Abstract
Background:
Sub-Saharan Africa is undergoing an epidemiological transition fueled by the interaction between infectious and cardiovascular diseases (CVD). Our cross-sectional study aimed to characterize the spectrum of abnormalities suggesting end-organ damage on electrocardiograms (ECG) and transthoracic echocardiograms (TTE) among older adults with CVD in rural South Africa.
Methods:
The prevalence of ECG and TTE abnormalities was estimated; Chi-square analyses and multivariable logistic regressions performed to test their association with sex, hypertension and other selected comorbidities.
Results:
Overall, 729 ECGs and 155 TTEs were completed, with 74 participants completing both. ECG evaluation showed high rates of left ventricular hypertrophy (LVH, 36.5%) and T wave abnormalities (13.6%). TTE evaluation showed high rates of concentric LVH (31.6%), with moderate-severe (56.8%) diastolic dysfunction. Participants with hypertension showed more cardiac remodeling on ECG by LVH (45.4% vs 22.1%, p<0.01), and TTE by concentric LVH (42.5% vs 8.2%, p<0.01) and increased left ventricular mass (LVM, 58.5% vs 20.4%, p<0.0001). In multivariable logistic regression, systolic blood pressure remained significantly associated with LVH on ECG (adjusted odds ratio 1.03 per mm Hg, 95% confidence interval 1.03–1.04, p<0.0001) and increased LVM on TTE (aOR 1.04 per mm Hg, 95% CI 1.01–1.06, p=0.001). Male participants (n=326, 40.2%) were more likely than females (n=484, 59.8%) to show ECG abnormalities like LVH (45% vs 30.8%, p<0.01), while females were more likely to show TTE abnormalities like concentric LVH (40.8% vs 13.5%, p<0.01) and increased LVM (58.4% vs 23.1%, p<0.0001). Similar results were confirmed in multivariable models.
Conclusions:
Our findings suggest that CVD are widespread in rural South Africa, with a larger burden of hypertensive heart disease than previously appreciated, and define the severity of end-organ damage that is already underway. Local health systems must adapt to face the growing burden of hypertension, as suboptimal rates of hypertension diagnosis and treatment may dramatically increase the heart failure burden.
Cardiovascular disease (CVD) represents the leading cause of death globally, taking 18 million lives annually. Notably, 75% of CVD-related deaths occur in low- and middle-income countries, where health systems are still focused on an “unfinished agenda” of infectious diseases like HIV and tuberculosis, and are unprepared to address their rapidly growing burden of CVD.1 Sub-Saharan Africa (SSA) is a particularly vulnerable region, accounting for 5.5% of global CVD-related deaths in 2013.1 The dynamic change of the CVD profile in Africa is the result of an epidemiological transition fueled by shifts in demographic, behavioral and treatment trends. Specifically, the widespread uptake of anti-retroviral therapy has significantly reduced HIV mortality in SSA, reversing falls in life expectancy. As a result, the aging population is facing an increase in cardiometabolic risk factors like hypertension (HTN), smoking and diabetes.1
This epidemiologic transition has a heterogeneous impact within SSA: some populations predominantly have CVDs resulting from communicable causes like rheumatic heart disease or HIV-related cardiomyopathy, others from noncommunicable causes like HTN.2 Our current understanding remains limited, because most studies struggle to generate data beyond tertiary hospitals in major cities. Currently, however, 60% of the SSA population lives in rural areas; up to 85% of this population may migrate to cities over the next decade, and many will return to rural areas via circular labour migration – ultimately generating a rural-urban continuum.3 Hence, the rural and migrating cohorts will represent an increasing proportion of the modern CVD profile of SSA.1 Even in South Africa, one of the most extensively studied countries in the continent, most CVD studies are focused on hospitalized peri-urban patients.4, 5
In South Africa, hypertensive heart disease is the predominant cardiovascular diagnosis and a precursor to stroke mortality.4, 6 However, there is a limited understanding of the clinical severity with which HTN affects the urban and rural populations. This is due to the scarce availability of fundamental technologies like electrocardiogram (ECG) and transthoracic echocardiogram (TTE) – which can detect silent abnormalities that predict adverse events in patients with HTN, heart failure (HF) or ischemic heart disease (IHD). Such contemporary data are needed for health systems to identify high-risk patient groups to prioritize and target with aggressive monitoring and treatment.7
To address this unmet need, in 2014 we began collecting epidemiologic data on a cohort of >5,000 individuals from the Health and Socio-Demographic Surveillance System (HDSS) established in the Agincourt sub-district, in rural South Africa,8 where we documented a high burden of HTN and obesity.6 However, the severity of this CVD burden – as evidenced by end-organ damage – has not been measured in this region. Therefore, in the current study we characterize the spectrum of ECG and TTE abnormalities that indicate advanced stages of CVD, and which could be used in future studies as a screening tool to identify individual at increased risk for future adverse cardiovascular outcomes.
Methods
Data Availability Statement
The authors encourage investigators interested in data sharing and collaboration to contact the corresponding author.
Study Population and Data Sources
The Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community (HAALSI) was launched in 2014 to investigate the impact of HIV and non-communicable diseases in an aging population of rural South Africa, which has been prospectively characterized by the HDSS since 1992.8 The HAALSI baseline survey was completed by 5,059 people aged ≥40 years who had permanently resided for at least 1 year in the Agincourt sub-district of rural Mpumalanga Province, which encompasses >120,000 people living in 20,000 households in an area of 450 km2 6, 9, where an annual census systematically updates denominator data with vital events (deaths, births, in/out migrations).8 Apart from the age cut-off, there were no other inclusion or exclusion criteria based on any demographic or clinical characteristics. Participants were randomly selected from the Agincourt population using the 2013 census database, stratifying on sex in order to achieve equal numbers of women and men.
Within the HAALSI cohort described above, a sub-sample of 2,000 participants aged 40–69 years was randomly selected to undergo clinic visits and laboratory testing.10 Among these 2,000 participants, ~35% were selected randomly for resting ECGs and ~10% by convenience sampling for TTEs – based on local availability of diagnostic machines and trained technicians to operate them in this resource-limited setting. Participants consented in the local language and received unique identification numbers. The study was approved by the institutional review boards of University of the Witwatersrand, Harvard T.H. Chan School of Public Health, and the Mpumalanga Provincial Research and Ethics Committee. We encourage investigators interested in data sharing, collaboration or replicating the study procedures to contact the corresponding author.
Patient Variables
Demographic characteristics, vital signs, laboratory measurements, cardiometabolic risk factors and comorbidities were collected in clinic or during home visits.6 Our study aimed to describe ECG and TTE abnormalities in the context of CVDs, with HTN defined as mean systolic blood pressure (SBP) ≥140 mmHg, mean diastolic blood pressure ≥ 90 mmHg, self-reported HTN or current use of hypertensive medication; IHD was defined using a validated subset of the Rose Angina Questionnaire 11 or as self-reported angina, use of anginal medications or prior heart attack; HF was defined as self-report or current treatment of HF (assessed only among patients who self-reported having a HF diagnosis), and obesity as body mass index (BMI) ≥30.0 Kg/m2.
ECG and TTE Variables
The same electrocardiograph (MAC 600, General Electric Healthcare, Chicago, USA) was used for all ECGs, and resting, 10-second standard simultaneous 12-lead ECGs were recorded for all participants. ECGs were manually interpreted in an independent fashion both by E.G.F. and T.V.J., who were blinded to the clinical or electrocardiographic data. Any discrepancies were reconciled and adjudicated as needed by a third reviewer (T.A.G.), who was also blinded. Major abnormalities from standard 12-lead ECGs were categorized using the Minnesota ECG Code (Supplementary Methods).12 To increase sensitivity in detecting left ventricular hypertrophy (LVH), we additionally included and combined the Sokolow-Lyon, Cornell and Peguero-Lo Presti.13 The QT interval was rate-corrected using the Bazett formula.
Two-dimensional and color Doppler TTE was performed by two accredited sonographers using the same echocardiograph (Vivid q, General Electric Healthcare, Chicago, USA), and were interpreted by F.P., who was blinded to clinical and electrocardiographic information. Parameters from two-dimensional and color Doppler echocardiography were categorized using the 2015 joint American Society of Echocardiography and European Association of Cardiovascular Imaging Guidelines (Supplementary Methods).14
Statistical Analysis
Descriptive statistics were calculated for patient characteristics and ECG/TTE parameters. Chi-square test of goodness of fit, with adjustments for small expected cell counts (n <5), was used to study the univariable relationship between cardiovascular diseases and selected ECG/TTE parameters,15–19 and to stratify parameters by sex. We further conducted multivariable analyses for selected ECG/TTE parameters that were significant at the univariable level and clinically meaningful. Statistical analyses were conducted using SAS Version 9.4 Software, with α-level=0.05.
Results
Characteristics of the Study Sample
Among HAALSI participants (n=5,059), 810 individuals completed either ECGs (n=729) or TTEs (n=155), with 74 subjects completing both (Figure 1). They had a mean age of 54.7 years and 59.9% were female, with mean SBP 136.6 (±23.3) mm Hg, mean BMI 28 (±10.0) kg/m2, with 27.9% (95% confidence interval: 26.7%−29.2%) overweight and 30% (27.7%−32.5%) obese individuals, >25% having ≥1 lipid abnormality, and 9.1% (8.3%−9.9%) reporting tobacco smoking. Compared to the larger HAALSI cohort described previously,6 individuals from the sub-set who completed ECG or TTE evaluation were often females and tended to be younger; the sub-set who completed ECG evaluation also had higher rates of HIV (Table 1). Overall, there was high prevalence of HTN and obesity in both sub-samples, but much lower prevalence of IHD and HF; since <10 participants with HF completed ECG/TTE, statistical analysis was not performed for HF.
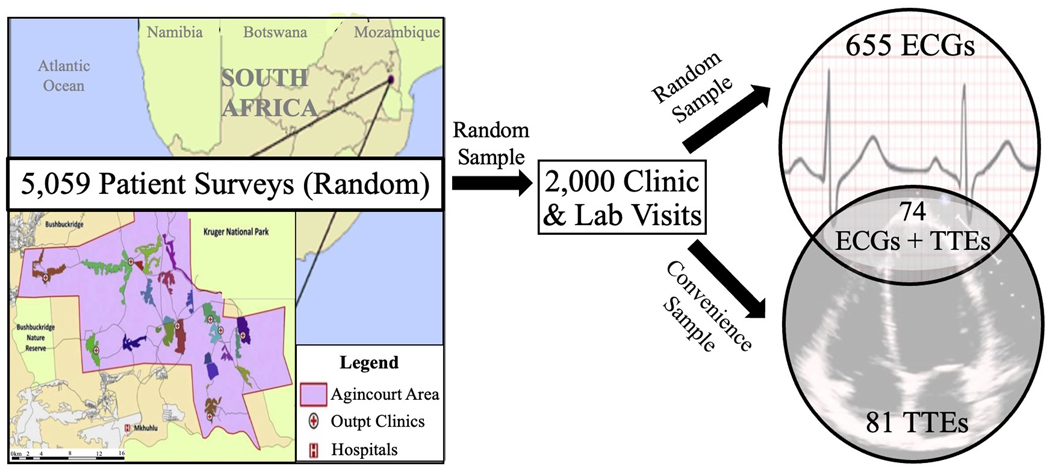
Figure 1. Flow Diagram of Patient Enrollment into the Study.
The insert on the left lower quadrant of the map represents the Agincourt study area (450 km2) in the North-East region of South Africa – where a longitudinal cohort of 5,059 people aged ≥40 years was first assembled in 2014 through random sampling from the 2013 census. From this initial cohort, a first sub-sample of 2,000 patients was randomly selected to undergo clinic visits and laboratory analyses to generate a cardiometabolic profile. Among those, another sub-set of 729 patients was randomly selected to undergo ECG evaluation. Finally, a sub-set of 155 patients was selected by convenience sampling (based on sonographer availability on site during clinic visits) to undergo TTE evaluation, with 74 patients completing both ECG and TTE evaluation. ECG, electrocardiogram; TTE, transthoracic echocardiogram.
Table 1:
Baseline Characteristics
| Full HAALSI Cohort (n=5,059) | Sub-Sample with ECG (n=729) | Sub-Sample with TTE (n=155) | |
|---|---|---|---|
| Demographic-Ethnic Characteristics | |||
| Age, years (Mean, ±SD) | 61.7 (±13.1) | 54.7 (±8.0) | 58.1(±6.8) |
| Female (N, %) | 2,714 (53.6) | 437 (59.9) | 103 (66.5) |
| Vital Signs | |||
| SBP, in mm Hg (Mean, ±SD) | 138.0 (±23.3) | 136.6 (±22.1) | 138.6 (±21.8) |
| DBP, in mm Hg (Mean, ±SD) | 82.1 (±12.7) | 84.0 (±12.5) | 84.1 (±12.5) |
| Heart Rate, in bpm (Mean, ±SD) | 73.5 (±12.0) | 73.6 (±11.9) | 72.7 (±11.3) |
| BMI, in kg/m2 (Mean, ±SD) | 27.5 (±10.0) | 28.0 (±7.1) | 27.9 (±6.9) |
| Laboratory Measurements | |||
| Hs-CRP, in mg/dL (Mean, IQR) | 3.3 (1.2, 4.3) | 3.4 (1.3, 4.4) | 3.3 (1.2, 4.2) |
| Cholesterol, in mmol/L (Mean, ±SD) | 4.2 (±1.3) | 4.2 (±1.2) | 4.2 (±1.3) |
| HDL, in mmol/L (Mean, ±SD) | 1.6 (±0.6) | 1.6 (±0.5) | 1.6 (±0.5) |
| LDL, in mmol/L (Mean, ±SD) | 2.1 (±1.5) | 2.1 (±1.0) | 1.9 (±1.1) |
| Triglycerides, in mmol/L (Mean, ±SD) | 1.8 (±1.6) | 1.8 (±1.0) | 2.8 (±6.2) |
| Blood Glucose, in mmol/L (Mean, IQR) | 6,7 (5.1, 7.2) | 6.6 (5.1, 7.1) | 7.1 (5.1, 7.2) |
| Risk Factors | N (%) [95%CI] | N (%) [95%CI] | N (%) [95% CI] |
| Current Tobacco Smoking | 460 (9.1) [8.3–9.9] | 66 (9.1) [7.2–11.4] | 14 (9.0) [5.4–14.8] |
| Alcohol Use | |||
| Drank at least once in last 30 days | 1,171 (23.2) [22.0–24.3] | 149 (20.4) [17.7–23.5] | 27 (17.4) [12.2–24.3] |
| Consumed >6 drinks in a day | 385 (7.6) [6.9–8.4] | 54 (7.4) [5.6–9.6] | 2 (1.3) [0.2–4.5] |
| BMI Categories | |||
| Underweight (<18.5 Kg/m2) | 339 (6.7) [6.0–7.4] | 41 (5.6) [4.1–7.5] | 9 (5.8) [3.1–11.1] |
| Normal (18.5–24.9 Kg/m2) | 1,791 (35.4) [34.1–36.7] | 228 (31.3) [28.4–35.2] | 51 (32.9) [26.3–41.2] |
| Overweight (25.0–29.9 Kg/m2) | 1,411 (27.9) [26.7–29.2] | 213 (29.2) [26.4–33.0] | 42 (27.1) [20.9–35.1] |
| Obese Grade 1 (30.0–34.9 Kg/m2) | 895 (17.7) [16.7–18.8] | 128 (17.6) [15.2–20.8] | 28 (18.1) [12.9–25.3] |
| Obese Grade 2 (35.0–39.9 Kg/m2) | 374 (7.4) [6.7–8.2] | 76 (10.4) [7.5–11.8] | 16 (10.3) [5.5–14.9] |
| Extreme Obesity (≥40.0 Kg/m2) | 248 (4.9) [4.3–5.5] | 41 (5.6) [4.3–7.8] | 9 (5.8) [3.1–11.0] |
| Total Dyslipidemia | |||
| High Cholesterol (≥ 6.21 mmol/L) | 334 (6.6) [5.3–7.3] | 41 (5.6) [4.1–7.6] | 9 (5.8) [2.7–10.7] |
| Low HDL (< 1.19 mmol/L) | 1,341 (26.5) [25.3–27.8] | 167 (22.9) [19.9–26.1] | 34 (21.9) [15.7–29.3] |
| High LDL (> 4.1 mmol/L) | 187 (3.7) [3.2–4.3] | 18 (2.5) [1.5–5.9] | 5 (3.2) [1.1–7.4] |
| High Triglycerides (>2.25 mmol/L) | 1,073 (21.2) [20.1–22.4] | 148 (20.3) [17.4–23.4] | 41 (26.5) [19.7–34.1] |
| Hs-CRP Risk Category | |||
| Low (<1 mg/L) | 971 (19.2) [18.1–20.3] | 128 (17.6) [14.9–20.5] | 26 (16.8) [11.3–23.6] |
| Intermediate (1–3 mg/L) | 2,145 (42.4) [41.0–43.8] | 259 (35.5) [32.1–39.1] | 60 (38.7) [31.0–46.9] |
| High (>3 mg/L) | 1,943 (38.4) [37.1–39.8] | 272 (37.3) [33.8–45.1] | 56 (36.1) [28.6–44.2] |
| Chronic Cardiovascular Conditions | N (%) [95%CI] | N (%)[95%CI] | N (%)[95%CI] |
| Hypertension | 473 (58.4) [57.0–59.8] | 398 (54.6) [50.9–58.3] | 96 (61.9) [54.0–69.3] |
| Ischemic Heart Disease | 87 (10.7) [9.9–11.6] | 86 (11.8) [9.5–14.4] | 15 (9.7) [5.9–15.5] |
| Heart Failure | 6 (0.8) [0.1–1.0] | 6 (0.8) [0.3–1.8] | 1 (0.7) [0.1–4.5] |
| Cerebrovascular Accident | 149 (2.95) [2.5–3.4] | 14 (1.9) [1.1–3.2] | 6 (3.9) [1.4–8.2] |
| Diabetes | 559 (11.05) [10.2–12.0] | 64 (8.8) [6.8–11.1] | 22 (14.2) [9.1–20.7] |
| Thyroid Disease | 36 (0.71) [0.5–1.0] | 3 (0.4) [0.1–1.2] | 0 (0) [0–0] |
| HIV | 1,048 (22.87) [21.7–24.1] | 206 (29.3) [26.1–32.8] | 32 (20.7) [14.6–27.9] |
ART, antiretroviral therapy, BMI, body mass index; DBP, diastolic, blood pressure; hs-CRP, high sensitivity C-reactive protein; HDL, high density lipoprotein; LDL, low density lipoprotein; SBP, systolic blood pressure.
Positive antibody test using dried blood spots
Presence of 3TC or FTC in lab test.
Spectrum of ECG Abnormalities
Overall, 47.6% (43.9%−51.3%) of participants (n=729) had at least one major ECG abnormality, with 36.5% (33.0%−40.1%) showing LVH, 13.6% (11.2%- 16.3%) T wave abnormalities and 7.8% (2.9%−12.0%) Q wave abnormalities (Table 2). ST segment abnormalities (<2%), ventricular conduction defects (<1%) and AF (<0.5%) were low Notably, 18.8% (16.0%−21.8%) had rate-corrected prolonged QT intervals. No participant had paced beats or high-degree atrioventricular blocks (Supplementary Table 1). Males (n=291) were significantly more likely to have major ECG abnormalities (57.4% vs 41.1%, p<0.01), which was driven by LVH (45% vs 30.8%, p<0.01) and Q wave abnormalities (10% vs 5.9%, p=0.04). Females (n=438) were significantly more likely to have prolonged QT intervals (28.8% vs 21%, p=0.02).
Table 2:
Prevalence of Electrocardiographic Abnormalities in Total Study Sample
| All (n=729) | Male (n=291) | Female (n=438) | p-value | |
|---|---|---|---|---|
| Major ECG Abnormalities | N (%) [95%CI] | N (%) [95%CI] | N (%) [95%CI] | |
| Any Major ECG Abnormality (composite) | 347 (47.6) [43.9–51.3] | 167 (57.4) [51.5–63.1] | 180 (41.1) [36.5–45.9] | <0.0001 |
| Left Ventricular Hypertrophy* | 266 (36.5) [33.0–40.1] | 131 (45.0) [39.2–50.9] | 135 (30.8) [26.5–35.4] | <0.0001 |
| Any Major Q Wave Abnormality | 55 (7.5) [5.7–9.7] | 29 (10.0) [6.8–14.0] | 26 (5.9) [3.9–8.6] | 0.040 |
| Any Major ST Segment Abnormality | 13 (1.8) [1.0–3.0] | 7 (2.4) [1.0–4.9] | 6 (1.4) [0.5–3.0] | 0.30 |
| Any Major T Wave Abnormality | 99 (13.6) [11.2– 16.3] | 48 (16.5) [12.4– 21.3] | 51 (11.6) [8.8–15.0] | 0.061 |
| Intra Ventricular Conduction Delay | 4 (0.6) [0.2–1.4] | 2 (0.7) [0.1–2.5] | 2 (0.5) [0.1–1.6] | 0.65 |
| Left Bundle Branch Block | 2 (0.3) [0.03–1.0] | 2 (0.7) [0.1–2.5] | 0 (0) [0–0] | 0.16 |
| Right Bundle Branch Block | 11 (1.5) [0.8–2.7] | 5 (1.7) [0.6–4.0] | 6 (1.4) [0.5–3.0] | 0.76 |
| Atrial Fibrillation | 2 (0.3) [0.03–1.0] | 1 (0.3) [0.01–1.9] | 1 (0.2) [0.01–1.3] | 1 |
| Other Conduction Defects | N (%) [95%CI] | N (%) [95%CI] | N (%) [95%CI] | |
| Prolonged QTc | 137 (18.8) [16.0–21.8] | 43 (14.8) [10.9–19.4] | 94 (21.5) [17.7–25.6] | 0.024 |
| Q Wave Abnormality Distribution** | N (%) [95%CI] | N (%) [95%CI] | N (%) [95%CI] | |
| Inferior Q Wave Abnormality | 18 (2.5) [1.5–3.9] | 7 (2.4) [1.0–4.9] | 11 (2.5) [1.3–4.5] | 0.93 |
| Anterior Q Wave Abnormality | 15 (2.1) [1.2–3.4] | 10 (3.4) [1.7–6.2] | 5 (1.1) [0.4–2.6] | 0.033 |
| Lateral Q Wave Abnormality | 23 (3.2) [0.20–4.7] | 13 (4.5) [2.4–7.5] | 10 (2.3) [1.1–4.2] | 0.099 |
| T Wave Abnormality Distribution** | N (%) [95%CI] | N (%) [95%CI] | N (%) [95%CI] | |
| Inferior T Wave Abnormality | 42 (5.8) [4.2–7.7] | 27 (9.3) [6.2–13.2] | 15 (3.4) [1.9–5.6] | 0.0010 |
| Anterior T Wave Abnormality | 54 (7.4) [5.6–9.6] | 19 (6.5) [4.0–10.0] | 35 (8.0) [5.6–10.9] | 0.46 |
| Lateral T Wave Abnormality | 54 (7.4) [5.6–9.6] | 26 (8.9) [5.9–12.8] | 28 (6.4) [4.3–9.1] | 0.20 |
ECG, Electrocardiogram; QTc, rate-corrected and sex-specific QT interval
Left Ventricular Hypertrophy was defined by the combination of sex-specific Sokolow-Lyon, Cornell and Peguero criteria
The distribution of each abnormality is not mutually exclusive.
Participants with HTN had significantly higher prevalence of major ECG abnormalities compared to participants without HTN (55.5% vs 35.0%, p<0.01), which was driven by LVH (45.4% vs 22.1%, p<0.01) and ST segment changes (17.4% vs 10.7%, p<0.01), as well as higher rates of prolonged QT interval (23.4% vs 11.4%, p<0.01). Older participants also showed higher rates of LVH, while participants with IHD and obesity showed no significant differences in ECG abnormalities (Table 3, Supplementary Tables 3 and 4).
Table 3:
Prevalence of Clinically Meaningful Electrocardiographic Abnormalities Among Participants with Cardiovascular Disease
| Hypertension | Ischemic Heart Disease | |||||
|---|---|---|---|---|---|---|
| Yes (n=449) | No (n=280) | Yes (n=86) | No (n=643) | |||
| ECG Variable | N (%) | N (%) | p-value* | N (%) | N (%) | p-value* |
| Any Major ECG Abnormality | 249 (55.5) | 98 (35.0) | <0.01 | 37 (43.0) | 310 (48.2) | 0.37 |
| Any Q Wave Abnormality | 32 (7.1) | 23 (8.2) | 0.59 | 3 (3.5) | 52 (8.1) | 0.19 |
| Any ST-T Segment Abnormality | 78 (17.4) | 30 (10.7) | 0.01 | 13 (15.1) | 95 (14.8) | 0.93 |
| Left Ventricular Hypertrophy** | 204 (45.4) | 62 (22.1) | <0.01 | 33 (38.4) | 233 (36.2) | 0.70 |
| Left Bundle Branch Block | 3 (0.7) | 2 (0.7) | 1.00 | 0 | 5 (0.8) | 1.00 |
| Right Bundle Branch Block | 9 (2.0) | 2 (0.7) | 0.22 | 1 (1.2) | 10 (1.6) | 1.00 |
| Atrial Fibrillation | 3 (0.7) | 0 | 0.29 | 0 | 3 (0.5) | 1.00 |
| Left Axis Deviation | 28 (6.2) | 10 (3.6) | 0.12 | 4 (4.7) | 34 (5.3) | 1.00 |
| Right Axis Deviation | 0 | 2 | 0.15 | 0 | 2 (0.3) | 1.00 |
| Prolonged QTc | 105 (23.4) | 32 (11.4) | <0.01 | 21 (24.4) | 116 (18.0) | 0.16 |
ECG, Electrocardiogram; QTc, rate-corrected and sex-specific QT interval
Chi-square probability or Fisher’s exact probability where any cell count ≤ 5
Left ventricular hypertrophy was defined by the combination of sex-specific Sokolow-Lyon, Cornell and Peguero criteria.
The multivariable associations confirmed the signals identified in the univariable associations for ECG abnormalities (Table 4): even after adjustment for age and other demographic variables, participants with HTN were significantly more likely to show evidence of LVH and prolonged QT interval on ECG. Similarly, male gender remained significantly associated LVH on ECG in the multivariable model, and female gender remained significantly associated with prolonged QT interval.
Table 4.
Multivariable Associations for Electrocardiographic Abnormalities
| Variables | Multivariable Association with LVH (n=592) | Multivariable Association with Prolonged QTc (n=592) | ||
|---|---|---|---|---|
| aOR (95% CI) | Adjusted p-value | aOR (95% CI) | Adjusted p-value | |
| Age☨ | 1.04 (1.01, 1.06) | 0.005 | 1.03 (1.01, 1.06) | 0.04 |
| Female | 0.61 (0.41, 0.92) | 0.02 | 1.68 (1.01, 2.82) | 0.05 |
| Diabetes | 0.89 (0.47, 1.69) | 0.72 | 0.48 (0.25, 0.92) | 0.03 |
| BMI☨ | 0.98 (0.95, 1.01) | 0.17 | 1.00 (0.96, 1.03) | 0.98 |
| SBP☨ | 1.03 (1.03, 1.04) | <0.0001 | 1.01 (1.01, 1.02) | 0.003 |
| Hs-CRP☨ | 1.04 (0.98, 1.11) | 0.17 | 1.02 (0.95, 1.10) | 0.57 |
| Total Cholesterol☨ | 1.01 (0.87, 1.17) | 0.93 | 1.06 (0.90, 1.26) | 0.93 |
aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; hs-CRP, high sensitivity C-reactive protein; LVH, left ventricular hypertrophy; SBP, systolic blood pressure; QTc, rate-corrected and sex-specific QT interval.
indicates continuous variables.
Spectrum of TTE Abnormalities
Overall, 10.4% (4.7%−19.7%) of participants (n=155) had ejection fraction (EF) <50%, with high prevalence of moderate (31%, 23.8%−38.9%) and severe (25.8%, 19.1%−33.4%) diastolic dysfunction (Table 5). Concentric LVH (31.6%, 24.4%−40.0%) was more prevalent than eccentric LVH (14.8%, 9.6%−21.4%); left ventricular dimensions and volumes were normal in systole and diastole for most participants (Supplementary Table 2). Almost all participants had collapsable inferior vena cava and normal pulmonary artery systolic pressures. The prevalence of valvular abnormalities was low, except mitral stenosis at 6.5% (3.1%−11.5%). Females were significantly more likely to have concentric LVH (40.8% vs 13.5%, p<0.01) and increased relative wall thickness (RWT, 70% vs 18.1%, p<0.01).
Table 5:
Prevalence of Echocardiographic Abnormalities in Total Study Sample
| Total (n=155) | Male (n=52) | Female (n=103) | ||
|---|---|---|---|---|
| Systolic Function | N (%) | N (%) | N (%) | p-value |
| EF, mean in % (±SD) | 58.3 (±8.6) | 57.6 (±8.4) | 58.7 (±8.6) | 1 |
| EF <40% | 5 (3.3) [1.1–7.4] | 1 (1.9) [0.1–10.3] | 4 (3.9) [1.1–9.7] | 0.48* |
| EF 41–49% | 11 (7.1) [3.6–12.3] | 6 (11.5) [4.4–23.4] | 5 (4.9) [1.6–11.0] | |
| EF ≥50% | 138 (89.6) [83.8–94.0] | 45 (29.2) [17.1–43.1] | 93 (60.4) [50.1–69.7] | |
| Diastolic Function | N (%) | N (%) | N (%) | p-value |
| Normal Diastolic Function | 2 (1.3) [0.2–4.6] | 1 (1.9) [0.1–10.3] | 1 (1.0) [0.03–5.3] | 0.87** |
| Mild Diastolic Dysfunction | 65 (41.9) [34.1–50.1] | 21 (40.4) [27.0–54.9] | 44 (42.7) [33.0–52.9] | |
| Moderate Diastolic Dysfunction | 48 (31.0) [23.8–38.9] | 11 (21.2) [11.1–34.7] | 37 (35.9) [26.7–46.0] | |
| Severe Diastolic Dysfunction | 40 (25.8) [19.1–33.4] | 19 (36.5) [23.6–51.0] | 21 (20.4) [13.1–29.5] | |
| Left Ventricular Indices in Diastole | N (%) | N (%) | N (%) | p-value |
| Concentric Hypertrophy | 49 (31.6) [24.4–40.0] | 7 (13.5) [5.6–25.8] | 42 (40.8) [31.2–51.0] | 0.0001 |
| Eccentric Hypertrophy | 23 (14.8) [9.6–21.4] | 5 (9.6) [3.2–21.0] | 18 (17.5) [10.7–26.2] | 0.19 |
| RWT, mean (±SD) | 0.49 (±0.10) | 0.46 (±0.10) | 0.50 (±0.10) | 0.098 |
| Normal RWT | 55 (35.5) [28.0–43.6] | 24 (46.2) [32.2–60.5] | 31 (30.1) [21.5–39.9] | 0.048 |
| High RWT | 100 (64.5) [56.4–72.0] | 28 (18.1) [8.2–30.3] | 72 (69.9) [60.1–78.6] | |
| IVSd, mean in mm (±SD) | 10.97 (±2.2) | 10.50(±2.2) | 11.20 (±2.1) | 0.055 |
| Normal IVSd | 49 (31.6) [24.4–40.0] | 26 (50.0) [35.8–64.2] | 23 (22.3) [35.8–64.2] | 0.0005 |
| High IVSd | 106 (68.4) [60.4–75.6] | 26 (50.0) [35.8–64.2] | 80 (77.7) [68.4–85.3] | |
| PWd, mean in mm (±SD) | 10.4 (±1.8) | 10.2 (±2.0) | 10.5 (±10.2) | 0.26 |
| Normal PWd | 63 (40.7) [32.8–48.8] | 32 (61.5) [47.0–74.7] | 31 (30.1) [21.5–39.9] | 0.0002 |
| High PWd | 92 (59.4) [51.2–67.2] | 20 (38.5) [25.3–53.0] | 72 (69.9) [25.3–53.0] | |
| LVM, mean in g (±SD) | 163.9 (±42.0) | 165.2 (±46.5) | 163.2 (±39.8) | 0.78 |
| Normal LVM | 83 (53.6) [45.4–61.6] | 40 (76.9) [63.2–87.5] | 43 (41.8) [32.1–51.9] | <0.0001 |
| High LVM | 72 (46.5) [38.4–54.6] | 12 (23.1) [12.5–36.8] | 60 (58.4) [48.1–67.9] | |
| LVMi, mean in g/m2 (±SD) | 90.9 (±21.1) | 93.3 (±23.2) | 89.6 (±19.9) | 0.31 |
| Normal LVMi | 84 (54.2) [46.0–62.2] | 33 (63.5) [49.0–76.4] | 51 (49.5) [40.0–59.5] | 0.10 |
| High LVMi | 71 (45.8) [37.8–54.0] | 19 (36.5) [23.6–51.0] | 52 (50.5) [40.5–60.5] | |
| Right Ventricle Parameters | N (%) | N (%) | N (%) | p-value |
| PASP, mean in mm Hg (±SD) | 22.8 (±6.6) | 22.3 (±7.2) | 23.0 (±6.4) | 0.65 |
| Normal PASP | 151 (97.4) [93.5–99.3] | 50 (96.2) [86.8–99.5] | 101 (98.1) [93.2–99.8] | 0.60 |
| High PASP | 4 (2.6) [0.07–6.5] | 2 (3.9) [0.05–13.2] | 2 (1.9) [0.2–6.8] | |
| Valvular Pathologies | N (%) | N (%) | N (%) | p-value |
| AR (moderate, severe) | 3 (1.9) [0.04–5.6] | 1 (1.9) [0.05–10.3] | 2 (1.9) [0.2–6.8] | 1 |
| No AR (including trace and mild) | 152 (98.1) [94.4–99.6] | 51 (98.1) [89.7– 99.9] | 101 (98.1) [93.2–99.8] | |
| MR (moderate, severe) | 3 (1.9) [0.4–5.6] | 0 (0) [0–0] | 3 (2.9) [0.6–8.3] | 0.55 |
| No MR (including trace and mild) | 152 (98.1) [94.4–99.6] | 52 (100) [98.7–100] | 100 (97.1) 91.7–99.4] | |
| MS (trace, mild, moderate, severe) | 10 (6.5) [3.1–11.5] | 5 (9.6) [3.2– 21.0] | 5 (4.9) [1.6–11.0] | 0.30 |
| No MS | 145 (93.6) [88.5–96.9] | 47 (90.4) [79.0–96.8] | 98 (95.2) [89.0–98.4] | |
| TR (moderate, severe) | 5 (3.2) [1.1–7.4] | 0 (0) [0–0] | 5 (4.9) [1.6–11.0] | 0.17 |
| No TR (including trace and mild) | 150 (96.8) [92.6–98.9] | 52 (100) [98.9–100] | 98 (95.2) [89.0–98.4] | |
| PR (moderate, severe) | 1 (0.7) [0.02–3.5] | 0 (0) [0–0] | 1 (1.0) [0.03– 5.3] | 1 |
| No PR (including trace and mild) | 154 (99.4) [96.5–99.9] | 52 (33.6) [20.3–47.1] | 102 (99.0) [94.7–99.9] |
EF, ejection fraction; TTE, transthoracic echocardiogram; RWT, relative wall thickness; IVSd, interventricular septum diameter; PWd, posterior wall diameter; LVM/LVMi, left ventricular mass/index; PASP, pulmonary artery systolic pressure; RV, right ventricle; AR, aortic regurgitation; MR, mitral regurgitation; MS, mitral stenosis; TR, tricuspid regurgitation; PR, pulmonary regurgitation.
This Chi-square test compared EF<40% and EF 41–49% (as a composite variable) versus EF ≥50%.
This Chi-square test compared normal diastolic function and mild diastolic dysfunction (as a composite variable) versus moderate and severe diastolic dysfunction (as a composite variable).
Participants with HTN had significantly higher rates of concentric LVH (42.5% vs 8.2%, p<0.01), higher left ventricular mass (LVM, 58.5% vs 20.4%, p<0.01) and LVM index (52.8% vs 30.6%, p<0.01) compared to patients without HTN, though diastolic dysfunction rates were similar (Table 6); participants with obesity had similar distribution of abnormalities, and younger participants had higher rates of diastolic dysfunction (Supplementary Tables 3 and 5). Participants with IHD were significantly more likely to have moderate diastolic dysfunction (60% vs 27.9%, p<0.01), higher thickness of the interventricular septum (93.3% vs 65.7%, p<0.01) and posterior wall (93.3% vs 55.7%, p<0.01).
Table 6:
Prevalence of Clinically Meaningful Echocardiographic Abnormalities Among Participants with Cardiovascular Disease
| Hypertension | Ischemic Heart Disease | |||||
|---|---|---|---|---|---|---|
| Yes (n=106) | No (n=49) | Yes (n=15) | No (n=140) | |||
| TTE Variable | N (%) | N (%) | p-value* | N (%) | N (%) | p-value* |
| Ejection Fraction <40% | 5 (4.7) | 0 | 0.18 | 0 | 5 (3.6) | 1.00 |
| Mod Diastolic Dysfunction | 29 (27.4) | 19 (38.8) | 0.15 | 9 (60) | 39 (27.9) | 0.01 |
| Sev Diastolic Dysfunction | 30 (28.3) | 10 (20.4) | 0.30 | 2 (13.3) | 38 (27.1) | 0.36 |
| Eccentric LVH | 17 (16.0) | 6 (12.2) | 0.54 | 0 | 23 (16.4) | 0.13 |
| Concentric LVH | 45 (42.5) | 4 (8.2) | <0.01 | 8 (53.3) | 41 (29.3) | 0.06 |
| High RWT | 73 (68.9) | 27 (55.1) | 0.09 | 14 (93.3) | 86 (61.4) | 0.02 |
| High LVM | 62 (58.5) | 10 (20.4) | <0.01 | 8 (53.3) | 64 (45.7) | 0.57 |
| High LVMi | 56 (52.8) | 15 (30.6) | 0.01 | 8 (53.3) | 63 (45.0) | 0.54 |
| High IVSd | 76 (71.7) | 30 (61.2) | 0.19 | 14 (93.3) | 92 (65.7) | 0.04 |
| High PWD | 73 (68.9) | 19 (38.8) | <0.01 | 14 (93.3) | 78 (55.7) | <0.01 |
| High LAVi | 7 (6.6) | 3 (6.1) | 1.00 | 0 | 10 (7.1) | 0.60 |
| High LVESD | 3 (2.8) | 1 (2.0) | 1.00 | 0 | 4 (2.9) | 1.00 |
| High LVEDD | 93 (87.7) | 41 (83.7) | 0.49 | 15 (100) | 119 (85) | 0.23 |
TTE, transthoracic echocardiogram; IVSd, interventricular septum diameter; PWd, posterior wall diameter; LVM/LVMi, left ventricular mass/index; LVH, left ventricular hypertrophy; LAVi, left atrium volume index; LVESD/ LVEDD, left ventricular end systolic/diastolic diameter; RWT, relative wall thickness.
Chi Square probability or Fisher’s exact probability where any cell count ≤ 5.
The multivariable associations confirmed some signals identified in the univariable associations for TTE abnormalities (Table 7): even after adjustment for age and other demographic variables, participants with HTN were significantly more likely to show evidence of high LVM on TTE, particularly among female participants. No significant association was identified with increased RWT at the multivariable level.
Table 7.
Multivariable Associations for Echocardiographic Abnormalities.
| Variables | Multivariable Association with High LVM (n=132) | Multivariable Association with High RWT (n=132) | ||
|---|---|---|---|---|
| aOR (95% CI) | Adjusted p-value | aOR (95% CI) | Adjusted p-value | |
| Age☨ | 1.06 (0.99, 1.13) | 0.062 | 1.03 (0.97, 1.09) | 0.38 |
| Female | 3.91 (1.33, 11.5) | 0.013 | 2.38 (0.92, 11.5) | 0.075 |
| Diabetes | 0.66 (0.20, 2.01) | 0.48 | 0.95 (0.32, 2.77) | 0.92 |
| BMI☨ | 1.06 (0.99, 1.14) | 0.118 | 0.98 (0.9q, 1.04) | 0.47 |
| SBP☨ | 1.04 (1.01, 1.06) | 0.001 | 1.01 (0.99, 1.03) | 0.17 |
| Hs-CRP☨ | 1.12 (0.98, 1.29) | 0.094 | 1.16 (0.99, 1.37) | 0.07 |
| Total Cholesterol☨ | 1.09 (0.78, 1.53) | 0.62 | 0.94 (0.70, 1.28) | 0.71 |
aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; hs-CRP, high sensitivity C-reactive protein; LVM, left ventricular mass; RWT, relative wall thickness; SBP, systolic blood pressure
indicates continuous variables.
Lastly, participants with LVH on ECG were not significantly more likely to demonstrate LVH on TTE (OR 1.79, 95% CI 0.92–3.51, data not shown).
Discussion
Hypertension has been identified as the predominant cardiovascular risk factor in our cohort and throughout SSA, with a disproportionately higher prevalence than other factors like diabetes or hyperlipidemia.1, 4, 6 Identifying high risk features among hypertensive patients, therefore, is fundamental to mitigate the impact of known complications like stroke and HF. Our work advances the field by documenting the severity of end-organ damage that is already underway among rural populations, in a region where data from fundamental cardiovascular technologies like ECG and TTE have traditionally been scarce. We found a high prevalence of participants meeting ECG criteria for LVH and other repolarization abnormalities consistent with HTN-induced ventricular remodeling. Many participants also met TTE criteria for myocardial wall thickening and concentric LVH, with >50% showing moderate to severe diastolic dysfunction. Lastly, we found that males show abnormalities predominantly on ECG, while females predominantly on TTE.
With regard to ECG abnormalities, there is wide variation in the prevalence of LVH (5–50%) reported in peri-urban settings within South Africa 4, 5 or other SSA countries.20 The LVH prevalence in our rural cohort was on the higher end of this spectrum (35%), possibly from applying more sensitive LVH criteria (Peguero-Lo Presti) that outperform traditional criteria including in African-Americans.13 Although the combined use of all LVH criteria may decrease the specificity of our findings, the goal of our study was to increase the sensitivity in detecting undiagnosed CVD that, if left untreated, would lead to higher morbidity and mortality in this population. While data on other major ECG abnormalities in SSA is minimal,5 we found that almost half of our sample presented ECG abnormalities that predict cardiovascular events. This mirrors data from contemporary cohorts in the U.S. 16, 21 and Europe.22 Alarmingly, these high-risk ECG patterns in our study were significantly more prevalent among participants with HTN, even after adjusting for age and other comorbidities in multivariable associations.
We similarly found a high prevalence of TTE abnormalities like concentric LVH (32%) and moderate/severe diastolic dysfunction (55%). Some of these TTE abnormalities, like increased LVM (46% of our TTE sub-sample) remained significantly associated with HTN even after adjusting for age and other comorbidities in multivariable models, which suggests that high-blood pressure may be contributing to end-organ remodeling. Our findings from this rural population align with data from peri-urban settings in South Africa and SSA, where a high HTN burden led to widespread LVH (40%) and diastolic dysfunction (25%), even in young patients with masked HTN.4, 7, 23 Notably, diastolic dysfunction in our rural sample is greater than contemporary reports from high-income countries in Europe (10–25%).24 Some variability is expected, since diastolic parameters vary across studies based on an evolving expert consensus. However, a more alarming interpretation must be considered: since LVH usually regresses with HTN treatment, our study population in South Africa may have a higher burden of uncontrolled HTN compared to these high-income countries, which is silently causing end-organ damage like diastolic dysfunction.
This interpretation is supported by the fact that South Africa has achieved low levels of HTN detection and treatment in urban areas;23, 25 similarly, our group found that <50% and <25% patients in rural areas achieved HTN awareness and control, respectively.26 Our data also builds on a growing body of evidence, which challenges the theory that CVDs in SSA are primarily the result of migration to urban areas and transition to the Western lifestyle.1, 25 This theory has been self-fulfilled as most existing studies focused on peri-urban hospitals.1 Our data suggests that HTN is already prevalent in rural populations, even among individuals not engaging in circular labour migration to urban areas.6, 9, 27 This is supported by the high prevalence of ventricular remodeling identified among older women, who may be exposed to urban lifestyles even if rural-dwelling – through the behaviors of household members. Notably, the prevalence of TTE features associated with HTN was much higher compared to those associated with rheumatic heart disease (like mitral stenosis), which is consistent with the epidemiological transition from communicable to noncommunicable forms of heart disease reported throughout urban SSA.1
The widespread end-organ damage in this rural cohort may result from suboptimal rates of diagnosis and treatment of HTN by local health systems, which are focused on an “unfinished agenda” of infectious diseases like HIV. Rural health systems are particularly vulnerable, because they are the most likely to be stressed by the growing CVD burden. A recent study in rural South Africa showed that migrant workers who become ill in urban areas usually return to rural communities to seek care and support.3 This relationship is well-established in the HIV literature throughout SSA and will likely apply to CVDs, especially when end-organ damage manifests as strokes or HF. Therefore, it is essential to equip health systems with population-based data to develop a tailored response to their CVD epidemic.
Our data on the sex-based differences in TTE and ECG abnormalities are particularly relevant for health system planning. We found that male participants are more likely to show LVH on ECG, while female participants are more likely to show concentric LVH and increased LVM on TTE; they also commonly show prolonged QT intervals, a less well-recognized marker of HTN that independently predicts mortality.15 The majority of these associations between sex and ECG/TTE abnormalities remained significant after adjusting for age and other comorbidities in multivariable models. Our group is the first to document such sex-based discrepancies in SSA, which are consistent with U.S. cohorts, where women with HTN show disproportionately lower QRS voltage per gram of LV mass. Despite having “electrically silent” ECGs, women still develop more concentric LVH.28 While these sub-analyses should be considered as hypothesis-generating, these results suggest that a strategic priority for SSA is to increase availability of TTEs, despite their high cost and training requirements. Monitoring based solely on ECG may underdiagnose a significant number of patients, especially females, and miss silent abnormalities that predict progression to HF, clinical decompensation and healthcare utilization. The lack of association between LVH by ECG and LVH by TTE (among participants who completed both tests) also supports the value of using both modalities as synergistic tools to acquire complementary information and possibly identify high-risk individuals. Our results align with recent studies in U.S. cohorts:29 for example, 57% of sudden cardiac death victims with ECG LVH showed no evidence of TTE LVH, and 84% of patients with TTE LVH showed no evidence of ECG LVH.30 While also hypothesis-generating, these findings in our cohort suggest that the two modalities may reflect distinct forms of electrical versus anatomic ventricular remodeling, with potentially different risk profiles – which will be formally explored in next waves of data collection and analysis.
This study must be interpreted in the context of its limitations. Randomized sampling, stratified by sex, was performed when assembling the larger HAALSI cohort, and was likely carried through to the ECG sub-set, which was also assembled via randomized sampling. The TTE sub-set, however, was assembled via convenience sampling and was one fifth as large as the ECG sub-set, thus it may not accurately represent the diversity of the larger HAALSI cohort. Due to the limited availability of trained medical sonographers in this rural area (who often had to commute from the nearest urban area on weekends), TTEs were only performed on the sub-set of individuals who happened to be in clinic when the sonographers were present on site. Nonetheless, the finding that approximately 50% of the participants who performed a TTE also had an ECG (i.e. 74 out of 155 participants) suggests that some level of randomization was achieved in the TTE sample, even via convenience sampling. This is further supported by the similarity in demographic and clinical characteristics between the larger HAALSI cohort and both the ECG and TTE sub-samples. Individuals completing ECG and TTE evaluation were often younger and female, likely because elderly patients have difficulty commuting to clinic, while male individuals have work-related conflicts, and thus had less opportunities to be asked to participate in the study. Individual with chronic comorbidities (such as HIV or diabetes) are instead more likely to present to clinic for routine follow-up or medication refills, thus having more opportunities to be asked to participate in the study. ECG and TTE abnormalities were defined using U.S. and European guidelines, as the African population is less characterized to date. Some abnormalities could represent a “normal variant” in the African population. Recent studies in healthy South Africans found that >10% would meet LVH criteria even without HTN.31 However, we employed the same criteria used in prior African cohorts. We further believe our results reflect true pathological states, because all average diastolic parameters are higher than the normative values recently established in a black African population, which will be used to update professional society guidelines, 32 as well as the normative values among healthy Nigerian individuals included in the World Alliance Societies of Echocardiography (WASE) Normal Values Study.33 In addition to HTN, obesity may influence pathological ventricular remodeling.34 However, the independent effect of obesity remains controversial, as some studies found no significant association with ventricular remodeling in the absence of HTN.35 Since obesity has been associated most strongly with eccentric LVH,34 the greater burden of concentric LVH in our population supports HTN as the predominant driver of end-organ damage. Furthermore, ECG abnormalities were associated with HTN but not with obesity in our sample, and the univariable association between obesity and high LVM did not remain significant after adjusting for age and other patient characteristics. This may be because the interaction between obesity and ventricular remodeling occurs through increased high blood pressure, which remained significantly associated with both ECG and TTE abnormalities in the multivariable models. Other cardiometabolic factors like sleep-disordered breathing also warrant further study, though they often interact with obesity.34
We also found a much lower prevalence of IHD and HF in our cohort, which reduced the power to identify associations between these conditions and ECG/TTE abnormalities. Unlike HTN, IHD and HF are less prevalent in SSA, underdiagnosed in resource-limited settings, and their manifestations less known to patients. In future studies, we plan to introduce laboratory and imaging assays (brain natriuretic peptide, wall motion abnormalities), in order to improve diagnosis of IHD and HF.
Conclusions
Our study uncovered the signs of a rapidly advancing epidemic of hypertensive heart disease in rural South Africa, which may be fueled by forces beyond traditional urbanization. The widespread prevalence of ECG and TTE abnormalities suggest that HTN remains largely uncontrolled in rural settings, and that local health systems are unprepared for the scale and speed of this epidemiological transition. While remaining responsive to chronic infectious conditions like HIV, it is essential for African health systems to start developing a targeted response. To do so, future studies from our rural cohort and established peri-urban cohorts throughout SSA should: 1) systematically accumulate TTE data in patients with and without CVD to define normative values and standardize detection of end-organ damage, and 2) understand which silent abnormalities most strongly predict HF, mortality and other adverse events. In these resource-limited settings, it will be essential to prioritize and target high-risk groups with aggressive monitoring and treatment.
Supplementary Material
WHAT IS KNOWN
Sub-Saharan Africa is undergoing an epidemiological transition from infectious to cardiovascular diseases, and local health systems need granular data to identify high-risk patient groups.
In South Africa, there is an established high burden of cardiometabolic conditions like hypertension and obesity, but few studies have characterized electrocardiographic and echocardiographic abnormalities that indicate advanced disease.
WHAT THE STUDY ADDS
Over 30% of our study population showed truly pathological cardiac remodeling, as evidenced by left ventricular hypertrophy on electrocardiogram (especially among males) and echocardiogram (especially among females).
Participants with hypertension were significantly more likely to show high rates of left ventricular hypertrophy and remodeling.
These findings support the severity of end-organ damage that is already underway in this rural population of South Africa, likely from the uncontrolled burden of hypertension, obesity and other cardiometabolic conditions.
Acknowledgments
Funding
This work was supported by a collaboration between the Harvard Center for Population and Development Studies from the Harvard T.H. Chan School of Public Health, the MRC/Wits Rural Public Health and Health Transitions Research Unit from the School of Public Health at the University of the Witwatersrand in South Africa, and the INDEPTH Network in Accra, Ghana. The HAALSI study, funded by the National Institute on Aging (P01 AG041710), is nested within the Agincourt Health and Demographic Surveillance System site, funded by the Department of Science and Innovation, the University of the Witwatersrand, and the Medical Research Council, South Africa, and previously the Wellcome Trust, UK (grants 058893/Z/99/A; 069683/Z/02/Z; 085477/Z/08/Z; 085477/B/08/Z). Research was also supported by an NIH supplement (3U54HG006938-03S1) to the AWI-Gen Collaborative Centre (1U54HG006938), an H3Africa Consortium member, to enable the integration of HAALSI and AWI-Gen research. The funding sources played no role in the design, analysis, nor interpretation of the data or in production of the manuscript.
Non-standard Abbreviations and Acronyms
- BMI
body mass index
- CVD
cardiovascular disease
- ECG
electrocardiogram
- HF
heart failure
- HIV
human immunodeficiency virus
- HTN
hypertension
- IHD
ischemic heart disease
- LVH
left ventricular hypertrophy
- LVM
left ventricular mass
- RWT
relative wall thickness
- SBP
systolic blood pressure
- SSA
Sub-Saharan Africa
- TTE
transthoracic echocardiogram
Footnotes
Conflict of Interest
All authors report no relevant conflict of interest.
References
- 1.Keates AK, Mocumbi AO, Ntsekhe M, Sliwa K and Stewart S. Cardiovascular disease in Africa: epidemiological profile and challenges. Nature reviews Cardiology. 2017;14:273–293. [DOI] [PubMed] [Google Scholar]
- 2.Sliwa K, Acquah L, Gersh BJ and Mocumbi AO. Impact of Socioeconomic Status, Ethnicity, and Urbanization on Risk Factor Profiles of Cardiovascular Disease in Africa. Circulation. 2016;133:1199–208. [DOI] [PubMed] [Google Scholar]
- 3.Clark SJ, Collinson MA, Kahn K, Drullinger K and Tollman SM. Returning home to die: circular labour migration and mortality in South Africa. Scandinavian journal of public health Supplement. 2007;69:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sliwa K, Wilkinson D, Hansen C, Ntyintyane L, Tibazarwa K, Becker A and Stewart S. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet (London, England). 2008;371:915–22. [DOI] [PubMed] [Google Scholar]
- 5.Peer N, Steyn K, Dennison CR, Levitt NS, Nyo MT, Nel JH, Commerford PJ, Fourie JM and Hill MN. Determinants of target organ damage in black hypertensive patients attending primary health care services in Cape Town: the Hi-Hi study. American journal of hypertension. 2008;21:896–902. [DOI] [PubMed] [Google Scholar]
- 6.Gaziano TA, Abrahams-Gessel S, Gomez-Olive FX, Wade A, Crowther NJ, Alam S, Manne-Goehler J, Kabudula CW, Wagner R, Rohr J, Montana L, Kahn K, Barnighausen TW, Berkman LF and Tollman S. Cardiometabolic risk in a population of older adults with multiple co-morbidities in rural south africa: the HAALSI (Health and Aging in Africa: longitudinal studies of INDEPTH communities) study. BMC public health. 2017;17:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekoba NP, Kruger R, Labuschagne P and Schutte AE. Left ventricular mass independently associates with masked hypertension in young healthy adults: the African-PREDICT study. Journal of hypertension. 2018;36:1689–1696. [DOI] [PubMed] [Google Scholar]
- 8.Kahn K, Collinson MA, Gómez-Olivé FX, Mokoena O, Twine R, Mee P, Afolabi SA, Clark BD, Kabudula CW, Khosa A, Khoza S, Shabangu MG, Silaule B, Tibane JB, Wagner RG, Garenne ML, Clark SJ and Tollman SM. Profile: Agincourt health and socio-demographic surveillance system. International journal of epidemiology. 2012;41:988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jardim TV, Witham MD, Abrahams-Gessel S, Gomez-Olive FX, Tollman S, Berkman L and Gaziano TA. Cardiovascular Disease Profile of the Oldest Adults in Rural South Africa: Data from the HAALSI Study (Health and Aging in Africa: Longitudinal Studies of INDEPTH Communities). Journal of the American Geriatrics Society. 2018;66:2151–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wade AN, Crowther NJ, Abrahams-Gessel S, Berkman L, George JA, Gómez-Olivé FX, Manne-Goehler J, Salomon JA, Wagner RG, Gaziano TA, Tollman SM and Cappola AR. Concordance between fasting plasma glucose and HbA(1c) in the diagnosis of diabetes in black South African adults: a cross-sectional study. BMJ Open. 2021;11:e046060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achterberg S, Soedamah-Muthu SS, Cramer MJ, Kappelle LJ, van der Graaf Y and Algra A. Prognostic value of the Rose questionnaire: a validation with future coronary events in the SMART study. European journal of preventive cardiology. 2012;19:5–14. [DOI] [PubMed] [Google Scholar]
- 12.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Littleton, MA: John Wright-PSG Inc; 1982. [Google Scholar]
- 13.Peguero JG, Lo Presti S, Perez J, Issa O, Brenes JC and Tolentino A. Electrocardiographic Criteria for the Diagnosis of Left Ventricular Hypertrophy. Journal of the American College of Cardiology. 2017;69:1694–1703. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W and Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European heart journal cardiovascular Imaging. 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 15.Soliman EZ, Shah AJ, Boerkircher A, Li Y and Rautaharju PM. Inter-relationship between electrocardiographic left ventricular hypertrophy and QT prolongation as predictors of increased risk of mortality in the general population. Circulation Arrhythmia and electrophysiology. 2014;7:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auer R, Bauer DC, Marques-Vidal P, Butler J, Min LJ, Cornuz J, Satterfield S, Newman AB, Vittinghoff E and Rodondi N. Association of major and minor ECG abnormalities with coronary heart disease events. Jama. 2012;307:1497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Neal WT, Mazur M, Bertoni AG, Bluemke DA, Al-Mallah MH, Lima JAC, Kitzman D and Soliman EZ. Electrocardiographic Predictors of Heart Failure With Reduced Versus Preserved Ejection Fraction: The Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association. 2017;6(6):e006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Simone G, Gottdiener JS, Chinali M and Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. European heart journal. 2008;29:741–7. [DOI] [PubMed] [Google Scholar]
- 19.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J and Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. Journal of the American College of Cardiology. 2001;37:1042–8. [DOI] [PubMed] [Google Scholar]
- 20.Sarr SA, Babaka K, Mboup MC, Fall PD, Dia K, Bodian M, Ndiaye MB, Kane A, Diao M and Ba SA. [Clinical, electrocardiographic and echocardiographic aspects in elderly hypertensive patients in Senegal]. The Pan African medical journal. 2016;25:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neal WT, Mazur M, Bertoni AG, Bluemke DA, Al-Mallah MH, Lima JAC, Kitzman D and Soliman EZ. Electrocardiographic Predictors of Heart Failure With Reduced Versus Preserved Ejection Fraction: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehtonen AO, Puukka P, Varis J, Porthan K, Tikkanen JT, Nieminen MS, Huikuri HV, Anttila I, Nikus K, Kahonen M, Jula A and Niiranen TJ. Prevalence and prognosis of ECG abnormalities in normotensive and hypertensive individuals. Journal of hypertension. 2016;34:959–66. [DOI] [PubMed] [Google Scholar]
- 23.Stewart S, Libhaber E, Carrington M, Damasceno A, Abbasi H, Hansen C, Wilkinson D and Sliwa K. The clinical consequences and challenges of hypertension in urban-dwelling black Africans: insights from the Heart of Soweto Study. International journal of cardiology. 2011;146:22–7. [DOI] [PubMed] [Google Scholar]
- 24.Kloch-Badelek M, Kuznetsova T, Sakiewicz W, Tikhonoff V, Ryabikov A, Gonzalez A, Lopez B, Thijs L, Jin Y, Malyutina S, Stolarz-Skrzypek K, Casiglia E, Diez J, Narkiewicz K, Kawecka-Jaszcz K and Staessen JA. Prevalence of left ventricular diastolic dysfunction in European populations based on cross-validated diagnostic thresholds. Cardiovascular ultrasound. 2012;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadiri S. Tackling cardiovascular disease in Africa. BMJ (Clinical research ed). 2005;331:711–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jardim TV, Reiger S, Abrahams-Gessel S, Gomez-Olive FX, Wagner RG, Wade A, Bärnighausen TW, Salomon J, Tollman S and Gaziano TA. Hypertension management in a population of older adults in rural South Africa. Journal of hypertension. 2017;35:1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Olive FX, Montana L, Wagner RG, Kabudula CW, Rohr JK, Kahn K, Barnighausen T, Collinson M, Canning D, Gaziano T, Salomon JA, Payne CF, Wade A, Tollman SM and Berkman L. Cohort Profile: Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa (HAALSI). International journal of epidemiology. 2018;47:689–690j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerdts E, Okin PM, de Simone G, Cramariuc D, Wachtell K, Boman K and Devereux RB. Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension (Dallas, Tex : 1979). 2008;51:1109–14. [DOI] [PubMed] [Google Scholar]
- 29.Aro AL and Chugh SS. Clinical Diagnosis of Electrical Versus Anatomic Left Ventricular Hypertrophy: Prognostic and Therapeutic Implications. Circulation Arrhythmia and electrophysiology. 2016;9:e003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayanan K, Reinier K, Teodorescu C, Uy-Evanado A, Chugh H, Gunson K, Jui J and Chugh SS. Electrocardiographic versus echocardiographic left ventricular hypertrophy and sudden cardiac arrest in the community. Heart Rhythm. 2014;11:1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sliwa K, Lee GA, Carrington MJ, Obel P, Okreglicki A and Stewart S. Redefining the ECG in urban South Africans: electrocardiographic findings in heart disease-free Africans. International journal of cardiology. 2013;167:2204–9. [DOI] [PubMed] [Google Scholar]
- 32.Nel S, Nihoyannopoulos P, Libhaber E, Essop MR, Ferreira Dos Santos C, Matioda H, Waterworth C, Grinter S, Meel R and Peters F. Echocardiographic Indices of the Left and Right Heart in a Normal Black African Population. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2020;33:358–367. [DOI] [PubMed] [Google Scholar]
- 33.Asch FM, Miyoshi T, Addetia K, Citro R, Daimon M, Desale S, Fajardo PG, Kasliwal RR, Kirkpatrick JN, Monaghan MJ, Muraru D, Ogunyankin KO, Park SW, Ronderos RE, Sadeghpour A, Scalia GM, Takeuchi M, Tsang W, Tucay ES, Tude Rodrigues AC, Vivekanandan A, Zhang Y, Blitz A and Lang RM. Similarities and Differences in Left Ventricular Size and Function among Races and Nationalities: Results of the World Alliance Societies of Echocardiography Normal Values Study. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2019;32:1396–1406.e2. [DOI] [PubMed] [Google Scholar]
- 34.Cuspidi C, Rescaldani M, Sala C and Grassi G. Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. Journal of hypertension. 2014;32:16–25. [DOI] [PubMed] [Google Scholar]
- 35.Maugeri A, Hruskova J, Jakubik J, Barchitta M, Lo Re O, Kunzova S, Medina-Inojosa JR, Agodi A, Sciacca S and Vinciguerra M. Independent Effects of Hypertension and Obesity on Left Ventricular Mass and Geometry: Evidence from the Cardiovision 2030 Study. J Clin Med. 2019;8:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors encourage investigators interested in data sharing and collaboration to contact the corresponding author.