Abstract
In mammals, plasma concentration of amino acids is affected by nutritional or pathological conditions. It has been well established that nutrients, and particularly amino acids, are involved in the control of gene expression. Here we examined the molecular mechanisms involved in the regulation of CHOP (a CCAAT/enhancer-binding protein [C/EBP]-related gene) expression upon amino acid limitation. We have previously shown that regulation of CHOP mRNA expression by amino acid concentration has both transcriptional and posttranscriptional components. We report the analysis of cis- and trans-acting elements involved in the transcriptional activation of the human CHOP gene by leucine starvation. Using a transient expression assay, we show that a cis-positive element is essential for amino acid regulation of the CHOP promoter. This sequence is the first described that can regulate a basal promoter in response to starvation for several individual amino acids and therefore can be called an amino acid response element (AARE). In addition, we show that the CHOP AARE is related to C/EBP and ATF/CRE binding sites and binds in vitro the activating transcription factor 2 (ATF-2) in starved and unstarved conditions. Using ATF-2-deficient mouse embryonic fibroblasts and an ATF-2-dominant negative mutant, we demonstrate that expression of this transcription factor is essential for the transcriptional activation of CHOP by leucine starvation. Altogether, these results suggest that ATF-2 may be a member of a cascade of molecular events by which the cellular concentration of amino acids can regulate mammalian gene expression.
The control of gene expression in multicellular organisms differs in many aspects from that operating in single-cell organisms and involves complex interactions of hormonal, neuronal, and nutritional factors. Although not as widely appreciated as the other factors, nutritional and metabolic signals play an important role in controlling gene expression in mammalian cells. It has been shown that major (carbohydrates, fatty acids, and sterols) or minor (minerals and vitamins) dietary constituents participate in the regulation of gene expression in response to nutritional changes (16, 20, 54, 60). Much less is known about the role of amino acids in the control of gene expression. The relative concentrations of amino acids are altered in response to various forms of stresses, such as sepsis, fevers, thermal burns, or malnutrition. For example, alteration of the amino acid concentration has been reported when there is a deficiency of any one of the essential amino acids, a dietary imbalance of amino acids, or an insufficient intake of protein (3).
The molecular mechanisms involved in the control of gene expression in response to amino acid availability have been extensively studied in yeast (24). In addition to specific controls of genes involved in the synthesis of individual amino acids, the yeast employs a general control process whereby multiple genes in nine different biosynthetic pathways are regulated by starvation of the cell for a single amino acid (25, 26). In mammalian cells, a few examples of enzymes, transporters, and mRNAs that are regulated by amino acid availability have been reported (32, 35). At the molecular level, the current understanding of amino acid-dependent control of gene expression is limited. It was reported that up-regulation of asparagine synthetase mRNA in response to amino acid limitation involves both transcriptional and posttranscriptional components (18). These authors defined a region of the asparagine synthetase promoter involved in transcriptional regulation in response to amino acid starvation but did not demonstrate transfer of amino acid responsiveness to a heterologous promoter.
Among the amino acid-regulated genes in mammalian cells, CHOP (also called GADD153) expression exhibits the greatest induction level (40). CHOP encodes a small nuclear protein that regulates certain aspects of the cell response to stress (62, 65). The induction of CHOP expression by stress agents is generally linked to the activation of an endoplasmic reticulum stress (ER stress), manifested as the accumulation of malfolded proteins in the endoplasmic reticulum (unfolded protein response) (61, 65). CHOP protein belongs to the CCAAT/enhancer-binding protein (C/EBP) family of transcription factors that have been implicated in the regulation of processes relevant to energy metabolism (43), cellular proliferation, and differentiation and expression of cell type-specific genes (7, 9, 56). By forming heterodimers with the members of the C/EBP family, CHOP protein can influence gene expression both as a dominant negative regulator of C/EBP binding to one class of DNA targets and by directing CHOP-C/EBP heterodimers to other sequences (5, 6, 14, 51, 55, 62).
It has been shown previously that leucine limitation in human cell lines leads to induction of CHOP mRNA and protein in a dose-dependent manner (8). In addition, the regulation of CHOP mRNA expression by leucine has both transcriptional and posttranscriptional components (8). The signaling pathway that links amino acid deprivation to CHOP expression is not known. However, it has been demonstrated that amino acid starvation regulates the expression of CHOP through a specific pathway that is distinct from the ER stress signaling cascade (31). In the present study, we report the characterization of cis- and trans-acting elements involved in the transcriptional activation of CHOP by amino acid deprivation. By deletion and mutation analysis of the CHOP promoter region, we characterize an amino acid response element (AARE). In addition, we show that the AARE binds activating transcription factor 2 in vitro and that this transcription factor is essential for the transcriptional activation of CHOP by leucine starvation.
MATERIALS AND METHODS
Cell culture and treatment conditions.
Cells were cultured at 37°C in Dulbecco's modified Eagle's medium F12 (DMEM F12) (Sigma) containing 10% (mouse embryonic fibroblasts [MEF], HeLa, and HepG2) or 20% (Caco-2) fetal bovine serum. When indicated, DMEM F12 lacking leucine was used. For other amino acid starvation experiments, MEM medium (Life Technologies, Inc.) was used. In all experiments involving amino acid starvation, 10% dialyzed calf serum was used. MEF deficient in C/EBPβ were a gift of David Ron (65). MEF deficient in ATF-2 were produced from decapitated, eviscerated day 14.5 ATF-20/0 embryos (39) using a 3T3 protocol until cells passed through crisis, typically by passage 18 (53).
DNA transfection and luciferase assay.
Cells were plated in 12-well dishes and transfected by the calcium phosphate coprecipitation method as described previously (8). Two micrograms of luciferase plasmid was transfected into the cells along with 0.1 μg of pCMV-βGal, a plasmid carrying the bacterial β-galactosidase gene fused to the human cytomegalovirus immediate-early enhancer-promoter region, as an internal control. In the experiments using cDNA expression plasmids, a mixture containing 1 μg of luciferase plasmid, 1 μg of cDNA expression plasmid, and 0.05 μg of pCMV-βGal was transfected in the cells. The total amount of plasmid DNA was adjusted to 2 μg by the addition of the control plasmid lacking the cDNA to be expressed. Cells were then exposed to the precipitate for 16 h, washed twice in phosphate-buffered saline (PBS), and then incubated with DMEM F12 containing 10% calf serum. Twenty-four hours after transfection, cells were starved for 16 h. After starvation, cells were harvested in 150 μl of lysis buffer (Promega) and centrifuged at 13,000 × g for 2 min. Twenty microliters of the supernatant was assayed for luciferase activity (PRODEMAT, Anduze, France). β-Galactosidase activity was measured as described previously (23). Relative luciferase activity was given as the ratio of relative light units to relative β-galactosidase units. All values are the means calculated from the results of at least three independent experiments.
RNA isolation and Northern blot analysis.
Total RNA was prepared as previously described (12). Northern blots were performed according to the procedure of Sambrook et al. (48). The membranes were UV cross-linked, and then prehybridization was carried out for 2 h at 55°C in 50% formamide–6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–5× Denhardt's reagent–0.5% sodium dodecyl sulfate (SDS)–0.1 mg of sonicated salmon sperm DNA/ml–10 μg of yeast tRNA/ml. The human CHOP cDNA (BH1), generously provided by N. J. Holbrook (46), was used as a probe. BH1 plasmid was linearized by PstI, and 32P-riboprobes were synthesized (48) using T7 RNA polymerase (Promega). Hybridization was carried out for 16 h at 55°C. The membranes were washed for 15 min at 55°C successively in 2× SSC containing 0.1% SDS, 0.5× SSC containing 0.1% SDS, and 0.1× SSC containing 0.1% SDS. Labeled bands were detected by autoradiography. Autoradiogram signals were visualized by using a PhosphorImager and IMAGEQUANT software (Molecular Dynamics). To control for variation in either the amount of RNA in different samples or loading errors, all blots were rehybridized with a DNA probe corresponding to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Relative CHOP mRNA was determined as the ratio of CHOP mRNA to GAPDH mRNA.
Antibodies.
Anti-C/EBPβ serum was generously provided by U. Schiebler. Anti-ATF-4 serum was a gift from D. Ron. Anti-ATF-1 (catalog number sc-241X), anti-ATF-2 (catalog number sc-6233X), anti-ATF-3 (catalog number sc-188X), and anti-c-Jun (catalog number sc-1694X) were purchased from Santa Cruz Biotechnologies.
Nuclear extract preparation.
Nuclear extracts were prepared from HeLa cells plated in 150-mm-diameter dishes. Cells were washed twice with ice-cold PBS, harvested with a rubber policeman, and centrifuged at 4°C. Cell pellets were resuspended in 10 volumes of ice-cold lysis buffer (20 mM HEPES [pH 7.9], 10 mM KCl, 3 mM MgCl2, 0.5 mM EDTA, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 1 μg of leupeptin/ml), incubated on ice for 5 min, and centrifuged 5 min at 2,000 × g. Nuclear pellets were resuspended in 2 volumes of ice-cold extract buffer (20 mM HEPES [pH 7.9], 450 mM KCl, 3 mM MgCl2, 0.5 mM EDTA, 25% glycerol, 1 mM DTT, 1 mM PMSF, 1 μg of leupeptin/ml, 0.5 mM spermidine, 0.15 mM spermine) and incubated on ice for 30 min. After centrifugation for 10 min at 14,000 × g, the supernatant was dialyzed for two 2-h periods against 100 volumes of dialysis buffer (20 mM HEPES [pH 7.9], 50 mM KCl, 0.2 mM EDTA, 10% glycerol, 1 mM DTT, 0.5 mM PMSF), frozen, and stored at −80°C.
Oligonucleotides.
Oligonucleotides were from Eurogentec. When double-stranded oligonucleotides were required, equal numbers of moles of complementary strands were heated to 90°C for 1 min and annealed by slow cooling to room temperature.
Gel mobility shift assays.
Probes for the gel mobility shift assay were obtained by labeling synthetic double-stranded oligonucleotides with T4 polynucleotide kinase (Eurogentec). HeLa nuclear extracts (5 μg) were preincubated for 10 min on ice in the presence of 3 μg of poly(dI-dC) in a reaction mixture containing 25 mM HEPES (pH 7.9), 60 mM KCl, 2.5 mM MgCl2, 0.1 mM EDTA, 0.75 mM DTT, 1 mM PMSF, and 5% glycerol. Fifty femtomoles (i.e., 100,000 cpm) of end-labeled oligonucleotides was then added and incubated for a further 10 min. Competition for specific complex formation was performed by preincubation of the extract with a 25-, 50-, or 100-fold molar excess of the unlabeled competitors. To test the effect of specific antibodies, 1 μl of antiserum (see above) was added to the incubation mixture on ice 2 h prior to the addition of the labeled probe. The samples were loaded onto a prerun, 16-cm-long, 1.5-mm-thick 4% acrylamide-bisacrylamide (29:1) gel, prepared in 1× TGE (25 mM Tris base, 190 mM glycine, 1 mM EDTA [pH 8.5]). Electrophoresis was carried out at 240 V in 1× TGE for 2 h at 4°C. Radioactive bands were visualized by using a PhosphorImager and IMAGEQUANT software (Molecular Dynamics). Each mobility shift experiment was repeated three times to confirm the reproducibility of the results.
Plasmid constructions.
All constructs containing deletions in the CHOP promoter were generated by PCR from cloned genomic DNA, using Pfu polymerase (Stratagene), primers, and antisense primers containing appropriate restriction sites at their 5′ end. Amplified fragments were then cloned into the pGL3-basic reporter construct (Promega) using the corresponding restriction sites. For 5′ CHOP promoter deletions (pCHOP-LUC series [see Fig. 1]), XhoI-ended primers and HindIII-ended antisense primers were used. For internal CHOP promoter deletions (pCHOP-LUCΔ series [see Fig. 2]), fragments of the 5′ part of the CHOP promoter were generated by using SstI-ended primers and MluI-ended antisense primers and fragments of the 3′ part by using XhoI-ended primers and HindIII-ended antisense primers.
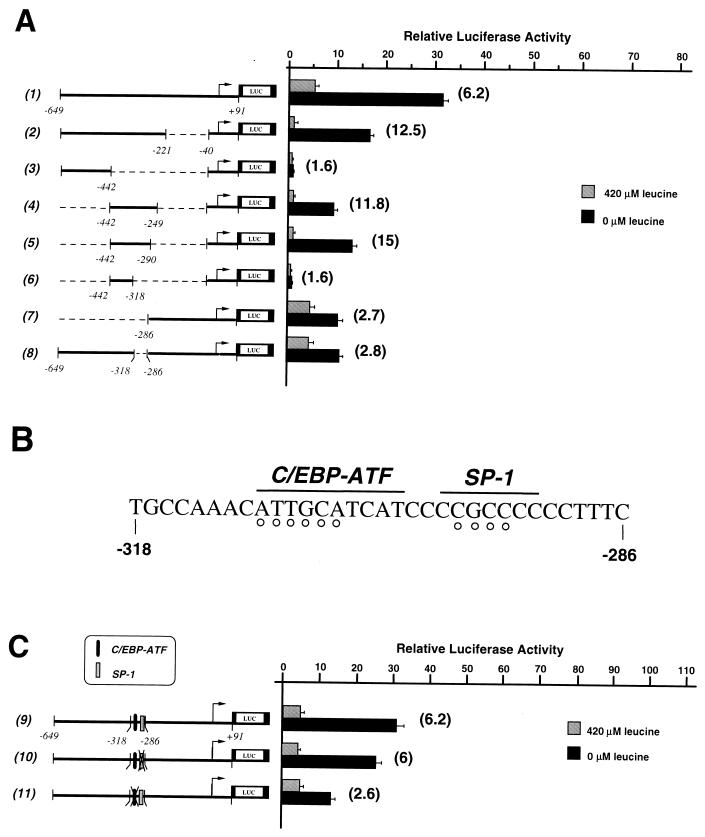
FIG. 1.
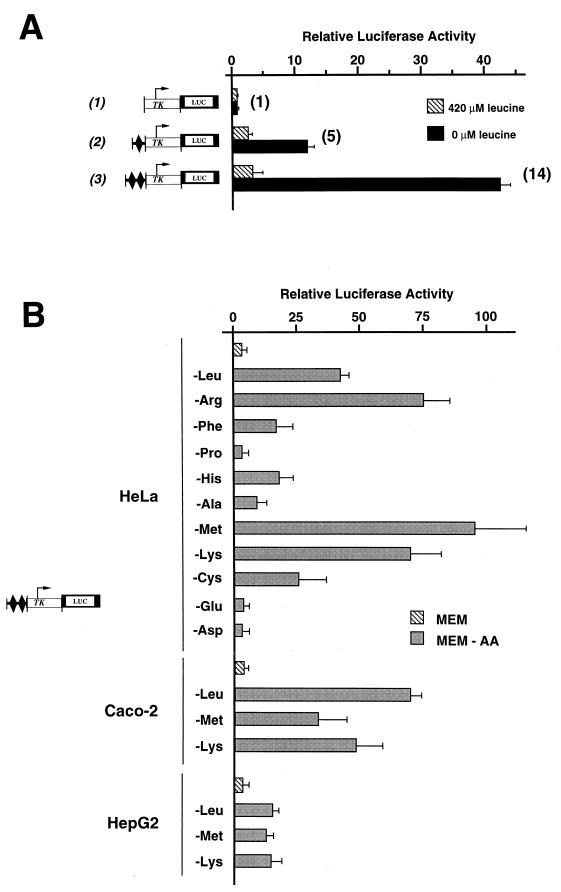
Effect of leucine limitation on 5′ deletions of the CHOP promoter. HeLa cells were transiently transfected with LUC constructs containing progressive 5′ deletions of the CHOP promoter, as described in Materials and Methods. Twenty-four hours after transfection, cells were incubated for 16 h in DMEM F12 (420 μM leucine) or in DMEM F12 lacking leucine (0 μM leucine) and then were harvested for preparation of cell extracts and determination of LUC activity. Relative LUC activities were determined as described in Materials and Methods. The relative fold induction, defined as the ratio of the relative LUC activity of leucine-starved cells to unstarved cells, is indicated in parentheses to the right of the bars.
FIG. 2.
Identification of an upstream cis-positive element involved in the activation of CHOP transcription by leucine starvation. HeLa cells were transiently transfected with LUC constructs containing internal 5′ deletions (A) or 5′ mutations (C) of the CHOP promoter as described in Materials and Methods. The experiments were carried out as described in the legend to Fig. 1. The relative fold induction, defined as the ratio of the relative LUC activity of leucine-starved cells to unstarved cells, is indicated in parentheses to the right of the bars. (B) Nucleotide sequence from −318 to −286 relative to the start site of CHOP transcription. The positions of the SP-1 and composite C/EBP-ATF sites are indicated. The open circles represent the positions of substitution mutations in either the SP-1 site or the C/EBP-ATF site.
The 5′ mutant promoter constructs (pCHOP-LUC-mt series [see Fig. 2C]) were generated by inserting wild-type or mutated oligonucleotides (−318 to −286; double-stranded form with MluI-XhoI-compatible ends [see Fig. 2B]) between the MluI and XhoI sites in the pCHOP-LUCΔ−318/−286 plasmid. Mutations in the 5′ CHOP sequence were made by substituting TAGA for GCCC (SP-1 mutant) or CAGATC for ATTGCA (C/EBP-ATF mutant) in both the sense and antisense orientations to create pCHOP-LUC-mt1 and pCHOP-LUC-mt2 constructs, respectively.
TATATK-LUC plasmid containing the minimal herpes simplex virus promoter for thymidine kinase (−40 to +50) was generated by PCR from cloned genomic DNA, using XhoI-ended primers and HindIII-ended antisense primers. The amplified fragment was then cloned into pGL3 using the XhoI and HindIII restriction sites. The 1XAARECHOP-TATATK-LUC mutant series was made by inserting wild-type or mutated oligonucleotides (−313 to −295; double-stranded form with SstI-XhoI-compatible ends [see Fig. 4A]) between the SstI and XhoI sites of TATATK-LUC plasmid. The mutation series in the CHOP AARE sequence was made by substituting CCT for AAC (mt3), CAG for ATT (mt4), ATG for GCA (mt5), GAC for TCA (mt6), GAA for TCC (mt7), AAT for CCG (mt8), and CTTATTGCATCATCCCCGC for AACATTGCATCAGAAAATC (mt9) in both the sense and antisense orientation. Plasmid 2XCHOPAARE-TATATK-LUC was constructed by inserting SstI-XhoI double-stranded oligonucleotides containing two iterations of the CHOP AARE sequence into TATATK-LUC plasmid. 2XC/EBP-TATATK-LUC, 2XFNCRE-TATATK-LUC, 2Xjun2TRE-TATATK-LUC, 2XE4ATF-TATATK-LUC, and 2XC/EBP-CRE-TATATK-LUC plasmids were constructed by inserting SstI-XhoI double-stranded oligonucleotides containing two iterations of the corresponding sequence (see Fig. 5A) into the SstI and XhoI sites of TATATK-LUC. All the luciferase plasmid constructs were sequenced before utilization using the U.S. Biochemicals Sequenase sequencing kit according to the manufacturer's instructions. Plasmids to express ATF-2 and the ATF-2Ala dominant negative mutant (see Fig. 9B) containing the chicken cytoplasmic β-actin promoter were a gift of S. Ishii (41, 49).
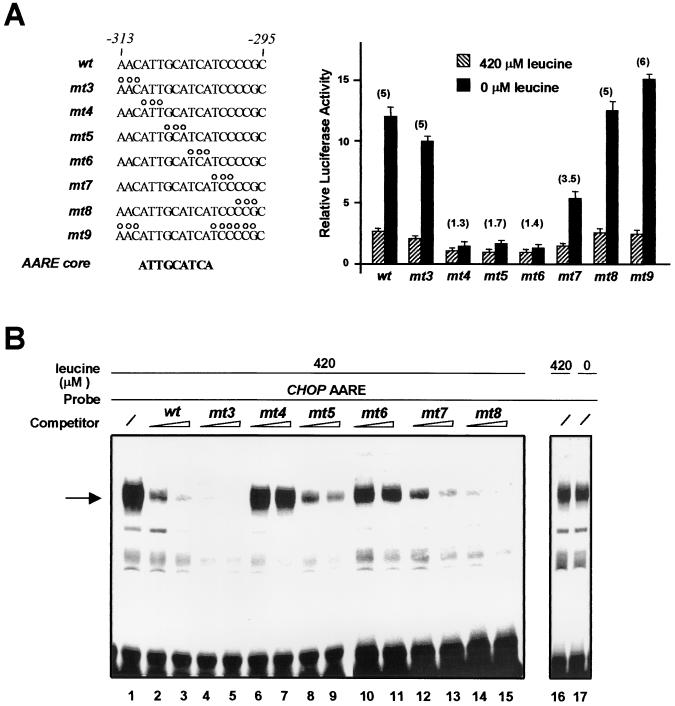
FIG. 4.
Identification of a CHOP AARE core sequence. (A) HeLa cells were transfected with LUC constructs containing a single copy of native or mutant CHOP AARE (−313 to −295) inserted 5′ to the TK promoter. The open circles represent the positions of substitution mutations in the 19-bp CHOP AARE sequence. The minimum AARE core sequence is shown in bold letters. Twenty-four hours after transfection, cells were incubated for 16 h in DMEM F12 (420 μM leucine) or in DMEM F12 lacking leucine (0 μM leucine) and then were harvested for preparation of cell extracts and determination of LUC activity. Relative LUC activities were determined as described in Materials and Methods. The relative fold induction, defined as the ratio of the relative LUC activity of leucine-starved cells to unstarved cells, is indicated in parentheses. Each data point represents the mean of at least three independent experiments performed in triplicate. (B) Gel mobility shift assays of nuclear extracts from HeLa cells incubated for 16 h in DMEM F12 (420 μM leucine) or in DMEM F12 lacking leucine (0 μM leucine) in the presence of the 19-bp CHOP AARE probe. The AARE probe carried sequences −313 to −295 (wild type). The 19-bp CHOP AARE and different mutated AARE oligonucleotides (mt3 to 8) were used as competitors at a 25- or 50-fold molar excess relative to the probe. The specific DNA-protein complex is indicated by an arrow.
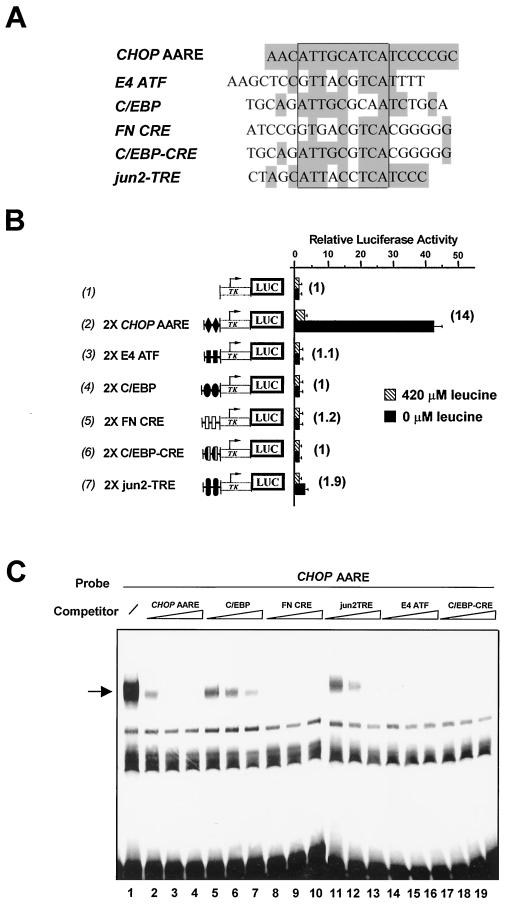
FIG. 5.
Comparison of CHOP AARE with C/EBP and ATF/CRE binding sites. (A) Sequence comparison of the CHOP AARE (−313 to −295) with the ATF binding site in the adenovirus E4 promoter (E4 ATF), a consensus C/EBP binding site (C/EBP), the ATF/CRE box of the fibronectin gene promoter (FN CRE), a chimeric C/EBP-CRE binding site (C/EBP-CRE), and the distal AP-1 binding site in the c-jun gene promoter (jun2-TRE). The position of the minimum AARE core sequence is boxed. Identical nucleotides are shaded in grey. (B) HeLa cells were transfected with LUC constructs containing two copies of the above-described sequences inserted 5′ to the TK promoter. Twenty-four hours after transfection, cells were incubated for 16 h in DMEM F12 (420 μM leucine) or in DMEM F12 lacking leucine (0 μM leucine) and then were harvested for preparation of cell extracts and determination of LUC activity. Relative LUC activities were determined as described in Materials and Methods. The relative fold induction, defined as the ratio of the relative LUC activity of leucine-starved cells to unstarved cells, is indicated in parentheses to the right of the bars. Each data point represents the mean of at least three independent experiments performed in triplicate. (C) Gel mobility shift assays of nuclear extracts from HeLa cells incubated for 16 h in DMEM F12 in the presence of the 19-bp CHOP AARE probe. The CHOP AARE carried sequences −313 to −295. Competing oligonucleotides (see panel A for sequence) were added at a 25-, 50-, and 100-fold molar excess relative to the probe. The specific DNA-protein complex is indicated by an arrow.
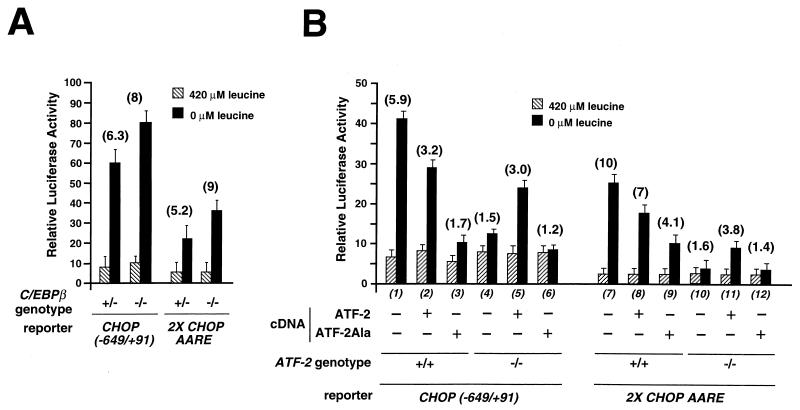
FIG. 9.
ATF-2 is essential for the induction of the CHOP promoter and AARE activity by leucine starvation. (A) Wild-type (+/+) or mutant (−/−) C/EBPβ MEF were transfected with LUC reporter constructs containing either the CHOP promoter region from −649 to +91 [CHOP (−649/+91)] or two copies of the 19-bp segment of the CHOP promoter including the AARE (−313 to −295) inserted 5′ to the TK promoter (2X CHOP AARE). (B) Wild-type (+/+) or mutant (−/−) ATF-2 MEF were transfected with a mixture containing CHOP (−649/+91) or 2X CHOP AARE LUC reporter constructs and a wild-type or a dominant negative form (ATF-2Ala [50]) of ATF-2 expression plasmids. Twenty-four hours after transfection, cells were incubated for 16 h in DMEM F12 (420 μM leucine) or in DMEM F12 lacking leucine (0 μM leucine) and then were harvested for preparation of cell extracts and determination of LUC activity. Relative LUC activities were determined as described in Materials and Methods. The relative fold induction, defined as the ratio of the relative LUC activity of leucine-starved cells to unstarved cells, is indicated in parentheses above the bars.
RESULTS
Transcriptional activation of CHOP by leucine starvation requires sequences located upstream from the start site.
To understand the regulation of gene expression by amino acids at a molecular level, we have studied the regulation of CHOP expression in response to leucine limitation because (i) leucine is an essential amino acid that is poorly utilized by HeLa cells during a 16-h incubation period (data not shown), and (ii) leucine, which is transported by system L, is rapidly equilibrated through the cell membrane (34, 42). It has been shown previously that regulation of CHOP transcription by leucine starvation is mediated through the promoter sequence situated between nucleotide position −954 and +91 (8). To identify amino acid-responsive elements within the CHOP promoter, a series of deletions in this region was created by PCR and fused to the coding region of the luciferase (LUC) reporter gene (Fig. 1). These constructs were transiently transfected into HeLa cells, and the response to leucine was determined by LUC assay, in starved and nonstarved conditions. Deletion to −649 had no significant effect on the activation of the CHOP promoter by leucine starvation (row 2). By contrast, deletion from −280 decreased the amino acid inducibility, suggesting that the region between −649 and −280 contains cis-positive elements involved in the transcriptional activation of CHOP by leucine starvation (row 3). Further deletions of the promoter from −221 to −40 resulted in a similar fold reduction in amino acid responsiveness and a progressive reduction of basal LUC activities (rows 4 to 6).
Localization of an upstream positive element involved in activation of CHOP transcription by leucine starvation.
To localize the positive elements involved in activation of CHOP transcription by leucine starvation, HeLa cells were transfected with a series of constructs containing internal deletions of the CHOP promoter (Fig. 2A). These deletions can be divided into two groups according to their level of amino acid inducibility. The first group includes three deletions that produced high levels of amino acid inducibility (rows 2, 4, and 5), while the second group of deletions led to reduced levels of induction (rows 3, 6, 7, and 8). These findings demonstrate that a 33-bp CHOP promoter region from −318 to −286 contains a cis-positive element that is essential for amino acid regulation. Close inspection of this 33-bp promoter sequence reveals the presence of a site that is similar to both the C/EBP consensus and the ATF/CRE-like sequence (referred to as a C/EBP-ATF composite site [15, 63]) and the presence of a putative SP-1 binding site (Fig. 2B). To determine the importance of each site in the leucine responsiveness of the CHOP promoter, we mutated these sites and assayed the LUC activity of the constructs. Figure 2C shows that mutation of the C/EBP-ATF site resulted in a decrease of amino acid responsiveness (row 11), while mutations in the SP-1 site did not affect the activation of the CHOP promoter by leucine starvation (row 10). These results show that the C/EBP-ATF composite site is essential for transcriptional activation of CHOP in response to leucine starvation.
The cis-positive element in the CHOP promoter is an AARE.
To determine whether the above-described CHOP cis-positive element could, by itself, render a heterologous promoter amino acid responsive, one or two copies of a 19-bp segment of the CHOP promoter from −313 to −295 containing the positive element were cloned 5′ of the minimal herpes simplex virus promoter for thymidine kinase (TK). As shown in Fig. 3A, a single copy of the CHOP-positive sequence was able to regulate the basal promoter in response to leucine (row 2). Furthermore, the leucine starvation-induced activity was enhanced synergistically by the presence of two copies of the positive element in the promoter (row 3).
FIG. 3.
The cis-positive element is an AARE. (A) HeLa cells were transfected with LUC constructs containing a single or two copies of a 19-bp segment of the CHOP promoter including the positive element (−313 to −295) inserted 5′ to the TK promoter. The 19-bp segment of the CHOP promoter is indicated by a black diamond. Twenty-four hours after transfection, cells were incubated for 16 h in DMEM F12 (420 μM leucine) or in DMEM F12 lacking leucine (0 μM leucine) and then were harvested for preparation of cell extracts and determination of LUC activity. (B) HeLa, HepG2, and Caco-2 cells were transfected with LUC constructs containing two copies of the 19-bp segment of the CHOP promoter including the positive element (−313 to −295) inserted 5′ to the TK promoter. Twenty-four hours after transfection, cells were incubated for 16 h in MEM control medium (MEM) or in MEM lacking one amino acid (MEM-AA) and then were harvested for preparation of cell extracts and determination of LUC activity. Relative LUC activities were determined as described in Materials and Methods. (A) The relative fold induction, defined as the ratio of the relative LUC activity of leucine-starved cells to unstarved cells, is indicated in parentheses to the right of the bars.
Starvation of other amino acids was tested for its ability to influence LUC activity driven by two copies of the CHOP-positive element (from −313 to −295) in front of the minimal TK promoter (Fig. 3B). The most potent omission of an amino acid for increasing the LUC activity level appeared to be that of methionine, arginine, lysine, or leucine. Deprivation of phenylalanine, histidine, alanine, and cysteine resulted in less dramatic but consistent increases of LUC activity. In contrast, proline, aspartate, and glutamate had no significant effects on the level of LUC activity. These results demonstrate that two copies of the CHOP-positive sequence can regulate a basal promoter in response to the omission of several individual amino acids. The extent of induction varied significantly according to the amino acid omitted, although only 3 of 11 amino acids tested had no effect. Fig. 3B also shows that the CHOP-positive sequence is functional in other human cell types, such as HepG2 and Caco-2, in which the CHOP gene is also regulated by amino acid starvation (8). Altogether these results demonstrate that the upstream positive element in the CHOP promoter can be considered an AARE.
The minimum AARE core sequence is 5′-ATTGCATCA-3′.
To pinpoint the exact nucleotides of the CHOP AARE that are involved in amino acid regulation, we scanned the −313 to −295 promoter region by site-directed mutagenesis. Each mutation consisted of a 3-bp substitution in a context of a single copy of the CHOP AARE sequence inserted upstream of the TK promoter (Fig. 4A). Mutations mt3 and mt8 did not affect the activation of the TK promoter construct by leucine starvation, and mutation mt7 caused a slight decrease in amino acid responsiveness. By contrast, mutations mt4, mt5, and mt6 resulted in a complete loss of the amino acid inducibility, suggesting that nine nucleotides are essential for conferring amino acid sensitivity. Furthermore, mutation mt9 demonstrates that the minimum core sequence able to render a promoter amino acid responsive is 5′-ATTGCATCA-3′.
To investigate whether the minimum core DNA sequence of the AARE mediated the regulation of the CHOP promoter through the binding of protein(s), gel mobility shift assays were performed with a 19-bp double-stranded probe (CHOP AARE) containing sequences −313 to −295 of the CHOP promoter (Fig. 4B). A major specific DNA-protein complex was detected after incubation of nonstarved HeLa nuclear extracts with the 32P-labeled CHOP AARE probe (lane 1). The abundance of this protein complex did not vary following leucine starvation (compare lanes 16 and 17). To identify the minimum sequence within the AARE that was capable of binding the proteins producing the gel retardation band, we introduced the substitution mutations described above in the 19-bp double-stranded oligonucleotide (see Fig. 4A) and performed gel mobility shift competition assays. The DNA-protein complex was highly competed by an excess of cold mt3 (Fig. 4B, lanes 4 and 5) and mt8 (lanes 14 and 15) mutated oligonucleotides, more weakly competed by mt5 (lanes 8 and 9) and mt7 (lanes 12 and 13) mutated oligonucleotides, and not competed by an equal amount of mt4 (lanes 6 and 7) and mt6 (lanes 10 and 11) oligonucleotides. Moreover, the DNA-protein complex failed to form when mt4, mt5, and mt6 mutated oligonucleotides were used as a probe (data not shown). These results demonstrate that the minimum sequence capable of binding proteins producing the gel retardation band corresponds to the core sequence of the CHOP AARE between −310 and −302.
CHOP AARE binds members of C/EBP and ATF protein families in vitro.
Sequence comparison of the CHOP AARE with database sequences did not reveal any perfect homology with known transcription factor binding sites. However, the minimum CHOP AARE core sequence 5′-ATTGCATCA-3′ showed some homology with binding sites of the C/EBP and ATF/CREB protein families and was referred to as a C/EBP-ATF composite site (15, 63) (Fig. 5A). To investigate whether DNA binding sites for C/EBP or ATF transcription factors could mediate amino acid inducibility, two copies of several candidate sites were cloned immediately upstream of the TK promoter (see Fig. 5A for sequences). The adenovirus E4-ATF binding site (E4 ATF) (27, 36) (Fig. 5B, row 3), a consensus C/EBP binding site (C/EBP) (50) (row 4), the cyclic AMP response element of the fibronectin gene (FN CRE) (45) (row 5), and a chimeric C/EBP-CRE site (C/EBP-CRE) (50) (row 6) could not render a basal promoter amino acid responsive. A low level of inducible promoter activity was observed with the distal AP-1 binding site in the c-jun gene promoter (jun2TRE) (58) (row 7), but five copies of this binding site did not produce higher amino acid responsiveness (data not shown). These findings demonstrate that previously identified binding sites for ATF or C/EBP are not able to mediate amino acid inducibility.
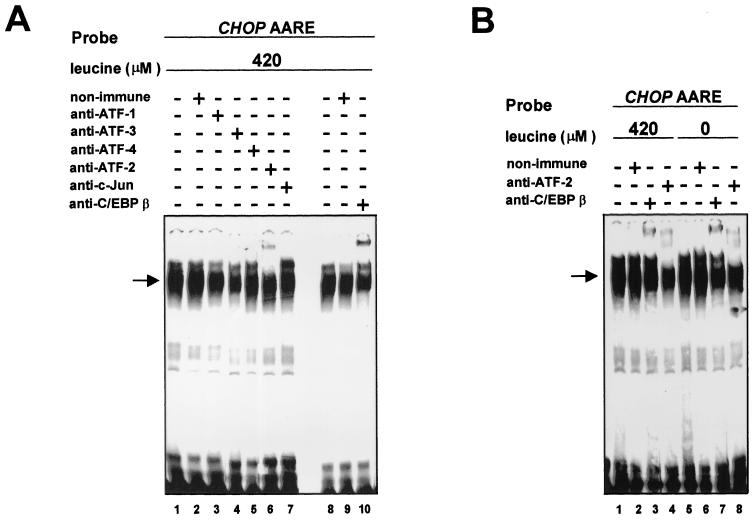
The results presented above do not exclude the possibility that members of the C/EBP and ATF/CREB protein families could be involved in the response to amino acid starvation. To determine whether proteins binding to the CHOP AARE could be related to C/EBP or ATF, gel mobility shift competition assays were carried out using the DNA binding sites of these families of transcription factors as competitors (see Fig. 5A for sequences). Oligonucleotides containing C/EBP (Fig. 5C, lanes 5 to 7), FN CRE (lanes 8 to 10), jun2TRE (lanes 11 to 13), E4 ATF (lanes 14 to 16), and C/EBP-CRE (lanes 17 to 19) abolished or greatly reduced the binding of the DNA-protein complex, suggesting that proteins binding to the CHOP AARE could be C/EBP or ATF related. To identify which members of the C/EBP and ATF transcription factor families bind in vitro to the CHOP AARE, gel mobility shift assays were carried out in the presence of specific antibodies (Fig. 6). All specific antibodies used in our experiments have been shown to supershift protein-DNA complexes containing their corresponding transcription factor in gel mobility shift analysis (data not shown). As shown in Fig. 6A, antibodies against ATF-2 (lane 6) (37) and C/EBPβ (lane 10) (9) supershifted the CHOP AARE-bound complex formed with nonstarved HeLa nuclear extracts, while the antibodies against ATF-1 (lane 3) (21), ATF-3 (lane 4) (11), ATF-4 (lane 5) (21), and c-Jun (lane 7) (27) did not affect the DNA-protein complex. Furthermore, the abundance of ATF-2 and C/EBPβ supershifted DNA-protein complexes did not vary with leucine starvation (Fig. 6B). These results show that ATF-2 and C/EBPβ transcription factors are able to bind in vitro to the CHOP AARE sequence in the presence and absence of leucine.
FIG. 6.
Presence of ATF-2 and C/EBPβ in the DNA-protein complex binding to the CHOP AARE. Supershift assays using specific antibodies to different proteins and nuclear extracts from HeLa cells incubated for 16 h in DMEM F12 (A) or DMEM F12 lacking leucine (B). HeLa nuclear extracts were first incubated with 1 μl of rabbit nonimmune serum or specific antiserum, and then the preincubation mixture was incubated with the 19-bp CHOP AARE probe as described in Materials and Methods. The 19-bp CHOP AARE carried sequences −313 to −295.
ATF-2 has a critical role in the transcriptional activation of CHOP by amino acids.
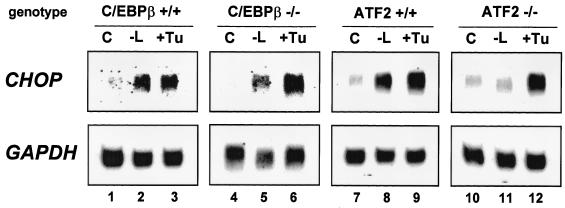
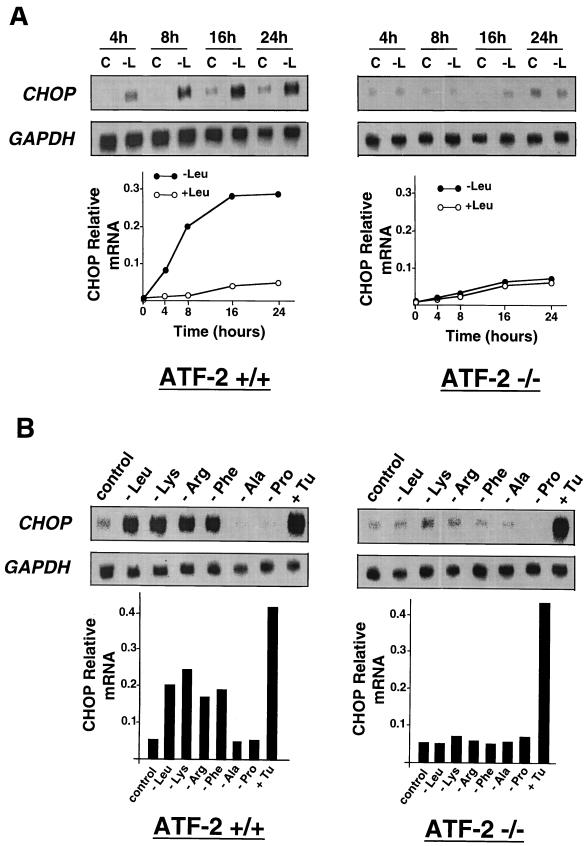
To assess the respective roles of ATF-2 and C/EBPβ in the transcriptional activation of CHOP by leucine, we first measured the effect of leucine starvation on CHOP mRNA expression in MEF deficient in ATF-2 or C/EBPβ (65) and in the corresponding wild-type cells. As shown in Fig. 7, CHOP exhibited a normal response to leucine starvation and to an agent (tunicamycin) that induces ER stress (31) in C/EBPβ+/+ (lanes 1 to 3) and ATF-2+/+ cells (lanes 7 to 9). Lack of C/EBPβ did not affect the induction of CHOP mRNA by leucine starvation and tunicamycin (lanes 4 to 6). By contrast, lack of ATF-2 resulted in a complete loss of the CHOP mRNA amino acid inducibility (lanes 10 and 11) but did not affect the induction of mRNA level by tunicamycin (lane 12). The result concerning the effect of the ATF-2 genotype on CHOP induction was strengthened by a kinetic analysis of the CHOP mRNA level in ATF-2+/+ and ATF-2−/− cells exposed to medium lacking leucine (Fig. 8A). CHOP mRNA was only detectable in ATF-2+/+ cells 4 h after starvation, and a maximum level was reached after 16 h. Moreover, as shown in Fig. 8B, the defect in endogenous CHOP induction in the ATF-2−/− cells extended to the response to deprivation of some other amino acids (lysine, arginine, and phenylalanine). Deprivation of these amino acids had been shown above to increase LUC activity from the CHOP AARE LUC reporter construct (see Fig. 3B). Taken together, these results provide evidence that ATF-2 is essential in the specific amino acid pathway that leads to the induction of CHOP mRNA.
FIG. 7.
CHOP mRNA level is induced by leucine starvation in C/EBPβ-deficient cells but not in ATF-2-deficient cells. Wild-type (+/+) or mutant (−/−) C/EBPβ or ATF-2 MEF were incubated for 16 h in DMEM F12 (C) or in DMEM F12 lacking leucine (−L) or for 6 h in medium containing 0.125 μg of tunicamycin (+Tu)/ml. Total RNA was extracted and Northern blot analysis was performed as described in Materials and Methods. The blots were hybridized with human probes corresponding to CHOP and GAPDH.
FIG. 8.
Lack of ATF-2 results in a complete loss of amino acid inducibility. (A) Wild-type (+/+) or mutant (−/−) MEF were incubated in DMEM F12 (+Leu) or in DMEM F12 lacking leucine (−Leu) and were harvested for RNA isolation after the indicated incubation times. (B) Wild-type (+/+) or mutant (−/−) MEF were incubated for 16 h in MEM (control) or in MEM lacking one amino acid or for 6 h in medium containing 0.125 μg of tunicamycin (+Tu)/ml and were harvested for RNA isolation. Northern blot analysis was performed as described in Materials and Methods. The blots were hybridized with human probes corresponding to CHOP and GAPDH. Quantification of the Northern blot analysis is given graphically, below the respective panels.
To more directly analyze the effects of ATF-2 and C/EBPβ on regulation of CHOP transcription by leucine, LUC constructs containing either the CHOP promoter from −649 to +91 or two copies of CHOP AARE inserted immediately upstream of the TK promoter were transiently transfected in C/EBPβ- or ATF-2-deficient MEF and in the corresponding wild-type cells. The response to leucine was determined by LUC assay in starved and nonstarved conditions. Lack of C/EBPβ did not significantly affect the amino acid inducibility of these constructs (Fig. 9A), confirming that although C/EBPβ binds in vitro to the CHOP AARE, this transcription factor is not essential for the transcriptional activation of CHOP by leucine starvation. On the other hand, lack of ATF-2 was found to dramatically decrease the amino acid responsiveness of both LUC constructs (Fig. 9B, bars 4 and 10). We examined the effect of an ATF-2 dominant negative mutant on leucine-induced activity of the CHOP promoter and the AARE. This mutant (ATF-2Ala), in which the three SAPK phosphorylation sites (Thr-69, Thr-71, and Ser-90) lying close to the N terminus are replaced by alanine, cannot be phosphorylated and therefore cannot mediate transcriptional activation (38, 49). Cotransfection of this mutant with LUC constructs in ATF-2+/+ cells significantly inhibited the increase in promoter activity due to leucine starvation (bars 3 and 9), demonstrating that a dominant negative form of ATF-2 can inhibit the stimulatory effect of leucine starvation on the AARE and promoter of CHOP. Moreover, cotransfection of the ATF-2 expression plasmid into ATF-2−/− cells resulted in a partial rescue of amino acid inducibility of the CHOP promoter (bar 5) as well as of the 2X CHOP AARE (bar 11). By contrast, in a similar experiment cotransfection of the ATF-2Ala dominant negative mutant was not able to rescue the amino acid inducibility (bars 6 and 12). These data demonstrate that ATF-2 expression is essential for induction of the CHOP promoter and AARE activity by leucine starvation, and they establish a vital role for ATF-2 in mediating amino acid responsiveness.
DISCUSSION
The mammalian plasma concentration of free amino acids shows striking alterations according to nutritional and pathological conditions (3, 13, 64). In recent years, evidence has accumulated that amino acids play an important role in controlling gene expression (30, 32). Nevertheless, the molecular mechanisms involved in the amino acid regulation of mammalian gene expression have not been elucidated to date. The work presented in this report focuses on the identification of the cis- and trans-acting elements which are involved in the transcriptional activation of CHOP in response to amino acid starvation.
The cis DNA sequence located upstream from the transcription start site (−313 to −295) is essential for amino acid activation of the CHOP promoter. This sequence is the first described that can regulate a basal promoter in response to starvation of several individual amino acids, in all human cell lines tested (HeLa, Caco-2, and HepG2). This DNA element is conserved in the CHOP gene in species other than humans, such as the hamster (46). Mutations affecting a stretch of nine nucleotides (AARE core) result in a loss of amino acid responsiveness. From these properties, this DNA sequence can be called an AARE. It appears that sequences outside the AARE core could also play an important role in achieving the full amino acid induction. Indeed, mutation mt7 outside the core sequence (see Fig. 4A) results in a slight decrease in amino acid inducibility, although the effect of this mutation appears to be inhibited in mutation mt9.
Sequences of the CHOP AARE region show some homology with the specific binding sites of the C/EBP and ATF/CREB transcription factor families (15, 63). All members of these families contain a DNA binding domain consisting of a cluster of basic amino acids and a leucine zipper region (b-ZIP domain) (33). They can form homodimers or heterodimers through their leucine zipper regions (17) and then bind to both ATF and C/EBP recognition sites (1, 4). Depending on the composition of the heterodimer, different sequence elements are preferentially recognized, leading to variable transcriptional effects (10, 58). Our present results demonstrate that although the CHOP AARE sequence binds C/EBPβ and ATF-2 in vitro, only ATF-2 has a critical role in the transcriptional activation of CHOP by leucine starvation. ATF-2 is capable of interacting with other b-ZIP proteins and binding to particular recognition sequences (22, 28, 29, 50). Our data suggest that ATF-2 could bind to the CHOP AARE as homodimer or as heterodimer with an unknown dimerization partner. cis-acting elements which are recognized by ATF-2 in association with other known transcription factors are not able to activate transcription in response to amino acid starvation. For example, the c-jun promoter contains two AP-1-like sites that are critical for its regulation by certain stresses and some other stimuli (2, 44, 57). In vitro binding studies reveal that in most cells examined, proximal (jun1TRE) and distal AP-1 (jun2TRE) sites bind heterodimers composed of c-Jun and ATF-2 (58, 59). Although the CHOP AARE shares significant similarities with the jun2TRE (Fig. 5A), c-Jun is not able to bind to the AARE in vitro (Fig. 6, lane 7). In addition, we demonstrate that jun2TRE sequences do not confer amino acid inducibility (Fig. 5B). Therefore, the association of ATF-2 and c-Jun does not mediate the response to amino acids. Further work will be required to determine whether another partner associated with ATF-2 is important in the mechanism of transcriptional activation by amino acid starvation.
We do not observe differences in the AARE binding activity of ATF-2 between leucine-starved and nonstarved conditions. The transactivating capacity of ATF-2 is activated via phosphorylation of N-terminal residues Thr-69, Thr-71, and Ser-90 by stress-activated protein kinases (19, 38, 59). We show that the ATF-2Ala dominant negative mutant in which these three residues cannot be phosphorylated inhibits full up-regulation of CHOP promoter activity induced by leucine starvation (see Fig. 9B). Moreover, when cotransfected into ATF-2−/− cells, this dominant negative mutant is not able to rescue the amino acid inducibility of the CHOP promoter. Taken together, these data suggest that the specific amino acid pathway that leads to transcriptional activation of CHOP may involve phosphorylation of prebound ATF-2 rather than an increase in ATF-2 binding (19).
The signaling pathways that recognize amino acid availability in mammalian cells have not been investigated as extensively as those in bacteria or yeast. We show that ATF-2 is essential in the amino acid pathway that leads to the induction of CHOP mRNA and confirm that this specific pathway is independent of the unfolded protein response. ATF-2 has been shown to be ubiquitously expressed, with the highest level of expression being observed in the brain (52), but the physiological role of ATF-2 remains poorly understood. Knockout mice generated by gene targeting exhibited lowered postnatal viability and growth, with a severe respiratory distress (39) or with a defect in endochondral ossification and a reduced number of cerebellar Purkinje cells (47). The data presented in this paper suggest that modulation of ATF-2 activity could also play an important role in the process of defense or adaptation to amino acid limitation that occurs in nutritional or pathological conditions.
The concept that amino acids can regulate gene expression has just started to emerge. It is now clear that amino acids can play an important role in the control of gene expression in concert with hormones. However, the underlying mechanisms have only begun to be discovered. Our results show that the transcriptional activity of ATF-2 is essential for the regulation of CHOP expression by amino acids. The physiological meaning of CHOP regulation by amino acids is not yet understood. Through its interaction with C/EBP transcription factors, CHOP may participate in the regulation of several mechanisms (43) during cellular response to amino acid limitation. For example, CHOP could play a crucial role in the regulation of nitrogen metabolism under amino acid control, although a direct role for CHOP in this pathway has yet to be demonstrated. During a cellular stress that perturbs function of the endoplasmic reticulum (ER stress), CHOP expression, in concert with a second signal, was found to be absolutely required for the activation of a set of previously undescribed genes referred to as DOCs (for downstream of CHOP) (62). We have hypothesized that CHOP may also mediate the induction of cellular DOC genes in the context of the amino acid response, but the identification of such genes remains to be done.
Defining the precise cascade of molecular events by which the cellular concentration of an individual amino acid regulates gene expression will be an important contribution to our understanding of metabolite control in mammalian cells. These studies will provide insight into the role of amino acids in the regulation of cellular functions like cell division, protein synthesis, and proteolysis.
ACKNOWLEDGMENTS
We are grateful to S. Ishii for the ATF-2 and ATF-2Ala expression plasmids and to U. Schiebler for providing anti-C/EBPβ serum. We thank V. Poli and D. Ron for the gift of the C/EBPβ-deficient MEF. We also thank D. Ron for the anti-ATF-4 serum.
This work was supported by grants from the Institut National de la Recherche Agronomique, the Fondation pour la Recherche Médicale, and the Arthritis Foundation (A.M.R.). C. Jousse is a recipient of a French M.E.N.S.R. predoctoral scholarship and a DANONE research scholarship.
REFERENCES
- 1.Alonso C R, Pesce C G, Kornblihtt A R. The CCAAT-binding proteins CP1 and NF-1 cooperate with ATF-2 in the transcription of the fibronectin gene. J Biol Chem. 1996;271:22271–22279. doi: 10.1074/jbc.271.36.22271. [DOI] [PubMed] [Google Scholar]
- 2.Angel P, Imagawa M, Chiu R, Stein B, Imbra R J, Ramsdorf H J, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis-acting element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 3.Baertl J M, Placko R P, Graham G G. Serum proteins and plasma free amino acids in severe malnutrition. Am J Clin Nutr. 1974;27:733–742. doi: 10.1093/ajcn/27.7.733. [DOI] [PubMed] [Google Scholar]
- 4.Bakker O, Parker M G. CAAT/enhancer binding protein is able to bind to ATF/CRE elements. Nucleic Acids Res. 1991;19:1213–1217. doi: 10.1093/nar/19.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barone M V, Crozat A Y, Tabaee A, Philipson L, Ron D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev. 1994;8:453–464. doi: 10.1101/gad.8.4.453. [DOI] [PubMed] [Google Scholar]
- 6.Batchvarova N, Wang X Z, Ron D. Inhibition of adipogenesis by the stress-induced protein CHOP. EMBO J. 1995;14:4654–4661. doi: 10.1002/j.1460-2075.1995.tb00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkenmeier E H, Gwynn B, Howard S, Jerry J, Gordon J I, Landschulz W H, McKnight S L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989;3:1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- 8.Bruhat A, Jousse C, Wang X-Z, Ron D, Ferrara M, Fafournoux P. Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J Biol Chem. 1997;272:17588–17593. doi: 10.1074/jbc.272.28.17588. [DOI] [PubMed] [Google Scholar]
- 9.Cao Z, Umek R M, McKnight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 10.Chatton B, Bocco J L, Goetz J, Gaire M, Lutz Y, Kedinger C. Jun and Fos heterodimerize with ATFa, a member of the ATF/CREB family, and modulate its transcriptional activity. Oncogene. 1994;9:375–385. [PubMed] [Google Scholar]
- 11.Chen B P C, Wolfgang C D, Hai T. Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol. 1996;16:1157–1168. doi: 10.1128/mcb.16.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 13.Fafournoux P, Remesy C, Demigné C. Fluxes and membrane transport of amino acids in rat liver under different protein diets. Am J Physiol. 1990;259:E614–E625. doi: 10.1152/ajpendo.1990.259.5.E614. [DOI] [PubMed] [Google Scholar]
- 14.Fawcett T W, Eastman H B, Martindale J L, Holbrook N J. Physical and functional association between GADD153 and CCAAT/enhancer-binding protein β during cellular stress. J Biol Chem. 1996;271:14285–14289. doi: 10.1074/jbc.271.24.14285. [DOI] [PubMed] [Google Scholar]
- 15.Fawcett T W, Martindale J L, Guyton K Z, Hai T, Holbrook N J. Complexes containing activating transcription (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339:135–141. [PMC free article] [PubMed] [Google Scholar]
- 16.Girard J, Perdereau D, Foufelle F, Prip-Buus C, Ferré P. Regulation of lipogenic enzyme gene expression by nutrients and hormones. FASEB J. 1994;8:36–42. doi: 10.1096/fasebj.8.1.7905448. [DOI] [PubMed] [Google Scholar]
- 17.Göttlicher M, Heck S, Herrlich P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J Mol Med. 1998;76:480–489. doi: 10.1007/s001090050242. [DOI] [PubMed] [Google Scholar]
- 18.Guerrini L, Gong S S, Mangasarian K, Basilico C. cis- and trans-acting elements involved in amino acid regulation of asparagine synthetase gene expression. Mol Cell Biol. 1993;13:3202–3212. doi: 10.1128/mcb.13.6.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Campbell D, Dérijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 20.Gurney A L, Pak E A, Liu J, Giralt M, McGrane M M, Patel Y M, Crawford D R, Nizielski S E, Savon S, Hanson R W. Metabolic regulation of gene transcription. J Nutr. 1994;124:1533S–1539S. doi: 10.1093/jn/124.suppl_8.1533S. [DOI] [PubMed] [Google Scholar]
- 21.Hai T, Liu F, Coukos W J, Green M R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding hetero-dimers. Genes Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- 22.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall C V, Jacob P E, Ringold M, Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2:101–109. [PubMed] [Google Scholar]
- 24.Hinnebusch A G. Mechanisms of gene regulation in the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Microbiol Rev. 1988;52:248–273. doi: 10.1128/mr.52.2.248-273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinnebusch A G. Translational control of GCN4: an in vivo barometer of initiation-factor activity. Trends Biochem Sci. 1994;19:409–414. doi: 10.1016/0968-0004(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 26.Hope I A, Struhl K. GCN4 protein, synthesized in vitro, binds HIS3 regulatory sequences: implications for general control of amino acid biosynthetic genes in yeast. Cell. 1985;43:177–188. doi: 10.1016/0092-8674(85)90022-4. [DOI] [PubMed] [Google Scholar]
- 27.Hurst H C, Jones N C. Identification of factors that interact with E1A-inducible adenovirus E3 promoter. Genes Dev. 1987;1:1132–1146. doi: 10.1101/gad.1.10.1132. [DOI] [PubMed] [Google Scholar]
- 28.Ivashkiv L B, Liou H C, Kara C J, Lamph W W, Verma I M, Glimcher L H. MXBP/CRE-BP2 and c-jun form a complex which binds to the cyclic AMP, but not the 12-O-tetracanoylphorbol-13-acetate, response element. Mol Cell Biol. 1990;10:1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivashkiv L B, Fleming M D, Glimcher L H. Dominant negative mutants of transcription factor mXBP (CRE-BP1, ATF2) New Biol. 1992;4:360–368. [PubMed] [Google Scholar]
- 30.Jousse C, Bruhat A, Ferrara M, Fafournoux P. Physiological concentration of amino acids regulates insulin-like-growth-factor-binding protein 1 expression. Biochem J. 1998;334:147–153. doi: 10.1042/bj3340147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jousse C, Bruhat A, Hardind H P, Ferrara M, Ron D, Fafournoux P. Amino acid limitation induces CHOP through a specific pathway independent on the unfolded protein response. FEBS Lett. 1999;448:211–216. doi: 10.1016/s0014-5793(99)00373-7. [DOI] [PubMed] [Google Scholar]
- 32.Jousse C, Bruhat A, Fafournoux P. Amino acid regulation of gene expression. Curr Opin Clin Nutr Metab Care. 1999;2:297–301. doi: 10.1097/00075197-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Karin M, Smeal T. Control of transcription factors by signal transduction pathways: the beginning of the end. Trends Biochem Sci. 1992;17:418–422. doi: 10.1016/0968-0004(92)90012-x. [DOI] [PubMed] [Google Scholar]
- 34.Kilberg M S, Stevens B R, Novak D A. Recent advances in mammalian amino acid transport. Annu Rev Nutr. 1993;13:137–165. doi: 10.1146/annurev.nu.13.070193.001033. [DOI] [PubMed] [Google Scholar]
- 35.Kilberg M S, Huston R G, Laine R O. Amino acid-regulated gene expression in eukaryotic cells. FASEB J. 1994;8:13–18. doi: 10.1096/fasebj.8.1.8299885. [DOI] [PubMed] [Google Scholar]
- 36.Lin Y S, Green M R. Interaction of a common cellular transcription factor, ATF, with regulatory elements in both E1a- and cyclic AMP-inducible promoters. Proc Natl Acad Sci USA. 1988;85:3396–3400. doi: 10.1073/pnas.85.10.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F, Green M R. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1A protein. Cell. 1990;61:1217–1224. doi: 10.1016/0092-8674(90)90686-9. [DOI] [PubMed] [Google Scholar]
- 38.Livingstone C, Patel G, Jones N. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maekawa T, Bernier F, Sato M, Nomura S, Singh M, Inoue Y, Tokunaga T, Imai H, Yokoyama M, Reimold A M, Glimcher L, Ishii S. Mouse ATF-2 null mutants display features of a severe type of meconium aspiration syndrome. J Biol Chem. 1999;274:17813–17819. doi: 10.1074/jbc.274.25.17813. [DOI] [PubMed] [Google Scholar]
- 40.Marten N W, Burke E J M, Hayden J, Straus D S. Effect of amino acid limitation on the expression of 19 genes in rat hepatoma cells. FASEB J. 1994;8:538–544. doi: 10.1096/fasebj.8.8.8181673. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda S, Maekawa T, Ishii S. Identification of the functional domains of the transcriptional regulator CRE-BP1. J Biol Chem. 1991;266:18188–18193. [PubMed] [Google Scholar]
- 42.McGivan J D, Pastor-Anglada M. Regulatory and molecular aspects of mammalian amino acid transport. Biochem J. 1994;299:321–334. doi: 10.1042/bj2990321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKnight S, Lane M D, Gluecksohn-Waelsch S. Is CCAAT/enhancer-binding protein a central regulator of energy metabolism? Genes Dev. 1989;3:2021–2024. doi: 10.1101/gad.3.12b.2021. [DOI] [PubMed] [Google Scholar]
- 44.Muegge K, Vila M, Gusella G L, Musso T, Herrlich P, Stein B, Durum S K. Interleukin 1 induction of the c-jun promoter. Proc Natl Acad Sci USA. 1993;90:7054–7058. doi: 10.1073/pnas.90.15.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muro A F, Bernath V A, Kornblihtt A R. Interaction of the −170 cyclic AMP response element with the adjacent CCAAT box in the human fibronectin gene promoter. J Biol Chem. 1992;267:12767–12774. [PubMed] [Google Scholar]
- 46.Park J S, Luethy J D, Wang M G, Fargnoli J, Fornace A J, Jr, McBride O W, Holbrook N J. Isolation, characterization and chromosomal localization of the human GADD153 gene. Gene. 1992;116:259–267. doi: 10.1016/0378-1119(92)90523-r. [DOI] [PubMed] [Google Scholar]
- 47.Reimold A M, Grusby M J, Kosaras B, Fries J W U, Mory R, Maniwa S, Clauss I M, Collins T, Sidman R L, Glimcher M J, Glimcher L H. Chondrodysplasia and neurological abnormalities in ATF-2 deficient mice. Nature. 1996;379:262–265. doi: 10.1038/379262a0. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. p. 7.46. [Google Scholar]
- 49.Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J Biol Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- 50.Shuman J D, Cheong J, Coligan J E. ATF-2 and C/EBPα can form a heterodimeric DNA binding complex in vitro. J Biol Chem. 1997;272:12793–12800. doi: 10.1074/jbc.272.19.12793. [DOI] [PubMed] [Google Scholar]
- 51.Sok J, Wang X-Z, Batchvarova N, Kuroda M, Harding H, Ron D. CHOP-dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol. 1999;19:495–504. doi: 10.1128/mcb.19.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeda J, Maekawa T, Sudo T, Seino Y, Imura H, Sato N, Tanaka C, Ishii S. Expression of CRE-BP1 transcriptional regulator binding to cyclic AMP response element in central nervous system, regenerating liver and human tumors. Oncogene. 1991;6:1009–1014. [PubMed] [Google Scholar]
- 53.Todaro G J, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Towle H C. Metabolic regulation of gene transcription in mammals. J Biol Chem. 1995;270:23235–23238. doi: 10.1074/jbc.270.40.23235. [DOI] [PubMed] [Google Scholar]
- 55.Ubeda M, Wang X-Z, Zinszner H, Wu I, Habener J F, Ron D. Stress-induced binding of the transcription factor CHOP to a novel DNA control element. Mol Cell Biol. 1996;16:1479–1489. doi: 10.1128/mcb.16.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Umek R M, Friedman A D, McKnight S L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- 57.Van Dam H, Offringa R, Meijer I, Stein B, Smits A M, Herrlich P, Bos J L, van der Eb A J. Differential effects of the adenovirus E1A oncogene on members of the AP-1 transcription factor family. Mol Cell Biol. 1990;10:5857–5864. doi: 10.1128/mcb.10.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Dam H, Duyndam M, Rottier R, Bosch A, de Vries-Smits L, Herrlich P, Zantema A, Angel P, van der Eb A J. Heterodimer formation of cJun and ATF-2 is responsible for induction of c-jun by the 243 amino acid adenovirus E1A protein. EMBO J. 1993;12:479–487. doi: 10.1002/j.1460-2075.1993.tb05680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaulont S, Kahn A. Transcriptional control of metabolic regulation genes by carbohydrates. FASEB J. 1994;8:28–35. doi: 10.1096/fasebj.8.1.8299888. [DOI] [PubMed] [Google Scholar]
- 61.Wang X-Z, Lawson B, Brewer J W, Zinszner H, Sanjay A, Mi L-J, Boorstein R, Kreibich G, Hendershot L M, Ron D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–4280. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X-Z, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, Ron D. Identification of novel stress-induced genes downstream of chop. EMBO J. 1998;17:3619–3630. doi: 10.1093/emboj/17.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolfgang C D, Chen B P C, Martindale J L, Holbrook N J, Hai T. gadd153/Chop10, a potential target gene of the transcriptional repressor ATF3. Mol Cell Biol. 1997;17:6700–6707. doi: 10.1128/mcb.17.11.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young W R, El-Khoury A E, Sanchez M, Castillo L. In: The biochemistry and physiology of protein and amino acid metabolism, with reference to protein nutrition. Niels C, Räihä R, editors. Vol. 33. 1994. pp. 1–28. Protein metabolism during infancy.Nestec Ltd., Vevey, Raven Press, Ltd., New York, N.Y. [Google Scholar]
- 65.Zinszner H, Kuroda M, Wang X-Z, Batchvarova N, Lightfoot R T, Remotti H, Stevens J L, Ron D. CHOP is implicated in programmed cell-death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]