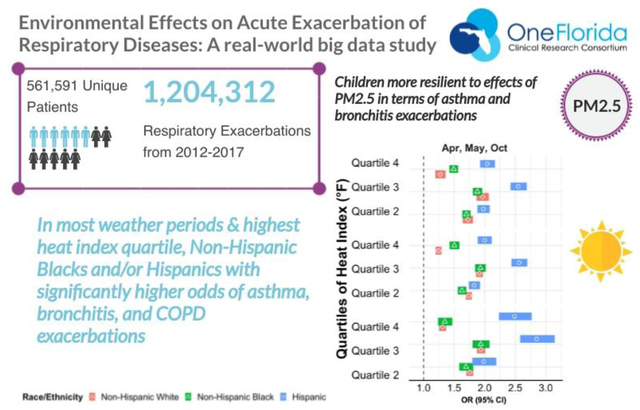
Graphical Abstract
Keywords: Asthma, Bronchitis, COPD, Heat Index, PM2.5, O3
1.0. Introduction
The Centers for Disease Control and Prevention (CDC) estimate that nearly 25 million Americans have asthma, and nearly 16 million Americans have chronic obstructive pulmonary disease (COPD) or bronchitis.1 Acute exacerbations of asthma, COPD, and bronchitis result in worsening symptoms which can lead to emergency department visits and hospitalizations. Management of asthma, bronchitis, and COPD and the associated morbidity and mortality from acute exacerbations cost the United States (US) healthcare system over $10 billion annually.2
Acute respiratory exacerbations are influenced by both intrinsic and extrinsic factors. A large body of literature has examined the effects of various environmental factors, including air pollution and allergens in inciting exacerbations for adults with asthma or COPD.3–7 In children with asthma, studies have identified associations between environmental exposures and asthma exacerbations; some of those exposures overlap with adult triggers, while others are distinct.8–10 For bronchitis, in addition to the risk from smoking, previous work has elucidated the effects of indoor pollutants and mold exposure on increasing exacerbation risk.11,12
However, translating the current knowledge of environmental triggers of acute respiratory exacerbations to preventative clinical practice has been limited mostly to allergen avoidance in children with asthma.13 Additionally, it is crucial to understand how environmental triggers affect different races and ethnicities, given that there are known racial, ethnic, and sex disparities in the severity of both chronic symptoms and acute exacerbations.14–18 In fact, conflicting results on pollutants’ effects from different studies could be explained by racial and ethnic differences, as well as the effects of different seasons, specifically the effect modifiers of temperature and heat index.
Therefore, the objective of this study was to leverage a large collection of real-world data (RWD) from the OneFlorida Data Trust, a repository of RWD with robust linked and longitudinal patient-level data for over 15 million (> 60%) Floridians. Linking OneFlorida with a well-curated external exposome database,19,20 we analyzed triggers for asthma, bronchitis, and COPD exacerbations by air pollution and extreme temperatures taking into account seasonal, sex, and race/ethnicity variables. Our hypothesis was that the effect of particulate matter exposure on respiratory exacerbations would be modified by temperature, either in magnitude and/or directionality of effect, especially at extremes of temperature. Leveraging those big data sources, our aim is to inform future precision medicine-based approaches to mitigation of environmental triggers of asthma, bronchitis, and COPD exacerbations.
2.0. Material and Methods
2.1. Study design
A case-crossover design was used to examine the associations of asthma, bronchitis, and COPD exacerbations with exposures to elevated heat index, PM2.5, and O3 prior to the outcome (acute exacerbation). This design allows us to control for many potential individual confounders (e.g., socioeconomic status, body mass index, tobacco use), since each case serves as its own control (e.g., assuming someone smoked throughout the time of the study, their inclusion as a case and their own control then controls for their tobacco use).21–23 We defined the outcome of interest as an acute care encounter for an asthma, bronchitis, or COPD exacerbation. We used a symmetric bidirectional approach to hazard and washout periods, as this is shown in the literature to better control for confounding.24 The hazard period was defined as starting from the day when the exacerbation occurred (lag day 0) through six days preceding the event (lag day 6). To account for the changes in exposures over time, we used a symmetric bidirectional approach with a two-week wash-out period to define two control periods: 1) starting from 15 days after the exacerbation through 21 days after the exacerbation, and 2) starting from 27 days preceding the exacerbation through 21 days preceding the exacerbation (Figure I). Previous studies have used similar lags in examining pollution exposure and exacerbations of COPD and asthma, and also found a minimum of 5–7 days exposure to high amounts of PM2.5 to be significant for bronchitis exacerbations.25–27
Figure I.
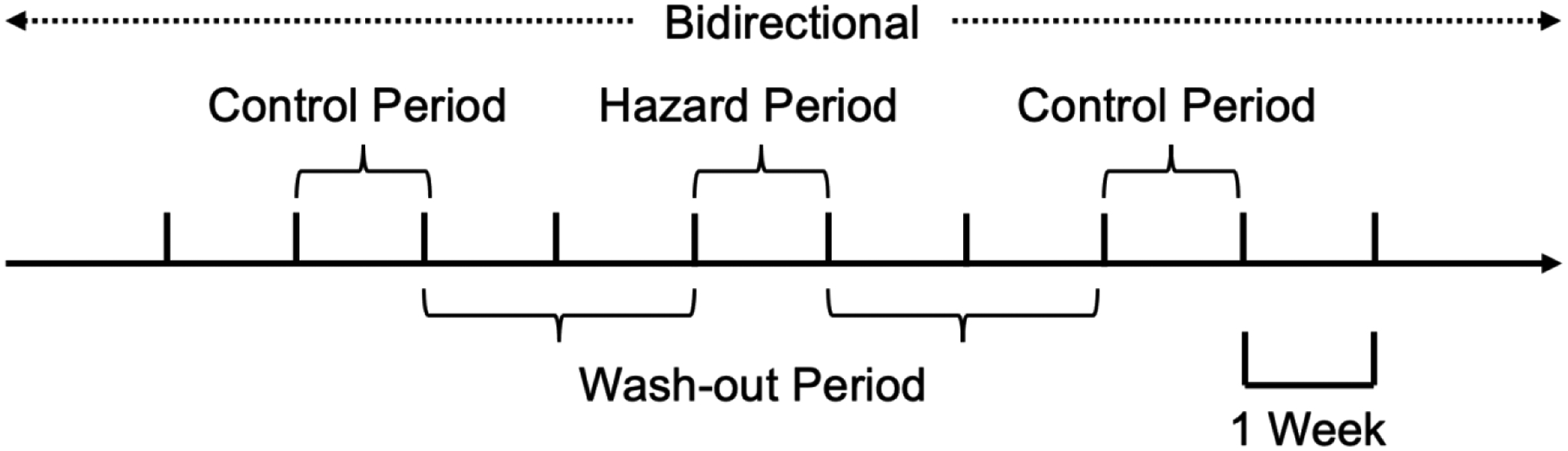
Graphical illustration of symmetric bidirectional approach to define hazard, control, and wash-out periods for each exacerbation.
Legend: Hazard Period = 6 days; Each wash-out period = 14 days; Each control period = 6 days
2.2. Study population
Electronic health records (EHR) data during 2012–2017 were obtained from the OneFlorida Clinical Research Consortium. The OneFlorida network is a clinical data research network in Florida and part of the national Patient Centered Clinical Research Network (PCORnet) funded by the Patient-Centered Outcomes Research Institute (PCORI).28 Partnered with 12 healthcare organizations, the OneFlorida network contains longitudinal patient-level EHRs linked with other data sources (e.g. administrative claims from Medicaid and Medicare (United States government health insurance programs), vital statistics, and cancer registries) of approximately 15 million individuals, covering over 60% of Florida’s population.29 OneFlorida follows the PCORnet Common Data Model (CDM) and is a Health Insurance Portability and Accountability Act (HIPAA) limited data set (i.e. except dates and locations, all other identifiers are removed). The University of Florida is the data coordinating center for OneFlorida, where the data are maintained and updated regularly with rigorous quality checks. A validated privacy-preserving record linkage process was employed to eliminate duplicate records of the same individuals obtained from different partners within the network.30
Acute care encounters for exacerbations of asthma, bronchitis and COPD were identified from OneFlorida based on the International Classification of Diseases, 9th/10th Revision, Clinical Modification (ICD-9/10-CM) codes for visits in a priori-defined settings (primary care visit, urgent care visit, ED visit, hospitalization). Codes indicating an asthma or COPD exacerbation were used for all those settings– see eTable I. If a patient had more than one encounter within the same hazard period, we counted that as one exacerbation. Unlike asthma and COPD, which have several ICD codes specific for acute exacerbations, bronchitis has very few exacerbation-specific codes. Additionally, clinicians often use the term acute bronchitis in real-world clinical settings (reflected by the OneFlorida real-world dataset) to represent non-specific bronchial inflammation, which can range from acute on chronic bronchitis, acute COPD exacerbations, or in younger patients, asthma exacerbations.31 Therefore we chose more broad bronchitis codes in combination with the selection of only healthcare encounters that would likely represent acute exacerbations of bronchitis (i.e., urgent care, ED visit, hospitalization). That approach was also used by Kowalska, et al to examine acute exacerbations of bronchitis.27 This study was approved by the authors’ institutional review board (UF IRB 202002779).
2.3. Exposure assessment
Well-validated gridded surface meteorological data, gridMET, from University of Idaho were obtained to estimate average daily temperature and relative humidity with a 2.5 arc minute (~4 km) spatial resolution.32, 33 For each 5-digit ZIP Code tabulation area (ZCTA5) in Florida, area-weighted daily average temperatures and relative humidity were calculated. To reflect the physiological effects of heat more accurately, we calculated the mean daily heat index (or apparent temperature) for each ZCTA5 using the formula from the National Weather Service based on average daily temperature and relative humidity.34 Daily PM2.5 and O3 data were obtained from the U.S. Environmental Protection Agency (EPA) and CDC National Environmental Public Health Tracking Network,35 which fuses monitoring data from the National Air Monitoring Stations/State and Local Air Monitoring Stations with 12 km gridded output from the Models-3/Community Multiscale Air Quality model. Daily estimates were obtained at the Census Tract level and area-weighted averages were calculated to estimate daily PM2.5 and O3 levels for each ZCTA5 in Florida. We then spatiotemporally linked the heat index, PM2.5 and O3 to each exacerbation based on ZIP code to generate the average exposure during the hazard and control periods.
2.4. Covariates
To assess whether the associations of extreme temperature, PM2.5 and O3 with exacerbations of asthma, bronchitis and COPD varied across patient groups, we considered age (i.e. <18 and >/=18 years which traditionally defines pediatric and adult research, clinical practice, including assessment, treatment, and risk prevention), sex (i.e. female and male), race/ethnicity (i.e. non-Hispanic Whites, non-Hispanic Blacks, and Hispanics), and neighborhood deprivation index (NDI) as potential effect modifiers.3, 36–38 The NDI is derived from 20 ZCTA5-level items from the 2013–2017 American Community Survey (ACS), reflecting wealth and income, education, occupation, and housing conditions as a summarized measure, which has been shown to reveal populations vulnerable to respiratory exacerbations.37, 39 The NDI was then spatiotemporally linked to each individual and categorized into quartiles. A higher NDI indicates a greater degree of socioeconomic deprivation, i.e., a more deprived neighborhood.
2.5. Statistical analysis
Characteristics of each exacerbation were examined. Each year was categorized into three periods: Florida’s hot period (i.e. with monthly average heat index >80°F, June to September), warm period (i.e. 70–80°F, April, May, and October), and cool period (i.e. ≤70°F, November to March). Stratified by the weather period, average heat index, PM2.5 and O3 exposures were calculated during the hazard and control periods for each outcome. We divided exposure to heat index, PM2.5, and O3 by quartile to account for potential non-linear associations between exposures and the exacerbation outcome, especially for temperature where non-linear associations are expected.40,41 Conditional logistic regression models were used to assess the associations between exacerbations and exposures of heat, PM2.5, and O3 in lag day 0 through lag day 6. Models for each exposure were adjusted for the other two exposures, with odds ratios (ORs) and 95% confidence intervals (95% CIs) reported. To examine whether the associations varied by season, we stratified the analyses by the three periods defined above. In addition, we also examined potential effect modifications by age, sex, race/ethnicity and NDI. We performed comparisons within strata (e.g., male vs. female), but not across strata and thus did not adjust for multiple comparisons. All statistical analyses were conducted using R 3.6.1 (Vienna, Austria).
3.0. Results
3.1. Sample population
A total of 1,204,312 exacerbations among 561,591 patients were identified from 2012–2017 OneFlorida data. We excluded 1,444 patients and 35,696 exacerbations with missing ZIP code information or ZIP codes outside Florida, respectively. We further excluded 18,666 hazard periods and 49,266 control periods of exacerbations with missing information on exposure. In total, 1,148,506 exacerbations among 533,446 patients were finally included in the analysis. Bronchitis had the highest number of exacerbation encounters (N=512,204; 44.6%), followed by asthma (N=351,365; 30.6%) then COPD (N=284,937; 24,8%). However, in the pediatric population, asthma exacerbations constituted 65.3% of encounters (N= 174,097). Overall, the demographics of patients with relevant encounters had a female predominance (59.1%) but was diverse with regards to race and ethnicity (25% non-Hispanic Black and 21.7% Hispanic). See Table I for complete patient demographics for the included asthma, bronchitis, and COPD exacerbation encounters.
Table I.
Characteristics of exacerbations of asthma, bronchitis and COPD in OneFlorida, 2012–2017, total n=1,148,506
| Asthma exacerbation (n=351,365) | Bronchitis exacerbation (n=512,204) | COPD exacerbation (n=284,937) | Overall | |
|---|---|---|---|---|
| n (%) | ||||
| Age at admission date (years) | ||||
| <18 | 174,097 (49.5) | 92,103 (18.0) | 290 (0.1) | 266,490 (23.2) |
| 18–29 | 41,648 (11.9) | 62,703 (12.2) | 733 (0.3) | 105,084 (9.1) |
| 30–39 | 35,187 (10.0) | 60,203 (11.8) | 4,685 (1.6) | 100,075 (8.7) |
| 40–49 | 32,130 (9.1) | 56,907 (11.1) | 23,059 (8.1) | 112,096 (9.8) |
| 50–59 | 32,026 (9.1) | 89,973 (17.6) | 86,385 (30.3) | 208,384 (18.1) |
| 60–69 | 19,282 (5.5) | 71,132 (13.9) | 81,369 (28.6) | 171,783 (15.0) |
| 70–79 | 10,157 (2.9) | 44,204 (8.6) | 50,816 (17.8) | 105,177 (9.2) |
| ≥80 | 6,838 (1.9) | 34,978 (6.8) | 37,600 (13.2) | 79,416 (6.9) |
| Missing | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) |
| Sex | ||||
| Female | 199,343 (56.7) | 317,694 (62.0) | 162,091 (56.9) | 679,128 (59.1) |
| Male | 152,021 (43.3) | 194,470 (38.0) | 122,804 (43.1) | 469,295 (40.9) |
| Missing | 1 (0.0) | 40 (0.0) | 42 (0.0) | 83 (0.0) |
| Race/ethnicity | ||||
| Non-Hispanic White | 79,573 (22.6) | 238,676 (46.6) | 175,100 (61.5) | 493,349 (43.0) |
| Non-Hispanic Black | 131,148 (37.3) | 113,024 (22.1) | 43,428 (15.2) | 287,600 (25.0) |
| Hispanic | 103,031 (29.3) | 110,256 (21.5) | 36,210 (12.7) | 249,497 (21.7) |
| Others | 16,385 (4.7) | 21,328 (4.2) | 9,471 (3.3) | 47,184 (4.1) |
| Missing | 21,228 (6.0) | 28,920 (5.6) | 20,728 (7.3) | 70,876 (6.2) |
| Regions | ||||
| Northwest | 12,450 (3.5) | 28,913 (5.6) | 18,715 (6.6) | 60,078 (5.2) |
| North Central | 24,963 (7.1) | 39,152 (7.6) | 25,842 (9.1) | 89,957 (7.8) |
| Northeast | 30,439 (8.7) | 44,419 (8.7) | 26,872 (9.4) | 101,730 (8.9) |
| Central | 112,449 (32.0) | 160,790 (31.4) | 81,082 (28.5) | 354,321 (30.9) |
| West Central | 47,232 (13.4) | 76,734 (15.0) | 47,971 (16.8) | 171,937 (15.0) |
| East Central | 19,901 (5.7) | 39,785 (7.8) | 20,216 (7.1) | 79,902 (7.0) |
| Southwest | 13,507 (3.8) | 20,221 (3.9) | 12,147 (4.3) | 45,875 (4.0) |
| Southeast | 90,424 (25.7) | 102,190 (20.0) | 52,092 (18.3) | 244,706 (21.3) |
Abbreviations: COPD, chronic obstructive pulmonary disease.
3.2. Heat Index
The average exposures to heat index, PM2.5, and O3 were very similar between hazard and control periods for asthma, bronchitis, and COPD exacerbations during the three weather periods measured for the duration of the study time period (2012–2017) (Table II). Overall, the highest average heat index was observed during June to September, while the highest exposure to PM2.5 was during November to March, and for O3, during the April, May, and October months. Given the similarity of the exposures between hazard and control periods, we then examined the association of average heat index, PM2.5, and O3 each separately with asthma, bronchitis, and COPD exacerbations, respectively, while controlling for the other two environmental variables, without taking into account those three weather periods. As continuous variables, there were no significant associations (Table III). However, examination of average heat index, PM2.5, and O3 as quartiles revealed that as average heat index rose, the odds of asthma, bronchitis, and COPD exacerbations decreased, and some of those comparisons were statistically significant (Table III). For example, increasing from the reference coldest heat index quartile, the odds of asthma exacerbations were 0.92 (95% confidence interval (CI) 0.90 – 0.93) for quartile 2, 0.87 (95% CI 0.86 – 0.89) for quartile 3, and 0.86 (95% CI 0.84 – 0.88) for quartile 4. The other significant finding shown in Table III was that as exposure to O3 increased, there was a slight increased odds of asthma exacerbations (from 1.01 (95% CI 1.00 – 1.03 for quartile 2 to 1.06 (95% CI 1.05 – 1.07) for quartile 3).
Table II.
Average exposure level of heat, PM2.5 and O3 among asthma, bronchitis and COPD exacerbations in OneFlorida, 2012–2017.
| Mean ± SD | ||||||
|---|---|---|---|---|---|---|
| Asthma exacerbation | Bronchitis exacerbation | COPD exacerbation | ||||
| Hazard period | Control period | Hazard period | Control period | Hazard period | Control period | |
| June to September | ||||||
| Heat index (°F) | 87.24±4.75 | 86.50±5.55 | 86.78±4.79 | 86.09±5.60 | 87.09±4.87 | 86.40±5.63 |
| PM2.5 (μg/m3) | 7.06±2.06 | 7.21±2.11 | 7.07±2.09 | 7.20±2.13 | 7.16±2.06 | 7.28±2.09 |
| O3 (ppb) | 30.99±5.85 | 32.10±7.18 | 31.31±5.87 | 32.44±7.30 | 31.15±5.88 | 32.28±7.31 |
| April, May and October | ||||||
| Heat index (°F) | 76.52±5.51 | 76.37±7.32 | 76.13±5.51 | 76.00±7.37 | 76.11±5.63 | 75.96±7.48 |
| PM2.5 (μg/m3) | 7.38±1.90 | 7.40±2.05 | 7.38±1.90 | 7.39±2.03 | 7.46±1.92 | 7.48±2.04 |
| O3 (ppb) | 41.69±8.21 | 40.02±8.31 | 41.91±8.14 | 40.23±8.24 | 41.79±8.22 | 40.24±8.24 |
| November to March | ||||||
| Heat index (°F) | 65.71±6.99 | 66.70±7.45 | 65.16±7.11 | 65.95±7.55 | 65.09±7.17 | 65.86±7.64 |
| PM2.5 (μg/m3) | 7.76±2.13 | 7.58±2.02 | 7.79±2.09 | 7.65±2.02 | 7.83±2.07 | 7.71±2.01 |
| O3 (ppb) | 37.61±6.21 | 37.47±6.49 | 37.47±6.28 | 37.55±6.47 | 37.45±6.30 | 37.54±6.50 |
Abbreviations: COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Table III.
Associations of exposome with asthma, bronchitis and COPD exacerbations in OneFlorida, 2012–2017, total n=1,148,506
| Adjusted OR (95% CI)a | |||
|---|---|---|---|
| Asthma exacerbation | Bronchitis exacerbation | COPD exacerbation | |
| Average Heat Index (°F) | |||
| Continuous | 0.99 (0.99, 0.99) | 0.99 (0.99, 0.99) | 1.00 (0.99, 1.00) |
| Quartiles | |||
| Quartile 1 (≤66.2) | Reference | Reference | Reference |
| Quartile 2 (66.2–73.4) | 0.92 (0.90, 0.93) | 0.95 (0.94, 0.96) | 0.97 (0.95, 0.98) |
| Quartile 3 (73.4–82.6) | 0.87 (0.86, 0.89) | 0.91 (0.90, 0.93) | 0.94 (0.92, 0.96) |
| Quartile 4 (>82.6) | 0.86 (0.84, 0.88) | 0.86 (0.85, 0.88) | 0.90 (0.88, 0.93) |
| PM2.5 (μg/m3) | |||
| Continuous | 1.01 (1.00, 1.01) | 1.01 (1.00, 1.01) | 1.00 (1.00, 1.01) |
| Quartiles | |||
| Quartile 1 (≤6.05) | Reference | Reference | Reference |
| Quartile 2 (6.05–7.27) | 1.00 (0.99, 1.01) | 1.01 (1.00, 1.02) | 1.00 (0.99, 1.02) |
| Quartile 3 (7.27–8.58) | 1.01 (1.00, 1.02) | 1.02 (1.01, 1.03) | 1.01 (1.00, 1.03) |
| Quartile 4 (>8.58) | 1.01 (1.00, 1.03) | 1.03 (1.02, 1.05) | 1.02 (1.00, 1.04) |
| O3 (ppb) | |||
| Continuous | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| Quartiles | |||
| Quartile 1 (<31.1) | Reference | Reference | Reference |
| Quartile 2 (31.1–36.4) | 1.01 (1.00, 1.03) | 0.98 (0.97, 0.99) | 0.99 (0.98, 1.00) |
| Quartile 3 (36.4–42.0) | 1.06 (1.05, 1.07) | 0.99 (0.98, 1.01) | 0.99 (0.97, 1.00) |
| Quartile 4 (>42.0) | 1.07 (1.05, 1.08) | 0.99 (0.98, 1.01) | 0.98 (0.96, 0.99) |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Models were adjusted for the other 2 exposome. ORs and 95% CIs for continuous variables were generated from continuous models, while those for categorical variables were from categorical models.
3.3. Weather Periods
We present all unstratified associations and associations stratified by NDI, age, sex, and race/ethnicity for the three environmental exposures from each of the three different weather periods for asthma, bronchitis, and COPD in eTables II, III, and IV, respectively. Across all three conditions in June to September and April, May, and October, the heat index rising from Quartile 1 to Quartile 2 to Quartile 3 conferred increasing odds of exacerbations in the unstratified analysis (eTables II–IV). Interestingly, in the extreme Quartile 4 of heat index the odds of exacerbation were often lower relative to Quartile 3. In the unstratified analysis of the November to March weather period, as heat index rose, the odds of exacerbations decreased. For example, the unstratified odds of a COPD exacerbation decreased from the quartile 1 reference to 0.63 (95% CI 0.62–0.65) in quartile 4, with all decreases by quartile statistically significant (eTable IV).
In unstratified analyses for PM2.5, increased exposure was associated with decreased odds of exacerbations for all three conditions except again during the colder weather period of November through March, when increasing exposure was associated with increased odds of an exacerbation. For unstratified analyses of O3 exposure, there was not a strong significant association with exacerbations except for the temperate April, May, and October months, where for all three diseases had increased odds of an exacerbation with increased O3 exposure.
3.4. PM2.5 & O3
Analyses stratified by age revealed differences between asthma and bronchitis exacerbations after exposure to PM2.5 in the three different weather periods (eTables II and III, grey shading), with pediatric patients’ odds of exacerbations significantly lower than adults (e.g., for asthma during June – September, pediatric odds 0.71 (95% CI 0.68–0.74) and adult odds 0.82 (95% CI 0.79–0.85). The only difference between exacerbations stratified by sex was lower female odds of bronchitis exacerbations in response to higher O3 exposure during the period of November through March (for quartile 3 odds were 0.92 (95% CI 0.91–0.94) for females and 0.98 (95% CI 0.61–1.01) for males, eTable III, grey shading). There were few statistical differences found between NDI subgroups (eTables II and III, grey shading). Interestingly, although NDI quartile 4 represents areas of high deprivation, we found decreased odds of asthma exacerbations in quartile 4 compared to quartiles 1–3 in April, May, and October (1.43 (95% CI 1.34–1.53) vs. 1.62 (95% CI 1.57–1.68)), and the same phenomenon was also observed for bronchitis exacerbations in the same weather period (eTables II and III).
3.5. Race / Ethnicity
There were several statistically significant differences in exacerbations with rising heat index for all three diseases when stratified by race and ethnicity (Figure IIa). For asthma, there were increasing odds of exacerbations for Non-Hispanic Blacks compared to Non-Hispanic Whites, and for Hispanics compared to both Non-Hispanic Whites and Non-Hispanic Blacks for the fourth heat index quartile during the April, May, and October months (Figure IIa and eTable II shaded areas). For bronchitis there were differences based off exposure to the heat index in all the weather periods, again with increasing odds of exacerbations for Non-Hispanic Blacks compared to Non-Hispanic Whites, and for Hispanics compared to both Non-Hispanic Whites and Non-Hispanic Blacks (eTable III shaded areas). For bronchitis exacerbations there were higher odds of exacerbations for Non-Hispanic Blacks compared to Non-Hispanic Whites and Non-Hispanic Blacks for quartile 3 of O3 exposure during November – March (eTable III shaded areas and Figure IIb). Lastly for COPD exacerbations there were multiple significant differences in odds of exacerbations by race/ethnicity in response to heat index and PM2.5 in various weather periods, all again with the same direction of effect – increasing odds of exacerbations for non-Hispanic Blacks and Hispanics compared to non-Hispanic Whites (eTable IV shaded areas and Figure IIc).
Figure IIa.
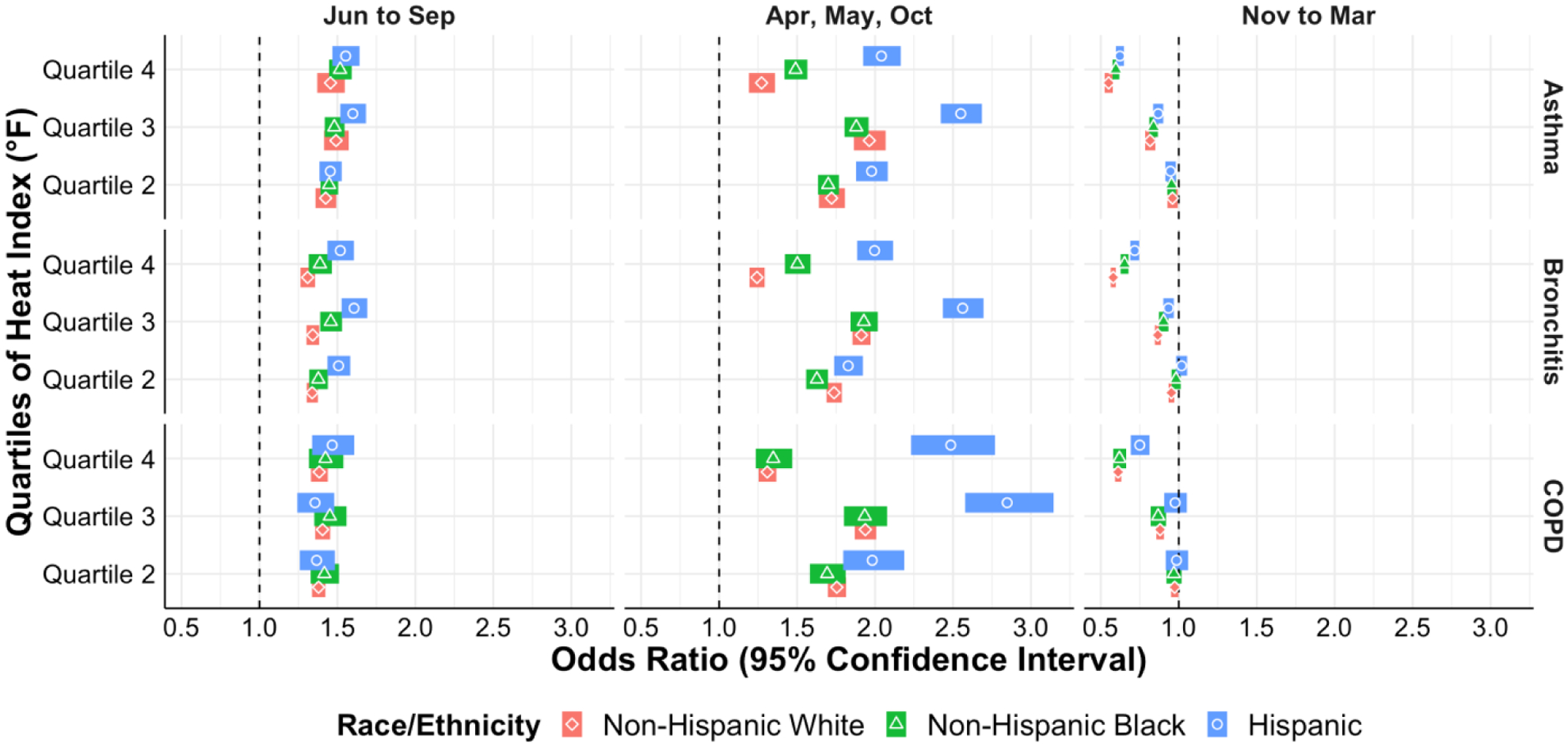
Odds of acute exacerbation of asthma, bronchitis, and COPD by heat index quartile stratified by race/ethnicity in different weather periods.
Figure IIb.
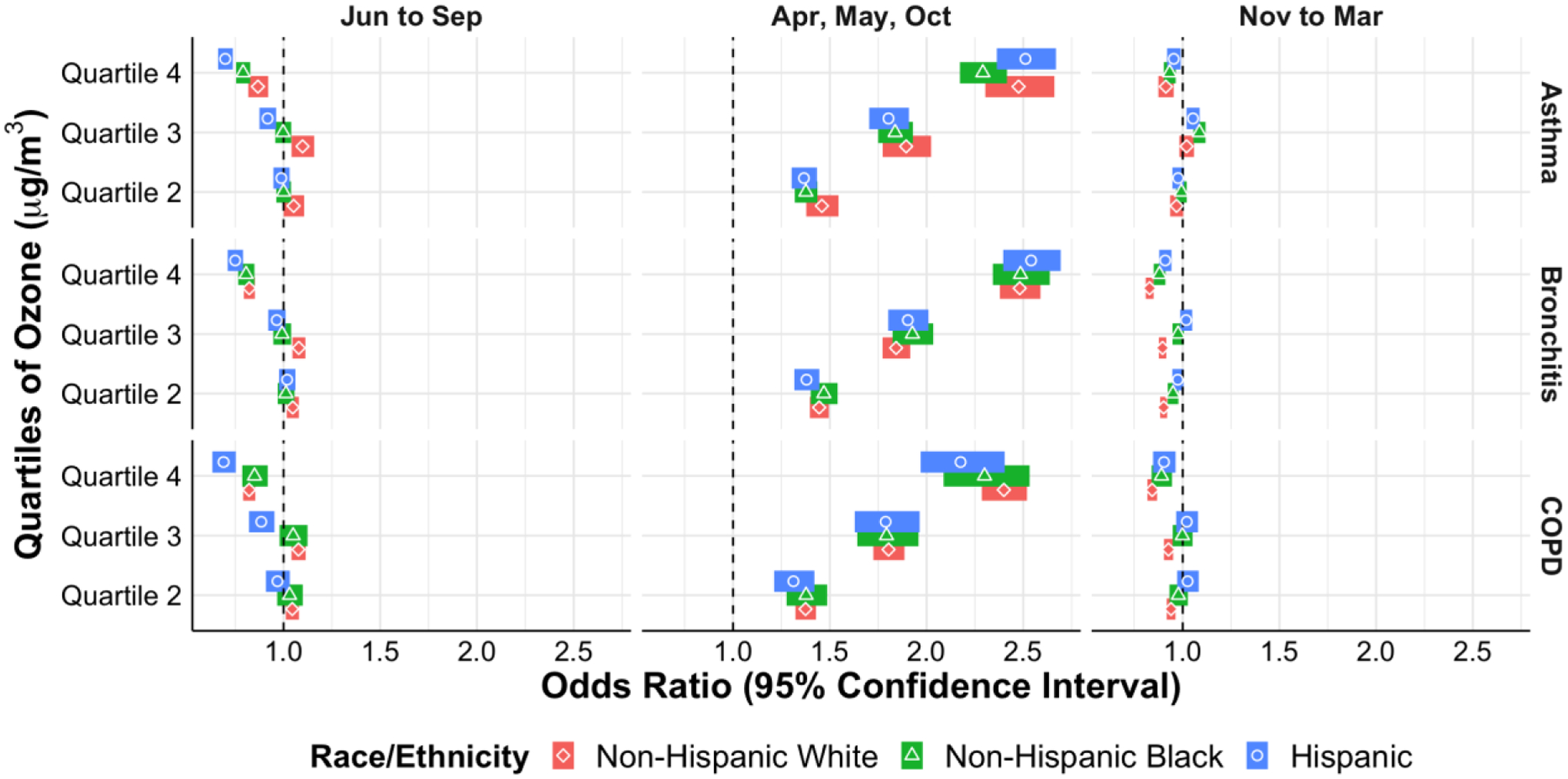
Odds of acute exacerbation of asthma, bronchitis, and COPD by O3 quartile stratified by race/ethnicity in different weather periods.
Figure IIc.
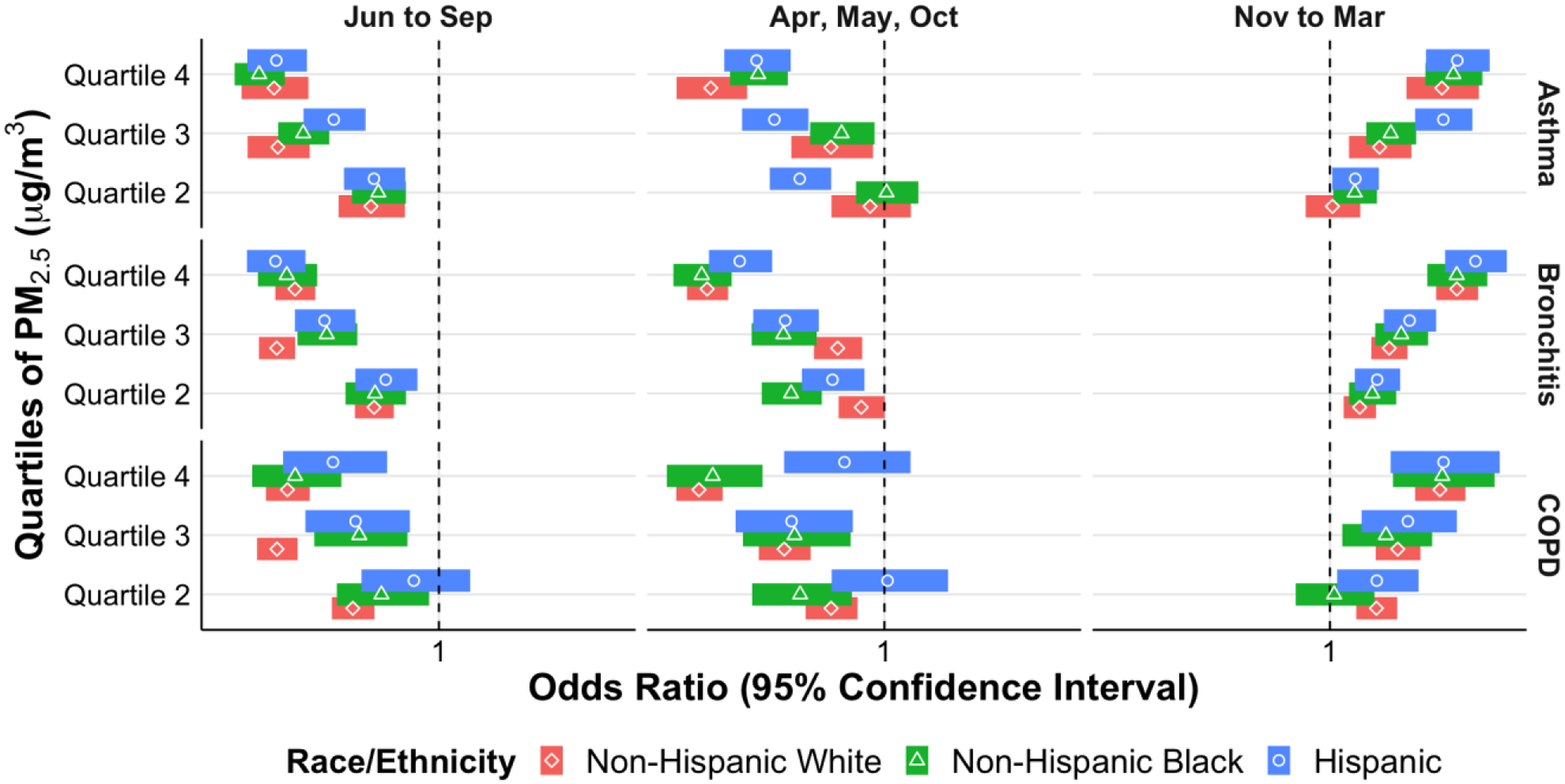
Odds of acute exacerbation of asthma, bronchitis, and COPD by PM2.5 quartile stratified by race/ethnicity in different weather periods.
4.0. Discussion
This study presents a comprehensive analysis of temperature and air pollution related to acute exacerbations for asthma, COPD, and bronchitis patients. In a diverse study population from the OneFlorida network, we found actionable information (e.g., useful for patients and clinicians) stratified by weather period, age, sex, race, and ethnicity that could aid in primary preventative care and exacerbation trigger avoidance. If validated in settings beyond Florida, these results could lead to future design of real-time or near real-time warning systems to those with chronic respiratory diseases of potentially dangerous environmental conditions with regards to heat index, PM2.5, and O3.
Our findings help clarify the potential cause of conflicting results from other studies. For example, a previous systematic review and meta-analysis found that O3 and PM2.5 exposure was associated with increased odds of an asthma-related ED visit or hospitalization (1.006–1.0009, 95% CI 1.006–1.011, and 1.023, 95% CI 1.015–1.031, respectively).3 That review did not account for seasonality or control for temperature, which we found to be particularly important in the directionality of particulate matter exposures’ effect. Specifically, we found increasing PM2.5 exposure was associated with decreased odds of asthma exacerbations (that decrease was more significant for children versus adults in the June to September period), except during November through March, where PM2.5 exposure was associated with increased odds of exacerbation (adults with more significant increases than children). Therefore, temperature may have a significant modifying (even “protective” during warmer temperatures) effect on particulate matter exposure consequences. Indeed, researchers in Taiwan found that increasing temperature had a seemingly protective effect for pediatric asthma exacerbations when examining the effects of PM2.5 exposure and that of four other pollutants.42 However, much larger geographic validation of that observation would need to be conducted before drawing conclusions.
It is well known that children have differential responses to environmental triggers than adults, and our asthma analysis stratified by age is further evidence of that.10 In addition, given that exposure to PM2.5 in Florida is much lower than that in other southeastern states,43 our findings are consistent with previous studies showing adverse health effects of PM2.5 exposure, even at very low levels.44–46 As for O3 exposure, we found association with increased odds of asthma exacerbations only in the April, May, and October months. Although O3 is an exposure of great interest, like PM2.5, its effects may be muted when considering other exposures, like temperature. Of note, a French study of 599 adults over 20 years identified 21 environmental factors associated with baseline lung function, but none were significant when controlling for multiple exposures.47 Our study controlled for other exposures and found significant associations with acute exacerbations of asthma. Future work to combine predictors of baseline pulmonary function and risk of acute exacerbations would be help take this and other more environmental research and individualize its implications to patients, caregivers, and clinicians.
To our knowledge this is the one of the largest studies of environmental triggers of COPD exacerbations. As with asthma, it has long been known that weather changes and environmental pollution, especially traffic-related air pollution, can trigger COPD exacerbations.4,6 More granular data from a case cross-over study of 168 patients with COPD from Massachusetts found strong associations of exacerbations with self-reported car/truck exhaust exposure, and more modest exposures with swings in outdoor temperature, with warmer weather less associated with exacerbations.5 Our study of 284,937 COPD exacerbation encounters revealed that higher heat index had increased association with exacerbations for the late spring, summer, and early fall months, but a decreased association during the relatively mild Florida winter. Increased heat index may be protective during colder seasons as a lower temperature at that time of year would indicate a more extreme cold, and it is widely recognized that extremely cold (and hot) temperatures are associated with exacerbations.48
Similar to COPD, this is the largest study to our knowledge of environmental triggers of acute bronchitis. Bronchitis lies on the reactive airway disease spectrum, and thus it is not surprising that we found mostly the same associations with heat index as we did with COPD and asthma. However, unlike COPD, which is a disease mostly of adults, bronchitis can afflict pediatric patients, and we found differential response by age to the effects of PM2.5 exposure, with children significantly less affected than adults during the April, May, and October months.
Of note, the associations of exacerbations with higher heat index exposure were significantly different by race/ethnicities, with Non-Hispanic Black and Hispanics having increased odds of exacerbations compared to Non-Hispanic whites. Although that effect’s statistical significance varied by month (eSupplement Tables II–IV), it was consistent in that the odds of exacerbations were always higher for Non-Hispanic Blacks and Hispanics than whites (and higher odds for Hispanics than Non-Hispanic Blacks). It is known that bronchodilator response (key to all three conditions studied because bronchodilators are first-line medications for exacerbations) varies by race/ethnicity. Various studies have found lower bronchodilator response in Non-Hispanic Black and Hispanic adults and children with asthma compared to Non-Hispanic Whites with asthma.49–51 A lower bronchodilator response can result in failure of outpatient treatment of an exacerbation, resulting in a healthcare visit that would be noted by our study. However, one would expect any difference in bronchodilator response to affect response to pollutants as well, not just increasing heat index. Yet we only found one statistically different response between Non-Hispanic Blacks and Non-Hispanic Whites in odds of bronchitis exacerbations after O3 exposure in November through March (eSupplement Table III). Other studies have found that early exposure to pollutants affects the development of asthma in minority children, therefore there may be a selection bias in the particulate matter results.52 An alternative is that total cumulative exposure to pollutants such as PM2.5 and O3 (likely to be higher in minority communities) affects epigenetic changes which result in less pulmonary resilience against heat index changes, thus when the effects of PM2.5 and O3 are controlled for (as we did in this study), racial/ethnic disparities are revealed.53, 54
Although we explored a limited number of environmental factors, this study supports the broad push to include more than just genomics in precision medicine and incorporate a host of environmental factors into future evidence-based advisements for individuals with asthma, bronchitis, and COPD.55 Specifically, our results have applications to the revolution in mHealth technology and wearable devices that measure a host of physiologic real-time variables, including silicone wristband technology that can measure environmental toxic exposures.56,57 More than current ‘pollen alerts’ and ‘heat index warnings,’ our results could provide the basis for personalized alerts for susceptible individuals based their age, sex, and race/ethnicity incorporating not just heat index and pollen, but also pollutants and the weather period. Specifically, our results showing significantly different effects in certain weather periods of heat index and PM2.5 and O3 exposure by race/ethnicity validates supports the large body of evidence of disparate impacts of respiratory diseases on Non-Hispanic Blacks and Hispanics compared to Non-Hispanic Whites for both chronic asthma and acute exacerbations.58 Further work to incorporate other external exposome factors (psychosocial, socioeconomic/social determinants of health, and other non-allergic environmental influences),59–62 combined with the internal exposome could be even more impactful in preventing exacerbations or reducing their frequency.61 Additionally, the growing body of evidence for environmental risk factors in the face of climate change could help spur future public health policy decision-making.63
This study has limitations to note. It is a study of patient encounters in Florida, and thus variations in the heat index will not be as cold or as variable from 1st to 4th quartile as other areas in the United States or the world. However, by dividing our results into three weather periods, other areas could attempt external validation of our results by comparing weather periods with similar absolute temperatures. Air pollution exposures (i.e., O3 and PM2.5) can be heterogeneously dispersed even within a smaller area (e.g., based on the location of a highway or factory), and in the absence of data on individual patient exposure to air pollution daily averages.
Additionally, we did not have information on both short-term and long-term exposure to allergens such as pollen, dust mites, and other chemicals (e.g., smoking) that can also play a role in triggering respiratory exacerbations. The hazard and washout/control periods were defined based on previous studies and our own clinical and research experience, but some patients may be symptomatic for greater than 6 days preceding an emergency encounter. Our identification of a healthcare encounter for a respiratory exacerbation was contingent upon ICD 9/10 codes by healthcare providers, which introduces a degree of uncertainty in whether the visit was truly for an exacerbation. However, we attempted to mitigate this as much as possible through our inclusion criteria which used both type of encounter (e.g., emergency department visit) and type of ICD code (e.g., ICD 9 493.92: Asthma, unspecified type, with (acute) exacerbation).
5.0. Conclusion
In conclusion, to our knowledge this is the largest study to date linking temperature and air pollution exposures to acute exacerbations of asthma, bronchitis, and COPD. We found new and nuanced information on how exposure to heat index, PM2.5 and O3 varies in magnitude and directionality by weather period, age, and especially race/ethnicity. These results represent a foundational step towards a future precision medicine beyond more static genomic data to incorporate race, ethnicity, sex, and seasonality to alert patients, caregivers, and clinicians of environmental conditions that can trigger acute exacerbations of respiratory disease, thereby decreasing future morbidity and mortality.
Supplementary Material
Highlights.
Environmental factors can trigger asthma, bronchitis, and COPD exacerbations
We used the OneFlorida clinical database has data from over 15 million Floridians
Hispanics and Blacks had higher odds of exacerbations with higher heat indices
Children had less odds of exacerbations with exposure to PM2.5 than adults
Acknowledgements:
Research reported in this publication was supported in part by R21ES032762 funded by the National Institute of Environmental Health Sciences (NIEHS); Dr. Fishe’s work in part by 1K23HL149991-01 funded by the National Heart, Lung, and Blood Institute; and in part by the OneFlorida Clinical Data Network, funded by the Patient-Centered Outcomes Research Institute #CDRN-1501- 26692; in part by the OneFlorida Cancer Control Alliance, funded by the Florida Department of Health’s James and Esther King Biomedical Research Program #4KB16; and in part by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Patient-Centered Outcomes Research Institute, its Board of Governors or Methodology Committee, the OneFlorida Clinical Research Consortium, the University of Florida’s Clinical and Translational Science Institute, the Florida Department of Health, or the National Institutes of Health.
Common Abbreviations/Acronyms:
- CDC
Centers for Disease Control and Prevention
- COPD
Chronic Obstructive Pulmonary Disease
- EHR
Electronic Health Record
- NDI
Neighborhood Deprivation Index
- RWD
Real-world Data
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to report.
Conflicts_Disclosures
All authors have no conflicts of interest or financial disclosures to declare.
6.0 References
- 1.Centers for Disease Control and Prevention. 2018. National Health Interview Survey. Available at: https://www.cdc.gov/copd/basics-about.html. Accessed March 3, 2021.
- 2.Syamlal G, Bhattacharya A, Dodd KE. Medical Expenditures Attributed to Asthma and Chronic Obstructive Pulmonary Disease Among Workers — United States, 2011–2015. MMWR Morb Mortal Wkly Rep. 2020;69:809–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X-y, Ding H, Jiang L-n, et al. Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10(9):e0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko FW, Chan KP, Hui DS, et al. Acute exacerbation of COPD. Respirology. 2016;21(7):1152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sama SR, Kriebel D, Gore RJ, et al. Environmental triggers of COPD symptoms: a case crossover study. BMJ Open Respir Res. 2017;4(1):e000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacNee W, Donaldson K. Exacerbations of COPD: environmental mechanisms. Chest. 2000;117(5 Suppl 2):390S–7S. [DOI] [PubMed] [Google Scholar]
- 7.Tackett KL, Atkins A. Evidence-based acute bronchitis therapy. J Pharm Pract. 2012;25(6):586–90. [DOI] [PubMed] [Google Scholar]
- 8.Dick S, Doust E, Cowie H, et al. Associations between environmental exposures and asthma control and exacerbations in young children: a systematic review. BMJ Open. 2014;4:e003827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanchongkittiphon W, Mendell MJ, Gaffin JM, et al. Indoor Environmental Exposures and Exacerbation of Asthma: An Update to the 2000 Review by the Institute of Medicine. Environ Helath Perspect. 2015;123:6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollenbach JP, Cloutier MM. Childhood Asthma Management and Environmental Triggers. Pediatr Clin N Am. 2015;62:1199–1214. [DOI] [PubMed] [Google Scholar]
- 11.Fisk WJ, Eliseeva EA, Mendell MJ. Association of residential dampness and mold with respiratory tract infections and bronchitis: a meta-analysis. Environ Health. 2010. November 15;9:72. doi: 10.1186/1476-069X-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brender JD, Pichette JL, Suarez L, et al. Health risks of residential exposure to polycyclic aromatic hydrocarbons. Arch Environ Health. 2003. February;58(2):111–8. [DOI] [PubMed] [Google Scholar]
- 13.D’Amato G, Ortega OPM, Annesi-Maesano I, D’Amato M. Prevention of Allergic Asthma with Allergen Avoidance Measures and the Role of Exposome. Curr Allergy Asthma Rep. 2020;20(8). DOI: 10.1007/s11882-020-0901-3. [DOI] [PubMed] [Google Scholar]
- 14.Vernon MK, Wiklund I, Bell JA, et al. What do we know about asthma triggers? A review of the literature. J Asthma. 2012;49(10):991–998. [DOI] [PubMed] [Google Scholar]
- 15.Gold DR, Rotnitzky A, Damokosh AI, et al. Race and gender differences in respiratory illness prevalence and their relationship to environmental exposures in children 7 to 14 years of age. Am Rev Respir Dis. 1993. July;148(1):10–8. [DOI] [PubMed] [Google Scholar]
- 16.Kirkpatrick dP Dransfield MT. Racial and sex differences in chronic obstructive pulmonary disease susceptibility, diagnosis, and treatment. Curr Opin Pulm Med. 2009. March;15(2):100–4. [DOI] [PubMed] [Google Scholar]
- 17.Akimbami L “Asthma prevalence, health care use and mortality: United States, 2003–05.” 2015. Available from: http://www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm. Accessed April 13, 2019
- 18.Gorina Y “QuickStats: asthma death rates, by race and age group – United States, 2007–2009. MMWR. Centers for Disease Control and Prevention. 2012. [Google Scholar]
- 19.Hu H, Zhao J, Savitz DA, et al. An external exposome-wide association study of hypertensive disorders of pregnancy. Environment international. 2020. August 1;141:105797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu H, Zheng Y, Wen X, et al. An external exposome-wide association study of COVID-19 mortality in the United States. Science of The Total Environment. 2021. January:144832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maclure M The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991; 133(2):144–53. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Zeger SL. On the equivalence of case-crossover and time series methods in environmental epidemiology. Biostatistics 2007;8(2):337–344. [DOI] [PubMed] [Google Scholar]
- 23.Janes H, Sheppard L, Lumley T. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology 2005; 16(6)717–26. [DOI] [PubMed] [Google Scholar]
- 24.Lee JT, Kim H, Schwartz J. Bidirectional case-crossover studies of air pollution: bias from skewed and incomplete waves. Environ Health Perspect. 2000;108(12):1107–1111. doi: 10.1289/ehp.001081107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pothirat C, Chaiwong W, Liwsrisakun C, et al. Acute effects of air pollutants on daily mortality and hospitalizations due to cardiovascular and respiratory diseases. J Thorac Dis. 2019;11(7):3070–3083. doi: 10.21037/jtd.2019.07.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipsett M, Hurley S, Ostro B. Air pollution and emergency room visits for asthma in Santa Clara County, California. Environ Health Perspect. 1997;105(2):216–222. doi: 10.1289/ehp.97105216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowalska M, Skrzypek M, Kowalski M, Cyrys J. Effect of NOx and NO2 Concentration Increase in Ambient Air to Daily Bronchitis and Asthma Exacerbation, Silesian Voivodeship in Poland. Int J Environ Res Public Health. 2020;17(3):754. Published 2020 Jan 24. doi: 10.3390/ijerph17030754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funding citations. (n.d.). Retrieved August 17, 2021, from https://onefloridaconsortium.org/research/funding-citations/
- 29.Shenkman E, Hurt M, Hogan W, et al. OneFlorida Clinical Research Consortium: linking a clinical and translational science institute with a community-based distributive medical education model. Academic Medicine. 2018;93(3):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleurence RL, Curtis LH, Califf RM, et al. Launching PCORnet, a national patient-centered clinical research network. J Am Med Inform Assoc. 2014;21(4):578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinkade S, Long NA. “Acute Bronchitis.” Am Fam Physician. 2016;94(7):560–565. [PubMed] [Google Scholar]
- 32.GRIDMET. Available at: http://www.climatologylab.org/gridmet.html. Accessed March 23, 2021.
- 33.Abatzoglou JT. Development of gridded surface meteorological data for ecological applications and modelling. International Journal of Climatology. 2013;33(1):121–131. [Google Scholar]
- 34.National Weather Service Heat Index Calculator. Available at: https://www.wpc.ncep.noaa.gov/html/heatindex.shtml. Accessed March 3, 2021.
- 35.US EPA. Fused Air Quality Predictions Using Downscaling. https://www.epa.gov/hesc/rsig-related-downloadable-data-files. Published 2016. Accessed 1/1/2021.
- 36.Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J. Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta-analysis. PLoS One. 2017. March 20;12(3):e0174050. doi: 10.1371/journal.pone.0174050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strickland MJ, Klein M, Flanders WD, Chang HH, Mulholland JA, Tolbert PE, Darrow LA. Modification of the effect of ambient air pollution on pediatric asthma emergency visits: susceptible subpopulations. Epidemiology. 2014. November;25(6):843–50. doi: 10.1097/EDE.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore E, Chatzidiakou L, Jones RL, et al. Linking e-health records, patient-reported symptoms and environmental exposure data to characterise and model COPD exacerbations: protocol for the COPE study. BMJ Open. 2016;6(7):e011330. Published 2016 Jul 13. doi: 10.1136/bmjopen-2016-011330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. Journal of Urban Health. 2006;83(6):1041–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Z, Crooks JL, Davies JM, Khan AF, Hu W, Tong S. The association between ambient temperature and childhood asthma: a systematic review. Int J Biometeorol. 2018. March;62(3):471–481. doi: 10.1007/s00484-017-1455-5. Epub 2017 Oct 11. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Yang J, Guo C, Chen PY, Ou CQ, Guo Y. Particulate matter modifies the magnitude and time course of the non-linear temperature-mortality association. Environ Pollut. 2015. January;196:423–30. doi: 10.1016/j.envpol.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Yu HR, Lin CR, Tsai JH, Hsieh YT, Tsai TA, Tsai CK, Lee YC, Liu TY, Tsai CM, Chen CC, Chang CH, Hsu TY, Niu CK. A Multifactorial Evaluation of the Effects of Air Pollution and Meteorological Factors on Asthma Exacerbation. Int J Environ Res Public Health. 2020. June 4;17(11):4010. doi: 10.3390/ijerph17114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Shi L, Lee M, et al. Long-term exposure to PM2. 5 and mortality among older adults in the southeastern US. Epidemiology (Cambridge, Mass.). 2017. March;28(2):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeVries R, Kriebel D, Sama S. Low level air pollution and exacerbation of existing copd: a case crossover analysis. Environmental Health. 2016. December;15(1):1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malecki K, Schultz AA, Bergmans RS. Neighborhood perceptions and cumulative impacts of low level chronic exposure to fine particular matter (PM2. 5) on cardiopulmonary health. International journal of environmental research and public health. 2018. January;15(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi L, Zanobetti A, Kloog I, et al. Low-concentration PM2. 5 and mortality: estimating acute and chronic effects in a population-based study. Environmental health perspectives. 2016. January;124(1):46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guillien A, Lepeule J, Seyve E, et al. Profile of exposures and lung function in adults with asthma: An exposome approach in the EGEA study. Environmental Research. 2020. DOI: 10.1016/j.envres.2020.110422. [DOI] [PubMed] [Google Scholar]
- 48.Kim J, Lim Y, Kim H. Outdoor temperature changes and emergency department visits for asthma in Seoul, Korea: A time-series study. Environ Res. 2014. November;135:15–20. doi: 10.1016/j.envres.2014.07.032. Epub 2014 Sep 27. [DOI] [PubMed] [Google Scholar]
- 49.Mak ACY, White MJ, Eckalbar WL, et al. “Whole-genome sequencing of pharamacogenetic drug response in racially diverse children with asthma.” Am J Respir Crit Care Med. 2018;197(12):1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padhukasahasram BK, Yang JJ, Levin AM, et al. “Gene-based association identifies SPATA13-AS1 as a pharmacogenomic predictor of inhaled short-acting beta-agonist response in multiple population groups.” Pharmacogenomics J. 2014;14(4):365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spear ML, Hu D, Pino-Yanes M, et al. “A genome-wide association and admixture mapping study of bronchodilator drug response in African Americans with asthma.” Pharmacogenomics J. 2018. DOI: 10.1038/s41397-018-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishimura KK, Galanter JM, Roth LA, Oh SS, Thakur N, Nguyen EA, Thyne S, Farber HJ, Serebrisky D, Kumar R, Brigino-Buenaventura E, Davis A, LeNoir MA, Meade K, Rodriguez-Cintron W, Avila PC, Borrell LN, Bibbins-Domingo K, Rodriguez-Santana JR, Sen Ś, Lurmann F, Balmes JR, Burchard EG. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013. August 1;188(3):309–18. doi: 10.1164/rccm.201302-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pratt GC, Vadali ML, Kvale DL, Ellickson KM. Traffic, air pollution, minority and socioeconomic status: addressing inequities in exposure and risk. Int J Environ Res Public Health. 2015. May 19;12(5):5355–72. doi: 10.3390/ijerph120505355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hajat A, Hsia C, O’Neill MS. Socioeconomic Disparities and Air Pollution Exposure: a Global Review. Curr Environ Health Rep. 2015;2(4):440–450. doi: 10.1007/s40572-015-0069-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agustí A, Bafadhel M, Beasley R, et al. Precision Medicine in Airway Diseases: Moving into Clinical Practice. Eur Respir J. 2017;50:1701655. DOI: 10.1183/13993003.01655-2017. [DOI] [PubMed] [Google Scholar]
- 56.Bui AAT, Hosseini A, Rocchio R, et al. Biomedical REAL-Time Health Evaluation (BREATHE): toward an mHealth informatics platform. JAMIA Open. 2020;3(2):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohlman D, Dixon HM, Kincl L, et al. Development of an environmental health tool linking chemical exposures, physical location and lung function. BMC Public Health. 2019;19(1):854. DOI: 10.1186/s12889-019-7217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortega VE, Meyers DA. Pharmacogenetics: implications of race and ethnicity on defining genetic profiles for personalized medicine. J Allergy Clin Immunol. 2014;133(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puranik S, Forno E, Bush A, Celedón JC. Predicting Severe Asthma Exacerbations in Children. Am J Respir Crit Care Med. 2017;194(7):854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oland AA, Booster GD, Bender BG. Psychological and lifestyle risk factors for asthma exacerbations and morbidity in children. World Allergy Organization Journal. 2017;10:35. DOI 10.1186/s40413-017-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez-Villamizar LA, Berney C, Villa-Roel C, et al. The role of socioeconomic position as an effect-modifier of the association between outdoor air pollution and children’s asthma exacerbations: an equity-focused systematic review. Rev Environ Health. 2016;31(3):297–309. [DOI] [PubMed] [Google Scholar]
- 62.Ioachimescu OC, Desai NS. Nonallergic Triggers and Comorbidities in Asthma Exacerbations and Disease Severity. Clin Chest Med. 2019;40:71–85. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian A, Khatri SB. The Exposome and Asthma. Clin Chest Med. 2019;40:107–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


