Abstract
Our goal was to evaluate the effects of EC refill fluids and EC exhaled aerosol residue (ECEAR) on cultured human keratinocytes and MatTek EpiDerm™, a 3D air liquid interface human skin model. Quantification of flavor chemicals and nicotine in Dewberry Cream and Churrios refill fluids was done using GC-MS. The dominant flavor chemicals were maltol, ethyl maltol, vanillin, ethyl vanillin, benzyl alcohol, and furaneol. Cytotoxicity was determined with the MTT and LDH assays, and inflammatory markers were quantified with ELISAs. Churrios was cytotoxic to keratinocytes in the MTT assay, and both fluids induced ROS production in the medium (ROS-Glo™) and in cells (CellROX). Exposure of EpiDerm™ to relevant concentrations of Dewberry Cream and Churrios for 4 or 24 hours caused secretion of inflammatory markers (IL-1α, IL-6, and MMP-9), without altering EpiDerm™ histology. Lab made fluids with propylene glycol (PG) or PG plus a flavor chemical did not produce cytotoxic effects, but increased secretion of IL-1α and MMP-9, which was attributed to PG. ECEAR derived from Dewberry Cream and Churrios did not produce cytotoxicity with Epiderm™, but Churrios ECEAR induced IL-1α secretion. These data support the conclusion that EC chemicals can cause oxidative damage and inflammation to human skin.
Keywords: Electronic cigarette, refill fluids, flavors, inflammation, oxidative stress
INTRODUCTION
Electronic cigarettes (ECs) have gained worldwide popularity since their introduction in 2004 (Trtchounian and Talbot, 2010; Grana et al., 2014; US Food and Drug Administration, 2020). There are four generations of ECs referred to as cig-a-like, cartomizer, mods/tanks, and pods (Williams et al., 2019; National Academies of Sciences, Engineering, and Medicine, 2018). Products from each generation consist of a cartridge/tank/pod with liquid, a battery, and a heating element (atomizer). Refill fluids and e-liquids contain solvents (propylene glycol (PG) and/or glycerol (G)), flavor chemicals, and nicotine. While the concentration of nicotine normally ranges from 0 to 60 mg/mL (National Academies of Sciences, Engineering, and Medicine, 2018; Omaiye et al., 2019a), an unlabeled bottle of DIY nicotine contained over 100 mg/mL (Davis et al., 2015). EC liquid emissions may also have metals, such as nickel and lead, originating from either the coil, joints or wires (Aherrera et al., 2017; Farsalinos et al., 2015; Goniewicz et al., 2014; Lerner et al., 2015a; Mikheev et al., 2016; Williams et al., 2013; 2017; 2020). The heating of e-liquids to generate aerosol causes formation of additional chemicals, such as acetaldehyde, many of which are toxicants (Goniewicz et al., 2014; Uchiyama et al., 2013; Kosmider et al., 2014; Hutzler et al., 2014). Aerosol chemicals that are exhaled by EC users settle on indoor surfaces where they accumulate as ECEAR (EC exhaled aerosol residue) (Khachatoorian et al. 2018; 2019).
As the chemicals in EC fluids, aerosols, and ECEAR have been identified, there has been increased interest in evaluating their effects on human health, as many are on the Food and Drug Administration’s (FDA) list of Harmful and Potentially Harmful Chemicals (e. g., acrolein, acetaldehyde, benzene) (FDA 2012). Most health-effect research on ECs has involved the respiratory system (Pisinger and Døssing, 2014; Hua et al., 2013; 2020). However, EC chemicals also come in direct contact with the skin through leakage, spills, and touching of surfaces contaminated with ECEAR (Trtchounian et al., 2010; Won Kim and Baum, 2015; Maina et al., 2016; Hughes and Hendrickson, 2019; EU Health Programme, 2016; Khachatoorian et al 2019).
In studies that mined health data from EC internet websites, EC users reported skin disorders that included irritation, itchy skin, rash with burning, and eczema (Hua et al., 2013; 2020). Refill fluids can contain high concentrations of flavor chemicals, which sometimes exceed 100 mg/mL (Behar et al., 2016). Flavor chemicals, such as diacetyl and acetoin, can cause rashes or the development of dermatitis, and are present in many e-fluids and aerosols (US Department of Labor, Occupational Safety and Health Administration 2009; Farsalinos et al., 2014; Melvin et al., 2020). Nicotine is also a skin irritant that leaks from refill fluid bottles or ECs (Maina et al., 2016) and can be absorbed through the skin (Zorin et al., 1999; Kuswahyuning and Roberts, 2014). Nicotine can remain in the skin even after vigorous hand washing (Maina et al., 2017). Nickel has also been reported in e-fluids and aerosols (Aherrera et al., 2017; Williams et al., 2013; Olmedo et al., 2018) and causes skin rashes in EC users (Maridet et al., 2015; Shim and Kosztyuova, 2018).
Even though ECEAR has accumulated in the environment for over 15 years, few studies have addressed its effects on human health. The skin is usually the first point of contact and the main route of ECEAR exposure. ECEAR contains nicotine, flavor chemicals, solvents, nicotine alkaloids, and tobacco specific nitrosamines (TSNAs) (Son et al., 2020; Khachatoorian et al., 2018; 2019; 2020; Bush and Goniewicz, 2015; Goniewicz and Lee, 2015; Sempio et al., 2019). ECEAR chemicals increased in concentration in a vape shop over a month-long period of monitoring, and concentrations of nicotine reached 108 mg/m2 in heavily used areas (Khachatoorian et al., 2019). ECEAR can also move through spaces and vents, collecting and accumulating on surfaces away from its point of origin (Khachatoorian et al., 2018). Therefore, ECEAR accumulation in indoor environments presents an opportunity for active and passive exposure, especially through the skin.
Understanding how EC fluids and ECEAR affect human skin is a critical knowledge gap. Our goal was to evaluate the responses of human keratinocytes and a 3D tissue model of the human epidermis to EC refill fluids, flavor chemicals, and ECEAR. We identified the flavor chemicals in two popular brands of refill fluid, tested these products and their flavor chemicals at relevant concentrations using both submerged cultures of human keratinocytes and a 3D air-liquid interface (ALI) model (EpiDerm™), and evaluated the effects of ECEAR extracts on these skin models.
METHODS
Refill fluids
Refill fluids were purchased at a local vape shop. Dewberry Cream by Kilo was chosen because it has a high total concentration of flavor chemicals (Hua et al., 2019) and high concentrations of widely used flavor chemicals, including vanillin, ethyl vanillin, maltol and ethyl maltol. Churrios by the Milk Man was chosen because cinnamon-flavored refill fluids adversely affect cultured cells and respiratory tissues in animals (Bahl et al 2013; Behar et al., 2016; Wavreil and Heggland, 2019; Clapp et al., 2019; Fetterman et al., 2018). Each refill fluid had a labeled nicotine concentration of 6 mg/mL. Dewberry Cream was labeled 70/30 glycerin/propylene glycol (VG/PG), and Churrios was labeled MAX VG.
Lab made refill fluids
Custom refill fluids were made in our lab using 70% PG plus one of the following flavor chemicals: 1.7 mg/mL maltol CAS: 118-71-8 (Sigma-Aldrich, St Louis, MO), 5.2 mg/mL ethyl maltol CAS: 4940-11-8 (Sigma-Aldrich, St Louis, MO), 7 mg/mL vanillin CAS: 121-33-5 (Sigma-Aldrich, St Louis, MO), 7.5 mg/mL ethyl vanillin CAS: 121-32-4 (Sigma-Aldrich, St Louis, MO), and 4.2 mg/mL benzyl alcohol CAS: 100-51-6 (Sigma-Aldrich, St Louis, MO). These concentrations of flavor chemicals were chosen to match their concentrations in Dewberry Cream and Churrios refill fluids. PG and ethyl maltol at the above concentrations were used to test dosing exposure protocols (Figure 4). All chemicals were >97% pure. We also confirmed purity using GC/MS.
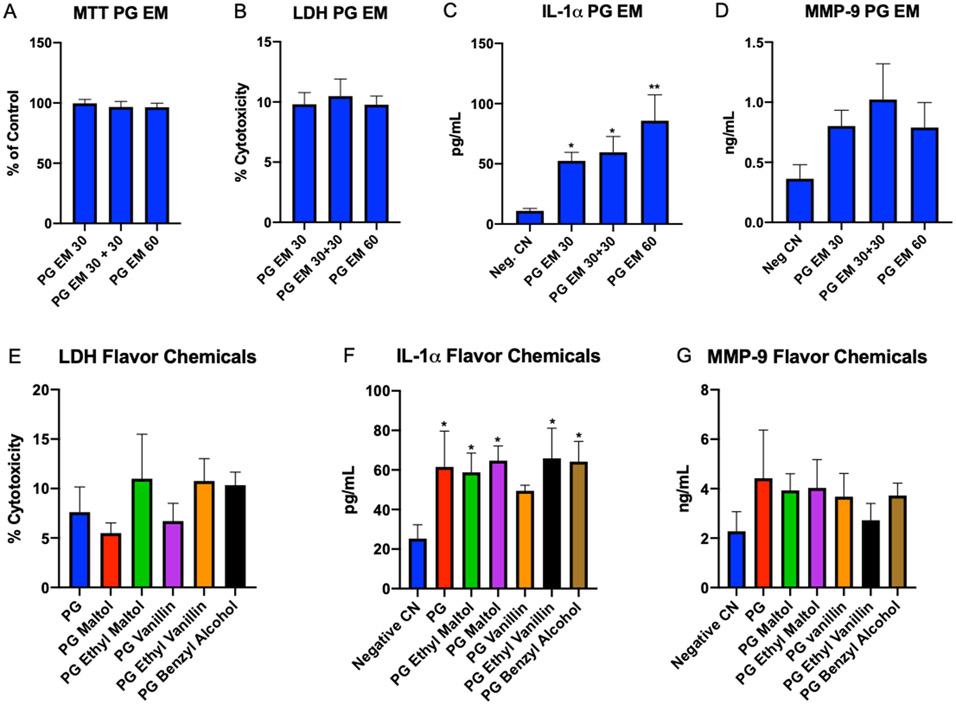
Figure 4: EpiDerm™ Exposed to Lab Made Refill Fluids for 4 hrs.
(A-D) EpiDerm™ exposed for 4 hrs. to PG/ethyl maltol (PG EM) using three protocols (1 = 30 μL, 2 = 30 μL + 30 μL, and 3 = 60 μL). (A) MTT assay, (B) LDH assay, (C) IL-1α secretion, (D) secretion of MMP-9. (E-G) EpiDerm™ exposed to lab made refill fluids with protocol 2 for 4 hrs. total. (E) LDH assay, (F) secretion of IL-1α, and (G) secretion of MMP-9. Each bar is the mean ± standard error of the mean for three independent experiments. * = p < 0.05; ** = p < 0.01.
Identification and quantification of flavor chemicals in refill fluids using GC/MS.
Dewberry Cream and Churrios refill fluids were analyzed by GC/MS. Internal standard-based calibration procedures similar to those described elsewhere were used (Tierney et al., 2016; Omaiye et al., 2019b; Brown et al, 2014), and analyses for 180 flavor-related target analytes and nicotine were performed with an Agilent (Santa Clara, CA) 5975C GCMS system. The capillary column used was a Restek (Bellefonte, PA) Rxi-624Sil MS (30 m long, 0.25 mm id, and 1.4 μm film thickness). For each refill fluid sample, 50 μL was dissolved in 950 μL of isopropanol (Fisher Scientific, Fair Lawn, New Jersey, USA). Prior to analysis, 20 μL of internal standard solution (2 μg/μL of 1,2,3-trichlorobenzene in isopropyl alcohol) was added into the 1 mL diluted refill samples, the aerosol and exhaled extract aliquots. 1 μL of the sample was injected into the GC/MS with a 10:1 split. The injector temperature was 235°C. The GC temperature program for all analyses was as follows: 40°C hold for 2 min; 10°C/min to 100°C; 12°C/min to 280°C and hold for 8 min at 280°C, then 10°C/min to 230°C. The MS was operated at electron ionization mode. The ion source temperature was 226°C. The scan range was from 34 to 400 amu. Each target analyte was quantitated using authentic standard material, and an internal standard (1,2,3-trichlorobenzene) normalized multipoint calibration. The limit of quantification was 10 μg/ml.
Culturing Keratinocytes
CCD 1106 KERTr (ATCC® CRL-2309™) transformed human keratinocytes were cultured on poly-L-lysine coated flasks using the supplier’s protocol in keratinocyte-Serum Free Medium (Gibco 17005-042) with added Keratinocytes Supplements (Gibco 37000-015) including Bovine Pituitary Extract (BPE; Gibco 13028-014) and human recombinant epidermal growth factor (EGF; Gibco 10450-013) further supplemented with a 35 ng/mL of human recombinant epidermal growth factor (EGF; BD cat# 354052). Cells were incubated in a 37°C/5% CO2/95% relative humidity incubator for 24 to 48 hours before processing the cells for experiments. In experiments, keratinocytes were dispersed into single cells and seeded at a density of 1,000 cells/well in a 96 well plate or 5,000 cells/well in an Ibidi μ-Slide 8 Well (Germany). Cells were counted using a hemocytometer.
Cytotoxicity of refill fluids using the MTT Assay
Refill fluids were tested for cytotoxicity using the MTT assay. The enzymatic reduction of 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to MTT-formazan is catalyzed by mitochondrial succinate dehydrogenase. Cells were seeded and incubated for 24 hrs, then exposed to refill fluids for 24 hrs. After exposures, 20 μl of 1 mg/mL of MTT reagent dissolved in phosphate buffered saline was added to each well in a 96 well plate for 2 hrs., then, formazan crystals were solubilized in dimethyl sulfoxide, and absorbance was read at 570 nm. For each refill fluid tested, three independent experiments were performed.
ROS-Glo™ H2O2 Assay
The Promega ROS-Glo™ H2O2 Assay (Madison, WI) was used to measure hydrogen peroxide in culture media, culture media plus refill fluid, and culture media plus refill fluid and cells. According to Promega, this kit is specific for hydrogen peroxide. The non-lytic protocol provided by the manufacturer was followed. Control reactions without cells were used to determine spontaneous hydrogen peroxide generation. The groups studied were medium only, medium with positive control (10 μM menadione), medium with 1% Dewberry Cream, and medium with 1% Churrios. Cells were seeded on a 96 well plate at 1000 cells/well and allowed to attach for 24 hrs., after which they were incubated in medium only, medium with the positive control, medium with 1% Dewberry Cream, and medium with 1% Churrios for 24 hrs. 20μl of H2O2 Substrate solution were added to all samples at 18 hrs. (final 6 hrs. of treatment). When treatment was done, 50μl of medium from each sample well was combined with 50μl of ROS-Glo™ Detection Solution in a separate plate and incubated for 20 minutes at room temperature. A Synergy™ HTX Multi-Mode Microplate Reader (BioTek, VT) was used to read relative luminescence units (RLU) (gain = 135, bottom optics). The experiment was done in triplicate, and each sample was done in duplicate; average luminescence is reported.
Live cell imaging
InVitrogen CellROX™ Green Reagent (Carlsbad, CA) was used to assay oxidative stress in cells exposed to Dewberry Cream and Churrios refill fluids. Cell ROX™ Green reagent is a dye that binds to DNA upon oxidation. For live imaging, cells were plated on μ-Slide Ibidi 8-well chambers (Ibidi) at approximately 5,000 cells/well. After 24 hrs. of attachment to the slide, cells were exposed to Dewberry Cream and Churrios refill fluids for 24 hrs. 50μM menadione was used as a positive control. Cells were exposed to 5 μM CellROX Green for 30 minutes, then rinsed three times with PBS (+). ThermoFisher NucBlue® (Waltham, MA) Live reagent (Hoechst® 33342 dye) was used to stain the nuclei of all cells. Cells were rinsed three times with PBS (+), and medium was added to each well. Fluorescent images were collected using a Nikon TI Eclipse inverted microscope equipped with a LiveCell temperature and CO2-regulating, heated stage (Pathology Devices Inc). Cells were imaged live using a Nikon Eclipse Ti-E microscope with a 37°C, 5% CO2, and 90% relative humidity-regulated stage top LiveCell Incubation Chamber (Pathology Devices, Inc., San Diego, CA). The images were collected using a Nikon 40x objective with 0.75 NA (model: CFI Plan Fluor). A high-resolution Andor Zyla VSC-04941 camera (Andor, Belfast, UK) was used to capture images. Excitation illumination was from a Nikon INTENSILIGHT C-HGFIE lamp. Nikon Elements was used to merge Hoechst® and CellROX™ Green images, and Fiji open-source software was used to sharpen images.
EpiDerm™ Culture
EpiDerm™ is a human 3D skin tissue model that can be exposed to chemicals at the air liquid interface (ALI), as would occur in a natural environment. EpiDerm™ exhibits human epidermal tissue structure and cellular morphology with uniformity and reproducibility. Its 3D structure consists of organized and proliferative basal cells, spinous and granular layers, and cornified epidermal layers that are mitotically and metabolically active. Exposures are applied to the apical side, while medium feeds the basal surface of the tissues. Twenty-four-well EpiDerm™ Skin cultures (Part No. EPI-100) from MatTek Corporation, Ashland, Massachusetts) were transferred from agarose to EPI-100-NMM medium and incubated at 37°C, 5% CO2, 95% relative humidity (RH) for 60 ± 5 min. Tissues were transferred to fresh medium and incubated at 37°C, 5% CO2, 95% RH overnight (20 hrs). The In Vitro EpiDerm™ Skin Irritation Test (EPI-200-SIT) (Kandárová et al., 2009), which has multiple validations from the European Centre for the Validation of Alternative Methods (ECVAM) and follows the Organization for Economic Co-operation and Development (OECD) TG 439 guidelines, was used to determine responses of EpiDerm™ to EC chemicals. According to the validated protocol, 30 μL of liquid test solution is applied to the surface of the tissues for 24 hrs. We followed this protocol and added an extra time point (4 hrs).
EpiDerm™ Exposure to Refill Fluids, Flavor Chemicals, and ECEAR
Three independent experiments were performed for each treatment using inserts that were exposed with the protocol described above. All experiments had a negative control (30 μl of DPBS) and a positive control (30 μl 5% SDS). For refill fluid experiment, EpiDerm™ tissues were exposed for 4 and 24 hrs to three concentrations (10%, 30%, and 100%) of Dewberry Cream or Churrios. When evaluating the exposure protocol, EpiDerm™ tissues were exposed to a PG ethyl maltol mixture (5.2 mg/mL of ethyl maltol in 70% PG) for 4 hrs. using one of three protocols: (1) 30 μL for 4 hrs, (2) 30 μL for 2 hours followed by another 30 μL for 2 additional hrs, or (3) 60 μL for 4 hrs. For the lab made refill fluid experiments, EpiDerm™ tissues were exposed to authentic standards of the dominant flavor chemicals in Dewberry Cream and Churrios with protocol 1. Finally, for the ECEAR experiments, EpiDerm™ tissues were exposed to a piece of paper towel containing ECEAR or 30 μL of ECEAR extract for 4 and 24 hrs. ECEAR paper towel was cut to 0.6 cm2 and applied to the apical side of tissues, then 30 μl of DPBS was applied on top of the paper towel to ensure adhesion to tissues for 4 or 24 hrs.
Cytotoxicity of Refill Fluids and Flavor Chemical using the MTT Assay with EpiDerm™
The enzymatic reduction of MTT was measured as described above with the following modifications. After each exposure, EpiDerm™ inserts were washed with PBS, loaded with 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide according to the MatTek EPI-200-SIT (Skin Irritation Test) protocol, then placed in a 24-well plate containing 300 μL of MTT (1 mg/mL) and incubated for 3 h at 37 °C, 5% CO2. After incubation, tissues were transferred into 24-well plates containing 2.0 mL of isopropyl alcohol (IPA) (Kandárová et al., 2009), and samples were read in a spectrophotometer at an absorbance of 570 nm. The percent of control was determined using the equation: % of control = OD (treated tissue)/OD (untreated tissue) x 100.
Histology of EpiDerm™ Tissues
EpiDerm™ tissues were exposed to DPBS (negative control), 5% sodium dodecyl sulfate (SDS positive control), 100% Dewberry Cream, or 100% Churrios refill fluid for 24 hours. Tissues were fixed in 10% paraformaldehyde overnight in 4 °C and shipped to MatTek for hematoxylin and eosin (H&E) staining (MatTek histology characterization sample preparation procedure). Histology slides were imaged using a DS-Fi1 color camera on a Nikon Eclipse TI inverted microscope using a 20× 0.45 NA (model: CFI Plan Apochromat VC 20X). Nikon Elements software was used to process images.
IL-1α, IL-6, and MMP-9 Secretion from EpiDerm™
After exposures to refill fluids, flavor chemicals, and ECEAR, media were collected from EpiDerm™ cultures, aliquoted into Eppendorf tubes, and stored at −80 °C for later analysis of IL-1α, IL-6, and MMP-9. R&D Systems Quantikine ELISA Human IL-1α/IL-1F1 kit, BioLegend® ELISA MAX™ Deluxe Set Human IL-6 kit, and R&D Systems Quantikine ELISA Human MMP-9 kit were used. Medium only was run to confirm no reaction with reagents. Standard curves were generated using a four-parameter logistic curve fit in GraphPad Prism software (GraphPad, San Diego, California, USA). Media from tissues were run in duplicate on each ELISA, and results were averaged. Each tissue was considered an independent experiment, and three independent experiments were done for each endpoint.
LDH Assay with EpiDerm™
EpiDerm™ cell media were collected from flavor chemical and ECEAR treatments and immediately assayed using the OPS Diagnostics Lactate Dehydrogenase Protocol. Tris-HCl (Sigma-Aldrich, T-3253) and Tris-base (Sigma-Aldrich, T4661) were combined to make TRIS, pH 8. Iodonitrotetrazolium chloride (INT, Sigma I-8377) dissolved in DMSO (Sigma D-8779), phenazine methosulfate (PMS, Sigma P-9625), nicotinamide adenine dinucleotide (NAD, Sigma N-0632), and lithium lactate (Sigma L-1500) were prepared before use and stored at −20°C. 50 μL of 100mM TRIS were added to 50 μl of 50 mM lithium lactate and 50 μl of PMS, INT, NAD. 50 μl of sample were added to each well and after 5 minutes, the absorbance was read on a Synergy™ HTX Multi-Mode Microplate Reader (BioTek, VT). The cytotoxicity was calculated:
Participant Recruitment for ECEAR Generation
A 21-year-old Asian male was recruited for the exhale/ECEAR generation portion of the study. The participant self-reported no use of combustible cigarettes during the study and was told to abstain from using ECs for 1 hour before reporting to our lab. The inclusion criteria were: (1) experienced EC users (at least 3 months) and (2) must use at least 3 mg of nicotine in his current EC. The participant would have been excluded if they were: (1) pregnant or breast feeding, (2) under the age of 18 or over 75 years, (3) a never-user of EC with nicotine, or (4) experiencing any medical conditions. The participant signed informed consent before admission into the study. The project was approved by the UCR Internal review Board (IRB # HS-12-023).
ECEAR Collection
In each 2-hour session on 5 different days, the participant exhaled ad libitum into tubing attached to an acrylic chamber. The EC was an Innokin iTaste MVP 3.0 battery with variable voltage (3V–9V) and wattage (6–30 watts) and with fresh unused Innokin iClear16D dual coil clearomizers (or tanks). A new tank was filled with 2 ml of either Dewberry Cream or Churrios before the first session and used throughout the 5 days. It was not refilled. The EC was set on 6 volts, 1.9 ohms, and 18.9 watts. The acrylic chamber was a rectangular box on wheels that had two ventilation holes. A 2 ft piece of Cole-Parmer Masterflex tubing L/S 18 tubing was attached to the hole on the side of the chamber to allow the participant to exhale into the chamber. A fresh piece of Bounty paper towel was placed on the floor of the chamber for 5 days of ECEAR collection. 5 days was chosen to model a standard work week and to collect sufficient ECEAR for subsequent EpiDerm™ experiments; however, the amount of ECEAR collected would be relatively small compared to what would accumulate in the home/office of an EC users or in a vape shop.
ECEAR Extraction
The paper towel was collected 20 minutes after the last exhale on day 5 and either extracted right away or cut into smaller pieces and stored in in a Ziploc bag inside a mylar bag in a −80°C freezer for EpiDerm™ exposure. To obtain ECEAR extract, paper towel was cut into small pieces for an extraction concentration of 0.1 g/mL of EpiDerm™ Assay Medium. 15 mL Falcon tubes with paper towel and medium were soaked for 1 hour then medium was transferred into 1.5 ml Eppendorf tubes and stored at −80°C for later EpiDerm™ exposures.
Data Analysis
All cytotoxicity assays were carried out using three independent experiments each with different passages of cells, and each experiment had triplicate points. Keratinocyte MTT and CellROX™ data were statistically analyzed with one-way analysis of variance (ANOVA), and each concentration was compared to the untreated control with Dunnett’s post hoc test using Prism software (GraphPad, CA). Data (EpiDerm™ MTT and ELISA) that did not satisfy the assumptions of ANOVA (normal distribution of data and homogeneity of variances) were transformed by Box-Cox transformation after which a one-way ANOVA was applied in MiniTab 17.0 (MiniTab Inc, PA). Dunnett’s post hoc test was used to compare exposures to negative controls. Means were considered significantly different when p values were ≤ 0.05. All graphs were made using GraphPad Prism 8.0 software (GraphPad, San Diego, California, USA).
RESULTS
Flavor Chemicals in Dewberry Cream and Churrios Refill Fluids
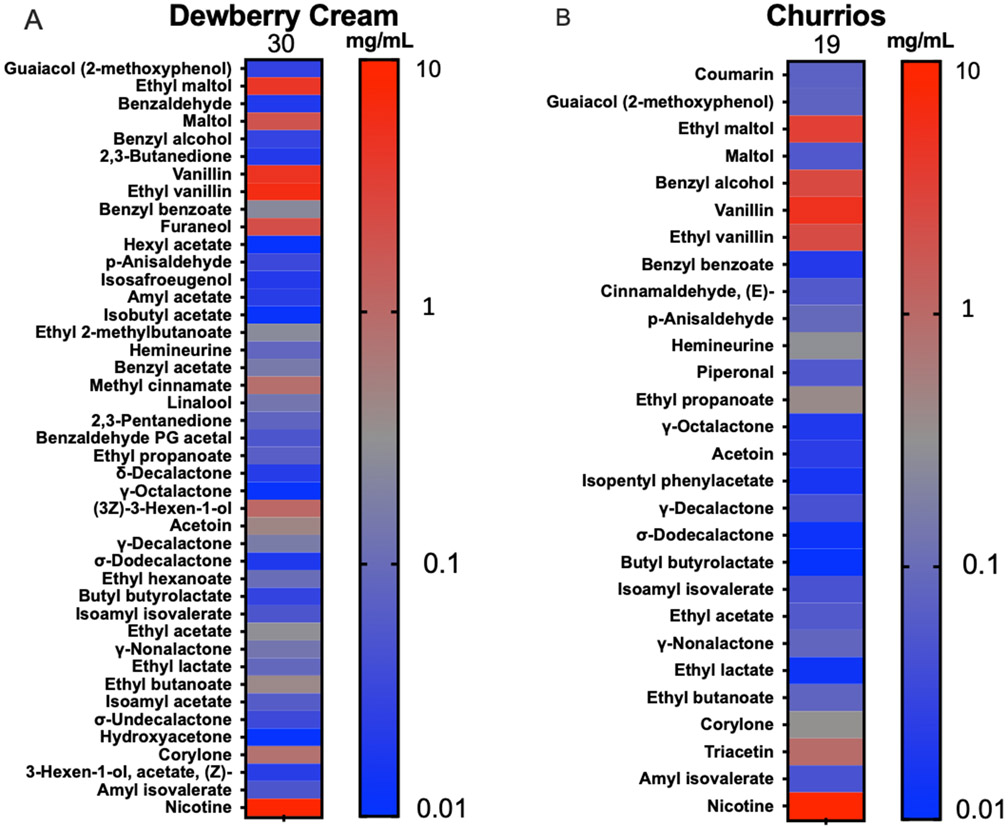
Of the 180 flavor chemicals on our target list, Dewberry Cream and Churrios had 30 and 19 mg/mL, respectively. Heatmaps show all the flavor chemicals and nicotine detected in the refill fluids (left y-axis), and the color gradient scale (right y-axis) shows their concentrations in mg/mL (Figure 1A, B). Total flavor chemicals are listed at the top of the heatmap columns. Flavor chemical concentrations can be found in Supplementary Table 1.
Figure 1: Flavor chemicals in Dewberry Cream and Churrios.
Heatmaps showing concentrations of flavor chemicals and nicotine in (A) Dewberry Cream and (B) Churrios refill fluids. Flavor chemicals are listed from high to low toxicity on the left y-axis as described by Hua et al., 2019. Right y-axis shows a gradient color map of chemical concentrations from 10 mg/mL to 0.01 mg/mL. The total concentration of flavor chemical is indicated above each column.
The flavor profile of Dewberry Cream is described by the manufacturer as mixed berries, honeydew, and cream. The total concentration of flavor chemicals in Dewberry Cream was 30 mg/mL (Figure 1A). Five flavor chemicals had concentrations > 1 mg/mL (7.5 mg/ml ethyl vanillin, 7 mg/mL vanillin, 5.2 mg/mL ethyl maltol, 1.7 mg/mL maltol, 1.6 mg/mL furaneol). Although labeled as 6 mg/mL, the measured nicotine concentration was 6.7 mg/mL.
The flavor profile of Churrios is described by the manufacturer as brown sugar, sweet cinnamon with fresh milk, and honey-infused cereal base. The total concentration of flavor chemicals in Churrios was 19 mg/mL (Figure 1B). Four flavor chemicals were present in Churrios at concentrations > 1 mg/mL (4.6 mg/mL ethyl maltol, 6.2 mg/mL vanillin, 2.2 mg/mL ethyl vanillin, and 4.2 mg/mL benzyl alcohol). Churrios contained 0.25 mg/mL of cinnamaldehyde, and although labeled as 6 mg/mL, the measured nicotine concentration was 7.8 mg/mL.
Cytotoxicity of Refill Fluids When Tested with Keratinocytes
The cytotoxicity of Dewberry Cream and Churrios refill fluids was measured with human keratinocytes using the MTT assay. Keratinocytes were exposed to 0.001, 0.01, 0.1, and 1% of Dewberry Cream or Churrios (Figure 2A). While Dewberry Cream did not significantly affect the cells at any concentration tested, there was a significant increase in cytotoxicity in the 1% Churrios group. In both the 0.01 and 0.1% Dewberry Cream and Churrios, there was an increase in mitochondrial reductase activity, although this was not significantly different than the untreated control.
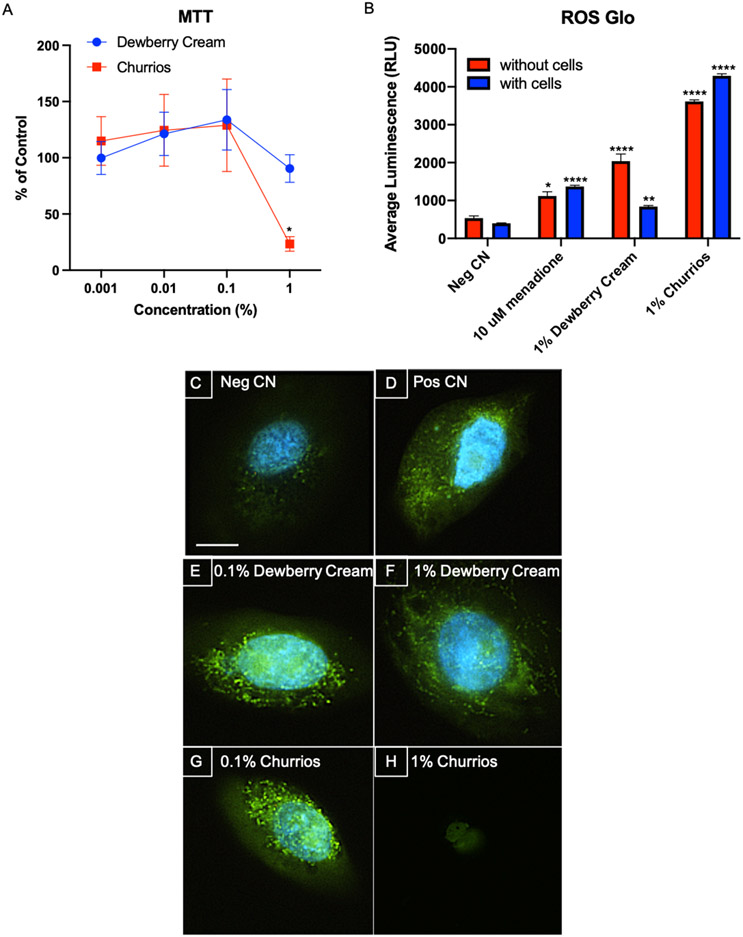
Figure 2: Cytotoxicity and Oxidative Stress in Keratinocytes Exposed to Dewberry Cream and Churrios Fluids for 24 hrs.
(A) The MTT assay y-axis shows the response of cells as a percentage of the untreated control. Each point is the mean ± standard error of the mean for three independent experiments. Dewberry Cream is in blue and Churrios is in red. (B) ROS-Glo™ experiment was done without cells (red) and with cells (blue) in wells. The y-axis shows the average luminescence (RLU). The negative CN is medium only, and the positive control is 10 μM of menadione. Each bar is the mean ± standard deviation for three independent experiments. (C-H) CellROX™ live cell imaging was done with a 40X objective. Representative images are shown. The negative CN is medium only and the positive control is 50 μM of menadione. The scale bar = 10 μm. * = p < 0.05; ** = p < 0.01; *** p < 0.001; **** = p < 0.0001.
Reactive Oxygen Species in Culture Medium and Cells
Extracellular H2O2 was measured in the culture medium, culture medium with refill fluids, and culture medium with refill fluids and cells using the ROS-Glo™ assay, in which H2O2 concentration is directly proportional to luminescence (Figure 2B). The negative control (culture medium only) was slightly luminescent, while the menadione positive control without cells had a significant elevation in luminescence. When no cells were present, addition of Dewberry Cream or Churrios to the culture medium produced a significant increase in luminescence, which was greater than that observed in the positive control. The increase was greater for Churrios than Dewberry Cream. Addition of cells had little effect on the positive and negative controls. However, addition of cells to the medium containing 1% Dewberry Cream significantly reduced luminescence. In contrast, addition of cells to the medium containing 1 % Churrios caused an elevation of luminescence.
Reactive oxygen species (ROS) were measured in cells using CellROX™ Green Reagent, which fluoresces upon oxidation by ROS and subsequent binding to DNA. In figures 2C-H, keratinocytes were exposed to 0.1% and 1% concentrations of Dewberry Cream or Churrios for 24 hours then assayed with CellROX™. The negative control was very weakly fluorescent (Figure 2C). The positive control, 50 μM menadione, was fluorescent, indicating ROS production (Figure 2D). Adding 0.1% and 1% Dewberry Cream or 0.1% Churrios to the culture medium increased CellROX™ fluorescence. However, cells exposed to 1% Churrios did not show fluorescence (Figure 2A).
Cytotoxicity of Refill Fluids When Tested with EpiDerm™
EpiDerm™ tissues were exposed to 10%, 30%, and 100% concentrations of either Dewberry Cream or Churrios refill fluids for 4 and 24 hours. These concentrations were chosen to mimic environmental exposure with the 100% concentration representing refill fluid that leaked/spilled onto the skin. Tissues were then subjected to the MTT assay to determine if exposures were cytotoxic (Figures 3A, B). No significant differences were seen between the untreated control group and the groups treated with Dewberry Cream or with 10 or 30% Churrios. There was a small, but significant, decrease in mitochondrial reductase activity for EpiDerm™ exposed to 100% Churrios for 24 hrs., but this may not be biologically significant as the decrease was very small and did not reach the ISO level of cytotoxicity (< 70% of the control) (Figure 3B).
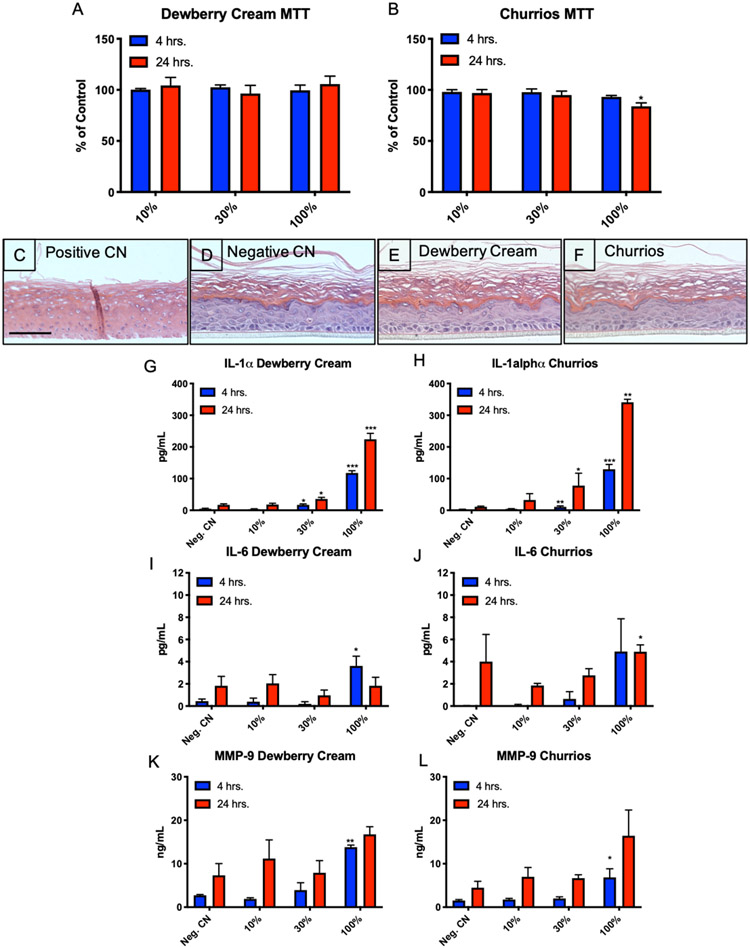
Figure 3: EpiDerm™ Exposed to Dewberry Cream and Churrios.
(A and B) MTT assay in which the y-axis shows the response of cells as a percentage of the untreated control. 4 hr. exposure is in blue, and 24 hr. exposure is in red. (C-F) Histological sections of EpiDerm 24 hours after treatment with DPBS (negative control), 5% SDS (positive control), or refill fluids. Scale bar = 100 μm. (G and H) Secretion of IL-1α, (I and J) secretion of IL-6, and (K and L) secretion of MMP-9. In all experiments, each point is the mean ± standard error of the mean for three independent tissues. * = p < 0.05; ** = p < 0.01; *** p < 0.001.
Histology of EpiDerm™ Exposed to Refill Fluids
Histological sections of EpiDerm™ exposed to refill fluids were evaluated microscopically. The positive control (treated with 5% SDS) lacked cellular integrity, did not have discrete cells, had empty gaps in the tissue, and lacked a clear outer cornified layer (Figure 3C). The negative control (treated with DPBS) (Figure 3D) had a clear stratum corneum, granulosum, spinosum, and basal layer. Dewberry Cream and Churrios exposed tissues (Figures 3E, F) were similar to the negative control. Distinct layers were observed within the tissues, and cells were intact, unlike the positive control.
Refill Fluids Induced Secretion of Inflammatory Proteins from EpiDerm™
IL-1α, IL-6 and MMP-9 are markers of an inflammatory response, and their secretion was examined in culture medium of EpiDerm™ treated with Dewberry Cream and Churrios (Figure 3G-L). IL-1α was significantly elevated in culture medium of tissues exposed to 30% and 100% Dewberry Cream or Churrios. Elevation of Il-1α was first observed at 4 hours of exposure, and it was further elevated at 24 hours.
IL-6 was elevated in culture medium of EpiDerm™ treated with 100% Dewberry Cream at 4 hours but was not significant in the 24-hour sample (Figure 3I). For EpiDerm™ treated with Churrios, IL-6 was elevated in both the 4- and 24-hour samples, but significance was found only in the 24-hour sample (Figure 3J).
Secreted matrix metallopeptidase 9 (MMP-9) was elevated by treatment with 100% Dewberry Cream at both 4 and 24 hours, although only the 4-hour treatment showed significance (Figure 3K). Similar results were obtained with 100% Churrios (Figure 3L). While the 100% Churrios sample at 24 hours was not significant, it had a p value of 0.063.
Effect of Exposure Protocol on Responses
Prior to evaluating individual flavor chemicals, EpiDerm™ was treated with PG containing 5.2 mg/mL of ethyl maltol (the concentration in Dewberry Cream refill fluid) using one of three protocols: (1) one-time exposure to 30 μl of PG/ethyl maltol, (2) two-time exposure to 30 μl of PG/ethyl maltol with exposures done 2 hours apart, and (3) one-time exposure to 60 μl of PG/ethyl maltol (Figures 4A-D). In each protocol, the endpoints were evaluated 4 hours after exposure. None of the exposure protocols produced an effect in the MTT or the LDH assay (Figures 4A, B), indicating treatments were not cytotoxic and were not killing the tissues. IL-1α and MMP-9 were elevated in all three exposure protocols. Elevation was significant for each protocol in the IL-1α assay. Significance was not reached in the MMP-9 assay for any group, although MMP-9 secretion was increased. We used the first protocol for subsequent exposures with authentic standards since it gave significance, and it follows the EpiDerm™ validated protocol.
Exposure of EpiDerm™ to Flavor Chemicals
To determine if PG or specific flavor chemicals in Dewberry Cream and Churrios induced cell death or an inflammatory response, EpiDerm™ tissues were exposed for 4 hours to either PG or PG containing a pure flavor chemical using exposure protocol #1 (Figure 4 E-G). In the LDH assay, the responses in each group were relatively low (~10% greater than the control) and were not significantly different than the untreated control. Therefore, treatments were interpreted to be non-cytotoxic. In contrast, all treatment groups, except PG vanillin, produced a significant increase in IL-1α secretion (Figure 4F). The p value for PG/vanillin was close to significant (0.08). MMP-9 was elevated in all treatment groups; however, none of the groups were significant compared to the negative control (Figure 4G).
Exposure of EpiDerm™ to ECEAR
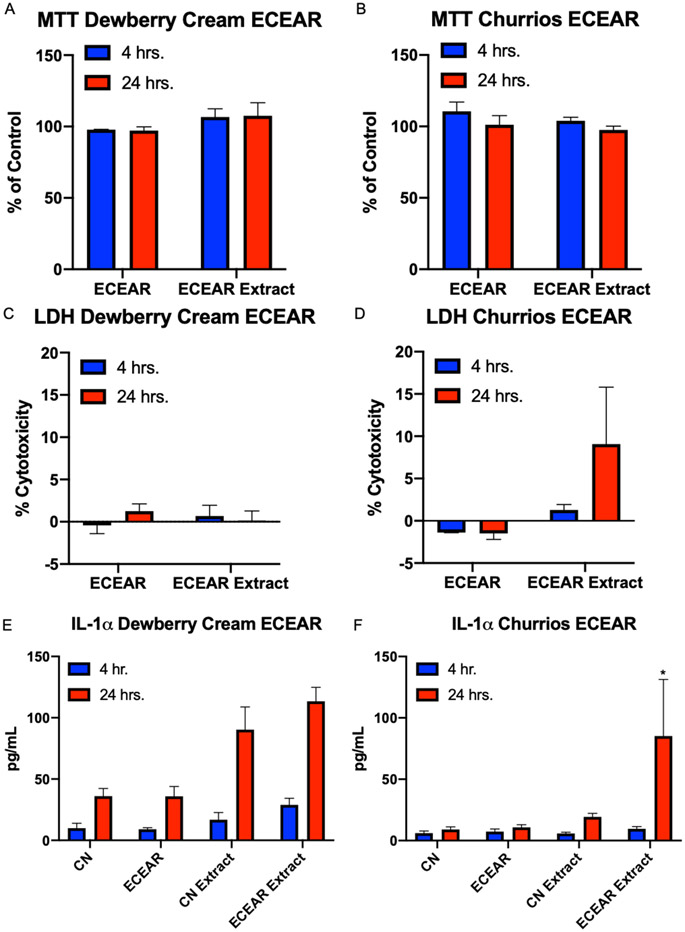
Experiments were done to determine if ECEAR, which builds up on surfaces where vaping occurs, could affect EpiDerm™ (Figure 5A-F). Exposures were done for both 4 and 24 hours using either ECEAR or ECEAR extracts. ECEAR treatments did not produce effects in the MTT or LDH assay when Dewberry Cream was used to create ECEAR (Figure 5A, C). Similar results were obtained with ECEAR made from Churrios, except that there was a small (10%) (non-significant) increase in LDH activity in EpiDerm™ treated with ECEAR extracts for 24 hours (Figure 5D). IL-1α secretion was increased in both Dewberry Cream and Churrios ECEAR extract samples treated for 4 and 24 hours, and significance was observed in the 24-hour Churrios extract (Figure 5E, F).
Figure 5: EpiDerm™ Exposed to ECEAR paper towel and ECEAR paper towel extract for 4 and 24 hrs.
(A and B) MTT assay, (C and D) LDH assay, and (E and F) secretion of IL-1α. Each tissue was exposed to ECEAR or 30 μL of ECEAR extract. Each bar is the mean ± standard error of the mean for three independent experiments. * = p < 0.05.
DISCUSSION
To our knowledge, this is the first study to test cellular responses of keratinocytes and EpiDerm™ tissues to refill fluids, their flavor chemicals, and ECEAR. Dermal exposure is of interest since the skin of EC users comes into direct contact with refill fluids through leakage and spills and through touching of surfaces with ECEAR. Non-users may be passively exposed via the skin when occupying indoor environments where ECEAR has been deposited. Both extracellular and intracellular ROS and secretion of inflammatory cytokines increased in keratinocytes treated with two refill fluids. Both refill fluids also induced secretion of inflammatory biomarkers from EpiDerm™. The release of inflammatory markers from EpiDerm™ was induced specifically by PG, but not flavor chemicals. At concentrations that were not cytotoxic in the MTT and LDH assays, Churrios ECEAR extract elevated IL-1α secretion from EpiDerm™.
Except for a small difference in total flavor chemical concentrations, our flavor chemical analysis of Dewberry Cream was in good agreement with prior studies (Hua et al., 2019; Khachatoorian et al., 2020). The small discrepancy is probably due to variations in manufacturing. Dewberry Cream was not cytotoxic to keratinocytes in the MTT assay at any concentration tested up to 1%. However, Hua et al. showed a decrease in mitochondrial reductase activity in the MTT assay using mouse neural stem cells (mNSC) at 1% refill fluid concentration (Hua et al., 2019). These differences between studies are likely related to the different cell types that were used (mNSC versus human keratinocytes). Stem cells are more sensitive to refill fluids than differentiated cells (fibroblasts), as shown previously (Bahl et al., 2012).
Churrios has not been analyzed previously; however, many other cinnamon-flavored refill fluids have been evaluated for cinnamaldehyde concentration, cytotoxicity, and cellular effects (Behar et al., 2014; Behar et al., 2016; Wavreil and Heggland, 2019; Clapp et al., 2019; Fetterman et al., 2018). Cinnamon-flavored refill fluids are among the most toxic of the many that have been tested in the MTT assay (Bahl et al., 2012; Behar et al., 2016). Their toxicity has been attributed to cinnamaldehyde, which produces an IC50 at 0.009 mg/mL when tested with human embryonic stem cells and human pulmonary fibroblasts (Behar et al., 2016). Cinnamaldehyde concentrations vary in cinnamon-flavored refill fluids (Behar et al., 2016), and Churrios contained a relatively low concentration of cinnamaldehyde (0.3 mg/mL), yet produced toxicity at 1% refill fluid concentration. Churrios also contained benzyl alcohol, which was not present in Dewberry Cream, and may have contributed to Churrios’ cytotoxicity. Benzyl alcohol was cytotoxic to mNSC and human lung epithelial cells (BEAS-2B) in the MTT assay at concentrations <1 mg/mL (Hua et al., 2019), which is considerably lower than the concentration we detected in Churrios refill fluid (4.2 mg/mL).
H2O2 concentration in the culture medium was affected by the refill fluids and keratinocytes. Dewberry Cream and Churrios increased H2O2 levels in the culture media. Similar elevation of ROS has been reported in other refill fluids and aerosols using different analytical methods (Goel et al., 2015; Lerner et al., 2015b; Muthumalage et al., 2018), and was associated with specific flavor chemicals, which all significantly increased ROS, except for vanillin (Muthumalage et al., 2018). In contrast, nicotine lowered the levels of H2O2 (Muthumalage et al., 2018). The decrease which we observed in H2O2 in the Dewberry Cream-containing culture medium with keratinocytes could be due to the release of antioxidants by the cells and/or the ability of aquaporin-3 to transport H2O2 into the cell for breakdown (Miller et al., 2010). This idea is supported by our observation that cells exposed to Dewberry Cream are still metabolically active in the MTT assay. Media containing Churrios had highly elevated levels of H2O2, which were not reduced by the addition of cells. This may be due to the reduced metabolic activity (MTT assay) of keratinocytes exposed to Churrios, which in turn reduced the antioxidizing capacity of the cells.
When labeled with CellROX™, the 0.1% and 1% Dewberry Cream and 0.1% Churrios exposed keratinocytes had mitochondrial fluorescence indicative of ROS. The lack of fluorescence in cells exposed to 1% Churrios was likely due to their decrease in mitochondrial activity, as shown in the MTT assay. Similar increases in ROS were observed in osteoblast-like MG-63 cells treated with cinnamon-flavored refill fluids (Wavreil and Heggland, 2019). In addition to our data, other lines of evidence support the idea that EC refill fluids/aerosols cause oxidative damage to cells. Mitochondrial protein oxidation occurred in mNSC exposed to EC liquids and aerosols in vitro (Zahedi et al., 2019), and superoxide was generated in menthol treated BEAS-2B cells in submerged cultures (Nair et al., 2020). In humans, urinary biomarkers of oxidative stress, 8-OHdG and 8-isoprostane, increased in EC users when compared to non-users (Sakamaki-Ching et al., 2020; Singh et al., 2019) and biomarkers of oxidative stress and inflammation (I-cell adhesion molecule, endothelial ROS, and C-reactive protein) increased in serum of nonsmoking subjects (smoking-naïve) in response to acute EC aerosol inhalation (Chatterjee et al., 2019).
An inflammatory response was seen in EpiDerm™ following exposure to Dewberry Cream and Churrios. Similarly, exposure of monocytes or BEAS-2B cells to refill fluids or EC aerosols induced secretion of biomarkers of inflammation (Lerner et al., 2015b; Muthumalage et al, 2018; 2019; Nair et al., 2020). Concentrations of refill fluids that produced an inflammatory response in Epiderm™ did not produce histological damage or a response in the MTT and LDH assays. Exposure of Epiderm™ to lab made refill fluids containing either PG or PG plus a flavor chemical further showed that PG, not the flavor chemicals, increased the secretion of MMP-9 and IL-1α from the EpiDerm™. This effect in EpiDerm™ agrees with work on gingival epithelial cells, which increased IL-6, IL-8, and MMP-9 secretion when exposed to PG/VG (Beklen and Uckan, 2020). While the concentrations of flavor chemicals we tested did not elevate interleukin secretion in EpiDerm™, flavor chemicals (diacetyl, cinnamaldehyde, acetoin, pentanedione, o-vanillin, maltol and coumarin) did so in monocytes (Muthumalage et al., 2018). The differences in the results between this study and ours could be due to the use of different cell types, flavor chemicals, and concentrations.
Although there are not many studies on the skin of EC users, it is probable that they experience similar effects to those caused by cigarette smoke and thirdhand smoke (THS). Cigarette smoking causes pre-mature aging of skin and slows wound healing, which are both related to increased levels of oxidative stress and extracellular metalloproteases (Frances, 1998; Ortiz, 2012; Silverstein, 1992). THS, tobacco smoke residue deposited on indoor surfaces after smoking has stopped, is analogous to ECEAR (Jacob et al., 2017). In mice, exposure to THS delayed closure and weakened cutaneous wounds, apparently due to an increase in keratins (Martins-Green, 2014). Future studies could address the effects of EC fluids and ECEAR on skin diseases, such as psoriasis and dermatitis, using EpiDerm™ models.
Our study has several limitations. There are many refill fluids with high flavor chemical and nicotine concentrations that could be evaluated in future studies. In the ECEAR study, we did not quantify the flavor chemicals or nicotine in the participant’s exhale; therefore we do not know if the participant was a mouth or lung inhaler, which would affect the concentration of chemicals in ECEAR (Khachatoorian et al., 2020). Other potential toxicants in refill fluids and ECEAR which we did not study, such as, nicotine, formaldehyde, and acetaldehyde, could also affect the skin. Longer ECEAR collection periods would likely produce stronger effects in EpiDerm™ experiments.
In conclusion, this study provides evidence that EC fluids and aerosol residues can adversely affect human skin. Human skin can be exposed to refill fluids due to leakage or spills and/or contact with ECEAR on indoor surfaces. For both refill fluids and ECEAR exposures, concentrations that produced an effect in the keratinocyte and EpiDerm™ assays were within the range users would receive due to spills or touching contaminated surfaces. Our study focused on two popular refill fluids, containing mostly sweet flavors with a relatively low nicotine concentration, available online and in shops. Churrios was more cytotoxic to keratinocytes than Dewberry Cream and both fluids increased extracellular and intracellular ROS. Our work demonstrated a clear increase in inflammatory marker secretion upon ALI exposure of 3D EpiDerm™ tissues to PG, which is present in most EC products. EC users and employees at vape shops should be aware that harm could be caused by handling and/or touching refill fluids, leaky ECs, or by touching ECEAR. Our data suggest a need for regulation of solvents in EC products and accumulation of ECEAR in indoor environments.
Supplementary Material
Acknowledgments:
We thank Tammy Nguyen for help with participant ECEAR collection and preliminary ECEAR collection and processing.
Funding:
This research was supported by Grant #26IR-0018 from the Tobacco-Related Disease Research Program of California (TRDRP), grant # R01ES029741 from the NIEHS and the FDA Center for Tobacco Products, and the Armenian Engineers and Scientists of America Scholarship. The content is solely the responsibility of the authors and does not necessarily represent the official views of TRDRP, NIH, or other granting agencies.
Footnotes
Competing financial interests: All authors declare that they have no actual or potential competing financial interest.
Declaration of Interests: The authors declare no competing interests.
REFERENCES
- Aherrera A, Olmedo P, Grau-Perez M, Tanda S, Goessler W, Jarmul S, Chen R, Cohen JE, Rule AM, Navas-Acien A (2017). The association of e-cigarette use with exposure to nickel and chromium: A preliminary study of non-invasive biomarkers. Env Re 159, 313–320. [DOI] [PubMed] [Google Scholar]
- Bahl V, Lin S, Xu N, Davis B, Wang Y-H, Talbot P (2012). Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reproductive Toxicology 34, 529–537. [DOI] [PubMed] [Google Scholar]
- Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P (2014). Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro 28, 198–208. [DOI] [PubMed] [Google Scholar]
- Behar RZ, Luo W, Lin SC, Wang Y, Valle J, Pankow JF, Talbot P (2016). Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control 25, ii94–ii102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beklen A, Uckan D (2020). Electronic cigarette liquid substances propylene glycol and vegetable glycerin induce an inflammatory response in gingival epithelial cells. Hum Exp Toxicol 2, 960327120943934. [DOI] [PubMed] [Google Scholar]
- Berner B, Mazzenga GC, Otte JH, Steffens RJ, Juang RH, Ebert CD (1989). Ethanol: water mutually enhanced transdermal therapeutic system II: skin permeation of ethanol and nitroglycerin. J Pharm Sci 78, 402–407. [DOI] [PubMed] [Google Scholar]
- Brown JE, Luo W, Isabelle LM, Pankow JF (2014). Candy flavoring in tobacco. N Engl J Med 2014;370:2250–2. [DOI] [PubMed] [Google Scholar]
- Bush D, Goniewicz ML (2015). A pilot study on nicotine residues in houses of electronic cigarette users, tobacco smokers, and non-users of nicotine-containing products. Int. J. Drug Policy 26, 609–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JM, Howell DN, White JR, Andrenyak DM, Layton ME, Roll JM (2014). Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob Control 23, 77–78. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Tao J-Q, Johncola A, Guo W, Caporale A, Langham MC, Wehrli FW (2019). Acute exposure to e-cigarettes causes inflammation and pulmonary endothelial oxidative stress in nonsmoking, healthy young subjects. Am J Physiol Lung Cell Mol Physiol 317, L155–L166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp PW, Lavrich KS, van Heusden CA, Lazarowski ER, Carson JL, Jaspers I (2019). Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am J Physiol Lung Cell Mol Physiol 316, L470–L486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B, Dang M, Kim J, Talbot P (2015). Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 17, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EU Health Programme (2016). Study on the identification of potential risks to public health associated with the use of refillable electronic cigarettes and development of technical specifications for refill mechanisms 1–102. [Google Scholar]
- Farsalinos KE, Kistler KA, Gillman G, Voudris V (2014). Evaluation of Electronic Cigarette Liquids and Aerosol for the Presence of Selected Inhalation Toxins. NICTOB 17, 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K, Voudris V, Poulas K (2015). Are Metals Emitted from Electronic Cigarettes a Reason for Health Concern? A Risk-Assessment Analysis of Currently Available Literature. Int. J. Environ. Res. Publ. Health 12, 5215–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, Robertson RM, Conklin DJ, Bhatnagar A, Hamburg NM (2018). Flavorings in Tobacco Products Induce Endothelial Cell Dysfunction. Arterioscler. Thromb. Vasc. Biol 38, 1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances C (1998). Smoker’s Wrinkles: Epidemiological and Pathogenic Considerations. Clinics in Dermatology 16, 565–570. [DOI] [PubMed] [Google Scholar]
- Frasch HF, Barbero AM (2016). In vitro human epidermal permeation of nicotine from electronic cigarette refill liquids and implications for dermal exposure assessment. Journal of Exposure Science & Environmental Epidemiology 27, 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelloni ML, Sabbione F, Jancic C, Bass JF, Keitelman I, Iula L, Oleastro M, Geffner JR, Trevani AS (2013). NADPH oxidase derived reactive oxygen species are involved in human neutrophil IL-1β secretion but not in inflammasome activation. Eur. J. Immunol 43, 3324–3335. [DOI] [PubMed] [Google Scholar]
- Goel R, Durand E, Trushin N, Prokopczyk B, Foulds J, Elias RJ, Richie JP Jr. (2015). Highly Reactive Free Radicals in Electronic Cigarette Aerosols. Chem Res Toxicol. 28, 1675–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Hajek P, McRobbie H, 2014. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction 109, 500–507. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Lee L, 2015. Electronic cigarettes are a source of thirdhand exposure to nicotine. Nicotine Tob Res. 17, 256–258. doi: 10.1093/ntr/ntu152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA, 2014. E-cigarettes: a scientific review. Circulation 129, 1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig-Do H-T, von Kleist-Retzow JC, Lanz K, Wickenhauser C, Kudin AP, Kunz WS, Wiesner RJ, Schauen M (2007). Human epidermal keratinocytes accumulate superoxide due to low activity of Mn-SOD, leading to mitochondrial functional impairment. J Invest Dermatol 127, 1084–1093. [DOI] [PubMed] [Google Scholar]
- Hua M, Alfi M, Talbot P (2013). Health-Related Effects Reported by Electronic Cigarette Users in Online Forums. J Med Internet Res 15, e59–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Omaiye EE, Luo W, McWhirter KJ, Pankow JF, Talbot P (2019). Identification of Cytotoxic Flavor Chemicals in Top-Selling Electronic Cigarette Refill Fluids. Sci Reports 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Sadah S, Hristidis V, Talbot P (2020). Health Effects Associated With Electronic Cigarette Use: Automated Mining of Online Forums. J Med Internet Res 22, e15684–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, Hendrickson RG (2019). An epidemiologic and clinical description of e-cigarette toxicity. Clin Toxicol (Phila) 57, 287–293. [DOI] [PubMed] [Google Scholar]
- Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A (2014). Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol 88, 1295–1308. [DOI] [PubMed] [Google Scholar]
- Jabaut J, Ather JL, Taracanova A, Poynter ME, Ckless K (2013). Mitochondria-targeted drugs enhance Nlrp3 inflammasome-dependent IL-1β secretion in association with alterations in cellular redox and energy status. Free Radical Biology and Medicine 60, 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P III, Benowitz NL, Destaillats H, Gundel L, Hang B, Martins-Green M, Matt GE, Quintana PJE, Samet JM, Schick SF, Talbot P, Aquilina NJ, Hovell MF, Mao J-H, Whitehead TP (2017). Thirdhand Smoke: New Evidence, Challenges, and Future Directions. Chem. Res. Toxicol 30, 270–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob SE, Scheman A, McGowan MA, (2018). Propylene Glycol. Dermatitis 29, 3–5. [DOI] [PubMed] [Google Scholar]
- Kandárová H, Hayden P, Klausner M, Kubilus J, Kearney P, Sheasgreen J, (2009). In vitro skin irritation testing: Improving the sensitivity of the EpiDerm™ skin irritation test protocol. Altern Lab Anim 37, 671–689. [DOI] [PubMed] [Google Scholar]
- Khachatoorian C, Jacob P III, Benowitz NL, Talbot P (2018). Electronic cigarette chemicals transfer from a vape shop to a nearby business in a multiple-tenant retail building. Tob Control. 28, 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachatoorian C, Jacob P, Sen A, Zhu Y, Benowitz NL, Talbot P (2019). Identification and quantification of electronic cigarette exhaled aerosol residue chemicals in field sites. Environmental Research 170, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachatoorian C, McWhirter KJ, Luo W, Pankow JF, Talbot P (2020). Tracking the Movement of Electronic Cigarette Flavor Chemicals and Nicotine from Refill Fluids to Aerosol, Lungs and Exhale. bioRxiv 24, 2020.11.13.382309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML (2014). Carbonyl Compounds in Electronic Cigarette Vapors: Effects of Nicotine Solvent and Battery Output Voltage. Nic Tob 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara-Bergstrom T, Knutson K, DeNoble LJ, Goates CY, (1990). Percutaneous absorption enhancement of an ionic molecule by ethanol-water systems in human skin. Pharm Res 7, 762–766. [DOI] [PubMed] [Google Scholar]
- Kuswahyuning R, Roberts MS (2014). Concentration Dependency in Nicotine Skin Penetration Flux from Aqueous Solutions Reflects Vehicle Induced Changes in Nicotine Stratum Corneum Retention. Pharm Res 31, 1501–1511. [DOI] [PubMed] [Google Scholar]
- Lane ME, (2013). Skin penetration enhancers. International Journal of Pharmaceutics 447, 12–21. [DOI] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, Done D, Kurtzman R, Ossip DJ, Robinson R, McIntosh S, Rahman I, (2015a). Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environmental Pollution 198, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I, (2015b). Vapors Produced by Electronic Cigarettes and E-Juices with Flavorings Induce Toxicity, Oxidative Stress, and Inflammatory Response in Lung Epithelial Cells and in Mouse Lung. PLoS ONE 10, e0116732–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Rahman I, (2016). Mitochondrial redox system, dynamics, and dysfunction in lung inflammation and COPD. International Journal of Biochemistry and Cell Biology 1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina G, Castagnoli C, Ghione G, Passini V, Adami G, Filon FL, Crosera M (2017). Skin contamination as pathway for nicotine intoxication in vapers. Toxicology in Vitro 41, 102–105. [DOI] [PubMed] [Google Scholar]
- Maina G, Castagnoli C, Passini V, Crosera M, Adami G, Mauro M, Filon FL (2016). Transdermal nicotine absorption handling e-cigarette refill liquids. Regul. Toxicol. Pharmacol 74, 31–33. [DOI] [PubMed] [Google Scholar]
- Malińska D, Więckowski MR, Michalska B, Drabik K, Prill M, Patalas-Krawczyk P, Walczak J, Szymański J, Mathis C, Toorn M, Luettich K, Hoeng J, Peitsch MC, Duszyński J, Szczepanowska J (2019). Mitochondria as a possible target for nicotine action. Journal of Bioenergetics and Biomembranes 51, 259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridet C, Atge B, Amici J-M, Taïeb A, Milpied B (2015). The electronic cigarette: the new source of nickel contact allergy of the 21st century? Contact Derm. 73, 49–50. [DOI] [PubMed] [Google Scholar]
- Martins-Green M, Adhami N, Frankos M, Valdez M, Goodwin B, Lyubovitsky J, Dhall S, Garcia M, Egiebor I, Martinez B, Green HW, Havel C, Yu L, Liles S, Matt G, Destaillats H, Sleiman M, Gundel LA, Benowitz N, Jacob P, Hovell M, Winickoff JP, Curras-Collazo M (2014). Cigarette smoke toxins deposited on surfaces: implications for human health. PLoS ONE 9, e86391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin MS, Avery KC, Ballentine RM, Flora JW, Gardner W, Karles GD, Pithawalla YB, Smith DC, Ehman KD, Wagner KA (2020). Formation of Diacetyl and Other α-Dicarbonyl Compounds during the Generation of E-Vapor Product Aerosols. ACS Omega 5, 17565–17575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI (2016). Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. NICTOB 18, 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EW, Dickinson BC, Chang CJ (2010). Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. PNAS. 107, 15681–15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I (2018). Inflammatory and Oxidative Responses Induced by Exposure to Commonly Used E-Cigarette Flavoring Chemicals and Flavored e-Liquids without Nicotine. Front Physiol 8, 733–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T, Lamb T, Friedman MR, Rahman I (2019). E-Cigarette Flavored Pods Induce Inflammation, Epithelial Barrier Dysfunction, and DNA Damage in Lung Epithelial Cells and Monocytes. Sci Reports 9: 19035–11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V, Tran M, Behar RZ, Zhai S, Cui X, Phandthong R, Wang Y, Pan S, Luo W, Pankow JF, Volz DC, Talbot P (2020). Menthol in electronic cigarettes: A contributor to respiratory disease? Toxicology and Applied Pharmacology 407, 115238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems, Eaton DL, Kwan LY, Stratton K, (2018). Public Health Consequences of E-Cigarettes. [Google Scholar]
- Olmedo P, Goessler W, Tanda S, Grau-Perez M, Jarmul S, Aherrera A, Chen R, Hilpert M, Cohen JE, Navas-Acien A, Rule AM (2018). Metal Concentrations in e-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environmental Health Perspectives 126:027010–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye EE, McWhirter KJ, Luo W, Pankow JF, Talbot P (2019a). High-Nicotine Electronic Cigarette Products: Toxicity of JUUL Fluids and Aerosols Correlates Strongly with Nicotine and Some Flavor Chemical Concentrations. Chem. Res. Toxicol 32, 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye EE, McWhirter KJ, Luo W, Tierney PA, Pankow JF, Talbot P (2019b). High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci Rep 9, 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A and Grando SA (2012). Smoking and the skin. Int J Dermatol, 51: 250–262. [DOI] [PubMed] [Google Scholar]
- Pavlou P, Rallis M, Deliconstantinos G, Papaioannou G, Grando SA (2009). In-vivo data on the influence of tobacco smoke and UV light on murine skin. Toxicol Ind Health 25, 231–239. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Døssing M (2014). A systematic review of health effects of electronic cigarettes. Preventive Medicine 69, 248–260. [DOI] [PubMed] [Google Scholar]
- Sakamaki-Ching S, Williams M, Hua M, Li J, Bates SM, Robinson AN, Lyons TW, Goniewicz ML, Talbot P (2020). Correlation between biomarkers of exposure, effect and potential harm in the urine of electronic cigarette users. BMJ Open Resp Res 7:e000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempio C, Lindley E, Klawitter J, Christians U, Bowler RP, Adgate JL, Allshouse W, Awdziejczyk L, Fischer S, Bainbridge J, Vandyke M, Netsanet R, Crume T, Kinney GL (2019). Surface Detection of THC Attributable to Vaporizer Use in the Indoor Environment. Sci Rep 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim TN, Kosztyuova T (2018). Allergic Contact Dermatitis to Electronic Cigarette. Dermatitis 29, 94–95. [DOI] [PubMed] [Google Scholar]
- Silverstein P (1992). Smoking and wound healing. The American Journal of Medicine 93, S22–S24. [DOI] [PubMed] [Google Scholar]
- Singh KP, Lawyer G, Muthumalage T, Maremanda KP, Khan NA, McDonough SR, Ye D, McIntosh S, Rahman I (2019). Systemic biomarkers in electronic cigarette users: implications for noninvasive assessment of vaping-associated pulmonary injuries. ERJ Open Res 5, 00182–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y, Giovenco DP, Delnevo C, Khlystov A, Samburova V, Meng Q (2020). Indoor Air Quality and Passive E-cigarette Aerosol Exposures in Vape-shops. Nicotine Tob Res. 1772–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele JJ, Hsieh SN, Briviba K, Sies H (1999). Protein oxidation in human stratum corneum: susceptibility of keratins to oxidation in vitro and presence of a keratin oxidation gradient in vivo. JID 113, 335–339. [DOI] [PubMed] [Google Scholar]
- Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF (2016). Flavour chemicals in electronic cigarette fluids. Tob Control 25, e10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trtchounian A, Talbot P (2010). Electronic nicotine delivery systems: is there a need for regulation? Tob Control 20, 47–52. [DOI] [PubMed] [Google Scholar]
- Trtchounian A, Williams M, Talbot P (2010). Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine Tob Res. 12, 905–912. [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Ohta K, Inaba Y, Kunugita N (2013). Determination of carbonyl compounds generated from the E-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci 29, 1219–1222. [DOI] [PubMed] [Google Scholar]
- US Department of Labor, Occupational Safety and Health Administration, Directorate of Technical Support and Emergency Management, Office of Science and Technology Assessment. “Occupational Exposure to Flavoring Substances: Health Effects and Hazard Control” April 1, 2009. Accessed December 7, 2020. https://www.osha.gov/dts/shib/shib10142010.html
- US Food and Drug Administration, (2012). Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List. Accessed January 27, 2021. https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/harmful-and-potentially-harmful-constituents-tobacco-products-and-tobacco-smoke-established-list
- US Food and Drug Administration, (2020). Enforcement priorities for electronic nicotine delivery systems (“ENDS”) and other deemed products on the market without premarket authorization 2020. [Google Scholar]
- Wavreil FDM, Heggland SJ (2019). Cinnamon-flavored electronic cigarette liquids and aerosols induce oxidative stress in human osteoblast-like MG-63 cells. Toxicology Reports 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander G, Norbäck D, Lindgren T (2001). Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med 58, 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P (2013). Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS ONE 8, e57987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Bozhilov K, Ghai S, Talbot P (2017). Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS ONE 12, e0175430–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Bozhilov KN, Talbot P (2019). Analysis of the elements and metals in multiple generations of electronic cigarette atomizers. Environmental Research 175, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Ventura J, Loza A, Wang Y, Talbot P (2020). Chemical Elements in Electronic Cigarette Solvents and Aerosols Inhibit Mitochondrial Reductases and Induce Oxidative Stress. Nicotine Tob Res. 22, S14–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Morita A, Tsuji T (2000). Alterations of extracellular matrix induced by tobacco smoke extract. Arch Dermatol Res 292, 188–194. [DOI] [PubMed] [Google Scholar]
- Zahedi A, Phandthong R, Chaili A, Leung S, Omaiye E, Talbot P (2019). Mitochondrial Stress Response in Neural Stem Cells Exposed to Electronic Cigarettes. ISCIENCE 16, 250–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorin S, Kuylenstierna F, Thulin H (1999). In vitro test of nicotine's permeability through human skin. Risk evaluation and safety aspects. The Annals of Occupational Hygiene 43, 405–413. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.