Abstract
Objective
The study determined the comparative renal functions on patients with diabetes treated with ACE inhibitors (ACEIs) plus either thiazide diuretics or calcium channel blockers (CCBs) in Northwestern Ethiopia.
Design
Retrospective cohort study design was employed to collect the data from medical records of patients with diabetes followed for 1–5 years (N=404).
Setting
The medical records of patients in chronic diabetic follow-up clinics of the hospital.
Participants
All the patients with diabetes medical records in Northwestern Ethiopian specialised hospital.
Main outcome measures
Exposures were ACEIs plus thiazide diuretics or CCBs collected from March to June 2020. Outcomes were defined as declining in estimated glomerular filtration rate (eGFR) values by ≥30% from the baseline recorded from 2015 to 2019. Descriptive and analytical statistics were illustrated to compare the study groups. Kaplan-Meier with log- rank test was used to plot the survival analyses curve. Potential factors substantially associated to renal events were examined using cox proportional hazards model.
Result
About 20% of patients developed renal events and significant numbers were from hydrochlorothiazide (HCT) users. The mean eGFR levels were significantly higher in patients on CCBs users over the follow-up years compared with HCT-based users. The CCBs users had an 18.8 mL/min/1.73 m2 higher in eGFR levels at the end of the follow-up period than HCT users (p<0.001). HCT users had shorter survival probability overtime to develop the outcomes compared with CCBs users (p=0.003). The CCBs-based regimen prevented risks of declining in renal function by 56.4% than HCT (p=0.001). Hazards of declining in eGFR levels were 93% higher for the patients with initial systolic blood pressure (SBP) levels were more than 150 mm Hg (p=0.006).
Conclusion
Compared with HCT, patients on CCBs had significantly prevented risks of renal events. However, both groups appeared with the same cardiovascular events. HCT-based regimen and higher initial SBP levels were significantly associated with eGFR reductions.
Keywords: diabetic nephropathy & vascular disease, diabetes & endocrinology, chronic renal failure
Strengths and limitations of this study.
This study can contribute significant changes on clinical decision-making processes on the existing clinical practices.
As in any retrospective observational study, there is potential confounders, and thus, this result should be interpreted cautiously.
It was a single-centred and used secondary data that potentially missed some important variables.
Besides, there was incapability to measure levels of adherence to the combined therapy.
Since no documentations on proteinuria and albuminuria to creatinine ratios, only estimated glomerular filtration rate values were used to characterise the renal function status.
Introduction
Diabetic kidney disease (DKD)/diabetic nephropathy is a microvascular complication of diabetes mellitus (DM).1 2 The surge in the occurrence of this complication has been obviously linked with the increase in DM prevalence.3 4 In 2018, WHO reported 422 million diabetic cases globally.5 Diabetes is growing in the sub-Saharan countries, for instance, it was 2.13 million in Ethiopia.6 Over 90% of the patients are type 2 DM (T2DM) type.7 DKD is commonly associated with T2DM8 and increases along with the surge in T2DM occurrences.9 Nearly 40% of patients with diabetes developed DKD.10 11 DKD is the leading causes of chronic kidney diseases (CKD),10 end-stage kidney disease, morbidity, mortality and increased treatment costs,12 13 which generally remain as a global public health threat.14 Patients with diabetes complicated with CKD have higher rates of mortality and increased risk of atherosclerotic cardiovascular diseases (ASCVDs) compared with either diabetes or CKD alone,15 and is common in T2DM.16 It has been evident that minor renal dysfunction accelerates mortality and ASCVD rates.13 17
It has been reported that the increase in blood pressure (BP),18 19 poor glycaemic controls and proteinuria as the major factors facilitating the occurrence of DKD.20 21 Patients with uncontrolled glucose measures and higher BP levels had 50% risk of developing CKD.21 However, glycaemic control alone reported not to reduce the risk of developing CKD among diabetes for the last 20 years.2 The target BP for patients with DM has always been controversial and kept changing.22 23 Achieving the desired BP levels with an appropriate combination of antihypertensives could potentially slow and prevent the progressions to DKD.13 24–26 In this regard, the combination of ACE inhibitors (ACEIs) with thiazide diuretics or calcium channel blockers (CCBs) is the first-line antihypertensive combination treatment currently. This is due to their credible benefits in reducing proteinuria and conserving estimated glomerular filtration rate (eGFR).24 27 28 Clinical trials recommended that patients with DM with high risk of increased BP requires an average of more than two different BP lowering agents to achieve the target BP to <130/80 mm Hg.25 29 However, only fewer reports compared the significant consequences of the combined therapies on renal events and ASCVDs incidences in patients with diabetes.30
Individuals with early onset of DKD are not likely to come with overt symptoms, and the symptoms are non-specific. The specific symptoms would be exhibited when the renal disease advances.1 Majority of patients die from cardiovascular diseases, ESRD and infections before having transplant.10 Different combinations of antihypertensive medications are known to prevent renal dysfunctions, ASCVDs and CKD complications; however, these comparative effectiveness between different combinations on outcomes such as renal functions and CV events have not yet been studied in Ethiopia. We hope that identifying better combinations of antihypertensive therapy between ACEIs plus either thiazide diuretics or CCBs for the renal event reductions in diabetes is important. Therefore, this study was aimed to determine the comparative renal outcomes of patients with diabetes treated with ACEIs plus either thiazide diuretics or CCBs.
Methods
Setting and design
This study was conducted in Northwestern Ethiopian comprehensive specialised hospital from 17 March 2020 to 19 June 2020. The hospital is serving for more than 5 million patients annually and 8000 of them were patients with DM.31 A hospital-based comparative retrospective cohort study was employed to determine the renal functions of diabetes taking combinations of thiazide diuretics or CCBs and ACEIs. Medical records of patients registered from January 2015 to December 2019 were included. The time at which the second BP lowering agent added was considered as an index date. The reviewed medical records had a minimum of 1 year and a maximum of 5 years follow-up documented data. Screening once a year is recommended to assess the eGFR levels in all diabetes and patients with comorbid hypertension.32
Sample
The sample size calculation assumed 50% prevalence of renal events among the exposed group. Using Epi-info, we considered 5% for two-tailed type-I error (Zα=1.96); 80% power; two-sided 95% confidence level and 0.35 HRs on renal events. The Fleiss WC calculated the sample size of 366. The final analysis comprised a total of 404 patients’ recordings after considering 10% contingency for the possible missed and lost data. Using simple random sampling technique, medical records that met the inclusion criteria were included with a 1:1 ratio in each group. Medical records of the patients were eligible in the study if they met the following inclusion criteria: (1) Patients with diabetes ≥18 years; (2) Had been diagnosed with diabetes and treated with the combinations of ACEIs plus either thiazide diuretics or CCBs and (3) Had serum creatinine (SCr) within 365 days prior to start initiations of second BP lowering agents. Whereas medical records with <1-year follow-up data and patients on dialysis, had previous kidney transplant or most recent eGFR <15 mL/min/1.73 m2 were excluded.
Outcome measurements
The first occurrence in declining the eGFR values (measured with ml/min/1.73 m2) by ≥30% from the baseline, initiation of dialysis or renal transplant was used as cut-off points to have the outcome/renal impairment (composite significant kidney event end points).30 33 34 All eGFR values were estimated from the SCr records using CKD Epidemiology Collaboration estimating equation.35
Operational definitions
In this study, thiazide diuretics mean hydrochlorothiazide (HCT); CCBs comprised either amlodipine or nifedipine; occurrence of renal events defined if the first records with ≥30% reductions in eGFR values measured by ml/min/1.73 m2 from the baseline; microvascular complications occurred if retinopathy, nephropathy and neuropathy diagnosed as per the clinicians workups; macrovascular or ASCVDs defined as the first diagnosis of acute myocardial infraction, acute coronary syndrome, transient ischaemic stroke, peripheral arterial disease, stroke and angina.
Data collection procedures and quality control technique
Three experienced clinical pharmacists collected the data after trained for 2 days regarding the data collection instrument. Diabetic patients’ medical recordings (logbooks) documented from January 2015 to December 2019 in the chronic follow-up clinic were used. Then, the lists reentered into Microsoft Office Excel 2013 and checked for duplications. Based on the lists, data (baseline SCr, and eGFR and other variables) were extracted from those medical archives retrospectively. The data abstraction format was prepared after reviewed different related clinical literatures on similar topics and some modifications were made that considered the local clinical settings. Pretest was done on 5% of the sample in the study area to ensure completeness of abstraction format. The medical records used for pretest were excluded from the final analyses. Then, an appropriate amendment was employed. The supervisor explicitly clarified the purpose of the study and data abstraction tool; and monitored the data collection closely.
Data processing and analyses
Data were entered EPI-INFO V.7 and analysed using the SPSS V.25. Both descriptive and analytical analyses were executed. Frequency tables, graphs and cross tabulations were used to present the descriptive findings. Further, Shapiro-Wilk test for the test of normality, and χ2 test were performed to compare the baseline sociodemographics and clinical characteristics, comorbidities, medications and dosage strengths differences between the comparative groups. Mann-Whitney U tests and independent t-test for median and mean differences (MD) were employed, respectively. The patients in both comparative study groups were previously treated for the combinations of either ACEIs plus CCBs or ACEIs plus thiazide diuretics and followed for the occurrence of the outcomes. Times from declining in eGFR levels and changes were estimated by means of the Kaplan-Meier procedure. The log-rank test and Cox-proportional hazard regression model were also used to test if there were significant changes in the renal functions between different sets of predictor variables. Recordings that were defaulted and lost in the retrospective follow-up period were considered as censored. Variables having p<0.05 were considered as statistically significant for the occurrence of reducing the eGFR measures.
Patient and public involvement
No patient involved.
Result
Sociodemographic and baseline characteristics of patients
The total sample size was 404 patients with diabetes, of whom half (50%) were taking combinations of ACEIs and HCT and the other half were on ACEIs and CCBs-based regimens. The mean (±SD) age of patients was 61.7 (±10.7) years and more than half (53.2%) were females. More than 90% of the study individuals were patients with T2DM. At baseline, the median blood pressure (BP) (systolic blood pressure (SBP)/diastolic blood pressure (DBP)) and total cholesterol levels were higher in patients taking CCBs-based regimen. Similarly, majority of CCBs-based regimen users were taking lipid lowering agents; and atorvastatin was the most prescribed one. Patients taking enalapril were overrepresented (98%) and almost both groups used it equally. Half of the patients had 90 mL/min/1.73 m2 eGFR levels at the baseline. Generally, at the baseline the medical records of the study subjects showed comparable sociodemographic and clinical characteristics (table 1). Metabolic syndrome (dyslipidaemia, obesity) (16.3% vs 11.4%) and macrovascular complications (8.4% vs 11.9%) were the most common comorbidities on HCT-based and CCBs-based users, respectively (online supplemental table 1). Among the participants, nearly one-third (29.5%) were taking Neutral Protamine Hagedorn (NPH), followed by combinations of NPH plus metformin (27.5%) (online supplemental table 2).
Table 1.
Sociodemographic and baseline characteristics of patients with diabetes receiving two classes of antihypertensive agents combined with ACEIs (N=404)
| Variables categories | HCT-based users (n=202) (%) | CCBs-based users (n=202) (%) | Total participant N (%) | P value |
| Sex | ||||
| Male | 85 (42.1) | 104 (51.5) | 189 (46.8) | 0.058 |
| Female | 117 (57.9) | 98 (48.5) | 215 (53.2) | |
| Age (in years) | ||||
| <Mean (61.7) | 100 (49.5) | 103 (51) | 203 (50.2) | 0.765 |
| ≥Mean (61.7) | 102 (50.5) | 99 (49) | 201 (49.8) | |
| Residence | ||||
| Urban | 60 (29.7) | 56 (27.7) | 116 (28.7) | 0.66 |
| Rural | 142 (70.3) | 146 (72.3) | 288 (71.3) | |
| DM type | ||||
| T1DM | 18 (8.9) | 20 (10.0) | 38 (9.4) | 0.733 |
| T2DM | 184 (91.1) | 182 (90.0) | 366 (90.6) | |
| DM duration (years) | ||||
| <10 (6–14) | 106 (52.5) | 106 (52.5) | 212 (52.2) | 1 |
| in Median (IQR) | ||||
| ≥10 (6–14) | 96 (47.5) | 96 (47.5) | 192 (47.5) | |
| HTN duration (years) | ||||
| <7 (5–12) | 114 (56.4) | 106 (52.5) | 220 (54.5) | 0.424 |
| In median (IQR) | ||||
| ≥7 (5–12) | 88 (43.6) | 96 (47.5) | 184 (45.5) | |
| Baseline FBS (mg/dL) | ||||
| <165 (126–208) | 106 (52.5) | 97 (48) | 203 (50.2) | 0.37 |
| In median (IQR) | ||||
| ≥165(126–208) | 96 (47.5) | 105 (52) | 201 (49.8) | |
| Baseline BP (mm Hg) | ||||
| <150(140–160) | 156 (77.2) | 127 (62.9) | 283 (70) | 0.002 |
| SBP in median (IQR) | ||||
| ≥150(140–160) | 46 (22.8) | 75 (37.1) | 121 (30) | |
| DBP | ||||
| <85(80–90) | 123 (60.9) | 90 (44.6) | 213 (52.7) | 0.001 |
| DBP | ||||
| ≥85(80–90) | 79 (39.1) | 112 (55.4) | 191 (47.3) | |
| Baseline lipid profiles (mg/dL): (median (IQR)) | ||||
| TC | ||||
| <177 (142–215) | 43 (21.3) | 44 (21.8) | 87 (21.5) | 0.904 |
| ≥177 (142–215) | 159 (78.7) | 158 (78.2) | 317 (78.5) | |
| TG | ||||
| <163(128–206) | 41 (20.3) | 50 (24.8) | 91 (22.5) | 0.284 |
| ≥163(128–206) | 161 (79.7) | 152 (75.2) | 313 (77.5) | |
| HDL | ||||
| <40 (30-49) | 18 (8.9) | 27 (13.4) | 45 (11.1) | 0.155 |
| ≥40 (30–49) | 184 (91.1) | 175 (86.6) | 359 (88.9) | |
| LDL | ||||
| <106 (79–126) | 12 (5.9) | 13 (6.4) | 25 (6.2) | 0.836 |
| ≥106 (79–126) | 190 (94.1) | 189 (93.6) | 379 (93.8) | |
| Baseline DM agents | ||||
| Metformin | 34 (16.8) | 42 (20.8) | 76 (18.8) | 0.084 |
| NPH | 45 (22.3) | 63 (31.2) | 108 (26.7) | |
| Glibenclamide | 6 (3) | 2 (0.1) | 8 (2) | |
| Metformin+glibinclamide | 81 (8.9) | 58 (28.7) | 139 (34.4) | |
| Metformin+NPH | 27 (13.4) | 27 (13.4) | 54 (13.4) | |
| Metformin+glibinclamide+NPH | 9 (4.5) | 10 (4.6) | 19 (4.7) | |
| Baseline lipid lowering agents | ||||
| Atorvastatin | 44 (21.8) | 62 (30.7) | 106 (26.2) | 0.011 |
| Simvastatin | 35 (17.3) | 21 (10.4) | 56 (13.4) | |
| Baseline ACEIs/ARBs type: enalapril | 197 (97.5) | 199 (98.5) | 396 (98) | |
| Candesartan | 2 (0.1) | 1 (0.5) | 3 (0.7) | |
| Losartan | 3 (1.5) | 1 (0.5) | 4 (1) | |
| Captopril | 0 | 1 (0.5) | 1 (0.2) | |
| Baseline eGFR (ml/min/1.73 m2) | ||||
| 15–30 | 1 (0.5) | 6 (3) | 7 (1.7) | |
| 30–45 | 3 (1.5) | 14 (6.9) | 17 (4.2) | ** |
| 45–60 | 8 (4) | 14 (6.9) | 22 (5.4) | |
| 60–90 | 83 (41.1) | 71 (35.1) | 154 (38.1) | |
| ≥90 | 107 (53) | 97 (48) | 204 (50.5) | |
Bold values indicated significant at p<0.05.
** Indicates Violated to achieve the χ2 assumptions.Bold values indicated significant at p<0.05
ACEIs, ACE inhibitors; ARB, Angiotensin Receptor Blocker; BP, blood pressure; CCBs, alcium channel blockers; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FBS, fasting blood sugar; HCT, hydrochlorothiazide; HDL, high density lipoprotein; HTN, hypertension; LDL, low density lipoprotein; NPH, Neutral Protamine Hagedorn; SBP, systolic blood pressure; TC, total cholesterol; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TG, total glyceride.
bmjopen-2020-048442supp001.pdf (113.1KB, pdf)
Mean eGFR values across different follow-up years in HCT and CCBs-based regimens
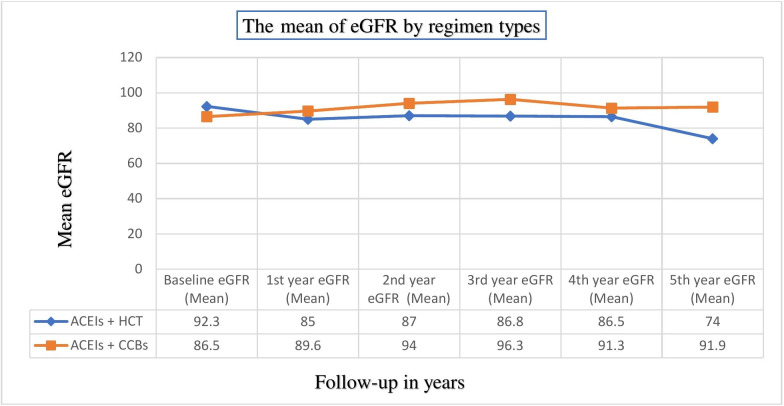
To compare the MD of eGFR values between groups, the scores recorded at every year were taken and the median (IQR) follow-up time was 24 (12–36) months. Before the combined regimens were initiated the mean (±SD) eGFR scores were higher in patients taking HCT-based regimens than CCBs-based users (92.3±21.4 vs 86.5±27.4 (ml/min/1.73 m2), p=0.019. However, after the combined therapies had started the mean eGFR levels were improving in CCBs-based users while there were failing in HCT-based groups throughout the follow-up years. The eGFR levels in CCBs user groups were higher in all times and statistically significant at second year (MD=6.92; p=0.026), third year (9.5; p=0.018) and fifth year (18.02; p=0.005) (measured with ml/min/1.73 m2) (figure 1).
Figure 1.
Comparisons of the mean of estimated glomerular filtration rate (eGFR) (ml/min/1.73 m2) at different follow-up years between the groups. ACEIs, ACE inhibitors; CCBs, calcium channel blockers; HCT, hydrochlorothiazide.
Median changes and last records of the eGFR levels among the studied groups
Regarding the changes in the median eGFR levels, substantial differences were observed between the groups. As a result, patients treated with the CCBs-based combinations had scored median changes of 18.8 mL/min/1.73 m2 higher compared with HCT-based combinations (5.9 vs (−12.9)), respectively, (p<0.001). Likewise, the CCBs combination users had significantly higher the last median filtration rates than their counter parts (15.1 mL/min/1.73 m2; p<0.001) (online supplemental table 3).
Incidence of estimated glomerular filtration rate reduction levels among patients
A total of 19.8% of participants’ eGFR levels declined by ≥30% from their baseline records and were higher from the HCT users (27.2%) than CCBs users (12.4%). The CCBs-based regimen had substantially reserved the renal functions (p<0.001). The risk ratio of getting in estimated filtration rate reductions in the CCBs-based regimen was 45.5% compared with HCT-based regimens and the estimated incidence rate of renal dysfunctions among the patients in both regimens was 62 per 1000 person-years.
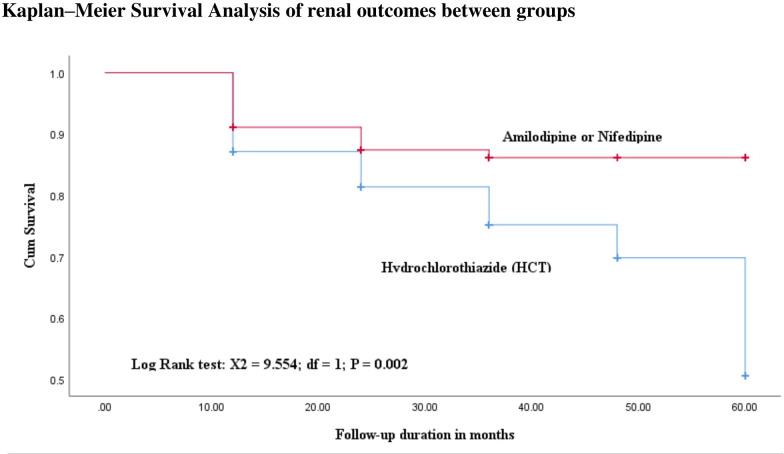
Kaplan-Meier survival analyses and factors affecting eGFR scores between groups
This study revealed that the predicted mean survival time to reduce in eGFR scores by ≥30% from the baseline levels was shorter in HCT-based group (49.6, SE 1.3) compared with CCBs-based treatments 53.8 (1.1). The hazards of getting reduced in eGFR scores were higher in the HCT-based therapies than CCBs users (figure 2). Multivariate Cox-regression analyses showed that the type of antihypertensive agents added on to ACEIs and higher baseline SBP (>150 mm Hg) were significantly associated to the occurrences of renal events. Hereby, individuals whose SBP levels were higher had an increased risk of declining in eGFR by 93%, with HR of 1.93, 95% CI 1.204 to 3.094, p=0.006. Similarly, patients in the CCBs group had a lower risk of significant failure in renal functions compared with those in the HCT group, with HR of 0.436, 95% CI 0.268 to 0.711, p=0.001) (table 2).
Figure 2.
Comparisons of Kaplan-Meier survival probability curves with diabetic subjects by combined class of CCBs medications added on to ACEIs compared with thiazide diuretics. ACEIs, ACE inhibitors; CCBs, calcium channel blockers.
Table 2.
HRs (95% CI) for renal events by combined class of calcium channel blocker medications added on to ACEIs compared with thiazide diuretics
| Variables | eGFR reduction by ≥30% from the baseline | CHR (95% CI) | P value | AHR (95% CI) | P value | |
| Yes | No | |||||
| Sex | ||||||
| Male | 29 | 160 | 1 | 1 | ||
| Female | 51 | 164 | 1.449 (0.917 to 2.289) | 0.112 | 1.444 (0.907 to 2.300) | 0.122 |
| Type of BP lowering agent added on ACEIs | ||||||
| HCT | 55 | 147 | 1 | 1 | ||
| CCBs | 25 | 177 | 0.497 (0.309 to 0.798) | 0.004 | 0.436 (0.268 to 0.711) | 0.001 |
| Aspirin (ASA) | ||||||
| No | 61 | 276 | 1 | 1 | ||
| Yes | 19 | 48 | 1.5 (0.896 to 2.511) | 0.123 | 1.422 (0.827 to 2.446) | 0.203 |
| DM duration | ||||||
| <9 years | 46 | 166 | 1 | 1 | ||
| ≥9 years | 34 | 158 | 0.755 (0.484 to 1.177) | 0.214 | 0.858 (0.544 to 1.356) | 0.513 |
| SBP baseline (mm Hg) | ||||||
| <150 | 48 | 235 | 1 | 1 | ||
| ≥150 | 32 | 89 | 1.816 (1.159 to 2.845) | 0.009 | 1.93 (1.204 to 3.094) | 0.006 |
| DBP last | ||||||
| <88 | 61 | 262 | 1 | 1 | ||
| ≥88 | 19 | 62 | 1.408 (0.840 to 2.361) | 0.194 | 1.592 (0.937 to 2.705) | 0.086 |
| Macrovascular (ASCVDs) complications | ||||||
| No | 68 | 309 | 1 | 1 | ||
| Yes | 12 | 15 | 2.464 (1.333 to 4.554) | 0.004 | 1.898 (0.866 to 4.161) | 0.109 |
Bold values indicated significant at p<0.05.
ACEIs, ACE inhibitors; AHR, adjusted HR; ASCVDs, atherosclerotic cardiovascular disease; BP, blood pressure; CCBs, calcium channel blockers; CHR, crude HR; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HCT, hydrochlorothiazide; SBP, systolic blood pressure.
Occurrences of secondary outcomes (ASCVDs) and predictors associated between groups
In the current study, the ASCVDs events were the secondary endpoints and occurred in about 6.7% of the study subjects. However, no significant incidence differences detected between the groups (online supplemental figure 1). Multivariate analyses identified that declining in renal functions were an important risk factor for the cardiovascular events, and the hazards of developing ASCVDs was 5.5 times higher in patients whose eGFR is reduced, with HR of 6.5, 95% CI 1.648 to 25.63; p=0.007) (table 3).
Table 3.
HRs (95% CI) for ASCVDs by combined class of CCBs medications added on to ACEIs compared with thiazide diuretics
| Variables | CHR (95% CI) | P value | AHR (95% CI) | P value |
| eGFR reduced by ≥30% from baseline | ||||
| No | 1 | 1 | ||
| Yes | 4.257 (1.98 to 19.148) | 0.000 | 6.499 (1.648 to 25.63) | 0.007 |
| SBP baseline (mm Hg) | ||||
| <150 | 1 | 1 | ||
| ≥150 | 3.681 (1.716 to 7.897) | 0.001 | 1.952 (0.508 to 7.496) | 0.33 |
| Metformin initial dose (mg) | ||||
| <1700 | 1 | 1 | ||
| ≥1700 | 2.639 (1.052 to 6.619) | 0.039 | 2.258 (0.596 to 8.559) | 0.231 |
| TC baseline (mg/dL) in median | ||||
| <178 | 1 | 1 | ||
| ≥178 | 0.478 (0.214 to 1.065) | 0.071 | 0.555 (0.129 to 2.384) | 0.429 |
| TG baseline (mg/dL) in median | ||||
| <165 | 1 | 1 | ||
| ≥165 | 2.267 (0.683 to 7.531) | 0.181 | 2.886 (0.293 to 28.43) | 0.364 |
| Baseline lipid lowering agent | ||||
| Atorvastatin | 1 | 1 | ||
| Simvastatin | 0. 154 (0.034 to 0.687) | 0.014 | 0.194 (0.033 to 1.150) | 0.071 |
| eGFR last in median | ||||
| <85 | 1 | 1 | ||
| ≥85 | 0.590 (0.269 to 1.292) | 0.187 | 0.952 (0.222 to 4.114) | 0.952 |
| NPH dose baseline (IU) | ||||
| <30 | 1 | * | * | |
| ≥30 | 0.302 (0.081 to 1.121) | 0.074 | ||
| HCT-based regimen | 1 | * | * | |
| CCBs-based regimen | 1.1 (0.515 to 2.349) | 0.806 | ||
Bold values indicated significant at p<0.05.
*Cells with empty AHR and p values, not fitted with the model.
ACEIs, ACE inhibitors; AHR, adjusted HR; ASCVDs, atherosclerotic cardiovascular diseases; CCBs, calcium channel blockers; CHR, crude HR; eGFR, estimated glomerular filtration rate; HCT, hydrochlorothiazide; NPH, Neutral Protamine Hagedorn; SBP, systolic blood pressure; TC, total cholesterol; TG, total glyceride.
Discussion
Our study suggested that patients in CCBs group had lower risks of significant renal events compared with HCT group. However, both regimens appeared with similar effects on the ASCVDs events. This 5-year follow-up study demonstrated that compared with HCT, CCBs-based regimens were associated with a lower risk of substantial renal events. However, both treatment regimens were found with similar threats of cardiovascular events. Therefore, the data in our finding suggested that when patients with diabetes need a second antihypertensive drugs added to ACEIs, the CCBs-based regimes are better to be included. These results are supported by the current guidelines developed by American diabetic association12 and American college of cardiology.36 Additionally, it is concurred with a recent finding in four integrated health systems of America30 that revealed CCBs had higher renal event protection effects than thiazide diuretics, but yet had the same effects on ASCVDs events. Furthermore, several randomised controlled trials disclosed that benazepril plus amlodipine had lower hazards of renal events compared with benazepril plus HCT.37–39
Differences in eGFR levels were noticed between the groups. After add-on therapy, patients treated with CCBs-based regimens had increased with a mean (±SD) eGFR scores from 86.5±27.4 to 91.9±23.5, while it was declined from 92.3±21.4 to 74±17 mL/min/1.73 m2 in HCT-based regimens. This finding could indicate that patients with diabetes treated with combinations of ACEIs plus CCBs had better mean of renal status in every follow-up year than their counter parts. It was agreed to a randomised double blind non-inferiority trial done in Taiwan40 and in Italy where significant rise was recorded after amlodipine was initiated.41 Fractions of filtration rates in the current study had improved with similar fashions to Taiwan and Italy; however, it was higher in this finding. The possible explanation for this could be patients in this study had treated for longer durations, whereas it was shorter in Taiwan and Italy. Generally, it is believed that chronic CCBs treatment would maintain reasonably pronounced and sustained afferent renal vasodilatations, which further results an improved in GFRs.41 Moreover, it might be due to different study designs, the former studies used controlled study designs that potentially handled possible confounders, compliance issues; and the purposes of the data recordings all these could prevent the overestimated eGFR values. Besides, these factors might be the reasons for higher filtration fraction rates levels observed in the current study.
Significantly higher positive median changes of eGFR (last minus initial) were documented in patients on CCBs group, whereas it was negative in HCT group (+5.9 vs (−12.7) mL/min/1.73 m2; p<0.001). This implies that at the end of follow-ups, every individual taking CCBs-based combinations had increased median eGFR levels by 5.9 mL/min/1.73 m2, while it was reducing by (−12.7 mL/min/1.73 m2) in HCT users. This was consistent with findings from Taiwan and Japanese patients,40 42 whose last eGFR levels on CCBs had an average of 15.4 mL/min/1.73 m2 more than HCT-based users. This result is agreed with the earlier findings.41 43 Changes in eGFR levels on this study is higher than found in other reports. This higher increment is probably due to patients in the current study had treated with combined therapy that strengthen synergistic renoprotection.
At the end of study, about 20% of the patients developed renal events and significant numbers were from HCT group. These results were coincided with the previous findings.30 37 In the current study, large number of patients developed events than the former reports. The discrepancy might be since the other studies used different study designs and they stopped studies early before the end of the follow-up. Moreover, most of the previous studies were done in well developed countries where there might be an adequate intervention have been implemented, so that significant numbers of patients possibly prevented from developing the events.
In our study, CCBs-based treatments prevented the risks of falling in renal function by 56.4%. Our results are consistent with those of the multicentre studies in USA and four European countries, in which CCBs-based sets reduced the hazards of kidney events compared with HCT users by 33% and 48%, respectively.30 37 Further, it is supported by several randomised trials.38 39 The possible underlining mechanisms of the calcium antagonists in reducing renal events might be work through maintaining the renal vasodilation effects that increases the eGFR scores; consequently a rose in filtration fractions. It was also stated as dihydropyridines CCBs principally associated with vasodilation of the preglomerular renal resistance vessels, by this means an increased eGFR level was observed.44 Conversely, thiazides related with unknown mechanisms of renal damages and associated with potential direct toxic effects through apoptosis of distal tubular cells. Moreover, thiazides are highly likely associated with chronic metabolic adverse effects and volume depletions which activates the renin angiotensin systems, swellings and inflammations of the kidneys.45 46 In our study, the CCBs-based treatments prevented the renal events more than previous studies. The possible reason for this could be the patients had been treated with CCBs based regimen for longer time and is associated with risk reductions.
Our results also declared that the hazards in falling renal events were about two times higher in patients whose initial SBP levels were >150 mm Hg, and is coincided to the previous reports.18 47 Elevated BP is associated with initiating of intraglomerular pressure that leads to impairing eGFR, microalbuminuria and proteinuria.
Study strengths and limitations
This study was done in the settings where the renal functions tests are not measured routinely in risky patients with diabetes treated with combined regimens. Therefore, the study findings could have greater implications for the clinical decision-making processes and the existing practices.
From the ACEIs drug classes almost all the studied patients were on enalapril, hence it might be difficult to conclude that the second antihypertensive agents are better to be added on to all ACEIs. Since it is retrospective observational study, we used secondary data that potentially missed some important variables and there could be uncontrolled confounders, thus, these results should be interpreted cautiously. It was also single-centred study and there was incapability to measure levels of adherence to the combined therapy. Moreover, because of no documentations on proteinuria and albuminuria to creatinine ratios, only eGFR values were used to characterise the renal function status.
Conclusion
Our findings recommended that when the patients with diabetes require an additional BP lowering agents to add on the ACEIs, the CCBs drug classes are better to be chosen for preventing the renal dysfunctions. Throughout the study times significantly higher mean estimated filtration rates were recorded in CCBs-based regimens than HCT users. Both regimens found with similar risks of ASCVD events. Elevated initial SBP levels were substantially linked with falling in eGFR levels. The renal function status of patients with diabetes would have better outcomes if monitored closely at least once a year.
Supplementary Material
Acknowledgments
We would like to acknowledge the Clinical Pharmacy department, University of Gondar, college of medicine and health sciences, medical directorate for inspiring us to do this project.
Footnotes
Contributors: AKN contributed to the study conceptualization and project administrations. MTT, EAM and MSA contributed to the study designs and clinical concepts. AKN and MTT contributed to the document writing. All authors contributed for editing final documents, data analyses, interpretation, preparing and reviewing the whole documents. AKN is the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All relevant materials and data supporting findings of this study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethical clearance was obtained from the Ethical Review Committee of the school of pharmacy and University of Gondar Institutional Review Board (IRB) with the approval number of SOPS/043/2020 and an official permission letter was obtained from University of Gondar, specialised referral hospital administrative office.
References
- 1.Knott. L. Diabetic Kidney Disease. Diabetic Kidney Disease Symptoms, Diagnosis & Complications Patient. Last edited 11 Mar 2020 Meets Patient’s editorial guidelines, 2020. [Google Scholar]
- 2.Sulaiman MK. Diabetic nephropathy: recent advances in pathophysiology and challenges in dietary management. Diabetol Metab Syndr 2019;11:7. 10.1186/s13098-019-0403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Global status report on non-communicable diseases. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 4.de Boer IH, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–9. 10.1001/jama.2011.861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . Diabetes. Geneva: WHO, 2018. [Google Scholar]
- 6.HEALTH, F.D.R.O.E.M.O . Guidelines on Clinical and Programmatic Management of Major Non Communicable Diseases. Participant’s Manual, 2016. [Google Scholar]
- 7.Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–81. 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 8.Ritz E, Rychlík I, Locatelli F, et al. End-Stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis 1999;34:795–808. 10.1016/S0272-6386(99)70035-1 [DOI] [PubMed] [Google Scholar]
- 9.Wu AYT, Kong NCT, de Leon FA, et al. An alarmingly high prevalence of diabetic nephropathy in Asian type 2 diabetic patients: the microalbuminuria prevalence (MAP) study. Diabetologia 2005;48:17–26. 10.1007/s00125-004-1599-9 [DOI] [PubMed] [Google Scholar]
- 10.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017;12:2032–45. 10.2215/CJN.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA consensus conference. Am J Kidney Dis 2014;64:510–33. 10.1053/j.ajkd.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 12.Marathe PH, Gao HX, Close KL. American diabetes association standards of medical care in diabetes 2017. J Diabetes 2017;9:320–4. 10.1111/1753-0407.12524 [DOI] [PubMed] [Google Scholar]
- 13.Collins AJ, Foley RN, Chavers B, et al. 'United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 2012;59:A7, e1. 10.1053/j.ajkd.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 14.Zhang X-X, Kong J, Yun K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in China: a meta-analysis of observational studies. J Diabetes Res 2020;2020:1–11. 10.1155/2020/2315607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005;16:489–95. 10.1681/ASN.2004030203 [DOI] [PubMed] [Google Scholar]
- 16.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035–8. 10.1001/jama.241.19.2035 [DOI] [PubMed] [Google Scholar]
- 17.Drury PL, Ting R, Zannino D, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the fenofibrate intervention and event lowering in diabetes (field) study. Diabetologia 2011;54:32–43. 10.1007/s00125-010-1854-1 [DOI] [PubMed] [Google Scholar]
- 18.Kumela Goro K, Desalegn Wolide A, Kerga Dibaba F, et al. Patient awareness, prevalence, and risk factors of chronic kidney disease among diabetes mellitus and hypertensive patients at Jimma University medical center, Ethiopia. Biomed Res Int 2019;2019:1–8. 10.1155/2019/2383508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raile K, Galler A, Hofer S, et al. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1c, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 2007;30:2523–8. 10.2337/dc07-0282 [DOI] [PubMed] [Google Scholar]
- 20.Ritz E. Diabetic nephropathy. Saudi J Kidney Dis Transpl 2006;17:481–90. [PubMed] [Google Scholar]
- 21.Eckardt K-U, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 2013;382:158–69. 10.1016/S0140-6736(13)60439-0 [DOI] [PubMed] [Google Scholar]
- 22.de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American diabetes association. Diabetes Care 2017;40:1273–84. 10.2337/dci17-0026 [DOI] [PubMed] [Google Scholar]
- 23.Grassi G, Mancia G, Nilsson PM. Specific blood pressure targets for patients with diabetic nephropathy? Diabetes Care 2016;39 Suppl 2:S228–33. 10.2337/dcS15-3020 [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association . Standards of medical care in diabetes-2017. Diabetes Care 2017;40:S1–112.27979885 [Google Scholar]
- 25.Saunders W. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease, 2007. [DOI] [PubMed] [Google Scholar]
- 26.KDOQI . KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007;49:S12–154. 10.1053/j.ajkd.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 27.Carey RM, Whelton PK, 2017 ACC/AHA Hypertension Guideline Writing Committee . Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American heart association hypertension guideline. Ann Intern Med 2018;168:351–8. 10.7326/M17-3203 [DOI] [PubMed] [Google Scholar]
- 28.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Hypertension 2018;71:1269–324. 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 29.Arauz-Pacheco C, Parrott MA, Raskin P, et al. Hypertension management in adults with diabetes. Diabetes Care 2004;27 Suppl 1:s65–7. 10.2337/diacare.27.2007.s65 [DOI] [PubMed] [Google Scholar]
- 30.Schroeder EB, Chonchol M, Shetterly SM, et al. Add-On antihypertensive medications to angiotensin-aldosterone system blockers in diabetes: a comparative effectiveness study. Clin J Am Soc Nephrol 2018;13:727–34. 10.2215/CJN.09510817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fasil A, Biadgo B, Abebe M. Glycemic control and diabetes complications among diabetes mellitus patients attending at University of Gondar Hospital, Northwest Ethiopia. Diabetes Metab Syndr Obes 2019;12:75–83. 10.2147/DMSO.S185614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes Association . Standards of medical care in diabetes-2019. Diabetes Care 2019;42. [Google Scholar]
- 33.Badve SV, Palmer SC, Hawley CM, et al. Glomerular filtration rate decline as a surrogate end point in kidney disease progression trials. Nephrol Dial Transplant 2016;31:1425–36. 10.1093/ndt/gfv269 [DOI] [PubMed] [Google Scholar]
- 34.Xue C, Zhou C, Yang B, et al. Comparison of efficacy and safety between benidipine and hydrochlorothiazide in fosinopril-treated hypertensive patients with chronic kidney disease: protocol for a randomised controlled trial. BMJ Open 2017;7:e013672. 10.1136/bmjopen-2016-013672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. J Am Coll Cardiol 2018;71:e127–248. 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 37.Bakris GL, Sarafidis PA, Weir MR, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (accomplish): a prespecified secondary analysis of a randomised controlled trial. Lancet 2010;375:1173–81. 10.1016/S0140-6736(09)62100-0 [DOI] [PubMed] [Google Scholar]
- 38.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med Overseas Ed 2008;359:2417–28. 10.1056/NEJMoa0806182 [DOI] [PubMed] [Google Scholar]
- 39.Weber MA, Bakris GL, Jamerson K, et al. Cardiovascular events during differing hypertension therapies in patients with diabetes. J Am Coll Cardiol 2010;56:77–85. 10.1016/j.jacc.2010.02.046 [DOI] [PubMed] [Google Scholar]
- 40.Lee I-T, Hung Y-J, Chen J-F, et al. Comparison of the efficacy and safety profiles of two fixed-dose combinations of antihypertensive agents, amlodipine/benazepril versus valsartan/hydrochlorothiazide, in patients with type 2 diabetes mellitus and hypertension: a 16-week, multicenter, randomized, double-blind, noninferiority study. Clin Ther 2012;34:1735–50. 10.1016/j.clinthera.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 41.Perinotto P, Ragni G, Serventi M, et al. Long term effects of amlodipine on renal haemodynamics and microalbuminuria in patients with essential hypertension. Clin Drug Investig 1997;13:36–41. 10.2165/00044011-199700131-00008 [DOI] [Google Scholar]
- 42.Nishiwaki M, Hosoai H, Ikewaki K, et al. Efficacy and effects on lipid metabolism of combination treatment with losartan + hydrochlorothiazide versus losartan + amlodipine: a 48-week prospective, multicenter, randomized, open-label trial. Clin Ther 2013;35:461–73. 10.1016/j.clinthera.2013.02.021 [DOI] [PubMed] [Google Scholar]
- 43.Rahman M, Pressel S, Davis BR, et al. Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). Arch Intern Med 2005;165:936–46. 10.1001/archinte.165.8.936 [DOI] [PubMed] [Google Scholar]
- 44.Loutzenhiser RD, Epstein M, Fischetti F, et al. Effects of amlodipine on renal hemodynamics. Am J Cardiol 1989;64:I122–8. 10.1016/0002-9149(89)90969-7 [DOI] [PubMed] [Google Scholar]
- 45.Reungjui S, Pratipanawatr T, Johnson RJ, et al. Do thiazides worsen metabolic syndrome and renal disease? the pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens 2008;17:470–6. 10.1097/MNH.0b013e328305b9a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez. T. 6 medications that can harm the kidneys. balancing the risks and benefits of medicines, 2019. [Google Scholar]
- 47.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (parade): a position paper of the National kidney Foundation. Am J Kidney Dis 1999;33:1004–10. 10.1016/S0272-6386(99)70442-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-048442supp001.pdf (113.1KB, pdf)
Data Availability Statement
Data are available on reasonable request. All relevant materials and data supporting findings of this study are available from the corresponding author on reasonable request.