Abstract
Purpose:
Little is known about the association between sarcoidosis and lymphoma. We aim to determine the prevalence of lymphoma in US sarcoidosis patients and compare the clinical characteristics of patients with and without lymphoma.
Methods:
Using a national registry-based study investigating 3,560 respondents to the Foundation for Sarcoidosis Research Sarcoidosis Advanced Registry for Cures Questionnaire (FSR-SARC) completed between June 2014 and August 2019, we identified patients who reported the diagnosis of lymphoma following sarcoidosis and randomly selected a computer-generated control sample of sarcoidosis patients with no reported lymphoma with a 2:1 ratio.
Results:
Among 3,560 patients with sarcoidosis, 43 (1.2%) reported developing lymphoma following their sarcoidosis diagnosis. Patients with lymphoma were more likely to be diagnosed with sarcoidosis at a younger age (median, IQR) 40 (27–50) vs 45 (34.8–56, p=0.017) years, were more likely to be African Americans (OR, 95% CI; 3.9 (1.6–9.6, P=0.002) and have low annual income (OR=2.7, 1.1–6.4 p=0.026). The sarcoidosis-lymphoma group were more likely to have salivary gland (16% vs 5%, p=0.026) (OR= 4; 1.1–14.5) and cutaneous (46% vs 23%, p=0.023) (OR=2.9;1.1–7.3) sarcoidosis. They also reported more chronic fatigue (42% vs 23%, p=0.029), chronic pain (37% vs 13%, p=0.001) and depression (42% vs 22%, p=0.019).
Conclusion:
The prevalence of lymphoma reported in sarcoidosis patients is higher than the general population which further supports the possible increased risk of lymphoma in sarcoidosis. Diagnosis of sarcoidosis at a younger age, African American race, cutaneous and salivary glands sarcoidosis were associated with lymphoma. Sarcoidosis patients who developed lymphoma reported higher disease burden and more non-organ specific manifestations.
Introduction
Sarcoidosis is a rare chronic multisystemic granulomatous disease of unknown etiology that is thought to result from exposure to specific environmental agents in a genetically susceptible host[1]. The link between sarcoidosis and lymphoma was first described in an incidence study by Bincker et al. in 1974 which reported an 11-times increased risk of lymphoma in sarcoidosis patients[2]. However, the methodology of this study was questioned and since then, the entity of sarcoidosis-lymphoma syndrome has been a topic of controversy[3]. In the majority of reported cases, the onset of sarcoidosis preceded the diagnosis of lymphoma. However, concurrent diagnosis of both conditions within one year[4] and the diagnosis of sarcoidosis following lymphoma[3–9] have been described. Additionally, sarcoid-like granulomatous reactions have been reported to occur in around 14% of Hodgkin’s lymphoma and 7% in non-Hodgkin’s lymphoma patients[9–11].
Both lymphoma and sarcoidosis can present with constitutional symptoms, hematological abnormalities, and similar organ involvement. Consequently, this can make the differentiation between these two disorders a challenge for the treating physician, and is especially true when the two disorders coexist. A high index of suspicion must be adopted to properly diagnose lymphoma in patients with sarcoidosis. Similarly, caution must be followed to spare patients with lymphoma from the potentially harmful chemotherapeutic agents that can be given for presumed lymphoma recurrence; while in fact they have undiagnosed sarcoidosis [3].
In this context, there is a paucity of data describing the clinical features of sarcoidosis patients who also develop lymphoma and most of the published studies are from the Scandinavian registries[2, 12–16]. For this reason, we aimed in this study to report the prevalence of self-reported lymphoma in patients with sarcoidosis in the United States. Our objective also included determining the demographic, clinical characteristics and organ involvement of the sarcoidosis patients who reported lymphoma in comparison with sarcoidosis patients who did not report developing lymphoma.
Methodology
Study Population:
We used a US national registry for sarcoidosis patients from the Foundation for Sarcoidosis Research: Sarcoidosis Advanced Registry for Cures Questionnaire (FSR-SARC)[17, 18]. The FSR enrolled English-speaking patients self-identifying as having sarcoidosis either by direct recruitment through treating physicians or via national and international organizations. They used a web-based questionnaire that includes 72 questions. This registry collects longitudinal, self-reported, and cross-sectional data related to demographics, diagnosis, manifestations, management, and the physical and psychosocial impact of sarcoidosis. We only included patient surveys completed between June 2014 and August 2019 who responded to the question “Have you developed lymphoma AFTER you were given the diagnosis of sarcoidosis”. We excluded patients who were reported to be deceased or had missing information regarding the question “is the patient living” as these cases answers were filled by their surrogates. The final cohort included 3,560 patients. Respondents were able to update their surveys longitudinally with time. Data from the most recent survey for each respondent was used for our analysis. The study was approved by the University of Florida Institutional Review Board (IRB no. 201902211).
Objectives and Data Management:
The primary objective of the study was to find the prevalence of self-reported lymphoma in patients with sarcoidosis. Secondary objectives were to determine the demographic, clinical characteristics and sarcoidosis organ involvement of the sarcoidosis patients who reported developing lymphoma. Patients were asked whether they developed lymphoma after the diagnosis of sarcoidosis. The responses “unsure” or “prefer not to answer” were labelled as missing information. Only complete cases were analyzed for each variable.
Multi-organ sarcoidosis was defined if the respondent reported three or more organs affected by sarcoidosis[19, 20]. Sarcoidosis-specific therapies were divided into five categories: 1) corticosteroids (prednisone, methylprednisolone, dexamethasone), 2) tumor necrosis factors (TNF) inhibitors (adalimumab, infliximab, etanercept, certolizumab, golimumab) 3) immunomodulatory agents (cyclophosphamide, chloroquine, hydroxychloroquine, azathioprine, mycophenolate, methotrexate, leflunomide), 4) rituximab, and 5) other systemic therapies (thalidomide, pentoxifylline, intravenous immunoglobulin, adrenocorticotropic hormone)[18].
Statistical analysis
Since only a minority of patients reported having lymphoma and to avoid skewed comparison with the rest of the patients due to large difference in the number of patients with and without lymphoma, we randomly selected a computer-generated control sample of sarcoidosis patients who did not report having lymphoma with a 2:1 ratio. We compared the data related to patient characteristics, diagnosis and treatment of sarcoidosis and data related to organ involvement and self-reported comorbidities developed after the diagnosis of sarcoidosis. The distribution of patient characteristics and outcomes were summarized as percentages for categorical variables, and medians with interquartile range (IQR) for continuous variables. We presented the categorical variables in the tables as nominators (positive cases) over denominator (complete cases for each variable). We used Mann-Whitney U to compare continuous variables and Chi-square test or Fisher’s exact test (as appropriate) for categorical variables. We displayed some of the results as bar charts. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 23.0 (released 2015, IBM Corp, Armonk, NY).
Results
Demographics and Baseline Characteristics
Among 3,560 US sarcoidosis patients identified for our analysis, 43 (1.2%) reported developing lymphoma after their diagnosis of sarcoidosis. The patients with self-reported lymphoma were generally middle-aged women and were younger at the time of sarcoidosis diagnosis than patients with no reported lymphoma (median, IQR) (40 (27–50) vs 45 (34.8–56, p=0.017). Although the majority of the respondents to this registry reported their race as white[18], 37% of the sarcoidosis patients with lymphoma reported themselves as African-Americans, a proportion that is significantly higher than the non-lymphoma patients (37% vs 13%, p=0.002) (Table 1). Put another way, patients who reported their race as African-American were four times more likely to report lymphoma than other races (OR=3.8, 95% CI: 1.5– 9.3, p=0.004). Sarcoidosis patients who developed lymphoma were more likely to have low-income compared to the control group (OR=2.7, 95% CI 1.1–6.4 p=0.026) with no difference in education status. Sarcoidosis patients with lymphoma were more likely to report sarcoidosis-related hospital admissions as compared to the control group (54% vs 34%, p=0.038). There was no statistically significant difference in sarcoidosis-related therapies received, except for rituximab which was reported more in the lymphoma group (29% vs 5%, p= 0.006) (Table 1).
Table 1:
Baseline demographics and clinical characteristics of sarcoidosis patients with self-reported lymphoma as compared to patients with no lymphoma*
| Sarcoidosis with lymphoma (n=43) | Sarcoidosis with no lymphoma (n=86) | p value | |
|---|---|---|---|
| Demographics | |||
| Age at time of sarcoidosis diagnosis (IQR) | 40 (27–50) | 45 (34.8–56) | 0.017 |
| Age at time of data collection (IQR) | 57.6 (51.8–65.9) | 57.2 (46.3–64.8) | 0.430 |
| Time since sarcoidosis diagnosis (IQR) | 15 (6.8–27.3) | 7 (4–13) | <0.001 |
| Women, n/total n (%) | 27/43 (63) | 62/86 (72) | 0.282 |
| Hispanic ethnicity, n/total n (%) | 4/40 (10) | 3/80 (3.8) | 0.168 |
| Race, n/total n (%) | |||
| African American | 15/42 (35.7) | 11/86 (12.7) | 0.004 |
| White | 26/42 (62) | 73/86 (85) | 0.005 |
| Other | 1/42 (2.4) | 2/86 (2.3) | 0.98 |
|
| |||
| Healthcare insurance, n/total n (%) | |||
| Private health insurance | 23/42 (54.8) | 51/85 (60) | 0.573 |
| Government insurance | 20/42 (47.6) | 25/85 (29.4) | 0.044 |
| No health insurance | 4/42 (9.5) | 16/85 (18.8) | 0.176 |
| Graduated college or university, n/total n (%) | 39/40 (97.5) | 71/71 (100) | 0.181 |
| Income, n/total n (%) | 0.026 | ||
| < 35K | 16/39 (41) | 14/68 (20.6) | |
| 35K-99.9K | 20/39 (51.3) | 38/68 (55.9) | |
| >100K | 3/39 (7.7) | 16/68 (23.5) | |
|
| |||
| Family history of sarcoidosis, n/total n (%) | 4/28 (14.3) | 10/72 (14) | 0.959 |
|
Ever admitted to hospital in relation to
sarcoidosis, n/total n (%) |
22/41 (54) | 28/82 (34) | 0.038 |
| Sarcoidosis-specific therapy, n/total n (%) § | |||
| Steroids | 37/42 (88.1) | 63/86 (73.3) | 0.057 |
| immunomodulatory agents | 27/34 (79.4) | 35/53 (66) | 0.179 |
| TNF inhibitors | 9/26 (34.6) | 6/41 (14.6) | 0.056 |
| Rituximab | 7/24 (29.1) | 2/41 (4.9) | 0.006 |
| Others | 3/24 (12.5) | 1/41 (2.4) | 0.138 |
Missing values were excluded for each variable. Control group was random computer-generated sample from sarcoidosis patients with no lymphoma
Steroids = prednisone, methylprednisolone and dexamethasone; immunomodulatory agents = hydroxychloroquine, chloroquine, methotrexate, azathioprine, leflunomide, mycophenolate and cyclophosphamide. TNF inhibitors = infliximab, adalimumab, certolizumab, golimumab and etanercept. Others = pentoxifyline, IVIG, thalidomide, adrenocorticotropic hormone.
TNF= tumor necrosis factor
Sarcoidosis Organ Involvement and Comorbidities
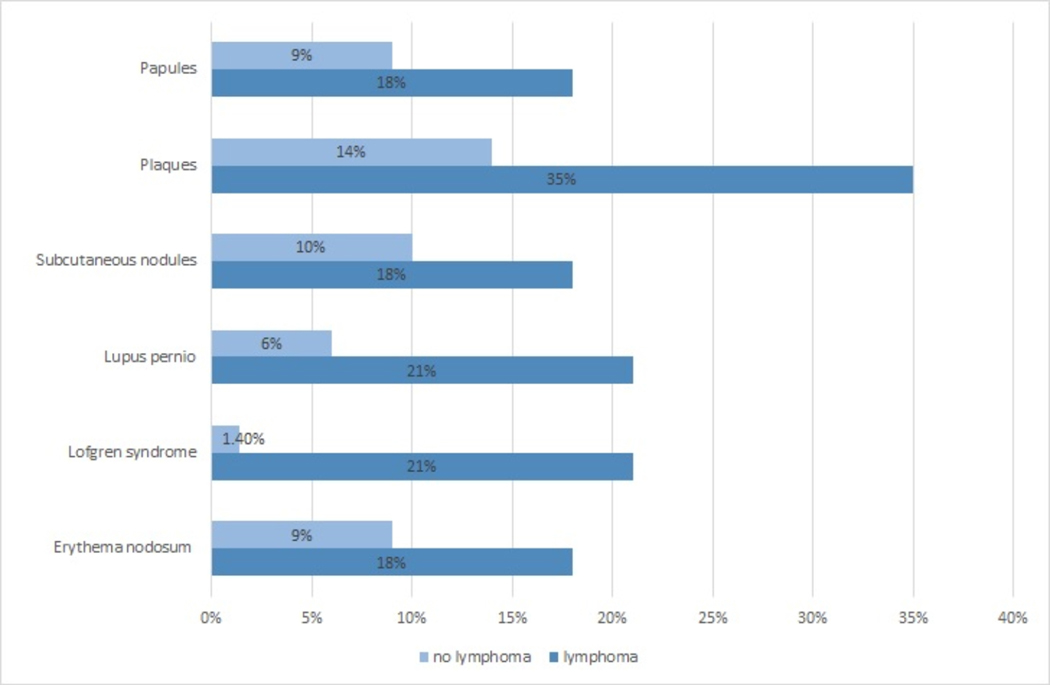
Around 60% of sarcoidosis patients with self-reported lymphoma reported multi-organ sarcoidosis involvement, which was not significantly different than the control group (58% vs 43%, p=0.1). The only organ systems that were reported to be more involved with sarcoidosis in the lymphoma patients were salivary gland (16% vs 5%, p=0.026) (OR= 4; 1.1–14.5) and cutaneous (46% vs 23%, p=0.023) (OR=2.9;1.1–7.3) sarcoidosis. There was no statistically significant difference in other organ systems sarcoidosis involvement (Table 2). Figure 1 shows specific skin manifestations reported by both groups.
Table 2:
Sarcoidosis involvement of other organs as reported by sarcoidosis patients with lymphoma as compared to those with no lymphoma*
| Organs involved, n/total n (%) | Lymphoma (n=43) | No lymphoma (n=86) | p value |
|---|---|---|---|
| Multi-organ sarcoidosis ≥3 organs† | 25/43 (58.1) | 36/84 (42.9) | 0.103 |
| Lungs | 28/42 (66.7) | 56/74 (75.7) | 0.297 |
| Bones or vertebrae | 5/31 (16.1) | 7/54 (13) | 0.687 |
| Brain or cranial nerves | 3/29 (10.3) | 9/51 (17.6) | 0.379 |
| Peripheral nerves | 13/32 (40.6) | 15/54 (27.8) | 0.219 |
| Central lymph nodes‡ | 21/37 (56.8) | 36/62 (58.1) | 0.899 |
| Peripheral lymph nodes§ | 16/33 (48.5) | 22/55 (40) | 0.437 |
| Eyes | 10/32 (31.3) | 14/53 (26.4) | 0.631 |
| Cardiac | 6/33 (18.2) | 4/54 (7.4) | 0.126 |
| Joints | 13/31 (41.9) | 14/56 (25) | 0.102 |
| Kidneys | 4/32 (12.5) | 6/51 (11.8) | 0.920 |
| Parotid or salivary glands | 7/43 (16.3) | 4/86 (4.7) | 0.026 |
| Stomach/intestines | 4/33 (12.1) | 5/50 (10) | 0.761 |
| Liver | 6/31 (19.4) | 7/55 (12.7) | 0.410 |
| Muscles | 7/32 (21.9) | 5/49 (10.2) | 0.148 |
| Sinuses | 4/32 (12.5) | 5/51 (9.8) | 0.701 |
| Skin | 16/35 (45.7) | 12/53 (22.6) | 0.023 |
Patients were asked whether or not the organ involvement is a confirmed diagnosis, suspected or if they were unsure. Prevalence presented in this table are based on confirmed diagnosis only as reported by patients. For this analysis, answers suspected or “not involved were regarded as “not involved”. “Unsure” or missing were regarded as missing values.
Multi-organ sarcoidosis defined if 3 or more organs were reported to be involved
lymph nodes in the chest and abdomen
Axillary, cervical and inguinal lymph nodes
Figure 1.
Self-reported skin manifestations in US sarcoidosis patients with and without lymphoma
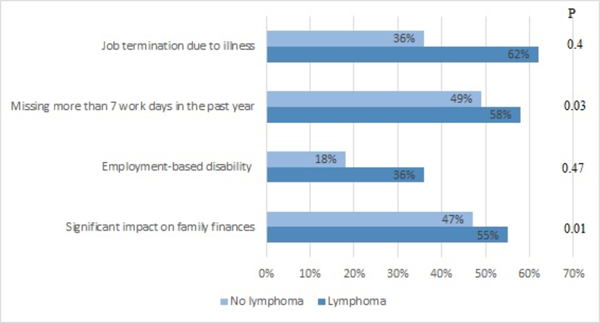
When comparing the comorbidities developed after the diagnosis of sarcoidosis was made, sarcoidosis patients with lymphoma reported more chronic fatigue (42% vs 23%, p=0.029), chronic pain (37% vs 13%, p=0.001), depression (42% vs 22%, p=0.019), fibromyalgia (26% vs 9%, p=0.012) and hypertension (37% vs 19%, p=0.021) when compared to the control group (Table 3). This translated into increased disease burden by reported missing more workdays and increased impact on family finances when compared to the control group (Figure 2).
Table 3:
Comorbidities related to sarcoidosis and its treatment as reported by sarcoidosis patients with lymphoma as compared to those with no lymphoma*
| Lymphoma (n=43) | No lymphoma (n=86) | P value | |
|---|---|---|---|
| Cushing’s disease | 2(4.7) | 3(3.5) | 0.747 |
| Diabetes mellitus | 5(11.6) | 18(21) | 0.193 |
| Hypercalcemia | 4(9.3) | 6(7) | 0.641 |
| Obesity | 18(42) | 30(35) | 0.440 |
| Chronic fatigue | 18(42) | 20(23.3) | 0.029 |
| Chronic pain syndrome | 16(37.2) | 11(12.8) | 0.001 |
| Congestive heart failure | 4(9.3) | 2(2.3) | 0.076 |
| Depression | 18(42) | 19(22.1) | 0.019 |
| Fibromyalgia | 11(26.2) | 8(9.3) | 0.012 |
| Hypertension | 16(37.2) | 16(18.6) | 0.021 |
Patients were asked whether they developed any of a list of comorbidities AFTER they were given the diagnosis of sarcoidosis
Figure 2.
Social impact of sarcoidosis in patients with and without lymphoma
Discussion
To our knowledge, this is the largest study in published literature to assess the prevalence of lymphoma and characteristics of sarcoidosis patients who later develop lymphoma. Our report shows that 1.2% of sarcoidosis patients reported developing lymphoma after being diagnosed with sarcoidosis. When compared to a control of sarcoidosis patients with no history of lymphoma, those with reported lymphoma were more likely to report their race as African-Americans, have lower annual income and were diagnosed with sarcoidosis at a somewhat younger age. Patients with lymphoma were more likely to have cutaneous and salivary gland sarcoidosis when compared to controls. Furthermore, these patients reported more sarcoidosis-related hospitalizations, chronic fatigue, chronic pain syndrome, and depression which all reflected on increased disease financial burden.
The prevalence of lymphoma in our study is less than what was previously published but is around 10 to 12 times more than what is reported in the general population with the same age group [21]. In a single center study from Italy reviewing 209 sarcoidosis patients, 10 patients (4.8%) were also diagnosed with lymphoma[22]. In another report from Germany, 15 out of 435 (3.4%) patients were diagnosed with lymphoma or leukemia[12]. Previous registry-based studies from the Scandinavia, reported an increased risk of non-Hodgkin’s and Hodgkin’s lymphoma in sarcoidosis patients that ranged from 2 to 11 times respectively when compared to the general population[23, 24]. It is possible that the self-reported nature of our study and the geographic variation may explain this discrepancy.
Although the majority of the participants in this registry reported their race as white[18], around 40% of the lymphoma group in our cohort reported their race as African-Americans, which was significantly more than the control group. In another cohort study of male veterans, the authors did not find inter-racial heterogeneity for the risk of lymphoma among sarcoidosis patients[25]. The discrepancy maybe related to the population studied and since it used the international classification of diseases codes to identify their cohort.
The increased likelihood of cutaneous sarcoidosis we found in the lymphoma group was previously reported by Alexandrescu et al, who found out that among 58 case reports published in the literature of cutaneous sarcoidosis associated malignancy, hematologic malignancies accounted for over 70% of cases and cutaneous sarcoidosis was present in over 50% of published sarcoidosis cases associated with cancer[26]. This finding may suggest that sarcoidosis cutaneous involvement may represent a marker for a specific sarcoidosis phenotype that is associated with lymphoma. [27]Furthermore, the lymphoma group in our study was four times more likely to report salivary gland sarcoidosis involvement. The chronic stimulation of B-cells with autoantigens is thought to be an important factor in the pathogenesis of primary salivary gland lymphoma in patients with Sjogren’s syndrome[27]. It is possible that sarcoidosis involvement of the salivary glands might increase the risk of primary salivary gland lymphoma with similar pathogenetic pathways; but this theory warrants further study.
There are few hypotheses that explain the link between sarcoidosis and lymphoma. The prolonged state of chronic inflammation in sarcoidosis patients with the production of inflammatory cytokines, such as macrophage migratory inhibiting factors, IL-6, TGB- β and TNF, may create a suitable microenvironment for malignancy causing lymphoma in the genetically predisposed sarcoidosis patient[28]. Interestingly, in our study, patients with lymphoma were roughly five years younger when the diagnosis of sarcoidosis was made. This might suggest that patients with more chronic form of sarcoidosis or those treated longer with immunosuppressive drugs are at increased risk of lymphoma. Another possibility is simply due to a selection bias related to a longer follow up period especially that the timing of lymphoma diagnosis following sarcoidosis diagnosis is unclear. Other proposed mechanisms for lymphoma-sarcoidosis syndrome include a common oncogenic infectious agent. For example, mycobacterium tuberculosis and propionibacterium acnes were proposed as a cause of sarcoidosis; analogous to that, viruses like Epstein-Barr virus, human-immune-deficiency-virus and human T-lymphotropic virus-1 were linked with lymphoma. Whether there is an unidentified common oncogenic pathogen is unclear. Some propose that the immune system in sarcoidosis has defective helper T-cells which causes a decreased protection against the tumorigenic viruses[29] and that a paradoxical decrease in the immune system of sarcoidosis despite the local increase at the site of granulomas may also contribute to the lymphoma development[28, 30]. Additionally, the prolonged use of immune suppressants in sarcoidosis patients may increase the risk for malignancy[4]. As expected, sarcoidosis patients who also had lymphoma, reported more chronic pain, chronic fatigue and depression. These findings stress the importance of keeping clinical vigilance when dealing with sarcoidosis patients and exploring other causes of non-organ related manifestations in this patient population. Additionally, sarcoidosis patients who developed lymphoma reported more disease-related hospitalizations and increased disease financial burden. This could be related to the complications of lymphoma and receiving chemotherapeutic agents for its treatment. Of note, patients in the sarcoidosis-lymphoma group reported more use of rituximab therapy. While there is inconsistent evidence for the use of rituximab in refractory sarcoidosis[31], it is likely that the increase in rituximab use in the lymphoma group was due to lymphoma-treatment rather than sarcoidosis treatment.
We acknowledge the limitations of our study that are related to the self-reported nature of this registry leading to non-differential misclassification of the outcome (lymphoma). It may have caused the lower prevalence of lymphoma in our cohort when compared to previous studies. Unfortunately, this bias is commonly noted in self-reported registry studies; however, the relatively large participant number in our study as compared to previously published literature might alleviate this bias. Additionally, we lacked data about patients who had lymphoma prior to their sarcoidosis diagnosis. The recruitment process of this registry may have led to a referral bias which may explain over-representation of white women in this registry. Furthermore, patients with more severe sarcoidosis, and chronic forms of sarcoidosis are more likely to be included in this registry but not self-remitting sarcoidosis. This could lead to a selection bias, since such patients may be at more risk for developing lymphoma. However, our study presents an important piece of evidence regarding the prevalence of lymphoma in US sarcoidosis patients and the clinical characteristics of the sarcoidosis phenotype that is linked to lymphoma.
Conclusion
In conclusion, lymphoma is prevalent in sarcoidosis patients and is associated with an increased disease burden. Sarcoidosis diagnosis at a younger age, African-American race, cutaneous sarcoidosis and involvement of salivary glands might be associated with increased likelihood of developing lymphoma in patients with sarcoidosis. It is important to have high clinical suspicion for lymphoma in sarcoidosis patients especially with these features. Further investigation is needed to investigate common pathogenesis and further define this phenotype.
Supplementary Material
Acknowledgment
The authors would like to thank the Foundation for Sarcoidosis Research for the use of FSR S.A.R.C. Patient Registry data to conduct this study.
Funding: Non-monetary support by Foundation for Sarcoidosis Research
Abbreviations
- aOR
adjusted odd ratio
- CI
confidence interval
- FSR
Foundation for Sarcoidosis Research
- FSR-SARC
Foundation for Sarcoidosis Research: Sarcoidosis Advanced Registry for Cures Questionnaire
- IQR
interquartile range
- IRB
Institutional Review Board
- TNF
tumor necrosis factors
- US
United States
Footnotes
Declarations:
Conflicts of interest: None of the authors have a conflict of interest.
Data availability Statement: Data are available upon request through the FSR S.A.R.C. Registry Committee. You can contact the committee at datarequests@stopsarcoidosis.org. Restrictions on access to data are to ensure patient privacy for all persons in the FSR S.A.R.C. Registry.
Code availability NA
References
- 1.(1999) Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 160:736–755. 10.1164/ajrccm.160.2.ats4-99 [DOI] [PubMed] [Google Scholar]
- 2.Brincker H, Wilbek E (1974) The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer 29:247–251. 10.1038/bjc.1974.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papanikolaou IC, Sharma OP (2010) The relationship between sarcoidosis and lymphoma. Eur Respir J 36:1207–1219. 10.1183/09031936.00043010 [DOI] [PubMed] [Google Scholar]
- 4.Chalayer É, Bachy E, Occelli P, et al. (2015) Sarcoidosis and lymphoma: a comparative study. QJM Int J Med 108:871–878. 10.1093/qjmed/hcv039 [DOI] [PubMed] [Google Scholar]
- 5.Kornacker M, Kraemer A, Leo E, Ho AD (2002) Occurrence of sarcoidosis subsequent to chemotherapy for non-Hodgkin’s lymphoma: report of two cases. Ann Hematol 81:103–105. 10.1007/s00277-001-0415-6 [DOI] [PubMed] [Google Scholar]
- 6.Zinzani PL, Tani M, Trisolini R, et al. (2007) Histological verification of positive positron emission tomography findings in the follow-up of patients with mediastinal lymphoma. Haematologica 92:771–777. 10.3324/haematol.10798 [DOI] [PubMed] [Google Scholar]
- 7.Gooneratne L, Nagi W, Lim Z, et al. (2008) Sarcoidosis and haematological malignancies: is there an association? Br J Haematol 141:260–262. 10.1111/j.1365-2141.2008.06986.x [DOI] [PubMed] [Google Scholar]
- 8.Maayan H, Ashkenazi Y, Nagler A, Izbicki G (2011) Sarcoidosis and lymphoma: case series and literature review. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG 28:146–152 [PubMed] [Google Scholar]
- 9.London J, Grados A, Fermé C, et al. (2014) Sarcoidosis Occurring After Lymphoma. Medicine (Baltimore) 93:. 10.1097/MD.0000000000000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadin ME, Glatstein E, Dorfman RF (1971) Clinicopathologic studies of 117 untreated patients subjected to laparotomy for the staging of Hodgkin’s disease. Cancer 27:1277–1294. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Dorfman RF (1974) Morphological studies of 84 untreated patients subjected to laparotomy for the staging of non-Hodgkin’s lymphomas. Cancer 33:657–674. [DOI] [PubMed] [Google Scholar]
- 12.Blank N, Lorenz H-M, Ho AD, Witzens-Harig M (2014) Sarcoidosis and the occurrence of malignant diseases. Rheumatol Int 34:1433–1439. 10.1007/s00296-014-2983-5 [DOI] [PubMed] [Google Scholar]
- 13.Askling J, Grunewald J, Eklund A, et al. (1999) Increased Risk for Cancer Following Sarcoidosis. Am J Respir Crit Care Med 160:1668–1672. 10.1164/ajrccm.160.5.9904045 [DOI] [PubMed] [Google Scholar]
- 14.Rømer FK, Hommelgaard P, Schou G (1998) Sarcoidosis and cancer revisited: a long-term follow-up study of 555 Danish sarcoidosis patients. Eur Respir J 12:906–912. 10.1183/09031936.98.12040906 [DOI] [PubMed] [Google Scholar]
- 15.Le Jeune I, Gribbin J, West J, et al. (2007) The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med 101:2534–2540. 10.1016/j.rmed.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 16.Ji J, Shu X, Li X, et al. (2009) Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol Off J Eur Soc Med Oncol 20:1121–1126. 10.1093/annonc/mdn767 [DOI] [PubMed] [Google Scholar]
- 17.Sarcoidosis Advanced Registry for Cures (FSR-SARC) - FSR. In: Found. Sarcoidosis Res. https://www.stopsarcoidosis.org/fsr-sarc/. Accessed 19 Apr 2020 [Google Scholar]
- 18.Alzghoul BN, Amer FN, Barb D, et al. (2021) Prevalence and Characteristics of Self-Reported Hypothyroidism and its Association with Non-Organ Specific Manifestations in US Sarcoidosis Patients: A Nationwide Registry Study. ERJ Open Res. 10.1183/23120541.00754-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauer WH, Stern BJ, Baughman RP, et al. (2017) High-Risk Sarcoidosis. Current Concepts and Research Imperatives. Ann Am Thorac Soc 14:S437–S444. 10.1513/AnnalsATS.201707-566OT [DOI] [PubMed] [Google Scholar]
- 20.Baughman RP, Teirstein AS, Judson MA, et al. (2001) Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 164:1885–1889. 10.1164/ajrccm.164.10.2104046 [DOI] [PubMed] [Google Scholar]
- 21.SEER*Explorer Application. https://seer.cancer.gov/explorer/application.html?site=86&data_type=5&graph_type=11&compareBy=sex&chk_sex_1=1&series=9&age_range=1&advopt_compprev_y_axis_var=1&advopt_display=2. Accessed 17 May 2021
- 22.Cerri S, Fontana M, Balduzzi S, et al. (2019) Clinical differences in sarcoidosis patients with and without lymphoma: a single-centre retrospective cohort analysis. Eur Respir J 54:. 10.1183/13993003.02470-2018 [DOI] [PubMed] [Google Scholar]
- 23.Fallah M, Liu X, Ji J, et al. (2014) Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol 25:2025–2030. 10.1093/annonc/mdu365 [DOI] [PubMed] [Google Scholar]
- 24.Fallah M, Liu X, Ji J, et al. (2014) Hodgkin lymphoma after autoimmune diseases by age at diagnosis and histological subtype. Ann Oncol 25:1397–1404. 10.1093/annonc/mdu144 [DOI] [PubMed] [Google Scholar]
- 25.Boffetta P, Rabkin CS, Gridley G (2009) A cohort study of cancer among sarcoidosis patients. Int J Cancer 124:2697–2700. 10.1002/ijc.24261 [DOI] [PubMed] [Google Scholar]
- 26.Alexandrescu DT, Kauffman CL, Ichim TE, et al. (2011) Cutaneous sarcoidosis and malignancy: An association between sarcoidosis with skin manifestations and systemic neoplasia. Dermatol Online J 17: [PubMed] [Google Scholar]
- 27.Travaglino A, Giordano C, Pace M, et al. (2020) Sjögren Syndrome in Primary Salivary Gland Lymphoma: A Systematic Review and Meta-Analysis. Am J Clin Pathol 153:719–724. 10.1093/ajcp/aqaa005 [DOI] [PubMed] [Google Scholar]
- 28.Bonifazi M, Bravi F, Gasparini S, et al. (2015) Sarcoidosis and cancer risk: systematic review and meta-analysis of observational studies. Chest 147:778–791. 10.1378/chest.14-1475 [DOI] [PubMed] [Google Scholar]
- 29.Brincker H (1986) The sarcoidosis-lymphoma syndrome. Br J Cancer 54:467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyara M, Amoura Z, Parizot C, et al. (2006) The immune paradox of sarcoidosis and regulatory T cells. J Exp Med 203:359–370. 10.1084/jem.20050648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweiss NJ, Lower EE, Mirsaeidi M, et al. (2014) Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J 43:1525–1528. 10.1183/09031936.00224513 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.