Abstract
Due to its pivotal role in autonomic networks, the vagus attracts continuous interest from both basic scientists and clinicians. In particular, recent advances in vagus nerve stimulation strategies and their application to pathological conditions beyond epilepsy provide a good opportunity to recall basic features of vagal peripheral and central anatomy. In addition to the “classical” vagal brainstem nuclei, i.e., dorsal motor nucleus, nucleus ambiguus and nucleus tractus solitarii, the spinal trigeminal and paratrigeminal nuclei come into play as targets of vagal afferents. On the other hand, the nucleus of the solitary tract receives and integrates not only visceral but also somatic afferents. Thus, the vagus system participates significantly in what may be defined as “somato-visceral interface”.
Keywords: Dorsal motor nucleus, nucleus ambiguus, nucleus tractus solitarii, paratrigeminal nucleus, auricular nerves, vagus nerve stimulation
1. Introduction
The vagus (the “wandering” nerve) is a major cranial nerve connecting the brain with neck, thoracic, abdominal and eventually also some pelvic organs. Although often introduced as the paradigmatic “parasympathetic” nerve, its visceral efferent component (and only this is referred to as parasympathetic) represents just one and not even the largest portion of its fibers. The vagus contains also branchiomotor (formerly called special visceral efferent) neurons and a quantitatively even more impressive afferent fiber contingent. It is this afferent component which is targeted by current methods of invasive (through electrodes wrapped around the cervical vagus) and non-invasive transcutaneous auricular (electrode on cymba conchae) or cervical (stimulation device over anterolateral neck skin) vagus nerve stimulation (VNS) for intractable epilepsy, depression and cluster headache (see Butt et al. 2020 for review; Silberstein et al. 2016). This review aims to briefly summarize the topography, peripheral innervation territories including the targets of preganglionic neurons and sensory structures as well as the brainstem nuclei of the vagus with only a short account of their major central connections. Special attention will be given to vagal innervation of the external ear and the upper aerodigestive tract, organs developmentally derived from foregut, pharyngeal arches, pouches and clefts where the somatic and visceral domains merge.
2. Vagus nerve: topography, branches, innervation territories, fiber spectrum
The rootlets of the vagus exit the medulla oblongata in the retro-olivary sulcus together with the root of the glossopharyngeal nerve and the cranial root of the accessory nerve. In rodents, the medullary exit and entrance of efferent and afferent vagal axons are separated into a ventral and dorsal root, respectively, a division which is not so evident in human. After piercing the dura, they leave the skull through the neural part (pars nervosa) of the jugular foramen, where the jugular or superior ganglion is located. The dural “sleeve” enveloping the vagal rootlets continues into the capsule of the jugular and nodose ganglia as well as the epi- and perineurium of the nerve, thereby significantly increasing its diameter. Immediately below the base of the skull, the large spindle-shaped nodose or inferior ganglion is formed. The vagus nerve continues its course through the neck located between the internal and, more caudally, common carotid artery and the internal jugular vein. On average, the nerve is found at the level of the laryngeal prominence at 3.5 cm lateral to the midline and 3.5 cm deep to the skin, with some variability (Hammer et al., 2018). Both blood vessels and vagus nerve, together with the ansa cervicalis profunda, are enveloped by the carotid sheath, in human a rather firm fascial structure which is anchored to the surrounding connective tissue (Fig. 1).
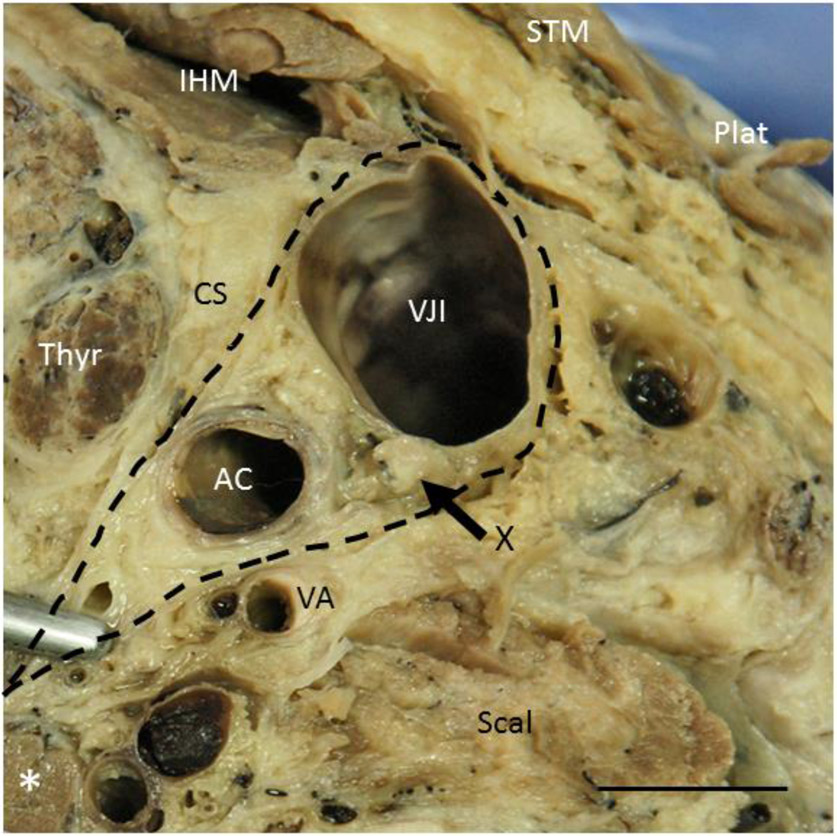
Fig. 1:
Transverse section through the antero-lateral quadrant of the neck of an embalmed body at level C7. The vagus nerve (X, arrow) is enwrapped together with the common carotid artery (AC) and the internal jugular vein (VJI) by the carotid sheath (CS, dashed). IHM, infrahyal muscles; Plat, Platysma; Scal, scalenus anterior muscle; STM, sternocleidomastoid muscle; Thyr, thyroid gland; VA, vertebral artery; asterisk, prevertebral muscle. Body donated to the Institute of Anatomy and Cell Biology, University of Erlangen-Nürnberg, for didactic, reasearch and postgraduate training purposes. Bar 1 cm
Average thickness of the human cervical vagus was variably described in post mortem studies between 2 mm (Pelot et al., 2020; Stakenborg et al., 2020) and 4.5 mm (Hammer et al., 2018), decreasing with age, but without sex or side differences. However, a study using high-resolution ultrasound revealed a larger cross-sectional area of the right versus left cervical vagus (Pelz et al., 2018). Five to seven fascicles were counted in the human cervical vagus (Hammer et al., 2018; Pelot et al., 2020; Stakenborg et al. 2020), while only one and as much as 46 fascicles were found in the mouse and pig cervical vagus, respectively (Pelot et al., 2020; Stakenborg et al., 2020).
2.1. Cranial and cervical branches
The first branch given off is the small ramus auricularis (auricular branch of the vagus nerve, ABVN), also called Arnold’s nerve (Fig. 2; Testut, 1922; for review see Butt et al., 2020). Leaving from the jugular ganglion, it enters the mastoid canaliculus, engaging in anastomotic connections with branches from the facial and glossopharyngeal nerves within the petrosal bone and innervates parts of the external ear; a branch of the ABVN reaches the dura of the posterior cranial fossa. Dissection studies on embalmed specimens provided a fairly detailed map of the innervation territories of the ABVN and other auricular nerves (greater auricular, auriculotemporal, lesser occipital) in human showing some overlap but also an autonomous ABVN area in the cymba conchae (Peuker and Filler, 2002).
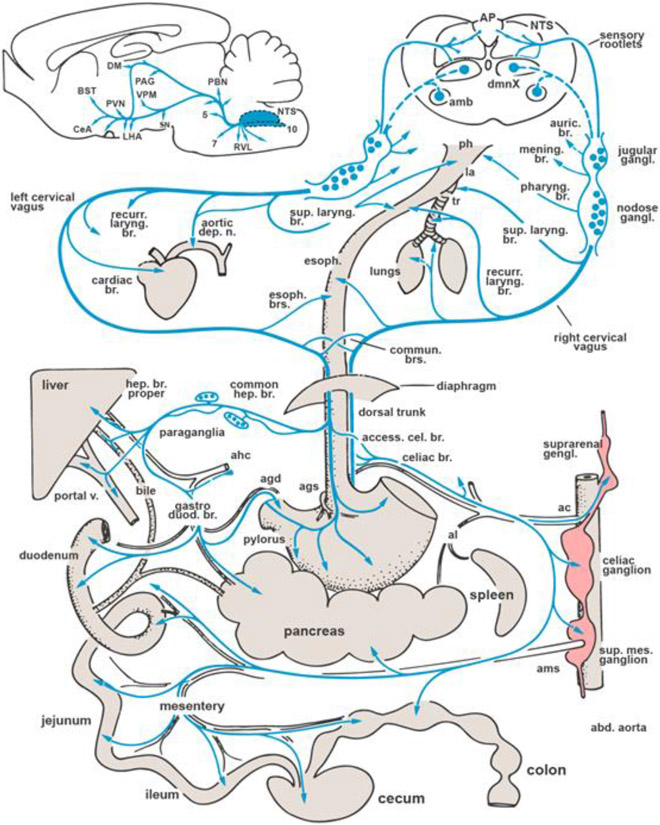
Fig. 2:
Schematic overview of the distribution of vagus nerve’s branches. Inset on top left summarizes the central distribution of vagal afferent information through the NTS. Abbreviations for periphery: ac, celiac artery; agd, right gastric artery; agd, left gastric artery; ahc, common hepatic artery; al, splenic artery; ams, superior mesenteric artery, la, larynx; ph, pharynx; tr, trachea. Abbreviations for brain areas: amb, nucleus ambiguus; AP, area postrema; BST, bed nucleus of stria terminalis; DM, dorsomedial thalamic nucleus; CeA, central nucleus of amygdala; LHA, lateral hypothalamic area; NTS, nucleus tractus solitarii; PAG, periaqueductal gray; PVN, paraventricular hypothalamic nucleus; PBN, parabrachial nuclei; RVL, rostroventrolateral medulla; SN, substantia nigra; VPM, ventroposteromedial thalamic nucleus; 5, trigeminal motor nucleus; 7, facial nucleus; 10/dmnX, dorsal vagal motor nucleus. Modified from Berthoud and Neuhuber, 2000, Auton. Neurosci. 85, 1-17.
At the level of the nodose ganglion, a branch is given off to the pharynx. At the distal pole of the nodose ganglion, the superior laryngeal nerve splits off, coursing medial to the carotid artery and, before piercing the thyrohyoid membrane, issues its external branch to the cricothyroid muscle and branches to the cervical esophagus and the cricopharyngeal muscle, the latter branches being endangered during thyroid surgery (Prades et al., 2009; Uludag et al., 2017; Fig. 2). It is also this area where connecting branches with the cervical sympathetic trunk, eventually providing some sympathetic postganglionics to the vagus (Yang et al., 1999; Verlinden et al., 2016), and with the hypoglossal nerve, leading afferent fibers from the tongue to their cell bodies in the jugular ganglion (Neuhuber and Mysicka, 1980 and references therein), are regularly observed.
Branching of the vagus within the carotid sheath at mid-cervical levels, the typical site where stimulating electrodes for invasive VNS are implanted, was observed in almost one-third of bodies in a post mortem dissection study, either uni- or bilaterally (Hammer et al., 2015). At low cervical levels, a superior cardiac branch was regularly observed (Kawashima, 2005).
2.2. Thoracic branches
The vagus enters the mediastinum between the subclavian vein and artery, on the right side giving off the recurrent laryngeal nerve around the artery. On the left, it abuts upon the anterolateral aspect of the aortic arch where the left recurrent laryngeal nerve winds around dorsally, bound by the ligamentum arteriosum (Botalli’s ligament). Inferior cardiac branches are given off by both recurrent nerves and thoracic cardiac branches split off distal to the recurrent nerve’s origin (Kawashima, 2005). The recurrent laryngeal nerves travel in a groove between trachea and esophagus, giving off branches to both organs, and eventually enter the larynx from below as the inferior laryngeal nerves (Fig. 2). Because of their close relationship to the inferior thyroid artery, they are vulnerable during thyroid surgery.
In its mediastinal course, the vagus crosses the principal bronchi dorsally, providing branches to the pulmonary plexus and follows the esophagus, embedded in its adventitia. Here, the right and left vagus form a plexus suggestive of fiber exchange between both sides (Fig. 2). Tracing and functional studies in rat indicated that this occurs for efferent and afferent fibers to different though small extents (Fox and Powley, 1985; Norgren and Smith, 1988; Horn and Friedman, 2005;).
2.3. Abdominal branches
Both left and right vagi enter the abdominal cavity as the anterior and posterior, respectively, trunk, together with the esophagus through its hiatus in the diaphragm. In some species, e.g., the ferret, the posterior trunk contained double as much axons than the anterior trunk (Asala and Bower, 1986) which was, however, not observed in the rat (Prechtl and Powley, 1990). The vagal trunks divide into two gastric and two celiac branches, each one from the anterior and posterior trunk, and one common hepatic branch from the anterior trunk (Fig. 2; Powley et al., 1983). However, terminology does not strictly correlate with innervation territories of the respective branches. In particular, the hepatic branch supplies, besides liver, biliary tract and portal vein, also the pancreas and duodenum which makes the interpretation of functional studies on the effects of “hepatic branch vagotomy” difficult (Berthoud and Neuhuber, 2019). The celiac branches serve primarily as a conduit for vagal efferent and afferent axons to the prevertebral plexus and from there along the branches of mesenteric arteries to the small and large intestines (Wang and Powley, 2007). However, anterograde tracing in rat demonstrated also presumed synaptic vagal preganglionic terminals on sympathetic postganglionic neurons of prevertebral ganglia (Berthoud and Powley, 1993).
The vagal efferent and afferent innervation extends well into the distal colon (equivalent to the descending colon in human) as demonstrated with anterograde tracing in rat (Berthoud et al., 1991; Wang and Powley, 2000). Anterograde tracing in female rats indicated vagal afferent innervation even of the uterus (Collins et al., 1999); it is unknown if there is also an efferent vagal influence on pelvic organs. There is no indication for vagal innervation of kidney, adrenal gland, lymphatic organs and adipose tissue (Cano et al., 2004; Berthoud et al., 2006; Giordano et al., 2006). Although it is possible that kindney, adrenal gland, adipose tissue and spleen are influenced by the vagus through a relay in prevertebral ganglia (Berthoud and Powley, 1993), this is controversially debated in particular for the spleen (Cano et al., 2001; Bratton et al., 2012; Kressel et al., 2020). Earlier data on vagal input to these organs rely on retrograde tracing notorious for diffusion artifacts (Fox and Powley, 1989).
2.4. Fiber spectrum
About 50 percent of axons in the cervical vagus in species of different body sizes (mouse, pig, human) are myelinated (Stakenborg et al., 2020). However, in this light microscopic study the proportion of unmyelinated fibers was underestimated as the neurofilament immunostaining demonstrated Remak bundles rather than individual unmyelinated axons. Remak units in the vagus nerve harbor on average two to four unmyelinated axons as demonstrated by electron microscopy (cat cervical vagus: Mei et al., 1980; ferret cervical vagus: Asala and Bower, 1986; rat abdominal vagus: Prechtl and Powley, 1990). Thus, a myelinated to unmyelinated axon ratio of one to five was calculated for the cat cervical vagus (Mei et al., 1980). Assuming a similar ratio also for the human cervical vagus, 20,000 myelinated axons (Stakenborg et al., 2020) were accompanied by some 100,000 unmyelinated axons. In human, about one half to two thirds of large myelinated fibers are motor axons of the recurrent laryngeal nerve, the others presumed afferent Aβ axons most likely from low threshold pulmonary mechanosensors; the numbers of thick myelinated axons in the thoracic vagus equals the number of this class of axons in the cervical vagus after subtracting the recurrent nerve’s number (Safi et al., 2016). These axons are presumably activated by current methods of invasive VNS (Evans et al., 2004). As there are very few or almost no muscle spindles in laryngeal muscles (Paulsen, 1958; Loucks et al., 2005) and thus almost no Aγ axons to be expected, small and medium sized myelinated axons in the cervical vagus are also most likely afferents or preganglionic cardioinhibitory efferents (see section 3.1.2). In contrast, more than 90 percent of axons in the subdiaphragmatic vagus are unmyelinated (99.5% in rat: Prechtl and Powley, 1990; 94% in human: Stakenborg et al., 2020, see caveat above), encompassing both preganglionic efferents and visceral afferents. The nature of the few myelinated fibers is unknown.
In the context of VNS strategies, knowledge of the anatomy of the vagus nerve’s non-neuronal components, which were hitherto poorly studied, is also important. A recent study found that the perineurium of vagal axon fascicles is thicker as compared to other peripheral nerves (Pelot et al., 2020). This has to be taken into account when defining stimulation parameters.
Blood supply to the cervical vagus is provided by branches of vertebral, inferior thyroid and other neighbouring arteries. The number of subepineurial arteries correlated positively with fascicle numbers in human (Hammer et al., 2018).
3. Efferent neurons
3.1. Preganglionic parasympathetic neurons
3.1.1. Dorsal motor nucleus
Most of the vagal preganglionic parasympathetic neurons are located in the dorsal motor nucleus (DMX), a flat sheeth of medium-sized neurons sandwiched between the nucleus of the solitary tract (NTS) dorsally and the hypoglossal nucleus ventrally (Fig. 3a). It extends from the level of the pyramidal decussation throughout the entire medulla oblongata. Together with the medial nucleus of the solitary tract, it bulges the caudal floor of the fourth ventricle forming the vagal trigone (trigonum nervi vagi); rostrally, it decreases in size and is displaced, together with the solitary tract and nucleus, ventro-laterally by the medial and inferior vestibular nuclei which increased in size in the dorsal medulla. The majority of DMX neurons are cholinergic preganglionics targeting the enteric nervous system from the esophagus down to the distal colon, including pancreatic ganglia (Fig. 3b; Berthoud and Powley, 1991; Berthoud et al., 1991). Surprisingly, DMX neurons project only to a negligible extent to the area of the rat liver pedicle (Berthoud et al., 1992), and the absence of cholinergic markers in intrahepatic nerve fibers, at least in rodents, argues against a significant parasympathetic cholinergic innervation of the liver (Arvidsson et al., 1997). DMX neurons project also to cardiac ganglia (retrograde tracing data in rhesus monkey: Hopkins and Armour, 1998; anterograde tracing data in rat: Cheng et al., 1999). However, it is unclear if they exert a negative chronotropic or rather a negative inotropic effect (Gourine et al., 2016). Preganglionic neurons of the rat DMX are viscerotopically arranged in longitudinal columns medio-laterally (Fox and Powley, 1985), which combines with a rostro-caudal somatotopy of visceroafferent terminals in the NTS (Altschuler et al., 1989) forming a lattice for reflex connections across organ borders (Powley et al., 1992). It is unknown how the somatotopy of the rat DMX relates to the subnuclei of the human DMX (Huang et al., 1993).
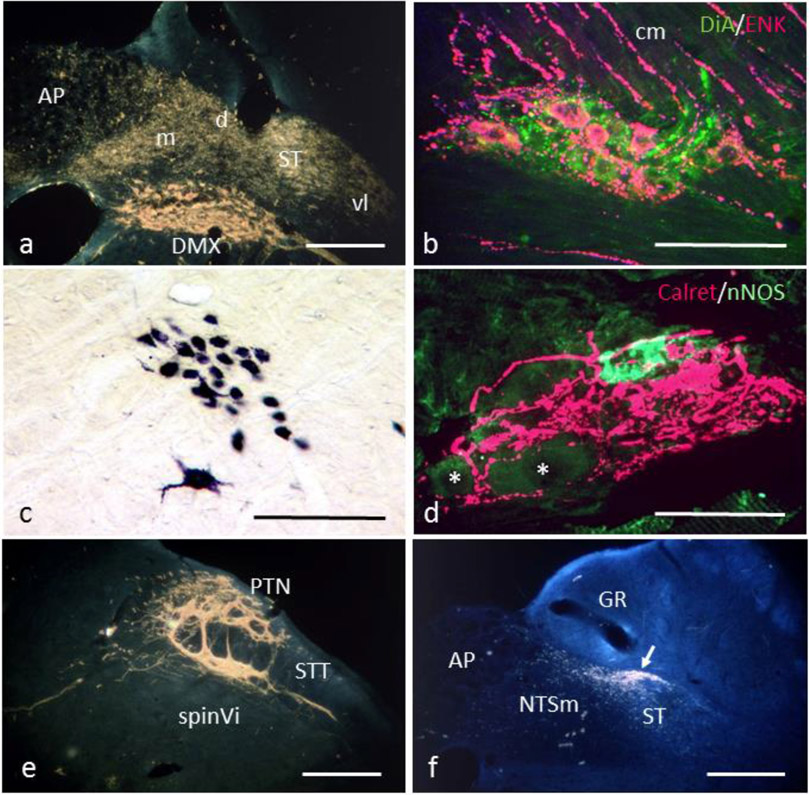
Fig. 3:
a. Retrogradely labeled neurons in the dorsal motor nucleus (DMX) and transganglionically labeled afferent terminals in the nucleus tractus solitarii (NTS) and area postrema (AP) upon horseradish peroxidase (HRP) application to the rat cervical vagus. d, m, vl, dorsal, medial, ventrolateral subnuclei of the NTS; ST, solitary tract. b. Preganglionic axons anterogradely labeled with DiA from the DMX (green) project to a myenteric ganglion in the rat pyloric antrum. Enkephalinergic myenteric neurons (ENK, red) project to the circular muscle layer (cm) Extended focus confocal image. c. Compact formation of the nucleus ambiguus labeled by HRP application to the rat cervical vagus. The large multipolar neuron belongs to the rostral semicompact ambiguus formation. d. Intraganglionic laminar endings (IGLEs, calretinin-positive, red) enwrapping a myenteric ganglion in the rat esophagus. A nitric oxide synthase (nNOS) positive myenteric neuron is labeled green, another two unlabeled neurons are indicated by asterisks. Extended focus confocal image. e. Dense afferent terminal labeling in the paratrigeminal (PTN) and interpolar spinal trigeminal nucleus (spinVi) upon wheat germ agglutinin-HRP injection into the rat jugular-nodose ganglion complex. STT, spinal trigeminal tract. f. Transganglionically HRP labeled terminals of rat aortic branch afferents concentrate in the dorsal NTS subnucleus (arrow). Note some terminal labeling also in dorsomedial subnucleus and area postrema. GR, nucleus gracilis. Hitherto unpublished micrographs from the authors’ previous work. Bars in a, c, e, f 200 μm, in b 100 μm, in d 50 μm
Besides cholinergic preganglionic neurons, the DMX harbours some subdiaphragmatically projecting neurons which display catecholaminergic (Yang et al., 1999; Tsukamoto et al., 2005) and nitrergic (Krowicki et al., 1997) markers. Furthermore, small neurons which do not project through the vagus nerve are considered interneurons or centrally projecting neurons (McLean and Hopkins, 1982; Jarvinen and Powley, 1999), some of them being GABAergic (Gao et al., 2009).
3.1.2. External formation of the nucleus ambiguus
In addition to the DMX, parasympathetic preganglionic neurons are also found in the external formation of the nucleus ambiguus (see section 3.2). They target postganglionic neurons in subepicardial ganglia of the heart and in the tracheobronchial tree. The axons of the cardioinhibitory ambiguus neurons are small myelinated in contrast to those from cardiac DMX neurons which are almost all unmyelinated (Cheng et al., 1999; Cheng and Powley, 2000; Gourine et al., 2016). Depending on species, the proportions of cardiac DMX and AMB preganglionic neurons vary, with more than 80 percent located in the cat’s and 60 percent in the monkey’s ambiguus nucleus (Hopkins and Armour, 1998; Taylor et al., 1999 for review). Interestingly, all vagal preganglionic neurons of the shark’s ambiguus homologue in the ventrolateral medulla innervate the heart with myelinated axons and account for 45 percent of cardiac preganglionic neurons in this phylogenetically old species, the other 55 percent residing in the DMX (Taylor et al., 2014). Thus, myelinated cardioinhibitory neurons are phylogenetically highly conserved and not a new trait specific of mammals as proposed by the polyvagal theory (Porges, 2001).
Both right and left ambiguus and dorsal motor nuclei, respectively, project to all cardiac ganglia as shown with anterograde tracing in rat (Cheng et al., 1999; Cheng and Powley, 2000). However, axons from the right DMX terminate slightly more often in ganglia close to the sinoatrial node whereas axons from left ambiguus neurons are slightly biased to ganglia in the vicinity of the atrioventricular node. It is unknown if this lateralization applies also to human and may explain the clinical notion that stimulation of the right vagus elicits bradycardia more readily (see Hammer et al., 2018 for references).
3.2. Branchiomotor neurons
Branchiomotor neurons projecting their axons mainly through the vagus but also the glossopharyngeal nerve and the cranial root of the accessory nerve are musculotopically grouped in the ambiguus nuclear complex (AMB) which extends ventrolaterally throughout the length of the medulla. The seminal study of Bieger and Hopkins (1987) demonstrated a rostro-caudal arrangement with the compact formation (AMBc, Fig. 3c) innervating striated muscle of the esophagus, the semicompact formation (AMBsc) the pharynx and the loose formation (AMBl) the intrinsic muscles of the larynx. The stylopharyngeus muscle, innervated by the glossopharyngeal nerve is represented in the most rostral portion of the AMB. The external formation (AMBext) with its loosely scattered preganglionic neurons lies largely ventral to the branchiomotor clusters. Different packing densities of motoneurons are also observed in the human AMB, although the rostro-caudal arrangement may be modified by a dorso-ventral gradient, the compact formation being located dorsal and the semicompact and loose formations ventral (Schwarzacher et al., 2011).
Remarkably, AMBc neurons target not only motor endplates in the striated esophageal muscle, but issue collaterals also to myenteric neurons which are known to co-innervate this unusual muscle fiber type (Neuhuber et al., 1994; Powley et al., 2013b). This preganglionic feature of branchiomotor AMBc neurons is one of the several ambiguities of the nucleus ambiguus.
Branchiomotor and preganglionic neurons of the AMB intermingle extensively with neurons of the ventral respiratory group complicating a cytoarchitectonic definition of the AMB (Ellenberger and Feldmann, 1990; Schwarzacher et al., 2011).
3.3. Neurons displaying catecholaminergic markers
The cervical and abdominal vagi contain fibers displaying catecholaminergic markers (tyrosine hydroxylase/TH and dopamine β-hydroxylase/DBH) (rat: Yang et al., 1999; human: Seki et al., 2014; Verlinden et al., 2016;). Some of them may be authentic sympathetic postganglionic “hitchhiking” fibers originating in the superior cervical ganglion (Yang et al., 1999; Verlinden et al., 2016). They are considered a possible source of unintended side effects of VNS. Although the functions of these fibers are unknown, a reasonable target are epineurial blood vessels of the vagus nerve itself (Fig. 4; Hammer et al., 2018). The proportions of “true” sympathetic postganglionic fibers was however considered minimal (Yang et al., 1999). As immunostaining for TH was used (Seki et al., 2014; Verlinden et al., 2016) and only a fraction of fibers co-stained for DBH (Verlinden et al., 2016), many of these “catecholaminergic” axons may be nodose primary afferents, since the nodose ganglion of rats contains numerous TH positive neurons which innervate the esophagus and stomach without being authentic catecholaminergic (Kummer et al., 1993). Another source are presumed dopaminergic neurons in the rat dorsal motor nucleus (Yang et al., 1999; Tsukamoto et al., 2005). Thus, it seems not very likely that cervical or abdominal VNS produces sympathetic side effects.
Fig. 4:
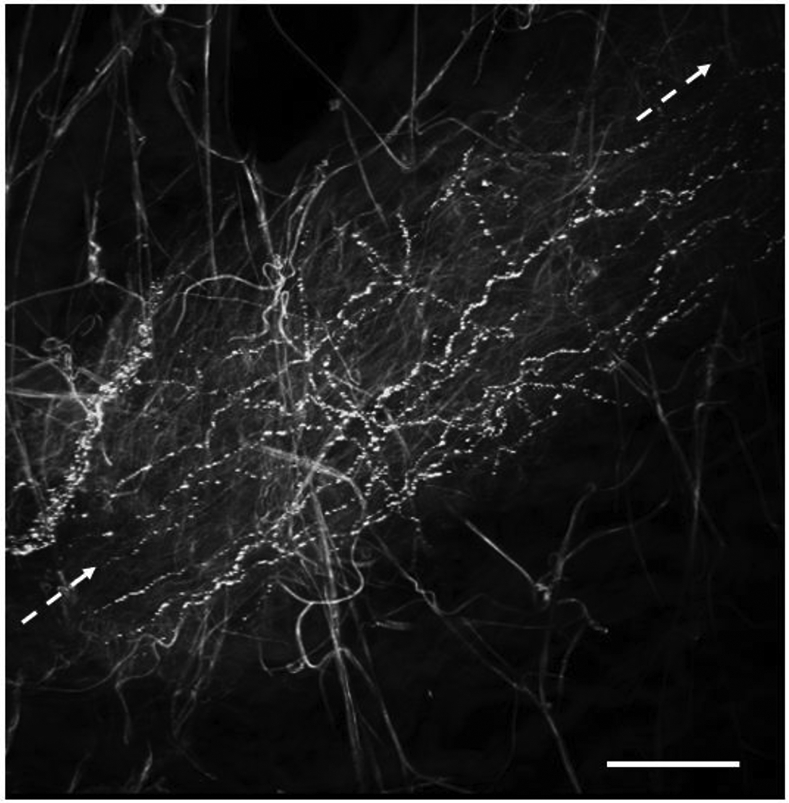
A small artery in the epineurium of the human cervical vagus (longitudinal axis of the vessel indicated by dashed arrows) enmeshed by a plexus of varicose TH positive sympathetic nerve fibers. Smooth-contoured connective tissue fibers display autofluorescence. Wholemount preparation; extended focus image of 13 stacked single optical sections, z-step 0.5 μm. Body donated to the Institute of Anatomy and Cell Biology, University of Erlangen-Nürnberg, for didactic, reasearch and postgraduate training purposes. Bar 100 μm
4. Afferent neurons
Primary afferent axons account for about 70 percent of fibers in the rat subdiaphragmatic vagus (Prechtl and Powley, 1990). The values for the cervical vagus were estimated between 50 to 70 percent in cat (Mei et al., 1980) and 80 percent in ferret (Asala and Bower, 1986). Pseudounipolar vagal primary afferent neurons reside in the jugular and nodose ganglia (Fig. 2). Neurons of the jugular ganglion originate from the cranial neural crest whereas nodose neurons are derived from an epibranchial placode (Baker and Bronner-Fraser, 2001). This differential origin is reflected by differences in peptide content, equipment with purinergic and growth factor receptors as well as transcriptomic differences. Thus, nodose ganglion neurons are typically non-peptidergic, express P2X2 and P2X3 receptors as well as TrkB, the high-affinity receptor for brain-derived neurotrophic factor (BDNF). Jugular ganglion neurons often contain peptides such as CGRP and express TrkA, the high affinity receptor for nerve growth factor (NGF) while another non-peptidergic subpopulation expresses TrkB (Wank and Neuhuber, 2001; Nassenstein et al., 2010). Likewise, transcriptomic analysis revealed significantly different molecular profiles, the jugular ganglion neurons being similar to the also neural crest derived dorsal root ganglion cells but completely different from placode derived nodose neurons (Kupari et al., 2019). Neurons expressing the vanilloid receptor TRPV1 were found in both jugular and nodose ganglia (Kim et al., 2020). These molecular differences most likely determine the functional properties of nodose and jugular neurons. Thus, neurons with a typical nociceptive phenotype are more commonly, though not exclusively found in the jugular rather than in the nodose ganglion (Kupari et al., 2019). Nevertheless, the esophagus is supplied with both crest- and placode-derived vagal C-fiber nociceptors (Surdenikova et al., 2012; Yu et al., 2014). TRPV1 expression correlates also with projections of vagal afferents to medial and dorsal (TRPV1+ afferents) and ventrolateral (TRPV1− afferents) areas of the nucleus tractus solitarii (Kim et al., 2020) as well as to sensitivity for certain inflammatory mediators (Zanos et al., 2018).
4.1. Visceral afferents
Vagal afferents from neck, thoracic and abdomino-pelvic viscera have their cell bodies in jugular and nodose ganglia. Afferents from the upper aerodigestive tract are represented in both ganglia, while afferents from subdiaphragmatic organs are almost confined to the nodose ganglion. Afferents from thoracic organs are both myelinated and unmyelinated, whereas afferents from subdiaphragmatic organs are almost exclusively unmyelinated.
4.1.1. Afferents from thoracic organs
Vagal pulmonary and cardiovascular afferents play vital roles in cardiorespiratory reflexes and coordination. Most prominent are thick myelinated pulmonary slowly and rapidly adapting mechanoreceptors (SARs and RARs, respectively) originating in bronchial smooth muscle and neuroepithelial bodies (NEBs) (Adriaensen et al., 2006; Chang et al., 2015; Brouns et al., 2021), some of which express Piezo2 (Nonomura et al., 2017). NEBs together with their associated vagal afferents may represent complex chemo- and mechanoreceptors. It is still debated whether NEBs or smooth muscle associated airway receptors (SMARs) represent the SARs which mediate the Hering-Breuer reflex (Brouns et al., 2021). Small myelinated and unmyelinated peptidergic and non-peptidergic pulmonary afferents are equally important as irritant receptors mediating the cough reflex, reacting to inflammatory stimuli or slowing breathing upon inhalation of toxins (Canning et al., 2004; Nassenstein et al., 2010; Krasteva et al., 2011; Chang et al., 2015; Driessen, 2019).
Aortic baroreceptor afferents are also partly myelinated (Krauhs, 1979) and utilize Piezo2 as transduction channel (Min et al., 2019). Nodose ganglion afferents from atria establish complex terminal structures around small intensely fluorescent cells of cardiac ganglia, in the myocardium including conduction fibers and in the endocardium (Cheng et al., 1997).
Among the small myelinated population are also mechanosensitive intraganglionic laminar endings (IGLEs) afferents from the esophagus involved in deglutition control (Raab and Neuhuber, 2007; Neuhuber and Bieger, 2013).
4.1.2. Gastrointestinal afferents
Vagal afferent structures in the gastrointestinal tract can be grouped into muscular and mucosal sensors. IGLEs which wrap around myenteric ganglia sandwiched between outer and inner layers of the tunica muscularis function as low-threshold mechanosensors (Fig. 3d; Neuhuber, 1987; Berthoud and Powley, 1992; Zagorodnyuk and Brookes, 2000; Zagorodnyuk et al., 2001). They extend throughout the vagal innervation territory from the esophagus to the distal colon (Wang and Powley, 2000) and mediate essential satiety signals (Bai et al., 2019). IGLEs form synaptic contacts with enteric neurons, express the vesicular glutamate transporters 1 and 2 (VGLUT1 and 2) as well as purine receptors P2X2/3, muscarinic and CGRP receptors (Hübsch et al., 2013; Horling et al., 2014; Raab and Neuhuber, 2007 for review); the functional significance of these features remains still to be elucidated. The other muscular sensors are the so-called intramuscular arrays (IMAs), found mainly in the outer muscle layer of the stomach and in sphincters (Berthoud and Powley, 1992; Kressel et al., 1994; Powley et al., 2013a, 2014). They are intricately related to interstitial cells of Cajal, a relation which is poorly understood (Powley et al., 2008). Vagal afferents in the mucosa display various distinct patterns which may relate to specific funtions (Berthoud et al., 1995b; Powley et al., 2011). In particular, close relationships to enteroendocrine cells (Berthoud and Patterson, 1996a; Kaelberer et al., 2018) and mucosal mast cells (Williams et al., 1997) are supposedly of functional relevance.
Anatomical and functional studies indicate some degree of lateralization of gastrointestinal vagal afferents. The ventral and dorsal halves of the rat stomach and duodenum are supplied by IGLEs connected to cell bodies in the left and right nodose ganglia, respectively; this lateralization vanishes analwards (Wang and Powley, 2000). Using optogenetic manipulation of nodose ganglion neurons in mice, gut induced reward was found to be mediated through right but not left vagal intestinal afferents (Han et al., 2018). This suggests side-specific channeling of some vagal afferents to the limbic forebrain.
In the rat pancreas, vagal afferents concentrate in islets (Neuhuber, 1989), most likely monitoring endocrine activity (Iwasaki et al., 2013; Makhmutova et al., 2021). Vagal afferents of the proper hepatic branch innervate the portal vein and biliary ducts. Within the rat liver, they distribute to periportal fields but are absent from the hepatic lobules (Berthoud et al., 1992).
4.1.3. Afferents from pelvic organs
Both retrograde and anterograde tracing studies in rat indicated vagal afferent supply of the uterus (Ortega-Villalobos et al., 1990; Collins et al., 1999). There is also evidence that patients with complete spinal cord lesion are able to sense cervico-vaginal stimulation (Komisaruk et al., 2004). In the intact organism, vagal and spinal afferents from uterus and vagina are interacting in the nucleus tractus solitarii (Hubscher and Berkley, 1995). Retrograde tracing in rat demonstrated vagal afferents also from the urinary bladder, confirmation by anterograde tracing from the nodose ganglion pending (Herrity et al., 2014).
4.1.4. Afferents from vagal paraganglia
All along their course, vagal branches contain paraganglia, most of them supplied by vagal afferent terminals (rat: Powley et al., 1983; Kummer and Neuhuber, 1989; Dahlqvist et al., 1994; Berthoud et al., 1995a; Berthoud and Patterson, 1996b; human: Plenat et al., 1988). A chemosensory function for pO2 monitoring was proposed analogous to carotid and aortic bodies. There are also indications for vagal paraganglia as sensors for inflammatory mediators (Goehler et al., 1999).
4.2. Somatic afferents
The external ear and the upper aerodigestive tract, i.e., soft palate, pharynx, larynx and partly also trachea forming the transition zone from the “somatic” oral and nasal cavities innervated by trigeminal afferents to the “visceral” organs, i.e., lower airways and gastrointestinal tract, are innervated to different extents by neural crest-derived jugular and placode-derived nodose sensory neurons. While the cell bodies of afferents in the vagal auricular branch (ABVN) were found only in the jugular ganglion, afferents from the other organs are distributed to both jugular and nodose ganglia and also to the petrosal ganglion of the glossopharyngeal nerve which in rat fuses with the jugular-nodose ganglionic complex (Altschuler et al., 1989). As to the sensory qualities mediated by these vagal “somatic” afferents, there is a shift from awareness of touch, temperature and pain to feelings of irritation and the urge to cough, depending on the distance from the dental ridge where a stimulus is applied.
4.3. Central termination sites of vagal afferents
The major central termination area of vagal afferents is the nucleus tractus solitarii (NTS) in the dorsal medulla (Figs 3a, 5). However, as detailed below in section 6.1, vagal afferents from organs in the somato-visceral transition zone terminate also in the spinal trigeminal and paratrigeminal nuclei (Fig. 3e; Nomura and Mizuno, 1984; Altschuler et al., 1989; Driessen, 2019). The NTS is a complex of several subnuclei organized in medial, dorsal and ventrolateral groups similar across species (rat: Altschuler et al., 1989; Herbert et al., 1990; cat: Loewy and Burton, 1978; human: McRitchie and Törk, 1993). Besides second order neurons relaying the glutamatergic primary afferents, the NTS contains excitatory glutamatergic and inhibitory GABA/glycinergic interneurons (Saha et al., 1999) and catecholaminergic neurons of the A2 and C2 groups. A number of hormones and peptides, e.g., oxytocin, ghrelin, CCK and neurokinins as well as nitric oxide modulate transmission in the NTS through their respective receptors (Colin et al., 2002; Atkinson et al., 2003; Boscan and Paton, 2005; Peters et al., 2008; Wan et al., 2008; Cui et al., 2011). In the NTS, most vagal afferents terminate in more than one subnucleus (Figs 3a, 5). There is some overlap of afferents from different organs already at the anatomical level which is even more pronounced when second-order NTS neurons activated by different specific stimuli are mapped (Paton and Kasparov, 2000). Nevertheless, gastrointestinal afferents dominate medial, gelatinous and commissural (Norgren and Smith, 1988, Altschuler et al., 1989), cardiovascular afferents dorsal (Fig. 3f; Ciriello and Calaresu, 1981) and afferents from the respiratory tract ventral, ventrolateral, intermediate, interstitial and commissural (Kalia and Richter, 1985; Altschuler et al., 1989; Hayakawa et al., 2001) subnuclei. The termination of e.g. single identified pulmonary SAR afferents in several subnuclei and their rostro-caudal extension over the entire NTS favor their access to functionally diverse NTS modules (Kalia and Richter, 1985). This principle may apply to some extent also to other vagal afferents. Afferents from the rat esophagus concentrate in the central subnucleus which in turn projects to AMBc, thus closing a deglutition reflex arc (Altschuler et al., 1989; Cunningham and Sawchenko, 1989). Likewise, pharyngeal afferents in rat are relayed by the interstitial NTS subnucleus to the AMBsc (Broussard et al., 1998).
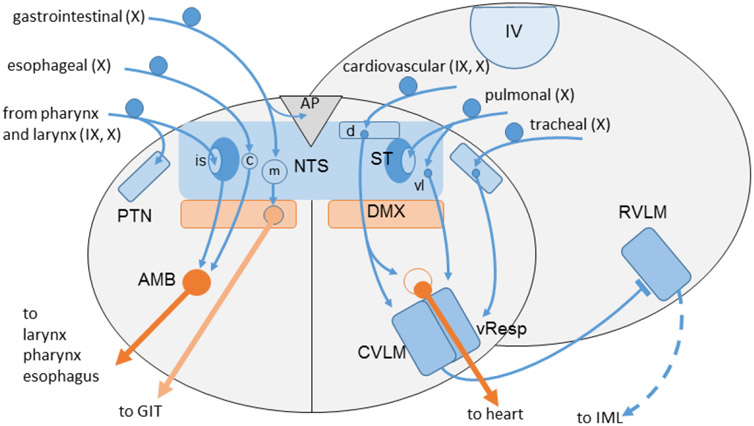
Fig. 5:
Schematic simplified summary of vagal (X) and glossopharyngeal (IX) afferent projections to the medulla oblongata and their major reflex connections to the dorsal motor nucleus (DMX), ambiguus nucleus (AMB; left: branchiomotor division; right: parasympathetic neurons of external formation) and the ventrolateral medulla. Note afferents from pharynx, larynx and trachea to the paratrigeminal nucleus (PTN). Arrows indicate excitatory (glutamatergic) connections; the bar on rostral ventrolateral medulla (RVLM) symbolizes the inhibitory GABAergic projection from caudal ventrolateral medulla (CVLM) to glutamatergic presympathetic neurons in the rostral ventrolateral medulla (RVLM). AP, area postrema; c, d, is, m, vl, central, dorsal, interstitial, medial, ventrolateral subnuclei of NTS; DMX, dorsal motor nucleus of vagus; IML, sympathetic intermediolateral nucleus; IV, fourth ventricle; vResp, ventral respiratory group; ST, solitary tract.
It is conceivable that nociceptive neurons of largely jugular origin terminate preferentially in trigeminal and paratrigeminal nuclei whereas non-nociceptive neurons of largely nodose origin terminate preferentially in the NTS. However, this difference is certainly not clear-cut as for example crest-derived and placode-derived putative nociceptive afferents from the cervical esophagus expressing the TRPV1 receptor (Surdenikova et al., 2012) appear to regularly terminate in the interstitial nucleus of the NTS whereas additional afferent terminal labeling in the paratrigeminal nucleus was inconsistent (Wank and Neuhuber, 2001).
There is also a significant vagal afferent projection to the area postrema from intestines and thoracic organs (Fig. 3a; Norgren and Smith, 1988).
5. Central connections of vagal nuclei
The NTS connects reciprocally to respiratory and vasomotor centers in the caudal and rostral ventrolateral medulla, communicating information from bronchopulmonary, baro- and chemosensors vital for respiratory and cardiovascular control (Blessing and Benarroch, 2012). In the ambiguus nuclear complex, both branchiomotor and cardioinhibitory preganglionic neurons are reflexly activated by secondary NTS neurons mediating deglutition and baroreceptor reflexes, respectively (Blessing and Benarroch, 2012; Neuhuber and Bieger, 2013). The medial NTS subnuclei are intricately connected to the DMX and the area postrema, thus forming the dorsal vagal complex, the medullary center of gastrointestinal regulation (Travagli and Anselmi, 2016). The different NTS subnuclei are engaged in complex reciprocal connections with the parabrachial nuclei and the Kölliker-Fuse nucleus (Herbert et al., 1990) and project to the locus coeruleus and periaqueductal gray (Van Bockstaele et al., 1999; Carrive and Morgan, 2012). Reciprocal connections link the NTS also to the paraventricular and posterior hypothalamus and the central nucleus of the amygdala. Most of these connections originate from separate neuron populations rather than collateralizing neurons (Hermes et al., 2006). Thus, being much more than a simple relay station, the NTS second-order and interneuronal network integrates and channels the signals from different peripheral sensors in highly specific ways to medullary, pontine, mesencephalic and prosencephalic autonomic centers for appropriate reflex and behavioural responses (Figs 2, 5).
6. Somato-visceral interface
Breathing, chewing, swallowing, speaking and singing require coordination of several muscles in the head, neck and trunk which is secured by afferent feedback via cranial nerves V, VII, IX and X as well as cervical spinal nerves. The territories supplied by these nerves comprise somatic (face, oral and nasal cavities, neck muscles and skin, diaphragm) and visceral (pharynx and esophagus, larynx, tracheobronchial tree and lung) domains. On the level of medullary and spinal nuclei, it is thus not surprising to find mutual terminations of vagal, trigeminal and cervical primary afferents in their “private” as well as “alien” nuclei, i.e., the NTS, trigeminal sensory nuclei and the cervical dorsal horn (Altschuler et al., 1989; Neuhuber and Zenker, 1989; Marfurt and Rajchert, 1991; Driessen, 2019). Thus, these nuclei are the central representatives of the somato-visceral interface on the afferent side. At the motor level, there is a sophisticated pontomedullary interneuronal premotor network in the parvocellular reticular formation which integrates trigeminal propriopceptive afferents and coordinates trigeminal, facial, glossopharyngeal, vagal and hypoglossal motor nuclei as well as neck muscle motoneurons (Dessem and Luo, 1999; Zhang et al., 2012). In turn, vagal and glossopharyngeal afferents from pharynx and larynx are relayed via premotor neurons in the NTS to trigeminal motor neurons (Oka et al. 2013). The ventral respiratory group projects to phrenic and intercostal nerve motoneurons as well as to cranial nerve motor nuclei conferring respiratory rhythmicity to their activity patterns (Ellenberger and Feldmann, 1990). The nucleus retroambiguus harbours another premotor neuron pool which integrates signals from vagal afferents and periaqueductal gray and projects to laryngeal motoneurons of AMBl (Suramanian et al., 2018).
6.1. Vagal afferents to non-vagal nuclei
As mentioned above, afferents of vagal branches innervating the external ear and upper aerodigestive tract terminate in the spinal trigeminal nucleus (medullary dorsal horn) and in the cervical dorsal horn, where they converge with trigeminal and cervical spinal afferents (Nomura and Mizuno, 1984; Sessle et al., 1986; Altschuler et al., 1989). A particulary interesting structure is the paratrigeminal nucleus (PTN), groups of neurons interspersed between the fiber bundles of the spinal trigeminal tract (Figs 3e, 5, 6). It receives input largely from jugular sensory neurons innervating soft palate, pharynx, larynx (Altschuler et al., 1989; Hayakawa et al., 2001) and trachea (McGovern et al., 2015) as well from the trigeminal area (Marfurt and Rajchert, 1991) and cervical cutaneous nerves (Neuhuber and Zenker, 1989) and projects to the NTS, ventrolateral medulla, parabrachial nuclei and thalamus (Saxon and Hopkins, 1998; Driessen et al., 2018). Besides a role in nociception, the PTN is also involved in respiratory reflexes (Driessen, 2019). Although not explicitely mentioned by Nomura and Mizuno in their landmark ABVN tracing study (1984), a closer look on their figures reveals ABVN afferent teminals also in the PTN.
Fig. 6:
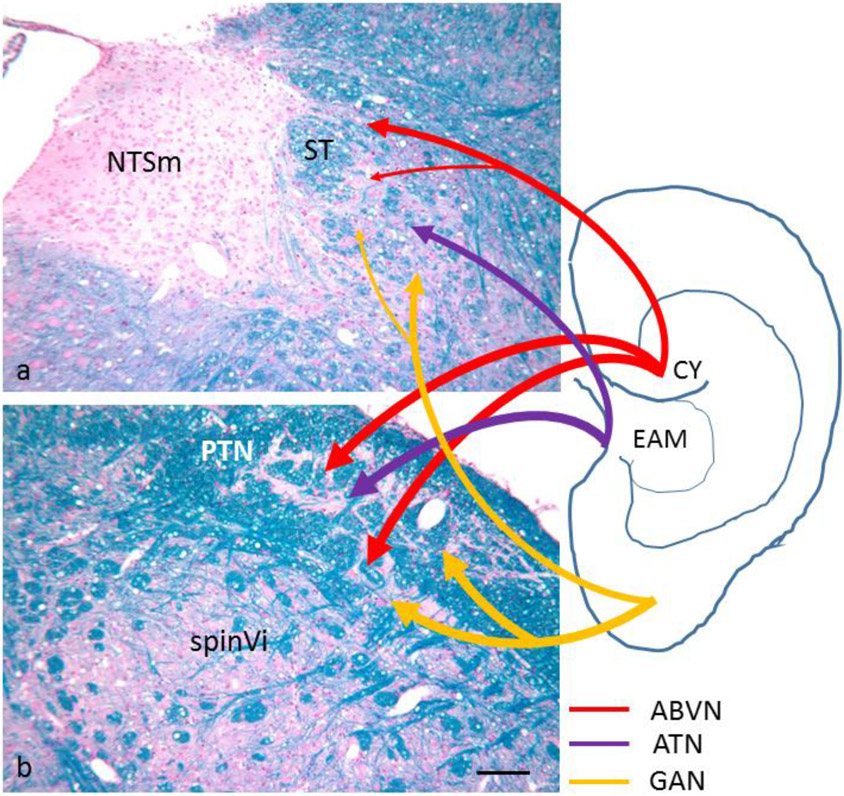
Schematic summary of brainstem projections of auricular nerve afferents as demonstrated by transganglionic tracing studies. All three nerves (ABVN, auricular branch of the vagus; ATN, auriculotemporal nerve; GAN, greater auricular nerve) project to spinal trigeminal, paratrigeminal and solitary tract nuclei. In the NTS, afferents terminate preferentially in dorsal and lateral subnuclei. CY, cymba conchae; EAM, external auditory meatus; NTSm, medial subnucleus of the nucleus tractus solitarii; PTN, paratrigeminal nucleus; spinVi, subnucleus interpolaris of the spinal trigeminal nucleus; ST, solitary tract. Micrographs are from rat dorso-medial (a) and dorso-lateral (b) medulla, Klüver-Barrera stain. Bar 100 μm
6.2. Non-vagal afferents to the nucleus tractus solitarii
On the other hand, the NTS receives primary trigeminal afferents, in particular from intraoral structures and the mandibular area (Jacquin et al., 1982; Takemura et al., 1987; Marfurt and Rajchert, 1991; Hayakawa et al., 2001). There are also projections from cervical cutaneous and auricular nerve afferents preferentially to dorsal and lateral areas of the NTS (Neuhuber and Zenker, 1989; for auricular nerve afferent projections see section 6.3.2 below).
Secondary afferents to the NTS originate in the spinal cord, spinal trigeminal and paratrigeminal nuclei (Menétrey and Basbaum 1987; Gamboa-Esteves et al., 2001; Ma et al., 2005). These pathways provide the NTS also with nociceptive input from somatic domains which can modulate visceral reflexes, e.g., the baroreceptorreflex (Boscan et al., 2002). In addition, there is even a pathway for proprioceptive afferents to the NTS. The intermediate nucleus, a small structure wedged between the lateral edges of the hypoglossal and vagal dorsal motor nuclei, receives neck muscle afferents (Neuhuber and Zenker, 1989; Edwards et al., 2015) and projects to the NTS (Edwards et al., 2007). Forelimb muscle afferents were shown to inhibit baroreceptor signals in the NTS via GABAergic interneurons (Potts et al., 2003).
6.3. Auricular nerves
6.3.1. Peripheral distribution
The auricle and the external auditory meatus (EAM) develop from derivatives of the first and second pharyngeal arches, although the contribution of each of them is debated (Anthwal and Thompson, 2016). The ectoderm-derived epidermal lining of the EAM continues covering the outer surface of the tympanic membrane while its inner aspect is covered by a squamous epithelium derived from the endoderm of the first pharyngeal pouch (Thompson and Tucker, 2013). Thus, somatic and visceral domains are in touch back-to-back. This development is reflected by a complex sensory innervation provided by trigeminal, facial, glossopharyngeal, vagal and cervical spinal nerves, besides sympathetic and parasympathetic periarterial fibers (Cakmak et al., 2018). Although there is consensus that the lateral aspect of the human auricle, which is the preferred site of transcutaneous auricular VNS, is supplied by the trigeminal auriculotemporal nerve, auricular branch of the vagus nerve (ABVN) and the greater auricular nerve from the plexus cervicalis, the exact innervation territories are debated and there is some overlap (Fig. 6; Peuker and Filler, 2002; see discussion in Butt et al., 2020). Nevertheless, the cymba conchae is said to be exclusively supplied by the ABVN. However, intrapetrosal anastomoses of the ABVN with facial and glossopharyngeal branches (Testut, 1922; Butt et al., 2020) suggest that non-vagal fibers intermingle with authentic vagal afferents in the terminal arborizations of the ABVN even in the cymba. The medial aspect of the auricle receives fibers from the lesser occipital nerve, the ABVN and the greater auricular nerve (Peuker and Filler, 2002). Tracing studies on the dog’s pinna, where a rostral, middle and caudal auricular nerve can be identified, indicated also a contribution by the facial nerve because tracer application resulted in retrogradely labeled neurons in the geniculate ganglion in addition to trigeminal, jugular and superior cervical spinal ganglia. More than one of these sensory ganglia contained labeled neurons upon tracer application to one particular nerve indicating anastomotic connections (Chien et al., 1996).
6.3.2. Central termination of auricular nerve afferents
In the quest to elucidate mechanisms of transcutaneous auricular VNS, central afferent terminations of auricular nerves are of great interest. Afferent terminals of all nerves were detected in the spinal trigeminal and partly also principal trigeminal nuclei, paratrigeminal nucleus, cuneate nucleus and upper cervical dorsal horn. Remarkably, all nerves in question send projections also to the NTS, in particular to its lateral, interstitial and dorsal but rarely to its medial subnuclei (Fig. 6; rat auriculotemporal nerve: Jacquin et al., 1982; cat ABVN: Nomura and Mizuno, 1984; rabbit greater auricular nerve: Liu and Hu, 1988; dog intrinsic pinna nerves: Chien et al., 1996; rat ABVN: He et al., 2013). In this respect, the ABVN is not more “vagal” than the other auricular nerves. Afferent projections to the (ventro-)lateral and interstitial NTS subnuclei are noteworthy as these subnuclei are the main termination site of myelinated vagal respiratory afferents (Kalia and Richter, 1985) which are the most likely target of invasive cervical VNS (Evans et al., 2004).
The densitiy of terminals of all auricular nerve afferents in the NTS is rather low. This may be due to the transganglionic tracing technique which probably labels only a fraction of afferent terminals. However, all auricular nerve afferents terminate even more densely in spinal trigeminal and paratrigeminal nuclei which may relay this input to the NTS (Fig. 6; Saxon and Hopkins, 1998; Menétrey and Basbaum, 1987). Thus, fMRI studies of auricular VNS stimulation in human found BOLD activity in both NTS and spinal trigeminal nucleus (Frangos et al., 2015). Rostral areas in which BOLD signals were recorded upon auricular VNS, e.g., parabrachial nuclei (PBN), locus coeruleus, periaqueductal gray and limbic subcortical and cortical structures (Frangos et al., 2015; Yakunina et al., 2016) may be activated via conjoined projections from trigeminal and paratrigeminal nuclei as well as from the NTS, because both NTS and trigeminal/paratrigeminal nuclei project to PBN (Herbert et al., 1990; Feil and Herbert, 1995), which are an important relay to the more rostral areas (Krukoff et al., 1992; Luppi et al., 1995). Thus, it would be surprising if this afferent neuronal chain can be activated specifically by stimulation of the ABVN only unless one proposes that its afferents were relayed preferentially over those of the other auricular nerves in a kind of “labeled vagus line”. Why stimulation with innocuous intensities outside the proposed specific ABVN territory in the cymba conchae, e.g., tragus or ear lobe, is reportedly less effective in activating all these nuclei (Frangos et al., 2015; Yakunina et al., 2017) is not easily explained. Earlobe stimulation resulted in BOLD activity signals in cuneate and spinal trigeminal nuclei but not in NTS or more rostral sites (Frangos et al., 2015), although greater auricular nerve afferents project to the NTS (Liu and Hu, 1988). On the other hand, cymba stimulation did not activate the cuneate nucleus (Frangos et al., 2015), although ABVN afferents project heavily to this site at least in cat (Nomura and Mizuno, 1984). One may speculate that low threshold ABVN afferents are preferentially channeled by the intricate interneuronal network of the NTS to rostral brainstem and forebrain sites where the beneficial effects are generated. A more parsimonious explanation may be found in different sensory innervation densities of cymba conchae and earlobe, respectively. Although a recent study mapped the distribution of perivascular autonomic nerves in the human auricle (Cakmak et al., 2018), estimates of sensory Aβ fibers which are the likely target of transcutaneous auricular VNS, are available for the cymba but not for the earlobe (Dabiri et al., 2020).
6.4. Transcutaneous cervical vagus stimulation
The question of what is exactly stimulated by transcutaneous VNS is even more urgent in case of transcutaneous cervical VNS using a hand-held device (e.g., Silberstein et al., 2016; Frangos and Komisaruk, 2017) which may stimulate low-threshold afferents. However, there are several sheets of tissue between the skin and the cervical vagus, which abund of low threshold sensors. Immediately below the low-threshold cutaneous sensors, the platysma is also densely supplied with low-threshold sensors, i.e., muscle spindles (May et al., 2018). Within the carotid sheath, the ansa cervicalis profunda containing bundles of low threshold proprioceptive axons from infrahyoid muscles lies superficially to the carotid artery, internal jugular vein and vagus nerve. It may require a sophisticated tuning of stimulus parameters in order to specifically activate the 200 to 400 (on average) thick myelinated afferents in the cervical vagus (Safi et al., 2016).
7. Conclusion
Innervating a large portion of our body and contributing significantly to homeostasis, the vagus represents an attractive target for therapeutic attempts to influence a variety of pathological conditions. The accessibility of its cervical and auricular branches encouraged the development of non-invasive vagus stimulation strategies. However, anastomoses of the ABVN with facial and glossopharyngeal nerves, the suspicion that transcutaneous cervical VNS may affect afferents other than vagal, the mutual distribution of all auricular nerve afferents to both “vagal” and “trigeminal” termination sites and the likely involvement of both NTS and trigeminal nuclei in mediating the stimulation effects question the appropriateness of the term “vagus nerve stimulation” for transcutaneous techniques.
Highlights.
Concise overview of vagal peripheral and central functional anatomy
The vagus as part of a viscero-somatic network
A critical appraisal of auricular nerve afferent projections
Acknowledgements
The authors greatly appreciate the assistance of Lukas Bochtler and Philip Eichhorn with photography and Hedwig Symowski for histological specimens. Work in the authors’ laboratories was supported by Deutsche Forschungsgemeinschaft grant NE 534/3-1 and National Institutes of Health grant DK047348.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriaensen D, Brouns I, Pintelon I, De Proost I, Timmermans JP, 2006. Evidence for a role of neuroepithelial bodies as complex airway sensors: comparison with smooth muscle-associated airway receptors. J. Appl. Physiol 101, 960–970. 10.1152/japplphysiol.00267.2006. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR, 1989. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J. Comp. Neurol 283, 248–268. [DOI] [PubMed] [Google Scholar]
- Anthwal N, Thompson H, 2016. The development of the mammalian outer and middle ear. J. Anat 228, 217–232. doi: 10.1111/joa.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Elde R, Meister B, 1997. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous system. J. Comp. Neurol 378, 454–467. [PubMed] [Google Scholar]
- Asala SA, Bower AJ, 1986. An electron microscopic study of vagus nerve composition in the ferret. Anat. Embryol 175, 247–253. [DOI] [PubMed] [Google Scholar]
- Atkinson L, Batten TFC, Corbett EKA, Sinfield JK, Deuchars J, 2003. Subcellular localization of neuronal nitric oxide synthase in the rat nucleus of the solitary tract in relation to vagal afferent inputs. Neuroscience 118, 115–122. doi: 10.1016/S0306-4522(02)00946-6. [DOI] [PubMed] [Google Scholar]
- Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, Madisen L, Zeng H, Krasnow MA, Knight ZA, 2019. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143. 10.1016/j.cell.2019.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M, 2001. Vertebrate cranial placodes I. embryonic induction. Devel. Biol 232, 1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL, 1991a. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol 260, R200–R207. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL, 1991b. Morphology and distribution of efferent vagal innervation of rat pancreas as revealed with anterograde transport of DiI. Brain Res. 553, 336–341. doi: 10.1016/0006-8993(91)90846-n. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL, 1992. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J. Comp. Neurol 319, 261–276. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Neuhuber WL, 1992. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat. Embryol 186, 431–442. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL, 1993. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J. Auron. Nerv. Syst 42, 153–170. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Neuhuber WL, 1995a. Vagal afferent innervation of rat abdominal paraganglia as revealed by anterograde DiI-tracing and confocal microscopy. Acta Anat. 152, 127–132. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Raybould HE, Neuhuber WL, 1995b. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat. Embryol 203–212. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, 1996a. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat. 156, 123–131. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, 1996b. Innervation of rat abdominal paraganglia by calretinin-like immunoreactive nerve fibers. Neurosci. Lett 210, 115–118. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL, 2000. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci 85, 1–17. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Fox EA, Neuhuber WL, 2006. Vagaries of adipose tissue innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol 291, R1240–R1242. doi: 10.1152/ajpregu.00428.2006. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL, 2019. Vagal mechanisms as neuromodulatory targets for the treatment of metabolic disease. Ann. N. Y. Acad. Sci doi: 10.1111/nyas.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieger D, Hopkins DA, 1987. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J. Comp. Neurol 262, 546–562. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Benarroch EE, 2012. Lower brainstem regulation of visceral, cardiovascular, and respiratory function, in: Mai JK, Paxinos G (Eds.), The Human Nervous System, 3rd ed. Academic Press, London, pp. 1058–1073. [Google Scholar]
- Boscan P, Kasparov S, Paton JFR, 2002. Somatic nociception activates NK1 receptors in the nucleus tractus solitarii to attenuate the baroreceptor cardiac reflex. Eur. J. Neurosci 16, 907–920. doi: 10.1046/j.1460-9568.2002.02131.x. [DOI] [PubMed] [Google Scholar]
- Boscan P, Paton JFR, 2005. Excitatory convergence of periaqueductal gray and somatic afferents in the solitary tract nucleus: role for neurokinin 1 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol 288, 262–269. doi: 10.1152/ajpregu.00328.2004. [DOI] [PubMed] [Google Scholar]
- Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM, 2012. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp. Physiol 97, 1180–1185. doi: 10.1113/expphysiol.2011.061531. [DOI] [PubMed] [Google Scholar]
- Brouns I, Verckist L, Pintelon I, Timmermans JP, Adriaensen D, 2021. The pulmonary neuroepithelial body microenvironment: a multifunctional unit in the airway epthelium. Adv. Anat. Embryol. Cell. Biol 233, 1–99. 10.1007/978-3-030-65817-5_1. [DOI] [PubMed] [Google Scholar]
- Broussard DL, Lynn RB, Wiedner EB, Altschuler SM, 1998. Solitarial premotor neuron projection to the rat esophagus and pharynx: implications for control of swallowing. Gastroenterology 114, 1268–1275. [DOI] [PubMed] [Google Scholar]
- Butt MF, Albusoda A, Farmer AD, Aziz Q, 2020. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J. Anat 236, 588–611. doi: 10.1111/joa.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak YO, Cotofana S, Jäger C, Morawski M, Sora MC, Werner M, Hammer N, 2018. Peri-arterial autonomic innervation of the human ear. Sci. Reports 8, 10469. doi: 10.1038/s41598-018-29839-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ, 2004. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J. Physiol 5557, 543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano G, Sved AF, Rinaman L, Rabin BS, Card JP, 2001. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J. Comp. Neurol 439, 1–18. [DOI] [PubMed] [Google Scholar]
- Cano G, Card JP, Sved AF, 2004. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J. Comp. Neurol 471, 462–481. doi: 10.1002/cne.20040. [DOI] [PubMed] [Google Scholar]
- Carrive P, Morgan MM, 2012. Periaqueductal gray, in: Mai JK, Paxinos G (Eds.), The Human Nervous System, 3rd ed. Academic Press, London, pp. 397–400. [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD, 2015. Vagal sensory neuron subtypes that differentially control breathing. Cell 161, 622–633. 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, Doyle FJ III, 1997. Vagal afferent innervation of the atria of the rat heart reconstructed with confocal microscopy. J. Comp. Neurol 381, 1–17. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, Doyle FJ III, 1999. Projections of the dorsal motor nucleus of the vagus to cardiac ganglia of rat atria: an anterograde tracing study. J. Comp. Neurol 410:320–341. [PubMed] [Google Scholar]
- Cheng Z, Powley TL, 2000. Nucleus ambiguus projections to cardiac gangia of rat atria: an anterograde tracing study. J. Comp. Neurol 424, 588–606. [PubMed] [Google Scholar]
- Chien CH, Shieh JY, Ling EA, Tan CK, Wen CY, 1996. The composition and central projections of the internal auricular nerves of the dog. J. Anat 189, 349–362. [PMC free article] [PubMed] [Google Scholar]
- Ciriello J, Calaresu FR, 1981. Projections from buffer nerves to the nucleus of the solitary tract: an anatomical and electrophysiological study in the cat. J. Auton. Nerv. Syst 3, 299–310. [DOI] [PubMed] [Google Scholar]
- Colin I, Blondeau C, Baude A, 2002. Neurokinin release in the rat nucleus of the solitary tract via NMDA and AMPA receptors. Neuroscience 115, 1023–1033. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Lin CE, Berthoud HR, Papka RE, 1999. Vagal afferents from the uterus and cervix provide direct connections to the brainstem. Cell Tissue Res. 295, 43–54. [DOI] [PubMed] [Google Scholar]
- Cui RJ, Li X, Appleyard SM, 2011. Ghrelin inhibits visceral afferent activation of catecholamine neurons in the solitary tract nucleus. J. Neurosci 31, 3484–3492. doi: 10.1523/JNEUROSCI.3187-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET Jr., Sawchenko PE, 1989. A circumscribed projection from the nucleus of the solitary tract to the nucleus ambiguus in the rat: anatomical evidence for somatostatin-28-immunoreactive interneurons subserving reflex control of esophageal motility. J. Neurosci 9, 1668–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri B, Kampusch S, Geyer SH, Le VH, Weninger WJ, Széles JC, Kaniusas E, 2020. High-resolution episcopic imaging for visualization of dermal arteries and nerves of the auricular cymba conchae in humans. Front. Neuroanat 14, 1–12. doi: 10.3389/fnana.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A, Neuhuber WL, Forsgren S, 1994. Innervation of laryngeal nerve paraganglia: an anterograde tracing and immunohistochemical study in the rat. J. Comp. Neurol 345, 440–446. [DOI] [PubMed] [Google Scholar]
- Dessem D, Luo P, 1999. Jaw-muscle spindle afferent feedback to the cervical spinal cord in the rat. Exp. Brain Res 128, 451–459. [DOI] [PubMed] [Google Scholar]
- Driessen AK, Farrell MJ, Dutschmann M, Stanic D, McGovern AE, Mazzone SB, 2018. Reflex regulation of breathing by the paratrigeminal nucleus via multiple bulbar circuits. Brain Struct. Funct 223, 4005–4022. doi: 10.1007/s00429-018-1732-z. [DOI] [PubMed] [Google Scholar]
- Driessen AK, 2019. Vagal afferent processing by the paratrigeminal nucleus. Front. Physiol 10, 1–7. doi: 10.3389/fphys.2019.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards IJ, Dallas ML, Poole SL, Milligan CJ, Yanagawa Y, Szabó G, Erdélyi F, Deuchars SA, Deuchars J, 2007. The neurochemically diverse intermedius nucleus of the medulla as a source of excitatory and inhibitory synaptic input to the nucleus tractus solitarii. J. Neurosci 27, 8324–8333. doi: 10.1523/JNEUROSCI.0638-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards IJ, Lall VK, Paton JF, Yanagawa Y, Szabó G, Deuchars SA, Deuchars J, 2015. Neck muscle afferents influence oromotor and cardiorespiratory brainstem neural circuits. Brain Struct. Funct 220, 1421–1436. doi: 10.1007/s00429-014-0734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, 1990. Subnuclear organization of the lateral tegmental field of the rat. I: nucleus ambiguus and ventral respiratory group. J. Comp. Neurol 294, 202–211. [DOI] [PubMed] [Google Scholar]
- Evans MS, Verma-Ahuja S, Naritku DK, Espinosa JA, 2004. Intraoperative human vagus nerve compound action potentials. Acta Neurol. Scand 110, 232–238. [DOI] [PubMed] [Google Scholar]
- Feil K, Herbert H, 1995. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kölliker-Fuse nuclei. J. Comp. Neurol 353, 506–528. [DOI] [PubMed] [Google Scholar]
- Fox EA, Powley TL, 1985. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 341, 269–282. [DOI] [PubMed] [Google Scholar]
- Fox EA, Powley TL, 1989. False-positive artifacts of tracer strategies distort autonomic connectivity maps. Brain Res. Brain Res. Rev 14, 53–77. [DOI] [PubMed] [Google Scholar]
- Frangos E, Ellrich J, Komisaruk BR, 2015. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 8, 624–636. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangos E, Komisaruk BR, 2017. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul. 10, 19–27. doi: 10.1016/j.brs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Gamboa-Esteves FO, Tavares I, Almeida A, Batten TFC, McWilliam PN, Lima D, 2001. Projection sites of superficial and deep spinal dorsal horn cells in the nucleus tractus solitarii of the rat. Brain Res. 921, 195–205. [DOI] [PubMed] [Google Scholar]
- Gao H, Glatzer NR, Williams KW, Derbenev AV, Liu D, Smith BN, 2009. Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res. 1291, 40–52. doi: 10.1016/j.brainres.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ, 2006. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol 291, R1243–R1255. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Nguyen KT, Lee JE, Tilders FJH, Maier SF, Watkins LR, 1999. Interleukin-1β in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J. Neurosci 19, 2799–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Machhada A, Trapp S, Spyer KM, 2016. Cardiac vagal preganglionic neurones: an update. Auton. Neurosci 199, 24–28. 10.1016/j.autneu.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Hammer N, Glätzner J, Feja C, Kühne C, Meixensberger J, Planitzer U, Schleifenbaum S, Tillmann BN, Winkler D, 2015. Human vagus nerve branching in the cervical region. PLoS ONE 10, e0118006. doi: 10.1371/journal.pone.0118006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer N, Löffler S, Cakmak YO, Ondruschka B, Planitzer U, Schultz M, Winkler D, Weise D, 2018. Cervical vagus nerve morphometry and vascularity in the context of nerve stimulation – a cadaveric study. Sci. Reports 8, 7997. doi: 10.1038/s41598-018-26135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Takanaga A, Maeda S, Seki M, Yajima Y, 2001. Subnuclear distribution of afferents from the oral, pharyngeal and laryngeal regions in the nucleus tractus solitarii of the rat: a study using transganglionic transport of cholera toxin. Neurosci. Res 39, 221–232. [DOI] [PubMed] [Google Scholar]
- He W, Jing XH, Zhu B, Zhu XL, Li L, Bai WZ, Ben H, 2013. The auriculo-vagal afferent pathway and its role in seizure suppression in rats. BMC Neuroscience 14, 85–93. doi: 10.1186/1471-2202-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB, 1990. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol 293, 540–580. [DOI] [PubMed] [Google Scholar]
- Hermes SM, Mitchell JL, Aicher SA, 2006. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Exp. Neurol 198, 539–551. doi: 10.1016/j.expneurol.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Herrity AN, Rau KK, Petruska JC, Stirling DP, Hubscher CH, 2014. Identification of bladder and colon afferents in the nodose ganglia of male rats. J. Comp. Neurol 522, 3667–3682. doi: 10.1002/cne.23629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins DA, Armour JA, 1998. Brainstem cells of origin of physiologically identified cardiopulmonary nerves in the rhesus monkey (Macaca mulatta). J. Auton. Nerv. Syst 68, 21–32. [DOI] [PubMed] [Google Scholar]
- Horling L, Bunnett NW, Messlinger K, Neuhuber WL, Raab M, 2014. Localization of receptors for calcitonin-gene-related peptide to intraganglionic laminar endings of the mouse esophagus: peripheral interaction between vagal and spinal afferents? Histochem. Cell Biol 141, 321–335. doi: 10.1007/s00418-013-1162-1. [DOI] [PubMed] [Google Scholar]
- Horn CC, Friedman MI, Thoracic cross-over pathways of the rat vagal trunks. Brain Res. 1060, 153–161. doi: 10.1016/j.brainres.2005.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XF, Törk I, Paxinos G, 1993. Dorsal motor nucleus of the vagus nerve: a cyto- and chemoarchitectonic study in the human. J. Comp. Neurol 330, 158–182. [DOI] [PubMed] [Google Scholar]
- Hübsch M, Neuhuber WL, Raab M, 2013. Muscarinic acetylcholine receptors in the mouse esophagus: focus on intraganglionic laminar endings (IGLEs). Neurogastroenterol. Motil 25, e560–e573. doi: 10.1111/nmo.12161. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Berkley KJ, 1995. Spinal and vagal influences on the responses of rat solitary nucleus neurons to stimulation of uterus, cervix and vagina. Brain Res. 702, 251–254. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Shimomura K, Kohno D, Dezaki K, Ayush EA, Nakabayashi H, Kubota N, Kadowaki T, Kakei M, Nakata M, Yada T, 2013. Insulin activates vagal afferent neurons including those innervating pancreas via insulin cascade and Ca2+ influx: its dysfunction in IRS2-ko mice with hyperphagic obesity. PLoS ONE 8, e67198.doi: 10.1371/journal.pone.0067198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin MF, Semba K, Rhoades RW, Egger MD, Trigeminal primary afferents project bilaterally to dorsal horn and ipsilaterally to cerebellum, reticular formation, and cuneate, solitary, supratrigeminal and vagal nuclei. Brain Res. 246, 285–291. [DOI] [PubMed] [Google Scholar]
- Jarvinen MK, Powley TL, 1999. Dorsal motor nucleus of the vagus neurons: a multivariate taxonomy. J. Comp. Neurol 403, 359–377. [PubMed] [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, Bohórquez DV, 2018. A gut-brain neural circuit for nutrient sensory transduction. Science 361, eaat5236.doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M, Richter D, 1985. Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitaries of the cat. I. a light microscopic analysis. J. Comp. Neurol 241, 503–520. [DOI] [PubMed] [Google Scholar]
- Kawashima T, 2005. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat. Embryol 209, 425–438. doi: 10.1007/s00429-005-0462-1. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hadley SH, Maddison M, Patil M, Cha B, Kollarik M, Taylor-Clark TE, 2020. Mapping of sensory nerve subsets within tha vagal ganglia and the brainstem using reporter mice for Pirt, TRPV1, 5-HT3, and Tac1 expression. eNeuro 7, 1–24. 10.1523/ENEURO.0494-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komisaruk BR, Whipple B, Crawford A, Grimes S, Liu WC, Kalnin A, Mosier K, 2004. Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves. Brain Res. 1024, 77–88. doi: 10.1016/j.brainres.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Mühlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, Baal N, Weihe E, Schütz B, Kotlikoff M, Ibanez-Tallon I, Kummer W, 2011. Cholinergic chemosensory cells in the trachea regulate breathing. PNAS 108, 9478–9483. doi: 10.1073/pnas.1019418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauhs JM, 1979. Structure of rat aortic baroreceptors and their relationship to connective tissue. J. Neurocytol 8, 401–414. doi: 10.1007/BF01214800. [DOI] [PubMed] [Google Scholar]
- Kressel M, Berthoud HR, Neuhuber WL, 1994. Vagal innervation of the rat pylorus: an anterograde tracing study using carbocyanine dyes and laser scanning confocal microscopy. Cell Tissue Res. 275, 109–123. [DOI] [PubMed] [Google Scholar]
- Kressel AM, Tsaaava T, Levine YA, Chang EH, Addorisio ME, Chang Q, Burbach BJ, Carnevale D, Lembo G, Zador AM, Andersson U, Pavlov VA, Chavan SS, Tracey KJ, 2020. Identification of a brainstem locus that inhibits tumor necrosis factor. PNAS 117, 29803–29810. doi: 10.1073/pnas.2008213117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ, 1997. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J. Comp. Neurol 377, 49–69. [DOI] [PubMed] [Google Scholar]
- Krukoff TL, Harris KH, Jhamandas JH, 1992. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer phaseolus vulgaris leukoagglutinin. Brain Res. Bull 30, 163–172. [DOI] [PubMed] [Google Scholar]
- Kummer W, Neuhuber WL, 1989. Vagal paraganglia in the rat. J. Electron. Microsc. Tech 12, 343–355. [DOI] [PubMed] [Google Scholar]
- Kummer W, Bachmann S, Neuhuber WL, Hänze J, Lang RE, 1993. Tyrosine-hydroxylase-containing vagal afferent neurons in the rat nodose ganglion are independent from neuropeptide-Y-containing populations and project to esophagus and stomach. Cell Tissue Res. 271, 135–144. [DOI] [PubMed] [Google Scholar]
- Kupari J, Häring M, Agirre E, Castelo-Branco G, Ernfors P, 2019. An atlas of vagal sensory neurons and their molecular specialization. Cell Reports 27, 2508–2523. doi: 10.1016/j.celrep.2019.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hu Y, 1988. The central projections of the great auricular nerve primary afferent fibers – an HRP transganglionic tracing method. Brain Res. 445, 205–210. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Burton H, 1978. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J. Comp. Neurol 181, 421–449. [DOI] [PubMed] [Google Scholar]
- Loucks TMJ, Poletto CJ, Saxon KG, Ludlow CL, 2005. Laryngeal muscle response to mechanical displacement of the thyroid cartilage in humans. J. Appl. Physiol 99, 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M, 1995. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with choleratoxin B subunit and phaseolus vulgaris leucoagglutinin. Neuroscience 65, 119–160. [DOI] [PubMed] [Google Scholar]
- Ma WL, Zhang WB, Feng G, Cai YL, 2005. Calbindin D28k-containing neurons in the paratrigeminal nucleus receive convergent nociceptive information and project to nucleus of the solitary tract in rat. Brain Res. 1038, 132–140. doi: 10.1016/j.brainres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Makhmutova M, Weitz J, Tamayo A, Pereira E, Boulina M, Almaca J, Rodriguez-Diaz R, Caicedo A, 2021. Pancreatic β-cells communicate with vagal neurons. Gastroenterology 160, 875–888. doi: 10.1053/j.gastro.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, Rajchert DM, 1991. Trigeminal primary afferent projections to “non-trigeminal” areas of the rat central nervous system. J. Comp. Neurol 303, 489–511. [DOI] [PubMed] [Google Scholar]
- May A, Bramke S, Funk RHW, May CA, 2018. The human platysma contains numerous muscle spindles. J. Anat 232, 146–151. doi: 10.1111/joa.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Hopkins DA, 1982. Ultrastructural identification of labeled neurons in the dorsal motor nucleus of the vagus nerve following injections of horseradish peroxidase into the vagus nerve and brainstem. J. Comp. Neurol 206, 243–252. [DOI] [PubMed] [Google Scholar]
- McRitchie DA, Törk I, 1993. The internal organization of the human solitary nucleus. Brain Res. Bull 31, 171–193. [DOI] [PubMed] [Google Scholar]
- Mei N, Condamin M, Boyer A, 1980. The composition of the vagus nerve of the cat. Cell Tissue Res. 209, 423–431. [DOI] [PubMed] [Google Scholar]
- Menétrey D, Basbaum AI, 1987. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J. Comp. Neurol 255, 439–450. [DOI] [PubMed] [Google Scholar]
- Min S, Chang RB, Prescott SL, Beeler B, Joshi NR, Strochlic DE, Liberles SD, 2019. Arterial baroreceptors sense blood pressure through decorated aortic claws. Cell Reports 29, 2192–2201. doi: 10.1016/j.celrep.2019.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Nandigama R, Bettner W, Undem BJ, 2010. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J. Physiol 588, 4769–4783. doi: 10.1113/jphysiol.2010.195339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhuber W, Mysicka A, 1980. Afferent neurons of the hypoglossal nerve of the rat as demonstrated by horseradish peroxidase tracing. Anat. Embryol 158, 349–360. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, 1987. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J. Auton. Nerv. Syst 20, 243–255. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, 1989. Vagal afferent fibers almost exclusively innervate islets in the rat pancreas as demonstrated by anterograde tracing. J. Auton. Nerv. Syst 29, 13–18. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, Wörl J, Berthoud HR, Conte B, 1994. NADPH-diaphorase-positive nerve fibers associated with motor endplates in the rat esophagus: evidence for co-innervation of striated muscle by enteric ganglia. Cell Tissue Res. 276, 23–30. [DOI] [PubMed] [Google Scholar]
- Neuhuber W, Bieger D, 2013. Brainstem control of deglutition: brainstem neural circuits and mediators regulating swallowing, in: Shaker R, Belafsky PC, Postma GN, Easterling C (Eds.), Principles of Deglutition. Springer, New York, pp. 89–113. [Google Scholar]
- Neuhuber WL, Zenker W, 1989. Central distribution of cervical primary afferents in the rat, with emphasis on proprioceptive projections to vestibular, perihypoglossal and upper thoracic spinal nuclei. J. Comp. Neurol 280, 231–253. [DOI] [PubMed] [Google Scholar]
- Nomura S, Mizuno N, 1984. Central distribution of primary afferent fibers in the Arnold’s nerve (the auricular branch of the vagus nerve): a transganglionic HRP study in the cat. Brain Res. 292, 199–205. [DOI] [PubMed] [Google Scholar]
- Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z Francisco AG, Ranade SS, Liberles SD, Patapoutian A, 2017. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541, 176–181. doi: 10.1038/nature20793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R, Smith GP, 1988. Central distribution of subdiaphragmatic vagal branches in the rat. J. Comp. Neurol 273, 207–223. [DOI] [PubMed] [Google Scholar]
- Oka A, Yamamoto M, Takeda R, Ohara H, Sato F, Akhter F, Haque T, Kato T, Sessle BJ, Takada K, Yoshida A, 2013. Jaw-opening and –closing premotoneurons in the nucleus of the solitary tract making contacts with laryngeal and pharyngeal afferent terminals in rats. Brain Res. 1540, 48–63. doi: 10.1016/j.brainres.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Ortega-Villalobos M, García-Bazán M, Solano-Flores LP, Ninomiya-Alarcón JG, Guevara-Guzmán R, Wayner MJ, 1990. Vagus nerve afferent and efferent innervation of the rat uterus: an electrophysiological and HRP study. Brain Res. Bull 25, 365–371. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Kasparov S, 2000. Sensory channel specific modulation in the nucleus of the solitary tract. J. Auton. Nerv. Syst 80, 117–129. [DOI] [PubMed] [Google Scholar]
- Paulsen K, 1958. Über Vorkommen und Zahl von Muskelspindeln in inneren Kehlkopfmuskeln des Menschen (M. cricoarythaenoideus dorsalis, M. cricothyreoideus). Z. Zellforsch 48, 349–355. [PubMed] [Google Scholar]
- Pelot NA, Goldhagen GB, Cariello JE, Musselman ED, Clissold KA, Ezzell JA, Grill WM, Quantified morphology of the cervical and subdiaphragmatic vagus nerves of human, pig, and rat. Front. Neurosci 14, 601479. doi: 10.3389/fnins.2020.601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz JO, Belau E, Henn P, Hammer N, Classen J, Weise D, 2018. Sonographic evaluation of the vagus nerves: protocol, reference values, and side-to-side differences. Muscle Nerve 57, 766–771. D oi: 10.1002/mus.25993. [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC, 2008. Oxytocin enhances cranial visceral afferent synaptic transmission in the solitary tract nucleus. J. Neurosci 28, 11731–11740. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuker ET, Filler TJ, 2002. The nerve supply of the human auricle. Clin. Anat 15, 35–37. [DOI] [PubMed] [Google Scholar]
- Plenat F, Leroux P, Floquet J, Floquet A, 1988. Intra and juxtavagal paraganglia: a topographical, histochemical, and ultrastructural study in the human. Anat. Rec 221, 743–753. [DOI] [PubMed] [Google Scholar]
- Porges SW, The polyvagal theory: phylogenetic substrates of a social nervous system. Int. J. Psychophysiol 42, 123–146. [DOI] [PubMed] [Google Scholar]
- Potts JT, Paton JFR, Mitchell JH, Garry MG, Kline G, Anguelov PT, Lee SM, 2003. Contraction-sensitive skeletal muscle afferents inhibit arterial baroreceptor signaling in the nucleus of the solitary tract: role of intrinsic GABA interneurons. Neuroscience 119, 201–214. doi: 10.1016/S0306-4522(02)00953-3. [DOI] [PubMed] [Google Scholar]
- Powley TL, Prechtl JC, Fox EA, Berthoud HR, 1983. Anatomical considerations for surgery of the rat abdominal vagus: distribution, paraganglia and regeneration. J. Auton. Nerv. Syst 9, 79–97. [DOI] [PubMed] [Google Scholar]
- Powley TL, Berthoud HR, Fox EA, Laughton W 1992. The dorsal vagal complex forms a sensory-motor lattice: the circuitry of gastrointestinal reflexes, in: Ritter S, Ritter RC, Barnes CD (Eds.), Neuroanatomy and physiology of abdominal vagal afferents. CRC Press, Boca Raton, pp. 55–79. [Google Scholar]
- Powley TL, Wang XY, Fox EA, Phillips RJ, Liu LWC, Huizinga JD, 2008. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol. Motil 20, 69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- Powley TL, Spaulding RA, Haglof SA, 2011. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J. Comp. Neurol 519, 644–660. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Baronowsky EA, Gilbert JM, Hudson CN, Martin FN, Mason JK, McAdams JL, Phillips RJ, 2013a. Vagal afferent innervation of the lower esophageal sphincter. Auton. Neurosci 177, 129–142. doi: 10.1016/j.autneu.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Mittal RK, Baronowsky EA, Hudson CN, Martin FN, McAdams JL, Mason JK, Phillips RJ, 2013b. Architecture of vagal motor units controlling striated muscle of esophagus: peripheral elements patterning peristalsis? Auton. Neurosci 179, 90–98. doi: 10.1016/j.autneu.2013.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Hudson CN, McAdams JL, Baronowsky EA, Martin FN, Mason JK, Phillips RJ, 2014. Organization of vagal afferents in pylorus: mechanoreceptors arrayed for high sensitivity and fine spatial resolution? Auton. Neurosci 183, 36–48. doi: 10.1016/j.autneu.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prades JM, Timoshenko AP, Asanau A, Gavid M, Benakki H, Dubois MD, Faye MB, Martin C, 2009. The cricopharyngeal muscle and the laryngeal nerves: contribution to the functional anatomy of swallowing. Morphologie 93, 35–41. doi: 10.1016/j.morpho.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Prechtl JC, Powley TL, 1990. The fiber composition of the abdominal vagus of the rat. Anat. Embryol 181, 101–115. [DOI] [PubMed] [Google Scholar]
- Raab M, Neuhuber WL, 2007. Glutamatergic functions of primary afferent neurons with special emphasis on vagal afferents. Int. Rev. Cytol 256, 223–275. doi: 10.1016/S0074-7696(07)56007-9. [DOI] [PubMed] [Google Scholar]
- Safi S, Ellrich J, Neuhuber W, 2016. Myelinated axons in the auricular branch of the human vagus nerve. Anat. Rec 299, 1184–1191. doi: 10.1002/ar.23391. [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TFC, McWilliam PN, 1999. Glycine-immunoreactive synaptic terminals in the nucleus tractus solitarii of the cat: ultrastructure and relationship to GABA-immunoreactive terminals. Synapse 33, 192–206. [DOI] [PubMed] [Google Scholar]
- Saxon DW, Hopkins DA, 1998. Efferent and collateral organization of paratrigeminal nucleus projections: an anterograde and retrograde fluorescent tracer study in the rat. J. Comp. Neurol 402, 93–110. [PubMed] [Google Scholar]
- Schwarzacher SW, Rüb U, Deller T, 2011. Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain 134, 24–35. doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- Seki A, Green HR, Lee TD, Hong LS, Tan J, Vinters HV, Chen PS, Fishbein MC, 2014. Sympathetic nerve fibers in human cervical and thoracic vagus nerves. Heart Rhythm. 11, 1411–1417. doi: 10.1016/j.hrthm.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle BJ, Hu JW, Amano N, Zhong G, 1986. Convergence of cutaneous, tooth pulp, visceral, neck and muscle afferents onto nociceptive and non-nociceptive neurones in trigeminal subnucleus caudalis (medullary dorsal horn) and its implications for referred pain. Pain 27, 219–235. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Mechtler LL, Kudrow DB, Calhoun AH, McClure C, Saper JR, Liebler EJ, Rubenstein Engel E, Tepper SJ, ACT1 Study Group, 2016. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache doi: 10.1111/head.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stakenborg N, Gomez-Pinilla PJ, Verlinden TJM, Wolthuis AM, D’Hoore A, Farré R, Herijgers P, Matteoli G, Boeckxstaens GE, 2020. Comparison between the cervical and abdominal vagus nerves in mice, pigs, and humans. Neurogastroenterol. Motil 32, e13889. doi: 10.1111/nmo.13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Huang ZG, Silburn PA, Balnave RJ, Holstege G, 2018. The physiological motor patterns produced by neurons in the nucleus retroambiguus in the rat and their modulation by vagal, peripheral chemosensory, and nociceptive stimulation. J. Comp. Neurol 526, 229–242. doi: 10.1002/cne.24318. [DOI] [PubMed] [Google Scholar]
- Surdenikova L, Ru F, Nassenstein C, Tatar M, Kollarik M, 2012. The neural crest- and placodes-derived afferent innervation of the mouse esophagus. Neurogastroenterol. Motil 24, e517–e525. doi: 10.1111/nmo.12002. [DOI] [PubMed] [Google Scholar]
- Takemura M, Sugimoto T, Sakai A, 1987. Topographic organization of central terminal region of different sensory branches of the rat mandibular nerve. Exp. Neurol 96, 540–557. doi: 10.1016/0014-4886(87)90217-2. [DOI] [PubMed] [Google Scholar]
- Taylor EW, Jordan D, Coote JH, 1999. Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol. Rev 79, 855–916. [DOI] [PubMed] [Google Scholar]
- Taylor EW, Leite CAC, Sartori MR, Wang T, Abe AS, Crossley DA II, 2014. The phylogeny and ontogeny of autonomic control of the heart and cardiorespiratory interactions in vertebrates. J. Exp. Biol 217, 690–703. doi: 10.1242/jeb.086199. [DOI] [PubMed] [Google Scholar]
- Testut L, 1922. Traité d’Anatomie Humaine, vol 3, septième ed. Librairie Octave Doin, Paris. [Google Scholar]
- Thompson H, Tucker AS, 2013. Dual origin of the epithelium of the mammalian middle ear. Science 339, 1453–1456. doi: 10.1126/science.1232862. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Anselmi L, 2016. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol 13, 389–401. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto K, Hayakawa T, Maeda S, Tanaka K, Seki M, Yamamura T, 2005. Projections to the alimentary canal from the dopaminergic neurons in the dorsal motor nucleus of the vagus of the rat. Auton. Neurosci 123, 12–18. doi: 10.1016/j.autneu.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Uludag M, Aygun N, Isgor A, 2017. Innervation oft he human cricopharyngeal muscle by the recurrent laryngeal nerve and external branch oft he superior laryngeal nerve. Langenbecks Arch Surg 402, 683–690. doi: 10.1007/s00423-016-1376-5. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Telegan P, 1999. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J. Comp. Neurol 412, 410–428. [DOI] [PubMed] [Google Scholar]
- Verlinden TJM, Rijkers K, Hoogland G, Herrler A, 2016. Morphology of the human cervical vagus nerve: implications for vagus nerve stimulation treatment. Acta Neurol. Scand 133, 173–182. doi: 10.1111/ane.12462. [DOI] [PubMed] [Google Scholar]
- Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA, 2008. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J. Neurosci 28, 4957–4966. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FB, Powley TL, 2000. Topographic inventories of vagal afferents in gastrointestinal muscle. J. Comp. Neurol 421, 302–324. [PubMed] [Google Scholar]
- Wang FB, Powley TL, 2007. Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res. 329, 221–230. doi: 10.1007/s00441-007-0413-7. [DOI] [PubMed] [Google Scholar]
- Wank M, Neuhuber WL, Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J. Comp. Neurol 435, 41–59. [DOI] [PubMed] [Google Scholar]
- Williams RM, Berthoud HR, Stead RH, 1997. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation 4, 266–270. doi: 10.1159/000097346. [DOI] [PubMed] [Google Scholar]
- Yakunina N, Kim SS, Nam EC, 2017. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation 20, 290–300. doi: 10.1111/ner.12541. [DOI] [PubMed] [Google Scholar]
- Yang M, Zhao X, Miselis RR, 1999. The origin of catecholaminergic nerve fibers in the subdiaphragmatic vagus nerve of rat. J. Auton. Nerv. Syst 76, 108–117. doi: 10.1016/s0165-1838(99)00014-4. [DOI] [PubMed] [Google Scholar]
- Yu X, Hu Y, Yu S, 2014. Effects of acid on vagal nociceptive afferent subtypes in guinea pig esophagus. Am. J. Physiol. Gastrointest. Liver Physiol 307, G471–G478. doi: 10.1152/ajpgi.00156.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJH, 2000. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J. Neurosci 20, 6249–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJH, 2001. Intraganglionic laminar endings are mechanotransduction sites of vagal tension receptors in the guinea-pig stomach. J. Physiol 534, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos TP, Silverman HA, Levy T, Tsaava T, Battinelli E, Lorraine PW, Ashe JM, Chavan SS, Tracey KJ, Bouton CE, 2018. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. PNAS 115, E4843–E4852. doi: 10.1073/pnas.1719083115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Luo P, Ro JY, Xiong H, 2012. Jaw muscle spindle afferents coordinate multiple orofacial motoneurons via common premotor neurons in rats: an electrophysiological and anatomical study. Brain Res. 1489, 37–47. doi: 10.1016/j.brainres.2012.10.021. [DOI] [PubMed] [Google Scholar]