Abstract
Background
The global COVID-19 pandemic is currently underway. A massive worldwide vaccination campaign is still underway, representing the most promising weapon available to stop the pandemic.
Methods and Results
However, research continues to investigate the most effective drug treatments to reduce and avoid the most serious complications caused by COVID-19 infection. Recently, new evidence of good therapeutic efficacy against COVID-19 has emerged for the antiviral Remdesivir and the immunomodulatory Baricitinib, also in combination. The first one showed SARS-CoV-2 antireplicative activity, the second one useful to reduce the hyperinflammatory state caused by cytokine storm in the most severe phases of the infection.
Conclusions
In this short communication we describe the molecular pharmacological mechanisms and the latest evidence for the use of these therapeutic agents in the treatment of COVID-19 infection.
Keywords: COVID-19, SARS-CoV-2, Drugs, Baricitinib/Remdesivir
Introduction
COVID-19 pandemic
The current pandemic of coronavirus disease 2019 (COVID-19) is ongoing, to date it has caused about 120 Mln infected and 2.66 Mln deaths [1–3]. A massive COVID-19 vaccination campaign worldwide is underway representing the most important hope to permanently stop this pandemic [4]. Infection in most cases has an asymptomatic or mildly symptomatic course. The most common clinical manifestations of COVID-19 infection include loss of sense of smell, fatigue, fever, cough, and dyspnea [5–7]. In severe cases, there may be pulmonary, cardiac, hepatic, and neurological lesions caused by an abnormal and dysregulated response of the inflammatory/immune system induced by a massive and sudden release of proinflammatory mediators such as cytokines, chemokines [8–11]. The coronavirus SARS-CoV-2 penetrates into cells via the glycoprotein ACE-2, which is expressed in several tissues of the body [12, 13]. ACE2 plays a key role in the renin-angiotensin RAS system [14]. A specific antiviral treatment directed against SARS-CoV-2 has not yet been identified. During this time, several pharmacological treatments have been proposed, for COVID-19 infection, including anti-inflammatory/immunomodulatory agents, anticoagulants, convalescent plasma, and antivirals indicated for other diseases [15–18]. Among the various antivirals, there are protease inhibitors and nucleotide or nucleoside analogs that inhibit viral RNA synthesis. Among these is Remdesivir. Epidemiologic evidence associates Remdesivir with decreased numbers of hospitalizations from COVID-19 and reduced disease duration [19–21]. Among the various immunomodulants used proposed to treat the hyperinflammatory state induced by a cytokine storm, which occurs in the most severe stages of infection, good efficacy has been associated with the use of baricitinib, a pharmacologic agent indicated for the treatment of moderate to severe active phase rheumatoid arthritis [22, 23].
Baricitinib and Remdesivir
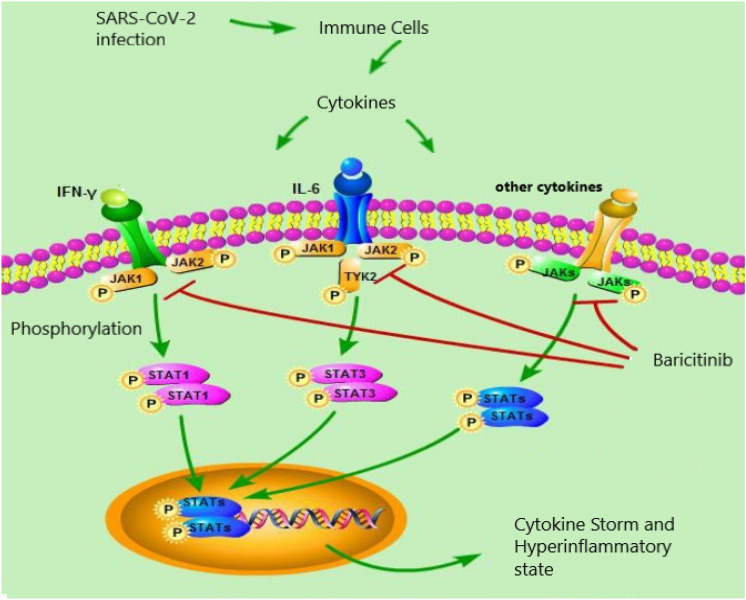
The pharmacological treatments proposed for COVID-19 infection are aimed at avoiding serious complications of the disease [24, 25]. Among immunomodulants, clinical evidence associates some efficacy to Baricitinib. Baricitinib is a drug indicated for the treatment of moderate to severe active phase rheumatoid arthritis in adult patients, the mechanism of action involves reversible inhibition of Janus Kinases (JAK), which are signal transducing enzymes induced by cytokines and growth factors involved in the inflammatory/immune response. JAKs are a family of enzymes with tyrosine kinase activity that mediate the transmission of signals induced by pro-inflammatory cytokines. The activated JAKs phosphorylate STAT, a transcription factor, which regulates the expression of proinflammatory genes (Fig. 1) [26]. This action of immunomodulation and control of inflammation may be of benefit in reducing the abnormal and dysregulated response that occurs in the advanced stages of COVID-19 disease, and has been seen to be responsible for severe organ injury. Recent evidence has also associated Baricitinib with an effect of inhibiting endocellular penetration of SARS-CoV-2. SARS-CoV-2 virus uses ACE2 as a cellular entry receptor. AP2-associated protein kinase-1 (AAK1) and cyclin G-associated kinase (GAK) are two key regulators of ACE2, mediating clathrin-dependent endocytosis [22]. Some evidence has shown that Baricitinib binds to AAK1 and GAK and blocks cellular penetration of SARS-CoV-2 [23]. Ultimately, Baricitinib is associated with a dual mechanism of anti-SARS-CoV-2 action, anti-inflammatory/immunomodulatory and antiviral. A study in an animal model demonstrated a major decrease in cytokines and chemokines in lung tissues, supporting the use of Baricitinib as a pharmacological treatment for the management of the hyperinflammatory state induced by the cytokine cascade in severe stages of COVID-19 infection [27] (Fig. 1).
Fig. 1.
Schematic representation mechanism of action Baricitinib: SARS-CoV-2 viral infection induces immune cells to release cytokines such as IFN-γ, IL-6 etc. Cytokines bind to receptors activating JAKs, which phosphorylate STATs. Activated STATs translocate into the nucleus and induce expression of cytokine-responsive genes inducing the cytokine storm that occurs in the most severe stages of COVID-19 infection
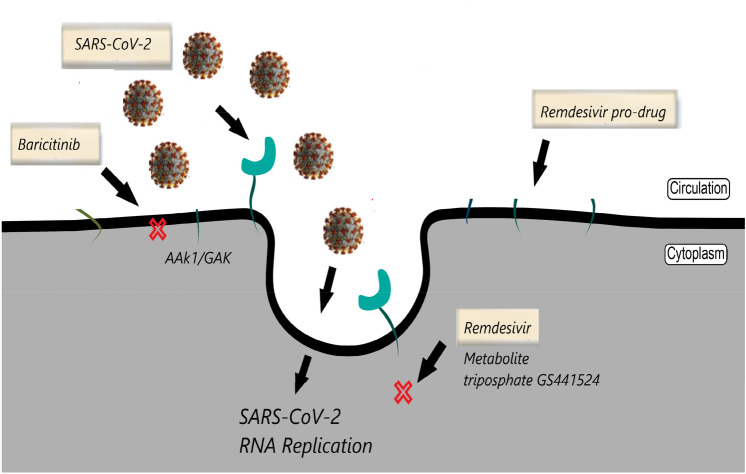
Several antiviral agents have been proposed to treat COVID-19 infection, most often showing conflicting and not fully elucidated clinical results. Evidence has associated some anti-SARS-CoV-2 efficacy with Remdesivir [28, 29]. Remdesivir is an antiviral pharmacological agent of the nucleotide analogue family, in vitro studies have shown some SARS-CoV-2 inhibition activity. Remdesivir is a pro-drug, which within cells is converted to the active metabolite nucleoside triphosphate (GS-443902), the active antiviral compound that acts through inhibition of viral RNA replication (Fig. 2). Several randomized trials have already been conducted in both hospitalized and non hospitalized patients. Recent studies associate Remdesivir with continuous and clinically significant improvements in COVID-19 [30] positive patients, resulting in reduced mortality and recovery time. While demonstrating some efficacy against SARS-CoV-2, therapeutic treatment with Remdesivir in COVID-19 patients requires additional data. Recently, evidence has emerged showing the Baricitinib/Remdesivir combination to be more effective than single administration of the two drugs. The pharmacological basis to explain these data, suggest a particular synergism, Baricitinib acts by inhibiting cell entry, Remdesivir prevents viral replication (Fig. 2). Thus, currently Baricitinib therapy is combined with antivirals such as Remdesivir, drug synergism appears to be beneficial in decreasing viral replication and viral load and regulating the cytokine storm [31]. A double-blind, randomized, placebo-controlled trial with a total of 1033 patients evaluated the combination of Baricitinib and Remdesivir in hospitalized adult COVID-19 patients. Results showed that the Baricitinib/Remdesivir combination was superior to single administration of Remdesivir in reducing COVID-19 hospitalization and disease time [32]. World Health Organization expert groups recommended mortality trials of four antiviral drugs—Remdesivir, hydroxychloroquine, lopinavir, and interferon beta-1a—in patients hospitalized with coronavirus disease 2019. At 405 hospitals in 30 countries, 11,330 adults underwent randomization; 2750 were assigned to receive Remdesivir, 954 to hydroxychloroquine, 1411 to lopinavir (without interferon), 2063 to interferon (including 651 to interferon plus lopinavir), and 4088 to no trial drug. These Remdesivir, hydroxychloroquine, lopinavir, and interferon regimens had little or no effect on hospitalized patients with Covid-19, as indicated by overall mortality, initiation of ventilation, and duration of hospital stay [33]. Another double-blind, randomized, placebo-controlled study of intravenous Remdesivir was conducted in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection. A total of 1062 patients underwent randomization. The Kaplan–Meier estimates of mortality were 6.7% with Remdesivir and 11.9% with placebo by day 15 and 11.4% with Remdesivir and 15.2% with placebo by day 29. The data show that Remdesivir was superior to placebo in shortening the time to recovery in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection [34].
Fig. 2.
Schematic representation of the SARS-CoV-2 antiviral mechanism of action of the Baricitinib/Remdesivir combination. Baricitinib binds to AAK1 and GAK and blocks cellular penetration of SARS-CoV-2. Remdesivir is activated in the triphosphate metabolite and inhibits viral replication
Discussions
In the fight against COVID-19 the main goals are to identify drug therapies capable of blocking the establishment of the infection. reduce the viral load and modulate the cytokine storm that induces the generalized hyperinflammatory response. SARS-CoV-2 infection is mainly characterized by two phases, the initial infection and viral replication, the cytokine storm and the abnormal inflammatory/immune response. The real challenge is to identify not only the most effective and safe treatments, but also the right timing of administration. Using an antiviral only in the late stages of infection might have less therapeutic efficacy, as might using an immunomodulatory in the early stages of infection where the immune system has to fight the viral infection. Evidence shows that the combination Baricitinib/Remdesivir seems to have positive effects in terms of reduction of hospitalization. The COVID-19 patient is a complex patient, the safety profile of the treatments used must be carefully monitored [35, 36]. Baracitinib at common doses can in some cases cause respiratory tract infections, with the risk of complicating the clinical scenario. Remdesivir is extensively metabolized to the pharmacologically active nucleoside triphosphate analog GS-443902 (formed intracellularly). The metabolic activation pathway involves hydrolysis operated by esterases, leading to the formation of an intermediate metabolite GS-704277. Cleavage of phosphoramidate followed by phosphorylation forms the active triphosphate GS-443902. Dephosphorylation of all phosphorylated metabolites can result in the formation of the nucleoside metabolite GS-441524, which cannot be efficiently rephosphorylated. Remdesivir is a substrate of enzymes such as CYP3A4, CYP2D6 and CYP2C8 involved in the metabolism of a large number of drugs used in the COVID-19 patient, and potential co-administration of inhibitors may lead to a potential increase in its levels. There are no particular drug interactions for the two drugs used in combination. Epidemiological studies associate Baricitinib/Remdesivir combination with greater efficacy against SARS-CoV-2 infection than single administration of Remdesivir, but more studies are needed to provide additional dose data and more appropriate timing of use.
Conclusion
The global COVID-19 pandemic is underway. A massive mass vaccination campaign has recently begun worldwide, yet research continues to identify the most effective treatments. Recent evidence demonstrates good efficacy in terms of reduced time to hospitalization and disease from COVID-19 to the Baricitinib/Remdesivir combination. Further clinical studies are needed to generate more evidence, such as the best timing and dosage of administration.
Author contributions
AV: Conceptualization, Writing—original draft, Methodology, Writing—original draft. FF: Writing—review and editing, Supervision, Validation. All authors read and approved the manuscript and all data were generated in-house and that no paper mill was used.
Funding
None.
Data availability
Full availability of data and materials.
Declarations
Conflict of interest
None of the authors have conflicts of interest to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
The authors consent to the publication of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Antonio Vitiello, Email: antonio.vitiello2@uslumbria1.it.
Francesco Ferrara, Email: f.ferrara@aslnapoli3sud.it, Email: ferrarafr@libero.it.
References
- 1.World health organization (WHO) (2021) https://www.who.int/emergencies/diseases/novelcoronavirus2019/situation-reports (Situation Reports March 2021). Accessed 31 Mar 2021
- 2.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2019;395(2020):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitiello A, Porta R, Pianesi L, Ferrara F. COVID-19 pandemic: vaccine and new monoclonal antibodies, point of view. Ir J Med Sci. 2021 doi: 10.1007/s11845-021-02584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus- infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019;382(2020):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorbalenya AE, et al. Severe acute respiratory syndrome-related coronavirus: the species and its viruses – a statement of the coronavirus study group. Microbiology. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- 8.Guarner J. Three emerging coronaviruses in two decades. Am J Clin Pathol. 2020;153:420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitiello A, Ferrara F. Colchicine and SARS-CoV-2: management of the hyperinflammatory state. Respir Med. 2021 doi: 10.1016/j.rmed.2021.106322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitiello A, LaPorta R, D’Aiuto V, Ferrara F. Pharmacological approach for the reduction of inflammatory and prothrombotic hyperactive state in COVID-19 positive patients by acting on complement cascade. Human Immunol. 2021 doi: 10.1016/j.humimm.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitiello A, Ferrara F, Pelliccia C, Granata G, La Porta R. Cytokine storm and colchicine potential role in fighting SARS-CoV-2 pneumonia. Ital J Med. 2020;14(2):88–94. [Google Scholar]
- 12.Vitiello A, Ferrara F. Correlation between renin-angiotensin system and severe acute respiratory syndrome coronavirus 2 infection: what do we know? Eur J Pharmacol. 2020;15(883):173373. doi: 10.1016/j.ejphar.2020.173373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitiello A, Pelliccia C, Ferrara F. Drugs acting on the renin–angiotensin system and SARS-CoV-2. Drug Discov Today. 2021 doi: 10.1016/j.drudis.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitiello A, Ferrara F. Therapeutic strategies for SARS-CoV-2 acting on ACE-2. Eur J Pharm Sci. 2021;1(156):105579. doi: 10.1016/j.ejps.2020.105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara F, Granata G, Pelliccia C, La Porta R, Vitiello A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2: anti-inflammatory and anti-fibrotic therapy could solve the lung complications of the infection? Eur J Clin Pharmacol. 2020;76(11):1615–1618. doi: 10.1007/s00228-020-02947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitiello A, Ferrara F. Pharmacological agents to therapeutic treatment of cardiac injury caused by Covid-19. Life Sci. 2020;1(262):118510. doi: 10.1016/j.lfs.2020.118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitiello A, La Porta R, Ferrara F. Sacubitril, valsartan and SARS-CoV-2. BMJ Evid Based Med. 2021 doi: 10.1136/bmjebm-2020-111497. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara F, Vitiello A. Efficacy of synthetic glucocorticoids in COVID-19 endothelites. Naunyn-Schmiedeberg Arch Pharmacol. 2021 doi: 10.1007/s00210-021-02049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorgensen SCJ, Kebriaei R, Dresser LD. Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacotherapy. 2020;40(7):659–671. doi: 10.1002/phar.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020;34:101615. doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitiello A, Ferrara F, La Porta R. Remdesivir and COVID-19 infection, therapeutic benefits or unnecessary risks? Ir J Med Sci. 2021 doi: 10.1007/s11845-020-02482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favalli EG, Biggioggero M, Maioli G, Caporali R. Baricitinib for COVID-19: a suitable treatment? Lancet Infect Dis. 2020;20(9):1012–1013. doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorgensen SCJ, Tse CLY, Burry L, Dresser LD. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacotherapy. 2020;40(8):843–856. doi: 10.1002/phar.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara F, Vitiello A. Potential pharmacological approach in the regulation of ACE-2 and DPP-IV in diabetic COVID-19 patient. Ital J Med. 2020 doi: 10.4081/itjm.2020.1435. [DOI] [Google Scholar]
- 25.Vitiello A, La Porta R, Ferrara F. Scientific hypothesis and rational pharmacological for the use of sacubitril/valsartan in cardiac damage caused by COVID-19. Med Hypotheses. 2021;147:110486. doi: 10.1016/j.mehy.2021.110486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titanji BK, Farley MM, Mehta A, Connor-Schuler R, Moanna A, Cribbs SK, et al. Use of Baricitinib in patients with moderate and severe COVID-19. Clin Infect Dis. 2020;29:ciaa879. doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoang TN, Pino M, Boddapati AK, Viox EG, Starke CE, Upadhyay AA, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021 doi: 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrara F, Porta R, D'Aiuto V, Vitiello A. Remdesivir and COVID-19. Ir J Med Sci. 2020;17:1–2. doi: 10.1007/s11845-020-02401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitiello A, Ferrara F. Remdesivir versus ritonavir/lopinavir in COVID-19 patients. Ir J Med Sci. 2020;18:1–2. doi: 10.1007/s11845-020-02440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCreary EK, Angus DC. Efficacy of Remdesivir in COVID-19. JAMA. 2020;324(11):1041–1042. doi: 10.1001/jama.2020.16337. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Zhang Y, Qiao W, Zhang J, Qi Z. Baricitinib a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19. Int Immunopharmacol. 2020;86:106749. doi: 10.1016/j.intimp.2020.106749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. ACTT-2 study group members Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO Solidarity Trial Consortium. Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S. Repurposed antiviral drugs for Covid-19 - Interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members Remdesivir for the treatment of Covid-19 - Final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrara F, Porta R, Santilli P, DAiuto V, Vitiello A, Are multiple sclerosis therapies safe in severe acute respiratory syndrome coronavirus 2 times? Indian J Pharmacol. 2020;52(5):441–442. doi: 10.4103/ijp.IJP_417_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitiello A, La Porta R, D’Aiuto V, et al. The risks of liver injury in COVID-19 patients and pharmacological management to reduce or prevent the damage induced. Egypt Liver J. 2021;11:11. doi: 10.1186/s43066-021-00082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Full availability of data and materials.