Abstract
Purpose:
The population of adolescent and young adult (AYA, ages 15-39 years) diffuse large B-cell lymphoma (DLBCL) survivors is growing, however long-term overall survival patterns and disparities are largely unknown.
Methods:
The current study utilized the Surveillance, Epidemiology, and End Results (SEER) registry to assess the impact of race/ethnicity, sex, socioeconomic status, and rurality on long-term survival in 5-year DLBCL survivors using an accelerated failure time model.
Results:
Included were 4,767 5-year survivors of AYA DLBCL diagnosed between the years 1980 and 2009 with a median follow-up time of 13.4 years. Non-Hispanic Black survivors had significantly worse long-term survival than non-Hispanic White survivors (Survival Time Ratio (STR): 0.53, p<0.0001). Male sex (STR: 0.57, p<0.0001) and older age at diagnosis were also associated with reduced long-term survival. There was no evidence that survival disparities improved over time.
Conclusions:
Racial disparities persist well into survivorship among AYA DLBCL survivors. Studies investigating specific factors associated with survival disparities are urgently needed to better address these disparities.
Keywords: Adolescent and Young Adult Oncology, AYA, Diffuse Large B-Cell lymphoma, racial disparities, survival
Introduction:
Each year there are approximately 90,000 new cancer diagnoses in the adolescent and young adult (AYA, ages 15-39 years) population in the United States and cancer incidence in this group has risen by about 30% over the past four decades.[1,2] Accompanying this increase has been an improvement in 5-year mortality to >80%,[3,4] leading to a growing population of survivors of AYA cancer. As the population of survivors grows, more data on long-term outcomes is needed. Compared with knowledge of long-term outcomes in the childhood cancer population,[5] data in the AYA survivor population is lacking. Prior studies have reported that long-term survival in AYA cancer survivors is compromised compared with the general population.[6–9]
Socioeconomic status (SES), rurality, race and ethnicity are known to impact 5-year survival in AYA cancer patients, with low SES neighborhood, rural area residence, and Black race associated with worse 5-year survival compared with high SES neighborhood metropolitan residence, and White race, respectively.[10–14] Initial studies have shown these factors can also impact longer-term survival. There is evidence that racial disparities in survival persist at up to 10 years of follow-up,[15,16] and a single institution study has found that low SES markers are associated with inferior survival at up to 15 years of follow-up in AYA cancer survivors.[17] Among 5-year AYA Hodgkin lymphoma survivors, race and SES have been found to impact survival at up to 30 years of follow-up.[18] Additional studies are needed assessing long-term survival disparities in other common AYA cancer types.
Diffuse large B cell lymphoma (DLBCL), is the most common subtype of non-Hodgkin lymphoma (NHL) and the most common lymphoid malignancy after classical Hodgkin lymphoma (HL) in the AYA population.[19] With treatment advances such as the addition of rituximab therapy, 5-year survival of AYA DLBCL has improved to about 80% in recent years.[20] To date, data on racial/ethnic and SES disparities in AYA DLBCL survival are sparse and even less is known about long-term mortality disparities. With incidence increases of NHL among AYAs outpacing the increased incidence of cancer in general in this population,[2] knowledge of long-term mortality disparities is important to guide survivorship care. Utilizing data from the Surveillance, Epidemiology, and End Results (SEER) database, the current retrospective analysis sought to characterize long-term mortality outcomes over time among 5-year survivors of AYA DLBCL, with a focus on race/ethnicity, SES, rurality, diagnosis age, sex, and disease stage at diagnosis.
METHODS
Diffuse large B-cell lymphoma data from SEER spanning 1975-2011 included data for 5,940 patients who were between the ages of 15-39 years at diagnosis and were alive 5 years after diagnosis. The final dataset included 4,767 patients after excluding those whose race and origin code were missing or those who were Native American (n=61), those missing county level statistics (n=1), those missing the Rural-Urban Continuum Code (n=46), those diagnosed prior to 1980 or later than 2009 (n=738), and those that did not have data on diagnosis stage (n=327). Native Americans were excluded due to insufficient sample size of this cohort and those diagnosed in 2010 or after were excluded due to insufficient follow-up time. A list of case IDs as well as the names of variables that were extracted from SEER can be found in Supplemental Table 1.
A county-level socioeconomic deprivation index (SES index) was defined based upon county-level variables previously defined by Truong et al.[21] Variables included level of poverty (P) based upon percentage with income below the 200th percentile of the poverty line, educational attainment (E) per the percentage obtaining less than a high school education, crowding (C) per the percentage with greater than one person per room, unemployment (U) per the percentage unemployed, levels of immigration (I) per the percentage of foreign-born, and language isolation (L) per the percentage of language isolation. The variables P, E, C, U, I, L, were each standardized as the difference from the mean divided by the standard deviation. The county SES index was then calculated as (((P + E + C + U)/4) + ((I + L)/2))/2. Since this is defined as a socioeconomic deprivation index, higher values are indicative of greater deprivation.
Continuous variables were summarized by decade and overall as mean, standard deviation, median, minimum and maximum. Discrete variables were summarized by decade and overall as count, percentage, mortality count, and mortality percentage. A time-to-event model was used to model the time (following 5 years survival) to death with relation to diagnosis decade, potential covariates including age at diagnosis (numeric years), rurality (numeric integer between 0 and 8), race and origin (Non-Hispanic White, Hispanic (All Races), Non-Hispanic Black, Non-Hispanic Asian or Pacific Islander), sex (female or male), Lymphoma Ann Arbor Stage (I, II, III, IV), and SES index. Interactions with decade of diagnosis were also considered. A Cox proportional hazards model was considered, but due to violations of the proportionality of hazards assumption, an accelerated failure time (AFT) model was selected instead.[22] The Weibull distribution was chosen as optimal due to lower Akaike Information Criterion (AIC) together with good fit of a Kaplan-Meier plot of model residuals to the model distribution; the Weibull distribution also has the advantage of allowing coefficients to be interpreted as hazard ratios. Based upon the AIC and beginning with a model including all variables and interactions with diagnosis decade, a reverse process of model selection excluded interactions followed by main effects variables where the exclusion yielded an improved model with lower AIC. The selection process eliminated all interactions from the model.
The optimal model covariates included diagnosis decade, age at diagnosis, rurality, race and origin, sex, and lymphoma Ann Arbor Stage. The final time-to-event accelerated failure time model of overall survival included these variables plus SES index. Differences among discrete variable levels were assessed by contrasts Tukey-adjusted p-values. Statistical analyses were performed using R statistical software (R Core Team, 2020, version 3.6.3). All statistical tests used two-sided alpha=.05. Survival modeling was performed using the “survival” package.[23,24] Assessment of differences among discrete variable levels in the accelerated failure time model were estimated using the “emmeans” package;[25] this includes adjusted means weighted proportionally to covariate marginal frequencies. Catseye plots were produced using the “catseyes” package.[26,27]
RESULTS
Patient Characteristics
The characteristics of 4,767 5-year survivors of AYA DLBCL included in the analysis are shown in Table 1. Forty one percent of the cohort was female. The median age of diagnosis was 32 years (range 15-39) and the median follow-up time was 8.4 years from 5-year survival (range 0.1 to 28.9 years). Sixty five percent were non-Hispanic White, 13% were non-Hispanic Black, 15% were Hispanic (all races), and 7% were non-Hispanic Asian or Pacific Islander. Among these survivors, 8% were diagnosed in the 1980s, 26% in the 1990s, and 66% in the 2000s. Grouping by stage, 39% of diagnoses were stage I, 26% were stage II, 11% were stage III, and 24% were stage IV. The overall mortality rate was 9%. Mortality rates were 25%, 13%, and 5% for those diagnosed in the 1980s, 1990s, and 2000s, respectively (these raw mortality rates are unadjusted for differential follow-up times among the decades). The county-level SES index, as well as the county-level variables which informed the SES index, are summarized in Table 1, where the mean rurality index was 0.6 (STD 1.4) and the mean SES index was 0.0 (STD 0.8), with higher SES deprivation index representing lower SES status.
Table 1.
Survivor Characteristics
| Characteristic | No. (%); n=4,767 |
|---|---|
| Sex | |
| Female | 1,964 (41.2) |
| Male | 2,803 (58.8) |
| Age at Diagnosis | |
| Mean ± STD | 30.2 ± 6.7 |
| Median(range) | 32 (15-39) |
| Follow-up Time | |
| Mean ± STD | 9.7 ± 6.3 |
| Median(range) | 8.4 (0.1-28.9) |
| Race/Ethnicity | |
| Non-Hispanic White | 3,088 (65.8) |
| Non-Hispanic Black | 603 (12.6) |
| Hispanic (All Races) | 734 (15.4) |
| Non-Hispanic Asian or Pacific Islander | 342 (7.2) |
| Decade of Diagnosis | |
| 1980s | 379 (8.0) |
| 1990s | 1,226 (25.7) |
| 2000s | 3,162 (66.3) |
| Stage | |
| I | 1,836 (38.5) |
| II | 1,247 (26.2) |
| III | 527 (11.0) |
| IV | 1,157 (24.3) |
| Rurality Index, Mean ± STD | 0.6 ± 1.4 |
| County % <200% Poverty | |
| Mean ± STD | 30.6 ± 8.9 |
| Median(range) | 28.3 (10.3-71.9) |
| County % HS Education | |
| Mean ± STD | 13.1 ± 5.6 |
| Median(range) | 12.1 (3.2-37.0) |
| County % Housed >1 per Room | |
| Mean ± STD | 4.9 ± 3.5 |
| Median(range) | 3.5 (0.0-13.0) |
| County % Unemployed | |
| Mean ± STD | 6.9 ± 2.0 |
| Median(range) | 6.8 (1.7-16.0) |
| County % Foreign Born | |
| Mean ± STD | 19.2 ± 11.4 |
| Median(range) | 19.4 (0.1-43.0) |
| County % Language Isolated | |
| Mean ± STD | 6.3 ± 4.2 |
| Median(range) | 5.6 (0.0-22.0) |
| SES Index | |
| Mean ± STD | 0.0 ± 0.8 |
| Median(range) | −0.1 (−2.0-2.6) |
Survival by Race/Ethnicity
Non-Hispanic Black survivors had significantly inferior long-term survival compared with non-Hispanic White survivors (Survival Time Ratio (STR): 0.53, p<0.0001) (Table 2). Non-Hispanic Black survivors also had significantly worse long-term survival compared with non-Hispanic Asian or Pacific Islander survivors, with non-Hispanic Asian or Pacific Islanders more than doubling the survival time of non-Hispanic Black survivors (STR: 2.29, p=0.014). While Hispanic and non-Hispanic Black survivors each experienced about 75% of the survival times as non-Hispanic White and Hispanic survivors, respectively, these did not reach statistical significance (STR: 0.74, p=0.23 and STR: 0.72, p=0.30). There was no evidence of change or improvement in racial disparities over time. Unadjusted Kaplan Meier curve reporting overall survival by race is shown in Figure 1.
Table 2.
Differences in Survival by Race and Ethnicity. These are estimated from the accelerated failure time survival model which included the covariates diagnosis decade, age at diagnosis, rurality, race and origin, sex, and Lymphoma Ann Arbor Stage. Tukey-adjusted p-values are shown.
| Race/Ethnicity Comparison | Survival Time Ratio (95% CI) | p-value |
|---|---|---|
| Non-Hispanic Black - Non-Hispanic White | 0.53 (0.40 - 0.70) | <0.0001 |
| Hispanic (All Races) - Non-Hispanic White | 0.74 (0.54 - 1.01) | 0.23 |
| Non-Hispanic Black - Hispanic (All Races) | 0.72 (0.50 - 1.04) | 0.30 |
| Non-Hispanic Asian or Pacific Islander – Non-Hispanic White | 1.21 (0.74 – 2.00) | 0.87 |
| Non-Hispanic Asian or Pacific Islander – Hispanic (All Races) | 1.65 (0.95-2.87) | 0.28 |
| Non-Hispanic Asian or Pacific Islander – Non-Hispanic Black | 2.29 (1.34 – 3.94) | 0.014 |
Figure 1.
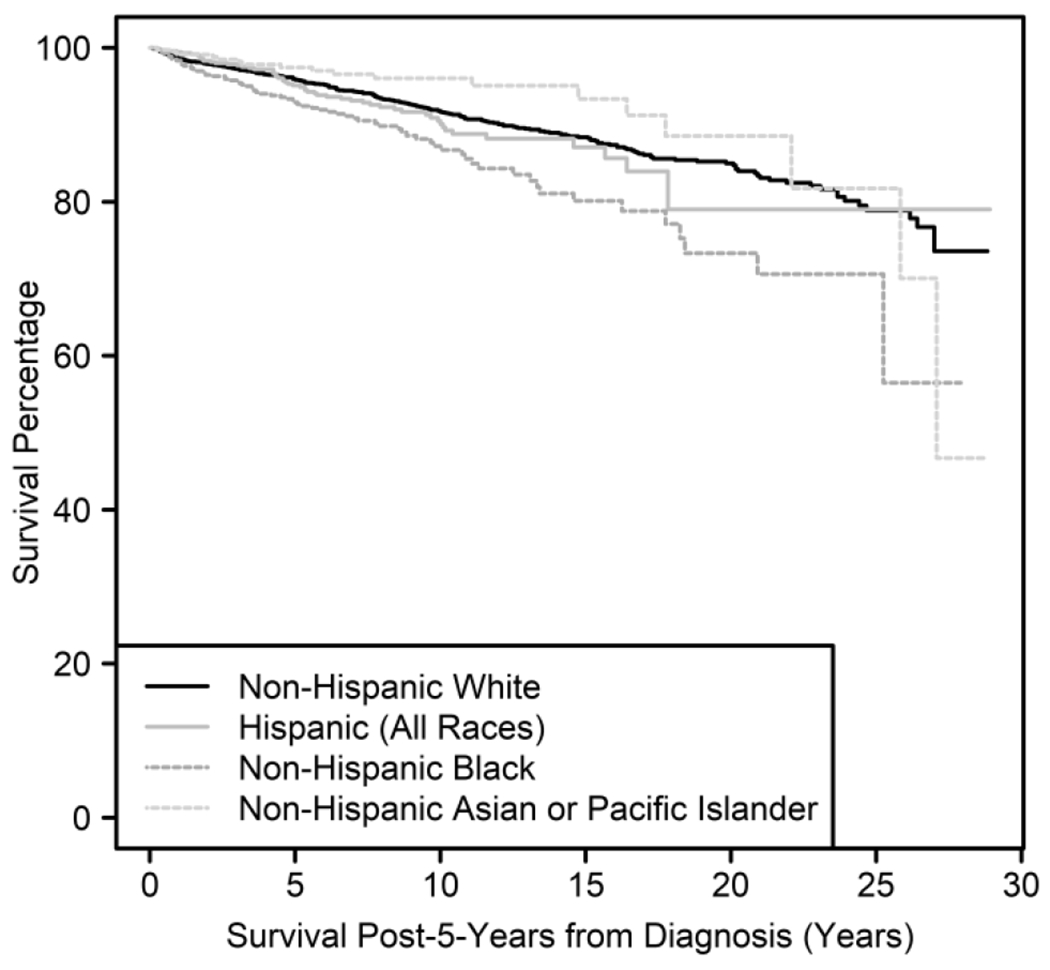
Kaplan Meier curve showing survival by race/ethnicity over time among 5-year survivors of AYA DLBCL.
Survival by SES and Rurality
The county-level SES index took into account county-level education, poverty, unemployment, household crowding, and immigration status. There was no evidence of significant association between long-term survival and SES index or rurality (Table 3).
Table 3.
Rates of Change in Survival by Age, and Rurality and SES Index. These are estimated from the accelerated failure time survival model which included the covariates diagnosis decade, age at diagnosis, race and origin, sex, Lymphoma Ann Arbor Stage, rurality, and SES index. Tukey-adjusted p-values are shown for SES index. Age at diagnosis and rurality p-values are unadjusted since these variables were not part of an interaction leading to multiple comparisons.
| Continuous Variable | Survival Time Ratio (95% CI) | p-value |
|---|---|---|
| Age at Diagnosis | 0.94 (0.92 - 0.96) | <0.0001 |
| Rurality | 0.95 (0.90 - 1.03) | 0.21 |
| SES Index | 1.10 (0.94 – 1.28) | 0.23 |
Survival by Sex, Age at Diagnosis, and Diagnosis Stage
With each increased year of age at diagnosis, long-term survival decreased by 6% (STR: 0.938, p<0.0001, Table 3). Compared with females, males attained 57% of females’ survival time (STR: 0.57, p<0.0001. Table 4). Higher stage at diagnosis had shorter long-term survival compared with earlier stages of diagnosis, however this did not reach statistical significance (Table 4). Compared with those diagnosed at stage I and stage II, those diagnosed at stage IV had 78% (STR: 0.78, p=0.19) and 72% (STR: 0.72, p=0.10) the survival times, respectively.
Table 4.
Differences in Survival by Sex, Stage, and Decade of Diagnosis. These are estimated from the accelerated failure time survival model which included the covariates diagnosis decade, age at diagnosis, race and origin, sex, Lymphoma Ann Arbor Stage, rurality, and SES index. Tukey-adjusted p-values are shown.
| Survival Time Ratio (95% CI) | p-value | |
|---|---|---|
| Sex Comparison | ||
| Male - Female | 0.57 (0.45 – 0.71) | <0.0001 |
| Stage Comparison | ||
| Stage II - Stage I | 1.09 (0.83 – 1.42) | 0.93 |
| Stage III - Stage I | 0.75 (0.55 – 1.03) | 0.28 |
| Stage IV - Stage I | 0.78 (0.61 – 1.00) | 0.19 |
| Stage III - Stage II | 0.69 (0.49 – 0.98) | 0.15 |
| Stage IV - Stage II | 0.72 (0.54 – 0.95) | 0.10 |
| Stage IV - Stage III | 1.04 (0.75 – 1.43) | 1 |
| Decade Comparison | ||
| 1990s – 1980s | 1.40 (1.07 – 1.84) | 0.042 |
| 2000s – 1980s | 1.68 (1.24 – 2.28) | 0.002 |
| 2000s – 1990s | 1.20 (0.95 – 1.52) | 0.29 |
Survival by Decade of Diagnosis
Long-term survival outcomes improved for those diagnosed in the 1990s compared with those diagnosed in the 1980s and those diagnosed in the 2000s compared to those diagnosed in the 1980s, but there were no significant long-term survival differences for those diagnosed in the 2000s compared with the 1990s (Table 4). A diagnosis in the 1990s conferred a 40% improvement in survival time compared with a diagnosis in the 1980s (STR: 1.40, p=0.042), and a there was a 68% improvement in survival time for those diagnosed in the 2000s compared with those diagnosed in the 1980s (STR: 1.68, p=0.002). Unadjusted Kaplan Meier curve reporting overall survival by decade of diagnosis is shown in Figure 2.
Figure 2.
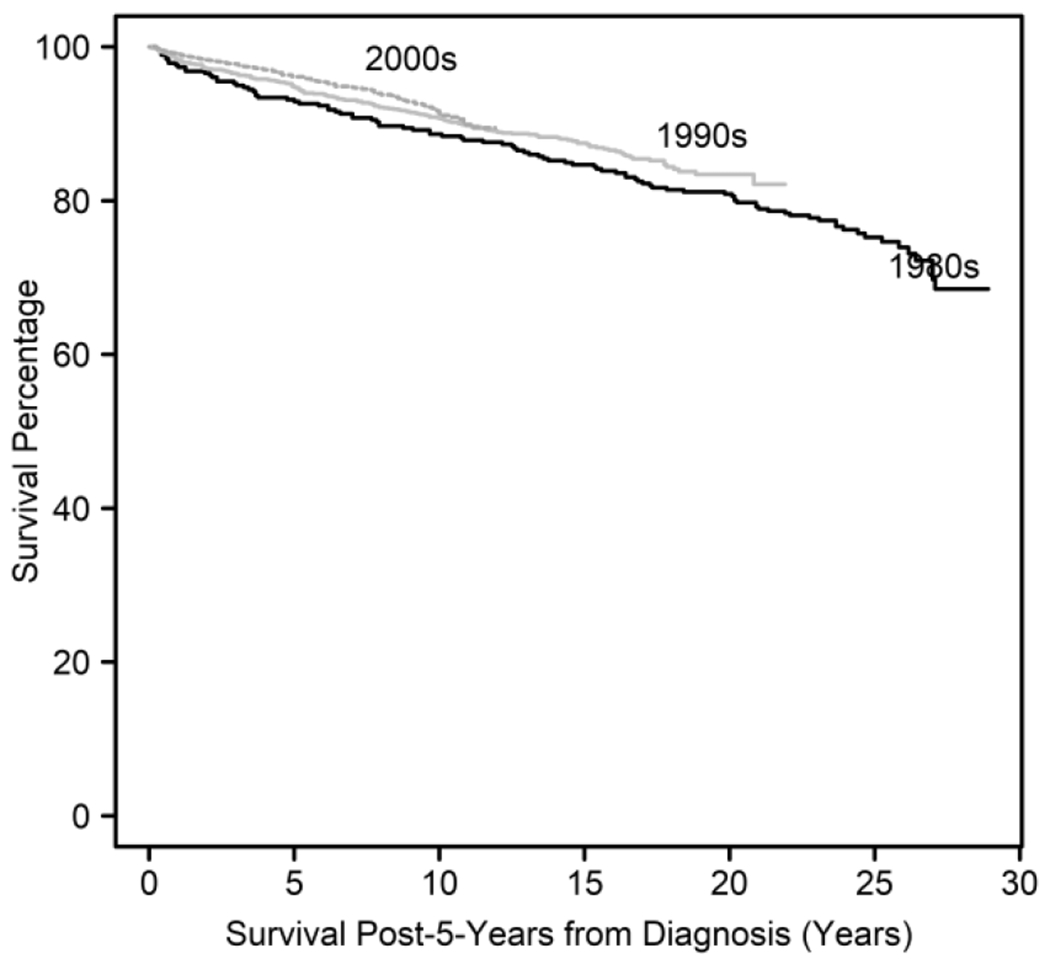
Kaplan Meier curve showing survival by decade of diagnosis over time among 5-year survivors of AYA DLBCL.
DISCUSSION
We found that racial disparities in mortality persist for decades after diagnosis among long-term survivors of DLBCL diagnosed as AYAs. The current study also found that younger age at diagnosis, female sex, and later decade of diagnosis were associated with favorable long-term survival outcomes, while stage at diagnosis did not impact long-term survival in this analysis. There was not clear evidence of rurality or SES impacting long-term survival in the AYA DLBCL survivor population.
The findings in the current study add to a growing body of literature describing racial disparities in survival among AYA cancer patients, and show that disparities between non-Hispanic Black and non-Hispanic White patients persist beyond the 5-year mortality timeframe that is most commonly studied.[10,13,28,29] Additionally, we found that non-Hispanic Black survivors had worse long-term survival than non-Hispanic Asian and Pacific Islander survivors. Notably, a recent population-based analysis demonstrated lower 5-year survival for non-Hispanic Black patients compared with non-Hispanic White patients across many cancer types including cervical, kidney or renal/pelvic, breast, non-Hodgkin lymphoma, Hodgkin lymphoma, leukemia, colorectal, thyroid and lung.[30] Data on racial disparities in mortality specifically in the AYA DLBCL population are sparse, as are data assessing disparities in long-term survival; a previous analysis that examined the impact of race/ethnicity on 5-year survival in AYA DLBCL patients found that Hispanic and non-Hispanic Black patients have a higher likelihood of mortality compared with non-Hispanic White patients.[20,30] There have been similar findings among AYA survivors of other hematological malignancies including leukemia and Hodgkin lymphoma, with inferior outcomes for non-Hispanic Black patients persisting at up to 30 years of follow-up.[15,18]
There are several possible contributing factors to the persistent survival differences between non-Hispanic Black and non-Hispanic White AYA DLBCL survivors. Compared with non-Hispanic White cancer survivors, non-Hispanic Black cancer survivors are less likely to report consistent and comprehensive follow-up care, such as seeing a specialist, adherence to prescribed medications, and adherence to preventive health services guidelines.[31–35] Insurance coverage, an important component of cancer and survivorship care, differs by race with a recent analysis finding a 12.4% gap in insurance coverage for non-White patients compared with White patients, even after full implementation of the Affordable Care Act.[36] In the young adult cancer survivor population, the Affordable Care Act reduced the proportion of uninsured overall, but did not change insurance enrollment among non-Hispanic Black survivors or survivors in low SES neighborhoods.[37] Additionally, modifiable risk factors for mortality differ by race/ethnicity. Non-Hispanic Black cancer survivors are more likely to be obese and less likely to meet physical activity guidelines compared with non-Hispanic white cancer survivors.[38–41] Structural racism and its impact on creating gaps in access to care and SES opportunity in the non-Hispanic Black population is also likely contributing to the elevated long-term mortality seen in non-Hispanic Black AYA DLBCL survivors.[42]
Unlike the difference found between non-Hispanic Black and non-Hispanic White AYA DLBCL survivors, we found no difference in long-term survival between Hispanic and non-Hispanic White survivors. It is possible that survivorship care for the Hispanic population aided in closing the survival gap, however, it is also possible that our cohort size limited our ability to detect survival differences between these populations in the second and third decade of survivorship. While past studies have found that Hispanic cancer survivors are less likely than non-Hispanic White survivors to engage in survivorship care,[35] there have been focused efforts on improving care in the Hispanic cancer survivor population and recent data have shown that Hispanic childhood cancer survivors are less likely than other race/ethnic groups to forego survivorship care.[43,44] Further studies are needed to identify differences in healthcare utilization in Hispanic and non-Hispanic White AYA DLBCL survivors. Finally, non-Hispanic Asian and Pacific Islander survivors of AYA DLBCL had similar long-term survival compared with Hispanic and non-Hispanic White survivors. Previous studies assessing disparities in late-effects among AYA cancer survivors have found that Asian and Pacific Islander survivors have similar risks of cardiac and respiratory late-effects as non-Hispanic White survivors and data have been mixed on risk of secondary malignancies.[45–47] Increased focus on survivorship care specific to the AYA Asian Pacific Islander population has potential to further improve outcomes in this population.[48]
Similar to race/ethnicity, SES has been consistently reported to impact multiple aspects of cancer care receipt and patient health, and lower SES status is associated with poorer survival in the AYA cancer population.[11,13,49,50] For example, an analysis among AYA DLBCL patients found that living in a neighborhood in the lowest SES index compared with living in a neighborhood with the highest SES index was associated with decreased 5-year survival.[20] In the current study, there was no evidence that SES at time of diagnosis impacted long-term survival among AYA DLBCL survivors. A limitation of our study is that SEER collects population level SES data, as opposed to individual level cancer and demographic data, which complicates interpretation of SES data in this population, especially as this data is collected at time of diagnosis and the focus of this analysis is on long-term survivorship and SES at time of diagnosis may not accurately reflect the impact of SES within the survivorship period on long-term outcomes. Lower SES, compared with higher SES has been shown to impact access to care including decreased use of subspecialty and survivorship care as well as an increased likelihood of diagnosis delays, which likely contribute to disparities in both short-term and long-term mortality.[51–54] While we did not find evidence that SES factors at diagnosis impact long-term survival in AYA DLBCL survivors, future studies should continue to explore these associations with a particular focus on longitudinal impact of SES and SES changes.
Similarly, though living in a rural or non-metropolitan area compared with living in an urban or metropolitan area has been found to impact both cancer care and risk factor burden,[55] the current study found no evidence of a rural-urban long-term survival difference in the AYA DLCBL survivor population. In contrast, among an older DLBCL population, rural and urban residence, compared with metropolitan, has been associated with inferior overall survival.[56] Like SES, in the SEER dataset rurality is captured at time of diagnosis and the AYA population tends to be one of the most mobile populations, thus for those who do move away from the area in which they received treatment, rurality designation at the time of diagnosis may not reflect the area in which individuals are living and receiving care as survivors. Additionally, compared with non-SEER areas, those captured within the SEER database are more urban and among the rural areas that are captured in SEER there are shorter travel times to care compared with rural areas outside of SEER, further limiting the conclusions that can be drawn.[57,58]
Younger age at diagnosis, female sex, and more recent decade of diagnosis were associated with long-term survival benefit among AYA DLBCL survivors. Inferior long-term survival among AYAs diagnosed at later ages likely reflects that these survivors will naturally accrue age-related comorbidities earlier into the survivorship period compared with those diagnosed at younger ages. Improvement in efficacy and toxicity of treatments used for DLBCL likely explains improved long-term survival for those AYA DLBCL survivors diagnosed in more recent decades.[59] Sex-based differences in long-term survival may reflect trends in the general population showing longer lifespans for females compared with males,[60] though the survival advantage of females compared with males in the current study was significantly more dramatic. This is particularly striking given that previous studies have not found sex-related differences in AYA DLBCL specific survival.[61] A study that included both young adult and older adult DLBCL patients found that females received less intensive chemotherapies and had fewer comorbidities compared with males,[62] which may lead to decreased treatment-related morbidity and mortality in females, though this needs to be confirmed in the AYA population. Additionally, while data is limited, studies have shown that male AYA cancer survivors have less frequent engagement in survivorship care compared with females,[63] and in the general population males are less likely than females to utilize preventative health care,[64] both of which likely contribute to decreased long-term survival in male compared with female AYA DLBCL survivors.
In addition to sociodemographic factors, cancer specific factors may also impact long-term survival. Our data suggested that higher stage at diagnosis was associated with inferior long-term survival, however this did not reach statistical significance. Survival for relapsed late-stage disease is poor, and it is likely that these patients may not live long enough into survivorship to see late-effects. Additionally, while recent advancements in gene expression profiling are leading to the development of precision guided therapies, treatment regimen has historically been similar for early and late stage disease, with more advanced disease stages receiving additional cycles.[65] Those with advanced disease who underwent longer treatment duration may have a higher burden of treatment related toxicities secondary to higher cumulative exposures. Some AYA DLBCL patients may receive radiation to large nodal areas including mediastinal radiation, known to increase risk of late cardiovascular mortality in a dose dependent relationship.[66] One limitation to the SEER data is lack of robust and reliable treatment data. Whether receipt and total dose of mediastinal radiation varied by diagnosis stage in the SEER AYA DLBCL population is unknown and an important factor in interpretation of differences in long-term survival by stage. The majority of AYA DLBCL survivors in the current analysis were treated prior to the approval of rituximab as a first line treatment for DLBCL. It is unknown whether the addition of rituximab impacts long-term survival differences by stage of diagnosis in AYA DLBCL survivors. Limited data have found no late toxicities associated with rituximab treatment,[67] though additional studies are needed, particularly among those exposed at a younger age.
The SEER registries are widely considered as a definitive source of cancer data in the U.S., however there are limitations to using this dataset. Limitations regarding SES data were discussed previously, most notably that SES data is collected on a population level. Census tract data is unavailable in SEER prior to the year 2000, and thus could not be used to study long-term survivors. Thus we used a county-level SES deprivation index, taking into account measures of income, education, employment, and household crowding, as well as immigration status and language isolation which also impact access to healthcare. Furthermore, SES status and patient location are not tracked over time, limiting conclusions that can be drawn.. Analyses including more refined SES data are needed to clarify the impact of patient level SES status on long-term survival and further studies are needed to assess the extent to which SES status impacts racial/ethnic survival disparities among long-term AYA cancer survivors. Rural populations are underrepresented SEER data, which may have limited our ability to capture rural-urban disparities in long-term mortality of AYA DLBCL survivors. Additionally, data on specific chemotherapy and radiation regimens are not available, thus the extent to which treatment variables contributed to long-term survival disparities is unknown. A strength of this study is the large sample size, particularly given the relatively low prevalence of AYA DLBCL. Length of follow-up time is also a significant strength.
The findings in the current study demonstrate that race and sex impact mortality outcomes among AYA DLBCL survivors at up three decades of follow-up. This data is unique in that it focuses on 5-year survivors and follow-up time extends beyond that previously assessed in the AYA DLBCL survivor population. As both cancer incidence and cure rates increase in the AYA population more focus is needed on improvement of long-term morbidity and mortality. While we identified high-risk subsets of AYA DLBCL survivors, future studies are needed to understand the mechanisms underlying racial and sex-based disparities in outcomes that persist well past initial cancer treatment.
Supplementary Material
Highlights:
Racial disparities in survival persist decades into AYA DLBCL survivorship
Male survivors have worse long-term survival than female survivors
Older age at diagnosis is associated with unfavorable long-term survival
Disparities have not improved over time
Funding:
This work was supported by the National Cancer Institute at the National Institutes of Health [grant numbers P30 CA016672 and R38-HL143612] and research support from the Archer Foundation and LyondellBasell (MR, JAL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement:
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Miller KD, Fidler-Benaoudia M, Keegan TH, et al. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020. November;70(6):443–459. [DOI] [PubMed] [Google Scholar]
- 2.Scott AR, Stoltzfus KC, Tchelebi LT, et al. Trends in Cancer Incidence in US Adolescents and Young Adults, 1973-2015. JAMA Netw Open. 2020. December 1;3(12):e2027738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Close AG, Dreyzin A, Miller KD, et al. Adolescent and young adult oncology-past, present, and future. CA Cancer J Clin. 2019. November;69(6):485–496. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Moke DJ, Tsai KY, et al. A Reappraisal of Sex-Specific Cancer Survival Trends Among Adolescents and Young Adults in the United States. J Natl Cancer Inst. 2019. May 1;111(5):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norsker FN, Pedersen C, Armstrong GT, et al. Late Effects in Childhood Cancer Survivors: Early Studies, Survivor Cohorts, and Significant Contributions to the Field of Late Effects. Pediatr Clin North Am. 2020. December;67(6):1033–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkman AM, Livingston JA, Merriman K, et al. Long-term survival among 5-year survivors of adolescent and young adult cancer. Cancer. 2020. August 15;126(16):3708–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson C, Smitherman AB, Nichols HB. Conditional relative survival among long-term survivors of adolescent and young adult cancers. Cancer. 2018. July 15;124(14):3037–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armenian SH, Xu L, Cannavale KL, et al. Cause-specific mortality in survivors of adolescent and young adult cancer. Cancer. 2020. May 15;126(10):2305–2316. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Wang F, Chen L, et al. Long-term cardiovascular disease mortality among 160 834 5-year survivors of adolescent and young adult cancer: an American population-based cohort study. Eur Heart J. 2020. November 6. [DOI] [PubMed] [Google Scholar]
- 10.Avila JC, Livingston JA, Rodriguez AM, et al. Disparities in Adolescent and Young Adult Sarcoma Survival: Analyses of the Texas Cancer Registry and the National SEER Data. J Adolesc Young Adult Oncol. 2018. December;7(6):681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moke DJ, Tsai K, Hamilton AS, et al. Emerging Cancer Survival Trends, Disparities, and Priorities in Adolescents and Young Adults: A California Cancer Registry-Based Study. JNCI Cancer Spectr. 2019. June;3(2):pkz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keegan TH, Grogan RH, Parsons HM, et al. Sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults in California. Thyroid. 2015. June;25(6):635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keegan TH, Clarke CA, Chang ET, et al. Disparities in survival after Hodgkin lymphoma: a population-based study. Cancer Causes Control. 2009. December;20(10):1881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keegan TH, DeRouen MC, Parsons HM, et al. Impact of Treatment and Insurance on Socioeconomic Disparities in Survival after Adolescent and Young Adult Hodgkin Lymphoma: A Population-Based Study. Cancer Epidemiol Biomarkers Prev. 2016. February;25(2):264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn JM, Keegan TH, Tao L, et al. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. 2016. September 1;122(17):2723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkman AM, Brewster AM, Jones LW, et al. Racial Differences in 20-Year Cardiovascular Mortality Risk Among Childhood and Young Adult Cancer Survivors. J Adolesc Young Adult Oncol. 2017. September;6(3):414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuglievan B, Berkman A, Dibaj S, et al. Impact of Lagtime, Health Insurance Type, and Income Status at Diagnosis on the Long-Term Survival of Adolescent and Young Adult Cancer Patients. J Adolesc Young Adult Oncol. 2020. July 14. [DOI] [PubMed] [Google Scholar]
- 18.Berkman AM, Andersen CR, Puthenpura V, et al. Impact of race, ethnicity and socioeconomic status over time on the long-term survival of adolescent and young adult Hodgkin lymphoma survivors. Cancer Epidemiol Biomarkers Prev. 2021. July 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum KA, Keller FG, Castellino S, et al. Incidence and outcomes of lymphoid malignancies in adolescent and young adult patients in the United States. Br J Haematol. 2018. November;183(3):385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrahao R, Ribeiro RC, Lichtensztajn DY, et al. Survival after diffuse large B-cell lymphoma among children, adolescents, and young adults in California, 2001-2014: A population-based study. Pediatr Blood Cancer. 2019. April;66(4):e27559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truong B, Green AL, Friedrich P, et al. Ethnic, Racial, and Socioeconomic Disparities in Retinoblastoma. JAMA Pediatr. 2015. December;169(12):1096–104. [DOI] [PubMed] [Google Scholar]
- 22.Cox D Regression Models and Life-Tables. Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- 23.Therneau TM, Grambsch PM. Modeling survival data : extending the Cox model. New York: Springer; 2000. (Statistics for biology and health). [Google Scholar]
- 24.Therneau TM. A Package for Survival Analysis in R. R package version 3.2-3 2020. Available from: https://CRAN.R-project.org/package=survival>
- 25.Lenth R, Buerkner P, Herve M, et al. Estimated Marginal Means, aka Least-Squares Means 2020. Available from: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf
- 26.Cumming G The new statistics: why and how. Psychol Sci. 2014. January;25(1):7–29. [DOI] [PubMed] [Google Scholar]
- 27.Anderson C catseyes: Create Catseye Plots Illustrating the Normal Distribution of the Means. R package version 0.2.5 2020. Available from: https://CRAN.R-project.org/package=catseyes
- 28.Alese OB, Jiang R, Zakka KM, et al. Analysis of racial disparities in the treatment and outcomes of colorectal cancer in young adults. Cancer Epidemiol. 2019. December;63:101618. [DOI] [PubMed] [Google Scholar]
- 29.Kahn JM, Kelly KM, Pei Q, et al. Survival by Race and Ethnicity in Pediatric and Adolescent Patients With Hodgkin Lymphoma: A Children’s Oncology Group Study. J Clin Oncol. 2019. November 10;37(32):3009–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy CC, Lupo PJ, Roth ME, et al. Disparities in Cancer Survival Among Adolescents and Young Adults: A Population-Based Study of 88 000 Patients. J Natl Cancer Inst. 2021. August 2;113(8):1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver KE, Rowland JH, Bellizzi KM, et al. Forgoing medical care because of cost: assessing disparities in healthcare access among cancer survivors living in the United States. Cancer. 2010. July 15;116(14):3493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Husain M, Nolan TS, Foy K, et al. An overview of the unique challenges facing African-American breast cancer survivors. Support Care Cancer. 2019. March;27(3):729–743. [DOI] [PubMed] [Google Scholar]
- 33.Lee M, Salloum RG. Racial and ethnic disparities in cost-related medication non-adherence among cancer survivors. J Cancer Surviv. 2016. June;10(3):534–44. [DOI] [PubMed] [Google Scholar]
- 34.Loomer L, Ward KC, Reynolds EA, et al. Racial and socioeconomic disparities in adherence to preventive health services for ovarian cancer survivors. J Cancer Surviv. 2019. August;13(4):512–522. [DOI] [PubMed] [Google Scholar]
- 35.Palmer NR, Geiger AM, Felder TM, et al. Racial/Ethnic disparities in health care receipt among male cancer survivors. Am J Public Health. 2013. July;103(7):1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courtemanche C, Marton J, Ukert B, et al. The three-year impact of the Affordable Care Act on disparities in insurance coverage. Health Serv Res. 2019. February;54 Suppl 1:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abrahao R, Maguire FB, Morris CR, et al. The influence of the Affordable Care Act-Dependent Care Expansion on insurance coverage among young cancer survivors in California: an updated analysis. Cancer Causes Control. 2021. January;32(1):95–101. [DOI] [PubMed] [Google Scholar]
- 38.Schootman M, Deshpande AD, Pruitt SL, et al. National estimates of racial disparities in health status and behavioral risk factors among long-term cancer survivors and non-cancer controls. Cancer Causes Control. 2010. September;21(9):1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrd DA, Agurs-Collins T, Berrigan D, et al. Racial and Ethnic Differences in Dietary Intake, Physical Activity, and Body Mass Index (BMI) Among Cancer Survivors: 2005 and 2010 National Health Interview Surveys (NHIS). J Racial Ethn Health Disparities. 2017. December;4(6):1138–1146. [DOI] [PubMed] [Google Scholar]
- 40.Nayak P, Paxton RJ, Holmes H, et al. Racial and ethnic differences in health behaviors among cancer survivors. Am J Prev Med. 2015. June;48(6):729–36. [DOI] [PubMed] [Google Scholar]
- 41.Paxton RJ, Phillips KL, Jones LA, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012. August 15;118(16):4024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Churchwell K, Elkind MSV, Benjamin RM, et al. Call to Action: Structural Racism as a Fundamental Driver of Health Disparities: A Presidential Advisory From the American Heart Association. Circulation. 2020. December 15;142(24):e454–e468. [DOI] [PubMed] [Google Scholar]
- 43.Baedke JL, Lindsey LA, James AS, et al. Forgoing needed medical care among long-term survivors of childhood cancer: racial/ethnic-insurance disparities. J Cancer Surviv. 2021. May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kronenfeld JP, Graves KD, Penedo FJ, et al. Overcoming Disparities in Cancer: A Need for Meaningful Reform for Hispanic and Latino Cancer Survivors. Oncologist. 2021. June;26(6):443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao C, Bhatia S, Xu L, et al. Incidence, Risk Factors, and Mortality Associated With Second Malignant Neoplasms Among Survivors of Adolescent and Young Adult Cancer. JAMA Netw Open. 2019. June 5;2(6):e195536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abrahao R, Huynh JC, Benjamin DJ, et al. Chronic medical conditions and late effects after acute myeloid leukaemia in adolescents and young adults: a population-based study. Int J Epidemiol. 2021. May 17;50(2):663–674. [DOI] [PubMed] [Google Scholar]
- 47.Keegan THM, Kushi LH, Li Q, et al. Cardiovascular disease incidence in adolescent and young adult cancer survivors: a retrospective cohort study. J Cancer Surviv. 2018. June;12(3):388–397. [DOI] [PubMed] [Google Scholar]
- 48.Ke Y, Tan CJ, Ng T, et al. Optimizing Survivorship Care Services for Asian Adolescent and Young Adult Cancer Survivors: A Qualitative Study. J Adolesc Young Adult Oncol. 2020. June;9(3):384–393. [DOI] [PubMed] [Google Scholar]
- 49.DeRouen MC, Parsons HM, Kent EE, et al. Sociodemographic disparities in survival for adolescents and young adults with cancer differ by health insurance status. Cancer Causes Control. 2017. August;28(8):841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lara J, Brunson A, Keegan TH, et al. Determinants of Survival for Adolescents and Young Adults with Urothelial Bladder Cancer: Results from the California Cancer Registry. J Urol. 2016. November;196(5):1378–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pisu M, Kenzik KM, Oster RA, et al. Economic hardship of minority and non-minority cancer survivors 1 year after diagnosis: another long-term effect of cancer? Cancer. 2015. April 15;121(8):1257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dreyer MS, Nattinger AB, McGinley EL, et al. Socioeconomic status and breast cancer treatment. Breast Cancer Res Treat. 2018. January;167(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desmond RA, Jackson BE, Waterbor JW. Disparities in Cancer Survivorship Indicators in the Deep South Based on BRFSS Data: Recommendations for Survivorship Care Plans. South Med J. 2017. March;110(3):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garner EF, Maizlin II, Dellinger MB, et al. Effects of socioeconomic status on children with well-differentiated thyroid cancer. Surgery. 2017. September;162(3):662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh GK, Williams SD, Siahpush M, et al. Socioeconomic, Rural-Urban, and Racial Inequalities in US Cancer Mortality: Part I-All Cancers and Lung Cancer and Part II-Colorectal, Prostate, Breast, and Cervical Cancers. J Cancer Epidemiol. 2011;2011:107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ritter AJ, Goldstein JS, Ayers AA, et al. Rural and urban patients with diffuse large B-cell and follicular lymphoma experience reduced overall survival: a National Cancer DataBase study. Leuk Lymphoma. 2019. July;60(7):1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuo TM, Mobley LR. How generalizable are the SEER registries to the cancer populations of the USA? Cancer Causes Control. 2016. September;27(9):1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herb J, Wolff R, McDaniel P, et al. Rural representation of the surveillance, epidemiology, and end results database. Cancer Causes Control. 2021. March;32(3):211–220. [DOI] [PubMed] [Google Scholar]
- 59.Kubuschok B, Held G, Pfreundschuh M. Management of diffuse large B-cell lymphoma (DLBCL). Cancer Treat Res. 2015;165:271–88. [DOI] [PubMed] [Google Scholar]
- 60.Collaborators GBDM. Global, regional, and national age-sex-specific mortality and life expectancy, 1950-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018. November 10;392(10159):1684–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki Y, Yano T, Suehiro Y, et al. Clinical characteristics and outcomes of diffuse large B-cell lymphoma in adolescents and young adults. Int J Hematol. 2018. August;108(2):161–166. [DOI] [PubMed] [Google Scholar]
- 62.Huang HH, Hsiao FY, Chen LJ, et al. Women with Diffuse Large B Cell Lymphoma Benefit More from Rituximab-Containing Chemotherapy. J Womens Health (Larchmt). 2019. February;28(2):203–211. [DOI] [PubMed] [Google Scholar]
- 63.Berg CJ, Stratton E, Esiashvili N, et al. Young Adult Cancer Survivors’ Experience with Cancer Treatment and Follow-Up Care and Perceptions of Barriers to Engaging in Recommended Care. J Cancer Educ. 2016. September;31(3):430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaidya V, Partha G, Karmakar M. Gender differences in utilization of preventive care services in the United States. J Womens Health (Larchmt). 2012. February;21(2):140–5. [DOI] [PubMed] [Google Scholar]
- 65.Mondello P, Nowakowski GS. Treatment of Aggressive B Cell Lymphomas: Updates in 2019. Curr Hematol Malig Rep. 2020. June;15(3):225–234. [DOI] [PubMed] [Google Scholar]
- 66.Ratosa I, Ivanetic Pantar M. Cardiotoxicity of mediastinal radiotherapy. Rep Pract Oncol Radiother. 2019. Nov-Dec;24(6):629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinelli G, Schmitz SF, Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol. 2010. October 10;28(29):4480–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


