Abstract
Taste buds consist of specialized epithelial cells which detect particular tastants and synapse onto the afferent taste nerve innervating the endorgan. The nature of the neurotransmitter released by taste cells onto the nerve fiber was enigmatic early in this century although neurotransmitters for other sensory receptor cell types, e.g. hair cells, photoreceptors, was known for at least a decade. A 1999 paper by Burnstock and co-workers (Bo et al. 1999) showing the presence of P2X receptors on the afferent nerves served as a springboard for research that ultimately led to the discovery of ATP as the crucial neurotransmitter in the taste system (Finger et al. 2005). Subsequent work showed that a subpopulation of taste cells utilize a unique release channel, CALHM1/3, to release ATP in a voltage-dependent manner. Despite these advances, several aspects of purinergic transmission in this system remain to be elucidated.
Keywords: Taste bud, geniculate ganglion, serotonin, CALHM1, ATP
Background:
The sense of taste starts with taste buds, which are assemblies of specialized, elongate epithelial cells arrayed across the tongue, palate and larynx. Each taste bud contains roughly 50–100 cells divisible into morphological and functional classes. The most plentiful population, comprising about 50% of the cells in a taste bud are glial-like cells resembling astrocytes in terms of morphology and molecular features(Kinnamon and Finger 2019). The sensory cells of taste buds are so-called “short” receptor cells, lacking an axon. In this sense they are akin to hair cells of the auditory, vestibular and lateral line systems as well as the photoreceptors of the retina. All of these short sensory cells form a synapse onto a neuron which conveys the signal onward into the CNS (Fig. 1).
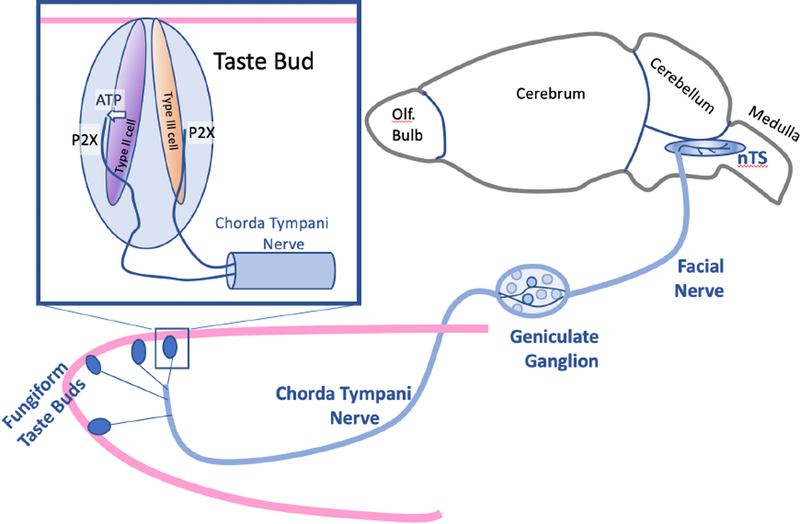
Fig. 1.
Semi-schematic diagram of the relationship between taste buds on the tip of the tongue (Fungiform Taste Buds) and their innervation by fibers of the chorda tympani nerve, a branch of the Facial nerve. The gustatory ganglion cells in the geniculate ganglion extend one branch centrally to terminate in the nuc. of the solitary tract (nTS) of the medulla, and the other branch peripherally, to innervate taste buds. Inset upper left: Each taste bud contains multiple elongate cells including glial-like Type 1 cells [not illustrated], and receptor cells some of which (Type II cells) release ATP through channel synapses to activate the P2X (P2X2 and P2X3 in mice) receptors on the sensory terminals of the afferent nerve fibers. Less clear is how Type III cells (for sour) release ATP to activate the P2X receptors on the afferent fibers.
The nature of the neurotransmitter between these short sensory cells and the next element in the transmission pathway was a matter of some conjecture before the late 1970s (Wu and Dowling 1978, Schessel and Highstein 1981) and 1980s (Sewell and Mroz 1987). Suggested candidates included acetycholine, catecholamines, GABA, and various amino acids (Sewell and Mroz 1987). Early candidates for the essential neurotransmitter in taste buds focused on acetycholine (DeHan and Graziadei 1973) and serotonin (Nada and Hirata 1976, Morimoto and Sato 1977).
The advent of immunohistochemistry and development of more specific pharmacological tools in the 1980s and 1990s led to more precise characterization of the neurotransmitters and receptors involved in transmission from the short sensory cells to the subsequent neuronal elements in the hair cell and retinal systems. In these systems, glutamate is the key neurotransmitter (Bobbin et al. 1981, Massey and Redburn 1987, Felix and Ehrenberger 1990, Kataoka and Ohmori 1996). Even in somatic sensation, glutamate is the first neurotransmitter utilized by the long-axon receptor cells of the dorsal root ganglia (Jessell et al. 1986, Radhakrishnan and Henry 1993). Hence it was not unreasonable to suggest that glutamate may serve as the afferent neurotransmitter for taste receptor cells as well. Curiously, attempts to localize glutamate or its processing enzymes to taste receptor cells (e.g. (Nagai et al. 1998, Lawton et al. 2000)) were largely not supportive of the hypothesis that taste cells utilize glutamate as a key neurotransmitter. A confound for interpretation of these results in the taste system is that some taste receptor cells respond to glutamate as a taste stimulus, so the presence of glutamate receptors in taste cells was not necessarily indicative of a role in neurotransmission (Brand et al. 1991, Lin and Kinnamon 1999, Caicedo et al. 2000, Eram and Michel 2005). Further, a few taste cells in each taste bud, in particular the Type III cells in mammalian taste buds, take up and release serotonin,, suggesting that this neurotransmitter might play a role in taste transmission (Morimoto and Sato 1977, Jain and Roper 1991, Nagai et al. 1998, Huang et al. 2005). Indeed, recent studies show that serotonin acting on neural 5HT3 receptors does participate in transmission of sour taste information in mice, but that these receptors are not necessary for transmission of taste information to the nervous system (Kaya et al. 2004, Larson et al. 2015). In addition, the scarcity of serotonin-accumulating cells suggested that other transmitters might be involved.
Serotonin had been localized only to one of the three principal types of taste cells in mammalian taste buds: the Type III cell (Nada and Hirata 1975). Type III cells are implicated in transduction and transmission of sour taste information (Huang et al. 2008) but not for other taste qualities, e.g. sweet, umami and bitter which rely on a PLC-mediated transduction cascade found in Type II receptor cells. Further, Type III, but not Type II, taste cells formed classical synapses onto afferent nerve fibers complete with an accumulation of presynaptic vesicles including some dense-cored vesicles typical of serotonergic transmission (Nada and Hirata 1977, Toyoshima et al. 1984). So the problem remained as to what the transmitter might be for the Type II taste cells. These receptor cells do not display typical synaptic junctions with nerve fibers, but rather show a specialized so-called “atypical mitochondrion” (Royer and Kinnamon 1988) closely apposed to the plasma membrane of the taste cell at its point of contact with the nerve fiber. How this arrangement might lead to regulated release of neurotransmitter was entirely unclear.
So by the mid-1990s, even though a primary neurotransmitter had been identified in all other sensory systems, the nature of the neurotransmitter in taste receptor cells remained enigmatic. Previous studies through that time had mostly eliminated potential neurotransmitter candidates rather than suggesting new possibilities.
Personal Reflection:
At about that time, one of us (TEF) was working with Prof. Thomas Dunwiddie at the Univ. Colorado School of Medicine to develop a slice preparation to study transmission of taste information from the sensory nerves to taste centers of the brainstem, e.g. (Finger and Dunwiddie 1992). Indeed, we established that glutamate was indeed the principal neurotransmitter utilized at the first synapse within the brainstem (Smeraski et al. 1998, Smeraski et al. 1999) but did not have the means to test the situation in regard to transmission from taste buds to sensory nerves. Dr. Dunwiddie had been studying purinergic transmission in the hippocampus for quite some time, e.g. (Dunwiddie and Hoffer 1980, Dunwiddie 1985, Masino et al. 2002) and our informal discussions often turned to the idea that perhaps taste buds used some form of purinergic transmission to communicate with the sensory nerves. Unfortunately, we did not then have the tools to investigate that possibility. This became a frequent point of discussion among the investigators of the Rocky Mountain Taste and Smell Center, including Drs. Restrepo, Barlow, John Kinnamon and Sue Kinnamon.
The seminal breakthrough in uncovering the neurotransmitter from taste buds was the paper in 1999 by Geoff Burnstock and colleagues (Bo et al. 1999) reporting the presence of P2X2 and P2X3 receptors in taste nerves. This led to our speculation that ATP might serve as a key neurotransmitter in this system, but how to test it? Looking at the list of key features of a neurotransmitter, demonstration of the presence of the purported neurotransmitter in the presynaptic element came to mind. But of course all cells have ATP. Nonetheless, we attempted experiments relying on quinacrine fluorescence as a surrogate for regions of ATP accumulation. While some taste cells do exhibit quinacrine puncta within their cytoplasm (e.g. (Kataoka et al. 2006)), their relationship to neurotransmission was still unclear.
After several futile attempts to demonstrate ATP release, we found a presentation by an associate of Dr. Burnstock, Debra Cockayne then of the Roche Bioscience Program at the 2002 Soc Neuroscience meeting (Orlando, FL) on P2X2/P2X3 double knockout mice. We realized that if ATP were a key neurotransmitter acting on neural P2X2 and P2X3 receptors, then these double-KO mice should be unable to taste – or unable to taste at least some taste qualities.
Excitedly, we approached Dr. Cockayne at the meeting and asked her if these mice could taste. She responded, “How would I know?”, they eat normally but have a high rate of pup mortality. We told her it was really simple: just offer the mice 2 water bottles – one with just plain water and the other containing sugar water. If they can taste they will drink more of the sugar water; it’s called a 2-bottle preference test. She replied most graciously that she would just send us the mice and we could test them ourselves…which we did, leading up to our 2005 paper in Science establishing ATP as the necessary neurotransmitter in the taste periphery (Fig. 2).
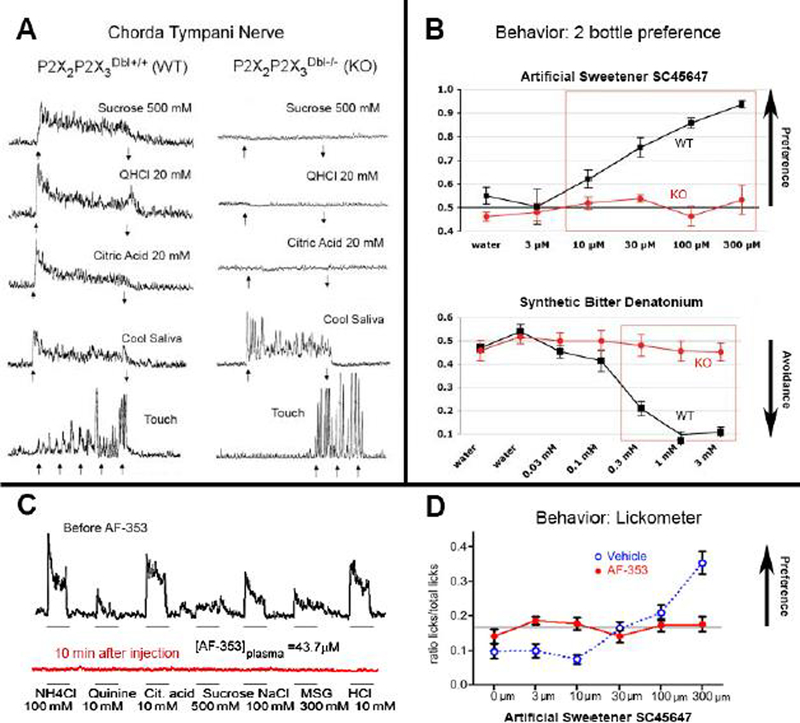
Fig. 2.
Genetic Deletion or Pharmacological Block of P2X3 and P2X2/P2X3 channels eliminates taste responses and behaviors. A. Taste nerve (chorda tympani) recordings comparing responses in P2X2/P2X3 double KO mice (Right column) with WT controls (Left column) on the same mixed background for selected taste substance (Sucrose = sweet; QHCl, Quinine HCl = bitter; Citric Acid = sour). Responses in the KO animals are absent although the KO nerves still respond to temperature and touch. B. Graphs comparing taste preference behavior in P2X2/P2X3 double KO and WT mice in aa 2 bottle preference test. The ordinate shows the ratio of tastant consumed compared to water. WT animals (black) strongly prefer the artificial sweetener and avoid the bitter compound, denatonium. In contrast, KO animals (red) show neither preference nor avoidance to the respective tastants. C. Nerve recordings in WT animals injected with the antagonist AF-353 which inhibits all P2X3 containing receptors. Top trace (black) shows responses to all tastants; the drug-treated animal shows virtually no taste responses (red) to any tastant. NH4Cl = ammonium chloride; Cit. Acid = citric acid, MSG = monosodium glutamate. D. Tastant-evoked behavior in WT mice treated either with vehicle or with AF-353. Vehicle-treated mice show increasing preference for the artificial sweetener whereas the AF-353-treated animals show no response to this sweetener. Panels A & B adapted from (Finger et al. 2005). Panels C & D adapted from (Vandenbeuch et al. 2015).
Another important group contributing to that landmark paper was the team of Vika Danilova and Goran Hellekant at the Univ. Wisconsin, who provided the expertise in peripheral taste nerve recording, which we had not then mastered. This is a powerful approach for assessing peripheral taste function because it is a direct readout of the output of the taste bud-taste nerve periphery. Unlike behavioral assays, nerve recording is not reliant on proper functioning of CNS structures involved in preference and motivational states. We arranged to ship the mice to the Hellekant laboratory once we generated sufficient double-KO animals. Shortly after the first lot went out, one of us (TEF) received a call from Dr. Danilova saying that something was wrong because she was unable to record any taste responses in the nerves of even the wildtype mice we had sent although responses to touch and temperature seemed intact. Well, we explained, that is indeed good news in that we had not yet sent any wildtype controls and that the first shipment was only the double knockout animals. Subsequent shipments of both wildtype and knockout mice established that indeed, Dr. Danilova could find taste responses in the wildtype mice but not in the knockouts and it is these data that appear in the 2005 Science article (Fig. 2A).
The Science:
Taste buds are complex sensory endorgans containing 3 morphologically and molecularly distinct types of cells with limited life spans. The cells of a taste bud are replaced continuously throughout life with an average replacement time of 14–30 days depending on cell type (Finger and Barlow 2021). The majority of cells in a taste bud are glial-like cells (Type I cells), which surround and isolate the other cell types in a taste bud (Yang et al. 2020). Type II cells rely on GPCR taste receptors for bitter, sweet or umami, coupled to a PLC-mediated signaling cascade to generate an action potential triggering release of neurotransmitter (Kinnamon and Finger 2019). Type III cells transduce sour (acids) via proton influx through the Otop1 ion channel (Liman and Kinnamon 2021) to depolarize the cell, trigger an action potential, and ultimately open voltage-gated Ca++ channels to allow for SNARE-protein mediated vesicular release of neurotransmitter.
Our investigation of the P2X2/P2X3 double KO mice (Finger et al. 2005) showed that these purinergic receptors were required for taste-mediated behavior and neural activity for all taste qualities, including both those mediated by Type II cells (bitter, sweet, umami) and those mediated by Type III cells (sour). Recent work shows that at least some components of salty taste are mediated by a unique cell population with some, but not all characteristics of Type II cells (Nomura et al. 2020). Salty tastes also were absent in the P2X2/P2X3 double KO mice and so must affect this novel cell population as well.
In the 2005 paper, we also showed that in taste epithelia from wildtype mice, we could measure taste-mediated ATP release by luciferin-luciferase assay. Curiously, the double-KO mice also showed reduced ATP release from the taste epithelia (Huang et al. 2011) but this may have been a secondary effect of the KO. Taste cells have no measurable expression of P2X3 and only low expression of P2X2. If absence of P2X2 were responsible for the loss of taste signaling, then a P2X2 single KO should be similarly debilitated. Such is not the case (Fig. S2 in (Finger et al. 2005)); P2X2 single KO mice show taste impairment but not to the extent of the P2X2/P2X3 double KO animals. Subsequent studies established that transmission of all taste qualities was eliminated by application of AF353, an antagonist for P2X3-containing receptors, either injected i.p. or applied directly to the tongue epithelium (Vandenbeuch et al. 2015) (Fig. 2C & D). This pharmacological blockade of P2X3-containing receptors does not result in decreases in taste-evoked ATP release again suggesting that the result from P2X2/P2X3 double-KO mice is a side-effect of the KO.
An important criterion for the establishment of a neurotransmitter candidate is clearance of the substance from the synaptic cleft. In the case of ATP, this clearance is mediated by ectonucleotidases. The presence of high levels of Mg++ activated ATPase in taste buds was noted back in the 1960s although the significance of this was unclear at the time (Iwayama and Nada 1967) although these investigators noted that the membrane-associated ectoATPase was absent at the interface between nerve fibers and the Type II or Type III taste cells (Akisaka and Oda 1977). The functional importance of this ectoATPase became clear once we identified ATP as the key neurotransmitter. In 2006, we molecularly identified the taste bud ectoATPase as NTPDase2 which is highly selective for ATP over ADP (Bartel et al. 2006). Genetic elimination of this ectoATPase results in perdurance of ATP in the taste epithelium and reduced responses to tastants, likely due to desensitization of the P2X receptors on the afferent nerves (Vandenbeuch et al. 2013).
Mechanism of ATP release:
Despite the clear demonstration that ATP is released from taste buds to activate P2X receptors on the afferent nerves, until 2013, the mechanism for ATP release was unclear. Type II taste cells do not express several components of the SNARE complex (Yang et al. 2004, Yang et al. 2007, Asano-Miyoshi et al. 2009) and contacts between Type II cells and nerve fibers lack synaptic vesicles. Despite this, the amount of ATP release was clearly related to the number of action potentials in the Type II taste cells (Murata et al. 2010). Several investigators suggested that ATP release might occur via a gated ion channel ((Huang et al. 2007, Romanov et al. 2007) but the identity of this channel was unclear.
The first physiological suggestion that ATP release might be non-vesicular was a study from Dr. Steve Roper’s laboratory showing that ATP release was blocked by carbenoxolone, a rather specific inhibitor of the ATP release channel Pannexin-1 (Panx1), which blocks ATP release in other systems (Ransford et al. 2009). Low concentrations of carbenoxolone blocked taste-evoked ATP release from isolated Type II cells. (Huang et al. 2007) suggesting that Pannexin 1was involved. Using immunocytochemistry and rt-PCR, they went on to show that Panx1 was expressed in nearly all Type II taste cells, and concluded, albeit prematurely, that Panx1 was the ATP release mechanism in the taste bud. Several other studies supported this conclusion, including studies showing carbenoxolone block of ATP signaling in slice preparations of taste buds (Dando and Roper 2009) and carbenoxolone block of tastant-evoked ATP release from single type II cells during loose patch recording (Murata et al. 2010). However, questions remained about whether the Panx1 channels had the right kinetics and voltage dependence to explain ATP release in taste buds (cf, (Romanov et al. 2008, Romanov et al. 2012) and none of the studies tested Panx1 knockout mice for effects on taste responses. Then in 2013 a seminal paper appeared suggesting a relatively new player, Calcium homeostasis modulator 1 (CALHM1), is the basis of taste-evoked ATP release (Taruno et al. 2013). CALHM1 is a large conductance, voltage-gated channel previously thought to be involved primarily in calcium homeostasis in hippocampal neurons (Ma et al. 2018). Taruno et al showed CALHM1 is expressed in taste buds in Type II taste cells, and that CALHM1 knockout mice have greatly diminished responses to bitter, sweet and umami tastants. However, responses were not totally abolished in the knockout and some features of the channel observed in heterologous systems were not consistent with CALHM1 being the only channel involved in the ATP release from taste buds.
Questions remained, however, about the role of Panx1, since it is expressed in the ATP releasing taste cells and ATP release is blocked by carbenoxolone, which does not affect ATP release via the CALHM channels, at least in heterologous systems. To determine if Panx1 might play some minor role in ATP release in taste buds, two independent labs obtained Panx1 knockout mice and tested them behaviorally and physiologically for deficits related to ATP release in a systems context (Tordoff et al. 2015, Vandenbeuch et al. 2015). However, in both studies, knockouts and wildtype mice had normal chorda tympani responses to all tastants and responded normally in behavioral experiments, confirming that Panx1 plays no direct role in ATP release in taste buds.
So, if pannexin is not involved, what accounts for the discrepancy between properties of CALHM1 and the release channel in taste buds? The discovery of another CALHM subunit in Type II taste cells, CALHM3, resolved this problem (Ma et al. 2018). When CALHM3 was co-expressed with CALHM1 in heterologous systems, the heteromeric channel had the appropriate kinetics and voltage-dependence to account for ATP release in taste buds. Further, double knockout of CALHM1 and CALHM3 completely eliminated responses to bitter, sweet and umami stimuli, confirming the channel as the ATP release channel of Type II cells in taste buds.
So why does carbenoxolone block ATP release in taste buds, if it does not block the CALHM1/3 channel? A recent study by one of us (TEF) in collaboration with the Romanov lab (Romanov et al. 2018) seems to resolve this question. Romanov et al showed that the CALHM1 channel lies on the membrane adjacent to “atypical” mitochondria (Fig. 3) and forms a signaling complex with the afferent nerves containing the P2X receptors. This signaling complex was termed a “channel synapse” by Taruno et al. (Taruno et al. 2021). The mitochondrion appears to be the immediate source of ATP for release by the CALHM1/3 channel and carbenoxolone inhibits the mitochondrial membrane potential, thereby depleting the mitochondrion of ATP resulting in inhibition of ATP release. Thus, the carbenoxolone inhibition is upstream of the ATP release channel and explains the block of ATP release observed by other investigators upon application of carbenoxolone to taste tissues
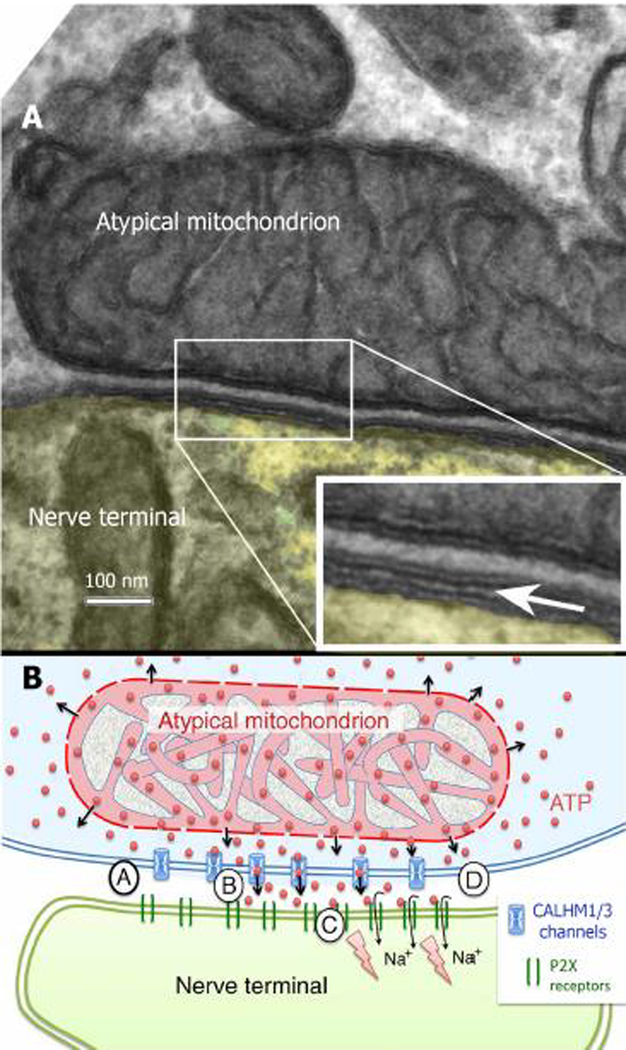
Fig. 3:
Micrograph (A) and Semi-schematic diagram (B) of the channel synapse wherein ATP release is regulated by the voltage-gated CALHM1/3 channels situated in the taste cell membrane. In rodents, a large “atypical” mitochondrion is tightly associated with the release complex with a relatively constant spacing of 20–30 nm between the mitochondrion and the plasma membrane. A. Arrow in the inset indicates the intercellular cleft. B. Circled letters AA-D indicate the sequence of events leading to ATP release. (A) At rest, the channel is closed; ATP diffuses freely from intermembrane space of the mitochondrion into the surrounding cytoplasm. (B) Action potentials in the taste cell gate open the CALHM1/3 channel to allow diffusion of ATP into the intracellular cleft where it (C) binds to P2X2/P2X3 receptors in the neural membrane to generate a post-synaptic potential, (D) The CALHM1/3 channel closes and extracellular ATP is degraded by the ectoATPase in the surrounding taste cells. Adapted from (Romanov et al. 2018)
So, if ATP is released from Type II cells via “channel synapses” and they are exclusively found in Type II taste cells, why is ATP also required for transmission from Type III taste cells, and where is it coming from? As mentioned earlier, Type III cells form conventional synapses with afferent nerve fibers and release serotonin via vesicles to activate 5-HT3a receptors on afferent nerve fibers (Larson et al. 2015) (Fig. 4A, B). However, although knockout or pharmacological blockade of 5-HTa reduces neural responses to acids, it does not eliminate them and ATP is still required for a full neural response. Yet ATP release has not been detected from identified Type III taste cells, although serotonin release can be detected with biosensor cells by either depolarization or acid stimulation of isolated Type III taste cells (Huang et al. 2007). Further, Type III cells do not contain the ATP vesicular transporter, VNUT, which would be required for transport of ATP into the serotonergic vesicles for co-release (Iwatsuki et al. 2009).
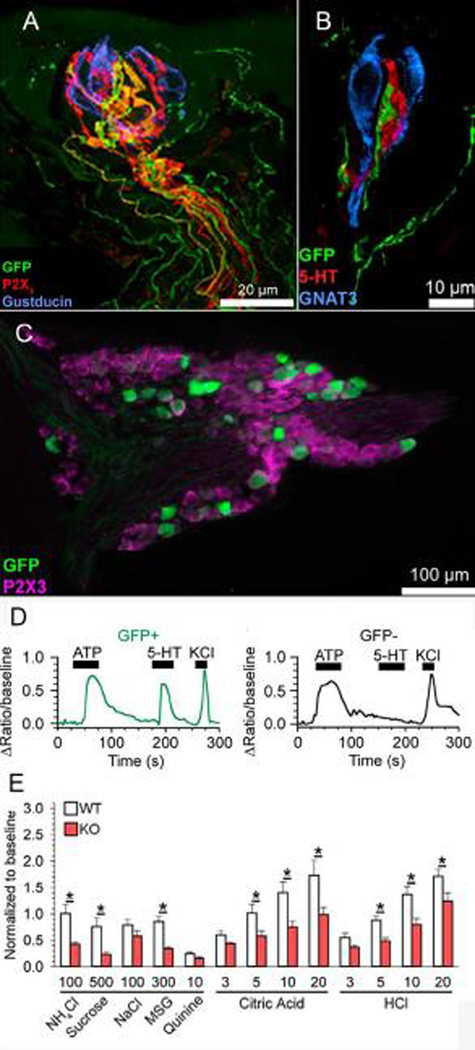
Figure 4.
Role of 5-HT3A in signaling from Type III taste cells to afferent fibers. A. Longitudinal Z stack of a fungiform taste bud from a 5-HT3a-GFP mouse. Intragemmal fibers expressing 5-HT3 (green) are also labeled with an antibody to P2X3 (red). Antibody against gustducin labels Type II taste cells (blue). B. Optical slices from a different fungiform taste bud, showing Type III cells stained with an antibody against 5-HT (red) and Type II cells labeled with an antibody against gustducin (blue). 5-HT3a-GFP fibers (green) preferentially contact the Type III taste cells. C. Optical section through a geniculate ganglion from a 5-HT3a-GFP mouse. All neurons are stained with an antibody against P2X3 (magenta), while a subset also stain with GFP (green). D. Calcium imaging recordings from geniculate ganglion neurons isolated from a 5-HT3a-GFP mouse. GFP+ neurons responded to both ATP and 5-HT, while GFP- neurons responded only to ATP. KCl was used as a index of cell viability. E. Chorda tympani recordings from 5-HT3a KO mice and their WT littermates. Responses in KO mice were reduced to all acids compared to their WT littermates. Modified from (Larson et al. 2015)
Another recent study suggests that Type II cells are not the source of ATP required for Type III cell transmission, since Skn-1a knockout mice, which completely lack Type II cells, have normal responses to sour stimuli (Larson et al. 2020). So if Type II cells are not the source of ATP required for Type III cell transmission, where is it coming from? Clearly this is one of the main unanswered questions remaining in the field and is a focus of current investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERRENCES:
- Akisaka T and Oda M (1977). “The fine structural localization of adenosine triphosphatase activity on the taste bud in the fungiform papillae of the rat.” Arch Histol Jpn 40(1): 63–72. [DOI] [PubMed] [Google Scholar]
- Asano-Miyoshi M, Hamamichi R and Emori Y (2009). “Synaptophysin as a probable component of neurotransmission occurring in taste receptor cells.” J Mol Histol 40(1): 59–70. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J and Finger TE (2006). “Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds.” J Comp Neurol 497(1): 1–12. PMC2212711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo X, Alavi A, Xiang Z, Oglesby I, Ford A and Burnstock G (1999). “Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds.” Neuroreport 10(5): 1107–1111. [DOI] [PubMed] [Google Scholar]
- Bobbin RP, Bledsoe SC Jr., Chihal DM and Morgan DN (1981). “Comparative actions of glutamate and related substances on the Xenopus laevis lateral line.” Comp Biochem Physiol C Comp Pharmacol 69c(1): 145–147. [DOI] [PubMed] [Google Scholar]
- Brand JG, Teeter JH, Kumazawa T, Huque T and Bayley DL (1991). “Transduction mechanisms for the taste of amino acids.” Physiol Behav 49(5): 899–904. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Kim KN and Roper SD (2000). “Glutamate-induced cobalt uptake reveals non-NMDA receptors in rat taste cells.” J Comp Neurol 417(3): 315–324. [PubMed] [Google Scholar]
- Dando R and Roper SD (2009). “Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels.” J Physiol 587(Pt 24): 5899–5906. PMC2808547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHan RS and Graziadei P (1973). “The innervation of frog’s taste organ: “a histochemical study”.” Life Sci 13(10): 1435–1449. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV (1985). “The physiological role of adenosine in the central nervous system.” Int Rev Neurobiol 27: 63–139. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV and Hoffer BJ (1980). “Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus.” Br J Pharmacol 69(1): 59–68. PMC2044173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eram M and Michel WC (2005). “Morphological and biochemical heterogeneity in facial and vagal nerve innervated taste buds of the channel catfish, Ictalurus punctatus.” J Comp Neurol 486(2): 132–144. [DOI] [PubMed] [Google Scholar]
- Felix D and Ehrenberger K (1990). “A microiontophoretic study of the role of excitatory amino acids at the afferent synapses of mammalian inner hair cells.” Eur Arch Otorhinolaryngol 248(1): 1–3. [DOI] [PubMed] [Google Scholar]
- Finger TE and Barlow LA (2021). “Cellular Diversity and Regeneration in Taste Buds.” Curr Opin Physiol 20: 146–153. PMC7888981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G and Kinnamon SC (2005). “ATP signaling is crucial for communication from taste buds to gustatory nerves.” Science 310(5753): 1495–1499. [DOI] [PubMed] [Google Scholar]
- Finger TE and Dunwiddie TV (1992). “Evoked responses from an in vitro slice preparation of a primary gustatory nucleus: the vagal lobe of goldfish.” Brain Res 580(1–2): 27–34. [DOI] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R and Roper SD (2008). “Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste.” J Physiol 586(12): 2903–2912. PMC2517205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Stone LM, Pereira E, Yang R, Kinnamon JC, Dvoryanchikov G, Chaudhari N, Finger TE, Kinnamon SC and Roper SD (2011). “Knocking out P2X receptors reduces transmitter secretion in taste buds.” J Neurosci 31(38): 13654–13661. PMC3188419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N and Roper SD (2007). “The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds.” Proc Natl Acad Sci U S A 104(15): 6436–6441. PMC1851090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D and Roper SD (2005). “Mouse taste buds use serotonin as a neurotransmitter.” J Neurosci 25(4): 843–847. PMC6725637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K and Uneyama H (2009). “Identification of the vesicular nucleotide transporter (VNUT) in taste cells.” Biochem Biophys Res Commun 388(1): 1–5. [DOI] [PubMed] [Google Scholar]
- Iwayama T and Nada O (1967). “Histochemically demonstrable ATPase activity in the taste buds of the rat.” Exp Cell Res 46(3): 607–608. [DOI] [PubMed] [Google Scholar]
- Jain S and Roper SD (1991). “Immunocytochemistry of gamma-aminobutyric acid, glutamate, serotonin, and histamine in Necturus taste buds.” J Comp Neurol 307(4): 675–682. [DOI] [PubMed] [Google Scholar]
- Jessell TM, Yoshioka K and Jahr CE (1986). “Amino acid receptor-mediated transmission at primary afferent synapses in rat spinal cord.” J Exp Biol 124: 239–258. [DOI] [PubMed] [Google Scholar]
- Kataoka S, Toyono T, Seta Y and Toyoshima K (2006). “Expression of ATP-gated P2X3 receptors in rat gustatory papillae and taste buds.” Arch Histol Cytol 69(4): 281–288. [DOI] [PubMed] [Google Scholar]
- Kataoka Y and Ohmori H (1996). “Of known neurotransmitters, glutamate is the most likely to be released from chick cochlear hair cells.” J Neurophysiol 76(3): 1870–1879. [DOI] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL and Herness S (2004). “A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes.” Am J Physiol Regul Integr Comp Physiol 286(4): R649–658. [DOI] [PubMed] [Google Scholar]
- Kinnamon SC and Finger TE (2019). “Recent advances in taste transduction and signaling.” F1000Res 8. PMC7059786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ED, Vandenbeuch A, Anderson CB and Kinnamon SC (2020). “Function, Innervation, and Neurotransmitter Signaling in Mice Lacking Type-II Taste Cells.” eNeuro 7(1). PMC7004487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ED, Vandenbeuch A, Voigt A, Meyerhof W, Kinnamon SC and Finger TE (2015). “The Role of 5-HT3 Receptors in Signaling from Taste Buds to Nerves.” J Neurosci 35(48): 15984–15995. PMC4666921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton DM, Furness DN, Lindemann B and Hackney CM (2000). “Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds.” Eur J Neurosci 12(9): 3163–3171. [DOI] [PubMed] [Google Scholar]
- Liman ER and Kinnamon SC (2021). “Sour taste: receptors, cells and circuits.” Curr Opin Physiol 20: 8–15. PMC7943026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W and Kinnamon SC (1999). “Physiological evidence for ionotropic and metabotropic glutamate receptors in rat taste cells.” J Neurophysiol 82(5): 2061–2069. [DOI] [PubMed] [Google Scholar]
- Ma J, Qi X, Yang C, Pan R, Wang S, Wu J, Huang L, Chen H, Cheng J, Wu R, Liao Y, Mao L, Wang FC, Wu Z, An JX, Wang Y, Zhang X, Zhang C and Yuan Z (2018). “Calhm2 governs astrocytic ATP releasing in the development of depression-like behaviors.” Mol Psychiatry 23(4): 883–891. [DOI] [PubMed] [Google Scholar]
- Ma Z, Taruno A, Ohmoto M, Jyotaki M, Lim JC, Miyazaki H, Niisato N, Marunaka Y, Lee RJ, Hoff H, Payne R, Demuro A, Parker I, Mitchell CH, Henao-Mejia J, Tanis JE, Matsumoto I, Tordoff MG and Foskett JK (2018). “CALHM3 Is Essential for Rapid Ion Channel-Mediated Purinergic Neurotransmission of GPCR-Mediated Tastes.” Neuron 98(3): 547–561 e510. PMC5934295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Diao L, Illes P, Zahniser NR, Larson GA, Johansson B, Fredholm BB and Dunwiddie TV (2002). “Modulation of hippocampal glutamatergic transmission by ATP is dependent on adenosine a(1) receptors.” J Pharmacol Exp Ther 303(1): 356–363. 2254338 [DOI] [PubMed] [Google Scholar]
- Massey SC and Redburn DA (1987). “Transmitter circuits in the vertebrate retina.” Prog Neurobiol 28(1): 55–96. [DOI] [PubMed] [Google Scholar]
- Morimoto K and Sato M (1977). “Is serotonin a chemical transmitter in the frog taste organ?” Life Sci 21(11): 1685–1695. [DOI] [PubMed] [Google Scholar]
- Murata Y, Yasuo T, Yoshida R, Obata K, Yanagawa Y, Margolskee RF and Ninomiya Y (2010). “Action potential-enhanced ATP release from taste cells through hemichannels.” J Neurophysiol 104(2): 896–901. PMC3774670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada O and Hirata K (1975). “The occurrence of the cell type containing a specific monoamine in the taste bud of the rabbit’s foliate papila.” Histochemistry 43(3): 237–240. [DOI] [PubMed] [Google Scholar]
- Nada O and Hirata K (1976). “Pharmaco-histochemical studies on a specific monoamine in the gustatory epithelia of the rabbit.” Histochemistry 50(2): 111–117. [DOI] [PubMed] [Google Scholar]
- Nada O and Hirata K (1977). “The monoamine-containing cell in the gustatory epithelium of some vertebrates.” Arch Histol Jpn 40 Suppl: 197–206. [DOI] [PubMed] [Google Scholar]
- Nagai T, Delay RJ, Welton J and Roper SD (1998). “Uptake and release of neurotransmitter candidates, [3H]serotonin, [3H]glutamate, and [3H]gamma-aminobutyric acid, in taste buds of the mudpuppy, Necturus maculosus.” J Comp Neurol 392(2): 199–208. [PubMed] [Google Scholar]
- Nomura K, Nakanishi M, Ishidate F, Iwata K and Taruno A (2020). “All-Electrical Ca(2+)-Independent Signal Transduction Mediates Attractive Sodium Taste in Taste Buds.” Neuron 106(5): 816–829 e816. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan V and Henry JL (1993). “Excitatory amino acid receptor mediation of sensory inputs to functionally identified dorsal horn neurons in cat spinal cord.” Neuroscience 55(2): 531–544. [DOI] [PubMed] [Google Scholar]
- Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE and Salathe M (2009). “Pannexin 1 contributes to ATP release in airway epithelia.” Am J Respir Cell Mol Biol 41(5): 525–534. PMC2778159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Bystrova MF, Rogachevskaya OA, Sadovnikov VB, Shestopalov VI and Kolesnikov SS (2012). “The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable.” J Cell Sci 125(Pt 22): 5514–5523. PMC3561859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Lasher RS, High B, Savidge LE, Lawson A, Rogachevskaja OA, Zhao H, Rogachevsky VV, Bystrova MF, Churbanov GD, Adameyko I, Harkany T, Yang R, Kidd GJ, Marambaud P, Kinnamon JC, Kolesnikov SS and Finger TE (2018). “Chemical synapses without synaptic vesicles: Purinergic neurotransmission through a CALHM1 channel-mitochondrial signaling complex.” Sci Signal 11(529). PMC5966022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF and Kolesnikov SS (2007). “Afferent neurotransmission mediated by hemichannels in mammalian taste cells.” EMBO J 26(3): 657–667. PMC1794384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Khokhlov AA and Kolesnikov SS (2008). “Voltage dependence of ATP secretion in mammalian taste cells.” J Gen Physiol 132(6): 731–744. PMC2585863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer SM and Kinnamon JC (1988). “Ultrastructure of mouse foliate taste buds: synaptic and nonsynaptic interactions between taste cells and nerve fibers.” J Comp Neurol 270(1): 11–24, 58–19. [DOI] [PubMed] [Google Scholar]
- Schessel DA and Highstein SM (1981). “Is transmission between the vestibular type I hair cell and its primary afferent chemical?” Ann N Y Acad Sci 374: 210–214. [DOI] [PubMed] [Google Scholar]
- Sewell WF and Mroz EA (1987). “Neuroactive substances in inner ear extracts.” J Neurosci 7(8): 2465–2475. PMC6568975 [PMC free article] [PubMed] [Google Scholar]
- Smeraski CA, Dunwiddie TV, Diao L and Finger TE (1998). “Excitatory amino acid neurotransmission in the primary gustatory nucleus of the goldfish Carassius auratus.” Ann N Y Acad Sci 855: 442–449. [DOI] [PubMed] [Google Scholar]
- Smeraski CA, Dunwiddie TV, Diao L and Finger TE (1999). “NMDA and non-NMDA receptors mediate responses in the primary gustatory nucleus in goldfish.” Chem Senses 24(1): 37–46. [DOI] [PubMed] [Google Scholar]
- Taruno A, Nomura K, Kusakizako T, Ma Z, Nureki O and Foskett JK (2021). “Taste transduction and channel synapses in taste buds.” Pflugers Arch 473(1): 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P and Foskett JK (2013). “CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes.” Nature 495(7440): 223–226. PMC3600154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Aleman TR, Ellis HT, Ohmoto M, Matsumoto I, Shestopalov VI, Mitchell CH, Foskett JK and Poole RL (2015). “Normal Taste Acceptance and Preference of PANX1 Knockout Mice.” Chem Senses 40(7): 453–459. PMC4635636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima K, Nada O and Shimamura A (1984). “Fine structure of monoamine-containing basal cells in the taste buds on the barbels of three species of teleosts.” Cell Tissue Res 235(3): 479–484. [DOI] [PubMed] [Google Scholar]
- Vandenbeuch A, Anderson CB and Kinnamon SC (2015). “Mice Lacking Pannexin 1 Release ATP and Respond Normally to All Taste Qualities.” Chem Senses 40(7): 461–467. PMC4635638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Anderson CB, Parnes J, Enjyoji K, Robson SC, Finger TE and Kinnamon SC (2013). “Role of the ectonucleotidase NTPDase2 in taste bud function.” Proc Natl Acad Sci U S A 110(36): 14789–14794. PMC3767538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Larson ED, Anderson CB, Smith SA, Ford AP, Finger TE and Kinnamon SC (2015). “Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice.” J Physiol 593(5): 1113–1125. PMC4358674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM and Dowling JE (1978). “L-aspartate: evidence for a role in cone photoreceptor synaptic transmission in the carp retina.” Proc Natl Acad Sci U S A 75(10): 5205–5209. PMC336294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Dzowo YK, Wilson CE, Russell RL, Kidd GJ, Salcedo E, Lasher RS, Kinnamon JC and Finger TE (2020). “Three-dimensional reconstructions of mouse circumvallate taste buds using serial blockface scanning electron microscopy: I. Cell types and the apical region of the taste bud.” J Comp Neurol 528(5): 756–771. PMC7041425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Ma H, Thomas SM and Kinnamon JC (2007). “Immunocytochemical analysis of syntaxin-1 in rat circumvallate taste buds.” J Comp Neurol 502(6): 883–893. [DOI] [PubMed] [Google Scholar]
- Yang R, Stoick CL and Kinnamon JC (2004). “Synaptobrevin-2-like immunoreactivity is associated with vesicles at synapses in rat circumvallate taste buds.” J Comp Neurol 471(1): 59–71. [DOI] [PubMed] [Google Scholar]