Abstract
We are considering a new COVID-19 model with an optimal control analysis when vaccination is present. Firstly, we formulate the vaccine-free model and present the associated mathematical results involved. Stability results for are shown. In addition, we frame the model with the vaccination class. We look at the mathematical results with the details of the vaccine model. Additionally, we are considering setting controls to minimize infection spread and control. We consider four different controls, such as prevention, vaccination control, rapid screening of people in the exposed category, and people who are identified as infected without screening. Using the suggested controls, we develop an optimal control model and derive mathematical results from it. In addition, the mathematical model with control and without control is resolved by the forward–backward Runge–Kutta method and presents the results graphically. The results obtained through optimal control suggest that controls can be useful for minimizing infected individuals and improving population health.
Keywords: Vaccination, Optimal control, COVID-19, Stability analysis, Real data, Numerical results
Introduction
The COVID-19 infection has affected most countries in the world and their economies. There are so many cases of infection, and the occurring of new infection waves that provided more cases than the previous wave. It is not yet sure, when this deadly infection will end from the population, although, many prevention mechanisms and other control measures were introduced to reduce the disease spread. Currently, most countries are experiencing the infection and death cases of COVID-19. Up to September 04, 2021, the total infected cases that reported were 220917130, that include the death cases which are 4571624 while the number of people that have been recovered from the COVID-19 infection are 197441726 [1]. Like other countries of the world, Pakistan is also facing this infection with so many infected and death cases. The total coronavirus cases reported in Pakistan till September 04, 2021, are 1175558, with 26,114 death cases, which makes Pakistan amongst at 31st position to be the affected country of the world. The rate of recovery from the infection in Pakistan is 2% and currently the recovered cases are 1,057,941. Now Pakistan is facing the fourth wave, and it is not determined yet when the peak of infection will occur [1]. The government of Pakistan is now again in action and making plan to seal some cities and other measures were suggested by implementing smart lockdown. In parts of Khyber Pakhtunkhwa and Punjab, the universities and schools, marriages halls etc. are closed for short time. The government policies together the implementations of Standard Operating Procedure (SOPs), proposed by World Health Organization (WHO), that is the vaccinations, prevention as using masks, washing hands, social distances can decrease the infection in population once it is followed properly.
The researchers, biologists and those working in medicine areas are continuously working to identify the effective vaccinations, preventions and treatment measure for the control of the coronavirus infection. Due to so many variants of this infection, the researchers are looking to have some more effective vaccine for the infection minimization. Literature suggests that many research articles in different perspectives are formulated and published regarding the infection minimization of this virus. If we speak out that related work on coronavirus through mathematical models, then we have a lot of models. The applications of mathematical models that can determine the peak of the infection and to determine the best possible way to control the infection is only be possible though mathematical models. For instant, the COVID-19 infection dynamics is studied through a mathematical model considered by the authors in [2], where the authors implemented different controls combinations to determine the best possible way for the infection minimization. The dynamics of COVID-19 infection using quarantined and isolation is proposed in [3]. The global perspective of the coronavirus model to the real data of Ghana, and its cost-effective analysis with environmental fluctuations, is considered in [4]. A nonlinear predictive control model and its management for the coronavirus infection is formulated in [5]. The use of drug resistance in coronavirus infection has been modeled and discussed in [6]. The spread tendency of the coronavirus infection in China, its modeling and prediction has been explored in [7]. The authors designed a mathematical model to understand the dynamical analysis of the COVID-19 infected cases in the Kingdom of Saudi Arabia has been investigated in [8]. A bacterial model to study the coronavirus infection with vaccine availability is considered in [9]. The modeling of coronavirus with vaccination, the efficacy of vaccine and its response has been discussed in [10]. The mathematical modeling of the SARS-COV-2 through an optimal control analysis is studied in [11]. The application of an optimal control modeling to the coronavirus infection using South African cases has been studied in [12]. The impact of control intervention on the modeling of coronavirus infection considering Malaysian infection cases is considered in [13]. There are many more interested work on the coronavirus infection and its dynamical analysis as well as controls of the infection, we refer the reader to see the above-mentioned works as well as [3], [14], [15], [16], [17] and the references therein.
With the above details investigations of the research articles on coronavirus infection, we aim to develop a new mathematical model for the transmission dynamics of coronavirus infection with vaccination and optimal control analysis. We considered the reported cases of COVID-19 from Pakistan and suggest a reasonable fitting to the cases. Further, we propose a set of controls combinations as describes already in details to study the control of the disease in Pakistan. We divide the rest of the work in this paper as follows: We study the modeling of the infection in Section “Formulations of the model” together with its stability analysis in details. We formulate a vaccination model and study the related work for the model in Section “Vaccination model”. In Section “Parameters estimations”, we investigate the data fitting results to the model. We formulate in details the modeling of the optimal control problems, and deriving the mathematics involved in Section “Optimal control Problem”. Numerical results are carried out in Section “Numerical simulation of optimal control model” with detailed discussion. Section “Conclusion” includes the details outcomes of the model and suggestions for infection control.
Formulations of the model
In order to formulate the model for the COVID-19, we describe the population of humans by and divide into five different compartments, the susceptible, , exposed , asymptomatic , infected and recovered, . The deterministic model that describes the transmission dynamics of COVID-19 in the community is shown by the following nonlinear differential equations:
| (1) |
In system (1), the growth rate of the susceptible population is shown by . The parameters for describe respectively, the rate at which the asymptomatic and symptomatic infected people transmits the COVID-19 infections to the susceptible individuals. Assuming that , is the contact rates of infected individuals with no clinical symptoms, and inflected individuals with clinical symptoms of COVID-19 respectively. The exposed individuals completing there incubations period shown by the parameter and becomes infected by joining the asymptomatic (with no clinical symptoms) class or symptomatic (with clinical symptoms) respectively, by , and . We do consider the death at the asymptomatic class, due to the reason, that it is difficult to identify that the person is infected with COVID-19, and the recovery is possible for the asymptomatic people and shown here in the model by . The symptomatic infected people die after getting the infection by while its recovery is shown by . The individuals in each compartment of the model die naturally at the rate .
Stability of the disease-free equilibria
Here, we explore the qualitative analysis of the model (1). For the qualitative analysis of the COVID-19 system given by (1), we need to study some basic properties of the model. The model analysis can be obtained in the feasible region given by the following:
The region given by is positively invariant (i.e., for every , the solutions start in remain in ), and attracts all the solutions of system (1). So the system given in (1) is mathematically and biologically well posed in the region .
Exploring the local asymptotic stability of the steady-states in the absence of infection, can be achieved by equal to zero the right side of the model (1), shown by:
The stability of the disease-free equilibrium can be explored considering the next generation method [18]. To use this technique, it is reasonable to consider the infected compartments of the system (1). The matrices and involves in the method are given by,
| (2) |
It follows from the above matrices
The following theorem demonstrates the local stability of the model (1).
Theorem 1
The COVID-19 model at the DFE is locally asymptotically stable if .
Proof
We begin by evaluating the system (1) at , and have
We have negative, while the other three eigenvalues can be computed through the following cubic equations:
The Routh–Hurwitz condition , can be verified as
So, upon satisfying the Routh–Hurwitz conditions, then, it will give three eigenvalues with negative real parts and hence the model (1) is locally asymptotically stable at the infection free equilibrium point for . □
Theorem 2
The model (1) at the disease-free equilibrium is globally asymptotically stable when .
Proof
To show the result, we begin by define the Lyapunov function given by
where , and are positive constants that can be obtained later. The derivative of is given by,
Using system (3), we have
Arranging the terms, then we have
Note than for every , and then choosing , , and , we have,
So, when , then , and when iff . Inserting in system (1) leads to for . Also, it can be shown that the largest invariant set is the disease-free equilibrium . Hence, follows the LaSalle’s Invariance Principle, the COVID-19 model given in (1) is globally asymptotically stable in if . □
Existence of equilibria
We obtain the conditions for the existence of the endemic equilibria. We denote it by , where
Using the above into
we get
where
Here and can be positive if . Further, , and if , then we can have a positive endemic equilibrium for the model (1), which shows the impossibility of backward bifurcation in the system (1).
Vaccination model
With the emergence of the new infectious diseases in the human population, the first attempt is to provide the prevention measure that introduced by the researcher’s to protect the people. Beside the prevention measure, the researchers look to discover the effective vaccination or treatment for the curtail of the infectious disease. It is known that no vaccine has 100% efficacy but the vaccine is effective and have been proven in the past against many disease. COVID-19 infection is one of these that gave more deaths and infected cases to the humans society and the passible way to get ride from this infection is the introducing of vaccine. The vaccine can reduce better the infection and can be useful in disease control. In this regard, we add the vaccine class to the model (1) and update the system as follows:
| (3) |
The susceptible individuals are vaccinated at the rate while the vaccine waning rate is . The parameter defines the vaccination efficacy against the COVID-19 infection in the susceptible vaccinated people. The rest of the parameters in (3) are the same as in (1) already defined above. The feasible region for the given vaccination model is given by
Stability of the disease-free equilibrium
A unique disease-free equilibrium for the model (3) exists and can be shown by
where
| (4) |
The basic reproduction number using the next generation approach in [18] for the system (3) can be obtained as follows:
where
where and are defined by (4).
Theorem 3
The COVID-19 vaccination model (3) at the disease-free equilibrium is locally asymptotically stable when .
Existence of endemic equilibria
We obtain the conditions for the existence of the endemic equilibria for the model (3). We denote it by , where
Substituting the above into
we get
| (5) |
where,
| (6) |
The quadratic equations (5) can be studied to identify the possible multiple equilibria for . The sign of can determine the possibility of equilibria and the bifurcation phenomenon. It is to be noted that is positive, and is positive when . The results can be summarized as follows:
Theorem 4
The COVID-19 system (3) with vaccination has:
- 1.
A unique endemic equilibrium exists if iff ,
- 2.
A unique endemic equilibrium exists when , and ,
- 3.
Two endemic equilibria exists if , and their respective discriminant is positive,
- 4.
No possibilities of equilibria otherwise.
Remark:
It can be observed from (1) of the result in 4 when , we have a positive endemic equilibrium. The third part (3) of Theorem 4 when gives the existence of possible backward bifurcation. To study the backward bifurcation for the vaccination model, we have to set , and then solving for the critical values of denoted by and having the results given by
by which we can see that a backward bifurcation can occur for such that . It should be noted that when there is a backward bifurcation phenomenon involved in the model, then it is hard to control the infection of the disease dynamics, since it requires to be reduce significantly the basic reproduction number of the model less than unity to get a unique disease-free equilibrium being stable globally asymptotically. Now, by discussing here that can we eradicate the backward bifurcation phenomenon from the given vaccination system?. For this, considering the model (3) when (perfect vaccine), the result below is claimed.
Theorem 5
The vaccination model given in (3) when , then the unique disease-free equilibrium of the model is globally asymptotically stable in whenever .
Proof
To show the above result, we need to define the Lyapunov function as follows:
The time differentiation of gives
Simplifying further, we have
Note that for every , and then choosing , , and , we have,
So, when , then , and iff . Inserting in system (3) leads to for . Also, it can be shown that the largest invariant set is the disease-free equilibrium . Hence, follows the LaSalle’s Invariance Principle, the COVID-19 model given in (3) is globally asymptotically stable in if . □
The above theorem indicates the impossibility of backward bifurcation in the vaccination model (3) when at . While the range in which the COVID-19 vaccination model give backward bifurcation phenomenon is .
Parameters estimations
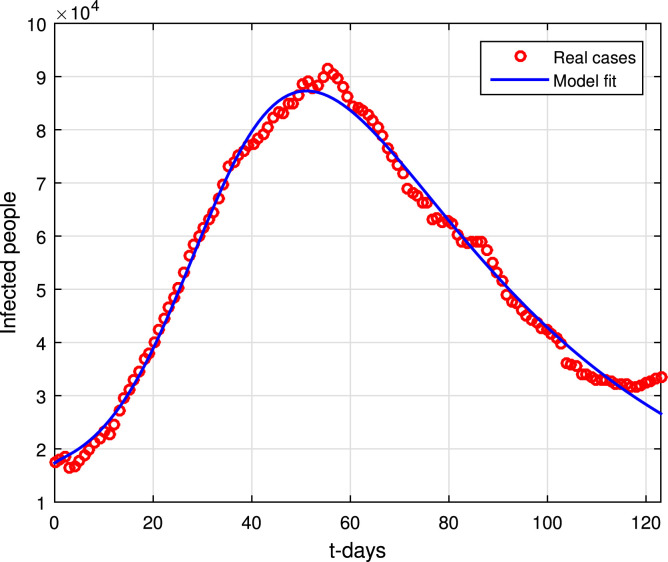
The estimations of the parameters for an epidemic model using the reported cases of an outbreak can determine effectively the disease actual dynamics and determine the present situation of the disease in a population. Here, we utilized the procedure of nonlinear least square curve fitting method for the proposed model (1) to have the realistic values of the parameters involved. In this regard, we have some of the parameters values that can be achieved or estimate, such include the natural death rate and birth rate given by and respectively. The reported cases here we consider are from March 06 to July 06, 2021 [1] for the data fitting. The cases are taken from the worldmeters and can be accessed easily though the link given in [1]. After utilizing the technique and the required result of data fitting has been depicted in Fig. 1. It can be seen that our model has best fitting to the reported data of COVID-19 infection in Pakistan. The parameters that obtained during the curve fitting analysis are shown in Table 1. For the given fitted data, the basic reproduction number is . The initial values of the parameters are considered as: is the initial population size of Pakistan, and in the disease absence, (subject to fitting). At march 06, the infected people are , (subject to data fitting) and .
Fig. 1.
Data versus model fitting, March, 06, 2021 till July 06, 2021.
Next, we performs the global sensitivity analysis for the COVID-19 infection model with and without vaccination.
Global sensitivity analysis
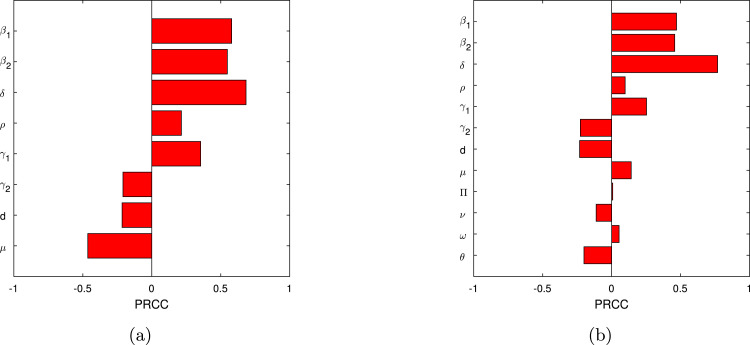
We perform the global sensitivity analysis in order to indemnify the parameters that has great impact on the basic reproduction number . Partial rank correlation coefficient (PRRC) is a useful method that provides the information about the parameters involved in the basic reproduction number and its impact that how the models parameters can increase or decrease the basic reproduction number. This method is reliable to determine monotonic and nonlinear association between the inputs and output results associated to the model. We give details of the sensitive parameters and the respective p-values are shown in Table 2 for model (1), while the graph is shown in Fig. 2. As it can be seen from Table 2 that one of the most sensitive parameters is , and the rest are , , , etc. The subgraph is given to shows the PRCC for , which describes that the vaccination can decrease better the basic reproduction number in comparison with model with no vaccination. The parameter that divide the individuals after completing the incubation period into asymptomatic and infected people. The rapid resting, and to identify the individuals with asymptomatic and symptomatic should be treated with care. This will decrease the infection spread further. Similarly, the parameters and can reduce the infection once the SOPs of WHO are followed with care (see Table 2).
Fig. 2.
Significance of parameters on and using PRCC for model (1), (3). (a) Reproduction number , Reproduction number .
Table 2.
PRCC values of with their p-values.
| Symbol | Definition | PRCC | p-values |
|---|---|---|---|
| Natural death rate | −0.4868 | 0.0000 | |
| Contact rate with no clinical symptoms | 0.5713 | 0.0000 | |
| Contact rate with clinical symptoms | 0.5473 | 0.0000 | |
| Incubation rate | 0.6228 | 0.0000 | |
| Proportion rate | 0.1603 | 0.3791 | |
| Recovery of asymptomatic individuals | 0.3688 | 0.0000 | |
| Recovery of symptomatic individuals | −0.2247 | 0.0000 | |
| Death rate of symptomatic individuals via infection | −0.2069 | 0.0000 |
Optimal control problem
We apply the optimal control theory to the COVID-19 model given in (3). We consider four different controls to minimize the infection of the coronavirus and their further spread in the community. These controls can be defined as: The control variable is defined to be the prevention/isolation control, minimizing the contact among the healthy and infected people, washing hands regularly, using sanitizers and masks. Further, avoid gathering and to restrict their travel where the cases are high. The second control is the vaccination control, all the individuals possibly to be vaccinated in order to reduce the spread further. The vaccination can best decrease the infection and its further spread, so it is suggested that the individuals should get vaccine to reduce the further risk. The control denotes the rapid testing of the individuals in the exposed stage, and to identify the asymptomatic and asymptomatic individuals. Upon identification through testing, the individuals should be isolated or restricted to their home etc to minimize the infection further. The control variable represents, the individuals that are not tested yet but identified the patients of COVID-19, either asymptomatic or asymptomatic, can be treated and should be restricted to their places or hospitals, quarantines, etc. So, should be regarded as a treatment control, while and should be considered the rate at which the asymptomatic and symptomatic individuals are treated. The above discussion, can leads to the following system of optimal control problem:
| (7) |
The state variables are non-negative with non-negative initial conditions. The main purpose is to minimize the function given by,
| (8) |
subject to the control system (7). The constants for describe the weight or the balancing constants, and is the final time. Here, the quadratic control functions is implemented because the controls cost of intervention is nonlinear. It means that there is no linear relationship among the cost of intervention among infected people and effects of intervention. The controls defined above are Lebesgue integrable functions and are bounded. We seek to have an optimal controls for , such that
where denoted to be the control set,
| (9) |
Characterization of an optimal control
The necessary conditions that an optimal controls should satisfy comes from the theory of Pontryagin’s Maximum Principle [20]. This principal converts the system (7), (8) into a problem of minimizing pointwise a Hamiltonian with respect to the controls . First, we have to write the Hamiltonian , given by
| (10) |
where for denote the adjoint variables associated to the state variables and . The system of adjoint equations can be obtained using the appropriate partial derivatives of with respected to the state variables.
Theorem 6
The given optimal controls and solutions of the control system (7) that minimizes over . Then there exists adjoint variables satisfying
(11) with the transversality conditions,
(12) The optimality condition is given by
(13) Furthermore, we have the controls ,
(14)
Proof
The results from Fleming and Rishel [21] gives the guarantee about the existence of an optimal control problem. The system governing the adjoint variables are achieved upon differentiation of , evaluated at the optimal controls. So, the adjoint system can be written as,
(15)
Numerical simulation of optimal control model
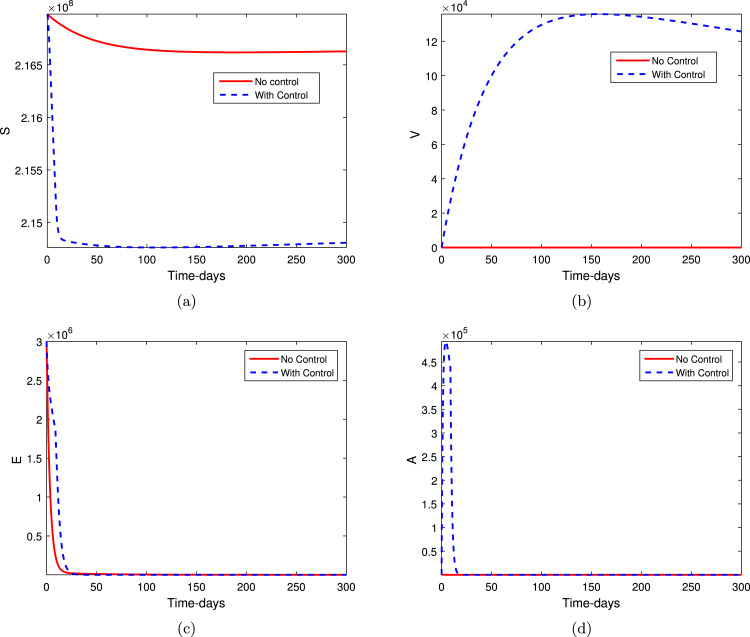
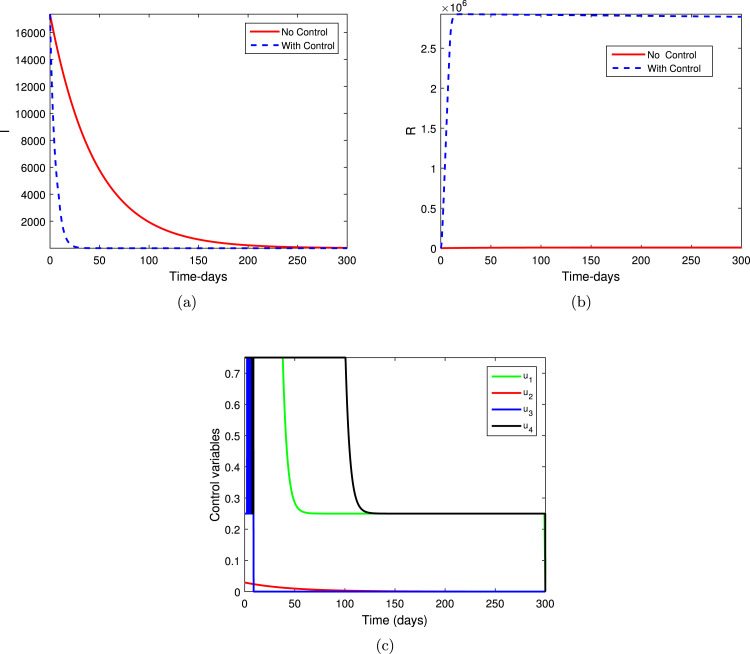
The optimal control problem (7) together with adjoint equation (15) and optimal control characterization (14) have been solved numerically and the results are obtained graphically. In simulations of the optimal control problem, the time unit is considered in days. The parameters values are taken from the system 1, where the parameters values of vaccination model are subjected to optimal control simulations are: , , , , . The numerical value of the weight constants are , , , , , , and . The initial values of the variables in simulations are considered to be , , , , , . We assumed that the initial values for vaccination and recovery is null, as there is no vaccinated or recovered individuals. The Runge–Kutta order four scheme has been instrumented to obtain the graphical results shown in Fig. 3, Fig. 4. The graphical results for an optimal control problem in comparison with no control system have been obtained with the realistic parameters values that shown in Table 1. It can be observed from the given graphical results that the controls are much effective for the disease eliminations. The subgraphs in Fig. 3 shows the dynamics of susceptible individuals, vaccinated, exposed and asymptomatic individuals, while Fig. 4 shows the infected, recovered and the control characterization. It can be seen that the controls considered in this numerical simulations are effective to decease the population of infected individuals and maximize the vacillated and recovered individuals. The social distances, wearing masks, using sanitizer to wash hands regularly, the vaccination to the individuals, the rapid testing, and the possible early treatment can best reduce the infection of coronavirus in the community. It is observed from our optimal controls results that the infection cases are decreasing faster by implementing the controls as defined above. In the future, the infected cases of COVID-19 and its burden on the population will decreases if the government should implement the SOPs and restrict the individuals to the risky places. As it is well-known that the COVID-19 infection provided great loss to the humans society and many developing countries are facing a big financial loss to his economy. Therefore, proper vaccinations of the individuals and preventions of the individuals from the infections should be a priority for the less developed countries in order to sustain their populations and its economy.
Table 1.
Estimated and fitted parameters values of the model.
| Symbol | Definition | Value | Source |
|---|---|---|---|
| Birth rate | Estimated | ||
| Natural death rate | [19] | ||
| Contact rate with no clinical symptoms | 0.9567 | Fitted | |
| Contact rate with clinical symptoms | 0.500 | Fitted | |
| Incubation rate | 0.2933 | Fitted | |
| Proportion rate | 0.9988 | Fitted | |
| Recovery of asymptomatic individuals | 0.6583 | Fitted | |
| Recovery of symptomatic individuals | 0.0100 | Fitted | |
| Death rate of symptomatic individuals via infection | 0.0119 | Fitted |
Fig. 3.
Numerical simulation of an optimal control system.
Fig. 4.
Numerical simulation of an optimal control system.
Conclusion
We formulated a mathematical model for the coronavirus infection with optimal control theory. For any disease in the humans populations need to be controls with the available controls that have been suggested by the researchers and experts working on the disease control and preventions. In light of the possible controls that are considered to be effective have been implemented and formulated an optimal control model. The four control variables have been considered in the form of preventions, using the standard principles suggested by the World Health Organization (WHO), the vaccination to the individuals, the rapid testing, and the early treatment to the infected people. We first considered the model without intervention and presented the model results in details. We presented the stability of the model at disease-free equilibrium with no control and found that the system is locally asymptotically stable when while it is globally asymptotically stable when . We then used the model and shown his fitting to real data reported from Pakistan for the third wave. We used the PRCC to obtained the global sensitivity analysis of the model. Some important parameters and its effect on the system have been shown graphically. We formulated a vaccination model by adding the vaccine class and some more parameters that important for vaccinations of the susceptible individuals. We also presented the useful results for the vaccination model in order to have no possibilities of backward bifurcation. When the vaccine is perfect then the model with vaccine is globally asymptotically stable whenever . The optimal control system is generated using four different controls and solved the optimality system and achieved the desired results that were necessary for the optimal control problem. The optimal control system in connection with on control together with optimal control characterization, and the adjoint equations, we obtained the results graphically. The archived results indicate the suggestions of the WHO, and other controls in the form of vaccination, rapid testing, and the early treatment to the infective can reduce better the infection.
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province (Grant No.: LQY18G030001). The authors acknowledge the support and funding of Research Center for Advanced Material Science (RCAMS) at King Khalid University through Grant No. RCAMS/KKU/009-21.
References
- 1.Coronavirus cases. https://www.worldometers.info/coronavirus/.
- 2.Ullah Saif, Khan Muhammad Altaf. Modeling the impact of non-pharmaceutical interventions on the dynamics of novel coronavirus with optimal control analysis with a case study. Chaos Solitons Fractals. 2020;139 doi: 10.1016/j.chaos.2020.110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan Muhammad Altaf, Atangana Abdon, Alzahrani Ebraheem, et al. The dynamics of COVID-19 with quarantined and isolation. Adv Difference Equ. 2020;2020(1):1–22. doi: 10.1186/s13662-020-02882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asamoah Joshua Kiddy K, Owusu Mark A, Jin Zhen, Oduro FT, Abidemi Afeez, Gyasi Esther Opoku. Global stability and cost-effectiveness analysis of COVID-19 considering the impact of the environment: using data from Ghana. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Péni Tamás, Csutak Balázs, Szederkényi Gábor, Röst Gergely. Nonlinear model predictive control with logic constraints for COVID-19 management. Nonlinear Dynam. 2020;102(4):1965–1986. doi: 10.1007/s11071-020-05980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matouk A.E. Complex dynamics in susceptible-infected models for COVID-19 with multi-drug resistance. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Deshun, Duan Li, Xiong Jianyi, Wang Daping. Modeling and forecasting the spread tendency of the COVID-19 in China. Adv Difference Equ. 2020;2020(1):1–16. doi: 10.1186/s13662-020-02940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alshammari Fehaid Salem. A mathematical model to investigate the transmission of COVID-19 in the Kingdom of Saudi Arabia. Comput Math Methods Med. 2020;2020 doi: 10.1155/2020/9136157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar Pushpendra, Erturk Vedat Suat, Murillo-Arcila Marina. A new fractional mathematical modelling of COVID-19 with the availability of vaccine. Results Phys. 2021;24 doi: 10.1016/j.rinp.2021.104213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acuña Zegarra Manuel Adrian, Díaz-Infante Saúl, Baca-Carrasco David, Olmos-Liceaga Daniel. COVID-19 Optimal vaccination policies: a modeling study on efficacy, natural and vaccine-induced immunity responses. Math Biosci. 2021;337 doi: 10.1016/j.mbs.2021.108614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asamoah Joshua Kiddy K., Bornaa C.S., Seidu Baba, Jin Zhen. Mathematical analysis of the effects of controls on transmission dynamics of SARS-CoV-2. Alex Eng J. 2020;59(6):5069–5078. [Google Scholar]
- 12.Gatyeni SP, Chukwu CW, Chirove Faraimunashe, Nyabadza F, et al. 2021. Application of optimal control to the dynamics of COVID-19 disease in South Africa; pp. 2008–2020. MedRxiv. Cold Spring Harbor Laboratory Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abidemi Afeez, Zainuddin Zaitul Marlizawati, Aziz Nur Arina Bazilah. Impact of control interventions on COVID-19 population dynamics in Malaysia: a mathematical study. Eur Phys J Plus. 2021;136(2):1–35. doi: 10.1140/epjp/s13360-021-01205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araz Seda İğret. Analysis of a Covid-19 model: optimal control, stability and simulations. Alex Eng J. 2021;60(1):647–658. [Google Scholar]
- 15.Asamoah Joshua Kiddy K, Jin Zhen, Sun Gui-Quan, Seidu Baba, Yankson Ernest, Abidemi Afeez, et al. Sensitivity assessment and optimal economic evaluation of a new COVID-19 compartmental epidemic model with control interventions. Chaos Solitons Fractals. 2021;146 doi: 10.1016/j.chaos.2021.110885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razzaq Oyoon Abdul, Rehman Daniyal Ur, Khan Najeeb Alam, Ahmadian Ali, Ferrara Massimiliano. Optimal surveillance mitigation of COVID’19 disease outbreak: Fractional order optimal control of compartment model. Results Phys. 2021;20 doi: 10.1016/j.rinp.2020.103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atangana Abdon. Modelling the spread of COVID-19 with new fractal-fractional operators: can the lockdown save mankind before vaccination? Chaos Solitons Fractals. 2020;136 doi: 10.1016/j.chaos.2020.109860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van den Driessche Pauline, Watmough James. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci. 2002;180(1–2):29–48. doi: 10.1016/s0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 19.Pakistan population. pp. 1950–2021. https://www.worldometers.info/worldpopulation/pakistan-population/.
- 20.Pontryagin Lev Semenovich. CRC Press; 1987. Mathematical theory of optimal processes. [Google Scholar]
- 21.Fleming Wendell H., Rishel Raymond W. Springer Science & Business Media; 2012. Deterministic and stochastic optimal control. Vol. 1. [Google Scholar]