Abstract
Background:
Left ventricular assist device (LVAD) therapy is an increasingly viable alternative for patients who are not candidates for heart transplantation or who are waiting for a suitable donor. We aimed to determine whether there is an association between gender, race/ethnicity, insurance coverage, and neighborhood income, and access to / outcomes of LVAD implantation. We further analyzed whether access to LVAD improved in states that did vs did not expand Medicaid.
Methods and Results:
Retrospective cohort study using State Inpatient Databases to identify patients 18–85 years of age admitted for heart failure, cardiogenic shock, or LVAD implantation from 2012–2015. Logistic regression analyses adjusting for age, all the sociodemographic factors above, medical comorbidities, and a hospital random effect were used to quantify odds of receipt of LVADs, as well as outcomes conditional on receiving an LVAD, for the sociodemographic groups of interest. A total of 925,770 patients were included; 3,972 (0.43%) received LVADs. After adjusting for age, comorbidities, and hospital effects, women (adjusted OR (aOR) 0.45 [0.41–0.49]), black patients (aOR 0.83 [0.74–0.92]) and Hispanic patients (aOR 0.74 [0.64–0.87]) were less likely to receive LVADs than whites. Medicare (aOR 0.79 [0.72–0.86]), Medicaid (aOR 0.52 [0.46–0.58]), and uninsured patients (aOR 0.17 [0.11–0.25]) were less likely to receive LVADs than the privately insured, and patients in low-income ZIP codes were less likely than those in higher-income areas (aOR 0.71 [0.65–0.77]). Among those who received LVADs, women (aOR 1.78 [1.38–2.30]), patients of unknown race or race other than white, black, or Hispanic (aOR 1.97 [1.42–2.74]), and uninsured patients (aOR 4.86 [1.92–12.28]) had higher rates of in-hospital mortality. Medicaid expansion was not associated with an increase in LVAD implantation.
Conclusions:
There are meaningful sociodemographic disparities in access and outcomes for LVAD implantation. Medicaid expansion was not associated with an increase in LVAD rates.
Over 6.5 million people in the United States were living with heart failure (HF) in 2017, and its prevalence continues to rise; estimates suggest that by 2030, over 8 million Americans will be affected by this condition.1 Heart failure is a highly morbid disease, with high rates of hospitalization and 5-year mortality rates approaching 50%.1 One important treatment option for end-stage HF is mechanical support, in particular the use of left ventricular assist device (LVAD) therapy. This technology, growing in use in recent years, can be implanted either as a bridge to transplant (BTT) or as destination therapy (DT). Recent data suggests that the one-year and two-year survival among patients receiving LVADs approach 80% and 70% respectively,1 making it an increasingly viable alternative for patients who are not candidates for heart transplantation or who are waiting for a suitable donor.
However, it is unclear whether access to such technological advances has been equitable. Many prior studies have demonstrated significant disparities in healthcare access and outcomes in the care of patients with cardiovascular disease.2–5 Disparities based on sociodemographic risk factors such as gender, race and ethnicity, poverty, and geography have been demonstrated for many cardiac procedures, including cardiac catheterization, coronary artery bypass grafting, and defibrillator implantation.6–16 One prior study used Interagency Registry of Mechanically Assisted Circulatory Support (INTERMACS) data to report LVAD use per population, and found that African-American patients had the highest rate of use and Hispanic and Asian patients the lowest.17 However, this finding could reflect differences in the prevalence of heart failure across the population, or differences in the receipt of LVADs among patients with heart failure.
Among these sociodemographic factors, one particularly important (and modifiable) factor that determines access to care is insurance coverage. Under the Affordable Care Act, 23 States and the District of Columbia expanded their Medicaid programs, granting new access to health insurance for millions of Americans. Such insurance expansion could have a significant impact on access to care. One prior study showed that Medicaid expansion improved access to heart transplant listings among African Americans,15 and has been associated with a host of improvements in cardiovascular risk factor management and outcomes,18–21 but to our knowledge there are no studies examining whether Medicaid expansion has contributed to better access to advanced medical therapies such as LVAD.
Therefore, in this study, we aimed to determine whether there is an association between sociodemographic risk factors, including gender, race/ethnicity, insurance coverage, and neighborhood income, and access to and outcomes of LVAD implantation among patients with heart failure. We further analyzed whether access to LVAD improved in states that did vs those that did not expand Medicaid.
Methods
Data
The data used for this study are available from the Healthcare Cost and Utilization Project (https://www.hcup-us.ahrq.gov/) under appropriate data use agreements; the investigators are not authorized to share data independently. Code can be obtained by contacting the corresponding author directly. We used data from the State Inpatient Databases (SID) provided by the Healthcare Cost and Utilization Project22 for Arizona, Arkansas, Colorado, Florida, Massachusetts, Maryland, Michigan, Nebraska, New Jersey, Nevada, New York, Vermont, Washington, Wisconsin, and Iowa. Data between January 1, 2012 and December 31, 2015 were analyzed. These states were chosen because they were the only states with complete data available in the SID during the study period. We focused on 2012 to 2015 because the SID was redesigned beginning in 2012, changing from all discharges at a sample of hospitals to a sample of discharges from all hospitals. Therefore, trend analysis across that timeline requires a different approach to analysis. We included data through 2015, which was the most recent available year for many of the states in our sample. Patient demographics (age, sex, race, insurance status, ZIP code income quartile) and medical comorbidities were obtained from the database. The American Hospital Association (AHA) Annual Survey Database was linked to the SID to obtain hospital characteristics, such as hospital state, zip code, urban versus rural location, medical school affiliation, number of beds, and type of hospital. We adhered to the HCUP-provided “Checklist for Working with the NIS” during our data analysis.
Patient cohort
We identified patients between 18 and 85 years of age who were admitted for heart failure or with cardiogenic shock from January 1, 2012 through December 31, 2015. International Classification of Diseases, Clinical Modification, 9th revision (ICD-9 CM) and International Classification of Diseases, Clinical Modification, 10th revision (ICD-10 CM) codes were used to identify patients who were admitted with heart failure (ICD-9 428.xx, ICD-10 I50.xx) as their primary diagnosis, or cardiogenic shock (ICD-9 785.51, ICD-10 R57.0) as a primary or secondary diagnosis. Patients missing information on hospital id, zip code income, or insurance status were excluded from analysis. All patients treated in Maryland were missing information on discharge status and were therefore excluded from the discharge to home analysis.
Primary predictors
Our primary predictors were gender, race, insurance status, and median income of patients’ residential ZIP code, coded as lowest quartile or top three quartiles. We used Elixhauser comorbidities for risk adjustment.
For the analyses of differences based on Medicaid expansion, we considered expansion states to be Arkansas, Arizona, Colorado, Massachusetts, Maryland, Michigan, New Jersey, Nevada, New York, Vermont, Washington, and Iowa; non-expansion states were Florida, Nebraska, and Wisconsin. We reached out to heart failure clinicians in each state to determine whether LVADs were covered under state Medicaid; Arkansas was the only state for which LVADs were not covered, and Nevada lacks an in-state LVAD center, but all other states covered LVADs at least under some circumstances.
Primary and Secondary Outcomes
Our primary outcome was LVAD implantation (ICD-9 procedure code 37.66, ICD-10-PCS 02HA0QZ). Our secondary outcomes were limited to patients that received LVADs, and were mortality and discharge home (versus to a skilled nursing facility, inpatient rehabilitation, or long-term care hospital) after index LVAD implantation admission.
Analyses
We compared baseline characteristics such as age, sex, race, income, insurance status, and Elixhauser comorbidities between patients who did versus did not receive LVADs. We also summarized hospital characteristics such as size, ownership, teaching status, and urban/rural location. These characteristics were unavailable for hospitals in Michigan. We calculated raw LVAD implantation rates for our primary predictors, including race/ethnicity (Caucasian, African American, Hispanic, other/unknown), insurance status (private, Medicaid, Medicare and other public insurances, uninsured), and median ZIP income (lowest quartile, top three quartiles). We then ran a series of regression models to determine the independent association between each of these predictors and our primary and secondary outcomes. First, we added age and clinical comorbidities defined using the Elixhauser method to each model. This model includes both the between-hospital and within-hospital differences in outcome associated with the predictor of interest – for example, if uninsured patients were less likely to receive LVADs because they systematically went to hospitals that didn’t provide that technology, these models would capture that difference. Next, we added hospital-level random effects to the model), along with all primary predictors (race, insurance status, and median zip income). These models narrow any observed differences to only the within-hospital portion of the effect, and therefore, for these models, only the subset of hospitals that implanted at least one VAD were included (n=86). These models capture the difference between, for example, insured and uninsured patients within the same hospital. We calculated median odds ratios from these fully adjusted models.
Lastly, we performed difference-in-difference analyses in order to assess the association between Medicaid expansion and LVAD implantation rates. First, to validate our use of difference-in-differences analyses, we did a test for parallel pre-trends in LVAD access in expansion versus non-expansion states using 2012–2013 data by quarter. The p value for difference in trends was 0.10, suggesting that difference in difference analyses were appropriate. We used generalized linear models adjusting for gender, age, and comorbidities with and without a hospital random effect, and again limited the random effects models to the 86 hospitals implanting LVADs during the study period.
All analyses were performed using SAS 9.4 (Cary, North Carolina). IRB approval was obtained from the Washington University Human Research Protection Office, which deemed this project non-human-subjects research given the de-identified nature of the data, and waived the requirement for informed consent.
Results
Sample, Patient, and Hospital Characteristics
A total of 925,770 patients were included in the analysis; 3,972 (0.43%) received LVADs. Compared to the group that did not receive LVADs, the LVAD recipients were younger (67.9% less than 65 years of age in the LVAD group versus 35.0% in the non-LVAD group, Table 1). Fewer women were in the LVAD group (21.6%) compared to the non-LVAD group (43.5%, p<0.001). The LVAD group had lower percentages of individuals who were white, black, or Hispanic compared to the non-LVAD group, and a higher proportion of patients whose race was recorded as other or unknown (19.9% versus 8.9%, p<0.001). The LVAD group had a higher count of Elixhauser comorbidities (5.82) compared to the non-LVAD group, on average (5.33, p<0.001). In terms of individual comorbidities, this group had a higher prevalence of heart failure, renal failure, and valvular heart disease, but a lower prevalence of chronic lung disease, diabetes, hypertension, and obesity.
Table 1.
Patient Characteristics
| Characteristics | Overall (n=925,770) | No LVAD (n=921,798) | LVAD (n=3,972) | Comparison | |||
|---|---|---|---|---|---|---|---|
| n/mean | %/SD | n/mean | %/SD | n/mean | %/SD | p | |
| Age | |||||||
| 18–34 | 14,691 | 1.6% | 14,380 | 1.6% | 311 | 7.8% | <0.001 |
| 35–44 | 31,793 | 3.4% | 31,428 | 3.4% | 365 | 9.2% | <0.001 |
| 45–54 | 94,990 | 10.3% | 94,193 | 10.2% | 797 | 20.1% | <0.001 |
| 55–64 | 183,556 | 19.8% | 182,333 | 19.8% | 1,223 | 30.8% | <0.001 |
| 65–74 | 264,280 | 28.5% | 263,233 | 28.6% | 1,047 | 26.4% | 0.002 |
| 75–84 | 336,460 | 36.3% | 336,231 | 36.5% | 229 | 5.8% | <0.001 |
| Female | 402,110 | 43.4% | 401,254 | 43.5% | 856 | 21.6% | <0.001 |
| Race | |||||||
| White | 577,050 | 62.3% | 574,798 | 62.4% | 2,252 | 56.7% | <0.001 |
| Black | 187,859 | 20.3% | 187,156 | 20.3% | 703 | 17.7% | <0.001 |
| Hispanic | 77,660 | 8.4% | 77,434 | 8.4% | 226 | 5.7% | <0.001 |
| Other/Unknown | 83,201 | 9.0% | 82,410 | 8.9% | 791 | 19.9% | <0.001 |
| Average number of Elixhauser Comorbidities | 5.34 | 2.01 | 5.33 | 2.01 | 5.82 | 2.21 | <0.001 |
| Heart Failure | 881,428 | 95.2% | 877,572 | 95.2% | 3,856 | 97.1% | <0.001 |
| Chronic Lung Disease | 374,023 | 40.4% | 373,257 | 40.5% | 766 | 19.3% | <0.001 |
| Diabetes | 458,152 | 49.5% | 456,746 | 49.5% | 1,406 | 35.4% | <0.001 |
| Hypertension | 730,374 | 78.9% | 727,981 | 79.0% | 2,393 | 60.2% | <0.001 |
| Obesity | 228,856 | 24.7% | 228,116 | 24.7% | 740 | 18.6% | <0.001 |
| Renal Failure | 383,395 | 41.4% | 381,647 | 41.4% | 1,748 | 44.0% | 0.001 |
| Valvular Heart Disease | 265,741 | 28.7% | 263,910 | 28.6% | 1,831 | 46.1% | <0.001 |
LVAD=left ventricular assist device
There were 1,303 hospitals in our sample. 132 of these hospitals were in Michigan, which does not allow linkage of its hospitals to the AHA dataset for hospital information, so these were excluded from our hospital characteristics table. Of the hospitals for which we had information, 510 were small in size (43.6%), 349 were medium (29.8%), and 312 were large (26.6%, Table 2). The majority of the hospitals were private, not-for-profit (66.7%), non-teaching hospitals (66.9%), and located in urban settings (65.8%).
Table 2.
Hospital Characteristics
| Characteristics | Number of Hospitals (N) | Percentage (%) |
|---|---|---|
| Size | ||
| Small | 510 | 43.6% |
| Medium | 349 | 29.8% |
| Large | 312 | 26.6% |
| Ownership | ||
| Government, nonfederal (public) | 217 | 18.5% |
| Private, not-for-profit | 781 | 66.7% |
| Private, investor owned (for-profit) | 173 | 14.8% |
| Teaching Status | ||
| Teaching Hospital | 391 | 33.4% |
| Non-teaching hospital | 783 | 66.9% |
| Urban/Rural | ||
| Urban | 771 | 65.8% |
| Rural | 400 | 34.2% |
A total of 682 patients were missing hospital id, 25,228 patients were missing zip code income, and 497 patients were missing insurance status. Additionally, among patients who received LVAD, 166 were missing discharge status.
Disparities in LVAD Implantation
Women had lower unadjusted rates of LVAD implantation compared to men (0.21% versus 0.60%, p<0.001, Table 3). Unadjusted rates of LVAD implantation were 0.39%, 0.37%, and 0.29% in white, black, and Hispanic patients, respectively, and 0.95% in those whose race was classified as other or unknown (p<0.001). Patients who were privately insured had higher unadjusted rates of LVAD implantation (1.18%) compared to patients who had Medicaid (0.47%), Medicare and other public insurance (0.29%), or no insurance coverage (0.09%, p<0.001). Patients in low-income ZIP codes had lower rates of LVAD implantation than those in higher-income areas (0.30% versus 0.49%, p<0.001).
Table 3.
Rate and Odds of Left Ventricular Assist Device Implantation, by Sociodemographic Risk Factor
| Raw Rate (%) | Raw Rate of LVAD Implantation | Risk-Adjusted Odds Ratio (95% CI)* | Fully Adjusted Odds Ratio (95% CI) † |
|---|---|---|---|
| Gender | |||
| Male | 0.60% | Reference | Reference |
| Female | 0.21% | 0.41 (0.38, 0.44) | 0.45 (0.41, 0.49) |
| Race | |||
| Caucasian | 0.39% | Reference | Reference |
| African American | 0.37% | 0.68 (0.53, 0.86) | 0.83 (0.74, 0.92) |
| Hispanic | 0.29% | 0.59 (0.44, 0.81) | 0.74 (0.64, 0.87) |
| Other/Unknown | 0.95% | 1.57 (0.95, 2.60) | 0.76 (0.68, 0.86) |
| Insurance Status | |||
| Private Insurance | 1.18% | Reference | Reference |
| Medicare | 0.29% | 0.64 (0.57, 0.72) | 0.79 (0.72, 0.86) |
| Medicaid | 0.47% | 0.37 (0.31, 0.45) | 0.52 (0.46, 0.58) |
| Uninsured | 0.09% | 0.07 (0.04, 0.12) | 0.17 (0.11, 0.25) |
| ZIP Code Median Income | |||
| Top Three Quartiles | 0.49% | Reference | Reference |
| Lowest Quartile | 0.30% | 0.62 (0.50, 0.77) | 0.71 (0.65, 0.77) |
| Median Odds Ratio (MOR) | N/A | N/A | 4.16 |
Model adjusts for age and Elixhauser comorbidities
Model adjusts for age, Elixhauser comorbidities, all listed sociodemographic risk factors, and a hospital random effect. Therefore, these odds ratios represent the within-hospital effect of the factor in question and limit the sample to only those 86 hospitals that performed LVADs.
After adjusting for age and comorbidities, women were less likely to receive LVADs compared to men (odds ratio [OR] 0.41, 95% confidence interval [CI] 0.38–0.44, Table 3), which remained similar after additionally adjusting for all sociodemographic risk factors we examined and adding a hospital random effect (fully-adjusted OR 0.45 [0.41–0.49]). Black patients were less likely to receive LVADs compared to white patients (risk-adjusted OR 0.68 [0.53–0.86], fully-adjusted OR 0.83 [0.74–0.92]). Patterns were similar for Hispanic patients (risk-adjusted OR 0.59 [0.44–0.81], fully-adjusted OR 0.74 [0.64–0.87]). Medicare patients (risk-adjusted OR 0.64 [0.57–0.72], fully-adjusted OR 0.79 [0.72–0.86]) and Medicaid patients (risk-adjusted OR 0.37 [0.31–0.45], fully-adjusted OR 0.52 [0.46–0.58]) were less likely to receive LVADs compared to privately insured patients. Uninsured patients were even less likely to receive LVADs compared to privately insured patients (risk-adjusted OR 0.07 [0.04–0.12], fully-adjusted OR 0.17 [0.11–0.25]). Patients in low-income ZIP codes were less likely to receive LVADs compared to those in higher-income areas (risk-adjusted OR 0.62 [0.50–0.77], fully-adjusted OR 0.71 [0.65–0.77]). The median odds ratio (MOR) for LVAD implantation was 4.16, suggesting that the site at which care was received was a larger predictor of whether LVAD was implanted than any specific clinical features of the patient, even among the subset of hospitals at which LVADs were implanted.
Outcomes of LVAD implantation
Among individuals who received LVADs, and after adjusting for age, Elixhauser comorbidities, insurance status, income, and adding a hospital-level random effect, women had higher in-hospital mortality rates compared to men (fully-adjusted OR 1.78 [1.38–2.30], Table 4). Patients of unknown race or race other than white, black, or Hispanic who received LVADs had higher in-hospital mortality rates compared to white patients (fully-adjusted OR 1.97 [1.42–2.74]). There were no significant differences in mortality between white, black, and Hispanic patients. Compared to patients who were privately insured, uninsured patients had higher in-hospital mortality (fully-adjusted OR 4.86 [1.92–12.28]). There were no differences in in-hospital mortality between Medicare patients or Medicaid patients compared to privately insured patients (fully-adjusted OR 0.92 [0.70–1.21], fully-adjusted OR 0.71 [0.46–1.10] respectively), or by ZIP income (fully-adjusted OR 0.88 [0.65–1.19]). The MOR was 1.75, suggesting substantial variability in mortality rates between hospitals even after accounting for measurable patient characteristics.
Table 4:
Relationship between Sociodemographic Risk Factors and Outcomes of LVAD implantation
| Unadjusted Mortality Rate | Adjusted Odds Ratio (95% CI) | Unadjusted Discharge Home Rate | Adjusted Odds Ratio (95% CI) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 10.0% | Reference | 24.4% | Reference |
| Female | 14.5% | 1.78 (1.38, 2.30) | 19.9% | 0.70 (0.55, 0.90) |
| Race | ||||
| Caucasian | 10.5% | Reference | 26.3% | Reference |
| African American | 7.7% | 0.89 (0.62, 1.29) | 21.7% | 0.80 (0.60, 1.07) |
| Hispanic | 10.2% | 1.18 (0.71, 1.96) | 26.5% | 0.96 (0.65, 1.42) |
| Other/Unknown | 15.6% | 1.97 (1.42, 2.74) | 16.0% | 0.88 (0.64, 1.20) |
| Insurance Status | ||||
| Private Insurance | 11.0% | Reference | 26.5% | Reference |
| Medicare | 11.7% | 0.92 (0.70, 1.21) | 19.2% | 0.79 (0.63, 1.00) |
| Medicaid | 6.6% | 0.71 (0.46, 1.10) | 30.5% | 1.05 (0.78, 1.43) |
| Uninsured | 34.6% | 4.86 (1.92, 12.28) | 34.6% | 0.56 (0.18, 1.78) |
| ZIP Code Median Income | ||||
| Top Three Quartiles | 11.7% | Reference | 23.7% | Reference |
| Lowest Quartile | 8.4% | 0.88 (0.65, 1.19) | 22.3% | 0.90 (0.71, 1.15) |
| Median Odds Ratio (MOR) | 1.75 | 5.28 | ||
Models adjust for age, Elixhauser comorbidities, all listed sociodemographic risk factors, and a hospital random effect. Therefore, the adjusted odds ratios represent the within-hospital differences in LVAD outcomes.
Women had a lower likelihood of being discharged home compared to men (fully-adjusted OR 0.70 [0.55, 0.90]). There was no significant difference in the likelihood of being discharged home based on race. While Medicare patients had lower odds of being discharged home than privately insured patients (fully-adjusted OR 0.79 [0.63 −1.00], Table 4), there were no statistical differences in the likelihood of being discharged home for Medicaid or uninsured patients compared to privately insured patients, nor based on ZIP income quartile. The MOR was 5.28, suggesting very wide variability in discharge practices between hospitals.
Association of Medicaid Expansion with LVAD Rates and Outcomes
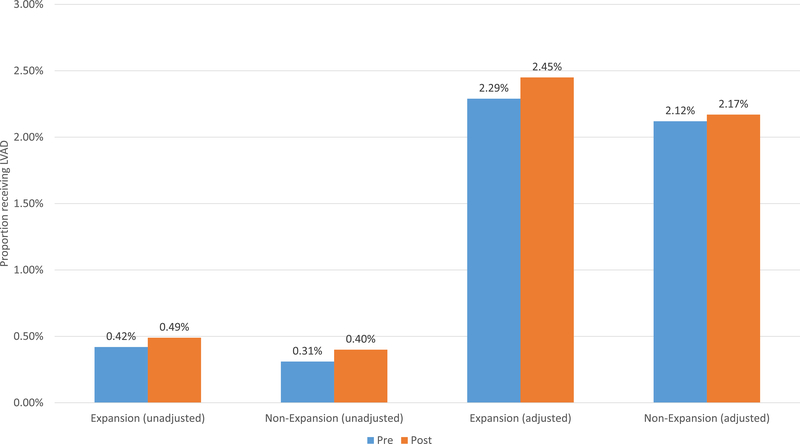
In Medicaid expansion states, the unadjusted LVAD implantation rate increased from 0.42% pre-expansion to 0.49% post-expansion (p=0.004); the unadjusted LVAD implantation rate in the non-expansion states did not change significantly (0.31% to 0.40%, p=0.14, difference in differences (DID) −0.02%, p=0.76, Figure 1). Results were similar after adjusting for age, gender, race, insurance status, income, Elixhauser comorbidities, and adding a hospital random effect (therefore limiting the denominator to patients at hospitals where at least one LVAD was implanted) (expansion states 2.29% to 2.45%, p=0.02, non-expansion states 2.12% to 2.17%, p=0.59, DID 0.11%, p=0.39).
Figure 1: Association Between Medicaid Expansion and Receipt of LVAD.
Adjusted models adjust for age, Elixhauser comorbidities, and a hospital random effect. Therefore, the adjusted analyses limit the sample only to hospitals that implanted at least one LVAD in the study period.
Discussion
In this national study of patients with heart failure or cardiogenic shock, we found significant differences in LVAD implantation based on sociodemographic risk factors, such as gender, race, insurance coverage, and income. However, among patients who received LVADs, clinical outcomes, such as in-hospital mortality, length-of-stay, and percentage discharged home were relatively similar across different sociodemographic groups, except for higher in-hospital mortality among women, patients of unknown or non-white, non-black, non-Hispanic race/ethnicity, and patients who were uninsured. We also found that Medicaid expansion was not associated with a differential increase in LVAD implantation among states that expanded versus those that did not, among the limited sample of states in this study.
We found that women, black and Hispanic patients, patients with Medicaid or who were uninsured, and those living in low-income ZIP codes, were less likely to receive LVADs than patients who were male, white, privately insured, or living in wealthier areas. It has long been known that women, minorities, and individuals living in poverty have less access to cardiovascular procedures, including implantable cardiac defibrillators, cardiac resynchronization therapy, stress testing, coronary artery bypass surgery, percutaneous coronary intervention following myocardial infarction, and cardiac transplantation.6–16 The reasons for this are complex, and include access to high-quality specialty care in both the inpatient and outpatient settings, as well as financial barriers to receiving costly therapies. Interestingly, the median odds ratios, even among hospitals that implanted LVADs, were very high, suggesting that patients’ odds of receiving an LVAD varied tremendously by hospital. This is likely in part due to referral patterns (ie the sickest patients concentrating at a small number of hospitals), but may also reflect significant underlying differences in practice patterns between centers.
It is also possible that differences are explained by clinicians’ assessment of patients’ resources. The care of LVAD patients not only requires technological skills and financial resources on the part of the hospital, but also requires more intense follow up and a great deal of social support on the part of the patient. Clinical decision making therefore often involves not only consideration of patients’ objective clinical status, but also involves assessment of patients’ social support and psychosocial status. Real – or perceived – differences among different racial and socioeconomic groups in these factors may contribute to clinician assessment of patients’ candidacy for LVAD therapy. Qualitative studies and work involving more granular clinical and socioeconomic data and considering the possibility of implicit bias could shed further light on why this might be the case. There may also be differences among these groups in patient preferences for LVAD implantation, and socioeconomic factors have been shown to be associated with these preferences; interestingly, lower income was previously found to be associated with a higher treatment preference for LVAD therapy,23 which is the opposite of the pattern we found. A better understanding of both clinician decision-making and patient preferences will be crucial to understanding whether the differences we document represent disparities or patient preference-concordant care.
Conditional on receiving LVADs, we found that black and Hispanic individuals had similar in-hospital mortality compared to white patients, as did patients with Medicaid insurance compared to those who were privately insured. The median odds ratio for mortality was high, also suggesting that the site of care plays a major role in outcomes. These findings may suggest that when patients have access to procedures and advanced technologies, race and ethnicity play a lesser role in certain outcomes. Indeed, one study looking at the impact of ethnicity on outcomes following coronary artery bypass surgery using Veterans Health Administration data – a group in which everyone has essentially the same coverage – showed no difference in 30-day and 6-month mortality between African-American and Caucasian patients.24 Another multi-institutional outcome analysis of patients undergoing LVAD implantation also found no difference in survival at 1, 3 or 5 years by race.25 However, women had higher mortality than men following LVAD implantation, in contrast to one prior study showing no long-term survival difference by sex, albeit in a smaller sample.25 This also highlights the importance of assuring equal access to emerging technologies in ensuring that medical advances serve to narrow, rather than widen, disparities in care.
Uninsured patients also fared particularly poorly after receiving LVADs, which could be related to underlying differences in disease, or to difficulties with access to care before LVAD implantation that contributed to more poorly controlled risk factors prior to the procedure. It is possible that these differences reflect unmeasured differences in underlying comorbidities and severity of illness, that these groups were sicker when they received their LVADs, or that post-operative care differed for one group versus another. In the case of the uninsured, it is also conceivable that uninsured patients are only qualifying for emergent LVAD implantation (such as acute fulminant heart failure in younger individuals), making it a different patient population and again raising the possibility of potential selection bias. Unfortunately, the SID database does not have physiologic variables to further delineate these possibilities. A different database with more clinical data, such as INTERMACS, could help elucidate the underlying factors contributing to these differences.
We hypothesized that Medicaid expansion would be associated with an increase in the rate of LVAD implantation in Medicaid expansion states. While we did see a significant increase in the raw rate of LVAD implantation after Medicaid expansion, there was no significant differential change during this time period between expansion states and non-expansion states. We also did not find a significant differential change in mortality, length of stay, or discharge location between expansion states and non-expansion states. There are several possible explanations for this. Because LVADs remain a relatively rare technology, and our sample size was low, we may have simply been underpowered to detect a difference. Additionally, coverage of expensive procedures like LVADs likely varies by state, and details on coverage at the state level are not readily publicly available. As a result, we were unable to stratify our analysis by level of Medicaid coverage of this procedure. Finally, while many LVADs are placed emergently, there are patients who are referred and evaluated as outpatients for LVAD implantation. Patients who have become newly eligible for LVAD coverage still need time to apply for Medicaid and to be referred for advanced heart failure therapy. Such a time lag could potentially reduce the ability to detect a significant change within our study period.
Our findings add to prior literature on disparities in LVADs. One prior study examining LVAD implantation between 2002–2003 found that Caucasians were more likely to receive LVADs compared to African Americans,26 which is consistent with our findings. Another study demonstrated that patients with lower socioeconomic status were more likely to be readmitted after LVAD implantation.27 Joshi et al examined the NIS database from 2004–2016 and found higher inpatient mortality in women compared to men, although this difference was more pronounced in the pulsatile-flow era (2004–2008) and was no longer present in the continuous-flow era (2009–2016). We suspect our findings differ from theirs because of differences in sample years and states as well as slightly different approaches to risk adjustment; Joshi et al elected to adjust for complications whereas we felt that these were likely on the causal pathway to poor outcomes and have not done so.28 To our knowledge there are no prior studies examining Medicaid expansion and LVAD implantation.
There are several limitations to our findings. First, there are inherent limitations in administrative database research, as we do not have access to more granular details of patient characteristics or clinical decision-making. As a result, we included all patients who were admitted with heart failure or cardiogenic shock, only a minority of whom would be expected to require LVAD implantation. We are also unable to adjust for patients’ clinical status, such as medications, hemodynamics, or ejection fraction, and these may be unmeasured confounders. Administrative data may also be subject to misclassification and missing data, which may vary by state. Second, as stated above, given the small number of LVAD implantations overall, and the limited number of states with longitudinal data available in the SID database, our analyses examining the LVAD implantation rate pre- and post-Medicaid expansion could be underpowered and should be considered exploratory. In particular, we had a small number of non-expansion states and they may not be a representative group against which to compare. Lack of evidence of an effect does not indicate evidence of lack of an effect. Third, while the majority of the states in our sample had Medicaid programs that covered LVAD in at least some situations, this is not the case nationwide, and our findings regarding Medicaid may therefore not generalize more broadly.
In summary, we found that patients who are women, racial/ethnic minorities, who have no insurance or Medicaid insurance, or who are from low-income zip codes were less likely to receive LVADs. Among patients who received LVADs, gender, unknown/other race, and insurance status were associated with poor outcomes. Among the states examined in this study, Medicaid expansion was not associated with a differential change in LVAD implantation rates. Further studies are needed to identify the factors underlying these differences in LVAD implantation, and to identify potential solutions if they do indeed reflect inappropriate disparities in care.
Supplementary Material
What is Known
Left ventricular assist device (LVAD) therapy is an increasingly viable alternative for patients who are not candidates for heart transplantation or who are waiting for a suitable donor.
Disparities based on sociodemographic risk factors such as gender, race and ethnicity, poverty, insurance coverage, and geography have been demonstrated for many cardiac procedures, but less is known about the relationship between these factors and LVAD implantation.
What the Study Adds
Women, Black patients, and Hispanic patients were less likely to receive LVADs than whites, and individuals with Medicare or Medicaid or who were uninsured were less likely to receive LVADs than those who were privately insured. Patients living in low-income ZIP codes were less likely to receive LVADs than those in wealthier areas.
Among patients receiving LVADs, women, patients of unknown race or race other than white, Black, or Hispanic, and uninsured patients had higher in-hospital mortality rates.
Medicaid expansion was not associated with an increase in LVAD rates.
Acknowledgments:
The authors acknowledge Kristine Huang, BA, for assistance with formatting and tables. She received no compensation for this work beyond that associated with employment.
Sources of Funding:
This project was funded in part by the Mentors in Medicine Program at Washington University in St Louis School of Medicine. The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ).
Disclosures:
Dr. Joynt Maddox receives research support from the National Heart, Lung, and Blood Institute (R01HL143421) (significant) and National Institute on Aging (R01AG060935) (significant), and previously did contract work for the US Department of Health and Human Services (significant).
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilic A, Higgins RS, Whitson BA, Kilic A. Racial disparities in outcomes of adult heart transplantation. Circulation. 2015;131:882–889. [DOI] [PubMed] [Google Scholar]
- 3.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. [DOI] [PubMed] [Google Scholar]
- 4.Mirvis DM, Graney MJ. Impact of race and age on the effects of regionalization of cardiac procedures in the Department of Veterans Affairs Health Care System. Am J Cardiol. 1998;81:982–987. [DOI] [PubMed] [Google Scholar]
- 5.Mirvis DM, Graney MJ. Variations in the use of cardiac procedures in the Veterans Health Administration. Am Heart J. 1999;137:706–713. [DOI] [PubMed] [Google Scholar]
- 6.Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med. 2001;135:352–366. [DOI] [PubMed] [Google Scholar]
- 7.Napoli AM, Choo EK, Dai J, Desroches B. Racial disparities in stress test utilization in an emergency department chest pain unit. Critical Pathways in Cardiology. 2013;12:9–13. [DOI] [PubMed] [Google Scholar]
- 8.Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm. 2009;6:325–331. [DOI] [PubMed] [Google Scholar]
- 9.Groeneveld PW, Heidenreich PA, Garber AM. Racial disparity in cardiac procedures and mortality among long-term survivors of cardiac arrest. Circulation. 2003;108:286–291. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez AF, Fonarow GC, Liang L, Al-Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–1532. [DOI] [PubMed] [Google Scholar]
- 11.Groeneveld PW, Heidenreich PA and Garber AM. Trends in implantable cardioverter-defibrillator racial disparity: the importance of geography. J Am Coll Cardiol. 2005;45:72–78. [DOI] [PubMed] [Google Scholar]
- 12.Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE and Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. New Engl J Med. 1999;340:609–616. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. J Am Coll Cardiol. 2006;47:417–424 [DOI] [PubMed] [Google Scholar]
- 14.Brown CP, Ross L, Lopez I, Thornton A, Kiros GE. Disparities in the receipt of cardiac revascularization procedures between blacks and whites: an analysis of secular trends. Ethn Dis. 2008;18:S2–112-117. [PMC free article] [PubMed] [Google Scholar]
- 15.Breathett K, Allen LA, Helmkamp L, Colborn K, Daugherty SL, Khazanie P, Lindrooth R, Peterson PN. The Affordable Care Act Medicaid Expansion Correlated With Increased Heart Transplant Listings in African-Americans But Not Hispanics or Caucasians. JACC Heart Fail. 2017;5:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Onofrio G, Safdar B, Lichtman JH, Strait KM, Dreyer RP, Geda M, Spertus JA, Krumholz HM. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: results from the VIRGO study. Circulation. 2015;131:1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breathett K, Allen LA, Helmkamp L, Colborn K, Daugherty SL, Blair IV, Jones J, Khazanie P, Mazimba S, McEwen M, Stone J, Calhoun E, Sweitzer NK, Peterson PN. Temporal trends in contemporary use of ventricular assist devices by race and ethnicity. Circ Heart Fail. 2018;11:e005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khatana SAM, Bhatla A, Nathan AS, Giri J, Shen C, Kazi DS, Yeh RW, Groeneveld PW. Association of Medicaid expansion with cardiovascular mortality. JAMA Cardiol. 2019;4:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wherry LR, Miller S. Early coverage, access, utilization, and health effects associated with the affordable care act medicaid expansions: a quasi-experimental study. Ann Intern Med. 2016;164:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman HW, Chen Z, Fonseca VA, McPhaul MJ. Surge in newly identified diabetes among medicaid patients in 2014 within Medicaid expansion states under the Affordable Care Act. Diabetes Care. 2015;38:833–7. [DOI] [PubMed] [Google Scholar]
- 21.Cole MB, Galarraga O, Wilson IB, Wright B, Trivedi AN. At federally funded health centers, Medicaid expansion was associated with improved quality of care. Health Aff (Millwood). 2017;36:40–48. [DOI] [PubMed] [Google Scholar]
- 22.Healthcare Cost and Utilization Project (HCUP). HCUP State Inpatient Databases (SID). 2005–2009.
- 23.Tchoukina I, Shah KB, Thibodeau JT, Estep JD, Lala A, Lanfear DE, Gilotra NA, Pamboukian SV, Horstmanshof DA, McNamara DM, Haas DC, Jorde UP, McLean RC, Cascino TM, Khalatbari S, Richards B, Yosef M, Spino C, Baldwin JT, Mann DL, Aaronson KD, Stewart GC. Impact of Socioeconomic Factors on Patient Desire for Early LVAD Therapy Prior to Inotrope Dependence. J Card Fail. 2020;26:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rumsfeld JS, Plomondon ME, Peterson ED, Shlipak MG, Maynard C, Grunwald GK, Grover FL, Shroyer ALW. The impact of ethnicity on outcomes following coronary artery bypass graft surgery in the Veterans Health Administration. JACC. 2002;40:17861793. [DOI] [PubMed] [Google Scholar]
- 25.Meeteren JV, Maltais S, Dunlay SM, Haglund NA, Beth Davis M, Cowger J, Shah P, Aaronson KD, Pagani FD, Stulak JM. A multi-institutional outcome analysis of patients undergoing left ventricular assist device implantation stratified by sex and race. J Heart Lung Transplant. 2017;36:64–70. [DOI] [PubMed] [Google Scholar]
- 26.Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res. 2009;152:111–117. [DOI] [PubMed] [Google Scholar]
- 27.Smith SA, Hasan AK, Binkley PF, Foraker RE. The impact of insurance and socioeconomic status on outcomes for patients with left ventricular assist devices. J Surg Res. 2014;191:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi AA, Lerman JB, Sajja AP, Dahiya G, Gokhale AV, Dey AK, Kyvernitakis A, Halbreiner MS, Bailey S, Alpert CM, Poornima IG, Murali S, Benza RL, Kanwar M, Raina A. Sex-based differences in left ventricular assist device utilization. Circ Heart Fail. 2019;12:e006082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.