Abstract
To initiate protein synthesis, a ribosome with bound initiator methionyl-tRNA must be assembled at the start codon of an mRNA. This process requires the coordinated activities of three translation initiation factors (IF) in prokaryotes and at least 12 translation initiation factors in eukaryotes (eIF). The factors eIF1A and eIF5B from eukaryotes show extensive amino acid sequence similarity to the factors IF1 and IF2 from prokaryotes. By a combination of two-hybrid, coimmunoprecipitation, and in vitro binding assays eIF1A and eIF5B were found to interact directly, and the eIF1A binding site was mapped to the C-terminal region of eIF5B. This portion of eIF5B was found to be critical for growth in vivo and for translation in vitro. Overexpression of eIF1A exacerbated the slow-growth phenotype of yeast strains expressing C-terminally truncated eIF5B. These findings indicate that the physical interaction between the evolutionarily conserved factors eIF1A and eIF5B plays an important role in translation initiation, perhaps to direct or stabilize the binding of methionyl-tRNA to the ribosomal P site.
The fundamental process of translation initiation has been conserved between prokaryotes and eukaryotes. The initiator Met-tRNA is bound to the small ribosomal subunit, and this complex is localized to the AUG start codon of an mRNA. Three translation initiation factors (IFs) have been identified in prokaryotes (reviewed in references 7 and 18). Factor IF2 is responsible for binding fMet-tRNAiMet to the 30S ribosomal subunit. Factor IF1 binds to the 30S subunit and protects the same region of the ribosome (A site) as the elongation factor EF-Tu–GTP–aminoacyl-tRNA complex (28). Although a unique function has not been attributed to IF1, it does promote IF2 activities (reviewed in references 7, 18, 22, and 31. Factor IF3 dissociates ribosomal complexes, presumably to generate a pool of small ribosomal subunits for translation initiation. In addition, IF3 has recently been implicated in the process of ribosome recycling following termination of translation (21). Translation initiation is a much more complex process in eukaryotes than in prokaryotes, and, as might be expected, a larger number of initiation factors are required in eukaryotes (27).
The binding of Met-tRNAiMet to the small 40S ribosomal subunit in eukaryotes is facilitated by factor eIF2. Factor eIF1A, formerly known as eIF4C, has been reported to function in subunit dissociation as well as promoting and stabilizing Met-tRNA binding to the 40S subunit (4, 9, 10, 41, 42). In addition, ribosomal toe-printing assays revealed that eIF1A is required for formation of a 48S preinitiation complex in which a 40S subunit with associated initiation factors and Met-tRNAiMet is bound at the AUG start codon of an mRNA following ribosome scanning (32).
Translation initiation factor eIF5B shows striking sequence similarity to prokaryotic IF2 and was previously referred to as yIF2 (11) or hIF2 (25) for yeast (Saccharomyces cerevisiae) or human IF2, respectively. It was reported recently that eIF5B plays an important role in the subunit joining step of protein synthesis (33). In yeast, eIF5B is nonessential; however, deletion of the FUN12 gene encoding eIF5B impairs translation initiation in vivo and in vitro and causes a severe slow-growth phenotype (11). This slow-growth phenotype can be partially suppressed by overexpression of tRNAiMet, suggesting that eIF5B is important for Met-tRNAiMet binding to ribosomes (11). Human eIF5B can functionally substitute for the yeast factor to promote growth in vivo and to restore translation initiation in extracts prepared from fun12Δ strains (25). Like prokaryotic IF2, eIF5B contains a consensus GTP-binding domain. The factor possesses ribosome-dependent GTPase activity that appears to be required for dissociation of the factor from 80S ribosomes following subunit joining (33). Consistent with an important role for GTP binding by eIF5B, mutations in the GTP-binding domain of human eIF5B impair translation in yeast and human cells (25, 44). Recently, Drosophila melanogaster eIF5B was reported to interact with the DEAD box RNA helicase VAS encoded by the gene vasa (8). Genetic interactions between mutations in VAS and eIF5B suggest that the proteins functionally interact; however, the role of this interaction in translation initiation is not known. An eIF5B/IF2 homolog has also been identified in Archaea, and we found that this protein can partially substitute for the yeast protein both in vivo and in vitro (25). Interestingly, archaeal and eukaryotic eIF1As show strong sequence similarity to prokaryotic IF1 (23). Thus, eIF5B/IF2 and eIF1A/IF1 form a pair of universally conserved translation initiation factors.
A common theme emerging from structural studies of translation factors is mimicry of tRNA. Comparisons of the crystal structures of the prokaryotic translation elongation factor EF-Tu–GTP–Phe-tRNAPhe ternary complex and elongation factor EF-G–GDP binary complex revealed that domains III and IV of EF-G bore strong resemblance to the anticodon stem and loop of the tRNA in the EF-G ternary complex (29, 30). In addition, the GTP-binding domains of EF-Tu and EF-G could be superimposed in the two structures (29, 30). Given that EF-Tu binds the aminoacyl-tRNA to the ribosomal A site, the structure of EF-G supported the idea that EF-G binds in the ribosomal A site to promote translocation of the peptidyl-tRNA from the A site to the P site during elongation. The structure of the translation termination factor eRF1 from humans also shows some similarities to that of a tRNA molecule; however, the tertiary structure of the putative tRNA-mimicking domains in eRF1 do not resemble that of the corresponding domains in EF-G (1, 14, 39). Likewise, the structural elements comprising the tRNA mimicry domain of the bacterial ribosome recycling factor RRF resemble neither those of EF-G nor those of eRF1 (36). Thus, proteins have developed multiple ways to mimic the structure of a tRNA.
Recently, Brock et al. (7) extended this molecular mimicry hypothesis to translation initiation. Based on amino acid sequence similarity between different segments of the C-terminal regions of EF-G and prokaryotic IF1 or IF2, they proposed that IF1 and IF2 interact with one another and together structurally mimic EF-G. Because Met-tRNAiMet is thought to be delivered directly to the ribosomal P site (16, 18), the notion that an IF1-IF2 complex structurally mimics EF-G and binds to the A site on the ribosome is appealing. According to this model, A site binding by the IF1-IF2 complex sterically blocks this site and directs the binding of Met-tRNAiMet to the P site. The fact that IF1 binding has been localized to the ribosomal A site supports this model (28). Protein cross-linking studies suggested that IF1 and IF2 can interact when bound to the 30S ribosomal subunit (6); however, a direct interaction between the isolated proteins off the ribosome has not been reported. We were intrigued by the molecular mimicry hypothesis for IF1 and IF2, and, based on the strong sequence conservation of eIF1A and eIF5B with IF1 and IF2, respectively, we decided to test the hypothesis that the eukaryotic factors interact.
MATERIALS AND METHODS
Plasmids.
A 3.95-kb SalI-SacI DNA fragment carrying the FUN12 gene encoding yeast eIF5B was excised from plasmid pC479 (11) and inserted between the same sites of pUC19 (45) and pRS426 (12) creating plasmids pC1039 and pC1070, respectively. A FLAG epitope (DYKDDDDK) was inserted in place of Glu-16 in eIF5B making use of a natural NspV site. With a 3′ primer that contained the NspV site and also encoded the FLAG epitope, an ∼0.43-kb SalI-NspV fragment containing the 5′ end of FUN12 was amplified by PCR and inserted between the same sites of pC1039 to generate FLAG-tagged FUN12 plasmid pC1041. The SalI-SacI fragment from pC1041 was subcloned into URA3 plasmids pRS316 (38) and pRS426 (12) creating low- and high-copy-number FLAG-FUN12 plasmids pC1005 and pC1064, respectively. The plasmids for expressing N-terminally truncated FLAG-tagged eIF5B378–1002 were constructed in three steps. First, an ∼2.4-kb fragment encoding eIF5B378–1002 was amplified by PCR using pC1039 as a template, a 5′ primer that introduced an NspV site, and a 3′ primer containing the SacI site present in pC1039. The PCR product was digested with NspV and SacI and inserted between the same sites of pC1041 creating plasmid pC1042. Finally, an ∼2.8-kb SalI-SacI fragment from pC1042 was subcloned to pRS316 and pRS426 generating low- and high-copy-number FLAG-FUN12 (378–1002) plasmids pC1043 and pC1007, respectively. An ∼1.63-kb NspV fragment from plasmid pC1070 containing the 5′ end of the FUN12 gene and flanking vector sequences was inserted in place of the corresponding fragment in pC1007 generating the high-copy-number URA3 plasmid pC1037 that expressed untagged N-terminally truncated eIF5B378–1002. Due to the manner in which the N-terminally truncated allele was generated, both eIF5B378–1002 and FLAG-eIF5B378–1002 contained the first 15 residues of the native eIF5B at their N termini.
The FUN12 alleles expressing C-terminally deleted forms of eIF5B were generated by PCR. The FUN12 sequences encoding eIF5B396–827 or eIF5B396–915 were amplified using a 5′ primer that included the native Eco47III site and a 3′ primer that introduced a BamHI site at the appropriate position. The PCR products were subcloned between the Eco47III and BamHI sites of pC1041 creating pC1092 (396–827) and pC1093 (396–915). The 3′ portions of FUN12 were amplified using a 3′ primer that included the SacI site present in pC1041 and 5′ primers that introduced a BamHI site at the codon for eIF5B residue 918 or 987. These PCR products were subcloned into the BamHI and SacI sites of pC1092 and pC1093 creating plasmids pC1050 (eIF5B1–827), pC1052 (eIF5B1–915), and pC1057 (eIF5BΔ828–918). Despite the nomenclature used, it should be noted that the eIF5B encoded on pC1050 and pC1052 contains the last 16 amino acids (residues 987 to 1002) of the native protein. To express these eIF5B mutants in yeast cells, SalI-SacI fragments containing the FUN12 alleles from pC1050, pC1052, and pC1057 were subcloned to plasmid pRS316 generating pC1051, pC1006, and pC1056, respectively. In addition, the same SalI-SacI fragments from pC1051 and pC1006 were subcloned to plasmid pRS426 creating plasmids pC1038 and pC1008, respectively. Finally, an Eco47III-SacI fragment from pC1051 was subcloned in place of the corresponding fragment of pC1043 creating FLAG-eIF5B378–827 expression plasmid pC1058.
A 1.3-kb DNA fragment containing the TIF11 gene encoding yeast eIF1A (43) was amplified by PCR using yeast genomic DNA as a template and primers that introduced a 5′ EcoRI site and a 3′ XbaI site. The PCR product was inserted between the EcoRI and XbaI sites of pBSII (Stratagene) creating plasmid pDSO3. A 1.1-kb BglII-XbaI fragment from pDSO3 was subcloned to the single-copy LEU2 and URA3 plasmids YCplac111 and YCplac33, respectively, and the high-copy-number LEU2 plasmid YEplac181 (17) generating TIF11 plasmids pDSO9, pDSO11, and pDSO23, respectively. Using PCR, a BamHI site was inserted immediately before the stop codon of the TIF11 gene on plasmid pDSO3 creating plasmid pDSO12-8. A triple-hemagglutinin (HA) tag was inserted in the BamHI site of pDSO12-8 generating plasmid pDSO26. Finally, a 1.1-kb BglI-XbaI fragment from pDSO26 was inserted in YEplac181 to create pDSO46, a high-copy-number LEU2 plasmid expressing eIF1A-HA. The DNA sequences of PCR-generated FUN12 and TIF11 genes were verified during construction of these plasmids.
For two-hybrid analyses, plasmids encoding fusion proteins between the GAL4 DNA binding domain or the GAL4 activation domain and various segments of eIF5B, as indicated in Fig. 1, were constructed by PCR using as primers oligonucleotides that introduced BamHI and PstI sites at the 5′ and 3′ ends, respectively, of FUN12 coding sequences. The PCR products were digested with BamHI and PstI and ligated with BamHI and PstI-digested pGBT9 (binding domain fusion) and pGAD424 (activation domain fusion) (plasmids from Clontech). To generate two-hybrid constructs containing eIF1A, PCR was used to insert an EcoRI site immediately following the first codon of the TIF11 allele in pDSO3 creating plasmid pDSO8. An EcoRI-XbaI fragment from pDSO8 encoding full-length eIF1A was ligated into similarly cut pGAD424 and pGBT9 generating plasmids pDSO14 and pDSO15, respectively.
FIG. 1.
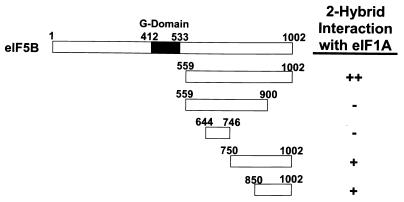
eIF1A interacts with the C terminus of eIF5B in the yeast two-hybrid assay. The schematic at the top depicts full-length yeast eIF5B. The remaining schematics depict the segments of eIF5B tested in the two-hybrid assays, with the numbers referring to the amino acid positions at the termini of the eIF5B fragments. The DNA fragments encoding the indicated portions of yeast eIF5B and full-length yeast eIF1A were inserted in the yeast two-hybrid activation domain vector pGAD424 and the DNA binding domain vector pGBT9. Yeast strain Y187 bearing pGAD424 derivatives was mated with Y190 bearing pGBT9 derivatives, and diploids were isolated on synthetic complete (SC)-Trp-Leu medium. The strength of the protein-protein interactions was measured by stimulation of the HIS3 reporter present in the diploids as assayed by growth on SC-Trp-Leu-His medium containing 30 mM 3-aminotriazole after 9 days at 30°C (++, robust growth; +, weak growth, but above background levels; −, background growth equivalent to that empty-vector controls that lack eIF5B and eIF1A sequences). Equivalent results were obtained when the eIF5B fragments were fused to the GAL4 DNA binding domain or to the activation domain. G-domain, GTP-binding domain.
To construct plasmids for bacterial expression of glutathione S-transferase (GST)–eIF5B fusions, DNA fragments containing the appropriate regions of the FUN12 open reading frame were amplified by PCR using oligonucleotide primers that introduce a 5′ BamHI site and a 3′ XhoI site. The PCR products were subcloned between the BamHI and XhoI sites of vector pGEX-4T-2 (Amersham Pharmacia Biotech) generating the following plasmids, which express the indicated fusion proteins: pC840, GST-eIF5B396–959; pC842, GST-eIF5B396–876; and pC955, GST-eIF5B745–1002. For bacterial expression of GST-eIF5B396–1002, a BamHI-XhoI fragment from yeast GST-eIF5B396–1002 expression plasmid pC485 (11) was subcloned to pGEX-4T-2 generating plasmid pC484. The GST-eIF1A expression plasmid pDSO41-1 was constructed in two steps. First, PCR was used to insert a BamHI site immediately following the first codon of TIF11 in pDSO3 creating plasmid pDSO7. Second, a BamHI-XbaI fragment from pDSO7 was inserted between the same sites of pGEX-4T-1 (Amersham Pharmacia Biotech) to generate plasmid pDSO41-1.
Yeast strains.
The fun12Δ strains J130 (MATa ura3-52 leu2-3 leu2-112 fun12::hisG) and J133 (MATα ura3-52 leu2-3 leu2-112 fun12::LEU2) were described previously (11). Strain J111 is identical to strain J130. Strain H1895 (MATa leu2-3 leu2-112 ura3-52 trp1-Δ63 gcn2Δ 〈GCN4-lacZ TRP1〉) in which the GCN4-lacZ allele is integrated at TRP1 was constructed by replacing the chromosomal GCN2 in strain H1642 with an unmarked gcn2Δ allele as described previously (15). The TIF11 gene was deleted in H1895 generating strain H2809 (MATa leu2-3 leu2-112 ura3-52 trp1-Δ63 gcn2Δ tif11Δ pDSO11 [TIF11, URA3] 〈GCN4-lacZ TRP1〉), as will be described elsewhere. Strain H2769 (KAY11; MATα his1-29 gcn2-508 ura3-52 leu2-3 leu2-112 tif34Δ-1 〈HIS4-lacZ ura3-52〉 YCpL-tif34-HA-1 [tif34-HA-1, LEU2]) is a derivative of strain KAY8 (2) in which plasmid YCpL-TIF34-HA carrying wild-type TIF34 is replaced with plasmid YCpL-tif34-HA-1 carrying a temperature-sensitive allele. Strain TB11B-4-1 (MATa ade1 leu2-3 leu2-112 ura3-52 prt1-1) was provided by G. Johnston. Strains Y187 (MATα gal4-Δ gal80-Δ his3-200 trp1-Δ901 ade2-101 ura3-52 leu2-3,112 met− URA3::GAL1-lacZ) and Y190 (MATa leu2-3,112 ura3-52 trp1-Δ901 his3-200 ade2-101 gal4-Δ gal80-Δ URA::GAL1-lacZ LSY2::GAL1-HIS3) were used for yeast two-hybrid analyses (20).
GST pull-down assays.
GST (pGEX-4T-2) and GST-eIF1A (pDSO41-1) fusions in Escherichia coli extracts were purified on glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech) in phosphate-buffered saline containing a complete protease inhibitor cocktail (Boehringer Mannheim), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 10 μg of pepstatin A/ml. Yeast cells expressing various forms of eIF5B were broken in lysis buffer (20 mM Tris-HCl [pH 7.4], 1 mM magnesium acetate, 100 mM KCl, 0.1% Triton X-100, complete protease inhibitor cocktail, 1 mM PMSF, and 10 μg of pepstatin A/ml) with acid-washed glass beads by 12 30-s agitations on a vortex mixer at 4°C, with 30 s on ice between mixing cycles. Whole-cell extracts (WCEs) were obtained by clearing the lysates by centrifugation. Postribosomal supernatants (PRSs) were obtained following centrifugation of the WCEs at 200,000 × g for 1 h.
For GST pull-down assays with WCEs or PRSs, the GST or GST-eIF1A fusions bound to glutathione beads were incubated with 1 mg of WCE or the equivalent volume of PRS prepared from the same WCE in 100 μl of lysis buffer. After incubation for 2 h at 4°C, protein complexes bound to the beads were washed three times with 400 μl of lysis buffer and then eluted by boiling for 5 min in red loading buffer (New England Biolabs). The eluted proteins were separated by [sodium dodecyl sulfate–4 to 20% polyacrylamide gel electrophoresis (SDS–4 to 20% PAGE; Novex) and transferred to nitrocellulose membranes. The GST fusion proteins were visualized by staining with Ponceau-S (0.5% in 1% acetic acid) followed by destaining in 1% acetic acid. The eIF5B proteins were visualized by probing the membranes with mouse anti-FLAG peptide antibodies (Sigma). The immune complexes were detected by enhanced chemiluminescence (ECL; Amersham).
For GST pull-down assays with purified, recombinant forms of eIF5B, the GST or GST-eIF1A fusions bound to glutathione beads were mixed with eIF5B proteins in protein storage buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10% glycerol, 5 mM dithiothreitol [DTT]). The reaction volumes were adjusted to 100 μl with lysis buffer and incubated for 2 h at 4°C. Protein complexes bound to the beads were washed three times with 400 μl of lysis buffer and then eluted by boiling for 5 min in red loading buffer (New England Biolabs). The eluted proteins were separated by SDS–4 to 20% PAGE (Novex) and visualized by staining with Coomassie blue followed by destaining with Gel-Clear solution (Novex) according to the vendor's protocol.
Purification of recombinant forms of eIF5B.
The GST-eIF5B fusion proteins were overexpressed in E. coli strain BL21(DE3)pLysS and purified by affinity chromatography using glutathione-Sepharose (Pharmacia). The fusion proteins were eluted in running buffer (25 mM Tris-HCl [pH 7.5], 100 mM NaCl, 5 mM DTT) containing 20 mM reduced glutathione and then dialyzed against running buffer. The eIF5B fragments were cleaved from the carrier protein using thrombin, and the carrier protein was removed by passage through a MonoS column (Amersham Pharmacia Biotech). Finally, the protein was extensively dialyzed against buffer containing 25 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10% glycerol, and 5 mM DTT.
Coimmunoprecipitation assays.
For coimmunoprecipitation experiments with eIF1A-HA, 800 μg of yeast WCEs was precipitated with 3 μl of monoclonal anti-HA antibodies (HA.11; Babco) bound to 25 μl of protein A-Sepharose beads in 300 μl of lysis buffer for 2 h at 4°C. Immune complexes attached to the beads were washed four times with 800 μl of lysis buffer and then eluted by boiling for 5 min in red loading buffer (New England Biolabs). Proteins were separated by SDS-PAGE and then electroblotted to nitrocellulose membranes. The eIF1A-HA protein was visualized by probing the membranes with polyclonal anti-HA antiserum (Babco), and eIF5B was visualized by probing the membrane with polyclonal antiserum raised against a GST-eIF5B396–1002 fusion protein. The immune complexes were detected by ECL (Amersham).
The coimmunoprecipitation experiments with FLAG-eIF5B378–1002 were conducted as described above for eIF1A-HA except that 30 μl of a 50% slurry of anti-FLAG affinity resin (Sigma) equilibrated in phosphate-buffered saline was mixed with 800 μg of WCE and lysis buffer in a total volume of 200 μl. The NaCl concentration in the binding reaction mixtures was then adjusted to 150 mM, and the reaction mixtures were incubated at 4°C for 2 h. Following washing, elution, SDS-PAGE, and transfer of the proteins to nitrocellulose membranes, eIF5B was detected by probing with the anti-eIF5B antiserum described above. eIF1A was visualized by probing the membrane with polyclonal antiserum raised against a GST-eIF1A fusion protein. The eIF2α, eIF2γ, and eIF3p90 proteins were detected using polyclonal anti-SUI2 (15), anti-GCD11 (19), and anti-PRT1 (13) antisera, respectively. The immune complexes were detected by ECL (Amersham).
RESULTS
Two-hybrid interaction between eIF5B and eIF1A.
We used yeast two-hybrid assays to identify and then localize the eIF1A-interacting region in eIF5B. A series of GAL4 DNA binding domain and GAL4 activation domain fusions containing various truncated fragments of yeast eIF5B were tested for interaction with the appropriate fusions containing the full-length 153-amino-acid yeast eIF1A (43) (Fig. 1). The results demonstrate that the C-terminal half of eIF5B (amino acids 559 to 1002), which lacks the GTP-binding domain, binds to eIF1A. Removal of the last 100 amino acids of this eIF5B fusion eliminated eIF1A binding, whereas eIF5B fusions containing either the C-terminal 250 or 150 residues also bound eIF1A (Fig. 1). Identical results were obtained whether the eIF5B fragments were expressed as GAL4 DNA binding domain or GAL4 activation domain fusions. We conclude that eIF1A and eIF5B can physically interact in vivo and that the C-terminal 100 to 250 residues of eIF5B are necessary and sufficient for this interaction.
The C terminus of eIF5B is critical for function in vivo.
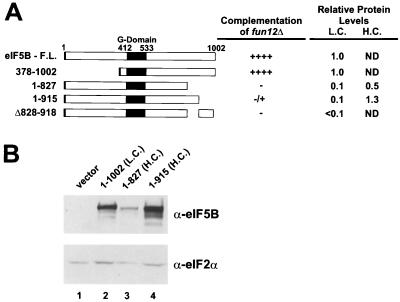
As the two-hybrid results indicated that the C-terminal 100 to 250 residues of eIF5B mediated the interaction with eIF1A, we examined the importance of these residues for eIF5B function in vivo. Yeast strains lacking the FUN12 gene encoding eIF5B exhibit a severe slow-growth phenotype. Previously, we reported that high-level expression of an N-terminally truncated form of yeast eIF5B (residues 378 to 1002), retaining only the GTP-binding and C-terminal domains, fully complemented the slow-growth phenotype of a fun12Δ strain (11). As shown in Fig. 2, when expressed from a low-copy-number plasmid under the control of the native FUN12 promoter, N-terminally truncated eIF5B378–1002 retained its complementing activity. When expressed in the same manner, the eIF5B1–915 mutant protein, lacking the last 77 residues, was severely impaired in its ability to complement the slow-growth phenotype of a fun12Δ strain (Fig. 2A). eIF5B proteins lacking the C-terminal 175 residues (eIF5B1–827) or an internal segment of 90 residues near the C terminus (eIF5BΔ828–918) were completely inactive (Fig. 2A). Because the expression levels of these C-terminally mutated proteins were significantly less than that of the wild type, we expressed eIF5B1–915 and eIF5B1–827 from high-copy-number plasmids. As indicated in Fig. 2B, when expressed from a high-copy-number plasmid, eIF5B1–915 was present at levels that were greater than those of wild-type eIF5B (amino acids 1 to 1002); however, the protein still failed to complement effectively the slow-growth phenotype of a fun12Δ strain (see Fig. 7A; data not shown). These results reveal that the C-terminal portion of eIF5B is necessary for activity in vivo, consistent with a functionally important interaction between eIF5B and eIF1A mediated by this segment of eIF5B.
FIG. 2.
The C terminus of yeast eIF5B is required for activity in vivo. (A) Schematic of yeast eIF5B. Shown are the full-length (F.L.) form and various truncated forms or a form with or an internal deletion. The numbers refer to the amino acid positions in the proteins. Large black box, conserved GTP-binding domain; small black box (left end), N-terminal FLAG epitope tag. The various eIF5B proteins were expressed under the control of the native FUN12 promoter on low-copy-number (L.C.) or high-copy-number (H.C.) plasmids in fun12Δ strain J111 and tested for the ability to complement the slow-growth phenotype of this strain. Growth rates were assessed by streaking transformants for single colonies on minimal medium. ++++, wild-type growth with visible colonies in streak-outs after 2 days; −, fun12Δ growth with visible colonies in streak-outs after 5 days; ±, slightly larger-sized colonies in streak-outs than observed with fun12Δ strains after 5 days. The relative expression levels of the eIF5B constructs expressed from low- or high-copy-number plasmids are indicated (ND, not determined). (B) Immunoblot analysis of eIF5B expression. WCEs of transformants of strain J111 expressing the indicated eIF5B construct or vector alone were prepared and subjected to immunoblot analysis using polyclonal antiserum raised against a GST-eIF5B396–1002 fusion protein (shown) as well as monoclonal anti-FLAG antibodies. To control for protein loading, the lower half of the blot was probed with antiserum specific for yeast eIF2α. Immune complexes were visualized by ECL.
FIG. 7.
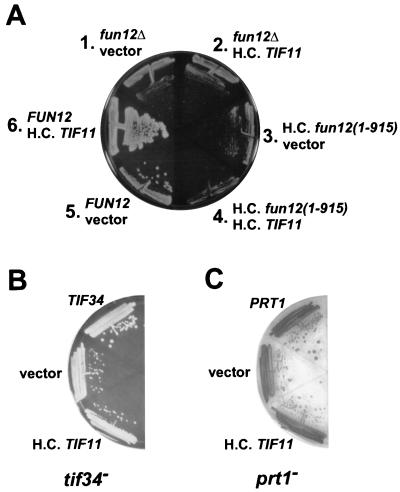
Overexpression of eIF1A strongly exacerbates the growth defect of a strain expressing C-terminally truncated eIF5B. (A) fun12Δ strain J130 was cotransformed with the high-copy-number (H.C.) LEU2 plasmid pDSO23 bearing the TIF11 gene encoding eIF1A (sectors 2, 4, and 6) or the empty vector pRS425 (sectors 1, 3, and 5) and either the low-copy-number URA3 plasmid pC1005 bearing a FUN12 gene encoding FLAG-tagged eIF5B (sectors 5 and 6), the empty vector pRS316 (sectors 1 and 2), or the high-copy-number plasmid pC1008 expressing the FLAG-tagged, C-terminally truncated eIF5B1–915 (sectors 3 and 4). Transformants were streaked on synthetic dextrose (SD) minimal medium and incubated for 5 days at 30°C. (B and C) Overexpression of eIF1A does not exacerbate eIF3 mutant strains. The temperature-sensitive tif34 (H2769; B) and prt1-1 (TP11B-4-1; C) strains were transformed with the high-copy-number TIF11 plasmid pDSO23, the empty vector pRS425, or the low-copy-number TIF34 (eIF3-p39, YCpU-TIF34 [2]) or PRT1 (eIF3-p90, pJA100 [34]) plasmid, as indicated. The transformants were streaked on SD minimal media with essential supplements and incubated for 5 days at 30°C.
The C-terminal region of eIF5B interacts directly with eIF1A in vitro.
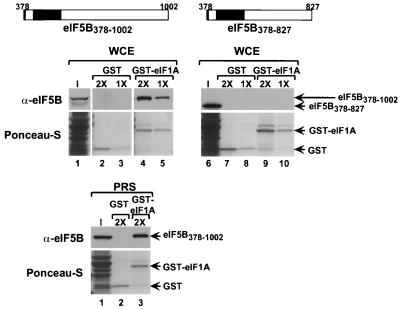
To confirm the eIF5B-eIF1A interaction detected in the two-hybrid assays, we carried out in vitro binding assays. Recombinant GST and GST fused to full-length yeast eIF1A (GST-eIF1A) were expressed in E. coli, bound to glutathione-Sepharose beads, and incubated with WCEs prepared from fun12Δ strains expressing either eIF5B378–1002 or eIF5B378–827 (Fig. 3, middle). The GST-eIF1A fusion, but not GST alone, bound high levels of eIF5B378–1002 (∼20% with the 2× concentration); however, no binding to eIF5B378–827 was observed (Fig. 3). As both eIF5B and eIF1A have been found to bind ribosomes (33, 41), it was possible that the interaction we detected was bridged by ribosomes and that the two factors do not interact directly. To test if the interaction was dependent on ribosomes, the WCEs were centrifuged at high speed to pellet the ribosomes and the binding assay was repeated using the PRS. Immunoblot analysis using antisera against the yeast ribosomal protein S22 confirmed that the PRS was depleted of ribosomes (data not shown). As shown in Fig. 3 (bottom), GST-eIF1A, but not GST, bound a significant fraction of eIF5B378–1002 present in the PRS. This result demonstrates that eIF5B and eIF1A can interact independently of the ribosome; however, it remains possible that the functionally important interaction between the two proteins occurs on the ribosome in vivo.
FIG. 3.
The C terminus of eIF5B is required for interaction with GST-eIF1A in a yeast WCE. (Top) Schematics of FLAG epitope-tagged eIF5B378–1002 and eIF5B378–827. Small black box (left end), FLAG epitope tag; large black box, GTP-binding domain. (Middle) The indicated GST (lanes 2, 3, 7, and 8) and GST-eIF1A (lanes 4, 5, 9, and 10) fusions attached to glutathione-Sepharose beads were incubated with WCEs from fun12Δ strain J111 expressing either N-terminally truncated eIF5B378–1002 (pC1043) (lanes 1 to 5) or N- and C-terminally truncated eIF5B378–827 (pC1058) (lanes 6 to 10) from low-copy-number plasmids. Both eIF5B proteins contain an N-terminal FLAG epitope tag. The 1× concentrations for GST and GST-eIF1A were 0.8 and 0.5 mM, respectively. The input (I) lanes represent 10% of the yeast extracts used for the binding assays. Following binding, the beads were pelleted and washed, and the bound proteins were analyzed by SDS-PAGE followed by electroblotting to nitrocellulose membranes. The GST fusion proteins were visualized by Ponceau-S staining (lower panels), and the eIF5B proteins were detected using anti-FLAG peptide antiserum and ECL (upper panels). (Bottom) The indicated GST (lane 2) and GST-eIF1A (lane 3) fusions attached to glutathione-Sepharose beads were incubated with PRSs from fun12Δ strain J111 expressing FLAG-tagged N-terminally truncated eIF5B378–1002 (pC1043). The concentrations of GST and GST-eIF1A proteins in the binding assays were 1.6 and 1.0 mM, respectively. The input lane represents 10% of the PRS used for the binding assays. The analysis of the binding reactions and visualization of the results were as described above.
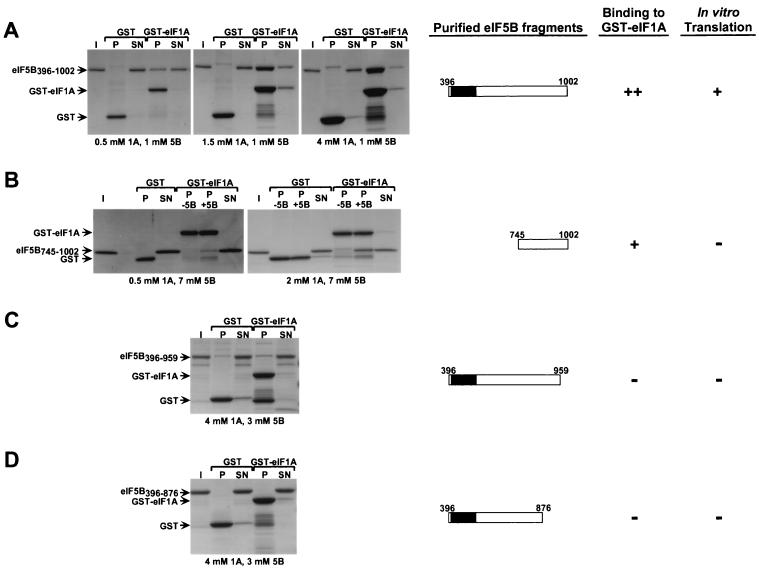
We next tested if eIF5B and eIF1A could interact directly. GST-eIF5B fusions containing various segments of eIF5B were expressed in E. coli and purified using glutathione-Sepharose resin. The eIF5B portion of the fusions was liberated by protease cleavage and then isolated from the GST fragment as described in Materials and Methods. GST and the GST-eIF1A fusion described previously containing full-length yeast eIF1A were also expressed in E. coli and isolated as described in Materials and Methods. The purified GST and GST-eIF1A fusion were bound to glutathione-Sepharose and incubated with the purified eIF5B fragments depicted in Fig. 4, and the resulting pellet and supernatant fractions were resolved by SDS-PAGE and visualized by Coomassie staining. The concentrations of GST-eIF1A and eIF5B were varied to optimize binding; the concentration of the GST control protein was roughly the same as that of GST-eIF1A in each experiment. Substantial amounts of the eIF5B396–1002 fragment bound to GST-eIF1A (Fig. 4A). At the highest concentration of GST-eIF1A, a majority of the eIF5B396–1002 was driven into the complex, whereas no detectable binding to GST occurred (Fig. 4A, right panel). These data show that eIF5B and eIF1A can interact in the absence of other proteins. In addition, the interaction was retained following treatment with micrococcal nuclease, indicating that RNA was not serving as a bridge for the interaction (data not shown). The eIF5B396–1002 fragment is similar to the minimal segment required for eIF5B function in vivo (Fig. 2).
FIG. 4.
eIF5B interacts directly with eIF1A. The indicated GST and GST-eIF1A fusion proteins expressed in E. coli were purified on glutathione-Sepharose beads and incubated with the following purified recombinant eIF5B fragments: eIF5B396–1002 (A), eIF5B745–1002 (B), eIF5B396–959 (C), and eIF5B396–876 (D). Following binding and washing, 10% of the supernatant fractions (SN) and 100% of the pellet fractions (P) were resolved by SDS–4 to 20% PAGE and visualized by Coomassie staining (left panels). The input (I) lanes contain 10% of the eIF5B fragments used in the binding assays. The concentrations of eIF5B and GST or GST-eIF1A proteins in the binding reaction mixtures are indicated below the results. Because breakdown products of the GST-eIF1A fusion protein comigrated with eIF5B745–1002, the pellet fractions of binding reaction mixtures lacking (P −5B) and containing (P +5B) eIF5B are presented (B). Schematic depictions of the eIF5B constructs and a qualitative summary of the results of these binding assays and of the in vitro translation assays shown in Fig. 5 are presented on the right.
Two-hybrid results (Fig. 1) indicated that the C-terminal 250 residues of eIF5B were sufficient to bind eIF1A. In accordance with that finding, purified eIF5B745–1002 was specifically bound by GST-eIF1A and not by GST (Fig. 4B). In this case, however, relatively high concentrations of the two proteins were required to form a large amount of the complex (Fig. 4B, right panel). Because several proteolytic fragments of GST-eIF1A had mobilities similar to that of eIF5B745–1002, we examined the pellet fractions from reaction mixtures that either lacked or contained eIF5B745–1002. The binding of eIF5B745–1002 is seen clearly above the background of GST-eIF1A proteolytic fragments in the right panel of Fig. 4B. Even though the concentration of eIF5B745–1002 used in these last binding assays was significantly higher than that of eIF5B396–1002 used in Fig. 4A, a smaller fraction of eIF5B745–1002 than of eIF5B396–1002 bound to eIF1A. Thus, while the C terminus of eIF5B is sufficient for binding to eIF1A, it appears that additional eIF5B segments contribute to the interaction. The C-terminally truncated fragments eIF5B396–959 and eIF5B396–876 failed to bind eIF1A even at relatively high concentrations of both proteins (Fig. 4C and D). (Even though a small fraction of eIF5B396–959 was recovered in the pellet with GST-eIF1A, a similar amount of eIF5B396–959 was precipitated with the GST control [Fig. 4C], suggesting that this eIF5B fragment may be sticky.) The failure to detect the binding of these C-terminally truncated eIF5B fragments to eIF1A supports the results of the two-hybrid and GST-pull-down experiments and indicates that the eIF5B C terminus plays a critical role in binding to eIF1A.
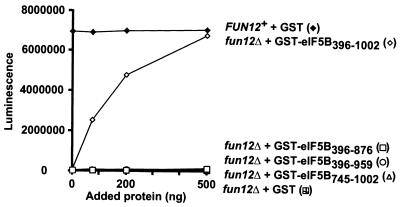
The various eIF5B fragments depicted in Fig. 4 were tested for the ability to rescue translation in extracts from a fun12Δ strain assayed using a luciferase reporter mRNA. As shown in Fig. 5 and reported previously (11), extracts from wild-type strains have approximately 20- to 100-fold-greater translational activity than extracts from fun12Δ strains (Fig. 5). The translational defect of the fun12Δ extract was complemented in a dose-dependent manner by addition of purified eIF5B396–1002 (Fig. 5). Despite its ability to interact with eIF1A (Fig. 4), eIF5B745–1002 was unable to promote protein synthesis, supporting the idea that the GTP-binding domain is essential for eIF5B function. The eIF5B396–876 and eIF5B396–959 proteins contain an intact GTP-binding domain but are defective for binding to eIF1A (Fig. 4). Translational activity in fun12Δ extracts supplemented with eIF5B396–876 or eIF5B396–959 was very low, comparable to the activity observed in extracts supplemented with the GST control protein (Fig. 5). Finally, when these same amounts of eIF5B fragments were added to extracts from wild-type cells, no effects on translation were observed (data not shown). The fact that the eIF5B fragments that fail to bind eIF1A are defective both in vivo and in these translation assays is consistent with the idea that the eIF5B-eIF1A interaction plays an important role in promoting protein synthesis in eukaryotic cells.
FIG. 5.
The C terminus of eIF5B is required for function in an in vitro translation system. Translation extracts were prepared as described previously (11) from fun12Δ strain J133 carrying low-copy-number FUN12 plasmid pC479 (FUN12+) or empty vector pRS316 (fun12Δ). Extracts were incubated with 200 ng of luciferase mRNA and the indicated amounts of highly purified recombinant GST or GST-eIF5B fusions. Translational activity was determined, as described previously (11), by measuring luminescence after a 15-min incubation at 26°C. Results are representative of at least two independent experiments.
Coimmunoprecipitation analysis reveals interaction between eIF5B and eIF1A in vivo.
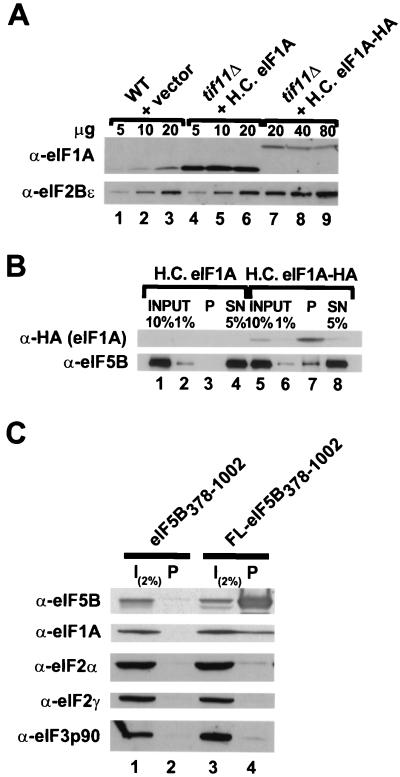
Having reconstituted interactions between purified GST-eIF1A and purified eIF5B (Fig. 4) and between bacterially expressed GST-eIF1A and native eIF5B in cell extracts (Fig. 3), we next examined whether we could coimmunoprecipitate eIF5B and eIF1A when both proteins were present in the cell at physiological levels. WCEs were prepared from isogenic strains expressing either native eIF1A or a triple-HA epitope-tagged form of eIF1A (eIF1A-HA; the HA tag is at the C terminus of eIF1A). When eIF1A-HA was expressed from a high-copy-number plasmid, its abundance was comparable to that of the endogenous eIF1A protein present in wild-type strains (Fig. 6A, upper panel; compare lanes 3 and 7). In addition, no growth defects in tif11Δ strains expressing the eIF1A-HA protein were observed (data not shown), indicating that the epitope does not interfere with eIF1A function in vivo. The WCEs were incubated with anti-HA antibodies prebound to protein A-Sepharose beads, and after extensive washing, the pellet and supernatant fractions were analyzed by SDS-PAGE and immunoblotting. As shown in Fig. 6B, approximately 20 to 40% of the eIF1A-HA was precipitated with the resin, whereas none of the untagged eIF1A was precipitated (Fig. 6B; compare lanes 7 and 3). Approximately 3% of the cellular eIF5B coprecipitated with eIF1A-HA (Fig. 6B, lane 7). Thus, we estimate that the percentage of cellular eIF5B in a complex with eIF1A, when corrected for the efficiency of eIF1A-HA recovery, was 7.5 to 15%.
FIG. 6.
eIF5B and eIF1A interact in vivo. (A) Immunoblot analysis of eIF1A and eIF1A-HA expression. WCEs were prepared from the wild-type (WT) strain H1895 and derivatives of the isogenic tif11Δ strain H2809 containing the high-copy-number (H.C.) plasmid pDSO23 (TIF11 LEU2) encoding eIF1A or pDSO46 (TIF11-HA LEU2) encoding eIF1A-HA. The indicated amounts of WCEs were subjected to SDS-PAGE and analyzed by immunoblotting using polyclonal antisera raised against yeast eIF2Bε (GCD6) or eIF1A. Immune complexes were visualized by ECL. (B) Coimmunoprecipitation of eIF5B with epitope-tagged eIF1A. WCEs were prepared from the tif11Δ strains described for panel A, which express either eIF1A or HA epitope-tagged eIF1A (eIF1A-HA) from high-copy-number plasmids. Aliquots containing 800 μg of protein were incubated with monoclonal anti-HA antibodies (HA.11; Babco) prebound to protein A-Sepharose beads, and, after being washed, the bound proteins were analyzed by immunoblotting using anti-HA and anti-eIF5B antisera, as indicated. Input lanes contain 1 or 10% of the starting amount of WCE, the pellet lanes (P) containing 100% of the immunoprecipitated fraction, and the supernatant lanes (SN) contain 5% of the reaction mixtures following removal of the pellet. (C) Coimmunoprecipitation of eIF1A with epitope-tagged eIF5B. WCEs were prepared from fun12Δ strain J111 expressing, from high-copy-number plasmids, eIF1A and either an untagged (pC1037) or FLAG epitope-tagged (FL; pC1007) form of N-terminally truncated eIF5B378–1002, as indicated. Aliquots containing 800 μg of protein were incubated with anti-FLAG affinity resin, and after being washed the bound proteins were analyzed by SDS-PAGE and immunoblotting using antisera specific for the proteins indicated at the left. The input (I) lanes containing 2% of the starting amount of WCE, and the pellet lanes contain the entire immunoprecipitated fraction.
To further examine the interaction between eIF5B and eIF1A, WCEs were prepared from the fun12Δ strain J111 overexpressing on high-copy-number plasmids both eIF1A and either untagged eIF5B378–1002 or the same protein bearing an N-terminal FLAG epitope tag (FL-eIF5B378–1002). Both the untagged and FLAG-tagged eIF5B proteins were functional in vivo (Fig. 2 and data not shown). The WCEs were incubated with anti-FLAG affinity resin, and after extensive washing, the pellet fractions were analyzed by SDS-PAGE and immunoblotting. As shown in Fig. 6C, approximately 1 to 2% of the eIF1A coprecipitated with FL-eIF5B378–1002. This interaction appeared to be specific in that no eIF1A was precipitated in extracts prepared from strains expressing untagged eIF5B. In addition, neither eIF2 (α and γ subunits) nor eIF3 (p90, PRT1 subunit) coprecipitated with FL-eIF5B378–1002 (Fig. 6C). The apparently low recovery of eIF1A in complex with FL-eIF5B378–1002 (Fig. 6C) compared to the recovery of eIF5B in complex with eIF1A-HA (Fig. 6B) may reflect differences in the relative abundances of the two factors. Consistent with this idea, quantitative immunoblot analyses indicate that eIF1A is roughly five- to sixfold more abundant than eIF5B in vivo (data not shown). Alternatively, the eIF5B N-terminal region (present only in Fig. 6B) may contribute to complex formation with eIF1A.
Genetic interaction between eIF5B and eIF1A.
The results thus far indicated that the C terminus of eIF5B was necessary for its ability to interact with eIF1A, for the support of translation in vitro, and for normal growth of yeast cells. There is evidence that the binding of IF1 and IF2 to ribosomes is coupled in bacterial cells and that release of IF2 from 70S ribosomes is dependent on IF1 (5, 18, 31). By analogy, we suggest that release of eIF1A and eIF5B from 80S initiation complexes in eukaryotic cells may also be coupled. If release of eIF1A from the ribosome is dependent on its interaction with eIF5B, then overexpression of eIF1A in yeast cells lacking eIF5B or expressing eIF5B mutants that fail to interact with eIF1A is expected to interfere with completion of 80S initiation complex formation. The TIF11 gene encoding yeast eIF1A was inserted into a high-copy-number plasmid and transformed into fun12Δ strains that lack eIF5B and carry either an empty vector, a low-copy-number plasmid that expresses wild-type eIF5B, or a high-copy-number plasmid that expresses eIF5B1–915. The results in Fig. 4 showing that removal of as few as 43 residues from the C terminus of eIF5B disrupts the interaction with eIF1A indicate that eIF5B1–915 would not bind to eIF1A. As shown previously (11, 25), fun12Δ strains exhibit a significant slow-growth phenotype (Fig. 7A; compare sectors 1 and 5). Introduction of the high-copy-number TIF11 plasmid, which overexpressed eIF1A ∼50-fold (Fig. 6A), slightly exacerbated the slow-growth phenotype of the fun12Δ strain (Fig. 7A, sector 2 versus sector 1), although this was not easily observed. A more significant exacerbation of the slow-growth phenotype of a fun12Δ strain was observed when eIF1A was overexpressed under the control of the yeast GAL1 promoter (D. S. Olsen and A. G. Hinnebusch, unpublished observation). Interestingly, overexpression of eIF1A severely exacerbated the slow growth of the strain expressing eIF5B1–915 (Fig. 7A, sector 4 versus sector 3). Thus, overexpression of eIF1A impaired growth in a strain expressing C-terminally truncated eIF5B1–915 more than it did in a strain lacking eIF5B entirely. As shown in Fig. 7B and C, overexpression of eIF1A did not exacerbate the slow-growth phenotype of strains mutated in the TIF34 (p39) and PRT1 (p90) subunits of yeast eIF3 (34). The strong exacerbation of the phenotype of fun12(1–915) versus that of fun12Δ and the lack of any effect in tif34 and prt1 mutants overexpressing eIF1A support our conclusion that eIF5B and eIF1A functionally interact during translation initiation. Moreover, the toxicity associated with overexpression of eIF1A in the eIF5B mutant strains is consistent with the model that release of eIF1A and eIF5B from 80S initiation complexes is coupled.
DISCUSSION
Based on a number of in vivo and in vitro assays, we report that eIF5B and eIF1A, the eukaryotic orthologs of the prokaryotic translation factors IF2 and IF1, physically interact. In addition, the growth defects observed in yeast cells expressing altered forms or levels of eIF1A and eIF5B indicate that these factors functionally interact. It should be noted that relatively high concentrations of the purified factors were necessary to detect complex formation in vitro. It is possible that complex formation between eIF5B and eIF1A in vivo is dependent on the binding of the factors to the ribosome or on contributions from other translation initiation factors that may interact with eIF1A and eIF5B. A few previous reports presented suggestive evidence for an interaction between eIF5B and eIF1A. Early purification studies indicated that eIF1A (then referred to as eIF4C) and eIF5B (then referred to as eIF5) copurified through several ammonium sulfate precipitation and ion-exchange chromatography steps (35). The two factors were finally resolved on sucrose density gradients containing 400 mM KCl. It was unclear whether this copurification indicated a physical interaction between the factors or, more simply, similar chemical properties of the two proteins. In light of our data, we interpret the copurification of eIF1A and eIF5B to reflect the physical and functional interaction between these factors.
Early studies examining cross-linking of bacterial translation factors to the ribosome revealed cross-links between IF1 and IF2, indicating that these factors are in close proximity when bound to the ribosome (6). In support of this idea, IF1 binding to the ribosome is stabilized by IF2 (18). In addition, stable binding of an N-terminally truncated form of IF2 containing the GTP-binding and C-terminal domains to the ribosome is dependent on IF1 (31). Finally, IF1 and IF2 were reported to function together to destabilize the binding of peptidyl-tRNAs to the ribosomal P site (22). Taken together, these data indicate that IF1 and IF2 physically and functionally interact on the ribosome. However, a direct interaction between these prokaryotic factors occurring independently of the ribosome has not been reported. We propose that eIF5B and eIF1A physically interact both on and off the ribosome, and we suggest that the ability of the eukaryotic factors to interact off the ribosome serves to increase translational efficiency in eukaryotic cells.
Interestingly, IF1 binding to the ribosome protects some of the same residues in rRNA that are protected upon binding the EF-Tu–GTP–aminoacyl-tRNA complex (28), suggesting that IF1 binds in the ribosomal A site. As the nuclear magnetic resonance structures of IF1 and eIF1A show extensive similarities with the proteins sharing a core antiparallel β-barrel (OB-fold) structure (3, 37), it is reasonable to propose a similar A site-binding function for eIF1A. eIF1A has been reported to promote ribosomal subunit dissociation and to stabilize Met-tRNAiMet binding to ribosomes (4, 27, 41, 42). More recently, it was reported that eIF1A acts catalytically to stimulate Met-tRNAiMet binding to the 40S subunit (9, 10). In accordance with this idea, eIF1A did not stably associate with the 40S subunit in sucrose gradient analyses (4, 10). However, in previous studies using gel filtration chromatography it was reported that eIF1A was present in 40S preinitation complexes (41). Thus, it appears that eIF1A binding to the 40S subunit is weak but perhaps can be stabilized in the presence of additional initiation factors. The association of eIF1A with the 40S subunit is consistent with the finding that eIF1A, together with eIF1, is necessary for proper positioning and stable binding of 48S preinitiation complexes (containing the 40S subunit, eIF2–GTP–Met-tRNAiMet, and associated factors) at the AUG codon of an mRNA (32).
The function of IF2 in translation is somewhat better understood than that of IF1. IF2 interacts with fMet-tRNAiMet through its C-terminal region (18, 40), and the factor is thought to promote Met-tRNAiMet binding to the small ribosomal subunit. In addition, IF2 increases the affinity of IF1 binding to the ribosome (18). The GTPase activity of IF2 is activated upon subunit joining, and it is thought that the GTPase activity is required for proper positioning of fMet-tRNAiMet in the ribosomal P site and for release of IF2 from the ribosome (16, 24, 26).
We showed previously that fun12Δ yeast strains lacking the IF2 ortholog eIF5B had a defect in translation initiation (11). The fact that the slow-growth phenotype of fun12Δ strains could be partially suppressed by overexpression of tRNAiMet (11) suggested that eIF5B resembles bacterial IF2 in promoting the binding of fMet-tRNAiMet to the small ribosomal subunit. However, we have not detected Met-tRNAiMet binding to yeast or human eIF5B off the ribosome, and the eukaryotic factors do not appear to promote Met-tRNAiMet binding to the ribosome (33; S. K. Choi and T. E. Dever, unpublished observations; T. V. Pestova, personal communication). Recently, we reported that eIF5B is required for ribosomal subunit joining (33). We also showed that eIF5B possesses ribosome-dependent GTPase activity and demonstrated that GTP hydrolysis is required for release of eIF5B from 80S ribosomes following subunit joining. Interestingly, release of IF2 following subunit joining in prokaryotes requires IF1 (5), raising the possibility that release of eIF1A and eIF5B will be coupled during subunit joining in eukaryotes. Our observation that overexpression of eIF1A exacerbates the growth defect in strains expressing eIF5B1–915 (Fig. 7) is consistent with this idea. We propose that one defect in fun12Δ strains or in strains expressing inactive eIF5B1–915 is an impaired release of eIF1A during subunit joining. Thus, overexpression of eIF1A would exacerbate this defect and lead to a more severe slow-growth phenotype.
Molecular mimicry by eIF5B and eIF1A in translation initiation?
The observation that elongation factor EF-G shows striking structural similarities to the EF-Tu–GTP–Phe-tRNAPhe complex led to the intriguing idea of molecular mimicry among translation factors and tRNA molecules (29, 30). It has been proposed that a complex of IF1 and IF2 mimics the structure of EF-G (7), with IF1 interacting with domain III of IF2. Our studies on eIF1A and eIF5B map the site of interaction to the C terminus of eIF5B, which corresponds to domain IV of IF2. Thus, our data are not consistent with the proposed model (7) for the IF1-IF2 complex mimicking EF-G. The observations that co-overexpression of IF1 and IF2 in certain E. coli strains slows growth and that in vitro these proteins cooperate to promote dissociation of peptidyl-tRNAs from translating ribosomes are both consistent with the idea that an IF1-IF2 complex can compete with the EF-Tu–GTP–aminoacyl-tRNA complex for binding to the ribosomal A site (22). As IF1 was shown to bind to the ribosomal A site (28), and in view of our data demonstrating that the IF1 and IF2 orthologs in eukaryotes directly interact, we favor the idea that the IF1-IF2 complex, and by extension the eIF1A-eIF5B complex in eukaryotes, binds to the A site and stabilizes or correctly positions Met-tRNAiMet binding in the ribosomal P site.
ACKNOWLEDGMENTS
We thank members of the Dever, Hinnebusch, and Burley laboratories for helpful discussions.
D.S.O. holds a National Research Council Research Associateship, and A.R.-M. was supported by a National Science Foundation Graduate Fellowship, The Rockefeller University, and the Burroughs Wellcome Fund. S.K.B. is an investigator in the Howard Hughes Medical Institute.
S.K.C. and D.S.O. contributed equally to this work.
REFERENCES
- 1.Aevarsson A, Brazhnikov E, Garber M, Zheltonosova J, Chirgadze Y, al-Karadaghi S, Svensson L A, Liljas A. Three-dimensional structure of the ribosomal translocase: elongation factor G from Thermus thermophilus. EMBO J. 1994;13:3669–3677. doi: 10.1002/j.1460-2075.1994.tb06676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano K, Phan L, Anderson J, Hinnebusch A G. Complex formation by all five homologues of mammalian translation initiation factor 3 subunits from yeast Saccharomyces cerevisiae. J Biol Chem. 1998;273:18573–18585. doi: 10.1074/jbc.273.29.18573. [DOI] [PubMed] [Google Scholar]
- 3.Battiste J B, Pestova T V, Hellen C U T, Wagner G. The eIF1A solution structure reveals a large RNA-binding surface important for scanning function. Mol Cell. 2000;5:109–119. doi: 10.1016/s1097-2765(00)80407-4. [DOI] [PubMed] [Google Scholar]
- 4.Benne R, Hershey J W B. The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J Biol Chem. 1978;253:3078–3087. [PubMed] [Google Scholar]
- 5.Benne R, Naaktgeboren N, Gubbens J, Voorma H O. Recycling of initiation factors IF-1, IF-2 and IF-3. Eur J Biochem. 1973;32:372–380. doi: 10.1111/j.1432-1033.1973.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 6.Boileau G, Butler P, Hershey J W, Traut R R. Direct cross-links between initiation factors 1, 2, and 3 and ribosomal proteins promoted by 2-iminothiolane. Biochemistry. 1983;22:3162–3170. doi: 10.1021/bi00282a020. [DOI] [PubMed] [Google Scholar]
- 7.Brock S, Szkaradkiewicz K, Sprinzl M. Initiation factors of protein biosynthesis in bacteria and their structural relationship to elongation and termination factors. Mol Microbiol. 1998;29:409–417. doi: 10.1046/j.1365-2958.1998.00893.x. [DOI] [PubMed] [Google Scholar]
- 8.Carrera P, Johnstone O, Nakamura A, Casanova J, Jackle H, Lasko P. VASA mediates translation through interaction with a Drosophila yIF2 homolog. Mol Cell. 2000;5:181–187. doi: 10.1016/s1097-2765(00)80414-1. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri J, Chowdhury D, Maitra U. Distinct functions of eukaryotic translation initiation factors eIF1A and eIF3 in the formation of the 40S ribosomal preinitiation complex. J Biol Chem. 1999;274:17975–17980. doi: 10.1074/jbc.274.25.17975. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhuri J, Si K, Maitra U. Function of eukaryotic translation initiation factor 1A (eIF1A) (formerly called eIF-4C) in initiation of protein synthesis. J Biol Chem. 1997;272:7883–7891. doi: 10.1074/jbc.272.12.7883. [DOI] [PubMed] [Google Scholar]
- 11.Choi S K, Lee J H, Zoll W L, Merrick W C, Dever T E. Promotion of Met-tRNAiMet binding to ribosomes by yIF2, a bacterial IF2 homolog in yeast. Science. 1998;280:1757–1760. doi: 10.1126/science.280.5370.1757. [DOI] [PubMed] [Google Scholar]
- 12.Christianson T W, Sikorski R S, Dante M, Shero J H, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 13.Cigan A M, Foiani M, Hannig E M, Hinnebusch A G. Complex formation by positive and negative translational regulators of GCN4. Mol Cell Biol. 1991;11:3217–3228. doi: 10.1128/mcb.11.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czworkowski J, Wang J, Steitz T A, Moore P B. The crystal structure of elongation factor G complexed with GDP, at 2.7 A resolution. EMBO J. 1994;13:3661–3668. doi: 10.1002/j.1460-2075.1994.tb06675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dever T E, Feng L, Wek R C, Cigan A M, Donahue T D, Hinnebusch A G. Phosphorylation of initiation factor 2α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 16.Dubnoff J S, Lockwood A H, Maitra U. Studies on the role of guanosine triphosphate in polypeptide chain initiation in Escherichia coli. J Biol Chem. 1972;247:2884–2894. [PubMed] [Google Scholar]
- 17.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 18.Gualerzi C O, Pon C L. Initiation of mRNA translation in prokaryotes. Biochemistry. 1990;29:5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- 19.Hannig E M, Cigan A M, Freeman B A, Kinzy T G. GCD11, a negative regulator of GCN4 expression, encodes the γ subunit of eIF-2 in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:506–520. doi: 10.1128/mcb.13.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 21.Karimi R, Pavlov M Y, Buckingham R H, Ehrenberg M. Novel roles for classical factors at the interface between translation termination and initiation. Mol Cell. 1999;3:601–609. doi: 10.1016/s1097-2765(00)80353-6. [DOI] [PubMed] [Google Scholar]
- 22.Karimi R, Pavlov M Y, Heurgue-Hamard V, Buckingham R H, Ehrenberg M. Initiation factors IF1 and IF2 synergistically remove peptidyl-tRNAs with short polypeptides from the P-site of translating Escherichia coli ribosomes. J Mol Biol. 1998;281:241–252. doi: 10.1006/jmbi.1998.1953. [DOI] [PubMed] [Google Scholar]
- 23.Kyrpides N C, Woese C R. Universally conserved translation initiation factors. Proc Natl Acad Sci USA. 1998;95:224–228. doi: 10.1073/pnas.95.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Teana A, Pon C L, Gualerzi C O. Late events in translation initiation. Adjustment of fMet-tRNA in the ribosomal P-site. J Mol Biol. 1996;256:667–675. doi: 10.1006/jmbi.1996.0116. [DOI] [PubMed] [Google Scholar]
- 25.Lee J H, Choi S K, Roll-Mecak A, Burley S K, Dever T E. Universal conservation in translation initiation revealed by human and archaeal homologs of bacterial translation initiation factor IF2. Proc Natl Acad Sci USA. 1999;96:4342–4347. doi: 10.1073/pnas.96.8.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luchin S, Putzer H, Hershey J W, Cenatiempo Y, Grunberg-Manago M, Laalami S. In vitro study of two dominant inhibitory GTPase mutants of Escherichia coli translation initiation factor IF2. Direct evidence that GTP hydrolysis is necessary for factor recycling. J Biol Chem. 1999;274:6074–6079. doi: 10.1074/jbc.274.10.6074. [DOI] [PubMed] [Google Scholar]
- 27.Merrick W C, Hershey J W B. The pathway and mechanism of eukaryotic protein synthesis. In: Hershey J W B, Matthews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 31–69. [Google Scholar]
- 28.Moazed D, Samaha R R, Gualerzi C, Noller H F. Specific protection of 16 S rRNA by translational initiation factors. J Mol Biol. 1995;248:207–210. doi: 10.1016/s0022-2836(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 29.Nissen P, Kjeldgaard M, Thirup S, Polekhina G, Reshetnikova L, Clark B F, Nyborg J. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science. 1995;270:1464–1472. doi: 10.1126/science.270.5241.1464. [DOI] [PubMed] [Google Scholar]
- 30.Nyborg J, Nissen P, Kjeldgaard M, Thirup S, Polekhina G, Clark B F C. Structure of the ternary complex of EF-Tu: macromolecular mimicry in translation. Trends Biochem Sci. 1996;21:81–82. [PubMed] [Google Scholar]
- 31.Palacios Moreno J M, Drskjotersen L, Kristensen J E, Mortensen K K, Sperling-Petersen H U. Characterization of the domains of E. coli initiation factor IF2 responsible for recognition of the ribosome. FEBS Lett. 1999;455:130–134. doi: 10.1016/s0014-5793(99)00858-3. [DOI] [PubMed] [Google Scholar]
- 32.Pestova T V, Borukhov S I, Hellen C U T. Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature. 1998;394:854–859. doi: 10.1038/29703. [DOI] [PubMed] [Google Scholar]
- 33.Pestova T V, Lomakin I B, Lee J H, Choi S K, Dever T E, Hellen C U T. The joining of ribosomal subunits in eukaryotes requires eIF5B. Nature. 2000;403:332–335. doi: 10.1038/35002118. [DOI] [PubMed] [Google Scholar]
- 34.Phan L, Zhang X, Asano K, Anderson J, Vornlocher H P, Greenberg J R, Qin J, Hinnebusch A G. Identification of a translation initiation factor 3 (eIF3) core complex, conserved in yeast and mammals, that interacts with eIF5. Mol Cell Biol. 1998;18:4935–4946. doi: 10.1128/mcb.18.8.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreier M H, Erni B, Staehelin T. Initiation of mammalian protein synthesis: purification and characterization of seven initiation factors. J Mol Biol. 1977;116:727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- 36.Selmer M, Al-Karadaghi S, Hirokawa G, Kaji A, Liljas A. Crystal structure of Thermotoga maritima ribosome recycling factor: a tRNA mimic. Science. 1999;286:2349–2352. doi: 10.1126/science.286.5448.2349. [DOI] [PubMed] [Google Scholar]
- 37.Sette M, van Tilborg P, Spurio R, Kaptein R, Paci M, Gualerzi C O, Boelens R. The structure of the translational initiation factor IF1 from E. coli contains an oligomer-binding motif. EMBO J. 1997;16:1436–1443. doi: 10.1093/emboj/16.6.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song H, Mugnier P, Das A K, Webb H M, Evans D R, Tuite M F, Hemmings B A, Barford D. The crystal structure of human eukaryotic release factor eRF1—mechanism of stop codon recognition and peptidyl-tRNA hydrolysis. Cell. 2000;100:311–321. doi: 10.1016/s0092-8674(00)80667-4. [DOI] [PubMed] [Google Scholar]
- 40.Spurio R, Brandi L, Caserta E, Pon C L, Gualerzi C O, Misselwitz R, Krafft C, Welfle K, Welfle H. The C-terminal subdomain (IF2 C-2) contains the entire fMet-tRNA binding site of initiation factor IF2. J Biol Chem. 2000;275:2447–2454. doi: 10.1074/jbc.275.4.2447. [DOI] [PubMed] [Google Scholar]
- 41.Thomas A, Goumans H, Voorma H O, Benne R. The mechanism of action of eukaryotic initiation factor 4C in protein synthesis. Eur J Biochem. 1980;107:39–45. doi: 10.1111/j.1432-1033.1980.tb04621.x. [DOI] [PubMed] [Google Scholar]
- 42.Trachsel H, Erni B, Schreier M H, Staehelin T. Initiation of mammalian protein synthesis: the assembly of the initiation complex with purified initiation factors. J Mol Biol. 1977;116:755–767. doi: 10.1016/0022-2836(77)90269-8. [DOI] [PubMed] [Google Scholar]
- 43.Wei C L, Kainuma M, Hershey J W. Characterization of yeast translation initiation factor 1A and cloning of its essential gene. J Biol Chem. 1995;270:22788–22794. doi: 10.1074/jbc.270.39.22788. [DOI] [PubMed] [Google Scholar]
- 44.Wilson S A, Sieiro-Vazquez C, Edwards N J, Iourin O, Byles E D, Kotsopoulou E, Adamson C S, Kingsman S M, Kingsman A J, Martin-Rendon E. Cloning and characterization of hIF2, a human homologue of bacterial translation initiation factor 2, and its interaction with HIV-1 matrix. Biochem J. 1999;342:97–103. [PMC free article] [PubMed] [Google Scholar]
- 45.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1984;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]