastomosis95,96. Studies on mice suggested that drinking a phosphate-based polymer can achieve the goal of preventing anastomotic leak by suppressing collagenase production in E. faecalis107. After the early phase, the production phase of healing is followed by the production of collagen by the fibroblasts, which increases the strength of the anastomosis39,40,92. For this reason, the production and deposition of collagen in the area of anastomosis is an important indicator of the adequacy of the healing process52,63. Raptis et al.23 found a significant reduction in the number of fibroblasts in the ileus group compared to the control group. In our study, on the 4th postoperative day, a reduced number of fibroblasts, compared with the control, was observed in the ileus subgroup and this difference was statistically significant. Similarly, on the 8th postoperative day in the ileus subgroup, a decreased number of fibroblasts was observed, compared to the control, and this difference was also statistically significant.
At the remodeling phase, collagenolysis progressively decreases, whereas collagen deposition, which begins on days 7-8 from the formation of anastomosis, increases and may take up to 90 days. The collagen fibres intertwine with each other and, in combination with the connective tissue, provide elasticity, durability and integrity. In the remodeling phase, the most important event is the collagen deposition and the gradual replacement of granular tissue with connective tissue, which consists of a network of collagen and elastic fibres38. Additionally, as the inflammatory elements recede, angiogenesis is disrupted, fibroblasts are produced, the old type III collagen degrades and the novel type I collagen is synthesized. The strength of the anastomosis is determined by the quality and amount of the newly formed collagen. This increases from the 2nd to the 6th week of healing, then stays constant and reaches its maximum in 2-3 years. At the same time, tissue remodeling (after three weeks), which lasts up to three years, can also cause anastomosis constriction after a significant period of time (up to six months). The end result is the formation of scarring connective tissue59,83,97. In experimental studies, occlusive ileus has been shown to increase the activity of metalloproteinases (between day 1 and day 5), which reduces the deposition of new collagen and increases the degradation of the old, both in the anastomosis region and away from it96,98,99. Raptis et al.23 found significant reduction in neocollagen formation in the ileus group compared to the control group. Also, Moran et al.100, in an experimental study on rats, after erythropoietin administration under ileus conditions, found that the formation of neo-collagen decreased in the ileus group compared to all other groups. However, in our study, we didn’t observe any significant differences in neocollagen synthesis among the experimental groups.
The mechanical strength of anastomosis is dependent on connective tissue cell proliferation, collagen molecule synthesis and collagen fibril formation. The synthesis and deposition of collagen are complex biological processes that are affected by a variety of factors. Experimental models are necessary to investigate the deposition of collagen during the anastomosis healing process. The most common method is to determine hydroxyproline, as a unit of total collagen count, in colorectal cells from the anastomosis site. Hydroxyproline is one of the key amino acids components of collagen of all types and is a product of proline hydroxylation metabolism. The main feature of this amino acid is that it is almost exclusively found in connective tissue collagen. The measurement of hydroxyproline is quantitative and does not give information on the quality of collagen and its identification in fractions (types I, III and IV). However, its measurement is now the only reliable quantitative method for determining collagen in the anastomosis region101. It was also found that obstructive ileus causes disorders in the structure of the intestinal wall, resulting in a decrease in the concentration of hydroxyproline63,101. Raptis et al.23 found that the ileus group had a decreased concentration of hydroxyproline relative to all other groups. In our study, we found that on the 4th postoperative day, after obstructive ileus, there was a decreased concentration of hydroxyproline compared to the control, but this difference was not statistically significant, while on the 8th postoperative day, there was a significant reduction in hydroxyproline concertation in the ileus group compared to the control.
Collagen is an abundant extracellular substance and determines the non-mechanical stability of connective tissue during healing. The most important types are type I collagen, which is responsible for the formation of mature collagen, and type III collagen, which occurs mainly during the early phase of healing and has less mechanical strength. Their content and proportion determine the mechanical strength and stability of the anastomosis. The total degradation of collagen is the result of the synergistic action of various MMPs46,60,66. Various experimental studies have shown that the intense activity of MMPs is one of the major pathogenetic factors of postoperative attenuation of the anastomosis. Other studies have found that the levels of MMPs on the suturing line increased during the first three days after anastomosis. In addition, other studies have shown that MMP-1 and MMP-13 are found in all patients with leakage, suggesting a potential predisposition for collagen metabolism disorder. These findings suggest that treatment with synthetic inhibitors of MMPs contributes to maintaining mature collagen fibres in the submucosal layer and, consequently, the integrity of anastomosis98,102.
Experimental studies have shown that ileus increases the activity of metalloproteinases, which reduces the deposition of new collagen in the anastomosis and increases the degradation of the old one. Their greater concentration and greater collagen degradation are observed near the staple line. The end result is a delay in the healing of anastomosis and an increased chance of leakage42,96,98. Collagen is resistant to the action of proteolytic enzymes and is degraded by collagenase. A significant increase in collagenase was found in the gastrointestinal tract following anastomosis in the colon96. Collagenase plays an important role in determining the integrity of anastomosis and the ability to hold sutures in the early postoperative days of healing. In addition, immunohistochemical studies have shown that collagenase and other metalloproteinases are proximal to the anastomosis stapling line. Collagenase belonging to the metalloproteinase family is considered to be responsible for the loss of mature collagen of the submucosal layer and the consequent breakdown of anastomosis103. This is supported by experimental data indicating maximum collagenolytic activity in anastomosis in rats on the 3rd postoperative day. Other experimental studies in rats using aprotinin, which is a protein inhibitor, showed a significant increase in the breakdown pressure of the anastomoses of the colon52. Collagenase is the major breakdown enzyme of mature collagen, belongs to the family of metalloproteinases and plays an important role in the integrity of anastomosis. The factors that affect the activity of collagenase are bacteria and the inflammatory reaction around the anastomosis. It has also been found that occlusive ileum increases the enzymatic activity of metalloproteinases and causes rapid degradation of collagen in the intestinal wall. Therefore, the collagenase concentration assay provides a better measure of the balance between collagenogenesis and collagenolysis28,46,60. In our study, on the 4th postoperative day in the ileus subgroup, the collagenase concentration decreased in comparison with the control group, but this difference was not statistically significant. On the 8th postoperative day, the ileus subgroup showed a higher collagenase concentration compared to control subgroup, but this difference was also not statistically significant.
CONCLUSION
As a high proportion of colorectal cancer cases initially manifest with obstructive ileus symptomatology, there is a need for improved outcomes and safety of colonic anastomosis. The balance of collagenolysis and collagenogenesis plays a decisive role in the healing of anastomoses. Anastomotic healing in the early period seems to be impaired following obstructing ileus conditions, as anastomotic dehiscence percentages did not significantly increase, the bursting pressure significantly decreased, and the majority of leakages occurred at the anastomotic site. This reduction was not affected by local inflammatory reaction, neocollagen concentration and collagenase activity, but rather by decreased neovascularization, reduced fibroblast presence and lower hydroxyproline concertation. Thus, extreme caution should be exercised in the construction of an anastomosis following obstructive ileus and there is a need for measures, either local or systemic, to enhance the anastomotic healing process. Future studies should focus on the delineation of the mechanisms of healing of colonic anastomoses and identify those factors that can be altered to minimize the incidence of the anastomosis rupture.
KEY POINTS
◊ The balance of collagenolysis and collagenogenesis plays a decisive role in the healing of anastomoses
◊ Obstructive ileus subgroup presented with decreased number of fibroblasts (production and deposition of collagen) and decreased concentration of hydroxyproline (reliable quantitative method for determining collagen)
◊ In obstructive ileus subgroup, higher collagenase concentration and lower neovascularization were observed
◊ The highest incidence of anastomosis rupture was seen after obstructive ileus conditions compared to the control group
◊ Future studies should focus on the delineation of the mechanisms of healing of colonic anastomoses and identifying those factors that can be altered to minimize the incidence of anastomosis rupture
Acknowledgments
The authors would like to acknowledge and thank Aristotle University of Thessaloniki for its continuous support. The authors would like to thank Dr. Haidich AB, Assistant Professor of Hygiene-Medical Statistics for reviewing the statistical methods used in this paper.
Footnotes
Conflict of interests: The authors declare no conflicts of interest.
Abbreviations: Systemic Inflammatory Reaction Syndrome (SIRS); Multidrug Disorder Syndrome (MODS); Fibroblast Growth Factor (FGF); Transforming Growth Factor-a (TGF-α); Vascular Endothelial Growth Factor (VEGF); Matrix Metalloproteinases (MMPs).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Progress in rectal cancer staging and treatment. Pramateftakis M G, Kanellos D, Vrakas G, Tsachalis Tau, Raptis D, Makrantonakis A, Koukouritaki Z, Kanellos I. Techniques in coloproctology. 2010;14 Suppl 1:S29–31. doi: 10.1007/s10151-010-0619-7. [DOI] [PubMed] [Google Scholar]
- 2.Anastomotic leakage following anterior resection for rectal cancer. Kanellos I, Vasiliadis K, Angelopoulos S, Tsachalis T, Pramateftakis M G, Mantzoros I, Betsis D. Techniques in coloproctology. 2004;8 Suppl 1:s79–81. doi: 10.1007/s10151-004-0119-8. [DOI] [PubMed] [Google Scholar]
- 3.Oncologic impact of anastomotic leakage after low anterior resection for rectal cancer. Mantzoros I. Techniques in coloproctology. 2010;14 Suppl 1:S39–41. doi: 10.1007/s10151-010-0633-9. [DOI] [PubMed] [Google Scholar]
- 4.Anastomotic leakage after colonic resection. Kanellos D. Techniques in coloproctology. 2010;14 Suppl 1:S43–4. doi: 10.1007/s10151-010-0621-0. [DOI] [PubMed] [Google Scholar]
- 5.Parameters of healing in approximative intestinal anastomosis. Rygl M, Novotna J, Herget J, Skaba R, Snajdauf J. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery ... [et al] = Zeitschrift fur Kinderchirurgie. 2009;19(1):25–9. doi: 10.1055/s-2008-1039010. [DOI] [PubMed] [Google Scholar]
- 6.Repair of full-thickness bowel injury. Witte Maria B, Barbul Adrian. Critical care medicine. 2003;31(8 Suppl):S538–46. doi: 10.1097/01.CCM.0000081436.09826.A4. [DOI] [PubMed] [Google Scholar]
- 7.[Prevention of anastomotic dehiscence in colorectal surgery]. Gainant A. Journal de chirurgie. 2000;137(1):45–50. [PubMed] [Google Scholar]
- 8.[Anastomotic leakage after traditional surgery of the colon and rectum]. Abete M, Ronchetti V, Casano A, Pescio G. Minerva chirurgica. 2003;58(2):167–74. [PubMed] [Google Scholar]
- 9.Anastomotic leakage after colorectal anastomosis. Moran B, Heald R. Seminars in surgical oncology. 2000;18(3):244–8. doi: 10.1002/(sici)1098-2388(200004/05)18:3<244::aid-ssu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Walker Kenneth G, Bell Stephen W, Rickard Matthew J F X, Mehanna Daniel, Dent Owen F, Chapuis Pierre H, Bokey E Leslie. Annals of surgery. 2004;240(2):255–9. doi: 10.1097/01.sla.0000133186.81222.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colonic healing after portal ischemia and reperfusion: an experimental study with oxidative stress biomarkers. Rolim Marcia Farias, Riger Cristiano J, Eleutherio Elis C A, Colão Cristiane da Fonseca, Pereira Gerson Cotta, Schanaider Alberto. Redox report : communications in free radical research. 2007;12(6):267–74. doi: 10.1179/135100007X239261. [DOI] [PubMed] [Google Scholar]
- 12.[Pathophysiology of ileus]. Roscher R, Lommel K. Zentralblatt fur Chirurgie. 1998;123(12):1328–33. [PubMed] [Google Scholar]
- 13.Colorectal cancer presenting as surgical emergencies. Cuffy Madison, Abir Farshad, Audisio Riccardo A, Longo Walter E. Surgical oncology. 2004;13(2-3):149–57. doi: 10.1016/j.suronc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Carcinoma of the colon caused intussusception presenting with obstructive ileus. Pavlidis Theodoros E. International journal of colorectal disease. 2007;22(3):337. doi: 10.1007/s00384-005-0746-1. [DOI] [PubMed] [Google Scholar]
- 15.Effect of antibiotics in fibrin sealant on healing colonic anastomoses in the rat. van der Ham A C, Kort W J, Weijma I M, van den Ingh H F, Jeekel H. The British journal of surgery. 1992;79(6):525–8. doi: 10.1002/bjs.1800790617. [DOI] [PubMed] [Google Scholar]
- 16.The effects of iloprost on colonic anastomotic healing in rats under obstructive ileus conditions. Galanopoulos Georgios, Raptis Dimitrios, Pramateftakis Manousos-Georgios, Mantzoros Ioannis, Kanellos Ioannis, Lazarides Charalambos. The Journal of surgical research. 2014;189(1):22–31. doi: 10.1016/j.jss.2014.01.052. [DOI] [PubMed] [Google Scholar]
- 17.The effects of the intraperitoneal administration of oxaliplatin and 5-FU on the healing of colonic anastomoses: an experimental study. Kanellos D, Pramateftakis M G, Mantzoros I, Zacharakis E, Raptis D, Despoudi K, Zaraboukas Th, Koliakos G, Lazaridis H. Techniques in coloproctology. 2011;15 Suppl 1:S111–5. doi: 10.1007/s10151-011-0754-9. [DOI] [PubMed] [Google Scholar]
- 18.The effect of glutamine and synbiotics on the healing of colonic anastomosis. Sapidis N, Tziouvaras C, Ioannidis O, Kalaitsidou I, Botsios D. Revista espanola de enfermedades digestivas : organo oficial de la Sociedad Espanola de Patologia Digestiva. 2014;106(4):255–62. [PubMed] [Google Scholar]
- 19.Effects of chronic corticosteroids and vitamin A on the healing of intestinal anastomoses. Phillips J D, Kim C S, Fonkalsrud E W, Zeng H, Dindar H. American journal of surgery. 1992;163(1):71–7. doi: 10.1016/0002-9610(92)90255-p. [DOI] [PubMed] [Google Scholar]
- 20.Histopathology of human intestinal anastomosis. Shomaf M. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2003;9(3):413–21. [PubMed] [Google Scholar]
- 21.A simplified method for the analysis of hydroxyproline in biological tissues. Reddy G K, Enwemeka C S. Clinical biochemistry. 1996;29(3):225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 22.BioGlue surgical adhesive for thoracic aortic repair during coagulopathy: efficacy and histopathology. Hewitt C W, Marra S W, Kann B R, Tran H S, Puc M M, Chrzanowski F A, Tran J L, Lenz S D, Cilley J H, Simonetti V A, DelRossi A J. The Annals of thoracic surgery. 2001;71(5):1609–12. doi: 10.1016/s0003-4975(01)02424-9. [DOI] [PubMed] [Google Scholar]
- 23.The effects of tacrolimus on colonic anastomotic healing in rats. Raptis D, Mantzoros I, Pramateftakis M G, Despoudi K, Zaraboukas T, Koliakos G, Kanellos I, Lazarides Ch. International journal of colorectal disease. 2012;27(3):299–308. doi: 10.1007/s00384-011-1337-y. [DOI] [PubMed] [Google Scholar]
- 24.The effects of intestinal ischemia on colonic motility in conscious rats. Suzuki Makoto, Takahashi Atsushi, Toki Fumiaki, Hatori Reiko, Tomomasa Takeshi, Morikawa Akihiro, Kuwano Hiroyuki. Journal of gastroenterology. 2008;43(10):767–73. doi: 10.1007/s00535-008-2224-3. [DOI] [PubMed] [Google Scholar]
- 25.The failed intraperitoneal colon anastomosis after colon resection. Kanellos I, Blouhos K, Demetriades H, Pramateftakis M G, Mantzoros I, Zacharakis E, Betsis D. Techniques in coloproctology. 2004;8 Suppl 1:s53–5. doi: 10.1007/s10151-004-0111-3. [DOI] [PubMed] [Google Scholar]
- 26.Management and causes of acute large-bowel obstruction. Lopez-Kostner F, Hool G R, Lavery I C. The Surgical clinics of North America. 1997;77(6):1265–90. doi: 10.1016/s0039-6109(05)70617-4. [DOI] [PubMed] [Google Scholar]
- 27.Intestinal obstruction. Stephenson James A., Singh Baljit. Surgery (Oxford) 2011;29(1):33-38. [Google Scholar]
- 28.Serosal injury in the equine jejunum and ascending colon after ischemia-reperfusion or intraluminal distention and decompression. Dabareiner R M, Sullins K E, White N A, Snyder J R. Veterinary surgery : VS. 2001;30(2):114–25. doi: 10.1053/jvet.2001.21393. [DOI] [PubMed] [Google Scholar]
- 29.Structural and neuronal changes in rat ileum after ischemia with reperfusion. Lindeström Lille-Mor, Ekblad Eva. Digestive diseases and sciences. 2004;49(7-8):1212–22. doi: 10.1023/b:ddas.0000037815.63547.08. [DOI] [PubMed] [Google Scholar]
- 30.Colorectal cancer: obstruction is an independent negative prognostic factor after radical resection. Zucchetti F, Negro F, Matera D, Bolognini S, Mafucci S. Annali italiani di chirurgia. 2002;73(4):421–5. [PubMed] [Google Scholar]
- 31.Management of malignant bowel obstruction. Ripamonti Carla Ida, Easson Alexandra M, Gerdes Hans. European journal of cancer (Oxford, England : 1990) 2008;44(8):1105–15. doi: 10.1016/j.ejca.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Surgical options for malignant left-sided colonic obstruction. Villar Jesus M, Martinez Ana P, Villegas Maria T, Muffak Karim, Mansilla Alfonso, Garrote Daniel, Ferron Jose A. Surgery today. 2005;35(4):275–81. doi: 10.1007/s00595-004-2931-1. [DOI] [PubMed] [Google Scholar]
- 33.Clinical and morphologic correlation after stapled transanal rectal resection for obstructed defecation syndrome. Dindo Daniel, Weishaupt Dominik, Lehmann Kuno, Hetzer Franc H, Clavien Pierre-Alain, Hahnloser Dieter. Diseases of the colon and rectum. 2008;51(12):1768–74. doi: 10.1007/s10350-008-9412-3. [DOI] [PubMed] [Google Scholar]
- 34.Emergency one-stage surgery for obstructing left-sided colorectal carcinomas. Huang Tsung-Jen, Wang Jaw-Yuan, Lee Li-Wei, Chen Fang-Ming, Chuan Chieh-Han, Chan Hon-Man, Hou Ming-Feng, Huang Che-Jen, Huang Yu-Sheng, Hsieh Jan-Sing. The Kaohsiung journal of medical sciences. 2002;18(7):323–8. [PubMed] [Google Scholar]
- 35.Anastomotic leakage following low anterior resection: results of a standardized diagnostic and therapeutic approach. Eckmann C, Kujath P, Schiedeck T H K, Shekarriz H, Bruch H-P. International journal of colorectal disease. 2004;19(2):128–33. doi: 10.1007/s00384-003-0498-8. [DOI] [PubMed] [Google Scholar]
- 36.Suture support: is it advantageous? Kjaergard H K. American journal of surgery. 2001;182(2 Suppl):15S–20S. doi: 10.1016/s0002-9610(01)00772-3. [DOI] [PubMed] [Google Scholar]
- 37.The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Carlson Mark A, Longaker Michael T. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2004;12(2):134–47. doi: 10.1111/j.1067-1927.2004.012208.x. [DOI] [PubMed] [Google Scholar]
- 38.Strength and failure of fibrin fiber branchpoints. Carlisle C R, Sparks E A, Der Loughian C, Guthold M. Journal of thrombosis and haemostasis : JTH. 2010;8(5):1135–8. doi: 10.1111/j.1538-7836.2010.03824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collagen reorganization in leech wound healing. Tettamanti Gianluca, Grimaldi Annalisa, Congiu Terenzio, Perletti Gianpaolo, Raspanti Mario, Valvassori Roberto, de Eguileor Magda. Biology of the cell. 2005;97(7):557–68. doi: 10.1042/BC20040085. [DOI] [PubMed] [Google Scholar]
- 40.Hierarchical structures in fibrillar collagens. Ottani V, Martini D, Franchi M, Ruggeri A, Raspanti M. Micron (Oxford, England : 1993) 2002;33(7-8):587–96. doi: 10.1016/s0968-4328(02)00033-1. [DOI] [PubMed] [Google Scholar]
- 41.The pathophysiology of intestinal damage: effects of luminal distention and ischemia. Snyder J R. The Veterinary clinics of North America. Equine practice. 1989;5(2):247–70. doi: 10.1016/s0749-0739(17)30587-4. [DOI] [PubMed] [Google Scholar]
- 42.Mesenteric microcirculatory dysfunctions and translocation of indigenous bacteria in a rat model of strangulated small bowel obstruction. Zanoni Fernando Luiz, Benabou Simon, Greco Karin Vicente, Moreno Ana Carolina Ramos, Cruz José Walber Miranda Costa, Filgueira Fernando Paranaiba, Martinez Marina Baquerizo, Figueiredo Luiz Francisco Poli de, Silva Maurício Rocha e, Sannomiya Paulina. Clinics (Sao Paulo, Brazil) 2009;64(9):911–9. doi: 10.1590/S1807-59322009000900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colonic healing after portal ischemia and reperfusion: an experimental study with oxidative stress biomarkers. MF Rolim, CJ Riger, EC Eleutherio, Cda F Colao, GC Pereira, A. Schanaider. https://pubmed.ncbi.nlm.nih.gov/17961298/ Redox Report. 2007;12(6) doi: 10.1179/135100007X239261. [DOI] [PubMed] [Google Scholar]
- 44.Clinical implications of ischaemia-reperfusion injury. Abela Christopher B., Homer-Vanniasinkham S. Pathophysiology : the official journal of the International Society for Pathophysiology. 2003;9(4):229–240. doi: 10.1016/s0928-4680(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 45.Induction of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression in rat brain after focal ischemia/reperfusion. Tanaka Ryota, Mochizuki Hideki, Suzuki Asuka, Katsube Nobuo, Ishitani Ryoichi, Mizuno Yoshikuni, Urabe Takao. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22(3):280–8. doi: 10.1097/00004647-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Gastrointestinal disorders of the critically ill. Systemic consequences of ileus. Madl Christian, Druml Wilfred. Best practice & research. Clinical gastroenterology. 2003;17(3):445–56. doi: 10.1016/s1521-6918(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 47.[Intra-abdominal hypertension and multiple organ dysfunction syndrome]. Serpytis Mindaugas, Ivaskevicius Juozas. Medicina (Kaunas, Lithuania) 2005;41(11):903–9. [PubMed] [Google Scholar]
- 48.Cell death/proliferation and alterations in glial morphology contribute to changes in diffusivity in the rat hippocampus after hypoxia-ischemia. Anderova Miroslava, Vorisek Ivan, Pivonkova Helena, Benesova Jana, Vargova Lydia, Cicanic Michal, Chvatal Alexandr, Sykova Eva. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31(3):894–907. doi: 10.1038/jcbfm.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Relationship between C-telopeptide pyridinoline cross-links (ICTP) and putative periodontal pathogens in periodontitis. Palys M D, Haffajee A D, Socransky S S, Giannobile W V. Journal of clinical periodontology. 1998;25(11 Pt 1):865–71. doi: 10.1111/j.1600-051x.1998.tb02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Generalized peritonitis due to perforated diverticulitis: Hartmann’s procedure or primary anastomosis? Trenti Loris, Biondo Sebastiano, Golda Thomas, Monica Millan, Kreisler Esther, Fraccalvieri Domenico, Frago Ricardo, Jaurrieta Eduardo. International Journal of Colorectal Disease. 2010;26(3):377-384. doi: 10.1007/s00384-010-1071-x. [DOI] [PubMed] [Google Scholar]
- 51.Nutrition, anabolism, and the wound healing process: an overview. Demling Robert H. Eplasty. 2009;9:e9. [PMC free article] [PubMed] [Google Scholar]
- 52.Inhibition of matrix metalloproteinases enhances breaking strength of colonic anastomoses in an experimental model. Syk I, Agren M S, Adawi D, Jeppsson B. The British journal of surgery. 2001;88(2):228–34. doi: 10.1046/j.1365-2168.2001.01649.x. [DOI] [PubMed] [Google Scholar]
- 53.Connective tissue growth factor mediates the profibrotic effects of transforming growth factor-beta produced by tubular epithelial cells in response to high glucose. Kobayashi Tatsuya, Inoue Tsutomu, Okada Hirokazu, Kikuta Tomohiro, Kanno Yoshihiko, Nishida Takashi, Takigawa Masaharu, Sugaya Takeshi, Suzuki Hiromichi. Clinical and experimental nephrology. 2005;9(2):114–21. doi: 10.1007/s10157-005-0347-x. [DOI] [PubMed] [Google Scholar]
- 54.Effect of routine fibrin glue use on the duration of air leaks after lobectomy. Fleisher A G, Evans K G, Nelems B, Finley R J. The Annals of thoracic surgery. 1990;49(1):133–4. doi: 10.1016/0003-4975(90)90371-c. [DOI] [PubMed] [Google Scholar]
- 55.Gelatin-resorcinol-formaldehyde-glutaraldehyde glue for sealing pulmonary air leaks during thoracoscopic operation. Nomori H, Horio H, Morinaga S, Suemasu K. The Annals of thoracic surgery. 1999;67(1):212–6. doi: 10.1016/s0003-4975(98)01184-9. [DOI] [PubMed] [Google Scholar]
- 56.Correlation between bursting pressure and breaking strength in colonic anastomosis. Durães Leonardo de Castro, Durães Eliana Ferreira Ribeiro, Lobato Luiz Felipe de Campos, Oliveira Paulo Gonçalves de, Sousa João Batista de. Acta cirurgica brasileira. 2013;28(6):447–52. doi: 10.1590/s0102-86502013000600008. [DOI] [PubMed] [Google Scholar]
- 57.Choice of hemostatic agent influences adhesion formation in a rat cecal adhesion model. Hoffmann Nathan E, Siddiqui Sameer A, Agarwal Shvetank, McKellar Stephen H, Kurtz Harold J, Gettman Matthew T, Ereth Mark H. The Journal of surgical research. 2009;155(1):77–81. doi: 10.1016/j.jss.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Collagen distribution and expression of matrix metalloproteinases 1 and 13 in patients with anastomotic leakage after large-bowel surgery. Stumpf Michael, Cao Wei, Klinge Uwe, Klosterhalfen Bernd, Kasperk Reinhard, Schumpelick Volker. Langenbeck's archives of surgery. 2002;386(7):502–6. doi: 10.1007/s00423-001-0255-9. [DOI] [PubMed] [Google Scholar]
- 59.Tissue repair, contraction, and the myofibroblast. Desmoulière Alexis, Chaponnier Christine, Gabbiani Giulio. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2005;13(1):7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 60.Proteolytic events of wound-healing--coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Steffensen B, Häkkinen L, Larjava H. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 2001;12(5):373–98. doi: 10.1177/10454411010120050201. [DOI] [PubMed] [Google Scholar]
- 61.Effect of fibrin sealant on the healing colonic anastomosis in the rat. van der Ham A C, Kort W J, Weijma I M, van den Ingh H F, Jeekel J. The British journal of surgery. 1991;78(1):49–53. doi: 10.1002/bjs.1800780117. [DOI] [PubMed] [Google Scholar]
- 62.Mechanical behavior of colonic anastomosis in experimental settings as a measure of wound repair and tissue integrity. Ekmektzoglou Konstantinos A, Zografos Georgios C, Kourkoulis Stavros K, Dontas Ismene A, Giannopoulos Panagiotis K, Marinou Katerina A, Poulakou Maria V, Perrea Despina N. World journal of gastroenterology. 2006;12(35):5668–73. doi: 10.3748/wjg.v12.i35.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collagen deposition and mechanical strength of colon anastomoses and skin incisional wounds of rats. Oxlund H, Christensen H, Seyer-Hansen M, Andreassen T T. The Journal of surgical research. 1996;66(1):25–30. doi: 10.1006/jsre.1996.0367. [DOI] [PubMed] [Google Scholar]
- 64.Tumour necrosis factor alpha and nuclear factor kappaB inhibit transcription of human TFF3 encoding a gastrointestinal healing peptide. Loncar M B, Al-azzeh E-d, Sommer P S M, Marinovic M, Schmehl K, Kruschewski M, Blin N, Stohwasser R, Gött P, Kayademir T. Gut. 2003;52(9):1297–303. doi: 10.1136/gut.52.9.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collagen fleeces do not improve colonic anastomotic strength but increase bowel obstructions in an experimental rat model. Schreinemacher Marc H, Bloemen Johanne G, van der Heijden Stijn J, Gijbels Marion J, Dejong Cornelis H, Bouvy Nicole D. International Journal of Colorectal Disease. 2011;26(6):729-735. doi: 10.1007/s00384-011-1158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wound healing of intestinal epithelial cells. Iizuka Masahiro. World Journal of Gastroenterology. 2011;17(17):2161. doi: 10.3748/wjg.v17.i17.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.[Leakages after surgery of the lower gastrointestinal tract]. Willis S, Stumpf M. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen. 2004;75(11):1071–8. doi: 10.1007/s00104-004-0895-8. [DOI] [PubMed] [Google Scholar]
- 68.Risk factors for clinical anastomotic leakage following the resection of sigmoid and rectal cancer. Rudinskaite Giedre, Tamelis Algimantas, Saladzinskas Zilvinas, Pavalkis Dainius. Medicina (Kaunas, Lithuania) 2005;41(9):741–6. [PubMed] [Google Scholar]
- 69.Intra-abdominal healing: gastrointestinal tract and adhesions. Munireddy Sanjay, Kavalukas Sandra L, Barbul Adrian. The Surgical clinics of North America. 2010;90(6):1227–36. doi: 10.1016/j.suc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Wound-healing trajectories. Steed David L. The Surgical clinics of North America. 2003;83(3):547–55, vi. doi: 10.1016/S0039-6109(02)00208-6. [DOI] [PubMed] [Google Scholar]
- 71.Skin: histology and physiology of wound healing. Gantwerker Eric A, Hom David B. Clinics in plastic surgery. 2012;39(1):85–97. doi: 10.1016/j.cps.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Molecular mechanisms of leukocyte recruitment: organ-specific mechanisms of action. Liu Lixin, Kubes Paul. Thrombosis and haemostasis. 2003;89(2):213–20. [PubMed] [Google Scholar]
- 73.Wound healing in denervated rat skin. Fukai Takao, Takeda Akira, Uchinuma Eijyu. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2005;13(2):175–80. doi: 10.1111/j.1067-1927.2005.130208.x. [DOI] [PubMed] [Google Scholar]
- 74.Mucosal repair in the gastrointestinal tract. Mammen Joshua M V, Matthews Jeffrey B. Critical care medicine. 2003;31(8 Suppl):S532–7. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- 75.Inflammatory processes in muscle injury and repair. Tidball James G. American journal of physiology. Regulatory, integrative and comparative physiology. 2005;288(2):R345–53. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 76.The physiology of wound healing. Singh Shailendra, Young Alistair, McNaught Clare-Ellen. Surgery (Oxford) 2017;35(9):473-477. [Google Scholar]
- 77.Intraperitoneally administered irinotecan with 5-fluorouracil impair wound healing of colonic anastomoses in a rat model: an experimental study. Pramateftakis M G, Kanellos D, Mantzoros I, Despoudi K, Raptis D, Angelopoulos S, Koliakos G, Zaraboukas Th, Lazaridis Charalambos. Techniques in coloproctology. 2011;15 Suppl 1:S121–5. doi: 10.1007/s10151-011-0755-8. [DOI] [PubMed] [Google Scholar]
- 78.Regulation of wound healing by growth factors and cytokines. Werner Sabine, Grose Richard. Physiological reviews. 2003;83(3):835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 79.Wound-healing perspectives. Li Wei, Dasgeb Bahar, Phillips Tania, Li Yong, Chen Mei, Garner Warren, Woodley David T. Dermatologic clinics. 2005;23(2):181–92. doi: 10.1016/j.det.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Growth factors and cytokines in wound healing. Barrientos Stephan, Stojadinovic Olivera, Golinko Michael S, Brem Harold, Tomic-Canic Marjana. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 81.A new concept of a multidisciplinary wound healing center and a national expert function of wound healing. Gottrup F, Holstein P, Jørgensen B, Lohmann M, Karlsmar T. Archives of surgery (Chicago, Ill. : 1960) 2001;136(7):765–72. doi: 10.1001/archsurg.136.7.765. [DOI] [PubMed] [Google Scholar]
- 82.A review of studies that examine the impact of infection on the normal wound-healing process. Penhallow K. Journal of wound care. 2005;14(3):123–6. doi: 10.12968/jowc.2005.14.3.26747. [DOI] [PubMed] [Google Scholar]
- 83.Wound healing: an overview of acute, fibrotic and delayed healing. Diegelmann Robert F, Evans Melissa C. Frontiers in bioscience : a journal and virtual library. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 84.Cellular, molecular and biochemical differences in the pathophysiology of healing between acute wounds, chronic wounds and wounds in the aged. Enoch S., Price P. https://www.researchgate.net/publication/288271556_Cellular_molecular_and_biochemical_differences_in_the_pathophysiology_of_healing_between_acute_wounds_chronic_wounds_and_wounds_in_the_aged World Wide Wounds. 2004 [Google Scholar]
- 85.Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Baum Christian L, Arpey Christopher J. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.] 2005;31(6):674–86; discussion 686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 86.Wound healing. Thomas D W, Harding K G. The British journal of surgery. 2002;89(10):1203–5. doi: 10.1046/j.1365-2168.2002.02181.x. [DOI] [PubMed] [Google Scholar]
- 87.Acute wounds. Ramasastry Sai S. Clinics in plastic surgery. 2005;32(2):195–208. doi: 10.1016/j.cps.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 88.Acute wound healing. Monaco JoAn L, Lawrence W.Thomas. Clinics in Plastic Surgery. 2003;30(1):1-12. doi: 10.1016/s0094-1298(02)00070-6. [DOI] [PubMed] [Google Scholar]
- 89.A study of metabolites as intermediate effectors in angiogenesis. Murray B, Wilson D J. Angiogenesis. 2001;4(1):71–7. doi: 10.1023/a:1016792319207. [DOI] [PubMed] [Google Scholar]
- 90.Macrophage migration inhibitory factor is induced by thrombin and factor Xa in endothelial cells. Shimizu Tadamichi, Nishihira Jun, Watanabe Hirokazu, Abe Riichiro, Honda Ayumi, Ishibashi Teruo, Shimizu Hiroshi. The Journal of biological chemistry. 2004;279(14):13729–37. doi: 10.1074/jbc.M400150200. [DOI] [PubMed] [Google Scholar]
- 91.The macrophage-activating lipopeptide-2 accelerates wound healing in diabetic mice. Deiters U, Barsig J, Tawil B, Mühlradt P F. Experimental dermatology. 2004;13(12):731–9. doi: 10.1111/j.0906-6705.2004.00233.x. [DOI] [PubMed] [Google Scholar]
- 92.Fibroblast migration on fibronectin requires three distinct functional domains. Clark Richard A F, An Jian-Qiang, Greiling Doris, Khan Azim, Schwarzbauer Jean E. The Journal of investigative dermatology. 2003;121(4):695–705. doi: 10.1046/j.1523-1747.2003.12484.x. [DOI] [PubMed] [Google Scholar]
- 93.Thrombin activatable fibrinolysis inhibitor (TAFI) at the interface between coagulation and fibrinolysis. Bouma Bonno N, Mosnier Laurent O. Pathophysiology of haemostasis and thrombosis. 2003;33(5-6):375–81. doi: 10.1159/000083832. [DOI] [PubMed] [Google Scholar]
- 94.Principles of early wound management. Wilson David A. The Veterinary clinics of North America. Equine practice. 2005;21(1):45–62, vi. doi: 10.1016/j.cveq.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 95.Matrix metalloproteinases: their function in tissue repair. Toy L W. Journal of wound care. 2005;14(1):20–2. doi: 10.12968/jowc.2005.14.1.26720. [DOI] [PubMed] [Google Scholar]
- 96.Collagenase-1 (MMP-1), matrilysin-1 (MMP-7), and stromelysin-2 (MMP-10) are expressed by migrating enterocytes during intestinal wound healing. Salmela M T, Pender S L F, Karjalainen-Lindsberg M-L, Puolakkainen P, Macdonald T T, Saarialho-Kere U. Scandinavian journal of gastroenterology. 2004;39(11):1095–104. doi: 10.1080/00365520410003470. [DOI] [PubMed] [Google Scholar]
- 97.The pathophysiology of wound repair. Theoret Christine L. The Veterinary clinics of North America. Equine practice. 2005;21(1):1–13. doi: 10.1016/j.cveq.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 98.Tissue repair and the dynamics of the extracellular matrix. Midwood Kim S, Williams Leyla Valenick, Schwarzbauer Jean E. The international journal of biochemistry & cell biology. 2004;36(6):1031–7. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 99.Collagen distribution and expression of matrix metalloproteinases 1 and 13 in patients with anastomotic leakage after large-bowel surgery. Stumpf M, Cao W, Klinge U, Klosterhalfen B, Kasperk R, Schumpelick V. https://pubmed.ncbi.nlm.nih.gov/11819107/ Langenbecks Arch Surg. 2002 doi: 10.1007/s00423-001-0255-9. [DOI] [PubMed] [Google Scholar]
- 100.The healing role of erythropoietin in the obstructive vs nonobstructive left colonic anastomosis. Faruquzzaman , Mazumder Saroj Kumar. Bratislavske lekarske listy. 2009;110(9):530–5. [PubMed] [Google Scholar]
- 101.Solubility of tissue hydroxyproline in experimental intestinal anastomoses. Hendriks T, Hesp W L, Klompmakers A A, Lubbers E J, de Boer H H. Experimental and molecular pathology. 1985;43(2):253–9. doi: 10.1016/0014-4800(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 102.Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Hashimoto Tadashi, Suzuki Yoshihisa, Tanihara Masao, Kakimaru Yoshimi, Suzuki Kyoko. Biomaterials. 2004;25(7-8):1407–14. doi: 10.1016/j.biomaterials.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 103.Matrix glycosaminoglycans in the growth phase of fibroblasts: more of the story in wound healing. Kosir M A, Quinn C C, Wang W, Tromp G. The Journal of surgical research. 2000;92(1):45–52. doi: 10.1006/jsre.2000.5840. [DOI] [PubMed] [Google Scholar]
- 104.A Human Cellular Model for Colorectal Anastomotic Repair: The Effect of Localization and Transforming Growth Factor-β1 Treatment on Collagen Deposition and Biomarkers. Türlü Ceylan, Willumsen Nicholas, Marando Debora, Schjerling Peter, Biskup Edyta, Hannibal Jens, Jorgensen Lars N, Ågren Magnus S. International journal of molecular sciences. 2021;22(4) doi: 10.3390/ijms22041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Inhibition of HIF-prolyl hydroxylases improves healing of intestinal anastomoses. Strowitzki Moritz J, Kimmer Gwendolyn, Wehrmann Julian, Ritter Alina S, Radhakrishnan Praveen, Opitz Vanessa M, Tuffs Christopher, Biller Marvin, Kugler Julia, Keppler Ulrich, Harnoss Jonathan M, Klose Johannes, Schmidt Thomas, Blanco Alfonso, Taylor Cormac T, Schneider Martin. JCI insight. 2021;6(8) doi: 10.1172/jci.insight.139191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Novel de novo synthesized phosphate carrier compound ABA-PEG20k-Pi20 suppresses collagenase production in Enterococcus faecalis and prevents colonic anastomotic leak in an experimental model. Wiegerinck M, Hyoju S K, Mao J, Zaborin A, Adriaansens C, Salzman E, Hyman N H, Zaborina O, van Goor H, Alverdy J C. The British journal of surgery. 2018;105(10):1368–1376. doi: 10.1002/bjs.10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Selective matrix metalloproteinase inhibition increases breaking strength and reduces anastomotic leakage in experimentally obstructed colon. Krarup Peter-Martin, Eld Mikkel, Jorgensen Lars Nannestad, Hansen Mark Berner, Ågren Magnus S. International journal of colorectal disease. 2017;32(9):1277–1284. doi: 10.1007/s00384-017-2857-x. [DOI] [PubMed] [Google Scholar]
- 108.Effect of omiganan on colonic anastomosis healing in a rat model of peritonitis. Lorenzi Teresa, Trombettoni Maria Michela Cappelletti, Ghiselli Roberto, Paolinelli Francesca, Gesuita Rosaria, Cirioni Oscar, Provinciali Mauro, Kamysz Wojciech, Kamysz Elzbieta, Piangatelli Cristiano, Castellucci Mario, Guerrieri Mario, Morroni Manrico. American journal of translational research. 2017;9(7):3374–3386. [PMC free article] [PubMed] [Google Scholar]
- 109.Effects of albumin/glutaraldehyde glue on healing of colonic anastomosis in rats. Despoudi Kalliopi, Mantzoros Ioannis, Ioannidis Orestis, Cheva Aggeliki, Antoniou Nikolaos, Konstantaras Dimitrios, Symeonidis Savvas, Pramateftakis Manousos George, Kotidis Efstathios, Angelopoulos Stamatis, Tsalis Konstantinos. World journal of gastroenterology. 2017;23(31):5680–5691. doi: 10.3748/wjg.v23.i31.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]


